
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Immunol., 07 March 2025
Sec. Inflammation
Volume 16 - 2025 | https://doi.org/10.3389/fimmu.2025.1526110
This article is part of the Research TopicThe Impact of Proteomics on Understanding Inflammatory and Infectious DiseasesView all articles
 Fuyuan Zhang1†
Fuyuan Zhang1† Congmin Xia1†
Congmin Xia1† Guang Yang2†
Guang Yang2† Biyue Shang1
Biyue Shang1 Guangrui Huang3
Guangrui Huang3 Kai Yuan3
Kai Yuan3 Hesong Wang3
Hesong Wang3 Xun Gong1*
Xun Gong1* Quan Jiang1*
Quan Jiang1*Objective: Qingre Huoxue Decoction (QRHXD) is a traditional Chinese herbal prescription widely used in clinical practice with significant therapeutic effects on RA; however, its mechanism of action remains unclear. This study aimed to investigate the efficacy and underlying mechanisms of QRHXD in treating RA through clinical research, multiomics approaches, and animal experiments.
Methods: We conducted a 24-week clinical study in which QRHXD was the primary treatment, collecting serum samples from patients before and after treatment for integrated proteomic and metabolomic analysis to identify potential therapeutic targets. Bioinformatics analysis of differentially expressed proteins (DEPs) and differential metabolites (DMs) was performed using hierarchical clustering, volcano plots, heat maps, Gene Ontology (GO), and Kyoto Encyclopaedia of Genes and Genomes (KEGG) analysis. To validate the identified therapeutic targets, we constructed a collagen-induced arthritis (CIA) mouse model.
Results: Clinical research has shown that QRHXD can improve clinical symptoms and relevant indicators in RA patients, including the disease activity score-28 (DAS28), C-reactive protein (CRP), erythrocyte sedimentation rate (ESR), tender joint count (TJC), swollen joint count (SJC), visual analogue scale (VAS), patient-reported outcome (PRO), and health assessment questionnaire (HAQ). Proteomics and metabolomics analysis identified 83 DEPs and 54 DMs, including 46 upregulated and 37 downregulated proteins, as well as 11 upregulated and 43 downregulated metabolites. KEGG enrichment analysis revealed that DEPs are primarily associated with fatty acid degradation, ferroptosis, glycerolipid metabolism, and related pathways. The identified DMs are primarily associated with the AMPK signalling pathway, FoxO signalling pathway, glycolysis/gluconeogenesis, MTOR signalling pathway, and so on. GO enrichment analysis indicated that the DEPs were mainly associated with apoptotic mitochondrial changes, protein modification processes, fatty-acyl-CoA binding, and so on. Integrated proteomics and metabolomics analyses revealed a significant increase in fructose-1,6-biphosphatase 1 (FBP1) levels and a reduction in AMP-activated protein kinase (AMPK) levels in patients with RA. QRHXD inhibited FBP1 and activated AMPK signalling. Animal experiments validated the findings from proteomics and metabolomics analyses, demonstrating that QRHXD could also delay bone destruction and reduce inflammatory factor levels in CIA mice.
Conclusion: QRHXD may reduce the disease activity of RA, attenuate the inflammatory response, and delay bone destruction by inhibiting FBP1 and activating the AMPK signalling pathway.
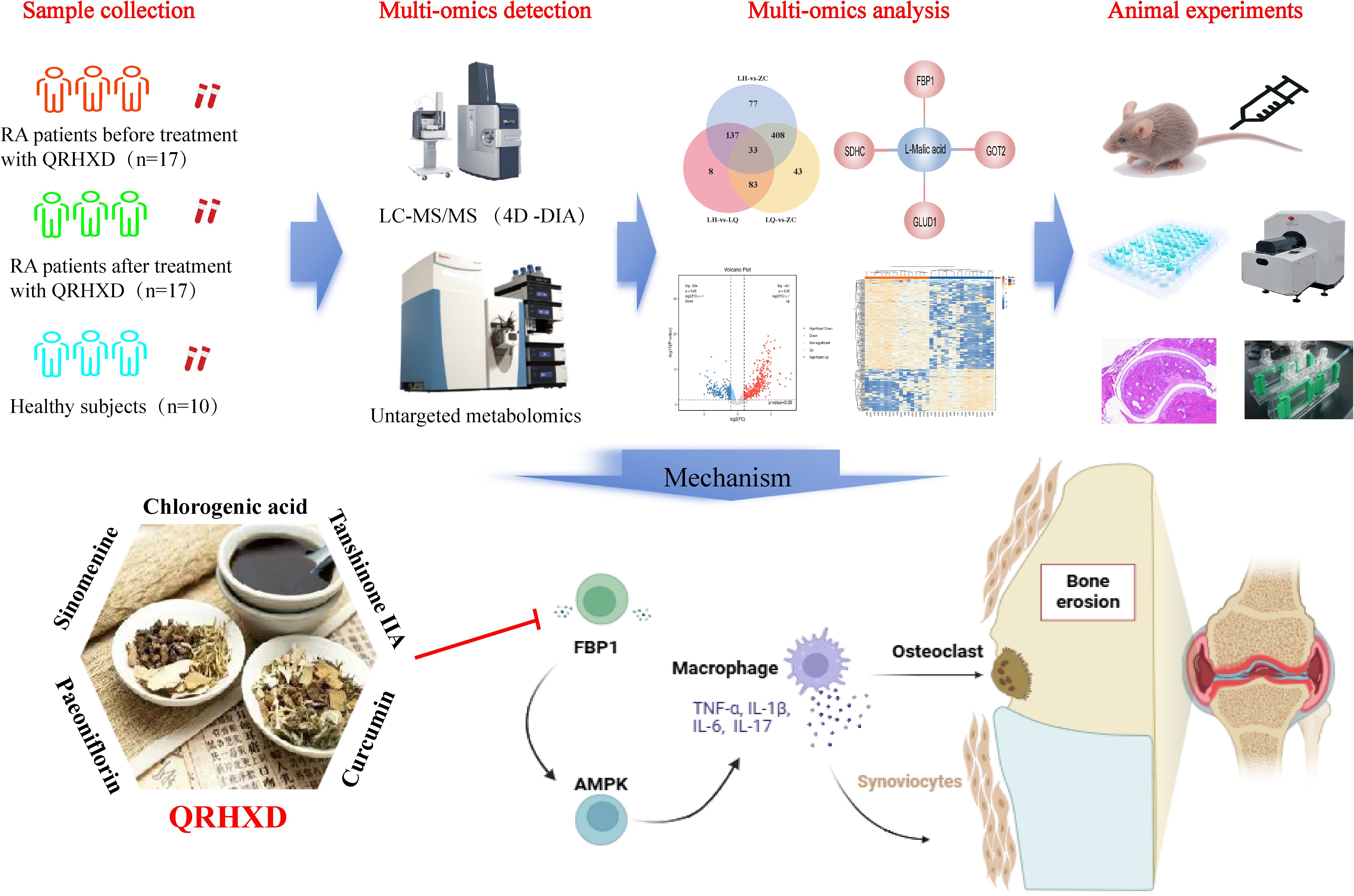
Graphical Abstract. QRHXD may reduce the disease activity of RA, attenuate the inflammatory response, and delay bone destruction by inhibiting FBP1 and activatingthe AMPK signalling pathway.
1. QRHXD can reduce disease activity and inhibit inflammatory response in RA patients.
2. High expression of FBP1 may be a key factor of high disease activity in RA.
3. QRHXD could modulate FBP1/AMPK signal pathway against RA.
Rheumatoid arthritis (RA) is a chronic, systemic autoimmune disease characterised by pathological changes such as synovitis and vascular opacification (1). With a global prevalence of 0.46% and an increasing annual incidence, RA has become a major cause of disability (2). Studies have shown that erosive joint changes are present in more than two-thirds of RA patients (3), while complications such as cardiovascular disease and interstitial lung disease significantly increase mortality (4). Early intervention and targeted treatment are necessary for controlling the disease progression, delaying bone destruction, and reducing complications (5).
Currently, the main treatments for RA include nonsteroidal anti-inflammatory drugs (NSAIDs), disease-modifying antirheumatic drugs (DMARDs), biologics, and glucocorticoids, which help slow disease progression, minimise tissue damage, and improve function (6). However, these drugs do not respond adequately in some patients and make treatment more difficult due to adverse effects (7). For example, methotrexate (MTX) is used as a first-line drug for the treatment of RA; however, up to 50% of patients treated with MTX do not achieve clinically satisfactory outcomes (8). TNF inhibitors such as etanercept, infliximab, and adalimumab are also widely used biologics for RA, and 30%–40% of RA patients do not achieve satisfactory outcomes (9). Therefore, it is essential to develop new and effective therapeutic strategies and medications to treat RA and reduce side effects.
Our team in China conducted a multicentre, 10,000-person prospective cohort study on RA, revealing that Chinese medicine (CM) can be used throughout the entire course of RA and exerts therapeutic effects (10). Qingre Huoxue Decoction (QRHXD), a traditional Chinese herbal prescription widely used in clinical practice, has demonstrated significant therapeutic effects on RA. QRHXD comprises 12 herbs: Atractylodes chinensis (Atractylodis Rhizoma), Phellodendron amurense (Phellodendri Chinensis Cortex), Smilax glabra Roxb (Smilacis glabrae rhizoma), Lonicera japonica Thunb (Lonicerae Japonicae Flos), Astragalus membranaceus (Fisch.) Bge. (Astragali Radix), Paeonia lactiflora Pall (Paeoniae Radix Rubra), Dioscorea futschauensis Uline ex R. Kunth (Dioscoreae Spongiosae Rhizoma), Salvia miltiorrhiza Bge. (Salviae Miltiorrhizae Radix Et Rhizoma), Curcuma phaeocaulis Val. (Curcumae Rhizoma), Sinomenium acutum (Thunb.) (Sinomenii Caulis), Polistes olivaceous (DeGeer) (Vespae Nidus), and Scolopendra subspinipes mutilans L. Koch (Scolopendra) (Table 1). We have previously reported that compound QRHXD can reduce RA-related disease activity and attenuate the inflammatory response in clinical settings (11). Additionally, QRHXD may help prevent bone destruction in RA patients (12). Pharmacological studies have shown that Tanshinone IIA, the main active component of QRHXD, reduces disease severity and mitigates bone loss in an adjuvant-induced arthritis (AIA) rat model (13). However, elucidating the intrinsic mechanism of QRHXD remains challenging due to its complex composition. Multiomics analysis provides a promising approach to uncovering mechanisms of action and identifying drug efficacy targets in inflammatory immune-based diseases (14).
