- 1Charité – Universitätsmedizin Berlin, corporate member of Freie Universität Berlin, Humboldt-Universität zu Berlin, Medizinische Klinik m. S. Hämatologie, Onkologie und Tumorimmunologie, Berlin, Germany
- 2Onkologische Schwerpunktpraxis Tiergarten, Berlin, Germany
- 3Berlin Institute of Health (BIH) at Charité Universitätsmedizin Berlin, BIH Center for Regenerative Therapies (BCRT), Berlin, Germany
- 4Institute of Medical Immunology, Charité-Universitätsmedizin Berlin, Berlin, Germany
- 5Department of Immunology, Labor Berlin-Charité Vivantes GmbH, Berlin, Germany
- 6German Cancer Consortium (DKTK) Partner Site Berlin, German Cancer Research Center (DKFZ), Heidelberg, Germany
- 7ECRC Experimental and Clinical Research Center, Charité - Universitätsmedizin Berlin, corporate member of Freie Universität Berlin and Humboldt Universität zu Berlin, Berlin, Germany
Introduction: Infections are a major cause of early morbidity and mortality in patients with multiple myeloma (MM) who are characterized by immunodeficiency secondary to disease. However, prospectively collected data on infection risk in this population are scarce. We aimed at identifying parameters in monoclonal gammopathy of undetermined significance (MGUS) and newly diagnosed MM (NDMM) patients with predictive power for early severe infections (SI).
Methods: We conducted a prospective study with newly diagnosed MGUS and NDMM patients. Besides clinical and laboratory data, immune parameters were collected at initial diagnosis before therapy initiation. Primary endpoint was the occurrence of SI within 12 months after diagnosis.
Results: 45% of patients developed infection, 26% with SI. Four main risk factors for SI were identified: ECOG ≥ 2 (p < 0.001), ISS stage II/III (p = 0.002), therapeutic intervention (p < 0.001), and elevated CD8+ TEMRA cells (p = 0.027). A risk score was compiled, enabling the stratification of patients with low or high risk for SI with a sensitivity of 92.9% and a specificity of 80%.
Conclusion: We developed a straightforward risk score that considers the relevance of T cell fitness in MGUS and NDMM patients and can help physicians to identify patients at risk of infection, thus enabling the implementation of timely and individualized prevention strategies.
1 Introduction
Improving the prediction of infections and establishing stronger correlations between laboratory markers and clinically relevant endpoints has been identified as a critical need by an international expert panel, emphasizing the importance of developing novel biomarkers (1). Monoclonal gammopathy of undetermined significance (MGUS) is a highly prevalent precancerous state in adults above 50 years of age, characterized by monoclonal plasma cell proliferation in the bone marrow and potential progression to multiple myeloma MM (2). The impairment of the immune system due to the underlying disease, particularly the humoral deficiency that accompanies both the diagnoses of MGUS and MM, plays a crucial role. It has been shown that MM patients, and to a lesser extent MGUS patients, exhibit significantly lower antibody titers against common pathogens compared to age-matched controls (3), which explains the increased risk of infections with encapsulated pathogens such as haemophilus influenzae and streptococcus pneumoniae. Additional immune abnormalities beyond hypogammaglobulinemia have been detected in MM patients (4, 5). Some of these findings have been associated with clinical manifestations: it has been suggested that patients who would develop infections early after initial diagnosis exhibited lower numbers of circulating CD19+ B-cells compared to those who remained infection free. Further, high CD19+ B-cell numbers have been associated with a decreased incidence, severity, and mortality from infections and with better overall survival (6, 7). Notably, antineoplastic therapy is also known to exacerbate pre-existing SID (4, 5, 8–10).
Several routine clinical and laboratory parameters, particularly those denoting aggressive disease, have been identified as predictors for infection risk, including international staging system (ISS) stage, low hemoglobin, low platelet count, high β2-microglobulin (β2-MG), elevated lactate dehydrogenase (LDH) and serum calcium levels (11–16).
Significant advances in therapeutic strategies in recent years have transformed MM into a chronic disease. Nevertheless, the early mortality rate of MM patients remains high (17). In addition to deaths due to progressive disease, heart and kidney failure, infections are one of the leading causes of early mortality, accounting for approximately 40% of early deaths in MM (18).
Here, we provide data from a prospective single-center trial analyzing clinical and laboratory characteristics of MGUS and newly diagnosed MM (NDMM) patients and propose an easy-to-use prognostic tool to identify patients at increased risk of early severe infections (SI). To the best of our knowledge, this is the first study combining a broad range of immune parameters with clinical features and routine laboratory tests in the prognostic analysis, thus capturing and ranking the impact of disease-related immune dysfunctions on infectious complications in MGUS and NDMM.
Our aim was to identify parameters in MGUS and NDMM patients that have a reliable predictive power for SI within the first year after diagnosis to facilitate the timely implementation of individualized prevention strategies.
2 Methods
2.1 Patients
64 patients with MGUS or NDMM who presented to our outpatient or inpatient center between 01/2019 – 09/2022 were prospectively enrolled. Clinical as well as an array of immunological parameters (Supplementary Table 1) and laboratory data (Supplementary Table 2) were investigated at time of study inclusion, in any case before the initiation of a specific therapy, including corticosteroids. Patients were monitored for infectious complications within the first year after initial diagnosis. All patients gave their informed consent, and the study was approved by the Ethics Committee of Charité-Universitätsmedizin Berlin.
2.2 Classifications
Infections were graded according to the Common Terminology Criteria for Adverse Events (CTCAE) v5.0; SI were classified as infections of CTCAE grade 3 or higher. In case of multiple SI, the most severe was considered for the analysis. Early SI were defined as severe infections occurring within one year after initial diagnosis. Clinical performance status of patients was categorized using the ECOG performance status (19). All NDMM patients were classified using the ISS from 2005 (20).
2.3 Experimental analyses
Enumeration and phenotyping of naïve and memory T-cell subsets was performed in EDTA whole blood samples using accredited test methods (DIN EN ISO 15189) at the Department of Immunology at Labor Berlin, as described previously (21). Briefly, the following mouse anti-human fluorescently-labelled monoclonal antibodies (all from Beckman Coulter, Krefeld, Germany) were used: CD3 APC-A750 (clone UCHT1), CD4 ECD (clone SCFI12T4D11), CD8 APC (clone B9.11), CD45RA Pacific-Blue (clone J33), and CCR7 PE (clone G043H7). Stained samples were acquired on a ten-color Navios EX flow cytometer and analyzed using Navios Software (Beckman Coulter). For gating strategy see Supplementary Figure 1. Interleukin-8 was measured from patients’ plasma after lysis of erythrocytes by immunoassay using an Immulite 1000 (Siemens, München, Germany) and Immulite Kit LK8P1 (Siemens, München, Germany).
2.4 Statistical analysis
Patients were grouped according to the occurrence of SI. Group 1: with no or non-severe infections, group 2: with SI (CTCAE ≥ 3). Data points that were >1.5 times the interquartile range below quartile one or >1.5 times the interquartile range above quartile three were considered outliers. The data set was cleaned from outliers by removing patients from the analysis with outliers in >50% of parameters (n = 3). Missing numerical values were imputed with the mean if absolute skewness was < 0.5, otherwise the median was used. Categorical features were imputed with the mode (highest frequency). Statistical significance of numerical variables was calculated using student’s t-test. Pearson’s correlation coefficients were used to calculate linear correlations among all significant numerical features for internal validation and cross-correlation. Statistical significance of categorical variables was calculated using chi-squared (χ2) test. P-values <0.05 were defined as statistically significant. 26 numerical variables met statistical significance threshold and were retained for expert review (Supplementary Table 1). Of these, clinically significant numerical parameters were selected and transformed into discrete variables by setting cut-offs according to laboratory reference values or, if not applicable, clinically feasible cut-off values. Statistically significant variables were selected by expert assessment based on their clinical and/or biological relevance to be included in a multiple logistic regression model. In addition, results of the correlation analysis were included in parameter selection. The final model included four variables. A scoring system was developed in which factors were assigned scores based on their coefficient in the multivariate logistic model. Based on the cumulative score, patients were categorized into groups with high (≥7 points) or low (<7 points) risk of severe infection.
