
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Immunol. , 13 February 2025
Sec. Nutritional Immunology
Volume 16 - 2025 | https://doi.org/10.3389/fimmu.2025.1507080
 Sujuan Ding1,2†
Sujuan Ding1,2† Ting Ye1,2†
Ting Ye1,2† Md. Abul Kalam Azad1,3
Md. Abul Kalam Azad1,3 Qian Zhu1,3
Qian Zhu1,3 Yating Liu1,3
Yating Liu1,3 Bie Tan2
Bie Tan2 Xiangfeng Kong1,2,3*
Xiangfeng Kong1,2,3*Maternal nutrition is one of the main factors regulating the growth and immunity of piglets. This study aimed to investigate the effects of maternal or maternal-offspring supplementation of antibiotics, probiotics, and synbiotics on the immunity of offspring (21, 65, and 125 day-old) in Bama mini-pigs. The results showed that adding antibiotics to maternal diets increased the plasma IFN-γ level of offspring piglets at 21 day-old. Compared with maternal supplementation, maternal-offspring supplementation of antibiotics decreased the IL-10 level in the spleen, probiotics decreased IL-2, IL-10, and TNF-α levels in the ileum, and synbiotics decreased IL-10 and IFN-γ levels in the ileum of offspring piglets. Moreover, maternal-offspring antibiotics supplementation increased the IL-1β level in the ileum, while probiotics supplementation increased the IL-1β level in the spleen of offspring piglets. Maternal antibiotics supplementation increased the TNF-α level in the ileum at 95 day-old compared with maternal probiotics and synbiotics supplementation. Maternal-offspring antibiotics supplementation increased the IL-1β level in the ileum compared with the probiotics supplementation, while synbiotics supplementation increased the IL-6 level in the ileum than the probiotics and antibiotics supplementation at 95 day-old. Moreover, maternal-offspring probiotics supplementation increased the IL-1β level in the spleen of offspring pigs, which was higher than the maternal probiotics supplementation. These findings suggest that the immune function of the offspring piglets varied depending on the specific approach used for probiotics and synbiotics supplementation.
The immature immune system and different common stressors at birth and weaning transition, including physiological, nutritional, and environmental factors, increase the risk of infection of piglets, which can seriously lead to intestinal and respiratory diseases, and even death (1). The nutritional and physiological status of sows during pregnancy and lactation is directly associated with the development and disease resistance of fetuses and neonates (2). During pregnancy and lactation, maternal nutrient intake shapes the development of the fetal immune system (3). In mammals, almost all nutrients, respiratory gases, excretory products, and exogenous drugs are transported through the umbilical cord during the development of the fetus (4). After birth, the foremost function of suckling is to support the essential nutrients of newborns. Piglets are born without accumulated brown fat, while breast milk can provide energy promptly (2). At the same time, colostrum also provides bioactive molecules such as immunoglobulins for piglets (5). Although maternal immunoglobulins cannot be transported through the placental barrier (6), these immunoglobulins are transferred to piglets through colostrum (7). In addition, maternal diets influence the maturation of the neonatal immune system by regulating the composition of colostrum and milk (8). Therefore, maternal nutrition is crucial for developing the immune function of offspring.
Probiotics are defined as “a living microbe that, when added in an adequate amount, is beneficial to the health of the host” and are considered an economically safe alternative to antibiotics to improve the host’s health (9). It is well-established that probiotics regulate the host’s immune system by stimulating immune functions and inhibiting pathogens. Research evidence indicated that probiotics can protect the host from different infectious and non-communicable diseases through their colonization and inhibition of pathogens (10). For instance, Lactobacillus salivarius has been found to improve immunomodulatory function by enhancing survival in the ileum through the competitive advantage conferred by its bacteriocins (11). Synbiotics, as a mixture of probiotics and prebiotics, enhance the survival and colonization of active microbial components in the gut by selectively stimulating growth and/or activating the metabolism of one or a limited number of health-promoting bacteria, thereby having beneficial effects on the host health (12). Currently, the primary purpose of using probiotics and synbiotics in the pig industry is to improve the overall health status of animals (13). However, when selecting dietary supplementation with probiotics, it is necessary to evaluate their potential beneficial effects and related metabolism. Moreover, determining synbiotics formulas is a complex and challenging task involving animal breed and growth stages.
China has rich resources of indigenous pig breeds that are highly adaptable to the environment, and their high-quality meat has attracted the interest of consumers. However, the current production management for indigenous pig breeds is not well-developed, which may be one of the most important reasons why the consumer acceptance of indigenous pig breeds is not widely established. The Bama mini-pig is a domestic pig breed in Bama County, Guangxi, China, which is a unique breed with long-term reproductive success (14). Bama mini-pigs have advantageous characteristics with red muscle, white fat, and delicious taste; however, the immune dysfunction and slow growth rate restrict the overall productivity of Bama mini-pigs (15). Thus, we hypothesized that maternal-offspring supplementation of probiotics and synbiotics might have positive regulatory effects on the immune function of offspring in Bama mini-pigs. Therefore, the present study was designed to evaluate the effects of maternal or maternal-offspring supplementation of probiotics and synbiotics on the immunity of offspring piglets.
Sixty-four Bama mini-pigs within their 3rd-5th parity were randomly allocated into four groups, and each group consisted of 16 replicates. Sows were fed either a basal diet (CON group) or a basal diet supplemented with 50 g/t·feed virginiamycin (ANT group), 200 mL/d·head probiotic culture solution (PRO group), and 500 g/t·diet xylo-oligosaccharide and 200 mL/d·head probiotic culture solution (SYN group), respectively. The feed additives were added to sow diets throughout pregnancy (three days after mating) and lactation periods. The probiotic fermentation liquid was provided by Hunan Lifeng Bio-technology Co., Ltd. (Changsha, China), containing Lactobacillus plantarum ≥ 1×108 CFU/mL and Saccharomyces cerevisiae ≥ 0.2×108 CFU/mL. Xylo-oligosaccharides (XOS, including xylobiose, xylotriose, and xylotetrose; >35%) was provided by Shandong Longlive Bio-technology Co., Ltd. (Shandong, China).
According to the management standards of the pig farm, sows were fed at 8:00 and 17:00 daily, with free access to drinking water. The feeding amount for each sow during pregnancy was 0.8 kg/d for 1−15 days, 1.0 kg/d for 16−30 days, 1.2 kg/d for 31−75 days, 1.5 kg/d for 76−90 days, and 2.0 kg/d for 91−110 days kg/d. The sows were fed 1.0 kg/d for one week before parturition, ad libitum three days after parturition, and 2.4 kg/d from delivery to weaning. After weaning (28 day-old), two piglets (one male and one female) close to the average body weight of the litter were selected from each litter for subsequent feeding experiments. Offspring piglets originated from the same treatment; two piglets from each litter and a total of four piglets from two litters were merged into one pen; each pen consisted of four piglets, and each group had eight pens.
Offspring piglets from the same maternal treatment group were randomly allocated into two treatments, including maternal supplementation and maternal-offspring supplementation. The feed additives supplementation levels for offspring piglets were as follows: 30 mL/d·head probiotics for PRO group and 250 g/t·feed XOS + 30 mL/d·head probiotics mixture for SYN group during 35−95 day-old; 60 mL/d·head probiotics for PRO group and 250 g/t·feed XOS + 60 mL/d·head probiotics mixture for SYN group during 96−125 day-old; 40 g/t·feed virginiamycin for ANT group during 35−125 day-old.
The composition and nutrient levels of the basal diet for sows and offspring piglets are presented in Tables 1 and 2. All pigs were raised in the same environment with the same feeding and management conditions. Breeding and management were carried out according to the standards of commercial pig farms.
