- Department of Neurology, Shandong Provincial Hospital Affiliated with Shandong First Medical University, Jinan, Shandong, China
Clinical management of the rare and complex overlapping syndrome of MOG-antibody disease and anti-NMDAR encephalitis (MNOS), which has an uncertain pathogenesis and a high risk of recurrence, is highly challenging. We describe the case of a 19 years-old female patient, who first complained of headache, fever, and irritability. After that, she experienced frequent seizures and mood disorders. The diagnosis of MNOS was verified through antibody tests and imaging. For the patient, intravenous immunoglobulin and high-dose methylprednisolone were effective as first-line immunotherapy. Long-term immunotherapy with oral prednisone and mycophenolate mofetil was used to prevent relapses. However, over six years, the patient had five relapses when the mycophenolate mofetil dosage was reduced. The patient’s condition stabilized after taking rituximab as second-line immunotherapy, with less than 1% of total lymphocytes being CD19+ cells. Eleven months later, the plasmablast ratio increased, and patients experienced new symptoms such as bilateral optic neuritis. After that, the patient got telitacicept injections regularly for 13 months, during which time her symptoms subsided, and there were no adverse effects or relapses. This case suggests that telitacicept may be a viable adjunct or sequential therapy option for the depletion of B cells in MNOS.
Introduction
Myelin oligodendrocyte glycoprotein antibody-associated disease (MOGAD) is an immune-mediated inflammatory demyelinating disorder of the central nervous system, recently found and defined by the presence of anti-MOG antibodies (1). Autoimmune encephalitis (AE) is a set of illnesses associated with antibodies against neuroglial antigens. The most common AE association is anti-NMDAR encephalitis, which accounts for around 54% to 80% of all AE cases (2). Previously, MOGAD and anti-NMDAR encephalitis were thought to be two independent illnesses, each associated with a different specific pathogenic mechanism. However, new researches revealed that these two disorders can coexist (3–6), leading to overlapping clinical symptoms. Currently, researchers classify patients with clinical and imaging features in common between the two diseases and coexistence of both anti-MOG and anti-NMDAR antibodies as having MOGAD and NMDAR encephalitis overlap syndrome (MNOS) (6).
MNOS is characterized by frequent relapses, and no standardized treatment protocol is available. In the acute phase, treatment of MNOS generally includes intravenous methylprednisolone (IVMP), intravenous immunoglobulin (IVIG), or plasmapheresis (PE). Long-term treatments like oral prednisone, mycophenolate mofetil (MMF), rituximab (RTX), and azathioprine (AZA) can be given to some individuals to avoid relapse following acute treatment (4, 6–9). RTX, an anti-CD20 chimeric monoclonal antibody, targets and depletes CD20-positive B cells, including initial, mature, and memory B cells. In addition, telitacicept, a novel recombinant fusion protein that is composed of the human IgG Fc component and the ligand-binding domain of the TACI receptor, neutralizes two critical cell signaling molecules, B lymphocyte stimulator (BLyS) and proliferation-inducing ligand (APRIL) (10). This action impedes the development and survival of plasma and mature B cells. To date, telitacicept has been proven to be effective as treatment strategy in neuromylitis optica spectrum disorder (NMOSD), Myasthenia gravis (MG), and other neuroimmunological conditions (11, 12). Here, we present a case of refractory MNOS where rituximab and telitacicept were used sequentially to limit clinical relapse successfully.
Case presentation
A 19-year-old female was admitted to the hospital in September 2016, presenting with headache, fever (up to 39°C), and irritability. The patient did not report any joint discomfort or any anomalies in the muscles or skin, and a physical examination, which included a neurological evaluation, revealed no significant results. Routine blood exams, including immunological tests, creatine kinase (CK) levels, rheumatoid factor, and antistreptolysin O, were all within normal limits, exlcuding systemic autoimmune rheumatic conditions. The cerebrospinal fluid (CSF) pressure was 190 mmH2O, and the examination showed increased leucocytes (130/mm³, 69% mononuclear) and normal protein levels (0.37g/L, normal range <0.4g/L). Polymerase chain reaction (PCR) analysis for Mycobacterium tuberculosis, herpes simplex virus (HSV), cytomegalovirus (CMV), Epstein-Barr virus (EBV), and cryptococcal DNA yielded negative results. The magnetic resonance imaging (MRI) revealed a hyperintense lesion in the right splenium of the corpus callosum, corona radiata, and basal ganglia, as observed on fluid-attenuated inversion recovery (FLAIR) imaging (Figure 1). These findings demonstrated that the patient met the diagnostic criteria for encephalitis of presumed autoimmune etiology established by the International Encephalitis Alliance in 2013 (13). She received acyclovir and methylprednisolone (500 mg/d for 3 days and 240 mg/d for 3 days), resulting in a swift improvement in symptoms. A subsequent CSF examination one month later revealed a decrease in pressure (178 mmH2O) and leucocyte count (24/mm³, 100% mononuclear). After discharge, the patient was prescribed 60 mg/day oral prednisone, which was methodically tapered until stop over three months.

