
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Immunol., 07 March 2025
Sec. Inflammation
Volume 16 - 2025 | https://doi.org/10.3389/fimmu.2025.1518339
This article is part of the Research TopicAdvances in Innate Immune Cells in chronic non-transmissible diseases: From Sensing to Effector Mechanisms in Inflammatory DiseasesView all 3 articles
Ulcerative colitis (UC) is an incurable autoimmune disease. Patients with UC endure the burden of recurrent flare-ups and face a substantial economic burden due to long-term medication. The complex etiology and unclear pathogenesis pose a significant challenge to the development of effective and curative treatments. Recent research indicates that local memory at the site of inflammatory intestinal mucosa in UC is closely associated with the persistent presence of tissue-resident memory T (TRM) cells. TRM cells, a subset of memory T cells, exhibit long-lived, low-migration characteristics. These cells reside in tissues, where they provide immediate immune protection while also contributing to chronic, localized inflammation. The presence of TRM cells in the inflamed intestinal mucosa of UC patients is a crucial factor in the recurrence of the disease. However, the process involved in the formation and differentiation of TRM cells within the intestinal mucosa remains poorly understood. Various surface markers, transcriptional networks, and signaling pathways regulate the formation and maintenance of TRM cells in the intestine. To further understand the role of TRM cells in UC pathogenesis, we have summarized the latest findings to pave the way for the development of future targeted therapies.
Ulcerative colitis (UC) is a chronic, recurrent and currently incurable inflammatory bowel disease (IBD) characterized by uncontrolled inflammation, which leads to damage to the bowel (1). Over the past decade, there has been a notable increase in the global prevalence of UC, particularly in developing countries and regions such as Asia and Eastern Europe (2, 3). Patients with UC experience a significantly reduced quality of life due to associated symptoms, including diarrhea or bloody stools, abdominal pain, and fecal urgency. Additionally, they bear a substantial physical, psychological and economic burden due to disease recurrence and the elevated risk of cancerization (4).
The treatment goals for UC are focused on achieving rapid endoscopic remission or combined endoscopic and histologic remission, collectively referred to as mucosal healing. In addition to conventional treatments, although significant advances in biological therapeutic strategies such as anti-tumor necrosis factor-α (TNF-α) drugs, anti-leukocyte adhesion molecule preparations, kinase inhibitors, interleukin (IL) -12 and IL-23 antagonists (5–9), maintaining clinical remission and preventing recurrence remains a substantial challenge for most UC patients, even after drug withdrawal. The recurrence of UC is often characterized by periodic remission and exacerbation, which can occur spontaneously or in response to drug treatment. A meta-analysis revealed that the overall risk of UC recurrence after discontinuing anti-TNF treatment is 38% (10). Apparently, current therapeutic measures remain insufficient to fully address these complexities (11). Therefore, it is imperative to gain a comprehensive understanding of the mechanisms underlying the recurrence and to develop effective prevention strategies.
Tissue-resident memory T (TRM) cells, a subtype of memory T cells, have been identified as a critical cell type in the immune system. Under physiological conditions, only 5%~10% of effector T cells could escape from apoptosis after clearing pathogens or antigens (12). These cells have the potential to transform into TRM cells through the induction of transforming growth factor-β (TGF-β) or IL-15 (13). Following this transforming, TRM cells are stably established in barrier organs or tissues, including the skin, gut, and respiratory mucosa. These cells provide rapid immune responses to reinfection without being activated by antigen-presenting cells (14, 15). However, growing evidence indicates that TRM cells may also target autoantigens or persistently exposed antigens, contributing to autoimmune diseases such as autoimmune hepatitis, rheumatoid arthritis and UC (16–18).
Current evidence indicates that TRM cells may play a central role in the mechanisms of intestinal inflammation and local recurrence in UC. The potent proinflammatory and tissue-resident properties of TRM cells may be responsible for the recurrent episodes and specific inflammation localization in UC patients (19, 20). Therefore, a detailed discussion of the cellular phenotype of TRM cells, as well as the underlying immunomodulatory and inflammation mechanisms is necessary.
Although without exact and consistent findings, various cell markers have been observed on TRM cells. We have summarized some of the key cell surface markers in the intestine based on recent studies.
CD103 and CD69 were defined as classic phenotypic markers on TRM cells (21) (Figure 1). CD103 (αE integrin), a receptor for E-Cadherin, is predominantly expressed on intestinal CD8+ TRM cells, where it promotes the residency of TRM cells (22). It is considered to be a CD8+ TRM-residency-specific molecule (23), as most CD4+ T cells do not express CD103 (24). In normal barrier tissues, TGF-β signaling promotes CD103 expression, ensuring the long-term maintenance of CD103+ CD8+ TRM cells in nonlymphoid barrier tissues (25). During intestinal inflammation, the expression of CD103 can be influenced by cytokines such as interferon (IFN) -β and IL-12. These inflammatory mediators may modulate TGF-β-mediated CD103 expression, potentially inhibiting or altering its induction in different cellular contexts (26). Since E-cadherin is primarily expressed in the intestinal epithelium, it may have limited impact on the lamina propria where CD103+ cells locate (27).