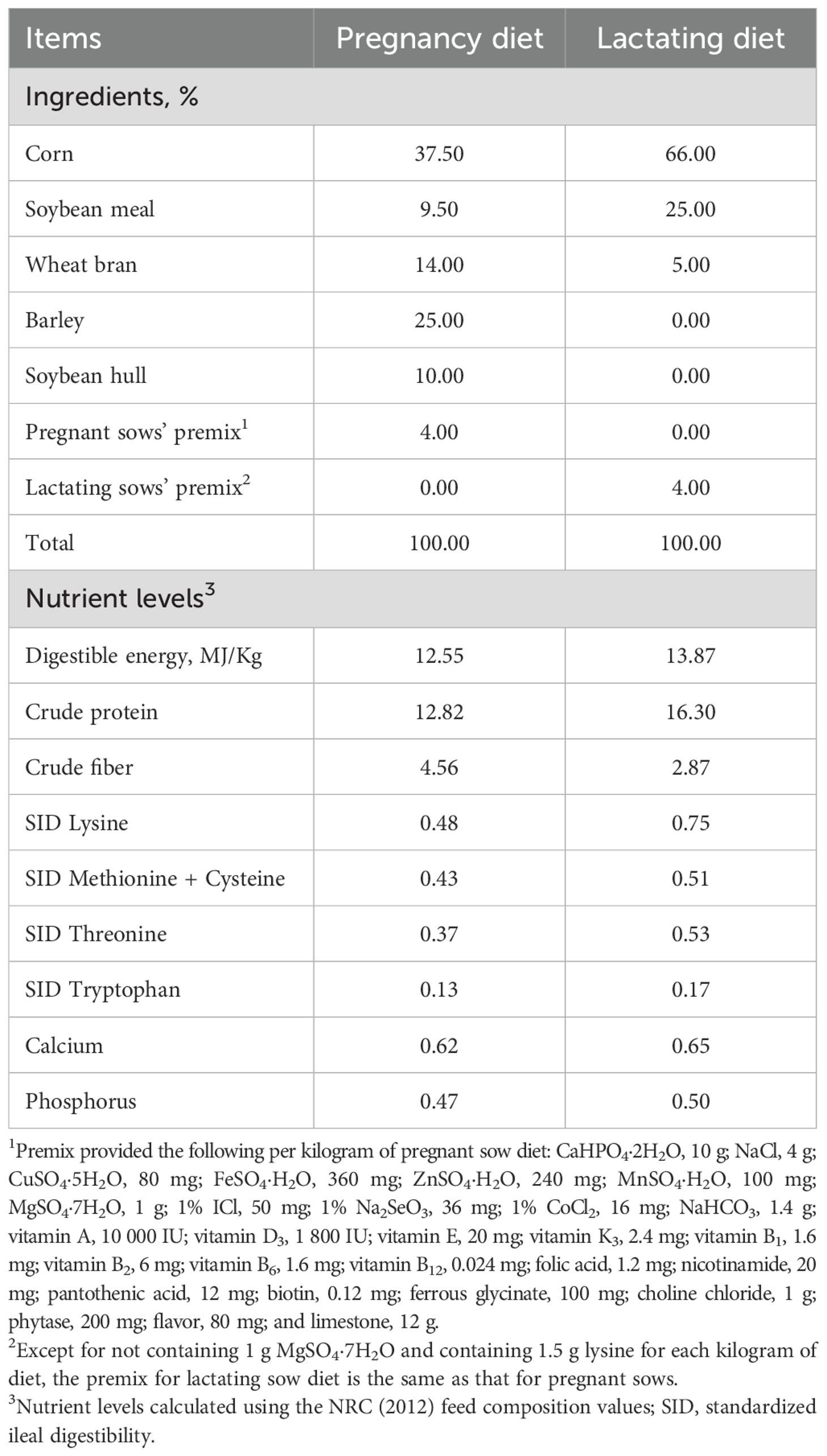
Table 1. Composition and nutrient levels of basal diets for sows during pregnancy and lactation (air-dried; %).
One piglet from each pen and eight piglets from each group at 21 day-old, 95 day-old (two months after weaning), and 125 day-old (three months after weaning) were selected for sampling. The body weight was measured after 12 h fasting. Blood samples (six piglets from each group) were collected into heparin anticoagulant tubes from the anterior vena cava, centrifuged at 4°C and 3500 × g for 10 min to obtain the plasma, and immediately stored at −80°C for immunocytokine assays. Experimental pigs were anesthetized by injection of Zoletil@ 50 (1 ml, 0.5 mg/kg; Beijing Lab Anim Tech Develop Co., Ltd., Beijing, China) at 21 day-old and the pigs at 95 and 125 day-old were euthanized using electrical stunning (120 V, 200 HZ) and exsanguination. After dissection, 0.5 g of spleen and liver tissues were collected from the same location, and the posterior end of the ileum (approximately 2 cm in length) was immediately removed and washed with phosphate buffer saline to remove the intestinal contents. Tissue samples were immediately preserved at −80°C for immunocytokine and gene expression analyses.
The immunoglobulin and immunocytokine levels in plasma, spleen, and ileum were determined with enzyme-linked immunosorbent assay (ELISA) kits. The testing protocols were followed as provided by the manufacturer’s instructions (Shanghai Kexing Trading Co., Ltd., Shanghai, China). The absorbance values (OD at 450 nm) were read on a microplate reader (Infinite M200 PRO, TECAN, Männedorf, Switzerland). The immunoglobulin and immunocytokine levels in plasma were expressed as the unit of volume. The total protein level in the spleen and ileum was measured using the BCA kit (Beyotime, Shanghai, China), and the final levels of immunoglobulins and immunocytokines in tissues were expressed as the unit of protein level.
The real-time quantitative PCR (RT-PCR) was used to detect the expression of immune-related genes in the ileum following the methods described previously (16). The total RNA from the ileal tissues was extracted with Trizol reagent (Accurate Biology, Changsha, China), and the agarose gel electrophoresis was used to detect the quality of the extracted RNA. According to the manufacturer’s instructions (Accurate Biology, Changsha, China), the extracted RNA was reverse-transcribed at 37°C for 15 min and 95°C for 5 s. An RT-PCR was conducted using the SYBR® Green Premix Pro Taq HS qPCR kit (Accurate Biology, Changsha, China) and LightCycle® 480 II Real-Time PCR system (Roche, Basel, Switzerland). The primers used in this study are presented in Supplementary Table S1. The PCR cycle conditions for RT-PCR were as follows: initial denaturation at 94°C for 30 s, denaturation at 94°C for 5 s and annealing at 55°C for 30 s with 40 cycles, and a final extension for 30 s at 72°C. The relative expression levels of the target gene and β-actin were calculated by comparing Ct values, and expressed by 2-ΔΔCt values (15).
All data are expressed as means with their standard error of the means (SEM). The individual piglets were considered the experimental unit. Statistical analyses were performed by two-way ANOVA for pig breed and day-old using the SPSS 26.0 software package (SPSS, Inc., Chicago, IL, USA). P-values < 0.05 were considered statistical significance.
The effects of maternal supplementation of probiotics and synbiotics on immunocytokines and immunoglobulins levels in the spleen and ileum of piglets at 21 day-old are presented in Tables 3 and 4. In the spleen, interleukin (IL)-6 and interferon (IFN)-γ levels were higher (P < 0.05) in the SYN group compared with the other three groups, while IL-6 level in the PRO group and IFN-γ level in the ANT group were higher (P < 0.05) compared with the CON group. The SYN group had higher (P < 0.05) IL-2 and tumor necrosis factor (TNF)-α levels than the CON and ANT groups, whereas the TNF-α level was higher (P < 0.05) in the PRO group compared with the CON group. Moreover, the IL-10 level was lower (P < 0.05) in the CON group, whereas the IL-1β level was higher (P < 0.05) in the SYN group when compared with the other groups (Table 3). However, there were no significant differences (P > 0.05) in the immunocytokine (Table 3) and immunoglobulin (Table 4) levels in the ileum of piglets at 21 day-old among the four groups.