Figure 1. Magnetic Resonance Imaging of the brain and spinal cord. (A, B) Brain MRI at onset showing abnormal signals on the right corona radiata and basal ganglia regions. (C, D) Brain MRI showing alterations on left temporal an occipital lobes with enhancement on FLAIR and T1 post-Gd during second attack. (E, F) Abnormal signal on T2/FLAIR in the right pons at the time of the patient’s third attack. (G, H) New lesion in the left parietal lobe with contrast-enhancement during fourth attack. (I, J) New lesions in the right frontal cortex with linear contrast-enhancement during fifth attack. (K) Abnormal signal in both optic nerves, following treatment with RTX at the time of the patient’s sixth attack. (L) Lesions after 1 year of treatment with telitacicept.
In May 2018, the patient was referred to our hospital due to recurrent headaches and fever. During hospitalization, she experienced daily recurrent generalized tonic-clonal seizures (GTCS). There were no noticeable abnormalities on the neurologic exam. Brain MRI scanning showed a new lesion in the left temporal and occipital lobes. The 24-hour video electroencephalogram (VEEG) identified an aberrant background rhythm marked by diffuse paroxysmal discharges within the theta frequency band. CMV DNA, EBV DNA, and varicella-zoster virus (VZV) nucleic acid were not detected in serum or CSF by PCR, and antibodies to HSV types I and II, EBV, CMV, and rubella virus were also negative by ELISA. Immunologic tests, such as antinuclear antibodies, antineutrophil cytoplasmic antibodies, and anticardiolipin antibodies (via ELISA), showed no abnormalities. In addition, we employed a commercially available immunoblotting assay (Euroimmun, Lübeck, Germany) to detect Ri, Yo, Hu, Ma2, Amphiphysin, and CV2 IgG in serum, and the results were likewise negative. Moreover, serum tests for anti-aquaporin-4 (AQP4), anti-myelin basic protein (MBP), anti-NMDAR, anti-MOG, anti-LGI1, anti-CASPR-2, anti-AMPA R1/R2, and anti-GABABR conducted via fixed cell-based assay (CBA) by Hangzhou Dian Medical Laboratory Center yielded negative results. The patient was treated with 500 mg of IVMP for five days with subsequent tapering (240 mg/d for three days and 120 mg/d for three days), and intravenous acyclovir. Her clinical symptoms improved following the treatment, and she was discharged from the hospital with a prescription for oral prednisone at a daily dose of 60 mg, followed by tapering.
In July 2018, following the reduction of the oral prednisone dose, the patient exhibited a flare-up of symptoms characterized by intermittent fever, mood instability, and unsteady gait. She was later referred to Peking Union Medical College Hospital, where neurological examination indicated left-sided limbs weakness, ataxia, and bilateral Babinski sign. The brain MRI showed a new lesion involving the right pons (Figure 1). CSF examination revealed normal pressure, white blood cell count, and protein levels. Laboratory tests identified positive MOG IgG in serum and NMDAR IgG in CSF, with titers of 1:100 and 1:10, respectively, as assessed by fixed CBA from Neurology Pathology Laboratory of Peking Union Medical College Hospital. Based on the patient’s clinical symptoms, antibody test results, and MRI, the patient meets the diagnostic criteria for both MOGAD and anti-NMDA receptor encephalitis (1, 14). Eventually, diagnosis of MNOS was made, according to the concept of Fan et al. (6). The patient received IVMP, initiating with 1,000 mg daily for 3 days, subsequently reducing to 500 mg daily for 3 days, and tapering to 250 mg and 120 mg daily over the following 6 days. Immunoglobulin was given at a dosage of 0.4 g/kg daily for 5 days after IVMP, alongside oral MMF at 0.5 g administered twice daily. This treatment led to clinical enhancement. Upon discharge, the patient was treated with oral prednisone at a dosage of 60 mg per day, gradually decreasing by 5 mg every one to two weeks, and MMF at 1 g per day.
In January 2019, the patient exhibited no additional aberrant symptoms and then discontinued prednisone and MMF was reduced to 0.75 g daily. After six months, MMF was further reduced to 0.5 g/day, at which point a new generalized motor seizure occurred. Increasing the MMF dose to 1 g/day resulted in no more seizures. The brain MRI revealed no new or enhanced lesions. In the subsequent two years, the patient experienced two analogous episodes, both coinciding with decreases in the MMF dosage. In 2021 and the initial quarter of 2022, the patient had two relapses after reduction in MMF dosage, with each episode linked to new lesions on the left parietal lobe and right frontal cortical regions (Figure 1). During the patient’s fifth attack, the patient experienced seizures again, and FLAIR showed new lesions on the right frontal cortical area, which were termed as FLAIR-hyperintense lesions in anti-MOG-associated encephalitis with seizures (FLAMES). Fixed CBAs for serum MOG IgG and CSF NMDAR IgG showed low positive results at titers of 1:10 and 1:1, respectively (Figure 2). After counseling with the patient, rituximab (RTX) treatment was initiated in April 2022, with a 500 mg infusion, followed by an additional 500 mg infusion two weeks later. After eleven months, the patient experienced bilateral optic neuritis. The peripheral blood CD19+/lymphocyte ratio was notably low, measuring only 0.06%, far below the threshold required for RTX re-treatment. However, the proportion of memory B lymphocytes and plasmablasts had increased to 4.15% and 0.46%, respectively. In March, 2023, the patient began treatment with telitacicept, initially at the dose of 160 mg/week for the first three months, and after repeated every 2-4 weeks. During the subsequent 16 months, the patient exhibited no additional relapses and reported no notable discomfort or adverse effects. The patient has been off prednisone for six months and has returned to normal daily activities. Figure 3 illustrates the disease progression over the past eight years.

