
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Immunol. , 20 February 2025
Sec. Microbial Immunology
Volume 16 - 2025 | https://doi.org/10.3389/fimmu.2025.1510948
The immunocompromised population is susceptible to Legionella pneumonia. The diagnosis and treatment of Legionella pneumonia in immunocompromised individuals are challenging clinical endeavors. Previous studies have identified Legionella pneumonia as a potential cause of organizing pneumonia (OP), however, the association between Legionella pneumonia and OP has not received enough clinical attention. We retrospectively evaluated a case involving Legionella micdadei infection and OP in a patient with myelodysplastic syndrome and concurrent Sweet syndrome. The diagnosis of Legionella micdadei pneumonia was confirmed through various methods: metagenomic next generation sequencing (mNGS), Giemsa-staining and fluorescence in situ hybridization of lung tissue, as well as serum immunofluorescence antibody testing. Histopathological analysis of lung tissue revealed OP. The patient was successfully treated with a combination of antibiotics and low-dose glucocorticoids. In immunocompromised individuals, mNGS was capable of detection non-Legionella pneumophila serogroup 1. The pathological examination is important for identifying secondary OP and provides the evidence for treatment with glucocorticoids.
Immunocompromised hosts are susceptible to Legionella pneumonia, which has been widely described (1). The mortality rate of Legionella pneumonia in immunocompromised hosts is up to 50%, significantly higher than the 5–33% rate observed in the general population (2). However, due to the limitations of available detection methods, the diagnosis of Legionella infection, especially non-Legionella pneumophila (Lp) serogroup 1(Lp1), in immunocompromised hosts is challenging. Legionella infection can cause pulmonary interstitial changes, which are thought to be among the factors that lead to death (3). Although organizing pneumonia (OP) secondary to Legionella pneumonia has been reported (4), a comprehensive understanding of the clinical implications of this association is still lacking. Furthermore, the effect of modifying treatment strategies for secondary OP on the prognosis of Legionella pneumonia remains unclear.
We herein report a rare case of a Legionella pneumonia patient who was suffering from myelodysplastic syndrome (MDS) accompanied by Sweet syndrome. The diagnosis of Legionella micdadei pneumonia was established using multiple methods, including metagenomic next-generation sequencing(mNGS). The aim of this report is to enhance the comprehension of the relationship between Legionella pneumonia and OP. Furthermore, the report provides additional insights into the management and treatment strategies for patients with Legionella pneumonia who exhibit suboptimal responses to antibiotic therapy.
A 58-year-old male Chinese sanitation worker initially presented to our fever clinic on March 30, 2021, reporting recurrent fever lasting for over a year. The fever typically occurred in the afternoon, with a daily maximum temperature between 37.5 and 37.6°C, without respiratory system symptoms or other accompanying symptoms. He was referred to a local hospital for medical evaluation and diagnosed with anemia. The patient was intermittently administered cephalosporins, traditional Chinese medicine, and dexamethasone. Despite achieving a normal temperature for approximately one week, his condition relapsed. The patient was committed to environmental sanitation duties. On March 24, 2021, the patient’s temperature reached a record high of 39.5°C and was accompanied by bloody sputum. Consequently, he sought medical attention at our fever clinic, where routine blood tests revealed anemia with a hemoglobin level of 75 g/L and thrombocytopenia with a platelet count of 59*10^9/L. Bone marrow aspiration revealed no abnormal primary cells, and the ratio of granulocytes to erythrocytes increased significantly. The next-generation sequencing of the marrow showed a gene mutation in the DNMT3A gene (with a 41.28% variant allele frequency). Therefore, the patient was diagnosed as having MDS. Additionally, chest CT revealed consolidation and ground-glass opacity in the right lung (Figure 1A) and elevated C-reactive protein (272.0 mg/L) and procalcitonin (0.93ng/mL). Despite receiving a two-week regimen of intravenous cephalosporin and levofloxacin, the patient’s fever persisted. Subsequently, the patient was transferred to the respiratory department on April 14, 2021. The patient presented with no prior medical history and explicitly denied any history of pulmonary disease. He had not undergone any previous chest X-ray examinations. Additionally, he denied any history of pet ownership or substance abuse. There was no family history of pulmonary disease or cancer reported. The blood gas analysis revealed type I respiratory failure with a PaO2 of 63.9 mmHg while receiving 2 L/min nasal oxygen therapy (oxygenation index: 220.34 mmHg). Serum 1-3-beta-D-glucan and galactomannan tests and interferon-gamma release assays yielded negative results. Sputum culture results were negative. The primary laboratory results are listed in Table 1.