3 Results
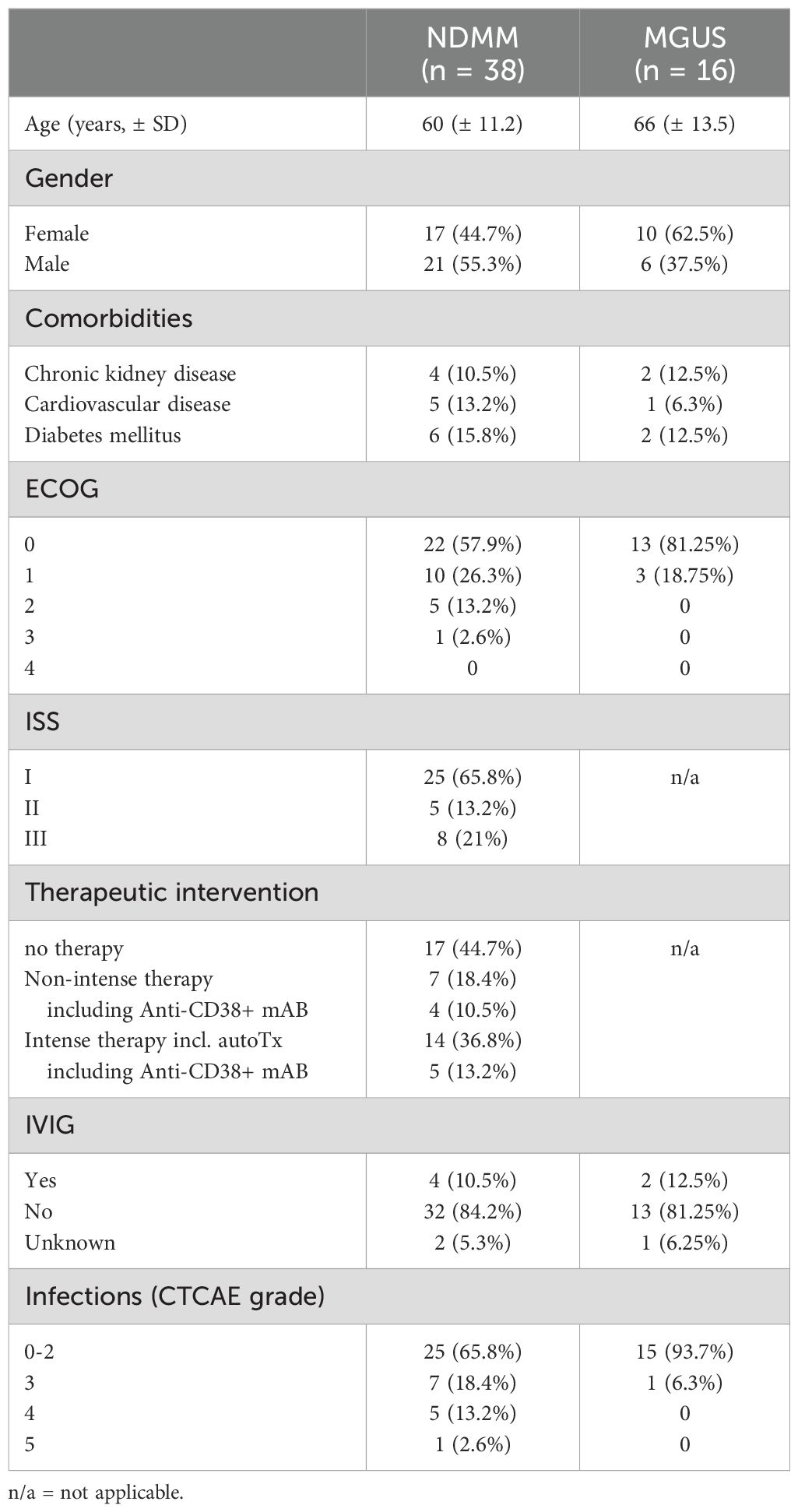
3.1 Patient characteristics
A total of 64 patients were enrolled in our analysis; seven patients were excluded due to loss of follow up or non-newly diagnosed disease; three patients were excluded during statistical analysis due to outliers in >50% of investigated parameters. Characteristics of analyzed patients are summarized in Table 1. We analyzed a total of 54 patients, 16 diagnosed with MGUS and 38 with NDMM irrespective of treatment intention (watch and wait, non-intensive or intensive therapy). Median age was 66 years (SD ± 13.5) in the MGUS cohort and 60 years (± 11.2) in the NDMM with even distribution by sex (27 males, 27 females). Most patients exhibited an ECOG 0-1, only 15.8% showed an ECOG of 2 or higher (Table 1). Concerning prognostic grading of NDMM, 65.8% of patients were categorized as ISS I, 13.2% as ISS II, and 21% as ISS III. In the NDMM cohort, 31.6% received no therapy; 39.5% of patients received a high-intensity therapy including autologous stem cell transplantation (autoTx) while 28.9% of patients received low-intensity treatment (Table 1). All NDMM patients received continuous pneumocystis jirovecii prophylaxis with sulfamethoxazole/trimethoprim as well as acyclovir in a kidney-function adjusted, prophylactic dose. Patients who underwent autoTx received ciprofloxacin 500 mg bidaily during neutropenia until reaching leukocytes >1/nl, continuous acyclovir prophylaxis in a kidney-function adjusted dose as well as pneumocystis jirovecii prophylaxis with sulfamethoxazole/trimethoprim after sufficient engraftment.
