
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Immunol., 26 February 2025
Sec. Cancer Immunity and Immunotherapy
Volume 16 - 2025 | https://doi.org/10.3389/fimmu.2025.1518647
This article is part of the Research TopicImmune Predictive and Prognostic Biomarkers in Immuno-Oncology: Refining the Immunological Landscape of CancerView all 12 articles
Background: The systemic inflammation response index (SIRI) as an immune marker, is associated with prognosis of urological malignancies(UM). However, the conclusion remains controversial. Therefore, the objective of this study was to conduct a meta-analysis to comprehensively evaluate the predictive value of SIRI in patients with UM.
Methods: A comprehensive search of PubMed, Web of Science, and EMBASE databases was performed for articles investigating the association between SIRI and UM. The search deadline was August 28, 2024. Survival outcome such as overall survival (OS), disease-free survival (DFS), progression-free survival (PFS), and recurrence-free survival (RFS) were analyzed.
Results: 15 studies from 13 articles involving 4985 patients were included in the meta-analysis. The results showed that increased SIRI was associated with poorer OS (HR: 2.16, 95% CI: 1.61-2.89) and DFS/PFS/RFS (HR: 3.56, 95% CI: 1.41-8.99). Subgroup analysis further confirmed the prognostic value of SIRI in urinary system cancer.
Urological malignancies(UM) has become a great threat to human health. Renal cell carcinoma, bladder cancer, and prostate cancer rank among the most common types of UM, together representing about 12.3% of cancer cases and 7.7% of cancer-related deaths (1). It is expected that incidence and mortality of urinary system cancer would continue to rise worldwide (2). Even with considerable progress in surgical techniques and medical interventions such as chemotherapy, radiotherapy and immunotherapy, the five-year survival rate of UM patients remains alarmingly low (3, 4). Therefore, there is an urgent requirement to identify novel biomarkers prognostic markers for UM.
Inflammation and immune status play important roles in tumor biology (5, 6). Inflammatory response promotes cell proliferation by upregulating cytokines and producing inflammatory mediators (7). Inflammation can also lead to malnutrition, leading to a poor prognosis for cancer patients (8). Inflammation can also impair immune cell function, leading to immunosuppression (9). Immune system restrained tumor cell proliferation, angiogenesis and metastasis through by down-regulating the production of cytokines and inflammatory mediators (10). Many blood indicators, such as neutrophil to lymphocyte ratio, platelet to lymphocyte ratio and albumin-globulin ratio, have been confirmed to predict the prognosis of patients with UM (11–13). However, due to limitations, they were not widely used.
Systemic inflammation response index(SIRI) as a prognostic marker, was first proposed by Qi et al. in 2016 and was composed of neutrophil x monocyte)/lymphocyte count (14). The SIRI score was widely used as a screening tool to assess inflammation and immune status in cancer patients. Many studies have confirmed that SIRI can be used to evaluate patient prognosis in different cancers including non-small cell lung cancer, gastric cancer, breast cancer, ovarian cancer and hepatocellular carcinoma (15–19). Compared with neutrophil to lymphocyte ratio (NLR) and platelet to lymphocyte ratio (PLR), SIRI had higher prediction accuracy (20, 21). The SIRI incorporates three different cells, and may better reflect the inflammatory and immune status of tumor patients. In UM, some studies found that SIRI was associated with prognosis (22, 23). However, the results were still disputed. Whether SIRI was a reliable prognostic marker for UM was unclear. Therefore, we conducted the meta-analysis to summarize the prognostic significance of SIRI in UM.
A comprehensive search of PubMed, Web of Science, and EMBASE databases was performed for articles investigating the association between SIRI and UM. The search deadline was August 28, 2024. The following search terms were used: “systemic inflammation response index” OR “system inflammation response index” OR “systemic inflammatory response index” AND cancer OR tumor OR carcinoma OR neoplasm OR malignancy OR tumor AND prognosis OR prognostic OR survival OR outcome. There were no language restrictions. The references for the selected studies were carefully checked for possible studies. This analysis followed the PRISMA guidelines(Supplementary Material).
The inclusion: (1) study evaluated the association between SIRI and survival outcomes in patients with UM. (2)provided adequate data to compute the hazard ratios (HRs) with its 95% confidence intervals (CIs). Exclusion criteria: (1) study with insufficient data for calculating HRs and 95% CIs. (2) abstracts, case reports, reviews or letters.
The process of extracting data was independently conducted by two researchers, and any differences were settled by discussions. The gathered data included the lead author’s name, publication year, treatment method, cancer type, analytical method and survival outcomes. The Newcastle-Ottawa Scale (NOS) was employed to assess the quality of the incorporated studies (24).
Meta-analysis was performed using STATA software. The aggregated data was evaluated by calculating HRs and 95% CIs. When I2 was less than 50%, a fixed effect model was used, and when I2 was more than 50%, a random effect model was used. Subgroup analysis was analyzed to further explore the predictive significance of SIRI in UM. The strength of the findings was assessed using sensitivity analysis. Begg’s test, Egger’s test, and the trim-and-fill methods were used to investigate publication bias (25–27).The p value less than 0.05 was considered statistically significant.
A total of 814 articles were obtained through a preliminary query of relevant databases. After the removal of 253 duplicates, a total of 561 publications were further evaluated. After a review of titles and abstracts, 229 articles were further removed. Finally, 15 studies from 13 articles with a total of 1066 patients were included in the meta-analysis (22, 23, 28–38). Figure 1 showed the literature search process.
Table 1 summarized the main features of the included studies. 14 studies reported OS data and 4 studies analyzed DFS/PFS/RFS data. A total of four different tumors were reported, including prostate cancer, renal cancer, bladder cancer and upper tract urothelial carcinoma. NOS scores for the included studies ranged from 6 to 7, indicating a high quality of each study (Supplementary Table S1).