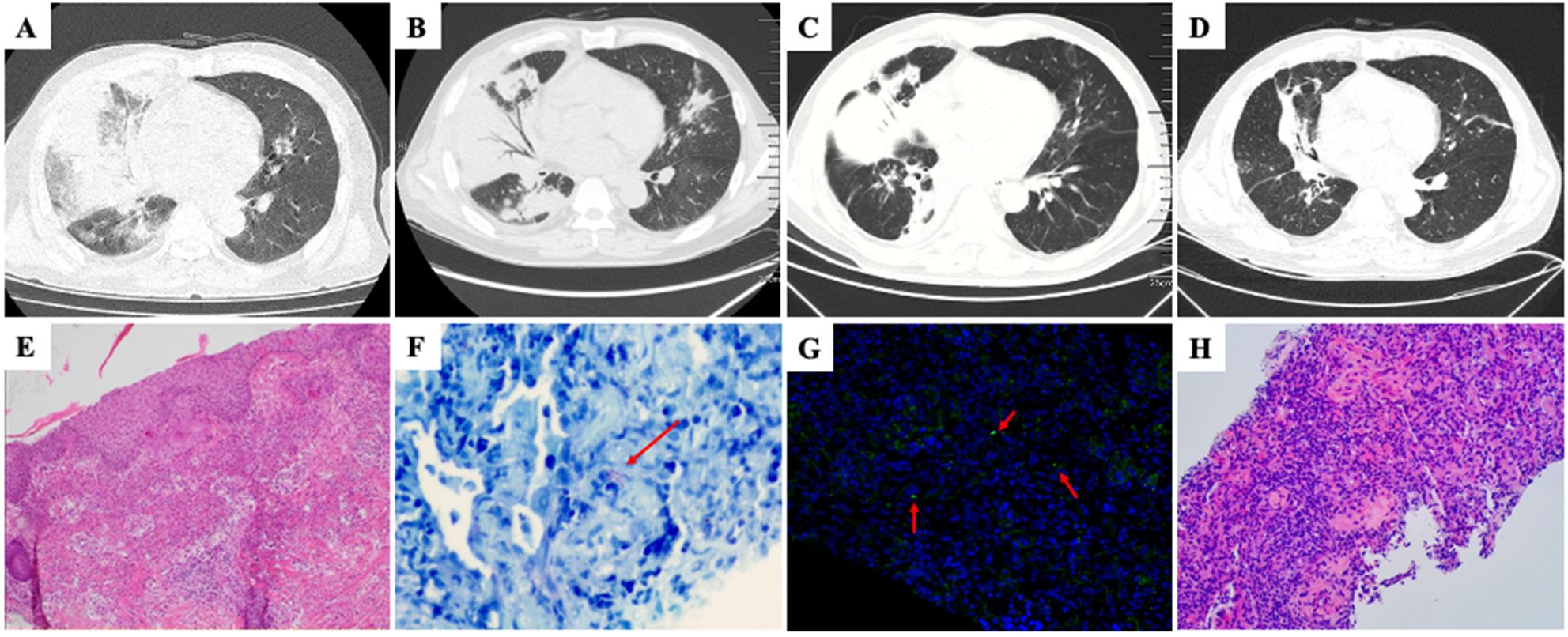
Figure 1. (A) Chest CT scan (March 30, 2021) showed multiple ground glass opacities and consolidation in right middle and lower lobes. (B) Post-admission chest CT scan, following a two-week course of antibiotic treatment, revealed a reduction in ground-glass opacities and an increase in consolidation. (C) Chest CT performed after five-day intensive antibiotics treatment for Legionella pneumonia showed decreased consolidation and reduced right lung volume. (D) Follow-up chest CT performed after seven weeks of glucocorticoid treatment showed markedly decreased extent of abnormal opacity in right lung. (E) Histopathological analysis of skin biopsy showed dense dermal infiltration of neutrophils without overt vasculitis (hematoxylin and eosin stain, magnification × 100). (F) Giemsa staining of lung tissue showed presence of intracellular slender bacilli (arrow) suggestive of Legionella (magnification × 400). (G) Specific identification of Legionella micdadei (arrow) in lung biopsy tissue by fluorescence in situ hybridization (magnification × 400). (H) Lung biopsy showed buds of granulation tissue occupying alveolar spaces (hematoxylin and eosin stain, magnification × 200).
