
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Immunol., 12 March 2025
Sec. Cancer Immunity and Immunotherapy
Volume 16 - 2025 | https://doi.org/10.3389/fimmu.2025.1526318
This article is part of the Research TopicCommunity Series in Methods in Cancer Immunity and Immunotherapy: Volume IIView all 7 articles
 Min Zhang†
Min Zhang† Yingying Wu†
Yingying Wu† Zhipeng Cheng
Zhipeng Cheng Lu Zhang
Lu Zhang Lin Liu
Lin Liu Fang Liu
Fang Liu Guohui Cui
Guohui Cui Linghui Xia
Linghui Xia Yu Hu
Yu Hu Heng Mei
Heng Mei Tao Guo*
Tao Guo* Jun Fang*
Jun Fang*Background: Relevant studies have demonstrated the poor treatment outcomes and prognosis for double-expressor diffuse large B cell lymphoma (DE-DLBCL) in the rituximab era. Zanubrutinib plus R-CHOP (rituximab, cyclophosphamide, doxorubicin/liposomal doxorubicin, vincristine, prednisone; ZR-CHOP) has shown efficacy in untreated non-GCB DLBCL patients with extranodal involvement. However, its efficacy in newly diagnosed DE-DLBCL remains uncertain.
Objective: This retrospective study sought to assess the efficacy and safety of ZR-CHOP in comparison to R-CHOP in treatment-naïve patients with DE-DLBCL.
Method: This study assessed 78 patients with newly diagnosed DE-DLBCL who were admitted between June 2017 and January 2024. Among them, 55 patients received the R-CHOP regimen, while 23 patients were treated with the ZR-CHOP regimen. The clinical characteristics were well balanced between the two groups.
Results: The complete response rates (CRR) were higher in the ZR-CHOP group than the R-CHOP group, regardless of whether patients completed 4 or 6 treatment cycles (P= 0.019; P= 0.025). ORR in the ZR-CHOP group showed a higher trend than that in the R-CHOP group (P= 0.624; P= 0.219). The median follow-up period was 23.3 months, and the predicted median progression free survival (PFS) in the R-CHOP group was 22.8 months, whereas the median PFS in the ZR-CHOP group was not reached. The 1-, 2-, and 3-year PFS rates in the ZR-CHOP group showed a beneficial trend compared with the R-CHOP group, but there was no statistical difference (P= 0.072). However, the PFS of the ZR-CHOP group was longer than that of the R-CHOP group in patients with Ki67 index >75% (P= 0.034) and p53 expression >50% (P= 0.0033). The predicted median overall survival (OS) in the ZR-CHOP and R-CHOP groups were not reached. The 1-, 2- and 3-year OS rates were not significantly different between the two groups (P= 0.29). The most common adverse event in both groups was hematotoxicity, but there was no significant difference in the incidence of all adverse events between the two groups.
Conclusion: First-line treatment with the ZR-CHOP regimen improved CRR in the untreated patients with DE-DLBCL and prolonged PFS in the Ki67 index >75% subgroup and the p53 expression >50% subgroup.
Diffuse large B cell lymphoma (DLBCL) is the most common type of non-Hodgkin’s lymphoma and is characterized by high aggressiveness and heterogeneity (1). Double-expressor diffuse large B cell lymphoma (DE-DLBCL) co-expresses MYC and BCL-2 as determined by immunohistochemistry (IHC) (2, 3). The revised 2016 WHO classification recommends cutoff values of 40% for MYC and 50% for BCL-2 expression as assessed by IHC (4). MYC and BCL-2 overexpression is likely attributable to gene amplification and posttranslational processes in the absence of chromosomal translocations (5–7). DE-DLBCL accounts for 20-35% of new DLBCL cases. It is associated with an aggressive clinical course and is more common in the activated B-cell (ABC) subtype (8–10). DE-DLBCL has demonstrated distinctive clinical features such as older age, advanced Ann Arbor stage, higher lactate dehydrogenase (LDH) level, higher Ki67 proliferation index, and higher international prognostic index (11–13).
R-CHOP, an anthracycline-based regimen, is widely used as the first-line treatment for DLBCL (14–16). Multiple studies have identified the double-expressor status as an adverse prognostic factor for response to R-CHOP in DLBCL (9, 17). Relapsed/refractory DE-DLBCL patients often have inferior outcomes after autologous stem cell transplantation (ASCT) (18). Additionally, DE-DLBCL demonstrated a 10% risk of central nervous system (CNS) relapse at 2 years (19). These data highlight that DE-DLBCL is associated with adverse outcomes. Therefore, new treatment regimens with increased efficacy need to be developed for untreated DE-DLBCL patients to achieve better remission and long-term survival.
Bruton tyrosine kinase (BTK) is an essential component of the B-cell receptor intracellular signaling pathway, mediating B-cell development, proliferation, and survival (20). Recently, BTK inhibitors have proven to be a successful strategy for managing B-cell malignancies due to their broad efficacy across a range of diseases, safety, and the convenience of oral administration. The first-generation BTK inhibitor, ibrutinib, rapidly became the standard of care for treating patients with certain subtypes of non-Hodgkin lymphoma and chronic lymphocytic leukemia (CLL) (21–24). According to the latest results, event-free survival (EFS) of DE-DLBCL treated with ibrutinib combined with R-CHOP was superior to those receiving R-CHOP alone (25).
Zanubrutinib is a novel small molecule oral BTK inhibitor that effectively targets BTK (26). In a phase 1/2 clinical study, zanubrutinib demonstrated promising safety and efficacy in patients with relapsed/refractory DLBCL (27). In a phase 2 clinical study, the ZR-CHOP was found to be safe and effective for treating newly diagnosed non-GCB DLBCL patients with extranodal involvement (28). Another study indicated that non-GCB DLBCL patients with CD79B mutations, higher TCL1A expression, or high MYC/BCL-2 expression have clinical benefits to zanubrutinib monotherapy or combination therapy (29). At present, the prospective study of ZR-CHOP in the treatment of DE-DLBCL has attracted much attention, but the detailed data have not been officially published. The purpose of this retrospective study is to compare the effectiveness and safety of the ZR-CHOP regimen with the R-CHOP regimen in the treatment of newly diagnosed DE-DLBCL.
This single-center, retrospective study evaluated the outcomes of patients with newly diagnosed DE-DLBCL who were treated with either the ZR-CHOP regimen or the R-CHOP regimen from June 2017 to January 2024, aiming to assess and compare the efficacy and safety of these two treatment protocols. The study was approved by the ethics committee and institutional review board of the Union Hospital Affiliated with Tongji Medical College, Huazhong University of Science and Technology (Ethics Approval No. UHCT240766). The study was conducted in compliance with the Declaration of Helsinki. As this study is retrospective and anonymous, informed consent was waived.
The inclusion criteria for patients were as follows: (1) no previous chemotherapy or targeted therapy; (2) pathologically confirmed DLBCL exhibiting co-expression of MYC (≥40%) and BCL2 (>50%) (2, 4); (3) availability of complete clinical and treatment information; (4) no involvement of central nervous system; (5) completion of at least four cycles of therapy.
Enrolled patients were divided into the ZR-CHOP regimen group and the R-CHOP regimen group. Zanubrutinib was administrated orally at a dose of 160 mg twice daily throughout the chemotherapeutic cycles. The R-CHOP regimen included both R-CHOP and R-miniCHOP. Some patients received liposomal doxorubicin instead of doxorubicin due to cardiac insufficiency, advanced age, and personal choice. The standard induction therapy for each group consisted of 6 cycles.