The expression levels of immune-related genes in the spleen and ileum of piglets at 21 day-old are presented in Table 5. The TNF-α expression was upregulated (P < 0.05) in the spleen of the PRO and SYN groups compared with the CON and ANT groups, while toll-like receptors (TLR)4 expression was upregulated (P < 0.05) in the spleen of the SYN groups than the other three groups. In the ileum, IL-2 and myeloid differentiation factor 88 (MyD88) expressions were upregulated (P < 0.05) in the SYN group than in the other three groups. Moreover, the nuclear factor kappa B (NF-κB) expression was downregulated (P < 0.05) in the PRO and SYN groups than the CON group, whereas it was even more downregulated (P < 0.05) in the PRO group compared with the ANT group.
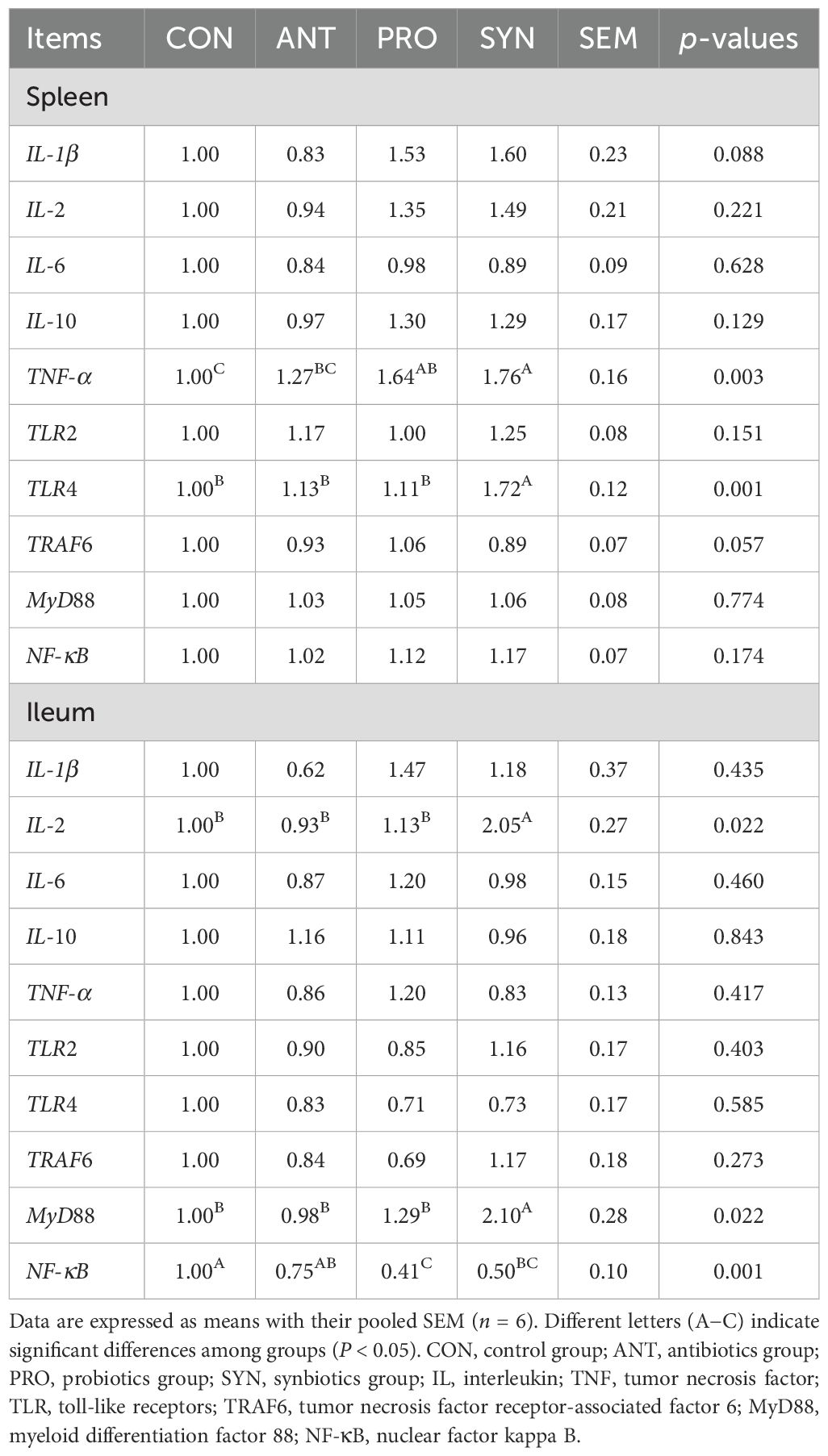
Table 5. Expression of immune-related genes in the spleen and ileum of offspring piglets at 21 day-old.
The effects of maternal-offspring supplementation of probiotics and synbiotics on plasma immunocytokines of offspring pigs at 95 and 125 day-old are shown in Figure 1. At 95 day-old, the maternal-offspring supplementation decreased (P < 0.05) the plasma IL-1β, IL-10, TNF-α, immunoglobulin (Ig)A, and IgM levels in the ANT group, as well as the plasma IL-10, IgA, and IgM levels in the SYN group, but increased (P < 0.05) the plasma IL-1β level in the PRO group compared with the offspring pigs in the maternal supplemented groups. Among the maternal supplemented groups, the PRO group had a lower (P < 0.05) plasma IL-1β level in relation to the other three groups, while plasma IL-10 and IgA levels in the PRO group were also lower (P < 0.05) compared with those in the CON and SYN groups. In addition, maternal synbiotics supplementation increased (P < 0.05) the plasma IgM level in comparison to the CON and PRO groups. Among the maternal-offspring supplemented groups, the SYN group had lower (P < 0.05) plasma IL-10, TNF-α, and IgA levels than the CON and PRO groups.
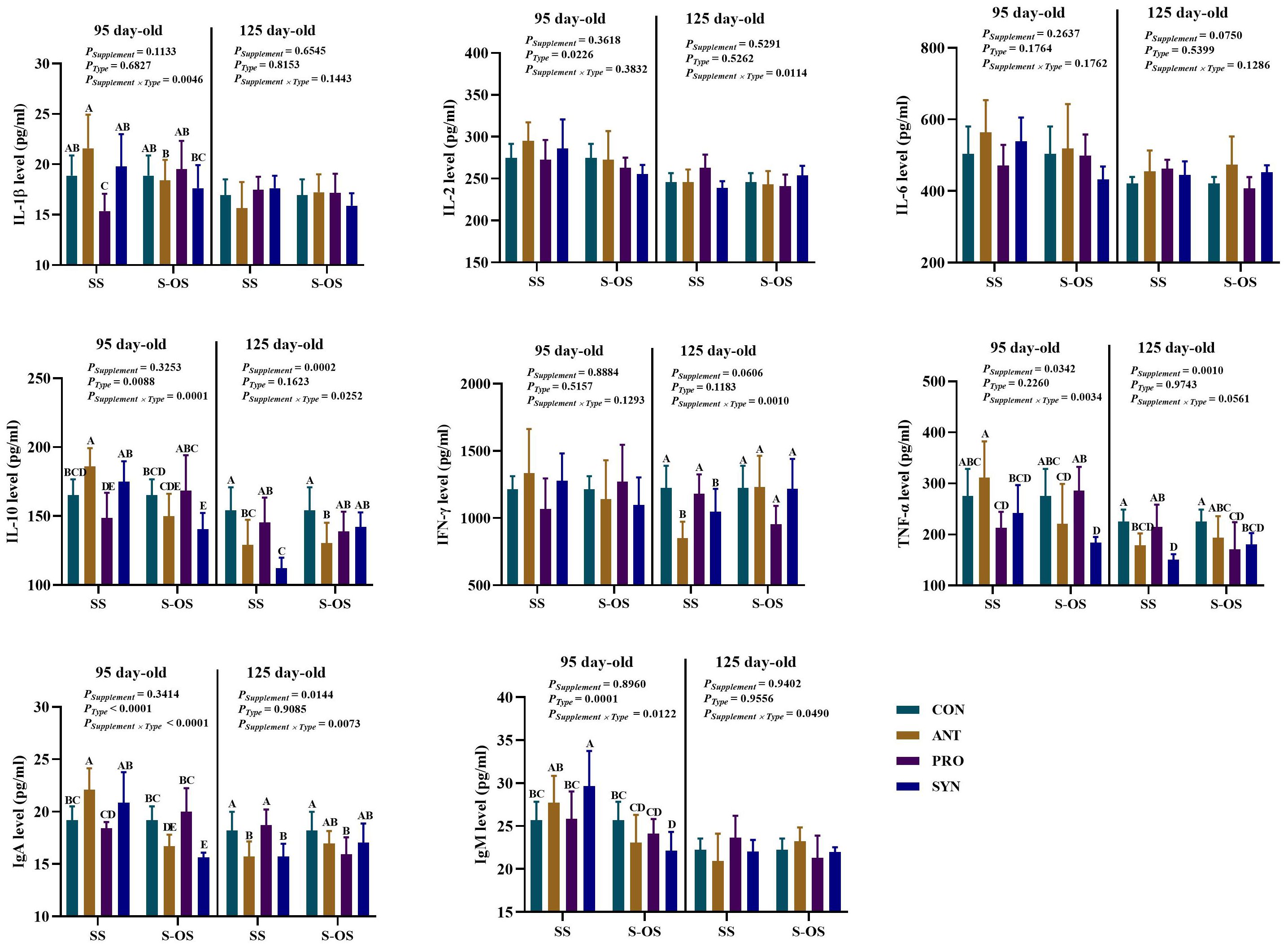
Figure 1. Effects of maternal-offspring supplementation of probiotics and synbiotics on plasma immunocytokines and immunoglobulins of offspring piglets at 95 and 125 day-old. Data are expressed as means with their pooled SEM (n = 6). Different letters (A−E) indicate significant differences among groups (P < 0.05). PSupplement: P-values of different kinds of additives in the diet, including no or added antibiotics, probiotics, or prebiotics; PType: P-values of different ways of supplement, including maternal supplementation and maternal-offspring supplementation; PSupplement × PType: P-values of different kinds of additives and different ways of supplement. CON, control group; ANT, antibiotic group; PRO, probiotics group; SYN, synbiotics group; SS, maternal supplementation; S-OS, maternal-offspring supplementation; IL, interleukin; IFN, interferon; TNF, tumor necrosis factor; Ig, immunoglobulin; sIgA, secretory immunoglobulin A.