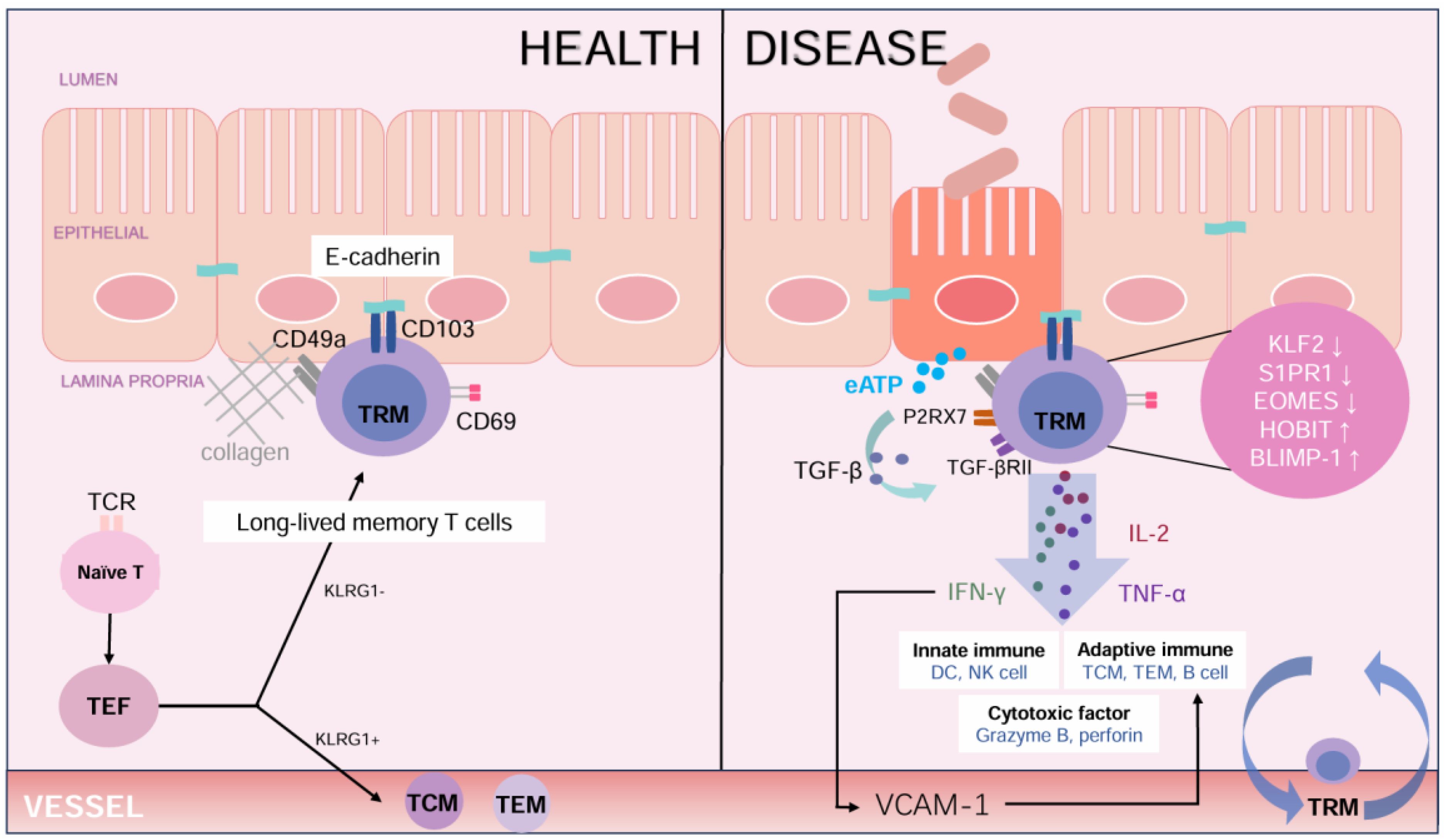
Figure 1. The role of TRM cells in maintaining intestinal health and contributing to disease. In healthy colon, TRM cells primarily express CD103 and CD69 to maintain local immunity and homeostasis. The binding of Ecadherin supports the retention of these cells, while cytokines such as TGF-β promote the development of CD103+TRM cells. In Ulcerative Colitis, TRM cells exhibit a proinflammatory phenotype, characterized by secreting IL-2, IFN-γ, and TNF-α. These cytokines activate both innate and adaptive immune responses and release cytotoxic factors like granzyme B and perforin. Molecular changes, such as decreased expression of KLF2 and increased expression of BLIMP-1 expression, enhance the inflammation response. Signals from extracellular ATP (eATP) and P2RX7 receptor exacerbate immune activation, contributing to tissue damage in UC. (TCR, T cell receptor; TEF, effector T cell; TCM, central memory T cell; TEM, effector memory T cell; TRM, tissue resident memory T cell; KLRG1, Killer cell Lectin-like Receptor G1; eATP, extracellular Adenosine Triphosphate; P2RX7, Purinergic Receptor P2X Ligand Gated Ion Channel 7; TGF-β, Transforming Growth Factor-β; TGF-βRII, Transforming Growth Factor-beta Receptor II; IL-2, Interleukin-2; IFN-γ, Interferon-γ; TNF-α, tumor necrosis factor-α; KLF2, Krüppel-like Factor 2; S1PR1, Sphingosine-1-phosphate Receptor 1; EOMES, Eomesodermin; HOBIT, Homeobox Protein Hox-11-Like; BLIMP-1, B Lymphocyte Induced Maturation Protein 1; VCAM-1, vascular cell adhesion molecule-1.).
CD69 serves as a marker of early T cell activation and is closely associated with T cell residency (28). During the development of TRM cells, TGF-β negatively regulates Forkhead Box Protein O1 (FoxOl) through the PI3K-Akt pathway, downregulating Kruppel-like factor 2 (KLF2) (29). KLF2 is a transcription factor critical for T cell trafficking, which directly downregulates sphingosine-1 phosphate receptor 1 (S1PR1) and indirectly upregulates CD69, preventing T cells egress from the intestine (30). The lack of S1PR1 is also a method to identify TRM cells. Compared with CD103, CD69 is a secondary marker, as CD69 is not an essential residency marker in the intestine of mice (31). However, in humans, CD69 is instrumental in distinguishing between TRM cells and circulating memory T cells (32).
Only 25% of TRM cells in the colon express both CD103 and CD69, TRM cells lacking CD103 or CD69 can also be found (33). Their generation depends on the location and whether they arise from local tissue infection (34), as these cells play a rapid response role in secondary infections (35). Compared to CD103+ TRM cells, intestinal CD103- TRM cells have been demonstrated to possess greater effector functions, including the ability to produce higher levels of granzyme A (36). CD103- TRM cells share similar transcriptional profiles with circulating T cells, rapidly producing IFN-γ, TNF-α, and IL-2 while retaining memory of infection within tissues after clearance (37, 38). The cytokine IFN-γ, largely regulated by signal transducer and activator of transcription 4 (STAT4), is a key driver of CD103-TRM cell differentiation (39). Some CD8+ CD103- TRM cells also express high levels of β2-integrin (40), while others express Killer Cell Lectin-like Receptor G1 (KLRG1), another receptor for E-Cadherin, which can compete with CD103 (41). Intestinal CD103- TRM within the intraepithelial layer are derived from KLRG1+T cells (42). These KLRG1+ T cells exhibit enhanced survival and developmental plasticity, enabling the generation of CD103- TRM (42).
Furthermore, CD49a, the α chain of integrin α1β1, is an immunomodulatory and adhesive molecule that interacts with type IV collagen in the lamina propria to establish tissue residency, promoting the accumulation of CD8+ TRM cells (43, 44) (Figure 1). CD49a expression is upregulated in CD69+ TRM cells, contributing to the adhesion between T cells and intestinal epithelial cells (45). This is critical for the retention of TRM cells in the gut.
CD161, also called Killer Cell Lectin-Like Receptor C1 (KLRC1), is a C-type lectin-like receptor expressed by adult CD8+ T cells (46). It is a subtype of CD8+ T cells that produces significant amounts of cytotoxic meditators, including granzyme B and perforin. Additionally, these cells express high levels of the transcription factors Eomesodermin (EOMES) and T-box expressed in T cells (T-bet) (47), which predominantly regulate the expression of cytotoxicity mediators and cytokine production (48). Intermediate levels of CD161 are highly expressed in the colon. In inflamed tissues of IBD patients, CD161 is enriched in CD103+ TRM cells, and the majority of CD161 in the colon co-expresses CD69 (47). CD161int CD8+T cells provide rapid immune protection against pathogens within the gut.
