- 1School of Medicine, Oregon Health & Science University, Portland, OR, United States
- 2Department of Surgery, Division of Vascular and Endovascular Surgery, Oregon Health & Science University, Portland, OR, United States
- 3Department of Pediatrics, Division of Gastroenterology, Oregon Health & Science University, Portland, OR, United States
- 4Division of Vascular Surgery, Research & Development, Portland Veterans Affairs (VA) Health Care System, Portland, OR, United States
Introduction: Post-thrombotic syndrome (PTS) is a chronic complication of deep vein thrombosis (DVT). Given its impact on vascular health, understanding risk factors for the development of PTS, as well as conditions such as metabolic syndrome that may contribute to vascular inflammation, is crucial. Metabolic syndrome is a constellation of factors that increase cardiovascular disease risk, insulin resistance, diabetes mellitus (DM), and cerebrovascular disease. Despite the established connection between metabolic syndrome and venous thromboembolism (VTE), the association between metabolic syndrome and PTS has yet to be explored.
Methods: A literature search identified studies regarding PTS and metabolic syndrome and the individual components of metabolic syndrome. A specialist performed the search, and studies were identified through PubMed, Ovid Medline, and Cochrane in accordance with PRISMA guidelines. Search terms included “post-thrombotic syndrome” and “metabolic syndrome” as well as “obesity,” “hyperglycemia,” “hypertension,” “dyslipidemia,” and “insulin resistance.” Two people independently screened articles and consolidated differences. Abstract-only studies, review articles, case studies, and conference abstracts were excluded. Case reports, literature reviews, and studies not discussing PTS were excluded. Prospective cohort, retrospective cohort, and case-control studies were included. All English-based studies that met inclusion criteria published before January 3rd, 2024, were included.
Results: 281 articles were initially identified. After abstract and title screening, 16 articles underwent full-text review. Of the 16 articles that underwent review, nine were included in the final analysis. Among the selected articles, eight out of nine mentioned obesity as a risk factor for developing PTS, making it the most common component mentioned. Hypertension, diabetes mellitus, hyperlipidemia, and low high-density lipoprotein (HDL) followed in prevalence. There was no noted difference between inflammatory markers in patients with and without PTS.
Conclusion: Metabolic syndrome and its components, individually and in association with PTS, are not commonly examined. Eight articles examined the association of obesity with the development of PTS. This review identified a strong association between obesity, particularly abdominal or visceral obesity, and the development of PTS. While the association between PTS and VTE is established, further research is needed to identify the role of metabolic syndrome in the development of PTS.
Introduction
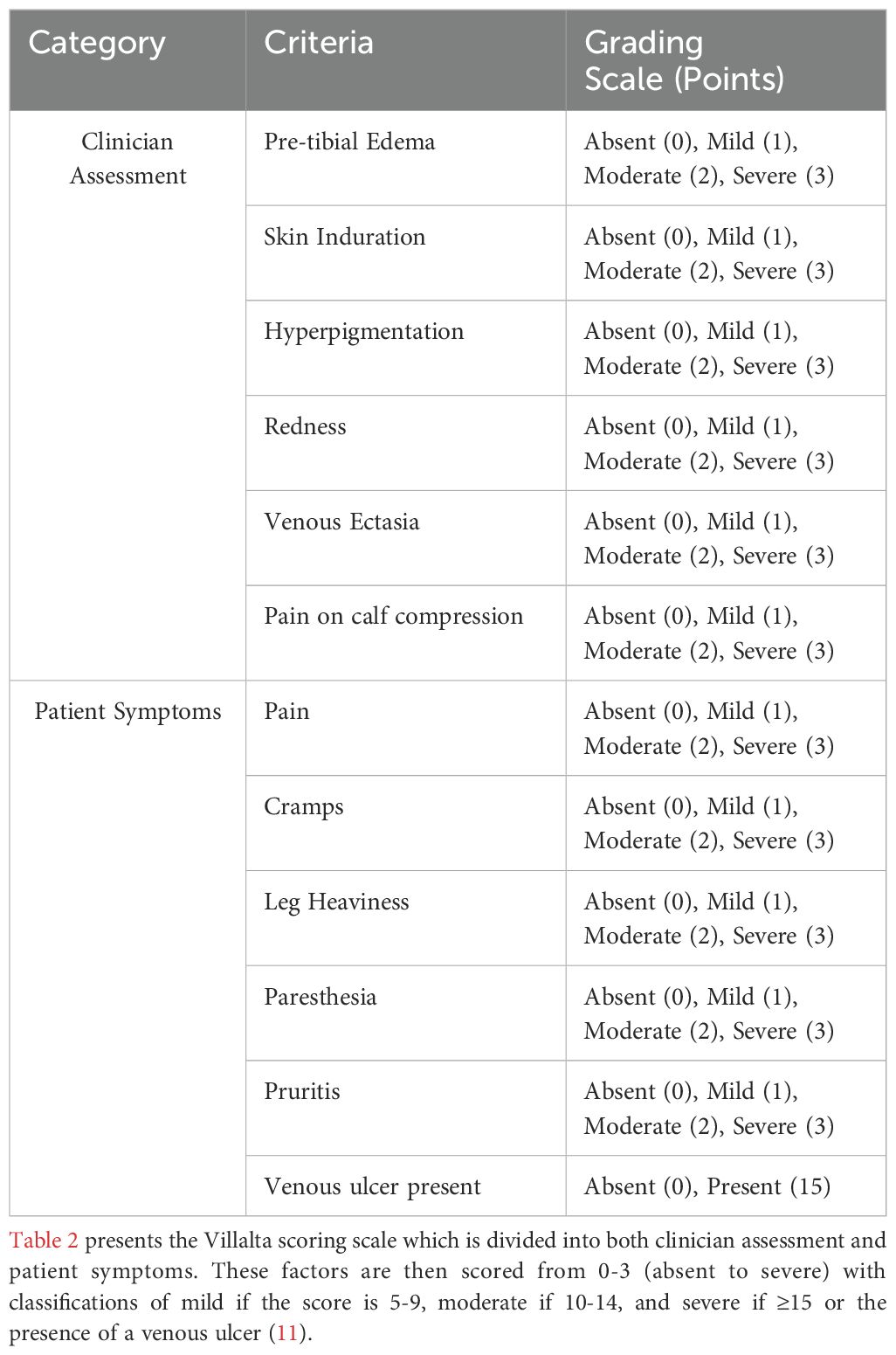
Post-thrombotic syndrome (PTS), a long-term complication of deep vein thrombosis (DVT), is marked by chronic symptoms such as persistent leg pain, swelling, venous ulcers, and skin changes. These symptoms, which worsen with activity, can significantly diminish patients’ quality of life and impose a considerable burden on healthcare systems. It has been observed that 20-50% of individuals with DVT develop PTS, a condition that arises from residual venous obstruction and valvular reflux (1). The diagnosis of PTS is primarily clinical, based on a patient’s history of DVT and the presence of characteristic signs and symptoms. Validated tools, such as the Villalta score, evaluate symptom severity and clinical signs to stratify PTS into mild, moderate, or severe categories (2). The Villalta score is a widely recognized disease scale used for diagnosing and grading the severity of PTS (2). It evaluates five symptoms (pain, heaviness, cramps, paresthesia, and pruritus) and six clinical signs (pretibial edema, hyperpigmentation, venous ectasia, skin induration, and redness), scoring each from 0 (absent) to 3 (severe). A total score of ≥5 confirms PTS, with scores of 5–9 indicating mild PTS, 10–14 moderate PTS, and ≥15 or the presence of a venous ulcer denoting severe PTS. This standardized system is valued for its ability to monitor disease progression and assess treatment outcomes (2). Risk factors for PTS can be categorized into three groups: those present at the time of DVT diagnosis (including increased age, elevated body mass index (BMI), pre-existing primary venous insufficiency, history of ipsilateral DVT, and proximal DVT location), those related to the initial treatment (notably inadequate anticoagulation), and those identified during follow-up (such as recurrent DVT, residual thrombosis on imaging, and persistent elevation of D-dimer levels). It is unclear what factors are most critical for mitigating the risk of PTS. For those already affected, treatment focuses on symptomatic management through compression therapy, promoting healthy lifestyle choices, and, if necessary, surgical intervention (1).
