
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
MINI REVIEW article
Front. Immunol. , 31 January 2025
Sec. Mucosal Immunity
Volume 16 - 2025 | https://doi.org/10.3389/fimmu.2025.1525928
This article is part of the Research Topic The Role of Mucosal Surfaces on Asthma and Respiratory Disease View all 6 articles
Goblet cell hypersecretion is a hallmark of airway inflammation and is driven by complex neuroimmune regulation involving submucosal glands and goblet cells. Although studies have focused on mast cell degranulation as a critical driver of nasal secretion, the role of goblet cells in this process is relatively under-researched. In allergic airway inflammation, goblet cells exhibit metaplasia and hypersecretion. However, allergen exposure does not directly trigger goblet cell degranulation, raising questions regarding the underlying mechanisms of these reactions. The activation of enteric neurons promotes goblet cell degranulation by stimulating the calcitonin gene-related peptide (CGRP)–receptor active modification protein-1 (RAMP1) axis. Meanwhile, airway goblet cells express various neuropeptide receptors, and their activation by neuropeptides such as substance P and CGRP induces mucus secretion, exacerbating allergic rhinitis-associated hypersecretion. Thus, although previously less recognised, the neuron–goblet cell signalling axis plays a critical role in allergic rhinitis mucus secretion. This review highlights current research on the neuroimmune mechanisms underlying goblet cell metaplasia and degranulation, focusing on allergic rhinitis, so as to guide clinical treatment strategies.
Goblet cells (GCs) are distributed across various organs, including the digestive tract, respiratory tract, and conjunctiva. Despite their distinct functions arising from evolutionary adaptations, GCs have common functions (1). Mucus and mucin produced by GCs and enterocytes are crucial components of the first line of defence, interacting with the immune system to maintain homeostasis (2). However, under pathological conditions, this interaction can result in various disease phenotypes. The two most prevalent diseases of the nasal mucosa are allergic rhinitis (AR) and chronic rhinosinusitis, both of which share common characteristics, including increased mucin 5AC (MUC5AC) and MUC5B secretion. Airway secretions originate primarily from GCs, glandular tissues, and vascular exudates (3). The current treatments for AR, including antihistamines, leukotriene antagonists, allergen immunotherapy, and biologics, primarily target traditional immunological pathways (4). In contrast, chronic rhinosinusitis management relies on pharmacological and surgical interventions, focusing less on the role of GCs. Therefore, a comprehensive investigation of GC function in different diseases and the composition of nasal secretions may reveal the fundamental mechanisms underlying these different hypersecretions.
Neuro-immunity encompasses the bidirectional communication pathways between the nervous and immune systems, vital for maintaining tissue homeostasis, combating infections, and modulating inflammatory responses (5). Neural sensitisation significantly increases acetylcholine release, enhancing GC secretion. Meanwhile, neuromedin U mediates eosinophil activation and increases the number of intestinal GCs, potentially impacting mucus secretion due to eosinophilic effects (6). Moreover, neuron–GC signalling via the calcitonin gene-related peptide (CGRP)–receptor active modification protein-1(RAMP1) axis protects against colitis (7).
In the airways, neural endings facilitate GC degranulation through various neuroimmune mechanisms, including those mediated by neuropeptides, neurotransmitters, and transient receptor potential (TRP) channels (8). As recently reported, current medications often prove insufficient for patients with AR exposed to physicochemical stimuli. Neuroimmune modulation of GCs likely contributes to this challenge. A deeper understanding of the neuroimmune mechanisms underlying GC metaplasia and degranulation in AR may provide valuable insights into novel therapeutic strategies for managing this condition.
The neuroimmune system is pivotal in the GC hypersecretion associated with AR. Although our understanding of the effects of GCs on AR remains limited, research on other systems provides valuable information. For example, voltage-gated sodium channel 1.8+ neurons are present near mucus-secreting GCs, where intestinal nerve–GC signalling may induce mucus secretion via the CGRP–RAMP1 axis (7). Additionally, intestinal neuronal cells release interleukin (IL)-18, which promotes mucin secretion (9). The specific mechanisms in the intestine are shown in Figure 1A. Mucosal barrier immunity is essential for maintaining the commensal microflora and combating infection by invasive bacteria, whereas tuft cell-derived acetylcholine regulates epithelial mucus secretion (10). Moreover, inhibiting TRP cation channel subfamily M member 4 (TRPM4) protein activity in cystic fibrosis cell lines abolished MUC5AC secretion (11). Finally, nerve–GC interactions promote allergic conjunctivitis through goblet cell-associated antigen passages (GAPs). The function of GAP as a novel therapeutic target for airway allergic inflammation warrants further investigation.
