
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Immunol. , 04 February 2025
Sec. Cancer Immunity and Immunotherapy
Volume 16 - 2025 | https://doi.org/10.3389/fimmu.2025.1508293
This article is part of the Research Topic Advancements in Immune Heterogeneity in Inflammatory Diseases and Cancer: New Targets, Mechanisms, and Strategies View all 9 articles
The use of immune checkpoint inhibitors (ICIs) often develops immune-related adverse events (irAEs). However, irAEs-induced multi-organ injuries remain a rare event. We herein report a case of multi-organ injuries induced by tislelizumab in a lung squamous cell carcinoma (LUSC) patient. A 68-year-old man had undergone neoadjuvant chemotherapy with paclitaxel, carboplatin, and tislelizumab. He presented with a 1-month history of nausea and poor appetite after the second dose of therapy. During investigations, rhabdomyolysis, liver, kidney, and thyroid damage were detected. After multi-disciplinary consultation, multi-organ injuries related to ICIs (striated muscle, liver, kidney, and thyroid) were considered to result from cumulated irAEs induced by tislelizumab. The patient was treated with levothyroxine, methylprednisolone, intravenous immunoglobulins, and continuous renal replacement therapy. After treatment, the patient recovered and was discharged from the hospital. The patient presented with multiple organ damage, not single immunity treatment adverse reactions, relatively rare. In clinical work, irAEs are likely not a single-system organ disorder and many kinds of attention need to be combined with the risk of multi-system damage.
Recent studies have focused on immune checkpoint inhibitors (ICIs). In patients with unresectable tumors, ICIs are used as the first-line therapy in a variety of fields, which has contributed to substantially improved survival. The use of these ICIs often develops immune-related adverse events (irAEs), which are becoming essential safety issues worthy of attention despite the exciting therapeutic prospects. Multi-organ injuries associated with the use of ICIs are rare compared with other irAEs.
Tislelizumab, a humanized IgG4 anti-programmed cell death protein-1 (PD-1) monoclonal antibody, was launched in China in December 2019. It prevents autoimmune responses by promoting the apoptosis of antigen-specific T cells in lymph nodes while reducing the apoptosis of regulatory T cells (1). Tislelizumab, in combination with paclitaxel and carboplatin, was approved as the first-line treatment option in patients with advanced squamous non-small-cell lung cancer in China in 2021 (2). A few adverse reactions to tislelizumab have been reported. The common adverse reactions include fatigue, rash, hypothyroidism, and elevated aminotransferase. We herein report a case of tislelizumab-associated rhabdomyolysis, liver, kidney, and thyroid damage in a lung squamous cell carcinoma (LUSC) patient. We present this case in accordance with the CARE reporting checklist.
