
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Immunol. , 28 February 2025
Sec. Cancer Immunity and Immunotherapy
Volume 16 - 2025 | https://doi.org/10.3389/fimmu.2025.1538635
Neutrophils, the most abundant myeloid cells in human peripheral blood, serve as the first defense line against infection and are also significantly involved in the initiation and progression of cancer. In colorectal cancer (CRC), neutrophils exhibit a dual function by promoting tumor events and exerting antitumor activity, which is related to the heterogeneity of neutrophils. The neutrophil extracellular traps (NETs), gut microbiota, and various cells within the tumor microenvironment (TME) are involved in shaping the heterogeneous function of neutrophils. This article provides an updated overview of the complex functions and underlying mechanisms of neutrophils in CRC and their pivotal role in guiding prognosis assessment and therapeutic strategies, aiming to offer novel insights into neutrophil-associated treatment approaches for CRC.
Colorectal cancer (CRC) is among the most prevalent malignant tumors worldwide, with its incidence ranking fourth and mortality ranking third, according to the latest global cancer data (1). The CRC encompasses hereditary, sporadic, and colitis-associated colorectal cancer (CAC) forms (2). Indeed, extensive epidemiological and experimental studies have consistently revealed that chronic inflammation plays a pivotal role in the initiation and progression of CRC (3–6). Depending on the stage at which inflammation affects CRC development, it can be categorized into three types: pre-tumor chronic inflammation, tumor-triggered inflammation, and treatment-induced inflammation. These processes activate tumor-promoting innate immune cells, inhibit anti-tumor adaptive immune cells, and establish an immunosuppressive tumor microenvironment (TME) (5).
Neutrophils serve as the primary line of defense against microbial infection by employing various mechanisms: (1) phagocytosis, wherein they engulf pathogenic microorganisms to form phagocytic vacuoles and generate toxic chemicals for microbial eradication (7); (2) degranulation, involving the controlled release of cytotoxic enzymes and proteases from intracellular vesicles to eliminate microorganisms (8); and (3) the formation of neutrophil extracellular traps (NETs), which are protein-DNA structures that ensnare and neutralize microorganisms (9). These processes encompass both oxidative and non-oxidative pathways that drive neutrophils to exert their antimicrobial effects with potent killing capabilities (10, 11). It has been traditionally believed that circulating neutrophils cannot proliferate after reaching maturity, leading to rapid depletion under steady-state conditions with a half-life of less than 1 day (12). However, it is important to note that neutrophils exhibit high plasticity and can transition into different functional states in various disease contexts, consequently impacting their lifespan (13). Recent studies have demonstrated that neutrophils recruited to tissues can undergo reverse migration back into the bloodstream after functioning and can even migrate to distant organs to induce inflammatory responses (14, 15).
Neutrophils not only play a crucial role in the body’s defense against infection, but also exert significant influence in tissue repair, autoimmune diseases, and tumor development (16–18). Due to the heterogeneity and plasticity of the tumor-associated neutrophils (TANs) within the TME, the precise subtype characterization of TAN has become a crucial research focus. Neutrophils can be polarized into TAN1 or TAN2 subtypes in the TME. TAN1 exhibits increased expression of immune-activating cytokines and chemokines, along with reduced arginase. While TAN2 demonstrates elevated expression of angiogenic factors, stroma-degrading enzymes, and substantial arginase, exerting immunosuppressive effects (19). The absence of widely recognized subgroup markers has hindered comprehension of neutrophil heterogeneity. Consequently, higher-dimensional techniques are needed for investigation. A study employed time-of-flight mass spectrometry to identify seven neutrophil clusters in human melanoma: the CD117+CD66b+ progenitor population and Cneut1 to Cneut6. It shows the heterogeneity in neutrophil differentiation. Notably, Cneut2 has the highest expression of CD101, CD10, and CD16, indicating its maturity (20). Furthermore, recent advancements in single-cell RNA sequencing (scRNA-seq) analysis have identified distinct TAN subsets in cancers. The four TAN subgroups in pancreatic ductal adenocarcinoma are designated as TAN1 to TAN4. TAN1 is characterized by elevated expression of VEGFA, PLAU, and LGALS3. TAN2 expresses inflammation-related genes NLRP3 and PDE4B. TAN3 is a transitional stage with high expression of transendothelial migration genes. TAN4 preferentially expresses interferon-stimulated genes, such as IFIT1, IFIT2, and ISG1 (21). The six TAN subgroups identified in primary liver cancer are Neu_01_MMP8, Neu_07_APOA2, Neu_08_CD74, Neu_09_IFIT1, Neu_10_SPP1, and Neu_11_CCL4. Subgroups Neu_09/10/11 are associated with a poorer prognosis. The expression of CD274, encoding PD-L1, is elevated in TANs, highest in the Neu_09_IFIT1 subgroup (22). To further investigate the tissue and phenotype plasticity of neutrophils, researchers integrated transcriptome data from 225 samples across 17 cancers. The analysis revealed substantial heterogeneity, characterized by ten distinct cell states, including S100A12+, HLA-DR+CD74+, VEGFA+SPP1+, TXNIP+, CXCL8+IL1B+, CXCR2+, IFIT1+, ISG15+, MMP9+, NFKBIZ+, HIF1A+, and ARG1+ neutrophils (23).
All these indicate that neutrophils are not single-function cells with terminal differentiation, but rather their differentiation and maturation trajectories can be significantly altered under specific conditions, leading to diverse functional outcomes. Here, we discuss neutrophils’ multifaceted contributions to CRC development, encompassing NET formation, interactions with gut microbiota, and intricate crosstalk within the TME. Furthermore, we discuss the inhibitory effect of neutrophils on CRC progression and their potential to guide the prognosis and treatment of CRC. We hope to provide novel perspectives for optimizing CRC treatment strategies.
Two independent researchers conducted a comprehensive search to identify the studies published in databases, such as PubMed and Embase, from January 1st, 2000, to November 30th, 2024. The following medical subject headings (MeSH) or keywords were used: “colorectal cancer,” or “colorectal carcinoma,” or “colon cancer,” or “colon carcinoma,” or “rectum cancer,” or “rectum carcinoma,” or “CRC,” and “neutrophil,” or “TAN,” or “inflammation”.
The inclusion criteria were as follows: (1) original experimental studies on the roles of neutrophils in the development of CRC, or sequencing analysis of neutrophils in CRC, or bioinformatics analysis, or meta-analysis. (2) full text in English; (3) studies with necessary ethical approvals.
The exclusion criteria were as follows: (1) studies involving human or animal subjects without ethical approval; (2) expert opinions or comments.
The above methods include the literature required for the main body of the review. In addition to CRC, we also referred to some relevant studies on other cancers to further discuss the function of neutrophils.
The classical process of intravascular neutrophil recruitment during inflammation involves five coordinated stages: tethering, rolling, adhesion, crawling, and transmigration (24). The entire process is regulated by a variety of inflammatory-related molecules, including chemoattractant subfamilies (e.g., chemokines and their receptors, chemotactic lipids, complement anaphylatoxins), cytokines, integrins, and cell adhesion molecules (24–26). In addition to the classical molecules mentioned above, a recent study discovered that neutrophil surface RNAs with glycan modifications facilitate neutrophil recruitment and promote neutrophil-endothelial interactions by binding to P-selectin on endothelial cells (27).
Tumor progression is always regarded as a chronic inflammatory process (28, 29), during which the recruitment pattern of neutrophils is consistent with physiological inflammation. Chemotactic compositions present in the TME mainly include CXCL8 family chemokines (e.g., CXCL1/2/3/5/6/7/8) (30), cytokine interleukin (e.g. IL-1β, IL-8, IL-17) (31–33), and the associated signaling pathways (e.g., TGF-β/Smad3) (34), responsible for neutrophil recruitment and polarization. In CRC, chemokines (e.g., CXCL1/2) and cytokines (e.g., IL-8, and TGFβ) (35–37) are also involved in regulating neutrophil enrichment to the TME and facilitating their polarization towards TAN2 phenotype, while IL-22 plays a role in recruiting beneficial neutrophils (38).
Neutrophils are increased in peripheral blood and tumors of patients with CRC, as compared to healthy donor peripheral blood and paired adjacent non-tumor colon tissue, and are associated with higher cancer stage and histological grade (39). The phenomenon has been found that ulcerative colitis patients with more neutrophil infiltration are at a higher risk of developing CAC (40). In both colitis and CAC models, neutrophil infiltration increased as the disease progressed, and neutrophils may promote the transition from colitis to CAC (40). The above studies suggest neutrophils are important in the development and progression of CRC.
NETs are extracellular network structures consisting of decondensed chromatin, histones, and granzymes (41). NETs serve to capture and eliminate extracellular pathogens, thereby contributing to the body’s defense against infections (42). Emerging research indicates that NETs also play an important role in non-communicable diseases, encompassing autoimmune diseases, allergic diseases, blood clotting disorders and cancers (43). Notably, NETs possess potent tumorigenic properties and contribute to the initiation, spread, and thrombotic complications associated with cancer (44). An Ewing sarcoma study first demonstrated that activation of intratumor TANs resulted in NETs releasing, which was associated with a poor prognosis (45). NETs can capture circulating tumor cells and wake up tumor cells in vivo (46–49). Indeed, NETs promote cancer progression through direct promotion of cell growth and facilitating tumor metastasis in CRC (Figure 1).
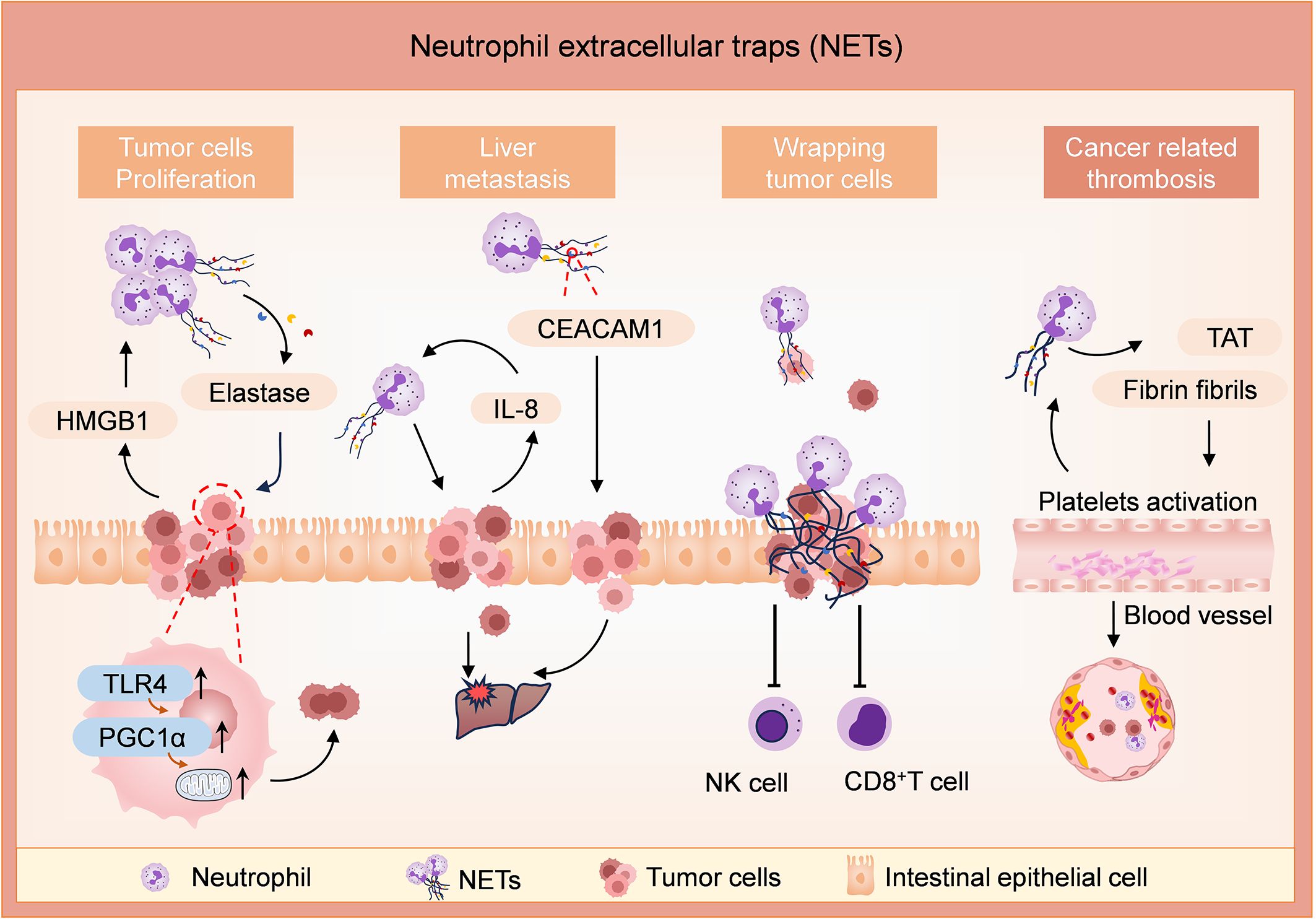
Figure 1. Roles of NETs in tumor development, metastasis, and thrombogenesis in CRC. Levels of NETs are enhanced by tumor-derived damage-associated molecular pattern proteins (such as HMGB1). NETs release elastase and promote tumor cell proliferation by enhancing their mitochondrial biogenesis in the TLR4/PGC1α-pathway. In addition, NETs enhance liver metastases in an IL-8 or CEACAM1-dependent way. The NET formation is enhanced by tumor cells-derived IL-8. CEACAM1, anchored on NETs, facilitates the adhesion and migration of tumor cells by weakening endothelial connections. NETs create an immunosuppressive niche and protect tumor cells from cytotoxicity mediated by CD8+ T cells and NK cells. NETs induce thrombogenesis by increasing the expression of TAT complexes and fibrinogen fibrils, and the procoagulant environment enhances the levels of NETs in turn.