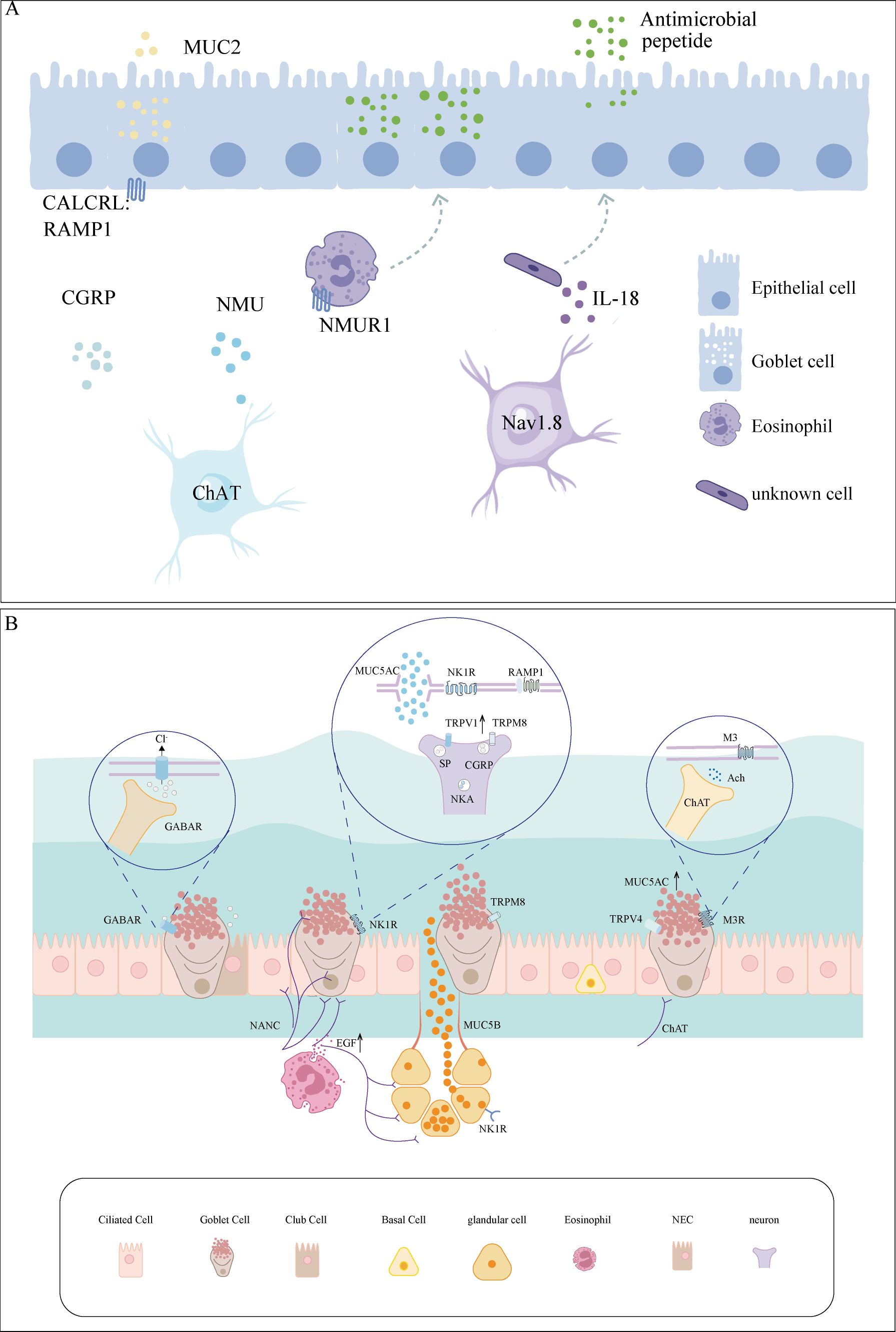
Figure 1. Neuro-GC interactions in the airway and intestine. (A) Immune crosstalk between neurons and GCs in the intestine. Intestinal neurons release CGRP and NMU, which directly or indirectly stimulate GCs, promoting MUC2 secretion. Nav1.8+ neuron-derived IL-18 orchestrates antimicrobial peptide secretion. (B) Immune crosstalk between neurons and GCs in the airway. The nerves, which predominantly consist of cholinergic and sensory nerve fibres, act upon GCs. External stimuli, such as temperature fluctuations and allergens, trigger the release of neurotransmitters and neuropeptides, which interact with receptors on GCs, including M3, NK1R, and GABAAR. CALCRL, calcitonin receptor-like receptor; CGRP, calcitonin gene-related peptide; ChAT, choline acetyltransferase; EGF, epidermal growth factor; GABAR, gamma-aminobutyric acid receptor; GC, goblet cell; M3, muscarinic type 3; MUC2, mucin 2; MUC5AC, mucin 5AC; NANC, non-adrenergic non-cholinergic; NKA, neurokinin A; NK1R, neurokinin-1 receptor; NMU, neuromedin U; NMUR1, neuromedin U receptor 1; RAMP1, receptor activity-modifying protein 1; SP, substance P.
In asthma, substance P produced by airway sensory neurons amplifies allergy-induced GC hyperplasia and MUC5AC hypersecretion (12). Similarly, activating the gamma-aminobutyric acid (GABA) type A receptor, a specific chloride channel, triggers mucin release. GABA secreted by neuroendocrine cells also promotes GC hyperplasia, resulting from the increased trans-differentiation of ciliated airway epithelial cells and club cells into mucin-producing GCs following ovalbumin challenge. GCs are often located near airway sensory neuron terminals and express receptors for various neuropeptides, including muscarinic acetylcholine receptor type 3 (M3), neurokinin-1 receptor, GABA type A receptor, and vasoactive intestinal peptide (VIP) receptor 1 (13). TRP melastatin subtype 8 (TRPM8), a calcium channel, modulates intracellular calcium levels and influences GC activity, leading to MUC5AC secretion, particularly during cold exposure (14). The specific details are illustrated in Figure 2A and Table 1.

