- 1Department of Pharmacy, Guangdong Pharmaceutical University, Guangzhou, China
- 2School of Life Sciences and Biopharmaceutics, Guangdong Pharmaceutical University, Guangzhou, China
- 3Department of Endodontics, Southern Medical University Stomatological Hospital, Guangzhou, China
Periodontitis is a significant global public health issue associated with the onset and progression of various systemic diseases, thereby requiring additional research and clinical attention. Although ferroptosis and cuproptosis have emerged as significant areas of research in the medical field, their precise roles in the pathogenesis of periodontitis remain unclear. We aim to systematically summarize the current research on ferroptosis and cuproptosis in periodontal disease and investigate the roles of glutathione pathway and autophagy pathway in connecting ferroptosis and cuproptosis during periodontitis. Further, we propose that a homeostatic imbalance of copper and iron, driven by periodontal pathogens, may contribute to elevated periodontal oxidative stress, representing a potential unifying link between ferroptosis and cuproptosis involved in periodontitis. This article presents a comprehensive overview of the molecular mechanisms underlying ferroptosis and cuproptosis in periodontitis, offering novel theoretical insights into its pathogenesis and potential therapeutic targets.
1 Introduction
Periodontitis is a multifaceted inflammatory oral disease initiated by pathogenic biofilms. This condition might lead to persistent destruction of the periodontium, characterized by periodontal inflammation and alveolar bone loss, and subsequently tooth loss. Periodontitis represents a significant global health threat, affecting individuals across all age groups and contributing to a considerable public health burden (1). Numerous studies have confirmed that periodontitis is not only limited to a significant oral health concern but also closely associated with the initiation and development of systemic or organ-specific diseases (2–8). However, the underlying molecular mechanisms of periodontitis remain poorly understood. Therefore, investigating its pathogenesis and uncovering novel therapeutic targets are pivotal for advancing safe and efficacious treatments for periodontitis, with profound clinical implications for oral and systemic health.
Recently, the concept of cell death has evolved to primarily encompass two categories, including necrosis and programmed cell death (PCD). PCD is a series of regulated cellular suicide mechanisms to maintain organismal homeostasis, including apoptosis, autophagy, necroptosis, pyroptosis (9–12). Several novel PCD pathways, such as ferroptosis and cuproptosis, have gradually become recent research hotspots in the medical field (13, 14). Although plenty of studies have found that PCD may be involved in the periodontal inflammatory response, the precise roles of ferroptosis and cuproptosis in the pathogenic mechanism of periodontitis have not yet been fully clarified. Therefore, the present article aims to systematically synthesize empirical findings that elucidate the critical function of ferroptosis and cuproptosis during periodontitis and investigate the roles of glutathione (GSH) pathway and autophagy pathway in linking ferroptosis and cuproptosis within periodontal inflammation. Furthermore, we suppose that a homeostatic imbalance of copper and iron, mediated by periodontal pathogens, may constitute a significant factor contributing to elevated periodontal oxidative stress, potentially representing a unifying mechanism that elucidates the intricate interconnection between the two forms of PCD during periodontitis. A better understanding of these pathogenic mechanisms may guide the development of innovative therapeutic approaches for periodontitis.
2 Ferroptosis in periodontitis
Ferroptosis, a novel form of PCD characterized by an iron-dependent oxidative imbalance in the intracellular microenvironment, is primarily triggered by the dysfunction of glutathione peroxidase 4 (GPX4), an important regulator of intracellular redox homeostasis (13). Iron balance serves a vital function in the redox cycle reactions to carry out normal biological functions, while intracellular iron overload can facilitate the generation of substantial quantities of reactive oxygen species (ROS) and lipid peroxidation through the Fenton reaction, leading to exaggerated inflammatory cascades that underlie the pathogenesis of inflammatory diseases (15).
Research has increasingly focused on the role of ferroptosis in periodontal inflammation. Extensive bioinformatics analyses have indicated that ferroptosis is involved in the etiology and progression of periodontitis (16–23). Both basic and clinical research has also unveiled common pathophysiological features of periodontitis and ferroptosis, such as oxidative stress and lipid peroxidation (24–27). Elevated iron levels are closely associated with the initiation and severity of periodontitis, indicating that alterations in iron metabolism may occur during periodontitis and thus provide possible positive feedback that reinforces periodontal damage (28–31). An excessive level of iron can exacerbate ROS generation within periodontal tissues and accelerate susceptibility to infection by periodontal pathogens, ultimately inducing cellular ferroptosis and subsequent periodontium damage (31–36). Therefore, iron-dependent oxidative stress likely underlies both periodontitis and ferroptosis. Nevertheless, the specific mechanisms by which ferroptosis contributes to periodontitis remain incompletely elucidated.
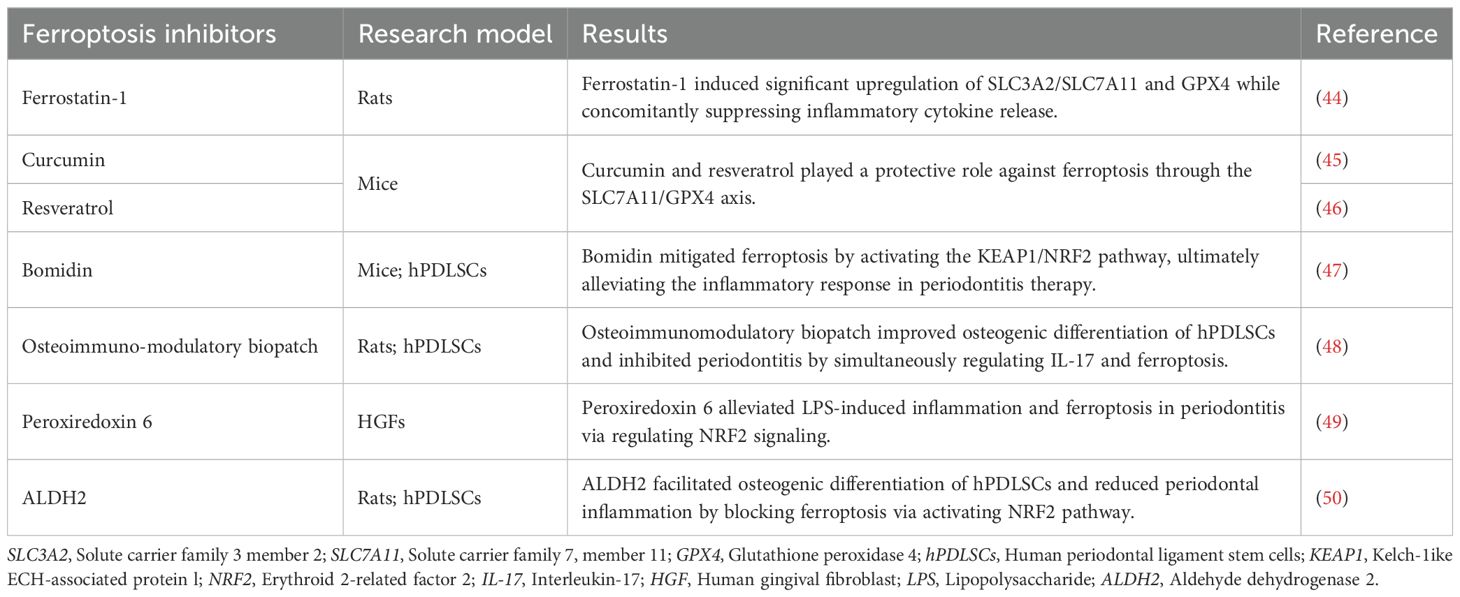
Recently, increasing evidence has revealed an association between periodontitis and ferroptosis, indicating that ferroptosis may be a crucial risk factor for the development and progression of periodontal inflammation. Butyrate, a short-chain fatty acid from periodontal pathogens, promoted nuclear receptor coactivator 4 (NCOA4)-mediated ferritinophagy and ferroptosis in periodontal ligament fibroblasts through p38/hypoxia inducible factor-1α (HIF-1α) signaling and bromodomain-containing protein 4 (BRD4)/cyclin-dependent kinase 9 (CDK9) complexes (37). Similarly, lncRNA LINC00616 aggravated ferroptosis in human periodontal ligament stem cells (hPDLSCs) via the microRNA-370/transferrin receptor axis (38). Activating transcription factor 3 (AFT3) inhibited osteogenic differentiation of lipopolysaccharide (LPS)-stimulated hPDLSCs by activating ferroptosis through the nuclear factor erythroid 2-related factor 2 (NRF2)/heme oxygenase 1 (HO-1) pathway (39). Other empirical studies have also found that ferroptosis acts as a catalyst in the progression of periodontitis in human gingival fibroblasts (HGFs) and murine models (40, 41). Moreover, IL-17 administration alleviated osteoblast ferroptosis and promoted osteogenic differentiation via the direct interaction of phosphorylated signal transducer and activator of transcription 3 (STAT3) with NRF2 in periodontitis models (42). Inhibition of zinc finger DHHC-type palmitoyl transferases 16 (ZDHHC16) facilitated osteogenic differentiation of dental pulp stem cells by inhibiting ferroptosis through cAMP-response element binding protein (CREB) pathway, suggesting a negative association between ferroptosis and alveolar bone repair (43). These findings suggest that ferroptosis may be a risk factor for periodontitis development. Appropriate inhibition of ferroptosis may have certain clinical application value in periodontitis treatment (Table 1) (44–50).