Figure 2. The antibody test results of serum and cerebrospinal fluid (CSF) during the patient’s fourth relapse. (A) CSF showing the binding to the surface of the cells expressing anti-NMDAR receptors (NMDAR) (titer 1:1). (B) Myelin oligodendrocyte glycoprotein (MOG) antibodies (titer 1:10) were detected in the serum.

Figure 3. Temporal variation of symptoms, MOG-IgG titers, NMDAR-IgG titers, EDSS, and mRS in relation with treatment status.
Review of the literature
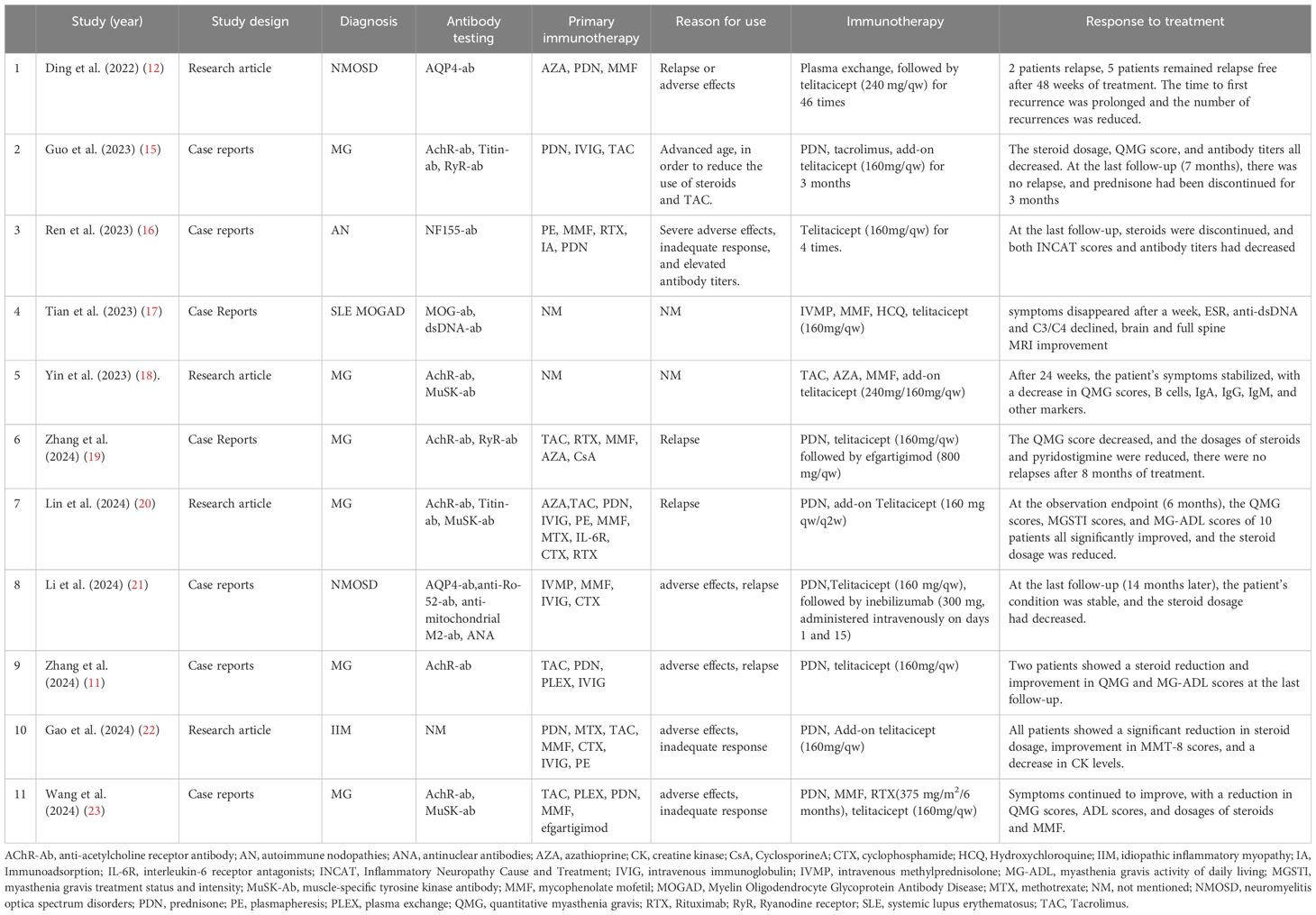
A literature review was conducted using PubMed, and 11 case reports and studies were identified regarding telitacicept in treating neuroimmune diseases (see Table 1). The studies demonstrated the notable efficacy of telitacicept in multiple autoimmune conditions. Six of the studies reviewed examined its use in MG, two in NMOSD, one in idiopathic inflammatory myopathy (IIM), one in autoimmune neuropathies (AN), and one in systemic lupus erythematosus (SLE) combined with MOGAD. In these instances, telitacicept was predominantly employed as an add-on or sequential therapy as a result of inadequate responses to prior treatments (Studies 3, 10, 11), notable adverse effects (Studies 1, 2, 3, 8, 9, 10, 11), or relapses after prolonged use of other treatments (Studies 1, 6, 7, 8, 9). Telitacicept exhibited significant efficacy, irrespective of its use as a monotherapy or in combination with other immunosuppressive agents. In the six MG studies, telitacicept facilitated disease stabilization, evidenced by enhancement in the Quantitative Myasthenia Gravis Score (QMG) and additional clinical scoring systems. The two NMOSD studies (1 and 8) demonstrated that telitacicept decreased disease relapses and improved symptoms. Several studies (2, 6, 7, 9, 11) found that telitacicept facilitated a reduction in the dosage of steroids and other immunosuppressants, consequently minimizing associated side effects. In patients with IIM, MOGAD, AN, telitacicept similarly resulted in significant clinical improvement and prolonged relapse intervals.