Figure 2. (A) Neuropeptides released from nerve endings and receptors on GCs are illustrated. Solid black lines highlight confirmed findings, while blue dashed lines represent findings yet to be validated. (B) Cellular signal transduction during GC differentiation. Muc5ac is the structural gene governing GC differentiation, and SPDEF, FOXA3, FOXA2, FOXJ1, NFκB, and ERK act as its transcription factors. The Notch signalling pathway is responsible for the differentiation of cells into secretory cells under normal conditions, while EGFR, and IL-4R are involved in the regulation of GC metaplasia in allergic rhinitis. ACh, acetylcholine; CALCRL, calcitonin receptor-like receptor; CGRP, calcitonin gene-related peptide; ER, endoplasmic reticulum; GABA, gamma-aminobutyric acid; GC, goblet cell; IP3, inositol trisphosphate; M3, muscarinic acetylcholine type 3; NCX2 (SLC8A2), solute carrier family 8 member A2; NKA, neurokinin A; NK1R, neurokinin 1R; RAMP1, receptor activity-modifying protein 1; SP, substance P; TRP, transient receptor potential vanilloid 1; VIP, vasoactive intestinal peptide. EGFR, epidermal growth factor receptor; FOXA2, forkhead box protein A2; GABA A, gamma-aminobutyric acid type A; GC, goblet cell; IL-13, interleukin 13; LEF-1, lymphoid enhancer-binding factor 1; MUC5AC, mucin 5AC; NICD, Notch intracellular domain; SPDEF, SAM-pointed domain containing ETS-like factor; STAT6, signal transducer and activator of transcription 6.
Allergens trigger GC metaplasia, and IL-13 is a critical factor driving airway allergic inflammation, GC proliferation, and metaplasia (15). IL-13 upregulates several genes involved in GC metaplasia, including SAM pointed domain-containing ETS transcription factor (SPDEF), forkhead box A2 (FOXA2), and MUC5AC (16–19). Specifically, it enhances SPDEF transcription, subsequently stimulating FOXA2 expression, leading to MUC5AC upregulation and stromal cell metaplasia in GCs (20).
IL-13 promotes GC metaplasia through multiple signalling pathways. For instance, circZNF652 and microRNA-141 are upregulated in patients with airway allergic inflammation, contributing to GC metaplasia by downregulating microRNA-452 and modulating IL-13 signalling (21, 22). In vivo, IL-13 increases MUC5AC transcription while suppressing FOXJ1, preventing GC apoptosis in AR (23). In vitro, IL-13 induces MUC5AC formation via the phosphatidylinositol 3-kinase and Janus kinases 1 (JAK1)-signal transducer and activator of transcription 6 (STAT6) pathways (24), as shown in Figure 2B. Thus, blocking IL-13 signalling and reducing GC metaplasia could enhance mucociliary clearance and restore the nasal epithelial structure (25).
Another important property of IL-13 is its ability to activate or sensitize peripheral sensory neurons. In addition to inflammatory factors’ responses, neurogenic inflammation mediated by neurotransmitters contributes to GC metaplasia regulation. The vagus nerve releases Substance P, which promotes GC metaplasia by interacting with the neurokinin-1 receptor (12). This induces GC metaplasia in the airways and promotes features such as GC hyperplasia (16). Neuroendocrine cells express GABA, and GABA type A receptors are upregulated in patients with airway allergic inflammation. GABA’s action on these receptors inhibits the SMAD pathway and promotes GC proliferation (26–28). The biological clock also helps regulate GC proliferation and differentiation (29). Animal studies (29, 30) have demonstrated more significant GC proliferation, metaplasia, and secretion in mice with disrupted circadian rhythms than those with normal rhythms. Furthermore, MUC5AC levels exhibit a circadian rhythm, indicating that it may be a therapeutic target for airway allergic inflammation. Thus, chronotherapeutics related to MUC5AC may exhibit enhanced efficacy with reduced side effects.
The airways involve three neural pathways, namely sympathetic (adrenergic), parasympathetic (cholinergic), and non-adrenergic non-cholinergic (NANC) (31). GC degranulation is primarily regulated by the cholinergic and NANC sensory nervous systems (32). In the nasal mucosa, NANC nerve fibres are predominantly found in the trigeminal nerve’s C fibres, which are particularly susceptible to direct activation by allergic mediators (31). Afferent C fibres often express TRP ion channels, which promote mucin synthesis and secretion by releasing neuropeptides and neurotransmitters (12).
The proximity of parasympathetic and sympathetic nerves to GCs underscores their critical role in regulating nasal secretion (33). In patients with AR, upregulation of M receptors in the nasal mucosal epithelium, vagus nerve, sensory nerve fibres, and lymphocytes facilitates acetylcholine signal transduction (34). Concurrently, a reduction in sympathetic α and β receptors leads to a neuroimmune imbalance, disrupting the typical functions of the sympathetic nervous system, which typically reduces secretions, and the parasympathetic nervous system, which promotes secretion. Parasympathetic neurons release acetylcholine, which acts directly on M3 receptors in GCs via the protein kinase C pathway, inducing MUC5AC secretion. Additionally, acetylcholine produced by tuft cells modulates epithelial fluid secretion (12, 35), highlighting the pivotal role of the cholinergic signal in mucus secretion. Moreover, clinical observations and studies have shown that a vidian neurectomy can disrupt the nerve–GC axis, demonstrating its potential for managing nasal hypersecretion in patients with AR (36).
