- 1Central Laboratory, Quanzhou First Hospital Affiliated to Fujian Medical University, Quanzhou, China
- 2The Cancer Center of The Fifth Affiliated Hospital of Sun Yat-Sen University, Zhuhai, China
Background: The clinical application of immune checkpoint blockade (ICB)-based neoadjuvant therapy has been approved in breast cancer since 2021. However, no studies have evaluated its efficacy and safety in randomized and non-randomized settings. Additionally, there exists controversy about which specific subpopulation can benefit from this management strategy.
Methods: We searched MEDLINE and EMBASE databases for prospective clinical trials of ICB-based neoadjuvant therapy in breast cancer. Information regarding pathological complete response (pCR), event-free survival (EFS), overall survival (OS), and treatment-related adverse event (TRAE) were pooled to estimate the efficacy and safety. Hazard ratio, relative risk (RR) and their 95% confidence intervals (CIs) were calculated.
Results: Among 22 eligible trials including 6134 women with resectable breast cancer, there were 11 randomized studies with 5574 patients. Pooled analysis on pCR (RR, 1.38; 95% CI, 1.20-1.58; P<0.001), EFS (hazard ratio, 0.67; 95% CI, 0.54-0.81; P<0.001), and OS (hazard ratio, 0.56; 95% CI, 0.35-0.91; P=0.01) revealed that ICB-based neoadjuvant therapy was associated with favorable outcomes over conventional treatment. Moreover, the benefits of EFS were independent of PD-L1 expression (Pinteraction=0.57) and pCR (Pinteraction=0.37) in neoadjuvant immunotherapy. However, combining ICB with conventional neoadjuvant treatment significantly increased the risk of high-grade TRAE (RR, 1.06; 95% CI, 1.01-1.12; P=0.03), serious TRAE (RR, 1.57; 95% CI, 1.26-1.94; P<0.001), treatment discontinuation due to TRAE (RR, 1.47; 95% CI, 1.14-1.90; P=0.003), and potentially fatal adverse event (RR, 2.25; 95% CI, 0.80-6.31; P=0.12).
Conclusion: The combination of ICB with conventional neoadjuvant treatment is associated with favorable clinical outcomes and importantly, increased grade 3+ toxicities. Clinicians should meticulously monitor patients to minimize the risk of treatment discontinuation in individuals with potentially curable breast cancer.
Introduction
Breast cancer (BC) is one commonly diagnosed malignancy and the second leading cause of death among women globally (1). Due to the systematic therapies and surgical advancements, the standard care of paradigm for BC has gradually shifted to neoadjuvant therapy (2). ICB has becomes the standard of care in many cancer types in the past decade (3). In 2021, the United States (US) Food and Drug Administration (FDA) approved the application of ICB-based neoadjuvant therapy for BC due to the improvement in pCR and EFS in KEYNOTE-522 (4). However, it is still unknown which specific subpopulations could benefit from neoadjuvant immunotherapy. Consequently, many studies focus on discovering new biomarkers to spare non-responders from TRAEs. For example, in lung cancer, the European Medicines Agency (EMA) has granted the application of nivolumab-based immunotherapy in the neoadjuvant setting exclusively for patients who exhibit tumor cell PD-L1 expression levels exceeding 1% (5). Interestingly, in BC, the role of PD-L1 expression in neoadjuvant immunotherapy has not been systematically examined.
Key clinical trials have exhibited heterogeneous outcomes for ICB, with some demonstrating significant efficacies resulting in the subsequent approvals, while some revealing safety concerns leading to the early termination. ICB have been associated with a range of morbidities affecting various organs, including cardiovascular, pulmonary, endocrine, neurological, and hepatic systems (6, 7). Additionally, up to 60% of melanoma patients undergoing ICB experience grade 3+ immune-related adverse event (irAE) (8). Occasionally, fatal adverse events (FAEs) are reported (9). Before exclusively relying on ICB, it is imperative to thoroughly understand the clinical implications and address the contentious issues associated with neoadjuvant immunotherapy. A pooled analysis of existing studies can yield critical and clinically relevant insights. Therefore, with recently accumulated evidence, here we systematically evaluate the efficacy and safety of ICB-based neoadjuvant treatment in breast cancer.
Method
Search strategy and selection criteria
We searched MEDLINE and EMBASE databases for published trials pertaining to neoadjuvant ICB, alone or in combination, in breast cancer from inception to September 2024 without language restriction. In addition, abstracts from European Society for Medical Oncology conference, American Society of Clinical Oncology conference, and American Association for Cancer Research conference were examined for updates on published trials. The keywords used for search included: breast cancer, clinical trial, immunotherapy, and PD-1/PD-L1. All researchers conducted the search independently, screened the titles and abstracts for relevance, and categorized the potential papers as excluded, included, and uncertain. For uncertain trials, the full-texts were examined to confirm the eligibility.
To be eligible, potential trials had to meet the following criteria: (1) study design: prospective studies irrespective of clinical phase; (2) population: enrolled more than 10 patients, over 18 years of age, had histologic confirmation resectable breast cancer; (3) intervention: at least one arm of patients were treated with neoadjuvant ICB irrespective of dosage or duration; (4) outcomes: pathology (pCR defined as ypT0/is ypN0), efficacy (EFS and OS), and toxicity (TRAE, irAE, serious TRAE, TRAE led to treatment discontinuation, and FAE). Pre-clinical papers, review articles, retrospective studies, editorials, comments, quality of life studies, and cost effectiveness analyses were excluded.
Data extraction and quality assessment
Relevant data were extracted independently by all investigators using a prespecified form. Extracted data were: (1) study information, including year of publication, first author, clinical phase, study design, definition of endpoints, neoadjuvant regimens, and the sample size; (2) baseline features of the enrolled patients, including age, stage, cancer subtype, and PD-L1 expression; (3) data on treatment-related outcomes, including pCR. The incidence of all-grade and high-grade TRAE, irAE, serious TRAE, and FAE were also recorded. Here, high-grade TRAE meant grade 3+ TRAE. When multiple publications of the same trial appeared, only the most recent or complete report was included.
Risk of bias was assessed by Cochrane risk of bias tool (10) for RCTs and the Joanna Briggs Institute checklist for single-arm trials.
Statistical analysis
Overall incidences were measured by random-effects or fixed-effects models depending on the heterogeneities. Outcome information from single-arm trials were pooled by an inverse variance random-effects meta-analysis using logit transformation. As for RCT data, we conducted a restricted maximum likelihood meta-analysis of hazard ratios for time-dependent data. Statistical heterogeneities among different studies were estimated by Cochrane’s Q statistic (11). I2 statistic was calculated to evaluate the extent of inconsistency contributable to the heterogeneity. The assumption of homogeneity was considered invalid for P< 0.05 and I2 > 25%.