Discussion
We present the first report of sequential treatment with rituximab and telitacicept in a patient with refractory MNOS. Titulaer’s initial report on MOGAD cases associated with anti-NMDA receptor encephalitis (8) led to a significant rise in related publications. In 2018, Fan et al. presented the concept of MNOS (6). After that, Hikari suggested that the presence of anti-NMDAR and anti-MOG antibodies in autoimmune encephalitis is a new clinical entity (24).
As understanding of this syndrome continues to evolve, universally accepted diagnostic criteria have yet to be established. Nevertheless, many case reports and clinical studies indicate that antibody positivity should be correlated with the clinical phenotype. Without corresponding clinical manifestations, diagnosing of the antibody-associated disease is not feasible, and the presence of antibodies should be regarded as an unspecific association. Several studies have found that when both anti-NMDA receptor and anti-MOG antibodies are present, the clinical symptoms are predominantly those of anti-NMDA receptor encephalitis (9, 24–26). These include cognitive deficits, psychosis, seizures, movement disorders, and so forth (27). Syndromes typical for MOGAD, including optic neuritis, myelitis, and acute disseminated encephalomyelitis, are relatively uncommon. The patient described in our report had several relapses, with lesions affecting the thalamus, pons, centrum semiovale, and cerebral cortex (Figure 1). Based on clinical and radiological characteristics, the patient sequentially exhibited features of anti-NMDAR encephalitis, FLAMES, and optic neuritis, which are consistent with the coexistence of anti-NMDAR encephalitis and MOGAD(conformed to the concept of MNOS by Fan (6)).
In the acute phase of MNOS, glucocorticoids, IVIG, and PE are frequently employed. Ding et al. reported a recurrence rate of 63.4% for MNOS, with 50.0% of patients experiencing relapses as encephalitis and 53.8% presenting as demyelinating events (28). A systematic review and meta-analysis involving 43 patients with dual positivity for MOG and NMDAR antibodies found that 15 of these patients experienced relapses (9). The findings indicate that MNOS exhibits a significantly elevated relapse rate. Given this, it is imperative to implement long-term immunotherapy in order to prevent relapses. The immunosuppressants utilized for long-term management correspond with the treatment strategies for anti-NMDAR encephalitis and MOGAD, such as prednisone, MMF, AZA, CTX, and RTX (3, 6, 29–33). In some patients with recurrent relapses, symptoms have been managed through the use of other immunosuppressants as a bridge. Of note, there are currently no reports of relapses in MNOS after treatment with rituximab.
MMF inhibits T and B lymphocyte proliferation by inhibiting inosine monophosphate dehydrogenase (34). This patient received MMF treatment in 2018. However, during drug tapering, relapses and symptom variations led us to switch to RTX. RTX depletes CD20-positive B cells through different mechanisms including antibody-dependent cellular cytotoxicity (ADCC) and complement-dependent cytotoxicity (CDC) (35, 36). During the eleven months after RTX administration, CD19 was utilized as a marker to evaluate B cell depletion (37). Despite low CD19+ B cells levels, the patient experienced bilateral optic neuritis, as evidenced by brain MRI (Figure 1). Later, she presented with headaches and blurred vision. A brain MRI scan revealed bilateral optic neuritis (Figure 1). At this point, serum anti-MOG IgG was tested and found to be negative, with a CD19-positive cell proportion of 0.06%. This implies that evaluating relapse risk exclusively using CD19+ B cells need to be more thorough and accurate (Figure 4). Future research is required to identify more precise biomarkers for monitoring relapse rates in B cell depletion therapy for neuroimmunological diseases.

Figure 4. The percentage of CD19+B cells, plasmablast, memory B cells during treatment with RTX and telitacicept.
Multiple factors may contribute to the persistent relapse recurrency of neuroimmune diseases despite use only RTX. Firstly, RTX is capable of eliminating short-lived plasma cells (SLPCs). However, it is ineffective in the eliminating of long-lived plasma cells (LLPCs) or plasmablasts in the bone marrow, as these cells lack CD20 expression (38–40). LLPCs can migrate to the bone marrow as antibody-secreting cells (ASCs). These cells can then proliferate and differentiate into short-lived plasma cells, extending antibody secretion (16). Secondly, repetitive RTX administration may reactivate specific autoreactive memory B cells resistant to RTX, enhancing production of pathogenic plasma cells and activating immune responses (41). Thirdly, patients who undergo multiple RTX treatments may develop anti-RTX antibodies, which can neutralize RTX activity, leading to a higher relapse rate and quicker B cell reconstitution (42, 43). Fourth, polymorphisms in the FCGR3A gene can influence the efficacy of RTX in B cells depletion, with the quantity of FCGR3A-158V allele copies correlating with RTX-induced ADCC (44). This study observed that the patient received only two RTX injections. Although the proportion of CD19+/lymphocytes remained low, there was a gradual increase in the proportion of memory B cells and plasmablasts. Thus, the patient’s higher relapse rate may be associated with the persistence of LLPCs, memory B cells, or the FCGR3A-158V variant allele copies.