NETs can directly modulate the metabolic program of tumor cells in murine models of metastatic CRC. Under a hypoxia state, tumor cells in metastatic CRC models release damage-associated molecular pattern proteins, such as HMGB1, which recruit neutrophils to the TME and trigger NET formation. Then, neutrophil elastase released by NETs activates TLR4 on tumor cells, leading to the upregulation of PGC1α, enhanced mitochondrial biogenesis, and accelerated tumor cell proliferation (50).
NETs promote liver metastasis in CRC in vivo and in vitro. A study showed that NETs were frequently detected in CRC tissue slices (37/85, 44%). It revealed a preferential localization of NETs in the central or front of invasion within CRC tissue sections and a significant correlation with tumor grade and lymph node metastasis. The purified NETs induce filamentous foot formation and cell migration in CRC cell lines, suggesting their potential to activate epithelial-mesenchymal transition-like processes and promote progression and metastasis in CRC (51). In vivo and in vitro investigations discovered that NETs promote liver metastasis in CRC by inducing the expression of IL-8 in tumor cells. Overexpression of IL-8 causes the activation of neutrophils to release more NETs, which further promotes liver metastasis (52). CEACAM1 is an important molecule on NETs, facilitating the adhesion and migration of colon tumor cells at metastatic sites by mediating the interaction between tumor cells and NETs, as well as weakening endothelial connections (53). NETs create an immunosuppressive niche to facilitate the growth and translocation of the tumor. NETs directly protect the tumor cells from the immune cells by shielding tumor cells from cytotoxicity mediated by CD8+ T cells and natural killer (NK) cells (54). In the mouse CRC model, NETs have been shown to cause dysfunction of T cell metabolism and function via the PD-1/PD-L1 signaling pathway, ultimately promoting tumor growth (55). Thus, NETs contribute to the development of CRC tumors in several ways.
Beyond the roles in the development and metastasis of CRC, NETs are involved in the formation of thrombin antithrombin (TAT) and blood vessels, thereby causing adverse clinical outcomes. There are few studies on how NETs affect angiogenesis in CRC. During angiogenesis, NETs support human endothelial cells’ proliferation and tubular capacity in vitro (56, 57). NETs in CRC individuals significantly increase the formation of TAT complexes and fibrinogen fibril, inducing endothelial cells to exhibit a procoagulant phenotype, resulting in enhanced platelets procoagulant activity by inducing phosphatidylserine exposure to platelets (58). Furthermore, platelets from patients with CRC stimulate healthy neutrophils to produce NETs (58). Thus, inhibition of NET is an important strategy for preventing tumor-related thrombosis in CRC patients.
The majority of studies have highlighted the pro-tumor effects of NETs but also show negative effects during tumor chemotherapy. For instance, research has demonstrated that chemotherapy-induced IL-1β can trigger NET formation, which subsequently activates the TGF-β signaling pathway in tumor cells, promoting epithelial-mesenchymal transition and chemotherapy resistance in breast cancer lung metastasis (59). However, a recent study has revealed an unexpected outcome: chemotherapy-induced NETs can inhibit CRC tumor growth. The combination of the glutaminase inhibitor CB-839 and 5-fluorouracil induces IL-8 expression in PiK3CA-mutated CRC cells, attracting neutrophils to tumor tissues, inducing NET formation and releasing Cathepsin G. Cathepsin G enters tumor cells through the cell surface protein RAGE, leading to mitochondrial translocation of pro-apoptotic BAX and triggering apoptosis in tumor cells (60). This study significantly enhances our understanding of the complex roles of neutrophils and NETs in anti-cancer therapy.
Previous studies have established a correlation between dysregulation of the gut microbiota and the development of CRC. The gut microbiota, closely associated with CRC, constitutes a crucial component of the TME. Certain bacteria, such as Fusobacterium nucleatum (F. nucleatum), Escherichia coli, and Peptostreptococcus spp, are presumed to be pro-carcinogenic in CRC and were found to be abundant in patients, while potentially protective bacteria, including Roseburia, Clostridium, and Bifidobacterium were decreased (4). Research has indicated that the gut microbiota can impact the development of CRC by releasing various metabolites, proteins, and macromolecules that interact with colon epithelial cells and immune cells (61).
Neutrophils, as an integral part of immune cells, are known to play a significant role in the onset and progression of CRC through their interactions with the gut microbiota (Figure 2). Using fluorescence in situ hybridization and double RNA sequencing technique, researchers also discovered a novel association between Bacteroides fragilis and neutrophil infiltration in CRC (62). CRC is more prevalent in individuals with obesity (63, 64). Indeed, A recent study demonstrated an enrichment of neutrophils in visceral adipose tissue (VAT) and the presence of bacteria, primarily Streptococcaceae and Ruminococaceae, in the VAT of obese individuals. It has been revealed that obesity-induced translocation of gut microbiota leads to infiltration of neutrophils in VAT, followed by activation by bacteria or their metabolites, resulting in pro-inflammatory and anti-apoptotic functions (65). Peptostreptococcus anaerobius has been found to promote the development of CRC and modulate tumor immunity. In ApcMin+/+ mice treated with Peptostreptococcus anaerobius, myeloid suppressor cells, tumor-associated macrophages, and TANs were significantly expanded, which are linked to chronic inflammation and tumor progression (66). Invariant natural killer T cells (iNKTs) represent an evolutionarily conserved subset of lymphocytes situated at the interface between innate and adaptive immunity. The study revealed that tumor-infiltrating iNKTs promoted tumorigenesis in CRC patients. F. nucleatum can induce iNKTs cell-mediated recruitment of neutrophils, which inhibit the adaptive immune response in cancer, by expressing IL-17 and GM-CSF (67). F. nucleatum infection has been shown to activate TGF-β, a critical signaling pathway that regulates TAN differentiation (68). Current findings indicate that CRC with a high abundance of F. nucleatum have increased neutrophil counts and elevated levels of NETs. F. nucleatum induces the formation and release of extensive NETs by activating TLR4-ROS and NOD1/2 signaling, thereby promoting the growth and metastasis of CRC (69).
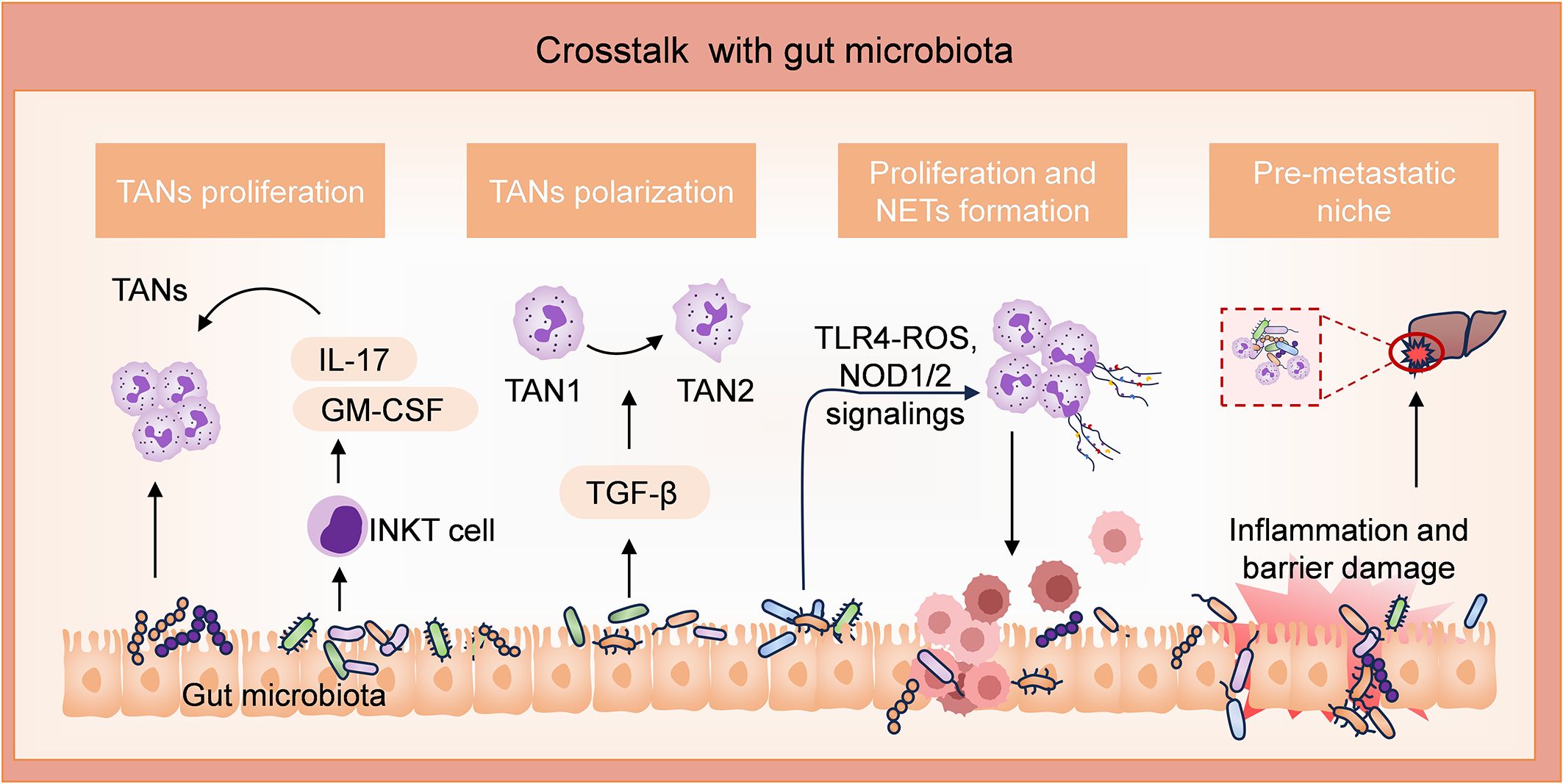
Figure 2. Involvements of neutrophils through interaction with gut microbiota in CRC. Peptostreptococcus anaerobius (P. anaerobius) and Fusobacterium nucleatum (F. nucleatum) possess pro-tumor activities. P. anaerobius promotes the proliferation of TANs. F. nucleatum enhances the expression of iNKT cell-derived IL-17 and GM-CSF, which further promotes the expansion of TANs. F. nucleatum also facilitates the differentiation of TANs to TAN2 by activating the TGF-β pathway. Furthermore, by activating TLR4-ROS and NOD1/2 signalings, F. nucleatum induces the formation and release of extensive NETs, thereby promoting tumor growth and metastasis. In addition, high levels of inflammation induced by the gut microbiota facilitate liver metastasis niche by compromising the intestinal barrier.
Bacteria translocation not only facilitates the proliferation of tumor cells at the primary site but also enhances tumor metastasis. Various bacterial strains have been demonstrated to activate innate and adaptive immune cells, thereby amplifying inflammation at the primary site and compromising intestinal barrier function (70). After damaging the barrier function of the gut, bacteria migrate from the lumen through the blood circulation to the liver, promoting the recruitment of neutrophils in metastatic lesions and establishing a pro-inflammatory immune microenvironment conducive to pre-metastatic niche formation in the liver (71, 72).
The TME consists of cellular components, including tumor cells, immune cells, endothelial cells, neurons, etc., as well as extracellular components such as extracellular matrix, extracellular vesicles, and chemokines (73–75). Further study showed that the TME was classified into five distinct states based on the scRNA-seq analysis: immune activation, immune suppression mediated by myeloid or stromal cells, immune exclusion, and immune residence phenotypes (22). Crosstalk between neutrophils and various components in the TME contributes to creating a favorable milieu for tumor initiation and advancement (Figure 3).