Significantly, periodontitis has also been demonstrated to be a potential risk factor for systemic diseases through activating ferroptosis in other organs. The association is largely attributed to the strong epidemiological link between periodontal pathogens and systemic disorders, mediated by the ability of oral microbiota to ectopically colonize distant tissues and induce systemic inflammation through aberrant secretion of proinflammatory cytokines. Xiong et al. underscored the critical roles of periodontal pathogenic bacteria in driving ferroptosis in lung tissue, aggravating chronic obstructive pulmonary disease (51). Porphyromonas gingivalis (P.g) could exacerbate alcoholic liver disease and nonalcoholic fatty liver disease in murine models, potentially mediated by ferroptosis activation in hepatocytes (52, 53). Additionally, indoxyl sulfate in the gingival crevicular fluid from mice with chronic kidney disease promoted ferroptosis-mediated osteogenic differentiation disorder in MC3T3-E1 cells via blocking the SLC7A11/GPX4 pathway (54).
In summary, ferroptosis is regarded a potential periodontal risk factor, associated with the incidence and severity of periodontitis and related systemic disease. A positive feedback loop may exist between periodontal pathogens and iron concentration in periodontitis. Periodontal pathogens induce iron overload to disrupt the antioxidant system in the periodontium by degrading iron-binding proteins, while elevated iron concentrations may further increase the susceptibility of periodontal tissue to infection and oxidative stress (28, 31, 34, 35, 55, 56). However, previous research has shown that Saikosaponin A attenuate alveolar bone resorption in experimental periodontitis rat models by promoting ferroptosis of osteoclasts via the NRF2/SLC7A11/GPX4 axis, indicating a protective role of ferroptosis against alveolar bone loss (57). Ferroptosis may exhibit different regulatory roles in periodontitis depending on the types of periodontal cells in which ferroptosis occurs. Notably, ferroptosis inhibition as a therapeutic approach for periodontitis is in the exploratory data analysis phase and warrants further study. Given the presence of similar characteristic markers (e.g., iron and ROS) involved in autophagy, apoptosis and pyroptosis, it remains unclear whether ferroptosis coexists with other types of PCD during periodontitis and their specific correlation in periodontal disease. Differences between animal models and humans have generally led to the perception that interventions of ferroptosis inhibitors on periodontitis in animals may not be reproducible in human. Besides, the intricate regulatory networks underlying ferroptosis remain incompletely characterized, which may limit the therapeutic efficacy of single-target inhibitors across diverse pathological conditions. Current pharmacological interventions targeting ferroptosis inhibition are hampered by insufficient tissue selectivity and nonspecific biodistribution, resulting in ineffective targeting of periodontal lesional microenvironments.
3 Cuproptosis in periodontitis
Copper is an essential trace metal for human health, acting as a catalytic cofactor in various enzyme-driven physiological processes. Cuproptosis is a recently discovered form of PCD triggered by excessive copper, which is highly correlated with mitochondrial respiration and protein lipoylation. Copper overload stimulates lipoylated protein aggregation and subsequent iron−sulfur cluster protein loss through direct binding to lipoylated components in the tricarboxylic acid (TCA) cycle, resulting in proteotoxic stress and subsequent cell death (14, 58–60). The discovery of cuproptosis may provide new insights into molecular mechanisms and therapeutic targets for various pathological conditions.
Growing evidence indicates potential crosstalk between cuproptosis and periodontitis. Significantly elevated copper levels in saliva and serum of periodontitis patients compared to healthy controls indicated disrupted copper homeostasis and a positive correlation with the periodontal index (61, 62). In addition, 11 cuproptosis-related hub genes associated with periodontitis were identified by bioinformatics analysis and further verified in periodontal tissue from periodontitis patients and healthy controls via real-time quantitative PCR and immunohistochemistry (63). Liu et al. also revealed that the intracellular copper concentration tripled in P.g LPS-treated HGFs compared to controls (64). Furthermore, age-associated differences in cuproptosis-related gene expression were noted in gingival tissue biopsies from experimental periodontitis macaque models (65). In contrast, copper chelator tetrathiomolybdate significantly reduced cuproptosis-associated marker levels in LPS-stimulated RAW 264.7 cells and murine periodontitis models, suggesting that cuproptosis inhibition could alleviate macrophage dysfunction in periodontitis (66). Similarly, curcumin gel decreased copper levels, proinflammatory cytokines, and clinical indicators in periodontitis patients (67).
Cuproptosis is considered a possible PCD contributing to periodontal inflammation, offering more comprehensive understanding of periodontitis pathogenesis. Dual immunomodulatory effect of copper complicates the interaction between pathogens and host (68, 69). Invading microorganisms must acquire copper from host to ensure an adequate supply of essential cuproenzymes during infection, whereas excessive copper has a substantial bactericidal effect through ROS production and enzyme mismetalation. Hence, we hypothesize that the host immunomodulation response to periodontal infection impacts copper homeostasis in the periodontium, leading to cuproptosis and initiation of periodontitis. Changes in the inflammatory microenvironment likely intensify copper-induced cytotoxicity and oxidative damage to periodontal tissue by further raising copper levels (70, 71). However, the characteristic markers of cuproptosis in periodontal tissues and its precise relationship with other types of PCD in periodontitis pathogenesis are ambiguous. In addition, further research is warranted to elucidate whether cuproptosis serves as a critical link connecting periodontitis and systemic diseases.
4 Crosstalk between ferroptosis and cuproptosis in periodontitis
Iron and copper are crucial metal elements involved in human physiological processes, such as cellular metabolism, oxygen transport, DNA and RNA synthesis, signaling transduction, and enzyme catalysis. A homeostatic imbalance of iron or copper plays a significant role in the pathogenesis and exacerbation of multiple diseases. Notably, increased intracellular copper levels may be important regulators of ferroptosis. Elesclomol-induced copper retention within mitochondria promoted oxidative stress and consequent ferroptosis in colorectal cancer cells via copper-transporting ATPase 1 (ATP7A) degradation and ROS accumulation (72). Copper-mediated GPX4 degradation drove autophagy-dependent ferroptosis via direct interaction of copper with GPX4 cysteines (Cys 107 and Cys 148) (73). Furthermore, consistent with the cuproptosis mechanism, blocking iron−sulfur cluster biogenesis could decrease mitochondrial lipoylation in brown adipose tissue, suggesting a regulatory role of iron in cuproptosis (74). Accumulating evidence has illuminated the mutual connections between ferroptosis and cuproptosis in various diseases (75–81). However, their interaction in periodontitis needs further clarification. This section describes the possible relevance of ferroptosis and cuproptosis in periodontitis pathogenesis (Figure 1A).