On evaluation at our institution, his body temperature was 36.5°C, with a pulse of 110 beats per minute, blood pressure of 98/72 mmHg, and a breath of 20 times/min. Hematology showed a hemoglobin level of 88 g/L, a white blood cell count of 7.06×109/L, 89.2% neutrophils, and 6.9% lymphocytes. Chest CT revealed a small mass (1.4 x 0.9 cm) in the anterior lobe of the left superior lung, bronchial wall truncation, and peripheral pleural stretch (Figure 1I).
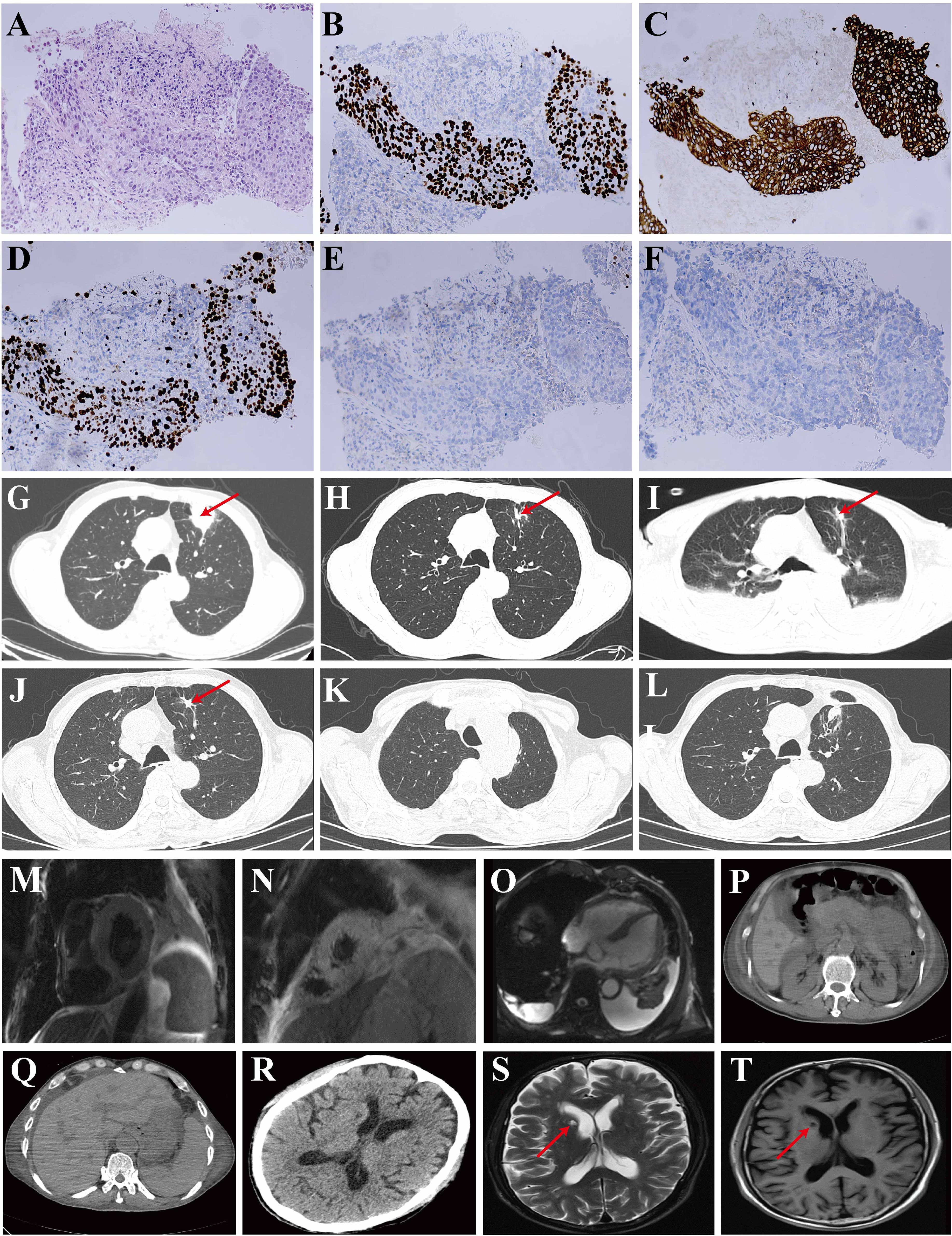
Figure 1. Pathological images of the lung biopsy and imageological examination. (A) Hematoxylin and Eosin (HE) staining; (B) P40 immunohistochemical staining; (C) Pan-cytokeratin (AE1/AE3) staining; (D) Ki-67 proliferation index showing expression levels up to 60% in tumor cells; (E) Thyroid Transcription Factor-1 (TTF-1) staining; (F) Napsin A staining. (G-L) Chest computed tomography (CT) scans: (G) Image acquired on December 5, 2023, revealing a soft tissue density shadow in the anterior segment of the left upper lobe, measuring approximately 3.4 x 3.1 cm (indicated by a red arrow); (H) Image acquired on February 20, 2024, demonstrating shrinkage of the soft tissue (indicated by a red arrow); (I) Image acquired on April 15, 2024, showing a small mass measuring 1.4 x 0.9 cm (indicated by a red arrow) in the anterior lobe of the left superior lung, with evidence of bronchial wall truncation, peripheral pleural stretch and bilateral pleural effusions; (J) Image acquired on July 9, 2024, pre-operatively; (K, L) Images acquired on August 27, 2024, post-operatively. (M-O) Cardiac MRI. (P, Q) Abdominal CT. (R) Head CT revealed encephalatrophy and demyelination in the white matter. (S, T) Head MRI. The brain MRI indicated old infarcts in the lateral ventricle, as denoted by the red arrow.
He had conjunctival icterus. Laboratory evaluation showed significantly deranged liver and renal function (Table 1). Evaluation for other causes was conducted. Pathogen investigations, including hepatitis A, B, C, and E, syphilis, human immunodeficiency virus, cytomegalovirus, Epstein-Barr virus serum, serum (1,3)-beta-D-glucan, serum galactomannan, and sputum culture were all negative. Additional autoimmune disease markers were also investigated. Antinuclear antibodies were 1:100 positive. Anti-neutrophil cytoplasmic antibody was within normal range. Urinalysis showed haematuria (24 red blood cells/high-power field) and proteinuria (1+). Renal ultrasound showed no issues. Abdominal CT indicated a slight decrease in liver density, with normal kidney results (Figures 1P, Q).
The muscle strength of the upper limbs was 3, and the lower limbs were 2. The ability of the patient to perform daily physical activities was limited, especially in the lower limbs. Serum levels of creatine kinase (CK) and lactate dehydrogenase (LDH) had increased (Table 1). Head CT revealed no new cerebral hemorrhage, and head magnetic resonance imaging (MRI) showed no new infarcts (Figures 1R-T). Rhabdomyolysis was considered based on the clinical manifestation above.
