
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Immunol. , 14 March 2025
Sec. Cancer Immunity and Immunotherapy
Volume 16 - 2025 | https://doi.org/10.3389/fimmu.2025.1530623
This article is part of the Research Topic Microbiota-Immunity Dynamics in Cancer: Mechanisms and Implications for Treatment Strategies View all articles
 Qianyi Zhou1†
Qianyi Zhou1† Yuxin An1†
Yuxin An1† Xiaomei Zhang2
Xiaomei Zhang2 Xia Xiao3
Xia Xiao3 Xue Bai3
Xue Bai3 Pengjiang Liu3
Pengjiang Liu3 Yedi Pu3
Yedi Pu3 Juanxia Meng3
Juanxia Meng3 Haibo Zhu3
Haibo Zhu3 Cuicui Lyu3
Cuicui Lyu3 Huan Zhang3
Huan Zhang3 Yu Zhang3
Yu Zhang3 Tianle Xie3
Tianle Xie3 Haotian Meng3
Haotian Meng3 Hairong Lyu3*
Hairong Lyu3*Purpose: CD19 chimeric antigen receptor T (CAR-T) cell therapy has shown promise in treating relapsed or refractory (R/R) B-cell acute lymphoblastic leukemia (B-ALL), but cytokine release syndrome (CRS) remains a significant side effect.
Methods: This retrospective cohort study investigated the use of tocilizumab for managing CAR-T-related CRS in 45 R/R B-ALL patients.
Results: Of these, 17 patients received tocilizumab, resulting in a significant reduction in the duration of grade 3 CRS compared to those who did not receive the drug. Additionally, 10 patients showed decreased cytokine levels.Importantly, tocilizumab did not impair CAR-T cell expansion or efficacy, nor did it increase the incidence of adverse events.
Conclusion: These findings suggest that tocilizumab may be an effective and safe strategy for mitigating CAR-T-related CRS in R/R B-ALL patients, potentially improving patient outcomes and survival.
CAR-T cell therapy is an effective emerging therapy for treating hematological malignancies. Up till now, six CAR-T cell products have been approved by the Food and Drug Administration (FDA) (1–8). Among them, CAR-T cells targeting CD19 for B-cell malignancies has been widely and maturely applied in clinical practice, achieving a complete remission rate up to 90% (9, 10).
However, CAR-T cells can cause certain side effects, among which cytokine release syndrome (CRS) is the most common and serious (11). The symptoms of CRS include fever, hypoxia, hypotension, and so on (12). The American Society for Transplantation and Cellular Therapy (ASTCT) graded CRS based on symptoms (13). The mechanism of CRS is complex and still not fully understood, which may be related to the release of inflammatory factors by various immunocytes (11). The initial cytokines released lead to further cytokine release after activation of immune cells such as macrophages. This results in a cytokine storm. Of these, IL-6 is considered the most critical cytokine and the serum IL-6 levels may correlate with the severity of CRS (14). Due to the important role of IL-6, current guidelines recommend the use of tocilizumab (anti-IL-6R) for the prevention and treatment of CRS caused by CAR-T cells (15). Currently, there are more studies on the prevention and treatment of CRS with tocilizumab in lymphoma and myeloma patients (16–18), while in B-ALL patients relevant research is less. In addition, few studies have evaluated the side effects of tocilizumab in the treatment of CAR-T cell-induced CRS (19).
In summary, we enrolled 45 B-ALL patients who received CAR-T cell infusion and retrospectively studied the efficacy and safety of tocilizumab in the treatment of CRS through patients’ symptoms and serum biomarkers. We consider that tocilizumab does not affect the proliferation and efficacy of CAR-T cells, and there are no serious side effects.
According to the inclusion/exclusion criterion of two clinical trials (ChiCTR-ONN-16009862 and ChiCTR1800015164), we conducted a retrospective analysis of 61 eligible patients with R/R B-ALL from April 2020 and October 2023. The exclusion criteria for follow-up were as follows (1): patients progressed before infusion (2); the follow-up time less than 28 days (3); the related data lost. In the end, 16 patients were excluded and 45 patients were enrolled in this study, 17 patients of which were treated with tocilizumab infusion (Figure 1).
This study protocol was reviewed and approved by the Ethics Committee of Tianjin First Central Hospital and was registered for clinical trials in the China Clinical Trial Registration Center. All participations signed written informed consent before enrollment.
The peripheral blood mononuclear cells were obtained from patients or healthy donors. CD3+ T cells were isolated by magnetic beads and cultured them in culture medium which contains CD3/CD28 stimulating beads. After activation, we transduce lentiviral vector including CD19 CAR into these cells. The CD19 CAR contained a 4-1BB costimulatory domain and a CD3-ζ signaling domain constructed as previously described (20). Finally, CAR-T cells were expanded and evaluated for transduction efficiency (approximately 50%). Prior to infusion (Day 0), all patients received lymphodepleting chemotherapies. (300mg/m2/d cyclophosphamide and 30mg/m2/d fludarabine for 3 to 2 days) (21).
CRS is the main toxicity observed in B-ALL patients treated with CAR-T cells. We graded CRS according to the ASTCT consensus (the grading criteria is detailed in Table 1). We classified it into mild CRS (grade 1-2 CRS) and severe CRS (grade 3-4 CRS). In addition to symptomatic treatments such as oxygen therapy, antipyretics and vasopressors, tocilizumab and corticosteroid are common drugs used in the treatment of CRS. We regarded fever that could not be explained by other causes and the increasing of cytokines as the beginning of CRS. However, the optimal timing of tocilizumab is not yet clear, and it is most commonly recommended for patients with symptoms such as refractory or persistent hypotension, hypoxia, etc. (22) In our study, tocilizumab or corticosteroid is given to patients as determined by the physicians, within 12 hours of the appearance of symptoms.
ICANS is the second observed side effect in R/R B-ALL patients treated with CD19 CAR-T. We recorded the different ICANS symptoms and the exact time of symptom appearance, then graded ICANS according to the ASTCT consensus. We also focused on the occurrence of hematological toxicity. In addition, impairment of liver and kidney function was also observed in a small percentage of patients, which were also recorded and evaluated. Finally, the infection of these patients was diagnosed based on clinical presentation and etiological examination.