Metabolic syndrome is a constellation of several disorders that may lead to an increased risk of developing cardiovascular disease, insulin resistance, diabetes mellitus (DM), and cerebrovascular disease (3). Metabolic syndrome is diagnosed if the patient has any three or more of the following conditions: waist circumference: >40 inches (males) & 35 inches (females), hypertension, >130/85 mmHg, or requiring drug treatment for elevated blood pressure, hyperglycemia: elevated fasting glucose >100 mg/dL or requiring drug treatment for elevated blood glucose, hypertriglyceridemia: elevated triglycerides >150 mg/dL or requiring drug treatment for elevated triglycerides, low high-density lipoprotein (HDL): <40 mg/dL (men) <50 mg/dL (women) or requiring drug treatment for low HDL cholesterol (4).
The etiology of metabolic syndrome is complex, involving genetic predisposition, corpulence with increased waist circumference, excess body mass, and a sedentary lifestyle. The root cause is the accumulation of excess adipose tissue, leading to tissue dysfunction and insulin resistance. The increased adipose tissue releases proinflammatory cytokines, including resistin, leptin, adiponectin, plasminogen activator inhibitor, and tumor necrosis factor, negatively affecting and modifying insulin sensitivity. Insulin resistance may be exacerbated by anomalies in the insulin receptor, diminished insulin production, and disruptions in the signaling cascade, impeding the body’s ability to utilize insulin efficiently. These impairments hinder the body’s ability to effectively use insulin, leading to a range of metabolic disturbances, notably metabolic syndrome, which can manifest as vascular injury and autonomic dysregulation (5, 6).
In the US, the prevalence of metabolic syndrome was estimated to be 93 million people in 2018 and rising (7). This multifaceted health condition increases the risk of many other disorders, particularly cardiovascular disease and type 2 DM (8). Moreover, metabolic syndrome has also been linked to VTE, which consists of DVT and pulmonary embolism (PE) (7). An extensive body of literature describes the relationship between metabolic syndrome and VTE, highlighting underlying causes of metabolic syndrome that can help mitigate the risk of this life-threatening condition.
Despite the purported connection between metabolic syndrome and VTE, little is known about the relation between metabolic syndrome and PTS (9). The pro-inflammatory environment that results from metabolic syndrome increases the lifetime risk of thrombosis (10). Given the health burden associated with PTS, it is essential to better understand their complex relationship and to identify potential risk factors that may contribute to the development of PTS. This systematic review seeks to explore and identify risk factors and underlying inflammatory mechanisms in patients with metabolic syndrome that may lead to the development of PTS.
Methods
A comprehensive literature search was performed to identify all studies that discussed the development of PTS and metabolic syndrome or the individual components of metabolic syndrome in accordance with PRISMA guidelines. The electronic search was performed by a specialist, and studies were identified through Pubmed, Ovid Medline, and Cochrane.
Inclusion and exclusion criteria
Only clinical articles specifically discussing PTS, metabolic syndrome, or its components in humans were included. Search terms included “post-thrombotic syndrome” and “metabolic syndrome” as well as “obesity,” “hyperglycemia,” “hypertension,” “dyslipidemia,” and “insulin resistance.” Articles discussing only chronic venous insufficiency and PTS in pediatric populations were excluded. Abstract-only studies, review articles, case studies, and conference abstracts were excluded. Case reports, literature reviews, and studies that did not directly discuss PTS were excluded. Prospective cohort, retrospective cohort, and case-control studies were included. All English-based studies that met the inclusion criteria published before January 3rd, 2024, were included.
Screening and extraction
Two reviewers independently screened articles by abstracts and titles and collaborated to consolidate their differences. Disagreements were resolved by discussion of the articles with a third reviewer. For potential articles, the full text was retrieved and reviewed independently. Once the included studies were finalized, data was collected regarding study characteristics, patient demographics, comorbidities, and clinical outcomes.
Results
There were 281 articles initially identified through our database search. After abstract and title screening, 16 articles underwent full-text review. Of the 16 articles that underwent full-text review, nine were included in the final analysis (Figure 1). The majority of the studies included were retrospective (n=5), followed by prospective cohort studies (n=3), and case-control studies (n=1). The characteristics of these papers are presented in Table 1. Of the nine studies included, three were multi-institutional. Across the studies included in this review, PTS was predominantly diagnosed using the Villalta score (Table 2). In addition to the Villalta score, some studies supplemented clinical assessment with imaging to detect residual thrombosis or venous obstruction. The Villalta score was used for diagnosis in 7 of the 9 included studies (12–18). Clinical examination was utilized to diagnose PTS in 6 of the 9 studies included (12–17). Ultrasonography was used in 4 studies (13–15, 19) and patient questionaries collecting data on symptoms and signs of PTS were utilized in 2 studies (13, 16). Color duplex sonography was utilized in three studies to objectively confirm DVT (12, 16, 19).

Figure 1. PRISMA flow diagram depicting the process of identifying, screening, and including studies in this systematic review.

Table 1. Characteristics of studies included in the systematic review of the association of post-thrombotic syndrome with metabolic syndrome and inflammation.
Sample sizes in the included studies ranged from 83 to 1,668 patients. A total of 6258 patients were included in the studies, 3030 (48%) of whom were male (Table 3). Ages reported ranged from 18 – 97, and the mean age of all patients was 52.93 ± 5.40 years. The mean BMI of all patients was 29.28 ± 2.11 kg/m2. Notably, all nine articles reference obesity as a risk factor for developing PTS, and obesity was noted in 2297 patients (37%). The average length of follow-up for these patients was 26.38 ± 27.81 months (range 7- 96 months).
Of the components of metabolic syndrome, obesity was the most common component noted in patients. This was followed by hypertension (n=629, 10%), DM (n=400, 6%), hypertriglyceridemia (n=268, 4%), and then low HDL (n=13, 0.2%). 1,774 patients (28%) were noted to have PTS.
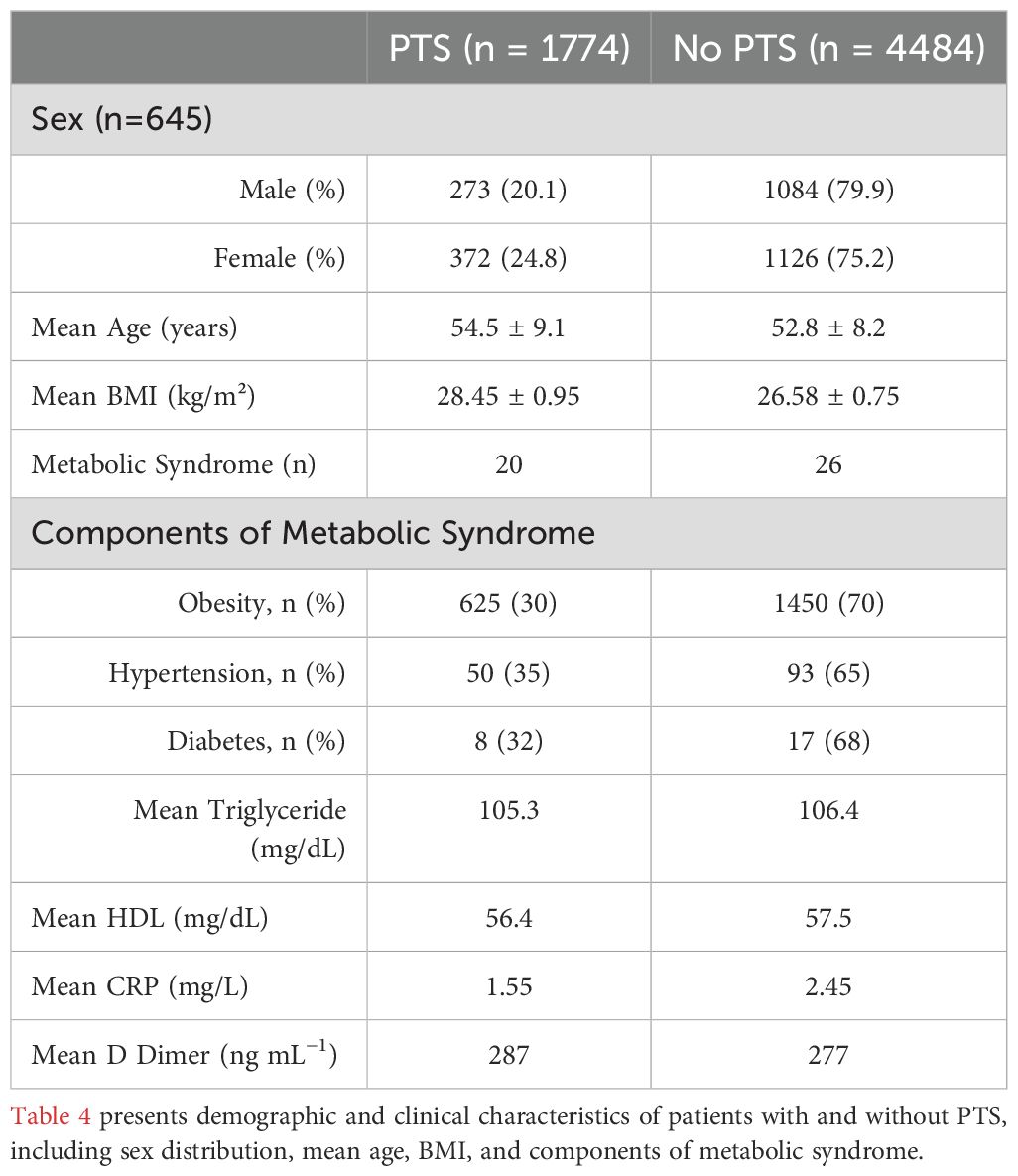
Of the 1774 patients with PTS, gender was reported for 645, of which 273 (42%) were men (Table 4). The average age for patients with PTS was 54.5 ± 9.1 years, and the average BMI was 28.45 ± 0.95 kg/m2, compared to an average age of 52.8 ± 8.2 years and BMI of 26.58 ± 0.75 kg/m2 for patients without PTS. Obesity was reported in 625 patients (35.2%) with PTS, as opposed to 1450 (34.7%) without PTS. A total of 747 patients were diagnosed with PTS using the Villalta scale. Among these, 135 patients had severe PTS, defined by a Villalta score of ≥ 15, while 662 were classified as having mild to moderate PTS. Among studies utilizing the Villalta scale, Rattazzi et al. (15) reported that patients with PTS had an average Villalta score of 7.5 ± 2.7, compared to 2.3 ± 1.1 in patients without PTS, highlighting the significant difference in clinical severity between these groups.