Chemical sensation in the nasal mucosa is mediated by NANC neurons located near specialised chemosensory cells. When sensory nerve endings in the epithelium detect inhaled irritants, local or axonal motor neurotransmission through collateral ‘sensory-efferent’ pathways triggers sensory neuropeptide release. The peptides most relevant to airway mucus secretion include Substance P, neurokinin A, and CGRP. The sensory nerves and the released neurotransmitters and neuropeptides initiate GC degranulation (31). Elevated Substance P, VIP, and CGRP levels in the nasal secretions and tears of patients with AR, in conjunction with increased Substance P and VIP levels in the nasal cavity, positively correlate with visual analogue scale scores (37). Substance P, a member of the kininase family, binds to the neurokinin-1 receptor and induces airway mucus secretion (12). Animal studies have shown that Substance P released by airway sensory neurons following ovalbumin sensitisation directly promotes excessive MUC5AC secretion and GC proliferation (12). Additionally, allergens activate sensory nerve endings and amplify central nervous system signalling via CGRP, increasing the efficacy of efferent nerve terminals. This may explain why vidian neurectomies yield bilateral benefits over unilateral surgical procedures. Although GC hypertrophy was observed in an AR rat model of post-nasal neurectomy, nasal secretion was reduced due to the depletion of nerve fibres, acetyltransferase, and neuropeptides (e.g., Substance P and CGRP) in the nasal mucosa (38). Although neuropeptides are thought to promote GC secretion, the underlying mechanisms warrant further investigation.
Clinical manifestations indicate that temperature changes often exacerbate nasal secretions in patients with AR, highlighting the role of heat- and cold-sensitive TRP channels. Channel proteins are expressed in sensory neurons, epithelial cells, and immune cells (39).TRPV1 mRNA expression is significantly upregulated in the nerves of a mouse model of asthma. Similarly, patients with AR had higher numbers of TRPV1-positive cells in the nasal mucosa than healthy controls. Whereas MUC5AC and mucin 5B secretion are reduced in an asthma mouse model with TRPV1 knockout (8). TRPM8 can be activated by cold stimuli or menthol, directly triggering MUC5AC secretion in epithelial cells and releasing specific amines and peptides from stromal cells (14, 40). This process facilitates mucus secretion through a paracrine mechanism, creating a positive feedback loop. Specifically, TRPM8 activation by cold stimulation induces GC degranulation through the protein kinase C pathway and Ca2+ influx (14, 40, 41). TRP cation channel subfamily V member 4 indirectly regulates GC degranulation by sensing mucus viscosity and controlling ciliary beating (42). ATP binds to the purinergic receptor P2Y2, activating inositol trisphosphate receptors in the endoplasmic reticulum, which increases the cytosolic Ca2+ concentration, leading to extracellular mucin secretion (43). ATP activation also triggers TRP cation channel subfamily M member 4/5 channels to regulate Ca2+ and promote Na+ entry, causing Na+/Ca2+ exchangers to switch modes, allowing Na+ efflux and additional Ca2+ influx to induce mucus secretion (11).
TRPV1 activation promotes cation influx across the cell membrane and sensory nerve membrane depolarisation (44). This depolarisation is amplified by voltage-gated sodium channels, which generate action potentials. TRPV1 mRNA expression is significantly increased in the neurons of a mouse model of asthma, rendering TRPV1+ nerve fibres highly sensitive (45). These effects are driven by action potentials generated through TRPV1 activation and neuropeptide release (e.g., Substance P). Alterations in the expression and function of these channels can increase MUC5B secretion (46). Thus, TRPV and TRPM subfamilies represent potential therapeutic targets for controlling GC hypersecretion, particularly in sensory nerves and epithelial cells associated with airway mucus secretion. The specific details are illustrated in Figure 1B.
Dupilumab, a monoclonal antibody targeting IL-13Ra and IL-4Ra in clinical trials, inhibits GC metaplasia and excessive mucus secretion (47). The heat-shock protein 90 inhibitor geldanamycin reverses IL-13-induced airway GC metaplasia and improves airway remodelling (48). In human airway epithelial cells, the anticholinergic agent tiotropium attenuates IL-13-induced GC metaplasia (49). Additionally, tiotropium suppresses TRPV1 neuronal activity independently of M3 receptor blockade (49). TRPV1 agonists such as capsaicin can treat atopic rhinitis by leveraging the principle that the nasal mucosa enters a refractory period after stimulation; however, they have not significantly improved outcomes for patients with identified allergens (50). Furthermore, neuropeptides secreted by the sensory nerves and neuroendocrine cells, such as CGRP, Substance P, and GABA, can induce GC degranulation (12, 31, 51). Therefore, targeting neurotransmitter receptors in GCs is a promising therapeutic strategy. For example, stapled peptides can disrupt Ca2+ signalling and reduce stimulated mucin secretion (52). Although not yet explored in AR, stapled peptides represent an ‘ideal’ strategy for disrupting Ca2+ signalling and reducing mucin secretion.
In addition to targeting inflammatory mediators and signalling pathways, treatment methods should aim to reverse persistent GC metaplasia. This approach includes epigenetic editing to silence genes such as SPDEF. MicroRNAs, such as miR-141, miR-205-5p, miR-92a, and circZNF652, also target MUC5AC to alleviate mucus hypersecretion caused by allergic airway inflammation and reduce the nasal mucosal epithelial remodelling (21, 22, 53). Blocking GC metaplasia is crucial for promoting mucosal barrier restoration (23). The specific targets are listed in Table 2.