3.2 Characteristics of early SI
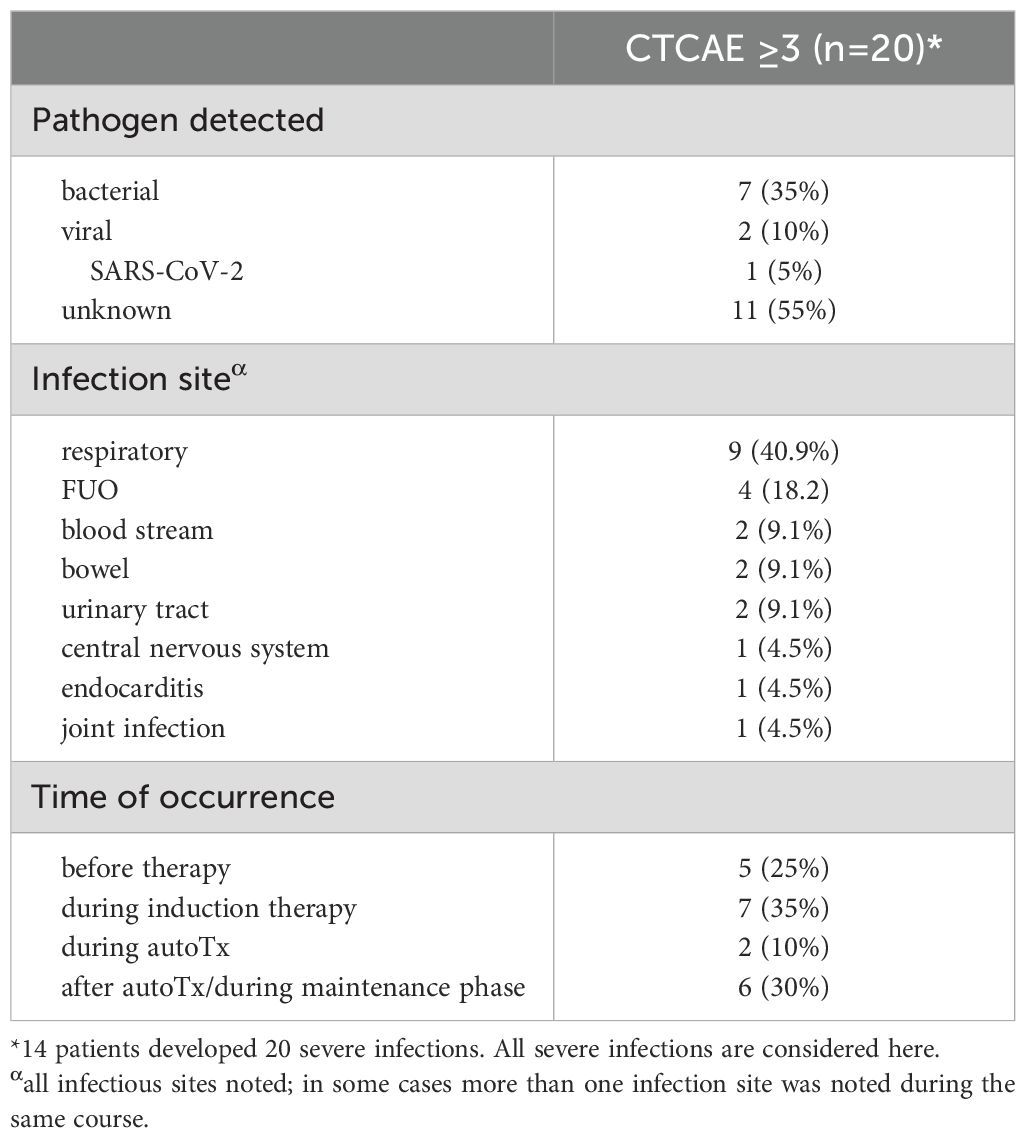
24 of 54 patients developed an infection within the first year of initial diagnosis, 14 patients experienced infections classified as severe (CTCAE 3 or higher) (Table 1). In these 14 patients, we documented a total of 20 infections during the observation period. SI occurred almost exclusively in the group of NDMM patients. Prevalence of early SI increased with advancing ISS stage. The majority of SI occurred before or during induction therapy (60% of SI). In most cases, no pathogen was identified. However, a bacterial infection was found in around one third of SI patients and a viral infection in 10% of SI patients. Most SI were respiratory tract infections (40.9%). Detailed information on all SI can be found in Table 2. Though study recruitment was in part conducted during the SARS-CoV-2 pandemic, we only observed one severe (CTCAE grade 3) SARS-CoV-2 infection in our cohort.
3.3 Clinical characteristics and biomarkers associated with early SI
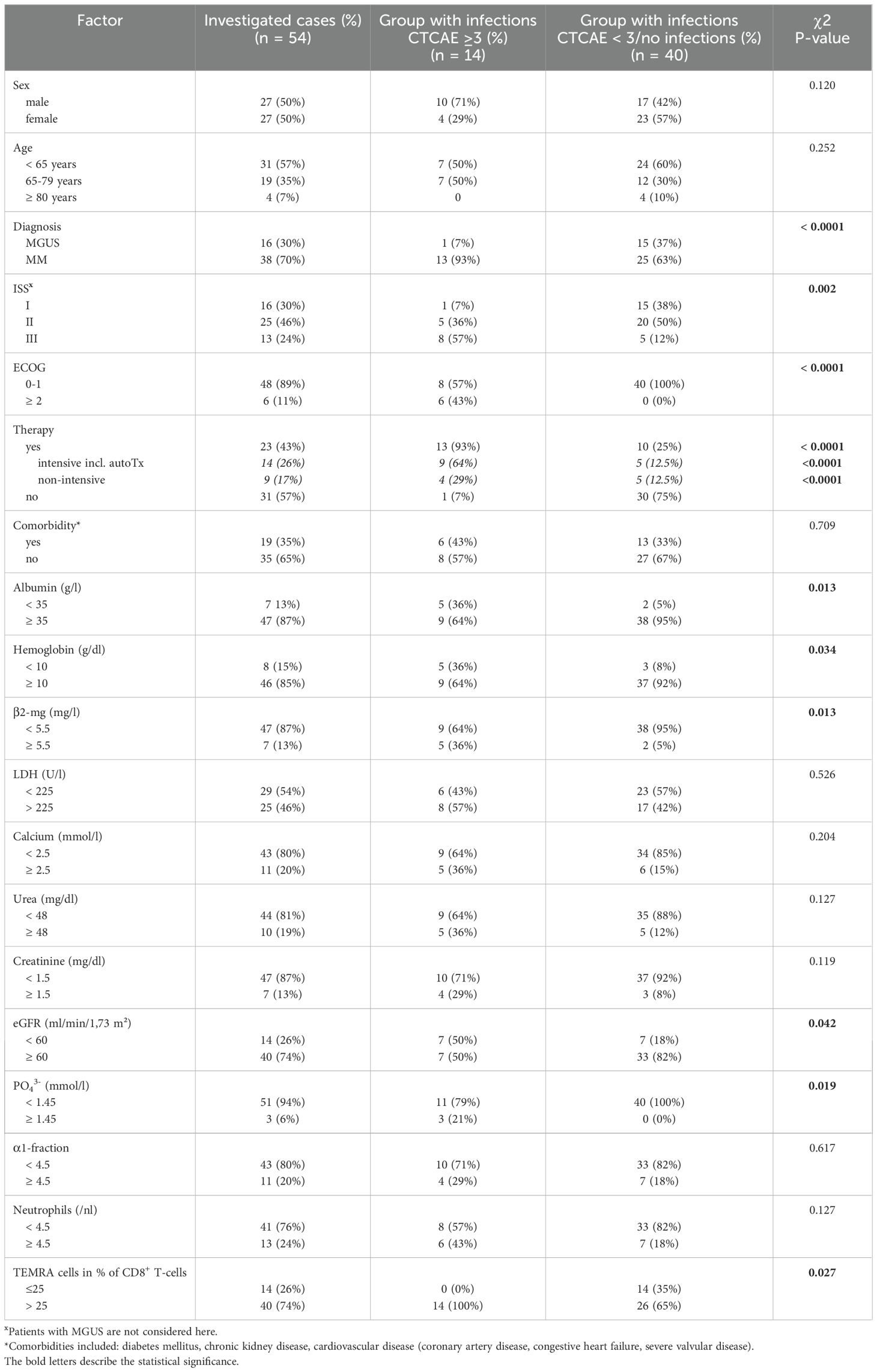
We aimed at identifying relevant parameters associated with SI occurring within the first year after diagnosis. We found 26 parameters to be significantly associated with the occurrence of SI (Table 3). The following parameters were selected for further evaluation due to clinical significance: albumin, hemoglobin, β2-MG, LDH, serum calcium, urea, creatinine, eGFR, inorganic phosphate, absolute neutrophil count, the alpha1-fraction of serum immunofixation, and CD8+ terminally differentiated memory T-cells [CD3+/CD8+/CD45RA+/CCR7-, which are often referred to as CD45RA+ effector-memory T-cells (TEMRA)]. For these laboratory parameters, clinically feasible and analytically implementable cut-off values were set (Table 4). In the univariate analysis, we identified NDMM diagnosis compared to MGUS (p < 0.001), ISS stage II and III (p = 0.002), ECOG ≥ 2 (p < 0.001), and therapeutic intervention (p < 0.001) as highly significant clinical parameters. The commencement of therapy was in any case associated with the occurrence of early SI, this effect was even more pronounced, when an intensive therapy regimen containing autoTx was administered (Figure 1A). Nine patients (23.7%) received a therapy containing an anti-CD38 antibody such as Isatuximab or Daratumumab. Four of these nine patients developed an SI, in the group receiving conventional therapy, ten out of 16 patients developed an SI. We found no significant differences in the occurrence of infections depending on the use of anti-CD38 antibody therapy (data not shown). Age, sex, and presence of comorbidities were not associated with the occurrence of early SI (Table 4). Key lab findings included albumin <35 g/l, anemia with hemoglobin <10 g/l, elevated β2-MG >5.5 mg/l, reduced estimated glomerular filtration rate (eGFR) <60 ml/min/1,73 m², and elevated serum phosphate (PO43-) >1.45 mmol/l, all tied to a higher SI risk (Table 4, Figure 1B). A comprehensive summary of all investigated parameters and their corresponding p-values, calculated using Student’s t-test, is provided in Supplementary Table 3.