Moreover, cardiac damage parameters were altered with elevated levels of myohemoglobin, troponin-T, creatine kinase-myocardial band (CK-MB), and B-type natriuretic peptide precursor (BNP) (Table 1). An electrocardiogram (ECG) showed paroxysmal atrial fibrillation. Echocardiography showed a normal left ventricular ejection fraction. Cardiac MRI indicates cardiac enlargement and interventricular septal thickening, with no significant abnormalities in myocardial signal or apparent dysfunction in the ventricles (Figures 1M-O).
Thyroid function evaluation showed a concentration of thyroid-stimulating hormone 41.2 mIU/L [normal range 0.27-4.2], free triiodothyronine 1.47 pmol/L [normal range 3.60-7.50], free thyroxine 4.74 pmol/L [normal range 12-22], suggesting hypothyroidism.
After multi-disciplinary consultation, given the absence of underlying cardiovascular diseases, evidence of virus or other pathogen infections, and a history of ICIs administration before hospitalization, as well as multiple organ involvement simultaneously, multi-organ injuries related to ICIs (striated muscle, liver, kidney, and thyroid) were considered to result from cumulated irAEs induced by tislelizumab. Then the patient received methylprednisolone (80 mg daily for nine days, tapering gradually after two months), intravenous immunoglobulins (IVIG) (15 g daily for four days), and continuous renal replacement therapy (CRRT), with improvement leading to recovery, and was discharged from our hospital in May 2024 (Figure 2). On follow-up in the outpatient, he had a lung cancer resection at the local hospital in July 2024. Postoperative chest CT found no metastasis (Figure 1J, pre-operative; Figures 1K, L, one month postoperatively).
In patients with LUSC undergoing treatment with ICIs, the most frequently observed irAEs are asthenia (10%), decreased appetite (11-19%), dermatologic toxicity (4-28.6%), diarrhea or colitis (8-11.4%), fatigue (16-33%), nausea (8.6-15%), and pyrexia (5-14.3%) (3). Severe adverse events, classified as Grade 3 or higher, are rare (3). This case report described a LUSC patient with multi-organ injuries (striated muscle, liver, kidney, and thyroid) induced by tislelizumab. To our knowledge, this is the first report of tislelizumab-associated multi-organ injuries with rhabdomyolysis, liver, kidney, and thyroid damage. The patient also received paclitaxel and carboplatin chemotherapy. Common side effects of paclitaxel include hypersensitivity, myelosuppression, bradycardia, hypotension, peripheral neuropathy, muscle and joint pain, nausea, diarrhea, mouth sores, and hair loss (4). Carboplatin’s main dose-limiting side effects are myelosuppression (thrombocytopenia) and peripheral neurotoxicity (5). Paclitaxel and carboplatin rarely cause cholestatic liver injury. Paclitaxel can elevate serum aminotransferase levels in 7% to 26% of patients, but levels exceed 5 times the upper limit of normal (ULN) in only 2% of high-dose cases (6). Similar rates of alkaline phosphatase (ALP) elevation and occasional mild bilirubin increases are observed (6). Paclitaxel is not convincingly linked to delayed, idiosyncratic liver injury with jaundice (6). While up to one-third of patients on carboplatin may experience mild, temporary increases in serum aminotransferase levels, significant liver injury from carboplatin is very rare and not well characterized (7). Carboplatin and pemetrexed usually present a low to moderate risk of transaminitis, typically without causing substantial liver injury or jaundice (8). Diagnosing drug-induced liver injury (DILI) can be customized based on the liver injury pattern, using the R-value: serum alanine aminotransferase (ALT)/ULN divided by ALP/ULN (9). This categorizes the injury as hepatocellular (R > 5), mixed (R = 2-5), or cholestatic (R < 2). To our knowledge, only one case of a patient with esophageal and thoracic squamous cell carcinoma receiving radiotherapy and concurrent chemotherapy with paclitaxel and cisplatin has been reported to develop cholestatic DILI (10). Checkpoint inhibitor-induced liver injury (CHILI), occurring in up to 25% of patients, is primarily treated with steroids (11). Lina Hountondji et al. conducted a multicenter cohort study with 117 patients to describe the clinical patterns of CHILI (12). CHILI was categorized as cholestatic (36.8%), hepatocellular (38.5%), or mixed (24.8%), with cholestatic and hepatocellular patterns being the most common. Steroid treatment was given to 79.5% of patients. Kazuyuki Mizuno et al. found a cholestatic pattern in 58.6% (17/29) of CHILI cases (13). Our patient showed a cholestatic liver injury pattern, marked by high ALP levels (R < 2) and jaundice. Abdominal ultrasound and CT ruled out biliary obstruction, aligning more closely with ICI-induced