Response to CAR-T cells was assessed by bone marrow morphological analysis, flow cytometry, and genetic testing on day 28. Complete response (CR) was defined as <5% bone marrow blasts in morphology regardless of cell count recovery. And it was further classified into MRD+CR or MRD-CR based on minimal residual disease (MRD). The efficacy of tocilizumab or corticosteroid was assessed by serial monitoring of temperature, ferritin, C-reactive protein (CRP) and cytokines (including IL-2, IL-6, IL-10, TNF-α (tumor necrosis factor-α), etc). The end of CRS was considered to be the disappearance of fever and a decrease in cytokines. Finally, we evaluated the progression-free survival (PFS) and overall survival (OS) of these two groups of patients (toci group and non-toci group).
The characteristics of patients were analyzed using descriptive statistics. All measurement data were described in terms of median and range and compared by using t tests or Mann-Whitney tests. Enumeration data were described as frequencies (%) and compared using chi-squared tests or Fisher’s exact tests. Statistical analyses were performed using the SPSS v26.0 software (Chicago, IL, USA) and the GraphPad Prism v9 software (GraphPad, La Jolla, CA, USA).
From April 2020 to October 2023, a total of 61 patients with R/R B-ALL were proposed to receive CAR-T cells. Among them,1 case experienced disease progression before receiving CAR-T therapy, eventually 60 patients received CAR-T therapy. Among these 60 cases, excluding 4 cases with follow-up time less than 28 days, and 11 cases with relevant data loss, finally 45 cases were included in our retrospective study. Baseline characteristics of all 45 patients and the subgroup analysis of 2 groups (toci group and non-toci group) were summarized (Table 2). Of the total 45 patients, 53.3% (24/45) were male, the median age was 36 years (range 8–67), and 84.8% (38/45) were high-risk phenotype or genotypes. The median number of therapy lines before CAR-T cell infusion was 2 (range, 1–3). As shown in the table, the two groups were very similar in terms of gender, genotypic risk level, previous treatments and dose of CAR-T cells. The differences were not statistically significant. However, the toci group had a higher tumor load, CRS grade and dose of corticosteroid with significant statistical difference. We also performed a logistic regression analysis of these potential factors (Supplementary Table S4). We found that none of the factors were statistically significant. In this study, there was insufficient evidence of an association between these factors and the use of tocilizumab in treatment.
All 45 patients received CD19 CAR-T cells. Of the total 45 patients, 88.9% (N=40) achieved MRD-CR, 8.9% (N=4) achieved MRD+CR, and 2.2% (N=1) achieved partial remission (PR) (Figure 2A). After infusion, 91.1% (41/45) of patients had various grades of CRS, including 7 patients in grade 1, 19 patients in grade 2, and 15 patients in grade 3. There were no grade 4 CRS events. The median duration of CRS in patients in toci group was 6.1 (range, 2-14) days. In comparison, the median duration in non-toci group was 5.5 (range, 0-12) days. And ICANS occurred in two patients (4.5%). One patient developed grade 3 ICANS characterized by syncope and vision decrease that lasted 7 days.
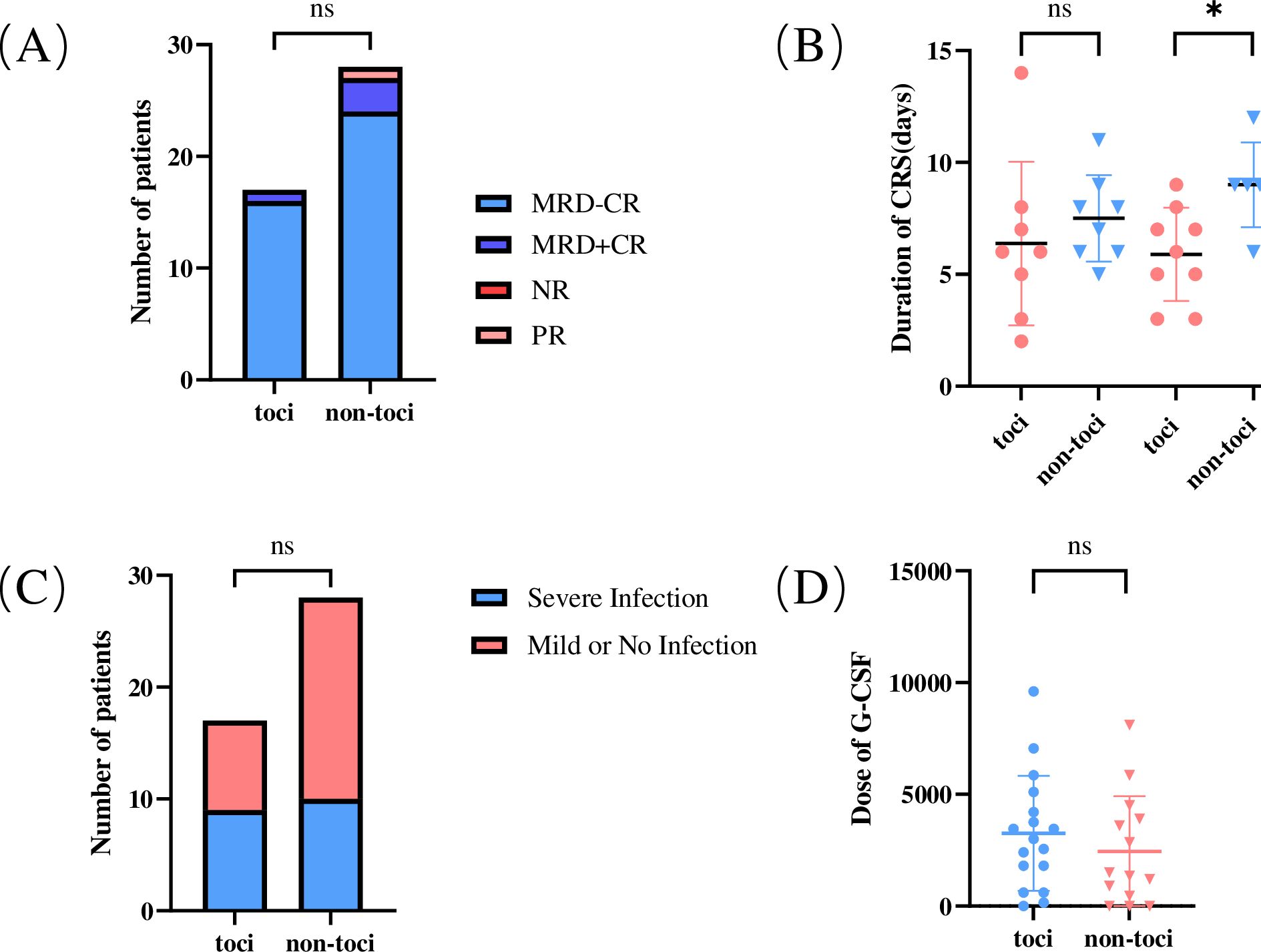
Figure 2. Efficacy and safety of using tocilizumab to treat CRS. (A) Comparison of efficacy between the two groups of patients at day 28 after receiving CD19 CAR-T therapy; (B) The duration of CRS between the two groups. We compared grade 1-2 CRS separately from grade 3 CRS; (C) Comparison of the incidence of serious infections in the two groups; (D) Comparison of the dose of G-CSF in the two groups, we regarded high doses of G-CSF as more severe hematologic toxicity. *p < 0.05. CR, complete response; MRD, minimal residual disease; PR, partial response; NR, non-remission. ns, no significance, P>0.05.