Rattazzi et al. (15) presented the only study that directly assessed the relationship between patients diagnosed with metabolic syndrome and PTS. 120 patients were evaluated, and of the 49 patients reported to have PTS, only 20 (41%) also had metabolic syndrome.
Inflammatory markers were evaluated in 4 of the 9 studies included (44%) (12–15). These comprised adiponectin, leptin, resistin, plasminogen activator inhibitor- 1, D-dimer, fibrinogen, and C-reactive protein (CRP) levels. Of patients with reported CRP, the average CRP for patients with PTS was 1.55 mg/L, compared to 2.45 mg/L for patients without. D-dimer was only reported in one study, and the average reported for patients with PTS was 287 ng mL−1, compared to 277 ng mL−1 for those without PTS (12). This difference was insignificant, and the reference range was (226-337) ng mL−1.
Discussion
PTS is a chronic condition that often develops following DVT. While the exact causes are not fully understood, the existing literature suggests inflammation plays a key role in its onset (20). A history of DVT contributes to the development of PTS, as the thrombus can damage valves, leading to venous obstruction and hypertension, which presents as pain, swelling, discoloration, and ulceration (21). The initial inflammation is responsible for the thrombus resolution by clot degradation, which simultaneously promotes collateral damage to the venous tissue and valves. This chronic inflammation may have a role in PTS (20). Inflammatory adipokines, such as leptin and adiponectin, play a significant role in this process. Elevated leptin levels promote platelet aggregation and enhance plasminogen activator inhibitor-1 (PAI-1), impairing fibrinolysis, while lower adiponectin levels mitigate these effects by reducing oxidative stress and promoting nitric oxide production. These adipokines have been shown to predict PTS independently of obesity, underscoring their unique contribution to venous pathophysiology (12). Individuals with chronic inflammation are at a higher risk of experiencing recurrent DVT. This hypercoagulable state may be attributed to the complex interactions between inflammatory processes and the coagulation system triggered by ongoing low-grade inflammation (22). Residual venous obstruction and underlying chronic inflammation are common findings in patients with PTS.
Metabolic syndrome is comprised of a combination of three or more of the following: obesity, hypertension, hyperglycemia, hypertriglyceridemia, and low HDL. Metabolic syndrome increases the risk of developing conditions such as cardiovascular disease, DM, and cerebrovascular disease. The underlying mechanism of metabolic syndrome involves a combination of genetics and lifestyle factors, with inflammation resulting from excess adipose tissue playing a crucial role. The components of metabolic syndrome and related inflammation can influence the development of PTS (Figure 2). Our discussion below breaks down the current literature regarding each component of metabolic syndrome and its association with the development of PTS.
Obesity
In our review, eight of the nine included studies discussed the relationship between obesity and the development of PTS (12–19). Ageno et al. (18) was the only study that did not directly mention obesity as a contributor—but rather BMI as a driving factor in the development of PTS. In Rattazzi et al.’s (15) assessment of metabolic syndrome and its components’ relationship with PTS, they discovered that obesity, measured by visceral adiposity, significantly increases the likelihood of developing PTS. Waist circumference, a marker of visceral fat, has been strongly correlated with PTS severity as measured by the Villalta score (15). This metric serves as both an indicator of obesity and a contributor to inflammatory pathways that exacerbate venous hypertension and remodeling, directly driving PTS progression (15). This finding reinforces obesity as a driving factor for PTS, even in the absence of other components of metabolic syndrome. Interestingly, one of the studies by El-Maynar et al. (23) found that although obesity contributes to the development of DVT and the pro-inflammatory state, the incidence of PTS was similar between the obese and non-obese groups. This suggests that while obesity increases the risk for DVT, it does not necessarily lead to a higher risk of developing PTS. The remaining six studies, however, further demonstrated that obesity is associated with an increased risk of PTS (12–14, 16, 17, 19). The relationship between obesity, inflammation, and coagulation emphasizes the prothrombotic state resulting from increased adipose tissue. Given this, it is understandable that obesity acts as a key risk factor for the development of thrombotic sequala, such as VTE and DVT, and the progression to PTS and appears to drive a localized inflammatory response (15).
Abdominal or visceral obesity, a marker of metabolic syndrome is reported to lead to stasis of blood, which is a precursor to DVT (24). The risk of developing recurrent DVT rises by 25% with every 10-point increase in BMI (25). This was reinforced in our review by El-Meynar et al. (23), whose study demonstrated that individuals with obesity are more prone to thrombotic events. Similarly, another study demonstrated a 2-fold increase in recurrent DVT with a BMI of over 30 compared to normal BMI (26). Obesity affects the coagulation cascade by decreasing fibrin degradation and increasing clotting factors (27). Additional findings further support this by demonstrating elevated levels of d-dimer, fibrinogen, coagulation factor VIII, and coagulation factor IX significantly correlated to BMI (28). These coagulation changes are likely mediated by the metabolic activity of adipose tissue, which secretes bioactive molecules that influence both systemic and localized hemostatic balance. This can be explained by the release of adipokines from excess fatty tissue that shifts the pendulum from an antithrombotic to a prothrombotic state (29). Obesity can also be considered a chronic low-grade inflammatory condition where the proinflammatory factors secreted by adipose tissue increase. Weight loss diminishes this proinflammatory state conferred by excess fatty tissue and clotting factors (30).
Hypertension
The role of hypertension in PTS remains unclear. El-Meynar et al. (23) noted that hypertension is more prevalent in obese patients and is one of the leading cardiovascular risk factors that contribute to DVT. Similarly, Rattazzi et al. (15) also addressed the relation between hypertension and its contribution to overall cardiovascular and thrombotic risk. However, in both aforementioned studies, it was noted that there is not an observed association between high blood pressure and the development of PTS, as rates of hypertension were equivalent between patients with and without PTS. Asim et al. (19) further concluded that while hypertension was prevalent in DVT patients (37%) and in those with recurrent DVT (38.4%), it was not a statistically significant independent risk factor for DVT recurrence.