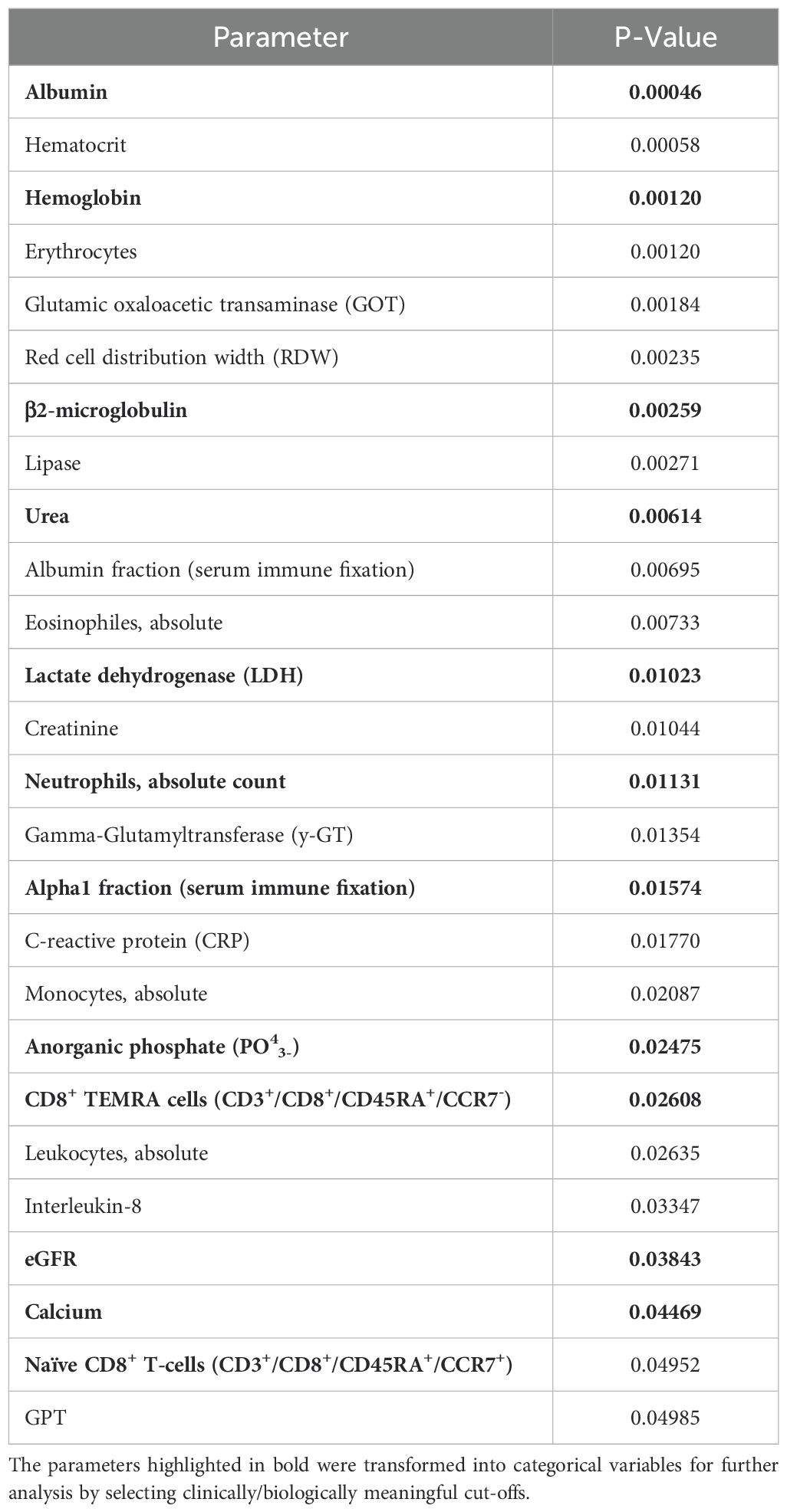
Table 3. All laboratory parameters significantly associated with occurrence of severe infections in MGUS and MM patients as determined by student’s t-test.
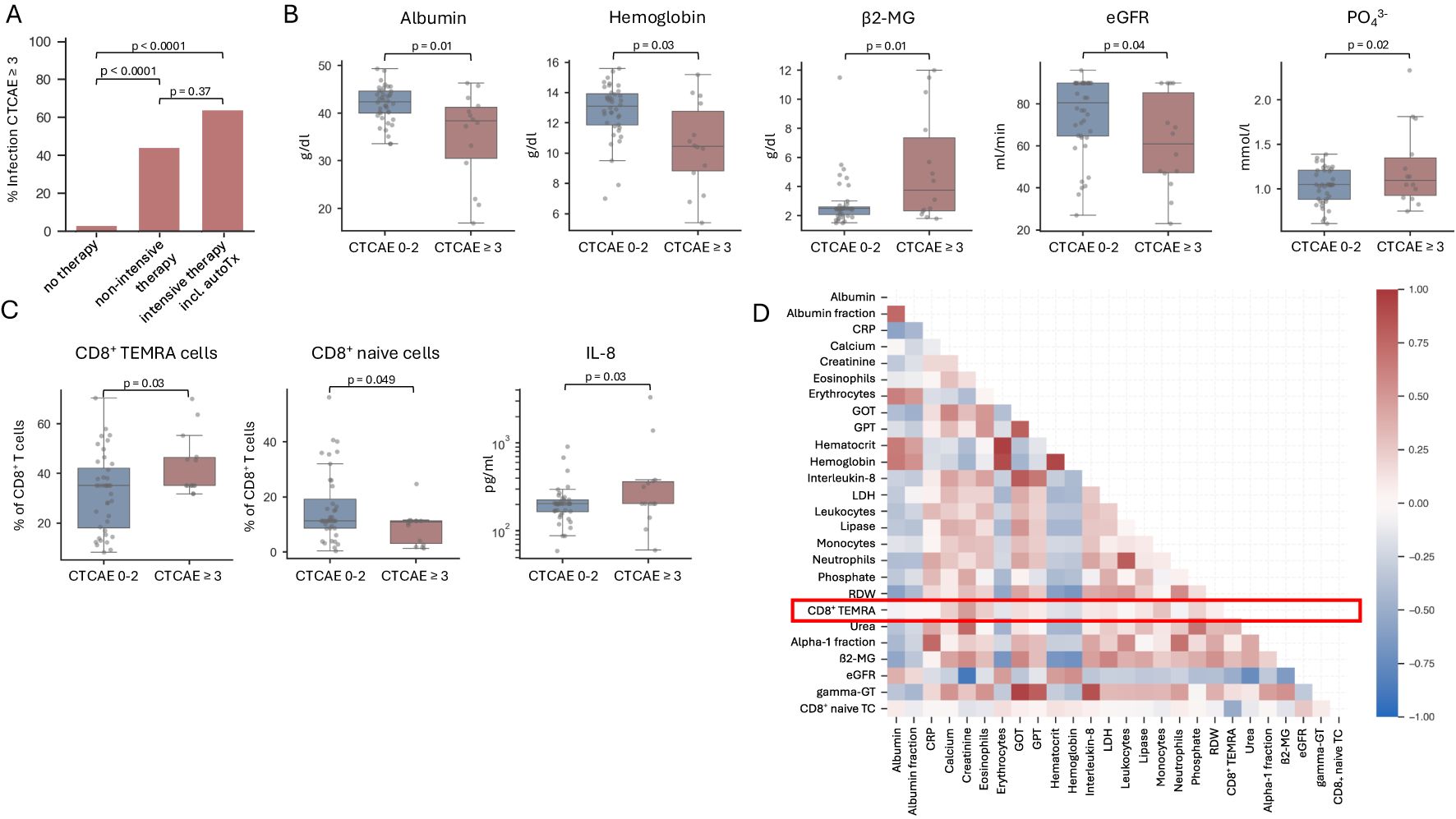
Figure 1. Risk factors associated with early severe infection in MGUS and NDMM patients. (A) Bar graph illustrating the percentage of severe infections (CTCAE 3 or higher) in MGUS and NDMM patients receiving no therapy, non-intensive therapy or intensive therapy including autologous stem cell transplantation within the first year after initial diagnosis. n=54. (B, C) Box plots showing parameters denoting aggressive disease and T cell exhaustion are associated with the occurrence of severe infections (CTCAE 3 or higher). Significance was calculated using a student’s t-test. n=54. (D) The figure depicts a correlation matrix of relevant laboratory parameters analyzed using Pearson’s correlation coefficient. The inclusion of parameters was based on their statistical significance, determined through prior analysis using a student’s t-test. n=54.
