- Department of Orthopaedic Medical Center, The Second Norman Bethune Hospital of Jilin University, Changchun, Jilin, China
Multiple myeloma (MM) is a malignant disease of plasma cells that accounts for approximately 10% of all hematological malignancies and is characterized by a clonal proliferation of malignant plasma cells in the bone marrow. Numerous therapeutic strategies, including proteasome inhibitors, immunomodulators, monoclonal antibodies against CD38 and autologous stem cell transplantation, have prolonged the median survival of MM patients. Nevertheless, almost all MM patients suffer disease relapses due to drug resistance and eventually die from MM or MM-related complications. Chimeric antigen receptor (CAR) T-cell therapy is a novel immunotherapy strategy for MM and has shown encouraging results in several clinical trials. However, the use of CAR T-cell therapy for the treatment of MM is still associated with several difficulties, including antigen escape, poor persistence, an immunosuppressive microenvironment, cytokine release syndrome, immune effector cell-associated neurotoxicity syndrome, CAR T-cell-associated encephalopathy syndrome, cytopenia, and infections. In this review, we describe in detail the target antigens of CAR T cells in MM. We also comprehensively discuss recent innovations in the development of CAR T cells to improve clinical efficacy and strategies to overcome the limitations of CAR T-cell therapy in MM.
1 Introduction
Multiple myeloma (MM) is a malignant disease of plasma cells that accounts for approximately 10% of all hematological malignancies (1). It is characterized by clonal proliferation of malignant plasma cells in the bone marrow associated with the overproduction of monoclonal immunoglobulin protein (known as M protein) and subsequent damage to internal organs (2). The main clinical manifestations of MM are osteoporosis, hypercalcemia, bone pain and pathological fractures. In the past, many therapeutics, including proteasome inhibitors, immunomodulators and monoclonal antibodies to CD38, have extended the median survival of MM patients from 3 to 6 years after initial diagnosis (3). Unfortunately, almost all MM patients eventually relapse (4). Moreover, with each new MM relapse, malignant plasma cells undergo clonal evolution and acquire new mutations that lead to disease progression and resistance to conventional treatments (5). In 2020, cancer statistics in the United States showed an MM related mortality rate of 39.76% (6). Therefore, new therapeutic approaches for the treatment of MM are urgently needed.
Chimeric antigen receptor (CAR) T cell therapy mediate tumor killing in several ways, including secretion of cytotoxic granules containing perforin and granzymes, production of pro-inflammatory cytokines such as IFN-g and TNF-a and activation of Fas/Fas ligand (Fas/FasL), elevation of circulating levels of serum cytokines (such as IL-15) and depletion of endogenous regulatory T cells (7). In recent years, CAR T cell therapy has been widely used to treat a number of malignancies, including MM, leukemia, ovarian cancer, breast cancer and osteosarcomas (8, 9). However, the clinical application of CAR T cell therapy for MM still faces several disadvantages, including antigen escape, poor persistence and an immunosuppressive microenvironment, cytokine release syndrome (CRS), immune effector cell-associated neurotoxicity syndrome (ICANS) and CAR T cell-related encephalopathy syndrome (10). In this review, we therefore describe in detail the structure of CAR T cells, the target antigens of CAR T cells in MM, the dilemmas of CAR T cell therapy for MM and the ways to solve these dilemmas.
2 CAR T cells
CAR is a genetically engineered chimeric receptor consisting of an extracellular single-chain variable fragment (scFv), a hinge region, a transmembrane structural domain, an intracellular costimulatory structural domain and a CD3ζ activating structural domain, which enables T cells to reorient towards target antigens, thereby producing a cytotoxic effect that is completely independent of the expression of the major histocompatibility complex of the target cell (11). In particular, scFv is a fusion protein consisting of variable regions, such as the heavy and light chains in monoclonal antibodies, which are responsible for recognizing specific antigens on the surface of tumor cells. The hinge region serves as a bridge connecting scFv to the transmembrane structural domain. The transmembrane structural domains have the ability to anchor CAR to the T cell membrane and facilitate mechanical signal transduction into the cell (12).Costimulatory structural domains include CD28, 4-1BB, ICOS and OX40; of these, CD28 and 4-1BB are the most commonly used costimulatory structural domains. CD3ζ is the most commonly used activation structural domain. It is located in the innermost part of the CAR and is able to trigger moderate secretion of IL-2, which plays a role in promoting T cell activation and tumor cell cleavage (13).
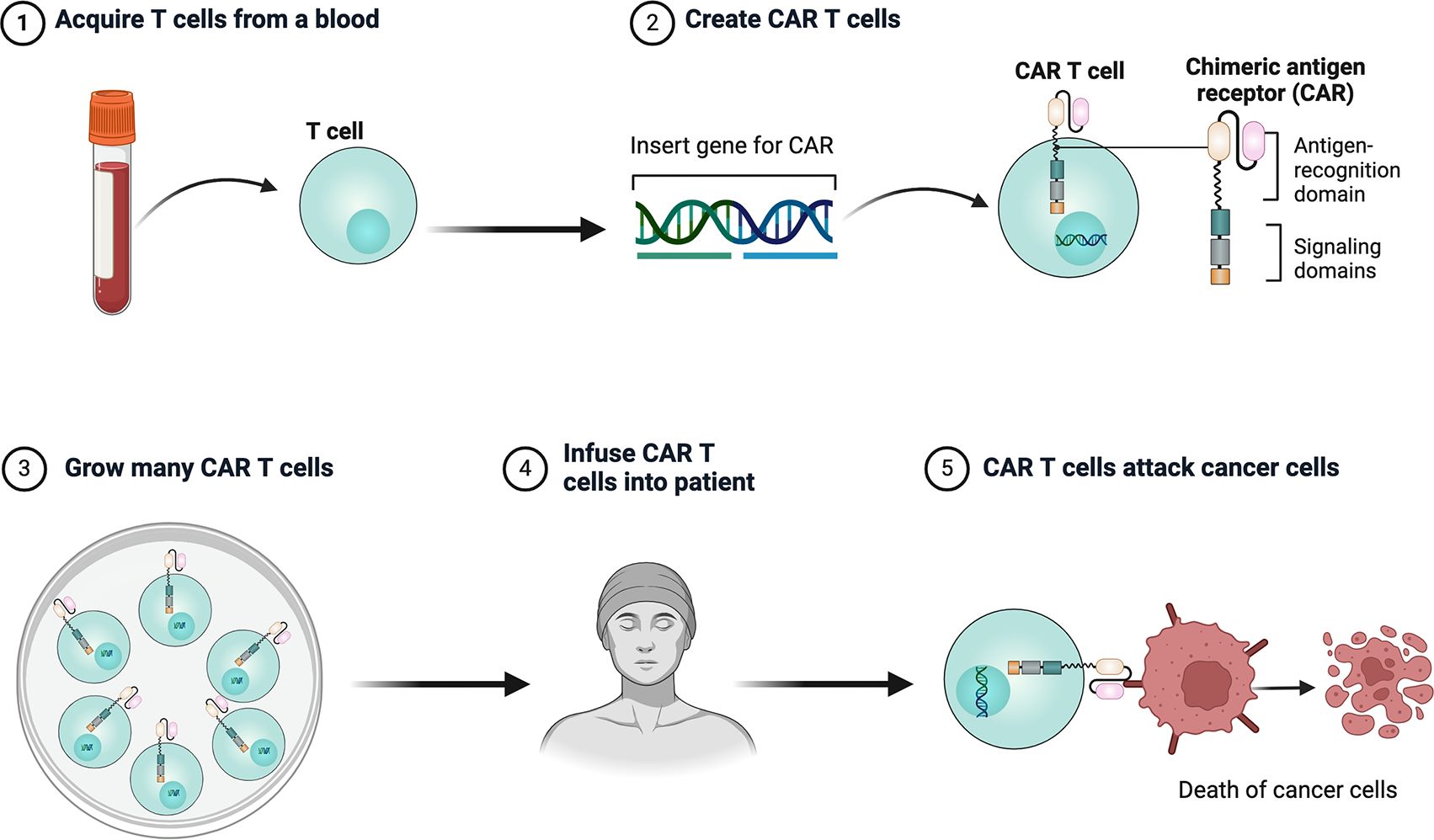
CAR T cell therapy involves modifying a patient’s own T cells to better recognize and attack cancer cells. First, T cells are collected from the patient, then genetically engineered in the lab to express a CAR that targets cancer cell markers. These modified T cells are then multiplied and infused back into the patient, where they target cancer cells by recognizing the target antigen (Figures 1, 2).