BLyS and APRIL are essential cytokines for the growth and development of B cells and plasma cells (45). Studies have demonstrated that BLyS and APRIL levels are significantly elevated in peripheral blood and CSF during the acute phases of NMOSD and anti-NMDAR encephalitis. These levels correlate with Expanded Disability Status Scale (EDSS) scores and disease prognosis (12, 46, 47). Moreover, BLyS levels increase notably in patients receiving multiple RTX infusions (48, 49). Despite consistently low CD19+/lymphocyte levels, the patient displayed increased proportions of plasmablasts, indicating heightened disease activity. Similar results have been reported in systemic lupus erythematosus (SLE) and IgG4-related diseases, emphasizing the potential of plasmablast surveillance to evaluate disease activity, which may be linked to BLyS and APRIL levels (50–53). Telitacicept inhibits the development and survival of plasmablasts and mature B cells by neutralizing BLyS and APRIL (10). Guan et al. described treating eight patients with refractory NMOSD utilizing telitacicept sequential with plasma exchange. Among them, five (63%) did not relapse within 48 weeks, while two patients (25%) experienced relapses at 45 and 234 days post-treatment, respectively. The interval between relapses was significantly extended, and the overall relapse frequency decreased markedly compared to pre-treatment levels (P < 0.001). Non-relapsed patients also showed a mean EDSS score reduction of 0.83 points (range 0.5–2) (54). Considering the evidence presented, telitacicept was chosen as a bridging therapy alongside RTX for our case.
The patient’s response to telitacicept was remarkable, with the EDSS and the modified Rankin Scale (mRS) scores dropping to 0 one month after initiating telitacicept (Figure 2). The observed clinical improvement correlated with a notable decrease in CD19+ B lymphocytes, plasmablasts, and memory B cells, all falling below the detection threshold. At the most recent follow-up, 16 months following the regular administration of telitacicept, the percentage of CD19+ B cells increased slightly to above 1%, while the proportion of plasmablasts and memory B cells remained low (Figure 4).
Our study has several limitations. First of all, the study is a case report with a small sample size; additional research with more cases is required to confirm the viability of telitacicept and rituximab treatment in succession. Additionally, extended follow-up is required to assess this treatment method’s long-term efficacy and safety. Furthermore, this study did not evaluate changes in BLyS or APRIL levels in serum or CSF, which could yield important insights into the mechanism of action. Also, we did not test the serum and CSF using fixed tissue-based assay (TBA). Despite these limitations, our study offers a promising new treatment option for patients with refractory MNOS and provides a potential bridging therapy for B cell depletion in neuroimmunological diseases. The potential of telitacicept to substitute traditional B cell depletion therapies or its role in conjunction with other immunotherapies for enhanced efficacy is a critical issue that necessitates further research.
Conclusion
This exploration presents a clinically significant opportunity for refractory MNOS patients, potentially reducing medical costs and minimizing drug-related side effects. The safety and efficacy of this therapy will be further evaluated to extend its application to other neuroimmune diseases, including autoimmune encephalitis and NMOSD, through the implementation of exploratory case series or clinical investigations.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving humans were approved by Research Ethics Committee of the Shandong Provincial Hospital affiliated to Shandong First Medical University. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. The animal study was approved by Research Ethics Committee of the Shandong Provincial Hospital affiliated to Shandong First Medical University. The study was conducted in accordance with the local legislation and institutional requirements. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article. Written informed consent was obtained from the participant/patient(s) for the publication of this case report.