Fourteen studies evaluated the correlation between elevated SIRI and OS. Given the significant heterogeneity (I²=89.6%), a random-effects model was applied. The pooled HR was 2.16 (95% CI: 1.61–2.89), indicating that UM patients with elevated SIRI s had poorer OS. The result was shown in Figure 2.
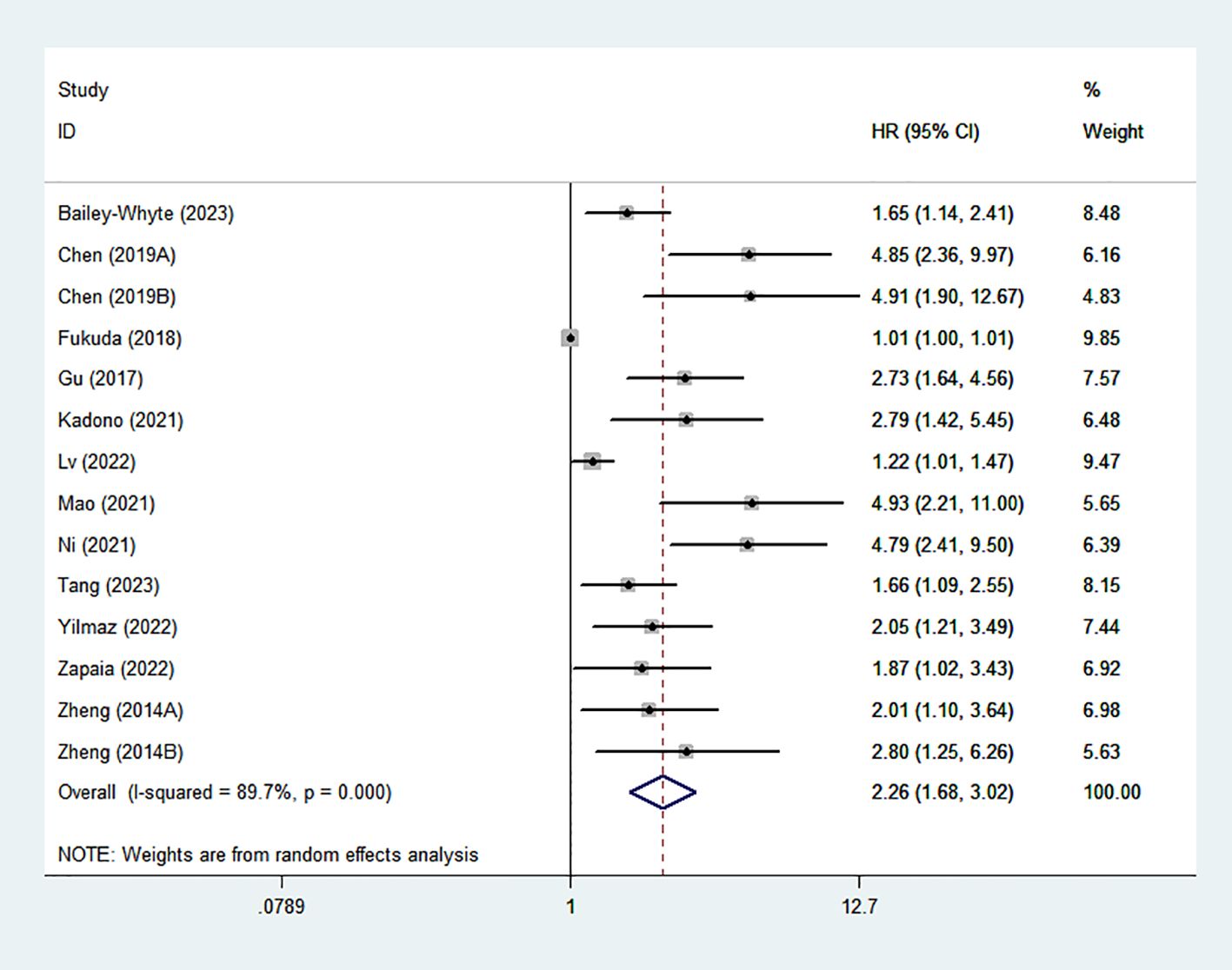
Figure 2. Forest plots of the relationship between SIRI and OS. SIRI, systemic inflammation response index; OS, overall survival;.
Subgroup analysis was further performed based on country, cancer type, treatment method and region (Table 2). The results showed that high SIRI predicted poor prognosis in both the surgical treatment group (HR:1.58; 95% CI:1.17-2.15) and the non-surgical treatment group (HR:2.02; 95% CI:3.00). Subgroup analysis also indicated that elevated SIRI mainly served as a poor prognostic marker in renal cell carcinoma, bladder cancer and upper tract urothelial carcinoma. In addition, subgroup analysis also showed that treatment method and cancer type may be the important source of heterogeneity.
Five studies reveled the relationship between SIRI and DFS/PFS/RFS. Owing to considerable diversity (I2 = 95.2%), a random-effects model was utilized. Our findings revealed a correlation between elevated SIRI and adverse DFS/PFS/RFS (HR: 3.56; 95% CI: 1.41–8.99) (Figure 3).