Hypertension is associated with a higher risk of VTE (31, 32). Hypertension may contribute to hemostasis, endothelium dysfunction, and vessel inflammation, leading to an increased risk of thrombosis and ultimately a pro-inflammatory state. Inflammation subsequently leads to the stiffening of blood vessels and vascular remodeling, and these processes play a critical role in the development of PTS (15, 33). Pro-inflammatory cytokines, including interleukin-6 (IL-6) and tumor necrosis factor-alpha (TNF-α), promote oxidative stress, leukocyte adhesion, and the recruitment of macrophages, which release matrix metalloproteinases (33). These enzymes degrade the extracellular matrix, contributing to structural alterations in the vascular wall. Additionally, endothelial cells, under inflammatory stress, increase the expression of adhesion molecules like vascular cell adhesion molecule 1 (VCAM-1) and intercellular adhesion molecule-1 (ICAM-1), further amplifying leukocyte infiltration and local inflammatory responses. This cascade perpetuates vascular remodeling through smooth muscle cell proliferation, collagen deposition, and impaired nitric oxide bioavailability, all of which contribute to vessel stiffening and venous dysfunction (33). In the context of metabolic syndrome, hypertension frequently coexists with other risk factors, such as obesity and hyperlipidemia, yet its direct role in PTS remains less clear, with systemic effects like inflammation and endothelial dysfunction taking precedence (23). Hypertension has also been linked to an increased risk of recurrent VTE, which indirectly elevates the risk of developing PTS by compounding venous damage and obstruction (16). Our results suggest that while hypertension and obesity often coexist in patients resulting in an increased risk of DVT, the role of hypertension in PTS may hinge on additional factors.
Hyperglycemia
Type 2 DM is a chronic disease of hyperglycemia and is a known risk factor for VTE, which includes PE and DVT (31, 34, 35). Three studies included in our review discussed the impact of DM. El-Meynar et al. (23) highlighted how DM, along with other metabolic conditions, contribute to the development of a prothrombotic state, thereby increasing the risk for DVT and PTS. While no direct association between the conditions was made, Asim et al. (19) identified DM as a comorbid risk factor for recurrent DVTs. Conversely, Rattazzi et al. (15) found no association between DM and the development of PTS.
Acute hyperglycemia is associated with a poorer prognosis in thrombosis (36). In DM, hyperglycemia may lead to a hypercoagulable state, and both acute and chronic hyperglycemia increase the risk for VTE (36). Galanaud et al. (14) shows that elevated fasting glucose levels, even without a diagnosis of diabetes, has been linked to heightened inflammatory and thrombotic states. This is measured by elevated levels of inflammatory markers such as CRP and IL-6, as well as thrombotic markers like D-dimer and fibrinogen. This suggests that glucose dysregulation may exacerbate vascular complications associated with PTS. In chronic hyperglycemia, many physiologic changes result in a prothrombotic state; this includes a decrease in activated partial thromboplastin time, prothrombin time, protein C, antithrombin, and fibrinolysis, and a paradoxical increase in clotting factors II, VII, VIII, fibrinogen, and tissue factor (37–41). Glucose metabolism dysfunction may also affect residual venous obstruction, contributing to long-term PTS progression (14). Correction of hyperglycemia, irrespective of treatment modality, reduced thrombotic events in patients with DM (42). Many hypotheses exist that try to explain the relationship between hyperglycemia and hypercoagulability (43). Elevated glucose and insulin increase levels of plasminogen activator inhibitor activity, which reduces tissue plasminogen activator and subsequent fibrinolysis (44). Exposure of advanced glycation end products to endothelial cells causes activation of the coagulation cascade, and procoagulant activity is seen (45). In type I DM, when insulin is absent, tissue factor and plasminogen activator inhibitor levels surge. These elevations are accompanied by malondialdehyde and protein carbonyl groups, which are surrogates for oxidative stress (46). These changes may lead to an overall pro-inflammatory and pro-thrombotic state but have not been shown to increase an individual’s risk for the development of PTS in the existing literature.
Hyperlipidemia
Four studies included in our review assessed lipid profiles. Asim et al. (19) observed that hyperlipidemia and dyslipidemia were once again noted to be more common in patients with recurrent DVT, with 34.9% of patients with recurrent DVT having dyslipidemia compared to 25.8% in single DVT cases. El-Meynar et al. (23) found that dyslipidemia and hypertriglyceridemia were more commonly observed in obese patients, suggesting that the disruption in fat metabolism was contributing to the prothrombic state. Similarly, Mrozinska et al. (12) noted that patients who developed PTS had higher triglyceride levels compared to those without, highlighting the potential role triglycerides may contribute to a prothrombotic environment. This was however contradicted in the study by Rattazzi et al. (15) who found no association between lipid levels and the development of PTS. In addition, no statistically significant differences were found between groups treated with a statin and those without (15).
Hypertriglyceridemia is recognized for elevating blood viscosity, which can lead to a procoagulant state (47). This effect is primarily linked to venous thrombosis. The pro-thrombotic effects of elevated triglycerides involve several mechanisms: enhanced platelet aggregation, reduced activity of antithrombin III and interactions with coagulation factors, a rise in proinflammatory markers, and endothelial dysfunction (48). In addition to increasing blood viscosity, triglycerides increase plasminogen activator inhibitor and factor VII (49). Triglyceride-rich lipoproteins (TRL) in the blood are considered risk factors and contribute to atherosclerosis and inflammation (49, 50). TRLs interact with coagulation factors, promote endothelial dysfunction, and contribute to chronic low-grade inflammation. This effect is largely mediated by increased expression of vascular adhesion molecules and tissue factors, as observed by multiple studies (15, 18, 23). These particles accumulate in the vessels and are not cleared as frequently (45). In the context of atherosclerosis, foam cells—lipid-laden macrophages—accumulate within arterial walls, contributing to plaque formation. These foam cells upregulate metalloproteinases, enzymes that degrade extracellular matrix components, leading to the erosion of the fibrous cap covering the plaque (48). This degradation weakens the cap, increasing the risk of plaque rupture and subsequent thrombus formation (2). Additionally, TRLs achieve this prothrombotic state by directly increasing intercellular adhesion molecule-1, vascular cell adhesion molecule-1, and tissue factor in endothelium lining (51). These molecules facilitate leukocyte adhesion and transmigration, further promoting inflammation and thrombogenesis (45, 48). Elevated blood levels of triglycerides result in aberrant platelet overactivity (52). Increases in triglycerides above 200 mg/dL have been associated with a decrease in antithrombin activity and increased platelet aggregation (52).
High-density lipoprotein
Rattazzi et al. (13) was the only study that assessed HDL levels and their impact on the development of PTS and found no significant association between low HDL and the development of PTS, however, it suggests that the atherogenic lipid profile, defined by low HDL and high triglycerides, could play a potential role in the development of PTS.
HDL is a lipoprotein, and increased levels of HDL confer protection against cardiovascular disease (53). Elevated HDL levels would reduce the risk of developing DVT (54). HDL is known to have antithrombotic effects on blood vessels (55, 56). Additionally, patients with low HDL levels were more likely to develop DVT (31). This antithrombotic effect may be explained by reducing platelet aggregation and activation of the coagulation cascade. HDL also increases the production of endothelial prostacyclin and nitric oxide, which inhibits platelet aggregation, deactivates clotting factors, and reduces tissue factor production (57). Ultimately, thrombin is decreased, which reduces fibrin conversion from fibrinogen, reducing platelet aggregation (58).
Inflammatory and metabolic associations
Inflammation plays a role in many components of metabolic syndrome, and this was discussed in several studies. Rattazzi et al. (15) compared the prevalence of metabolic syndrome in patients with and without diagnosed PTS. While no significant difference in the overall prevalence of metabolic syndrome was found between the two groups, a strong association between visceral adiposity and both the presence and severity of PTS was identified, suggesting that visceral fat, rather than metabolic syndrome, is more closely linked to PTS. This association was further emphasized by a linear correlation between the Villalta score, which measures PTS severity, and waist circumference, a marker of visceral obesity, underscoring the role of localized fat accumulation in driving inflammation and PTS development (15). This was the only article that assessed the two chronic conditions together (15).