The patient presented with flat papules of dark purplish-red coloration distributed on both upper limbs, neck, front chest, back shoulder, and buttocks. Histopathological examination of the skin revealed Sweet syndrome (Figure 1E).
The patient was treated with ubenimex and stanozolol for MDS. Nevertheless, following a two-week course of antibiotic therapy, the patient’s body temperature persisted, and a subsequent review of CT scans revealed a reduction in ground-glass opacities and an increase in consolidation (Figure 1B). Subsequently, a percutaneous aspiration lung biopsy was performed. mNGS of lung tissue detected Legionella micdadei. Based on the literature (5–7), the fluorescence in situ hybridization (FISH) probe for Legionella micdadei was prepared as follows: FAM-AGCTGATTGGTTAATAGCCCAATCGG-FAM. Giemsa staining (Figure 1F) and FISH-probe of lung tissue (Figure 1G) confirmed Legionella micdadei infection. In addition, the patient’s serum immunofluorescent antibody to Legionella micdadei was positive, whereas the Legionella urinary antigen was negative. Legionella micdadei pneumonia was confirmed. The patient received a regimen of moxifloxacin and tigecycline for the management of Legionella pneumonia.
Despite undergoing five-day intensive antibiotic treatment for Legionella pneumonia, the patient experienced recurrent episodes of high fever. Repeated full blood count testing a white cell count was 8.6× 109/L with a neutrophil ratio of 76.4%, and the CRP level decreased to 70.4mg/L. Additionally, chest CT showed that the consolidation had decreased alongside a reduction in the right lung volume, suggesting the possibility of OP (Figure 1C). Subsequently, pathological examination of the patient’s lung tissue revealed OP changes (Figure 1H). Therefore, the patient was administered a ten-day course of intravenous methylprednisolone at a daily dose of 40 mg in conjunction with antibiotics. The patient’s temperature normalized on the first day of methylprednisolone treatment. Consequently, the intravenous methylprednisolone was replaced with oral prednisone tablets (40 mg daily). The patient was discharged without complications. Subsequently, the prednisone dosage was gradually decreased by 5 mg every two weeks post-discharge, with no reported adverse effects observed during the treatment period. A chest CT scan performed after seven weeks of glucocorticoid treatment revealed notable resolution of the pulmonary radiographic findings (Figure 1D).
To the best of our knowledge, this is the first report of a patient with MDS and Sweet syndrome diagnosed with Legionella micdadei pneumonia and OP.