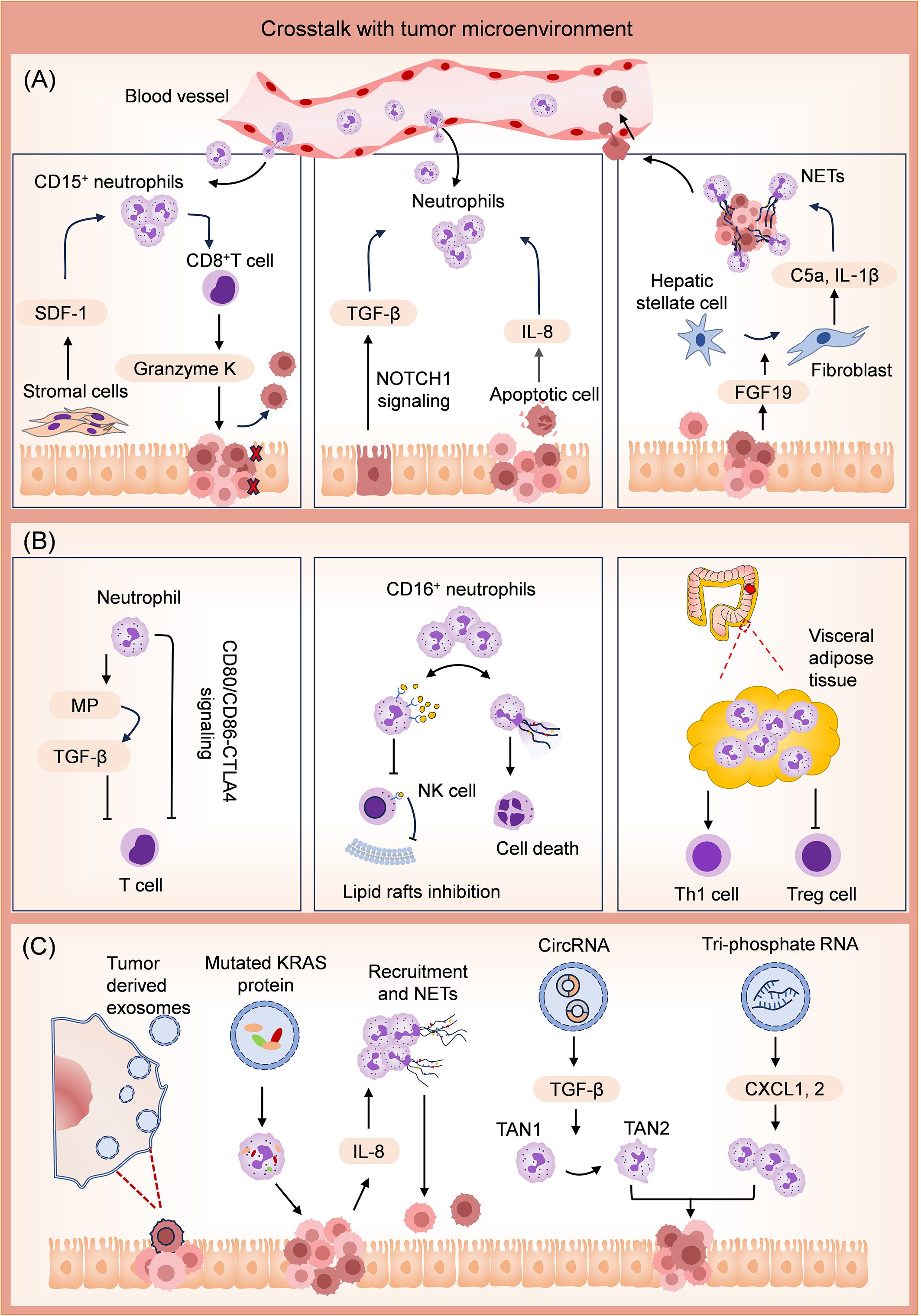
Figure 3. Mechanisms for tumor promotion effects of neutrophils through crosstalk with the TME. (A) Crosstalk with several types of cells in TME. The stromal cells facilitate the accumulation of CD15+ neutrophils by releasing stromal cell-derived factor-1 (SDF-1). In this case, CD8+ T cells produce Granzyme K, diminishing E-cadherin on the intestinal epithelium. Activation of Epithelial NOTCH1 promotes TGFβ-dependent neutrophil recruitment. Apoptotic tumor cells release several neutrophil chemokines and attract neutrophils into the tumor. Tumor cells promote polarization of hepatic stellate cells into inflammatory cancer-associated fibroblasts by releasing fibroblast growth factor 19 (FGF19). These fibroblasts facilitate colonizing tumor cells in the liver by promoting neutrophil infiltration and NET release in liver metastasis niches. (B) Crosstalk with other immune cells in TME. Neutrophils suppress the activities of T cells by releasing metalloproteinase (MP) to activate the TGF-β. Neutrophils also promote T-cell exhaustion via CD80/CD86-CTLA4 signaling. CD16+ neutrophils competitively inhibit cholesterol intake of NK cells by activating the CD16/TAK1/NF-κB axis. Moreover, CD16+ neutrophils can directly induce NK cell death by releasing NETs. In visceral adipose tissue, neutrophils upregulate the level of proinflammatory CD4+ Th1 cells and reduce the level of anti-inflammatory Treg cells. The above activities of neutrophils promote the formation of an immunosuppressive TME. (C) Crosstalk with the tumor cell-derived exosomes. Exosomes can transfer mutated KRAS protein into neutrophils, stimulate IL-8 secretion of CRC cells, promote neutrophil recruitment, and induce NET formation. Exosomal circRNA promotes the differentiation of TAN1 to TAN2 neutrophils. Exosomal tri-phosphate RNA has been discovered to attract neutrophils through chemokine CXCL1 and CXCL2 secretion.
Communication between various cells in the TME and neutrophils promotes CRC development. The stromal cell-derived factor-1 (CXCL12/SDF-1) facilitates the accumulation of neutrophils expressing high levels of CD15 in CRC tumors, in which case, CD8+ T cells produce high levels of Granzyme K, subsequently diminishing E-cadherin on the intestinal epithelium and promotes tumor progression (76). Epithelial NOTCH1 signaling drives metastasis through TGFβ-dependent neutrophil recruitment, which always leads to poor prognostic human CRC subtypes (CMS4 and CRIS-B) (77). Apoptotic tumor cells release a number of neutrophil chemokines, such as IL-8, which strongly attract neutrophils into the tumor (52). The neighboring macrophages facilitate neutrophil activation to accelerate the development of an immunosuppressive TME, potentially contributing to tumor recurrence after chemotherapy-induced apoptosis (35). FGF19 has been identified as a tumorigenic gene in human cancers (78–80). It activates the autocrine of IL-1α through the FGFR4-JAK2-STAT3 pathway, leading to the polarization of hepatic stellate cells into inflammatory tumor-associated fibroblasts (81). These fibroblasts play a role in promoting neutrophil infiltration and NET release in liver metastasis niches by secreting complement C5a and IL‐1β, thereby facilitating the colonization of CRC cells (81). In addition, TANs enhance the migration of CRC cells through CD98hc-xCT via secreting anterior gradient-2 (82).
Neutrophils also influence the state of other immune cells, forming an immunosuppressive TME. T cell‐mediated anti-tumor immune response correlates with favorable disease outcome in cancer immunotherapy. High level of T cell infiltration in CRC is the basis of favorable immunotherapy response (83, 84). Neutrophils from CRC patients suppressed T cell activity via activation of latent TGFβ by neutrophil‐secreted metalloproteinase for creating an immunosuppressive TME in vitro (85). Sui et al. also discovered that in high microsatellite instability (MSI-H) CRC, increased neutrophil infiltration promotes T cell exhaustion by activating CD80/CD86-CTLA4 signaling, which is associated with an immunosuppressive status (86). Activation of the CD16/TAK1/NF-κB axis in CD16+ neutrophils up-regulates scavenger receptors for cholesterol intake, thereby enabling these neutrophils to block the formation of NK lipid rafts and transduction of anti-tumor signals by competitively inhibiting cholesterol intake of NK cells. Furthermore, it was found that CD16+ neutrophils can directly induce NK cell death by releasing NETs (87). Transcriptome analysis revealed elevated levels of inflammation-related factors and antiapoptotic proteins in VAT neutrophils of obese individuals with CRC. The increase in VAT neutrophils was subsequently accompanied by an upregulation of proinflammatory CD4+ Th1 cells and a decrease in anti-inflammatory Treg cells (65).
Exosomes derived from CRC cells are effective vectors for regulating neutrophil activity. Exosomes loading with mutated KRAS protein can transfer mutated KRAS protein into neutrophils and stimulate IL-8 secretion of CRC cells, thereby accelerating neutrophils recruitment and NET formation, eventually leading to CRC deterioration (88). CircPACRGL is an exosomal circular RNA, promoting differentiation of TAN1 to TAN2 neutrophils via miR-142-3p/miR-506-3p-TGF-β axis, further promoting CRC cells proliferation, migration and invasion (89). Exosomal tri-phosphate RNA, derived from CRC stem cells, has been discovered to attract neutrophils through secretion of CXCL1 and CXCL2, as well as to prolong the survival of neutrophils by inducing the expression of IL-1β via the NF-κB pathway (90).
The direct carcinogenic effect of neutrophils in CRC is associated with generating reactive oxygen species. Excessive H2O2 from myeloid cells triggers the mutation of genomic DNA and promotes the metaplasia of intestinal epithelial cells. H2O2 also induces intestinal epithelial cells to secrete cytokines and chemokines through the TNFα autocrine ring to recruit myeloid cells and form positive feedback conducive to tumor development (91).
Neutrophils play a role in modulating the landscape of DNA repair. In low-grade CRC, neutrophils promote deficiency in homologous recombination by down-regulating RAD51 in a Mir-155-dependent manner, thereby impeding tumor growth. However, in advanced CRC, neutrophil-mediated genotoxicity resulting from the accumulation of double-strand breaks induces non-homologous end-joining repair, facilitating tumor survival and proliferation (92).
The angiogenic functions of TANs are primarily associated with two well-established pro-angiogenic factors, VEGF and MMP9 (93–95). The transcriptomic study revealed the enrichment of pathways conducive to angiogenesis and development in TANs. Bioinformatics analysis and functional validation demonstrated that osteopontin and Mmp14, another two angiogenic factors, exhibit the most significant induction effect, effectively regulating endothelial cell activity in CRC. The CRC niche drives pro-angiogenic transcriptional programming in TANs (96), offering a novel strategy for CRC treatment based on the pro-angiogenic properties of TANs.
Most studies on the role of neutrophils in tumors have primarily reported their promotion of tumor development; however, they indeed possess anti-tumor functionality in CRC (Figure 4). This is intricately associated with the complex TME, tumor stage, and neutrophil heterogeneity.