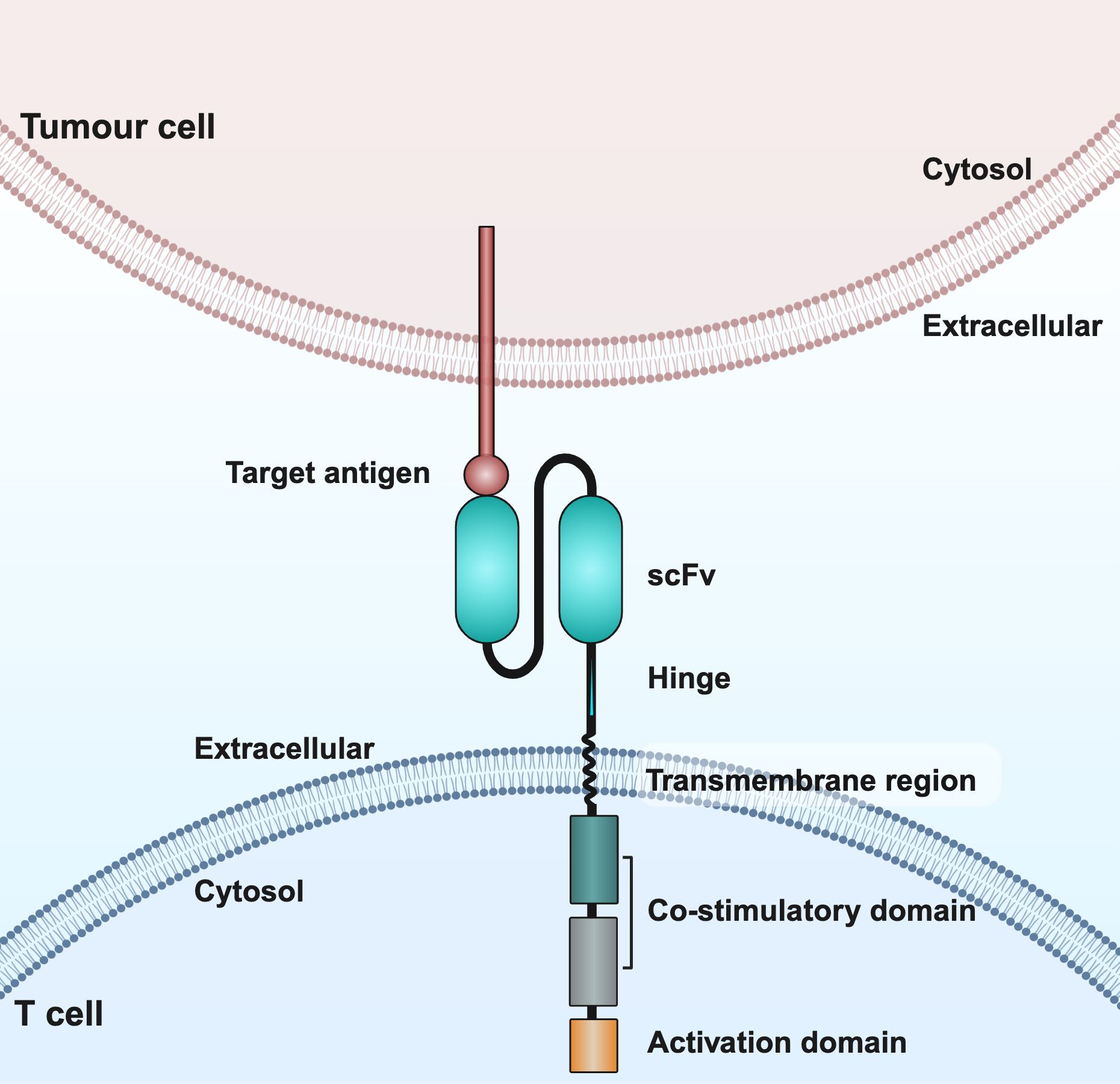
Figure 2. Schematic structure of CAR T cells. CAR consists of an extracellular structural domain, a transmembrane region, and an intracellular structural domain. scFv, single-chain variable fragment.
3 Target antigens
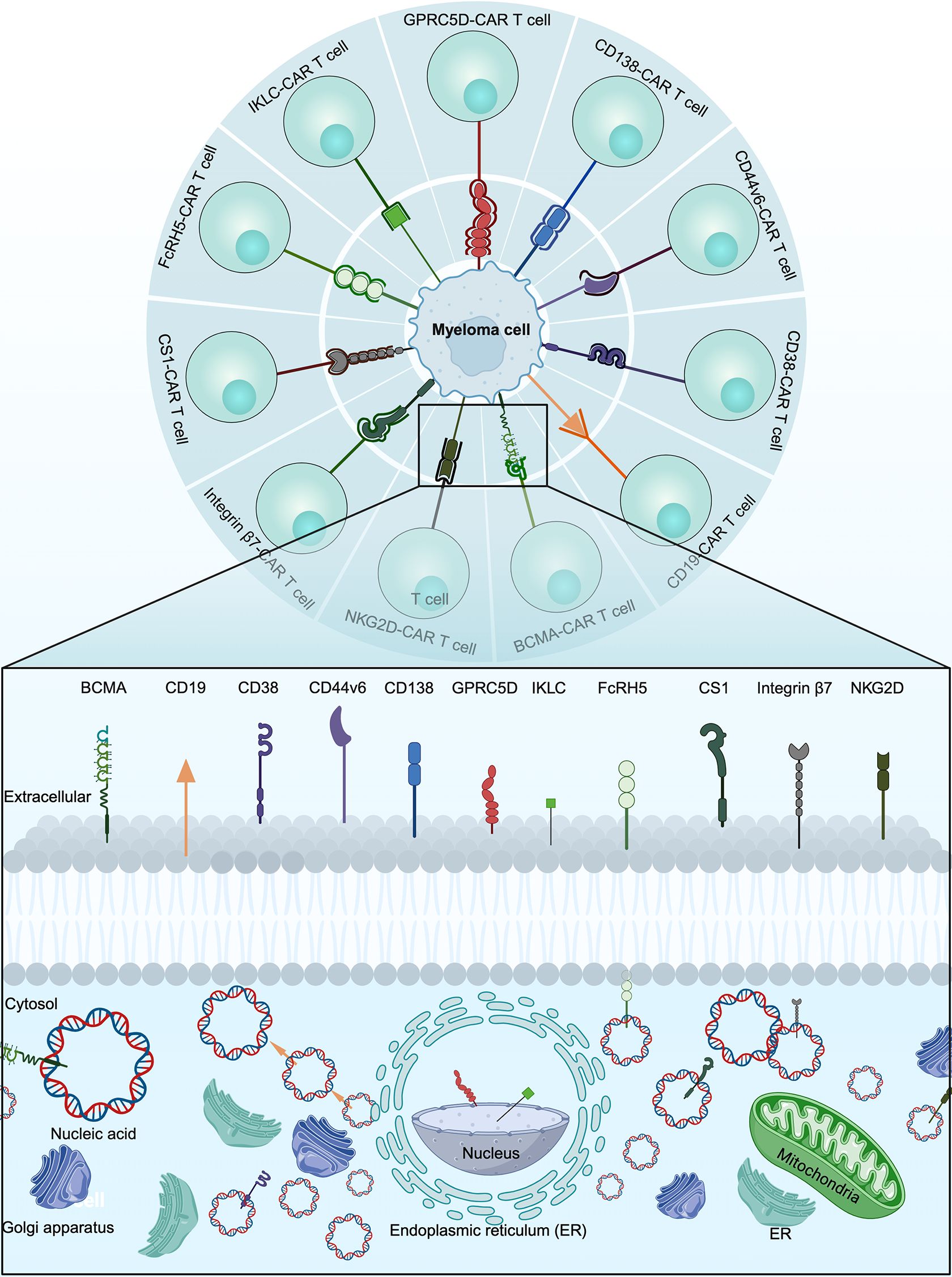
The most important determinant for the success of CAR T cell therapy is the choice of target antigen (14). The optimal CAR T cell target antigen is one that is consistently expressed on the surface of malignant cells but not on the surface of non-malignant cells (15). A variety of target antigens, including B-cell maturation antigen (BCMA), CD19, CD38, CD44v6, CD138, G protein‐coupled receptor, class C, group 5, member D (GPRC5D), immunoglobulin kappa light chain (IKLC), Fc receptor-homologue 5 (FcRH5), signaling lymphocyte activation molecule member 7 (SLAMF7), integrin β7 and natural killer group 2 member D (NKG2D), were investigated for the treatment of MM (Figure 3).
3.1 BCMA
BCMA is a cell surface protein expressed mainly on plasma cells, mature B lymphocytes and most MM cells (16). BCMA is the most attractive target antigen for CAR T cell therapy for MM, and BCMA-CAR T cell therapy has achieved unprecedented responses in recurrent or refractory MM patients, bringing new hope for these patients (17). In untreated and relapsed MM patients, the expression rate of BCMA on the surface of tumor cells was 100% (18). Currently, two BCMA-CAR T cell products, idecabtagene vicleucel (ide-cel) (19) and ciltacabtagene autoleucel (cilta-cel) (9), have been approved by the US Food and Drug Administration (FDA) for the treatment of recurrent or refractory MM.
Cilta-cel is a CAR T cell that expresses two antigen recognition regions that target BCMA. A phase II clinical trial showed that 72% of patients showed a durable antitumor response after infusion of cilta-cel (9). A phase III, randomized, open-label trial found that a single cilta-cel infusion resulted in a lower risk of disease progression or death than standard care in lenalidomide-refractory patients with MM who had received one to three previous therapies (NCT04181827) (20).