liver injury. Recently, numerous guidelines have been developed for managing irAEs (11, 14–16). Treatment recommendations depend on the affected organ(s), CTCAE grade, and patient comorbidities. Immunosuppression, primarily with high-dose corticosteroids, is the cornerstone of treatment. For severe toxicities, guidelines advise tapering corticosteroids over 4-6 weeks, resulting in extended immunosuppression. Subsequently, treatment with methylprednisolone confirmed the cause, leading to near-complete liver recovery in a month. Kidney issues were evident through proteinuria, hematuria, and a rise in serum creatinine. High CK, LDH, and aspartate transaminase (AST)/ALT ratios, along with reduced muscle strength, indicated rhabdomyolysis. Rhabdomyolysis due to paclitaxel/carboplatin is rare, with only one reported case of myositis after paclitaxel-based chemotherapy in an HIV-negative Kaposi’s sarcoma patient (17). Other instances involve high doses of paclitaxel/ifosfamide/carboplatin/etoposide or carboplatin/etoposide/ifosfamide (18, 19). Our case involved standard doses of paclitaxel and carboplatin, making rhabdomyolysis unlikely. While rhabdomyolysis is associated with the development of acute kidney injury, the risk remains low in non-traumatic instances when serum CK levels are below 15,000-20,000 U/L (20). In this case, the patient exhibited an increase in CK levels, reaching up to 1,553 U/L, which may suggest a reduced likelihood of acute kidney injury secondary to rhabdomyolysis.
The adverse effects associated with statin therapy encompass cramps, myalgia, weakness, and, less frequently, rhabdomyolysis (21). The administration of statins in individuals with hypothyroidism poses substantial risks. It is probable that these mechanisms interact synergistically in hypothyroid patients receiving statins, increasing the likelihood of myopathy, particularly at elevated statin dosages (22). To date, there have been reports of rhabdomyolysis occurring in cases of undiagnosed hypothyroidism (20). These patients exhibited muscle symptoms within days to weeks following the initiation of statin therapy. The symptoms resolved upon the discontinuation of statins and the correction of hypothyroidism through thyroxine replacement (20). The normalization of CK levels after hypothyroidism treatment confirmed that hypothyroidism was the primary cause of rhabdomyolysis (20). In our case, the patients were prescribed atorvastatin calcium on November 2023, during which liver, renal, and thyroid functions remained stable. Following the administration of a second dose of tislelizumab on February 24, 2024, the patient developed hypothyroidism and was subsequently prescribed levothyroxine. In March 2024, the patient discontinued statin therapy due to abnormalities in liver function. By April 2024, the patient exhibited CK abnormalities. The persistence of abnormal CK levels, despite more than two months of levothyroxine treatment and over a month since the discontinuation of statins, indicated that statins were not the causative factor for rhabdomyolysis in this hypothyroid patient.
Among irAEs, ICIs-induced cardiovascular toxicity remains a significant concern. Cardiovascular toxicities can present as myocarditis, complete heart block, atrial fibrillation, ventricular arrhythmia, and heart failure (23). Diagnosing ICIs-related myocarditis is complex, requiring a thorough evaluation of clinical symptoms and test results. Diagnosis primarily relies on cardiac biomarkers (such as troponin, BNP, ECG, echocardiography, cardiac MRI, and, when necessary, myocardial biopsy (24). As a gold standard for diagnosis, myocardial biopsy is limited due to its invasive nature. Despite elevated myohemoglobin, troponin-T, CK-MB, and BNP levels, our case report debates the extent of cardiac involvement. A CK-MB to CK ratio exceeding 6% is considered specific for myocardial injury, whereas a ratio below 6% is indicative of skeletal muscle damage or non-cardiac etiologies (25). In the present case, the CK-MB to CK ratio was observed to be less than 2%, suggesting that the elevation in CK-MB is likely secondary to rhabdomyolysis. Furthermore, troponin-T levels also appear to be elevated in a nonspecific manner. The elevation in BNP is potentially attributable to atrial fibrillation. Additionally, it is noteworthy that BNP levels may also be elevated in numerous cancer patients due to inflammation associated with the malignancy itself (24). Given the patient’s compromised general condition, a cardiac MRI was performed on the 16th day post-admission, following more than 11 days of steroid therapy. The administration of steroids may have partially influenced the myocardial damage detected by the MRI. Therefore, myocardial involvement was not well defined.