The neuroimmune regulatory mechanisms of GCs in other systems, such as the lower airways and intestines, have been extensively studied, including pharmacological interventions. However, the upper airways have not received adequate attention. Developing nasal sprays for delivering therapeutic agents, such as exosomes or capsaicin, or targeting genes in the nasal mucosa could be beneficial. For instance, mesenchymal stem cells overexpressing IL−10 significantly reduce the number of GCs in an allergic airway inflammation model (55). Additionally, GCs are poorly studied outside of their secretory role, and their associations with various diseases suggest the need to explore these extra functions further (56). The potential role of GCs in immune surveillance suggests that they could serve as new targets for treating allergic diseases.
The role of nerve–GC signalling requires consideration in airway allergic diseases. Increased nasal secretion in AR is closely associated with GC metaplasia and degranulation, orchestrated by a complex network of cytokines, neurotransmitters, neuropeptides, and their respective receptors, including TRP channels. However, the precise functional mechanisms remain unclear. Zhao (57) developed and validated a chemical approach to induce high-purity GCs. Their use in functional experiments on neuropeptides and their receptors will help elucidate specific signalling pathways. In addition, introducing a hydrocarbon-stapled peptide conjugated with cell-penetrating peptides into cultured human airway epithelial cells can inhibit stimulated secretion without affecting basal secretion (54). Further breakthroughs in AR could provide multidimensional approaches for treating mucus hypersecretion diseases, including asthma, chronic obstructive pulmonary disease, and cystic fibrosis.
Although the functions of GCs in the gut, conjunctiva, and airways are distinct under pathological conditions, they all imprint the central nervous system, which regulates peripheral diseases (58). For instance, similar to the storage of immune memory in the insular cortex following dextran sodium sulphate-induced colitis, DBH+ neurons in the solitary tract nucleus modulate airway hyperresponsiveness in asthma (59, 60). However, phenotypic changes in peripheral neurons during airway inflammation and their impact on GC function remain largely unknown. Thus, we introduced the nose–brain axis (61), building on pioneering research in related fields. Adaptations of the central nervous system in AR and their effects on GC metaplasia and degranulation are not fully understood. Shifting the research focus from peripheral to central mechanisms offers a transformative perspective on AR pathogenesis.
GCs are not merely mucus barriers but also immune barriers, acting as the first line of defence against airway mucosal immunity. Previous research has been limited to the immunological aspects; however, future basic and clinical studies should focus on the neuroimmunological regulation of GCs. Current treatments for airway hypersecretion primarily target immune factors such as IL-13. More in-depth research on GCs is urgently needed, and new treatment strategies specifically targeting GCs are essential.
XZ: Writing – original draft, Writing – review & editing. FC: Writing – original draft, Writing – review & editing. HD: Resources, Visualization, Writing – review & editing. SF: Visualization, Writing – review & editing. CZ: Writing – review & editing, Conceptualization, Funding acquisition.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was supported by the National Natural Science Foundation of China (grant numbers 81870707, 82171119, 82201263) and the National Key R&D Program of China (grant number 2023YFC2507900).
The authors thank Yanjie Wang, Xueping Qi, Luyao Wang, Haoxiang Zhang, Yanting Zhang for revising our manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declare that no Generative AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
AR, allergic rhinitis; CGRP, calcitonin gene-related peptide; FOXA2, forkhead box A2; GABA, gamma-aminobutyric acid; GC, goblet cell; MUC5AC, mucin 5AC; NANC, non-adrenergic non-cholinergic; SPDEF, SAM pointed domain containing ETS transcription factor; TRP, transient receptor potential; TRPM8, TRP melastatin subtype 8; VIP, vasoactive intestinal peptide.
1. Nyström EEL, Martinez-Abad B, Arike L, Birchenough GMH, Nonnecke EB, Castillo PA, et al. An intercrypt subpopulation of goblet cells is essential for colonic mucus barrier function. Science. (2021) 372(6539):eabb1590. doi: 10.1126/science.abb1590