A phase II clinical trial showed that 73% of patients with relapsed and refractory myeloma were effectively treated with ide-cel, and 33% achieved complete remission (19). Recently, an international, open-label, phase 3 trial reported that ide-cel therapy significantly prolonged progression-free survival and improved response as compared with standard regimens in patients with triple-class-exposed relapsed or refractory MM who had received two to four regimens (including immunomodulatory agents, proteasome inhibitors, and daratumumab) previously. The toxicity of ide-cel was consistent with previous reports (NCT03651128) (21).
BCMA-CAR T cell therapy has demonstrated robust therapeutic activity in clinical trials, but toxicities remain a significant concern for a subset of patients. Thus, fully humanized scFv, which reduces immunogenicity, was developed. Researchers developed a fully human BCMA-specific CAR, CT103A, to improve clinical efficacy (22). In a phase 1 trial, CT103A was found to be safe and highly active in patients with RRMM, even at lower doses (1 and 3 × 106 CAR+ T cells/kg) with minimal side effects, including CRS and neurotoxicity (22). Patients who relapsed after prior murine BCMA CAR T cell therapy also benefited from CT103A (22). In a phase 1 clinical trials, Yang et al. generated CT053 with an optimized, fully-human scFv, and demonstrated that CT053 had strong efficacy and a good safety profile when administered to relapsed or refractory MM patients (23). Moreover, to enhance CAR T cell therapy and overcome PD-L1-mediated suppression, Pornpimon et al. developed anti-BCMA-CAR5-T cells, which incorporate three costimulatory domains and the ability to secrete anti-PD-L1 scFv. These cells were generated by fusing anti-BCMA-CAR4-T cells, which contain a human anti-BCMA scFv and costimulatory domains (CD28, 4-1BB, CD27) linked to CD3ζ, with anti-PD-L1 scFv. Anti-BCMA-CAR5-T cells demonstrated specific cytotoxicity against BCMA-expressing targets, enhanced CAR-T cell proliferation, and sustained CAR expression after antigen re-stimulation. Additionally, they lowered PD-1 levels and reduced PD-L1 expression on target cells, resulting in improved antitumor activity, better proliferation, and reduced T-cell exhaustion in MM models (24). Taken together, BCMA is a potential and promising target antigen for CAR-T cell therapy for MM.
3.2 CD19
CD19 is a B lymphocyte-specific surface antigen that belongs to the immunoglobulin superfamily and is predominantly expressed in mature cells of the B-cell lineage (25). Although only a small proportion of plasma cells express CD19, importantly, studies have found that CD19+ multiple myeloma patients have a poor prognosis, characterized by a tendency to relapse and short survival (26). An early phase study by Garfall et al. found that ten patients received CD19-CAR T cells after high‐dose melphalan and autologous stem cell transplantation, and two patients had significantly longer PFS with CD19-CAR T cells plus autologous stem cell transplantation compared to their previous autologous stem cell transplantation (27). Therefore, they concluded that CD19-CAR T cells may improve the duration of response to standard therapies for MM by targeting and eliciting secondary immune responses against myeloma-spreading cells (27). In addition, Shi et al. reported the results of a phase 1/2 study of bispecific CAR T cells targeting CD19 and BCMA in relapsed or refractory MM. The study demonstrated that bispecific CD19-BCMA CAR T cells were feasible, safe, and effective in patients with relapsed or refractory MM. Future prospective and multicenter clinical trials with larger sample sizes and longer follow-up are needed to evaluate the long-term impact of CD19-BCMA CAR T cell therapy on patient outcomes (28). Thus, CD19 is a potential, promising, valuable and important target antigen for CAR T cell therapy for MM.
3.3 CD38
CD38 is a transmembrane glycoprotein associated with calcium regulation, signal transduction and cell adhesion (29). More than 90% of MM patients express CD38 on malignant plasma cells (30). Daratumumab and isatuximab are two CD 38 antibodies which FDA approved by FDA (31). Preclinical evidence that CD38-CAR T cells are effective in the treatment of myeloma (32). Besides, CD38-CAR T cells are being investigated in an ongoing clinical trial (NCT03464916). A phase 1/2 trial is investigating dual-targeted tandem CD38‐BCMA-CAR T cells in recurrent or refractory MM (NCT03767751) (33). In addition, studies have also shown the downregulation of CD38 expression in recurrent or refractory MM, raising concerns about adverse resistance (34). However, it is undeniable that CD38 is a potential and promising target antigen for CAR T cell therapy for MM.
3.4 CD44v6
CD44, the hyaluronan receptor, is a class I membrane glycoprotein that is overexpressed in hematological and epithelial tumors and can be expressed in both the canonical form (CD44s) and a variety of isoforms (CD44v), which include CD44v6 (35). CD44v6 expression correlates with chromosomal band 13q14 deletions, a well-known risk factor in MM. CD44v6 is frequently expressed in advanced, high-risk MM, and CD44v6 positivity is seen in 17% of stage I leukemia MM and 43% of stage II/III leukemia MM or plasma cell leukemia (36). Moreover, Monica et al. found that CD44v6-CAR T cells have an inhibitory effect on myeloma cell proliferation without damage to hematopoietic stem cells, because CD44v6 is essential for myeloma cell growth (37). Thus, CD44v6 is a potential and promising target antigen for CAR T cell therapy for MM.
3.5 CD138
CD138 is a known cell surface antigen on myeloma cells, and its expression has been positively associated with plasma cell proliferation and survival in MM (38). CD138 is also highly expressed in normal tissues, raising concerns that anti‐CD138 therapies may be toxic outside the tumor. In the phase 1 dose‐escalation study (NCT01886976), autologous CD138-CAR T cell monotherapy was used to treat five patients with recurrent or refractory MM. In four of them, the disease remained stable for more than three months, and in one advanced-stage patient, the number of myeloma cells in the peripheral blood decreased from 10.5% to < 3%. No patient showed a complete response, and no intolerable toxic effects were observed during treatment (39). Thus, CAR T cell therapy targeting CD138 is safe and feasible. It has potential antitumor activity in vivo, but the sample size of this clinical trial was too small. Thus, further studies with CD138-CAR T cell therapy for MM are needed to fully evaluate its effects.
3.6 GPRC5D
GPRC5D has emerged as a novel target for CAR T‐cell therapy in recurrent or refractory MM. GPRC5D expression is restricted to malignant plasma cells and hair follicles, making it an attractive therapeutic target (40). MCARH109, a novel CAR T cell construct targeting GPRC5D, showed promising efficacy in a phase 1 trial in patients with heavily treated recurrent or refractory MM, even in those who had previously progressed on BCMA‐targeted therapy. At the time of the last update in September 2022, a total of 17 patients had received MCARH109, 12 of whom received the maximum tolerated dose. Of these, seven patients (58%) had a partial response or better, with three patients (25%) achieving complete remission. Of note, of the six patients who had previously received BCMA therapy, three patients (50%) achieved a partial response or better. With a median follow‐up of 10.1 months, six of the 12 patients (50%) with a partial response or better remained progression-free (40). An updated analysis of the phase 1 trial of MCARH109 (GPRC5D-CAR T cell therapy for MM, NCT04555551) showed no new serious adverse events at a median follow-up of 37 months, with 71% of patients responding, a median response duration of 8.6 months, a 3-year overall survival estimate of 59%, and an association between an activated T-cell phenotype at apheresis and response, while possible GPRC5D loss was observed in 60% of patients at relapse (41). A phase 2, open label, multicenter study of BMS-986393, a GPRC5D-CAR T Cell therapy in adult participants with relapsed or refractory MM (QUINTESSENTIAL) (NCT06297226) is ongoing. Although existing clinical trial studies targeting the GPRC5D antigen have small sample sizes, they have also demonstrated its feasibility as an MM antigen target, making GPRC5D-CAR T cells a promising treatment for MM patients.
3.7 κ-light chain
Only mature B cells and malignant cells derived from mature B cells express surface immunoglobulins with a κ-light chain. Therefore, CAR T cells targeting immunoglobulin light chains (κ or λ) have been used to lyse MM cells expressing a specific light chain while avoiding cytotoxicity to healthy mature B cells expressing other light chains (42). Ramos et al. developed a second-generation CAR T cell (κ-CAR T) that targets the κ-light chain, and a phase 1 clinical trial showed that four of seven MM patients responded to infusion of κ-CAR T cells and achieved disease stabilization for more than 24 months or overall improvement in disease symptoms. Moreover, the CAR T cell product was well tolerated, with no patients experiencing severe CRS (43). Thus, CAR T cells targeting the immunoglobulin κ light chain are a safe and effective treatment for patients with relapsed or refractory MM.
3.8 FcRH5
FcRH5 can first be detected in progenitor B cells and is maintained on normal plasma cells (44). However, FcRH5 expression is upregulated on malignant plasma cells in MM compared to normal plasma cells. Jiang et al. found in a preclinical study that FcRH5 CAR T cells triggered antigen-specific activation, cytokine secretion and cytotoxicity against MM cells. Bispecific FcRH5-BCMA-CAR T cells efficiently recognized MM cells expressing FcRH5 and/or BCMA and showed enhanced efficacy compared to monospecific CAR T cells (45). In addition, cevostamab is a bispecific FcRH5-CD3 antibody that facilitates the T cell-directed killing of myeloma cells. The phase 1 clinical trial (NCT03275103) showed that cevostamab monotherapy in patients with heavily pretreated relapsed or refractory MM continued to have clinically meaningful activity and manageable safety without seriously damaging vital organs or tissues (46). Thus, FcRH5-CAR T cells may represent a promising therapeutic avenue for MM.
