
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Immunol., 28 February 2025
Sec. Nutritional Immunology
Volume 16 - 2025 | https://doi.org/10.3389/fimmu.2025.1512293
This article is part of the Research TopicImmunonutrition: Bridging Precision Nutrition and Modern MedicineView all 9 articles
Background: Magnesium is an essential immune nutrient for the body, and recent studies have found that it plays an important role in osteoarthritis (OA). Magnesium depletion score(MDS) is a new method for evaluating the magnesium status of the body. Our objective is to explore the association between MDS and the incidence of OA, as well as the relationship between MDS and mortality in patients with OA.
Methods: Eligible participants were obtained from NHANES from 2005 to 2018. Logistic regression models were employed to evaluate the link between MDS and the incidence of OA. Cox regression models were employed to evaluate the link between MDS and mortality among OA patients. In addition, restricted cubic spline was utilized to explore the correlation between MDS and the incidence of OA, as well as the relationship between MDS and mortality in patients with OA. Subgroup analysis were adopted in order to ensure the credibility of the results in different subgroups, including age, gender, race, education level, BMI, smoking, diabetes and hypertension.
Results: 19,394 individuals qualified for analysis, including 3,256 OA patients. After excluding missing follow-up data, 630 all-cause deaths and 172 cardiovascular deaths (CVDs) were observed in 3,250 OA patients. The individuals with OA had higher levels of MDS. In the logistic regression model, MDS was positively related to OA (MDS≥3 vs. MDS=0, OR =1.83 (1.46-2.30, P<0.001)). Besides, a positive association was observed between MDS and all-cause mortality [MDS≥3 vs. MDS=0, HR =2.56 (1.49-4.41, P<0.001)] and CVDs [MDS≥3 vs. MDS=0, HR =3.00 (1.13-7.98, P=0.01)] in cox regression models. In addition, a 1-unit rise in MDS was significantly linked to an increased risk of mortality. Restricted cubic spline indicated a positive relationship between MDS and incidence and mortality of OA. Subgroup analysis demonstrated that the results are stable in different subgroups.
Conclusions: MDS is positively correlated with the incidence and mortality of OA. Optimizing the nutritional status of magnesium may bring benefits to OA patients.
Osteoarthritis(OA) is a degenerative condition of the joints marked by stiffness, pain, and deformities (1). OA is particularly prevalent among middle-aged and elderly populations, affecting approximately 595 million people worldwide (2). OA affects about 14% of American adults. In addition, it is the second leading cause of labor loss, second only to cardiovascular disease. OA causes medical expenses and other economic losses of up to about $125 billion annually (3). Patients with OA might face an increased risk of mortality in comparison to the overall population (4–6). Although the prevention and surgical treatment measures have been effectively implemented, the incidence and mortality of OA are still difficult to control.
Recently, some studies have found that mineral elements are crucial in the development of OA (7, 8). Magnesium is one of the most important and abundant trace elements in cells. It is an auxiliary factor in enzymatic reactions (9). Furthermore, many metabolic reactions in the human body, including the production of ATP and the maintenance of normal mitochondrial function, rely on the participation of magnesium (10). Magnesium supplementation can reduce joint cartilage damage, apoptosis, and promote chondrocyte generation (11, 12). Moreover, administering intra-articular magnesium injections can help reduce pain in both OA rats and patients (13, 14). Therefore, magnesium plays an important role in OA.The National Institutes of Health (NIH) in the United States pointed out that assessing magnesium levels in the body is very difficult due to the fact that most magnesium remains in tissues or cells. The detection of serum magnesium in clinical practice is not accurate (15). Due to the lack of an effective method for evaluating the magnesium status of the body at present, the significance of magnesium deficiency in OA has been largely neglected.
The magnesium depletion score (MDS) serves as an effective instrument for evaluating the body’s magnesium levels. An increase in MDS indicates severe magnesium deficiency in the individual. Fan et al. found that MDS has greater value in evaluating the magnesium status of the body compared with serum magnesium. Furthermore, high MDS may indicate an inflammatory state which is linked to increased long-term mortality in individuals (16). In addition, other studies also revealed that an elevation in MDS is related to an increased risk of abdominal aortic calcification, cardiovascular disease and diabetes retinopathy (17–19). Nonetheless, the relationship between MDS and the incidence and mortality of OA remains ambiguous. Therefore, our objective is to explore the association between MDS and the incidence of OA, as well as the relationship between MDS and mortality in patients with OA.
Data for this research were sourced from the National Health and Nutrition Examination Survey (NHANES) database (www.cdc.gov/nchs/nhanes.com).
Part I: The inclusion criteria are as follows: The participants from NHANES between 2005 and 2018. The exclusion criteria are as follows: participants younger than 40 years, participants without OA information and those missing data on MDS.
Part II: Based on Part I, we have developed inclusion and exclusion criteria. The inclusion criteria are as follows: participants with OA. The exclusion criteria are as follows: those missing data on follow-up information.