In this case, the patient initially presented with hyperthyroidism, which subsequently progressed to persistent hypothyroidism. Endocrine events are among the most prevalent toxicities associated with ICIs, affecting up to 40% of patients undergoing treatment (26). The thyroid gland is the most frequently affected endocrine organ by ICIs, typically presenting as hypothyroidism, which may be preceded by transient thyrotoxicosis due to thyroiditis (26). In certain cases, patients may develop persistent primary hypothyroidism (27). The underlying pathophysiological mechanism is believed to involve immune-mediated acute inflammation, leading to the destruction of the thyroid gland (28). The activation of T cells, along with the involvement of various antibodies and cytokines, plays a crucial role in both the initiation and progression of the disease (28). The patient had abnormal thyroid function in the early stage. Thyroid damage may serve as an indicator of potential multi-organ injuries, necessitating heightened vigilance among physicians for complications in other organs both at the time of diagnosis and during follow-up.
ICIs have transformed the treatment landscape for a variety of solid tumors and hematological malignancies, thereby increasing the benefits for more cancer patients. However, an increasing number of adverse reactions caused by ICIs have also been reported. ICIs can induce irAEs, and up to 70% of patients undergoing ICIs therapy can have irAEs (29). These toxic effects are thought to be mediated by autoreactive T cells no longer kept in check by feedback mechanisms. Limited data are available to estimate the overall multi-organ injury incidence; two independent analyses described multi-organ irAEs occurring at a rate of roughly 5%, and a separate study found an incidence of 9% (14). They are generally manageable with high-dose glucocorticoids but can be fatal in some cases (30).
The multi-organ injuries of irAEs events in available case reports are shown in Table 2 (30–40). All the patients with multiple organ injuries are male. The patient’s age ranged from 33 to 75 years, with an average age of 64 years old. Most complications occur within the first or second cycles of ICIs exposure. They are generally manageable with glucocorticoids and IVIG. As the first treatment, most of the patients were administered high-dose methylprednisolone (approximately 1-2 mg/kg/day). To our knowledge, only 5 cases of tislelizumab-induced multi-organ injuries have been reported to date (Table 2) (34–37, 39). Two are generally manageable with methylprednisolone, and the remaining three are manageable with methylprednisolone plus IVIG. Though the Society for Immunotherapy of Cancer (SITC) consensus definitions for irAEs terminology have been established for managing and treating individual organ irAEs, more experience should be needed in treating multiple-organs irAEs (14). In our case, the patient had multi-organ injuries after two cycles of tislelizumab. He also received methylprednisolone and IVIG, which is similar to that of previous reports. Therefore, effective management of severe irAEs from tislelizumab, including multi-organ injuries, can be partly achieved through methylprednisolone and immunoglobulins. Cases 5 and 6 described liver damage with only increased serum ALT and AST (34, 35). In contrast, our patient had severe hepatic damage with an increase of total bilirubin more than 14.5 times higher than the upper limit of normal. To our knowledge, such severe hepatic damage has not been reported. Case 6 reported kidney damage, characterized by an increase in creatinine levels up to 136 μmol/L. In contrast, our patient exhibited a creatinine increase to 432 μmol/L and required CRRT, indicating a more severe condition than that observed in Case 6. For instances of Grade 3 or 4 toxicity, defined as creatinine levels exceeding three times the baseline or greater than 4.0 mg/dL, inpatient hemodialysis should be considered, and steroid treatment initiated at a dosage of 1-2 mg/kg/day. Our patient experienced creatinine levels exceeding four times the baseline and underwent CRRT; such severe renal damage is uncommon.
All patients recovered except for two patients who died (Table 2). The correlation between multi-organ irAEs and survival outcomes has been studied. A multi-center study revealed that patients experiencing ICIs-associated myocarditis exhibited multi-organ irAEs, with a notably higher incidence of severe myocarditis, increased mortality, and poorer prognosis compared to patients with pure myocarditis (41). However, little is known about the specific prognosis of co-occurrence patterns of irAEs. Recently, Guihong Wan et al. conducted a retrospective multicohort study about the co-occurrence patterns of and survival outcomes after multi-organ irAEs among recipients of ICIs (42). Co-occurring irAEs were decomposed into seven factors across organs, including endocrine, cutaneous, respiratory, gastrointestinal, hepatic, musculoskeletal, and neurological. It found that patients dominated by endocrine and cutaneous irAEs were associated with improved survival at the 6-month landmark time point. At the same time, the other clusters either had unfavorable (respiratory) or neutral survival outcomes (gastrointestinal, musculoskeletal, hepatic, and neurological). These findings contribute to a deeper understanding of the potential biological mechanisms underlying irAEs across various organs.
As the clinical use of immunotherapy continues to increase, understanding and managing its unique toxicity becomes critical. The most urgent problem in clinical practice is minimizing the problems caused by irAEs while maximizing the therapeutic benefit of ICIs. It is also important to realize that more patients with irAEs of the treatment with ICIs will be admitted; multi-disciplinary consultation is essential because of the difficulty of early recognition and optimal treatment of these possible lethal side effects. Clinicians should fully understand the diversity and severity of adverse reactions to immunotherapy drugs, improve the ability to make early diagnoses and treatment, and pay attention to medication details so that these drugs can play a better role and bring more clinical benefits to patients.
