
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Immunol. , 14 February 2025
Sec. Microbial Immunology
Volume 16 - 2025 | https://doi.org/10.3389/fimmu.2025.1524563
This article is part of the Research Topic Immune Tolerance Dual Role: Advancements in Cancer and Autoimmune Diseases View all 7 articles
 Yanguang Yang1,2
Yanguang Yang1,2 Xinli Shi1,2*
Xinli Shi1,2*Hepatocellular carcinoma (HCC) is the most frequently occurring type of liver tumor and is considered one of the most common primary malignant neoplasms. The prognosis for HCC is dismal because of its complicated etiology and high level of medication resistance. Immunotherapy is presently regarded as one of the most effective therapeutic options for HCC; nevertheless, because of the disturbance of intestinal flora, immunotherapy shows low antitumor efficacy. An increasing body of research indicates that intestinal flora, particularly Akkermansia muciniphila (A. muciniphila), is vital for the treatment of tumors. Studies have demonstrated that the diminished effectiveness of immunotherapy in cancer patients is associated with a reduction in A. muciniphila levels, suggesting that increasing A. muciniphila levels significantly enhance the efficacy of immunotherapy. A. muciniphila functions as a gut probiotic and can treat and prevent a wide range of illnesses, including cancer. Consequently, preserving A. muciniphila abundance is enough to prevent and lower the danger of developing cancer disorders. In this review, we critically evaluate the current body of research on A. muciniphila, with a primary focus on its biological properties and functions. The different illnesses that A. muciniphila treats were then discussed, particularly the way it works with liver cancer. This review aims to give a novel treatment plan for patients with HCC as well as a theoretical foundation for improving HCC immunotherapy.
Liver cancer is the most prevalent type of primary tumor, ranking third in cancer mortality and sixth in incidence, with hepatocellular carcinoma (HCC) being its main type (1). Presently, hepatectomy is the main radical treatment for patients with early liver cancer, and radiofrequency ablation can be selected for patients who cannot be operated (2). Transcatheter arterial chemoembolization (TACE) is mainly used in patients with mid-term HCC (2). When TACE treatment fails in monkeys, systemic treatment will be recommended (3). Systemic treatment is suitable for most patients with HCC in intermediate and advanced stages. Current first-line treatment includes atezolizumab combined with bevacizumab, sorafenib, and lenvatinib (2). In recent years, the advent of immunotherapies, including immune checkpoint inhibitors (ICIs), has brought new hope to cancer patients, such as anti-programmed cell death 1 (PD-1)/L1 antibodies, anticytotoxic T lymphocyte-associated antigen-4 (CTLA-4) antibodies, and anti-lymphocyte-activating gene-3 (LAG3) antibodies (4–6). A study reported that the median overall survival (mOS) of atezolizumab combined with bevacizumab was longer than that of sorafenib (7). Although much work has been done to enhance responses and eliminate toxicities, the response of HCC patients to ICI remains unsatisfactory.
In recent years, there is growing evidence that there is a correlation between intestinal flora and the efficacy of tumor immunotherapy (5, 8). Studies have shown that after receiving ICI treatment, analysis of fecal samples from patients has found a decrease in the diversity and richness of intestinal flora, which is associated with low response rates and survival rates in patients (9, 10). For example, disorders of intestinal flora may promote tumor progression, through mechanisms including the production of toxic metabolites, induction of an inflammatory environment, and suppression of antitumor immunity (10). Thus, in mouse models of tumors, mice receiving ICI had reduced antitumor effects after antibiotic treatment (11). However, intestinal flora regulates the efficacy and toxicity of cancer immunotherapy, and the most researched is improving immunotherapy and its associated side effects (10). The most renowned study involved the transplantation of fecal matter from patients who had shown a positive response to immunotherapy into germ-free mice, revealing that these mice exhibited enhanced immunity, significantly inhibited tumor growth, and enhanced the effectiveness of anti-PD-L1 therapy (12, 13). Therefore, targeted regulation of intestinal flora is one of the effective ways to improve the efficacy of immunotherapy in tumors.
Akkermansia muciniphila (A. muciniphila), a bacterium that inhabits the intestinal mucus layer, is recognized as the most prevalent lytic bacteria in the intestines of healthy hosts and plays a crucial role in maintaining the intestinal barrier, promoting mucus production, and regulating mucus layer thickness (14, 15). The specific mechanism is that A. muciniphila reduces intestinal permeability, increases the expression of tight junction proteins, and increases the number of goblet cells that produce mucins (16–18). Furthermore, A. muciniphila has been implicated in the progression of various diseases, including inflammatory bowel diseases (19, 20), aging-related conditions (21), obesity (22, 23), type 2 diabetes (T2D) (24, 25), cardiovascular disorders (26, 27), non-alcoholic fatty liver disease (NAFLD) (28, 29), and cancers (11). Another study showed that whether A. muciniphila is live or pasteurized, oral administration is safe for human volunteers (30). In 2021, the European Food Safety Authority (EFSA) affirmed the safety of pasteurized A. muciniphila in adults and authorized its usage as a food supplement (31). Consequently, elucidating the molecular and immune response mechanisms of A. muciniphila in disease contexts, as well as determining safe supplementation strategies for A. muciniphila during disease states, is crucial for the exploration of innovative therapeutic approaches aimed at enhancing prevention and treatment outcomes.
This review seeks to clarify the connection between A. muciniphila and HCC, particularly in its role as an adjuvant in immunotherapy, while also elucidating the possible mechanisms behind the antitumor effects of A. muciniphila. Therefore, this review seeks to propose an innovative treatment strategy for patients with HCC while providing a theoretical framework to enhance immunotherapy approaches for HCC.
A. muciniphila, a common intestinal anaerobic bacterium, was first successfully isolated in 2004 (15). It is widely distributed in the intestines of humans and animals and has the largest number in the cecum, accounting for 3%–5% of the human microbiome (15, 32). A. muciniphila relies solely on mucin as its primary source of carbon, nitrogen, and energy, thereby supplying energy to epithelial cells (33). The main function of A. muciniphila involves the breakdown of mucus, facilitated by a range of mucolytic enzymes encoded in its genetic material (34, 35). In addition, A. muciniphila and its functional active components, including metabolites, metabolic enzymes, and outer membrane proteins, effectively improve intestinal barrier function by regulating metabolism and enhancing tight junctions (36–38). Interestingly, A. muciniphila commenced colonization and exhibited rapid growth and enrichment in the human intestines during infancy (39). However, its abundance progressively declined with aging and the progression of disease (39, 40). Therefore, A. muciniphila supplementation has demonstrated therapeutic benefits for a range of diseases, including liver diseases, colitis, obesity, and diabetes (40). At present, there are many traditional Chinese medicine formulas, individual Chinese medicinal materials, and extracts to increase the number of A. muciniphila, including Si-Ni-San (41), Liangxue guyuan yishen decoction (42), wolfberry (43), berberine (44), and Dendrobium officinale polysaccharide (45). In summary, increasing the abundance of A. muciniphila has become one of the effective treatment measures for many diseases.
Akkermansia muciniphila is classified within the Akkermansia genus of the Verrucomicrobia (15, 34). Moreover, in addition to A. muciniphila, this study also includes other species within the genus Akkermansia, such as massiliensis (46), biwaensis (47), ignis (48), durhamii, and others with less precise descriptions (49). This bacterium is characterized as gram-negative, non-motile, and non-spore-forming, and the long axis of the cell changes with the medium. In the mucin medium, the strain exhibits a diameter of 640 nm and a length of 690 nm; however, in brain–heart infusion (BHI), the diameter increases to 830 nm with a corresponding length of 1 μm (15). It predominantly exists as individual cells or in pairs, with chain formation being a rare occurrence (15). Interestingly, the strictly anaerobic A. muciniphila demonstrates a notable capacity for survival when subjected to atmospheric conditions. After 24 h in a standard air environment, its survival rate is observed to be 25%, while after 48 h, this rate declines significantly to merely 1% (33). Studies have demonstrated that A. muciniphila can be cultured in various media, including BHI, porcine mucin, human mucin combined with BHI medium, Colombia medium, and tryptic soy broth (15, 50–52). The medium utilized by our research group comprised 2.45 g of BHI, 0.3 g of mucin, 100 ml of a 0.3% L-cysteine solution, and autoclaved distilled water, which were mixed uniformly (43). Furthermore, A. muciniphila is cultivated at temperatures ranging from 20°C to 40°C and a pH of 5.5 to 8.0, with optimal growth conditions identified as 37°C and a pH of 6.5 (15). Notably, the bacterial growth is inhibited when the pH falls below 5.5 or exceeds 8.0. In the agar base medium, the A. muciniphila colony displayed a circular morphology, appeared white in color, and had a diameter of 0.7 mm (15).
A. muciniphila is recognized as one of the most prevalent bacterial species, comprising over 1% of the overall fecal microbiota (53). Fluctuations in its abundance are closely associated with various factors including age, diet, body weight, disease states, and immune status (54). Similar to other intestinal bacteria, A. muciniphila predominantly colonizes the outer layer of intestinal mucus; its development is maintained by carbon and nitrogen produced by goblet cells to maintain the dynamic balance of the mucus layer (55–57). A study published in 2007 revealed that A. muciniphila is capable of colonizing the intestines of infants early in life, achieving levels comparable to those found in adults within 1 year, and subsequently experiencing a gradual decline in abundance among the elderly population (39). Interestingly, recent studies have revealed that A. muciniphila is not only present in the human intestine but is also detected in the nasopharynx and breast milk, where it utilizes human milk oligosaccharides as a carbon source (58–60). This finding offers robust evidence supporting the early colonization of A. muciniphila within the intestinal microbiota. Furthermore, A. muciniphila is not only colonized in the human intestines but is also present in the intestinal microbiota of various other animals, including rats (61), mice (62), horses (63), rex rabbits (64), guinea pigs (65), calves (66), rock ptarmigans (67), zebrafish (68), and Burmese pythons (69). These findings demonstrate the extensive colonization of A. muciniphila across various animal models and provide foundational research to support its applications.
The initial gene sequencing of A. muciniphila ATCC BAA-835 was accomplished in 2011, revealing a complete genome comprising a circular chromosome of 2,664,102 bp and encompassing a total of 2,176 predicted protein-coding sequences, which collectively exhibit an overall coding capacity of 88.8% (35). Twenty-two A. muciniphila species were isolated from the feces of people in southern China, which had more than 99% consistency with strain ATCC BAA-835, and 12 subtypes were distinguished by enterobacterial repetitive intergenic consensus (ERIC-PCR) (70). Additionally, A. muciniphila in the human gut utilized over 2,000 genomes from humans and various other animals for a comprehensive population genomic analysis, resulting in the delineation of five distinct candidate species (71). In 2017, a total of 39 A. muciniphila samples derived from human and mice feces were sequenced to elucidate the genomic structure, leading to the identification of three distinct species-level A. muciniphila populations (AmI, AmII, and AmIII) through phylogenetic analysis (72). Furthermore, through an integrative approach combining genomic, molecular biology, and traditional microbiological methods, A. muciniphila has identified at least four species-level groups within its systems (AmI to AmIV) exhibiting different functional characteristics (73). Subsequent investigations indicated that the AmII strain possessed a gene responsible for vitamin B12 synthesis. In the absence of vitamin B12, the AmII strain was able to produce acetate and propionate, whereas the AmI strain produced acetate and succinate. Interestingly, the abundance of AmI strains was greatest among children and adolescents; however, antibiotic-treated mice exhibited a higher quantity of AmII and AmIV strains compared to AmI strains (74).
In the contemporary research landscape, an escalating volume of investigations has been centered on A. muciniphila and its specific derivatives, encompassing pasteurized A. muciniphila, the Amuc series, extracellular vesicles (AmEVs), short-chain fatty acids (SCFAs), and metabolic enzymes (Figure 1).