CD39 and CD73 are two regulatory markers expressed by TRM cells, involved in nucleotide-metabolizing that regulate immunity and inflammation (49). After the release of the inflammatory signal adenosine triphosphate (ATP), CD39 converts ATP to adenosine monophosphate (AMP), which is subsequently dephosphorylated by CD73 to produce adenosine (50). These enzymes facilitate the transition from ATP-driven, proinflammatory immune cell activity an adenosine-mediated, anti-inflammatory states. As these markers are typically expressed at high levels on regulatory T cells (51), reduced levels of CD39 with IBD may indicate a compromised regulatory function. Studies have shown that CD39 and CD73 are highly expressed on CD103+ CD8+ TRM cells, suggesting that these cells possess an immunosuppressive function (51). Human TRM cells expressing CD39 and CD73 may contribute to the maintenance of intestinal immune homeostasis.
Although CD69 and CD103 are considered the most prominent surface markers of TRM cells, they are not specific to TRM cells. Currently, the study of cell surface markers is not comprehensive, other potential markers of intestinal TRM cells include CD44 and CD101 (40). In particular, the specific TRM subsets in UC patients need to be further refined and studied.
Multiple metabolic pathways are involved in the generation and function of TRM cells. At present, a greater number of studies have been conducted on TRM cells in the small intestine than in the colon. Most of the findings were derived from experimental lymphocytic choriomeningitis virus (LCMV) mouse infection and clinical research in patients.
Purinergic Receptor P2X, Ligand Gated Ion Channel 7 (P2RX7) has recently been identified as a key signaling molecule associated with the formation of TRM cells. Given the abundant antigenic substances and microbiota in the colon, extracellular adenosine triphosphate (eATP) from the colon microbiota activates the P2RX7 (52), which enhances the sensitivity of TGF-β in CD8+ TRM cells (53). TGF-β has been proven to contribute to the development of TRM cell characteristics and the establishment of transcriptional networks (54). In the intestine, naive CD8+T cells are activated and differentiated in response to antigenic stimulation. TGF-β in the intestine environment accelerates the apoptosis of short-lived effector cells while promoting the rapid formation of memory precursor cells (55). This process occurs through the simultaneous downregulation of KLF2 and SIPR1, along with the upregulation of CD69 and CD103 (56) (Figure 1). The signaling molecule Smad3 also contributes to CD103 regulation (57).
Additionally, P2RX7 is an ion channel that responds not only to eATP but also to Nicotinamide Adenine Dinucleotide (NAD+), playing a role in cell death via the ART2.2/P2RX7 pathway (58). Given its dual function, the role of P2RX7 in the development and function of TRM cells warrants further investigation. CD38 is an enzyme expressed on T cells that exerts regulatory effects on both the metabolism of NAD+ (59) and the development of TRM cells. CD38 serves as a marker distinguishing TRM cells from circulating memory T cells across various organs. Deletion of CD38 has been observed to impact TGF-β-dependent CD103+ TRM cell subpopulations in epithelial tissues (60).
Newly published research (61) has revealed that depletion of nutrient-dependent lysosomal signaling nodes activates the transcription factor EB (Tfeb), and deficiency in Follicular Lymphoma Overexpressed Gene (Flcn) enhances protective responses in intestinal TRM cells, with the Flcn-Tfeb axis mechanistically inhibiting retinoic acid-induced CCR9 expression to promote TGF-β-mediated lineage differentiation programs. Subsequently, the expression of CCR9 contributes to the differentiation of intestinal CD103+ TRM cells via retinoic acid signaling in mesenteric lymph nodes (62).
Although many new pathways are being discovered, studies on colonic TRM cells remain limited compared to those on small intestine TRM cells. Most of the mechanism-related studies have been conducted in mice and require further validation due to the heterogeneity of TRM cells.
The development, maintenance, migration, and function of intestinal TRM cells are regulated by a complex network of transcription factors. The transcriptional factors Blimp-1/Hobit and Eomes/T-bet play crucial but distinct roles in this process.
Blimp-1 and Hobit are transcription factors involved in TRM cell generation and regulation (63), encoded by Zfp683 and PRDM1 (64), respectively. They exhibit a high degree of overlap in their genomic binding sites, homologous sequences, and synergistic action that collectively drive T cell lineage differentiation (65). Approximately 30% of the genes related to tissue-resident functions in TRM cells are regulated by Blimp-1 and Hobit (64). Blimp-1 and Hobit suppress the expression of Klf2, S1pr1, T-cell transcription factor (Tcf), and C-C motif chemokine receptor 7 (Ccr7), thereby contributing to the residency of TRM cells in tissues. Ccr7 is a critical regulator of lymphocyte egress from peripheral tissues, which is usually absent in TRM cells (64). The core transcription factor Runx3 could induce chromatin accessibility and regulate Blimp-1 (66, 67). In CD8+T cells, Blimp-1 knockout alone is insufficient to induce granzyme B expression (68). However, Hobit is responsible for maintaining granzyme B expression, and in conjunction with Blimp-1, sustains the cytotoxic function of TRM cells (69). Following antigen rechallenge, TRM cells may experience a decrease in Hobit expression, leading to the formation of circulating memory cells (70). The development and maintenance of intestinal CD8+ TRM cells are impaired in mice with a double knockout Blimp-1 and Hobit, resulting in alleviated colitis (19). In a mouse model of colitis, the Hobit- Blimp-1- CD4+ TRM cells lead to reduced chemokines expression, impaired leukocyte recruitment, and decreased expression of proinflammatory molecules (71).
The T-box transcription factors Eomes and T-bet are involved in the complex regulatory mechanisms that control TRM cell differentiation and maintenance in the colon. Eomes is a key transcription factor to the differentiation and effector function of CD8+ T cells (72), inducing the production of proinflammatory substances such as IFN-γ, granzyme B, and perforin (73). Compared to the small intestine, the number and function of colonic TRM cells do not depend on EOMES (33). This phenomenon may be attributed to the distinct microenvironment present in the small intestine and colon. Ectopic expression of Eomes in a mouse model enhances the pathological properties of CD8+ T cells, supporting that Eomes promote inflammatory and pathological states in CD8+ TRM cells (73, 74). The coordinated downregulation of Eomes and T-bet promotes the generation of CD8+ CD103+ TRM cells and enhances their responsiveness to TGF-β signaling (75). A recent study (76) discovered that depletion of T-bet, a key molecule along with Hypermethylated in cancer 1 (Hic1) in regulating intestinal TRM cell differentiation, partially restored Hic1 expression and significantly rescued the formation of intestinal CD103+ TRM cells in the absence of TGF-β receptor signaling.