3.9 CS1/SLAM7
CS1 is a cell surface glycoprotein of the lymphocyte signaling activation molecule F7 (SLAM7) family that is strongly expressed on myeloma cells and weakly expressed in normal tissue cells (47). To confirm the feasibility of CS1-CAR T cells for the treatment of MM, researchers examined the effect of CS1-CAR T cells on various MM cells, and the data showed that CS1-CAR T cells had antigen-dependent killing effects. Interestingly, CS1-CAR T cells had a beneficial therapeutic effect in dexamethasone-resistant MM patients (48). It is also the target of the fully humanized elotuzumab, which is currently FDA-approved for the treatment of recurrent or refractory MM. Preclinical data on CS1-CAR T cell therapy have shown superior efficacy compared to elotuzumab (49), and it is currently being investigated in clinical trials (NCT03958656 and NCT03710421). In Europe, the phase 1/2 trial CARAMBA (EudraCT identifier 2019‐001264‐30) is investigating a novel CS1-CAR made with virus‐free, advanced Sleeping Beauty Transposon technology, and the results are eagerly awaited (50).
3.10 Integrin β7
Integrins play an important role in the establishment, survival, proliferation and drug resistance of MM cells in the bone marrow (51). Hosen et al. found that activated integrin β7 was highly expressed in the cells of patients with MM, even when they had received treatment. These results suggest that CAR T cell therapies targeting activated integrin β7 have the potential to benefit patients with relapsed or refractory MM (52). Hosen et al. screened more than 10,000 monoclonal antibody clones against MM and identified MMG49 as an MM-specific monoclonal antibody that specifically recognizes a subset of integrin β7 molecules. The MMG49 epitope in the N-terminal region of the β7-chain is presumably inaccessible in the resting integrin conformation but exposed in the active conformation. Increased expression and constitutive activation of integrin β7 resulted in high MMG49 reactivity on MM cells, whereas MMG49 binding was barely detectable in other cell types, including normal integrin β7+ lymphocytes. T cells transduced with MMG49-derived CAR exerted anti-MM activity without damaging normal hematopoietic cells (53). Thus, MMG49-CAR T cell therapy is promising for MM, and a receptor protein with a rare but physiologically relevant conformation may serve as a target for cancer immunotherapy.
3.11 NKG2D
NKG2D is an activating receptor expressed on NK cells, invariant NKT cells, γδ T cells, CD8+ T cells and a small fraction of CD4+ T cells. NKG2D ligands are expressed on MM but not on healthy tissue (54). In addition, NKG2D ligands are also expressed on immunosuppressive cells, such as regulatory T cells and myeloid-derived suppressor cells, making NKG2D-CAR T cell therapy an attractive option in hematological malignancies and solid tumors (55). Importantly, human NKG2D-CAR T cells do not respond in vitro to autologous peripheral blood mononuclear cells or bone marrow from healthy donors (56). Studies by Sentman and colleagues in mice demonstrated the efficacy of NKG2D-CAR T cells in eradicating established MM, lymphomas and ovarian cancers and inducing autologous immunity that protects against tumor rechallenge even after NKG2D-CAR T cells were no longer detectable (57). The phase 1 study by Baumeister et al. showed that the produced NKG2D-CAR T cells have functional activity against autologous tumor cells in vitro. However, to increase clinical activity, modifications may be required to increase CAR T cell expansion and target density (57). In addition, CYAD-01 is an autologous CAR T cell product based on the NKG2D receptor, which binds eight ligands overexpressed in a variety of hematological malignancies but is largely absent on nonneoplastic cells. A phase 1 clinical trial (NCT03018405) showed that treatment with a CYAD-01 multiple infusion regimen is well tolerated without preconditioning, albeit without durability outside of patients being converted to allogeneic hematopoietic stem cell transplantation (56). These phases 1 data support the proof of concept of targeting NKG2D ligands with CAR T cell therapy. Further clinical trials with NKG2D-CAR T cells are warranted, possibly via combinatorial antigen-targeted approaches to enhance antitumor activity.
3.12 Others
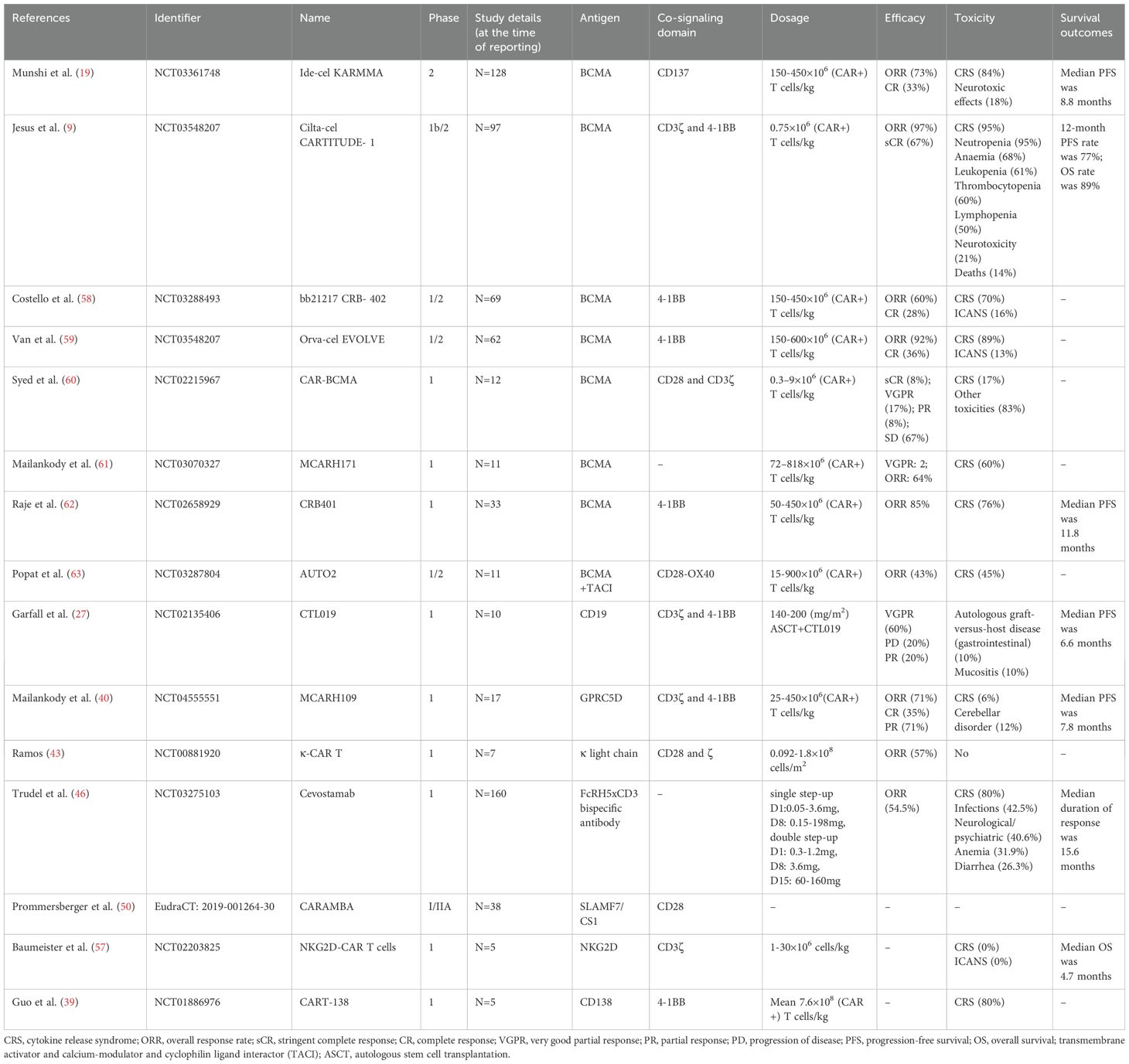
Many clinical trials exploring the safety and efficacy of CAR T cell therapy for MM are currently either underway or completed (Table 1) (Supplementary File 1).
Pediatric MM is rare with only approximately 0.3% of cases diagnosed before the age of 30 (64). CAR T cell therapy for pediatric MM is an emerging treatment with ongoing research focused on addressing the unique challenges in children. Key targets for CAR T cells include BCMA, GPRC5D, and CD38, with the aim of improving the targeting and killing of malignant plasma cells. However, pediatric MM presents with more aggressive disease characteristics, and the immune system in children may respond differently to treatment, requiring tailored protocols for safety. Further research is needed to refine these therapies for pediatric patients and enhance long-term outcomes.