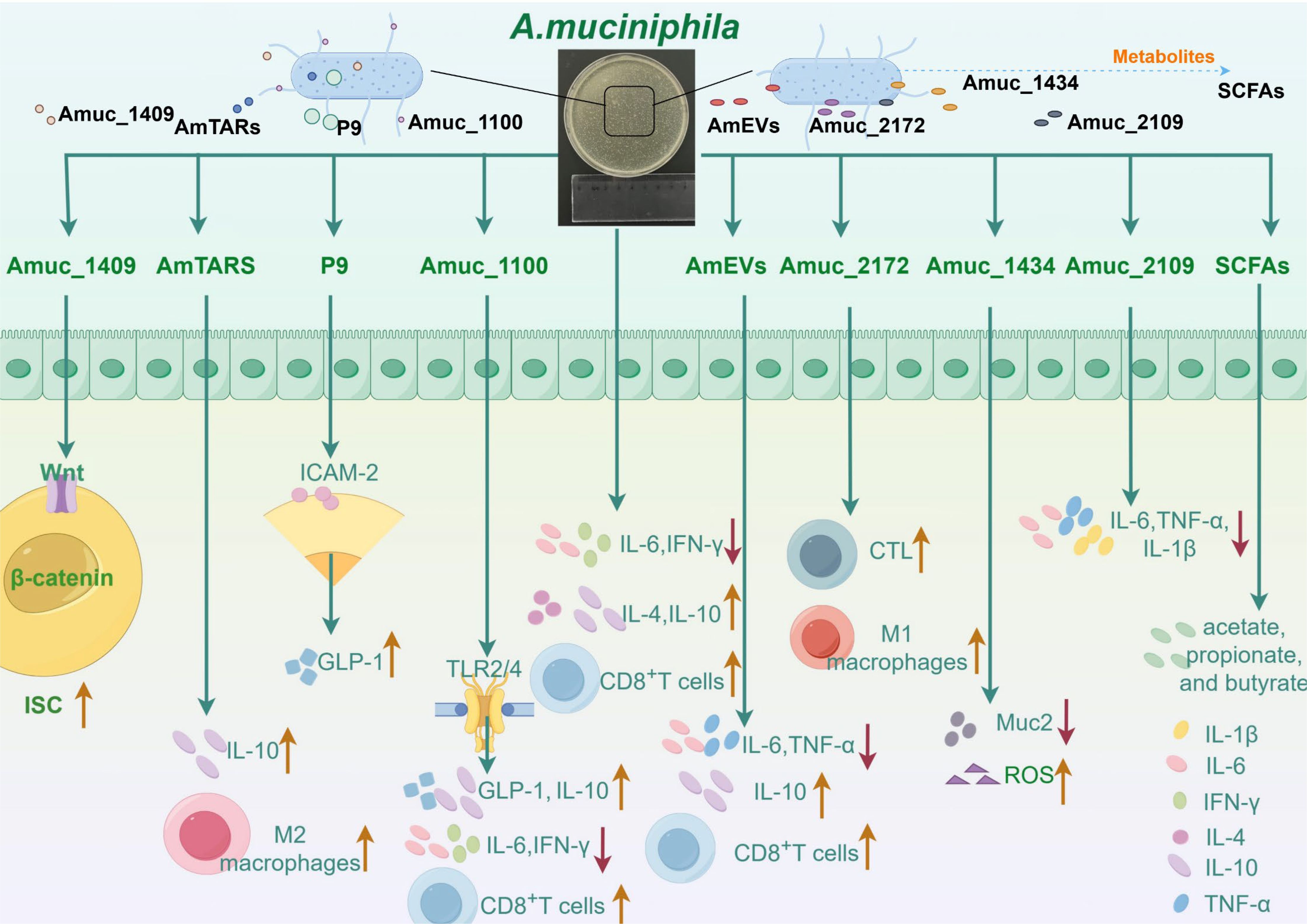
Figure 1. The ways in which Akkermansia muciniphila and its active proteins interact with the immune system are complex and varied. Akkermansia muciniphila has been shown to reduce the levels of interleukin-6 (IL-6) and interferon-γ (IFN-γ) in mice with abdominal aortic aneurysms. Moreover, A. muciniphila simultaneously increased the concentrations of interleukin-4 (IL-4) and interleukin-10 (IL-10), thereby enhancing the capacity of A549 and NCI-H1395 cells to recruit CD8+ T cells. Amuc_1100 mitigates inflammatory responses by decreasing IFN-γ and IL-6 levels in both the spleen and pancreas. Furthermore, Amuc_1100 interacts with Toll-like receptor 2 (TLR2) and modestly promotes glucagon-like peptide-1 (GLP-1) production by L cells, thus reinforcing gut barrier function. It also plays a role in the host intestinal immune response through activation of TLR2 and TLR4, leading to increased IL-10 production. Additionally, Amuc_1100 exhibits potent antitumor effects by augmenting both the quantity and functionality of CD8+ T cells. Studies suggest that P9 interacts with intercellular adhesion molecule 2 (ICAM-2), leading to the activation of the glucagon-like peptide-1 receptor (GLP-1R) pathway, which in turn enhances GLP-1 production. Moreover, AmTARS facilitates M2 macrophage polarization while specifically interacting with TLR2 to induce IL-10 production. Moreover, Amuc_1409 regulates intestinal stem cell (ISC) activity via Wnt/β-catenin signal transduction pathways. Extracellular vesicles derived from A. muciniphila (AmEVs) significantly lower serum TNF-α and IL-6 levels while elevating IL-10 concentrations, thereby ameliorating liver damage and fibrosis in murine models with hepatic injury. Studies have demonstrated that these extracellular vesicles can serve as an immunotherapeutic agent against prostate cancer progression primarily through activating effector states in CD8+ T cells alongside promoting macrophage polarization toward an M1 phenotype. Furthermore, Amuc_2172 enhances transcriptional expression as well as secretion levels of heat shock protein 70 (HSP70), subsequently increasing CD8 cytotoxic T lymphocyte (CTL) numbers and activities within colorectal cancer contexts. In another investigation, it was revealed that Amuc_2172 induces M1 macrophage polarization; however, this effect is reversed by puerarin treatment which improves ulcerative colitis outcomes. Lastly, Amuc_1434 promotes apoptosis along with degradation processes involving mucin 2 (Muc2) within LS174T cells while inducing intracellular reactive oxygen species (ROS). Recent findings suggest that Amuc_2109 reduces TNF-α, IL-1β, and IL-6 levels alongside downregulating NLR family pyrin domain 3 (NLRP3) expression while enhancing gut barrier integrity—thereby improving DSS-induced colitis symptoms. Short-chain fatty acids (SCFAs)—the primary metabolites produced by A. muciniphila—are predominantly composed of acetate, propionate, and butyrate.
Current research indicates that live A. muciniphila modulates the intestinal immune response and fortifies intestinal barrier function, participating in the immune system and enhancing the intestinal barrier through the production of SCFAs and acetate (16, 75). Furthermore, due to its capacity to degrade intestinal mucus, A. muciniphila may exert an indirect influence on both innate and adaptive immune responses (76). A. muciniphila is frequently correlated with inflammation-related cytokines. Studies reported that during liver injury treatment, A. muciniphila combined with inosine increased the number of regulatory T (Treg) but reduced T helper (Th) 1 (77). During lactation, A. muciniphila levels in breast milk are positively correlated with TNF-α and IFN-γ levels but negatively correlated with interleukin-10 (IL-10) and interleukin-4 (IL-4) levels (59). In contrast, A. muciniphila was observed to decrease the expression of interleukin-33 (IL-33) and the levels of interleukin-6 (IL-6) and IFN-γ in mice with abdominal aortic aneurysm, while simultaneously increasing the concentrations of IL-4 and IL-10 (26). Additionally, our previous research demonstrated that during dihydroartemisinin (DHA) treatment, A. muciniphila increased the population of CD8+ T cells in both the spleen and tumor niche of liver cancer model mice (78). Similarly, A. muciniphila enhances the capacity of A549 and NCI-H1395 cells to recruit CD8+ T cells and augments the cytotoxicity of CD8+ T cells (79). Interestingly, in Lewis lung cancer mouse models treated with anti-PD-1 therapy, A. muciniphila activates the MHC-II–pDC pathway, reduces the expression of CXCL3 in cancer-associated fibroblasts, stimulates CD8+ T-cell activation, and enhances their functionality compared to pasteurized A. muciniphila and Amuc_1100 (80). The combination of A. muciniphila and cisplatin inhibits the growth of lung cancer in mice, with the underlying mechanism involving an increase in serum expression of IFN-γ, IL-6, and TNF-α and a reduction in the number of Treg cells (81). In another study, A. muciniphila improved the inflammation of chronic colitis induced by dextran sulfate sodium (DSS) in mice but did not increase the ability of Treg cells to differentiate from the CD4+ T-cell population (82). Treatment with A. muciniphila alleviates liver tissue damage by upregulating serotonin (5-HT) expression and enhances cognitive function through the upregulation of brain-derived neurotrophic factor (BDNF) expression in the gut–liver–brain axis (83). Currently, A. muciniphila supplementation has been established as a novel therapeutic strategy across multiple disease models and is considered safe for oral administration in humans, thereby earning recognition as a new generation of probiotic bacteria.
Additionally, the EFSA has verified the safety of pasteurized A. muciniphila as a new food item. Numerous studies indicate that live A. muciniphila exhibits comparable therapeutic effects to those of pasteurized A. muciniphila, with some evidence suggesting that the latter may even demonstrate superior efficacy (84–87). For instance, both pasteurized A. muciniphila and live A. muciniphila restore damaged intestinal structure, improve liver function, and reduce the number of Treg cells in the spleen, but pasteurized A. muciniphila is more effective at improving glucose tolerance (87). Pasteurized A. muciniphila significantly reduced fat accumulation and dyslipidemia, thereby ameliorating obesity-related comorbidities (30). Additionally, another study demonstrated that pasteurized A. muciniphila enhances GLP-1 production to improve glucose homeostasis and alleviate symptoms associated with T2D in mice (24). Interestingly, pasteurized A. muciniphila improves colitis-related tumorigenesis by regulating CD8 cytotoxic T lymphocytes (CTLs) (88). A recent report showed that pasteurized A. muciniphila improved symptoms associated with irritable bowel syndrome in mice by reducing colon hypersensitivity reactions while enhancing intestinal barrier function (89). These lines of evidence suggest that pasteurized A. muciniphila also has a therapeutic effect on some diseases and is hopeful of clinical application.
Amuc_1100, a particular outer membrane protein extracted from A. muciniphila, was first isolated and characterized in 2017 (30). It has been demonstrated to interact with Toll-like receptor 2 (TLR2) and slightly promote glucagon-like peptide-1 (GLP-1) production via L cells, thereby enhancing intestinal microecology and improving metabolic outcomes in murine models of obesity and diabetes (30, 90). Furthermore, Amuc_1100 participates in the host intestinal immune response by activating TLR2 and TLR4 to produce IL-10 (91). Amuc_1100 demonstrates an antitumor effect in lung adenocarcinoma by inhibiting the JAK/STAT signaling pathway and enhancing both the quantity and activity of CD8+ T cells (79). Both A. muciniphila and Amuc_1100 ameliorate colitis by diminishing the infiltration of macrophages and CTLs in the colon; however, they also impede tumor progression by enhancing the population of CTLs within this tissue (88). Simultaneously, A. muciniphila and Amuc_1100 downregulate the expression of nodule receptor protein 3 (NLRP3) and TLR4/nuclear factor κB (NF-κB), inhibiting the production of inflammatory cytokines and thereby improving NAFLD (28). Furthermore, Amuc_1100 pretreatment attenuates the inflammatory response of acute pancreatitis by inhibiting the NF-κB signaling pathway, reducing the levels of proinflammatory cytokines, including TNF-α, IL-1β, IFN-γ, and IL-6, in both the spleen and pancreas, and decreasing the inflammatory infiltration of Ly6C+ macrophages and neutrophils within the spleen (92). Moreover, Amuc_1100 has been demonstrated to promote the proliferation of anti-inflammatory M2 macrophages while reducing the number of proinflammatory M1 macrophages in periodontitis, particularly by increasing IL-10 levels and decreasing TNF-α levels (93). Interestingly, a nanodrug formulation containing Amuc_1100 has been demonstrated to mitigate doxorubicin-induced cardiotoxicity by enhancing the intestinal barrier function, enhancing the synthesis of butyric and valeric acids, and ultimately regulating lymphocyte homeostasis in both the spleen and heart (94). Therefore, Amuc_1100 could become a new option for metabolic diseases, inflammation-related diseases, and immunotherapy. Furthermore, in 2022, research demonstrated that a phospholipid derived from the cell membranes of A. muciniphila exhibits immunomodulatory properties and facilitates the release of TNF-α and IL-6 (95). These findings are solely derived from in-vitro trials, and the outcomes of in-vivo research remain ambiguous; therefore, an in-depth investigation is required to verify these findings.
P9, an 84-kDa protein, was initially identified as being released through A. muciniphila, which significantly stimulates GLP-1 production and enhances thermogenesis in brown adipose tissue, thereby ameliorating glucose regulation in mice fed a high-fat diet (HFD) (96). Moreover, the underlying mechanism involves the binding of P9 to intercellular adhesion molecule 2 (ICAM-2), leading to the activation of the glucagon-like peptide-1 receptor (GLP-1R) pathway and IL-6. In a distinct study, recombinant Lactococcus lactis NZP9 was shown to secrete P9 heterologously, thereby stimulating NCI-H716 cells to produce GLP-1 as well (97). Furthermore, threonyl-tRNA synthetase (AmTARS) derived from A. muciniphila serves an essential function in monitoring and regulating immune response. AmTARS facilitates the polarization of M2 macrophages and engages in specific interactions with TLR2 (98). This interaction focuses on cAMP response element-binding (CREB), ultimately resulting in the production of IL-10. Concurrently, AmTARS promotes the restoration of IL-10-positive macrophages, elevates serum IL-10 levels, and mitigates inflammation in colitis mice (98). In a related study published this year, it was demonstrated that the protein Amuc_1409, secreted by A. muciniphila, serves an essential function in enhancing intestinal health through the modulation of intestinal stem cell (ISC) activity (52). Mechanistically, Amuc_1409 promotes the dissociation of the E-cadherin/β-catenin complex via its interaction with E-cadherin, thereby activating Wnt/β-catenin signaling and regulating ISC activity (52). Notably, Amuc_1409 significantly increases ISC populations across diverse experimental models, including isolated intestinal organoids and instances of chemically induced intestinal damage as well as natural aging in male mice.