Among the maternal supplemented groups, the ANT and SYN groups had lower (P < 0.05) plasma IL-10 and IgA levels compared with the CON and PRO groups, whereas the SYN group had a lower (P < 0.05) plasma TNF-α level compared with the CON and PRO groups. In the maternal-offspring supplemented groups, plasma IL-10 level in the ANT group, TNF-α level in the SYN group, and IgA level in the PRO group were lower (P < 0.05) compared with the CON group.
The immunocytokines level in the spleen of offspring pigs at 95 and 125 day-old is shown in Figure 2. At 95 day-old, maternal-offspring supplementation decreased (P < 0.05) the IL-10 level in the ANT group and the IFN-γ level in the SYN group compared with the maternal supplemented groups. Among the maternal supplemented groups, the PRO group had a lower (P < 0.05) IL-10 level than that in the CON and ANT groups, while IFN-γ and TNF-α levels were lower (P < 0.05) compared with the other three groups. Among the maternal-offspring supplemented groups, IL-10 and TNF-α levels in the CON group were higher, and IFN-γ level in the SYN group was lower, when compared with the other three groups (P < 0.05).
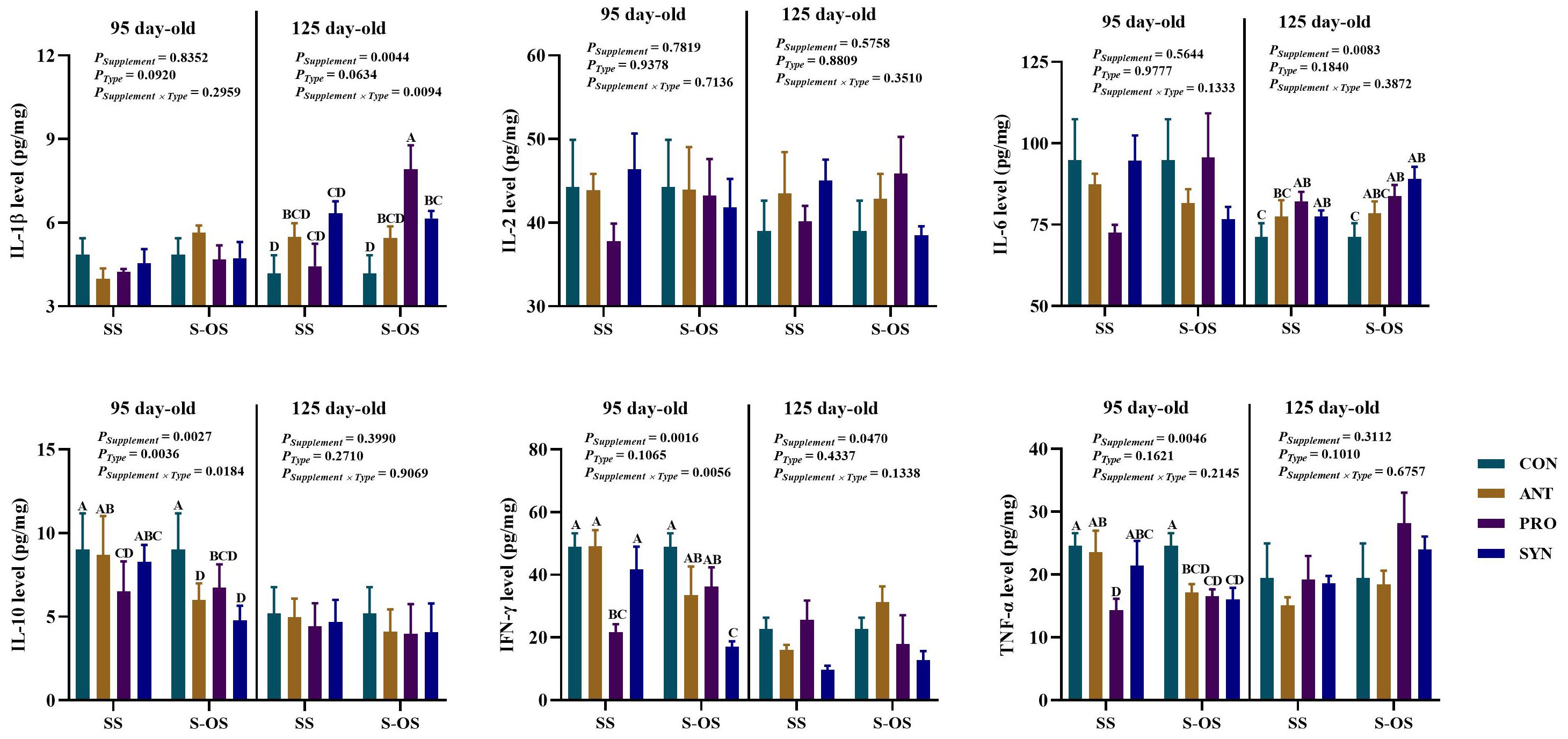
Figure 2. Effects of maternal-offspring supplementation of probiotics and synbiotics on splenic immunocytokines of offspring piglets at 95 and 125 day-old. Data are expressed as means with their pooled SEM (n = 6). Different letters (A−D) indicate significant differences among groups (P < 0.05). PSupplement: P-values of different kinds of additives in the diet, including no or added antibiotics, probiotics, or prebiotics; PType: P-values of different ways of supplement, including maternal supplementation and maternal-offspring supplementation; PSupplement × PType: P-values of different kinds of additives and different ways of supplement. CON, control group; ANT, antibiotic group; PRO, probiotics group; SYN, synbiotics group; SS, maternal supplementation; S-OS, maternal-offspring supplementation; IL, interleukin; IFN, interferon; TNF, tumor necrosis factor.
At 125 day-old, maternal-offspring probiotics supplementation increased (P < 0.05) the IL-1β level in the spleen of offspring pigs compared with the maternal-supplemented PRO group. Among the maternal supplemented groups, the PRO and SYN groups had a higher (P < 0.05) IL-6 level in contrast to the CON group.