The success of CAR T-cell therapy has led researchers to explore engineering other immune cells, such as natural killer (NK) cells, NKT cells (65), macrophages (66), and neutrophils (67), for therapeutic use. CAR-NK cell therapy, in particular, has shown promising results in clinical trials. While these immune cells may have fewer concerns regarding graft-versus-host disease, making them potential off-the-shelf products, they face limitations like short lifespans, limited proliferation, and an inability to form memory cells. Additionally, T cells can be engineered to target tumors using tumor-neoantigen-specific TCRs (68), offering an advantage over CARs by targeting not only membrane antigens but also intracellular neoantigens in the context of MHC complexes (69).
4 Challenges
4.1 CAR preparation failure
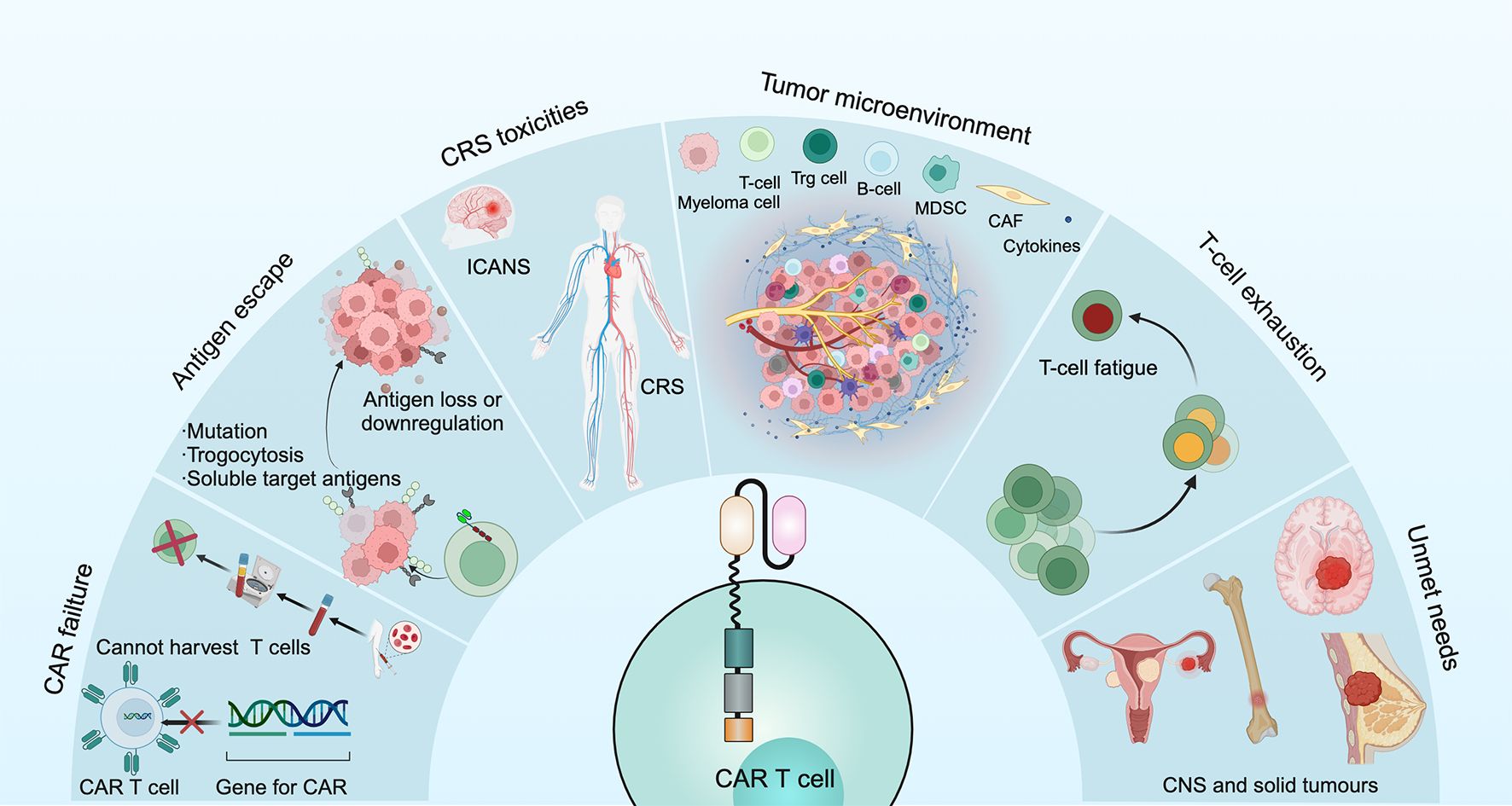
CAR T cell failures have several causes: for some patients, the CAR T cell product cannot be successfully manufactured or the generated CAR T cells do not expand sufficiently (either during manufacturing in vitro or after administration in vivo); in other patients, the problem of limited persistence in vivo is a potential mechanism underlying disease relapse (70) (Figure 4).
4.2 Tumor antigen escape
Myeloma cells can undergo loss or downregulation of target antigens, which occurs through 2 main mechanisms. One mechanism is that T cells can actively transfer antigens from MM cells to T cells through trogocytosis, which not only downregulates the number of target antigens on MM cells but also promotes T cell cytotoxicity (71). Another mechanism is the cleavage of BCMA by γ-secretase in myeloma cells, leading to a short extracellular structural domain of BCMA allowing direct shedding and an increase in soluble BCMA, thus binding these antigens to CAR T cells and limiting their effectiveness (72) (Figure 4).
4.3 CRS and ICANS
Roex et al. investigated the safety of CAR T cell therapy in 639 patients with MM and found that the incidences of CRS and ICANS were 80.3% and 10.5%, respectively (73).
CRS is a systemic inflammatory response syndrome triggered by T cell activation and mediated by a variety of cytokines, such as IFN-γ, TNF-α, IL-8, IL-10 and macrophage colony-stimulating factor. Common symptoms include fever, chills, tachycardia, tachypnoea, myalgia, and it can lead to hypoxia, cardiac arrhythmias, renal failure, liver damage, coagulopathy and multiorgan failure (74). Morgan et al. reported serious adverse events, even fatal, caused by CRS (75). According to the CRS grading system of Lee et al, the toxicity of CRS is classified as 1-5 from mild to severe (74). The treatment of CRS varies significantly based on severity. Mild CRS (Grade 1 or 2) is managed with supportive care, including hydration, antipyretics, and monitoring. Moderate CRS (Grade 2) is treated with tocilizumab, which blocks IL-6, along with supportive care as needed. Severe CRS (Grade 3 or 4) requires higher doses or repeated tocilizumab, corticosteroids like dexamethasone, and intensive care, including mechanical ventilation and organ support (22, 63, 76). Pre-emptive tocilizumab may be used for high-risk patients, and early detection and intervention are crucial for improving outcomes.
ICANS is a neurological complication that can occur as a result of certain immunotherapies, particularly CAR T-cell therapy and other adoptive T-cell therapies. It is considered one of the key complications associated with these treatments, and its severity can range from mild to life-threatening. The severity of ICANS was dose- and tumor burden-dependent and correlated positively with the severity of CRS (33) (Figure 4). ICANS occurs when CAR T-cells, while targeting and destroying cancer cells, release inflammatory cytokines such as IL-6 and other immune mediators. These substances not only affect the tumor but also impact the brain and nervous system, leading to neurotoxicity (77). The presentation of ICANS can vary widely but commonly includes cognitive impairment, weakness or paralysis, seizures, speech difficulties, headache, and changes in behavior (78). ICANS is classified by severity from grade 1 to grade 4. Grade 1 involves mild confusion and transient neurological symptoms; grade 2 includes noticeable confusion or agitation without major functional impairment; grade 3 consists of severe symptoms like confusion, disorientation, or seizures that affect daily activities; grade 4 represents life-threatening conditions, such as coma or continuous seizures, requiring intensive care (79). Risk factors for ICANS include severe CRS, older age, pre-existing neurological conditions, and certain CAR T-cell characteristics (2, 10, 80). Treatment focuses on controlling inflammation and managing symptoms with corticosteroids, tocilizumab, supportive care, and symptomatic treatment (71). The prognosis varies; mild to moderate cases often resolve with intervention, but severe cases may result in long-term impairment or be life-threatening if not treated promptly.
4.4 Tumor microenvironment
MM has a remarkably complex bone marrow microenvironment involved in promoting tumor growth, immune escape and drug resistance (81). The bone marrow microenvironment accumulates various immunosuppressive cells, including regulatory T- and B-cells, myeloid derived suppressor cells, tumor associate macrophages, dysfunctional dendritic cells as well as mesenchymal stromal cells and osteoclasts, which can reduce the anti-MM efficacy of CAR T cells (82). Moreover, metabolism has been shown to be important for the differentiation and functional expression of T cells. In the tumor microenvironment, tumor cells compete with T cells for the use of glucose, thus suppressing T cell function (83). In addition, high concentrations of potassium ions released by dead tumor cells strongly inhibit T cell function (84). The decrease in specific nutrients and the accumulation of metabolic waste thus lead to a change in the microenvironment and adversely affect T cell function (Figure 4).