Tocilizumab was applied to 17 patients, including 8 patients with grade 2 CRS and 9 patients with grade 3 CRS. The administration of tocilizumab was mostly within 12 hours after the onset of CRS-related symptoms, and a repeated dose of tocilizumab was given if the symptoms were not significantly relieved 8 hours after infusion. One to six doses of tocilizumab were used to suppress CRS with median dose of 480 (range, 160–800) mg. The dosing range for tocilizumab was 24-48 hours. Corticosteroids were used in 25 patients, and the cumulative methylprednisolone-equivalent corticosteroid dose was 100 mg (range, 7.5–302.5). A total of 10 patients in the non-toci group were treated with corticosteroid only, including 1 patient with grade 1 CRS, 3 patients with grade 2 CRS and 6 patients with grade 3 CRS. There were no cases of ICANS, nor any instances of grade 4 CRS or higher. In combination with the clinical symptoms, tocilizumab was not chosen due to the physician’s decision and the financial constraints of a part of the patients.
Clinical symptoms of CRS in patients after CAR-T cells infusion include unexplained fever, hypotension and hypoxemia. These symptoms are the main basis for grading according to ASTCT criteria. Accordingly, the clinical presentation of the patients was documented and the duration of CRS was calculated, with particular attention to the toci group. For grade 3 CRS, we found that the duration in the toci group was significantly shorter than non-toci group, and the difference was statistically significant. But there was no significant difference in duration between the two groups in grade 1-2 CRS (Figure 2B).
The serum cytokine levels were correlated with CRS levels, so we continuously monitored changes of serum cytokine levels for 17 patients in the toci group. (Figure 3) 58.8% (10/17) patients exhibited a reduction in cytokine levels following tocilizumab treatment. Meanwhile, their clinical symptoms associated with CRS were markedly alleviated. We consider that this illustrates a substantial therapeutic role of tocilizumab in these patients.
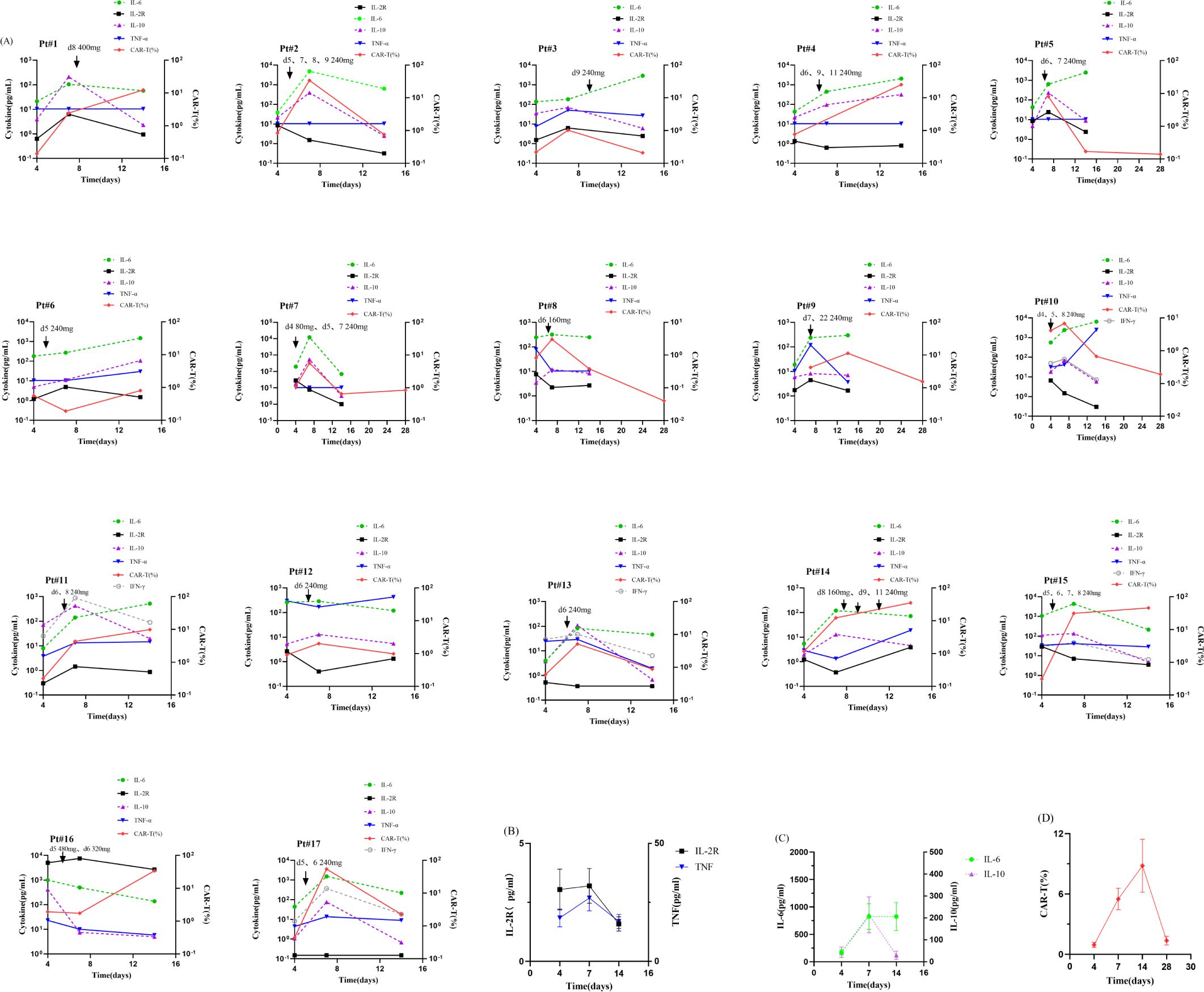
Figure 3. (A) Cytokines, CAR-T ratio of toci group patients. (B-D) The overall trends of Cytokines, CAR-T ratio in toci group. We consider the time of the first CAR-T cell infusion as day 0. The Cytokines included IL-2R, IL-6, IL-10 and TNF-α. A few patients also included data about IFN-γ.