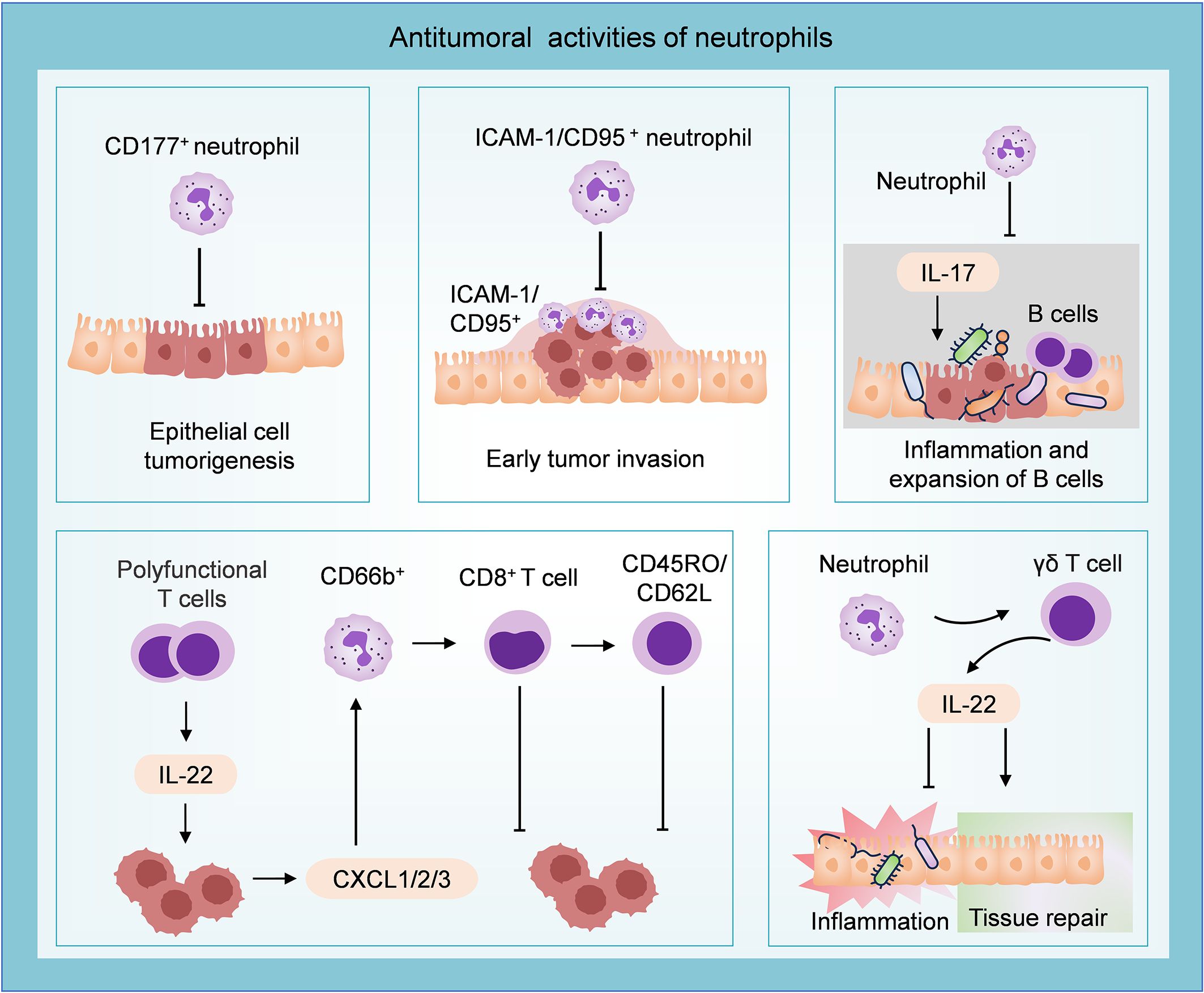
Figure 4. Antitumoral role of neutrophils in CRC. CD177+ neutrophils can inhibit epithelial cell tumorigenesis. Neutrophils characterized by ICAM-1 and CD95 infiltrate the invasive edge and restrain the development of early colon tumors. Neutrophils inhibit tumor growth by suppressing IL-17-mediated bacteria-dependent inflammatory response and the expansion of B cells. T cell-derived IL-22 stimulates the secretion of CXCL1/2/3 from tumor cells, then recruits CD66b+ neutrophils. These CD66b+ neutrophils inhibit tumor progression by augmenting the CD8+ T cell response and expanding the “central memory” population. Neutrophils could modulate the gut microbiome-related intestinal inflammation and activate an IL-22-dependent tissue repair approach.
Neutrophils possess high plasticity and can transit into different anti-tumor roles. Studies have demonstrated a significant increase of CD177+ neutrophils in CAC and CRC tissues. Furthermore, patients with high infiltration of CD177+ neutrophils (>8 cells/HPF in CRC sections) exhibited improved 5-year overall survival (OS, 80.4% versus 69.3%, P = 0.007) and 5-year disease-free survival (DFS, 81.7% versus 71.6%, P = 0.010). Subsequent investigation confirmed the ability of CD177+ neutrophils to inhibit epithelial cell tumorigenesis (97). Additionally, a study reported that TANs with high levels of ICAM-1 and CD95 displayed an anti-tumor phenotype and were found to infiltrate the invasive edge of early colon tumors, suggesting their potential role in combating the disease at an early stage (98). The strong colocalization of HLA-DR+ neutrophils and CD8+ T cells in COAD indicates a spatial association between antigen-presenting neutrophils and T cells. HLA-DR+ neutrophils can widely trigger T cell activation, antigen reactivity, and cytotoxicity, promoting T cell response and playing a synergistic role in immunotherapy (23). Transcriptome analysis revealed dysregulation of genes involved in antimicrobial and inflammatory processes in neutrophil-deficient colon tumors. The mechanism study demonstrated that polymorphonuclear neutrophils in early colon tumors inhibited the proliferation and aggressiveness of tumor cells by suppressing IL-17-mediated bacteria-dependent inflammatory response and the expansion of B cells in the colon (99).
IL-22 is generated by various immune cells in innate and adaptive immune systems, including group 3 innate lymphoid cells, NK cells, and CD8+ T cells (100, 101). Combined survival analysis revealed that the protective performance of IL-22 in CRC relied on the presence of neutrophils. IL-22 can stimulate the secretion of chemokines CXCL1, CXCL2, and CXCL3 by colon tumor cells for recruiting neutrophils. IL-22 augmented the T cell response through the recruitment of beneficial neutrophils (CD66b+ cells) and contributed to favorable 5-year OS (58% versus 43%, P = 0.004) (38). Co-cultivation with CD66b+ neutrophils enhanced the response of CD8+ T cells to the T cell receptors, leading to increased activation and proliferation of CD8+ T cells, and expanding the population of cells exhibiting the “central memory” phenotype characterized by CD45RO/CD62L expression (102). A recent study has demonstrated that neutrophil-deficient mice (Csf3r−/− mice) showed higher susceptibility to carcinogenesis. Neutrophils may confer protection against intestinal inflammation and CAC by modulating the gut microbiome and initiating activation of an γδ T cells-derived IL-22-dependent tissue repair approach (103). IL-22 has been demonstrated to accelerate the development of CRC (104–106), which contradicts the previously mentioned association between IL-22+ cell infiltration and improved prognosis. This indicates that the interaction between neutrophils and IL-22 within the established TME exerts multifaceted effects on tumor progression.
Given the multifaceted roles of neutrophils in the pathogenesis and progression of CRC, this review provides several novel insights for developing clinical treatment strategies for CRC patients. Neutrophils are essential components of the host’s defense against infection (24), and their depletion could result in significant immunosuppression. Although existing studies indicate that neutrophils contribute to tumor progression, clinical trials targeting neutrophils remain limited due to concerns about inducing neutropenia. The lack of widely recognized subgroup markers hinders a comprehensive understanding of the heterogeneity of neutrophils, making therapies targeting specific subgroups of neutrophils difficult. Therefore, most research has focused on inhibiting neutrophil-associated molecules known to promote tumor growth and aggressiveness (107). Therefore, we mainly review the clinical studies of key molecules or pathways related to neutrophil function. While not directly depleting neutrophils, these interventions can elucidate the effects of neutrophil-associated targeted or combination therapies on CRC, highlighting their potential to guide clinical therapies.
The importance of the CXCR1/2-IL8 axis in neutrophil chemotaxis and the multiple pro-tumor functions mediated by this axis (35–37) make CXCR1/2-IL8 a desirable therapeutic target. Other classical cytokines such as G-CSF, TGF-β, and VEGF are closely related to the functional status of neutrophils during tumor development (68, 108, 109) and are also important therapeutic targets in CRC treatment. Researchers have found that neutrophils from CRC samples strongly express Bv8/PROK2, while inhibition of G-CSF or Bv8/PROK2 can improve the efficacy of anti-VEGF antibodies and prevent the emergence of drug resistance. The G-CSF/Bv8/PROK2 axis holds potential as a therapeutic target for CRC when combined with anti-VEGF agents (110). In both colitis and CAC models, neutrophil infiltration and pSTAT3 expression increased as the disease progressed. This suggests that neutrophils may promote the transition from colitis to CRC through the JAK/STAT pathway (40). Several clinical trials have explored these targets in depth (Table 1).
Studies have shown neutrophils participate in the inhibitory immune microenvironment through the CD80/CD86-CTLA4 pathway, resulting in poor response to immune checkpoint inhibitors (ICIs) in MSI-H patients. In a mouse CRC model, blocking the CD80/CD86-CTLA4 axis under inflammatory conditions improves tumor response to PD-1 blocking (86). The PD1/PD-L1 axis is commonly associated with immune escape and tumor progression. Studies of mouse breast cancer have revealed that tumor cells expressing PD-1 inhibit the cytotoxicity of neutrophils and enhance their metastatic potential through the PD-L1/PD-1 axis. PD-L1+ neutrophils are less cytotoxic than PD-L1 neutrophils, and blocking the PD-L1/PD-1 interaction can enhance the anti-tumor cytotoxicity of neutrophils (111). PD-L1+ neutrophils in HCC patients can effectively inhibit the proliferation and activation of T cells, and blocking PD-L1 can partially reverse this inhibition (112). In a mouse model of CRC, tumor-derived G-CSF induces neutrophils to express PD-L1 through the STAT3 pathway, and neutrophils promote tumor by inhibiting anti-tumor immunity of NK cells dependent on PD-L1/PD-1. Neutrophil depletion combined with anti-PD-L1 can improve the survival of the mice with CRC (113). These strongly suggest that the PD1/PD-L1 axis is an important therapeutic target under conditions of neutrophil infiltration, and targeting TANs may be an important strategy to prevent PD1/PD-L1-associated tumor immune evasion.
Other functions of TANs are currently under investigation in preclinical studies. PAD4 expressed in neutrophils plays an important role in forming NETs (114). Studies on nude mouse xenograft models have shown that GSK484 (PAD4 inhibitor) can improve the radiosensitivity of CRC, inhibit NET formation, and inhibit tumor growth (115). F. nucleatum infection can induce PD-L1 expression by activating the NF- κB/STAT3 signaling in neutrophils, and CX3CR1+PD-L1+ neutrophils infiltration promotes CRC metastasis and weakens the efficacy of immunotherapy. Treatment with doxycycline eliminated F. nucleatum intracellular, thereby reducing CX3CR1+PD-L1+ neutrophils populations and slowing F. nucleatum-promoted tumor growth and metastasis in mice (116). This suggests that the combination of antibiotics and ICIs may benefit such patients.
It should be noted that it has been reported that the combination of anti-TGF-β and anti-PD-L1 therapies may lead to tumor resistance, which has been linked to the upregulation of CR-related metabolic pathways in mice (117). It is recommended that researchers and clinicians should employ a multifaceted approach to assess patient status and develop a precise medical strategy when formulating combination drug regimens targeting TAN.
Multivariate survival analysis found that the high levels (more than 60 per TMA spot) of intratumoral CD66b+ neutrophil was an independent risk factor for adverse overall patient survival (hazard ratio [HR]: 2.040; 95% confidence interval [CI]: 1.186–3.843; P = 0.010) (118). Multivariate Cox analysis demonstrated that high levels of intra-tumoral CD66b+ neutrophils are significantly associated with decreased OS and DFS (119). Interestingly, a separate study reported that co-infiltration of CD66b+ neutrophils and CD8+ T cells is associated with a more favorable prognosis compared to sole infiltration by CD8+ T cells in CRC, potentially due to the complex interplay between these immune cells (102). However, the investigation into the prognostic role of neutrophil infiltration at intra-tumoral subsites in CRC revealed that low levels (semi-quantitatively score, no/sporadic) of neutrophil infiltration in the front of the tumor is an independent prognostic factor for the poor prognosis of patients with early colon cancer (HR: 2.32, 95% CI: 1.45-3.73, P <.001) (120). These ostensibly contradictory findings suggest that the prognostic role of neutrophils is multifaceted and may be influenced by their infiltration sites within the TME and their functional status.
The neutrophil-to-lymphocyte ratio (NLR) has been utilized in various types of tumors as a prognostic indicator for poor survival, and it can be employed to inform immune-related treatment strategies and predict clinical outcomes (121–124). Colon cancer patients with a high NLR (> 3.0) exhibit poor 5-year OS (87.0% versus 94.5%, P = 0.042) and 5-year relapse-free survival (RFS) (77.9% versus 87.8%, P = 0.032) (125). The high NLR serves as a crucial prognostic indicator for advanced colon cancer, particularly in the case of left-sided colon cancer (5-year OS: 86.4% versus 95.2%, P = 0.014; 5-year RFS:79.2% versus 87.3%, P = 0.047) (125). Multivariate analysis showed that increased NLR (> 5.0) in CRC liver metastasis was associated with poor 5-year OS (27% versus 47%, P < 0.01) and 5-year DFS (6% versus 37%, P < 0.01) (126). Low baseline NLR (<5.11, HR: 0.42, 95% CI: 0.21-0.84, P = 0.014) and early NLR reduction after two courses of immunotherapy (HR: 0.33, 95% CI: 0.18-0.61, P < 0.001) were significantly associated with better outcomes in CRC patients. Among them, patients with a low baseline NLR and an early decline in NLR exhibited the longest median OS (n = 53, median OS = 29.63 months) (127). Further analysis showed that a combination of NLR and tumor mutation burden provided additional predictive capacity (127). Currently, several clinical studies have investigated the practical clinical significance, including the exploration of NLR (NCT05673343, NCT06495827), combination of NLR with C-reactive Protein (NCT05129046), and neutrophils-circulating tumor cells analysis (NCT05793775) in diagnosis and prediction of advanced CRC.