AmEVs, another component of A. muciniphila, demonstrate decreased levels in T2D patients and DSS mice models (99). Furthermore, in HFD-induced diabetic mice, AmEVs enhanced intestinal tight junction function and reduced the intestinal permeability of lipopolysaccharide (LPS)-treated Caco-2 cells (99). Similarly, AmEVs restored the gut microbiota of HFD/carbon tetrachloride (CCL4) mice to levels comparable to those of normal mice, improved intestinal permeability, reduced serum levels of TNF-α and IL-6, and elevated IL-10, thereby ameliorating liver damage and fibrosis in HFD/CCL4 mice (100). AmEVs can function as immunotherapeutics to impede the progression of prostate cancer, primarily by activating the effector state of CD8+ T cells and facilitating the polarization of macrophages toward the M1 phenotype (101). AmEVs have also demonstrated protective effects against hypertension; specifically, they enhance the expression of genes involved in vasoconstriction and vasodilation within the kidney, while simultaneously increasing the number of Treg cells and tending to reduce the production of proinflammatory cytokines (102, 103). On the other hand, AmEVs have also demonstrated potential for treating preeclampsia (PE) through AmEV transfer from the gastrointestinal tract to the placenta (104). Molecular investigations indicate that the therapeutic efficacy of AmEVs in managing PE is mediated through activation of the epidermal growth factor receptor (EGFR)-phosphoinositide 3-kinases (PI3K)/protein kinase B (AKT) signaling, thereby regulating trophoblast invasion into spiral arteries (SpA). Moreover, Amuc_2172, a recently identified probiotic enzyme derived from A. muciniphila, is present in AmEVs secreted by this bacterium. Research has demonstrated that Amuc_2172 functions as an acetyltransferase on Lys14 of histone H3 (H3K14ac), thereby enhancing the transcription and secretion of heat shock protein 70 (HSP70) and subsequently increasing both the quantity and activity of CTLs in colorectal cancer (CRC) (105). Finally, the researchers developed a bioengineering method to prepare macrophage membrane-encapsulated coated Amuc_2172 (CoAmuc_2172), which was proven to be a safe and reliable CRC treatment strategy, improving the clinical transformation value of the research. In another study, Amuc_2172 was shown to induce polarization in M1 macrophages, but puerarin reversed this change, thereby improving ulcerative colitis (106). Similarly, Amuc_1434, which is also derived from AmEVs, has been reported to enhance the adhesion of the colon cancer cell line LS174T that expresses high levels of mucin 2 (Muc2) and also facilitated the degradation of Muc2 in colon cancer cells (107). Subsequently, in-depth studies demonstrated that Amuc_1434 inhibits the viability of LS174T cells by the apoptotic pathway (108). The specific mechanism is that Amuc_1434 inhibited the proliferation and blocked the cell cycle of LS174T cells, promoted the apoptosis of CRC cell LS174T cells, and also induced the production of intracellular reactive oxygen species (ROS), damaged the mitochondrial membrane potential, and caused mitochondrial dysfunction of LS174T cells (108). Although AmEVs have demonstrated significant therapeutic potential for hypertension, gastrointestinal disorders, and tumor immunotherapy, its cultivation remains constrained as a derivative of A. muciniphila, with the inability to scale up production being a primary factor that impedes its development.
Amuc_2109, a β-N-acetylhexosaminidase, is classified within the GH family 3 enzymes, secreted via A. muciniphila. Furthermore, Amuc_2109 exhibits enzymatic activity at pH levels above 4.0 and temperatures ranging from 20°C to 80°C, demonstrating its high survival rate in the intestinal environment (109). A recent study demonstrated that Amuc_2109 reduces the levels of TNF-α, IL-1β, and IL-6, as well as NLRP3 expression, while enhancing intestinal barrier function and improving DSS-induced colitis (110). SCFAs are the primary intestinal metabolites of A. muciniphila, with acetate, propionate, and butyrate as its principal components (111). SCFAs have been demonstrated to alleviate intestinal inflammation and cognitive dysfunction (112). A. muciniphila elevates serum levels of acetate and butyrate in sleep-deprived mice, while a mixture of SCFAs containing acetate and butyrate inhibits microglial synaptic phagocytosis and neuronal synapse loss, thereby ameliorating cognitive dysfunction induced by sleep deprivation (113). In addition, recent studies have shown that acetic acid derived from A. muciniphila plays an anti-aging role not only in Caenorhabditis elegans but also in naturally aging mice (114). On the other hand, studies have shown that acetic acid supports the growth and proliferation of tumor cells, leads to reduced infiltration of CD8+ T cells in tumor tissues, and promotes immune escape of tumor cells (115). Fucose fermentation enhances the production and metabolism of propionic acid in A. muciniphila, thereby facilitating the intestinal stem cell-mediated development of the intestinal epithelium (116). Other studies have shown that propionic acid plays an anti-inflammatory role and enhances intestinal barrier function by binding to the G-protein-coupled receptor 43 (GPR43) receptor (117, 118). Furthermore, the supplementation of butyric acid significantly mitigates pathological damage to lung tissue and improves the inflammatory response in mice with acute lung injury by restoring intestinal butyric acid production (119). Notably, A. muciniphila possesses the capability to synthesize γ-aminobutyric acid (GABA) in acidic environments, primarily through its genome encoding glutamate decarboxylase (120). In summary, the identification of these metabolites from A. muciniphila clarifies the relationship between A. muciniphila and health, elucidates the molecular mechanisms underlying its effects, and serves as a reference for future investigations into other probiotics.
Traditional Chinese medicine is abundant in natural bioactive compounds and demonstrates remarkable therapeutic efficacy by holistically regulating the homeostasis of various organs within the body (Table 1). Notably, numerous studies have indicated that traditional Chinese medicine can effectively restore intestinal barrier function and maintain microecological balance, mainly by growing the levels of A. muciniphila (121–123). Our prior research has shown that the number of A. muciniphila can be enhanced by 4.1-fold when cultured in a wolfberry decoction, and in-vivo experiments have further certified that wolfberry supplementation increases the abundance of A. muciniphila in both male and female mice (43). Further investigations demonstrated that a synergistic combination of wolfberry, yams, and chrysanthemums markedly increased the levels of A. muciniphila both in vivo and in vitro, with their polysaccharide mixtures displaying a similar effect (124). Furthermore, polysaccharides derived from D. officinale, Atractylodes chinensis, and Poria cocos promote the proliferation of intestinal microorganisms, including Akkermansia, Lactobacillus, and Bifidobacterium (45, 125, 126). In a mouse model of drug-induced liver injury, we similarly demonstrated that wolfberry significantly augmented the abundance of A. muciniphila by approximately twofold (127). Recent studies further demonstrated that wolfberry, Platycodon grandiflorus root extract, and Polygonatum sibiricum rhizome aqueous extracts enhance intestinal barrier function by elevating the abundance of A. muciniphila in HFD rats (128–130). Hypericum perforatum L. enhances the gut microbiome by elevating the levels of A. muciniphila, thereby contributing to an improvement in depressive symptoms (131). Interestingly, berberine is a promising prebiotic that indirectly elevates A. muciniphila levels by stimulating mucin secretion in the gut (44). Furthermore, traditional Chinese medicine prescriptions also address diseases associated with disruptions in intestinal homeostasis by enhancing the abundance of A. muciniphila (42, 132–135). Research has demonstrated that both Jinqi Jiangtang tablets and Shouhuitongbian can ameliorate T2D by enhancing the population of beneficial bacteria, particularly A. muciniphila (134, 135). Furthermore, the synergistic effect of Astragalus membranaceus and Salvia miltiorrhiza enhanced the number of A. muciniphila and improved diabetic nephropathy (122). Zexie-Baizhu decoction boosts the number of beneficial bacteria, especially A. muciniphila; regulates the composition of intestinal flora; and improves NAFLD (133). Shuangshen granules also increase the number of beneficial bacteria in the intestine, mainly A. muciniphila and Limosilactobacillus reuteri, while inhibiting the NF-κB pathway and thus inhibiting lung metastasis (132). The predominant dosage forms of traditional Chinese medicine are primarily oral. Upon entering the intestine, the active components of traditional Chinese medicine effectively interact with intestinal flora, which in turn influences the metabolic transformation of these compounds. This interaction may enhance the absorption of active ingredients, improve bioavailability, bolster intestinal barrier function, promote probiotic proliferation, and inhibit the growth of pathogenic bacteria. These mechanisms may constitute the primary pathways through which traditional Chinese medicine exerts its therapeutic effects. These results indicate the promise of traditional Chinese medicine as a prebiotic and provide new perspectives on its molecular mechanisms. In addition to the aforementioned factors, dietary interventions can also enhance the levels of Akkermansia, including dietary polyphenols and FODMAP (136). FODMAP refers to fermentable oligosaccharides, disaccharides, monosaccharides, and polyols, which are small carbohydrates that many people cannot digest. A randomized controlled trial demonstrated that oats possess prebiotic potential, significantly increasing the abundance of A. muciniphila, Dialister, Butyrivibrio, and Paraprevotella while concurrently reducing total cholesterol levels (137). Furthermore, a diverse array of compounds has been shown to significantly promote the growth of A. muciniphila in axenic culture, including metformin (138), tryptophan (139), fructo-oligosaccharides (22, 140), and betaine (141). Interestingly, the study demonstrated that the combination of probiotics Lactobacillus rhamnosus (L. rhamnosus) LMG S-28148 and Bifidobacterium animalis (B. animalis) LMG P-28149 significantly enhanced the abundance of A. muciniphila, likely attributable primarily to the influence of the latter (142). In contrast, the administration of Bifidobacterium bifidum G9-1 inhibited the growth of A. muciniphila, thus preventing the reduction of jejunal goblet cells in a mouse model of small intestinal loss (143). Noteworthy, broad-spectrum antibiotic treatment also increases the amount of A. muciniphila in the gut, especially vancomycin (144–146). While these findings collectively demonstrate an enhancement in the numbers of A. muciniphila, the underlying mechanisms of action differ among them, necessitating further investigation to determine any potential commonalities.
Intestinal flora constitutes a crucial component of the human intestinal microbial ecosystem and serves an essential function in liver physiology and pathology via the gut–liver axis (147). Numerous studies have shown that intestinal flora facilitates the onset and progression of HCC by influencing intestinal barrier function, exacerbating liver inflammation, and undermining antitumor responses. The primary factors implicated in this process include dysbiosis, increased intestinal permeability, microbe-associated molecular patterns (MAMPs), and their metabolites (147, 148). Therefore, dysregulation of the intestinal microbiota is a key factor in promoting the progression of HCC.
Intestinal permeability is compromised at all stages of HCC development, allowing MAMPs to translocate from the intestine into the liver via the portal vein. This process promotes the onset and progression of HCC through signaling pathways mediated by receptors on hepatocyte surfaces (149). Furthermore, as HCC progresses, the biosynthetic pathways of LPS are upregulated, leading to a gradual increase in serum LPS levels during liver injury, which correlates with the Th1/Th17 proinflammatory phenotype (150). LPS, a common proinflammatory MAMP, constitutes the principal element of the outer membrane. LPS translocates into the bloodstream as a result of alterations in intestinal permeability, subsequently binding to TLR4 on hepatocyte surfaces. This interaction triggers the production of numerous inflammatory cytokines, thereby exacerbating the occurrence of liver cancer and facilitating hepatic cell regeneration (151–153). Studies found that high levels of LPS were detected in the peripheral blood of mice with N-nitrosodiethylamine (DEN)-induced HCC, and long-term application of low-toxic doses of LPS increased the volume and number of tumors in HCC (154). In another study, serum endotoxin levels were used as a relevant diagnostic biomarker for NAFLD and were used to detect and determine disease stage (155). The use of antibiotic regimens to reduce LPS or knock out the TLR4 gene on hepatocytes in animal models of HCC to inhibit HCC growth confirmed the important role of the LPS–TLR4 axis in the occurrence and development of HCC (154). Metagenomic sequencing has elucidated alterations in the intestinal microbiota of patients with chronic liver disease and HCC, characterized by an addition in pathogenic bacteria and a reduction in beneficial bacterial populations (156, 157). For instance, among patients with liver cirrhosis, there was a notable rise in the abundance of Enterobacter within pathogenic taxa, while the numbers of Lactobacillus and Akkermansia, among beneficial taxa, were diminished (158, 159). Similarly, the abundance of Phascolarctobcterium, Gemella, Enterococcus, and Streptococcus increased in the intestinal flora of patients with HCC, and the levels of Collinsella and Akkermansia reduced (159). A separate study demonstrated that A. muciniphila was significantly diminished in both patients with non-alcoholic steatohepatitis (NASH)-associated HCC and in murine models (160). However, increased abundance of A. muciniphila has been reported in patients with esophageal adenocarcinoma and CRC (161–163). The level of A. muciniphila in tumor patients changes depending on the tumor site, but this does not affect the beneficial bacterial status of A. muciniphila. Supplementing A. muciniphila still inhibits tumor growth in HCC and CRC. In HCC induced by NASH, the proliferation of Clostridium in the intestine results in an increased synthesis of the bacterial metabolite deoxycholic acid (DCA) in the bloodstream. DCA primarily induces the production of the senescence-associated secretory phenotype (SASP) in hepatic stellate cells (HSCs) via enterohepatic circulation, which subsequently secretes various inflammatory and protumor factors within the liver or activates the mTOR signaling pathway, thereby facilitating the progression of NASH-induced HCC (149, 164). In addition to DCA, SCFAs not only serve as an energy source for intestinal epithelial cells but also modulate the permeability of the intestinal mucosa, resulting in increased harmful leakage within the intestine and thereby facilitating the progression of HCC (165, 166). Consequently, intestinal flora is anticipated to emerge as one of the most promising therapeutic targets for HCC. Finally, metagenomic analysis elucidated the correlation between species diversity and abundance within the intestinal microbiota and clinical responses as well as adverse reactions to immunotherapy. This study confirmed the potential of intestinal microbiota as a biomarker for liver cancer immunotherapy and identified new targets for modulating immunotherapy responses and mitigating adverse effects (167) (Figure 2).