This finding was further supported by Mrozinska et al. (12), who found that lower adiponectin, which has anti-thrombotic effects, and higher leptin, which promotes platelet aggregation, measured three months after DVT, despite obesity or inflammatory states, independently predicted the development of PTS. In the article, leptin was identified as a key pro-inflammatory and pro-thrombotic adipokine, promoting platelet aggregation and enhancing the production of PAI-1, which impairs fibrinolysis. Conversely, adiponectin was shown to protect against thrombosis by reducing platelet activation and mitigating oxidative stress through the promotion of endothelial nitric oxide production. This imbalance of adipokines suggests that inflammation and thrombosis in PTS are influenced by adipose tissue beyond general obesity metrics. This interplay between adipokines and inflammation highlights how obesity-driven chronic inflammatory states may exacerbate the risk of PTS, even in patients without overt metabolic syndrome. All four studies that discussed inflammatory markers concluded that inflammatory markers such as CRP and D-dimer do not predict the development of PTS (12–15).
Ende-Verhaar et al. (13) investigated the role of inflammation over an 8-year period in patients diagnosed with PTS and found that, although CRP is an inflammatory marker, it did not have a significant predictive value for PTS when compared to factors such as obesity and thrombus location. This study highlighted that obesity, particularly visceral obesity, and recurrent DVT were stronger drivers of PTS than chronic systemic inflammation alone. The findings suggest that inflammatory biomarkers like CRP may reflect generalized inflammation without capturing localized processes, such as endothelial dysfunction and venous remodeling, that are critical to PTS. Tick et al. (16) expanded on this concept, emphasizing that venous valvular incompetence and persistent venous obstruction create a localized inflammatory state that is significant in PTS progression. Obesity amplified these effects, increasing the 1-year cumulative incidence of PTS from 22% in normal-weight patients to 34% in obese individuals (16).
Metabolic syndrome is also linked to a hypofibrinolytic and procoagulant state. Clot lysis time is considerably increased in patients with metabolic syndrome compared to healthy controls (59). Metabolic syndrome may alter the production of adipokines due to excess adipose tissue, which leads to low-grade chronic inflammation responsible for endothelial dysfunction, vascular remodeling, and thrombosis (24, 25, 60). This aligns with the observations of Rattazzi et al. (15) and Mrozinska et al. (12), who identified visceral fat and adipokine dysregulation as pivotal factors in PTS pathogenesis. The pathophysiology of idiopathic VTE may involve all the components of metabolic syndrome, which may also serve as a link between atherosclerosis and VTE (31).
Inflammation is crucial in thrombus formation, mediated by inflammatory pathway activation and recruitment of platelets and leukocytes. Thrombosis and inflammation are distinct systems but have significant overlap (61). Under physiological circumstances, inflammation activates the coagulation system, a component of the body’s natural reaction to pathogens, ultimately suppressing their spread through blood. This process, known as immunothrombosis, involves leukocytes and platelets that activate the coagulation cascade (62). In PTS, this inflammatory response may persist or become dysregulated, leading to chronic endothelial activation, venous hypertension, and impaired fibrinolysis, which drive the progression of post-thrombotic changes. Galanaud et al. (14) underscored that residual venous obstruction, a hallmark of PTS, is exacerbated by chronic inflammation and inadequate anticoagulation, further worsening venous stasis and thrombus burden.
Inflammation activates thrombin, which in turn affects endothelial cells, smooth muscle cells, and platelets. Protease-activated receptors are also actuated by thrombin. This increases endothelium expression of vascular cell adhesion molecules, intracellular adhesion molecules, P-selectin, and E-selectin. These molecules facilitate leukocyte and platelet adhesion, amplifying local inflammation and thrombus stabilization, which may persist in PTS. Chemokine production by the vascular endothelium is also increased, including monocyte chemotactic protein-1, platelet-derived growth factor, and interleukins (60). Platelets also express CD40 ligands, which activate leukocytes that have the CD40 receptor (60). It was noted by Asim et al. (19) that recurrent DVT makes matters complex as there are increased inflammatory markers and abnormal coagulation profiles in patients with repeated thrombotic episodes which lead to higher rates of PTS. This interplay further highlights the significant overlap between vascular remodeling, inflammation, and thrombosis in the context of metabolic syndrome and PTS. Addressing the underlying inflammation could provide preventative therapeutic opportunities.
Follow up periods
Long-term follow-up periods beyond one year provided valuable insights into the progression and complications of PTS. Of the studies included, eight of the nine had follow-up periods of one year or greater (12, 13, 15–19, 23). Ageno et al. (18) observed that the cumulative incidence of PTS increased progressively over time, reaching 30.3% by eight years, with symptoms such as leg heaviness, varicose veins, and venous ulcers becoming more pronounced in some patients. Interestingly, while some individuals improved in their PTS classification, others experienced worsening symptoms, with 13 patients in Ageno et al.’s (18) cohort progressing from moderate to severe PTS over eight years. Tick et al. (16) reported that severe PTS occurred in 7% of patients within one year and noted that women, obese individuals, and those with proximal DVT faced higher risks, reflecting the impact of these risk factors on disease severity. Spiezia et al. (17) found that obesity and iliofemoral DVT were significant predictors of PTS over a three-year follow-up, and patients with severe PTS often presented with persistent leg swelling, pain, and venous ulcers despite treatment. Rattazzi et al. (15) also emphasized the impact of excess adipose tissue on venous health through the link between visceral adiposity and persistent venous inflammation and obstruction, as observed in patients assessed more than two years after their initial DVT diagnosis. El-Menyar et al. (23) highlighted recurrent DVT as a significant complication associated with PTS, noting that while the link was strong initially, its impact diminished after adjusting for confounders, such as the duration of anticoagulation therapy. Overall, longer follow-up periods help in understanding the progression of disease as well as the impact of risk factors in patient outcomes.
Potential risk factors
Several studies highlighted key risk factors for PTS, emphasizing the interaction between metabolic syndrome, vascular inflammation, and other clinical features (12–19, 23). Visceral adiposity, obesity, and elevated BMI were consistently identified as risk factors for PTS and associated with earlier onset of PTS. However, while weight gain post-DVT was less directly correlated with PTS development, it remains an important metabolic consideration due to its broader implications for vascular health (18). Reduced physical activity was also implicated, as it can hinder a muscle’s ability to efficiently pump blood, compounding venous insufficiency and increases the risk of PTS, although detailed assessments of activity levels were limited (13). Proximal thrombus location and recurrent DVT were identified as significant contributors, with both factors worsening venous damage and elevating the risk of PTS progression (16). Demographic factors, such as higher BMI and female sex, were linked to increased PTS risk, though the impact of sex differences diminished over longer follow-up periods (17). Inadequate anticoagulation is a significant risk factor in the development of PTS. Subtherapeutic anticoagulation or residual venous obstruction due to poor INR control, as highlighted by Galanaud et al. (14), significantly elevated the risk of developing PTS. This was in part due to patient adherence to prescribed anticoagulants. These risk factors remain areas for potential improvement in order to enhance patient outcomes.
Protective factors
Protective factors for PTS were less commonly reported. Compression stockings were noted as a potential intervention in some studies, but their effectiveness in preventing PTS remains inconsistent. While studies like Tick et al. (16) reported widespread use, PTS still developed in 25% of cases within one year despite daily compliance, suggesting limited efficacy in some populations. Conversely, Ende-Verhaar et al. (13) noted potential benefits when compression stockings were used consistently over extended periods, but the results varied across follow-up durations. Both Ende-Verhaar et al. (13) and Tick et al. (16) identified older age (>60 years) as a protective factor, with older individuals demonstrating a significantly lower risk of developing PTS compared to younger patients (<30 years). As previously mentioned, effective anticoagulation remains a key protective risk factor for PTS (14). In addition, weight management remains a key preventative measure despite the “obesity paradox” demonstrated by El-Menyar et al. (23), where a lower mortality rate was suggested for patients with obesity despite their inherent increased risk for DVT and PTS. Lastly, symptom improvement over time, as noted by Ende-Verhaar et al. (13), highlights the potential for natural resolution in some cases.
Medical treatment options
While there is limited direct evidence regarding the potential effects of ongoing treatments for PTS, there are a few studies that have explored this topic. Rattazzi et al. (15) emphasized that weight loss strategies could play a significant role in mitigating PTS severity by reducing visceral adiposity. Pharmacological treatments, such as statins and antihypertensive therapies, were also mentioned as potential confounders in PTS progression, but their specific impact on metabolic syndrome parameters was not definitively assessed (15). As mentioned previously, the dysregulation of adipokines is implicated in chronic venous disease and PTS. These may be a possible target for treatment through lifestyle or medication management which could benefit both conditions (12).