Therefore, we conducted a 24-week clinical study in which QRHXD served as the primary treatment, collecting serum samples from patients before and after treatment with QRHXD for integrated proteomic and metabolomic analyses to identify potential therapeutic targets. Furthermore, a collagen-induced arthritis (CIA) mouse model was constructed to validate QRHXD’s efficacy and targets using Western blotting (WB), micro-CT, haematoxylin-eosin (HE), and other methods, with the goal of identifying new therapeutic methods for treating RA.
QRHXD was prepared and supplied by Sichuan New Green Pharmaceutical Technology Development Co. Ltd., while MTX was supplied by Shanghai Xinyi Pharmaceutical Co. Ltd. Collagen-Type II bovine (batch number: 20022) and Complete Freund’s Adjuvant (batch number: 7009) were purchased from Beijing BioDee Biotechnology Co. Ltd. AMPK antibody (batch number: AF6423) and p-AMPK antibody (batch number: AF3423) were obtained from Affinity Biosciences and the actin antibody (batch number: GB15003) was purchased from Wuhan Servicebio Technology Co. Ltd. The HE kit (batch number: G1120) was acquired from Beijing Solarbio Science & Technology Co. Ltd. Additionally, the human fructose-1,6-bisphosphatase-1 (FBP1) antibody (batch number: A11664), FBP1 kit (batch number: RK10840), and multifactor kit (batch number: RK04321) were purchased from ABclonal Technology Co. Ltd.
The active ingredients of QRHXD were determined using liquid chromatography-mass spectrometry/mass spectrometry (LC-MS/MS). A total of 0.1 g of the prescribed extract was added to 20 mL of methanol and sonicated for 30 min until completely dissolved. Next, 1 mL of the extract was diluted to a volume of 10 mL, filtered through a 0.22-µm microporous filter membrane, and analysed using the Agilent 1260 Liquid Chromatography System, which includes two solvent delivery systems, a degasser, a column oven, an autosampler, and a workstation. The chromatographic column used was an Agilent Poroshell 120 SB C18 (100 mm × 2.1 mm, 2.7 μm), with a mobile phase A consisting of water containing 0.1% ammonium formate and mobile phase B being acetonitrile. The flow rate was set to 0.3 mL/min. The elution gradient was as follows: 0.00 min, 10% B; 0.50 min, 20% B; 7.50 min, 45% B; 8.50 min, 90% B; 12.00 min, 90% B; 12.10 min, 10% B; and 15.00 min, 10% B.
The MS data were collected using an Applied Biosystems API 4500 Qtrap liquid mass spectrometry system in MRM mode. An ESI source was used with simultaneous scanning of positive and negative ions. The MS parameters were as follows: ion source temperature, 500°C; ion source voltage, 5,500 V/− 4,500 V; atomising gas, N2; collision gas CAD, 6 psi; curtain gas CUR, 35 psi; atomising gas GS1, 50 psi; and auxiliary gas GS2, 50 psi. The declustering potential (DP) and collision energy (CE) were configured as listed in Table 2. The precursor and product ions for these compounds were set accordingly (Table 2).
Previously, we conducted a multicentre, randomised, double-blinded, controlled trial lasting 24 weeks to evaluate the efficacy of QRHXD against RA (ClinicalTrials.gov ID: NCT04170504). A total of 204 patients were randomly assigned in a 1:1 ratio to receive either QRHXD or placebo, in addition to MTX as the basic treatment. As a single centre, Guang’anmen Hospital was responsible for recruiting 18 RA patients for each of the treatment and control groups between January 2019 and December 2021. In this study, we collected serum samples from 18 RA patients in the treatment group before and after enrolment for proteomics and metabolomics analyses. Additionally, 10 healthy individuals were recruited as the control group. All participants voluntarily signed informed consent forms. The study was approved by the ethics committee of Guang’anmen Hospital (No. 2019-073-KY-01).
The inclusion criteria were as follows: (1) patients diagnosed with RA based on the 2010 American College of Rheumatology (ACR)/European League Against Rheumatism (EULAR) RA classification criteria (15); (2) age over 18 years; (3) DAS-28 score exceeding 3.2; (4) not taking NSAIDs in the last 4 weeks; and (5) not taking glucocorticosteroids in the last 4 weeks or taking oral hormone doses that had stabilised at less than 10 mg/day.
The exclusion criteria were as follows: (1) patients with serious diseases affecting other organs; (2) those with other autoimmune diseases, such as desiccation syndrome; (3) individuals who are pregnant, breastfeeding, or have a recent birth plan; (4) those allergic to MTX or Sinomenium acutum (Thunb.); (5) patients previously treated with DMARDs (except MTX) or biologics that were discontinued for less than 4 weeks; (6) patients previously treated with leflunomide (LEF) that was discontinued for less than 12 weeks; (7) any other condition that investigators deem unsuitable for inclusion in this clinical trial.
The QRHXD granules were packaged in 10 g bags. Eighteen patients received QRHXD twice daily for 24 weeks (taken by brewing one bag with boiled water and consumed at 0.5 h after breakfast and dinner). This treatment was combined with a base medication of 10 mg of MTX taken once weekly. Blood samples were collected from all subjects in the morning after overnight fasting. Serum was obtained after centrifugation at 3,000 rpm for 15 min, then aliquoted into serum tubes and stored at − 80°C. Serum samples from 17 patients were collected, as one patient’s sample was deemed invalid.
Each serum sample (150 µL) was thawed at room temperature and transferred to a 1.5-mL Eppendorf tube. A mixture of 400 µL of methanol and 200 µL of acetonitrile was then added. The samples were vortexed for 1 min, subjected to ultrasonication for 10 min in an ice-water bath, and stored overnight at − 40°C. Following extraction, the samples were centrifuged at 4°C (12,000 rpm) for 10 min. A total of 150 µL of the supernatant was collected and filtered through a 0.22-µm organic phase pinhole filter into an LC injection vial for LC-MS analysis.
Metabolic profiling was conducted in both ESI-positive and ESI-negative ion modes using an ACQUITY UPLC I-Class plus coupled with a Waters ACQUITY UPLC I-Class plus/Thermo QE plus, which was equipped with a heated electrospray ionisation (ESI) source. The analysis was performed on ACQUITY UPLC HSS T3 column (100 mm × 2.1 mm, 1.8 µm) maintained at 45°C. The mobile phase consisted of A-water (containing 0.1% formic acid) and B-acetonitrile, with a flow rate of 0.35 mL/min and an injection volume of 3 µL. The elution gradient was as follows: 0 min, 5% B; 2 min, 5% B; 4 min, 30% B; 8 min, 50% B; 10 min, 80% B; 14 min, 100% B; 15 min, 100% B; 15.1 min, 5% B; and 16 min, 5% B. The mass range was set from 100 to 1,200 m/z, with a primary MS scan resolution of 70,000, a secondary MS scan resolution of 17,500, and collision energies of 10, 20, and 40 eV, respectively. The mass spectrometer was operated according to normal standards.
The original data were baseline filtered, peak identified, integrated, retention time corrected, peak aligned, and normalised using Progenesis QI V2.3 software. Compound characterisation was performed using the Human Metabolome Database (HMDB), Lipidmaps (V2.3), Metlin, and self-built databases, based on accurate mass-charge ratios (M/z), secondary fragmentation, and isotopic distributions. After characterising the compounds, the positive and negative ion data were combined and imported into the R package for principal component analysis (PCA) to assess the overall distribution and stability among samples. Group differences were analysed using orthogonal partial least squares discriminant analysis (OPLS-DA) and partial least squares discriminant analysis (PLS-DA). A t-test was conducted to determine the statistical significance of these differences, and differential metabolites (DMs) were identified based on VIP values greater than 1.0 and p-values less than 0.05.
Low-abundance proteins in the serum samples were enriched using the EasyPept DeeP Kit for protein enrichment and pretreatment (Omicron, Shanghai, China). Following the manufacturer’s protocol, 1 mg (40 µL) of magnetic nanoparticle suspension was utilised, and the magnetic beads were isolated via magnetic separation to remove the supernatant. After several washes, the magnetic beads were resuspended, and 100 µL of serum was added. The mixture was then incubated at 37°C for 1 h with 360° rotation using a flip mixer. The supernatant was subsequently separated via magnetic separation, followed by the addition of 300 µL of washing solution and gentle shaking for 5 min. This washing step was repeated three times. The proteins were enzymatically hydrolysed to generate peptides, followed by reductive alkylation and desalting. The peptide concentration of each sample was determined using a Nanodrop.
All analyses were conducted using a TimsTOF Pro mass spectrometer equipped with an EASY-nLCTM 1200 system. The data-independent acquisition (DIA) liquid phase elution gradient was as follows: 0 min, 5% B; 45 min, 27% B; 50 min, 46% B; 55 min, 100% B; and 60 min, 100% B. The DIA mass spectrometry scanning parameters included a capillary voltage of 1.4 kV, a dry temperature of 180°C, a drying gas rate of 3.0 L/min, a mass range of 100–1,700 m/z, an ion mobility between 0.7 and 1.3 Vs/cm², and a collision energy range of 20–59 eV.
The DIA original data were processed using Spectronaut Pulsar 17.5 software, with parameter settings and analysis conducted according to the user manual. Differentially expressed proteins (DEPs) were identified based on thresholds of a fold change > 2 or < 0.5 and a p-value < 0.05. Furthermore, the Kyoto Encyclopedia of Genes and Genomes (KEGG) and Gene Ontology (GO) enrichment analyses were performed to annotate the functions and pathways of the DEPs. Interaction analysis between DEPs was performed using String (https://string-db.org/).