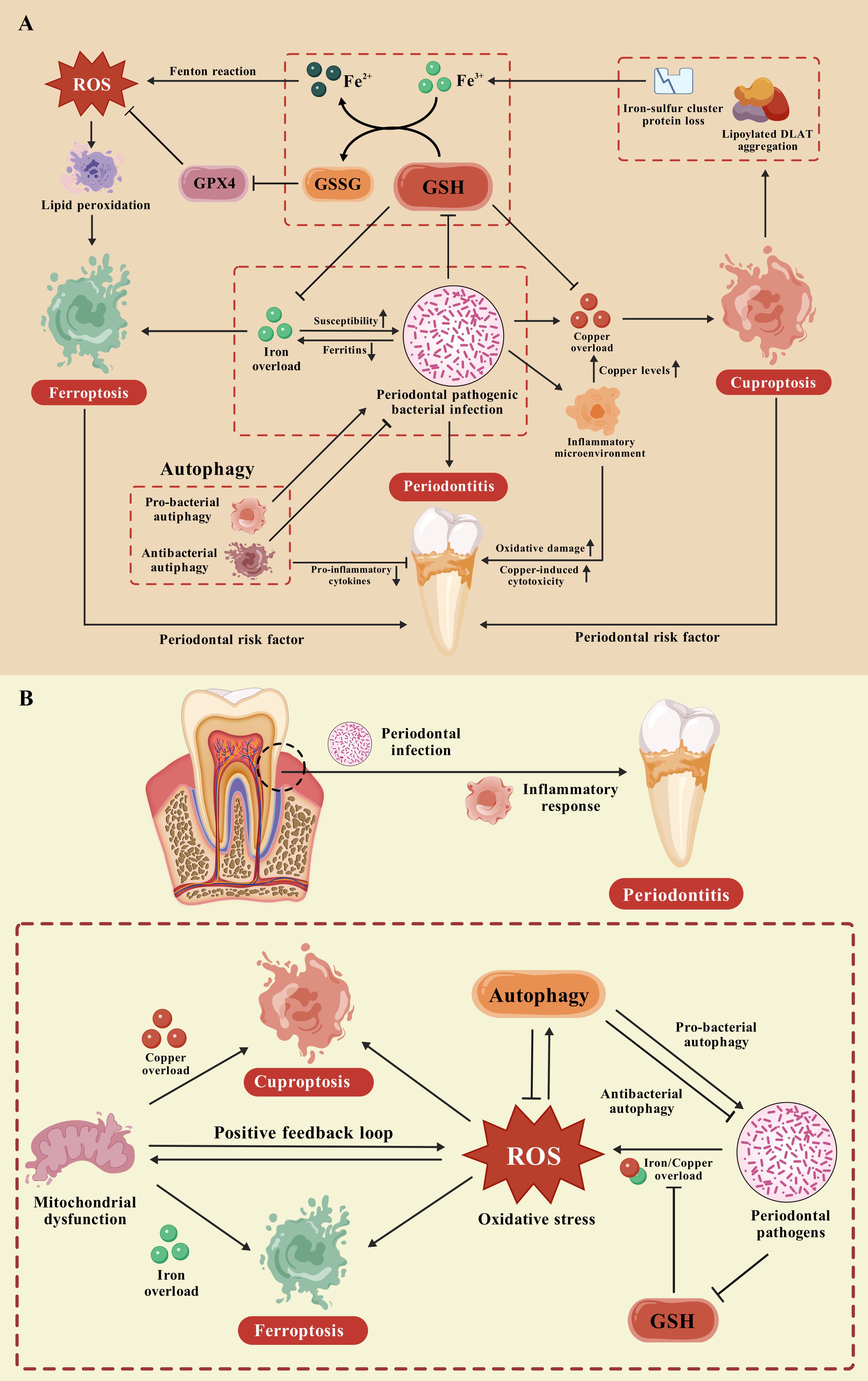
Figure 1. The potential crosstalk between ferroptosis and cuproptosis in periodontitis. (A) As possible periodontal risk factors, ferroptosis and cuproptosis are regarded as positively associated with the incidence and severity of periodontitis. A positive feedback loop exists between periodontal pathogens and iron concentration during periodontitis, ultimately triggering ferroptosis in the periodontium. The immunomodulatory responses to periodontal infection affect copper homeostasis in the periodontium, potentially resulting in the occurrence of cuproptosis and periodontitis. The increased inflammatory microenvironment may exacerbate copper toxicity and oxidative damage to periodontal tissue through further elevating copper levels. Notably, a self-accelerating cycle of ferroptosis and cuproptosis with robust proinflammatory potential may arise because of GSH consumption under attack by periodontal pathogens. Besides, autophagy exhibits both protective and pathological effects through pro-bacterial and antibacterial autophagy, ultimately regulating both ferroptosis and cuproptosis during periodontal inflammation. (B) The GSH pathway and autophagy pathway are closely related to alterations in oxidative stress levels during periodontitis. A homeostatic imbalance of copper and iron mediated by periodontal pathogens may contribute significantly to elevated periodontal oxidative stress. This phenomenon represents a unifying mechanism that elucidates the intricate interconnection between ferroptosis and cuproptosis in periodontitis.
4.1 GSH pathway
Copper/iron chelator GSH may be a central signal mediator of both ferroptosis and cuproptosis, albeit with distinct roles in these processes. In ferroptosis, GSH functions as a cofactor for GPX4 activation to mitigate lipid peroxidation and ROS accumulation, whereas it serves as a copper chaperone to inhibit lipoylated protein aggregation in the TCA cycle in cuproptosis (13, 14). GSH synthesis suppresses both ferroptosis and cuproptosis, suggesting a potential unifying mechanism that clarifies their interconnection (13, 82, 83). Conversely, SLC7A11 blockade boosted the susceptibility of hepatocellular carcinoma cells to disulfiram/copper treatment, inducing both ferroptosis and cuproptosis via GSH depletion (84). More recent evidence also suggests that concurrent induction of these processes via GSH depletion may be a promising cancer treatment (85–87). Ferroptosis activators sorafenib and erastin could promote cuproptosis in primary liver cancer cells through elevating copper-dependent lipoylated protein aggregation via inhibiting GSH synthesis and mitochondria-dependent ferredoxin 1 (FDX1) protein degradation (88). Similar results were obtained in myelodysplastic syndromes cell lines (89).
GSH, a common biomarker of oxidative stress, may act as the intersection of ferroptosis and cuproptosis during periodontitis (26, 45, 90–92). Nevertheless, their crosstalk has not been established in periodontitis. We hypothesize that a self-accelerating cycle of ferroptosis and cuproptosis with robust proinflammatory potential may arise from GSH consumption under periodontal pathogen attack. Pathogen-stimulated iron overload induces ferroptosis through GSH depletion and mitochondrial dysfunction, resulting in excess intracellular copper and subsequent cuproptosis in the periodontium. Cuproptosis initiates lipoylated protein aggregation, iron–sulfur cluster protein loss, and ultimately the release of free Fe3+. The reaction between Fe3+ and GSH could further deactivates the GSH/GPX4 pathway through the transformation of GSH into oxidative GSH (GSSG) and provides abundant Fe2+ for the Fenton reaction, which accelerates ferroptosis in periodontal tissue. Consequently, targeting GSH may represent a novel approach to periodontitis treatment through simultaneous inhibition of ferroptosis and cuproptosis in periodontal cells. However, whether GSH pathway is involved in other PCD pathways in periodontitis and which pathway dominates periodontitis deserve further study.
4.2 Autophagy pathway
Autophagy is a complex cellular process contributing to the phagocytosis and degradation of various substrates, which maintains cellular homeostasis under normal physiological conditions and evokes autophagic cell death under pathological circumstances. Ferroptosis and cuproptosis are usually accompanied by autophagy activation, suggesting its role as a common hub for these processes. Multiple studies have demonstrated that selective autophagy plays a crucial role in the initiation of ferroptosis. Autophagy could act as a catalyst for ferroptosis to amplify iron-dependent lipid peroxidation and ROS accumulation through degradation of anti-ferroptotic factors, such as ferritin, GPX4, lipid droplets, and cadherin 2 (73, 93–99). The precise regulatory mechanism linking cuproptosis and autophagy remains incompletely understood. However, disruption in copper homeostasis can regulate the autophagy process through multiple pathways, triggering either a protective response or autophagic cell death based on stimulus strength and substrate properties (100–105). Moreover, cuproptosis improved chemosensitivity to docetaxel in prostate cancer cell lines by hindering autophagy via the dihydrolipoamide S-acetyltransferase (DLAT)/mammalian target of rapamycin (mTOR) pathway (106). The cuproptosis key gene ferredoxin-1 (FDX1) and its related genes were positively correlated with the expression of autophagy marker genes via recent bioinformatics analysis. Additionally, FDX1-mediated blockade of mitophagy also indicated a possible interplay between FDX1-mediated cuproptosis and autophagy (107, 108).
Existing data support the importance of autophagy as a bidirectional regulator of periodontitis pathogenesis, including cellular protection against apoptosis, enhancement of angiogenesis in periodontal tissues, promotion of other types of PCD, and regulation of alveolar bone homeostasis (109, 110). IL-17A-mediated iron metabolism prompted ferritin expression in osteoblasts, eventually bolstering osteogenic differentiation and alveolar bone repair via autophagy activation in murine periodontitis models. In addition, LPS-induced cuproptosis impeded autophagosome biogenesis and mitophagy in macrophages during periodontitis (66). Therefore, autophagy plays a significant role in both ferroptosis and cuproptosis, potentially serving as a central hub linking them in periodontal inflammation.
Autophagy exhibits both protective and pathological effects in periodontitis, which may constitute a significant factor contributing to the intricate influence of autophagy on ferroptosis and cuproptosis. This phenomenon is likely attributable to different responses of autophagy to bacterial infections, depending on the types of infected cells and periodontal pathogens (111). Autophagy can not only prevent periodontal infection and production of pro-inflammatory cytokines but also establish a unique microenvironment conducive to the replication and immune evasion of periodontal pathogens. Furthermore, excessive accumulation of iron and copper induced by periodontal pathogens could damage the antioxidant system in the periodontium, ultimately resulting in ferroptosis and cuproptosis during periodontitis. Thus, diverse responses of autophagy to periodontal infection may exert multifaceted effects on ferroptosis and cuproptosis through modulating the oxidative stress in periodontitis. Nevertheless, the specific regulatory mechanisms by which autophagy influences ferroptosis and cuproptosis in periodontitis remain elusive. Moreover, the precise relationship between ROS and autophagy initiation in periodontitis warrants further exploration for a comprehensive understanding of the association between ferroptosis and cuproptosis.