Publication bias was evaluated through visual inspection of Begg’s funnel plots (12). All analysis was conducted by Stata version 12.0 and MedCalc 18.2.1. Two-sided P<0.05 was considered statistically significant.
Results
Baseline characteristics
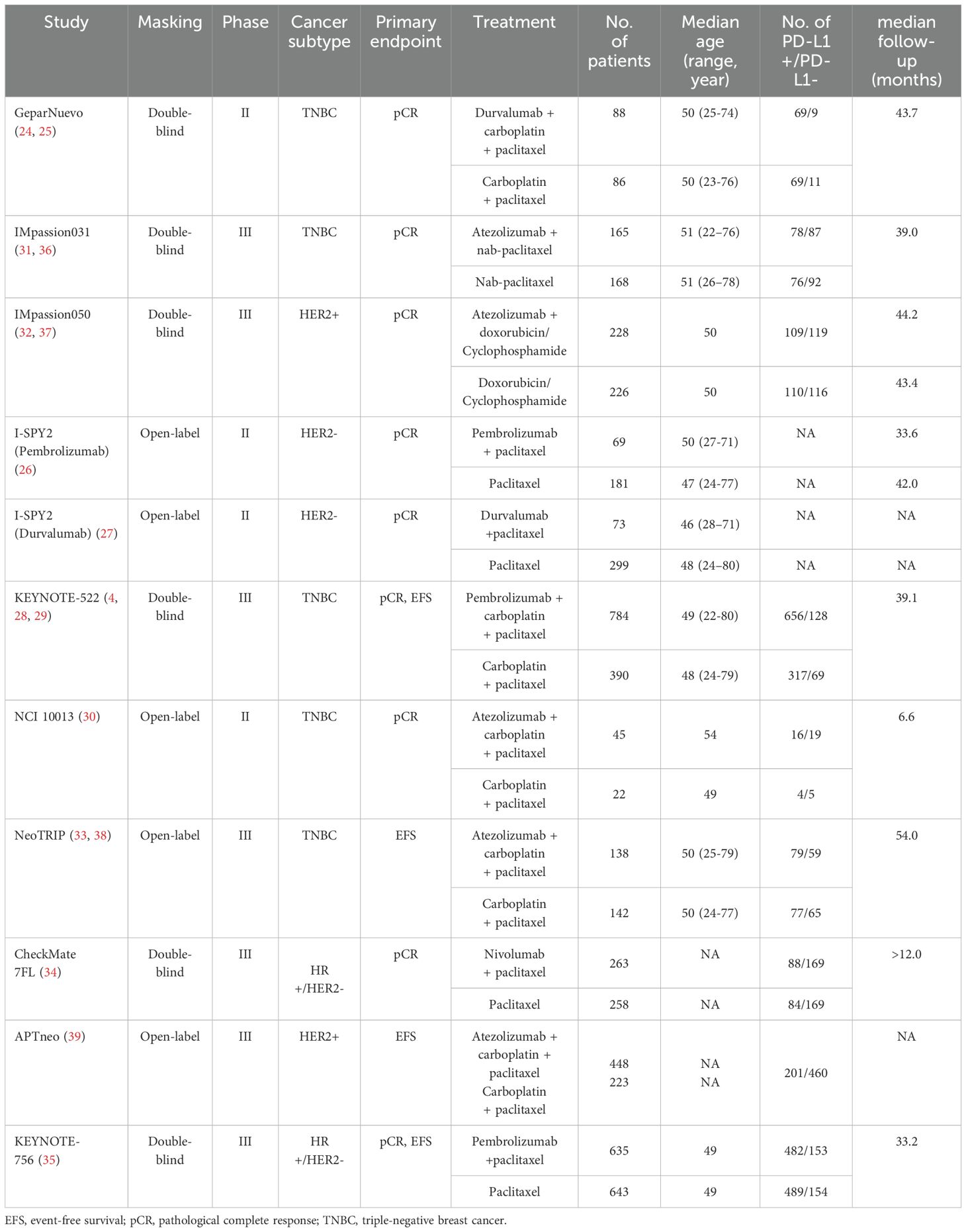
1097 relevant articles were discovered from the initial search (Supplementary Figure S1). After careful review, a total of 22 publications (4, 13–33) and 6 abstracts (34–39) met inclusion criteria, including 22 trials and 6 follow-up studies (4, 24, 29, 36–38), from which we extracted 33 arms. Overall, 6134 patients were included in these 22 trials (median age range, 45-65 years) (Table 1, Supplementary Table S1). There were 1709 women treated with pembrolizumab, 1091 with atezolizumab, 321 with nivolumab, 218 with durvalumab, 70 with toripalimab, 59 with camrelizumab, and 10 with adebrelimab, and the rest 2656 patients were in control arm. The PD-L1 expression status were recorded in 14 trials (4, 16, 18, 21–25, 28–39), and the detection methods were summarized in Supplementary Table S2. Among them, 957 patients with PD-L1 positive tumors and 656 women with PD-L1 negative BC were treated with neoadjuvant ICB, 798 patients with PD-L1 positive tumors and 497 women with PD-L1 negative BC were in the control arms.
Totally, 11 RCTs were included here, namely GeparNuevo (24, 25), I-SPY2 (Durvalumab) (27), IMpassion031 (31, 36), IMpassion050 (32, 37), KEYNOTE-522 (4, 28, 29), I-SPY2 (Pembrolizumab) (26), NCI 10013 (30), NeoTRIP (33, 38), CheckMate 7FL (34), APTneo (39), and KEYNOTE-756 (35). The enrolled patients on each trial ranged from 67 to 1278. Four trials were phase II RCTs (24–27, 30), and seven were phase III studies (4, 28, 29, 31–39). The primary endpoint was pCR in all RCTs except NeoTRIP (33, 38) and APTneo (39), EFS was co-primary endpoint in KEYNOTE-522 (4, 28, 29) and KEYNOTE-756 (35). The median follow-up on each trial ranged from 6.6 months (30) to 54.0 months (33, 38). Five trials were conducted on 2028 patients with TNBC (4, 24, 25, 28–31, 33, 36, 38), two RCTs on 1799 women with HR+/HER2- BC (34, 35), two studies on 622 patients with HER2- tumors (26, 27), and two trial on 1125 women with HER2+ BC (32, 37). Across 5574 patients included in these RCTs, 2936 patients (52.7%) were treated with ICB, 2638 patients (47.3%) were in the control arms.
Generally speaking, low risks of bias were confirmed in most RCTs (Supplementary Figure S2), the major issue was lack of blinding as five studies (26, 27, 30, 33, 38, 39) were open-labelled. For non-RCTs, bias concerns were usually associated with inadequate length of follow-up (Supplementary Table S3).