The diagnosis of Legionella pneumonia frequently encounters obstacles stemming from both underdiagnosis and delayed diagnosis, primarily attributable to the absence of easily accessible diagnostic tools for the early detection of all Legionella serogroups and species capable of causing infections. In addition to distinct clinical manifestations, a detailed environmental exposure history is a crucial factor in the diagnostic process and warrants careful attention. The patient had been engaged in sanitation duties and had a history of environmental exposure to sewage, which may have been the origin of his infection with Legionella micdadei. The Legionella micdadei is the second most common species associated with Legionella pneumonia (8). This species has been found to be associated with more immunocompromised patients and was not identified by the first-line screening test, Legionella urinary antigen. Therefore, it is critical to use specific methods to identify various Legionella species, especially in immunocompromised individuals. Recently, mNGS has emerged as a valuable tool for the identification of difficult-to-culture pathogens. Through the use of advanced nucleic acid detection and molecular techniques, mNGS offers heightened sensitivity, specificity, and expedited pathogen detection capacity (9). Advances in pathogen identification technology will reveal the etiological pathogens of pneumonia in immunocompromised populations.
OP is characterized by a pattern of lung tissue repair, which often occurs as a result of a specific injury (such as infection, connective tissue disorder, aspiration, and drug toxicity) or from an unknown source (cryptogenic) (10). The predominant etiology of secondary OP is an infection. Legionella includes a host of pathogens that can cause OP (10). Previous studies of Legionella pneumonia have shown that its pathological alterations include diffuse alveolar damage, fibrin deposition, and fibrosis in severe cases (3, 11). Pulmonary interstitial changes due to Legionella pneumonia have been recognized for some time and can lead to disease progression and potentially fatal outcomes (3). However, Legionella pneumonia-associated pulmonary interstitial changes have not received sufficient clinical attention.
Moreover, a previous study described the occurrence of OP in patients with MDS accompanied by Sweet syndrome (12). Sweet Syndrome, also known as “acute febrile neutrophilic dermatosis,” is often linked to malignant hematological conditions such as MDS, likely driven by autoimmunity and immunodeficiency (13). Interestingly, Sweet syndrome and OP can precede, coincide, or follow each other during MDS progression (12). The mechanisms underlying these conditions require further investigation.
The causes of OP formation in this patient were complex and likely related to both Legionella pneumonia and underlying MDS and Sweet syndrome. The initial chest CT results revealed a relatively sharp-bordered consolidation intermixed with ground-glass opacity, consistent with the chest imaging characteristics of acute Legionella pneumonia (14). Therefore, we speculated that Legionella infection was a significant factor in the development of OP in this patient, with MDS and Sweet syndrome potentially exerting a secondary facilitating effect.
The patient experienced a sustained fever despite treatment with a combination of antibiotics. Subsequent pathological findings indicated a diagnosis of OP, with symptoms and imaging showing improvement following glucocorticoid therapy. Therefore, in cases of Legionella pneumonia patients where clinical manifestations persist despite appropriate anti-Legionella treatment and imaging indicates the possibility of OP, active histopathological examination is essential to adjust the treatment regimen and improve the prognosis.
The number of immunocompromised individuals is steadily increasing, highlighting the increasing importance of recognizing the latent risk of Legionella pneumonia. mNGS has important application value in the detection of rare pathogens of pneumonia in immunocompromised individuals. For patients with Legionella pneumonia who do not respond well to a combination of antibiotic treatments, pathological analysis can aid in identifying potential secondary OP and provide evidence for treatment with glucocorticoid.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving humans were approved by Ethics Committee of Shengjing Hospital of China Medical University. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article. Written informed consent was obtained from the participant/patient(s) for the publication of this case report.