Nevertheless, 41.2% (7/17) patients still showed elevated serum level of IL-6 after application of tocilizumab. Concurrently, their clinical manifestations showed no significant improvement. This may be the result of a number of factors. First, serum level of IL-6 is affected by CAR-T cell expansion. Pt 4 and Pt 11 applied tocilizumab at day 6、9、11 and day 6、8, respectively, while they had persistent expansion of CAR-T cell until day 15. Secondly, the acute graft-versus-host disease after infusion of donor-derived CAR-T cell also has some effects. Pt 5 developed acute intestinal GVHD and her symptoms resolved after treatment with ruxolitinib. Subsequently her serum level of IL-6 also exhibited a reduction. Finally, infection is also a very important influencing factor. Pt 3、6、9、10 showed different sites of infection, such as pneumonia, cholecystitis, etc. After adjustment for antibiotics and supportive care, Pt 、9、10 were stable after infection control, while Pt 6 died of respiratory failure due to severe lung infection.
Overall, the majority of 7 patients had a transient and mild increase in serum levels of IL-6. No patients developed ICANS due to elevated serum IL-6 levels. And it is not clear how tocilizumab affects the levels of other cytokines.
Although tocilizumab is effective in relieving symptoms of severe CRS, the effect of tocilizumab on CAR-T cell expansion and efficacy is controversial. In our study, 94.12%(n=16)of the toci group and 85.71%(n=24)of the non toci group obtained MRD-CR. There was no statistically significant difference in the CR rate between toci and non-toci groups (p=0.616). In addition, there was no statistical difference in PFS (p=0.350) and OS (p=0.273) between the two groups (Figure 4). Meanwhile, tocilizumab didn’t affect the expansion of CAR-T cells (Supplementary Figure S1).
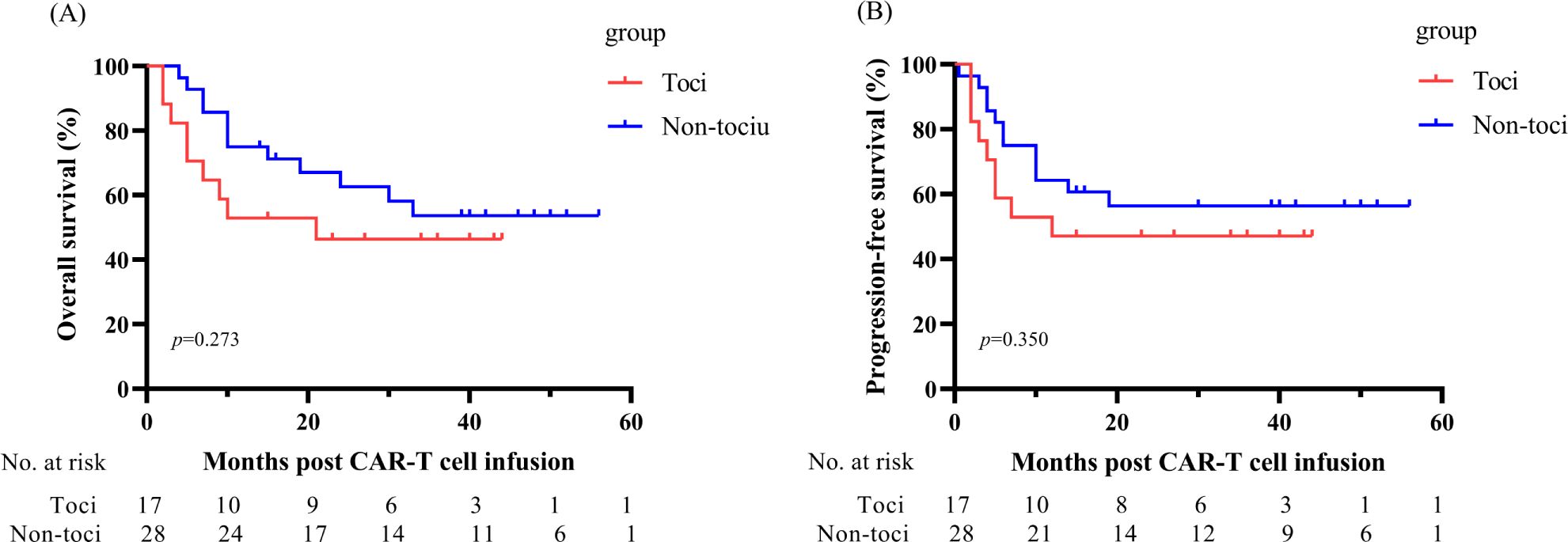
Figure 4. Relationship between receipt of tocilizumab with OS (A) and PFS (B) in patients with acute B-cell lymphoblastic leukemia after CD19 CAR-T therapy. OS, overall survival; PFS, progression-free survival.