The powerful proinflammatory properties and abnormal activation of TRM cells in the colon may explain the pathogenesis of UC flare-ups. In the colon, pathogens in the intestinal lumen are captured by antigen-presenting cells, such as macrophages and dendritic cells in the mucosal layer, which subsequently activate naïve T cells. These naïve T cells differentiate into effector T cells and memory T cell subsets, which migrate to sites of intestinal inflammation in response to cell adhesion molecules, including chemokine receptors. Among these subsets, memory T cells can be generated during the early stages of the immune response and at various stages of differentiation. In the later phase of the inflammatory response, some memory T cells return via the bloodstream, while others are retained locally in the intestinal epithelium or lamina propria. Upon encountering the same antigen, CD4+ and CD8+ TRM cells are rapidly activated, proliferate, and generate cytotoxic responses. If this immune response is not properly regulated, extensive immune cell crosstalk occurs, leading to intestinal mucosal damage and chronic intestinal inflammation.
In this way, TRM cells in UC are primarily categorized into CD4+ and CD8+ subsets, which are consistent with the general classification of T cells. CD8+ TRM cells are mainly located in the intestinal epithelium, but they can also be found in the lamina propria, where CD4+ TRM cells mainly exist (77). It was found in IBD patients that, CD4+ TRM cells produce more IL-17 than circulating CD4+ T cells (78). IL-17 is an important proinflammatory cytokine that can induce a variety of cells, such as epithelial cells, fibroblasts and endothelial cells, to produce proinflammatory cytokines and chemokines, such as IL-6, IL-8, TNF-α, etc., thereby aggravating intestinal inflammation. The number of CD4+ TRM cells in the lamina propria of colonic mucosa of active UC patients is significantly higher than that of healthy people and is closely related to the frequency of clinical recurrence of UC (19). So CD4+ TRM cells mainly play a proinflammatory role in UC, depletion of these cells in mice with transfer colitis resulted in significant alleviation of colitis flare-ups.
The research of CD8+ TRM cells in UC remain limited. A study on healthy participants showed that CD103+ CD8+ TRM cells in the gut directly kill infected or damaged cells through cytotoxic mechanisms involving granzyme B and perforin, and they mainly express IFN-γ, IL-2, and TNF-α upon activation (51). Single-cell sequencing analysis revealed significant heterogeneity in T-cell subsets between healthy controls and UC patients. In UC patients, Eomes-mediated pathologic CD8+ TRM cells were expanded and the KLRG1+EOMES+ITGB2+ subpopulation was enriched (74). The target genes of Eomes, including IFN-γ, granzyme A and KLRG1, displayed enhanced inflammatory and cytolytic characteristics (74). In the inflamed mucosa of CD patients, there was a relative decrease in the number of CD103+CD8+ TRM cells and an increase in the number of KLRG1+CD8+TRM cells (79). One subset of these cells exhibited a stronger response to T-cell receptor stimulation, while the other subset demonstrated enhanced cytotoxic potential, proliferative capacity and expression of T helper cell 17 genes (79). A clinical study analyzed that in both CD4+ and CD8+ TRM cells, the proportion of CD103+ TRM cells in IBD is reduced during the inflammation phase, indicating that CD103+ TRM cells may be involved in maintaining mucosal homeostasis and regulating the immune response (80, 81). Earlier experiments in mice showed that CD103+ CD8+ T cells could alleviate transfer ileitis and inhibit the proliferation of CD4+ T cells through TGF-β (82). Immunomodulatory markers such as CD39 and CD73 are mainly expressed on the surface of some CD8+ TRM cells (82). Thus, CD8+ CD103+ TRM cells may support homeostasis in noninflamed gut. Additionally, the expression of CD69+TRM cells mRNA and proinflammatory cytokines, including IFN-γ, IL-13, IL-17A and TNF-α, has been found to be elevated during the active phase of the disease (19). Therefore, it is worthwhile to investigate the potential of CD103+ TRM cells in maintaining intestinal immune homeostasis.
Emerging evidence demonstrated that TRM cells present a new potential target for the treatment of recurrent UC (71). CD8+ TRM cells, activated by IFN-γ, have been implicated in immune checkpoint inhibitor-associated colitis and have been shown to respond to JAK inhibitors, making them a promising target for it (83). New therapies targeting T cell trafficking, including the α4β7 antagonist peptide PTG-100 (84), anti-β7 integrin antibody Etrolizumab (85), the α4-integrin antagonist AJM300 (86), and sphingosine-1-phosphate (S1P) receptor modulator Ozanimod (87), are closely related to the residency and recirculation of TRM cells. Etrolizumab, which selectively targets α4β7 and αEβ7 integrins, is designed to impede the intestinal homing of inflammatory T cells. This consequently helps their residency in the intestinal mucosa, including the surface molecule CD103 (88). In a phase 3 randomized controlled trial, Etrolizumab demonstrated superior efficacy and safety compared to adalimumab and placebo in patients with moderately to severely active UC (89). Ozanimod, an S1P receptor modulator, has been shown to prevent lymphocyte mobilization to inflammatory sites. Furthermore, the drug has been found to be more effective than a placebo in improving endoscopic and mucosal healing in patients with moderately to severely active UC (90). The P2X7 receptor antagonist AZD9056 induced clinical remission in patients with moderate-to-severe CD, but failed to result in a notable reduction in serum C-reactive protein and fecal calprotectin (91). The calmodulin phosphatase pathway plays a pivotal role in P2RX7 signaling, which is implicated in the generation of TRM cells (92). Inhibitors of this pathway are employed in the induction therapy for acute severe UC, despite their dual function (92). This outcome presents a challenge for future clinical research on the P2RX7 receptor. In summary, the identification of pathogenic TRM cells represents a promising opportunity for the development of potential therapeutic strategies for the management of IBD.
In conclusion, TRM cells play a crucial role in both innate and adaptive immune systems by providing local immune defense in the intestine under normal physiological conditions. In pathological states, abnormally activated TRM cells have been observed to trigger severe intestinal inflammation and localized inflammatory flare-ups. The advent of single-cell transcriptomics, T-cell receptor library analysis and mass spectrometry technology provides the possibility to further elucidate the pathophysiological mechanisms of TRM cells at the cellular level. In light of these findings, we summarize the role of different phenotypes of TRM cells in the development of UC, with a particular focus on UC recurrence. We also provided an overview of the regulatory factors involved in the formation, differentiation, residence, migratory and recycling of TRM cells, and identified the potential of specific targets for the treatment of UC recurrence. In sum, it can be proposed that similar to the two sides of a coin, different subgroups of intestinal TRM cells exhibit both protective and pathogenic functions. The amplification and migration of abnormal TRM cells are the key targets in UC recurrence and are involved in triggering systemic symptoms. However, limitations remain. The inconsistency in results between human and animal studies on TRM cells is attributed to the tissue specificity of TRM cells and differences in the clinical backgrounds of patients. To identify the optimal balance between the protective and pathogenic functions of TRM cells, extensive studies are necessary to uncover the detailed characteristics and functions of TRM cell subpopulations, which is essential for the development of effective UC therapies.