4.5 T cell exhaustion
T cell exhaustion is a critical obstacle to achieving a sustained killing effect after CAR T cell therapy. According to data from the clinical trial with ide-cel, CAR T cells were detectable up to 3 months after infusion and then began to decline. By 20 months after infusion, they were only detectable in 12% of patients (62). T cell persistence is negatively affected by a number of factors, including ongoing chemotherapy and auto-immunogenicity (85). Dancy et al. demonstrated that the composition of T cells changes over time when patients receive multiple chemotherapies. Moreover, CAR T cells can be eliminated by the patient’s own immune system due to their auto-immunogenicity (86) (Figure 4).
4.6 Unmet needs
Additionally, the outcomes of CAR T cell therapy in pediatric patients with lymphoma and in patients with central nervous system (CNS) involvement remain an area of ongoing investigation. Notably, such therapies currently have limited efficacy in patients with solid tumors, and approaches to optimize response are being explored (70).
5 Strategies for overcoming obstacles
5.1 Optimizing the CAR structure
Optimizing the CAR structure is an effective way to overcome antigen escape after CAR T cell therapy, which can be achieved by targeting more than one antigen on cancer cells at a time. According to the composition of CAR, scholars have developed only heavy chains CAR (87), tandem CAR (88), bicistronic CAR (89), cotransduction CAR (8), long hinges CAR (90), hinge clusters CAR (90), armored CAR (80), commutative CAR (80), knock-out CAR (80), and gated CAR (80) by engineering extracellular recognition domains, transmembrane domains, and intracellular structural domains. In addition, researchers have found that CAR T cells made from early memory T cells and γδ T cells are also an effective way to improve the function of CAR T cells (80). On the other hand, CAR-NK cells are also an effective anti-tumor immunotherapy (80) (Figure 5).
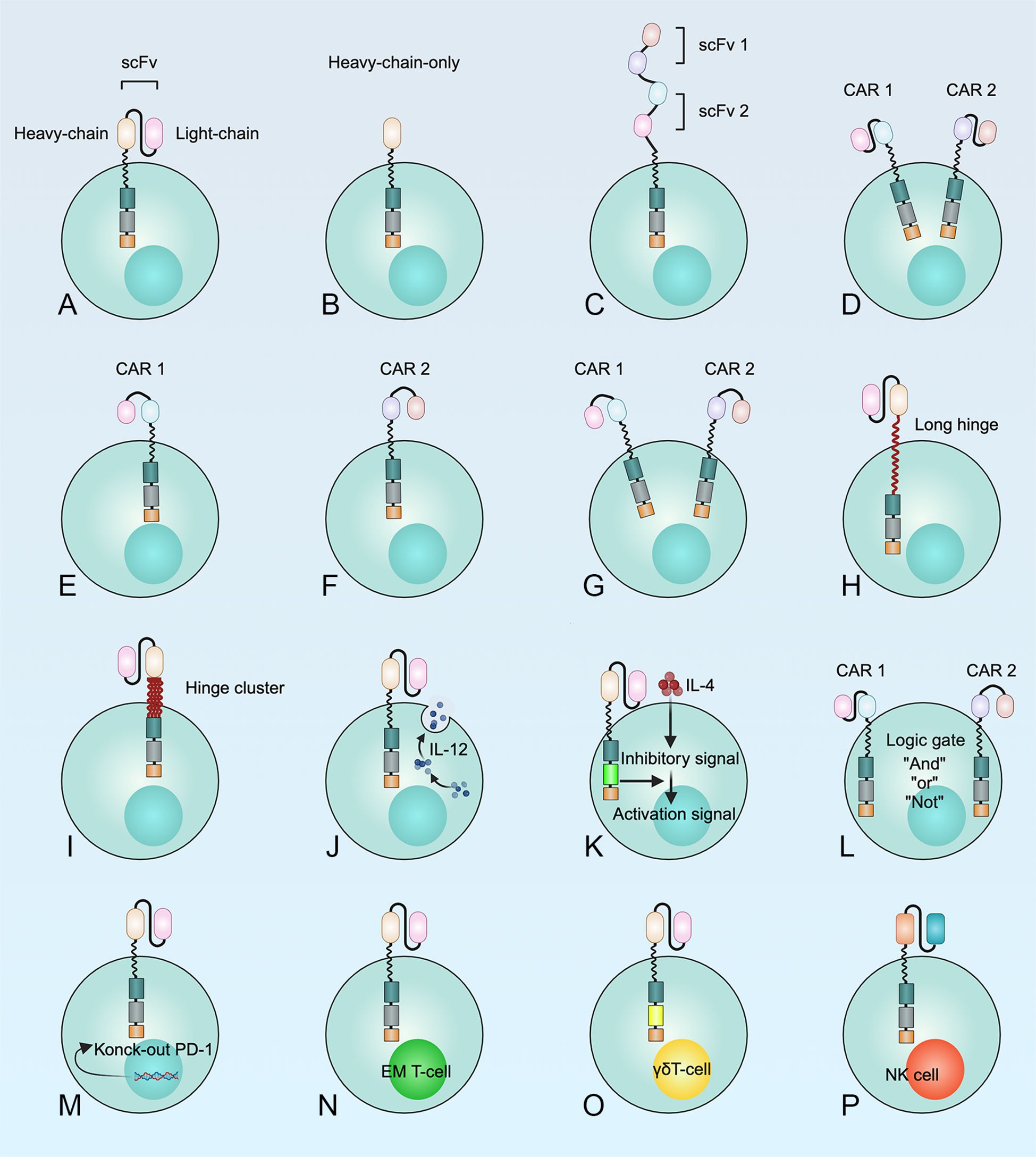
Figure 5. Multiple methods for the development of CAR T cell structures to optimize the therapeutic efficacy of CAR T cells in MM. (A) T cell expressing a monospecific CAR; (B) CAR T cells with only one heavy chain; (C) Tandem CAR T cells; (D) T cells transduced with a bicistronic CAR construct encoding two separate monospecific CARs, resulting in the simultaneous expression of both CARs; (E-G) Dual transduction describes the simultaneous transduction of an ex vivo T cell with two different CAR vectors; (H) Prolonged or (I) clustered hinge regions can improve recognition and binding of target antigens by CAR T cells; (J) “Armored” CAR T cells; (K) “Commuting” CAR T cells; (L) “Gated” CAR T cells. “AND-gate”, “OR-gate” and “NOT -gate” were developed to reduce the risk of “on-target, off-tumor” toxicities or "off-target" detection; (M) Knock-out of the immune checkpoint; (N) CAR early memory T cells; (O) CAR γδT cells; (P) CAR-NK cells.
A phase 1 study showed that tandem therapy with BCMA-CD38-CAR T cells had an overall efficacy of 87.5% in treating patients with MM. Four patients needed tocilizumab for CRS grade 3~4, but none of them reported neurotoxicity (91). Thus, the tandem strategy with two antigens could prevent tumor recurrence. Moreover, preclinical data have shown that bispecific BCMA-CD19 CAR T cells effectively eliminate myeloma cells both in vitro and in vivo. The first human clinical trial demonstrated the superior safety and efficacy of bispecific BCMA-CD19- CAR T cells in the treatment of relapsed and refractory MM (92). Coadministration of CAR T cells is feasible in patients with relapsed or refractory MM (93). Yan et al. found that 95% of patients had remission after sequential infusion of BCMA-CAR T cells and CD19-CAR T cells in relapsed or refractory MM (93).
5.2 Improving safety
A perfect CAR T cell should have a strong antitumor effects but should not cause severe CRS and neurotoxic reactions. Recently, Ying et al. developed a new CD19-CAR (derived from the CD19-BBz prototype with costimulatory 4-1BB and CD3ζ structural domains) that contains longer extracellular and intracellular sequences derived from CD8α compared to conventional CD19-CAR. A clinical trial showed that CD19-BBz-CAR T cell therapy for patients with B-cell lymphoma had a complete remission rate of 54.5% (6/11) but a CRS incidence of 0% (94). These results suggest that the CAR T cell structure will continue to be optimized so that patients may not experience serious adverse effects in the future. However, further in-depth studies are needed.
5.3 Preventing T cell exhaustion
The long persistence of CAR T cells is an essential protection against disease relapse. CAR T cells can be administered as a series of infusions to prevent T cell exhaustion (17). Kagoya et al. placed the cytoplasmic domain of the truncated IL2 receptor-β chain and the STAT3 conjugate into a CAR construct named 28-deltaIL2RB-z (YXXQ) CAR. They found that YXXQ CAR could activate the JAK/STAT pathway when cultured with tumor antigen-positive cells and showed a longer persistence time and a more pronounced antitumor effect than conventional CAR T cells in vivo (95). In addition, CAR T cells produced from different subpopulations of T cells may have different proliferation abilities and persistence. In particular, CAR T cells made from early memory T cells have good proliferation ability and persistence (96). Therefore, different approaches can prolong the persistence of CAR T cells. This would improve the antitumor effect and is necessary for clinical implementation.
5.4 Concomitant therapies
Another approach to overcome antigen loss following CAR T cell therapy is co-administration of chemotherapeutic agents (97), checkpoint inhibitors (98), localized radiotherapy, vaccines (99), other immunotherapies (100), autologous hematopoietic stem cell transplantation or localized cryoablation (2). This approach could lead to epitope spreading, which may counteract immune escape (Figure 6). Therefore, optimizing the CAR structure and integrating it with other therapies could prove to be a proficient strategy to combat antigen evasion and enhance the efficacy of tumor suppression.