4.3 The potential unifying mechanism linking ferroptosis and cuproptosis in periodontitis
The GSH pathway and autophagy pathway are closely associated with alterations in oxidative stress levels during periodontitis. Oxidative stress can induce and interact with autophagy, whereas autophagy mitigates oxidative stress and confers cellular protection against oxidative damage in the periodontium. GSH functions as a critical antioxidant in the cellular defense against oxidative stress and is converted to GSSG through the action of GSH peroxidase in response to oxidative stress. A reduced GSH/GSSG ratio typically indicates the presence of oxidative stress and is widely recognized as a significant biomarker for the oxidative damage associated with periodontitis. In addition, increasing evidence suggests that mitochondrial dysfunction is implicated in the onset and progression of periodontitis. Fluctuations in mitochondrial function represent critical signaling events and serve as direct indicators of cellular responses to periodontal pathogen infection. Diverse manifestations of mitochondrial dysfunction, including oxidative stress, mitophagy, mitochondria-mediated apoptosis, and metabolic disorders, are commonly observed in periodontal disease (112–117). Interestingly, oxidative stress frequently drives mitochondrial dysfunction, which in turn aggravates oxidative stress through ROS overgeneration (118). Mitochondrial iron and copper are widely recognized as critical regulators of mitochondrial electron transport chain function and various other mitochondrial processes. Disruption of mitochondrial metal homeostasis can result in excessive ROS generation and induce ferroptosis and cuproptosis (119–122). Therefore, increased oxidative stress is likely to be a key factor in the shared pathophysiological mechanisms of ferroptosis and cuproptosis in periodontitis.
As previously discussed, the possibility that a positive feedback loop exists between periodontal infection and iron levels has been raised. Periodontal pathogens may trigger iron overload by degrading iron-binding proteins and destroy the antioxidant system in periodontal tissue, whereas increased iron concentrations further enhance the vulnerability to infection by periodontal pathogens and aggravate oxidative damage. Similarly, periodontal infection facilitates copper overload in the periodontal microenvironment to ensure an adequate supply of essential cuproenzymes, thereby intensifying periodontal oxidative damage and exacerbating the local inflammatory milieu. Additionally, extensive crosstalk occurs between copper and iron in various physiological and pathological processes; consequently, either iron imbalance or copper imbalance may simultaneously modulate both ferroptosis and cuproptosis through copper−iron interactions (72–74, 123–125). We infer that a homeostatic imbalance of copper and iron, which is mediated by periodontal pathogens, may constitute a significant factor contributing to elevated periodontal oxidative stress. This phenomenon potentially represents a unifying mechanism that elucidates the intricate interconnection between ferroptosis and cuproptosis in periodontitis (Figure 1B).
Antioxidant and antibacterial therapies are expected to become effective treatment options against ferroptosis and cuproptosis in periodontitis. Several natural antioxidant agents have been demonstrated to be promising approaches against oxidative damage in the context of periodontitis. Quercetin has been shown to decrease oxidative damage in periodontal ligament cells through the activation of NRF2 signaling while also reducing alveolar bone loss in murine models of experimental periodontitis (126). Silibinin has demonstrated significant anti-inflammatory and antioxidative effects against periodontitis both in vitro and in vivo by downregulating the expression of NF-κB and NLRP3 while upregulating NRF2 expression (127). Similarly, resveratrol can prevent alveolar bone loss in an experimental rat model of periodontitis by ameliorating the production of circulating ROS via the NRF2/HO-1 axis (128). The local administration of curcumin gel also exerts an antioxidant effect on attenuating ligature-induced periodontitis in diabetic rats (129). In addition, novel treatment alternatives against periodontal pathogens, such as zinc materials, probiotics, N-chlorotaurine and antimicrobial photodynamic therapy, are being explored (130–134).
5 Conclusions and perspective
In conclusion, these findings revealed the involvement of ferroptosis and cuproptosis in the pathogenesis of periodontitis. Although the cause-and-effect relationship between ferroptosis and cuproptosis remains unclear, we propose several potential associations of these two forms of PCD in periodontitis, including the GSH pathway and the autophagy pathway. Elevated oxidative stress may constitute a unifying mechanism underlying the complex interrelationship between ferroptosis and cuproptosis in periodontitis. Antioxidative and antibacterial therapies may serve as efficacious treatment modalities for periodontal damage through inhibition of ferroptosis and cuproptosis.
Future research is imperative to address the following issues. The biomarkers of ferroptosis and cuproptosis in periodontal disease, as well as their precise relationships with other forms of PCD in periodontitis pathogenesis, remain poorly understood. The overlapping characteristic markers (e.g., iron, copper, p53, ROS, and inflammatory mediators) underscore the potential crosstalk among different PCD forms during periodontitis. Targeting shared nodes may yield synergistic therapeutic effects, offering novel combinatorial regimens to halt periodontitis progression. Furthermore, the disparity between animals and humans has contributed to the perception that the effects of ferroptosis inhibitors or cuproptosis inhibitors on periodontitis observed in animals may not be reliably replicated in humans. The potential effects of inhibiting ferroptosis and cuproptosis in periodontitis on the proliferation and dissemination of periodontal pathogenic bacteria necessitate further investigation.
Elucidating the roles of ferroptosis and cuproptosis in periodontitis may yield novel insights for future investigations to explore the specific pathogenic mechanisms underlying periodontitis.
Author contributions
TZ: Funding acquisition, Writing – original draft. FL: Writing – review & editing. PW: Writing – review & editing. YC: Funding acquisition, Supervision, Writing – review & editing. RZ: Supervision, Writing – review & editing. XL: Funding acquisition, Supervision, Writing – original draft.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. The review article was supported by the National Natural Science Foundation of China (82404478), the Guangzhou Municipal Science and Technology Bureau (2023A04J1164) and the Stomatology Hospital of Southern Medical University (PY2023039).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Albandar JM. Disparities and social determinants of periodontal diseases. Periodontology 2000. (2024) 00:1–20. doi: 10.1111/prd.12547
2. Bhuyan R, Bhuyan SK, Mohanty JN, Das S, Juliana N, Juliana IF. Periodontitis and its inflammatory changes linked to various systemic diseases: A review of its underlying mechanisms. Biomedicines. (2022) 10:2659. doi: 10.3390/biomedicines10102659