Moreover, tocilizumab does not increase the incidence of side effects. There were 52.9%(n=9)of patients in toci group and 35.7%(n=10)of patients in non-toci group developed serious infections. The differences were not statistically significant (p=0.353) (Figure 2C). In terms of hematological toxicity, we also compared the dosage of granulocyte colony stimulating factor (G-CSF) used between two groups to further verify the safety of tocilizumab, but the results still showed no statistically significant difference (p=0.227) (Figure 2D). In the Supplementary Materials, we also demonstrated that tocilizumab has no significant effect on liver and kidney function (Supplementary Figure S2).
CD19 CAR-T therapy has strong effects on R/R B-ALL patients in various disease states and is a promising immunotherapy (9, 23, 24). More and more R/R B-ALL patients are choosing CAR-T therapy for treatment. However, CAR-T therapy also has certain side effects, among which CRS is the most common and potentially life-threatening side effect (25). Consistent with previous conclusion, the determining factor that influenced the severity of CRS was tumor burden (26). National Comprehensive Cancer Network (NCCN) guidelines recommend the use of tocilizumab for the prevention and treatment of CRS caused by CAR-T cells (15). Tocilizumab is an antagonist of IL-6 receptors, which inhibits the IL-6 pathway by binding to IL-6R, thereby inhibiting inflammation (27). There are many researches on using tocilizumab for the prevention and treatment of CAR-T related CRS in patients with myeloma and lymphoma (16–18). B-ALL patients may have fewer reports due to their complex disease conditions, and due to the price, it is more likely to use tocilizumab for therapeutic rather than prevention of CRS in actual clinical practice. And the side effects of using tocilizumab to treat CRS have not yet been determined.
Our study enrolled 45 R/R B-ALL patients who received CD19 CAR-T cell therapy. We divided the patients into two cohorts based on whether or not they have received tocilizumab, with 17 patients receiving tocilizumab treatment for CRS. According to relevant guidelines and our center’s experience, tocilizumab is mainly administered within 12 hours after the onset of CRS-related symptoms (15). If there is no significant improvement after 8 hours of infusion, we will give patients a second dose. We compared baseline characteristics based on age, gender, high-risk phenotype or genotypes, lines of therapy, previous allogeneic hematopoietic stem cell transplantation (allo-HSCT), tumor burden, doses of CAR-T cells, and grade of CRS. Among them, tumor burden and the grade of CRS were higher in tocilizumab infusion cohort.
We evaluate the use of tocilizumab based on clinical symptoms such as high fever, hypoxemia, hypotension, and apply the ASTCT grading system to grade patients’ CRS. Moreover, we evaluated the efficacy of CAR-T cells on day 28 and long-term survival. In this trial, there was no significant difference in efficacy and long-term survival between 2 groups. This is consistent with the conclusions of several studies on the prevention and treatment of CAR-T related CRS by using tocilizumab (28, 29).
In terms of CRS duration, we compared patients with different grades of CRS separately. There was no statistically significant difference in the duration of CRS between toci cohort patients and non-toci cohort patients in grade 2. However, the use of tocilizumab in grade 3 can shorten the duration of CRS. Moreover, from the statistical perspective, using tocilizumab to treat CRS did not affect the expansion of CAR-T cells in patient’s body, although it may be associated with the peak expansion of CAR-T cells (30).
Regarding the potential side effects of using tocilizumab for treatment, our study shows that there is no significant difference between the two groups of patients (toci group and non-toci group) in terms of severe infection, hematological toxicity, liver function damage, and kidney function damage. Previous clinical trials have shown that tocilizumab may lead to neutropenia in patients (31), but researchers do not consider it to be a form of myelosuppression (32). Our study also considers that the infusion of tocilizumab for the treatment of CRS in B-ALL patients after CAR-T therapy has no hematological toxicity. Besides, due to the blockade of IL-6 receptors by tocilizumab, peripheral blood IL-6 levels may increase. Some studies also suggest that it may cause damage to the blood-brain barrier, allowing inflammatory factors and CAR-T cells to enter the central nervous system, thereby potentially increasing the incidence of ICANS (27, 33–35). Although, in our study, no patients experienced ICANS due to a significant increase in peripheral blood IL-6 levels by using tocilizumab. In these associations, if there is a sharply increase in IL-6 levels or no relief in CRS symptoms after the using of tocilizumab, our team will use corticosteroid as early as possible for these patients to avoid life-threatening adverse events. Some guidelines also have similar views: for severe CRS patients, it is recommended to use dexamethasone 10mg/6h for 1-3 days (20mg/6h for 3-7 days in severe cases) after treatment with tocilizumab (8mg/kg, 800mg max) is ineffective; the preferred drug treatment for ICANS is dexamethasone or methylprednisolone (15). In our center, for patients with severe ICANS after CAR-T therapy, we also used intrathecal injection of dexamethasone, which achieved excellent therapeutic effects. Corticosteroids have a wider range of immunosuppressive effects than tocilizumab, and the risk of reducing CAR-T cell expansion and efficacy may be higher [although many studies have shown that the use of corticosteroid did not affect the progression-free survival of infusion patients (36, 37)]. Meanwhile, the CAR-T expansion of few patients in our study was affected after corticosteroid infusion (Supplementary Figure S3), which may be related to prior treatment regimens. This conclusion is consistent with the findings of several other studies indicating that corticosteroids do not influence the efficacy of CAR-T cells (28, 38, 39). However, when we choose corticosteroid therapy, attention should be paid to the administration time and duration (40–42). We still consider tocilizumab infusion as the first treatment for CRS.
The limitation of our research is that it was a single-center retrospective cohort design with a small number of participants and multiple interfering factors (eg., the infusion time of tocilizumab is mostly based on physician experience within 12 hours after symptom appearance).