3.4 Association of TEMRA cell levels with early SI in MGUS and NDMM patients
Interestingly, we found that patients with SI had higher CD8+ TEMRA cells (CD3+/CD8+/CD45RA+/CCR7-) relative to all CD8+ T-cells at time of first diagnosis as compared to those who did not develop early SI (Figure 1C). Furthermore, we observed a significant increase in the inflammatory-mediating chemokine IL-8 in patients with SI (Figure 1C). Of note, when investigating NDMM patients separately, we observed a strong trend towards higher CD8+ TEMRAs (p = 0.05017) as well as elevated IL-8 levels (p = 0.05036) in patients developing SI (Supplementary Figure 2). To further analyze the subgroup of patients with elevated CD8+ TEMRAs at initial diagnosis, we have analyzed potential differences between patients with low and high CD8+ TEMRAs concerning other laboratory parameters and clinical characteristics. Aside from the incidence of severe infections, no significant differences were observed. Interestingly, the shift towards high CD8+ TEMRAs led to an overall reduction in naïve, effector memory, and central memory T-cells. Data on additional T-cell and B-cell populations as well as cytokines are summarized in Supplementary Figures 3–5 and Supplementary Table 3.
3.5 CD8+ TEMRA cells as an independent risk factor for SI in MGUS and MM patients
Using a correlation matrix of Pearson’s coefficients for all parameters significantly associated with the occurrence of SI, our analysis confirmed correlations between markers of advanced disease, such as those indicating impaired kidney function and hematopoiesis, along with β2-MG and LDH (Figure 1D). Intriguingly, CD8+ TEMRA cells and consequently CD8+ naïve T-cells did not demonstrate significant correlations with other parameters. Consequently, CD8+ TEMRA cells merit recognition as an independent risk factor for SI.
3.6 Individual risk prediction for SI in MGUS and NDMM patients by multiple logistic regression modeling
To discern the risk of early SI in MGUS and NDMM patients, a logistic regression model was employed. Four key variables emerged as paramount predictors of early SI: therapeutic intervention, ISS stage, ECOG, and the relative abundance of CD8+ TEMRA cells. The model achieved an overall accuracy of 88%, with a recall of 75%, and an area under the curve (AUC) of 0.96 (Figure 2A). A risk score, titled PREDICT-SID (Prediction of secondary immunodeficiency in MGUS and NDMM), was developed based on four primary risk factors identified in the study: induction of MM therapy, ECOG, ISS stage, and CD8+ TEMRAs. Each risk factor was converted into a point value according to the coefficient (Figure 2B). Additive scoring was used to classify patients into two risk groups: low (0-6 points) and high risk (≥7 points). The PREDICT-SID score shows a sensitivity of 92% and a specificity of 80%. Furthermore, we compared PREDICT-SID against two existing risk scores (15, 16), both based on retrospective analyses; characteristics are summarized in Table 5. First, we evaluated infection risk for each patient in our cohort using the published scores by Dumontet et al. (16) and Mai et al. (15), calculating an individual risk score based on each patient’s clinical and laboratory parameters according to each model. We then assessed the performance of each score in predicting SI by comparing the predicted risk classifications with the observed outcomes. This comparison allowed us to compute sensitivity and specificity via a confusion matrix: in our cohort, the Dumontet et al. score, which incorporates ECOG, β2-MG, hemoglobin, and LDH, yielded a sensitivity of 42.9% and specificity of 97.5%, while the Mai et al. score, which considers platelet count, ECOG, ISS, and age, achieved a sensitivity of 78.6% and specificity of 42.4% (Figure 2C). Secondly, the risk stratifiers from the two published scores were incorporated into a logistic regression analysis of our dataset, and the area under the receiver operating characteristic (ROC) curve was calculated for both scores, showing an inferior ROC and AUC of the two published scores (Figure 2D) when compared to PREDICT-SID (Figure 2A).
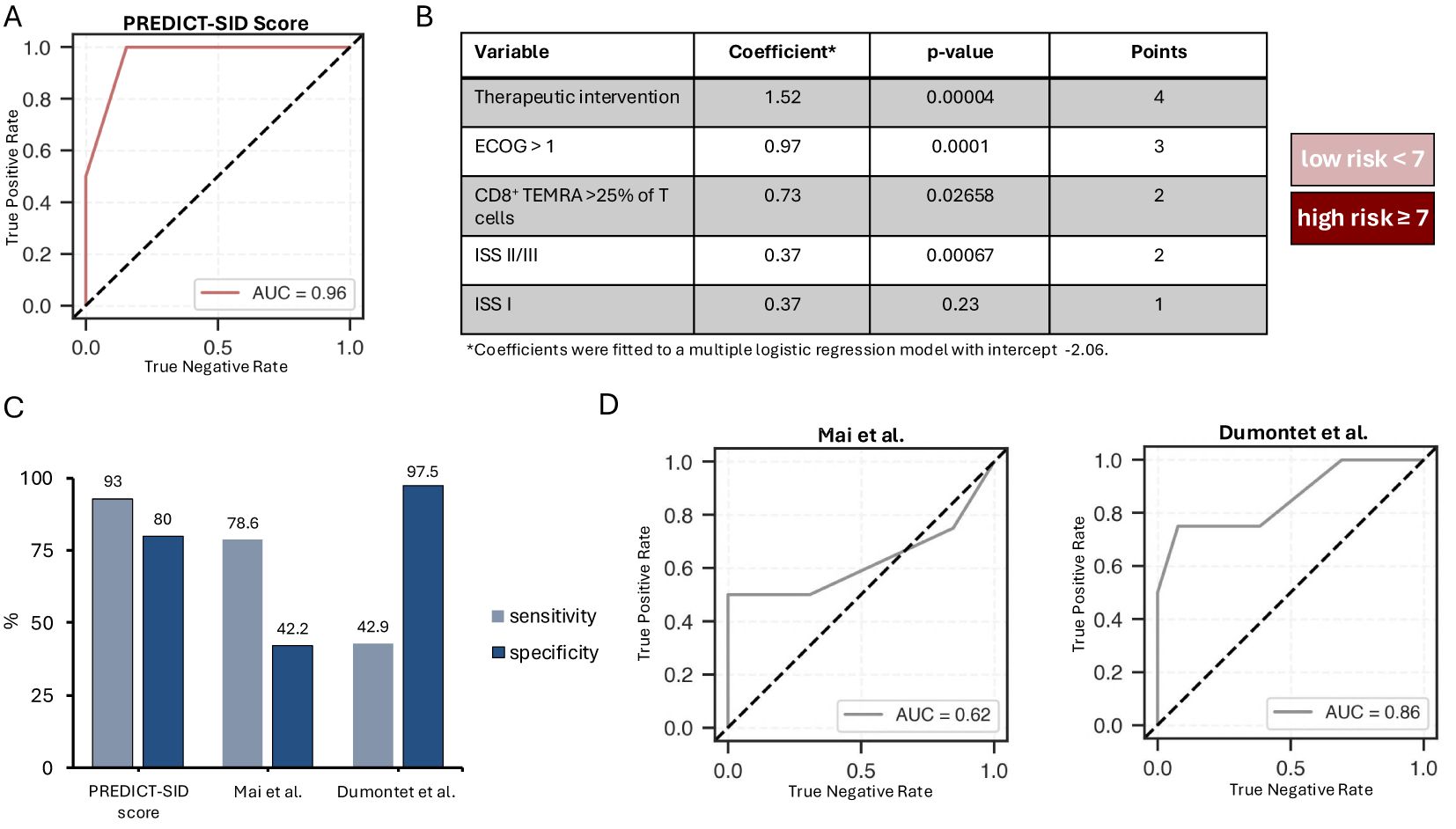
Figure 2. PREDICT score for the stratification of MGUS and NDMM patients at high risk of early severe infection. (A) Assessment of the presented multiple logistic regression model depicting the receiver operating characteristic (ROC) curve and the area under der ROC curve (AUC). (B) Summary of the variables included in the PREDICT risk score with presentation of their coefficients, p-values and the points assigned to the variables; patients with a score <7 are at low, patients with a score >= 7 are at high risk for severe infections (CTCAE 3 or higher. (C) Bar graph imaging the sensitivity and specificity of the presented risk score as well as two other published risk scores when applied to our dataset. (D) Assessment of the multiple logistic regression model considering the risk stratifiers proposed by Mai et al. and Dumontet et al., depicting the receiver operating characteristic (ROC) curve and the area under the curve (AUC).