Further research on neutrophil-related genes in CRC patients identified 17 genes significantly associated with OS. Based on these 17 genes, a prognostic risk score (PRS) system was developed. Patients in the high-PRS group were more likely to experience tumor recurrence and metastasis. However, high-PRS group patients showed improved response to immunotherapy, possibly due to increased tumor mutation burden and MSI. Therefore, the PRS can serve as a prognostic model for guiding individualized treatment for colon cancer patients (128).
Neutrophil infiltration can also predict the response of CRC patients to the drug therapies. A study revealed that neutrophil infiltration increases in stage I-III CRC but decreases significantly in stage IV, possibly indicating immune escape in advanced disease. In stage III CRC, high TAN infiltration is linked to a favorable response to a 5-fluorouracil-based chemotherapy regimen (better DFS, HR: 0.42, p = 0.01), suggesting that evaluating TAN infiltration may help identify patients who would benefit from this therapy (129). Due to neutrophil-associated immunosuppression, local inflammatory conditions in MSI-H CRC can hinder tumor response to ICIs, potentially leading to ICI resistance. It has been demonstrated that inflammation infiltration and high NLR (> 3.0) are clinical features of adverse ICI reactions in MSI-H CRC (86).
CRC is characterized by chronic inflammation, which is thought to function in tumor progression and metastasis. The neutrophils are vital for forming the inflammatory TME via the action of NETs, their interaction with the gut microbiota, and crosstalk with other cells such as tumor cells, T cells, and macrophages. However, neutrophils exhibit a dual role in cancers, which may be achieved through two mechanisms (13, 18, 23): (1) Neutrophils themselves possess heterogeneity, with different subtypes performing distinct functions; (2) Neutrophils undergo reprogramming within the TME. The function of neutrophils might result from a combination of these two mechanisms, which requires more evidence.
In CRC, TANs play a crucial role in the immune evasion mechanism during therapy by shaping an immunosuppressive microenvironment and promoting tumor resistance to immune checkpoint therapy through inflammation response. Targeting the molecules of the pathways involved in TANs-mediated tumor progression and therapeutic resistance can lead to the development of multiple synergistic therapeutic strategies. Several approaches have been explored for targeting TANs (107, 130): (1) Inhibition of TANs enrichment, proliferation, and polarization (131); (2) Suppression of neutrophil-induced immune escape, especially enhancing reaction of tumor cells to ICIs (132); (3) Inhibition of other key molecules associated with neutrophil function (133); (4) Utilization of neutrophils as drug delivery carriers based on their innate inflammatory response sensitivity and ability to cross physical barriers (134, 135). These pathways alone or in combination may bring breakthroughs in treating CRC.
The anti-tumor effect of neutrophils in CRC should not be disregarded, and it is worth considering amplifying the antagonistic effect of specific neutrophils subtypes to control the progression of CRC. Furthermore, a study has demonstrated that in successful immunotherapy for mice with lung tumors, neutrophils characterized by interferon-stimulated genes rapidly accumulate in tumor tissues and exhibit an anti-tumor phenotype (136). Another perspective suggests that T cells can eliminate tumor cells with high antigen density, while neutrophils kill tumor antigen escape variants, and immunotherapy with the combination of the two can eliminate highly heterogeneous tumors (137). Therefore, immunotherapy that induces anti-tumor T cells can be combined with therapy that optimizes the anti-tumor function of neutrophils, which may lead to more durable tumor control following treatment.
Neutrophils remain technical challenges in study due to their fragility, short lifespan, and low RNA content. Although mouse models are commonly employed for studying human tumors, they do not accurately reflect the in vivo conditions. Research has demonstrated that human neutrophils selectively release active neutrophilic elastase to eliminate various cancer cells while sparing non-cancer cells; however, this property is absent in mouse neutrophils (138). Therefore, there is a pressing need to develop reliable research techniques for studying human-derived TANs to obtain more credible insights into the mechanisms by which TANs regulate cancers. To simulate the in vivo environment more accurately, we propose that organ-on-a-chip represents an advanced model for in vitro research of neutrophils. It is feasible to recreate a TME on a microfluidic chip, presenting significant application potential (139). Intravital microscopy (IVM) combined with fluorescent neutrophil reporter mice enabled real-time visualization of neutrophil dynamics in tumors. Commonly used in vivo imaging techniques include confocal and multiphoton microscopy. Imaging techniques can broaden our understanding of how neutrophils are involved in cancer development, providing a powerful tool for neutrophilic research (140). The microbiome is involved in the establishment of the TME. The microbiome can trigger the immune system response directly or influence the functional state of immune cells via metabolic processes and metabolites (141). The combination of microbiome and immunotherapy is also a direction that researchers should keep exploring (142). Proteomic, biomechanical, and functional analyses can elucidate neutrophil heterogeneity in systemic lupus erythematosus, offering another possible method to investigate neutrophil heterogeneity in CRC (143).
In recent years, scRNA-seq technology has been utilized for the analysis of TAN characteristics in tumors, revealing the heterogeneous subtypes within the TME and elucidating the role of each subtype in tumor development and response to therapy (21, 22). Despite extensive studies on consensus molecular subtype classification of CRC (144–146), there is a lack of comprehensive analysis on TAN subtypes using scRNA-seq, which could provide valuable insights into assessing CRC prognosis and treatment based on TAN heterogeneity. The following directions can be considered for scRNA-seq of neutrophils from CRC patients: (1) Identify the TAN subtypes in human CRC; (2) Characterize molecular markers specific to each TAN subtype, with a focus on the pro-tumor subtype; (3) Investigate the differentiation pathways that lead to the development of pro-tumor neutrophil subtype; (4) Examine the metabolic reprogramming in tumor-promoting TANs and evaluate how these metabolic changes significantly influence immune cell function; (5) Analyze the interactions between specific TAN subtypes and other immune cells such as T cells and NK cells; (6) Based on these findings, explore strategies to block key molecules involved in the differentiation pathway of pro-tumor neutrophils. For instance, inhibiting upstream regulators and disrupting the active metabolic programs of TANs can suppress the pro-tumor functions and immunosuppressive activities of neutrophils, thereby identifying potential TAN-related therapeutic targets for CRC.
ScRNA-seq research of neutrophils poses several challenges due to their biological characteristics, functional heterogeneity, and technical limitations. Neutrophils are fragile and short-lived cells (with a half-life of 7 to 10 hours) (147) and have low mRNA content(0.33 μg per million cells) (148), challenging the isolation of neutrophils and the subsequent extraction of RNA. In a recent study, the lack of crucial biomarkers could restrict the accuracy of identifying the cells among neutrophil subsets with similar transcriptomic characteristics (149). ScRNA-seq is also insufficient to fully capture the spatiotemporal characteristics of neutrophils. The transcriptomes of neutrophils can vary depending on their activation states and microenvironment, making it difficult to comprehensively describe their transcriptional landscapes at the single-cell level (21). Existing datasets lack spatial information regarding tissue distribution and cell-cell interactions; fortunately, rapidly evolving spatial profiling technologies may address these limitations (150). Other inherent limitations of scRNA-seq technology should not be overlooked, including its limited sensitivity, scale, accuracy, and noise introduced by single-cell RNA preamplification (151). Overcoming these challenges requires a robust experimental design, advanced computational methodologies, and a profound understanding of neutrophil characteristics.
XW: Conceptualization, Writing – review & editing, Investigation, Methodology, Validation, Visualization, Writing – original draft. SH: Investigation, Methodology, Visualization, Writing – review & editing. XG: Investigation, Methodology, Writing – review & editing. SL: Writing – review & editing, Validation. QZ: Validation, Writing – review & editing, Investigation. JX: Methodology, Writing – review & editing. YL: Conceptualization, Supervision, Writing – review & editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declare that no Generative AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Bray F, Laversanne M, Sung H, Ferlay J, Siegel RL, Soerjomataram I, et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2024) 74:229–63. doi: 10.3322/caac.21834
2. Arif AA, Chahal D, Ladua GK, Bhang E, Salh B, Rosenfeld G, et al. Hereditary and inflammatory bowel disease-related early onset colorectal cancer have unique characteristics and clinical course compared with sporadic disease. Cancer Epidemiol Biomarkers Prev. (2021) 30:1785–91. doi: 10.1158/1055-9965.Epi-21-0507
3. Mizuno R, Kawada K, Itatani Y, Ogawa R, Kiyasu Y, Sakai Y. The role of tumor-associated neutrophils in colorectal cancer. Int J Mol Sci. (2019) 20:529. doi: 10.3390/ijms20030529
4. Janney A, Powrie F, Mann EH. Host-microbiota maladaptation in colorectal cancer. Nature. (2020) 585:509–17. doi: 10.1038/s41586-020-2729-3
5. Schmitt M, Greten FR. The inflammatory pathogenesis of colorectal cancer. Nat Rev Immunol. (2021) 21:653–67. doi: 10.1038/s41577-021-00534-x
6. Gros B, Kaplan GG. Ulcerative colitis in adults: A review. Jama. (2023) 330:951–65. doi: 10.1001/jama.2023.15389
7. Nordenfelt P, Tapper H. Phagosome dynamics during phagocytosis by neutrophils. J Leukoc Biol. (2011) 90:271–84. doi: 10.1189/jlb.0810457
8. Eichelberger KR, Goldman WE. Manipulating neutrophil degranulation as a bacterial virulence strategy. PloS Pathog. (2020) 16:e1009054. doi: 10.1371/journal.ppat.1009054
9. Burgener SS, Schroder K. Neutrophil extracellular traps in host defense. Cold Spring Harb Perspect Biol. (2020) 12:a037028. doi: 10.1101/cshperspect.a037028