The evaluation of efficacy for nodular lymphoma was based on the Lugano 2014 criteria (30, 31). All patients were assessed for efficacy using enhanced computed tomography (CT) or positron emission tomography-computed tomography (PET/CT) scans. Prognostic stratification was conducted using the International Prognostic Index (IPI) and the National Comprehensive Cancer Network IPI (NCCN-IPI) (32, 33). The evaluation included complete response (CR), partial response (PR), stable disease (SD), and disease progression (PD) (34). The objective response rate (ORR) was calculated as the sum of the complete response rate (CRR) and partial response rate. Progression-free survival (PFS) was defined as the interval from diagnosis to the first occurrence of disease progression, death from any cause, or the last follow-up. Overall survival (OS) was defined as the duration from diagnosis to death or the last follow-up. Adverse events (AEs) were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events (version 5.0).
Data processing was carried out using Statistical Application System Software (SPSS) version 25.0 and R Studio version 4.4.1. Classification of case characteristics and treatment response rates were compared between groups using the “Tableone” package. OS and PFS were estimated using the Kaplan−Meier method and compared using the log-rank test. A P-value of <0.05 was considered statistically significant.
A total of 78 patients were included in the study, with a median follow-up of 23.3 months (range: 3.7–84.5 months) until May 2024. In the ZR-CHOP regimen group, two patients who achieved PR and one patient who experienced PD transitioned to alternative treatments during induction therapy. These treatments included zanubrutinib plus VR-DA-EPOCH (venetoclax, rituximab, dose-adjusted etoposide, prednisone, vincristine, doxorubicin and cyclophosphamide), zanubrutinib plus R-EPOCD (rituximab, etoposide, prednisone, vincristine, cyclophosphamide and liposomal doxorubicin), as well as zanubrutinib plus polatuzumab vedotin and zuberitamab (anti-CD20 antibody). In the R-CHOP regimen group, two patients achieving PR and six patients experiencing PD received alternative treatments, including R-GDP (rituximab with gemcitabine, dexamethasone, cisplatin), R-EPOCH (rituximab with etoposide, prednisone, vincristine, cyclophosphamide, doxorubicin), pola-R-CHP (polatuzumab vedotin plus rituximab, cyclophosphamide, doxorubicin and prednisolone), orelabrutinib plus R-CHOP and Chimeric antigen receptor (CAR)-T cell therapy (Figure 1).
There were no significant differences between the two groups, in term of age, gender, Ann Arbor staging, B symptoms, Eastern Cooperative Oncology Group (ECOG) score, LDH count, β2-microglobulin (β2-MG), IPI, NCCN-IPI, bone marrow involvement, extranodal involvement, bulky disease (≥7.5cm), cell of origin (Hans algorithm), expression of MYC and p53, as well as Ki67 index (Table 1).
Twenty-three patients in the ZR-CHOP group and 55 patients in the R-CHOP group completed at least four cycles of therapy. The CRR was significantly higher in the ZR-CHOP group (82.6% vs. 50.9%, P = 0.019) (Figure 2A). After six cycles of treatment, twenty patients in the ZR-CHOP group and 46 patients in the R-CHOP group were evaluated. The CRR remained significantly higher in the ZR-CHOP regimen group (95% vs. 65.2%, P = 0.025) (Figure 2B). However, there was no statistically significant difference in ORR between the two groups (four cycles: 95.7% vs. 89.1%, P= 0.624; six cycles: 100% vs. 87%, P= 0.219).
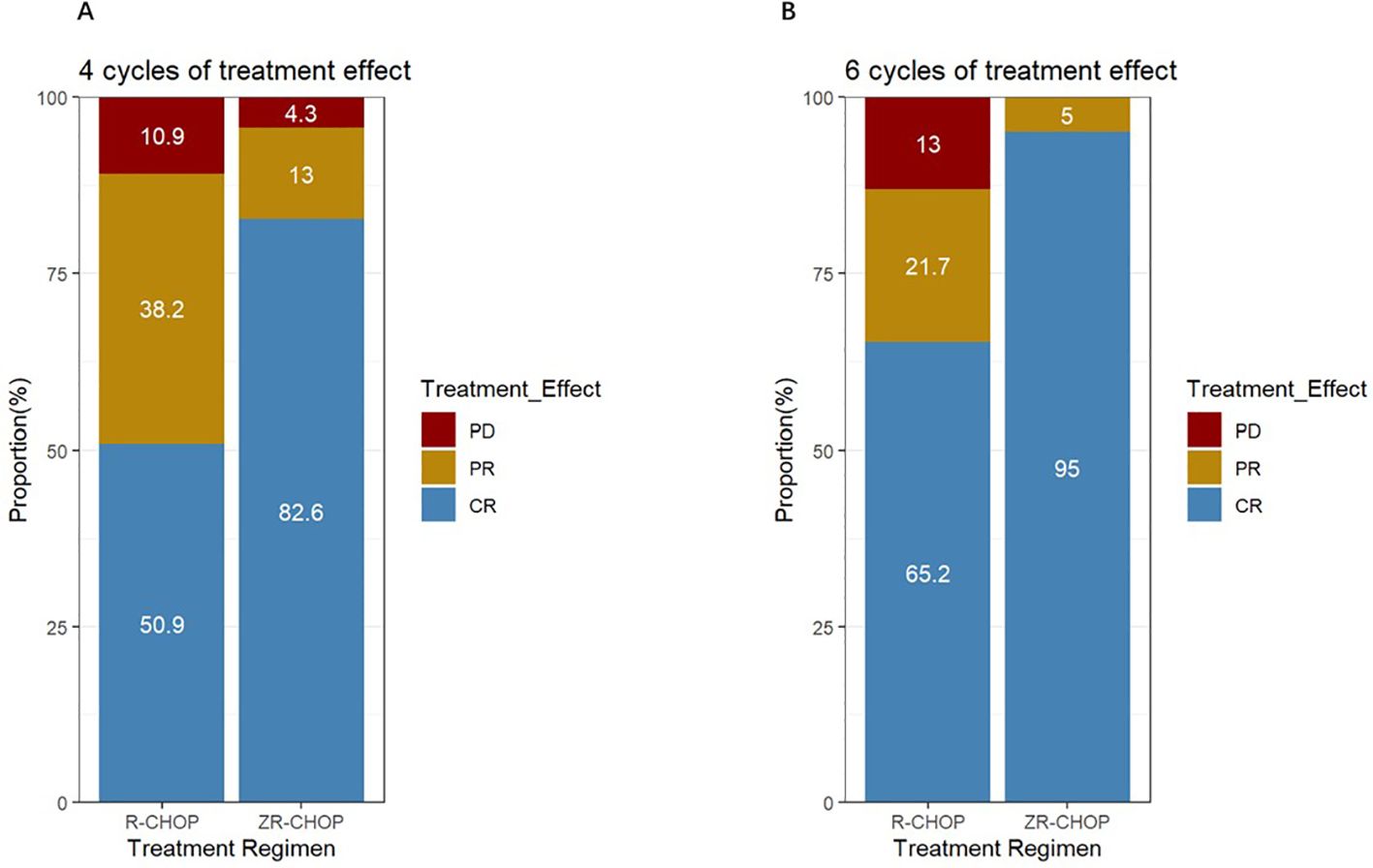
Figure 2. Comparisons of treatment efficacy between the two groups after 4 cycles (A) and 6 cycles (B) of treatment.