Author contributions
JZ: Data curation, Formal analysis, Methodology, Resources, Writing – original draft. MH: Data curation, Visualization, Writing – original draft. CW: Data curation, Supervision, Visualization, Writing – review & editing. SG: Resources, Supervision, Validation, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Banwell B, Bennett JL, Marignier R, Kim HJ, Brilot F, Flanagan EP, et al. Diagnosis of myelin oligodendrocyte glycoprotein antibody-associated disease: International MOGAD Panel proposed criteria. Lancet Neurol. (2023) 22:268–82. doi: 10.1016/S1474-4422(22)00431-8
2. Ren H, Fan S, Zhao Y, Guan H. The changing spectrum of antibody-mediated encephalitis in China. J Neuroimmunol. (2021) 361:577753. doi: 10.1016/j.jneuroim.2021.577753
3. Zhou J, Tan W, Tan SE, Hu J, Chen Z, Wang K. An unusual case of anti-MOG CNS demyelination with concomitant mild anti-NMDAR encephalitis. J Neuroimmunol. (2018) 320:107–10. doi: 10.1016/j.jneuroim.2018.03.019
4. Zhou L, ZhangBao J, Li H, Li X, Huang Y, Wang M, et al. Cerebral cortical encephalitis followed by recurrent CNS demyelination in a patient with concomitant anti-MOG and anti-NMDA receptor antibodies. Mult Scler Relat Disord. (2017) 18:90–2. doi: 10.1016/j.msard.2017.09.023
5. Sarigecili E, Cobanogullari MD, Komur M, Okuyaz C. A rare concurrence: Antibodies against Myelin Oligodendrocyte Glycoprotein and N-methyl-d-aspartate receptor in a child. Mult Scler Relat Disord. (2019) 28:101–3. doi: 10.1016/j.msard.2018.12.017
6. Fan S, Xu Y, Ren H, Guan H, Feng F, Gao X, et al. Comparison of myelin oligodendrocyte glycoprotein (MOG)-antibody disease and AQP4-IgG-positive neuromyelitis optica spectrum disorder (NMOSD) when they co-exist with anti-NMDA (N-methyl-D-aspartate) receptor encephalitis. Mult Scler Relat Disord. (2018) 20:144–52. doi: 10.1016/j.msard.2018.01.007
7. Ren Y, Chen X, He Q, Wang R, Lu W. Co-occurrence of anti-N-methyl-D-aspartate receptor encephalitis and anti-myelin oligodendrocyte glycoprotein inflammatory demyelinating diseases: A clinical phenomenon to be taken seriously. Front Neurol. (2019) 10:1271. doi: 10.3389/fneur.2019.01271
8. Titulaer MJ, Höftberger R, Iizuka T, Leypoldt F, McCracken L, Cellucci T, et al. Overlapping demyelinating syndromes and anti–N-methyl-D-aspartate receptor encephalitis. Ann Neurol. (2014) 75:411–28. doi: 10.1002/ana.24117
9. Zhang S, Yang Y, Liu W, Li Z, Li J, Zhou D. Clinical characteristics of anti-N-methyl-d-aspartate receptor encephalitis overlapping with demyelinating diseases: A review. Front Immunol. (2022) 13:857443. doi: 10.3389/fimmu.2022.857443
10. Dhillon S. Telitacicept: first approval. Drugs. (2021) 81:1671–5. doi: 10.1007/s40265-021-01591-1
11. Zhang Z, Wang Z, Du X, Huang X, Zhang Y. Refractory generalized myasthenia gravis treated successfully with telitacicept: two cases report. J Neurol. (2024) 271:584–8. doi: 10.1007/s00415-023-12036-y
12. Ding J, Jiang X, Cai Y, Pan S, Deng Y, Gao M, et al. Telitacicept following plasma exchange in the treatment of subjects with recurrent neuromyelitis optica spectrum disorders: A single-center, single-arm, open-label study. CNS Neurosci Ther. (2022) 28:1613–23. doi: 10.1111/cns.13904
13. Venkatesan A, Tunkel AR, Bloch KC, Lauring AS, Sejvar J, Bitnun A, et al. Case definitions, diagnostic algorithms, and priorities in encephalitis: consensus statement of the international encephalitis consortium. Clin Infect Dis. (2013) 57:1114–28. doi: 10.1093/cid/cit458
14. Graus F, Titulaer MJ, Balu R, Benseler S, Bien CG, Cellucci T, et al. A clinical approach to diagnosis of autoimmune encephalitis. Lancet Neurol. (2016) 15:391–404. doi: 10.1016/S1474-4422(15)00401-9
15. Guo Q, Huang Y, Wang F, Fang L. Case Report: Telitacicept in severe myasthenia gravis: a case study with multiple autoantibodies. Front Immunol. (2023) 14:1270011. doi: 10.3389/fimmu.2023.1270011
16. Ren Y, Chen S, Yang H. Case Report: Telitacicept in treating a patient with NF155+ autoimmune nodopathy: a successful attempt to manage recurrent elevated sero-anti-NF155 antibodies. Front Immunol. (2023) 14:1279808. doi: 10.3389/fimmu.2023.1279808
17. Tian M, Tang L. Good efficacy achieved by telitacicept, corticosteroids and immunosuppressants in the treatment of SLE combined with MOG-AD. Rheumatol Adv Pract. (2023) 7:rkad088. doi: 10.1093/rap/rkad088
18. Yin J, Zhao M, Xu X, Zhang M, Xu Z, Li Z, et al. A multicenter, randomized, open-label, phase 2 clinical study of telitacicept in adult patients with generalized myasthenia gravis. Eur J Neurol. (2024) 31:e16322. doi: 10.1111/ene.16322