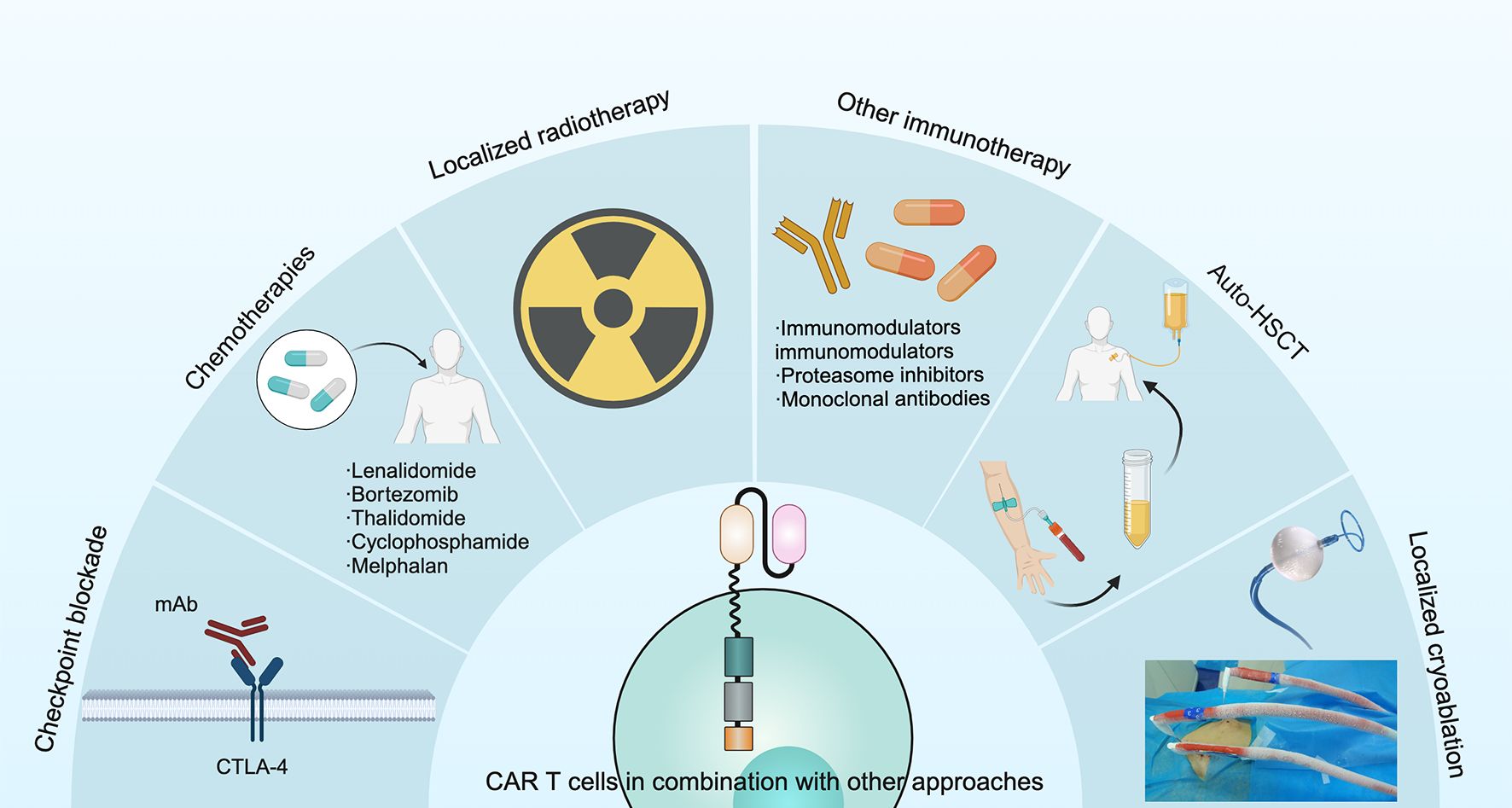
Figure 6. Several approaches have been used in combination with CAR T cells to improve treatment outcomes in MM, including checkpoint blockade, chemotherapies, localized radiotherapy, other immunotherapies, auto-HSCT and localized cryoablation.
6 Future of CAR T cell therapy
The future of CAR T-cell therapy is promising, with ongoing advancements focused on enhancing efficacy by targeting a broader range of cancers, reducing toxicity, developing off-the-shelf solutions, combining with other therapies, expanding applications to various conditions, and improving manufacturing scalability, all of which aim to make it more accessible and effective in personalized treatment.
7 Conclusions
CAR T cell therapy represents a new approach to the treatment of MM. Various target antigens, including BCMA, CD19, CD38, CD44v6, CD138, GPRC5D, kappa light chain, FcRH5, CS1, integrin β7, and NKG2D, have been used to engineer CAR T cells to kill myeloma cells, with encouraging results. However, CAR T cell therapy for MM still faces many challenges, such as tumor antigen escape, CRS, ICANS, an inhibitory tumor microenvironment and poor persistence of T cells in vivo. Accordingly, researchers have proposed many strategies to optimize CAR T cells, including updating the structure of CARs, improving the tumor microenvironment, promoting the durability of CAR T cells and increasing the safety of CAR T cells. In short, CAR T cell therapy is a novel, alternative and promising treatment for MM.
Author contributions
TY: Writing – original draft. JJ: Writing – original draft. MW: Writing – original draft.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This research was supported by Jilin Provincial Science and Technology Department Project (YDZJ202301ZYTS032), Jilin Province Health Science and Technology Capability Enhancement Project (2023JC012), Jilin University Bethune Plan Project (2024B09) and Jilin Province Higher Education Research Project (JGJX2021D28).
Acknowledgments
We thank Le Qi, Ran Meng, Zhijia Ma and Qingzhe Jia for identifying relevant literature in the manuscript and for editing the manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2025.1499590/full#supplementary-material
References
1. Röllig C, Knop S, Bornhäuser M. Multiple myeloma. Lancet. (2015) 385:2197–208. doi: 10.1016/S0140-6736(14)60493-1
2. Zhang X, Zhang H, Lan H, Wu J, Xiao Y. CAR-T cell therapy in multiple myeloma: Current limitations and potential strategies. Front Immunol. (2023) 14:1101495. doi: 10.3389/fimmu.2023.1101495
3. Facon T, Kumar S, Plesner T, Orlowski RZ, Moreau P, Bahlis N, et al. Daratumumab plus lenalidomide and dexamethasone for untreated myeloma. N Engl J Med. (2019) 380:2104–15. doi: 10.1056/NEJMoa1817249
4. Furukawa Y, Kikuchi J. Molecular basis of clonal evolution in multiple myeloma. Int J Hematol. (2020) 111:496–511. doi: 10.1007/s12185-020-02829-6
5. Ilic D, Liovic M. Industry updates from the field of stem cell research and regenerative medicine in February 2020. Regenerative Med. (2020) 15:1689–94. doi: 10.2217/rme-2020-0033
6. Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2021. CA Cancer J Clin. (2021) 71:7–33. doi: 10.3322/caac.21654
7. Kochenderfer JN, Somerville RP, Lu T, Shi V, Bot A, Rossi J, et al. Lymphoma remissions caused by anti-CD19 chimeric antigen receptor T cells are associated with high serum interleukin-15 levels. J Clin Oncol. (2017) 35:1803–13. doi: 10.1200/JCO.2016.71.3024
8. Mikkilineni L, Kochenderfer JN. CAR T cell therapies for patients with multiple myeloma. Nat Rev Clin Oncol. (2021) 18:71–84. doi: 10.1038/s41571-020-0427-6
9. Jesus GB, Deepu M, Saad ZU, Andrzej J, Mounzer A, Adam DC, et al. Ciltacabtagene autoleucel, a B-cell maturation antigen-directed chimeric antigen receptor T-cell therapy in patients with relapsed or refractory multiple myeloma (CARTITUDE-1): a phase 1b/2 open-label study. Lancet. (2021) 398:314–24. doi: 10.1016/S0140-6736(21)00933-8
10. Zhou D, Wang Y, Cheng H, Zhu L, Chen W, Li H, et al. Factors associated with infection events after chimeric antigen receptor T-cell therapy for relapsed or refractory multiple myeloma. J Infect Chemother. (2023) 29:179–85. doi: 10.1016/j.jiac.2022.10.012
11. Weinkove R, George P, Dasyam N, McLellan AD. Selecting costimulatory domains for chimeric antigen receptors: functional and clinical considerations. Clin Trans Immunol. (2019) 8:e1049. doi: 10.1002/cti2.2019.8.issue-5
12. Boettcher M, Joechner A, Li Z, Yang SF, Schlegel P. Development of CAR T cell therapy in children–A comprehensive overview. J Clin Med. (2022) 11:2158. doi: 10.3390/jcm11082158
13. Curran KJ, Pegram HJ, Brentjens RJ. Chimeric antigen receptors for T cell immunotherapy: current understanding and future directions. J Gene Med. (2012) 14:405–15. doi: 10.1002/jgm.v14.6
14. Brudno JN, Kochenderfer JN. Recent advances in CAR T-cell toxicity: Mechanisms, manifestations and management. Blood Rev. (2019) 34:45–55. doi: 10.1016/j.blre.2018.11.002
15. Sadelain M, Rivière I, Riddell S. Therapeutic T cell engineering. Nature. (2017) 545:423–31. doi: 10.1038/nature22395
16. Berahovich R, Zhou H, Xu S, Wei Y, Guan J, Guan J, et al. CAR-T cells based on novel BCMA monoclonal antibody block multiple myeloma cell growth. Cancers. (2018) 10:323. doi: 10.3390/cancers10090323
17. Cohen AD, Garfall AL, Stadtmauer EA, Melenhorst JJ, Lacey SF, Lancaster E, et al. B cell maturation antigen-specific CAR T cells are clinically active in multiple myeloma. J Clin Invest. (2019) 129:2210–21. doi: 10.1172/JCI126397
18. Seckinger A, Delgado JA, Moser S, Moreno L, Neuber B, Grab A, et al. Target expression, generation, preclinical activity, and pharmacokinetics of the BCMA-T cell bispecific antibody EM801 for multiple myeloma treatment. Cancer Cell. (2017) 31:396–410. doi: 10.1016/j.ccell.2017.02.002
19. Munshi NC, Anderson LD, Shah N, Madduri D, Berdeja J, Lonial S, et al. Idecabtagene vicleucel in relapsed and refractory multiple myeloma. (2021) 384:705–16. doi: 10.1056/NEJMoa2024850