The immune-related gene expressions in the spleen of offspring pigs at 95 and 125 day-old are shown in Figure 3. At 95 day-old, maternal-offspring probiotics supplementation upregulated (P < 0.05) the IL-1β expression compared with the maternal-supplemented PRO group. Among the maternal supplemented groups, the IL-1β expression was downregulated (P < 0.05) in the PRO and SYN groups compared with the CON group.
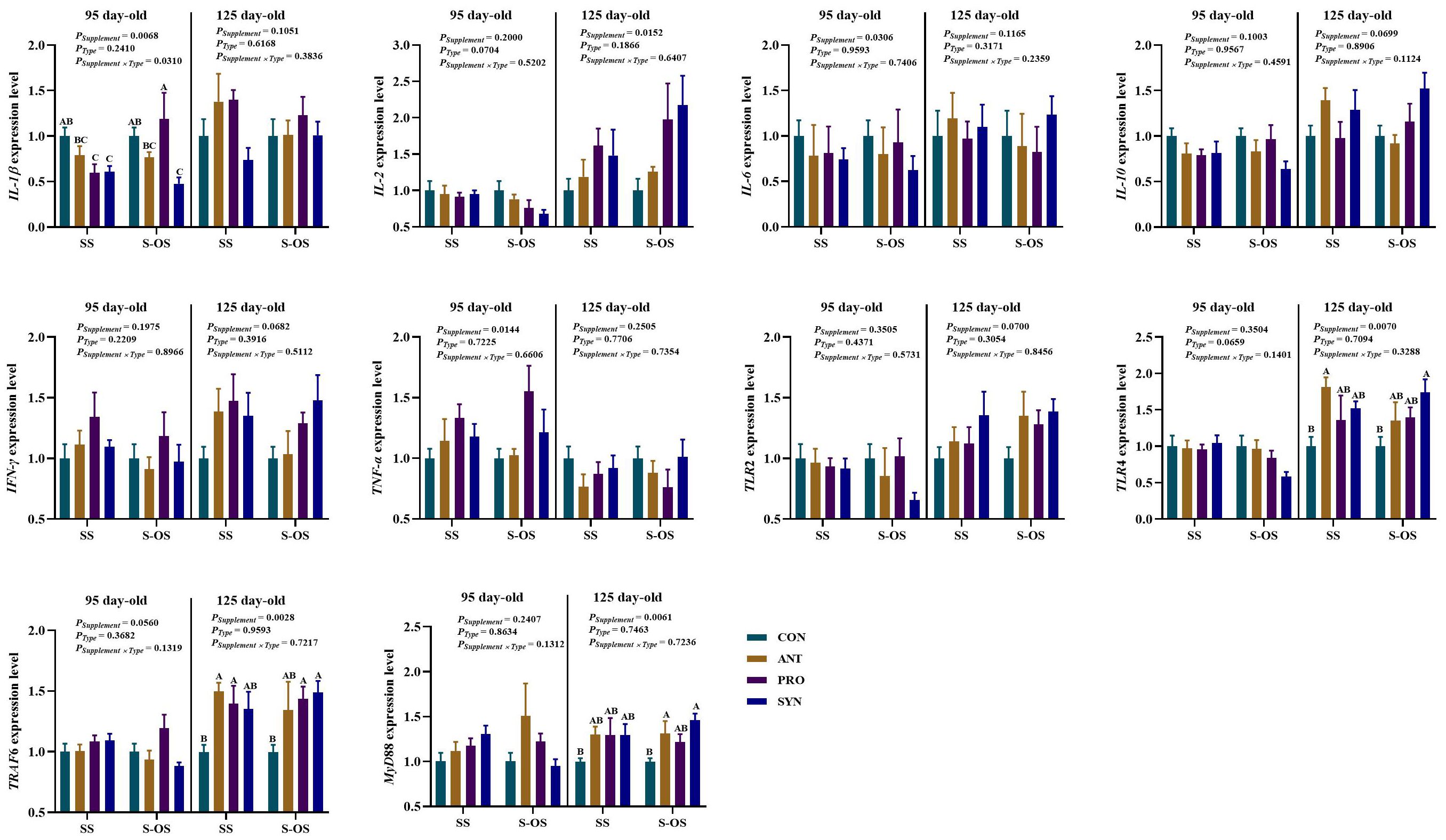
Figure 3. Expression of splenic immune-related genes of offspring piglets at 95 and 125 day-old. Data are expressed as means with their pooled SEM (n = 6). Different letters (A−C) indicate significant differences among groups (P < 0.05). PSupplement: P-values of different kinds of additives in the diet, including no or added antibiotics, probiotics, or prebiotics; PType: P-values of different ways of supplement, including maternal supplementation and maternal-offspring supplementation; PSupplement × PType: P-values of different kinds of additives and different ways of supplement. CON, control group; ANT, antibiotic group; PRO, probiotics group; SYN, synbiotics group; SS, maternal supplementation; S-OS, maternal-offspring supplementation; IL, interleukin; TNF, tumor necrosis factor; TLR, toll-like receptors; TRAF6, tumor necrosis factor receptor-associated factor 6; MyD88, myeloid differentiation factor 88; NF-κB, nuclear factor kappa B.
At 125 day-old, maternal antibiotics and probiotics supplementation upregulated (P < 0.05) the tumor necrosis factor receptor-associated factor 6 (TRAF6) expression compared with the maternal-supplemented CON group. Among the maternal-offspring supplemented groups, the TLR4 expression was upregulated (P < 0.05) in the SYN group in comparison to the CON group. Moreover, the MyD88 expression was upregulated (P < 0.05) in the ANT group compared with the CON group. However, there were no significant differences (P > 0.05) in immune-related gene expressions in the spleen of offspring pigs between maternal and maternal-offspring supplemented groups at 125 day-old.
The effects of maternal-offspring supplementation of probiotics and synbiotics on ileal immunoglobulins of offspring pigs at 95 and 125 day-old are shown in Figure 4. At 95 day-old, maternal-offspring supplementation decreased (P < 0.05) the IgA level in the ANT group and IgM level in the SYN group compared to the maternal-supplemented groups. Among maternal supplementation groups, the ANT group had a higher (P < 0.05) IgA level compared with the other three groups, the SYN group had a lower (P < 0.05) IgM level than the CON and PRO groups, and the PRO group had a higher (P < 0.05) IgM level than the ANT group.

Figure 4. Effects of maternal-offspring supplementation of probiotics and synbiotics on ileal immunoglobulins of offspring piglets at 95 and 125 day-old. Data are expressed as means with their pooled SEM (n = 6). Different letters (A−D) indicate significant differences among groups (P < 0.05). PSupplement: P-values of different kinds of additives in the diet, including no or added antibiotics, probiotics, or prebiotics; PType: P-values of different ways of supplement, including maternal supplementation and maternal-offspring supplementation; PSupplement × PType: P-values of different kinds of additives and different ways of supplement. CON, control group; ANT, antibiotic group; PRO, probiotics group; SYN, synbiotics group; SS, maternal supplementation; S-OS, maternal-offspring supplementation; Ig, immunoglobulin; sIgA, Secretory immunoglobulin A.
At 125 day-old, maternal supplementation of antibiotics and synbiotics increased (P < 0.05) the IgM level of offspring pigs compared with the maternal-supplemented CON group. Moreover, maternal-offspring supplementation of synbiotics increased (P < 0.05) the IgM level of offspring pigs compared with the maternal-offspring CON group.
The immunocytokines level in the ileum of offspring pigs at 95 and 125 day-old is shown in Figure 5. At 95 day-old, maternal-offspring probiotics supplementation decreased (P < 0.05) the IL-2, IL-10, and TNF-α levels compared with the maternal supplementation of probiotics. Among maternal supplementation groups, the PRO group had a higher (P < 0.05) IL-2 level compared with the other three groups, as well as the IL-10 level compared with the SYN group, while the ANT group had a higher (P < 0.05) TNF-α level compared with the other three groups.