The MDS calculation comprised the consolidation of four separate scores: (1) the use of diuretics at present received 1 point; (2) using a proton pump inhibitor (PPI) also earned 1 point; (3) an estimated glomerular filtration rate (eGFR) ranging from 60 mL/min/1.73 m² to less than 90 mL/min/1.73 m² was assigned 1 point, whereas an eGFR below 60 mL/min/1.73 m² received 2 points; (4) heavy alcohol consumption (defined as more than 1 drink per day for women and over 2 drinks per day for men) was allocated 1 point (18).
Demographic information encompasses age, gender, ethnicity, education levels, body mass index (BMI) and poverty income ratio (≤1.30, 1.31–3.49 and ≥3.50). Physical activity data was obtained through a questionnaire survey. Laboratory tests include total cholesterol (TC) (mmol/L), glycated hemoglobin (HbA1c) (%), calcium (mmol/L) and phosphorus (mmol/L). Dietary factors (magnesium intake, calcium intake, phosphorus intake, vitamin D intake), smoking status and comorbidities (hypertension and diabetes) are also included. Dietary data is sourced from a dietary recall survey. Oxidative stress is closely related to the occurrence and development of OA. Antioxidant diet may be an easily accepted treatment strategy. Based on previously published articles on OA, we also calculated the composite dietary antioxidant index (CDAI). It comprises a composite score of six dietary antioxidants: vitamins A, C, and E, as well as selenium, zinc, and carotenoids (20). The criteria for diagnosing hypertension include: SBP ≥ 140 mmHg or DBP ≥ 90 mmHg and the patients using antihypertensive medications (21). The criteria used for diagnosing diabetes include: doctor diagnosis as diabetes, HbA1c ≥ 6.5%, fasting glucose ≥ 7.0mmol/L, random blood glucose ≥ 11.1mmol/L, 2h OGTT blood glucose ≥ 11.1mmol/L, or the administration of diabetes medications and insulin therapy (22).
The mortality statistics were connected up to December 31, 2019 (https://www.cdc.gov/nchs/data-linkage/mortality.htm). Outcomes were divided into categories of all-cause deaths and CVDs. Death causes were classified according to ICD-10 codes, with CVDs specifically identified using codes 100-109, 111, 113, and 120-151 (23).
NHANES is conducted in a complex multi-stage sampling design. Moreover, NHANES is conducted in two-year cycles and includes a representative sample of the U.S. population. Standard error of mean (SEM) reflects the representativeness of sample mean to population mean. Weighted percentages can better represent the overall population. Therefore, SEM and weighted percentage might be more appropriate. Initially, continuous variables were represented as means corresponding standard error of the mean (mean ± SEM) and categorical variables were presented as means [95% confidence intervals (CI)]. The differences between the two groups were compared using chi-square tests and independent-sample t tests. P values < 0.05 were recognized statistically significant. The purpose of regression analysis is to observe the degree of correlation between the dependent variable and the independent variable after adjusting for various confounding factors. To examine the relationship between MDS and the incidence of OA, weighted logistic regression analyses were conducted. Three models were developed: unadjusted, Model I, and Model II. Model I was adjusted for age, sex, and race. Model II was adjusted for age, sex, ethnicity, education levels, BMI, smoking, HbA1c, TC, hypertension, DM, calcium, phosphorus, phosphorus intake, calcium intake, magnesium intake, CDAI, vitamin D intake, physical activity and poverty income ratio. The restricted cubic spline(RCS) curve can more vividly observe the relationship between the dependent variable and the independent variable. RCS analysis was employed to investigate the link between MDS and the incidence of OA. The purpose of subgroup analysis is to divide the study population into different groups based on certain characteristics (such as age, gender, comorbidities, etc.) to observe whether experimental variables have different effects in these different groups. Additionally, subgroup analysis was conducted to determine if the relationship between MDS and the incidence of OA remained consistent across various groups. These subgroups include age, gender, race, education level, BMI, smoking, diabetes and hypertension.
Additionally, weighted Kaplan-Meier curves and log-rank tests were utilized to examine the cumulative survival differences among different MDS groups. Cox regression analysis was carried out to investigate the link between MDS and mortality with OA. The variables included in the model are consistent with cox regression analysis model. Similarly, RCS was employed to investigate the relationship between MDS and mortality. Additionally, subgroup analysis was performed to further verify the robustness of the findings.
Besides, sensitivity analysis was also used to further validate our results. In many studies, weighted and unweighted results may be inconsistent. Consequently, we conducted unweighted Cox regression to carry out sensitivity analysis. Additionally, we also excluded OA patients who died within two years to further analyze the link between MDS and mortality.