Beyond metabolic syndrome, the role of DVT treatment and anticoagulation therapy in managing inflammation is essential. Coagulation factors, such as thrombin and factor Xa, not only contribute to clot formation but also act as mediators of inflammation by activating protease-activated receptors (PARs) and promoting cytokine release (63). The use of factor Xa inhibitors, such as rivaroxaban and apixaban, has been shown to suppress inflammatory pathways by inhibiting PAR signaling, potentially reducing cytokine-driven endothelial damage (64). Similarly, warfarin, while primarily an anticoagulant, has demonstrated secondary effects on inflammatory markers like IL-6 and CRP (64). Low-molecular-weight heparins (LMWH) have been highlighted for their additional anti-inflammatory properties, which are thought to influence PTS prevention by reducing venous wall inflammation, improving endothelialization, and reducing fibrosis. Studies have suggested that LMWH may also lead to better rates of venous recanalization compared to traditional Vitamin K antagonists (VKAs) by acting on inflammatory markers involved in thrombosis pathways (65, 66). Lastly, compression therapy, as mentioned previously, is often recommended as a first-line option for managing PTS, despite studies showing varying efficacy (65, 66). Based on these findings it seems that anticoagulation therapy influences both thrombus formation and inflammatory pathways, providing a multifaceted benefit.
Surgical treatment options
In addition to prevention and medical management, surgical treatment options following DVT have been explored and researched with the hopes of reducing the risk of PTS. Open thrombectomy and endovascular thrombolysis are two options aimed at restoring venous patency and reducing venous hypertension however their efficacy in preventing PTS remains uncertain (67). Open thrombectomy, while previously utilized, is no longer considered routine treatment for DVT and has been largely replaced by less invasive procedures such as catheter-directed therapies, which often provide comparable efficacy with reduced morbidity and surgical complications (68). Catheter-directed therapies were further evaluated in the Acute Venous Thrombosis: Thrombus Removal with Adjunctive Catheter-directed Thrombolysis (ATTRACT) trial, a large multicenter randomized study that investigated whether pharmacomechanical catheter-directed thrombolysis could prevent the development of PTS in patients diagnosed with acute proximal DVT. This trial however found no significant reduction in PTS rates among patients treated with catheter-directed thrombolysis compared to anticoagulation alone (67). While short-term improvements in pain and swelling in patients with DVT were observed, these benefits did not translate into long-term prevention of PTS, and an increased risk of bleeding and serious complications such as intracranial hemorrhage was noted (67). The Catheter-directed Venous Thrombolysis Trial (CaVenT), a prospective, multicenter, randomized controlled trial, also evaluated the use of catheter-directed thrombolysis in patients with proximal DVT to assess its potential in reducing PTS incidence (69). Interestingly, the CaVenT study determined that traditional anticoagulation with additional catheter-directed thrombolysis did result in a clinically significant reduction of PTS in patients however it similarly reported a heightened risk of bleeding complications (69).
Considering these findings, use of surgical intervention remains largely controversial and is not recommended solely for the prevention of PTS and alternative medical management, such as compression therapy and anticoagulation remain first line (67). Careful patient consideration and individualized decision-making is crucial when contemplating surgical management for patients with DVT.
Future directions
To our knowledge, this is the first systematic review addressing the association between metabolic syndrome and its components and the development of PTS. Currently, there is limited information investigating this relationship, however data exists suggesting that the association between the two conditions are likely driven by changes in inflammatory and prothrombotic factors. As there are current gaps in knowledge, larger retrospective studies with longer follow-up periods would aid in our understanding of the relationship between metabolic syndrome components and PTS. In addition, sub-analysis of patient populations can help in stratifying associations by age, gender, and additional comorbidities. A meta-analysis may be considered in the future.
Limitations
This systematic review was limited by the relatively small number of studies that have investigated the association between metabolic syndrome and its components, as well as PTS. Despite collecting and analyzing all available studies, the total number of studies was small. Additionally, while this review included 9 articles, only one article discussed metabolic syndrome as a whole and its association with PTS, while the remaining articles discussed the individual components. This limits our ability to draw definitive conclusions about the relationship between metabolic syndrome as a whole and PTS but instead focuses on the individual components that may be seen in patients with metabolic syndrome. There was also high heterogenicity in the studies included, limiting our ability to generalize findings. The assessment for PTS was performed at different follow-up times in all studies, which introduced variability and could impact the analyses. Finally, while included studies likely were referencing type 2 diabetes mellitus, it was not explicitly distinguished from Type 1, and this can be explored in future investigations.
Conclusion
While only one existing study directly investigates metabolic syndrome and its association with PTS, several papers highlight how the individual components of metabolic syndrome increase inflammation and, thereby, the risk of thrombosis. The association between PTS and VTE is established, however, further research is needed to clarify the role of metabolic syndrome and its components in combination in the development of PTS. Nonetheless, this systematic review identified a strong association between obesity, particularly abdominal obesity, and the development of PTS.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Author contributions
SA: Conceptualization, Data curation, Investigation, Methodology, Resources, Validation, Writing – original draft, Writing – review & editing. YA: Conceptualization, Data curation, Investigation, Methodology, Resources, Validation, Writing – original draft, Writing – review & editing. AE: Conceptualization, Resources, Supervision, Validation, Writing – original draft, Writing – review & editing. MB: Methodology, Validation, Writing – original draft, Writing – review & editing. IW: Writing – original draft, Writing – review & editing. AH: Writing – review & editing. KN: Conceptualization, Data curation, Investigation, Methodology, Resources, Validation, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. The publication of this article was supported by the OHSU McGriff Scholar’s Fund.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Kahn SR. The post-thrombotic syndrome. Hematol Am Soc Hematol Educ Program. (2016) 2016:413–8. doi: 10.1182/asheducation-2016.1.413
2. Melaku L, Dabi A. The cellular biology of atherosclerosis with atherosclerotic lesion classification and biomarkers. Bull Natl Res Cent. (2021) 45:225. doi: 10.1186/s42269-021-00685-w
3. Swarup S, Ahme I, Grigorova Y, et al. Metabolic syndrome. In: StatPearls. Treasure Island (FL): StatPearls Publishing (2025).