In conclusion, ICI treatment may be a “double-edged sword.” Our patient presented with multiple organ damage, not single immunity treatment adverse reactions, relatively rare. In clinical work, irAEs are likely not a single-system organ disorder and many kinds of attention need to be combined with the risk of multi-system damage. More clinical data are still required to feature tislelizumab.
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
The studies involving humans were approved by the Ethics Committee of the West China Hospital of Sichuan University (approval number: 2024-573). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
MY: Data curation, Writing – original draft. NH: Writing – review & editing. LS: Writing – review & editing. LY: Writing – review & editing. HT: Writing – review & editing.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This research was funded by the National Key Research and Development Program of China (No. 2022YFC2304800), the 1.3.5 project for disciplines of excellence, West China Hospital, Sichuan University (No. ZYGD23030), and the Science and Technology project of the Health Commission of Sichuan Province (No. 23LCYJ027).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be constructed as a potential conflict of interest.
The reviewer WY declared a shared affiliation, with no collaboration, with the authors, MY, NH, LY, HT, to the handling editor at the time of the review.
The author(s) declare that no Generative AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Song Y, Gao Q, Zhang H, Fan L, Zhou J, Zou D, et al. Treatment of relapsed or refractory classical Hodgkin lymphoma with the anti-PD-1, tislelizumab: results of a phase 2, single-arm, multicenter study. Leukemia. (2020) 34:533–42. doi: 10.1038/s41375-019-0545-2
2. Wang J, Lu S, Yu X, Hu Y, Sun Y, Wang Z, et al. Tislelizumab plus chemotherapy vs chemotherapy alone as first-line treatment for advanced squamous non–small-cell lung cancer. JAMA Oncol. (2021) 7:709–17. doi: 10.1001/jamaoncol.2021.0366
3. Kujtan L, Kancha RK, Gustafson B, Douglass L, Ward CR, Buzard B, et al. Squamous cell carcinoma of the lung: improving the detection and management of immune-related adverse events. Expert Rev Anticancer Ther. (2022) 22:203–13. doi: 10.1080/14737140.2022.2029414
4. Marupudi NI, Han JE, Li KW, Renard VM, Tyler BM, Brem H. Paclitaxel: a review of adverse toxicities and novel delivery strategies. Expert Opin Drug Saf. (2007) 6:609–21. doi: 10.1517/14740338.6.5.609
5. Oun R, Moussa YE, Wheate NJ. The side effects of platinum-based chemotherapy drugs: a review for chemists. Dalton Trans. (2018) 47:6645–53. doi: 10.1039/c8dt90088d
6. National Institute of Diabetes and Digestive and Kidney Diseases. Paclitaxel, in: LiverTox: Clinical and Research Information on Drug-Induced Liver Injury (2012). Available online at: https://www.ncbi.nlm.nih.gov/books/NBK548093 (Accessed 7 Sep, 2020).
7. National Institute of Diabetes and Digestive and Kidney Diseases. Carboplatin, in: LiverTox: Clinical and Research Information on Drug-Induced Liver Injury (2012). Available online at: https://www.ncbi.nlm.nih.gov/books/NBK548565 (Accessed 15 Sep, 2020).
8. Cancer Research UK. Pemetrexed and carboplatin (2024). Available online at: https://www.cancerresearchuk.org/about-cancer/treatment/drugs/pemetrexed-carboplatin (Accessed 15 Jan, 2024).
9. Chalasani NP, Maddur H, Russo MW, Wong RJ, Reddy KR. ACG clinical guideline: diagnosis and management of idiosyncratic drug-induced liver injury. Am J Gastroenterol. (2021) 116:878–98. doi: 10.14309/ajg.0000000000001259
10. Qiu G, Su Y-j, Wang M-m, Yan Y-d. A case of cholestatic drug-induced liver injury caused by chemotherapy with paclitaxel and cisplatin. Chin J Drug Appl Monit. (2019) 16:124–6.