The participants from NHANES between 2005 and 2018 (n = 70,190), we eliminated subjects younger than 40 years (n = 43,908), subjects without OA information (n = 3,211), those missing data on magnesium depletion score (n = 3,677). Consequently, the cross-sectional analysis sample comprised 19,394 participants. Detailed information of the screening process is shown in Figure 1. 19,394 adults were screened for this cross-sectional study, including 3,256 OA patients. The clinical baseline characteristics of non-OA and OA participants are presented in Table 1, including age, gender, ethnicity, educational levels, BMI, HbA1c, TC, calcium, phosphorus, phosphorus intake, calcium intake and magnesium intake, smoking, hypertension, diabetes. Individuals in the OA group tend to be older (63.57 ± 0.26vs.55.72 ± 0.16), with a higher likelihood of being female (64.85%vs.49.09%), white(84.05% vs.70.77%), and former smokers (51.32%vs.46.28%), as well as a greater proportion of those who have hypertension (64.06%vs.45.73%), and diabetes (23.56%vs.18.01%)(P<0.001). We also found differences between laboratory examination measurements (HbA1c, TC, serum calcium and phosphorus) and dietary factors (phosphorus intake, calcium intake and magnesium intake) (P < 0.001). Supplementary Table S1 presents the baseline characteristics of the participants categorized by their MDS levels. Compared with the MDS=0 group, patients in the MDS≥3 group were older (68.65 ± 0.35vs.50.61 ± 0.17), had higher BMI (30.77 ± 0.22vs.29.25 ± 0.12), HbA1c (6.01 ± 0.03vs.5.77 ± 0.02), and had a larger proportion of smoking (51.09%vs.44.55%), hypertension (86.64%vs.33.39%) and diabetes (34.11%vs.16.04%). We can also find that the incidence of OA gradually increases with the increase of MDS. In addition, patients in the OA group have a higher level of MDS (1.47 ± 0.02vs.1.01 ± 0.01). We found that OA patients had higher use of diuretics (25.56%vs.14.14%) and PPIs (21.82%vs.9.42%), as well as lower eGFR (Table 2), which are components of MDS.
Additionally, a positive correlation was presented between MDS and the incidence of OA with an OR of 1.22 (95%CI: 1.14-1.30) in logistic regression analysis (Table 3). Compared with the group with MDS=0, the group with MDS≥3 has a higher incidence of OA (OR = 1.83, 95%CI: 1.46-2.30) in Model II. The use of unweighted multiple analysis as a sensitivity analysis also confirmed this result (Supplementary Table S3).
Figure 2 showed a non-linear positive link between MDS and the incidence of OA (p for overall < 0.001, p for non-linear = 0.04). Moreover, in various subgroups such as age, sex, smoking, and DM, positive relationships were observed between MDS and the incidence of OA (Supplementary Figure S1).
Subgroup analysis showed that the results were significant and stable. This relationship is slightly weakened in elderly people over 60 years old but still statistically significant (P<0.001) (Figure 3).
Second, we excluded individuals without OA (n = 16,138) and those missing data on follow-up information (n = 6). Consequently, the sample comprised 3,250 participants. A total of 630 deaths from all causes and 172 fatalities due to CVDs were recorded until December 31, 2019 (Figure 1). 3,250 participants were involved in the cohort study, including 630 all-cause deaths and 172 CVDs. As illustrated in Supplementary Table S2, the data were categorized into four groups based on MDS levels. In comparison to the MDS≥3 group, individuals in the MDS=0 category exhibit a greater percentage of all-cause mortality (30.46%vs.6.79%) and CVDs (9.65%vs.1.93%) (P<0.001). Individuals in the MDS≥3 cohort tend to be older (70.29 ± 0.51vs.55.77 ± 0.46) and have a higher likelihood of being female (74.09%vs.66.60%) and white (88.20%vs.74.72%), as well as a greater prevalence of hypertension (89.00%vs.46.98%) and diabetes (35.96%vs.21.18%) (P<0.001). The differences were also found between laboratory examination measurements (HbA1c, TC and phosphorus) and dietary factors (phosphorus intake, calcium intake and magnesium intake) (P < 0.001).
Weighted Kaplan-Meier curves along with log-rank tests were employed to analyze the differences in cumulative survival across various MDS groups. The group with MDS≥3 has the highest all-cause mortality rate. The group with MDS=1 or MDS=2 has a moderate survival rate, while the group with MDS=0 have the highest survival rate (long-rank P<0.001) (Figure 4A). Similar death patterns are also reflected in CVDs (Figure 4B).

Figure 4. Kaplan-Meier survival analysis curves for mortality in different MDS groups. (A) all-cause mortality; (B) cardiovascular mortality.
A positive association was observed between MDS and all-cause mortality with an HR of 1.44 (95%CI:1.27-1.62) (Table 4) and cardiovascular mortality with an HR of 1.64 (95%CI: 1.33-2.02) (Table 5) in adjusted cox regression analysis. Besides, MDS was also positively related to all-cause mortality [MDS≥3 vs. MDS=0, HR =2.56 (1.49-4.41, P<0.001)] and CVDs [MDS≥3 vs. MDS=0, HR =3.00 (1.13-7.98, P=0.01)] as a categorical variable. Sensitivity analysis also confirmed this finding after excluding OA patients who died within two years (Supplementary Table S4).