4. Huang PL. A comprehensive definition for metabolic syndrome. Dis Model Mech. (2009) 2:231–7. doi: 10.1242/dmm.001180
5. Catharina AS, Modolo R, Ritter AMV, Sabbatini AR, Lopes HF, Moreno Junior H, et al. Metabolic syndrome-related features in controlled and resistant hypertensive subjects. Arq Bras Cardiol. (2018) 110:514–21. doi: 10.5935/abc.20180076
6. Cozma A, Sitar-Taut A, Orăşan O, Leucuta D, Alexescu T, Stan A, et al. Determining factors of arterial stiffness in subjects with metabolic syndrome. Metab Syndr Relat Disord. (2018) 16:490–6. doi: 10.1089/met.2018.0057
7. Ay C, Tengler T, Vormittag R, Simanek R, Dorda W, Vukovich T, et al. Venous thromboembolism–a manifestation of the metabolic syndrome. Haematologica. (2007) 92:374–80. doi: 10.3324/haematol.10828
8. Li W, Song F, Wang X, Wang D, Chen D, Yue W, et al. Relationship between metabolic syndrome and its components and cardiovascular disease in middle-aged and elderly Chinese population: a national cross-sectional survey. BMJ Open. (2019) 9:e027545. doi: 10.1136/bmjopen-2018-027545
9. Stewart LK, Kline JA. Metabolic syndrome increases risk of venous thromboembolism recurrence after acute pulmonary embolism. Ann Am Thorac Soc. (2020) 17:821–8. doi: 10.1513/AnnalsATS.201907-518OC
10. Borgel D, Bianchini E, Lasne D, Pascreau T, Saller F. Inflammation in deep vein thrombosis: a therapeutic target? Hematology. (2019) 24:742–50. doi: 10.1080/16078454.2019.1687144
11. Kahn SR, Partsch H, Vedantham S, Prandoni P, Kearon C. Subcommittee on Control of Anticoagulation of the Scientific and Standardization Committee of the International Society on Thrombosis and Haemostasis. Definition of post-thrombotic syndrome of the leg for use in clinical investigations: a recommendation for standardization. J Thromb Haemost. (2009) 7(5):879–83. doi: 10.1111/j.1538-7836.2009.03294.x
12. Mrozinska S, Cieslik J, Broniatowska E, Undas A. Elevated leptin and decreased adiponectin independently predict the post-thrombotic syndrome in obese and non-obese patients. Sci Rep. (2018) 8:6938. doi: 10.1038/s41598-018-25135-y
13. Ende-Verhaar YM, Tick LW, Klok FA, Huisman MV, Rosendaal FR, le Cessie S, et al. Post-thrombotic syndrome: Short and long-term incidence and risk factors. Thromb Res. (2019) 177:102–9. doi: 10.1016/j.thromres.2019.03.003
14. Galanaud JP, Holcroft CA, Rodger MA, Kovacs MJ, Betancourt MT, Wells PS, et al. Predictors of post-thrombotic syndrome in a population with a first deep vein thrombosis and no primary venous insufficiency. J Thromb Haemost. (2013) 11:474–80. doi: 10.1111/jth.12106
15. Rattazzi M, Callegari E, Sponchiado A, Galliazzo S, Pagliara V, Villalta S, et al. Visceral obesity, but not metabolic syndrome, is associated with the presence of post-thrombotic syndrome. Thromb Res. (2015) 136:225–8. doi: 10.1016/j.thromres.2015.05.019
16. Tick LW, Kramer MH, Rosendaal FR, Faber WR, Doggen CJ. Risk factors for post-thrombotic syndrome in patients with a first deep venous thrombosis. J Thromb Haemost. (2008) 6:2075–81. doi: 10.1111/j.1538-7836.2008.03180.x
17. Spiezia L, Campello E, Simion C, Poretto A, Dalla Valle F, Simioni P. Risk factors for post-thrombotic syndrome in patients with a first proximal deep venous thrombosis treated with direct oral anticoagulants. Angiology. (2022) 73:649–54. doi: 10.1177/00033197211070889
18. Ageno W, Piantanida E, Dentali F, Steidl L, Mera V, Squizzato A, et al. Body mass index is associated with the development of the post-thrombotic syndrome. Thromb Haemost. (2003) 89:305–9.
19. Asim M, Al-Thani H, El-Menyar A. Recurrent deep vein thrombosis after the first venous thromboembolism event: A single-institution experience. Med Sci Monit. (2017) 23:2391–9. doi: 10.12659/msm.901924
20. Shbaklo H, Holcroft CA, Kahn SR. Levels of inflammatory markers and the development of the post-thrombotic syndrome. Thromb Haemost. (2009) 101:505–12. doi: 10.1160/TH08-08-0511
21. Vedantham S. Valvular dysfunction and venous obstruction in the post-thrombotic syndrome. Thromb Res. (2009) 123 Suppl 4:S62–5. doi: 10.1016/S0049-3848(09)70146-X
22. Colling ME, Tourdot BE, Kanthi Y. Inflammation, infection and venous thromboembolism. Circ Res. (2021) 128:2017–36. doi: 10.1161/CIRCRESAHA.121.318225
23. El-Menyar A, Asim M, Al-Thani H. Obesity paradox in patients with deep venous thrombosis. Clin Appl Thromb Hemost. (2018) 24:986–92. doi: 10.1177/1076029617727858
24. Summer R, Walsh K, Medoff BD. Obesity and pulmonary arterial hypertension: Is adiponectin the molecular link between these conditions? Pulm Circ. (2011) 1:440–7. doi: 10.4103/2045-8932.93542
25. El Husseny MW, Mamdouh M, Shaban S, Ibrahim Abushouk A, Zaki MM, Ahmed OM, et al. Adipokines: potential therapeutic targets for vascular dysfunction in type II diabetes mellitus and obesity. J Diabetes Res. (2017) 2017:8095926. doi: 10.1155/2017/8095926
26. Ageno W, Prandoni P, Romualdi E, Ghirarduzzi A, Dentali F, Pesavento R, et al. The metabolic syndrome and the risk of venous thrombosis: a case–control study. J Thromb Haemost. (2006) 4:1914–8. doi: 10.1111/j.1538-7836.2006.02132.x
27. Prandoni P, Bilora F, Marchiori A, Bernardi E, Petrobelli F, Lensing AWA, et al. An association between atherosclerosis and venous thrombosis. New Engl J Med. (2003) 348:1435–41. doi: 10.1056/NEJMoa022157
28. Eichinger S, Hron G, Bialonczyk C, Hirschl M, Minar E, Wagner O, et al. Overweight, obesity, and the risk of recurrent venous thromboembolism. Arch Internal Med. (2008) 168:1678–83. doi: 10.1001/archinte.168.15.1678
29. Darvall KA, Sam RC, Silverman SH, Bradbury AW, Adam DJ. Obesity and thrombosis. Eur J Vasc Endovasc Surg. (2007) 33:223–33. doi: 10.1016/j.ejvs.2006.10.006
30. Meade TW, Ruddock V, Stirling Y, Chakrabarti R, Miller GJ. Fibrinolytic activity, clotting factors, and long-term incidence of ischaemic heart disease in the Northwick Park Heart Study. Lancet. (1993) 342:1076–9. doi: 10.1016/0140-6736(93)92062-X
31. Ageno W, Becattini C, Brighton T, Selby R, Kamphuisen PW. Cardiovascular risk factors and venous thromboembolism: a meta-analysis. Circulation. (2008) 117:93–102. doi: 10.1161/CIRCULATIONAHA.107.709204
32. Goldhaber SZ, Grodstein F, Stampfer MJ, Manson JE, Colditz GA, Speizer FE, et al. A prospective study of risk factors for pulmonary embolism in women. Jama. (1997) 277:642–5. doi: 10.1001/jama.1997.03540320044033
33. Renna NF, de Las Heras N, Miatello RM. Pathophysiology of vascular remodeling in hypertension. Int J Hypertens. (2013) 2013:808353. doi: 10.1155/2013/808353
34. Bai J, Ding X, Du X, Zhao X, Wang Z, Ma Z. Diabetes is associated with increased risk of venous thromboembolism: a systematic review and meta-analysis. Thromb Res. (2015) 135:90–5. doi: 10.1016/j.thromres.2014.11.003
35. Petrauskiene V, Falk M, Waernbaum I, Norberg M, Eriksson JW. The risk of venous thromboembolism is markedly elevated in patients with diabetes. Diabetologia. (2005) 48:1017–21. doi: 10.1007/s00125-005-1715-5
36. Lemkes BA, Hermanides J, Devries JH, Holleman F, Meijers JC, Hoekstra JB. Hyperglycemia: a prothrombotic factor? J Thromb Haemost. (2010) 8:1663–9. doi: 10.1111/j.1538-7836.2010.03910.x
37. Carmassi F, Morale M, Puccetti R, De Negri F, Monzani F, Navalesi R, et al. Coagulation and fibrinolytic system impairment in insulin dependent diabetes mellitus. Thromb Res. (1992) 67:643–54. doi: 10.1016/0049-3848(92)90068-L
38. Yudkin JS. Abnormalities of coagulation and fibrinolysis in insulin resistance. Evidence for a common antecedent? Diabetes Care. (1999) 22 Suppl 3:C25–30.
39. Ceriello A, Quatraro A, Dello Russo P, Marchi E, Barbanti M, Milani MR, et al. Protein C deficiency in insulin-dependent diabetes: a hyperglycemia-related phenomenon. Thromb Haemost. (1990) 64:104–7.