11. Haanen J, Obeid M, Spain L, Carbonnel F, Wang Y, Robert C, et al. Management of toxicities from immunotherapy: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann Oncol. (2022) 33:1217–38. doi: 10.1016/j.annonc.2022.10.001
12. Hountondji L, Ferreira De Matos C, Lebossé F, Quantin X, Lesage C, Palassin P, et al. Clinical pattern of checkpoint inhibitor-induced liver injury in a multicentre cohort. JHEP Rep. (2023) 7;5:100719. doi: 10.1016/j.jhepr.2023.100719
13. Mizuno K, Ito T, Ishigami M, Ishizu Y, Kuzuya T, Honda T, et al. Real world data of liver injury induced by immune checkpoint inhibitors in Japanese patients with advanced Malignancies. J Gastroenterol. (2020) 55:653–61. doi: 10.1007/s00535-020-01677-9
14. Naidoo J, Murphy C, Atkins MB, Brahmer JR, Champiat S, Feltquate D, et al. Society for Immunotherapy of Cancer (SITC) consensus definitions for immune checkpoint inhibitor-associated immune-related adverse events (irAEs) terminology. J Immunother Cancer. (2023) 11:e006398. doi: 10.1136/jitc-2022-006398
15. Schneider BJ, Naidoo J, Santomasso BD, Lacchetti C, Adkins S, Anadkat M, et al. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: ASCO guideline update. J Clin Oncol. (2021) 39:4073–126. doi: 10.1200/JCO.21.01440
16. Thompson JA, Schneider BJ, Brahmer J, Zaid MA, Achufusi A, Armand P, et al. NCCN guidelines® Insights: management of immunotherapy-related toxicities, version 2.2024. J Natl Compr Canc Netw. (2024) 22:582–92. doi: 10.6004/jnccn.2024.0057
17. Fardet L, Stoebner PE, Bachelez H, Descamps V, Kerob D, Meunier L, et al. Treatment with taxanes of refractory or life-threatening Kaposi sarcoma not associated with human immunodeficiency virus infection. Cancer. (2006) 106:1785–9. doi: 10.1002/cncr.21791
18. Sokolova A, Chan O, Ullah W, Hamdani AA, Anwer F. Delayed rhabdomyolysis with paclitaxel, ifosfamide, carboplatin, and etoposide regimen: a case report. J Med Case Rep. (2017) 11:100. doi: 10.1186/s13256-017-1272-9
19. Miki T, Mizutani Y, Akaza H, Ozono S, Tsukamoto T, Terachi T, et al. Long-term results of first-line sequential high-dose carboplatin, etoposide and ifosfamide chemotherapy with peripheral blood stem cell support for patients with advanced testicular germ cell tumor. Int J Urol. (2007) 14:54–9. doi: 10.1111/j.1442-2042.2006.01655.x
20. Chiang W-F, Chan J-S, Hsiao P-J, Lin S-H. Case report: Rhabdomyolysis and kidney injury in a statin-treated hypothyroid patient–kill two birds with one stone. Front Med. (2022) 9:1046330. doi: 10.3389/fmed.2022.1046330
21. Safitri N, Alaina MF, Pitaloka DAE, Abdulah R. A narrative review of statin-induced rhabdomyolysis: molecular mechanism, risk factors, and management. Drug Healthc Patient Saf. (2021) 13:211–9. doi: 10.2147/dhps.s333738
22. Renteria M, Jilani M, Brockman MJ, Davis HE. Inflammatory myositis following statin use in a patient with untreated hypothyroidism. Cureus. (2023) 15:e48463. doi: 10.7759/cureus.48463
23. Nishikawa T, Kunimasa K, Ohta-Ogo K, Ikeda Y, Yasui T, Shioyama W, et al. Sinus node dysfunction co-occurring with immune checkpoint inhibitor-associated myocarditis. Intern Med. (2022) 61:2161–5. doi: 10.2169/internalmedicine.8575-21
24. Pi JK, Chen XT, Zhang YJ, Chen XM, Wang YC, Xu JY, et al. Insight of immune checkpoint inhibitor related myocarditis. Int Immunopharmacol. (2024) 143:113559. doi: 10.1016/j.intimp.2024.113559
25. Al-Hadi HA, Fox KA. Cardiac markers in the early diagnosis and management of patients with acute coronary syndrome. Sultan Qaboos Univ Med J. (2009) 9:231–46.