XW: Visualization, Writing – original draft. JZ: Conceptualization, Writing – original draft. LF: Writing – review & editing. XT: Conceptualization, Writing – review & editing.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was supported by the National Natural Science Foundation of China (No. 82341233), China Academy of Chinese Medical Sciences Innovation Fund (NO.CI2021A01012), and Specialized Project of Xiyuan Hospital, China Academy of Chinese Medical Sciences for the Construction of Integrated Platform for Chinese Medicine Clinical Research (XYZX0405-34).
We declare that we do not have any commercial or associative interest that represents a conflict of interest in connection with the work submitted.
The author(s) declare that no Generative AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Chen B, Zhong J, Li X, Pan F, Ding Y, Zhang Y, et al. Efficacy and safety of ivarmacitinib in patients with moderate-to-severe, active, ulcerative colitis: A phase II study. Gastroenterology. (2022) 163:1555–68. doi: 10.1053/j.gastro.2022.08.007
2. Mak WY, Zhao M, Ng SC, Burisch J. The epidemiology of inflammatory bowel disease: East meets west. J Gastroenterol Hepatol. (2020) 35:380–9. doi: 10.1111/jgh.14872
3. Vegh Z, Kurti Z, Lakatos PL. Epidemiology of inflammatory bowel diseases from west to east. J Dig Dis. (2017) 18:92–8. doi: 10.1111/1751-2980.12449
4. Segal JP, LeBlanc J-F, Hart AL. Ulcerative colitis: an update. Clin Med (Lond). (2021) 21:135–9. doi: 10.7861/clinmed.2021-0080
5. Hindryckx P, Jairath V, D’Haens G. Acute severe ulcerative colitis: from pathophysiology to clinical management. Nat Rev Gastroenterol Hepatol. (2016) 13:654–64. doi: 10.1038/nrgastro.2016.116
6. Narula N, Wong ECL, Marshall JK, Colombel J-F, Dulai PS, Reinisch W. Comparative efficacy for infliximab vs vedolizumab in biologic naive ulcerative colitis. Clin Gastroenterol Hepatol. (2022) 20:1588–97.e3. doi: 10.1016/j.cgh.2021.07.038
7. Sandborn WJ, Danese S, Leszczyszyn J, Romatowski J, Altintas E, Peeva E, et al. Oral ritlecitinib and brepocitinib for moderate-to-severe ulcerative colitis: results from a randomized, phase 2b study. Clin Gastroenterol Hepatol. (2023) 21:2616–28.e7. doi: 10.1016/j.cgh.2022.12.029
8. Ma C, Panaccione R, Xiao Y, Khandelwal Y, Murthy SK, Wong ECL, et al. REMIT-UC: real-world effectiveness and safety of tofacitinib for moderate-to-severely active ulcerative colitis: A Canadian IBD research consortium multicenter national cohort study. Am J Gastroenterol. (2023) 118:861–71. doi: 10.14309/ajg.0000000000002129
9. Sands BE, Sandborn WJ, Panaccione R, O’Brien CD, Zhang H, Johanns J, et al. Ustekinumab as induction and maintenance therapy for ulcerative colitis. N Engl J Med. (2019) 381:1201–14. doi: 10.1056/NEJMoa1900750
10. Gisbert JP, Marín AC, Chaparro M. The risk of relapse after anti-TNF discontinuation in inflammatory bowel disease: systematic review and meta-analysis. Am J Gastroenterol. (2016) 111:632–47. doi: 10.1038/ajg.2016.54
11. Krugliak Cleveland N, Torres J, Rubin DT. What does disease progression look like in ulcerative colitis, and how might it be prevented? Gastroenterology. (2022) 162:1396–408. doi: 10.1053/j.gastro.2022.01.023
12. Krueger PD, Osum KC, Jenkins MK. CD4+ memory T-cell formation during type 1 immune responses. Cold Spring Harbor Perspect Biol. (2021) 13:a038141. doi: 10.1101/cshperspect.a038141
13. Szabo PA, Miron M, Farber DL. Location, location, location: Tissue resident memory T cells in mice and humans. Sci Immunol. (2019) 4:eaas9673. doi: 10.1126/sciimmunol.aas9673
14. Guggino G, Rizzo A, Mauro D, Macaluso F, Ciccia F. Gut-derived CD8+ tissue-resident memory T cells are expanded in the peripheral blood and synovia of SpA patients. Ann Rheum Dis. (2021) 80:e174. doi: 10.1136/annrheumdis-2019-216456
15. Park SL, Buzzai A, Rautela J, Hor JL, Hochheiser K, Effern M, et al. Tissue-resident memory CD8+ T cells promote melanoma-immune equilibrium in skin. Nature. (2019) 565:366–71. doi: 10.1038/s41586-018-0812-9
16. Sasson SC, Gordon CL, Christo SN, Klenerman P, Mackay LK. Local heroes or villains: tissue-resident memory T cells in human health and disease. Cell Mol Immunol. (2020) 17:113–22. doi: 10.1038/s41423-019-0359-1
17. Gao A, Zhao W, Wu R, Su R, Jin R, Luo J, et al. Tissue-resident memory T cells: The key frontier in local synovitis memory of rheumatoid arthritis. J Autoimmun. (2022) 133:102950. doi: 10.1016/j.jaut.2022.102950
18. Corridoni D, Antanaviciute A, Gupta T, Fawkner-Corbett D, Aulicino A, Jagielowicz M, et al. Single-cell atlas of colonic CD8+ T cells in ulcerative colitis. Nat Med. (2020) 26:1480–90. doi: 10.1038/s41591-020-1003-4
19. Zundler S, Becker E, Spocinska M, Slawik M, Parga-Vidal L, Stark R, et al. Hobit- and Blimp-1-driven CD4+ tissue-resident memory T cells control chronic intestinal inflammation. Nat Immunol. (2019) 20:288–300. doi: 10.1038/s41590-018-0298-5
20. Neurath MF. Cytokines in inflammatory bowel disease. Nat Rev Immunol. (2014) 14:329–42. doi: 10.1038/nri3661
21. Christo SN, Evrard M, Park SL, Gandolfo LC, Burn TN, Fonseca R, et al. Discrete tissue microenvironments instruct diversity in resident memory T cell function and plasticity. Nat Immunol. (2021) 22:1140–51. doi: 10.1038/s41590-021-01004-1
22. Schenkel JM, Masopust D. Tissue-resident memory T cells. Immunity. (2014) 41:886–97. doi: 10.1016/j.immuni.2014.12.007
23. Keller HR, Ligons DL, Li C, Hwang S, Luckey MA, Prakhar P, et al. The molecular basis and cellular effects of distinct CD103 expression on CD4 and CD8 T cells. Cell Mol Life Sci. (2021) 78:5789–805. doi: 10.1007/s00018-021-03877-9