Figure 5 showed a linear positive correlation between MDS and all-cause mortality (p for overall < 0.001, p for non-linear = 0.783) and CVDs (P for overall < 0.001, P for non-linear = 0.092) among OA individuals.
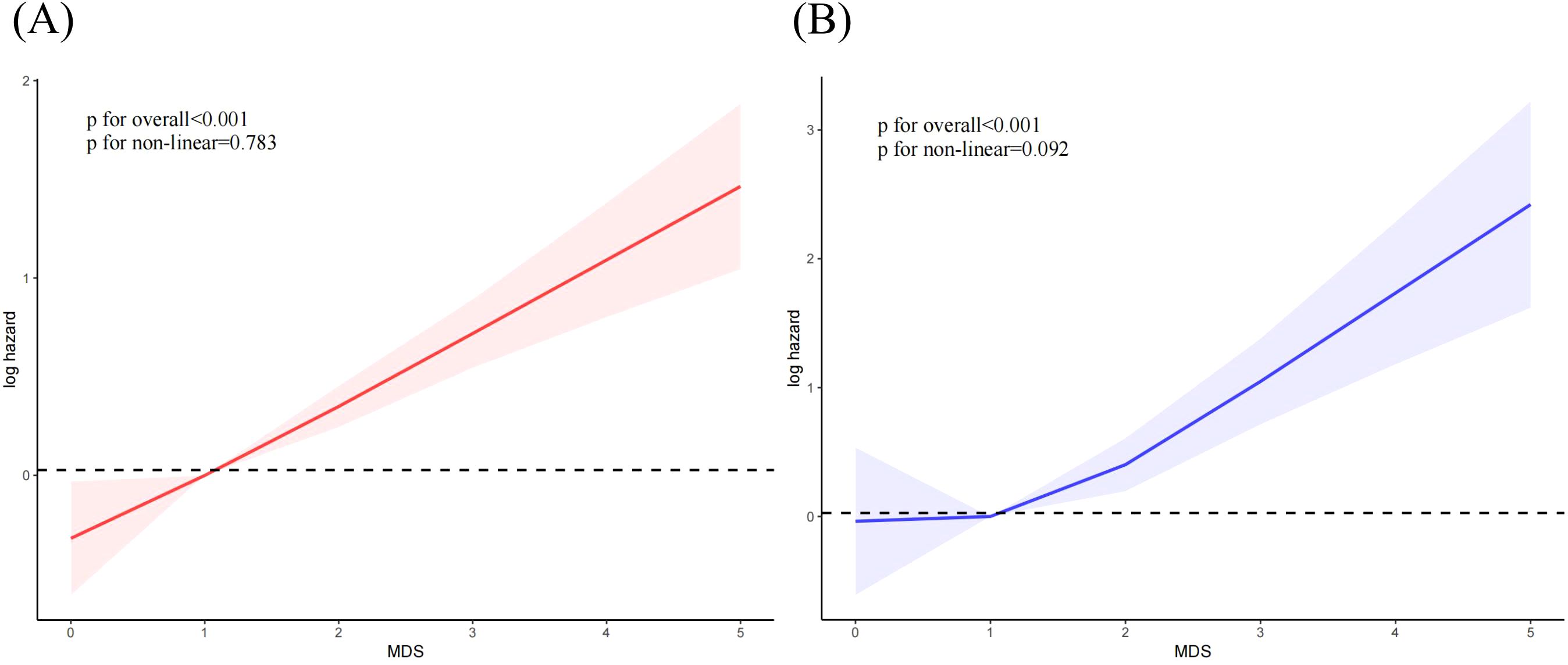
Figure 5. The correlation between MDS and mortality among OA individuals. (A) all-cause mortality; (B) cardiovascular mortality.
Subgroup analysis indicated that the results were significant and stable in most subgroups. Notably, this relationship was only significant in individuals aged 60 and above in the age subgroup (P<0.001) (Supplementary Figure S2).
Our study indicates a positive correlation between MDS and the incidence of OA among US middle aged and elderly people. Subgroup analysis indicated that the results were significant and stable in most subgroups. Moreover, there was a positive correlation between MDS and the mortality of OA.
Magnesium plays an important role in inflammatory diseases and has good anti-inflammatory effects. Research has found that giving mice a high magnesium diet can reduce levels of inflammatory factors (IL-1β, TNF-α and IL-6) in the body, alleviate joint inflammation and joint damage. Mechanistically, magnesium increases the number of Foxp3+Treg cells in an IL-10-dependent manner mediated by gut microbiota (24). The latest research has also found that magnesium can effectively reduce or even reverse the degeneration of cartilage tissue. Magnesium can enhance the proliferation and chondrogenic differentiation of bone marrow mesenchymal stem cells, and has the potential to promote joint cartilage regeneration. In addition, magnesium can also inhibit programmed cell death of chondrocytes, thereby protecting joint cartilage. For osteoclasts, magnesium can inhibit their generation and bone degradation functions (25). Therefore, magnesium has significant potential in the treatment of arthritis. The evaluation of MDS may have significant implications in OA.