Efficacy
For 3468 enrolled patients treated by neoadjuvant immunotherapy, 1689 pCRs were observed (incidence, 49%; 95% CI, 41%-57%; Figure 1). Of note, the pCR rates were different among various subtypes of BC, with the highest occurred in HER2+ BC (60%, 95% CI, 56%-63%), and the lowest in HR+/HER2- BC (24%, 95% CI, 19%-28%). The frequency of pCRs in TNBC was 58% (95% CI, 54%-61%). Compared with PD-L1 negative BC (34%, 95% CI, 22%-47%; Supplementary Figure S3), the pCR rate in PD-L1 positive tumors (65%, 95% CI, 51%-78%) was almost doubled. For patients treated with anti-PD-1 inhibitors, the pCR rate was 45% (95% CI, 32%-57%), similar with patients treated with anti-PD-L1 inhibitors (54%; 95% CI, 49%-60%; Supplementary Figure S4).

Figure 1. The pooled pCR rates of TNBC, HR+/HER2- BC, HER2- BC, and BC overall in patients treated with ICB-based neoadjuvant regimens. The vertical dot line indicates the overall pCR rate. BC, breast cancer; HER2, human epidermal growth factor receptor 2; HR, hormone receptor; ICB, immune checkpoint inhibitor; pCR, pathological complete response; TNBC, triple negative breast cancer.
Based on 11 RCTs with 5547 patients, ICB-based neoadjuvant therapy was associated with significant increased pCRs (RR, 1.38; 95% CI, 1.20-1.58; P<0.001; Figure 2A). Interestingly, the increased pCRs were observed in TNBC (RR, 1.38; 95% CI, 1.14-1.67) and HR+/HER2- tumors (RR, 1.68; 95% CI, 1.41-2.01), but not in HER2+ BC (RR, 1.06; 95% CI, 0.96-1.18). Moreover, the pCR rate were significant improved for both PD-L1 positive tumors (RR, 1.39; 95% CI, 1.15-1.69; P <0.001; Figure 2B) and PD-L1 negative BC (RR, 1.35; 95% CI, 1.05-1.73; P =0.01; Figure 2C), and the benefits were similar between these two subgroups (Pinteraction=0.89).

Figure 2. The pooled relative risk of pathological complete response in BC patients treated with ICB-based neoadjuvant regimens. (A) The relative risk of pCR in TNBC, HR+/HER2- BC, HER2- BC, and BC overall. (B) The relative risk of pCR in patients with PD-L1 positive BC. (C) The relative risk of pCR in patients with PD-L1 negative tumors. The vertical dot line equals 1. BC, breast cancer; ICB, immune checkpoint inhibitor; pCR, pathological complete response; RR, relative risk.
For 2385 patients from 5 RCTs, combining ICB with conventional neoadjuvant therapy was associate with favorable EFS (hazard ratio, 0.67; 95% CI, 0.54-0.81; P <0.001; Figure 3A). Additionally, similar EFS benefits were observed in patients with pCR and patients without pCR (Pinteraction=0.37; Figure 3B), or patients with PD-L1+ BC and patients with PD-L1- BC (Pinteraction=0.57; Figure 3C).

Figure 3. The pooled hazard ratio of survival in BC patients treated with ICB-based neoadjuvant regimens. (A) The pooled hazard ratio of EFS in patients treated with ICB-based neoadjuvant regimens. (B) The pooled EFS in patients with and without pCR after treated with ICB-based neoadjuvant regimens. (C) The pooled hazard ratio of EFS in patients with PD-L1 positive BC and patients with PD-L1 negative tumors. (D) The pooled overall survival in patients treated with ICB-based neoadjuvant regimens. The vertical dot line equals 1. BC, breast cancer; EFS, event-free survival; ICB, immune checkpoint inhibitor.
In 1681 women from 3 RCTs, neoadjuvant immunotherapy was associated with favorable overall survival (hazard ratio, 0.56; 95% CI, 0.35-0.91; P=0.01; Figure 3D). Of note, further investigations were needed to confirm this result since the median follow-ups were 39.1 months, 39.0 months, and 43.7 months for KEYNOTE 522, IMpassion031, and GeparNuevo, respectively.
Safety
Overall, in BC patients treated by neoadjuvant immunotherapy, the incidence of any-grade TRAE was 99% (95% CI, 98%-100%; Supplementary Figure S5); high-grade TRAE, 55% (95% CI, 45%-65%; Supplementary Figure S6); any-grade irAE, 34% (95% CI, 24%-45%; Supplementary Figure S7); high-grade irAE, 9% (95% CI, 7%-11%; Supplementary Figure S8); serious TRAE, 22% (95% CI, 17%-27%; Supplementary Figure S9); treatment discontinuation due to TRAEs, 18% (95% CI, 13%-22%; Supplementary Figure S10). 12 FAEs were recorded among 3135 patients (incidence, 0.48%; 95% CI, 0.27%-0.78%). The reasons of deaths were septic shock (n=2), myocardial infarction (n=2), alveolitis (n=1), pneumonitis (n=1), hepatitis (n=1), and unknown reasons (n=5).
The incidences of any-grade TRAE in ICB arm and control arm were similar (RR, 0.99; 95% CI, 0.99-1.00; P=0.20; Supplementary Figure S11). However, combining ICB with conventional neoadjuvant therapy significantly increased the risk of high-grade TRAE (RR, 1.06; 95% CI, 1.01-1.12; P=0.03; Figure 4A), serious TRAE (RR, 1.57; 95% CI, 1.26-1.94; P<0.001; Figure 4B), and treatment discontinuation due to TRAE (RR, 1.47; 95% CI, 1.14-1.90; P=0.003; Figure 4C). FAE was also increased although the difference was not statistically significant (RR, 2.25; 95% CI, 0.80-6.31; P=0.12; Figure 4D). As expected, compared with control, ICB-based neoadjuvant therapy was associated with more any-grade irAE (RR, 2.69; 95% CI, 1.40-5.16; P=0.003; Figure 5A), and high-grade irAE (RR, 3.62; 95% CI, 1.47-8.90; P=0.005; Figure 5B).