Forty female DBA mice (6–8 weeks old, 18 g ± 22 g) were obtained from Beijing Vital River Laboratory Animal Technology Co. Ltd. (Licence No. SYXK [Beijing] 2023-0011). The mice were housed in an SPF-grade animal facility at a constant temperature of 24°C, under a 12-h light/dark cycle, with free access to food and water. The experimental mice were first adaptively fed for 1 week, after which the animals were subjected to modelling, pharmacological interventions, and specimen sampling in accordance with the principles of animal welfare. This experiment was approved by the ethics committee of Guang’anmen Hospital, China Academy of Chinese Medical Sciences (No. IACUC-GAMH-2019-001).
The QRHXD solution for mice was prepared by dissolving QRHXD granules in hot water to obtain medicinal juice at concentrations of 1.35, 2.7, and 5.4 g/mL, which was then stored at − 20°C in the dark. The MTX solution was prepared by dissolving 5 mg of MTX in 84 mL of saline, to achieve a final concentration of 0.06 mg/mL, and the mixture was stored at − 20°C in the dark. For collagen preparation, bovine type II collagen and complete Fuchs’ adjuvant were mixed thoroughly at a 1:1 ratio on ice using a three-way tube until fully emulsified, resulting in an antigen concentration of 2 mg/mL.
The mice were initially immunised by subcutaneous injection of 0.2 mL of antigen into the root of the tail after 1 week of adaptive feeding. A booster injection of 0.2 mL of antigen was administered subcutaneously at the same site on day 21 days. The appearance of redness and swelling in the foot and paw after day 21 indicated successful modelling.
After successful modelling, 40 mice were randomly divided into the normal group, model group, MTX group (1.2 mg/kg/week), QRHXD low-dose group (QRHXD-L, 13.5 g/kg/day), QRHXD medium-dose group (QRHXD-M, 27 g/kg/day), QRHXD high-dose group (QRHXD-H, 54 g/kg/day), with 8 mice in each group. The normal group and model group were given 0.2 mL of saline by gavage, once per day; the QRHXD group was given 0.2 mL of QRHXD solution by gavage, once per day; the MTX group was given 0.2 mL of MTX solution by gavage, twice per week.
Four weeks after administration, the mice were anaesthetised, and blood was collected from the eyeballs in centrifuge tubes. Then, the blood was centrifuged at 3,000 rpm for 15 min, and the supernatant was collected in a clean centrifuge tube and frozen at − 80°C for the inflammatory cytokine assay. Both the hind feet, liver, and kidney were removed from a clean environment. The right foot was frozen at − 80°C for WB detection; the left foot was placed in a paraformaldehyde tube for histopathological testing and micro-CT testing.
Starting from the initial immunisation, the ankle joint leg diameters of both hind limbs of the mice were measured and averaged every 7 days using Vernier callipers. Additionally, joint swelling was assessed twice a week for arthritis index (AI) scoring. The AI scoring criteria were as follows: on a scale of 0–4, no inflamed joints were scored as 0, the presence of inflamed toe joints was scored as 1, both toe joints and inflamed metatarsals were scored as 2, joints below the inflamed ankle were scored as 3, and whole-foot inflamed joints, including the ankle joints, were scored as 4. The scores of the mouse limbs were recorded and summed each time, with a maximum cumulative score of 16 points.
The left hindfoot, liver, and kidney of each mouse were fixed in universal tissue fixative for 48 h and then decalcified for 6 weeks. After complete decalcification, the tissues were paraffin-embedded, sectioned, HE-stained, observed under a light microscope, and photographed.
The left hind feet of the mice were removed from the tissue fixative, positioned using a polystyrene tube to ensure alignment with the centre of rotation, and placed in a micro-CT machine. The region of interest (ROI) in the camera’s field of view was located, the pixel size was set, the filter was corrected, the voltage was adjusted, and scanning begins was initiated. Subsequently, 3D image reconstruction and analysis were performed. The bone surface area (BS), bone volume (BV), BS/BV, number of trabeculae (Tb.N), thickness of the trabeculae (Tb.Th), and degree of separation of the trabeculae (Tb.Sp) were analysed across different groups of mice.
Mouse serum was removed from a − 80°C refrigerator, incubated at room temperature, and processed according to the kit instructions to detect interleukin (IL)-1β, IL-6, IL-10, IL-17, and tumour necrosis factor α (TNF-α) levels.
The synovial tissues of the right hind foot of each mouse were collected, dissociated, and lysed with RIPA buffer. Protein concentration was determined simultaneously. SDS-PAGE gels (10%) were used for protein electrophoresis, and the separated proteins were transferred to PVDF membranes and blocked with 5% nonfat milk at room temperature. The target proteins were detected using primary antibodies. After being washed three times with TBST, the membranes were incubated with secondary antibodies. β-Actin served as an internal reference.
The data are presented as means ± SDs and were analysed using GraphPad Prism 10.0. One-way ANOVA was performed to compare different groups. A p-value of < 0.05 was considered statistically significant.
LC/MS-MS was utilised to establish the quality control of QRHXD and identify its compounds. Clear chromatographic separation and strong mass spectral responses were achieved for each target compound using the optimised LC-MS/MS conditions. For example, chlorogenic acid had a retention time of 2.95 min and a quantitative signal-to-noise ratio exceeding 10. By comparing QRHXD chromatograms with standards, nine active ingredients were identified: chlorogenic acid, sinomenine, paeoniflorin, calycosin-7-O-glucoside, astilbin, atractylodin, berberine hydrochloride, curcumin, and tanshinone IIA. Full details are presented in Table 2; Figure 1.
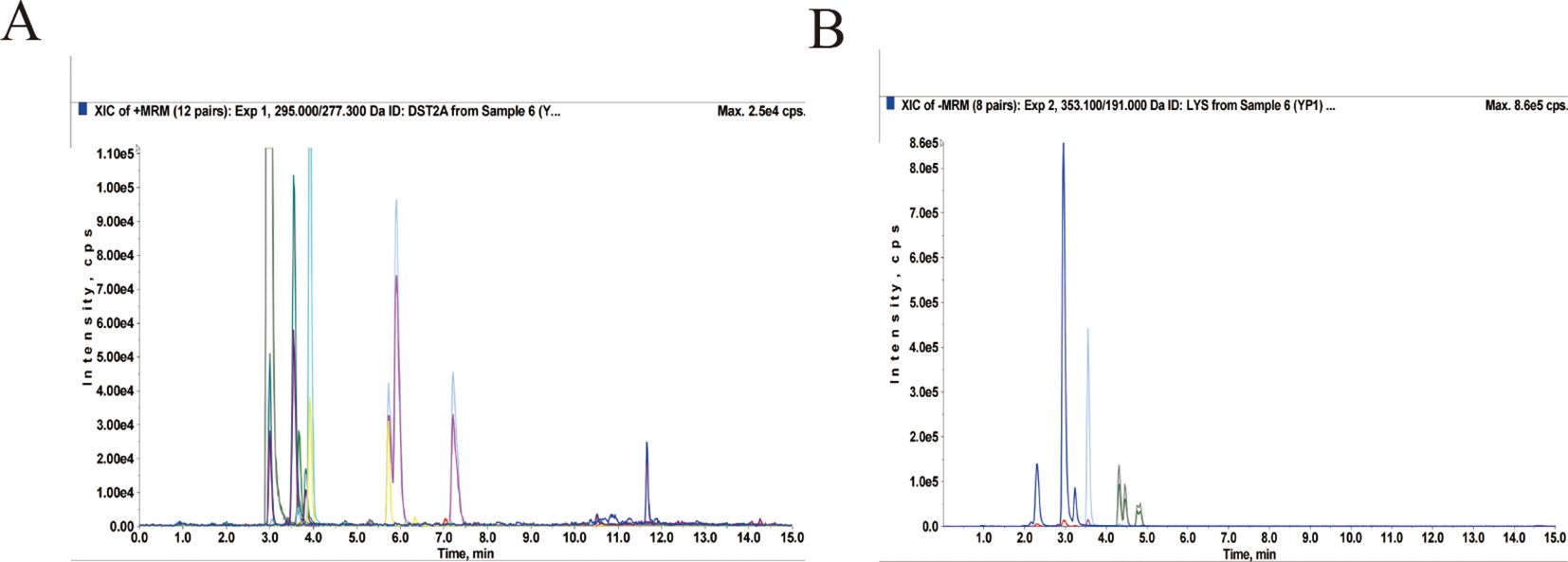
Figure 1. Total ion chromatogram of QRHXD in positive ion mode (A) and negative ion mode (B) of LC-MS/MS.
As shown in Table 3, there was no significant difference (p > 0.05) among the LQ group (baseline), LH group (patients after 24 weeks of treatment), and ZC group (healthy adults) in terms of age, sex, height, weight, temperature (T), pulse (P), respiration (R), systolic blood pressure (SBP), diastolic blood pressure (DBP), white blood cell (WBC), red blood cell (RBC), haemoglobin (HGB), aspartate aminotransferase (AST), alanine aminotransferase (ALT), platelet (PLT), blood urea nitrogen (BUN), and creatinine (Cr) levels. These findings indirectly indicate that QRHXD has satisfactory safety. As shown in Table 4, significant differences (p < 0.05) were observed between the LQ and LH groups in the primary outcome, disease activity score-28 (DAS28), as well as in the secondary outcomes, including the visual analogue scale (VAS) score, C-reactive protein (CRP) level, erythrocyte sedimentation rate (ESR), tender joint count (TJC), swollen joint count (SJC), patient-reported outcome (PRO), and health assessment questionnaire (HAQ). However, no significant differences (p > 0.05) were found in the rheumatoid factor (RF) score. These findings suggest that QRHXD can reduce RA disease activity, alleviate clinical symptoms, and mitigate the inflammatory response.
We analysed the collected serum samples via DIA proteomics. PCA (Figure 2A) revealed that the samples were well aggregated within the same group, with pronounced differences between groups. A total of 3,305 proteins were identified in this study, with their relative abundance spanning five orders of magnitude. The highest protein level was observed for apolipoprotein A4 (APOA4), while the lowest was for apolipoprotein E (APOE) (Figure 2B). Figures 2C–E show 177 upregulated and 84 downregulated proteins in the LH group compared with the LQ group, 339 upregulated and 228 downregulated proteins in the LQ group compared with the ZC group, and 401 upregulated and 254 downregulated proteins in the LH group compared with the ZC group. We then performed Venn intersection analysis on the DEPs and found that 83 proteins differed in both the LH and LQ groups, while there was no difference in the LH and ZC groups, suggesting that these proteins may be therapeutic targets of QRHXD (Figure 2G).