2. Pelaseyed T, Bergstrom JH, Gustafsson JK, Ermund A, Birchenough GM, Schutte A, et al. The mucus and mucins of the goblet cells and enterocytes provide the first defence line of the gastrointestinal tract and interact with the immune system. Immunol Rev. (2014) 260:8–20. doi: 10.1111/imr.12182
3. Tu Y, Liu J, Li T, Zhou X, Tan KS, Ong HH, et al. Mucus composition abnormalities in sinonasal mucosa of chronic rhinosinusitis with and without nasal polyps. Inflammation. (2021) 44:1937–48. doi: 10.1007/s10753-021-01471-6
4. Subspecialty Group of Rhinology EBoCJoOH, Neck S, Subspecialty Group of Rhinology SoOH, Neck Surgery CMA. Chinese guideline for diagnosis and treatment of allergic rhinitis (2022, revision). Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. (2022) 57:106–29. doi: 10.3760/cma.j.cn115330-20211228-00828
5. Zeng W, Yang F, Shen WL, Zhan C, Zheng P, Hu J. Interactions between central nervous system and peripheral metabolic organs. Sci China Life Sci. (2022) 65:1929–58. doi: 10.1007/s11427-021-2103-5
6. Li Y, Liu S, Zhou K, Wang Y, Chen Y, Hu W, et al. Neuromedin U programs eosinophils to promote mucosal immunity of the small intestine. Science. (2023) 381:1189–96. doi: 10.1126/science.ade4177
7. Yang D, Jacobson A, Meerschaert KA, Sifakis JJ, Wu M, Chen X, et al. Nociceptor neurons direct goblet cells via a CGRP-RAMP1 axis to drive mucus production and gut barrier protection. Cell. (2022) 185:4190–205.e25. doi: 10.1016/j.cell.2022.09.024
8. Klimek L, Werminghaus P, Bergmann C, Hagemann J, Huppertz T, Barhold F, et al. Neuroimmunology of allergic rhinitis: Part 1: Cellular and humoral basic principles. HNO. (2023) 71:337–46. doi: 10.1007/s00106-023-01292-z
9. Jarret A, Jackson R, Duizer C, Healy ME, Zhao J, Rone JM, et al. Enteric nervous system-derived IL-18 orchestrates mucosal barrier immunity. Cell. (2020) 180:50–63 e12. doi: 10.1016/j.cell.2019.12.016
10. Billipp TE, Fung C, Webeck LM, Sargent DB, Gologorsky MB, Chen Z, et al. Tuft cell-derived acetylcholine promotes epithelial chloride secretion and intestinal helminth clearance. Immunity. (2024) 57:1243–59.e8. doi: 10.1016/j.immuni.2024.03.023
11. Cantero-Recasens G, Butnaru CM, Brouwers N, Mitrovic S, Valverde MA, Malhotra V. Sodium channel TRPM4 and sodium/calcium exchangers (NCX) cooperate in the control of Ca(2+)-induced mucin secretion from goblet cells. J Biol Chem. (2019) 294:816–26. doi: 10.1074/jbc.RA117.000848
12. Talbot S, Doyle B, Huang J, Wang JC, Ahmadi M, Roberson DP, et al. Vagal sensory neurons drive mucous cell metaplasia. J Allergy Clin Immunol. (2020) 145:1693–6 e4. doi: 10.1016/j.jaci.2020.01.003
13. Rogers DF. Motor control of airway goblet cells and glands. Respir Physiol. (2001) 125:129–44. doi: 10.1016/s0034-5687(00)00209-7
14. Baumlin N, Silswal N, Dennis JS, Niloy AJ, Kim MD, Salathe M. Nebulized menthol impairs mucociliary clearance via TRPM8 and MUC5AC/MUC5B in primary airway epithelial cells. Int J Mol Sci. (2023) 24(2):1694. doi: 10.3390/ijms24021694
15. Yu H, Li Q, Kolosov VP, Perelman JM, Zhou X. Interleukin-13 induces mucin 5AC production involving STAT6/SPDEF in human airway epithelial cells. Cell Commun Adhes. (2010) 17:83–92. doi: 10.3109/15419061.2010.551682
16. Zhao J, Minami Y, Etling E, Coleman JM, Lauder SN, Tyrrell V, et al. Preferential generation of 15-HETE-PE induced by IL-13 regulates goblet cell differentiation in human airway epithelial cells. Am J Respir Cell Mol Biol. (2017) 57:692–701. doi: 10.1165/rcmb.2017-0031OC
17. Turner J, Roger J, Fitau J, Combe D, Giddings J, Heeke GV, et al. Goblet cells are derived from a FOXJ1-expressing progenitor in a human airway epithelium. Am J Respir Cell Mol Biol. (2011) 44:276–84. doi: 10.1165/rcmb.2009-0304OC
18. Gregorieff A, Stange DE, Kujala P, Begthel H, van den Born M, Korving J, et al. The ets-domain transcription factor Spdef promotes maturation of goblet and paneth cells in the intestinal epithelium. Gastroenterology. (2009) 137:1333–45.e1-3. doi: 10.1053/j.gastro.2009.06.044
19. Jackson ND, Everman JL, Chioccioli M, Feriani L, Goldfarbmuren KC, Sajuthi SP, et al. Single-cell and population transcriptomics reveal pan-epithelial remodelling in type 2-high asthma. Cell Rep. (2020) 32(1):107872. doi: 10.1016/j.celrep.2020.107872
20. Rajavelu P, Chen G, Xu Y, Kitzmiller JA, Korfhagen TR, Whitsett JA. Airway epithelial SPDEF integrates goblet cell differentiation and pulmonary Th2 inflammation. J Clin Invest. (2015) 125:2021–31. doi: 10.1172/JCI79422
21. Wang X, Xu C, Cai Y, Zou X, Chao Y, Yan Z, et al. CircZNF652 promotes the goblet cell metaplasia by targeting the miR-452-5p/JAK2 signalling pathway in allergic airway epithelia. J Allergy Clin Immunol. (2022) 150:192–203. doi: 10.1016/j.jaci.2021.10.041