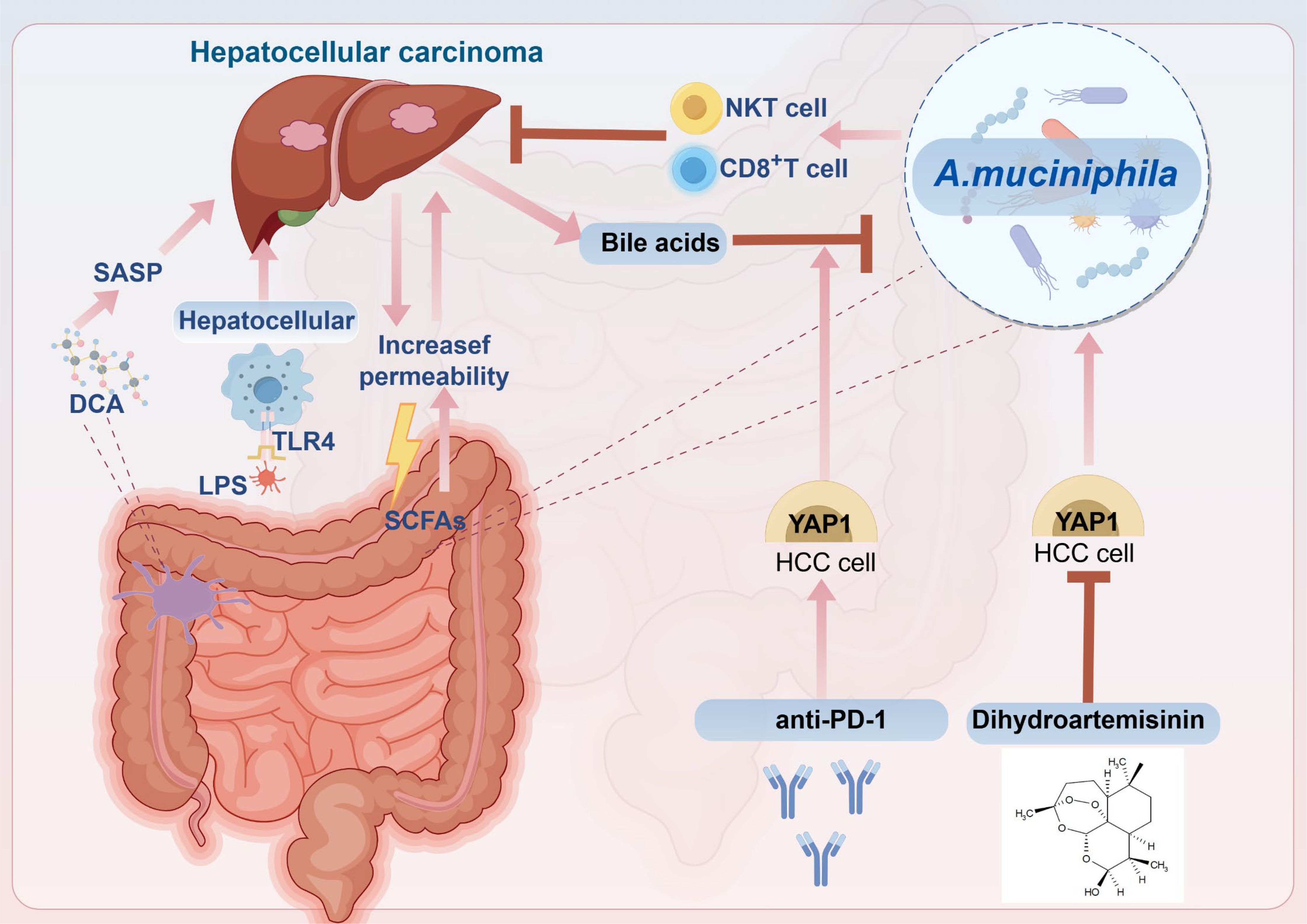
Figure 2. The correlation between hepatocellular carcinoma (HCC) and intestinal flora and the mechanism of A. muciniphila in treating HCC. Studies have shown that HCC promotes the increase of intestinal permeability, resulting in the production and release of large quantities of lipopolysaccharide (LPS). LPS binds to Toll-like receptor 4 (TLR4) on the surface of hepatocytes, leading to the production of various proinflammatory cytokines, thus promoting the occurrence and development of HCC. In addition, deoxycholic acid (DCA) derived from intestinal bacteria promotes the production of senescence-associated secretory phenotype (SASP), which leads to the progression of HCC. In our previous study, we found that anti-PD-1 promotes the expression of YAP1, thereby synergizing with bile acids to inhibit the growth of A. muciniphila, but dihydroartemisinin (DHA) can inhibit the expression of YAP1, thereby increasing the abundance of A. muciniphila, and the activity and number of CD8+ T cells sensitize the antitumor effect of anti-PD-1 in HCC.
Recent investigations indicate that the presence of specific intestinal microbiota and diverse bacterial populations is essential for effective antitumor immunotherapy (5, 167, 168). A. muciniphila has been demonstrated to enhance the response of tumor immunotherapy by inducing immunoglobulin G1 antibodies and promoting antigen-specific T-cell activation (169). Notably, research has shown that A. muciniphila is associated with favorable therapeutic outcomes in immunotherapy across various solid tumors, including HCC (11, 170), non-small cell lung cancer (NSCLC) (171), metastatic renal cell carcinoma (172), and CRC (173). Research has demonstrated that the response to ICIs influences the variety and structure of gut microbiota in fecal samples (11). Mice that responded improved the antitumor effectiveness of anti-PD-1 treatment, whereas FMT from non-responding mice did not yield comparable effects. Furthermore, oral administration of A. muciniphila improves the efficacy of anti-PD-1 in ICI non-responders, suggesting that A. muciniphila may play a potential role in regulating the clinical efficacy of ICIs (11). Therefore, increasing the abundance of A. muciniphila in the intestine or supplementing A. muciniphila appropriately is expected to become a new strategy for adjuvant HCC immunotherapy. Moreover, A. muciniphila effectively inhibited the progression of NASH to HCC, primarily by enhancing liver natural killer T (NKT) cell populations and reducing macrophage infiltration; additionally, A. muciniphila facilitated the cytotoxic activity of NKT cells against HepG2 cells (160). Furthermore, A. muciniphila enhances the efficacy of anti-PD-1 therapy in HCC mouse models by promoting apoptosis of HCC tumor cells and increasing the proportion of CD8+ T cells within the tumor microenvironment (TME) (170). In general, A. muciniphila exerts antitumor effects in HCC mainly by enhancing the number and activity of tumor killer cells and inhibiting the infiltration of immunosuppressive cells.
Similarly, supplementing A. muciniphila in the intestine enhanced the intestinal barrier function of mice, promoted intestinal homeostasis, improved antitumor immune function, and activated the activity of CD8+ T cells in the TME of ovarian cancer (174). Interestingly, fecal metagenomic analysis of patients with NSCLC or RCC who exhibited poor responses to anti-PD-1 antibody treatment revealed that those with partial response or stable disease had a higher abundance of A. muciniphila compared to patients experiencing disease progression (11). A recent study involving microbiome analysis of fecal samples from patients with advanced NSCLC demonstrated that A. muciniphila was associated with improved objective response rates and overall survival in multivariate analyses (175). Furthermore, utilizing 16S rDNA sequencing, it was observed that A. muciniphila facilitated an increase in its abundance within the bloodstream and tumor tissue, thereby inhibiting the progression of lung cancer in murine models (176). Another study demonstrated that the abundance of A. muciniphila is also reduced in patients with CRC. A. muciniphila induces the polarization of M1 macrophages via the TLR2/NLRP3 pathway, thereby inhibiting the progression of CRC (177). Likewise, A. muciniphila inhibits tryptophan metabolism through the aryl hydrocarbon receptor (AhR)/β-catenin signaling pathway to prevent the development of CRC (178). Moreover, the active components derived from A. muciniphila can also exhibit antitumor effects. AmEVs inhibit the growth of prostate cancer by enhancing the proportion of activated CD8+ T cells and M1 macrophages, while concurrently suppressing the polarization of M2 macrophages (101). Another A. muciniphila-derived component, Amuc_2172, inhibits the progression of CRC by enhancing the transcription and secretion of HSP70 to promote the immune response of CTLs (105). Both A. muciniphila and Amuc_1100 ameliorate colitis primarily by reducing the infiltration of macrophages and CTLs. Further investigations have demonstrated that they prevent colitis-related tumors by increasing the number of CTLs in the colon (88). Therefore, both A. muciniphila and its active compounds are candidate antitumor strategies, especially in tumor immunotherapy.
On the other hand, A. muciniphila can also improve the antitumor efficacy of chemotherapeutic drugs. For example, A. muciniphila enhances the antitumor effect of cisplatin in Lewis lung cancer mice, increasing the levels of IFN-γ, IL-6, and TNF-α by reducing the proportion of CD4+ CD25+ Foxp3+ Treg in the peripheral blood and spleen of mice (81). Studies have shown that after FOLFOX (oxaliplatin, fluorouracil, and calcium folinate) intervention, the abundance of A. muciniphila increases significantly and is positively correlated with treatment effect (179). It can also improve the antitumor effect of FOLFOX on colon cancer. Furthermore, pentadecanoic acid derived from A. muciniphila inhibits glycolysis by antagonizing the activity of far upstream element binding protein 1 (FUBP1), thereby enhancing the sensitivity of gastric cancer to oxaliplatin (180). These findings expand the potential applications of A. muciniphila and further substantiate its anticancer effects.
Likewise, interventions utilizing traditional Chinese medicine to enhance A. muciniphila colonization in the host represent a promising strategy for cancer treatment. Our previous study demonstrated that DHA inhibits YAP1 expression in a mouse model of liver cancer and enhances the accumulation of A. muciniphila to improve the therapeutic response to PD-1 inhibitor treatment (78). The combination of A. muciniphila with DHA, followed by its combination with the PD-1 inhibitor, resulted in an increase in the quantity and activation of CD8+ T cells within liver tumors. Quercetin combined with anti-PD-1 antibodies increases the levels in Dubosiella and Akkermansia and improves intestinal flora and macrophage immunity, thereby reshaping the TME of HCC (181). Furthermore, Shuangshen particles inhibit lung metastasis of lung cancer by increasing the abundance of A. muciniphila, enhancing the polarization of tumor-associated macrophages (TAMs), and suppressing the activation of the NF-κB signaling pathway (132). Similarly, another article showed that Huoxue Yiqi Recipe-2 increased A. muciniphila levels and thus enhanced the therapeutic effect of PD-L1 antibodies, thereby inhibiting the growth of lung cancer (182). Sini decoction inhibits the onset and progression of colitis-associated colon cancer in mice by upregulating the numbers of A. muciniphila, Bifidobacterium in the intestine, and CD8+ T cells, while downregulating CD4+ T cells, IL-6, and TNF-α (183). In addition, studies have shown that intervention with ginsenoside compound K restores intestinal flora to normal, partially increasing the abundance of A. muciniphila to prevent the progression of colitis-related colon cancer (184). It is reported that ginsenoside Rh4 also inhibits the progression of CRC by increasing the diversity of intestinal flora, especially the abundance of A. muciniphila, improving intestinal microbiota disorders and impaired intestinal barrier function caused by CRC (185). In short, traditional Chinese medicine and its active ingredients can treat cancer by increasing the colonization of A. muciniphila.
To sum up, as a standard for the next generation of probiotics, A. muciniphila is colonized in the intestinal mucus layer, participates in maintaining intestinal homeostasis and immune regulation to maintain host health, and is negatively related to the progress of various diseases, including metabolic-related diseases and tumors. On the contrary, studies have shown that A. muciniphila can also promote the progression of certain diseases. The mechanism behind this still needs further exploration and analysis to provide a reference for a detailed evaluation of A. muciniphila clinical application. In addition, the existing technology cannot cultivate A. muciniphila on a large scale. For A. muciniphila to be used on a large scale, the cultivation of A. muciniphila is a limitation. Although it has been shown that certain compounds, traditional Chinese medicines, and their monomers can enhance the levels of A. muciniphila, research on large-scale cultivation has not been reported, and the technology for large-scale preparation of A. muciniphila still needs further exploration. At present, most research on A. muciniphila focuses on in-vitro and rodent experiments, so clinical applications still require a lot of research, especially on side effects and safety. Although existing studies have displayed that oral A. muciniphila is safe and effective (30, 186, 187), it has not been officially approved as a food additive in China. In addition, the European Food Safety Authority has also verified the safety of pasteurized A. muciniphila as a new food. There are many studies showing that live A. muciniphila has the same therapeutic effect as pasteurized A. muciniphila, and even pasteurized A. muciniphila shows better effects (84–87), and other compounds derived from A. muciniphila have also shown therapeutic effects on certain diseases, indicating that research on the active ingredients of A. muciniphila is promising and further studies are needed. In terms of tumor immunotherapy, A. muciniphila has demonstrated strong immune activity and sensitizing effect, which provides favorable evidence for the use of A. muciniphila in tumor treatment. Overall, A. muciniphila is a powerful target for disease intervention, especially in tumor immunotherapy.
YY: Conceptualization, Writing – original draft. XS: Funding acquisition, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was financially supported by the National Natural Science Foundation of China (82274315), the Shanxi Provincial Administration of Traditional Chinese Medicine Innovation Team (zyytd2024021), the Key Research Office of Shanxi Province Administration of Traditional Chinese Medicine (zyyyjs2024035), and the Foundation for High-level Talents of Shanxi University of Chinese Medicine (2023RC03).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declare that no Generative AI was used in the creation of this manuscript.
A6ll claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
AmEVs, A. muciniphila extracellular vesicles; AmTARS, A. muciniphila threonyl-tRNA synthetase; CCL4, carbon tetrachloride; CREB, cAMP answer element-binding; DCA, deoxycholic acid; DEN, N-nitrosodiethylamine; ERIC-PCR, enterobacterial repetitive intergenic consensus; FUBP1, far upstream element binding protein 1; GLP-1, glucagon-like peptide-1; GPR43, G-protein-coupled receptor 43; H3K14ac, acetyltransferase on Lys14 of histone H3; HFD, high-fat diet; HSCs, hepatic stellate cells; HSP70, heat shock protein 70; ICAM-2, intercellular adhesion molecule 2; ICI, immune checkpoint inhibitor; ISC, intestinal stem cell; LPS, lipopolysaccharide; MAMPs, microbe-associated molecular patterns; mOS, median overall survival; Muc2, mucin 2; NAFLD, non-alcoholic fatty liver disease; NASH, non-alcoholic steatohepatitis; NKT, natural killer T; NLRP3, NLR family pyrin domain 3; NSCLC, non-small cell lung cancer; ROS, reactive oxygen species; SCFAs, short-chain fatty acids; SASP, senescence-associated secretory phenotype; TACE, transcatheter arterial chemoembolization; T2D, type 2 diabetes; TLR2, Toll-like receptor 2; TME, tumor microenvironment; TME, tumor microenvironment.