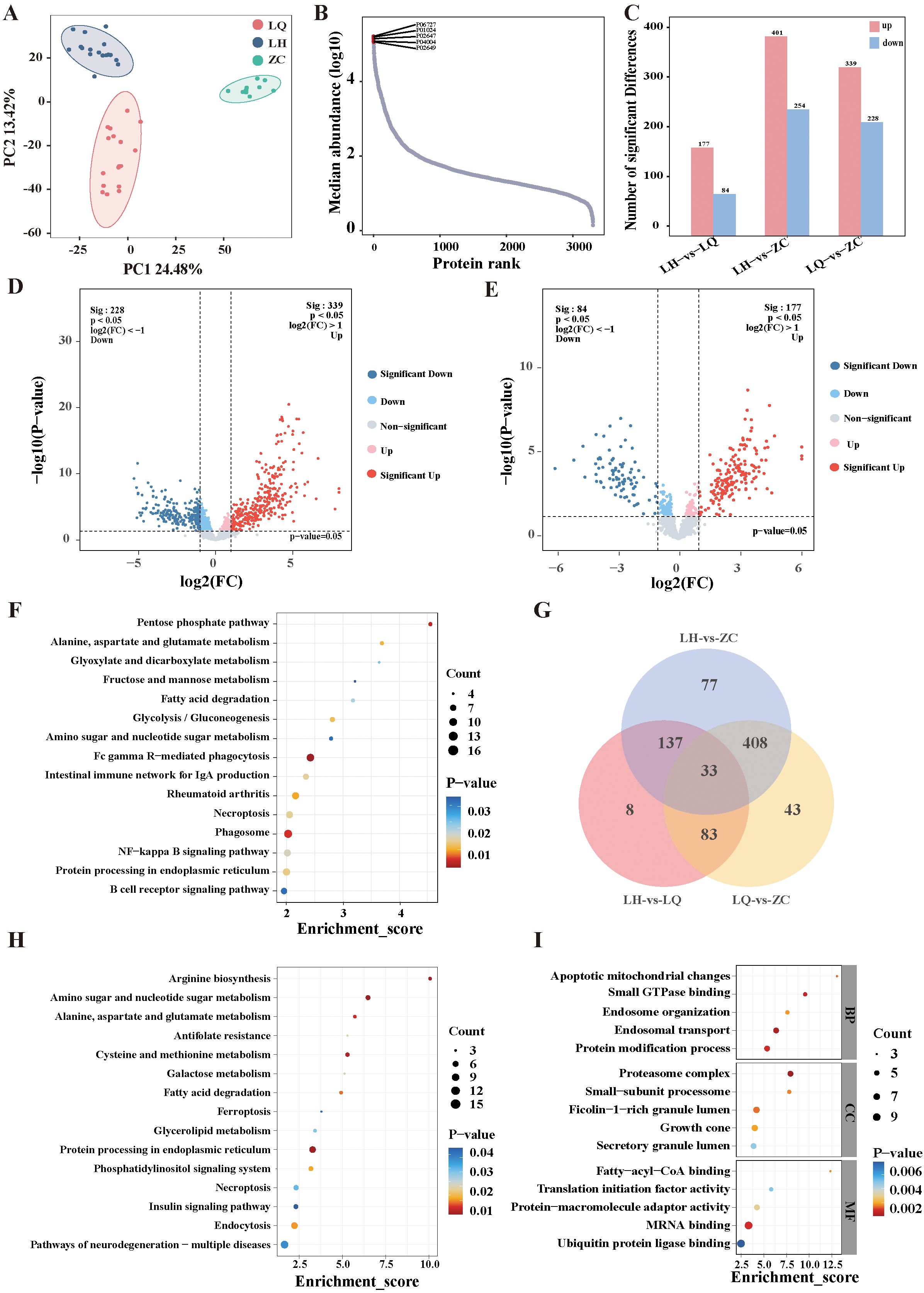
Figure 2. Proteomic analysis of RA patients before treatment with QRHXD (LQ Group), RA patients after treatment with QRHXD (LH Group) and healthy subjects (ZC Group). (A) Principal component analysis (PCA) of differentially expressed proteins (DEPs). (B) Relative abundance of proteins spans five orders of magnitude. (C) DEPs statistics for the 3 groups. (D) DEPs volcano plots of the LQ Group and ZC Group. (E) DEPs volcano plots of the LH Group and LQ Group. (F) KEGG enrichment analysis of DEPs between LQ Group and ZC Group. (G) Venn intersection analysis of DEPs in 3 groups. (H) KEGG enrichment analysis of DEPs between LH Group and LQ Group. (I) GO enrichment analysis of DEPs between LH Group and LQ Group.
The functions and biological pathways of the DEPs were analysed using KEGG and GO pathway analyses. KEGG pathway analysis revealed that pathogenic proteins associated with RA were primarily involved in the B-cell receptor signalling pathway; protein processing in the endoplasmic reticulum; NF-kappa B signalling pathway; phagosome; necroptosis; rheumatoid arthritis; intestinal immune network for IgA production; Fc gamma R-mediated phagocytosis; amino sugar and nucleotide sugar metabolism; glycolysis/gluconeogenesis; fatty acid degradation; fructose and mannose metabolism; glyoxylate and dicarboxylate metabolism; alanine, aspartate, and glutamate metabolism; and the pentose phosphate pathway (Figure 2F). The therapeutic proteins of QRHXD were involved principally in pathways of neurodegeneration—multiple diseases; endocytosis; insulin signalling pathway; necroptosis; phosphatidylinositol signalling system; protein processing in the endoplasmic reticulum; glycerolipid metabolism; ferroptosis; fatty acid degradation; galactose metabolism; cysteine and methionine metabolism; antifolate resistance; alanine, aspartate, and glutamate metabolism; amino sugar and nucleotide sugar metabolism; and arginine biosynthesis (Figure 2H). GO analysis revealed that the DEPs between the LH and LQ groups were associated mainly with protein modification process, endosomal transport, endosome organisation, small GTPase binding, apoptotic mitochondrial changes, growth cone, ficolin-1-rich granule lumen, small-subunit processome, proteasome complex, ubiquitin protein ligase binding, MRNA binding, protein-macromolecule adaptor activity, translation initiation factor activity, and fatty-acyl-CoA binding (Figure 2I).
The PCA model diagram indicated that the QC samples were closely clustered, demonstrating satisfactory detection stability (Figure 3A). DMs between the groups were distinguished using partial least squares discriminant analysis (PLS-DA), which revealed significant differences among groups (Figure 3B). The metabolomics analysis identified 70 upregulated and 133 downregulated metabolites in the LH group compared with the LQ group, 199 upregulated and 107 downregulated metabolites in the LQ group compared with the ZC group, and 175 upregulated and 98 downregulated metabolites in the LQ group compared with the ZC group (Figures 3C–E). A subsequent Venn intersection analysis of the DMs identified 54 metabolites that differed in both the LH and LQ groups, with no differences observed between the LH and ZC groups, suggesting that these metabolites may serve as therapeutic targets of QRHXD (Figure 3F).
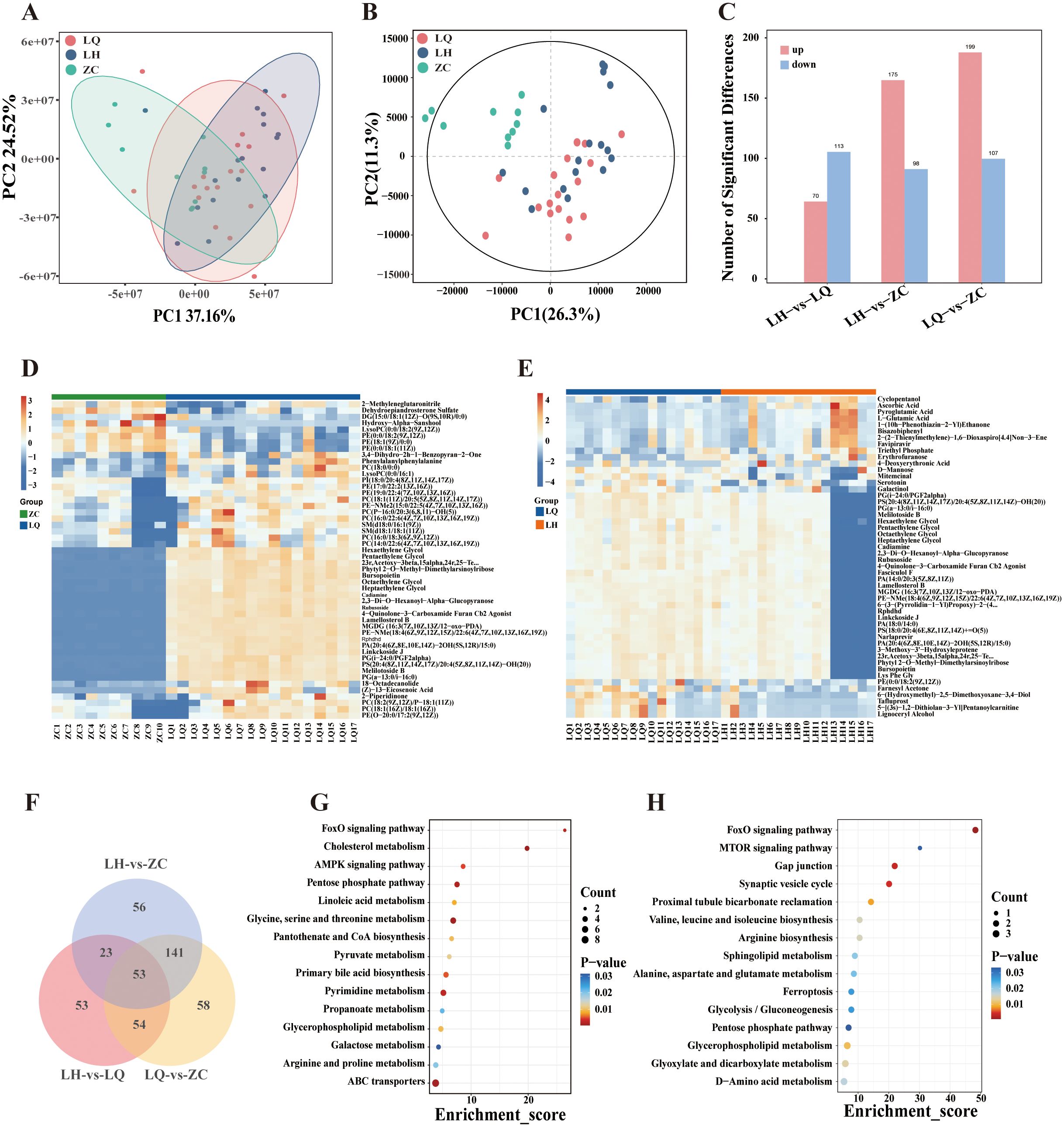
Figure 3. Metabolomics analysis of RA patients before treatment with QRHXD (LQ Group), RA patients after treatment with QRHXD (LH Group) and healthy subjects (ZC Group). (A) Principal component analysis (PCA) of differential metabolites (DMs). (B) Partial Least Squares Discriminant Analysis (PLS-DA) of DMs. (C) DMs statistics for the 3 groups. (D) Cluster heatmap of DMs between LQ Group and ZC Group. (E) Cluster heatmap of DMs between LH Group and LQ Group. (F) Venn intersection analysis of DMs between 3 groups. (G) KEGG enrichment analysis of DMs between LQ Group and ZC Group. (H) KEGG enrichment analysis of DMs between LH Group and LQ Group..