After four cycles of therapy, the complete response rates (CRRs) were significantly higher in the ZR-CHOP regimen group for patients with B symptoms (100% vs. 42.1%; P= 0.043), elevated LDH (84.6% vs. 45.5%; P= 0.037), p53 expression >50% (85.7% vs. 11.1%; P= 0.013), and Ki67 index >75% (86.7% vs. 51.4%; P= 0.042)(Figure 3). Additionally, the ZR-CHOP group exhibited a trend of higher CRRs in the other subgroups, including age ≥60 years (77.8% vs. 56.5%; P= 0.477), Ann Arbor stage of III and IV (83.3% vs. 45.5%; P= 0.055), bone marrow invasion (75% vs. 47.1%; P= 0.652), IPI score of 3-5 (72.7% vs. 42.9%; P= 0.186), extramedullary infiltration (80% vs. 50%; P= 0.112), MYC expression ≥50% (72.7% vs. 48.6%; P= 0.288), and non-GCB subtype (81.2% vs. 55.3%; P= 0.134).
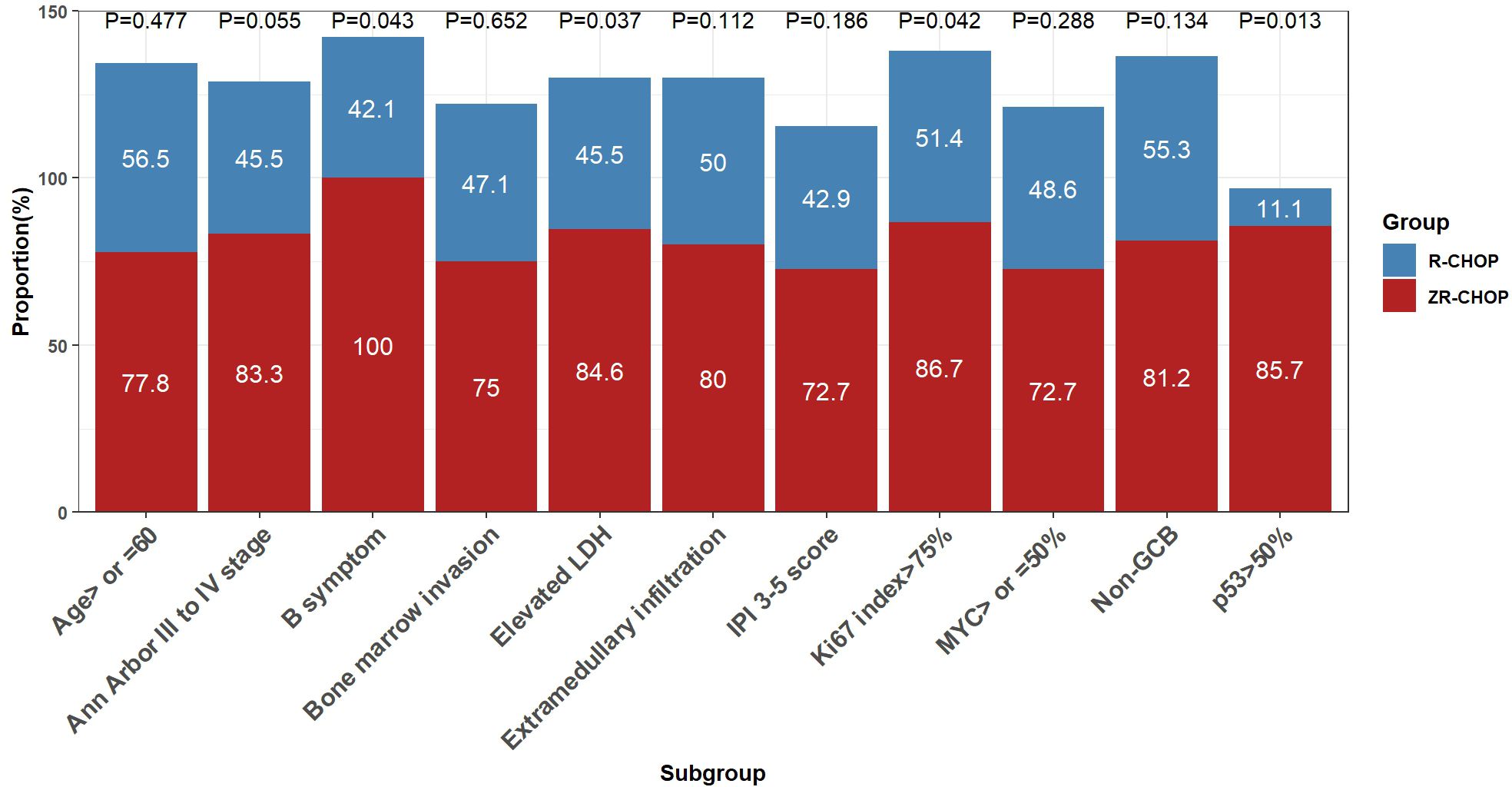
Figure 3. Comparisons of treatment efficacy among subgroups within the two groups after 4 cycles of therapy.
After six cycles of therapy, the CRRs were significantly higher in the ZR-CHOP regimen group for the elevated LDH subgroup (100% vs. 57.1%; P= 0.026) (Figure 4). Furthermore, there was a trend toward higher CRRs in certain subgroups within the ZR-CHOP group, including age ≥60 (87.5% vs. 61.9%; P= 0.377), Ann Arbor staging system III to IV (90.9% vs. 57.1%; P= 0.102), B symptoms (100% vs. 56.2%; P= 0.148), bone marrow invasion (75% vs. 66.7%; P= 1), IPI score of 3-5 (88.9% vs. 54.2%; P= 0.15), extramedullary infiltration (92.3% vs. 64%; P= 0.161), MYC expression ≥50% (100% vs. 60%; P= 0.083), p53 expression >50% (85.7% vs. 50%; P= 0.565), Ki67 index >75% (92.9% vs. 64.3%; P= 0.107) as well as non-GCB subtype (92.9% vs. 67.6%; P= 0.142).