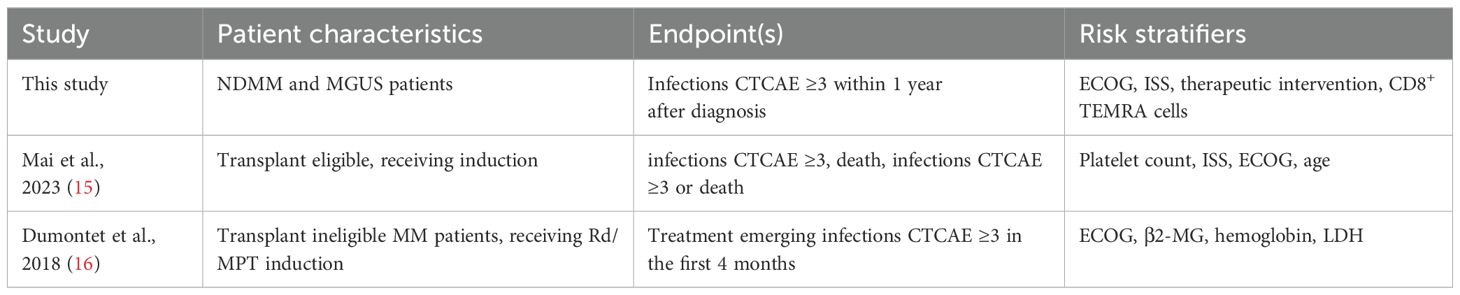
Table 5. Comparison of risk scores for the assessment of risk for severe infections MM and MGUS patients.
4 Discussion
In this prospective observational study, we investigated the risk factors associated with early SI in MGUS and NDMM patients linking performance status, markers of advanced disease, therapeutic intervention, and TEMRA cells to an increased SI risk within the first year after diagnosis.
Our findings underscore the clinically imminent and substantial burden of infections in this patient population, with 46% of patients experiencing infections of any severity and over a quarter of patients developing SI (CTCAE ≥ 3) within the first year. These results align with previous studies showing that NDMM patients have an early infection risk ranging widely between 11 and 78% (11, 15, 16, 22), depending largely on the performance status and/or transplant eligibility of the analyzed patient cohort. The majority of infections occurred during the first 3-12 months following MM diagnosis (15, 17, 22–24), contributing significantly to early morbidity and mortality. MGUS patients, though typically less immunocompromised than NDMM, also face heightened infection risks due to humoral insufficiency (3, 25). For instance, a population-based study reported a twofold increased risk of bacterial and viral infections in MGUS patients, including significantly higher risks for pneumonia and septicemia (26). Furthermore, the elevated susceptibility to infection has been highlighted in studies of infection-related outcomes, such as increased morbidity and mortality following COVID-19 infection (27, 28). Recommendations published by the European Myeloma Network in 2014 also highlighted the increased risk of infections and the associated higher mortality in MGUS patients compared to the general population (29). These observations highlight the need for early risk stratification in MGUS and NDMM patients, allowing for individualized preventive measures to be implemented at diagnosis, potentially reducing the incidence of serious infections and improving patient outcomes in this vulnerable population.
In the presented study, markers of advanced disease, e.g. ISS stages II and III, low albumin and hemoglobin levels, elevated β2-MG, impaired renal function, and antineoplastic therapy were found to be strongly associated with SI. We did not find an increase in infections in patients being treated with anti-CD38 antibodies. However, the small number of patients receiving therapy including anti-CD38 antibodies must be acknowledged as a limitation. The influence of antineoplastic therapy on immune competence of MM patients has been manifoldly described (4, 5, 8–10, 30). In our study, the start of antineoplastic therapy was among the most significant contributors to the risk of SI. This effect was even more pronounced when intensive treatment including autoTx was performed. This underscores the delicate balance between disease control and immunosuppression in MM treatment, necessitating careful consideration of infection prevention strategies during treatment, especially during induction therapy.
Consistent with prior analyses, we confirmed a poor performance status, as measured by ECOG score, to be strongly associated with SI, emphasizing the impact of overall health and functional physical capacity on infection incidence in NDMM and MGUS patients (15, 16, 22).
Our study uniquely assessed immunological parameters’ influence on infection in MGUS and NDMM (Supplementary Table 1). Patients with SI exhibited increased CD8+ TEMRA cells and IL-8 levels compared to patients who do not suffer from infectious complications. TEMRA cells are antigen experienced and are usually considered to be terminally differentiated T-cells that may arise as a result of prolonged antigen exposure, such as that seen in chronic infection with cytomegalovirus or Epstein-Barr virus (31). TEMRA cells are characterized by low proliferative capacity as well as high sensitivity to apoptosis (32). It has been proposed that prolonged antigen exposure leads to a distortion in the TCR repertoire, evidenced by the oligoclonality observed in TEMRA cells (33), potentially resulting in compromised defense against new infections (34). CD8+ TEMRA cells have also been found to accumulate in the bone marrow of MM patients and were characterized to be functionally severely impaired, displaying features of exhaustion and senescence (35). The authors of the study hypothesized that this T-cell-mediated secondary immunodeficiency is driven by myeloma cells and can be partly cured by treating the underlying disease.
The observed higher abundance of TEMRA and fewer naïve CD8+ T-cells in patients developing early SI may reflect a reduced capability to recognize and adequately response to new pathogens, contributing to the increased susceptibility to infections, as has been reported elsewhere (34). The problematic higher frequencies of TEMRA cells in some patients can be also relevant in the context of modern therapeutic strategies, such as bispecific antibodies and CAR-T cell therapies, which rely on the functional competency of T-cells for efficacy (36, 37). Understanding the baseline immunological status, including TEMRA cells and more generally T-cell dysfunction, thus holds dual significance: it aids in identifying high-risk patients for early SI and might inform the stratification for T-cell engaging immunotherapies.