In clinical practice, serum magnesium was often used to assess magnesium status. A study involving 2855 patients revealed that serum magnesium concentration was negatively linked to the incidence of imaging knee OA (26). Similarly, a meta-analysis showed that elevated serum magnesium levels correlate with a reduced incidence of OA, but this relationship is significantly affected by serum magnesium concentration (27). The possible reason is that serum magnesium may not comprehensively reflect the magnesium status of the body. Especially in cases of chronic magnesium deficiency, serum magnesium may still remain at normal levels due to the body’s compensation (28, 29). While serum magnesium is utilized in clinical practice, a clear correlation has yet to be established between serum magnesium concentrations and systemic magnesium levels or the concentrations found in particular tissues. In addition, the serum contain only holds 0.3% of the body’s total magnesium, and most of the rest remain in the organization (30). The National Institutes of Health (NIH) in the United States has pointed out that assessing magnesium levels is difficult due to the fact that most magnesium is present in cells or bones. When determining magnesium deficiency, this may lead to misleading blood test results. More than 80% of serum magnesium undergoes filtration and reabsorption in the kidneys. MDS incorporates pathophysiological factors influencing the renal reabsorption capability. Research has found that compared to serum magnesium, MDS has greater value in predicting the body’s magnesium status (16). Furthermore, the four risk factors included in MDS (current use of diuretics and PPIs, heavy alcohol consumption, and kidney function) are easily assessable in clinical practice.
Evaluating urinary magnesium offers an additional approach for determining the body’s magnesium levels, but it is easily influenced by diet, medication, and kidney disease (31). And for people with limited mobility, monitoring 24-hour urinary magnesium is difficult to widely implement (32). The evaluation of magnesium tolerance is considered the benchmark for determining the magnesium levels within the body. However, its application is severely limited due to the complexity of its operation and its unsuitability for patients with renal dysfunction (33). MDS is a practical tool for assessing the magnesium status of the body (16). Therefore, exploring its relationship with diseases may provide important guidance for clinical practice.
Magnesium deficiency is very common in middle-aged and elderly populations, with reasons including insufficient magnesium intake and increased excretion caused by various medications (34). In the cross-sectional study, our study found that this relationship between MDS and the incidence of OA is slightly weakened in elderly people over 60 years old but still statistically significant (P<0.001). We consider that the difference in magnesium deficiency levels between non-OA and OA patients in the elderly population may gradually narrow. This may be one of the possible reasons for the occurrence of this result. Additionally, this link between MDS and mortality was only significant in individuals aged 60 and above in the cohort study. Elderly OA patients often have multiple comorbidities and a higher proportion of diuretic use, often accompanied by renal dysfunction. This may partially explain the significant relationship between MDS and all-cause mortality in elderly OA patients. Therefore, in OA patients over 60 years old, MDS may be a better tool for assessing prognosis. Furthermore, we also observed that MDS is significantly associated with the incidence of OA and the death of OA patients in the subgroups of hypertension and diabetes (P<0.001). However, no significant interaction was observed between the two subgroups. This shows that the effect of magnesium deficiency on OA is not affected by hypertension and diabetes.
MDS consists of four elements that influence renal reabsorption: alcohol intake, the usage of diuretics, the use of PPIs, and kidney function (16). PPI mainly causes magnesium deficiency in the body by affecting the intestinal reabsorption of magnesium (35). Some patients who use PPIs in clinical practice may experience hypomagnesemia, but this phenomenon can be alleviated by supplementing magnesium or discontinuing medication. Recent studies have shown that the use of PPIs may accelerate the progression of OA (36), possibly through drug-induced magnesium deficiency, which is consistent with our research findings. Alcohol is deemed crucial for magnesium excretion. The mechanism may be that excessive alcohol use cause renal tubular damage, resulting in an increase in urinary magnesium (37). Magnesium deficiency may trigger systemic inflammatory reactions and promote the production of inflammatory mediators by chondrocytes and synovial cells, ultimately damaging the synthesis of articular cartilage (38). Omeprazole is a classic proton pump inhibitor. Omeprazole may cause an increase in segmental pH value in the small intestine after inhibiting gastric acid secretion, leading to a decrease in Mg2+ dissolution and reabsorption (39). Another presumed mechanism for the diminished absorption of magnesium by intestinal epithelial cells involves the PPIs-induced inhibition of transient receptor potential melastatin-6 (TRPM6) and TRPM7 channels. These factors lead to magnesium deficiency, which triggers joint inflammation and promotes the progression of OA (40). The kidneys are the most important organ for magnesium reabsorption, and the body’s magnesium balance depends on the involvement of the kidneys (41). Many diuretics also act on the renal tubules and inhibit magnesium reabsorption (42). For example, thiazide diuretics can act on the renal tubules, significantly reducing the reabsorption of magnesium in the body and serum magnesium concentration, indirectly inducing joint inflammation and the occurrence of OA (43).