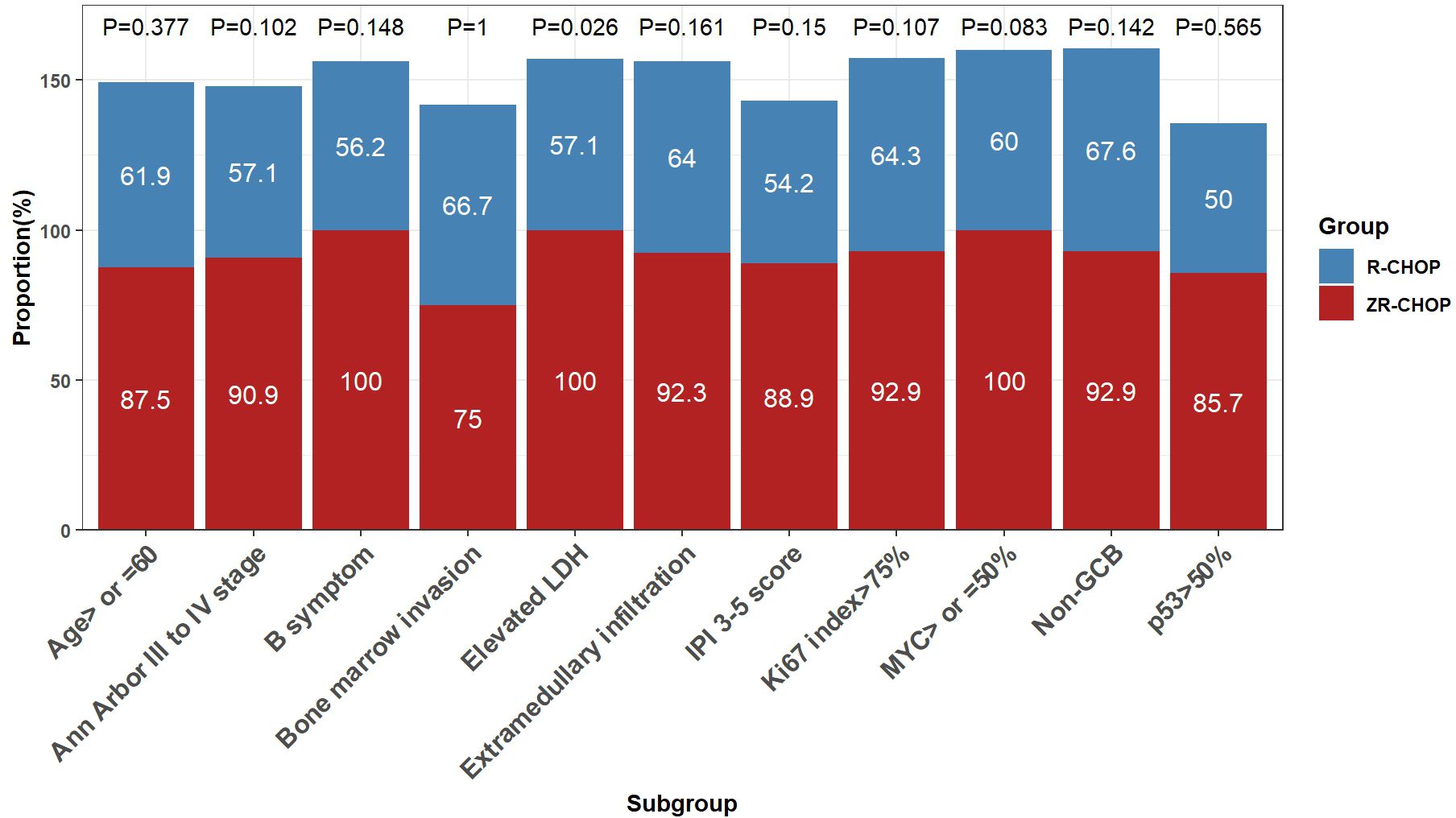
Figure 4. Comparisons of treatment efficacy among subgroups within the two groups after 6 cycles of therapy.
In the ZR-CHOP regimen group, the 1-, 2- and 3-year PFS rates were 86.1%, 77.5%, and 77.5%, respectively, while the OS rates for these time points were consistently at 95.2%. In the R-CHOP regimen group, the PFS rates the 1, 2 and 3 year were 71.6%, 47.6%, and 47.6%, respectively, while the OS rates for these intervals were 92%, 81.1% and 81.1%, respectively. The predicted median PFS was 22.8 months in the R-CHOP group, whereas the median PFS was not reached in the ZR-CHOP group. Additionally, the predicted median OS was not reached in both groups. Although the PFS and OS were longer in the ZR-CHOP regimen group compared to the R-CHOP regimen group, the differences were not statistically significant (P= 0.072; P= 0.29) (Figures 5A, B).
PFS was longer in the ZR-CHOP regimen group compared to the R-CHOP regimen group in several subgroups, including patients with age ≥60 (P= 0.12), Ann Arbor staging system III to IV (P= 0.17), IPI score of 3-5 (P= 0.23), elevated β2-MG (P= 0.33), elevated LDH (P= 0.29), extramedullary infiltration (P= 0.074), and non-GCB subtype (P= 0.33). However, these differences were not statistically significant. Notably, there were significant differences in the Ki67 index >75% subgroup (P= 0.034) and the p53 expression >50% subgroup (P= 0.0033) between the two treatment groups. These results are illustrated in Figure 6.
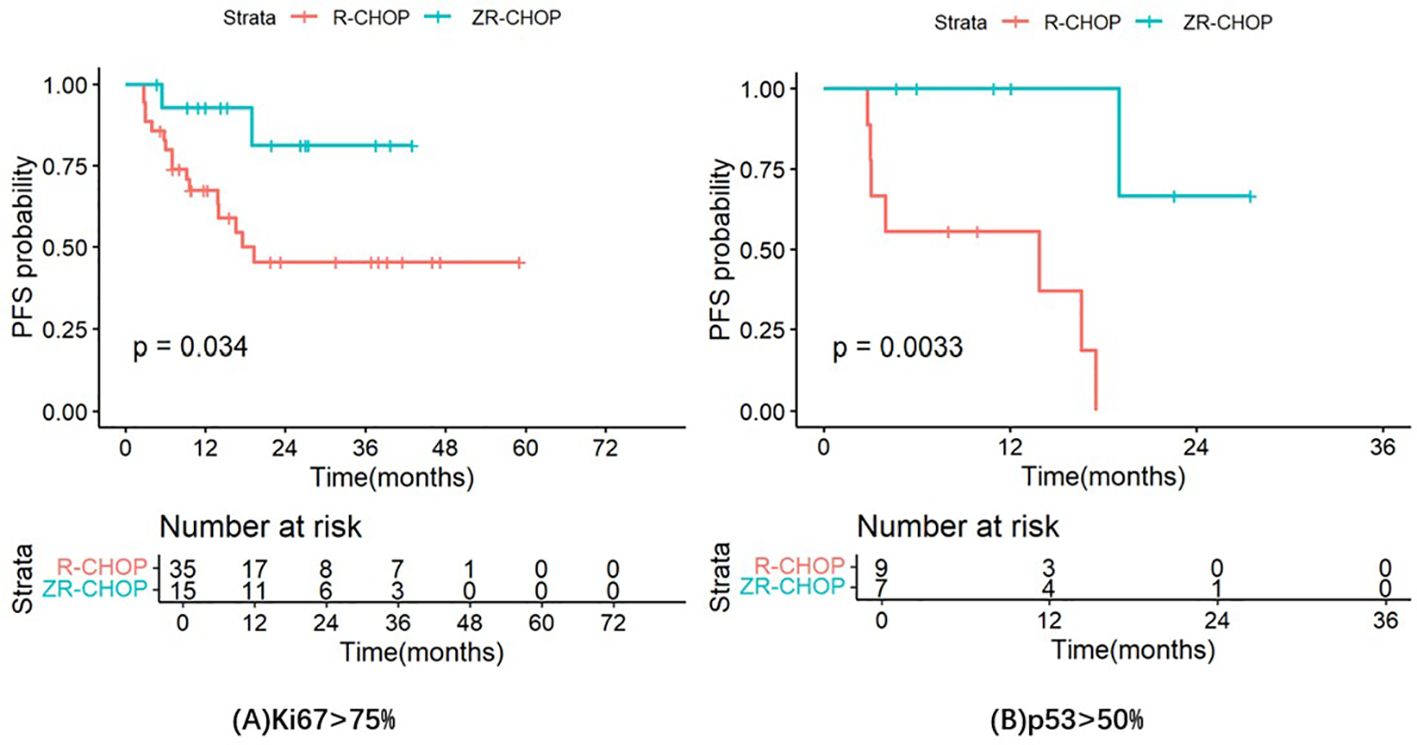
Figure 6. Comparisons of PFS in the Ki67 index >75% subgroup (A) and the p53 expression >50% subgroup (B) between the two treatment groups.