22. Siddiqui S, Johansson K, Joo A, Bonser LR, Koh KD, Le Tonqueze O, et al. Epithelial miR-141 regulates IL-13-induced airway mucus production. JCI Insight. (2021) 6:139019. doi: 10.1172/jci.insight.139019
23. Tyner JW, Kim EY, Ide K, Pelletier MR, Roswit WT, Morton JD, et al. Blocking airway mucous cell metaplasia by inhibiting EGFR antiapoptosis and IL-13 transdifferentiation signals. J Clin Invest. (2006) 116:309–21. doi: 10.1172/JCI25167
24. Shen H, Wei H, Jiang J, Yao H, Jia Y, Shen J, et al. Effects of 101BHG-D01, a novel M receptor antagonism, on allergic rhinitis in animal models and its mechanism. Eur J Pharmacol. (2023) 955:175902. doi: 10.1016/j.ejphar.2023.175902
25. Kanoh S, Tanabe T, Rubin BK. IL-13-induced MUC5AC production and goblet cell differentiation is steroid resistant in human airway cells. Clin Exp Allergy. (2011) 41:1747–56. doi: 10.1111/j.1365-2222.2011.03852.x
26. Barrios J, Patel KR, Aven L, Achey R, Minns MS, Lee Y, et al. Early life allergen-induced mucus overproduction requires augmented neural stimulation of pulmonary neuroendocrine cell secretion. FASEB J. (2017) 31:4117–28. doi: 10.1096/fj.201700115R
27. Feldman MB, Wood M, Lapey A, Mou H. SMAD signalling restricts mucous cell differentiation in human airway epithelium. Am J Respir Cell Mol Biol. (2019) 61:322–31. doi: 10.1165/rcmb.2018-0326OC
28. Barrios J, Kho AT, Aven L, Mitchel JA, Park JA, Randell SH, et al. Pulmonary neuroendocrine cells secrete gamma-aminobutyric acid to induce goblet cell hyperplasia in primate models. Am J Respir Cell Mol Biol. (2019) 60:687–94. doi: 10.1165/rcmb.2018-0179OC
29. Cheng FL, An YF, Xue JM, Wang YJ, Ding XW, Zhang YT, et al. Circadian rhythm disruption exacerbates Th2-like immune response in murine allergic airway inflammation. Int Forum Allergy Rhinol. (2022) 12:757–70. doi: 10.1002/alr.22914
30. Kim HK, Kim HJ, Kim JH, Kim TH, Lee SH. Asymmetric expression level of clock genes in left vs. right nasal mucosa in humans with and without allergies and in rats: Circadian characteristics and possible contribution to nasal cycle. PloS One. (2018) 13:e0194018. doi: 10.1371/journal.pone.0194018
31. Pavon-Romero GF, Serrano-Perez NH, Garcia-Sanchez L, Ramirez-Jimenez F, Teran LM. Neuroimmune pathophysiology in asthma. Front Cell Dev Biol. (2021) 9:663535. doi: 10.3389/fcell.2021.663535
32. Ha EV, Rogers DF. Novel therapies to inhibit mucus synthesis and secretion in airway hypersecretory diseases. Pharmacology. (2016) 97:84–100. doi: 10.1159/000442794
33. Specian RD, Neutra MR. Mechanism of rapid mucus secretion in goblet cells stimulated by acetylcholine. J Cell Biol. (1980) 85:626–40. doi: 10.1083/jcb.85.3.626
34. Ikeda K, Yokoi H, Saito T, Kawano K, Yao T, Furukawa M. Effect of resection of the posterior nasal nerve on functional and morphological changes in the inferior turbinate mucosa. Acta Otolaryngol. (2008) 128:1337–41. doi: 10.1080/00016480801935525
35. Billipp TE, Fung C, Webeck LM, Sargent DB, Gologorsky MB, McDaniel MM, et al. Tuft cell-derived acetylcholine regulates epithelial fluid secretion. bioRxiv. (2023) 03.17.533208. doi: 10.1101/2023.03.17.533208
36. Niu X, Chen Y, Zhou T, Xiao H. Endoscopic vidian and vidian-branch neurectomy for refractory allergic rhinitis: A systematic review. Int Forum Allergy Rhinol. (2024) 14:679–94. doi: 10.1002/alr.23259
37. Meng Y, Lu H, Wang C, Wang Y, Meng N, Yang K, et al. Naso-ocular neuropeptide interactions in allergic rhinoconjunctivitis, rhinitis, and conjunctivitis. World Allergy Organ J. (2021) 14:100540. doi: 10.1016/j.waojou.2021.100540
38. Nishijima H, Kondo K, Toma-Hirano M, Iwasaki S, Kikuta S, Fujimoto C, et al. Denervation of nasal mucosa induced by posterior nasal neurectomy suppresses nasal secretion, not hypersensitivity, in an allergic rhinitis rat model. Lab Invest. (2016) 96:981–93. doi: 10.1038/labinvest.2016.72
39. Banner KH, Igney F, Poll C. TRP channels: emerging targets for respiratory disease. Pharmacol Ther. (2011) 130:371–84. doi: 10.1016/j.pharmthera.2011.03.005
40. Li M, Li Q, Yang G, Kolosov VP, Perelman JM, Zhou XD. Cold temperature induces mucin hypersecretion from normal human bronchial epithelial cells in vitro through a transient receptor potential melastatin 8 (TRPM8)–mediated mechanism. J Allergy Clin Immunol. (2011) 128:626–34.e5. doi: 10.1016/j.jaci.2011.04.032
41. Azizli E, Dilber M. Do products containing menthol exacerbate allergic rhinitis? A narrative review. Eur Rev Med Pharmacol Sci. (2022) 26:61–4. doi: 10.26355/eurrev_202212_30484
42. Velasco E, Delicado-Miralles M, Hellings PW, Gallar J, Van Gerven L, Talavera K. Epithelial and sensory mechanisms of nasal hyperreactivity. Allergy. (2022) 77:1450–63. doi: 10.1111/all.15259