KEGG enrichment analyses of DMs clearly demonstrated that metabolic processes are closely associated with statistically significant pathway maps. The top 15 statistically significant pathways of the pathogenic DMs are presented in Figure 3G and include ABC transporters; arginine and proline metabolism; galactose metabolism; glycerophospholipid metabolism; propanoate metabolism; pyrimidine metabolism; primary bile acid biosynthesis; pyruvate metabolism; pantothenate and CoA biosynthesis; glycine, serine, and threonine metabolism; linoleic acid metabolism; pentose phosphate pathway; AMPK signalling pathway; cholesterol metabolism; and FoxO signalling pathway. The therapeutic metabolites of QRHXD were principally involved in d-amino acid metabolism; glyoxylate, and dicarboxylate metabolism; glycerophospholipid metabolism; the pentose phosphate pathway; glycolysis/gluconeogenesis; ferroptosis, alanine, aspartate, and glutamate metabolism; sphingolipid metabolism; arginine biosynthesis; valine, leucine, and isoleucine biosynthesis; proximal tubule bicarbonate reclamation; the synaptic vesicle cycle; gap junctions; the MTOR signalling pathway; and the FoxO signalling pathway (Figure 3H).
A total of 83 DEPs and 54 DMs were entered into MetaboAnalyst (https://www.metaboanalyst.ca/), and the results revealed a biological between L-malic acid and FBP1 (Figure 4A). In addition, KEGG enrichment analysis of the 83 DEPs and 54 DMs revealed that proteins and metabolites with similar biological relationships clustered into several pathways, including the glucagon signalling pathway, AMPK signalling pathway, glycolysis/gluconeogenesis, galactose metabolism, amino sugar and nucleotide sugar metabolism, insulin signalling pathway, diabetic cardiomyopathy, pentose phosphate pathway, nonalcoholic fatty liver disease, and starch and sucrose metabolism (Table 5). Since FBP1 is a key protein in the association analysis and plays a crucial role in the AMPK pathway, we considered FBP1 and AMPK to be central to the pathogenesis of RA and potential therapeutic targets of QRHXD. To further verify this, we examined FBP1 and AMPK in CIA mice.
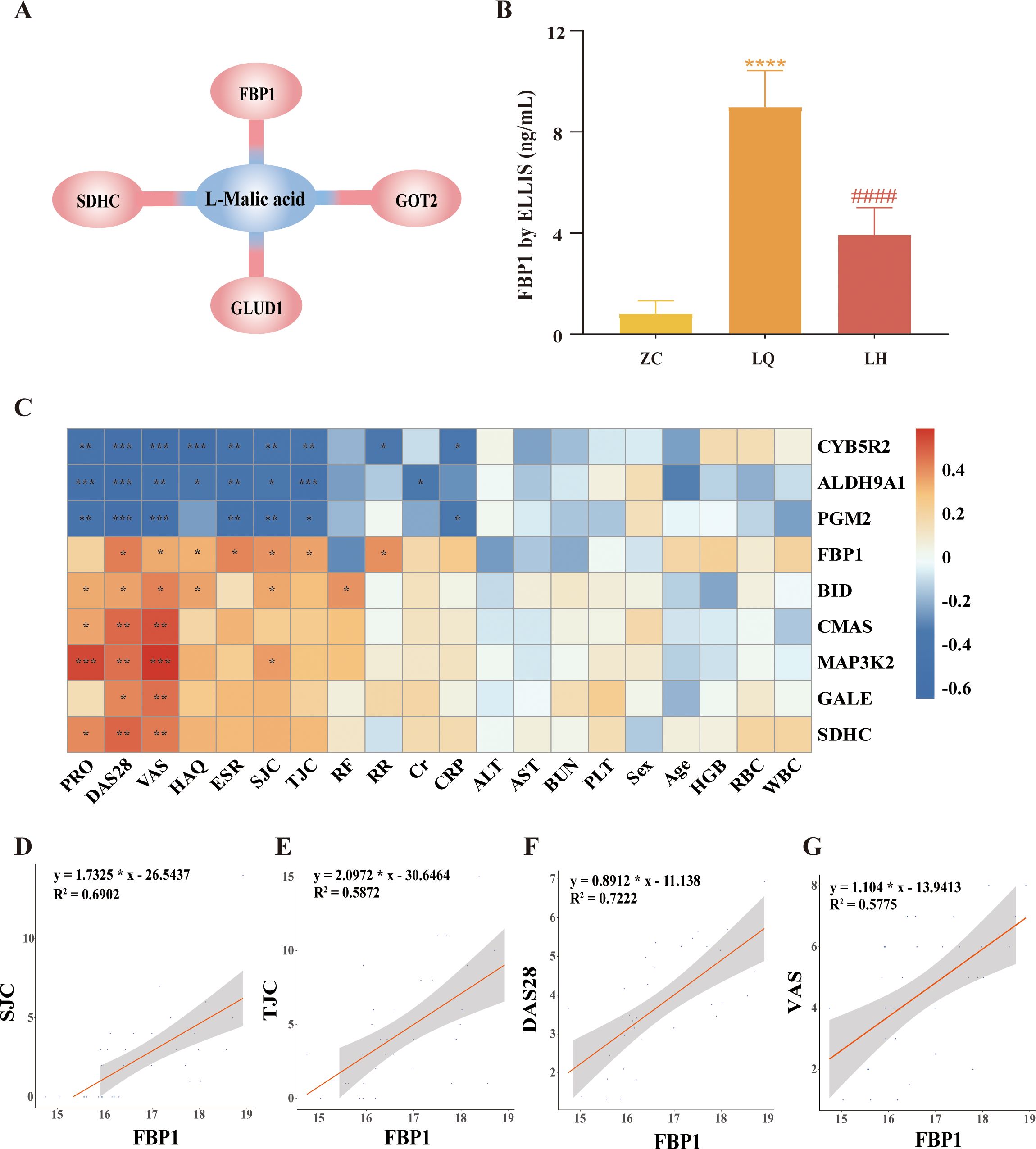
Figure 4. Integrated analysis of differentially expressed proteins (DEPs) and differential metabolites (DMs). (A) L-Malic acid and FBP1 are biologically related. (B) Validation of FBP1 in human serum by ELISA. ****p<0.0001, compared with ZC Group. ####p<0.0001, compared with LQ Group. (C) Heatmap of clustering of 9 DEPs with clinical indicators. *p<0.05, **p<0.01, ***p<0.001. (D) Spearman analysis of FBP1 and swollen joint count (SJC). (E) Spearman analysis of FBP1 and tender joint count (TJC). (F) Spearman analysis of FBP1 and the disease activity score-28 (DAS-28). (G) Spearman analysis of FBP1 and visual analogue scale (VAS).
Furthermore, we validated FBP1 levels in human serum samples using ELISA. The results showed that FBP1 was significantly higher in the LQ group compared to the ZC group and significantly lower in the LH group compared to the LQ group (Figure 4B). These findings were consistent with the multiomics analysis described earlier. Moreover, we performed Spearman’s correlation analysis between the target protein FBP1 and clinical indicators of RA patients. As shown in Figures 4C–G, elevated FBP1 levels were significantly associated with increased DAS-28, VAS, TJC, and SJC in RA patients. These results suggest that increased FBP1 may be a key factor contributing to high disease activity in RA. At the same time, QRHXD treatment was able to inhibit FBP1 expression in RA patients, potentially reducing disease activity.
To evaluate the efficacy and mechanism of QRHXD, we constructed animal experiments using CIA model mice. CIA model mice developed more severe arthritis than normal control mice. QRHXD and MTX significantly reduced toe swelling and arthritis (Figures 5A, B), decreased inflammatory factors such as IL-1β, IL-6, IL-17, and TNF-α, and increased the anti-inflammatory factor IL-10 (Figures 5C–G). Additionally, QRHXD-H demonstrated superior efficacy compared to MTX, QRHXD-M, and QRHXD-L. Moreover, HE staining of the ankle joints revealed that, in the model group, the joint space narrowed, the articular surface was less smooth or even collapsed, and numerous infiltrating inflammatory cells and vascular opacification were observed. Compared with those in the model group, synovial hyperplasia, and joint stenosis were less severe in the QRHXD and MTX groups, with QRHXD-H showing the most pronounced improvement. Notably, HE staining of the livers and kidneys of CIA mice indicated that QRHXD has favourable safety (Figure 5H).