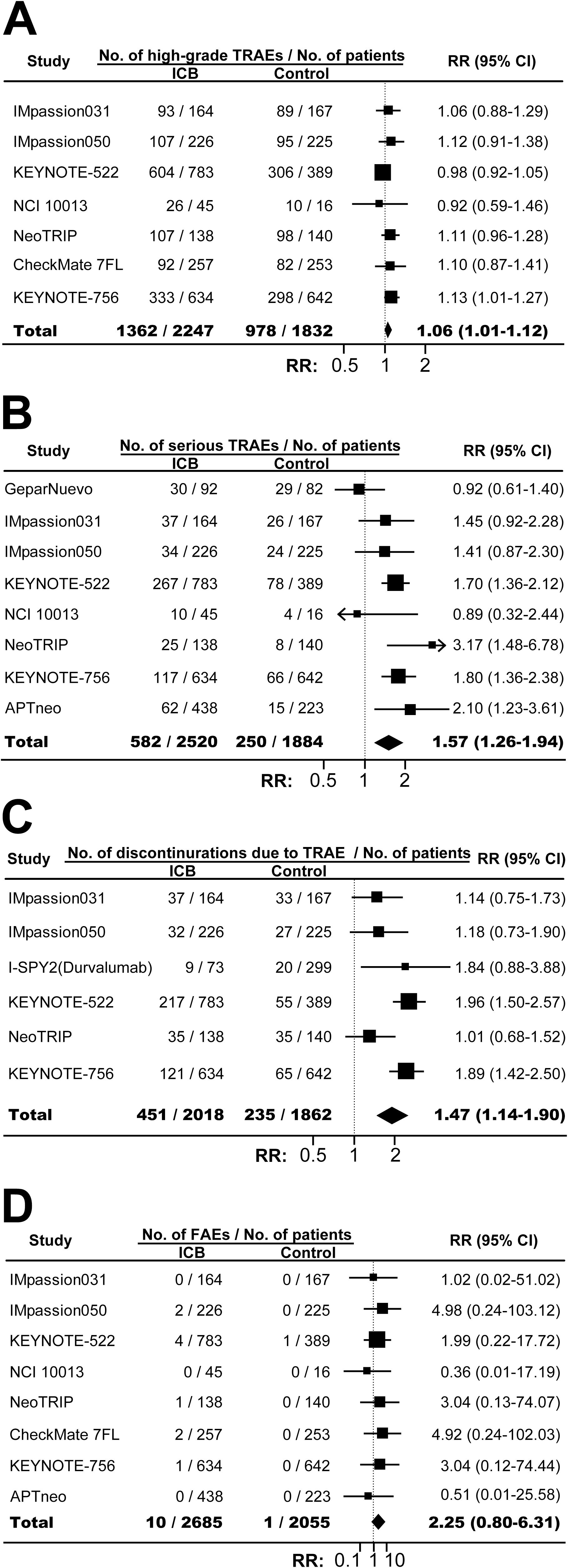
Figure 4. The overall relative risks of toxicities in BC patients treated with ICB-based neoadjuvant regimens. (A) The pooled relative risk of high-grade TRAE. (B) The pooled relative risk of serious TRAE. (C) The pooled relative risk of treatment discontinuration due to TRAE. (D) The pooled relative risk of treatment-related fatal adverse events. The vertical dot line equals 1. BC, breast cancer; ICB, immune checkpoint inhibitor; FAE, fatal adverse events; RR, relative risk; TRAE, treatment-related adverse events.
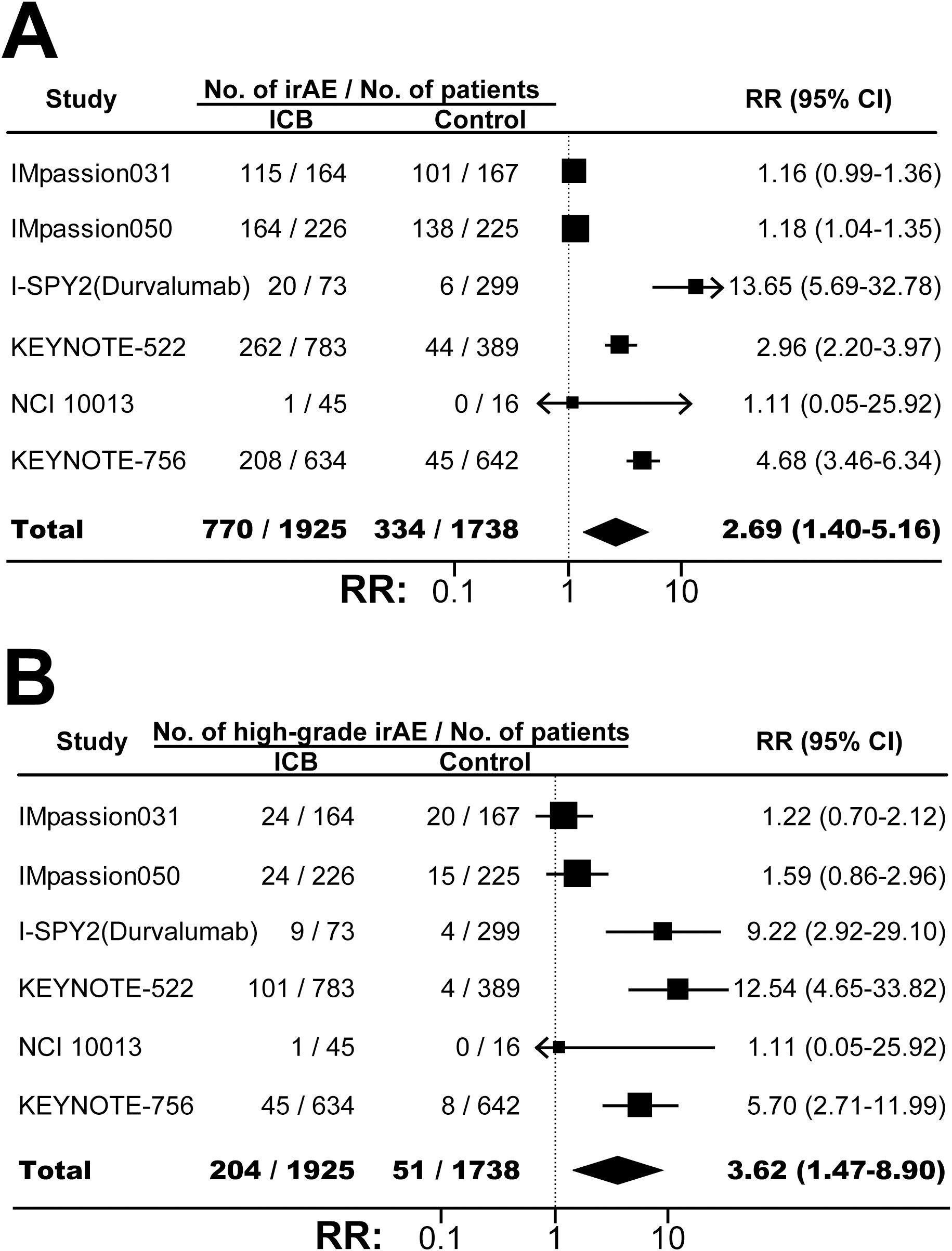
Figure 5. The pooled relative risks of any-grade immune-related adverse events (A) and high-grade immune-related adverse events (B) in BC patients treated with ICB-based neoadjuvant regimens. The vertical dot line equals 1. ICB, immune checkpoint inhibitor; irAE, immune-related adverse event; RR, relative risk.