3. Wu Q, Zhang W, Lu Y, Li H, Yang Y, Geng F, et al. Association between periodontitis and inflammatory comorbidities: The common role of innate immune cells, underlying mechanisms and therapeutic targets. Int Immunopharmacol. (2024) 128:111558. doi: 10.1016/j.intimp.2024.111558
4. Ryder MI, Shiboski C, Yao TJ, Moscicki AB. Current trends and new developments in HIV research and periodontal diseases. Periodontol 2000. (2020) 82:65–77. doi: 10.1111/prd.12321
5. Hajishengallis G, Chavakis T. Local and systemic mechanisms linking periodontal disease and inflammatory comorbidities. Nat Rev Immunol. (2021) 21:426–40. doi: 10.1038/s41577-020-00488-6
6. Baima G, Minoli M, Michaud DS, Aimetti M, Sanz M, Loos BG, et al. Periodontitis and risk of cancer: Mechanistic evidence. Periodontology 2000. (2024) 96:83–94. doi: 10.1111/prd.12540
7. Shinjo T, Nishimura F. The bidirectional association between diabetes and periodontitis, from basic to clinical. Japanese Dental Sci review. (2024) 60:15–21. doi: 10.1016/j.jdsr.2023.12.002
8. Carra MC, Rangé H, Caligiuri G, Bouchard P. Periodontitis and atherosclerotic cardiovascular disease: A critical appraisal. Periodontology 2000. (2023) 00:1–34. doi: 10.1111/prd.12528
9. Newton K, Strasser A, Kayagaki N, Dixit VM. Cell death. Cell. (2024) 187:235–56. doi: 10.1016/j.cell.2023.11.044
10. Yuan J, Ofengeim D. A guide to cell death pathways. Nat Rev Mol Cell Biol. (2024) 25:379–95. doi: 10.1038/s41580-023-00689-6
11. Christgen S, Tweedell RE, Kanneganti TD. Programming inflammatory cell death for therapy. Pharmacol Ther. (2022) 232:108010. doi: 10.1016/j.pharmthera.2021.108010
12. Yu P, Zhang X, Liu N, Tang L, Peng C, Chen X. Pyroptosis: mechanisms and diseases. Signal Transduct Target Ther. (2021) 6:128. doi: 10.1038/s41392-021-00507-5
13. Jiang X, Stockwell BR, Conrad M. Ferroptosis: mechanisms, biology and role in disease. Nat Rev Mol Cell Biol. (2021) 22:266–82. doi: 10.1038/s41580-020-00324-8
14. Chen L, Min J, Wang F. Copper homeostasis and cuproptosis in health and disease. Signal transduction targeted Ther. (2022) 7:378. doi: 10.1038/s41392-022-01229-y
15. Chen Y, Fang ZM, Yi X, Wei X, Jiang DS. The interaction between ferroptosis and inflammatory signaling pathways. Cell Death Dis. (2023) 14:205. doi: 10.1038/s41419-023-05716-0
16. Chen J, Ou L, Liu W, Gao F. Exploring the molecular mechanisms of ferroptosis-related genes in periodontitis: a multi-dataset analysis. BMC Oral Health. (2024) 24:611. doi: 10.1186/s12903-024-04342-2
17. Fu Y, Xu T, Guo M, Lv W, Ma N, Zhang L. Identification of disulfidptosis- and ferroptosis-related transcripts in periodontitis by bioinformatics analysis and experimental validation. Front Genet. (2024) 15:1402663. doi: 10.3389/fgene.2024.1402663
18. Xu Z, Tan R, Li X, Pan L, Ji P, Tang H. Development of a classification model and an immune-related network based on ferroptosis in periodontitis. J Periodontal Res. (2023) 58:403–13. doi: 10.1111/jre.13100
19. Zhang C, Xue P, Ke J, Cai Q. Development of ferroptosis-associated ceRNA network in periodontitis. Int Dent J. (2023) 73:186–94. doi: 10.1016/j.identj.2022.05.004
20. Pan S, Li Y, He H, Cheng S, Li J, Pathak JL. Identification of ferroptosis, necroptosis, and pyroptosis-associated genes in periodontitis-affected human periodontal tissue using integrated bioinformatic analysis. Front Pharmacol. (2022) 13:1098851. doi: 10.3389/fphar.2022.1098851
21. Li X, Chen T, Fu Y, Yang B, Lin X, Hou J, et al. Mechanism and functional verification of genes by virulence factors of P. gingivalis in ferroptosis. Arch Oral Biol. (2024) 163:105965. doi: 10.1016/j.archoralbio.2024.105965
22. Zhang S, Jin H, Da J, Zhang K, Liu L, Guo Y, et al. Role of ferroptosis-related genes in periodontitis based on integrated bioinformatics analysis. PloS One. (2022) 17:e0271202. doi: 10.1371/journal.pone.0271202
23. Torres A, Michea MA, Végvári Á, Arce M, Pérez V, Alcota M, et al. A multi-platform analysis of human gingival crevicular fluid reveals ferroptosis as a relevant regulated cell death mechanism during the clinical progression of periodontitis. Int J Oral Sci. (2024) 16:43. doi: 10.1038/s41368-024-00306-y
24. Tóthová L, Celec P. Oxidative stress and antioxidants in the diagnosis and therapy of periodontitis. Front Physiol. (2017) 8:1055. doi: 10.3389/fphys.2017.01055
25. Ma F, Luo S, Lu C, Jiang X, Chen K, Deng J, et al. The role of Nrf2 in periodontal disease by regulating lipid peroxidation, inflammation and apoptosis. Front Endocrinol (Lausanne). (2022) 13:963451. doi: 10.3389/fendo.2022.963451
26. Gou H, Chen X, Zhu X, Li L, Hou L, Zhou Y, et al. Sequestered SQSTM1/p62 crosstalk with Keap1/NRF2 axis in hPDLCs promotes oxidative stress injury induced by periodontitis. Free Radic Biol Med. (2022) 190:62–74. doi: 10.1016/j.freeradbiomed.2022.08.001
27. Veljovic T, Djuric M, Mirnic J, Gusic I, Maletin A, Ramic B, et al. Lipid peroxidation levels in saliva and plasma of patients suffering from periodontitis. J Clin Med. (2022) 11:3617. doi: 10.3390/jcm11133617
28. Boyer E, Le-Gall-David S, Martin B, Fong SB, Loréal O, Deugnier Y, et al. Increased transferrin saturation is associated with subgingival microbiota dysbiosis and severe periodontitis in genetic haemochromatosis. Sci Rep. (2018) 8:15532. doi: 10.1038/s41598-018-33813-0
29. Costa SA, Moreira ARO, Costa CPS, Carvalho Souza SF. Iron overload and periodontal status in patients with sickle cell anaemia: A case series. J Clin Periodontol. (2020) 47:668–75. doi: 10.1111/jcpe.13284
30. Guo W, Zhao Y, Li H, Lei L. NCOA4-mediated ferritinophagy promoted inflammatory responses in periodontitis. J Periodontal Res. (2021) 56:523–34. doi: 10.1111/jre.12852
31. Mukherjee S. The role of crevicular fluid iron in periodontal disease. J Periodontol. (1985) 56:22–7. doi: 10.1902/jop.1985.56.11s.22
32. Leung KP, Folk SP. Effects of porphyrins and inorganic iron on the growth of Prevotella intermedia. FEMS Microbiol Lett. (2002) 209:15–21. doi: 10.1111/j.1574-6968.2002.tb11103.x
33. DeCarlo AA, Paramaesvaran M, Yun PL, Collyer C, Hunter N. Porphyrin-mediated binding to hemoglobin by the HA2 domain of cysteine proteinases (gingipains) and hemagglutinins from the periodontal pathogen Porphyromonas gingivalis. J Bacteriol. (1999) 181:3784–91. doi: 10.1128/jb.181.12.3784-3791.1999
34. Goulet V, Britigan B, Nakayama K, Grenier D. Cleavage of human transferrin by Porphyromonas gingivalis gingipains promotes growth and formation of hydroxyl radicals. Infect Immun. (2004) 72:4351–6. doi: 10.1128/iai.72.8.4351-4356.2004
35. Guan SM, Nagata H, Shizukuishi S, Wu JZ. Degradation of human hemoglobin by Prevotella intermedia. Anaerobe. (2006) 12:279–82. doi: 10.1016/j.anaerobe.2006.09.001
36. Ke JY, Cen WJ, Zhou XZ, Li YR, Kong WD, Jiang JW. Iron overload induces apoptosis of murine preosteoblast cells via ROS and inhibition of AKT pathway. Oral Dis. (2017) 23:784–94. doi: 10.1111/odi.12662
37. Zhao Y, Li J, Guo W, Li H, Lei L. Periodontitis-level butyrate-induced ferroptosis in periodontal ligament fibroblasts by activation of ferritinophagy. Cell Death Discovery. (2020) 6:119. doi: 10.1038/s41420-020-00356-1
38. Wang H, Qiao X, Zhang C, Hou J, Qi S. Long non-coding RNA LINC00616 promotes ferroptosis of periodontal ligament stem cells via the microRNA-370/transferrin receptor axis. Bioengineered. (2022) 13:13070–81. doi: 10.1080/21655979.2022.2076508