Figure 5. Effects of maternal-offspring supplementation of probiotics and synbiotics on ileal immunocytokines of offspring piglets at 95 and 125 day-old. Data are expressed as means with their pooled SEM (n = 6). Different letters (A−C) indicate significant differences among groups (P < 0.05). PSupplement: P-values of different kinds of additives in the diet, including no or added antibiotics, probiotics, or prebiotics; PType: P-values of different ways of supplement, including maternal supplementation and maternal-offspring supplementation; PSupplement × PType: P-values of different kinds of additives and different ways of supplement. CON, control group; ANT, antibiotic group; PRO, probiotics group; SYN, synbiotics group; SS, maternal supplementation; S-OS, maternal-offspring supplementation; IL, interleukin; IFN, interferon; TNF, tumor necrosis factor.
Among the maternal supplementation groups, the SYN group had higher (P < 0.05) IL-1β and IL-6 levels compared with the CON and PRO groups, and the ANT group had a higher (P < 0.05) IL-10 level than the other three groups. Among the maternal-offspring supplementation groups, the ANT group had a higher (P < 0.05) IL-1β level than the CON and PRO groups, and the SYN group had a higher (P < 0.05) IL-6 level compared with the other three groups.
The immune-related gene expressions in the ileum of offspring pigs at 95 and 125 day-old are shown in Figure 6. At 95 day-old, maternal antibiotics and synbiotics supplementation downregulated (P < 0.05) the IL-10 expression compared with the maternal CON group. Maternal-offspring antibiotics and probiotics supplementation downregulated (P < 0.05) the IL-10 expression compared with the maternal-offspring CON group.
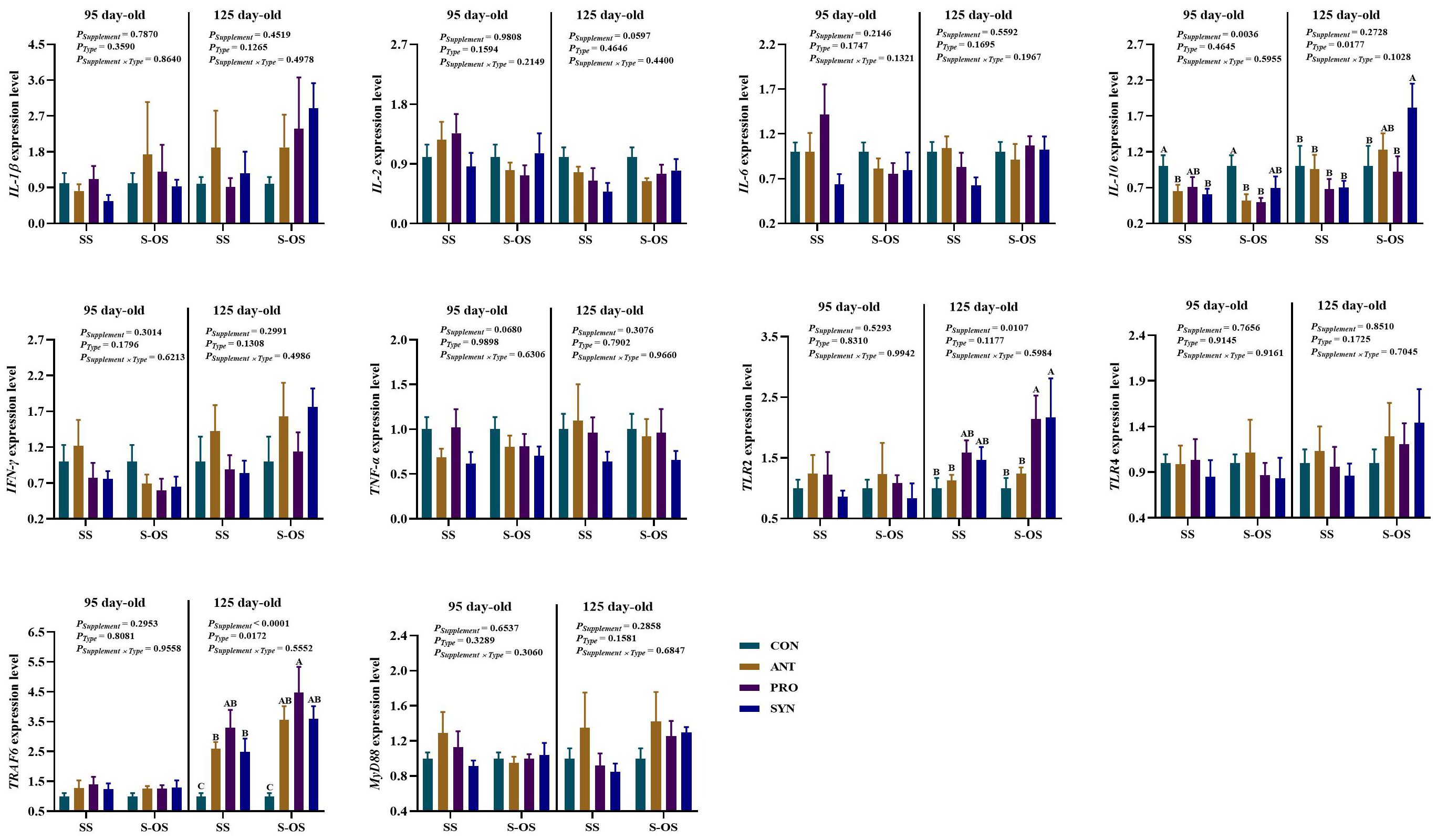
Figure 6. Expression of ileal immune-related genes of pig at 95 and 125 day-old. Data are expressed as means with their pooled SEM (n = 6). Different letters (A−C) indicate significant differences among groups (P < 0.05). PSupplement: P-values of different kinds of additives in the diet, including no or added antibiotics, probiotics, or prebiotics; PType: P-values of different ways of supplement, including maternal supplementation and maternal-offspring supplementation; PSupplement × PType: P-values of different kinds of additives and different ways of supplement. CON, control group; ANT, antibiotic group; PRO, probiotics group; SYN, synbiotics group; SS, maternal supplementation; S-OS, maternal-offspring supplementation; IL, interleukin; TNF, tumor necrosis factor; TLR, toll-like receptors; TRAF6, tumor necrosis factor receptor-associated factor 6; MyD88, myeloid differentiation factor 88; NF-κB, nuclear factor kappa B.
At 125 day-old, maternal-offspring synbiotics supplementation upregulated (P < 0.05) the IL-10 expression compared with the maternal synbiotics supplementation. Maternal antibiotics, probiotics, and synbiotics supplementation upregulated (P < 0.05) the TRAF6 expression compared with the CON group. Among the maternal-offspring supplementation groups, the IL-10 expression was upregulated (P < 0.05) in the SYN group compared with the CON and PRO groups, as well as the TLR2 expression in the PRO and SYN groups compared with the CON and ANT groups. Moreover, maternal-offspring antibiotics, probiotics, and synbiotics supplementation upregulated (P < 0.05) the TRAF6 expression compared with the CON group.
Maternal nutrition during pregnancy and lactation plays crucial roles in the overall health status and the postnatal growth and development of offspring, in turn, to the development of the pig industry. Probiotics, as a potential feed additive for swine nutrition, can enhance immunity and reduce the incidence of diarrhea in piglets at early stages of age (17). Moreover, maternal probiotics and synbiotics supplementation could also play an important role in the immune development of offspring (12, 18). Therefore, this study evaluated the effects of maternal and maternal-offspring supplementation of probiotics and synbiotics on the immune function of offspring piglets. The results indicated that different supplemental approaches have differences in the development of immune function in offspring piglets at different stages of age.