No significant asymmetry was identified by visual inspection of Begg’s funnel plot(Supplementary Figure S12).
Discussion
This study, based on 22 prospective trials involving 6134 patients, estimated the efficacy and safety, and address contentious issues associated with neoadjuvant immunotherapy in breast cancer. Specifically, we examined the pathological complete responses overall and across various molecular phenotypes, investigated long-term outcomes of ICB beyond pCR, evaluated the predictive powers of PD-L1 expression and pCR status as biomarkers, and demonstrated the high risk of toxicities arising from the ICB-based neoadjuvant treatment regimens.
Our study indicated that, compared with conventional neoadjuvant treatment, neoadjuvant immunotherapy could increase the pCR rates in patients with HR+/HER2- and triple-negative breast cancer significantly, but not in HER2+ tumors. Similarly, previous results from KATE2 (40), PANACEA (41), JAVELIN Solid Tumor trial (42), CCTG IND.229 (43), and DS8201-A-U105 (44) also showed poor outcomes of immunotherapy in HER2+ breast cancer with metastatic diseases. Currently, the interactions between anti-tumor immunity and HER2 expression were not fully understood (45). Future studies involving novel immunotherapeutic inhibitors and innovative conjugate antibodies were essential to achieve a more thorough understanding of this specific subtype of BC.
In breast cancer, ICB-based neoadjuvant settings improved the pathological and survival outcomes irrespective of PD-L1 expression status. Similar results had been previously reported in other tumors, such as lung cancer (46). Indeed, it had been well-established PD-L1 expression status was an imperfect biomarker in patient selection and prognostication (7, 47). It appeared more critical to explore the complex interactions between immune cells and cancer cells within the tumor microenvironment, rather than exclusively concentrating on PD-L1 expression. As indicated by the cancer immunogram (48), the efficacy of immunotherapy was influenced by PD-L1 expression as well as a multitude of unrelated characteristics, such as the immune status, the “foreignness” of tumor, the presence of other inhibitory mechanisms, the activity of infiltrated CD8+ T cell, and the sensitivity of tumor cells to immune cells. Furthermore, our analysis suggested that among patients undergoing surgery, women that received neoadjuvant ICB had better survival outcomes compared with their counterparts. This finding indicated that the benefits of immunotherapy extended across different stages of disease responses, highlighting its potential in managing breast cancer beyond traditional tumor pathology assessments. On the other hand, since there was no difference in the EFS benefits among BC patients with and without pCR, pCR, might be an imperfect surrogacy for clinical outcomes in neoadjuvant ICB settings.
This study were notable as previous studies failed to reveal the significant association between ICB-based neoadjuvant therapy and toxicities in breast cancer (49, 50). Here we utilized the most up-to-date data to perform this meta-analysis. With more patients included, our study enhanced the statistical power with more robust and reliable outcomes evaluations. Here, our results indicated that high-grade TRAEs occurred in over 50% patients who were treated with the ICB-based neoadjuvant settings. Furthermore, the overall incidence of serious TRAE was 22%, which was doubled the incidence of serious TRAE associated with nivolumab monotherapy (11.2%) (6). The significantly increased grade 3+ TRAEs underscored the necessity for further investigation into patient-specific risk factors that contribute to ICB-related toxicities. Such insights could be pivotal in refining patient selection criteria to mitigate the risk of potentially mortality. Of note, previous studies on patients with advanced-stage solid tumors revealed a potential association between the appearance of TRAEs and favorable survivals (3, 9). However, this concept remained ambiguous in the neoadjuvant context, where toxicities could delay surgery and the consequence clinical outcomes (51).
This study had some limitations. First, while a pooled study with overall survival as the primary outcome should be ideal, it took a prolonged period to obtain sufficient OS information even breast cancer is an aggressive disease. Accordingly, the included trials primarily focus on pCR and EFS rather than OS. Second, the low incidence of treatment-related FAE limited the statistical power to identify any significant differences in this outcome. Third, this study employed a trial-level aggregate meta-analysis of available data, hence the heterogeneity across various trials might not be fully elucidated. The risk factors associated with efficacy and toxicities, the variations in cancer subtypes and antibody structures, dose, and duration of ICB across the included RCTs, were not fully accounted for. Consequently, further examinations should be carried out once individual patient information become available. Of note, safety outcomes were not usually the major target in RCT with a large number of patients, which could diminish the potential publication bias. Fourth, the expressions of PD-L1 in eligible trials were evaluated through several different approaches. Although all these detection methods were approved, the inter-assay discordance was reported (52). This variability could potentially influence the conclusions derived from our analysis.
In summary, for patients with breast cancer, ICB-based neoadjuvant treatment was associated with favorable outcomes, as well as significantly increased grade 3+ toxicities. Additionally, patients with PD-L1 negative BC and patients failed to achieve pCR may also benefit from neoadjuvant immunotherapy.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author/s.
Author contributions
YY: Formal analysis, Investigation, Methodology, Resources, Software, Writing – original draft, Writing – review & editing. ZZ: Data curation, Formal analysis, Methodology, Project administration, Resources, Software, Validation, Visualization, Writing – original draft, Writing – review & editing. HZ: Conceptualization, Funding acquisition, Investigation, Supervision, Writing – original draft, Writing – review & editing. BZ: Conceptualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was funded by National Natural Science Foundation of China (No. 82373367).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2025.1537926/full#supplementary-material
Supplementary Figure 1 | Flow-chart diagram of selected clinical trials included in our study.
Supplementary Figure 2 | Risk of bias of eligible randomized trials assessed by Cochrane risk of bias tool.
Supplementary Figure 3 | The pooled pCR rates in patients with PD-L1 positive BC and patients with PD-L1 negative BC. The vertical dot line indicates the overall pCR rate in patients with PD-L1 positive BC and patients with PD-L1 negative BC. BC, breast cancer; pCR, pathological complete response.
Supplementary Figure 4 | The pooled pCR rates in BC patients treated with anti-PD-L1-based and anti-PD-1-based neoadjuvant settings. The vertical dot line indicates the overall pCR rate in patients treated with anti-PD-L1-based and anti-PD-1-based neoadjuvant settings. BC, breast cancer; pCR, pathological complete response.
Supplementary Figure 5 | The pooled any-grade TRAE rate in BC patients treated with ICB-based neoadjuvant regimens. The vertical dot line indicates the overall any-grade TRAE rate. BC, breast cancer; ICB, immune checkpoint inhibitor; TRAE, treatment-related adverse event.
Supplementary Figure 6 | The pooled high-grade TRAE rate in BC patients treated with ICB-based neoadjuvant regimens. The vertical dot line indicates the overall high-grade TRAE rate. BC, breast cancer; ICB, immune checkpoint inhibitor; TRAE, treatment-related adverse event.