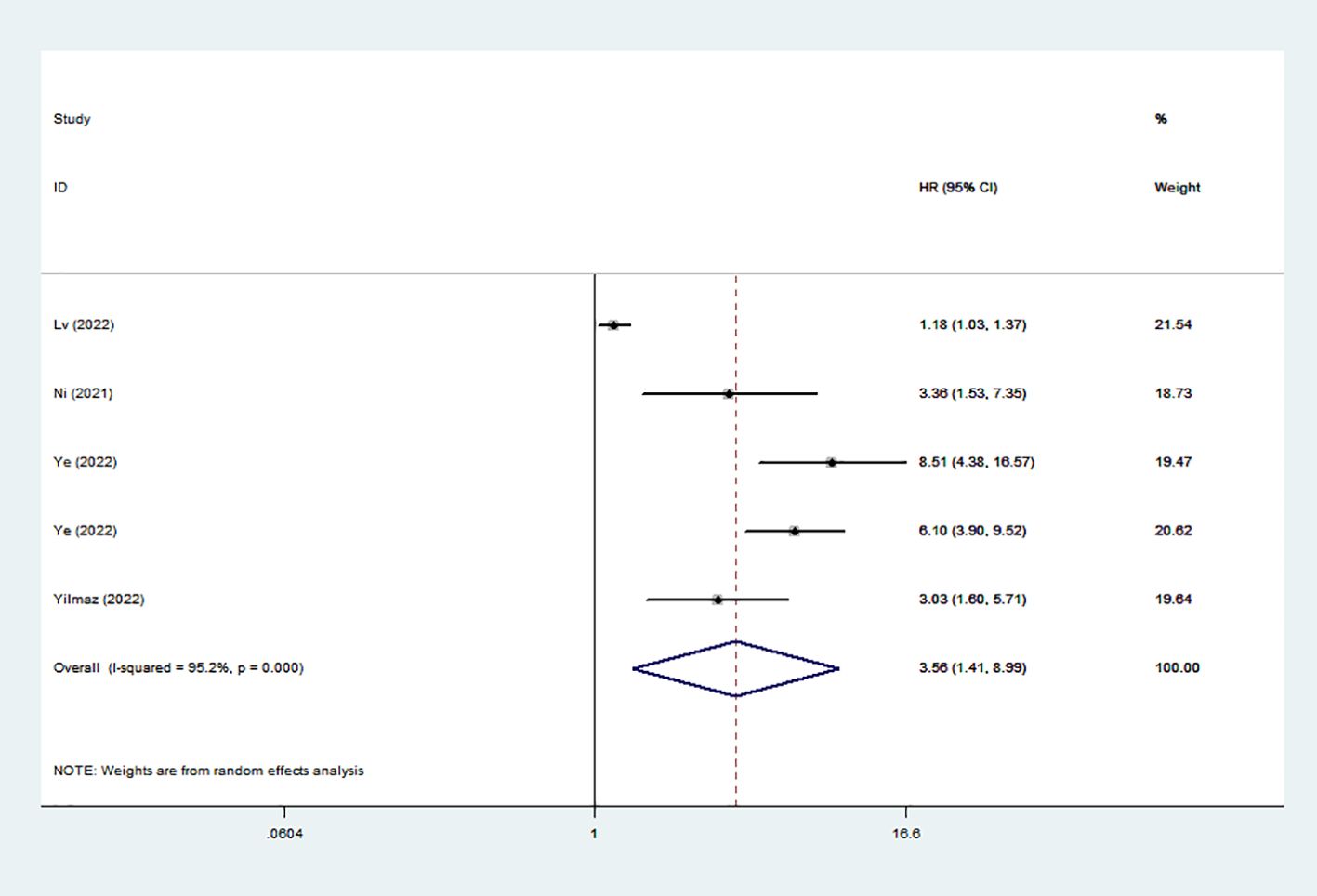
Figure 3. Forest plots of the relationship between SIRI and DFS/PFS/RFS. SIRI, systemic inflammation response index; DFS/PFS/RFS, disease-free survival/progression-free survival/recurrence-free survival.
Sensitivity analysis was performed by sequentially deleting one study. The results showed that no study had significant influence on the results of comprehensive analysis, indicating that the results of meta-analysis were stable and reliable (Figure 4A, B).
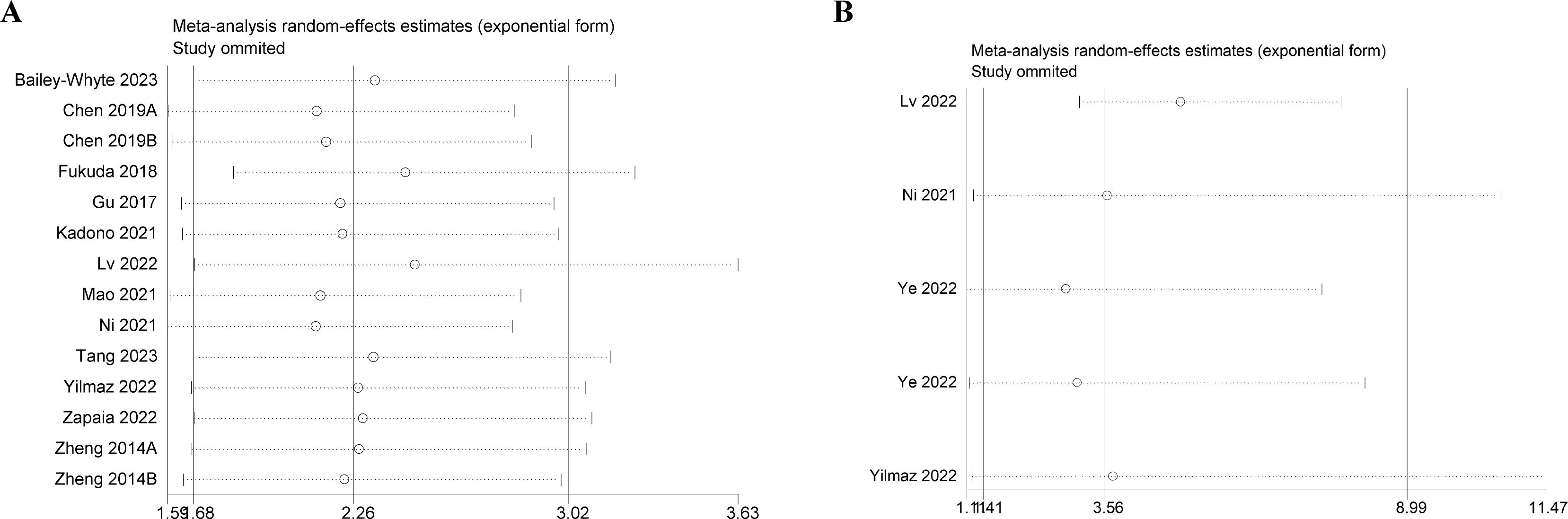
Figure 4. Sensitivity analysis. (A) Sensitivity analysis for OS. (B) Sensitivity analysis for DFS/PFS/RFS. OS, overall survival; DFS/PFS/RFS, disease-free survival/progression-free survival/recurrence-free survival.