40. Colwell JA, Nesto RW. The platelet in diabetes: focus on prevention of ischemic events. Diabetes Care. (2003) 26:2181–8. doi: 10.2337/diacare.26.7.2181
41. Ferroni P, Basili S, Falco A, Davì G. Platelet activation in type 2 diabetes mellitus. J Thromb Haemost. (2004) 2:1282–91. doi: 10.1111/j.1538-7836.2004.00836.x
42. Osende JI, Badimon JJ, Fuster V, Herson P, Rabito P, Vidhun R, et al. Blood thrombogenicity in type 2 diabetes mellitus patients is associated with glycemic control. J Am Coll Cardiol. (2001) 38:1307. doi: 10.1016/S0735-1097(01)01555-8
43. Ceriello A. Coagulation activation in diabetes mellitus: the role of hyperglycaemia and therapeutic prospects. Diabetologia. (1993) 36:1119–25. doi: 10.1007/BF00401055
44. Pandolfi A, Iacoviello L, Capani F, Vitacolonna E, Donati MB, Consoli A. Glucose and insulin independently reduce the fibrinolytic potential of human vascular smooth muscle cells in culture. Diabetologia. (1996) 39:1425–31. doi: 10.1007/s001250050594
45. Min C, Kang E, Yu SH, Shinn SH, Kim YS. Advanced glycation end products induce apoptosis and procoagulant activity in cultured human umbilical vein endothelial cells. Diabetes Res Clin Pract. (1999) 46:197–202. doi: 10.1016/S0168-8227(99)00094-7
46. Iorio A, Federici MO, Mourvaki E, Ferolla P, Piroddi M, Stabile A, et al. Impaired endothelial antithrombotic activity following short-term interruption of continuous subcutaneous insulin infusion in type 1 diabetic patients. Thromb Haemost. (2007) 98:635–41.
47. Liang HJ, Zhang QY, Hu YT, Liu GQ, Qi R. Hypertriglyceridemia: a neglected risk factor for ischemic stroke? J Stroke. (2022) 24:21–40. doi: 10.5853/jos.2021.02831
48. Safarova MS, Gupta K. Severe hypertriglyceridemia as a cause of aortic thrombus with peripheral embolic complications. Kans J Med. (2022) 15:298–301. doi: 10.17161/kjm.vol15.17006
49. Griffin JH, Fernández JA, Deguchi H. Plasma lipoproteins, hemostasis and thrombosis. Thromb Haemost. (2001) 86:386–94.
50. Ginsberg HN, Packard CJ, Chapman MJ, Borén J, Aguilar-Salinas CA, Averna M, et al. Triglyceride-rich lipoproteins and their remnants: metabolic insights, role in atherosclerotic cardiovascular disease, and emerging therapeutic strategies-a consensus statement from the European Atherosclerosis Society. Eur Heart J. (2021) 42:4791–806. doi: 10.1093/eurheartj/ehab551
51. Doi H, Kugiyama K, Oka H, Sugiyama S, Ogata N, Koide SI, et al. Remnant lipoproteins induce proatherothrombogenic molecules in endothelial cells through a redox-sensitive mechanism. Circulation. (2000) 102:670–6. doi: 10.1161/01.CIR.102.6.670
52. Man FH, Nieuwland R, van der Laarse A, Romijn F, Smelt AH, Gevers Leuven JA, et al. Activated platelets in patients with severe hypertriglyceridemia: effects of triglyceride-lowering therapy. Atherosclerosis. (2000) 152:407–14. doi: 10.1016/S0021-9150(99)00485-2
53. Razavi AC, Jain V, Grandhi GR, Patel P, Karagiannis A, Patel N, et al. Does elevated high-density lipoprotein cholesterol protect against cardiovascular disease? J Clin Endocrinol Metab. (2024) 109:321–32. doi: 10.1210/clinem/dgad406
54. Huang Y, Ge H, Wang X, Zhang X. Association between blood lipid levels and lower extremity deep venous thrombosis: A population-based cohort study. Clin Appl Thrombosis/Hemost. (2022) 28:10760296221121282. doi: 10.1177/10760296221121282
55. Ben-Aicha S, Badimon L, Vilahur G. Advances in HDL: much more than lipid transporters. Int J Mol Sci. (2020) 21(3):732. doi: 10.3390/ijms21030732
56. Holy EW, Besler C, Reiner MF, Camici GG, Manz J, Beer JH, et al. High-density lipoprotein from patients with coronary heart disease loses anti-thrombotic effects on endothelial cells: impact on arterial thrombus formation. Int J Mol Sci. (2020) 21(3):732. doi: 10.3390/ijms21030732
57. Van der Stoep M, Korporaal SJ, Van Eck M. High-density lipoprotein as a modulator of platelet and coagulation responses. Cardiovasc Res. (2014) 103:362–71. doi: 10.1093/cvr/cvu137
58. Mineo C, Deguchi H, Griffin JH, Shaul PW. Endothelial and antithrombotic actions of HDL. Circ Res. (2006) 98:1352–64. doi: 10.1161/01.RES.0000225982.01988.93
59. Stubblefield WB, Alves NJ, Rondina MT, Kline JA. Variable resistance to plasminogen activator initiated fibrinolysis for intermediate-risk pulmonary embolism. PloS One. (2016) 11:e0148747. doi: 10.1371/journal.pone.0148747
60. Molica F, Morel S, Kwak BR, Rohner-Jeanrenaud F, Steffens S. Adipokines at the crossroad between obesity and cardiovascular disease. Thromb Haemost. (2015) 113:553–66. doi: 10.1160/TH14-06-0513
61. Stark K, Massberg S. Interplay between inflammation and thrombosis in cardiovascular pathology. Nat Rev Cardiol. (2021) 18:666–82. doi: 10.1038/s41569-021-00552-1
62. Nicolai L, Leunig A, Brambs S, Kaiser R, Weinberger T, Weigand M, et al. Immunothrombotic dysregulation in COVID-19 pneumonia is associated with respiratory failure and coagulopathy. Circulation. (2020) 142:1176–89. doi: 10.1161/CIRCULATIONAHA.120.048488
63. Zuo P, Zuo Z, Wang X, Chen L, Zheng Y, Ma G, et al. Factor Xa induces pro-inflammatory cytokine expression in RAW 264.7 macrophages via protease-activated receptor-2 activation. Am J Transl Res. (2015) 7:2326–34.
64. Schiffer S, Schwers S, Heitmeier S. The effect of rivaroxaban on biomarkers in blood and plasma: a review of preclinical and clinical evidence. J Thromb Thrombol. (2023) 55:449–63. doi: 10.1007/s11239-023-02776-z
65. Makedonov I, Kahn SR, Galanaud JP. Prevention and management of the post-thrombotic syndrome. J Clin Med. (2020) 9:923. doi: 10.3390/jcm9040923
66. Palacios FS, Rathbun SW. Medical treatment for postthrombotic syndrome. Semin Intervent Radiol. (2017) 34:61–7. doi: 10.1055/s-0036-1597765
67. Machin M, Salim S, Tan M, Onida S, Davies AH, Shalhoub J. Surgical and non-surgical approaches in the management of lower limb post-thrombotic syndrome. Expert Rev Cardiovasc Ther. (2021) 19:191–200. doi: 10.1080/14779072.2021.1876563
68. Koopmann MC, McLafferty RB. Advances in operative thrombectomy for lower extremity venous thrombosis. Surg Clin North Am. (2018) 98:267–77. doi: 10.1016/j.suc.2017.11.005
69. Enden T, Haig Y, Kløw NE, Slagsvold CE, Sandvik L, Ghanima W, et al. Long-term outcome after additional catheter-directed thrombolysis versus standard treatment for acute iliofemoral deep vein thrombosis (the CaVenT study): a randomised controlled trial. Lancet (London England). (2012) 379:31–8. doi: 10.1016/S0140-6736(11)61753-4
Keywords: post-thrombotic syndrome, metabolic syndrome, deep vein thrombosis, venous thromboembolism, inflammation, vascular, obesity
Citation: Alturky S, Ashfaq Y, Elhance A, Barney M, Wadiwala I, Hunter AK and Nguyen KP (2025) Association of post-thrombotic syndrome with metabolic syndrome and inflammation - a systematic review. Front. Immunol. 16:1519534. doi: 10.3389/fimmu.2025.1519534
Received: 30 October 2024; Accepted: 06 February 2025;
Published: 28 March 2025.
Edited by:
Steven Philip Grover, University of North Carolina at Chapel Hill, United StatesReviewed by:
Ronaldo Go, Rutgers, The State University of New Jersey, United StatesSmriti Sharma, Medical University of Vienna, Austria
Copyright © 2025 Alturky, Ashfaq, Elhance, Barney, Wadiwala, Hunter and Nguyen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sara Alturky, YWx0dXJreUBvaHN1LmVkdQ==
†These authors have contributed equally to this work
 Sara Alturky
Sara Alturky Yusuf Ashfaq1†
Yusuf Ashfaq1† Ishaq Wadiwala
Ishaq Wadiwala Khanh P. Nguyen
Khanh P. Nguyen