Patients in both groups experienced comparable levels of adverse events (AEs), with hematological toxicities being the most prevalent side effect. Table 2 provides a summary of the AEs reported in both groups, including hematological AEs, pulmonary infection, atrial fibrillation, hemorrhage, hyperuricemia, elevated creatinine, alanine aminotransferase (ALT) or aspartate aminotransferase (AST) elevation, nausea, apositia, diarrhea and fatigue. However, there was no statistical difference between the two groups.
The new generation of BTK inhibitors, zanubrutinib, in the treatment of DE-DLBCL is worth exploring, and relevant prospective studies are currently in progress. Our study is the first to retrospectively compare the efficacy and safety of ZR-CHOP with R-CHOP for untreated DE-DLBCL. The results of this study showed a higher CR rate in the ZR-CHOP regimen group compared to the R-CHOP regimen group (4 cycles of treatment: 82.6% vs. 50.9%, P= 0.019; 6 cycles of treatment: 95% vs. 65.2%, P= 0.025). ORR was higher in the ZR-CHOP group than that in the R-CHOP group, but not statistically significant (95.7% vs. 89.1%, P= 0.624; 100% vs. 87%, P= 0.219). In the PHOENIX trial (25), the CRR was 64.9% in the DE-DLBCL patients treated with R-CHOP (similar to 65.2% in this study), and 67.5% in those treated with ibrutinib plus R-CHOP. In our study, the CRR of DE-DLBCL patients treated with ZR-CHOP was 95%, suggesting that zanubrutinib may have a better CRR than ibrutinib for DE-DLBCL. In the ALPINE trial (35), zanubrutinib performed better than ibrutinib in patients with relapsed/refractory CLL. ORR remained higher in zanubrutinib compared with ibrutinib (85.6% vs. 75.4%; RR: 1.13 [95% CI, 1.05-1.22]). The observed differences in efficacy between ibrutinib and zanubrutinib can be attributed to their distinct molecular structures. Firstly, zanubrutinib features a different ring system in the middle of its structure, altering the molecule’s planarity (36). Less planar structures often exhibit better solubility, which may explain why the plasma drug exposure of zanubrutinib is eight times greater than that of ibrutinib (37). Additionally, zanubrutinib shows a high and stable occupancy rate as a BTK inhibitor in peripheral blood mononuclear cells and lymph nodes (38). Moreover, zanubrutinib has a lower maximum half inhibitory concentration (IC50) compared to ibrutinib, indicating stronger inhibitory activity (39). Secondly, unlike ibrutinib, zanubrutinib lacks a pyrimidine ring (36). This absence reduces its ability to bind to kinases other than BTK, resulting in enhanced target selectivity and fewer adverse events. Consequently, the rate of treatment discontinuation due to adverse events or disease progression is lower for patients receiving zanubrutinib compared to those treated with ibrutinib (35). Therefore, the study of zanubrutinib in the treatment of DE-DLBCL deserves further exploration. Our study suggests that ZR-CHOP enables more DE-DLBCL patients to achieve CR, increasing the depth of remission.
In this study, ZR-CHOP regimen tended to prolong PFS and OS compared with R-CHOP regimen. The PFS rate of DE-DLBCL patients treated with ZR-CHOP was higher than that of R-CHOP, even though the difference was not significant (3-year PFS rates: 77.5% vs. 47.6%, P= 0.072). In addition, the PFS rate of R-CHOP in the treatment of DE-DLBCL (3-year PFS rates was 47.6%) was consistent with previous studies (5-year PFS rate was 44%; 5-year PFS rate was 46%) (11, 19). Similarly, the OS rate in the ZR-CHOP group tended to be higher than that in the R-CHOP group, but there was no significant statistical significance (3-year OS rates: 95.2% vs. 81.1%, P= 0.29). However, DE-DLBCL patients treated with R-CHOP had a higher OS rate (3-year OS rate was 81.1%) than in these studies (5-year OS rate was 39%; 5-year OS rate was 52%) (11, 19). The possible reasons for the difference in OS rate between our study and the prospective studies include the insufficient number of enrolled patients, the limited follow-up time, and the switch to other treatment after poor response to previous therapy. Therefore, ZR-CHOP has the potential to improve the prognosis of DE-DLBCL patients, especially in term of PFS.
It is clear that p53 overexpression (more than 50%) is associated with poor prognosis in DLBCL patients (5-year PFS of DLBCL with p53 expression >50% was 28%; 5-year OS of DLBCL with p53 expression >50% was 46%) (40–46). However, there is a lack of research data on the treatment response and progonosis of DE-DLBCL with strong p53 expression. In our study, the CR rates of DE-DLBCL patients with p53 expression >50% and p53 expression ≤50%, treated with R-CHOP, were 11.1% and 58.7% (P= 0.025), and the 1-year PFS rates were 55.6% and 74.8% (P= 0.00054), respectively. Therefore, DE-DLBCL with strong p53 expression had worse response and PFS than DLBCL with strong p53 expression. The CRR of DE-DLBCL patients with p53 expression >50% in the ZR-CHOP group was significantly higher than that in the R-CHOP group after 4 courses of treatment (85.7% vs. 11.1%; P= 0.013), but there was no statistical significance after 6 courses of treatment (85.7% vs. 50%; P= 0.565). In fact, some patients were switched to alternative medications due to poor treatment response, which led to a decrease in the number of patients on subsequent treatment and might diminish the difference between the two treatment groups after 6 courses. In addition, DE-DLBCL patients with p53 expression >50% had significantly longer PFS treated with ZR-CHOP than those treated with R-CHOP (1-year PFS rate: 100% vs. 55.6%, P= 0.0033). Therefore, the treatment response and prognosis of DE-DLBCL patients with p53 expression >50% are poorer; however, the ZR-CHOP regimen can assist these patients in achieving earlier CR and significantly enhancing both CRR and PFS.