24. Beura LK, Fares-Frederickson NJ, Steinert EM, Scott MC, Thompson EA, Fraser KA, et al. CD4+ resident memory T cells dominate immunosurveillance and orchestrate local recall responses. J Exp Med. (2019) 216:1214–29. doi: 10.1084/jem.20181365
25. Mackay LK, Rahimpour A, Ma JZ, Collins N, Stock AT, Hafon M-L, et al. The developmental pathway for CD103(+)CD8+ tissue-resident memory T cells of skin. Nat Immunol. (2013) 14:1294–301. doi: 10.1038/ni.2744
26. Bergsbaken T, Bevan MJ, Fink PJ. Local inflammatory cues regulate differentiation and persistence of CD8+ Tissue-resident memory T cells. Cell Rep. (2017) 19:114–24. doi: 10.1016/j.celrep.2017.03.031
27. Lutter L, Roosenboom B, Brand EC, Ter Linde JJ, Oldenburg B, van Lochem EG, et al. Homeostatic function and inflammatory activation of Ileal CD8+ Tissue-resident T cells is dependent on mucosal location. Cell Mol Gastroenterol Hepatol. (2021) 12:1567–81. doi: 10.1016/j.jcmgh.2021.06.022
28. Mackay LK, Braun A, Macleod BL, Collins N, Tebartz C, Bedoui S, et al. Cutting edge: CD69 interference with sphingosine-1-phosphate receptor function regulates peripheral T cell retention. J Immunol. (2015) 194:2059–63. doi: 10.4049/jimmunol.1402256
29. Skon CN, Lee J-Y, Anderson KG, Masopust D, Hogquist KA, Jameson SC. Transcriptional downregulation of S1pr1 is required for the establishment of resident memory CD8+ T cells. Nat Immunol. (2013) 14:1285–93. doi: 10.1038/ni.2745
30. Beura LK, Wijeyesinghe S, Thompson EA, Macchietto MG, Rosato PC, Pierson MJ, et al. T cells in nonlymphoid tissues give rise to lymph-node-resident memory T cells. Immunity. (2018) 48:327–38.e5. doi: 10.1016/j.immuni.2018.01.015
31. Walsh DA, da Silva HB, Beura LK, Peng C, Hamilton SE, Masopust D, et al. The functional requirement for CD69 in establishment of resident memory CD8+ T cells varies with tissue location. J Immunol. (2019) 203:946–55. doi: 10.4049/jimmunol.1900052
32. Kumar BV, Ma W, Miron M, Granot T, Guyer RS, Carpenter DJ, et al. Human tissue-resident memory T cells are defined by core transcriptional and functional signatures in lymphoid and mucosal sites. Cell Rep. (2017) 20:2921–34. doi: 10.1016/j.celrep.2017.08.078
33. Lin YH, Duong HG, Limary AE, Kim ES, Hsu P, Patel SA, et al. Small intestine and colon tissue-resident memory CD8+ T cells exhibit molecular heterogeneity and differential dependence on Eomes. Immunity. (2023) 56:207–23.e8. doi: 10.1016/j.immuni.2022.12.007
34. Bergsbaken T, Bevan MJ. Proinflammatory microenvironments within the intestine regulate the differentiation of tissue-resident CD8+ T cells responding to infection. Nat Immunol. (2015) 16:406–14. doi: 10.1038/ni.3108
35. Masopust D, Soerens AG. Tissue-resident T cells and other resident leukocytes. Annu Rev Immunol. (2019) 37:521–46. doi: 10.1146/annurev-immunol-042617-053214
36. Fung HY, Teryek M, Lemenze AD, Bergsbaken T. CD103 fate mapping reveals that intestinal CD103- tissue-resident memory T cells are the primary responders to secondary infection. Sci Immunol. (2022) 7:eabl9925. doi: 10.1126/sciimmunol.abl9925
37. Ge C, Monk IR, Pizzolla A, Wang N, Bedford JG, Stinear TP, et al. Bystander activation of pulmonary trm cells attenuates the severity of bacterial pneumonia by enhancing neutrophil recruitment. Cell Rep. (2019) 29:4236–44.e3. doi: 10.1016/j.celrep.2019.11.103
38. Schenkel JM, Fraser KA, Beura LK, Pauken KE, Vezys V, Masopust D. Resident memory CD8 T cells trigger protective innate and adaptive immune responses. Science. (2014) 346:98–101. doi: 10.1126/science.1254536
39. Fung HY, Espinal AM, Teryek M, Lemenze AD, Bergsbaken T. STAT4 increases the phenotypic and functional heterogeneity of intestinal tissue-resident memory T cells. Mucosal Immunol. (2023) 16:250–63. doi: 10.1016/j.mucimm.2023.03.002
40. FitzPatrick MEB, Provine NM, Garner LC, Powell K, Amini A, Irwin SL, et al. Human intestinal tissue-resident memory T cells comprise transcriptionally and functionally distinct subsets. Cell Rep. (2021) 34:108661. doi: 10.1016/j.celrep.2020.108661
41. Bartolomé-Casado R, Landsverk OJB, Chauhan SK, Richter L, Phung D, Greiff V, et al. Resident memory CD8 T cells persist for years in human small intestine. J Exp Med. (2019) 216:2412–26. doi: 10.1084/jem.20190414
42. Herndler-Brandstetter D, Ishigame H, Shinnakasu R, Plajer V, Stecher C, Zhao J, et al. KLRG1+ Effector CD8+ T cells lose KLRG1, differentiate into all memory T cell lineages, and convey enhanced protective immunity. Immunity. (2018) 48:716–29.e8. doi: 10.1016/j.immuni.2018.03.015
43. Ray SJ, Franki SN, Pierce RH, Dimitrova S, Koteliansky V, Sprague AG, et al. The collagen binding alpha1beta1 integrin VLA-1 regulates CD8 T cell-mediated immune protection against heterologous influenza infection. Immunity. (2004) 20:167–79. doi: 10.1016/s1074-7613(04)00021-4
44. Cheuk S, Schlums H, Gallais Sérézal I, Martini E, Chiang SC, Marquardt N, et al. CD49a expression defines tissue-resident CD8+ T cells poised for cytotoxic function in human skin. Immunity. (2017) 46:287–300. doi: 10.1016/j.immuni.2017.01.009
45. Zhang N, Bevan MJ. Transforming growth factor-β signaling controls the formation and maintenance of gut-resident memory T cells by regulating migration and retention. Immunity. (2013) 39:687–96. doi: 10.1016/j.immuni.2013.08.019
46. Takahashi T, Dejbakhsh-Jones S, Strober S. Expression of CD161 (NKR-P1A) defines subsets of human CD4 and CD8 T cells with different functional activities. J Immunol. (2006) 176:211–6. doi: 10.4049/jimmunol.176.1.211
47. Fergusson JR, Hühn MH, Swadling L, Walker LJ, Kurioka A, Llibre A, et al. CD161(int)CD8+ T cells: a novel population of highly functional, memory CD8+ T cells enriched within the gut. Mucosal Immunol. (2016) 9:401–13. doi: 10.1038/mi.2015.69