1. Bray F, Laversanne M, Sung H, Ferlay J, Siegel RL, Soerjomataram I, et al. Global cancer statistics 2022: globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2024) 74:229–63. doi: 10.3322/caac.21834
2. Xie DY, Zhu K, Ren ZG, Zhou J, Fan J, Gao Q. A review of 2022 chinese clinical guidelines on the management of hepatocellular carcinoma: updates and insights. Hepatobiliary Surg Nutr. (2023) 12:216–28. doi: 10.21037/hbsn-22-469
3. Kudo M, Matsui O, Izumi N, Kadoya M, Okusaka T, Miyayama S, et al. Transarterial chemoembolization failure/refractoriness: jsh-lcsgj criteria 2014 update. Oncology. (2014) 87 Suppl 1:22–31. doi: 10.1159/000368142
4. Liu Y, Xun Z, Ma K, Liang S, Li X, Zhou S, et al. Identification of a tumour immune barrier in the hcc microenvironment that determines the efficacy of immunotherapy. J Hepatol. (2023) 78:770–82. doi: 10.1016/j.jhep.2023.01.011
5. Lu Y, Yuan X, Wang M, He Z, Li H, Wang J, et al. Gut microbiota influence immunotherapy responses: mechanisms and therapeutic strategies. J Hematol Oncol. (2022) 15:47. doi: 10.1186/s13045-022-01273-9
6. Wang M, Du Q, Jin J, Wei Y, Lu Y, Li Q. Lag3 and its emerging role in cancer immunotherapy. Clin Transl Med. (2021) 11:e365. doi: 10.1002/ctm2.365
7. Finn RS, Qin S, Ikeda M, Galle PR, Ducreux M, Kim TY, et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. New Engl J Med. (2020) 382:1894–905. doi: 10.1056/NEJMoa1915745
8. Zhou CB, Zhou YL, Fang JY. Gut microbiota in cancer immune response and immunotherapy. Trends Cancer. (2021) 7:647–60. doi: 10.1016/j.trecan.2021.01.010
9. Zitvogel L, Ma Y, Raoult D, Kroemer G, Gajewski TF. The microbiome in cancer immunotherapy: diagnostic tools and therapeutic strategies. Science. (2018) 359:1366–70. doi: 10.1126/science.aar6918
10. Xie J, Liu M, Deng X, Tang Y, Zheng S, Ou X, et al. Gut microbiota reshapes cancer immunotherapy efficacy: mechanisms and therapeutic strategies. Imeta. (2024) 3:e156. doi: 10.1002/imt2.156
11. Routy B, Le Chatelier E, Derosa L, Duong CPM, Alou MT, Daillère R, et al. Gut microbiome influences efficacy of pd-1-based immunotherapy against epithelial tumors. Science. (2018) 359:91–7. doi: 10.1126/science.aan3706
12. Matson V, Fessler J, Bao R, Chongsuwat T, Zha Y, Alegre ML, et al. The commensal microbiome is associated with anti-pd-1 efficacy in metastatic melanoma patients. Science. (2018) 359:104–8. doi: 10.1126/science.aao3290
13. Gopalakrishnan V, Spencer CN, Nezi L, Reuben A, Andrews MC, Karpinets TV, et al. Gut microbiome modulates response to anti-pd-1 immunotherapy in melanoma patients. Science. (2018) 359:97–103. doi: 10.1126/science.aan4236
14. Cani PD, de Vos WM. Next-generation beneficial microbes: the case of akkermansia muciniphila. Front Microbiol. (2017) 8:1765. doi: 10.3389/fmicb.2017.01765
15. Derrien M, Vaughan EE, Plugge CM, de Vos WM. Akkermansia muciniphila gen. Nov., sp. Nov., a human intestinal mucin-degrading bacterium. Int J Syst Evol Microbiol. (2004) 54:1469–76. doi: 10.1099/ijs.0.02873-0
16. Sparfel L, Ratodiarivony S, Boutet-Robinet E, Ellero-Simatos S, Jolivet-Gougeon A. Akkermansia muciniphila and alcohol-related liver diseases. A systematic review. Mol Nutr Food Res. (2024) 68:e2300510. doi: 10.1002/mnfr.202300510
17. Ouyang J, Lin J, Isnard S, Fombuena B, Peng X, Marette A, et al. The bacterium akkermansia muciniphila: A sentinel for gut permeability and its relevance to hiv-related inflammation. Front Immunol. (2020) 11:645. doi: 10.3389/fimmu.2020.00645
18. Hiippala K, Jouhten H, Ronkainen A, Hartikainen A, Kainulainen V, Jalanka J, et al. The potential of gut commensals in reinforcing intestinal barrier function and alleviating inflammation. Nutrients. (2018) 10:988. doi: 10.3390/nu10080988
19. Gaifem J, Mendes-Frias A, Wolter M, Steimle A, Garzón MJ, Ubeda C, et al. Akkermansia muciniphila and parabacteroides distasonis synergistically protect from colitis by promoting ilc3 in the gut. mBio. (2024) 15:e0007824. doi: 10.1128/mbio.00078-24
20. Yilmaz O, Okullu SO, Catakci M, Elmas MA, Pinheiro Y, Arbak S, et al. Akkermansia muciniphila improves chronic colitis-induced enteric neuroinflammation in mice. Neurogastroenterol Motil. (2024) 36:e14745. doi: 10.1111/nmo.14745
21. Zeng SY, Liu YF, Liu JH, Zeng ZL, Xie H, Liu JH. Potential effects of akkermansia muciniphila in aging and aging-related diseases: current evidence and perspectives. Aging Dis. (2023) 14:2015–27. doi: 10.14336/ad.2023.0325
22. Everard A, Belzer C, Geurts L, Ouwerkerk JP, Druart C, Bindels LB, et al. Cross-talk between akkermansia muciniphila and intestinal epithelium controls diet-induced obesity. Proc Natl Acad Sci U.S.A. (2013) 110:9066–71. doi: 10.1073/pnas.1219451110
23. Hong MG, Lee Y, Chung WS, Seo JG, Lee SN. Supplementation with heat-killed akkermansia muciniphila eb-amdk19 counteracts diet-induced overweight in beagles. Arch Anim Nutr. (2024) 78(3):254–72. doi: 10.1080/1745039x.2024.2397221
24. Niu H, Zhou M, Ji A, Zogona D, Wu T, Xu X. Molecular mechanism of pasteurized akkermansia muciniphila in alleviating type 2 diabetes symptoms. J Agric Food Chem. (2024) 72:13083–98. doi: 10.1021/acs.jafc.4c01188
25. Zhang J, Ni Y, Qian L, Fang Q, Zheng T, Zhang M, et al. Decreased abundance of akkermansia muciniphila leads to the impairment of insulin secretion and glucose homeostasis in lean type 2 diabetes. Adv Sci (Weinh). (2021) 8:e2100536. doi: 10.1002/advs.202100536
26. He X, Bai Y, Zhou H, Wu K. Akkermansia muciniphila alters gut microbiota and immune system to improve cardiovascular diseases in murine model. Front Microbiol. (2022) 13:906920. doi: 10.3389/fmicb.2022.906920
27. Xiao X, Wu Y, Jie Z, Lin L, Li Y, Hu W, et al. Akkermansia muciniphila supplementation improves hyperlipidemia, cardiac function, and gut microbiota in high fat fed apolipoprotein E-deficient mice. Prostaglandins Other Lipid Mediat. (2024) 175:106906. doi: 10.1016/j.prostaglandins.2024.106906
28. Qu D, Chen M, Zhu H, Liu X, Cui Y, Zhou W, et al. Akkermansia muciniphila and its outer membrane protein amuc_1100 prevent high-fat diet-induced nonalcoholic fatty liver disease in mice. Biochem Biophys Res Commun. (2023) 684:149131. doi: 10.1016/j.bbrc.2023.149131
29. Rao Y, Kuang Z, Li C, Guo S, Xu Y, Zhao D, et al. Gut akkermansia muciniphila ameliorates metabolic dysfunction-associated fatty liver disease by regulating the metabolism of L-aspartate via gut-liver axis. Gut Microbes. (2021) 13:1–19. doi: 10.1080/19490976.2021.1927633
30. Plovier H, Everard A, Druart C, Depommier C, Van Hul M, Geurts L, et al. A purified membrane protein from akkermansia muciniphila or the pasteurized bacterium improves metabolism in obese and diabetic mice. Nat Med. (2017) 23:107–13. doi: 10.1038/nm.4236
31. Turck D, Bohn T, Castenmiller J, De Henauw S, Hirsch-Ernst KI, Maciuk A, et al. Safety of pasteurised akkermansia muciniphila as a novel food pursuant to regulation (Eu) 2015/2283. Efsa J. (2021) 19:e06780. doi: 10.2903/j.efsa.2021.6780
32. Li L, Li M, Chen Y, Yu Z, Cheng P, Yu Z, et al. Function and therapeutic prospects of next-generation probiotic akkermansia muciniphila in infectious diseases. Front Microbiol. (2024) 15:1354447. doi: 10.3389/fmicb.2024.1354447
33. Ouwerkerk JP, van der Ark KCH, Davids M, Claassens NJ, Finestra TR, de Vos WM, et al. Adaptation of akkermansia muciniphila to the oxic-anoxic interface of the mucus layer. Appl Environ Microbiol. (2016) 82:6983–93. doi: 10.1128/aem.01641-16
34. Geerlings SY, Kostopoulos I, de Vos WM, Belzer C. Akkermansia muciniphila in the human gastrointestinal tract: when, where, and how? Microorganisms. (2018) 6:75. doi: 10.3390/microorganisms6030075
35. van Passel MW, Kant R, Zoetendal EG, Plugge CM, Derrien M, Malfatti SA, et al. The genome of akkermansia muciniphila, a dedicated intestinal mucin degrader, and its use in exploring intestinal metagenomes. PloS One. (2011) 6:e16876. doi: 10.1371/journal.pone.0016876
36. Liu MJ, Yang JY, Yan ZH, Hu S, Li JQ, Xu ZX, et al. Recent findings in akkermansia muciniphila-regulated metabolism and its role in intestinal diseases. Clin Nutr. (2022) 41:2333–44. doi: 10.1016/j.clnu.2022.08.029
37. Cheng D, Xie MZ. A review of a potential and promising probiotic candidate-akkermansia muciniphila. J Appl Microbiol. (2021) 130:1813–22. doi: 10.1111/jam.14911
38. Zhai Q, Feng S, Arjan N, Chen W. A next generation probiotic, akkermansia muciniphila. Crit Rev Food Sci Nutr. (2019) 59:3227–36. doi: 10.1080/10408398.2018.1517725
39. Collado MC, Derrien M, Isolauri E, de Vos WM, Salminen S. Intestinal integrity and akkermansia muciniphila, a mucin-degrading member of the intestinal microbiota present in infants, adults, and the elderly. Appl Environ Microbiol. (2007) 73:7767–70. doi: 10.1128/aem.01477-07
40. Zhao Q, Yu J, Hao Y, Zhou H, Hu Y, Zhang C, et al. Akkermansia muciniphila plays critical roles in host health. Crit Rev Microbiol. (2023) 49:82–100. doi: 10.1080/1040841x.2022.2037506
41. Cai Y, Li X, Han Q, Bai J, Zheng Q, Sun R, et al. Si-ni-san improves experimental colitis by favoring akkermensia colonization. J Ethnopharmacol. (2023) 305:116067. doi: 10.1016/j.jep.2022.116067
42. Yan Z, Li Y, Xia T, Wang K, Liao Z, Zhang L, et al. Revitalizing gut health: liangxue guyuan yishen decoction promotes akkermansia muciniphila -induced intestinal stem cell recovery post-radiation in mice. Phytomedicine. (2024) 132:155888. doi: 10.1016/j.phymed.2024.155888
43. Liu Y, Xue Y, Zhang Z, Ji J, Li C, Zheng K, et al. Wolfberry enhanced the abundance of akkermansia muciniphila by yap1 in mice with acetaminophen-induced liver injury. FASEB J. (2023) 37:e22689. doi: 10.1096/fj.202200945R
44. Dong C, Yu J, Yang Y, Zhang F, Su W, Fan Q, et al. Berberine, a potential prebiotic to indirectly promote akkermansia growth through stimulating gut mucin secretion. BioMed Pharmacother. (2021) 139:111595. doi: 10.1016/j.biopha.2021.111595
45. Wu W, Zhao Z, Zhao Z, Zhang D, Zhang Q, Zhang J, et al. Structure, health benefits, mechanisms, and gut microbiota of dendrobium officinale polysaccharides: A review. Nutrients. (2023) 15:4901. doi: 10.3390/nu15234901
46. Ndongo S, Armstrong N, Raoult D, Fournier PE. Reclassification of eight akkermansia muciniphila strains and description of akkermansia massiliensis sp. Nov. And candidatus akkermansia timonensis, isolated from human feces. Sci Rep. (2022) 12:21747. doi: 10.1038/s41598-022-25873-0
47. Kobayashi Y, Kawahara T, Inoue S, Kohda N. Akkermansia biwaensis sp. Nov., an anaerobic mucin-degrading bacterium isolated from human faeces. Int J Syst Evol Microbiol. (2023) 73. doi: 10.1099/ijsem.0.005697
48. Kelly C, Jawahar J, Davey L, Everitt JI, Galanko JA, Anderson C, et al. Spontaneous episodic inflammation in the intestines of mice lacking hnf4a is driven by microbiota and associated with early life microbiota alterations. mBio. (2023) 14:e0150423. doi: 10.1128/mbio.01504-23
49. Panzetta ME, Valdivia RH. Akkermansia in the gastrointestinal tract as a modifier of human health. Gut Microbes. (2024) 16:2406379. doi: 10.1080/19490976.2024.2406379
50. Li Z, Hu G, Zhu L, Sun Z, Jiang Y, Gao MJ, et al. Study of growth, metabolism, and morphology of akkermansia muciniphila with an in vitro advanced bionic intestinal reactor. BMC Microbiol. (2021) 21:61. doi: 10.1186/s12866-021-02111-7
51. Niu H, Zhou M, Zogona D, Xing Z, Wu T, Chen R, et al. Akkermansia muciniphila: A potential candidate for ameliorating metabolic diseases. Front Immunol. (2024) 15:1370658. doi: 10.3389/fimmu.2024.1370658