In addition, we found that the use of diuretics and decreased renal function are risk factors for mortality in OA patients. Previous studies have shown that renal dysfunction is an independent risk factor for mortality (44). Besides, middle-aged and elderly people are often accompanied by other diseases, including cardiovascular disease, hypertension, diabetes, etc. It is understandable that those who take diuretics face an increased risk of mortality.
Despite the advantage of NHANES large sample research, our study still has some limitations. First, we cannot identify a causal association due to the type of cross-sectional study. Second, the results from the study mainly apply to the American population. Third, while the study utilizes MDS, the reliance on indirect markers (diuretic and PPI use) may not fully capture magnesium deficiency. we are unable to compare their advantages and disadvantages due to the lack of serum magnesium and urinary magnesium. Large prospective studies are needed in the future to further validate the role of MDS in evaluating magnesium deficiency. Finally, there may still be other confounding factors affecting the results although many variables are included.
MDS is positively correlated with the incidence and mortality of OA. Optimizing the nutritional status of magnesium may bring benefits to OA patients.
Publicly available datasets were analyzed in this study. This data can be found here: https://www.cdc.gov/nchs/nhanes/.
The data is accessible to the public (found in the NHANES database), therefore there is no need for an ethical approval statement or informed consent for the study.
RM: Writing – original draft. CZ: Writing – original draft. JL: Data curation, Methodology, Writing – original draft. JR: Data curation, Writing – original draft. HH: Data curation, Writing – original draft. GW: Writing – review & editing. YD: Writing – review & editing. XL: Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by grants from Dalian Medical University Interdisciplinary Research Cooperation Project Team Funding (JCHZ2023010).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declare that no Generative AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2025.1512293/full#supplementary-material
Supplementary Figure 1 | The correlation between MDS and the incidence of OA in different subgroups. (A) Age; (B) Sex; (C) Smoke; (D) DM.
Supplementary Figure 2 | Subgroup analysis of MDS with mortality among OA individuals. (A) all-cause mortality; (B) cardiovascular mortality.
1. Castagno S, Birch M, van der Schaar M, McCaskie A. Predicting rapid progression in knee osteoarthritis: a novel and interpretable automated machine learning approach, with specific focus on young patients and early disease. Ann Rheum Dis. (2024), ard–2024-225872. doi: 10.1136/ard-2024-225872
2. GBD 2021 Osteoarthritis Collaborators. Global, regional, and national burden of osteoarthritis, 1990-2020 and projections to 2050: a systematic analysis for the global burden of disease study 2021. Lancet Rheumatol. (2023) 5(9):e508–22. doi: 10.1016/S2665-9913(23)00163-7
3. Chen N, Fong DYT, Wong JYH. Health and economic outcomes associated with musculoskeletal disorders attributable to high body mass index in 192 countries and territories in 2019. JAMA Netw Open. (2023) 6(1):e2250674. doi: 10.1001/jamanetworkopen.2022.50674
4. Losina E, Walensky RP, Reichmann WM, Holt HL, Gerlovin H, Solomon DH, et al. Impact of obesity and knee osteoarthritis on morbidity and mortality in older americans. Ann Intern Med. (2011) 154(4):217–26. doi: 10.7326/0003-4819-154-4-201102150-00001
5. Mendy A, Park J, Vieira ER. Osteoarthritis and risk of mortality in the USA: a population-based cohort study. Int J Epidemiol. (2018) 47(6):1821–9. doi: 10.1093/ije/dyy187
6. Barbour KE, Lui LY, Nevitt MC, Murphy LB, Helmick CG, Theis KA, et al. Hip osteoarthritis and the risk of all-cause and disease-specific mortality in older women: A population-based cohort study. Arthritis Rheumatol. (2015) 67(7):1798–805. doi: 10.1002/art.39113
7. Zhou J, Liu C, Sun Y, Francis M, Ryu MS, Grider A, et al. Genetically predicted circulating levels of copper and zinc are associated with osteoarthritis but not with rheumatoid arthritis. Osteoarthritis Cartilage. (2021) 29(7):1029–35. doi: 10.1016/j.joca.2021.02.564