Some studies suggest that high Ki67 index had significant adverse prognostic effects in DLBCL, but the specific cut-off value is not uniform at present (47–52). Salles et al. found that the OS of DLBCL patients with Ki67 index ≤75% was significantly longer than that of patients with Ki67 index >75% (P<0.05) (53). In a study of R-CHOP in DLBCL patients, elevated Ki67 index seems to be associated with inferior EFS in patients with DLBCL treated with R-CHOP (P= 0.012) (54). The CR rates of DLBCL patients with Ki67 ≥85% and Ki67 <85% were 69.6% and 81.6%, and the 2-year PFS rates were 44.3% and 74.1%, respectively. In our study, the CR rates of DE-DLBCL patients with Ki67 >75% and Ki67 ≤75%, receiving R-CHOP, were 64.3% and 66.7% (P= 1), and the 2-year PFS rates were 45.4% and 52.8% (P= 0.74), respectively. The data of the high Ki67 group are similar to the results of the above study (54), and the efficacy and prognosis of the high Ki67 group were worse than those of the low Ki67 group. In DE-DLBCL patients with Ki67 index ≤75%, there was no significant difference in CRR between the ZR-CHOP group and the R-CHOP group after 4 courses (75% vs. 50%; P= 0.432) or 6 courses of treatment (100% vs. 66.7%; P= 0.276). The CRR of DE-DLBCL patients with Ki67 index >75% in the ZR-CHOP group was significantly higher than that in the R-CHOP group after 4 courses of treatment (86.7% vs. 51.4%; P= 0.042), but there was no statistical significance after 6 courses of treatment (92.9% vs. 64.3%; P= 0.107). The potential reasons are analogous to those observed in the strong p53 expression group, including a limited sample size and treatment modifications in certain patients. DE-DLBCL patients with Ki67 index >75% treated with ZR-CHOP had significantly longer PFS than those treated with R-CHOP (1-year PFS rate: 92.9% vs. 67.5%, P= 0.034). Therefore, ZR-CHOP may have better efficacy and improve prognosis for DE-DLBCL with Ki67 >75%, and delay disease progression in this population.
The adverse events (AEs) associated with the ZR-CHOP and R-CHOP regimens were comparable. Hematologic toxicity was the predominant AE in the ZR-CHOP group, whereas pulmonary infections (39.1%) and fatigue (34.8%) were the most common non-hematologic AEs. Notably, there were no instances of atrial fibrillation and fewer hemorrhagic events (4.3%) in the ZR-CHOP group. Furthermore, the incidence of grade 3–4 neutropenia and grade 3–4 thrombocytopenia was lower in the ZR-CHOP group compared to that in the R-CHOP group. As the treatment of the ZR-CHOP group occurred over the past three years, a greater number of patients opted for certain costly medications to prevent AEs due to enhanced awareness regarding treatment options, such as thrombopoietin receptor agonists and pegylated recombinant human granulocyte colony-stimulating factor, which may contribute to reducing and mitigating AE occurrences in this population.
This is the first retrospective study to compare the efficacy and safety of ZR-CHOP and R-CHOP in the treatment of DE-DLBCL. Compared with R-CHOP, zanubrutinib plus R-CHOP effectively increased the CR rate of DE-DLBCL and showed a tendency to delay disease progression, especially in DE-DLBCL with Ki67 index >75% or p53 expression >50%. These evidences support zanubrutinib plus R-CHOP as a first-line treatment option for untreated DE-DLBCL. This study has the limitations of small number of cases and short follow-up time, so large-scale prospective clinical studies are needed to further confirm the efficacy and safety of ZR-CHOP in DE-DLBCL.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving humans were approved by the Ethics Committee of Union Hospital, Tongji Medical College, Huazhong University of Science and Technology. The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants’ legal guardians/next of kin because this study is retrospective and anonymous.
MZ: Data curation, Formal Analysis, Project administration, Validation, Visualization, Writing – original draft, Writing – review & editing, Methodology. YW: Formal Analysis, Methodology, Project administration, Validation, Writing – original draft, Writing – review & editing, Data curation, Visualization. ZC: Conceptualization, Writing – review & editing. LZ: Conceptualization, Writing – review & editing. LL: Conceptualization, Writing – review & editing. FL: Conceptualization, Writing – review & editing. GC: Conceptualization, Writing – review & editing. LX: Supervision, Writing – review & editing. YH: Supervision, Writing – review & editing. HM: Supervision, Writing – review & editing. TG: Funding acquisition, Project administration, Resources, Supervision, Writing – review & editing. JF: Funding acquisition, Project administration, Resources, Supervision, Writing – review & editing.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was supported by the National Natural Science Foundation of China (81974008), and the Joint Fund Project of Hubei Provincial Natural Science Foundation of China (2024AFD430).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declare that no Generative AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Teras LR, DeSantis CE, Cerhan JR, Morton LM, Jemal A, Flowers CR. 2016 US lymphoid Malignancy statistics by world health organization subtypes. Ca-Cancer J Clin. (2016) 66:443–59. doi: 10.3322/caac.21357
2. Tavakkoli M, Barta SK. 2024 Update: Advances in the risk stratification and management of large B-cell lymphoma. Am J Hematol. (2023) 98:1791–805. doi: 10.1002/ajh.27075
3. Alaggio R, Amador C, Anagnostopoulos I, Attygalle AD, Araujo IBD, Berti E, et al. The 5th edition of the world health organization classification of haematolymphoid tumours: lymphoid neoplasms. Leukemia. (2022) 36:1720–48. doi: 10.1038/s41375-022-01620-2
4. Swerdlow SH, Campo E, Pileri SA, Harris NL, Stein H, Siebert R, et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood. (2016) 127:2375–90. doi: 10.1182/blood-2016-01-643569
5. Schuetz JM, Johnson NA, Morin RD, Scott DW, Tan K, Ben-Nierah S, et al. BCL2 mutations in diffuse large B-cell lymphoma. Leukemia. (2012) 26:1383–90. doi: 10.1038/leu.2011.378
6. Karube K, Campo E. MYC alterations in diffuse large B-cell lymphomas. Semin Hematol. (2015) 52:97–106. doi: 10.1053/j.seminhematol.2015.01.009
7. Horn H, Ziepert M, Becher C, Barth TFE, Bernd HW, Feller AC, et al. MYC status in concert with BCL2 and BCL6 expression predicts outcome in diffuse large B-cell lymphoma. Blood. (2013) 121:2253–63. doi: 10.1182/blood-2012-06-435842