Passive immunity can be derived from sows to suckling piglets, as several active antibodies derived from sow colostrum and milk could contribute to the development of active immunity to protect the offspring piglets from different diseases (19). Maternal nutritional strategies during pregnancy and lactation have positive impacts on the immunity of offspring piglets via transferring the immune molecules through colostrum (20). In the present study, maternal probiotics supplementation increased the splenic IL-6 level of offspring piglets compared with the CON group at 21 day-old, while maternal/maternal-offspring synbiotics supplementation increased the splenic IL-1β level at 95 day-old and IL-6 and IFN-γ levels at 95 day-old compared with the ANT group. A previous study also reported that adding amoxicillin to the sow diet from the 10th to the 21st day after delivery affected the umami perception and ghrelin regulation in the offspring piglets (21). Research evidence indicated that Bacillus-based probiotics supplementation in sow diets increased IL-10 and IgM concentrations in serum and ileum and decreased the TNF-α level in the ileum of offspring piglets, and concluded that the probiotics supplementation protected the intestinal health of piglets by forming biofilm structures in the digestive tract and villi structures (22). Probiotics can improve the host intestinal immune system by enhancing cell-mediated immunity, antibody production, and stimulating T-cell migration (23). Therefore, our findings suggest that maternal antibiotics supplementation adversely affects the immune function, while probiotics supplementation may enhance the anti-inflammatory ability of offspring piglets. Although certain combinations of synbiotics may enhance host immunity synergistically, it is necessary to assess the compatibility of these combinations and their impact on the metabolic molecules of sows and their offspring.
The spleen, as the largest secondary lymphoid organ, is an important site for immune response (24). The physiological position of the spleen allows it to filter pathogens and abnormal cells in the blood and promote low-probability interactions between antigen-presenting cells and homologous lymphocytes (25). Thus, we further evaluated the effects of maternal and maternal-offspring supplementation of probiotics and synbiotics on the splenic immunity of offspring piglets. Our findings showed that maternal-offspring synbiotics supplementation decreased the IL-10 and IFN-γ levels in the spleen of offspring piglets compared with the maternal synbiotics supplementation at 95 day-old, whereas maternal-offspring probiotics supplementation increased the IL-1β level in the spleen of offspring piglets compared with the maternal probiotics supplementation at 125 day-old. IL-10 is a typical anti-inflammatory cytokine and plays an important role in maintaining immune homeostasis (26), whereas IL-1β is a potential pro-inflammatory cytokine that plays a defensive role against host infections and injuries (27). Moreover, a previous study found that IL-10 plays an important regulatory role in piglets infected with porcine circovirus type 2 (28). The research evidence has also shown that MyD88-dependent IL-1β secretion in the spleen leads to neutrophil exosmosis and downregulates ZO-2 expression to increase endothelial cell permeability (29). Thus, maternal probiotics and synbiotics supplementation presented more beneficial for the immune response of spleen function of offspring piglets at 95 and 125 day-old than that of the maternal-offspring probiotics and synbiotics supplementation.
The small intestine is composed of a single layer of intestinal epithelial cells, which not only plays an important role in the digestion and absorption of nutrients, but also is the largest immune organ of the body, affecting the body’s defense mechanisms (30). Probiotics provide strong protection for the body health by competing with pathogenic bacteria for ecological niches and nutrients and enhancing the intestinal immune system (31, 32). In the present study, maternal-offspring probiotics supplementation decreased the IL-2, IL-10, and TNF-α levels in the ileum of offspring piglets at 95 day-old, but maternal-offspring antibiotics increased the IL-1β level in the ileum at 125 day-old compared with the maternal-supplemented groups. In addition, maternal probiotics and synbiotics supplementation decreased the TNF-α level in the ileum of offspring piglets at 95 day-old compared with the maternal antibiotics supplementation. However, maternal-offspring antibiotics supplementation increased the IL-1β level in the ileum of offspring piglets at 125 day-old compared with the maternal-offspring supplemented CON and PRO groups. Previously, it has been reported that soluble proteins p40 and p75 in the supernatant of Lactobacillus rhamnosus GG culture solution could inhibit apoptosis and barrier damage induced by TNF-α in colonic epithelial cells (33). Daily intake of Lactobacillus plantarum JL01 (0.5 × 1010 CFU) may regulate immunocytokine levels in piglets by downregulating IL-1β expression, reducing the accumulation of succinic acid and palmitic acid, and increasing the accumulation of docosahexaenoic acid and tauroursodeoxycholic acid, thereby enhancing immune function in weaned piglets (34). Consistent with the above findings, our findings indicate that both maternal supplementation and the maternal-offspring supplementation of probiotics were more effective in enhancing the ileal immune function of offspring Bama mini-pigs.
In summary, the immune function of the offspring piglets varied depending on the specific approach used for probiotics and synbiotics supplementation. Compared with the maternal-offspring probiotics and synbiotics supplementation, the maternal supplementation of these feed additives showed more beneficial for spleen function at 95 and 125 day-old. However, maternal-offspring probiotics supplementation was more effective in enhancing ileal immune function than the maternal probiotics supplementation in Bama mini-pigs. These findings provide a physiological and biochemical basis for explaining the stress resistance of Bama mini-pigs and provide technical guidance for the application of probiotics and synbiotics in the immune regulation of “maternal-offspring integration”, which may contribute to the development and utilization of local mini-pig breeds.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
The animal experiments were approved by the Animal Care Committee of the Institute of Subtropical Agriculture, Chinese Academy of Sciences (ISA-2018-071). The study was conducted in accordance with the local legislation and institutional requirements.
XK: Funding acquisition, Writing – review & editing. SD: Data curation, Funding acquisition, Writing – original draft. TY: Data curation, Methodology, Writing – review & editing. MA: Writing – review & editing. QZ: Methodology, Writing – review & editing. YL: Methodology, Writing – review & editing. BT: Methodology, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was jointly supported by the Youth Foundation of Guangxi Natural Science Foundation (2021JJB130431), National Key Research and Development Program (No. 2021YFD1300205-2), and Hunan Provincial Science and Technology Department (2020NK2004, 2021JJ30008).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declare that no Generative AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2025.1507080/full#supplementary-material
1. Azad MAK, Wang H, Yang H, Tan B, Zhou S, Guan G. Effects of dietary carboxymethyl pachyman on oxidative stress and inflammation in weaned piglets challenged with diquat. Anim Feed Sci Technol. (2021) 276:114922. doi: 10.1016/j.anifeedsci.2021.114922
2. Li Q, Yang S, Zhang X, Liu X, Wu Z, Qi Y, et al. Maternal nutrition during late gestation and lactation: Association with immunity and the inflammatory response in the offspring. Front Immunol. (2021) 12:758525. doi: 10.3389/fimmu.2021.758525
3. Macpherson AJ, de Agüero MG, Ganal-Vonarburg SC. How nutrition and the maternal microbiota shape the neonatal immune system. Nat Rev Immunol. (2017) 17:508517. doi: 10.1038/nri.2017.58
4. Schirmbeck GH, Sizonenko S, Sanches EF. Neuroprotective role of lactoferrin during early brain development and injury through lifespan. Nutrients. (2022) 14:2923. doi: 10.3390/nu14142923
5. Theil PK, Lauridsen C, Quesnel H. Neonatal piglet survival: Impact of sow nutrition around parturition on fetal glycogen deposition and production and composition of colostrum and transient milk. Animal. (2014) 8:102130. doi: 10.1017/S1751731114000950
6. Socha-Banasiak A, Pierzynowski S, Woliński J, Grujic D, Boryczka M, Grzesiak P, et al. The pig as a model for premature infants − The importance of immunoglobulin supplementation for growth and development. J Biol Regulators Homeostatic Agents. (2017) 31(1):87–92.
7. Rooke JA, Bland IM. The acquisition of passive immunity in the new-born piglet. Livest Prod Sci. (2002) 78:1323. doi: 10.1016/S0301-6226(02)00182-3
8. Nguyen TV, Yuan L, Azevedo MS, Jeong KI, Gonzalez AM, Saif LJ. Transfer of maternal cytokines to suckling piglets: In vivo and in vitro models with implications for immunomodulation of neonatal immunity. Vet Immunol Immunop. (2007) 117:23648. doi: 10.1016/j.vetimm.2007.02.013
9. FAO. Guidelines for evaluation of probiotics in food. Report of a joint FAO/WHO working group on drafting gidelines for the evaluation of probiotics in food. London, Ontario, Canada: MDC Publishers Sdn Bhd. (2002).