39. Lu H, Zheng Y, Wang D. ATF3 affects osteogenic differentiation in inflammatory hPDLSCs by mediating ferroptosis via regulating the Nrf2/HO-1 signaling pathway. Tissue Cell. (2024) 89:102447. doi: 10.1016/j.tice.2024.102447
40. Xing L, Dong W, Chen Y, Dai W, Xiao X, Liu Z, et al. Fibroblast ferroptosis is involved in periodontitis-induced tissue damage and bone loss. Int Immunopharmacol. (2023) 114:109607. doi: 10.1016/j.intimp.2022.109607
41. Tang Y, Su S, Yu R, Liao C, Dong Z, Jia C, et al. Unraveling ferroptosis in osteogenic lineages: implications for dysregulated bone remodeling during periodontitis progression. Cell Death Discovery. (2024) 10:195. doi: 10.1038/s41420-024-01969-6
42. Bao J, Wang Z, Yang Y, Yu X, Yuan W, Sun W, et al. Interleukin-17 alleviates erastin-induced alveolar bone loss by suppressing ferroptosis via interaction between NRF2 and p-STAT3. J Clin Periodontol. (2024) 51:233–50. doi: 10.1111/jcpe.13898
43. Liu W, Yu W, Zhou L, Ling D, Xu Y, He F. Inhibition of ZDHHC16 promoted osteogenic differentiation and reduced ferroptosis of dental pulp stem cells by CREB. BMC Oral Health. (2024) 24:388. doi: 10.1186/s12903-024-04107-x
44. Fu E, Kuo CY, Hsia YJ, Huang YM, Tseng HH, Fu MW, et al. Role of ferroptosis in periodontitis: An animal study in rats. J periodontal Res. (2023) 58:1031–40. doi: 10.1111/jre.13165
45. Wang Y, Lin H, Huang W, Liu Z, Chen Z, Zhao X, et al. Curcumin attenuates periodontal injury via inhibiting ferroptosis of ligature-induced periodontitis in mice. Int J Mol Sci. (2023) 24:9835. doi: 10.3390/ijms24129835
46. Li Y, Huang Z, Pan S, Feng Y, He H, Cheng S, et al. Resveratrol alleviates diabetic periodontitis-induced alveolar osteocyte ferroptosis possibly via regulation of SLC7A11/GPX4. Nutrients. (2023) 15:2115. doi: 10.3390/nu15092115
47. Wu W, Li G, Dong S, Huihan Chu C, Ma S, Zhang Z, et al. Bomidin attenuates inflammation of periodontal ligament stem cells and periodontitis in mice via inhibiting ferroptosis. Int immunopharmacology. (2024) 127:111423. doi: 10.1016/j.intimp.2023.111423
48. Liu S, Wang W, Chen Z, Wu P, Pu W, Li G, et al. An osteoimmunomodulatory biopatch potentiates stem cell therapies for bone regeneration by simultaneously regulating IL-17/ferroptosis signaling pathways. Advanced Sci (Weinheim Baden-Wurttemberg Germany). (2024) 11:e2401882. doi: 10.1002/advs.202401882
49. Yang WY, Meng X, Wang YR, Wang QQ, He X, Sun XY, et al. PRDX6 alleviates lipopolysaccharide-induced inflammation and ferroptosis in periodontitis. Acta odontologica Scandinavica. (2022) 80:535–46. doi: 10.1080/00016357.2022.2047780
50. Chen J, Hu C, Lu X, Yang X, Zhu M, Ma X, et al. ALDH2 alleviates inflammation and facilitates osteogenic differentiation of periodontal ligament stem cells in periodontitis by blocking ferroptosis via activating Nrf2. Funct Integr Genomics. (2024) 24:184. doi: 10.1007/s10142-024-01465-1
51. Xiong K, Yang P, Wei W, Li J, Cui Y, Li Y, et al. Periodontitis contributes to COPD progression via affecting ferroptosis. BMC Oral Health. (2023) 23:664. doi: 10.1186/s12903-023-03397-x
52. Yao C, Lu L, Lan D, Zhu X, Li X, Gao Y, et al. Porphyromonas gingivalis as a promotor in the development of the alcoholic liver disease via ferroptosis. Microbes Infect. (2024) 26:105250. doi: 10.1016/j.micinf.2023.105250
53. Yao C, Lan D, Li X, Wang Y, Qi S, Liu Y. Porphyromonas gingivalis is a risk factor for the development of nonalcoholic fatty liver disease via ferroptosis. Microbes Infect. (2023) 25:105040. doi: 10.1016/j.micinf.2022.105040
54. Chen H, Zhou Y, Liu Y, Zhou W, Xu L, Shang D, et al. Indoxyl sulfate exacerbates alveolar bone loss in chronic kidney disease through ferroptosis. Oral Dis. (2024) 31:264–77. doi: 10.1111/odi.15050
55. Lewis JP. Metal uptake in host-pathogen interactions: role of iron in Porphyromonas gingivalis interactions with host organisms. Periodontol 2000. (2010) 52:94–116. doi: 10.1111/j.1600-0757.2009.00329.x
56. Smalley JW, Byrne DP, Birss AJ, Wojtowicz H, Sroka A, Potempa J, et al. HmuY haemophore and gingipain proteases constitute a unique syntrophic system of haem acquisition by Porphyromonas gingivalis. PloS One. (2011) 6:e17182. doi: 10.1371/journal.pone.0017182
57. Li TQ, Liu Y, Feng C, Bai J, Wang ZR, Zhang XY, et al. Saikosaponin A attenuates osteoclastogenesis and bone loss by inducing ferroptosis. Front Mol biosciences. (2024) 11:1390257. doi: 10.3389/fmolb.2024.1390257
58. Ge EJ, Bush AI, Casini A, Cobine PA, Cross JR, DeNicola GM, et al. Connecting copper and cancer: from transition metal signalling to metalloplasia. Nat Rev Cancer. (2022) 22:102–13. doi: 10.1038/s41568-021-00417-2
59. Yang L, Yang P, Lip GYH, Ren J. Copper homeostasis and cuproptosis in cardiovascular disease therapeutics. Trends Pharmacol Sci. (2023) 44:573–85. doi: 10.1016/j.tips.2023.07.004
60. Tsang T, Davis CI, Brady DC. Copper biology. Curr Biol. (2021) 31:R421–r7. doi: 10.1016/j.cub.2021.03.054
61. Romano F, Castiblanco A, Spadotto F, Di Scipio F, Malandrino M, Berta GN, et al. ICP-mass-spectrometry ionic profile of whole saliva in patients with untreated and treated periodontitis. Biomedicines. (2020) 8:354. doi: 10.3390/biomedicines8090354
62. Dommisch H, Kuzmanova D, Jönsson D, Grant M, Chapple I. Effect of micronutrient malnutrition on periodontal disease and periodontal therapy. Periodontol 2000. (2018) 78:129–53. doi: 10.1111/prd.12233
63. Liu S, Ge J, Chu Y, Cai S, Wu J, Gong A, et al. Identification of hub cuproptosis related genes and immune cell infiltration characteristics in periodontitis. Front Immunol. (2023) 14:1164667. doi: 10.3389/fimmu.2023.1164667
64. Liu N, He Y, Chen X, Qiu G, Wu Y, Shen Y. Changes in cuproptosis-related gene expression in periodontitis: An integrated bioinformatic analysis. Life Sci. (2024) 338:122388. doi: 10.1016/j.lfs.2023.122388
65. Ebersole JL, Kirakodu SS, Nguyen LM, Gonzalez OA. Transcriptomic features of programmed and inflammatory cell death in gingival tissues. Oral Dis. (2024) 00:1–2. doi: 10.1111/odi.14939
66. Zhang L, Tsai IC, Ni Z, Chen B, Zhang S, Cai L, et al. Copper chelation therapy attenuates periodontitis inflammation through the cuproptosis/autophagy/lysosome axis. Int J Mol Sci. (2024) 25:5890. doi: 10.3390/ijms25115890
67. Mohammad CA. Efficacy of curcumin gel on zinc, magnesium, copper, IL-1β, and TNF-α in chronic periodontitis patients. BioMed Res Int. (2020) 2020:8850926. doi: 10.1155/2020/8850926
68. Focarelli F, Giachino A, Waldron KJ. Copper microenvironments in the human body define patterns of copper adaptation in pathogenic bacteria. PloS Pathog. (2022) 18:e1010617. doi: 10.1371/journal.ppat.1010617
69. Andrei A, Öztürk Y, Khalfaoui-Hassani B, Rauch J, Marckmann D, Trasnea PI, et al. Cu homeostasis in bacteria: the ins and outs. Membranes (Basel). (2020) 10:242. doi: 10.3390/membranes10090242
70. Pereira TC, Campos MM, Bogo MR. Copper toxicology, oxidative stress and inflammation using zebrafish as experimental model. J Appl Toxicol. (2016) 36:876–85. doi: 10.1002/jat.3303
71. Jiang C, Wu B, Xue M, Lin J, Hu Z, Nie X, et al. Inflammation accelerates copper-mediated cytotoxicity through induction of six-transmembrane epithelial antigens of prostate 4 expression. Immunol Cell Biol. (2021) 99:392–402. doi: 10.1111/imcb.12427