8. Yao H, Xu J, Wang J, Zhang Y, Zheng N, Yue J, et al. Combination of magnesium ions and vitamin c alleviates synovitis and osteophyte formation in osteoarthritis of mice. Bioact Mater. (2020) 6(5):1341–52. doi: 10.1016/j.bioactmat.2020.10.016
9. Bravo M, Simón J, González-Recio I, Martinez-Cruz LA, Goikoetxea-Usandizaga N, Martínez-Chantar ML. Magnesium and liver metabolism through the lifespan. Adv Nutr. (2023) 14(4):739–51. doi: 10.1016/j.advnut.2023.05.009
10. Volpe SL. Magnesium in disease prevention and overall health. Adv Nutr. (2013) 4(3):378S–83S. doi: 10.3945/an.112.003483
11. Li Y, Yue J, Yang C. Unraveling the role of mg(++) in osteoarthritis. Life Sci. (2016) 147:24–9. doi: 10.1016/j.lfs.2016.01.029
12. Kuang X, Chiou J, Lo K, Wen C. Magnesium in joint health and osteoarthritis. Nutr Res. (2021) 90:24–35. doi: 10.1016/j.nutres.2021.03.002
13. Yao H, Xu JK, Zheng NY, Wang JL, Mok SW, Lee YW, et al. Intra-articular injection of magnesium chloride attenuates osteoarthritis progression in rats. Osteoarthritis Cartilage. (2019) 27(12):1811–21. doi: 10.1016/j.joca.2019.08.007
14. He Y, He H, Li X, Lei G, Xie D, Wang Y. Intra-articular magnesium plus bupivacaine is the most effective and safe postoperative analgesic option following knee arthroscopy: A network meta-analysis. Arthroscopy. (2022) 38(10):2897–2908.e18. doi: 10.1016/j.arthro.2022.03.013
15. Zhou Z, Yao X. The kidney reabsorption-related magnesium depletion score is associated with cardiovascular disease and longitudinal mortality in diabetic kidney disease patients. Diabetol Metab Syndr. (2025) 17(1):38. doi: 10.1186/s13098-025-01598-8
16. Fan L, Zhu X, Rosanoff A, Costello RB, Yu C, Ness R, et al. Magnesium depletion score (MDS) predicts risk of systemic inflammation and cardiovascular mortality among US adults. J Nutr. (2021) 151(8):2226–35. doi: 10.1093/jn/nxab138
17. Lu J, Li H, Wang S. The kidney reabsorption-related magnesium depletion score is associated with increased likelihood of abdominal aortic calcification among US adults. Nephrol Dial Transplant. (2023) 38(6):1421–9. doi: 10.1093/ndt/gfac218
18. Ye L, Zhang C, Duan Q, Shao Y, Zhou J. Association of magnesium depletion score with cardiovascular disease and its association with longitudinal mortality in patients with cardiovascular disease. J Am Heart Assoc. (2023) 12(18):e030077. doi: 10.1161/JAHA.123.030077
19. Chen Y, Xiang X, Wu Y, Han S, Huang Z, Wu M. Magnesium depletion score predicts diabetic retinopathy risk among diabetes: Findings from NHANES 2005-2018. Biol Trace Elem Res. (2023) 201(6):2750–6. doi: 10.1007/s12011-022-03384-3
20. Jiang W, Li J, Li H. Association between the composite dietary antioxidant index and all-cause mortality in individuals with osteoarthritis via NHANES data. Sci Rep. (2024) 14(1):30387. doi: 10.1038/s41598-024-81871-4
21. Zheng X, Zou P, Zeng C, Liu J, He Y. Increased levels of urine volatile organic compounds are associated with hypertension risk. J Hypertens. (2025) 43(1):136–44. doi: 10.1097/HJH.0000000000003878
22. Song J, Ma R, Yin L. Associations between estimated glucose disposal rate and arterial stiffness and mortality among US adults with non-alcoholic fatty liver disease. Front Endocrinol (Lausanne). (2024) 15:1398265. doi: 10.3389/fendo.2024.1398265
23. Chang Q, Zhu Y, Liu Z, Cheng J, Liang H, Lin F, et al. Replacement of sedentary behavior with various physical activities and the risk of all-cause and cause-specific mortality. BMC Med. (2024) 22(1):385. doi: 10.1186/s12916-024-03599-2
24. Laragione T, Harris C, Azizgolshani N, Beeton C, Bongers G, Gulko PS. Magnesium increases numbers of Foxp3+ treg cells and reduces arthritis severity and joint damage in an IL-10-dependent manner mediated by the intestinal microbiome. EBioMedicine. (2023) 92:104603. doi: 10.1016/j.ebiom.2023.104603
25. Zheng L, Zhao S, Li Y, Xu J, Yan W, Guo B, et al. Engineered MgO nanoparticles for cartilage-bone synergistic therapy. Sci Adv. (2024) 10(10):eadk6084. doi: 10.1126/sciadv.adk6084
26. Zeng C, Wei J, Li H, Yang T, Zhang FJ, Pan D, et al. Relationship between serum magnesium concentration and radiographic knee osteoarthritis. J Rheumatol. (2015) 42(7):1231–6. doi: 10.3899/jrheum.141414