8. Sarkozy C, Traverse-Glehen A, Coiffier B. Double-hit and double-protein-expression lymphomas: aggressive and refractory lymphomas. Lancet Oncol. (2015) 16:e555–67. doi: 10.1016/s1470-2045(15)00005-4
9. Hu SM, Xu-Monette ZY, Tzankov A, Green T, Wu L, Balasubramanyam A, et al. MYC/BCL2 protein coexpression contributes to the inferior survival of activated B-cell subtype of diffuse large B-cell lymphoma and demonstrates high-risk gene expression signatures: a report from The International DLBCL Rituximab-CHOP Consortium Program. Blood. (2013) 121:4021–31. doi: 10.1182/blood-2012-10-460063
10. Xu-Monette ZY, Wei L, Fang XS, Au QY, Nunns H, Nagy M, et al. Genetic subtyping and phenotypic characterization of the immune microenvironment and MYC/BCL2 double expression reveal heterogeneity in diffuse large B-cell lymphoma. Clin Cancer Res. (2022) 28:972–83. doi: 10.1158/1078-0432.Ccr-21-2949
11. Johnson NA, Slack GW, Savage KJ, Connors JM, Ben-Neriah S, Rogic S, et al. Concurrent expression of MYC and BCL2 in diffuse large B-cell lymphoma treated with rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone. J Clin Oncol. (2012) 30:3452–9. doi: 10.1200/Jco.2011.41.0985
12. Sung HJ, Kim D, Yoon DH, Cho HYW, Huh J, Suh CW, et al. Clinicopathologic and genetic features of the starry-sky pattern in double-expressor diffuse large B-cell lymphoma. Hum Pathol Sep. (2023) 139:106–16. doi: 10.1016/j.humpath.2023.07.008
13. Rungwittayatiwat S, Boonsakan P, Chantrathammachart P, Puavilai T, Pukiat S, Phusanti S, et al. Treatment outcomes and clinical relevance in patients with double expressor DLBCL. Mediterr J Hematol I. (2021) 13:e2021063. doi: 10.4084/MJHID.2021.063
14. Coiffier B, Lepage E, Brière J, Herbrecht R, Tilly H, Bouabdallah R, et al. CHOP chemotherapy plus rituximab compared with CHOP alone in elderly patients with diffuse large-B-cell lymphoma. New Engl J Med. (2002) 346:235–42. doi: 10.1056/NEJMoa011795
15. Feugier P, Van Hoof A, Sebban C, Solal-Celigny P, Bouabdallah R, Fermé C, et al. Long-term results of the R-CHOP study in the treatment of elderly patients with diffuse large B-cell lymphoma: A study by the groupe d’Etude des lymphomes de l’adulte. J Clin Oncol. (2005) 23:4117–26. doi: 10.1200/Jco.2005.09.131
16. Sehn LH, Salles G. Diffuse large B-cell lymphoma. New Engl J Med. (2021) 384:842–58. doi: 10.1056/NEJMra2027612
17. Abdulla M, Laszlo S, Triumf J, Hedström G, Berglund M, Enblad G, et al. A population-based study of cellular markers in R-CHOP treated diffuse large B-cell lymphoma patients. Acta Oncol. (2016) 55:1126–31. doi: 10.1080/0284186x.2016.1189093
18. Herrera AF, Mei M, Low L, Kim HT, Griffin GK, Song JY, et al. Relapsed or refractory double-expressor and double-hit lymphomas have inferior progression-free survival after autologous stem-cell transplantation. J Clin Oncol. (2017) 35:24–31. doi: 10.1200/jco.2016.68.2740
19. Savage KJ, Slack GW, Mottok A, Sehn LH, Villa D, Kansara R, et al. Impact of dual expression of MYC and BCL2 by immunohistochemistry on the risk of CNS relapse in DLBCL. Blood. (2016) 127:2182–8. doi: 10.1182/blood-2015-10-676700
20. Fowler N, Davis E. Targeting B-cell receptor signaling: changing the paradigm. Hematol-Am Soc Hemat. (2013) 2013:553–60. doi: 10.1182/asheducation-2013.1.553
21. Noy A, de Vos S, Thieblemont C, Martin P, Flowers CR, Morschhauser F, et al. Targeting Bruton tyrosine kinase with ibrutinib in relapsed/refractory marginal zone lymphoma. Blood. (2017) 129:2224–32. doi: 10.1182/blood-2016-10-747345
22. Barr PM, Owen C, Robak T, Tedeschi A, Bairey O, Burger JA, et al. Up to 8-year follow-up from RESONATE-2: first-line ibrutinib treatment for patients with chronic lymphocytic leukemia. Blood Adv. (2022) 6:3440–50. doi: 10.1182/bloodadvances.2021006434
23. Novak U, Fehr M, Schaer S, Dreyling M, Schmidt C, Derenzini E, et al. Combined therapy with ibrutinib and bortezomib followed by ibrutinib maintenance in relapsed or refractory mantle cell lymphoma and high-risk features: a phase 1/2 trial of the European MCL network (SAKK 36/13). Eclinicalmedicine. (2023) 64:102221. doi: 10.1016/j.eclinm.2023.102221
24. Dimopoulos MA, Tedeschi A, Trotman J, García-Sanz R, Macdonald D, Leblond V, et al. Phase 3 trial of ibrutinib plus rituximab in waldenstrom’s macroglobulinemia. New Engl J Med. (2018) 378:2399–410. doi: 10.1056/NEJMoa1802917
25. Johnson PWM, Balasubramanian S, Hodkinson B, Shreeve SM, Sun SV, Srinivasan S, et al. Clinical impact of ibrutinib plus R-CHOP in untreated DLBCL coexpressing BCL2 and MYC in the phase 3 PHOENIX trial. Blood Adv. (2023) 7:2008–17. doi: 10.1182/bloodadvances.2022009389
26. Tam CS, Muñoz JL, Seymour JF, Opat S. Zanubrutinib: past, present, and future. Blood Cancer J. (2023) 13:141. doi: 10.1038/s41408-023-00902-x
27. Yang HY, Xiang B, Song YQ, Zhang HL, Zhao WL, Zou DH, et al. Zanubrutinib monotherapy for relapsed or refractory non-germinal center diffuse large B-cell lymphoma. Blood Adv. (2022) 6:1629–36. doi: 10.1182/bloodadvances.2020003698
28. Geng HZ, Jia SX, Zhang Y, Li JQ, Yang Q, Zeng LY, et al. Efficacy and safety of zanubrutinib plus R-CHOP in treatment of non-GCB DLBCL with extranodal involvement. Front Immunol. (2023) 14:1219167. doi: 10.3389/fimmu.2023.1219167
29. Liu Y, Ma XP, Wu XK, Hou XF, Jin W, Fu L, et al. Zanubrutinib is effective in non-germinal-center B-cell-like diffuse large B-cell lymphoma with mutated CD79B, high TCL1A expression, or over- expressed MYC/BCL-2. Leukemia Lymphoma. (2024) 65:1079–89. doi: 10.1080/10428194.2024.2343779
30. Cheson BD, Fisher RI, Barrington SF, Cavalli F, Schwartz LH, Zucca E, et al. Recommendations for initial evaluation, staging, and response assessment of hodgkin and non-hodgkin lymphoma: the lugano classification. J Clin Oncol. (2014) 32:3059. doi: 10.1200/Jco.2013.54.8800
31. Barrington SF, Mikhaeel NG, Kostakoglu L, Meignan M, Hutchings M, Müeller SP, et al. Role of imaging in the staging and response assessment of lymphoma: consensus of the international conference on Malignant lymphomas imaging working group. J Clin Oncol. (2014) 32:3048. doi: 10.1200/Jco.2013.53.5229
32. Zhou Z, Sehn LH, Rademaker AW. An enhanced International Prognostic Index (NCCN-IPI) for patients with diffuse large B-cell lymphoma treated in the rituximab era (vol 123, pg 837, 2014). Blood. (2014) 124:982–2. doi: 10.1182/blood-2013-09-524108
33. Ruppert AS, Dixon JG, Salles G, Wall A, Cunningham D, Poeschel V, et al. International prognostic indices in diffuse large B-cell lymphoma: a comparison of IPI, R-IPI, and NCCN-IPI. Blood. (2020) 135:2041–8. doi: 10.1182/blood.2019002729