10. Burn GL, Foti A, Marsman G, Patel DF, Zychlinsky A. The neutrophil. Immunity. (2021) 54:1377–91. doi: 10.1016/j.immuni.2021.06.006
11. Winterbourn CC, Kettle AJ, Hampton MB. Reactive oxygen species and neutrophil function. Annu Rev Biochem. (2016) 85:765–92. doi: 10.1146/annurev-biochem-060815-014442
12. Lahoz-Beneytez J, Elemans M, Zhang Y, Ahmed R, Salam A, Block M, et al. Human neutrophil kinetics: modeling of stable isotope labeling data supports short blood neutrophil half-lives. Blood. (2016) 127:3431–8. doi: 10.1182/blood-2016-03-700336
13. Hedrick CC, Malanchi I. Neutrophils in cancer: heterogeneous and multifaceted. Nat Rev Immunol. (2022) 22:173–87. doi: 10.1038/s41577-021-00571-6
14. Hirao H, Nakamura K, Kupiec-Weglinski JW. Liver ischaemia-reperfusion injury: a new understanding of the role of innate immunity. Nat Rev Gastroenterol Hepatol. (2022) 19:239–56. doi: 10.1038/s41575-021-00549-8
15. Skopelja-Gardner S, Tai J, Sun X, Tanaka L, Kuchenbecker JA, Snyder JM, et al. Acute skin exposure to ultraviolet light triggers neutrophil-mediated kidney inflammation. Proc Natl Acad Sci U S A. (2021) 118:e2019097118. doi: 10.1073/pnas.2019097118
16. Castanheira FVS, Kubes P. Neutrophils and NETs in modulating acute and chronic inflammation. Blood. (2019) 133:2178–85. doi: 10.1182/blood-2018-11-844530
17. Herrero-Cervera A, Soehnlein O, Kenne E. Neutrophils in chronic inflammatory diseases. Cell Mol Immunol. (2022) 19:177–91. doi: 10.1038/s41423-021-00832-3
18. Xiong S, Dong L, Cheng L. Neutrophils in cancer carcinogenesis and metastasis. J Hematol Oncol. (2021) 14:173. doi: 10.1186/s13045-021-01187-y
19. Jaillon S, Ponzetta A, Di Mitri D, Santoni A, Bonecchi R, Mantovani A. Neutrophil diversity and plasticity in tumour progression and therapy. Nat Rev Cancer. (2020) 20:485–503. doi: 10.1038/s41568-020-0281-y
20. Zhu YP, Eggert T, Araujo DJ, Vijayanand P, Ottensmeier CH, Hedrick CC. CyTOF mass cytometry reveals phenotypically distinct human blood neutrophil populations differentially correlated with melanoma stage. J Immunother Cancer. (2020) 8:e000473. doi: 10.1136/jitc-2019-000473
21. Wang L, Liu Y, Dai Y, Tang X, Yin T, Wang C, et al. Single-cell RNA-seq analysis reveals BHLHE40-driven pro-tumour neutrophils with hyperactivated glycolysis in pancreatic tumour microenvironment. Gut. (2023) 72:958–71. doi: 10.1136/gutjnl-2021-326070
22. Xue R, Zhang Q, Cao Q, Kong R, Xiang X, Liu H, et al. Liver tumour immune microenvironment subtypes and neutrophil heterogeneity. Nature. (2022) 612:141–7. doi: 10.1038/s41586-022-05400-x
23. Wu Y, Ma J, Yang X, Nan F, Zhang T, Ji S, et al. Neutrophil profiling illuminates anti-tumor antigen-presenting potency. Cell. (2024) 187:1422–39.e24. doi: 10.1016/j.cell.2024.02.005
24. Liew PX, Kubes P. The neutrophil’s role during health and disease. Physiol Rev. (2019) 99:1223–48. doi: 10.1152/physrev.00012.2018
25. Metzemaekers M, Gouwy M, Proost P. Neutrophil chemoattractant receptors in health and disease: double-edged swords. Cell Mol Immunol. (2020) 17:433–50. doi: 10.1038/s41423-020-0412-0
26. Vandendriessche S, Cambier S, Proost P, Marques PE. Complement receptors and their role in leukocyte recruitment and phagocytosis. Front Cell Dev Biol. (2021) 9:624025. doi: 10.3389/fcell.2021.624025
27. Zhang N, Tang W, Torres L, Wang X, Ajaj Y, Zhu L, et al. Cell surface RNAs control neutrophil recruitment. Cell. (2024) 187:846–60.e17. doi: 10.1016/j.cell.2023.12.033
28. Iyengar NM, Gucalp A, Dannenberg AJ, Hudis CA. Obesity and cancer mechanisms: tumor microenvironment and inflammation. J Clin Oncol. (2016) 34:4270–6. doi: 10.1200/jco.2016.67.4283
29. Afify SM, Hassan G, Seno A, Seno M. Cancer-inducing niche: the force of chronic inflammation. Br J Cancer. (2022) 127:193–201. doi: 10.1038/s41416-022-01775-w
30. de Oliveira S, Rosowski EE, Huttenlocher A. Neutrophil migration in infection and wound repair: going forward in reverse. Nat Rev Immunol. (2016) 16:378–91. doi: 10.1038/nri.2016.49
31. Cho NW, Guldberg SM, Nabet BY, Yu JZ, Kim EJ, Hiam-Galvez KJ, et al. T cells instruct immune checkpoint inhibitor therapy resistance in tumors responsive to IL-1 and TNFα Inflammation. Cancer Immunol Res. (2024) 13(2):229–44. doi: 10.1158/2326-6066.Cir-24-0416
32. Jing W, Wang G, Cui Z, Li X, Zeng S, Jiang X, et al. Tumor-neutrophil cross talk orchestrates the tumor microenvironment to determine the bladder cancer progression. Proc Natl Acad Sci U S A. (2024) 121:e2312855121. doi: 10.1073/pnas.2312855121
33. Zhang Y, Chandra V, Riquelme Sanchez E, Dutta P, Quesada PR, Rakoski A, et al. Interleukin-17-induced neutrophil extracellular traps mediate resistance to checkpoint blockade in pancreatic cancer. J Exp Med. (2020) 217:e20190354. doi: 10.1084/jem.20190354
34. Chung JY, Tang PC, Chan MK, Xue VW, Huang XR, Ng CS, et al. Smad3 is essential for polarization of tumor-associated neutrophils in non-small cell lung carcinoma. Nat Commun. (2023) 14:1794. doi: 10.1038/s41467-023-37515-8
35. Schimek V, Strasser K, Beer A, Göber S, Walterskirchen N, Brostjan C, et al. Tumour cell apoptosis modulates the colorectal cancer immune microenvironment via interleukin-8-dependent neutrophil recruitment. Cell Death Dis. (2022) 13:113. doi: 10.1038/s41419-022-04585-3
36. Casasanta MA, Yoo CC, Udayasuryan B, Sanders BE, Umaña A, Zhang Y, et al. Fusobacterium nucleatum host-cell binding and invasion induces IL-8 and CXCL1 secretion that drives colorectal cancer cell migration. Sci Signal. (2020) 13:eaba9157. doi: 10.1126/scisignal.aba9157
37. Verbeke H, Struyf S, Laureys G, Van Damme J. The expression and role of CXC chemokines in colorectal cancer. Cytokine Growth Factor Rev. (2011) 22:345–58. doi: 10.1016/j.cytogfr.2011.09.002
38. Tosti N, Cremonesi E, Governa V, Basso C, Kancherla V, Coto-Llerena M, et al. Infiltration by IL22-producing T cells promotes neutrophil recruitment and predicts favorable clinical outcome in human colorectal cancer. Cancer Immunol Res. (2020) 8:1452–62. doi: 10.1158/2326-6066.Cir-19-0934
39. Toor SM, Syed Khaja AS, El Salhat H, Bekdache O, Kanbar J, Jaloudi M, et al. Increased levels of circulating and tumor-infiltrating granulocytic myeloid cells in colorectal cancer patients. Front Immunol. (2016) 7:560. doi: 10.3389/fimmu.2016.00560
40. Zhang C, Zhang J, Zhang Y, Song Z, Bian J, Yi H, et al. Identifying neutrophil-associated subtypes in ulcerative colitis and confirming neutrophils promote colitis-associated colorectal cancer. Front Immunol. (2023) 14:1095098. doi: 10.3389/fimmu.2023.1095098
41. Brinkmann V, Reichard U, Goosmann C, Fauler B, Uhlemann Y, Weiss DS, et al. Neutrophil extracellular traps kill bacteria. Science. (2004) 303:1532–5. doi: 10.1126/science.1092385
42. Branzk N, Lubojemska A, Hardison SE, Wang Q, Gutierrez MG, Brown GD, et al. Neutrophils sense microbe size and selectively release neutrophil extracellular traps in response to large pathogens. Nat Immunol. (2014) 15:1017–25. doi: 10.1038/ni.2987
43. Herre M, Cedervall J, Mackman N, Olsson AK. Neutrophil extracellular traps in the pathology of cancer and other inflammatory diseases. Physiol Rev. (2023) 103:277–312. doi: 10.1152/physrev.00062.2021
44. Adrover JM, McDowell SAC, He XY, Quail DF, Egeblad M. NETworking with cancer: The bidirectional interplay between cancer and neutrophil extracellular traps. Cancer Cell. (2023) 41:505–26. doi: 10.1016/j.ccell.2023.02.001
45. Berger-Achituv S, Brinkmann V, Abed UA, Kühn LI, Ben-Ezra J, Elhasid R, et al. A proposed role for neutrophil extracellular traps in cancer immunoediting. Front Immunol. (2013) 4:48. doi: 10.3389/fimmu.2013.00048
46. Albrengues J, Shields MA, Ng D, Park CG, Ambrico A, Poindexter ME, et al. Neutrophil extracellular traps produced during inflammation awaken dormant cancer cells in mice. Science. (2018) 361:eaao4227. doi: 10.1126/science.aao4227
47. Cools-Lartigue J, Spicer J, McDonald B, Gowing S, Chow S, Giannias B, et al. Neutrophil extracellular traps sequester circulating tumor cells and promote metastasis. J Clin Invest. (2013) 123:3446–58. doi: 10.1172/jci67484
48. Najmeh S, Cools-Lartigue J, Rayes RF, Gowing S, Vourtzoumis P, Bourdeau F, et al. Neutrophil extracellular traps sequester circulating tumor cells via β1-integrin mediated interactions. Int J Cancer. (2017) 140:2321–30. doi: 10.1002/ijc.30635
49. Ren J, He J, Zhang H, Xia Y, Hu Z, Loughran P, et al. Platelet TLR4-ERK5 axis facilitates NET-mediated capturing of circulating tumor cells and distant metastasis after surgical stress. Cancer Res. (2021) 81:2373–85. doi: 10.1158/0008-5472.Can-20-3222
50. Yazdani HO, Roy E, Comerci AJ, van der Windt DJ, Zhang H, Huang H, et al. Neutrophil extracellular traps drive mitochondrial homeostasis in tumors to augment growth. Cancer Res. (2019) 79:5626–39. doi: 10.1158/0008-5472.Can-19-0800
51. Stehr AM, Wang G, Demmler R, Stemmler MP, Krug J, Tripal P, et al. Neutrophil extracellular traps drive epithelial-mesenchymal transition of human colon cancer. J Pathol. (2022) 256:455–67. doi: 10.1002/path.5860
52. Yang L, Liu L, Zhang R, Hong J, Wang Y, Wang J, et al. IL-8 mediates a positive loop connecting increased neutrophil extracellular traps (NETs) and colorectal cancer liver metastasis. J Cancer. (2020) 11:4384–96. doi: 10.7150/jca.44215
53. Rayes RF, Vourtzoumis P, Bou Rjeily M, Seth R, Bourdeau F, Giannias B, et al. Neutrophil extracellular trap-associated CEACAM1 as a putative therapeutic target to prevent metastatic progression of colon carcinoma. J Immunol. (2020) 204:2285–94. doi: 10.4049/jimmunol.1900240
54. Teijeira Á, Garasa S, Gato M, Alfaro C, Migueliz I, Cirella A, et al. CXCR1 and CXCR2 chemokine receptor agonists produced by tumors induce neutrophil extracellular traps that interfere with immune cytotoxicity. Immunity. (2020) 52:856–71.e8. doi: 10.1016/j.immuni.2020.03.001
55. Kaltenmeier C, Yazdani HO, Morder K, Geller DA, Simmons RL, Tohme S. Neutrophil extracellular traps promote T cell exhaustion in the tumor microenvironment. Front Immunol. (2021) 12:785222. doi: 10.3389/fimmu.2021.785222
56. Bergers G, Brekken R, McMahon G, Vu TH, Itoh T, Tamaki K, et al. Matrix metalloproteinase-9 triggers the angiogenic switch during carcinogenesis. Nat Cell Biol. (2000) 2:737–44. doi: 10.1038/35036374
57. Aldabbous L, Abdul-Salam V, McKinnon T, Duluc L, Pepke-Zaba J, Southwood M, et al. Neutrophil extracellular traps promote angiogenesis: evidence from vascular pathology in pulmonary hypertension. Arterioscler Thromb Vasc Biol. (2016) 36:2078–87. doi: 10.1161/atvbaha.116.307634
58. Zhang Y, Wang C, Yu M, Zhao X, Du J, Li Y, et al. Neutrophil extracellular traps induced by activated platelets contribute to procoagulant activity in patients with colorectal cancer. Thromb Res. (2019) 180:87–97. doi: 10.1016/j.thromres.2019.06.005
59. Mousset A, Lecorgne E, Bourget I, Lopez P, Jenovai K, Cherfils-Vicini J, et al. Neutrophil extracellular traps formed during chemotherapy confer treatment resistance via TGF-β activation. Cancer Cell. (2023) 41:757–75.e10. doi: 10.1016/j.ccell.2023.03.008