The evaluation of publication bias was performed using Begg’s test and Egger’s test. The p-values of Begg’s test and Egger’s test for OS were 0.059 and 0, respectively (Figure 5A). There was some publication bias for OS. However, trim-and-fill methods proved that the result was not affected by publication bias (HR: 2.26, 95% CI: 1.68–3.02) (Figure 5B). The p-values of Begg’s test and Egger’s test for DFS/PFS/RFS were 1 and 0.051, respectively (Figure 5C), suggesting there was no publication bias.
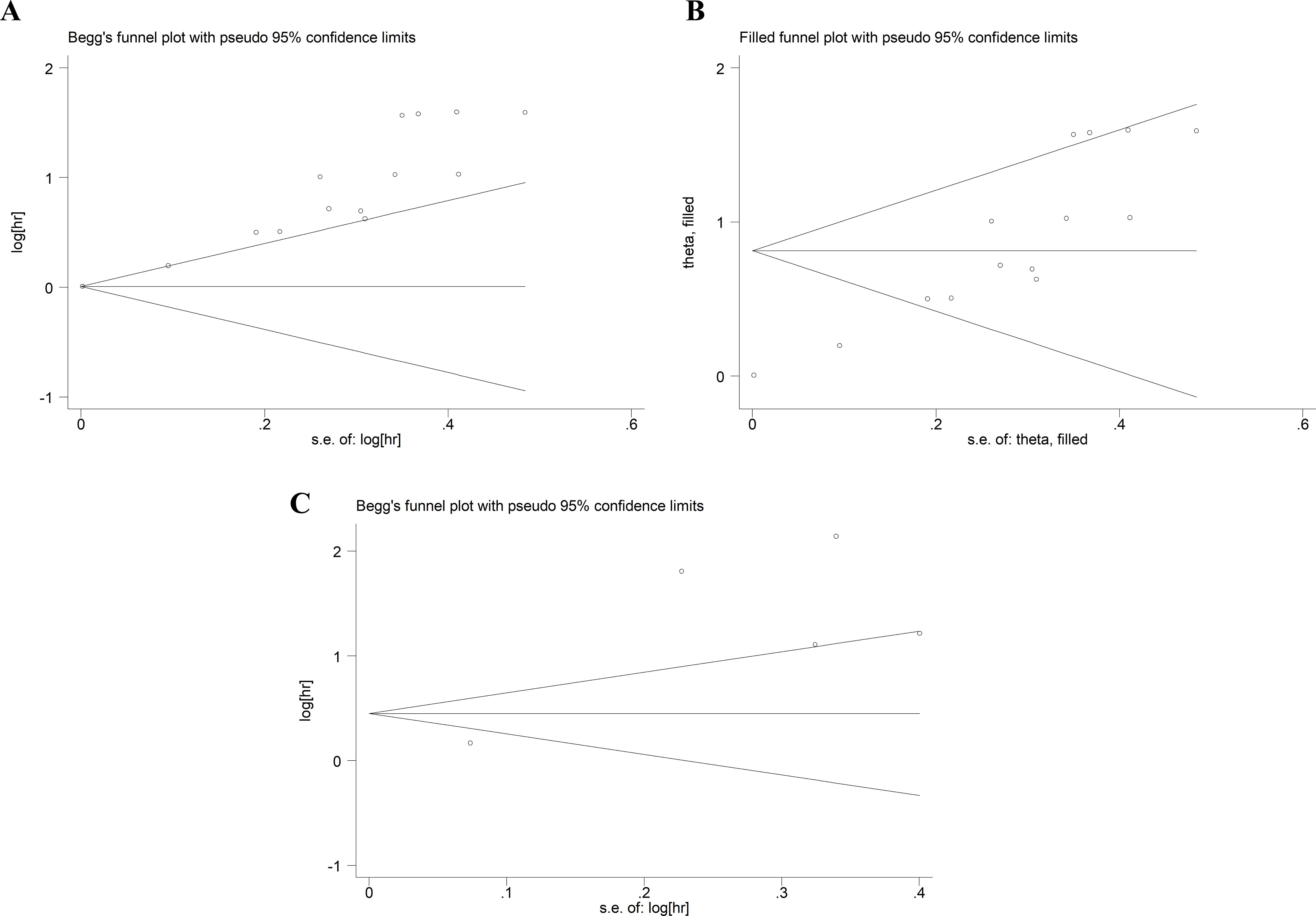
Figure 5. Publication bias. (A) Publication bias for OS. (B) The trim-and-fill method tested the OS data. (C) Publication bias for DFS/PFS/RFS. OS, overall survival; DFS/PFS/RFS, disease-free survival/progression-free survival/recurrence-free survival.
No studies have fully evaluated the prognostic significance of SIRI in patients with UM. 15 studies involving 4985 patients were included in the meta-analysis. The results showed that high SIRI was associated with adverse OS and DFS/PFS/RFS in patients with UM. Subgroup analysis showed that the prognostic value of SIRI in UM was not affected by treatment method. In addition, subgroup analysis also revealed that the prognostic value of SIRI was generalizable regardless of Asian or European-American populations. Furthermore, SIRI demonstrated better prognostic value in renal cell carcinoma, bladder cancer and upper tract urothelial carcinoma.