72. Gao W, Huang Z, Duan J, Nice EC, Lin J, Huang C. Elesclomol induces copper-dependent ferroptosis in colorectal cancer cells via degradation of ATP7A. Mol Oncol. (2021) 15:3527–44. doi: 10.1002/1878-0261.13079
73. Xue Q, Yan D, Chen X, Li X, Kang R, Klionsky DJ, et al. Copper-dependent autophagic degradation of GPX4 drives ferroptosis. Autophagy. (2023) 19:1982–96. doi: 10.1080/15548627.2023.2165323
74. Tajima K, Ikeda K, Chang HY, Chang CH, Yoneshiro T, Oguri Y, et al. Mitochondrial lipoylation integrates age-associated decline in brown fat thermogenesis. Nat Metab. (2019) 1:886–98. doi: 10.1038/s42255-019-0106-z
75. Song J, Ren K, Zhang D, Lv X, Sun L, Deng Y, et al. A novel signature combing cuproptosis- and ferroptosis-related genes in sepsis-induced cardiomyopathy. Front Genet. (2023) 14:1170737. doi: 10.3389/fgene.2023.1170737
76. Shen Y, Li D, Liang Q, Yang M, Pan Y, Li H. Cross-talk between cuproptosis and ferroptosis regulators defines the tumor microenvironment for the prediction of prognosis and therapies in lung adenocarcinoma. Front Immunol. (2022) 13:1029092. doi: 10.3389/fimmu.2022.1029092
77. Li Y, Fang T, Shan W, Gao Q. Identification of a novel model for predicting the prognosis and immune response based on genes related to cuproptosis and ferroptosis in ovarian cancer. Cancers (Basel). (2023) 15:579. doi: 10.3390/cancers15030579
78. Zhao C, Zhang Z, Jing T. A novel signature of combing cuproptosis- with ferroptosis-related genes for prediction of prognosis, immunologic therapy responses and drug sensitivity in hepatocellular carcinoma. Front Oncol. (2022) 12:1000993. doi: 10.3389/fonc.2022.1000993
79. Li Y, Wang RY, Deng YJ, Wu SH, Sun X, Mu H. Molecular characteristics, clinical significance, and cancer immune interactions of cuproptosis and ferroptosis-associated genes in colorectal cancer. Front Oncol. (2022) 12:975859. doi: 10.3389/fonc.2022.975859
80. Wang T, Jiang X, Lu Y, Ruan Y, Wang J. Identification and integration analysis of a novel prognostic signature associated with cuproptosis-related ferroptosis genes and relevant lncRNA regulatory axis in lung adenocarcinoma. Aging (Albany NY). (2023) 15:1543–63. doi: 10.18632/aging.204561
81. Ma Q, Hui Y, Huang BR, Yang BF, Li JX, Fan TT, et al. Ferroptosis and cuproptosis prognostic signature for prediction of prognosis, immunotherapy and drug sensitivity in hepatocellular carcinoma: development and validation based on TCGA and ICGC databases. Transl Cancer Res. (2023) 12:46–64. doi: 10.21037/tcr-22-2203
82. Xue Q, Kang R, Klionsky DJ, Tang D, Liu J, Chen X. Copper metabolism in cell death and autophagy. Autophagy. (2023) 19:2175–95. doi: 10.1080/15548627.2023.2200554
83. Luo Y, Yan P, Li X, Hou J, Wang Y, Zhou S. pH-sensitive polymeric vesicles for GOx/BSO delivery and synergetic starvation-ferroptosis therapy of tumor. Biomacromolecules. (2021) 22:4383–94. doi: 10.1021/acs.biomac.1c00960
84. Zhang P, Zhou C, Ren X, Jing Q, Gao Y, Yang C, et al. Inhibiting the compensatory elevation of xCT collaborates with disulfiram/copper-induced GSH consumption for cascade ferroptosis and cuproptosis. Redox Biol. (2024) 69:103007. doi: 10.1016/j.redox.2023.103007
85. Bai J, Zhang X, Zhao Z, Sun S, Cheng W, Yu H, et al. CuO nanozymes catalyze cysteine and glutathione depletion induced ferroptosis and cuproptosis for synergistic tumor therapy. Small. (2024) 20:e2400326. doi: 10.1002/smll.202400326
86. Zhang Y, Zhang N, Xing J, Sun Y, Jin X, Shen C, et al. In situ hydrogel based on Cu-Fe(3)O(4) nanoclusters exploits oxidative stress and the ferroptosis/cuproptosis pathway for chemodynamic therapy. Biomaterials. (2024) 311:122675. doi: 10.1016/j.biomaterials.2024.122675
87. Huang H, Guo H, Liu J, Ni C, Xia L, Cao X, et al. Dendrimer/metal-phenolic nanocomplexes encapsulating CuO(2) for targeted magnetic resonance imaging and enhanced ferroptosis/cuproptosis/chemodynamic therapy by regulating the tumor microenvironment. Acta Biomater. (2024) 183:252–63. doi: 10.1016/j.actbio.2024.05.035
88. Wang W, Lu K, Jiang X, Wei Q, Zhu L, Wang X, et al. Ferroptosis inducers enhanced cuproptosis induced by copper ionophores in primary liver cancer. J Exp Clin Cancer Res. (2023) 42:142. doi: 10.1186/s13046-023-02720-2
89. Gao Y, Jin F, Zhang P, Zheng C, Zheng X, Xie J, et al. Elesclomol-copper synergizes with imidazole ketone erastin by promoting cuproptosis and ferroptosis in myelodysplastic syndromes. BioMed Pharmacother. (2024) 175:116727. doi: 10.1016/j.biopha.2024.116727
90. Kluknavská J, Krajčíková K, Bolerázska B, Mašlanková J, Ohlasová J, Timková S, et al. Possible prognostic biomarkers of periodontitis in saliva. Eur Rev Med Pharmacol Sci. (2021) 25:3154–61. doi: 10.26355/eurrev_202104_25724
91. Dos Santos VRN, Frazão DR, Ferreira RO, Mendes PFS, Baia-da-Silva DC, Souza-Monteiro D, et al. Açaí (Euterpe oleracea mart.) attenuates oxidative stress and alveolar bone damage in experimental periodontitis in rats. Antioxidants (Basel). (2022) 11:1902. doi: 10.3390/antiox11101902
92. de Araújo Silva DN, Silva NTD, Sena IAA, Azevedo M, Júnior F, Silva R, et al. Efficacy of antimicrobial photodynamic therapy with chloro-aluminum phthalocyanine on periodontal clinical parameters and salivary GSH and MDA levels in patients with periodontitis. Photodiagnosis Photodyn Ther. (2020) 31:101843. doi: 10.1016/j.pdpdt.2020.101843
93. Liu J, Kuang F, Kroemer G, Klionsky DJ, Kang R, Tang D. Autophagy-dependent ferroptosis: machinery and regulation. Cell Chem Biol. (2020) 27:420–35. doi: 10.1016/j.chembiol.2020.02.005
94. Zhou B, Liu J, Kang R, Klionsky DJ, Kroemer G, Tang D. Ferroptosis is a type of autophagy-dependent cell death. Semin Cancer Biol. (2020) 66:89–100. doi: 10.1016/j.semcancer.2019.03.002
95. Lee S, Hwang N, Seok BG, Lee S, Lee SJ, Chung SW. Autophagy mediates an amplification loop during ferroptosis. Cell Death disease. (2023) 14:464. doi: 10.1038/s41419-023-05978-8
96. Xie Y, Kang R, Klionsky DJ, Tang D. GPX4 in cell death, autophagy, and disease. Autophagy. (2023) 19:2621–38. doi: 10.1080/15548627.2023.2218764
97. Hou W, Xie Y, Song X, Sun X, Lotze MT, Zeh HJ 3rd, et al. Autophagy promotes ferroptosis by degradation of ferritin. Autophagy. (2016) 12:1425–8. doi: 10.1080/15548627.2016.1187366
98. Bai Y, Meng L, Han L, Jia Y, Zhao Y, Gao H, et al. Lipid storage and lipophagy regulates ferroptosis. Biochem Biophys Res Commun. (2019) 508:997–1003. doi: 10.1016/j.bbrc.2018.12.039
99. Chen X, Song X, Li J, Zhang R, Yu C, Zhou Z, et al. Identification of HPCAL1 as a specific autophagy receptor involved in ferroptosis. Autophagy. (2023) 19:54–74. doi: 10.1080/15548627.2022.2059170
100. Wan F, Zhong G, Ning Z, Liao J, Yu W, Wang C, et al. Long-term exposure to copper induces autophagy and apoptosis through oxidative stress in rat kidneys. Ecotoxicology Environ safety. (2020) 190:110158. doi: 10.1016/j.ecoenv.2019.110158
101. Guo H, Ouyang Y, Yin H, Cui H, Deng H, Liu H, et al. Induction of autophagy via the ROS-dependent AMPK-mTOR pathway protects copper-induced spermatogenesis disorder. Redox Biol. (2022) 49:102227. doi: 10.1016/j.redox.2021.102227
102. Tsang T, Posimo JM, Gudiel AA, Cicchini M, Feldser DM, Brady DC. Copper is an essential regulator of the autophagic kinases ULK1/2 to drive lung adenocarcinoma. Nat Cell Biol. (2020) 22:412–24. doi: 10.1038/s41556-020-0481-4