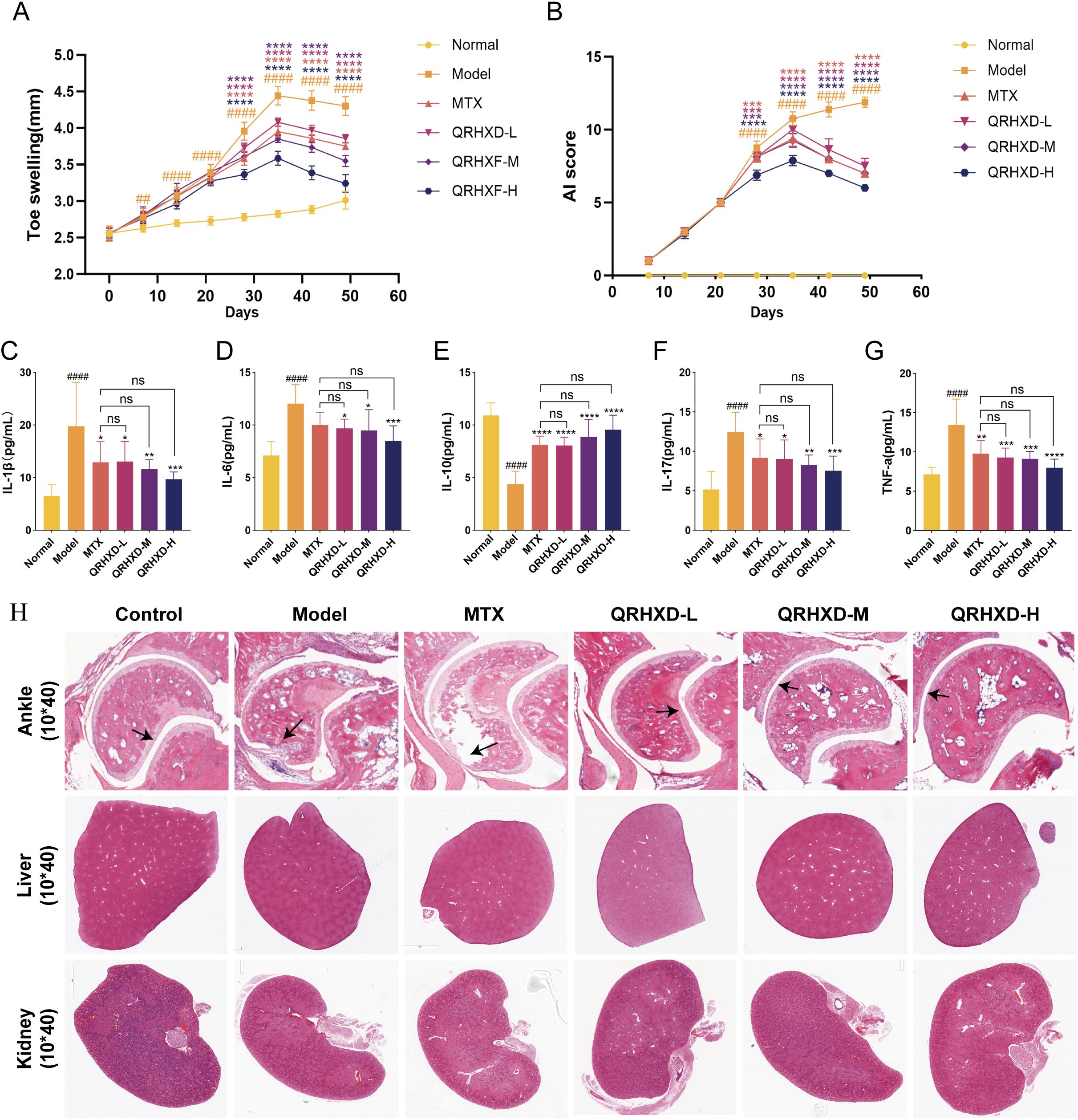
Figure 5. Effect of QRHXD on arthritis, toe swelling, synovial proliferation and inflammatory cytokines in CIA mice. (A) The degree of swelling of the toes. (B) Arthritis Index. (C–G) IL-10, IL-1β, IL-6, IL-17, and TNF-α in serum of mice. (H) HE of the ankle, liver and kidney in mice. ##p<0.05, ####p<0.0001, compared with Normal Group. *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001, compared with Model Group. nsp>0.05, compared with MTX Group.
In addition, we assessed the protective effect of QRHXD against bone destruction using micro-CT scanning of the ankle joints in CIA mice and by collecting bone indices, including BV, BS, BS/BV, Tb.N, Tb.Th, and Tb.Sp. The results revealed that mice in the model group exhibited rough articular surfaces, joint deformation, and severe bone destruction. In this group, BS, BS/BV, and Tb.Sp were elevated, while BV, Tb.N, and Tb.Th were decreased. QRHXD reversed these effects and reduced bone destruction, with QRHXD-H demonstrating greater efficacy (Figures 6A–G). To confirm the effect of QRHXD on the FBP1/AMPK signalling pathway, we performed a WB analysis of mouse synovial membranes. The results revealed that FBP1 was elevated, while AMPK and p-AMPK were reduced in the model group. In addition, FBP1 levels decreased, and AMPK and p-AMPK levels increased in the QRHXD and MTX groups. Moreover, these proteins were significantly elevated in the QRHXD-H group (Figures 6H–K).
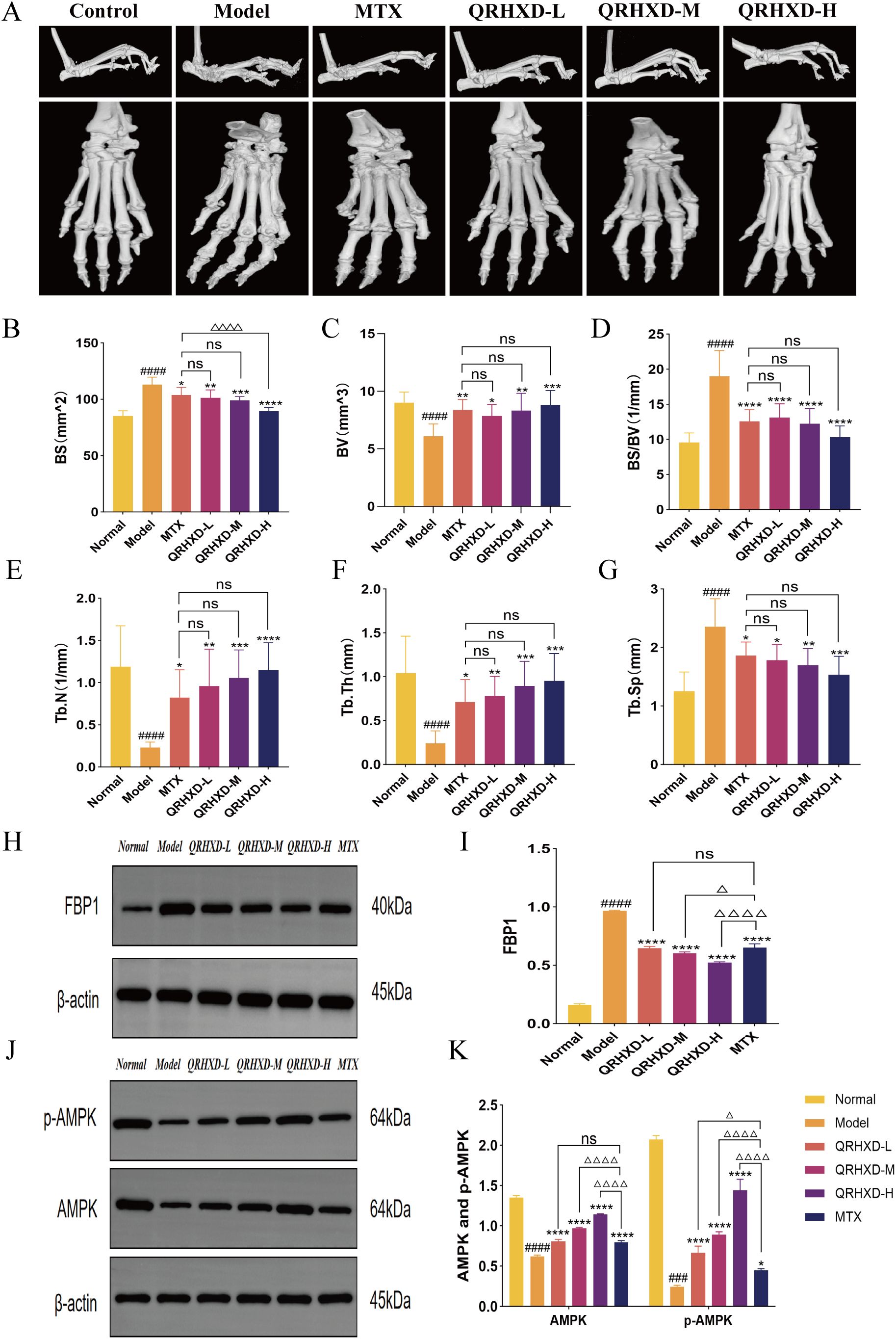
Figure 6. Assessment of bone destruction in mice and WB validation of target proteins. (A) Micro-CT scan of mice ankle joints. (B–G) The indexes of BV, BS, BS/BV, Tb.N, Tb.Th, and Tb.Sp in mice ankle joints. (H, I) Proteins express of FBP1. (J, K) Proteins express of AMPK and p-AMPK. ####p<0.0001, compared with Normal Group. *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001, compared with Model Group. nsp>0.05, compared with MTX Group. △p<0.05, △△△△p<0.0001, compared with MTX Group.
RA is an autoimmune disease characterised by progressive, irreversible joint damage, which can lead to permanent disability (16). Its prevalence and the number of affected people with disabilities continue to rise each year (17). Lifelong treatment is often necessary, and there is a consensus that early intervention and up-to-date therapeutic approaches are essential for controlling disease activity and delaying functional decline (18). The treatment goal is to reduce disease activity by at least 50% within 3 months and achieve remission or low disease activity status within 6 months (19). Currently, RA is primarily treated with DMARDs such as MTX, as well as biologics, including TNF-α inhibitors, IL-6 inhibitors, and JAK inhibitors. Despite advancements in disease management strategies and newer medications, a significant proportion of patients still experience uncontrolled disease (20). Additionally, the adverse effects of these treatments pose challenges for clinicians. For example, MTX can lead to elevated liver enzymes, pneumonia, and gastrointestinal discomfort; TNF inhibitors have been associated with demyelinating lesions; and JAK inhibitors may increase the risk of herpes zoster (21).