Increasing evidenced suggested that systemic inflammation significantly influences cancer growth, recurrence and progression, thereby affecting patient survival (6, 39). The systemic inflammation may precede malignant transformation, implying that an inflammatory microenvironment could facilitate tumor development (40). In addition, the emergence and progression of cancers were linked to interactions within the immune system (41). Research displayed obvious correlation between changes in regulatory T cells and tumor-related macrophages in non-muscle-invasive bladder cancer (NMIBC) patients and poor survival outcomes (42). Additionally, intravesical bacillus calmette-guerin immunotherapy increased the CD4+ T cell population more effectively than intravesical chemotherapy (43).Studies showed that viruses, especially HPV, may be a risk factor for urothelial carcinoma of the bladder (44–46). HPV infection caused inflammation and immune disorders in patients, thus promoting the occurrence of tumors (44).Targeting the progression of cancer by modulating specific inflammatory cytokines or immune cell has emerged as a promising therapeutic approach.
SIRI effectively predicted prognosis by evaluating the inflammatory and immune status of cancer patients. However, the specific mechanism that why SIRI determined the prognosis of cancer patients was unclear. We explained this phenomenon by analyzing SIRI composition parameters.
The critical role of neutrophils within the tumor’s immune microenvironment has garnered significant interest (47). Inflammation is essential for triggering tumor development through the damage of healthy tissues, and neutrophils played the crucial role in the process (48). Neutrophils entered different organs via CXCR2 ligands and performed immunosuppressive functions in the tumor microenvironment (49, 50). The reactive oxygen species and angiogenic factors produced by neutrophils could affect tumor initiation, progression and metastasis (51, 52). In addition, neutrophils can also promote the proliferation and differentiation of tumor cells by inhibiting lymphocyt-mediated cytolysis (53). Many studies confirmed that high blood neutrophils were closely related to poor prognosis of tumor patients (54).
At various stages of tumor development, monocytes are attracted by inflammatory mediators into the tumor microenvironment to exert specific immune functions (55). Studies showed that monocytes can differentiate into tumor-associated macrophages, which could degrade the extracellular matrix, induce immunosuppression, tumor angiogenesis and increase the likelihood of tumor metastasis (56). Data suggested that the prognosis of tumor patients with increased blood monocytes was generally adverse (57).
As an important part of immune system, lymphocytes act as an important role in immune defense. Lymphocytes could inhibit tumor progression by directly inhibiting tumor cell proliferation (58). Lymphocytes could activate cell-mediated immune responses and stimulate cytokine release to promote tumor lysis (59). However, a decrease in the numbers of lymphocytes can lead to immune dysfunction and immune dysfunction and immunosuppression. Evidence revealed that lymphocytopenia predicted significantly lower survival time in a variety of tumors, including UM (6, 60, 61).
High SIRI indicated high neutrophils or monocytes count and low lymphocytes counts, which reflected significant systemic inflammation and immunosuppression in tumor patients. Therefore, it was not difficult to understand that why high SIRI was associated with poor prognosis in patients with urinary tract tumors.
There were some defects in the study. Firstly, all included articles were retrospective studies. Secondly, most of the included studies were from China. Therefore, more large-scale studies from different regions were needed to further evaluate the prognostic value of SIRI in UM patients. Thirdly, Some urinary tumors, such as testicular cancer, was not evaluated. Fourth, our study was the grouping of different tumor types into a single category of urological malignancies. These cancers had distinct biological behaviors and prognostic factors, which may contribute to the observed heterogeneity (62–64). Finally, we were unable to assess the association between SIRI and clinicopathological features due to lack of data.
Our study also had some strengths. Firstly, this was the first meta-analysis to assess the prognostic value of SIRI in UM. Secondly, the sensitivity analysis confirmed that the results of the meta-analysis were stable. Thirdly, subgroup analysis further proved the prognostic value of SIRI. Fourth, the results of OS were not affected by publication bias through trim-and-fill method.
Our study suggested that elevated SIRI was associated with poorer survival outcomes in UM patients. SIRI acts as an accessible serum indicator, offering a dynamic assessment of prognosis and treatment outcomes in UM patients. Clinicians can use SIRI to quickly assess UM patient’s nutritional status, immune function, and prognosis, providing guidance for individualized treatment. However, due to the limitations, further prospective studies were needed to validate our results.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author/s.