52. Kang EJ, Kim JH, Kim YE, Lee H, Jung KB, Chang DH, et al. The secreted protein amuc_1409 from akkermansia muciniphila improves gut health through intestinal stem cell regulation. Nat Commun. (2024) 15:2983. doi: 10.1038/s41467-024-47275-8
53. Mo C, Lou X, Xue J, Shi Z, Zhao Y, Wang F, et al. The influence of akkermansia muciniphila on intestinal barrier function. Gut Pathog. (2024) 16:41. doi: 10.1186/s13099-024-00635-7
54. Ottman N, Huuskonen L, Reunanen J, Boeren S, Klievink J, Smidt H, et al. Characterization of outer membrane proteome of akkermansia muciniphila reveals sets of novel proteins exposed to the human intestine. Front Microbiol. (2016) 7:1157. doi: 10.3389/fmicb.2016.01157
55. Derrien M, Belzer C, de Vos WM. Akkermansia muciniphila and its role in regulating host functions. Microb Pathog. (2017) 106:171–81. doi: 10.1016/j.micpath.2016.02.005
56. Derrien M, Van Baarlen P, Hooiveld G, Norin E, Müller M, de Vos WM. Modulation of mucosal immune response, tolerance, and proliferation in mice colonized by the mucin-degrader akkermansia muciniphila. Front Microbiol. (2011) 2:166. doi: 10.3389/fmicb.2011.00166
57. Reunanen J, Kainulainen V, Huuskonen L, Ottman N, Belzer C, Huhtinen H, et al. Akkermansia muciniphila adheres to enterocytes and strengthens the integrity of the epithelial cell layer. Appl Environ Microbiol. (2015) 81:3655–62. doi: 10.1128/aem.04050-14
58. Kostopoulos I, Elzinga J, Ottman N, Klievink JT, Blijenberg B, Aalvink S, et al. Akkermansia muciniphila uses human milk oligosaccharides to thrive in the early life conditions in vitro. Sci Rep. (2020) 10:14330. doi: 10.1038/s41598-020-71113-8
59. Collado MC, Laitinen K, Salminen S, Isolauri E. Maternal weight and excessive weight gain during pregnancy modify the immunomodulatory potential of breast milk. Pediatr Res. (2012) 72:77–85. doi: 10.1038/pr.2012.42
60. Santee CA, Nagalingam NA, Faruqi AA, DeMuri GP, Gern JE, Wald ER, et al. Nasopharyngeal microbiota composition of children is related to the frequency of upper respiratory infection and acute sinusitis. Microbiome. (2016) 4:34. doi: 10.1186/s40168-016-0179-9
61. Luo Y, Zhang Y, Han X, Yuan Y, Zhou Y, Gao Y, et al. Akkermansia muciniphila prevents cold-related atrial fibrillation in rats by modulation of tmao induced cardiac pyroptosis. EBioMedicine. (2022) 82:104087. doi: 10.1016/j.ebiom.2022.104087
62. Garcia-Mazcorro JF, Lage NN, Mertens-Talcott S, Talcott S, Chew B, Dowd SE, et al. Effect of dark sweet cherry powder consumption on the gut microbiota, short-chain fatty acids, and biomarkers of gut health in obese db/db mice. PeerJ. (2018) 6:e4195. doi: 10.7717/peerj.4195
63. Rodriguez C, Taminiau B, Brévers B, Avesani V, Van Broeck J, Leroux A, et al. Faecal microbiota characterisation of horses using 16 rdna barcoded pyrosequencing, and carriage rate of clostridium difficile at hospital admission. BMC Microbiol. (2015) 15:181. doi: 10.1186/s12866-015-0514-5
64. Zeng B, Han S, Wang P, Wen B, Jian W, Guo W, et al. The bacterial communities associated with fecal types and body weight of rex rabbits. Sci Rep. (2015) 5:9342. doi: 10.1038/srep09342
65. Hildebrand F, Ebersbach T, Nielsen HB, Li X, Sonne SB, Bertalan M, et al. A comparative analysis of the intestinal metagenomes present in Guinea pigs (Cavia porcellus) and humans (Homo sapiens). BMC Genomics. (2012) 13:514. doi: 10.1186/1471-2164-13-514
66. Derakhshani H, De Buck J, Mortier R, Barkema HW, Krause DO, Khafipour E. The features of fecal and ileal mucosa-associated microbiota in dairy calves during early infection with mycobacterium avium subspecies paratuberculosis. Front Microbiol. (2016) 7:426. doi: 10.3389/fmicb.2016.00426
67. Ushida K, Segawa T, Tsuchida S, Murata K. Cecal bacterial communities in wild Japanese rock ptarmigans and captive svalbard rock ptarmigans. J Vet Med Sci. (2016) 78:251–7. doi: 10.1292/jvms.15-0313
68. Roeselers G, Mittge EK, Stephens WZ, Parichy DM, Cavanaugh CM, Guillemin K, et al. Evidence for a core gut microbiota in the zebrafish. Isme J. (2011) 5:1595–608. doi: 10.1038/ismej.2011.38
69. Costello EK, Gordon JI, Secor SM, Knight R. Postprandial remodeling of the gut microbiota in burmese pythons. Isme J. (2010) 4:1375–85. doi: 10.1038/ismej.2010.71
70. Guo X, Zhang J, Wu F, Zhang M, Yi M, Peng Y. Different subtype strains of akkermansia muciniphila abundantly colonize in southern China. J Appl Microbiol. (2016) 120:452–9. doi: 10.1111/jam.13022
71. Karcher N, Nigro E, Punčochář M, Blanco-Míguez A, Ciciani M, Manghi P, et al. Genomic diversity and ecology of human-associated akkermansia species in the gut microbiome revealed by extensive metagenomic assembly. Genome Biol. (2021) 22:209. doi: 10.1186/s13059-021-02427-7
72. Guo X, Li S, Zhang J, Wu F, Li X, Wu D, et al. Genome sequencing of 39 akkermansia muciniphila isolates reveals its population structure, genomic and functional diverisity, and global distribution in mammalian gut microbiotas. BMC Genomics. (2017) 18:800. doi: 10.1186/s12864-017-4195-3
73. Kirmiz N, Galindo K, Cross KL, Luna E, Rhoades N, Podar M, et al. Comparative genomics guides elucidation of vitamin B(12) biosynthesis in novel human-associated akkermansia strains. Appl Environ Microbiol. (2020) 86:e02117-19. doi: 10.1128/aem.02117-19
74. Becken B, Davey L, Middleton DR, Mueller KD, Sharma A, Holmes ZC, et al. Genotypic and phenotypic diversity among human isolates of akkermansia muciniphila. mBio. (2021) 12:e00478-21. doi: 10.1128/mBio.00478-21
75. Lukovac S, Belzer C, Pellis L, Keijser BJ, de Vos WM, Montijn RC, et al. Differential modulation by akkermansia muciniphila and faecalibacterium prausnitzii of host peripheral lipid metabolism and histone acetylation in mouse gut organoids. mBio. (2014) 5:e01438-14. doi: 10.1128/mBio.01438-14
76. Derrien M, van Passel MW, van de Bovenkamp JH, Schipper RG, de Vos WM, Dekker J. Mucin-bacterial interactions in the human oral cavity and digestive tract. Gut Microbes. (2010) 1:254–68. doi: 10.4161/gmic.1.4.12778
77. Wei L, Pan Y, Guo Y, Zhu Y, Jin H, Gu Y, et al. Symbiotic combination of akkermansia muciniphila and inosine alleviates alcohol-induced liver injury by modulating gut dysbiosis and immune responses. Front Microbiol. (2024) 15:1355225. doi: 10.3389/fmicb.2024.1355225
78. Zhang Z, Shi X, Ji J, Guo Y, Peng Q, Hao L, et al. Dihydroartemisinin increased the abundance of akkermansia muciniphila by yap1 depression that sensitizes hepatocellular carcinoma to anti-pd-1 immunotherapy. Front Med. (2023) 17:729–46. doi: 10.1007/s11684-022-0978-2
79. Xu Y, Tan X, Yang Q, Fang Z, Chen W. Akkermansia muciniphila outer membrane protein regulates recruitment of cd8(+) T cells in lung adenocarcinoma and through jak-stat signalling pathway. Microb Biotechnol. (2024) 17:e14522. doi: 10.1111/1751-7915.14522
80. Zhu Z, Huang J, Zhang Y, Hou W, Chen F, Mo YY, et al. Landscape of tumoral ecosystem for enhanced anti-pd-1 immunotherapy by gut akkermansia muciniphila. Cell Rep. (2024) 43:114306. doi: 10.1016/j.celrep.2024.114306
81. Chen Z, Qian X, Chen S, Fu X, Ma G, Zhang A. Akkermansia muciniphila enhances the antitumor effect of cisplatin in lewis lung cancer mice. J Immunol Res. (2020) 2020:2969287. doi: 10.1155/2020/2969287
82. Zhai R, Xue X, Zhang L, Yang X, Zhao L, Zhang C. Strain-specific anti-inflammatory properties of two akkermansia muciniphila strains on chronic colitis in mice. Front Cell Infect Microbiol. (2019) 9:239. doi: 10.3389/fcimb.2019.00239
83. Kang EJ, Cha MG, Kwon GH, Han SH, Yoon SJ, Lee SK, et al. Akkermansia muciniphila improve cognitive dysfunction by regulating bdnf and serotonin pathway in gut-liver-brain axis. Microbiome. (2024) 12:181. doi: 10.1186/s40168-024-01924-8
84. Grajeda-Iglesias C, Durand S, Daillère R, Iribarren K, Lemaitre F, Derosa L, et al. Oral administration of akkermansia muciniphila elevates systemic antiaging and anticancer metabolites. Aging. (2021) 13:6375–405. doi: 10.18632/aging.202739
85. Ashrafian F, Keshavarz Azizi Raftar S, Shahryari A, Behrouzi A, Yaghoubfar R, Lari A, et al. Comparative effects of alive and pasteurized akkermansia muciniphila on normal diet-fed mice. Sci Rep. (2021) 11:17898. doi: 10.1038/s41598-021-95738-5
86. Ashrafian F, Keshavarz Azizi Raftar S, Lari A, Shahryari A, Abdollahiyan S, Moradi HR, et al. Extracellular vesicles and pasteurized cells derived from akkermansia muciniphila protect against high-fat induced obesity in mice. Microbial Cell factories. (2021) 20:219. doi: 10.1186/s12934-021-01709-w
87. Choi Y, Bose S, Seo J, Shin JH, Lee D, Kim Y, et al. Effects of live and pasteurized forms of akkermansia from the human gut on obesity and metabolic dysregulation. Microorganisms. (2021) 9:2039. doi: 10.3390/microorganisms9102039
88. Wang L, Tang L, Feng Y, Zhao S, Han M, Zhang C, et al. A purified membrane protein from akkermansia muciniphila or the pasteurised bacterium blunts colitis associated tumourigenesis by modulation of cd8(+) T cells in mice. Gut. (2020) 69:1988–97. doi: 10.1136/gutjnl-2019-320105
89. Meynier M, Daugey V, Mallaret G, Gervason S, Meleine M, Barbier J, et al. Pasteurized akkermansia muciniphila improves irritable bowel syndrome-like symptoms and related behavioral disorders in mice. Gut Microbes. (2024) 16:2298026. doi: 10.1080/19490976.2023.2298026
90. Cani PD, Knauf C. A newly identified protein from akkermansia muciniphila stimulates glp-1 secretion. Cell Metab. (2021) 33:1073–5. doi: 10.1016/j.cmet.2021.05.004
91. Ottman N, Reunanen J, Meijerink M, Pietilä TE, Kainulainen V, Klievink J, et al. Pili-like proteins of akkermansia muciniphila modulate host immune responses and gut barrier function. PloS One. (2017) 12:e0173004. doi: 10.1371/journal.pone.0173004
92. Wang LJ, Jin YL, Pei WL, Li JC, Zhang RL, Wang JJ, et al. Amuc_1100 pretreatment alleviates acute pancreatitis in a mouse model through regulating gut microbiota and inhibiting inflammatory infiltration. Acta Pharmacol Sin. (2024) 45:570–80. doi: 10.1038/s41401-023-01186-4
93. Mulhall H, DiChiara JM, Deragon M, Iyer R, Huck O, Amar S. Akkermansia muciniphila and its pili-like protein amuc_1100 modulate macrophage polarization in experimental periodontitis. Infect Immun. (2020) 89:e00500-20. doi: 10.1128/iai.00500-20
94. Li Z, Xing J, Ma X, Zhang W, Wang C, Wang Y, et al. An orally administered bacterial membrane protein nanodrug ameliorates doxorubicin cardiotoxicity through alleviating impaired intestinal barrier. Bioact Mater. (2024) 37:517–32. doi: 10.1016/j.bioactmat.2024.03.027
95. Bae M, Cassilly CD, Liu X, Park SM, Tusi BK, Chen X, et al. Akkermansia muciniphila phospholipid induces homeostatic immune responses. Nature. (2022) 608:168–73. doi: 10.1038/s41586-022-04985-7
96. Yoon HS, Cho CH, Yun MS, Jang SJ, You HJ, Kim JH, et al. Akkermansia muciniphila secretes a glucagon-like peptide-1-inducing protein that improves glucose homeostasis and ameliorates metabolic disease in mice. Nat Microbiol. (2021) 6:563–73. doi: 10.1038/s41564-021-00880-5
97. Di W, Zhang Y, Zhang X, Han L, Zhao L, Hao Y, et al. Heterologous expression of P9 from akkermansia muciniphila increases the glp-1 secretion of intestinal L cells. World J Microbiol Biotechnol. (2024) 40:199. doi: 10.1007/s11274-024-04012-z
98. Kim SM, Park S, Hwang SH, Lee EY, Kim JH, Lee GS, et al. Secreted akkermansia muciniphila threonyl-trna synthetase functions to monitor and modulate immune homeostasis. Cell Host Microbe. (2023) 31:1021–37.e10. doi: 10.1016/j.chom.2023.05.007