60. Li Y, Wu S, Zhao Y, Dinh T, Jiang D, Selfridge JE, et al. Neutrophil extracellular traps induced by chemotherapy inhibit tumor growth in murine models of colorectal cancer. J Clin Invest. (2024) 134:e175031. doi: 10.1172/jci175031
61. Wong CC, Yu J. Gut microbiota in colorectal cancer development and therapy. Nat Rev Clin Oncol. (2023) 20:429–52. doi: 10.1038/s41571-023-00766-x
62. Kvich L, Fritz BG, Zschach H, Terkelsen T, Raskov H, Høst-Rasmussen K, et al. Biofilms and core pathogens shape the tumor microenvironment and immune phenotype in colorectal cancer. Gut Microbes. (2024) 16:2350156. doi: 10.1080/19490976.2024.2350156
63. Bardou M, Barkun AN, Martel M. Obesity and colorectal cancer. Gut. (2013) 62:933–47. doi: 10.1136/gutjnl-2013-304701
64. Bardou M, Rouland A, Martel M, Loffroy R, Barkun AN, Chapelle N. Review article: obesity and colorectal cancer. Aliment Pharmacol Ther. (2022) 56:407–18. doi: 10.1111/apt.17045
65. Shantaram D, Hoyd R, Blaszczak AM, Antwi L, Jalilvand A, Wright VP, et al. Obesity-associated microbiomes instigate visceral adipose tissue inflammation by recruitment of distinct neutrophils. Nat Commun. (2024) 15:5434. doi: 10.1038/s41467-024-48935-5
66. Long X, Wong CC, Tong L, Chu ESH, Ho Szeto C, Go MYY, et al. Peptostreptococcus anaerobius promotes colorectal carcinogenesis and modulates tumour immunity. Nat Microbiol. (2019) 4:2319–30. doi: 10.1038/s41564-019-0541-3
67. Lattanzi G, Strati F, Díaz-Basabe A, Perillo F, Amoroso C, Protti G, et al. iNKT cell-neutrophil crosstalk promotes colorectal cancer pathogenesis. Mucosal Immunol. (2023) 16:326–40. doi: 10.1016/j.mucimm.2023.03.006
68. Fridlender ZG, Sun J, Kim S, Kapoor V, Cheng G, Ling L, et al. Polarization of tumor-associated neutrophil phenotype by TGF-beta: “N1” versus “N2” TAN. Cancer Cell. (2009) 16:183–94. doi: 10.1016/j.ccr.2009.06.017
69. Kong X, Zhang Y, Xiang L, You Y, Duan Y, Zhao Y, et al. Fusobacterium nucleatum-triggered neutrophil extracellular traps facilitate colorectal carcinoma progression. J Exp Clin Cancer Res. (2023) 42:236. doi: 10.1186/s13046-023-02817-8
70. Yu LC. Microbiota dysbiosis and barrier dysfunction in inflammatory bowel disease and colorectal cancers: exploring a common ground hypothesis. J BioMed Sci. (2018) 25:79. doi: 10.1186/s12929-018-0483-8
71. Brescia P, Rescigno M. The gut vascular barrier: a new player in the gut-liver-brain axis. Trends Mol Med. (2021) 27:844–55. doi: 10.1016/j.molmed.2021.06.007
72. Bertocchi A, Carloni S, Ravenda PS, Bertalot G, Spadoni I, Lo Cascio A, et al. Gut vascular barrier impairment leads to intestinal bacteria dissemination and colorectal cancer metastasis to liver. Cancer Cell. (2021) 39:708–24.e11. doi: 10.1016/j.ccell.2021.03.004
73. Wu T, Dai Y. Tumor microenvironment and therapeutic response. Cancer Lett. (2017) 387:61–8. doi: 10.1016/j.canlet.2016.01.043
74. Xiao Y, Yu D. Tumor microenvironment as a therapeutic target in cancer. Pharmacol Ther. (2021) 221:107753. doi: 10.1016/j.pharmthera.2020.107753
75. Wang Y, Zhong X, He X, Hu Z, Huang H, Chen J, et al. Liver metastasis from colorectal cancer: pathogenetic development, immune landscape of the tumour microenvironment and therapeutic approaches. J Exp Clin Cancer Res. (2023) 42:177. doi: 10.1186/s13046-023-02729-7
76. Tiberti S, Catozzi C, Croci O, Ballerini M, Cagnina D, Soriani C, et al. GZMK(high) CD8(+) T effector memory cells are associated with CD15(high) neutrophil abundance in non-metastatic colorectal tumors and predict poor clinical outcome. Nat Commun. (2022) 13:6752. doi: 10.1038/s41467-022-34467-3
77. Jackstadt R, van Hooff SR, Leach JD, Cortes-Lavaud X, Lohuis JO, Ridgway RA, et al. Epithelial NOTCH signaling rewires the tumor microenvironment of colorectal cancer to drive poor-prognosis subtypes and metastasis. Cancer Cell. (2019) 36:319–36.e7. doi: 10.1016/j.ccell.2019.08.003
78. Xie M, Lin Z, Ji X, Luo X, Zhang Z, Sun M, et al. FGF19/FGFR4-mediated elevation of ETV4 facilitates hepatocellular carcinoma metastasis by upregulating PD-L1 and CCL2. J Hepatol. (2023) 79:109–25. doi: 10.1016/j.jhep.2023.02.036
79. Chen X, Chen J, Feng W, Huang W, Wang G, Sun M, et al. FGF19-mediated ELF4 overexpression promotes colorectal cancer metastasis through transactivating FGFR4 and SRC. Theranostics. (2023) 13:1401–18. doi: 10.7150/thno.82269
80. Chia L, Wang B, Kim JH, Luo LZ, Shuai S, Herrera I, et al. HMGA1 induces FGF19 to drive pancreatic carcinogenesis and stroma formation. J Clin Invest. (2023) 133:e151601. doi: 10.1172/jci151601
81. Li C, Chen T, Liu J, Wang Y, Zhang C, Guo L, et al. FGF19-induced inflammatory CAF promoted neutrophil extracellular trap formation in the liver metastasis of colorectal cancer. Adv Sci (Weinh). (2023) 10:e2302613. doi: 10.1002/advs.202302613
82. Tian S, Chu Y, Hu J, Ding X, Liu Z, Fu D, et al. Tumour-associated neutrophils secrete AGR2 to promote colorectal cancer metastasis via its receptor CD98hc-xCT. Gut. (2022) 71:2489–501. doi: 10.1136/gutjnl-2021-325137
83. Ribas A, Wolchok JD. Cancer immunotherapy using checkpoint blockade. Science. (2018) 359:1350–5. doi: 10.1126/science.aar4060
84. Topalian SL, Forde PM, Emens LA, Yarchoan M, Smith KN, Pardoll DM. Neoadjuvant immune checkpoint blockade: A window of opportunity to advance cancer immunotherapy. Cancer Cell. (2023) 41:1551–66. doi: 10.1016/j.ccell.2023.07.011
85. Germann M, Zangger N, Sauvain MO, Sempoux C, Bowler AD, Wirapati P, et al. Neutrophils suppress tumor-infiltrating T cells in colon cancer via matrix metalloproteinase-mediated activation of TGFβ. EMBO Mol Med. (2020) 12:e10681. doi: 10.15252/emmm.201910681
86. Sui Q, Zhang X, Chen C, Tang J, Yu J, Li W, et al. Inflammation promotes resistance to immune checkpoint inhibitors in high microsatellite instability colorectal cancer. Nat Commun. (2022) 13:7316. doi: 10.1038/s41467-022-35096-6
87. Zhang Y, Wang Z, Lu Y, Sanchez DJ, Li J, Wang L, et al. Region-specific CD16(+) neutrophils promote colorectal cancer progression by inhibiting natural killer cells. Adv Sci (Weinh). (2024) 11:e2403414. doi: 10.1002/advs.202403414
88. Shang A, Gu C, Zhou C, Yang Y, Chen C, Zeng B, et al. Exosomal KRAS mutation promotes the formation of tumor-associated neutrophil extracellular traps and causes deterioration of colorectal cancer by inducing IL-8 expression. Cell Commun Signal. (2020) 18:52. doi: 10.1186/s12964-020-0517-1
89. Shang A, Gu C, Wang W, Wang X, Sun J, Zeng B, et al. Exosomal circPACRGL promotes progression of colorectal cancer via the miR-142-3p/miR-506-3p- TGF-β1 axis. Mol Cancer. (2020) 19:117. doi: 10.1186/s12943-020-01235-0
90. Hwang WL, Lan HY, Cheng WC, Huang SC, Yang MH. Tumor stem-like cell-derived exosomal RNAs prime neutrophils for facilitating tumorigenesis of colon cancer. J Hematol Oncol. (2019) 12:10. doi: 10.1186/s13045-019-0699-4
91. Canli Ö, Nicolas AM, Gupta J, Finkelmeier F, Goncharova O, Pesic M, et al. Myeloid cell-derived reactive oxygen species induce epithelial mutagenesis. Cancer Cell. (2017) 32:869–83.e5. doi: 10.1016/j.ccell.2017.11.004
92. Bui TM, Butin-Israeli V, Wiesolek HL, Zhou M, Rehring JF, Wiesmüller L, et al. Neutrophils alter DNA repair landscape to impact survival and shape distinct therapeutic phenotypes of colorectal cancer. Gastroenterology. (2021) 161:225–38.e15. doi: 10.1053/j.gastro.2021.03.027
93. Nozawa H, Chiu C, Hanahan D. Infiltrating neutrophils mediate the initial angiogenic switch in a mouse model of multistage carcinogenesis. Proc Natl Acad Sci U S A. (2006) 103:12493–8. doi: 10.1073/pnas.0601807103
94. Jablonska J, Leschner S, Westphal K, Lienenklaus S, Weiss S. Neutrophils responsive to endogenous IFN-beta regulate tumor angiogenesis and growth in a mouse tumor model. J Clin Invest. (2010) 120:1151–64. doi: 10.1172/jci37223
95. Christoffersson G, Vågesjö E, Vandooren J, Lidén M, Massena S, Reinert RB, et al. VEGF-A recruits a proangiogenic MMP-9-delivering neutrophil subset that induces angiogenesis in transplanted hypoxic tissue. Blood. (2012) 120:4653–62. doi: 10.1182/blood-2012-04-421040
96. Bui TM, Yalom LK, Ning E, Urbanczyk JM, Ren X, Herrnreiter CJ, et al. Tissue-specific reprogramming leads to angiogenic neutrophil specialization and tumor vascularization in colorectal cancer. J Clin Invest. (2024) 134:e174545. doi: 10.1172/jci174545
97. Zhou G, Peng K, Song Y, Yang W, Shu W, Yu T, et al. CD177+ neutrophils suppress epithelial cell tumourigenesis in colitis-associated cancer and predict good prognosis in colorectal cancer. Carcinogenesis. (2018) 39:272–82. doi: 10.1093/carcin/bgx142
98. Vadillo E, Mantilla A, Aguilar-Flores C, De-León-Rodríguez SG, Vela-Patiño S, Badillo J, et al. The invasive margin of early-stage human colon tumors is infiltrated with neutrophils of an antitumoral phenotype. J Leukoc Biol. (2023) 114:672–83. doi: 10.1093/jleuko/qiad123
99. Triner D, Devenport SN, Ramakrishnan SK, Ma X, Frieler RA, Greenson JK, et al. Neutrophils restrict tumor-associated microbiota to reduce growth and invasion of colon tumors in mice. Gastroenterology. (2019) 156:1467–82. doi: 10.1053/j.gastro.2018.12.003
100. Duhen T, Geiger R, Jarrossay D, Lanzavecchia A, Sallusto F. Production of interleukin 22 but not interleukin 17 by a subset of human skin-homing memory T cells. Nat Immunol. (2009) 10:857–63. doi: 10.1038/ni.1767
101. Qin WZ, Chen LL, Pan HF, Leng RX, Zhai ZM, Wang C, et al. Expressions of IL-22 in circulating CD4+/CD8+ T cells and their correlation with disease activity in SLE patients. Clin Exp Med. (2011) 11:245–50. doi: 10.1007/s10238-011-0134-9
102. Governa V, Trella E, Mele V, Tornillo L, Amicarella F, Cremonesi E, et al. The interplay between neutrophils and CD8(+) T cells improves survival in human colorectal cancer. Clin Cancer Res. (2017) 23:3847–58. doi: 10.1158/1078-0432.Ccr-16-2047
103. Carnevale S, Ponzetta A, Rigatelli A, Carriero R, Puccio S, Supino D, et al. Neutrophils mediate protection against colitis and carcinogenesis by controlling bacterial invasion and IL22 production by γδ T cells. Cancer Immunol Res. (2024) 12:413–26. doi: 10.1158/2326-6066.Cir-23-0295
104. Sabat R, Ouyang W, Wolk K. Therapeutic opportunities of the IL-22-IL-22R1 system. Nat Rev Drug Discovery. (2014) 13:21–38. doi: 10.1038/nrd4176
105. Huber S, Gagliani N, Zenewicz LA, Huber FJ, Bosurgi L, Hu B, et al. IL-22BP is regulated by the inflammasome and modulates tumorigenesis in the intestine. Nature. (2012) 491:259–63. doi: 10.1038/nature11535
106. Ruan HX, Qin XN, Huang W, Lin L. IL-22 activates the PI3K-AKT pathway to promote colorectal cancer cell proliferation and metastasis. Discovery Oncol. (2024) 15:317. doi: 10.1007/s12672-024-01169-9
107. Que H, Fu Q, Lan T, Tian X, Wei X. Tumor-associated neutrophils and neutrophil-targeted cancer therapies. Biochim Biophys Acta Rev Cancer. (2022) 1877:188762. doi: 10.1016/j.bbcan.2022.188762