27. Wu Z, Yang J, Liu J, Lian K. The relationship between magnesium and osteoarthritis of knee: A MOOSE guided systematic review and meta-analysis. Med (Baltimore). (2019) 98(45):e17774. doi: 10.1097/MD.0000000000017774
28. Adomako EA, Yu ASL. Magnesium disorders: Core curriculum 2024. Am J Kidney Dis. (2024) 83(6):803–15. doi: 10.1053/j.ajkd.2023.10.017
29. Fiorentini D, Cappadone C, Farruggia G, Prata C. Magnesium: Biochemistry, nutrition, detection, and social impact of diseases linked to its deficiency. Nutrients. (2021) 13(4):1136. doi: 10.3390/nu13041136
30. Jomova K, Makova M, Alomar SY, Alwasel SH, Nepovimova E, Kuca K, et al. Essential metals in health and disease. Chem Biol Interact. (2022) 367:110173. doi: 10.1016/j.cbi.2022.110173
31. Abbasi S, Mohebbi M, Mousavi Vahed SH, Dadgar Moghaddam M, Afiat M, Nematy M, et al. Comparison of magnesium status using 24-h urine magnesium content and magnesium fraction excretion in PCOS with non-PCOS control women: a cross-sectional study. Biol Trace Elem Res. (2023) 201(12):5601–6. doi: 10.1007/s12011-023-03626-y
32. Lo Piano F, Corsonello A, Corica F. Magnesium and elderly patient: the explored paths and the ones to be explored: a review. Magnes Res. (2019) 32(1):1–15. doi: 10.1684/mrh.2019.0453
33. Costello R, Rosanoff A, Nielsen F, West C. Perspective: Call for re-evaluation of the tolerable upper intake level for magnesium supplementation in adults. Adv Nutr. (2023) 14(5):973–82. doi: 10.1016/j.advnut.2023.06.008
34. Dominguez LJ, Veronese N, Barbagallo M. Magnesium and the hallmarks of aging. Nutrients. (2024) 16(4):496. doi: 10.3390/nu16040496
35. Chamniansawat S, Suksridechacin N, Thongon N. Current opinion on the regulation of small intestinal magnesium absorption. World J Gastroenterol. (2023) 29(2):332–42. doi: 10.3748/wjg.v29.i2.332
36. Zeng C, Neogi T, Chan AT, Wei J, Misra D, Lu N, et al. Proton pump inhibitor therapy and risk of knee replacement surgery: a general population-based cohort study. Osteoarthritis Cartilage. (2022) 30(4):559–69. doi: 10.1016/j.joca.2021.12.010
37. Rivlin RS. Magnesium deficiency and alcohol intake: mechanisms, clinical significance and possible relation to cancer development (a review). J Am Coll Nutr. (1994) 13(5):416–23. doi: 10.1080/07315724.1994.10718430
38. Xu H, Kang JH, Choi SE, Park DJ, Kweon SS, Lee YH, et al. Increased alcohol intake is associated with radiographic severity of knee and hand osteoarthritis in men. Sci Rep. (2024) 14(1):12648. doi: 10.1038/s41598-024-63559-x
39. Gommers LMM, Hoenderop JGJ, de Baaij JHF. Mechanisms of proton pump inhibitor-induced hypomagnesemia. Acta Physiol (Oxf). (2022) 235(4):e13846. doi: 10.1111/apha.13846
40. Cao S, Wei Y, Yue Y, Li G, Wang H, Lin J, et al. Omeprazole and risk of osteoarthritis: insights from a mendelian randomization study in the UK biobank. J Transl Med. (2024) 22(1):504. doi: 10.1186/s12967-024-05255-y
41. de Baaij JH, Hoenderop JG, Bindels RJ. Magnesium in man: implications for health and disease. Physiol Rev. (2015) 95(1):1–46. doi: 10.1152/physrev.00012.2014
42. Katopodis P, Karteris E, Katopodis KP. Pathophysiology of drug-induced hypomagnesaemia. Drug Saf. (2020) 43(9):867–80. doi: 10.1007/s40264-020-00947-y
43. Wei J, Neogi T, Terkeltaub R, Fenves AZ, Zeng C, Misra D, et al. Thiazide diuretics and risk of knee replacement surgery among patients with knee osteoarthritis: a general population-based cohort study. Osteoarthritis Cartilage. (2019) 27(10):1454–61. doi: 10.1016/j.joca.2019.05.020
Keywords: osteoarthritis, NHANES, magnesium depletion score, all-cause mortality, cardiovascular mortality
Citation: Ma R, Zhang C, Liu J, Ren J, Huang H, Wang G, Ding Y and Li X (2025) Associations of magnesium depletion score with the incidence and mortality of osteoarthritis: a nationwide study. Front. Immunol. 16:1512293. doi: 10.3389/fimmu.2025.1512293
Received: 16 October 2024; Accepted: 11 February 2025;
Published: 28 February 2025.
Edited by:
Helena Sá, Unidade Local de Saúde de Coimbra, PortugalReviewed by:
Liyun He, Sun Yat-sen University Cancer Center (SYSUCC), ChinaCopyright © 2025 Ma, Zhang, Liu, Ren, Huang, Wang, Ding and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yanchun Ding, eWFuY2h1bmRpbmcwODgwQDE2My5jb20=; Xia Li, bGl4aWE0MTZAMTYzLmNvbQ==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.