WM: Conceptualization, Data curation, Formal analysis, Methodology, Software, Writing – original draft, Writing – review & editing. RL: Conceptualization, Data curation, Formal analysis, Methodology, Software, Writing – original draft, Writing – review & editing. XL: Conceptualization, Data curation, Formal analysis, Methodology, Software, Writing – original draft. JY: Project administration, Supervision, Writing – review & editing. WW: Project administration, Supervision, Writing – review & editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declare that no Generative AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2025.1518647/full#supplementary-material
1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2018) 68:394–424. doi: 10.3322/caac.21492
2. Mariotto AB, Yabroff KR, Shao Y, Feuer EJ, Brown ML. Projections of the cost of cancer care in the United States: 2010-2020. J Natl Cancer Inst. (2011) 103:117–28. doi: 10.1093/jnci/djq495
3. Peyton CC, Tang D, Reich RR, Azizi M, Chipollini J, Pow-Sang JM, et al. Sexton WJ et al: Downstaging and Survival Outcomes Associated With Neoadjuvant Chemotherapy Regimens Among Patients Treated With Cystectomy for Muscle-Invasive Bladder Cancer. JAMA Oncol. (2018) 4:1535–42. doi: 10.1001/jamaoncol.2018.3542
4. Kaur I, Doja MN, Ahmad T. Time-range based sequential mining for survival prediction in prostate cancer. J BioMed Inform. (2020) 110:103550. doi: 10.1016/j.jbi.2020.103550
5. Ferro M, Tataru OS, Fallara G, Fiori C, Manfredi M, Claps F, et al. Lazzeri M et al: Assessing the influence of smoking on inflammatory markers in bacillus Calmette Guérin response among bladder cancer patients: a novel machine-learning approach. Minerva Urol Nephrol. (2024). doi: 10.23736/S2724-6051.24.05876-2
6. Russo P, Marino F, Rossi F, Bizzarri FP, Ragonese M, Dibitetto F, et al. Iacovelli R et al: Is Systemic Immune-Inflammation Index a Real Non-Invasive Biomarker to Predict Oncological Outcomes in Patients Eligible for Radical Cystectomy? Med (Kaunas). (2023) 59:2063. doi: 10.3390/medicina59122063
7. Singh N, Baby D, Rajguru JP, Patil PB, Thakkannavar SS, Pujari VB. Inflammation and cancer. Ann Afr Med. (2019) 18:121–6. doi: 10.4103/aam.aam_56_18
8. Nakamura K, Smyth MJ. Targeting cancer-related inflammation in the era of immunotherapy. Immunol Cell Biol. (2017) 95:325–32. doi: 10.1038/icb.2016.126
9. Goldberg EL, Shaw AC, Montgomery RR. How inflammation blunts innate immunity in aging. Interdiscip Top Gerontol Geriatr. (2020) 43:1–17. doi: 10.1159/000504480
10. Abbott M, Ustoyev Y. Cancer and the immune system: the history and background of immunotherapy. Semin Oncol Nurs. (2019) 35:150923. doi: 10.1016/j.soncn.2019.08.002
11. Mano R, Baniel J, Shoshany O, Margel D, Bar-On T, Nativ O, et al. Neutrophil-to-lymphocyte ratio predicts progression and recurrence of non-muscle-invasive bladder cancer. Urol Oncol. (2015) 33:67.e61–67. doi: 10.1016/j.urolonc.2014.06.010
12. Chandrasekaran D, Sundaram S, Maheshkumar K, Kathiresan N, Padmavathi R. Preoperative neutrophil-lymphocyte ratio/platelet-lymphocyte ratio: A potential and economical marker for renal cell carcinoma. J Cancer Res Ther. (2022) 18:1635–9. doi: 10.4103/jcrt.JCRT_482_20
13. Tobing E, Tansol C, Tania C. Albumin-globulin ratio (AGR) as independent predictor of poor survival in renal cell carcinoma: A systematic review and meta-analysis. Arab J Urol. (2024) 22:219–26. doi: 10.1080/20905998.2024.2352954
14. Qi Q, Zhuang L, Shen Y, Geng Y, Yu S, Chen H, et al. A novel systemic inflammation response index (SIRI) for predicting the survival of patients with pancreatic cancer after chemotherapy. Cancer. (2016) 122:2158–67. doi: 10.1002/cncr.v122.14
15. Zuo R, Zhu F, Zhang C, Ma J, Chen J, Yue P, et al. The response prediction and prognostic values of systemic inflammation response index in patients with advanced lung adenocarcinoma. Thorac Cancer. (2023) 14:1500–11. doi: 10.1111/1759-7714.14893
16. Yazici H, Yegen SC. Is systemic inflammatory response index (SIRI) a reliable tool for prognosis of gastric cancer patients without neoadjuvant therapy? Cureus. (2023) 15:e36597. doi: 10.7759/cureus.36597
17. Zhu M, Chen L, Kong X, Wang X, Fang Y, Li X, et al. The systemic inflammation response index as an independent predictor of survival in breast cancer patients: A retrospective study. Front Mol Biosci. (2022) 9:856064. doi: 10.3389/fmolb.2022.856064
18. Huang H, Wu K, Chen L, Lin X. Study on the application of systemic inflammation response index and platelet-lymphocyte ratio in ovarian Malignant tumors. Int J Gen Med. (2021) 14:10015–22. doi: 10.2147/IJGM.S346610
19. Xu L, Yu S, Zhuang L, Wang P, Shen Y, Lin J, et al. Systemic inflammation response index (SIRI) predicts prognosis in hepatocellular carcinoma patients. Oncotarget. (2017) 8:34954–60. doi: 10.18632/oncotarget.16865
20. Ding Y, Liu Z, Li J, Niu W, Li C, Yu B. Predictive effect of the systemic inflammation response index (SIRI) on the efficacy and prognosis of neoadjuvant chemoradiotherapy in patients with locally advanced rectal cancer. BMC Surg. (2024) 24:89. doi: 10.1186/s12893-024-02384-5
21. Li KJ, Zhang ZY, Sulayman S, Shu Y, Wang K, Ababaike S, et al. Prognostic value of combined systemic inflammation response index and prognostic nutritional index in colorectal cancer patients. World J Gastrointest Surg. (2024) 16:3794–805. doi: 10.4240/wjgs.v16.i12.3794