108. Mouchemore KA, Anderson RL. Immunomodulatory effects of G-CSF in cancer: Therapeutic implications. Semin Immunol. (2021) 54:101512. doi: 10.1016/j.smim.2021.101512
109. Yao C, Wu S, Kong J, Sun Y, Bai Y, Zhu R, et al. Angiogenesis in hepatocellular carcinoma: mechanisms and anti-angiogenic therapies. Cancer Biol Med. (2023) 20:25–43. doi: 10.20892/j.issn.2095-3941.2022.0449
110. Itatani Y, Yamamoto T, Zhong C, Molinolo AA, Ruppel J, Hegde P, et al. Suppressing neutrophil-dependent angiogenesis abrogates resistance to anti-VEGF antibody in a genetic model of colorectal cancer. Proc Natl Acad Sci U S A. (2020) 117:21598–608. doi: 10.1073/pnas.2008112117
111. Yajuk O, Baron M, Toker S, Zelter T, Fainsod-Levi T, Granot Z. The PD-L1/PD-1 axis blocks neutrophil cytotoxicity in cancer. Cells. (2021) 10:1510. doi: 10.3390/cells10061510
112. He G, Zhang H, Zhou J, Wang B, Chen Y, Kong Y, et al. Peritumoural neutrophils negatively regulate adaptive immunity via the PD-L1/PD-1 signalling pathway in hepatocellular carcinoma. J Exp Clin Cancer Res. (2015) 34:141. doi: 10.1186/s13046-015-0256-0
113. Sun R, Xiong Y, Liu H, Gao C, Su L, Weng J, et al. Tumor-associated neutrophils suppress antitumor immunity of NK cells through the PD-L1/PD-1 axis. Transl Oncol. (2020) 13:100825. doi: 10.1016/j.tranon.2020.100825
114. Thiam HR, Wong SL, Wagner DD, Waterman CM. Cellular mechanisms of NETosis. Annu Rev Cell Dev Biol. (2020) 36:191–218. doi: 10.1146/annurev-cellbio-020520-111016
115. Wang B, Su X, Zhang B, Pan S. GSK484, an inhibitor of peptidyl arginine deiminase 4, increases the radiosensitivity of colorectal cancer and inhibits neutrophil extracellular traps. J Gene Med. (2023) 25:e3530. doi: 10.1002/jgm.3530
116. Chen F, Guo S, Li Y, Lu Y, Liu L, Chen S, et al. Fusobacterium nucleatum-driven CX3CR1(+) PD-L1(+) phagocytes route to tumor tissues and reshape tumor microenvironment. Gut Microbes. (2025) 17:2442037. doi: 10.1080/19490976.2024.2442037
117. Nair R, Lannagan TRM, Jackstadt R, Andrusaite A, Cole J, Boyne C, et al. Co-inhibition of TGF-β and PD-L1 pathways in a metastatic colorectal cancer mouse model triggers interferon responses, innate cells and T cells, alongside metabolic changes and tumor resistance. Oncoimmunology. (2024) 13:2330194. doi: 10.1080/2162402x.2024.2330194
118. Rao HL, Chen JW, Li M, Xiao YB, Fu J, Zeng YX, et al. Increased intratumoral neutrophil in colorectal carcinomas correlates closely with Malignant phenotype and predicts patients’ adverse prognosis. PloS One. (2012) 7:e30806. doi: 10.1371/journal.pone.0030806
119. Zhu B, Luo J, Jiang Y, Yu L, Liu M, Fu J. Prognostic significance of nomograms integrating IL-37 expression, neutrophil level, and MMR status in patients with colorectal cancer. Cancer Med. (2018) 7:3682–94. doi: 10.1002/cam4.1663
120. Wikberg ML, Ling A, Li X, Öberg Å, Edin S, Palmqvist R. Neutrophil infiltration is a favorable prognostic factor in early stages of colon cancer. Hum Pathol. (2017) 68:193–202. doi: 10.1016/j.humpath.2017.08.028
121. Zhou Y, Wei Q, Fan J, Cheng S, Ding W, Hua Z. Prognostic role of the neutrophil-to-lymphocyte ratio in pancreatic cancer: A meta-analysis containing 8252 patients. Clin Chim Acta. (2018) 479:181–9. doi: 10.1016/j.cca.2018.01.024
122. Maeda T, Hiura A, Uehara J, Toyoshima R, Nakagawa T, Yoshino K. Neutrophil-to-lymphocyte ratio is associated with survival and sentinel lymph node positivity in invasive cutaneous squamous cell carcinoma: A retrospective study. J Am Acad Dermatol. (2022) 86:615–20. doi: 10.1016/j.jaad.2021.10.033
123. Han QY, Zhang X, Zhang JG, Zhou WJ, Chen QY, Chen YY, et al. Pre-operative neutrophil-to-lymphocyte ratio is an independent prognostic factor in patients with gastric cancer. Int Immunopharmacol. (2022) 113:109371. doi: 10.1016/j.intimp.2022.109371
124. Zhang W, Tan Y, Li Y, Liu J. Neutrophil to Lymphocyte ratio as a predictor for immune-related adverse events in cancer patients treated with immune checkpoint inhibitors: a systematic review and meta-analysis. Front Immunol. (2023) 14:1234142. doi: 10.3389/fimmu.2023.1234142
125. Mazaki J, Katsumata K, Kasahara K, Tago T, Wada T, Kuwabara H, et al. Neutrophil-to-lymphocyte ratio is a prognostic factor for colon cancer: a propensity score analysis. BMC Cancer. (2020) 20:922. doi: 10.1186/s12885-020-07429-5
126. Lin N, Li J, Yao X, Zhang X, Liu G, Zhang Z, et al. Prognostic value of neutrophil-to-lymphocyte ratio in colorectal cancer liver metastasis: A meta-analysis of results from multivariate analysis. Int J Surg. (2022) 107:106959. doi: 10.1016/j.ijsu.2022.106959
127. Ouyang H, Xiao B, Huang Y, Wang Z. Baseline and early changes in the neutrophil-lymphocyte ratio (NLR) predict survival outcomes in advanced colorectal cancer patients treated with immunotherapy. Int Immunopharmacol. (2023) 123:110703. doi: 10.1016/j.intimp.2023.110703
128. Yang Y, Lu C, Li L, Zheng C, Wang Y, Chen J, et al. Construction and multicohort validation of a colon cancer prognostic risk score system based on big data of neutrophil-associated differentially expressed genes. J Cancer. (2024) 15:2866–79. doi: 10.7150/jca.94560
129. Galdiero MR, Bianchi P, Grizzi F, Di Caro G, Basso G, Ponzetta A, et al. Occurrence and significance of tumor-associated neutrophils in patients with colorectal cancer. Int J Cancer. (2016) 139:446–56. doi: 10.1002/ijc.30076
130. Zhang J, Gu J, Wang X, Ji C, Yu D, Wang M, et al. Engineering and targeting neutrophils for cancer therapy. Adv Mater. (2024) 36:e2310318. doi: 10.1002/adma.202310318
131. Xie Y, Zhou T, Li X, Zhao K, Bai W, Hou X, et al. Targeting ESE3/EHF with nifurtimox inhibits CXCR2(+) neutrophil infiltration and overcomes pancreatic cancer resistance to chemotherapy and immunotherapy. Gastroenterology. (2024) 167:281–97. doi: 10.1053/j.gastro.2024.02.046
132. Teijeira A, Garasa S, Ochoa MC, Villalba M, Olivera I, Cirella A, et al. IL8, neutrophils, and NETs in a collusion against cancer immunity and immunotherapy. Clin Cancer Res. (2021) 27:2383–93. doi: 10.1158/1078-0432.Ccr-20-1319
133. Yoshimoto M, Kagawa S, Kajioka H, Taniguchi A, Kuroda S, Kikuchi S, et al. Dual antiplatelet therapy inhibits neutrophil extracellular traps to reduce liver micrometastases of intrahepatic cholangiocarcinoma. Cancer Lett. (2023) 567:216260. doi: 10.1016/j.canlet.2023.216260
134. Su Y, Gao J, Dong X, Wheeler KA, Wang Z. Neutrophil-mediated delivery of nanocrystal drugs via photoinduced inflammation enhances cancer therapy. ACS Nano. (2023) 17:15542–55. doi: 10.1021/acsnano.3c02013
135. Zhao Y, Li M, Guo Y, Jin J, Pei F, Wang W, et al. Neutrophil hitchhiking nanoparticles enhance bacteria-mediated cancer therapy via NETosis reprogramming. J Control Release. (2024) 367:661–75. doi: 10.1016/j.jconrel.2024.01.068
136. Gungabeesoon J, Gort-Freitas NA, Kiss M, Bolli E, Messemaker M, Siwicki M, et al. A neutrophil response linked to tumor control in immunotherapy. Cell. (2023) 186:1448–64.e20. doi: 10.1016/j.cell.2023.02.032
137. Hirschhorn D, Budhu S, Kraehenbuehl L, Gigoux M, Schröder D, Chow A, et al. T cell immunotherapies engage neutrophils to eliminate tumor antigen escape variants. Cell. (2023) 186:1432–47.e17. doi: 10.1016/j.cell.2023.03.007
138. Cui C, Chakraborty K, Tang XA, Zhou G, Schoenfelt KQ, Becker KM, et al. Neutrophil elastase selectively kills cancer cells and attenuates tumorigenesis. Cell. (2021) 184:3163–77.e21. doi: 10.1016/j.cell.2021.04.016
139. Glaser DE, Curtis MB, Sariano PA, Rollins ZA, Shergill BS, Anand A, et al. Organ-on-a-chip model of vascularized human bone marrow niches. Biomaterials. (2022) 280:121245. doi: 10.1016/j.biomaterials.2021.121245
140. Yam AO, Jakovija A, Gatt C, Chtanova T. Neutrophils under the microscope: neutrophil dynamics in infection, inflammation, and cancer revealed using intravital imaging. Front Immunol. (2024) 15:1458035. doi: 10.3389/fimmu.2024.1458035
141. Lam KC, Araya RE, Huang A, Chen Q, Di Modica M, Rodrigues RR, et al. Microbiota triggers STING-type I IFN-dependent monocyte reprogramming of the tumor microenvironment. Cell. (2021) 184:5338–56.e21. doi: 10.1016/j.cell.2021.09.019
142. Sepich-Poore GD, Zitvogel L, Straussman R, Hasty J, Wargo JA, Knight R. The microbiome and human cancer. Science. (2021) 371:eabc4552. doi: 10.1126/science.abc4552
143. Bashant KR, Aponte AM, Randazzo D, Rezvan Sangsari P, Wood AJ, Bibby JA, et al. Proteomic, biomechanical and functional analyses define neutrophil heterogeneity in systemic lupus erythematosus. Ann Rheum Dis. (2021) 80:209–18. doi: 10.1136/annrheumdis-2020-218338
144. Joanito I, Wirapati P, Zhao N, Nawaz Z, Yeo G, Lee F, et al. Single-cell and bulk transcriptome sequencing identifies two epithelial tumor cell states and refines the consensus molecular classification of colorectal cancer. Nat Genet. (2022) 54:963–75. doi: 10.1038/s41588-022-01100-4
145. Khaliq AM, Erdogan C, Kurt Z, Turgut SS, Grunvald MW, Rand T, et al. Refining colorectal cancer classification and clinical stratification through a single-cell atlas. Genome Biol. (2022) 23:113. doi: 10.1186/s13059-022-02677-z
146. Mouradov D, Greenfield P, Li S, In EJ, Storey C, Sakthianandeswaren A, et al. Oncomicrobial community profiling identifies clinicomolecular and prognostic subtypes of colorectal cancer. Gastroenterology. (2023) 165:104–20. doi: 10.1053/j.gastro.2023.03.205
147. Shaul ME, Fridlender ZG. Tumour-associated neutrophils in patients with cancer. Nat Rev Clin Oncol. (2019) 16:601–20. doi: 10.1038/s41571-019-0222-4
148. Wigerblad G, Cao Q, Brooks S, Naz F, Gadkari M, Jiang K, et al. Single-cell analysis reveals the range of transcriptional states of circulating human neutrophils. J Immunol. (2022) 209:772–82. doi: 10.4049/jimmunol.2200154
149. Garrido-Trigo A, Corraliza AM, Veny M, Dotti I, Melón-Ardanaz E, Rill A, et al. Macrophage and neutrophil heterogeneity at single-cell spatial resolution in human inflammatory bowel disease. Nat Commun. (2023) 14:4506. doi: 10.1038/s41467-023-40156-6
150. Moffitt JR, Lundberg E, Heyn H. The emerging landscape of spatial profiling technologies. Nat Rev Genet. (2022) 23:741–59. doi: 10.1038/s41576-022-00515-3
Keywords: neutrophils, colorectal cancer, gut microbiota, tumor microenvironment, therapies, prognosis
Citation: Wang X, He S, Gong X, Lei S, Zhang Q, Xiong J and Liu Y (2025) Neutrophils in colorectal cancer: mechanisms, prognostic value, and therapeutic implications. Front. Immunol. 16:1538635. doi: 10.3389/fimmu.2025.1538635
Received: 03 December 2024; Accepted: 04 February 2025;
Published: 28 February 2025.
Edited by:
Arumugam Jayakumar, University of Texas MD Anderson Cancer Center, United StatesReviewed by:
Federica Rubbino, Humanitas Research Hospital, ItalyCopyright © 2025 Wang, He, Gong, Lei, Zhang, Xiong and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yang Liu, bGl1eWFuZ19AaHVzdC5lZHUuY24=
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.