19. Zhang C, Lin Y, Kuang Q, Li H, Jiang Q, Yang X. Case report: A highly active refractory myasthenia gravis with treatment of telitacicept combined with efgartigimod. Front Immunol. (2024) 15:1400459. doi: 10.3389/fimmu.2024.1400459
20. Lin J, Li Y, Gui M, Bu B, Li Z. Effectiveness and safety of telitacicept for refractory generalized myasthenia gravis: a retrospective study. Ther Adv Neurol Disord. (2024) 17:17562864241251476. doi: 10.1177/17562864241251476
21. Li F, Sui X, Pan X, Liu C, Xie L, Zhao H, et al. Neuromyelitis optica spectrum disorder with ultra-longitudinally extensive transverse myelitis: A case report and literature review. Heliyon. (2024) 10:e39687. doi: 10.1016/j.heliyon.2024.e39687
22. Gao H, Lin J, Yang M, Gui M, Ji S, Bu B, et al. Telitacicept add-on therapy in refractory idiopathic inflammatory myopathy: insights from a pilot study. Rheumatol Oxf Engl. (2024), keae601. doi: 10.1093/rheumatology/keae601
23. Wang J, Zheng H, Wei J, Wu J, Feng Z, Chen X, et al. Telitacicept in combination with B-cell depletion therapy in MuSK antibody-positive myasthenia gravis: a case report and literature review. Front Immunol. (2024) 15:1456822. doi: 10.3389/fimmu.2024.1456822
24. Kondo H, Takeuchi Y, Niwa J, Yoshida K, Takemura N, Hosoyama S, et al. Efficacy of steroid therapy in the acute stage of anti-NMDAR and anti-MOG antibody overlapping encephalitis: a case report and literature review. Front Immunol. (2024) 15:1392992. doi: 10.3389/fimmu.2024.1392992
25. Zhao C, Liu P, Zhao D, Ding J, Zhang G, Li H, et al. Coexistence of myelin oligodendrocyte glycoprotein immunoglobulin G and neuronal or glial antibodies in the central nervous system: A systematic review. Brain Sci. (2022) 12:995. doi: 10.3390/brainsci12080995
26. Wang M, Tan J, Zhou Z, Wang Y, Bako SY, Yang Y, et al. Relapsing MOG-IgG-associated diseases coexisting with anti-NMDAR encephalitis: a case report and literature review. J Integr Neurosci. (2022) 21:82. doi: 10.31083/j.jin2103082
27. Dalmau J, Lancaster E, Martinez-Hernandez E, Rosenfeld MR, Balice-Gordon R. Clinical experience and laboratory investigations in patients with anti-NMDAR encephalitis. Lancet Neurol. (2011) 10:63–74. doi: 10.1016/S1474-4422(10)70253-2
28. Ding J, Li X, Tian Z. Clinical features of coexisting anti-NMDAR and MOG antibody-associated encephalitis: A systematic review and meta-analysis. Front Neurol. (2021) 12:711376. doi: 10.3389/fneur.2021.711376
29. Kim HW, Lamb C, Jamali S, Lopez Chiriboga AS. Mystery Case: Anti-NMDAR encephalitis with overlapping demyelinating syndrome. Neurology. (2020) 94(17):e1866–9. doi: 10.1212/WNL.0000000000009320
30. Li S, Wang M, Li H, Wang J, Zhang Q, Zhou D, et al. Case report: overlapping syndrome of anti-NMDAR encephalitis and MOG inflammatory demyelinating disease in a patient with human herpesviruses 7 infection. Front Immunol. (2022) 13:799454. doi: 10.3389/fimmu.2022.799454
31. Yang JX, Yang MM, Han YJ, Gao CH, Cao J. FLAIR-hyperintense lesions in anti-MOG-associated encephalitis with seizures overlaying anti-N-methyl-D-aspartate receptor encephalitis: a case report and literature review. Front Immunol. (2023) 14:1149987. doi: 10.3389/fimmu.2023.1149987
32. Berek K, Grams A, Uprimny C, Prieschl M, Ramberger M, Unterberger I, et al. Anti-NMDA receptor encephalitis and MOG-associated demyelination – a case report with long-term follow-up and a systematic review. BMC Neurol. (2022) 22:434. doi: 10.1186/s12883-022-02974-x
33. Guzmán J, Vera F, Soler B, Uribe-San-Martin R, García L, Del-Canto A, et al. Myelin Oligodendrocyte Glycoprotein Antibody-Associated Disease (MOGAD) in Chile: lessons learned from challenging cases. Mult Scler Relat Disord. (2023) 69:104442. doi: 10.1016/j.msard.2022.104442
34. Staatz CE, Tett SE. Clinical pharmacokinetics and pharmacodynamics of mycophenolate in solid organ transplant recipients. Clin Pharmacokinet. (2007) 46:13–58. doi: 10.2165/00003088-200746010-00002
35. Dalakas MC. Immunotherapy in myasthenia gravis in the era of biologics. Nat Rev Neurol. (2019) 15:113–24. doi: 10.1038/s41582-018-0110-z
36. Smith MR. Rituximab (monoclonal anti-CD20 antibody): mechanisms of action and resistance. Oncogene. (2003) 22:7359–68. doi: 10.1038/sj.onc.1206939
37. Kim HJ, Kim SH, Xu J, Cheng Xx, Xu Jr. Responsiveness to reduced dosage of rituximab in Chinese patients with neuromyelitis optica. Neurology. (2014) 82:546–7. doi: 10.1212/01.wnl.0000444161.12981.ba
38. Nguyen DC, Joyner CJ, Sanz I, Lee FE-H. Factors affecting early antibody secreting cell maturation into long-lived plasma cells. Front Immunol. (2019) 10:2138. doi: 10.3389/fimmu.2019.02138.