Supplementary Figure 7 | The pooled any-grade irAE rate in BC patients treated with ICB-based neoadjuvant regimens. The vertical dot line indicates the overall any-grade irAE rate. BC, breast cancer; ICB, immune checkpoint inhibitor; irAE, immune-related adverse event.
Supplementary Figure 8 | The pooled high-grade irAE rate in BC patients treated with ICB-based neoadjuvant regimens. The vertical dot line indicates the overall any-grade irAE rate. BC, breast cancer; ICB, immune checkpoint inhibitor; irAE, immune-related adverse event.
Supplementary Figure 9 | The pooled serious TRAE rate in BC patients treated with ICB-based neoadjuvant regimens. The vertical dot line indicates the overall high-grade TRAE rate. BC, breast cancer; ICB, immune checkpoint inhibitor; TRAE, treatment-related adverse event.
Supplementary Figure 10 | The pooled incidence of treatment discontinuation due to TRAE in BC patients treated with ICB-based neoadjuvant regimens. The vertical dot line indicates the overall treatment discontinuation rate. BC, breast cancer; ICB, immune checkpoint inhibitor; TRAE, treatment-related adverse event.
Supplementary Figure 11 | The pooled relative risk of any-grade TRAE in BC patients treated with ICB-based neoadjuvant regimens. The vertical dot line indicates 1. BC, breast cancer; ICB, immune checkpoint inhibitor; RR, relative risk; TRAE, treatment-related adverse event.
Supplementary Figure 12 | Begg’s funnel plot for the publication bias test. Each circle represents a separate trial for the indicated association. Vertical line, mean effect size.
References
1. Loibl S, Poortmans P, Morrow M, Denkert C, Curigliano G. Breast cancer. Lancet. (2021) 397:1750–69. doi: 10.1016/S0140-6736(20)32381-3
2. Zhao B, Zhao H, Zhao J. Impact of hormone receptor status on the efficacy of HER2-targeted treatment. Endocr Relat Cancer. (2018) 25:687–97. doi: 10.1530/ERC-18-0029
3. Zhang Z, Pan Q, Lu M, Zhao B. Intermediate endpoints as surrogates for outcomes in cancer immunotherapy: a systematic review and meta-analysis of phase 3 trials. EClinicalMedicine. (2023) 63:102156. doi: 10.1016/j.eclinm.2023.102156
4. Shah M, Osgood C, Amatya A, Fiero M, Pierce W, Nair A, et al. FDA approval summary: pembrolizumab for neoadjuvant and adjuvant treatment of patients with high-risk early-stage triple-negative breast cancer. Clin Cancer Res. (2022) 28:5249–53. doi: 10.1158/1078-0432.CCR-22-1110
5. Sorin M, Prosty C, Ghaleb L, Nie K, Katergi K, Shahzad M, et al. Neoadjuvant chemoimmunotherapy for NSCLC: A systematic review and meta-analysis. JAMA Oncol. (2024) 10:621–33. doi: 10.1001/jamaoncol.2024.0057
6. Zhao B, Zhao H, Zhao J. Serious adverse events and fatal adverse events associated with nivolumab treatment in cancer patients: Nivolumab-related serious/fatal adverse events. J Immunother Cancer. (2018) 6:101. doi: 10.1186/s40425-018-0421-z
7. Zhao B, Zhao H, Zhao J. Efficacy of PD-1/PD-L1 blockade monotherapy in clinical trials. Ther Adv Med Oncol. (2020) 12:1758835920937612. doi: 10.1177/1758835920937612
8. Lozano AX, Chaudhuri A, Nene A, Bacchiocchi A, Earland N, Vesely M, et al. T cell characteristics associated with toxicity to immune checkpoint blockade in patients with melanoma. Nat Med. (2022) 28:353–62. doi: 10.1038/s41591-021-01623-z
9. Zhao B, Zhao H, Zhao J. Fatal adverse events associated with programmed cell death protein 1 or programmed cell death-ligand 1 monotherapy in cancer. Ther Adv Med Oncol. (2020) 12:1758835919895753. doi: 10.1177/1758835919895753
10. Higgins JP, Altman D, Gotzsche P, Juni P, Moher D, Oxman A, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. Bmj. (2011) 343:d5928. doi: 10.1136/bmj.d5928
11. Higgins J, Thompson S, Deeks J, Altman D. Measuring inconsistency in meta-analyses. Bmj. (2003) 327:557–60. doi: 10.1136/bmj.327.7414.557
12. Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. (1994) 50:1088–101. doi: 10.2307/2533446
13. Dieci MV, Guarneri V, Tosi A, Bisagni G, Musolino A, Spazzapan S, et al. Neoadjuvant chemotherapy and immunotherapy in luminal B-like breast cancer: results of the phase II GIADA trial. Clin Cancer Res. (2022) 28:308–17. doi: 10.1158/1078-0432.CCR-21-2260
14. Zhang C, Liu Y, Wang X, Bi Z, Qiu P, Qiao G, et al. Clinical efficacy and biomarker analysis of neoadjuvant camrelizumab plus chemotherapy for early-stage triple-negative breast cancer: a experimental single-arm phase II clinical trial pilot study. Int J Surg. (2024) 110:1527–36. doi: 10.1097/JS9.0000000000001011
15. Chen G, Gu X, Xue J, Zhang X, Yu X, Zhang Y, et al. Effects of neoadjuvant stereotactic body radiotherapy plus adebrelimab and chemotherapy for triple-negative breast cancer: A pilot study. Elife. (2023) 12. doi: 10.7554/eLife.91737.sa2
16. Ahn HK, Sim S, Suh K, Kim M, Jeong J, Kim J, et al. Response rate and safety of a neoadjuvant pertuzumab, atezolizumab, docetaxel, and trastuzumab regimen for patients with ERBB2-positive stage II/III breast cancer: the neo-PATH phase 2 nonrandomized clinical trial. JAMA Oncol. (2022) 8:1271–7. doi: 10.1001/jamaoncol.2022.2310
17. Schmid P, Salgado R, Park Y, Munoz-Couselo E, Kim S, Sohn J, et al. Pembrolizumab plus chemotherapy as neoadjuvant treatment of high-risk, early-stage triple-negative breast cancer: results from the phase 1b open-label, multicohort KEYNOTE-173 study. Ann Oncol. (2020) 31:569–81. doi: 10.1016/j.annonc.2020.01.072
18. Fasching PA, Hein A, Kolberg H, Haberle L, Uhrig S, Rubner M, et al. Pembrolizumab in combination with nab-paclitaxel for the treatment of patients with early-stage triple-negative breast cancer - A single-arm phase II trial (NeoImmunoboost, AGO-B-041). Eur J Cancer. (2023) 184:1–9. doi: 10.1016/j.ejca.2023.01.001