To translate our findings into clinical practice, we have created a straightforward risk score, PREDICT-SID, that can be used to identify MGUS and NDMM patients at high risk of early SI. By combining clinical patient and routine laboratory characteristics, and, for the first time, immunological parameters, we created a compact prediction model based on four parameters: ISS stage, ECOG, MM therapy, and CD8+ TEMRA cells.
Our model, unlike previously published scores (15, 16) that focused solely on advanced disease markers and performance, incorporates an immune dysregulation marker, achieving superior model performance with a sensitivity of 93%, a specificity of 80%, and an AUC of 0.96. The discrepancies in the results obtained by the scoring systems are most likely due to the underlying differences in the characteristics of the patients studied. Mai et al. only included patients who were eligible for autoTx, while Dumontet et al. included patients who were not eligible for transplantation and were therefore likely to be in poorer general health. In contrast, the present study included both patients eligible for transplantation and those not eligible for transplantation, as well as MGUS patients under a watch and wait strategy.
Despite the good performance of the model, our study is limited by its sample size and single-center design with a heterogeneous patient cohort including MGUS and NDMM patients, all with different treatment indications and eligibility. However, a notable strength of the present study lies in its prospective design and the assessment of immunologic characteristics including T-cell subset characterization and cytokine profiling, allowing the clinical relevance of these characteristics to be determined.
Our findings underscore the complexity of infection risk in MGUS and NDMM patients and highlight the need for a multifaceted approach to risk assessment. We were able to show that if patient risk factors e.g. poor performance status, advanced disease, and elevated TEMRA cell levels concur with the need for antineoplastic therapy, the risk of a potentially life-threatening infection rises significantly. Integrating risk stratification, like the PREDICT-SID score, into clinical care could aid in early intervention and tailored treatment strategies, reducing ‘overtreatment’. As we move forward, prospective validation of our model and further exploration of T-cell dysfunctions in MGUS and MM will be crucial in refining our understanding of infection risk dynamics and optimizing patient care.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Ethics Committee of Charité – University Medicine Berlin. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
ET: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Software, Supervision, Visualization, Writing – original draft, Writing – review & editing. DB: Data curation, Project administration, Writing – original draft, Writing – review & editing. CH: Data curation, Project administration, Writing – original draft, Writing – review & editing. IB: Data curation, Project administration, Supervision, Writing – original draft, Writing – review & editing. AN: Data curation, Project administration, Writing – original draft, Writing – review & editing. PS: Formal analysis, Methodology, Writing – original draft, Writing – review & editing. CM: Data curation, Formal analysis, Investigation, Writing – original draft, Writing – review & editing. LB: Resources, Supervision, Writing – original draft, Writing – review & editing. MF: Formal analysis, Investigation, Methodology, Resources, Supervision, Writing – original draft, Writing – review & editing. I-KN: Conceptualization, Formal analysis, Funding acquisition, Investigation, Resources, Supervision, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was supported by Octapharma AG (grant number IS-II-DE-02) and Baxalta US Inc, now part of Takeda (grant number IIR-DEU-001589/IISR-2017-104155). The funding sources had no role in the design of the study, the collection, analysis, and interpretation of the data, the writing of the manuscript, or the decision to submit the manuscript for publication. Further, the project was supported by the Berlin Institute of Health at Charité -Universitätsmedizin Berlin, Berlin, Germany (BIH) and the Stiftung Charité (BIH Johanna Quandt funding).
Acknowledgments
We thank all the participating patients and their families/caregivers. ET was a fellowship holder in the BIH Charité Junior Clinician Scientist Program funded by the Charité Universitätsmedizin Berlin and the Berlin Institute of Health (BIH).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2025.1532645/full#supplementary-material
References
1. Jolles S, Giralt S, Kerre T, Lazarus HM, Mustafa SS, Papanicolaou GA, et al. Secondary antibody deficiency in chronic lymphocytic leukemia and non-Hodgkin lymphoma: Recommendations from an international expert panel. Blood Rev. (2023) 58:101020. doi: 10.1016/j.blre.2022.101020
2. Abeykoon JP, Tawfiq RK, Kumar S, Ansell SM. Monoclonal gammopathy of undetermined significance: evaluation, risk assessment, management, and beyond. Fac Rev. (2022) 11:34. doi: 10.12703/r/11-34
3. Pilarski LM, Joy Andrews E, Mant MJ, Ruether BA. Humoral immune deficiency in multiple myeloma patients due to compromised B-cell function. J Clin Immunol. (1986) 6:491–501. doi: 10.1007/BF00915255
4. Kay NE, Leong T, Bone N, Kyle RA, Greipp PR, Van Ness B, et al. T-helper phenotypes in the blood of myeloma patients on ECOG phase III trials E9486/E3A93: T-helper Cells in Untreated and Treated Myeloma Patients. Br J Haematol. (1998) 100:459–63. doi: 10.1046/j.1365-2141.1998.00609.x
5. Schütt P, Brandhorst D, Stellberg W, Poser M, Ebeling P, Müller S, et al. Immune parameters in multiple myeloma patients: influence of treatment and correlation with opportunistic infections. Leuk Lymphoma. (2006) 47:1570–82. doi: 10.1080/10428190500472503
6. Kay NE, Leong T, Kyle RA, Greipp P, Billadeau D, Van Ness B, et al. Circulating blood B cells in multiple myeloma: analysis and relationship to circulating clonal cells and clinical parameters in a cohort of patients entered on the Eastern Cooperative Oncology Group phase III E9486 clinical trial. Blood. (1997) 90:340–5. doi: 10.1182/blood.V90.1.340.340_340_345
7. Kay NE, Leong TL, Bone N, Vesole DH, Greipp PR, Van Ness B, et al. Blood levels of immune cells predict survival in myeloma patients: results of an Eastern Cooperative Oncology Group phase 3 trial for newly diagnosed multiple myeloma patients. Blood. (2001) 98:23–8. doi: 10.1182/blood.V98.1.23
8. Teh BW, Harrison SJ, Worth LJ, Thursky KA, Slavin MA. Infection risk with immunomodulatory and proteasome inhibitor–based therapies across treatment phases for multiple myeloma: A systematic review and meta-analysis. Eur J Cancer. (2016) 67:21–37. doi: 10.1016/j.ejca.2016.07.025
9. Heck C, Steiner S, Kaebisch EM, Frentsch M, Wittenbecher F, Scheibenbogen C, et al. CD4+ T cell dependent B cell recovery and function after autologous hematopoietic stem cell transplantation. Front Immunol. (2021) :12:736137. doi: 10.3389/fimmu.2021.736137
10. Ying L, YinHui T, Yunliang Z, Sun H. Lenalidomide and the risk of serious infection in patients with multiple myeloma: a systematic review and meta-analysis. Oncotarget. (2017) 8:46593–600. doi: 10.18632/oncotarget.16235
11. Lin C, Shen H, Zhou S, Liu M, Xu A, Huang S, et al. Assessment of infection in newly diagnosed multiple myeloma patients: risk factors and main characteristics. BMC Infect Dis. (2020) 20:699. doi: 10.1186/s12879-020-05412-w
12. Sørrig R, Klausen TW, Salomo M, Vangsted A, Gimsing P. Risk factors for infections in newly diagnosed Multiple Myeloma patients: A Danish retrospective nationwide cohort study. Eur J Haematol. (2019) 102:182–90. doi: 10.1111/ejh.2019.102.issue-2
13. Sørrig R, Klausen TW, Salomo M, Vangsted A, Gimsing P. Risk factors for blood stream infections in multiple myeloma: A population-based study of 1154 patients in Denmark. Eur J Haematol. (2018) 101:21–7. doi: 10.1111/ejh.13066
14. Shang Y, Wang W, Liang Y, Kaweme NM, Wang Q, Liu M, et al. Development of a risk assessment model for early grade ≥ 3 infection during the first 3 months in patients newly diagnosed with multiple myeloma based on a multicenter, real-world analysis in China. Front Oncol. (2022) 12:772015. doi: 10.3389/fonc.2022.772015
15. Mai EK, Hielscher T, Bertsch U, Salwender HJ, Zweegman S, Raab MS, et al. Predictors of early morbidity and mortality in newly diagnosed multiple myeloma: data from five randomized, controlled, phase III trials in 3700 patients. Leukemia. (2023). https://www.nature.com/articles/s41375-023-02105-6.