34. Cheson BD, Pfistner B, Juweid ME, Gascoyne RD, Specht L, Horning SJ, et al. Revised response criteria for Malignant lymphoma. J Clin Oncol. (2007) 25:579–86. doi: 10.1200/Jco.2006.09.2403
35. Brown JR, Eichhorst B, Lamanna N, O'Brien SM, Tam CS, Qiu L, et al. Sustained benefit of zanubrutinib vs ibrutinib in patients with R/R CLL/SLL: final comparative analysis of ALPINE. Blood. (2024) 144(26):2706–17. doi: 10.1182/blood.2024024667
36. Guo Y, Liu Y, Hu N, Yu D, Zhou C, Shi G, et al. Discovery of zanubrutinib (BGB-3111), a novel, potent, and selective covalent inhibitor of bruton’s tyrosine kinase. J Med Chem. (2019) 62:7923–40. doi: 10.1021/acs.jmedchem.9b00687
37. Advani RH, Buggy JJ, Sharman JP, Smith SM, Boyd TE, Grant B, et al. Bruton tyrosine kinase inhibitor ibrutinib (PCI-32765) has significant activity in patients with relapsed/refractory B-cell Malignancies. J Clin Oncol. (2013) 31:88–94. doi: 10.1200/jco.2012.42.7906
38. Tam CS, Trotman J, Opat S, Burger JA, Cull G, Gottlieb D, et al. Phase 1 study of the selective BTK inhibitor zanubrutinib in B-cell Malignancies and safety and efficacy evaluation in CLL. Blood. (2019) 134:851–9. doi: 10.1182/blood.2019001160
39. Ou YC, Tang Z, Novotny W, Cohen A, Wang K, Liu L, et al. Rationale for once-daily or twice-daily dosing of zanubrutinib in patients with mantle cell lymphoma. Leuk Lymphoma. (2021) 62:2612–24. doi: 10.1080/10428194.2021.1929961
40. Bouroumeau A, Bussot L, Bonnefoix T, Fournier C, Chapusot C, Casasnovas O, et al. c-MYC and p53 expression highlight starry-sky pattern as a favourable prognostic feature in R-CHOP-treated diffuse large B-cell lymphoma. J Pathol Clin Res. (2021) 7:604–15. doi: 10.1002/cjp2.223
41. Wang XJ, Medeiros LJ, Bueso-Ramos CE, Tang GL, Wang S, Oki Y, et al. P53 expression correlates with poorer survival and augments the negative prognostic effect of MYC rearrangement, expression or concurrent MYC/BCL2 expression in diffuse large B-cell lymphoma. Modern Pathol. (2017) 30:194–203. doi: 10.1038/modpathol.2016.178
42. Xu-Monette ZY, Wu L, Visco C, Tai YC, Tzankov A, Liu WM, et al. Mutational profile and prognostic significance of TP53 in diffuse large B-cell lymphoma patients treated with R-CHOP: report from an International DLBCL Rituximab-CHOP Consortium Program Study. Blood. (2012) 120:3986–96. doi: 10.1182/blood-2012-05-433334
43. Xu-Monette ZY, Moller MB, Tzankov A, Montes-Moreno S, Hu WW, Manyam GC, et al. MDM2 phenotypic and genotypic profiling, respective to TP53 genetic status, in diffuse large B-cell lymphoma patients treated with rituximab-CHOP immunochemotherapy: a report from the International DLBCL Rituximab-CHOP Consortium Program. Blood. (2013) 122:2630–40. doi: 10.1182/blood-2012-12-473702
44. Visco C, Canal F, Parolini C, Andreoli A, Ambrosetti A, Krampera M, et al. The impact of P53 and P21(waf1) expression on the survival of patients with the germinal center phenotype of diffuse large B-cell lymphoma. Haematologica. (2006) 91:687–90.
45. Winter JN, Li S, Aurora V, Variakojis D, Nelson B, Krajewska M, et al. Expression of p21 protein predicts clinical outcome in DLBCL patients older than 60 years treated with R-CHOP but not CHOP: a prospective ECOG and Southwest Oncology Group correlative study on E4494. Clin Cancer Res. (2010) 16:2435–42. doi: 10.1158/1078-0432.Ccr-09-1219
46. Farinha P, Sehn L, Skinnider B, Wu L, Patten N, Truong S, et al. Strong p53 expression is an independent predictor of outcome in De Novo diffuse large B cell lymphoma (DLBCL) treated with either CHOP or CHOP-R. Blood. (2006) 108:244a–a. doi: 10.1182/blood.V108.11.812.812
47. Wan MD, Zhang W, Huang H, Fang XJ, Chen YC, Tian Y, et al. Development and validation of a novel prognostic nomogram for advanced diffuse large B cell lymphoma. Clin Exp Med. (2024) 24:64. doi: 10.1007/s10238-024-01326-y
48. Zeng MC, Jia QJ, Chen JJ. Enhanced prognostic evaluation of diffuse large B-cell lymphoma: A comprehensive surveillance study incorporating Epstein-Barr virus infection status and immunohistochemical markers. J Med Virol. (2024) 96:e29834. doi: 10.1002/jmv.29834
49. Yimpak P, Bumroongkit K, Tantiworawit A, Rattanathammethee T, Aungsuchawan S, Daroontum T. Immunohistochemistry-based investigation of MYC, BCL2, and Ki-67 protein expression and their clinical impact in diffuse large B-cell lymphoma in upper Northern Thailand. PloS One. (2024) 19:e0307253. doi: 10.1371/journal.pone.0307253
50. Sadeghipour A, Taha SR, Zadeh MS, Kosari F, Babaheidarian P, Fattahi F, et al. Expression and clinical significance of ki-67, CD10, BCL6, MUM1, c-MYC, and EBV in diffuse large B cell lymphoma patients. Appl Immunohisto M M. (2024) 32:309–21. doi: 10.1097/Pai.0000000000001208
51. Huber F, Zwickl-Traxler E, Pecherstorfer M, Singer J. Evaluation of ki-67 as a prognostic marker in diffuse large B-cell lymphoma-A single-center retrospective cohort study. Curr Oncol. (2021) 28:4521–9. doi: 10.3390/curroncol28060383
52. Li ZM, Huang JJ, Xia Y, Zhu YJ, Zhao W, Wei WX, et al. High Ki-67 expression in diffuse large B-cell lymphoma patients with non-germinal center subtype indicates limited survival benefit from R-CHOP therapy. Eur J Haematol. (2012) 88:510–7. doi: 10.1111/j.1600-0609.2012.01778.x
53. Salles G, de Jong D, Xie WL, Rosenwald A, Chhanabhai M, Gaulard P, et al. Prognostic significance of immunohistochemical biomarkers in diffuse large B-cell lymphoma: a study from the Lunenburg Lymphoma Biomarker Consortium. Blood. (2011) 117:7070–8. doi: 10.1182/blood-2011-04-345256
Keywords: zanubrutinib, Bruton’s tyrosine kinase inhibitor, newly diagnosed DE-DLBCL, Ki67 index, p53 expression
Citation: Zhang M, Wu Y, Cheng Z, Zhang L, Liu L, Liu F, Cui G, Xia L, Hu Y, Mei H, Guo T and Fang J (2025) Zanubrutinib plus R-CHOP improves the treatment effect of newly diagnosed diffuse large B cell lymphoma with double expression of MYC and BCL-2. Front. Immunol. 16:1526318. doi: 10.3389/fimmu.2025.1526318
Received: 11 November 2024; Accepted: 24 February 2025;
Published: 12 March 2025.
Edited by:
Magdalena Plebanski, RMIT University, AustraliaReviewed by:
Ida Franiak-Pietryga, Poseida Therapeutic, United StatesCopyright © 2025 Zhang, Wu, Cheng, Zhang, Liu, Liu, Cui, Xia, Hu, Mei, Guo and Fang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tao Guo, Z3VvdGFvMTk2OEAxNjMuY29t; Jun Fang, anVuZmFuZ3R5eEBodXN0LmVkdS5jbg==
†These authors share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.