19. Jerusalem G, Prat A, Salgado R, Reinisch M, Saura C, Ruiz-Borrego M, et al. Neoadjuvant nivolumab + palbociclib + anastrozole for oestrogen receptor-positive/human epidermal growth factor receptor 2-negative primary breast cancer: Results from CheckMate 7A8. Breast. (2023) 72:103580. doi: 10.1016/j.breast.2023.103580
20. He M, Hao S, Ma L, Xiu B, Yang B, Wang Z, et al. Neoadjuvant anthracycline followed by toripalimab combined with nab-paclitaxel in patients with early triple-negative breast cancer (NeoTENNIS): a single-arm, phase II study. EClinicalMedicine. (2024) 74:102700. doi: 10.1016/j.eclinm.2024.102700
21. Wang C, Liu Z, Chen X, Qiao J, Lu Z, Li L, et al. Neoadjuvant camrelizumab plus nab-paclitaxel and epirubicin in early triple-negative breast cancer: a single-arm phase II trial. Nat Commun. (2023) 14:6654. doi: 10.1038/s41467-023-42479-w
22. Foldi J, Silber A, Reisenbichler E, Singh K, Fischbach N, Persico J, et al. Neoadjuvant durvalumab plus weekly nab-paclitaxel and dose-dense doxorubicin/cyclophosphamide in triple-negative breast cancer. NPJ Breast Cancer. (2021) 7:9. doi: 10.1038/s41523-021-00219-7
23. Sharma P, Stecklein S, Yoder R, Staley J, Schwensen K, O’Dea A, et al. Clinical and biomarker findings of neoadjuvant pembrolizumab and carboplatin plus docetaxel in triple-negative breast cancer: neoPACT phase 2 clinical trial. JAMA Oncol. (2024) 10:227–35. doi: 10.1001/jamaoncol.2023.5033
24. Loibl S, Schneeweiss A, Huober J, Braun M, Rey J, Blohmer J, et al. Neoadjuvant durvalumab improves survival in early triple-negative breast cancer independent of pathological complete response. Ann Oncol. (2022) 33:1149–58. doi: 10.1016/j.annonc.2022.07.1940
25. Loibl S, Untch M, Burechardi N, Huober J, Sinn B, Blohmer J, et al. A randomised phase II study investigating durvalumab in addition to an anthracycline taxane-based neoadjuvant therapy in early triple-negative breast cancer: clinical results and biomarker analysis of GeparNuevo study. Ann Oncol. (2019) 30:1279–88. doi: 10.1093/annonc/mdz158
26. Nanda R, Liu M, Yau C, Shatsky R, Pusztai L, Wallace A, et al. Effect of pembrolizumab plus neoadjuvant chemotherapy on pathologic complete response in women with early-stage breast cancer: an analysis of the ongoing phase 2 adaptively randomized I-SPY2 trial. JAMA Oncol. (2020) 6:676–84. doi: 10.1001/jamaoncol.2019.6650
27. Pusztai L, Yau C, Wolf D, Han H, Du L, Wallace A, et al. Durvalumab with olaparib and paclitaxel for high-risk HER2-negative stage II/III breast cancer: Results from the adaptively randomized I-SPY2 trial. Cancer Cell. (2021) 39:989–998.e5. doi: 10.1016/j.ccell.2021.05.009
28. Schmid P, Cortes J, Dent R, Pusztai L, McArthur H, Kummel S, et al. Event-free survival with pembrolizumab in early triple-negative breast cancer. N Engl J Med. (2022) 386:556–67. doi: 10.1056/NEJMoa2112651
29. Pusztai L, Denkert C, O’Shaughnessy J, Cortes J, Dent R, McArthur H, et al. Event-free survival by residual cancer burden with pembrolizumab in early-stage TNBC: exploratory analysis from KEYNOTE-522. Ann Oncol. (2024) 35:429–36. doi: 10.1016/j.annonc.2024.02.002
30. Ademuyiwa FO, Gao F, Street C, Chen I, Northfelt D, Wesolowski R, et al. A randomized phase 2 study of neoadjuvant carboplatin and paclitaxel with or without atezolizumab in triple negative breast cancer (TNBC) - NCI 10013. NPJ Breast Cancer. (2022) 8:134. doi: 10.1038/s41523-022-00500-3
31. Mittendorf EA, Zhang H, Barrios C, Saji S, Jung K, Hegg R, et al. Neoadjuvant atezolizumab in combination with sequential nab-paclitaxel and anthracycline-based chemotherapy versus placebo and chemotherapy in patients with early-stage triple-negative breast cancer (IMpassion031): a randomised, double-blind, phase 3 trial. Lancet. (2020) 396:1090–100. doi: 10.1016/S0140-6736(20)31953-X
32. Huober J, Barrios C, Niikura N, Jarzab M, Chang Y, Huggins-Puhalla S, et al. Atezolizumab with neoadjuvant anti-human epidermal growth factor receptor 2 therapy and chemotherapy in human epidermal growth factor receptor 2-positive early breast cancer: primary results of the randomized phase III IMpassion050 trial. J Clin Oncol. (2022) 40:2946–56. doi: 10.1200/JCO.21.02772
33. Gianni L, Huang C, Egle D, Bermejo B, Zamagni C, Thill M, et al. Pathologic complete response (pCR) to neoadjuvant treatment with or without atezolizumab in triple-negative, early high-risk and locally advanced breast cancer: NeoTRIP Michelangelo randomized study. Ann Oncol. (2022) 33:534–43. doi: 10.1016/j.annonc.2022.02.004
34. Loi S, Curigliano G, Salgado R, Romero Diaz R, Delaloge S, Rojas C, et al. LBA20 A randomized, double-blind trial of nivolumab (NIVO) vs placebo (PBO) with neoadjuvant chemotherapy (NACT) followed by adjuvant endocrine therapy (ET) &xb1; NIVO in patients (pts) with high-risk, ER+ HER2&x2212; primary breast cancer (BC). Ann Oncol. (2023) 34:S1259–60. doi: 10.1016/j.annonc.2023.10.010
35. Cardoso F, McArthur H, Schmid P, Cortes J, Harbeck N, Telli M, et al. LBA21 KEYNOTE-756: Phase III study of neoadjuvant pembrolizumab (pembro) or placebo (pbo) + chemotherapy (chemo), followed by adjuvant pembro or pbo + endocrine therapy (ET) for early-stage high-risk ER+/HER2&x2013; breast cancer. Ann Oncol. (2023) 34:S1260–1. doi: 10.1016/j.annonc.2023.10.011