22. Ye K, Xiao M, Li Z, He K, Wang J, Zhu L, et al. Preoperative systemic inflammation response index is an independent prognostic marker for BCG immunotherapy in patients with non-muscle-invasive bladder cancer. Cancer Med. (2023) 12:4206–17. doi: 10.1002/cam4.v12.4
23. Lv Z, Feng HY, Wang T, Ma X, Zhang X. Preoperative systemic inflammation response index indicates poor prognosis in patients treated with resection of renal cell carcinoma with inferior vena cava tumor thrombus. Urol Oncol. (2022) 40:167.e169–167.e119. doi: 10.1016/j.urolonc.2021.11.030
24. Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. (2010) 25:603–5. doi: 10.1007/s10654-010-9491-z
25. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. Bmj. (1997) 315:629–34. doi: 10.1136/bmj.315.7109.629
26. Liu R, Shen Y, Cui J, Ma W, Wang J, Chen C, et al. Association between glucose to lymphocyte ratio and prognosis in patients with solid tumors. Front Immunol. (2024) 15:1454393. doi: 10.3389/fimmu.2024.1454393
27. Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. (1994) 50:1088–101. doi: 10.2307/2533446
28. Bailey-Whyte M, Minas TZ, Dorsey TH, Smith CJ, Loffredo CA, Ambs S. Systemic inflammation indices and association with prostate cancer survival in a diverse patient cohort. Cancers (Basel). (2023) 15:1869. doi: 10.3390/cancers15061869
29. Fukuda H, Takagi T, Kondo T, Shimizu S, Tanabe K. Predictive value of inflammation-based prognostic scores in patients with metastatic renal cell carcinoma treated with cytoreductive nephrectomy. Oncotarget. (2018) 9:14296–305. doi: 10.18632/oncotarget.24507
30. Gu L, Ma X, Wang L, Li H, Chen L, Li X, et al. Prognostic value of a systemic inflammatory response index in metastatic renal cell carcinoma and construction of a predictive model. Oncotarget. (2017) 8:52094–103. doi: 10.18632/oncotarget.10626
31. Kadono Y, Kawaguchi S, Nohara T, Shigehara K, Izumi K, Kamijima T, et al. Nakagawa R et al: Blood Cell Count Biomarkers Predicting Efficacy of Pembrolizumab as Second-line Therapy for Advanced Urothelial Carcinoma. Anticancer Res. (2021) 41:1599–606. doi: 10.21873/anticanres.14921
32. Mao W, Sun S, He T, Jin X, Wu J, Xu B, et al. Systemic inflammation response index is an independent prognostic indicator for patients with renal cell carcinoma undergoing laparoscopic nephrectomy: A multi-institutional cohort study. Cancer Manag Res. (2021) 13:6437–50. doi: 10.2147/CMAR.S328213
33. Ni J, Wang K, Zhang H, Xie J, Xie J, Tian C, et al. Liang C et al: Prognostic Value of the Systemic Inflammatory Response Index in Patients Undergoing Radical Cystectomy for Bladder Cancer: A Population-Based Study. Front Oncol. (2021) 11:722151. doi: 10.3389/fonc.2021.722151
34. Tang Y, Shao Y, Hu X, Ren S, Li X. Validation and comparison of prognostic value of different preoperative systemic inflammation indices in non-metastatic renal cell carcinoma. Int Urol Nephrol. (2023) 55:2799–807. doi: 10.1007/s11255-023-03724-9
35. Zapała Ł, Ślusarczyk A, Garbas K, Mielczarek Ł, Ślusarczyk C, Zapała P, et al. Complete blood count-derived inflammatory markers and survival in patients with localized renal cell cancer treated with partial or radical nephrectomy: a retrospective single-tertiary-center study. Front Biosci (Schol Ed). (2022) 14:5. doi: 10.31083/j.fbs1401005
36. Zheng Y, Chen Y, Chen J, Chen W, Pan Y, Bao L, et al. Combination of systemic inflammation response index and platelet-to-lymphocyte ratio as a novel prognostic marker of upper tract urothelial carcinoma after radical nephroureterectomy. Front Oncol. (2019) 9:914. doi: 10.3389/fonc.2019.00914
37. Chen Z, Wang K, Lu H, Xue D, Fan M, Zhuang Q, et al. Systemic inflammation response index predicts prognosis in patients with clear cell renal cell carcinoma: a propensity score-matched analysis. Cancer Manag Res. (2019) 11:909–19. doi: 10.2147/CMAR.S186976
38. Yilmaz H, Cinar NB, Avci IE, Telli E, Uslubas AK, Teke K, et al. The systemic inflammation response index: An independent predictive factor for survival outcomes of bladder cancer stronger than other inflammatory markers. Urol Oncol. (2023) 41:256.e251–256.e258. doi: 10.1016/j.urolonc.2022.11.011
39. Diakos CI, Charles KA, McMillan DC, Clarke SJ. Cancer-related inflammation and treatment effectiveness. Lancet Oncol. (2014) 15:e493–503. doi: 10.1016/S1470-2045(14)70263-3
40. Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. (2008) 454:436–44. doi: 10.1038/nature07205
41. Wigner P, Grębowski R, Bijak M, Saluk-Bijak J, Szemraj J. The interplay between oxidative stress, inflammation and angiogenesis in bladder cancer development. Int J Mol Sci. (2021) 22:4483. doi: 10.3390/ijms22094483
42. Miyake M, Tatsumi Y, Gotoh D, Ohnishi S, Owari T, Iida K, et al. Itami Y et al: Regulatory T Cells and Tumor-Associated Macrophages in the Tumor Microenvironment in Non-Muscle Invasive Bladder Cancer Treated with Intravesical Bacille Calmette-Guérin: A Long-Term Follow-Up Study of a Japanese Cohort. Int J Mol Sci. (2017) 18. doi: 10.3390/ijms18102186
43. Kates M, Nirschl T, Sopko NA, Matsui H, Kochel CM, Reis LO, et al. McConkey DJ et al: Intravesical BCG Induces CD4(+) T-Cell Expansion in an Immune Competent Model of Bladder Cancer. Cancer Immunol Res. (2017) 5:594–603. doi: 10.1158/2326-6066.CIR-16-0267