48. Cruz-Guilloty F, Pipkin ME, Djuretic IM, Levanon D, Lotem J, Lichtenheld MG, et al. Runx3 and T-box proteins cooperate to establish the transcriptional program of effector CTLs. J Exp Med. (2009) 206:51–9. doi: 10.1084/jem.20081242
49. Antonioli L, Pacher P, Vizi ES, Haskó G. CD39 and CD73 in immunity and inflammation. Trends Mol Med. (2013) 19:355–67. doi: 10.1016/j.molmed.2013.03.005
50. Yegutkin GG. Nucleotide- and nucleoside-converting ectoenzymes: Important modulators of purinergic signalling cascade. Biochim Biophys Acta. (2008) 1783:673–94. doi: 10.1016/j.bbamcr.2008.01.024
51. Noble A, Durant L, Hoyles L, Mccartney AL, Man R, Segal J, et al. Deficient resident memory T cell and CD8 T cell response to commensals in inflammatory bowel disease. J Crohn’s Colitis. (2019) 14:525. doi: 10.1093/ecco-jcc/jjz175
52. Longhi MS, Moss A, Jiang ZG, Robson SC. Purinergic signaling during intestinal inflammation. J Mol Med (Berl). (2017) 95:915–25. doi: 10.1007/s00109-017-1545-1
53. Borges Da Silva H, Peng C, Wang H, Wanhainen KM, Ma C, Lopez S, et al. Sensing of ATP via the purinergic receptor P2RX7 promotes CD8+ Trm cell generation by enhancing their sensitivity to the cytokine TGF-β. Immunity. (2020) 53:158–71.e6. doi: 10.1016/j.immuni.2020.06.010
54. Qiu Z, Chu TH, Sheridan BS. TGF-β: many paths to CD103+ CD8 T cell residency. Cells. (2021) 10:989. doi: 10.3390/cells10050989
55. Sheridan BS, Pham Q-M, Lee Y-T, Cauley LS, Puddington L, Lefrançois L. Oral infection drives a distinct population of intestinal resident memory CD8+ T cells with enhanced protective function. Immunity. (2014) 40:747–57. doi: 10.1016/j.immuni.2014.03.007
56. Bai A, Hu H, Yeung M, Chen J. Kruppel-like factor 2 controls T cell trafficking by activating L-selectin (CD62L) and sphingosine-1-phosphate receptor 1 transcription. J Immunol. (2007) 178:7632–9. doi: 10.4049/jimmunol.178.12.7632
57. Mokrani M, Klibi J, Bluteau D, Bismuth G, Mami-Chouaib F. Smad and NFAT pathways cooperate to induce CD103 expression in human CD8 T lymphocytes. J Immunol. (2014) 192:2471–9. doi: 10.4049/jimmunol.1302192
58. Borges da Silva H, Wang H, Qian LJ, Hogquist KA, Jameson SC. ARTC2.2/P2RX7 signaling during cell isolation distorts function and quantification of tissue-resident CD8+ T cell and iNKT subsets. J Immunol. (2019) 202:2153–63. doi: 10.4049/jimmunol.1801613
59. Li W, Liang L, Liao Q, Li Y, Zhou Y. CD38: An important regulator of T cell function. BioMed Pharmacother. (2022) 153:113395. doi: 10.1016/j.biopha.2022.113395
60. Evrard M, Becht E, Fonseca R, Obers A, Park SL, Ghabdan-Zanluqui N, et al. Single-cell protein expression profiling resolves circulating and resident memory T cell diversity across tissues and infection contexts. Immunity. (2023) 56:1664–80.e9. doi: 10.1016/j.immuni.2023.06.005
61. Raynor JL, Collins N, Shi H, Guy C, Saravia J, Ah Lim S, et al. CRISPR screens unveil nutrient-dependent lysosomal and mitochondrial nodes impacting intestinal tissue-resident memory CD8+ T cell formation. Immunity. (2024) 57(11):2597–2614.e13. doi: 10.1016/j.immuni.2024.09.013
62. Qiu Z, Khairallah C, Chu TH, Imperato JN, Lei X, Romanov G, et al. Retinoic acid signaling during priming licenses intestinal CD103+ CD8 TRM cell differentiation. J Exp Med. (2023) 220:e20210923. doi: 10.1084/jem.20210923
63. Parga-Vidal L, Behr FM, Kragten NAM, Nota B, Wesselink TH, Kavazović I, et al. Hobit identifies tissue-resident memory T cell precursors that are regulated by Eomes. Sci Immunol. (2021) 6:eabg3533. doi: 10.1126/sciimmunol.abg3533
64. Mackay LK, Minnich M, Kragten NAM, Liao Y, Nota B, Seillet C, et al. Hobit and Blimp1 instruct a universal transcriptional program of tissue residency in lymphocytes. Science. (2016) 352:459–63. doi: 10.1126/science.aad2035
65. Kallies A, Nutt SL. Terminal differentiation of lymphocytes depends on Blimp-1. Curr Opin Immunol. (2007) 19:156–62. doi: 10.1016/j.coi.2007.01.003
66. Wang D, Diao H, Getzler AJ, Rogal W, Frederick MA, Milner J, et al. The Transcription factor Runx3 establishes chromatin accessibility of cis-regulatory landscapes that drive memory cytotoxic T lymphocyte formation. Immunity. (2018) 48:659–74.e6. doi: 10.1016/j.immuni.2018.03.028
67. B FM, C A, S R, van G KPJM. Armed and ready: transcriptional regulation of tissue-resident memory CD8 T cells. Front Immunol. (2018) 9:1770. doi: 10.3389/fimmu.2018.01770
68. Kallies A, Xin A, Belz GT, Nutt SL. Blimp-1 transcription factor is required for the differentiation of effector CD8+ T cells and memory responses. Immunity. (2009) 31:283–95. doi: 10.1016/j.immuni.2009.06.021
69. Kragten NAM, Behr FM, Vieira Braga FA, Remmerswaal EBM, Wesselink TH, Oja AE, et al. Blimp-1 induces and Hobit maintains the cytotoxic mediator granzyme B in CD8 T cells. Eur J Immunol. (2018) 48:1644–62. doi: 10.1002/eji.201847771
70. Behr FM, Parga-Vidal L, Kragten NAM, Van Dam TJP, Wesselink TH, Sheridan BS, et al. Tissue-resident memory CD8+ T cells shape local and systemic secondary T cell responses. Nat Immunol. (2020) 21:1070–81. doi: 10.1038/s41590-020-0723-4
71. Pathophysiology of inflammatory bowel diseases. N Engl J Med. (2020) 383:2652–64. doi: 10.1056/NEJMra2002697
72. Pritchard GH, Kedl RM, Hunter CA. The evolving role of T-bet in resistance to infection. Nat Rev Immunol. (2019) 19:398–410. doi: 10.1038/s41577-019-0145-4
73. Pearce EL, Mullen AC, Martins GA, Krawczyk CM, Hutchins AS, Zediak VP, et al. Control of effector CD8+ T cell function by the transcription factor Eomesodermin. Science. (2003) 302:1041–3. doi: 10.1126/science.1090148