In summary, despite some limitations, we have confirmed the efficacy and safety of early use of tocilizumab to treat CRS caused by CD19 CAR-T cells in B-ALL patients. CAR-T cells are a highly efficient weapon for treating hematological malignancies, and CRS is one of its unavoidable side effects. Timely treatment after the appearance of CRS related symptoms (such as fever, hypotension, hypoxemia, etc.) is necessary to benefit patients with hematological malignancies and prolong their survival time.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving humans were approved by the Ethics Committee of Tianjin First Central Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
QZ: Formal Analysis, Methodology, Writing – original draft. YA: Formal Analysis, Methodology, Writing – original draft. XZ: Resources, Supervision, Writing – review & editing. XX: Resources, Supervision, Writing – review & editing. XB: Resources, Supervision, Writing – review & editing. PL: Funding acquisition, Supervision, Writing – review & editing. YP: Resources, Supervision, Writing – review & editing. JM: Resources, Supervision, Writing – review & editing. HaZ: Resources, Supervision, Writing – review & editing. CL: Resources, Supervision, Writing – review & editing. HuZ: Resources, Supervision, Writing – review & editing. YZ: Resources, Supervision, Writing – review & editing. TX: Resources, Supervision, Writing – review & editing. HM: Resources, Supervision, Writing – review & editing. HL: Formal Analysis, Methodology, Resources, Writing – review & editing.
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the General Project of the National Natural Science Foundation of China#1 under Grant 81970180; the Science and Technology Project of Tianjin Municipal Health Committee#2 under Grant TJWJ2022QN030; the Key projects of Tianjin Applied Basic Research and Multi-Investment Fund#3 under Grant 21JCZDJC01240; the Science and Technology Project of Tianjin Municipal Health Committee#4 under Grant TJWJ2022XK018; Tianjin Key Medical Discipline (Specialty) Construction Project#5 under Grant TJYXZDXK-056B; Tianjin Municipal Natural Science Foundation#6 under Grant 22JCQNJC00820; Tianjin Municipal Science and Technology Commission#7 under Grant 21JCQNJC00070; Tianjin Health Research Project#8 under Grant TJWJ2023QN027; Tianjin Health Bureau Project#9 under Grant ZC20074; and Tianjin Key Medical Discipline(Specialty) Construction Project#10 under Grant TJWJ2023XK010.
All authors declare that the research was conducted without any commercial or financial relationships which could be construed as a potential conflict of interest.
The author(s) declare that no Generative AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2025.1530623/full#supplementary-material
CAR-T, chimeric antigen receptor T; R/R B-ALL, relapsed or refractory acute B-lymphoblastic leukemia; CRS, cytokine release syndrome; ICANS, immune effector cell-associated neurotoxicity syndrome; FDA, Food and Drug Administration; ASTCT, American Society for Transplantation and Cellular Therapy; CR, complete response; MRD, minimal residual disease; CRP, C-reactive protein; PFS, progression-free survival; OS, overall survival; TNF-α, tumor necrosis factor-α; PR, partial remission; G-CSF, granulocyte colony stimulating factor; allo-HSCT, allogeneic hematopoietic stem cell transplantation; NCCN, National Comprehensive Cancer Network.
1. Neelapu SS, Locke FL, Bartlett NL, Lekakis LJ, Miklos DB, Jacobson CA, et al. Axicabtagene ciloleucel CAR T-cell therapy in refractory large B-cell lymphoma. New Engl J Med. (2017) 377:2531–44. doi: 10.1056/NEJMoa1707447
2. Maude SL, Laetsch TW, Buechner J, Rives S, Boyer M, Bittencourt H, et al. Tisagenlecleucel in children and young adults with B-cell lymphoblastic leukemia. New Engl J Med. (2018) 378:439–48. doi: 10.1056/NEJMoa1709866
3. Schuster SJ, Bishop MR, Tam CS, Waller EK, Borchmann P, McGuirk JP, et al. Tisagenlecleucel in adult relapsed or refractory diffuse large B-cell lymphoma. New Engl J Med. (2019) 380:45–56. doi: 10.1056/NEJMoa1804980
4. Abramson JS, Palomba ML, Gordon LI, Lunning MA, Wang M, Arnason J, et al. Lisocabtagene maraleucel for patients with relapsed or refractory large B-cell lymphomas (TRANSCEND NHL 001): a multicentre seamless design study. Lancet (London England). (2020) 396:839–52. doi: 10.1016/S0140-6736(20)31366-0
5. Berdeja JG, Madduri D, Usmani SZ, Jakubowiak A, Agha M, Cohen AD, et al. Ciltacabtagene autoleucel, a B-cell maturation antigen-directed chimeric antigen receptor T-cell therapy in patients with relapsed or refractory multiple myeloma (CARTITUDE-1): a phase 1b/2 open-label study. Lancet (London England). (2021) 398:314–24. doi: 10.1016/S0140-6736(21)00933-8
6. Munshi NC, Anderson LD Jr., Shah N, Madduri D, Berdeja J, Lonial S, et al. Idecabtagene vicleucel in relapsed and refractory multiple myeloma. New Engl J Med. (2021) 384:705–16. doi: 10.1056/NEJMoa2024850