YYC: Validation, Writing – original draft. SL: Validation, Writing – original draft. YL: Validation, Writing – original draft. XZ: Data curation, Investigation, Writing – review & editing. RZ: Conceptualization, Supervision, Writing – review & editing. YC: Conceptualization, Project administration, Supervision, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by the National Natural Science Foundation of China (No: 81170009).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declare that no Generative AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Rello J, Allam C, Ruiz-Spinelli A, Jarraud S. Severe Legionnaires' Disease. Ann Intensive Care. (2024) 14:51. doi: 10.1186/s13613-024-01252-y
2. Guo Z, Zuo A, Liu X, Jiang Y, Yang S, Lu D. Multiple pulmonary cavities in an immunocompetent patient: A case report and literature review. Front Med (Lausanne). (2024) 11:1329381. doi: 10.3389/fmed.2024.1329381
3. Chastre J, Raghu G, Soler P, Brun P, Basset F, Gibert C. Pulmonary fibrosis following pneumonia due to acute Legionnaires' Disease. Clinical Ultrastructural Immunofluorescent Study Chest. (1987) 91:57–62. doi: 10.1378/chest.91.1.57
4. Haroon A, Higa F, Hibiya K, Haranaga S, Yara S, Tateyama M, et al. Organizing pneumonia pattern in the follow-up Ct of legionella-infected patients. J Infect Chemother. (2011) 17:493–8. doi: 10.1007/s10156-010-0205-y
5. Cross KE, Mercante JW, Benitez AJ, Brown EW, Diaz MH, Winchell JM. Simultaneous detection of legionella species and L. Anisa, L. Bozemanii, L. Longbeachae and L. Micdadei using conserved primers and multiple probes in a multiplex real-time Pcr assay. Diagn Microbiol Infect Dis. (2016) 85:295–301. doi: 10.1016/j.diagmicrobio.2016.03.022
6. Fernández-Cruz A, Marín M, Castelo L, Usubillaga R, Martín-Rabadán P, Bouza E. Legionella micdadei, a new cause of prosthetic joint infection. J Clin Microbiol. (2011) 49:3409–10. doi: 10.1128/jcm.00770-11
7. Whiley H, Taylor M, Bentham R. Detection of legionella species in potting mixes using fluorescent in situ hybridisation (Fish). J Microbiol Methods. (2011) 86:304–9. doi: 10.1016/j.mimet.2011.05.023
8. Htwe TH, Khardori NM. Legionnaire's disease and immunosuppressive drugs. Infect Dis Clin North Am. (2017) 31:29–42. doi: 10.1016/j.idc.2016.10.003
9. Maurer FP, Christner M, Hentschke M, Rohde H. Advances in rapid identification and susceptibility testing of bacteria in the clinical microbiology laboratory: implications for patient care and antimicrobial stewardship programs. Infect Dis Rep. (2017) 9:6839. doi: 10.4081/idr.2017.6839
10. King TE Jr., Lee JS. Cryptogenic organizing pneumonia. N Engl J Med. (2022) 386:1058–69. doi: 10.1056/NEJMra2116777
11. Hürter T, Rumpelt HJ, Ferlinz R. Fibrosing alveolitis responsive to corticosteroids following Legionnaires' Disease pneumonia. Chest. (1992) 101:281–3. doi: 10.1378/chest.101.1.281
12. Tzelepis E, Kampolis CF, VlaChadami I, Moschovi M, Alamani M, Kaltsas G. Cryptogenic organizing pneumonia in Sweet's syndrome: case report and review of the literature. Clin Respir J. (2016) 10:250–4. doi: 10.1111/crj.12206
13. Joshi TP, Friske SK, Hsiou DA, Duvic M. New practical aspects of Sweet syndrome. Am J Clin Dermatol. (2022) 23:301–18. doi: 10.1007/s40257-022-00673-4
Keywords: Legionella pneumonia, Legionella micdadei, organizing pneumonia, immunocompromised, Sweet syndrome
Citation: Chen Y, Liang S, Lu Y, Zhou X, Zheng R and Chen Y (2025) Case Report: First report of Legionella micdadei pneumonia and organizing pneumonia in a patient with myelodysplastic and Sweet syndromes. Front. Immunol. 16:1510948. doi: 10.3389/fimmu.2025.1510948
Received: 05 November 2024; Accepted: 06 February 2025;
Published: 20 February 2025.
Edited by:
Theocharis Konstantinidis, Democritus University of Thrace, GreeceReviewed by:
Gioulia Romanidou, Sismanogleio General Hospital, GreeceCopyright © 2025 Chen, Liang, Lu, Zhou, Zheng and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yu Chen, Y2hlbnl1c3lAaG90bWFpbC5jb20=; Rui Zheng, emhlbmdyQHNqLWhvc3BpdGFsLm9yZw==
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.