74. Boland BS, He Z, Tsai MS, Olvera JG, Omilusik KD, Duong HG, et al. Heterogeneity and clonal relationships of adaptive immune cells in ulcerative colitis revealed by single-cell analyses. Sci Immunol. (2020) 5:eabb4432. doi: 10.1126/sciimmunol.abb4432
75. Mackay LK, Wynne-Jones E, Freestone D, Pellicci DG, Mielke LA, Newman DM, et al. T-box transcription factors combine with the cytokines TGF-β and IL-15 to control tissue-resident memory T cell fate. Immunity. (2015) 43:1101–11. doi: 10.1016/j.immuni.2015.11.008
76. Wang L, Mishra S, Fan KK-H, Quon S, Li G, Yu B, et al. T-bet deficiency and Hic1 induction override TGF-β-dependency in the formation of CD103+ intestine-resident memory CD8+ T cells. Cell Rep. (2024) 43:114258. doi: 10.1016/j.celrep.2024.114258
77. Mueller SN, Mackay LK. Tissue-resident memory T cells: local specialists in immune defence. Nat Rev Immunol. (2016) 16:79–89. doi: 10.1038/nri.2015.3
78. Hegazy AN, West NR, Stubbington MJT, Wendt E, Suijker KIM, Datsi A, et al. Circulating and tissue-resident CD4+ T cells with reactivity to intestinal microbiota are abundant in healthy individuals and function is altered during inflammation. Gastroenterology. (2017) 153:1320–37.e16. doi: 10.1053/j.gastro.2017.07.047
79. Bottois H, Ngollo M, Hammoudi N, Courau T, Bonnereau J, Chardiny V, et al. KLRG1 and CD103 expressions define distinct intestinal tissue-resident memory CD8 T cell subsets modulated in Crohn’s disease. Front Immunol. (2020) 11:896. doi: 10.3389/fimmu.2020.00896
80. Roosenboom B, Wahab PJ, Smids C, Groenen MJM, van Koolwijk E, van Lochem EG, et al. Intestinal CD103+CD4+ and CD103+CD8+ T-cell subsets in the gut of inflammatory bowel disease patients at diagnosis and during follow-up. Inflammatory Bowel Diseases. (2019) 25:1497–509. doi: 10.1093/ibd/izz049
81. Smids C, Horjus Talabur Horje CS, Drylewicz J, Roosenboom B, Groenen MJM, van Koolwijk E, et al. Intestinal T cell profiling in inflammatory bowel disease: linking T cell subsets to disease activity and disease course. J Crohns Colitis. (2018) 12:465–75. doi: 10.1093/ecco-jcc/jjx160
82. Ho J, Kurtz CC, Naganuma M, Ernst PB, Cominelli F, Rivera-Nieves J. A CD8+/CD103high T cell subset regulates TNF-mediated chronic murine ileitis. J Immunol (Baltim Md, : 1950). (2008) 180:2573–80. doi: 10.4049/jimmunol.180.4.2573
83. Sasson SC, Slevin SM, Cheung VTF, Nassiri I, Olsson-Brown A, Fryer E, et al. Interferon-gamma-producing CD8+ Tissue resident memory T cells are a targetable hallmark of immune checkpoint inhibitor-colitis. Gastroenterology. (2021) 161:1229–44.e9. doi: 10.1053/j.gastro.2021.06.025
84. Sandborn WJ, Mattheakis LC, Modi NB, Pugatch D, Bressler B, Lee S, et al. PTG-100, an oral α4β7 antagonist peptide: preclinical development and phase 1 and 2a studies in ulcerative colitis. Gastroenterology. (2021) 161:1853–64.e10. doi: 10.1053/j.gastro.2021.08.045
85. Peyrin-Biroulet L, Hart A, Bossuyt P, Long M, Allez M, Juillerat P, et al. Etrolizumab as induction and maintenance therapy for ulcerative colitis in patients previously treated with tumour necrosis factor inhibitors (HICKORY): a phase 3, randomised, controlled trial. Lancet Gastroenterol Hepatol. (2022) 7:128–40. doi: 10.1016/S2468-1253(21)00298-3
86. Matsuoka K, Watanabe M, Ohmori T, Nakajima K, Ishida T, Ishiguro Y, et al. AJM300 (carotegrast methyl), an oral antagonist of α4-integrin, as induction therapy for patients with moderately active ulcerative colitis: a multicentre, randomised, double-blind, placebo-controlled, phase 3 study. Lancet Gastroenterol Hepatol. (2022) 7:648–57. doi: 10.1016/S2468-1253(22)00022-X
87. Sandborn WJ, Feagan BG, D’Haens G, Wolf DC, Jovanovic I, Hanauer SB, et al. Ozanimod as induction and maintenance therapy for ulcerative colitis. N Engl J Med. (2021) 385:1280–91. doi: 10.1056/NEJMoa2033617
88. Rosenfeld G, Parker CE, MacDonald JK, Bressler B. Etrolizumab for induction of remission in ulcerative colitis. Cochrane Database Syst Rev. (2015) 2015:CD011661. doi: 10.1002/14651858.CD011661.pub2
89. Rubin DT, Dotan I, DuVall A, Bouhnik Y, Radford-Smith G, Higgins PDR, et al. Etrolizumab versus adalimumab or placebo as induction therapy for moderately to severely active ulcerative colitis (HIBISCUS): two phase 3 randomised, controlled trials. Lancet Gastroenterol Hepatol. (2022) 7:17–27. doi: 10.1016/S2468-1253(21)00338-1
90. S Nh, R N. Ozanimod for Ulcerative Colitis. The New England journal of medicine. N Engl J Med;. (2022) 386(2):194. doi: 10.1056/NEJMc2117224
91. Eser A, Colombel J-F, Rutgeerts P, Vermeire S, Vogelsang H, Braddock M, et al. Safety and efficacy of an oral inhibitor of the purinergic receptor P2X7 in adult patients with moderately to severely active Crohn’s disease: A randomized placebo-controlled, double-blind, phase IIa study. Inflammation Bowel Dis. (2015) 21:2247–53. doi: 10.1097/MIB.0000000000000514
Keywords: tissue-resident memory T cells, ulcerative colitis, inflammatory bowel disease, recurrence, immune
Citation: Wang X, Zhang J, Fang L and Tang X (2025) Angel and devil: the protective immunity and pathogenic inflammation of tissue resident memory T cells in ulcerative colitis. Front. Immunol. 16:1518339. doi: 10.3389/fimmu.2025.1518339
Received: 28 October 2024; Accepted: 14 February 2025;
Published: 07 March 2025.
Edited by:
Agnes Lehuen, Institut National de la Santé et de la Recherche Médicale (INSERM), FranceReviewed by:
Hiroshi Nakase, Sapporo Medical University, JapanCopyright © 2025 Wang, Zhang, Fang and Tang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xudong Tang, dHhkbHlAc2luYS5jb20=; Jiaqi Zhang, empxNDA1QDE2My5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.