7. Shah BD, Ghobadi A, Oluwole OO, Logan AC, Boissel N, Cassaday RD, et al. KTE-X19 for relapsed or refractory adult B-cell acute lymphoblastic leukaemia: phase 2 results of the single-arm, open-label, multicentre ZUMA-3 study. Lancet (London England). (2021) 398:491–502. doi: 10.1016/S0140-6736(21)01222-8
8. Ying Z, Yang H, Guo Y, Li W, Zou D, Zhou D, et al. Relmacabtagene autoleucel (relma-cel) CD19 CAR-T therapy for adults with heavily pretreated relapsed/refractory large B-cell lymphoma in China. Cancer Med. (2021) 10:999–1011. doi: 10.1002/cam4.v10.3
9. Lee DW, Kochenderfer JN, Stetler-Stevenson M, Cui YK, Delbrook C, Feldman SA, et al. T cells expressing CD19 chimeric antigen receptors for acute lymphoblastic leukaemia in children and young adults: a phase 1 dose-escalation trial. Lancet (London England). (2015) 385:517–28. doi: 10.1016/S0140-6736(14)61403-3
10. Gardner RA, Finney O, Annesley C, Brakke H, Summers C, Leger K, et al. Intent-to-treat leukemia remission by CD19 CAR T cells of defined formulation and dose in children and young adults. Blood. (2017) 129:3322–31. doi: 10.1182/blood-2017-02-769208
11. Schubert ML, Schmitt M, Wang L, Ramos CA, Jordan K, Müller-Tidow C, et al. Side-effect management of chimeric antigen receptor (CAR) T-cell therapy. Ann oncology: Off J Eur Soc Med Oncol. (2021) 32:34–48. doi: 10.1016/j.annonc.2020.10.478
12. Neelapu SS. Managing the toxicities of CAR T-cell therapy. Hematological Oncol. (2019) 37 Suppl 1:48–52. doi: 10.1002/hon.2595
13. Gutgarts V, Jain T, Zheng J, Maloy MA, Ruiz JD, Pennisi M, et al. Acute kidney injury after CAR-T cell therapy: low incidence and rapid recovery. Biol Blood marrow transplantation: J Am Soc Blood Marrow Transplantation. (2020) 26:1071–6. doi: 10.1016/j.bbmt.2020.02.012
14. Norelli M, Camisa B, Barbiera G, Falcone L, Purevdorj A, Genua M, et al. Monocyte-derived IL-1 and IL-6 are differentially required for cytokine-release syndrome and neurotoxicity due to CAR T cells. Nat Med. (2018) 24:739–48. doi: 10.1038/s41591-018-0036-4
15. Thompson JA, Schneider BJ, Brahmer J, Achufusi A, Armand P, Berkenstock MK, et al. Management of immunotherapy-related toxicities, version 1.2022, NCCN clinical practice guidelines in oncology. J Natl Compr Cancer Network: JNCCN. (2022) 20:387–405. doi: 10.6004/jnccn.2022.0020
16. Caimi PF, Pacheco Sanchez G, Sharma A, Otegbeye F, Ahmed N, Rojas P, et al. Prophylactic tocilizumab prior to anti-CD19 CAR-T cell therapy for non-hodgkin lymphoma. Front Immunol. (2021) 12:745320. doi: 10.3389/fimmu.2021.745320
17. Kadauke S, Myers RM, Li Y, Aplenc R, Baniewicz D, Barrett DM, et al. Risk-adapted preemptive tocilizumab to prevent severe cytokine release syndrome after CTL019 for pediatric B-cell acute lymphoblastic leukemia: A prospective clinical trial. J Clin oncology: Off J Am Soc Clin Oncol. (2021) 39:920–30. doi: 10.1200/JCO.20.02477
18. Banerjee R, Marsal J, Huang CY, Lo M, Kambhampati S, Kennedy VE, et al. Early time-to-tocilizumab after B cell maturation antigen-directed chimeric antigen receptor T cell therapy in myeloma. Transplant Cell Ther. (2021) 27:477.e1–.e7. doi: 10.1016/j.jtct.2021.03.004
19. Zhang Y, Zhou F, Wu Z, Li Y, Li C, Du M, et al. Timing of tocilizumab administration under the guidance of IL-6 in CAR-T therapy for R/R acute lymphoblastic leukemia. Front Immunol. (2022) 13:914959. doi: 10.3389/fimmu.2022.914959
20. Cao J, Wang G, Cheng H, Wei C, Qi K, Sang W, et al. Potent anti-leukemia activities of humanized CD19-targeted Chimeric antigen receptor T (CAR-T) cells in patients with relapsed/refractory acute lymphoblastic leukemia. Am J hematology. (2018) 93:851–8. doi: 10.1002/ajh.25108
21. Wang J, Zhang M, Lyu H, Guo R, Xiao X, Bai X, et al. Low-dose administration of prednisone has a good effect on the treatment of prolonged hematologic toxicity post-CD19 CAR-T cell therapy. Front Immunol. (2023) 14:1139559. doi: 10.3389/fimmu.2023.1139559
22. Garcia Borrega J, Gödel P, Rüger MA, Onur ÖA, Shimabukuro-Vornhagen A, Kochanek M, et al. In the eye of the storm: immune-mediated toxicities associated with CAR-T cell therapy. HemaSphere. (2019) 3:e191. doi: 10.1097/HS9.0000000000000191
23. Lu W, Wei Y, Cao Y, Xiao X, Li Q, Lyu H, et al. CD19 CAR-T cell treatment conferred sustained remission in B-ALL patients with minimal residual disease. Cancer immunology immunotherapy: CII. (2021) 70:3501–11. doi: 10.1007/s00262-021-02941-4
24. Wan X, Yang X, Yang F, Wang T, Ding L, Song L, et al. Outcomes of anti-CD19 CAR-T treatment of pediatric B-ALL with bone marrow and extramedullary relapse. Cancer Res Treat. (2022) 54:917–25. doi: 10.4143/crt.2021.399
25. Hirayama AV, Turtle CJ. Toxicities of CD19 CAR-T cell immunotherapy. Am J Hematol. (2019) 94:S42–s9. doi: 10.1002/ajh.v94.S1
26. Wei J, Liu Y, Wang C, Zhang Y, Tong C, Dai G, et al. The model of cytokine release syndrome in CAR T-cell treatment for B-cell non-Hodgkin lymphoma. Signal transduction targeted Ther. (2020) 5:134. doi: 10.1038/s41392-020-00256-x
27. Kotch C, Barrett D, Teachey DT. Tocilizumab for the treatment of chimeric antigen receptor T cell-induced cytokine release syndrome. Expert Rev Clin Immunol. (2019) 15:813–22. doi: 10.1080/1744666X.2019.1629904