103. Yu Z, Zhou R, Zhao Y, Pan Y, Liang H, Zhang JS, et al. Blockage of SLC31A1-dependent copper absorption increases pancreatic cancer cell autophagy to resist cell death. Cell proliferation. (2019) 52:e12568. doi: 10.1111/cpr.12568
104. Polishchuk EV, Merolla A, Lichtmannegger J, Romano A, Indrieri A, Ilyechova EY, et al. Activation of autophagy, observed in liver tissues from patients with wilson disease and from ATP7B-deficient animals, protects hepatocytes from copper-induced apoptosis. Gastroenterology. (2019) 156:1173–89.e5. doi: 10.1053/j.gastro.2018.11.032
105. Peña KA, Kiselyov K. Transition metals activate TFEB in overexpressing cells. Biochem J. (2015) 470:65–76. doi: 10.1042/bj20140645
106. Wen H, Qu C, Wang Z, Gao H, Liu W, Wang H, et al. Cuproptosis enhances docetaxel chemosensitivity by inhibiting autophagy via the DLAT/mTOR pathway in prostate cancer. FASEB journal: Off Publ Fed Am Societies Exp Biol. (2023) 37:e23145. doi: 10.1096/fj.202300980R
107. Lu H, Zhou L, Zhang B, Xie Y, Yang H, Wang Z. Cuproptosis key gene FDX1 is a prognostic biomarker and associated with immune infiltration in glioma. Front Med. (2022) 9:939776. doi: 10.3389/fmed.2022.939776
108. Sun B, Ding P, Song Y, Zhou J, Chen X, Peng C, et al. FDX1 downregulation activates mitophagy and the PI3K/AKT signaling pathway to promote hepatocellular carcinoma progression by inducing ROS production. Redox Biol. (2024) 75:103302. doi: 10.1016/j.redox.2024.103302
109. Yang Y, Huang Y, Li W. Autophagy and its significance in periodontal disease. J periodontal Res. (2021) 56:18–26. doi: 10.1111/jre.12810
110. İnan S, Barış E. The role of autophagy in odontogenesis, dental implant surgery, periapical and periodontal diseases. J Cell Mol Med. (2024) 28:e18297. doi: 10.1111/jcmm.18297
111. Mostowy S. Autophagy and bacterial clearance: a not so clear picture. Cell Microbiol. (2013) 15:395–402. doi: 10.1111/cmi.12063
112. Liu J, Wang X, Xue F, Zheng M, Luan Q. Abnormal mitochondrial structure and function are retained in gingival tissues and human gingival fibroblasts from patients with chronic periodontitis. J periodontal Res. (2022) 57:94–103. doi: 10.1111/jre.12941
113. Sun X, Mao Y, Dai P, Li X, Gu W, Wang H, et al. Mitochondrial dysfunction is involved in the aggravation of periodontitis by diabetes. J Clin periodontology. (2017) 44:463–71. doi: 10.1111/jcpe.12711
114. Liu J, Zeng J, Wang X, Zheng M, Luan Q. P53 mediates lipopolysaccharide-induced inflammation in human gingival fibroblasts. J periodontology. (2018) 89:1142–51. doi: 10.1002/jper.18-0026
115. Li X, Wang X, Zheng M, Luan QX. Mitochondrial reactive oxygen species mediate the lipopolysaccharide-induced pro-inflammatory response in human gingival fibroblasts. Exp Cell Res. (2016) 347:212–21. doi: 10.1016/j.yexcr.2016.08.007
116. Jiang K, Li J, Jiang L, Li H, Lei L. PINK1-mediated mitophagy reduced inflammatory responses to Porphyromonas gingivalis in macrophages. Oral diseases. (2023) 29:3665–76. doi: 10.1111/odi.14286
117. Bullón P, Román-Malo L, Marín-Aguilar F, Alvarez-Suarez JM, Giampieri F, Battino M, et al. Lipophilic antioxidants prevent lipopolysaccharide-induced mitochondrial dysfunction through mitochondrial biogenesis improvement. Pharmacol Res. (2015) 91:1–8. doi: 10.1016/j.phrs.2014.10.007
118. Jiang W, Wang Y, Cao Z, Chen Y, Si C, Sun X, et al. The role of mitochondrial dysfunction in periodontitis: From mechanisms to therapeutic strategy. J periodontal Res. (2023) 58:853–63. doi: 10.1111/jre.13152
119. Tang D, Chen X, Kroemer G. Cuproptosis: a copper-triggered modality of mitochondrial cell death. Cell Res. (2022) 32:417–8. doi: 10.1038/s41422-022-00653-7
120. Tsvetkov P, Coy S, Petrova B, Dreishpoon M, Verma A, Abdusamad M, et al. Copper induces cell death by targeting lipoylated TCA cycle proteins. Sci (New York NY). (2022) 375:1254–61. doi: 10.1126/science.abf0529
121. Battaglia AM, Chirillo R, Aversa I, Sacco A, Costanzo F, Biamonte F. Ferroptosis and cancer: mitochondria meet the “Iron maiden” Cell death. Cells. (2020) 9:1505. doi: 10.3390/cells9061505
122. Tadokoro T, Ikeda M, Ide T, Deguchi H, Ikeda S, Okabe K, et al. Mitochondria-dependent ferroptosis plays a pivotal role in doxorubicin cardiotoxicity. JCI Insight. (2020) 5:e132747. doi: 10.1172/jci.insight.132747
123. Collins JF, Prohaska JR, Knutson MD. Metabolic crossroads of iron and copper. Nutr Rev. (2010) 68:133–47. doi: 10.1111/j.1753-4887.2010.00271.x
124. Doguer C, Ha JH, Collins JF. Intersection of iron and copper metabolism in the mammalian intestine and liver. Compr Physiol. (2018) 8:1433–61. doi: 10.1002/cphy.c170045
125. Jiayi H, Ziyuan T, Tianhua X, Mingyu Z, Yutong M, Jingyu W, et al. Copper homeostasis in chronic kidney disease and its crosstalk with ferroptosis. Pharmacol Res. (2024) 202:107139. doi: 10.1016/j.phrs.2024.107139
126. Wei Y, Fu J, Wu W, Ma P, Ren L, Yi Z, et al. Quercetin prevents oxidative stress-induced injury of periodontal ligament cells and alveolar bone loss in periodontitis. Drug design Dev Ther. (2021) 15:3509–22. doi: 10.2147/dddt.S315249
127. Li X, Zhou R, Han Y, Zeng J, Shi L, Mao Y, et al. Silibinin attenuates experimental periodontitis by downregulation of inflammation and oxidative stress. Oxid Med Cell longevity. (2023) 2023:5617800. doi: 10.1155/2023/5617800
128. Bhattarai G, Poudel SB, Kook SH, Lee JC. Resveratrol prevents alveolar bone loss in an experimental rat model of periodontitis. Acta biomaterialia. (2016) 29:398–408. doi: 10.1016/j.actbio.2015.10.031
129. Mohammad CA, Ali KM, Sha AM, Gul SS. Antioxidant effects of curcumin gel in experimental induced diabetes and periodontitis in rats. BioMed Res Int. (2022) 2022:7278064. doi: 10.1155/2022/7278064
130. Zhang Q, Lou C, Li H, Li Y, Zhang H, Li Z, et al. Zinc hybrid polyester barrier membrane accelerates guided tissue regeneration. J Controlled release: Off J Controlled Release Society. (2024) 368:676–90. doi: 10.1016/j.jconrel.2024.03.005
131. Li Y, Xu C, Mao J, Mao L, Li W, Liu Z, et al. ZIF-8-based nanoparticles for inflammation treatment and oxidative stress reduction in periodontitis. ACS Appl materials interfaces. (2024) 16:36077–94. doi: 10.1021/acsami.4c05722
132. Homayouni Rad A, Pourjafar H, Mirzakhani E. A comprehensive review of the application of probiotics and postbiotics in oral health. Front Cell infection Microbiol. (2023) 13:1120995. doi: 10.3389/fcimb.2023.1120995
133. Kowalczyk K, Coraça-Huber DC, Wille-Kollmar W, Berktold M, Nagl M. Activity of N-chlorotaurine against periodontal pathogens. Int J Mol Sci. (2024) 25:8357. doi: 10.3390/ijms25158357
Keywords: periodontitis, ferroptosis, cuproptosis, oxidative stress, glutathione, autophagy
Citation: Zheng T, Lu F, Wu P, Chen Y, Zhang R and Li X (2025) Ferroptosis and cuproptosis in periodontitis: recent biological insights and therapeutic advances. Front. Immunol. 16:1526961. doi: 10.3389/fimmu.2025.1526961
Received: 12 November 2024; Accepted: 05 February 2025;
Published: 24 February 2025.
Edited by:
Junqi Huang, Jinan University, ChinaReviewed by:
Kai Yang, Wuhan University, ChinaLyu Qiqi, Singapore General Hospital, Singapore
Shuheng Huang, Sun Yat-sen University, China
Copyright © 2025 Zheng, Lu, Wu, Chen, Zhang and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yangan Chen, Y2hlbnlhbmdhbjdAMTYzLmNvbQ==; Rongxin Zhang, cnh6aGFuZ0BnZHB1LmVkdS5jbg==; Xin Li, MTA3MDM1NzY4QHFxLmNvbQ==
 Tengyi Zheng1
Tengyi Zheng1 Rongxin Zhang
Rongxin Zhang Xin Li
Xin Li