99. Chelakkot C, Choi Y, Kim DK, Park HT, Ghim J, Kwon Y, et al. Akkermansia muciniphila-derived extracellular vesicles influence gut permeability through the regulation of tight junctions. Exp Mol Med. (2018) 50:e450. doi: 10.1038/emm.2017.282
100. Keshavarz Azizi Raftar S, Ashrafian F, Yadegar A, Lari A, Moradi HR, Shahriary A, et al. The protective effects of live and pasteurized akkermansia muciniphila and its extracellular vesicles against hfd/ccl4-induced liver injury. Microbiol Spectr. (2021) 9:e0048421. doi: 10.1128/Spectrum.00484-21
101. Luo ZW, Xia K, Liu YW, Liu JH, Rao SS, Hu XK, et al. Extracellular vesicles from akkermansia muciniphila elicit antitumor immunity against prostate cancer via modulation of cd8(+) T cells and macrophages. Int J Nanomedicine. (2021) 16:2949–63. doi: 10.2147/ijn.S304515
102. Kim JY, Kim CW, Oh SY, Jang S, Yetunde OZ, Kim BA, et al. Akkermansia muciniphila extracellular vesicles have a protective effect against hypertension. Hypertens Res. (2024) 47:1642–53. doi: 10.1038/s41440-024-01627-5
103. Olarinoye ZY, Kim CW, Kim JY, Jang S, Kim I. Differential gene expression in the kidneys of shr and wky rats after intravenous administration of akkermansia muciniphila-derived extracellular vesicles. Sci Rep. (2024) 14:20056. doi: 10.1038/s41598-024-69757-x
104. Chen Y, Ou Z, Pang M, Tao Z, Zheng X, Huang Z, et al. Extracellular vesicles derived from akkermansia muciniphila promote placentation and mitigate preeclampsia in a mouse model. J Extracell Vesicles. (2023) 12:e12328. doi: 10.1002/jev2.12328
105. Jiang Y, Xu Y, Zheng C, Ye L, Jiang P, Malik S, et al. Acetyltransferase from akkermansia muciniphila blunts colorectal tumourigenesis by reprogramming tumour microenvironment. Gut. (2023) 72:1308–18. doi: 10.1136/gutjnl-2022-327853
106. Tao Q, Liang Q, Fu Y, Qian J, Xu J, Zhu Y, et al. Puerarin ameliorates colitis by direct suppression of macrophage M1 polarization in dss mice. Phytomedicine. (2024) 135:156048. doi: 10.1016/j.phymed.2024.156048
107. Meng X, Wang W, Lan T, Yang W, Yu D, Fang X, et al. A purified aspartic protease from akkermansia muciniphila plays an important role in degrading muc2. Int J Mol Sci. (2019) 21:72. doi: 10.3390/ijms21010072
108. Meng X, Zhang J, Wu H, Yu D, Fang X. Akkermansia muciniphila aspartic protease amuc_1434* Inhibits human colorectal cancer ls174t cell viability via trail-mediated apoptosis pathway. Int J Mol Sci. (2020) 21:3385. doi: 10.3390/ijms21093385
109. Qian K, Yang W, Chen X, Wang Y, Zhang M, Wang M. Functional and structural characterization of a gh3 β-N-acetylhexosaminidase from akkermansia muciniphila involved in mucin degradation. Biochem Biophys Res Commun. (2022) 589:186–91. doi: 10.1016/j.bbrc.2021.12.022
110. Qian K, Chen S, Wang J, Sheng K, Wang Y, Zhang M. A β-N-acetylhexosaminidase amuc_2109 from akkermansia muciniphila protects against dextran sulfate sodium-induced colitis in mice by enhancing intestinal barrier and modulating gut microbiota. Food Funct. (2022) 13:2216–27. doi: 10.1039/d1fo04094d
111. He J, Zhang P, Shen L, Niu L, Tan Y, Chen L, et al. Short-chain fatty acids and their association with signalling pathways in inflammation, glucose and lipid metabolism. Int J Mol Sci. (2020) 21:6356. doi: 10.3390/ijms21176356
112. Mei L, Wang J, Hao Y, Zeng X, Yang Y, Wu Z, et al. A comprehensive update on the immunoregulatory mechanisms of akkermansia muciniphila: insights into active ingredients, metabolites, and nutrient-driven modulation. Crit Rev Food Sci Nutr. (2024), 1–18. doi: 10.1080/10408398.2024.2416481
113. Li N, Tan S, Wang Y, Deng J, Wang N, Zhu S, et al. Akkermansia muciniphila supplementation prevents cognitive impairment in sleep-deprived mice by modulating microglial engulfment of synapses. Gut Microbes. (2023) 15:2252764. doi: 10.1080/19490976.2023.2252764
114. Ma J, Liu Z, Gao X, Bao Y, Hong Y, He X, et al. Gut microbiota remodeling improves natural aging-related disorders through akkermansia muciniphila and its derived acetic acid. Pharmacol Res. (2023) 189:106687. doi: 10.1016/j.phrs.2023.106687
115. Wang J, Yang Y, Shao F, Meng Y, Guo D, He J, et al. Acetate reprogrammes tumour metabolism and promotes pd-L1 expression and immune evasion by upregulating C-myc. Nat Metab. (2024) 6:914–32. doi: 10.1038/s42255-024-01037-4
116. Duan C, Wu J, Wang Z, Tan C, Hou L, Qian W, et al. Fucose promotes intestinal stem cell-mediated intestinal epithelial development through promoting akkermansia-related propanoate metabolism. Gut Microbes. (2023) 15:2233149. doi: 10.1080/19490976.2023.2233149
117. Müller M, Hernández MAG, Goossens GH, Reijnders D, Holst JJ, Jocken JWE, et al. Circulating but not faecal short-chain fatty acids are related to insulin sensitivity, lipolysis and glp-1 concentrations in humans. Sci Rep. (2019) 9:12515. doi: 10.1038/s41598-019-48775-0
118. He KY, Lei XY, Wu DH, Zhang L, Li JQ, Li QT, et al. Akkermansia muciniphila protects the intestine from irradiation-induced injury by secretion of propionic acid. Gut Microbes. (2023) 15:2293312. doi: 10.1080/19490976.2023.2293312
119. Shen J, Wang S, Xia H, Han S, Wang Q, Wu Z, et al. Akkermansia muciniphila attenuated lipopolysaccharide-induced acute lung injury by modulating the gut microbiota and scfas in mice. Food Funct. (2023) 14:10401–17. doi: 10.1039/d3fo04051h
120. Konstanti P, Ligthart K, Fryganas C, Constantinos P, Smidt H, de Vos WM, et al. Physiology of Γ-aminobutyric acid production by akkermansia muciniphila. Appl Environ Microbiol. (2024) 90:e0112123. doi: 10.1128/aem.01121-23
121. Che Q, Luo T, Shi J, He Y, Xu DL. Mechanisms by which traditional chinese medicines influence the intestinal flora and intestinal barrier. Front Cell Infect Microbiol. (2022) 12:863779. doi: 10.3389/fcimb.2022.863779
122. Shen Z, Cui T, Liu Y, Wu S, Han C, Li J. Astragalus membranaceus and salvia miltiorrhiza ameliorate diabetic kidney disease via the “Gut-kidney axis. Phytomedicine. (2023) 121:155129. doi: 10.1016/j.phymed.2023.155129
123. Chu N, Chan JCN, Chow E. Pharmacomicrobiomics in western medicine and traditional chinese medicine in type 2 diabetes. Front Endocrinol (Lausanne). (2022) 13:857090. doi: 10.3389/fendo.2022.857090
124. Lu J, Gong Y, Gao Y, Yang Y, Zhang Y, Zhang Z, et al. Wolfberry, yam, and chrysanthemum polysaccharides increased intestinal akkermansia muciniphila abundance and hepatic yap1 expression to alleviate dili. FASEB J. (2023) 37:e23286. doi: 10.1096/fj.202301388R
125. Sun J, Jiang Y, Wang B, Yang J, Chen Y, Luo H, et al. Structural characterization of the polysaccharides from atractylodes chinensis (Dc.) koidz. And the protective effection against alcohol-induced intestinal injury in rats. Int J Biol Macromol. (2024) 282:136641. doi: 10.1016/j.ijbiomac.2024.136641
126. Xu H, Wang S, Jiang Y, Wu J, Chen L, Ding Y, et al. Poria cocos polysaccharide ameliorated antibiotic-associated diarrhea in mice via regulating the homeostasis of the gut microbiota and intestinal mucosal barrier. Int J Mol Sci. (2023) 24:1423. doi: 10.3390/ijms24021423
127. Lu J, Gao Y, Gong Y, Yue Y, Yang Y, Xiong Y, et al. Lycium barbarum L. Balanced intestinal flora with yap1/fxr activation in drug-induced liver injury. Int Immunopharmacol. (2024) 130:111762. doi: 10.1016/j.intimp.2024.111762
128. Jeong E, Eun S, Chae S, Lee S. Prebiotic potential of goji berry (Lycium barbarum) in improving intestinal integrity and inflammatory profiles via modification of the gut microbiota in high-fat diet-fed rats. J Med Food. (2024) 27:704–12. doi: 10.1089/jmf.2024.k.0031
129. Ou X, Chen J, Li B, Yang Y, Liu X, Xu Z, et al. Multiomics reveals the ameliorating effect and underlying mechanism of aqueous extracts of polygonatum sibiricum rhizome on obesity and liver fat accumulation in high-fat diet-fed mice. Phytomedicine. (2024) 132:155843. doi: 10.1016/j.phymed.2024.155843
130. Luo Z, Xu W, Yuan T, Shi C, Jin T, Chong Y, et al. Platycodon grandiflorus root extract activates hepatic pi3k/pip3/akt insulin signaling by enriching gut akkermansia muciniphila in high fat diet fed mice. Phytomedicine. (2023) 109:154595. doi: 10.1016/j.phymed.2022.154595
131. Jiang ZM, Wang FF, Zhao YY, Lu LF, Jiang XY, Huang TQ, et al. Hypericum perforatum L. Attenuates depression by regulating akkermansia muciniphila, tryptophan metabolism and nfκb-nlrp2-caspase1-il1β Pathway. Phytomedicine. (2024) 132:155847. doi: 10.1016/j.phymed.2024.155847
132. Li J, Shi B, Ren X, Hu J, Li Y, He S, et al. Lung-intestinal axis, shuangshen granules attenuate lung metastasis by regulating the intestinal microbiota and related metabolites. Phytomedicine. (2024) 132:155831. doi: 10.1016/j.phymed.2024.155831
133. Shi J, Liu Y, Zhang Z, Zhong X, Cao Y, Ni H, et al. Zexie-baizhu decoction ameliorates non-alcoholic fatty liver disease through gut-adipose tissue crosstalk. J Ethnopharmacol. (2024) 337:118700. doi: 10.1016/j.jep.2024.118700
134. Cao Y, Yao G, Sheng Y, Yang L, Wang Z, Yang Z, et al. Jinqi jiangtang tablet regulates gut microbiota and improve insulin sensitivity in type 2 diabetes mice. J Diabetes Res. (2019) 2019:1872134. doi: 10.1155/2019/1872134
135. Wang T, Liao H, Lin J, Zhang M, Chen B, Yin R, et al. Antidiabetic action of the chinese formula shouhuitongbian and the underlying mechanism associated with alteration of gut microbiota. Phytomedicine. (2024) 129:155575. doi: 10.1016/j.phymed.2024.155575
136. Zhou K. Strategies to promote abundance of akkermansia muciniphila, an emerging probiotics in the gut, evidence from dietary intervention studies. J Funct Foods. (2017) 33:194–201. doi: 10.1016/j.jff.2017.03.045
137. Xu D, Feng M, Chu Y, Wang S, Shete V, Tuohy KM, et al. The prebiotic effects of oats on blood lipids, gut microbiota, and short-chain fatty acids in mildly hypercholesterolemic subjects compared with rice: A randomized, controlled trial. Front Immunol. (2021) 12:787797. doi: 10.3389/fimmu.2021.787797
138. Wu H, Esteve E, Tremaroli V, Khan MT, Caesar R, Mannerås-Holm L, et al. Metformin alters the gut microbiome of individuals with treatment-naive type 2 diabetes, contributing to the therapeutic effects of the drug. Nat Med. (2017) 23:850–8. doi: 10.1038/nm.4345
139. Yin J, Song Y, Hu Y, Wang Y, Zhang B, Wang J, et al. Dose-dependent beneficial effects of tryptophan and its derived metabolites on akkermansia in vitro: A preliminary prospective study. Microorganisms. (2021) 9:1511. doi: 10.3390/microorganisms9071511
140. Reid DT, Eller LK, Nettleton JE, Reimer RA. Postnatal prebiotic fibre intake mitigates some detrimental metabolic outcomes of early overnutrition in rats. Eur J Nutr. (2016) 55:2399–409. doi: 10.1007/s00394-015-1047-2
141. Ribo S, Sánchez-Infantes D, Martinez-Guino L, García-Mantrana I, Ramon-Krauel M, Tondo M, et al. Increasing breast milk betaine modulates akkermansia abundance in mammalian neonates and improves long-term metabolic health. Sci Transl Med. (2021) 13:eabb0322. doi: 10.1126/scitranslmed.abb0322
142. Alard J, Lehrter V, Rhimi M, Mangin I, Peucelle V, Abraham AL, et al. Beneficial metabolic effects of selected probiotics on diet-induced obesity and insulin resistance in mice are associated with improvement of dysbiotic gut microbiota. Environ Microbiol. (2016) 18:1484–97. doi: 10.1111/1462-2920.13181