10. Zoetendal EG, Collier CT, Koike S, Mackie RI, Gaskins HR. Molecular ecological analysis of the gastrointestinal microbiota: A review. J Nutr. (2004) 134:46572. doi: 10.1093/jn/134.2.465
11. Walsh MC, Gardiner GE, Hart OM, Lawlor PG, Daly M, Lynch B, et al. Predominance of a bacteriocin-producing Lactobacillus salivarius component of a five-strain probiotic in the porcine ileum and effects on host immune phenotype. FEMS Microbiol Ecol. (2008) 64:31727. doi: 10.1111/j.1574-6941.2008.00454.x
12. Markowiak P, Śliżewska K. The role of probiotics prebiotics and synbiotics in animal nutrition. Gut Pathog. (2018) 10:21. doi: 10.1186/s13099-018-0250-0
13. Kenny M, Smidt H, Mengheri E, Miller B. Probiotics - Do they have a role in the pig industry? Animal. (2011) 5:46270. doi: 10.1017/S175173111000193X
14. Zhu X, Zhong Y, Ge Y, Lu K, Lu S. CRISPR/Cas9-mediated generation of Guangxi Bama minipigs harboring three mutations in α-synuclein causing Parkinson’s disease. Sci Rep. (2018) 8:12420. doi: 10.1038/s41598-018-30436-3
15. Wang K, Hu C, Tang W, Azad MAK, Zhu Q, He Q, et al. The enhancement of intestinal immunity in offspring piglets by maternal probiotic or synbiotic supplementation is associated with the alteration of gut microbiota. Front Nutr. (2021) 8:686053. doi: 10.3389/fnut.2021.686053
16. Ding S, Cheng Y, Azad MAK, Zhu Q, Huang P, Kong X. Developmental changes of immunity and different responses to weaning stress of Chinese indigenous piglets and Duroc piglets during suckling and weaning periods. Int J Mol Sci. (2022) 23:15781. doi: 10.3390/ijms232415781
17. Ali MS, Lee EB, Hsu WH, Suk K, Sayem SAJ, Ullah HMA, et al. Probiotics and postbiotics as an alternative to antibiotics: An emphasis on pigs. Pathogens. (2023) 12:874. doi: 10.3390/pathogens12070874
18. Gray LE, O’Hely M, Ranganathan S, Sly PD, Vuillermin P. The maternal diet gut bacteria and bacterial metabolites during pregnancy influence offspring asthma. Frontier Immunol. (2017) 8:365. doi: 10.3389/fimmu.2017.00365
19. Cheng Y, Azad MAK, Ding S, Liu Y, Blachier F, Ye T, et al. Metabolomics analysis reveals the potential relationship between sow colostrum and neonatal serum metabolites in different pig breeds. Mol Nutr Food Res. (2023) 67:e2200677. doi: 10.1002/mnfr.202200677
20. Benzoni G, Foresti F, Archetti IL, Coceva G, Guyonvarch A, Alborali L. Specific and non-specific immunity of piglets from sows fed diets containing specific fatty acids in field conditions. J Anim Physiol Anim Nutr. (2013) 97:9961005. doi: 10.1111/jpn.12014
21. Trevisi P, Luise D, Correa F, Messori S, Mazzoni M, Lallès JP, et al. Maternal antibiotic treatment affects offspring gastric sensing for umami taste and ghrelin regulation in the pig. J Anim Sci Biotechnol. (2021) 12:31. doi: 10.1186/s40104-021-00557-3
22. Konieczka P, Ferenc K, Jørgensen JN, Hansen LHB, Zabielski R, Olszewski J, et al. Feeding Bacillus-based probiotics to gestating and lactating sows is an efficient method for improving immunity gut functional status and biofilm formation by probiotic bacteria in piglets at weaning. Anim Nutr. (2023) 13:361372. doi: 10.1016/j.aninu.2023.03.003
23. Azizi AFN, Uemura R, Omori M, Sueyoshi M, Yasuda M. Effects of probiotics on growth and immunity of piglets. Animals. (2022) 12:1786. doi: 10.3390/ani12141786
24. Lewis SM, Williams A, Eisenbarth SC. Structure and function of the immune system in the spleen. Sci Immunol. (2019) 4:eaau6085. doi: 10.1126/sciimmunol.aau6085
25. den Haan JM, Kraal G. Innate immune functions of macrophage subpopulations in the spleen. J Innate Immun. (2012) 4:43745. doi: 10.1159/000335216
26. Brockmann L, Soukou S, Steglich B, Czarnewski P, Zhao L, Wende S, et al. Molecular and functional heterogeneity of IL-10-producing CD4(+) T cells. Nat Commun. (2018) 9:5457. doi: 10.1038/s41467-018-07581-4
27. Dinarello CA. Biologic basis for interleukin-1 in disease. Blood. (1996) 87:2095147. doi: 10.1182/blood.V87.6.2095.bloodjournal8762095
28. Crisci E, Ballester M, Domínguez J, Segalés J, Montoya M. Increased numbers of myeloid and lymphoid IL-10 producing cells in spleen of pigs with naturally occurring postweaning multisystemic wasting syndrome. Vet Immunol Immunop. (2010) 136:30510. doi: 10.1016/j.vetimm.2010.03.008
29. Hsiao HM, Fernandez R, Tanaka S, Li W, Spahn JH, Chiu S, et al. Spleen-derived classical monocytes mediate lung ischemia-reperfusion injury through IL-1β. J Clin Invest. (2018) 128:28332847. doi: 10.1172/JCI98436
30. Blikslager AT, Moeser AJ, Gookin JL, Jones SL, Odle J. Restoration of barrier function in injured intestinal mucosa. Physiol Rev. (2007) 87:54564. doi: 10.1152/physrev.00012.2006
31. Zhao Y, Dong BR, Hao Q. Probiotics for preventing acute upper respiratory tract infections. Cochrane Database Systematic Rev. (2022) 8:CD006895. doi: 10.1002/14651858.CD006895.pub4
32. Barbieri N, Herrera M, Salva S, Villena J, Alvarez S. Lactobacillus rhamnosus CRL1505 nasal administration improves recovery of T-cell mediated immunity against pneumococcal infection in malnourished mice. Benef Microbes. (2017) 8:393405. doi: 10.3920/BM2016.0152
33. Yan F, Cao H, Cover TL, Whitehead R, Washington MK, Polk DB. Soluble proteins produced by probiotic bacteria regulate intestinal epithelial cell survival and growth. Gastroenterology. (2007) 132:56275. doi: 10.1053/j.gastro.2006.11.022
34. Geng T, He F, Su S, Sun K, Zhao L, Zhao Y, et al. Probiotics Lactobacillus rhamnosus GG ATCC53103 and Lactobacillus plantarum JL01 induce cytokine alterations by the production of TCDA DHA and succinic and palmitic acids and enhance immunity of weaned piglets. Res Vet Sci. (2021) 137:5667. doi: 10.1016/j.rvsc.2021.04.011
Keywords: Bama mini-pigs, immunity, maternal-offspring, probiotics, synbiotics
Citation: Ding S, Ye T, Azad MAK, Zhu Q, Liu Y, Tan B and Kong X (2025) Effects of maternal-offspring supplementation of probiotics and synbiotics on the immunity of offspring Bama mini-pigs. Front. Immunol. 16:1507080. doi: 10.3389/fimmu.2025.1507080
Received: 07 October 2024; Accepted: 27 January 2025;
Published: 13 February 2025.
Edited by:
Susana Salva, CONICET Reference Centre for Lactobacilli (CERELA), ArgentinaReviewed by:
Eric Amenyogbe, University of Environment and Sustainable Development, GhanaCopyright © 2025 Ding, Ye, Azad, Zhu, Liu, Tan and Kong. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiangfeng Kong, bm5reGZAaXNhLmFjLmNu
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.