CM, with its long history, remains a treatment approach worth exploring. As an herbal compound, QRHXD has demonstrated a good efficacy against RA and a favourable safety profile. In this study, 17 RA patients showed improvements in DAS-28, HAQ, PRO, VAS, TJC, SJC, ESR, CRPF, and other clinical indicators after 24 weeks of QRHXD treatment, indicating that the formula effectively reduced RA disease activity, alleviated inflammation, and regulated somatic function. In addition, QRHXD helped delay RA-induced bone destruction. In CIA model mice, QRHXD intervention led to improvements in micro-CT results, bone indexes, histological evaluation (HE), and inflammatory factor levels, with the QRHXD-H group exhibiting the most pronounced efficacy.
Moreover, to identify the unknown components of the herbal formula, we conducted LC-MS/MS analysis of QRHXD and found that it contained various bioactive compounds, including chlorogenic acid, sinomenine, paeoniflorin, calycosin-7-O-glucoside, astilbin, atractylodin, berberine hydrochloride, curcumin, and tanshinone IIA. Numerous studies have demonstrated that these components can alleviate disease activity and mitigate bone destruction in individuals with RA. For instance, chlorogenic acid has been shown to reduce B-cell activating factor (BAFF) and TNF-α levels, inhibit the apoptosis of MH7A cells, and slow the progression of arthritis in CIA mice (22). A review suggested that sinomenine and paeoniflorin can modulate endoplasmic reticulum stress to exert anti-inflammatory effects, making them potential therapeutic agents for RA (23). Further experiments demonstrated that sinomenine downregulates the Src/FAK/P130Cas signalling pathway to inhibit macrophage migration, thereby exerting anti-inflammatory effects (24). Paeoniflorin has been shown to regulate the balance of the inflammatory-immune system, contributing to the treatment of AIA in rats (25). Additionally, astilbin may demonstrate satisfactory efficacy against RA by activating the A2AAR/adenosine system and inhibiting ERK/nuclear factor-kappa B (NF-kB)/STAT signalling pathways (26). Atractylodin has been shown to significantly downregulate CD40, CD80, and CD86 in the spleen, inhibit dendritic cell activation and proinflammatory cytokine secretion, and reduce joint swelling in CIA mice (27). A meta-analysis conducted by Kou reported that curcumin supplementation improved inflammation levels and clinical symptoms in RA patients (28). Moreover, animal studies have demonstrated that curcumin can inhibit the activation of the PI3K/AKT signalling pathway, suppress TNF-α, IL-6, and IL-17 expression; and ameliorate joint inflammation in CIA rats (29). In our preliminary study on tanshinone IIA, we found that it could reduce the accumulation of reactive oxygen species (ROS), inhibit osteoclast (OC) differentiation, and mitigate bone loss in AIA rats (13).
As a key protein in the glycolysis/gluconeogenesis and AMPK signalling pathways, FBP1 influences OC differentiation through glucose metabolism (30) and plays a role in programmed cell death, which is closely linked to hypoxia, inflammation, immunity, and iron death (31, 32). FBP1 is a rate-limiting enzyme in the gluconeogenic pathway and is elevated during starvation. On one hand, it participates in gluconeogenesis to maintain blood glucose; on the other hand, it inhibits the insulin pathway, reducing lipid synthesis and sugar consumption. These dual functions are coordinated to integrate metabolic and signalling pathways at the molecular level (33). FBP1 regulates recombinant axis inhibition protein (AXIN) to influence AMPK signalling. Liver kinase B1 (LKB1), an initiator of AMPK, activates the downstream catabolic pathway by binding to vacuolar-type ATPase (v-ATPase) with AXINs on the lysosomal membrane. When extracellular glucose and intracellular FBP1 levels decrease, fructose-1,6-bisphosphatase (FBP) is activated, promoting the dynamic binding of v-ATPase and AXINs (34). Moreover, FBP can bind to transient receptor potential vanilloid (TRPV) channels, inhibiting calcium ion channel activity, which may ultimately activate the AMPK signalling pathway (35).
AMPK is a crucial pathway for glycolysis/gluconeogenesis, serving as a key regulator of metabolic homeostasis. When the body experiences hypoxia or energy deficiency, adenosine diphosphate (ADP) binds to the γ-subunit of AMPK, while LKB1 phosphorylates threonine 172 on the α-subunit, activating the AMPK pathway. This activation further phosphorylates downstream genes involved in glucose metabolism, lipid metabolism, transcription factor production, mitochondrial homeostasis, and cellular autophagy (36). Additionally, AMPK plays a role in the maturation and differentiation of OC and osteoblasts (OB), contributing to the regulation of RA-related bone destruction. AMPK is involved in osteoclast metabolism through immune signalling pathways such as NF-kB. Activated AMPK inhibits receptor activator of nuclear kappa-B ligand (RANKL)-induced OC differentiation by reducing the expression of the cellular oncogene Fos (c-Fos) and recombinant nuclear factor of activated T cells, cytoplasmic 1 (NFATc1) (37). Additionally, AMPK can silence Beclin1 (BCN1), induce cellular autophagy, and promote OB differentiation and angiogenesis (38).
QRHXD regulates the AMPK signalling pathway to treat RA by inhibiting FBP1. In this study, we found that FBP1 was significantly overexpressed in the serum of RA patients, accompanied by the downregulation of AMPK signalling, as revealed through a combined multiomics analysis of serum. This may be due to the increased energy consumption in RA patients, where glucose is insufficient to meet metabolic demands, leading to the active activation of FBP1 for glucose synthesis. In contrast, after treatment with QRHXD, the inflammatory state and immune disorders in RA patients were alleviated, reducing the body’s energy consumption and rendering FBP1 inactive.
In conclusion, on the basis of proteomics, metabolomics, and animal experiments, this study demonstrated that QRHXD could reduce disease activity and the inflammatory response in RA patients while delaying bone destruction in CIA mice, by inhibiting FBP1 and activating the AMPK signalling pathway. This study provides new insight into the treatment of RA with QRHXD. However, it has several limitations. Although we identified the active ingredients of QRHXD, the mechanism underlying their effects on RA remains unclear. In the future, we will conduct drug target-molecule docking experiments with QRHXD to elucidate the “pharmacological component-disease molecule-signalling pathway” communication network of QRHXD against RA.
The mass spectrometry proteomics data have been deposited to the ProteomeXchange Consortium (https://proteomecentral.proteomexchange.org) via the iProX partner repository with the dataset identifier PXD054129. The metabolomics data have been deposited to the OMIX database (https://www.cncb.ac.cn/) with dataset identifier OMIX006902.
The studies involving humans were approved by the ethics committee of Guang’anmen Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. The animal study was approved by the ethics committee of Guang’anmen Hospital. The study was conducted in accordance with the local legislation and institutional requirements. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
FZ: Conceptualization, Data curation, Investigation, Methodology, Software, Validation, Visualization, Writing – original draft. CX: Funding acquisition, Supervision, Writing – original draft. GY: Writing – original draft. BS: Validation, Writing – original draft. GH: Data curation, Writing – original draft. KY: Formal analysis, Writing – original draft. HW: Validation, Writing – original draft. XG: Project administration, Writing – review & editing. QJ: Conceptualization, Funding acquisition, Resources, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was supported by the National Natural Science Foundation of China (No. 82230121); Clinical Research Operating Expenses of Centralised High-Level Chinese Medicine Hospitals—Centralised High-Level Chinese Medicine Hospital Clinical Research and Achievement Translation Capacity Enhancement Project (No. HLCMHPP2023087); and the Centralised Public Welfare Scientific Research Institutes Basic Scientific Research Operational Expenses Topics (No. ZZ16-XRZ-038). We thank the Shanghai Luming Biological Technology Co. Ltd. (Shanghai, China) for providing proteomics and metabolomics services.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declare that no Generative AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
AMPK, AMP-activated protein kinase; CIA, collagen-induced arthritis; CM, Chinese medicine; DEPs, differentially expressed proteins; DIA, data-independent acquisition; DMARDs, disease-modifying antirheumatic drugs; DMs, differential metabolites; FBP1, fructose-1,6-biphosphatase 1; GO, Gene Ontology; HE, haematoxylin–eosin; WB, Western blot; KEGG, Kyoto Encyclopaedia of Genes and Genomes; LC-MS/MS, liquid chromatography-mass spectrometry; LEF, leflunomide; IL, interleukin; LH, RA patients after treatment with QRHXD; LQ, RA patients before treatment with QRHXD; MTX, methotrexate; NSAIDs, nonsteroidal anti-inflammatory drugs; OB, osteoblasts; OC, osteoclasts; QRHXD, Qingre Huoxue Decoction; RA, rheumatoid arthritis; TNF-α, tumour necrosis factor-α; ZC, healthy adults.