44. Sarier M, Sepin N, Keles Y, Imir L, Emek M, Demir M, et al. Is there any association between urothelial carcinoma of the bladder and human papillomavirus? A case-control study. Urol Int. (2020) 104:81–6. doi: 10.1159/000500467
45. Sarier M, Usta SS, Turgut H, Öztürk SA, Soylu A, Emek M, et al. Prognostic value of HPV DNA in urothelial carcinoma of the bladder: A preliminary report of 2-year follow-up results. Urol J. (2021) 19:45–9. doi: 10.22037/uj.v18i.6429
46. Sarier M. Association between human papillomavirus and urothelial carcinoma of the bladder. Rev Assoc Med Bras (1992). (2022) 68:551–2. doi: 10.1590/1806-9282.20220154
47. Coffelt SB, Wellenstein MD, de Visser KE. Neutrophils in cancer: neutral no more. Nat Rev Cancer. (2016) 16:431–46. doi: 10.1038/nrc.2016.52
48. Elinav E, Nowarski R, Thaiss CA, Hu B, Jin C, Flavell RA. Inflammation-induced cancer: crosstalk between tumours, immune cells and microorganisms. Nat Rev Cancer. (2013) 13:759–71. doi: 10.1038/nrc3611
49. Jamieson T, Clarke M, Steele CW, Samuel MS, Neumann J, Jung A, et al. Nibbs RJ et al: Inhibition of CXCR2 profoundly suppresses inflammation-driven and spontaneous tumorigenesis. J Clin Invest. (2012) 122:3127–44. doi: 10.1172/JCI61067
50. Katoh H, Wang D, Daikoku T, Sun H, Dey SK, Dubois RN. CXCR2-expressing myeloid-derived suppressor cells are essential to promote colitis-associated tumorigenesis. Cancer Cell. (2013) 24:631–44. doi: 10.1016/j.ccr.2013.10.009
51. Deryugina EI, Zajac E, Juncker-Jensen A, Kupriyanova TA, Welter L, Quigley JP. Tissue-infiltrating neutrophils constitute the major in vivo source of angiogenesis-inducing MMP-9 in the tumor microenvironment. Neoplasia. (2014) 16:771–88. doi: 10.1016/j.neo.2014.08.013
52. Shojaei F, Singh M, Thompson JD, Ferrara N. Role of Bv8 in neutrophil-dependent angiogenesis in a transgenic model of cancer progression. Proc Natl Acad Sci U.S.A. (2008) 105:2640–5. doi: 10.1073/pnas.0712185105
53. Guthrie GJ, Roxburgh CS, Farhan-Alanie OM, Horgan PG, McMillan DC. Comparison of the prognostic value of longitudinal measurements of systemic inflammation in patients undergoing curative resection of colorectal cancer. Br J Cancer. (2013) 109:24–8. doi: 10.1038/bjc.2013.330
54. Ocana A, Nieto-Jiménez C, Pandiella A, Templeton AJ. Neutrophils in cancer: prognostic role and therapeutic strategies. Mol Cancer. (2017) 16:137. doi: 10.1186/s12943-017-0707-7
55. Movahedi K, Laoui D, Gysemans C, Baeten M, Stangé G, Van den Bossche J, et al. tumor microenvironments contain functionally distinct subsets of macrophages derived from Ly6C(high) monocytes. Cancer Res. (2010) 70:5728–39. doi: 10.1158/0008-5472.CAN-09-4672
56. Qian BZ, Pollard JW. Macrophage diversity enhances tumor progression and metastasis. Cell. (2010) 141:39–51. doi: 10.1016/j.cell.2010.03.014
57. Feng F, Zheng G, Wang Q, Liu S, Liu Z, Xu G, et al. Low lymphocyte count and high monocyte count predicts poor prognosis of gastric cancer. BMC Gastroenterol. (2018) 18:148. doi: 10.1186/s12876-018-0877-9
58. Liu A, Xia Y, Li W, Zhang G, Liu Y, Ye S, et al. The predictive value of changes in the absolute counts of peripheral lymphocyte subsets for progression and prognosis in breast cancer patients. Contrast Media Mol Imaging. (2022) 2022:3444360. doi: 10.1155/2022/3444360
59. Koliaraki V, Prados A, Armaka M, Kollias G. The mesenchymal context in inflammation, immunity and cancer. Nat Immunol. (2020) 21:974–82. doi: 10.1038/s41590-020-0741-2
60. Tatara T, Suzuki S, Kanaji S, Yamamoto M, Matsuda Y, Hasegawa H, et al. Lymphopenia predicts poor prognosis in older gastric cancer patients after curative gastrectomy. Geriatr Gerontol Int. (2019) 19:1215–9. doi: 10.1111/ggi.v19.12
61. Dai S, Zeng H, Liu Z, Jin K, Jiang W, Wang Z, et al. Intratumoral CXCL13(+)CD8(+)T cell infiltration determines poor clinical outcomes and immunoevasive contexture in patients with clear cell renal cell carcinoma. J Immunother Cancer. (2021) 9:e001823. doi: 10.1136/jitc-2020-001823
62. Grob G, Rogers D, Pandolfo SD, Vourganti S, Buscarini M, Mehrazin R, et al. Oncologic outcomes following radical nephroureterectomy for upper tract urothelial carcinoma: a literature review. Transl Androl Urol. (2023) 12:1351–62. doi: 10.21037/tau-22-882
63. Pandolfo SD, Wu Z, Campi R, Bertolo R, Amparore D, Mari A, et al. Outcomes and techniques of robotic-assisted partial nephrectomy (RAPN) for renal hilar masses: A comprehensive systematic review. Cancers (Basel). (2024) 16:693. doi: 10.3390/cancers16040693
Keywords: systemic inflammation response index, urological malignancies, biomarker, prognosis, meta-analysis
Citation: Ma W, Liu R, Li X, Yu J and Wang W (2025) Significant association between systemic inflammation response index and prognosis in patients with urological malignancies. Front. Immunol. 16:1518647. doi: 10.3389/fimmu.2025.1518647
Received: 28 October 2024; Accepted: 11 February 2025;
Published: 26 February 2025.
Edited by:
David Garcia-Illescas, Vall d’Hebron University Hospital, SpainReviewed by:
Mehmet Sarier, University of Istinye, TürkiyeCopyright © 2025 Ma, Liu, Li, Yu and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Weixing Wang, d2FuZ3d4QHdodS5lZHUuY24=; Jia Yu, eW9nYXFxMTE2QHdodS5lZHUuY24=; Rongqiang Liu, NDI1NDc1NTMxQHFxLmNvbQ==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.