36. Barrios C, Harbeck N, Zhang H, Saji S, Jung K, Hegg R, et al. LBA1 Final analysis of the placebo-controlled randomised phase III IMpassion031 trial evaluating neoadjuvant atezolizumab (atezo) plus chemotherapy (CT) followed by open-label adjuvant atezo in patients (pts) with early-stage triple-negative breast cancer (eTNBC). ESMO Open. (2023) 8. doi: 10.1016/j.esmoop.2023.101571
37. Huober J, Barrios C, Niikura N, Jarzab M, Chang Y, Huggins-Puhalla S, et al. 127P Atezolizumab (A) + pertuzumab + trastuzumab (PH) + chemotherapy (CT) in HER2-positive early breast cancer (HER2+ eBC): Final results of the phase III IMpassion050 trial. ESMO Open. (2024) 9. doi: 10.1016/j.esmoop.2024.103115
38. Gianni L, Huang C, Egle D, Bermejo B, Zamagni C, Thill M, et al. LBA19 Event-free survival (EFS) analysis of neoadjuvant taxane/carboplatin with or without atezolizumab followed by an adjuvant anthracycline regimen in high-risk triple negative breast cancer (TNBC): NeoTRIP Michelangelo randomized study. Ann Oncol. (2023) 34:S1258–9. doi: 10.1016/j.annonc.2023.10.009
39. Gianni L, Munzone E, Mansutti M, Bianchini G, Izarzuzaga Y, Caremoli E, et al. Abstract LBO1-02: Pathologic complete response (pCR) of neoadjuvant therapy with or without atezolizumab in HER2-positive, early high-risk and locally advanced breast cancer: APTneo Michelangelo randomized trial. Cancer Res. (2024) 84:LBO1–02-LBO1-02. doi: 10.1158/1538-7445.SABCS23-LBO1-02
40. Emens LA, Esteva F, Beresford M, Saura C, De Laurentiis M, Kim S, et al. Trastuzumab emtansine plus atezolizumab versus trastuzumab emtansine plus placebo in previously treated, HER2-positive advanced breast cancer (KATE2): a phase 2, multicentre, randomised, double-blind trial. Lancet Oncol. (2020) 21:1283–95. doi: 10.1016/S1470-2045(20)30465-4
41. Loi S, Giobbie-Hurder A, Gombos A, Bachelot T, Hui R, Curigliano G, et al. Pembrolizumab plus trastuzumab in trastuzumab-resistant, advanced, HER2-positive breast cancer (PANACEA): a single-arm, multicentre, phase 1b-2 trial. Lancet Oncol. (2019) 20:371–82. doi: 10.1016/S1470-2045(18)30812-X
42. Dirix LY, Takacs I, Jerusalem G, Nikolinakos P, Arkenau H, Forero-Torres A, et al. Avelumab, an anti-PD-L1 antibody, in patients with locally advanced or metastatic breast cancer: a phase 1b JAVELIN Solid Tumor study. Breast Cancer Res Treat. (2018) 167:671–86. doi: 10.1007/s10549-017-4537-5
43. Chia S, Bedard P, Hilton J, Amir E, Gelmon K, Goodwin R, et al. A phase ib trial of durvalumab in combination with trastuzumab in HER2-positive metastatic breast cancer (CCTG IND.229). Oncologist. (2019) 24:1439–45. doi: 10.1634/theoncologist.2019-0321
44. Ogitani Y, Hagihara K, Oitate M, Naito H, Agatsuma T. Bystander killing effect of DS-8201a, a novel anti-human epidermal growth factor receptor 2 antibody-drug conjugate, in tumors with human epidermal growth factor receptor 2 heterogeneity. Cancer Sci. (2016) 107:1039–46. doi: 10.1111/cas.2016.107.issue-7
45. Moragon S, Hernando C, Martinez-Martinex M, Tapia M, Orgega-Morillo B, Lluch A, et al. Immunological landscape of HER-2 positive breast cancer. Cancers (Basel). (2022) 14. doi: 10.3390/cancers14133167
46. Banna G, Hassan M, Signori A, Guinta E, Maniam A, Anpalakhan S, et al. Neoadjuvant chemo-immunotherapy for early-stage non-small cell lung cancer: A systematic review and meta-analysis. JAMA Netw Open. (2024) 7:e246837. doi: 10.1001/jamanetworkopen.2024.6837
47. Shen X, Zhao B. Efficacy of PD-1 or PD-L1 inhibitors and PD-L1 expression status in cancer: meta-analysis. Bmj. (2018) 362:k3529. doi: 10.1136/bmj.k3529
48. Blank C, Haanen J, Ribas A, Schumacher T. CANCER IMMUNOLOGY. The “cancer immunogram. Science. (2016) 352:658–60. doi: 10.1126/science.aaf2834
49. Tarantino P, Gandini S, Trapani D, Criscitiello C, Curigliano G. Immunotherapy addition to neoadjuvant chemotherapy for early triple negative breast cancer: A systematic review and meta-analysis of randomized clinical trials. Crit Rev Oncol Hematol. (2021) 159:103223. doi: 10.1016/j.critrevonc.2021.103223
50. Chen R, Yu Y, Zhang J, Song C, Wang C. Efficacy and safety of neoadjuvant therapy for HR-positive/HER2-negative early breast cancer: a Bayesian network meta-analysis. Expert Rev Anticancer Ther. (2024) 24:599–611. doi: 10.1080/14737140.2024.2350105
51. Shankar B, Zhang J, Naqash A, Forde P, Feliciano J, Marrone K, et al. Multisystem immune-related adverse events associated with immune checkpoint inhibitors for treatment of non-small cell lung cancer. JAMA Oncol. (2020) 6:1952–6. doi: 10.1001/jamaoncol.2020.5012
52. Pang J, Castles B, Byrne D, Button P, Hendry S, Lakhani S, et al. SP142 PD-L1 scoring shows high interobserver and intraobserver agreement in triple-negative breast carcinoma but overall low percentage agreement with other PD-L1 clones SP263 and 22C3. Am J Surg Pathol. (2021) 45:1108–17. doi: 10.1097/PAS.0000000000001701
Keywords: neoadjuvant therapy, immune checkpoint blockade, breast cancer, toxicity, pathologic complete response, event free survival
Citation: Ye Y, Zhang Z, Zhao H and Zhao B (2025) A system review of neoadjuvant immune checkpoint blockade for breast cancer. Front. Immunol. 16:1537926. doi: 10.3389/fimmu.2025.1537926
Received: 02 December 2024; Accepted: 27 February 2025;
Published: 27 March 2025.
Edited by:
Emanuela Marcenaro, University of Genoa, ItalyReviewed by:
Sungchan Gwark, Ewha Womans University Seoul Hospital, Republic of KoreaLubna Chaudhary, Medical College of Wisconsin, United States
Copyright © 2025 Ye, Zhang, Zhao and Zhao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hong Zhao, ZG9jdG9yaG9uZ3poYW9AMTI2LmNvbQ==; Bin Zhao, ZG9jdG9yYmluemhhb0AxMjYuY29t
†These authors have contributed equally to this work
 Yanle Ye1†
Yanle Ye1† Bin Zhao
Bin Zhao