1. Di Matteo A, Bathon JM, Emery P. Rheumatoid arthritis. Lancet. (2023) 402:2019–33. doi: 10.1016/S0140-6736(23)01525-8
2. Almutairi K, Nossent J, Preen D, Keen H, Inderjeeth C. The global prevalence of rheumatoid arthritis: a meta-analysis based on a systematic review. Rheumatol Int. (2021) 41:863–77. doi: 10.1007/s00296-020-04731-0
3. McInnes IB, Schett G. Pathogenetic insights from the treatment of rheumatoid arthritis. Lancet. (2017) 389:2328–37. doi: 10.1016/S0140-6736(17)31472-1
4. Holmqvist M, Ljung L, Askling J. Mortality following new-onset Rheumatoid Arthritis: has modern Rheumatology had an impact? Ann Rheum Dis. (2018) 77:85–91. doi: 10.1136/annrheumdis-2017-212131
5. Burmester GR, Pope JE. Novel treatment strategies in rheumatoid arthritis. Lancet. (2017) 389:2338–48. doi: 10.1016/S0140-6736(17)31491-5
6. Burgers LE, Raza K, van-der-Helm-van-Mil AH. Window of opportunity in rheumatoid arthritis - definitions and supporting evidence: from old to new perspectives. RMD Open. (2019) 5:e000870. doi: 10.1136/rmdopen-2018-000870
7. Nagy G, Roodenrijs NMT, Welsing PMJ, Kedves M, Hamar A, van der Goes MC, et al. EULAR points to consider for the management of difficult-to-treat rheumatoid arthritis. Ann Rheum Dis. (2022) 81:20–33. doi: 10.1136/annrheumdis-2021-220973
8. Friedman B, Cronstein B. Methotrexate mechanism in treatment of rheumatoid arthritis. Joint Bone Spine. (2019) 86:301–7. doi: 10.1016/j.jbspin.2018.07.004
9. Takahashi S, Saegusa J, Onishi A, Morinobu A. Biomarkers identified by serum metabolomic analysis to predict biologic treatment response in rheumatoid arthritis patients. Rheumatol (Oxford). (2019) 58:2153–61. doi: 10.1093/rheumatology/kez199
10. Gong X, Liu WX, Li D, Peng QW, Xia CM, Chang T, et al. China rheumatoid arthritis registry of patients with Chinese medicine (CERTAIN): Rationale, design, and baseline characteristics of the first 11,764 enrollees. Phytomedicine. (2022) 104:154236. doi: 10.1016/j.phymed.2022.154236
11. Gong X, Liu WX, Tang XP, Wang J, Liu J, Huang QC, et al. Traditional chinese medicine qingre huoxue treatment vs. the combination of methotrexate and hydroxychloroquine for active rheumatoid arthritis: A multicenter, double-blind, randomized controlled trial. Front Pharmacol. (2021) 12:679588. doi: 10.3389/fphar.2021.679588
12. Jiang Q, Zhou XY, Wang L, Yu W, Wang P, Cao W, et al. A one-year evaluation of radiographic progression in patients with rheumatoid arthritis treated by Qingre Huoxue Decoction (). Chin J Integr Med. (2012) 18:256–61. doi: 10.1007/s11655-011-0793-0
13. Peng Q, Wang J, Han M, Zhao M, Li K, Lu T, et al. Tanshinone IIA inhibits osteoclastogenesis in rheumatoid arthritis via LDHC-regulated ROS generation. Chin Med. (2023) 18:54. doi: 10.1186/s13020-023-00765-1
14. Bennike TB. Advances in proteomics: characterization of the innate immune system after birth and during inflammation. Front Immunol. (2023) 14:1254948. doi: 10.3389/fimmu.2023.1254948
15. Aletaha D, Neogi T, Silman AJ, Funovits J, Felson DT, Bingham CO 3rd, et al. 2010 rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Ann Rheum Dis. (2010) 69:1580–8. doi: 10.1136/ard.2010.138461
16. Jeganathan N, Nguyen E, Sathananthan M. Rheumatoid arthritis and associated interstitial lung disease: mortality rates and trends. Ann Am Thorac Soc. (2021) 18:1970–7. doi: 10.1513/AnnalsATS.202102-115OC
17. Almutairi KB, Nossent JC, Preen DB, Keen HI, Inderjeeth CA. The prevalence of rheumatoid arthritis: A systematic review of population-based studies. J Rheumatol. (2021) 48:669–76. doi: 10.3899/jrheum.200367
18. Yu Z, Lu B, Agosti J, Bitton A, Corrigan C, Fraenkel L, et al. Implementation of treat-to-target for rheumatoid arthritis in the US: analysis of baseline data from a randomized controlled trial. Arthritis Care Res (Hoboken). (2018) 70:801–6. doi: 10.1002/acr.23343
19. Aletaha D, Smolen JS. Diagnosis and management of rheumatoid arthritis: A review. JAMA. (2018) 320:1360–72. doi: 10.1001/jama.2018.13103
20. Sanchez-Piedra C, Sueiro-Delgado D, Garcia-Gonzalez J, Ros-Vilamajo I, Prior-Espanol A, Moreno-Ramos MJ, et al. Changes in the use patterns of bDMARDs in patients with rheumatic diseases over the past 13 years. Sci Rep. (2021) 11:15051. doi: 10.1038/s41598-021-94504-x
21. Rutherford AI, Patarata E, Subesinghe S, Hyrich KL, Galloway JB. Opportunistic infections in rheumatoid arthritis patients exposed to biologic therapy: results from the British Society for Rheumatology Biologics Register for Rheumatoid Arthritis. Rheumatol (Oxford). (2018) 57:997–1001. doi: 10.1093/rheumatology/key023
22. Fu X, Lyu X, Liu H, Zhong D, Xu Z, He F, et al. Chlorogenic acid inhibits BAFF expression in collagen-induced arthritis and human synoviocyte MH7A cells by modulating the activation of the NF-kappaB signaling pathway. J Immunol Res. (2019) 2019:8042097. doi: 10.1155/2019/8042097
23. Li RZ, Guan XX, Wang XR, Bao WQ, Lian LR, Choi SW, et al. Sinomenine hydrochloride bidirectionally inhibits progression of tumor and autoimmune diseases by regulating AMPK pathway. Phytomedicine. (2023) 114:154751. doi: 10.1016/j.phymed.2023.154751
24. Li JM, Yao YD, Luo JF, Liu JX, Lu LL, Liu ZQ, et al. Pharmacological mechanisms of sinomenine in anti-inflammatory immunity and osteoprotection in rheumatoid arthritis: A systematic review. Phytomedicine. (2023) 121:155114. doi: 10.1016/j.phymed.2023.155114
25. Li H, Cao XY, Dang WZ, Jiang B, Zou J, Shen XY. Total Glucosides of Paeony protects against collagen-induced mouse arthritis via inhibiting follicular helper T cell differentiation. Phytomedicine. (2019) 65:153091. doi: 10.1016/j.phymed.2019.153091
26. Ma Y, Gao Z, Xu F, Liu L, Luo Q, Shen Y, et al. A novel combination of astilbin and low-dose methotrexate respectively targeting A(2A)AR and its ligand adenosine for the treatment of collagen-induced arthritis. Biochem Pharmacol. (2018) 153:269–81. doi: 10.1016/j.bcp.2018.01.033
27. Chuang CH, Cheng YC, Lin SC, Lehman CW, Wang SP, Chen DY, et al. Atractylodin suppresses dendritic cell maturation and ameliorates collagen-induced arthritis in a mouse model. J Agric Food Chem. (2019) 67:6773–84. doi: 10.1021/acs.jafc.9b01163
28. Kou H, Huang L, Jin M, He Q, Zhang R, Ma J. Effect of curcumin on rheumatoid arthritis: a systematic review and meta-analysis. Front Immunol. (2023) 14:1121655. doi: 10.3389/fimmu.2023.1121655
29. Xu Z, Shang W, Zhao Z, Zhang B, Liu C, Cai H. Curcumin alleviates rheumatoid arthritis progression through the phosphatidylinositol 3-kinase/protein kinase B pathway: an in vitro and in vivo study. Bioengineered. (2022) 13:12899–911. doi: 10.1080/21655979.2022.2078942
30. Zhang Y, Fang M, Yang Z, Qin W, Guo S, Ma J, et al. GATA binding protein 4 regulates tooth root dentin development via FBP1. Int J Biol Sci. (2020) 16:181–93. doi: 10.7150/ijbs.36567
31. Xiong X, Zhang J, Li A, Dai L, Qin S, Wang P, et al. GSK343 induces programmed cell death through the inhibition of EZH2 and FBP1 in osteosarcoma cells. Cancer Biol Ther. (2020) 21:213–22. doi: 10.1080/15384047.2019.1680061
32. Ma J, Guo Z, Yang X, Zhu Y. Exploration of various roles of hypoxia genes in osteosarcoma. Sci Rep. (2022) 12:18293. doi: 10.1038/s41598-022-17622-0
33. Gu L, Zhu Y, Watari K, Lee M, Liu J, Perez S, et al. Fructose-1,6-bisphosphatase is a nonenzymatic safety valve that curtails AKT activation to prevent insulin hyperresponsiveness. Cell Metab. (2023) 35:1009–1021 e1009. doi: 10.1016/j.cmet.2023.03.021
34. Zhang CS, Hawley SA, Zong Y, Li M, Wang Z, Gray A, et al. Fructose-1,6-bisphosphate and aldolase mediate glucose sensing by AMPK. Nature. (2017) 548:112–6. doi: 10.1038/nature23275
35. Li M, Zhang CS, Zong Y, Feng JW, Ma T, Hu M, et al. Transient receptor potential V channels are essential for glucose sensing by aldolase and AMPK. Cell Metab. (2019) 30:508–524 e512. doi: 10.1016/j.cmet.2019.05.018
36. Herzig S, Shaw RJ. AMPK: guardian of metabolism and mitochondrial homeostasis. Nat Rev Mol Cell Biol. (2018) 19:121–35. doi: 10.1038/nrm.2017.95
37. Salminen A, Kauppinen A, Kaarniranta K. AMPK activation inhibits the functions of myeloid-derived suppressor cells (MDSC): impact on cancer and aging. J Mol Med (Berl). (2019) 97:1049–64. doi: 10.1007/s00109-019-01795-9
Keywords: rheumatoid arthritis, bone destruction, proteomics, metabolomics, Chinese medicine, FBP1, AMPK
Citation: Zhang F, Xia C, Yang G, Shang B, Huang G, Yuan K, Wang H, Gong X and Jiang Q (2025) Multiomics analysis of human serum and animal experiments reveals the protective mechanism of Qingre Huoxue Decoction against rheumatoid arthritis. Front. Immunol. 16:1526110. doi: 10.3389/fimmu.2025.1526110
Received: 11 November 2024; Accepted: 17 February 2025;
Published: 07 March 2025.
Edited by:
Victor Corasolla Carregari, State University of Campinas, BrazilReviewed by:
Bradley J. Smith, State University of Campinas, BrazilCopyright © 2025 Zhang, Xia, Yang, Shang, Huang, Yuan, Wang, Gong and Jiang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Quan Jiang, OTk0Mjc5ODU3QHFxLmNvbQ==; Xun Gong, Z29uZ3h1bjgyNjlAMTYzLmNvbQ==
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.