16. Dumontet C, Hulin C, Dimopoulos MA, Belch A, Dispenzieri A, Ludwig H, et al. A predictive model for risk of early grade ≥ 3 infection in patients with multiple myeloma not eligible for transplant: analysis of the FIRST trial. Leukemia. (2018) 32:1404–13. doi: 10.1038/s41375-018-0133-x
17. Blimark C, Holmberg E, Mellqvist UH, Landgren O, Bjorkholm M, Hultcrantz M, et al. Multiple myeloma and infections: a population-based study on 9253 multiple myeloma patients. Haematologica. (2015) 100:107–13. doi: 10.3324/haematol.2014.107714
18. Terebelo H, Srinivasan S, Narang M, Abonour R, Gasparetto C, Toomey K, et al. Recognition of early mortality in multiple myeloma by a prediction matrix: TEREBELO et al. Am J Hematol. (2017) 92:915–23. doi: 10.1002/ajh.24796
19. Oken MM, Creech RH, Tormey DC, Horton J, Davis TE, McFadden ET, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol. (1982) 5:649–55. doi: 10.1097/00000421-198212000-00014
20. Greipp PR, Miguel JS, Durie BGM, Crowley JJ, Barlogie B, Bladé J, et al. International staging system for multiple myeloma. J Clin Oncol. (2005) 23:3412–20. doi: 10.1200/JCO.2005.04.242
21. Klehmet J, Staudt M, Ulm L, Unterwalder N, Meisel A, Meisel C. Circulating lymphocyte and T memory subsets in glucocorticosteroid versus IVIG treated patients with CIDP. J Neuroimmunol. (2015) 283:17–22. doi: 10.1016/j.jneuroim.2015.03.023
22. Encinas C, Hernandez-Rivas JÁ, Oriol A, Rosiñol L, Blanchard MJ, Bellón JM, et al. A simple score to predict early severe infections in patients with newly diagnosed multiple myeloma. Blood Cancer J. (2022) 12:68. doi: 10.1038/s41408-022-00652-2
23. Augustson BM, Begum G, Dunn JA, Barth NJ, Davies F, Morgan G, et al. Early mortality after diagnosis of multiple myeloma: analysis of patients entered onto the United Kingdom medical research council trials between 1980 and 2002—Medical research council adult leukaemia working party. J Clin Oncol. (2005) 23:9219–26. doi: 10.1200/JCO.2005.03.2086
24. Pratt G, Goodyear O, Moss P. Immunodeficiency and immunotherapy in multiple myeloma. Br J Haematol. (2007) 138:563–79. doi: 10.1111/j.1365-2141.2007.06705.x
25. Tete SM, Bijl M, Sahota SS, Bos NA. Immune defects in the risk of infection and response to vaccination in monoclonal gammopathy of undetermined significance and multiple myeloma. Front Immunol. (2014). doi: 10.3389/fimmu.2014.00257/abstract
26. Kristinsson SY, Tang M, Pfeiffer RM, Bjorkholm M, Goldin LR, Blimark C, et al. Monoclonal gammopathy of undetermined significance and risk of infections: a population-based study. Haematologica. (2012) 97:854–8. doi: 10.3324/haematol.2011.054015
27. Ashruf OS, Orozco Z, Kaelber DC. Risk and severity of COVID-19 infection in monoclonal gammopathy of undetermined significance: A 3-year propensity matched cohort study. Clin Lymphoma Myeloma Leuk. (2023) 23:626–32. doi: 10.1016/j.clml.2023.04.010
28. Ho M, Zanwar S, Buadi FK, Ailawadhi S, Larsen J, Bergsagel L, et al. Risk factors for severe infection and mortality in COVID-19 and monoclonal gammopathy of undetermined significance. Blood. (2022) 140:1997–2000. doi: 10.1182/blood.2022017616
29. Van De Donk NWCJ, Palumbo A, Johnsen HE, Engelhardt M, Gay F, Gregersen H, et al. The clinical relevance and management of monoclonal gammopathy of undetermined significance and related disorders: recommendations from the European Myeloma Network. Haematologica. (2014) 99:984–96. doi: 10.3324/haematol.2013.100552
30. Nucci M, Anaissie E. Infections in patients with multiple myeloma in the era of high-dose therapy and novel agents. Clin Infect Dis. (2009) 49:1211–25. doi: 10.1086/605664
31. Appay V, Dunbar PR, Callan M, Klenerman P, Gillespie GMA, Papagno L, et al. Memory CD8+ T cells vary in differentiation phenotype in different persistent virus infections. Nat Med. (2002) 8:379–85. doi: 10.1038/nm0402-379
32. Geginat J, Lanzavecchia A, Sallusto F. Proliferation and differentiation potential of human CD8+ memory T-cell subsets in response to antigen or homeostatic cytokines. Blood. (2003) 101:4260–6. doi: 10.1182/blood-2002-11-3577
33. Miron M, Meng W, Rosenfeld AM, Dvorkin S, Poon MML, Lam N, et al. Maintenance of the human memory T cell repertoire by subset and tissue site. Genome Med. (2021) 13:100. doi: 10.1186/s13073-021-00918-7
34. Cornberg M. Narrowed TCR repertoire and viral escape as a consequence of heterologous immunity. J Clin Invest. (2006) 116:1443–56. doi: 10.1172/JCI27804
35. Zelle-Rieser C, Thangavadivel S, Biedermann R, Brunner A, Stoitzner P, Willenbacher E, et al. T cells in multiple myeloma display features of exhaustion and senescence at the tumor site. J Hematol OncolJ Hematol Oncol. (2016) 9:116. doi: 10.1186/s13045-016-0345-3
36. Cho SF, Yeh TJ, Anderson KC, Tai YT. Bispecific antibodies in multiple myeloma treatment: A journey in progress. Front Oncol. (2022) 12:1032775. doi: 10.3389/fonc.2022.1032775
Keywords: Multiple Myeloma, MGUS, infection, risk score, secondary immunodeficiency
Citation: Tranter E, Busch D, Heck C, Blau IW, Nogai A, Schiele P, Meisel C, Bullinger L, Frentsch M and Na I-K (2025) Advanced disease and CD8+ TEMRA cells predict severe infections in multiple myeloma. Front. Immunol. 16:1532645. doi: 10.3389/fimmu.2025.1532645
Received: 22 November 2024; Accepted: 24 January 2025;
Published: 12 February 2025.
Edited by:
Sumit Kumar Hira, University of Burdwan, IndiaReviewed by:
Monica Bocchia, University of Siena, ItalyJonas Loetscher, University Hospital of Basel, Switzerland
Copyright © 2025 Tranter, Busch, Heck, Blau, Nogai, Schiele, Meisel, Bullinger, Frentsch and Na. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Eva Tranter, ZXZhLnRyYW50ZXJAY2hhcml0ZS5kZQ==
†These authors have contributed equally to this work and share last authorship
 Eva Tranter
Eva Tranter David Busch1
David Busch1 Clarissa Heck
Clarissa Heck Axel Nogai
Axel Nogai Christian Meisel
Christian Meisel Il-Kang Na
Il-Kang Na