28. Costa BA, Flynn J, Nishimura N, Devlin SM, Farzana T, Rajeeve S, et al. Prognostic impact of corticosteroid and tocilizumab use following chimeric antigen receptor T-cell therapy for multiple myeloma. Blood Cancer J. (2024) 14:84. doi: 10.1038/s41408-024-01048-0
29. Locke FL, Neelapu SS, Bartlett NL, Lekakis LJ, Jacobson CA, Braunschweig I, et al. Tocilizumab prophylaxis following axicabtagene ciloleucel in relapsed or refractory large B-cell lymphoma. Transplant Cell Ther. (2024) 30:1065–79. doi: 10.1016/j.jtct.2024.08.018
30. Biasco L, Izotova N, Rivat C, Ghorashian S, Richardson R, Guvenel A, et al. Clonal expansion of T memory stem cells determines early anti-leukemic responses and long-term CAR T cell persistence in patients. Nat cancer. (2021) 2:629–42. doi: 10.1038/s43018-021-00207-7
31. Grange S, Schmitt C, Banken L, Kuhn B, Zhang X. Thorough QT/QTc study of tocilizumab after single-dose administration at therapeutic and supratherapeutic doses in healthy subjects. Int J Clin Pharmacol Ther. (2011) 49:648–55. doi: 10.5414/CP201549
32. Shovman O, Shoenfeld Y, Langevitz P. Tocilizumab-induced neutropenia in rheumatoid arthritis patients with previous history of neutropenia: case series and review of literature. Immunologic Res. (2015) 61:164–8. doi: 10.1007/s12026-014-8590-4
33. Freyer CW, Porter DL. Cytokine release syndrome and neurotoxicity following CAR T-cell therapy for hematologic Malignancies. J Allergy Clin Immunol. (2020) 146:940–8. doi: 10.1016/j.jaci.2020.07.025
34. Gust J, Hay KA, Hanafi LA, Li D, Myerson D, Gonzalez-Cuyar LF, et al. Endothelial activation and blood-brain barrier disruption in neurotoxicity after adoptive immunotherapy with CD19 CAR-T cells. Cancer discovery. (2017) 7:1404–19. doi: 10.1158/2159-8290.CD-17-0698
35. Wang X, Zhang B, Zhang Q, Zhou H, Sun Q, Zhou Y, et al. Impact of tocilizumab on anti-CD19 chimeric antigen receptor T-cell therapy in B-cell acute lymphoblastic leukemia. Cancer. (2024) 130:2660–9. doi: 10.1002/cncr.v130.15
36. Oluwole OO, Bouabdallah K, Muñoz J, De Guibert S, Vose JM, Bartlett NL, et al. Prophylactic corticosteroid use in patients receiving axicabtagene ciloleucel for large B-cell lymphoma. Br J haematology. (2021) 194:690–700. doi: 10.1111/bjh.17527
37. Gardner RA, Ceppi F, Rivers J, Annesley C, Summers C, Taraseviciute A, et al. Preemptive mitigation of CD19 CAR T-cell cytokine release syndrome without attenuation of antileukemic efficacy. Blood. (2019) 134:2149–58. doi: 10.1182/blood.2019001463
38. Lakomy T, Akhoundova D, Nilius H, Kronig MN, Novak U, Daskalakis M, et al. Early Use of Corticosteroids following CAR T-Cell Therapy Correlates with Reduced Risk of High-Grade CRS without Negative Impact on Neurotoxicity or Treatment Outcome. Biomolecules. (2023) 13(2):382. doi: 10.3390/biom13020382
39. Strati P, Ahmed S, Furqan F, Fayad LE, Lee HJ, Iyer SP, et al. Prognostic impact of corticosteroids on efficacy of chimeric antigen receptor T-cell therapy in large B-cell lymphoma. Blood. (2021) 137:3272–6. doi: 10.1182/blood.2020008865
40. Liu Q, Qian W, Zhao W. Chinese expert consensus on prevention and treatment of immunotherapeutic and molecular targeted agents-related infections in patients with hematological Malignancies (2021 version). Zhonghua xue ye xue za zhi = Zhonghua xueyexue zazhi. (2021) 42:717–27. doi: 10.3760/cma.j.issn.0253-2727.2021.09.002
41. Hayden PJ, Roddie C, Bader P, Basak GW, Bonig H, Bonini C, et al. Management of adults and children receiving CAR T-cell therapy: 2021 best practice recommendations of the European Society for Blood and Marrow Transplantation (EBMT) and the Joint Accreditation Committee of ISCT and EBMT (JACIE) and the European Haematology Association (EHA). Ann oncology: Off J Eur Soc Med Oncol. (2022) 33:259–75. doi: 10.1016/j.annonc.2021.12.003
42. Tu S, Luo X, Mei H, Hu Y, Liu Y, Li P, et al. Recommendations for the timing, dosage, and usage of corticosteroids during cytokine release syndrome (CRS) caused by chimeric antigen receptor (CAR)-T cell therapy for hematologic Malignancies. Chin Med J. (2024) 137:2681–3. doi: 10.1097/CM9.0000000000003379
Keywords: chimeric antigen receptor T (CAR-T) cell, tocilizumab, cytokine release syndrome (CRS), efficacy and safety, acute B-lymphoblastic leukemia (B-ALL)
Citation: Zhou Q, An Y, Zhang X, Xiao X, Bai X, Liu P, Pu Y, Meng J, Zhu H, Lyu C, Zhang H, Zhang Y, Xie T, Meng H and Lyu H (2025) Efficacy and safety of tocilizumab in managing cytokine release syndrome after CD19 CAR-T therapy for relapsed or refractory B-cell acute lymphoblastic leukemia. Front. Immunol. 16:1530623. doi: 10.3389/fimmu.2025.1530623
Received: 19 November 2024; Accepted: 27 February 2025;
Published: 14 March 2025.
Edited by:
Xin Zhou, Stanford University, United StatesReviewed by:
Walter Hanel, The Ohio State University, United StatesCopyright © 2025 Zhou, An, Zhang, Xiao, Bai, Liu, Pu, Meng, Zhu, Lyu, Zhang, Zhang, Xie, Meng and Lyu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hairong Lyu, MTg4MDIyMzU5MzlAMTYzLmNvbQ==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.