26. Wright JJ, Powers AC, Johnson DB. Endocrine toxicities of immune checkpoint inhibitors. Nat Rev Endocrinol. (2021) 17:389–99. doi: 10.1038/s41574-021-00484-3
27. Ohara N, Kobayashi M, Ohashi K, Ito R, Ikeda Y, Kawaguchi G, et al. Isolated adrenocorticotropic hormone deficiency and thyroiditis associated with nivolumab therapy in a patient with advanced lung adenocarcinoma: a case report and review of the literature. J Med Case Rep. (2019) 13:88. doi: 10.1186/s13256-019-2002-2
28. Karaviti D, Kani ER, Karaviti E, Gerontiti E, Michalopoulou O, Stefanaki K, et al. Thyroid disorders induced by immune checkpoint inhibitors. Endocrine. (2024) 85:67–79. doi: 10.1007/s12020-024-03718-2
29. Martins F, Sofiya L, Sykiotis GP, Lamine F, Maillard M, Fraga M, et al. Adverse effects of immune-checkpoint inhibitors: epidemiology, management and surveillance. Nat Rev Clin Oncol. (2019) 16:563–80. doi: 10.1038/s41571-019-0218-0
30. Charles J, Giovannini D, Terzi N, Schwebel C, Sturm N, Masson D, et al. Multi-organ failure induced by Nivolumab in the context of allo-stem cell transplantation. Exp Hematol Oncol. (2019) 8:8. doi: 10.1186/s40164-019-0132-2
31. Shah N, Jacob J, Househ Z, Shiner E, Baird L, Soudy H. Unchecked immunity: a unique case of sequential immune-related adverse events with Pembrolizumab. J Immunother Cancer. (2019) 7:247. doi: 10.1186/s40425-019-0727-5
32. Matull J, Livingstone E, Wetter A, Zimmer L, Zaremba A, Lahner H, et al. Durable complete response in a melanoma patient with unknown primary, associated with sequential and severe multi-organ toxicity after a single dose of CTLA-4 plus PD-1 blockade: A case report. Front Oncol. (2020) 10:592609. doi: 10.3389/fonc.2020.592609
33. Mintjens-Jager EMW, Vos ME, Kats-Ugurlu G, Hospers GAP, Rutgers A, van Meurs M. Severe mesenteric ischemia with multiple organ failure in a patient previously treated with a humanized monoclonal antibody against programmed death receptor-1 (pembrolizumab), a case of pembrolizumab associated catastrophic antiphospholipid syndrome? SAGE Open Med Case Rep. (2020) 8:2050313X20972225. doi: 10.1177/2050313x20972225
34. Deng C, Yang M, Jiang H, Wang R, Yang Z, Sun H, et al. Immune-related multiple-organs injuries following ICI treatment with tislelizumab in an advanced non-small cell lung cancer patient: A case report. Front Oncol. (2021) 11:664809. doi: 10.3389/fonc.2021.664809
35. Hu X, Wei Y, Shuai X. Case report: glucocorticoid effect observation in a ureteral urothelial cancer patient with ICI-associated myocarditis and multiple organ injuries. Front Immunol. (2021) 12:799077. doi: 10.3389/fimmu.2021.799077
36. Wang S, Peng D, Zhu H, Min W, Xue M, Wu R, et al. Acetylcholine receptor binding antibody–associated myasthenia gravis, myocarditis, and rhabdomyolysis induced by tislelizumab in a patient with colon cancer: A case report and literature review. Front Oncol. (2022) 12:1053370. doi: 10.3389/fonc.2022.1053370
37. Zhou Y, Xue H, Lu C, Zhang Y, Wu Q, Zhang J, et al. Treatment of tislelizumab-induced toxic epidermal necrolysis and agranulocytosis: A case report and literature review. Curr Drug Saf. (2024). doi: 10.2174/0115748863297885240604111018
38. Brazel D, Lee S, Mahadevan A, Warnecke B, Parajuli R. Multiorgan failure from nivolumab and ipilimumab: A case report and literature review. Cureus. (2023) 15:e41781. doi: 10.7759/cureus.41781
39. Deng Y, Huang M, Deng R, Wang J. Immune checkpoint inhibitor-related adrenal hypofunction and Psoriasisby induced by tislelizumab: A case report and review of literature. Medicine. (2024) 103:e37562. doi: 10.1097/md.0000000000037562
40. Squicciarini T, Villani R, Apollonio B, Fucci L, Zambetti M, Rossini M, et al. Case report: Is severe toxicity the price to pay for high sensitivity to checkpoint inhibitors immunotherapy in desmoplastic melanoma? Front Immunol. (2024) 15:1369531. doi: 10.3389/fimmu.2024.1369531
41. Xie X, Wang L, Li Y, Xu Y, Wu J, Lin X, et al. Multi-organ immune-related adverse event is a risk factor of immune checkpoint inhibitor-associated myocarditis in cancer patients: A multi-center study. Front Immunol. (2022) 13:879900. doi: 10.3389/fimmu.2022.879900
Keywords: lung squamous cell carcinoma, immune-related adverse events, immune checkpoint inhibitors, tislelizumab, multi-organ injuries, case report
Citation: Yuan M, Han N, Shu L, Yan L and Tang H (2025) Case report: Multi-organ injuries induced by tislelizumab. Front. Immunol. 16:1508293. doi: 10.3389/fimmu.2025.1508293
Received: 09 October 2024; Accepted: 13 January 2025;
Published: 04 February 2025.
Edited by:
Yongqiang Zhang, Henan Academy of Innovations in Medical Science, ChinaReviewed by:
Alexandre O. Gérard, Centre Hospitalier Universitaire de Nice, FranceCopyright © 2025 Yuan, Han, Shu, Yan and Tang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Libo Yan, eWFubGlib0B3Y2hzY3UuY24=; Hong Tang, dGFuZ2hvbmc2MTk4QHdjaHNjdS5jbg==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.