43. Kim K, Kim HJ, Binas B, Kang JH, Chung IY. Inflammatory mediators ATP and S100A12 activate the NLRP3 inflammasome to induce MUC5AC production in airway epithelial cells. Biochem Biophys Res Commun. (2018) 503:657–64. doi: 10.1016/j.bbrc.2018.06.057
44. Binshtok AM, Bean BP, Woolf CJ. Inhibition of nociceptors by TRPV1-mediated entry of impermeant sodium channel blockers. Nature. (2007) 449:607–10. doi: 10.1038/nature06191
45. Trankner D, Hahne N, Sugino K, Hoon MA, Zuker C. Population of sensory neurons essential for asthmatic hyperreactivity of inflamed airways. Proc Natl Acad Sci U.S.A. (2014) 111:11515–20. doi: 10.1073/pnas.1411032111
46. Alenmyr L, Herrmann A, Hogestatt ED, Greiff L, Zygmunt PM. TRPV1 and TRPA1 stimulation induces MUC5B secretion in the human nasal airway in vivo. Clin Physiol Funct Imaging. (2011) 31:435–44. doi: 10.1111/j.1475-097X.2011.01039.x
47. Wipperman MF, Gayvert KM, Atanasio A, Wang CQ, Corren J, Covarrubias A, et al. 410 Differential modulation of allergic rhinitis nasal transcriptome by dupilumab and allergy 411 immunotherapy. Allergy. (2024) 79:894–907. doi: 10.1111/all.16001
48. Pezzulo AA, Tudas RA, Stewart CG, Buonfiglio LGV, Lindsay BD, Taft PJ, et al. HSP90 inhibitor geldanamycin reverts IL-13- and IL-17-induced airway goblet cell metaplasia. J Clin Invest. (2019) 129:744–58. doi: 10.1172/JCI123524
49. Kistemaker LE, Hiemstra PS, Bos IS, Bouwman S, van den Berge M, Hylkema MN, et al. Tiotropium attenuates IL-13-induced goblet cell metaplasia of human airway epithelial cells. Thorax. (2015) 70:668–76. doi: 10.1136/thoraxjnl-2014-205731
50. Van Gerven L, Steelant B, Hellings PW. Nasal hyperreactivity in rhinitis: A diagnostic and therapeutic challenge. Allergy. (2018) 73:1784–91. doi: 10.1111/all.13453
51. Piao X, Jiang SH, Wang JN, Wu J, Xu WC, Li LQ, et al. Pingchuan formula attenuates airway mucus hypersecretion via regulation of the PNEC-GABA-IL13-Muc5ac axis in asthmatic mice. BioMed Pharmacother. (2021) 140:111746. doi: 10.1016/j.biopha.2021.111746
52. Lai Y, Fois G, Flores JR, Tuvim MJ, Zhou Q, Yang K, et al. Inhibition of calcium-triggered secretion by hydrocarbon-stapled peptides. Nature. (2022) 603:949–56. doi: 10.1038/s41586-022-04543-1
53. Dai J, Ma B, Wen X, Yang Z, Yue Y. Upregulation of miR-92a contributes to blocking goblet cell metaplasia by targeting MUC5AC in asthma. J Recept Signal Transduct Res. (2020) 40:613–9. doi: 10.1080/10799893.2020.1781172
54. Jaramillo AM, Piccotti L, Velasco WV, Delgado ASH, Azzegagh Z, Chung F, et al. Different Munc18 proteins mediate baseline and stimulated airway mucin secretion. JCI Insight. (2019) 4:4–124815. doi: 10.1172/jci.insight.124815
55. Kuang PP, Liu XQ, Li CG, He BX, Xie YC, Wu ZC, et al. Mesenchymal stem cells overexpressing interleukin-10 prevent allergic airway inflammation. Stem Cell Res Ther. (2023) 14:369. doi: 10.1186/s13287-023-03602-2
56. Tang M, Mei J, Sun M, Ma K, Zhao A, Fu X. An optimized method to visualize the goblet cell-associated antigen passages and identify goblet cells in the intestine, conjunctiva, and airway. Immunobiology. (2022) 227:152260. doi: 10.1016/j.imbio.2022.152260
57. Zhao A, Qin H, Sun M, Tang M, Mei J, Ma K, et al. Chemical conversion of human epidermal stem cells into intestinal goblet cells for modelling mucus-microbe interaction and therapy. Sci Adv. (2021) 7(16):eabb2213. doi: 10.1126/sciadv.abb2213
58. Kimura M, Ando T, Kume Y, Fukase S, Matsuzawa M, Kashiwagi K, et al. A nerve-goblet cell association promotes allergic conjunctivitis through rapid antigen passage. JCI Insight. (2023) 8(21):e168596. doi: 10.1172/jci.insight.168596
59. Koren T, Re Y, Amer M, Krot M, Boshnak N, Ben-Shaanan TL, et al. Insular cortex neurons encode and retrieve specific immune responses. Cell. (2021) 184:5902–15.e17. doi: 10.1016/j.cell.2021.10.013
60. Su Y, Xu J, Zhu Z, Chin J, Xu L, Yu H, et al. Brainstem Dbh(+) neurons control allergen-induced airway hyperreactivity. Nature. (2024) 631:601–9. doi: 10.1038/s41586-024-07608-5
Keywords: airway inflammation, allergic rhinitis, goblet cell, mucus secretion, metaplasia, neuroimmune regulation
Citation: Zhu X, Cheng F, Duan H, Fu S and Zhao C (2025) Novel insights into the study of goblet cell hypersecretion in allergic rhinitis. Front. Immunol. 16:1525928. doi: 10.3389/fimmu.2025.1525928
Received: 10 November 2024; Accepted: 06 January 2025;
Published: 31 January 2025.
Edited by:
Hiroshi Wakao, Dokkyo Medical University, JapanReviewed by:
Hontian Wang, Capital Medical University, ChinaCopyright © 2025 Zhu, Cheng, Duan, Fu and Zhao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Changqing Zhao, ZmFoeWpAMTI2LmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.