39. Barr TA, Shen P, Brown S, Lampropoulou V, Roch T, Lawrie S, et al. B cell depletion therapy ameliorates autoimmune disease through ablation of IL-6–producing B cells. J Exp Med. (2012) 209:1001–10. doi: 10.1084/jem.20111675
40. Krumbholz M, Derfuss T, Hohlfeld R, Meinl E. B cells and antibodies in multiple sclerosis pathogenesis and therapy. Nat Rev Neurol. (2012) 8:613–23. doi: 10.1038/nrneurol.2012.203
41. Crickx E, Chappert P, Sokal A, Weller S, Azzaoui I, Vandenberghe A, et al. Rituximab-resistant splenic memory B cells and newly engaged naive B cells fuel relapses in patients with immune thrombocytopenia. Sci Transl Med. (2021) 13:eabc3961. doi: 10.1126/scitranslmed.abc3961
42. Bai Y, Li W, Yan C, Hou Y, Wang Q. Anti-rituximab antibodies in patients with refractory autoimmune nodopathy with anti-neurofascin-155 antibody. Front Immunol. (2023) 14:1121705. doi: 10.3389/fimmu.2023.1121705
43. Development of anti-rituximab antibodies in rituximab-treated patients: Related parameters & consequences - PMC. Available online at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9707678/.
44. Robinson JI, Md Yusof MY, Davies V, Wild D, Morgan M, Taylor JC, et al. Comprehensive genetic and functional analyses of Fc gamma receptors influence on response to rituximab therapy for autoimmunity. eBioMedicine. (2022) 86:104343. doi: 10.1016/j.ebiom.2022.104343
45. Shi F, Xue R, Zhou X, Shen P, Wang S, Yang Y. Telitacicept as a BLyS/APRIL dual inhibitor for autoimmune disease. Immunopharmacol Immunotoxicol. (2021) 43:666–73. doi: 10.1080/08923973.2021.1973493
46. Wang H, Wang K, Zhong X, Qiu W, Dai Y, Wu A, et al. Cerebrospinal fluid BAFF and APRIL levels in neuromyelitis optica and multiple sclerosis patients during relapse. J Clin Immunol. (2012) 32:1007–11. doi: 10.1007/s10875-012-9709-9
47. Deng B, Liu XN, Li X, Zhang X, Quan C, Chen XJ. Raised cerebrospinal fluid BAFF and APRIL levels in anti-N-methyl-d-aspartate receptor encephalitis: Correlation with clinical outcome. J Neuroimmunol. (2017) 305:84–91. doi: 10.1016/j.jneuroim.2017.01.012
48. Chen X, Zhao Q, Hou Y, Jiang J, Zhong W, Wang W, et al. Pharmacokinetics, pharmacodynamics, short term efficacy and safety of RCT-18, a novel BLyS/APRIL fusion protein, in patients with rheumatoid arthritis. Br J Clin Pharmacol. (2016) 82:41–52. doi: 10.1111/bcp.12908
49. Ehrenstein MR, Wing C. The BAFFling effects of rituximab in lupus: danger ahead? Nat Rev Rheumatol. (2016) 12:367–72. doi: 10.1038/nrrheum.2016.18
50. Md Yusof MY, Shaw D, El-Sherbiny YM, Dunn E, Rawstron AC, Emery P, et al. Predicting and managing primary and secondary non-response to rituximab using B-cell biomarkers in systemic lupus erythematosus. Ann Rheum Dis. (2017) 76:1829–36. doi: 10.1136/annrheumdis-2017-211191
51. Lin W, Zhang P, Chen H, Chen Y, Yang H, Zheng W, et al. Circulating plasmablasts/plasma cells: a potential biomarker for IgG4-related disease. Arthritis Res Ther. (2017) 19:25. doi: 10.1186/s13075-017-1231-2
52. Vinuesa CG, Sanz I, Cook MC. Dysregulation of germinal centres in autoimmune disease. Nat Rev Immunol. (2009) 9:845–57. doi: 10.1038/nri2637
53. Raina A, Yadav D, Krasinskas AM, McGrath KM, Khalid A, Sanders M, et al. Evaluation and management of autoimmune pancreatitis: experience at a large US center. Am J Gastroenterol. (2009) 104:2295–306. doi: 10.1038/ajg.2009.325
Keywords: NMDAR encephalitis (NMDARE), telitacicept, rituximab, treatment, MOGAD
Citation: Zhang J, Hu M, Wang C and Guo S (2025) Successful sequential therapy with rituximab and telitacicept in refractory Anti-NMDA receptor encephalitis and MOG-associated demyelination: a case report and literature review. Front. Immunol. 16:1509143. doi: 10.3389/fimmu.2025.1509143
Received: 10 October 2024; Accepted: 22 January 2025;
Published: 06 February 2025.
Edited by:
Alvino Bisecco, University of Campania Luigi Vanvitelli, ItalyReviewed by:
Stefano Masciocchi, Asst degli Spedali Civili di Brescia, ItalyMario Risi, University of Campania Luigi Vanvitelli, Italy
Copyright © 2025 Zhang, Hu, Wang and Guo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chunjuan Wang, amltMTAyMkAxMjYuY29t; Shougang Guo, Z3Vvc2hvdWdhbmcxMTI0QDE2My5jb20=
 Jingliang Zhang
Jingliang Zhang Minzhe Hu
Minzhe Hu Chunjuan Wang
Chunjuan Wang Shougang Guo
Shougang Guo