143. Yoshihara T, Oikawa Y, Kato T, Kessoku T, Kobayashi T, Kato S, et al. The protective effect of bifidobacterium bifidum G9-1 against mucus degradation by akkermansia muciniphila following small intestine injury caused by a proton pump inhibitor and aspirin. Gut Microbes. (2020) 11:1385–404. doi: 10.1080/19490976.2020.1758290
144. Dubourg G, Lagier JC, Armougom F, Robert C, Audoly G, Papazian L, et al. High-level colonisation of the human gut by verrucomicrobia following broad-spectrum antibiotic treatment. Int J Antimicrob Agents. (2013) 41:149–55. doi: 10.1016/j.ijantimicag.2012.10.012
145. Hansen CH, Krych L, Nielsen DS, Vogensen FK, Hansen LH, Sørensen SJ, et al. Early life treatment with vancomycin propagates akkermansia muciniphila and reduces diabetes incidence in the nod mouse. Diabetologia. (2012) 55:2285–94. doi: 10.1007/s00125-012-2564-7
146. Sonomoto K, Song R, Eriksson D, Hahn AM, Meng X, Lyu P, et al. High-fat-diet-associated intestinal microbiota exacerbates psoriasis-like inflammation by enhancing systemic Γδ T cell il-17 production. Cell Rep. (2023) 42:112713. doi: 10.1016/j.celrep.2023.112713
147. Tripathi A, Debelius J, Brenner DA, Karin M, Loomba R, Schnabl B, et al. The gut-liver axis and the intersection with the microbiome. Nat Rev Gastroenterol Hepatol. (2018) 15:397–411. doi: 10.1038/s41575-018-0011-z
148. Yu LX, Schwabe RF. The gut microbiome and liver cancer: mechanisms and clinical translation. Nat Rev Gastroenterol Hepatol. (2017) 14:527–39. doi: 10.1038/nrgastro.2017.72
149. Yoshimoto S, Loo TM, Atarashi K, Kanda H, Sato S, Oyadomari S, et al. Obesity-induced gut microbial metabolite promotes liver cancer through senescence secretome. Nature. (2013) 499:97–101. doi: 10.1038/nature12347
150. Behary J, Raposo AE, Amorim NML, Zheng H, Gong L, McGovern E, et al. Defining the temporal evolution of gut dysbiosis and inflammatory responses leading to hepatocellular carcinoma in mdr2 -/- mouse model. BMC Microbiol. (2021) 21:113. doi: 10.1186/s12866-021-02171-9
151. Sandahl TD, Grønbaek H, Møller HJ, Støy S, Thomsen KL, Dige AK, et al. Hepatic macrophage activation and the lps pathway in patients with alcoholic hepatitis: A prospective cohort study. Am J Gastroenterol. (2014) 109:1749–56. doi: 10.1038/ajg.2014.262
152. Petrasek J, Mandrekar P, Szabo G. Toll-like receptors in the pathogenesis of alcoholic liver disease. Gastroenterol Res Pract. (2010) 2010:710381. doi: 10.1155/2010/710381
153. Dapito DH, Mencin A, Gwak GY, Pradere JP, Jang MK, Mederacke I, et al. Promotion of hepatocellular carcinoma by the intestinal microbiota and tlr4. Cancer Cell. (2012) 21:504–16. doi: 10.1016/j.ccr.2012.02.007
154. Yu LX, Yan HX, Liu Q, Yang W, Wu HP, Dong W, et al. Endotoxin accumulation prevents carcinogen-induced apoptosis and promotes liver tumorigenesis in rodents. Hepatology. (2010) 52:1322–33. doi: 10.1002/hep.23845
155. Soppert J, Brandt EF, Heussen NM, Barzakova E, Blank LM, Kuepfer L, et al. Blood endotoxin levels as biomarker of nonalcoholic fatty liver disease: A systematic review and meta-analysis. Clin Gastroenterol Hepatol. (2023) 21:2746–58. doi: 10.1016/j.cgh.2022.11.030
156. Scarpellini E, Scarlata GGM, Santori V, Scarcella M, Kobyliak N, Abenavoli L. Gut microbiota, deranged immunity, and hepatocellular carcinoma. Biomedicines. (2024) 12:1797. doi: 10.3390/biomedicines12081797
157. Bajaj JS, Heuman DM, Hylemon PB, Sanyal AJ, White MB, Monteith P, et al. Altered profile of human gut microbiome is associated with cirrhosis and its complications. J Hepatol. (2014) 60:940–7. doi: 10.1016/j.jhep.2013.12.019
158. Huang L, Yu Q, Peng H, Zhen Z. Alterations of gut microbiome and effects of probiotic therapy in patients with liver cirrhosis: A systematic review and meta-analysis. Medicine. (2022) 101:e32335. doi: 10.1097/md.0000000000032335
159. Ponziani FR, Bhoori S, Castelli C, Putignani L, Rivoltini L, Del Chierico F, et al. Hepatocellular carcinoma is associated with gut microbiota profile and inflammation in nonalcoholic fatty liver disease. Hepatology. (2019) 69:107–20. doi: 10.1002/hep.30036
160. Li T, Lin X, Shen B, Zhang W, Liu Y, Liu H, et al. Akkermansia muciniphila suppressing nonalcoholic steatohepatitis associated tumorigenesis through cxcr6(+) natural killer T cells. Front Immunol. (2022) 13:1047570. doi: 10.3389/fimmu.2022.1047570
161. Snider EJ, Compres G, Freedberg DE, Khiabanian H, Nobel YR, Stump S, et al. Alterations to the esophageal microbiome associated with progression from barrett’s esophagus to esophageal adenocarcinoma. Cancer Epidemiol Biomarkers Prev. (2019) 28:1687–93. doi: 10.1158/1055-9965.Epi-19-0008
162. Baxter NT, Zackular JP, Chen GY, Schloss PD. Structure of the gut microbiome following colonization with human feces determines colonic tumor burden. Microbiome. (2014) 2:20. doi: 10.1186/2049-2618-2-20
163. Osman MA, Neoh HM, Ab Mutalib NS, Chin SF, Mazlan L, Raja Ali RA, et al. Parvimonas micra, peptostreptococcus stomatis, fusobacterium nucleatum and akkermansia muciniphila as a four-bacteria biomarker panel of colorectal cancer. Sci Rep. (2021) 11:2925. doi: 10.1038/s41598-021-82465-0
164. Yamada S, Takashina Y, Watanabe M, Nagamine R, Saito Y, Kamada N, et al. Bile acid metabolism regulated by the gut microbiota promotes non-alcoholic steatohepatitis-associated hepatocellular carcinoma in mice. Oncotarget. (2018) 9:9925–39. doi: 10.18632/oncotarget.24066
165. Mirzaei R, Afaghi A, Babakhani S, Sohrabi MR, Hosseini-Fard SR, Babolhavaeji K, et al. Role of microbiota-derived short-chain fatty acids in cancer development and prevention. BioMed Pharmacother. (2021) 139:111619. doi: 10.1016/j.biopha.2021.111619
166. Lee WJ, Hase K. Gut microbiota-generated metabolites in animal health and disease. Nat Chem Biol. (2014) 10:416–24. doi: 10.1038/nchembio.1535
167. Mao J, Wang D, Long J, Yang X, Lin J, Song Y, et al. Gut microbiome is associated with the clinical response to anti-pd-1 based immunotherapy in hepatobiliary cancers. J Immunother Cancer. (2021) 9:e003334. doi: 10.1136/jitc-2021-003334
168. Xie Y, Liu F. The role of the gut microbiota in tumor, immunity, and immunotherapy. Front Immunol. (2024) 15:1410928. doi: 10.3389/fimmu.2024.1410928
169. Ansaldo E, Slayden LC, Ching KL, Koch MA, Wolf NK, Plichta DR, et al. Akkermansia muciniphila induces intestinal adaptive immune responses during homeostasis. Science. (2019) 364:1179–84. doi: 10.1126/science.aaw7479
170. Lan X, Ma J, Huang Z, Xu Y, Hu Y. Akkermansia muciniphila might improve anti-pd-1 therapy against hcc by changing host bile acid metabolism. J Gene Med. (2024) 26:e3639. doi: 10.1002/jgm.3639
171. He D, Li X, An R, Wang L, Wang Y, Zheng S, et al. Response to pd-1-based immunotherapy for non-small cell lung cancer altered by gut microbiota. Oncol Ther. (2021) 9:647–57. doi: 10.1007/s40487-021-00171-3
172. Salgia NJ, Bergerot PG, Maia MC, Dizman N, Hsu J, Gillece JD, et al. Stool microbiome profiling of patients with metastatic renal cell carcinoma receiving anti-pd-1 immune checkpoint inhibitors. Eur Urol. (2020) 78:498–502. doi: 10.1016/j.eururo.2020.07.011
173. Xu X, Lv J, Guo F, Li J, Jia Y, Jiang D, et al. Gut microbiome influences the efficacy of pd-1 antibody immunotherapy on mss-type colorectal cancer via metabolic pathway. Front Microbiol. (2020) 11:814. doi: 10.3389/fmicb.2020.00814
174. Wang Z, Qin X, Hu D, Huang J, Guo E, Xiao R, et al. Akkermansia supplementation reverses the tumor-promoting effect of the fecal microbiota transplantation in ovarian cancer. Cell Rep. (2022) 41:111890. doi: 10.1016/j.celrep.2022.111890
175. Derosa L, Routy B, Thomas AM, Iebba V, Zalcman G, Friard S, et al. Intestinal akkermansia muciniphila predicts clinical response to pd-1 blockade in patients with advanced non-small-cell lung cancer. Nat Med. (2022) 28:315–24. doi: 10.1038/s41591-021-01655-5
176. Zhu Z, Cai J, Hou W, Xu K, Wu X, Song Y, et al. Microbiome and spatially resolved metabolomics analysis reveal the anticancer role of gut akkermansia muciniphila by crosstalk with intratumoral microbiota and reprogramming tumoral metabolism in mice. Gut Microbes. (2023) 15:2166700. doi: 10.1080/19490976.2023.2166700
177. Fan L, Xu C, Ge Q, Lin Y, Wong CC, Qi Y, et al. A. Muciniphila suppresses colorectal tumorigenesis by inducing tlr2/nlrp3-mediated M1-like tams. Cancer Immunol Res. (2021) 9:1111–24. doi: 10.1158/2326-6066.Cir-20-1019
178. Zhang L, Ji Q, Chen Q, Wei Z, Liu S, Zhang L, et al. Akkermansia muciniphila inhibits tryptophan metabolism via the ahr/β-catenin signaling pathway to counter the progression of colorectal cancer. Int J Biol Sci. (2023) 19:4393–410. doi: 10.7150/ijbs.85712
179. Hou X, Zhang P, Du H, Chu W, Sun R, Qin S, et al. Akkermansia muciniphila potentiates the antitumor efficacy of folfox in colon cancer. Front Pharmacol. (2021) 12:725583. doi: 10.3389/fphar.2021.725583
180. Xu Q, Gao J, Zhao R, Li H, Cui H, Yuan Z, et al. Akkermansia muciniphila-derived pentadecanoic acid enhances oxaliplatin sensitivity in gastric cancer by modulating glycolysis. Pharmacol Res. (2024) 206:107278. doi: 10.1016/j.phrs.2024.107278
181. Wu R, Xiong J, Zhou T, Zhang Z, Huang Z, Tian S, et al. Quercetin/anti-pd-1 antibody combination therapy regulates the gut microbiota, impacts macrophage immunity and reshapes the hepatocellular carcinoma tumor microenvironment. Front Biosci (Landmark Ed). (2023) 28:327. doi: 10.31083/j.fbl2812327
182. Teng L, Wang K, Chen W, Wang YS, Bi L. Hyr-2 plays an anti-lung cancer role by regulating pd-L1 and akkermansia muciniphila. Pharmacol Res. (2020) 160:105086. doi: 10.1016/j.phrs.2020.105086
183. Wang Y, Zhang X, Li J, Zhang Y, Guo Y, Chang Q, et al. Sini decoction ameliorates colorectal cancer and modulates the composition of gut microbiota in mice. Front Pharmacol. (2021) 12:609992. doi: 10.3389/fphar.2021.609992
184. Shao L, Guo YP, Wang L, Chen MY, Zhang W, Deng S, et al. Effects of ginsenoside compound K on colitis-associated colorectal cancer and gut microbiota profiles in mice. Ann Transl Med. (2022) 10:408. doi: 10.21037/atm-22-793
185. Bai X, Duan Z, Deng J, Zhang Z, Fu R, Zhu C, et al. Ginsenoside rh4 inhibits colorectal cancer via the modulation of gut microbiota-mediated bile acid metabolism. J Adv Res. (2024). doi: 10.1016/j.jare.2024.06.028
186. Depommier C, Everard A, Druart C, Plovier H, Van Hul M, Vieira-Silva S, et al. Supplementation with akkermansia muciniphila in overweight and obese human volunteers: A proof-of-concept exploratory study. Nat Med. (2019) 25:1096–103. doi: 10.1038/s41591-019-0495-2
187. Perraudeau F, McMurdie P, Bullard J, Cheng A, Cutcliffe C, Deo A, et al. Improvements to postprandial glucose control in subjects with type 2 diabetes: A multicenter, double blind, randomized placebo-controlled trial of a novel probiotic formulation. BMJ Open Diabetes Res Care. (2020) 8:e001319. doi: 10.1136/bmjdrc-2020-001319
Keywords: hepatocellular carcinoma, Akkermansia muciniphila, immunotherapy, traditional Chinese medicine, intestinal flora
Citation: Yang Y and Shi X (2025) Big lessons from the little Akkermansia muciniphila in hepatocellular carcinoma. Front. Immunol. 16:1524563. doi: 10.3389/fimmu.2025.1524563
Received: 07 November 2024; Accepted: 30 January 2025;
Published: 14 February 2025.
Edited by:
Chunjing Wang, University College London, United KingdomReviewed by:
Junfeng Zhang, Nanjing University of Chinese Medicine, ChinaCopyright © 2025 Yang and Shi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xinli Shi, c3hsc3Vuc2hpbmVAc2luYS5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.