20. San-Miguel J, Dhakal B, Yong K, Spencer A, Anguille S, Mateos MV, et al. Cilta-cel or standard care in lenalidomide-refractory multiple myeloma. N Engl J Med. (2023) 389:335–47. doi: 10.1056/NEJMoa2303379
21. Rodriguez-Otero P, Ailawadhi S, Arnulf B, Patel K, Cavo M, Nooka AK, et al. Ide-cel or standard regimens in relapsed and refractory multiple myeloma. N Engl J Med. (2023) 388:1002–14. doi: 10.1056/NEJMoa2213614
22. Wang D, Wang J, Hu G, Wang W, Xiao Y, Cai H, et al. A phase 1 study of a novel fully human BCMA-targeting CAR (CT103A) in patients with relapsed/refractory multiple myeloma. Blood. (2021) 137:2890–901. doi: 10.1182/blood.2020008936
23. Yang M, Zhang W, Yu K, Wang P, Jiang H, Chen L, et al. A novel BCMA CAR-T-cell therapy with optimized human scFv for treatment of relapsed/refractory multiple myeloma: results from phase I clinical trials. Haematologica. (2022) 107:1960–5. doi: 10.3324/haematol.2022.280629
24. Yuti P, Sawasdee N, Natungnuy K, Rujirachaivej P, Luangwattananun P, Sujjitjoon J, et al. Enhanced antitumor efficacy, proliferative capacity, and alleviation of T cell exhaustion by fifth-generation chimeric antigen receptor T cells targeting B cell maturation antigen in multiple myeloma. BioMed Pharmacother. (2023) 168:115691. doi: 10.1016/j.biopha.2023.115691
25. Ishikawa H, Tsuyama N, Mahmoud MS, Fujii R, Abroun S, Liu S, et al. CD19 expression and growth inhibition of tumours in human multiple myeloma. Leukemia lymphoma. (2002) 43:613–6. doi: 10.1080/10428190290012146
26. Garfall AL, Maus MV, Hwang WT, Lacey SF, Mahnke YD, Melenhorst JJ, et al. Chimeric antigen receptor T cells against CD19 for multiple myeloma. N Engl J Med. (2015) 373:1040–7. doi: 10.1056/NEJMoa1504542
27. Garfall AL, Stadtmauer EA, Hwang WT, Lacey SF, Melenhorst JJ, Krevvata M, et al. Anti-CD19 CAR T cells with high-dose melphalan and autologous stem cell transplantation for refractory multiple myeloma. JCI Insight. (2018) 3. doi: 10.1172/jci.insight.120505
28. Shi M, Wang J, Huang H, Liu D, Cheng H, Wang X, et al. Bispecific CAR T cell therapy targeting BCMA and CD19 in relapsed/refractory multiple myeloma: a phase I/II trial. Nat Commun. (2024) 15:3371. doi: 10.1038/s41467-024-47801-8
29. Deaglio S, Mehta K, Malavasi F. Human CD38: a (r)evolutionary story of enzymes and receptors. Leukemia Res. (2001) 25:1–12. doi: 10.1016/S0145-2126(00)00093-X
30. Leo R, Boeker M, Peest D, Hein R, Bartl R, Gessner JE, et al. Multiparameter analyses of normal and Malignant human plasma cells: CD38++, CD56+, CD54+, cIg+ is the common phenotype of myeloma cells. Ann Hematology. (1992) 64:132–9. doi: 10.1007/BF01697400
31. Morandi F, Horenstein AL, Costa F, Giuliani N, Pistoia V, Malavasi F. CD38: A target for immunotherapeutic approaches in multiple myeloma. Front Immunol. (2018) 9:2722. doi: 10.3389/fimmu.2018.02722
32. Drent E, Groen RW, Noort WA, Themeli M, Lammerts van Bueren JJ, Parren PW, et al. Pre-clinical evaluation of CD38 chimeric antigen receptor engineered T cells for the treatment of multiple myeloma. Haematologica. (2016) 101:616–25. doi: 10.3324/haematol.2015.137620
33. Parikh RH, Lonial S. Chimeric antigen receptor T-cell therapy in multiple myeloma: A comprehensive review of current data and implications for clinical practice. (2023) 73:275–85. doi: 10.3322/caac.21771
34. Tembhare P, Yuan C, Korde N, Maric I, Landgren O. Antigenic drift in relapsed extramedullary multiple myeloma: plasma cells without CD38 expression. Leukemia lymphoma. (2012) 53:721–4. doi: 10.3109/10428194.2011.623257
35. Zöller M. CD44: can a cancer-initiating cell profit from an abundantly expressed molecule? Nat Rev Cancer. (2011) 11:254–67. doi: 10.1038/nrc3023
36. Liebisch P, Eppinger S, Schöpflin C, Stehle G, Munzert G, Döhner H, et al. CD44v6, a target for novel antibody treatment approaches, is frequently expressed in multiple myeloma and associated with deletion of chromosome arm 13q. Haematologica. (2005) 90:489–93.
37. Casucci M, Nicolis di Robilant B, Falcone L, Camisa B, Norelli M, Genovese P, et al. CD44v6-targeted T cells mediate potent antitumor effects against acute myeloid leukemia and multiple myeloma. Blood. (2013) 122:3461–72. doi: 10.1182/blood-2013-04-493361
38. McCarron MJ, Park PW, Fooksman DR. CD138 mediates selection of mature plasma cells by regulating their survival. Blood. (2017) 129:2749–59. doi: 10.1182/blood-2017-01-761643
39. Guo B, Chen M, Han Q, Hui F, Dai H, Zhang W, et al. CD138-directed adoptive immunotherapy of chimeric antigen receptor (CAR)-modified T cells for multiple myeloma. J Cell Immunother. (2016) 2:28–35. doi: 10.1016/j.jocit.2014.11.001
40. Mailankody S, Devlin SM, Landa J, Nath K, Diamonte C, Carstens EJ, et al. GPRC5D-targeted CAR T cells for myeloma. New Engl J Med. (2022) 387:1196–206. doi: 10.1056/NEJMoa2209900
41. Jurgens EM, Firestone RS, Chaudhari J, Hosszu K, Devlin SM, Shah UA, et al. Phase I trial of MCARH109, a G protein-coupled receptor class C group 5 member D (GPRC5D)-targeted chimeric antigen receptor T-cell therapy for multiple myeloma: an updated analysis. J Clin Oncol. (2024) 2024:JCO2401785. doi: 10.1200/JCO-24-01785
42. Matsui W, Huff CA, Wang Q, Malehorn MT, Barber J, Tanhehco Y, et al. Characterization of clonogenic multiple myeloma cells. Blood. (2004) 103:2332–6. doi: 10.1182/blood-2003-09-3064
43. Ramos CA, Savoldo B, Torrano V, Ballard B, Zhang H, Dakhova O, et al. Clinical responses with T lymphocytes targeting Malignancy-associated κ light chains. J Clin Invest. (2016) 126:2588–96. doi: 10.1172/JCI86000
44. Polson AG, Zheng B, Elkins K, Chang W, Du C, Dowd P, et al. Expression pattern of the human FcRH/IRTA receptors in normal tissue and in B-chronic lymphocytic leukemia. Int Immunol. (2006) 18:1363–73. doi: 10.1093/intimm/dxl069
45. Jiang D, Huang H, Qin H, Tang K, Shi X, Zhu T, et al. Chimeric antigen receptor T cells targeting FcRH5 provide robust tumour-specific responses in murine xenograft models of multiple myeloma. Nat Commun. (2023) 14:3642. doi: 10.1038/s41467-023-39395-4
46. Trudel S, Cohen AD, Krishnan AY, Fonseca R, Spencer A, Berdeja JG, et al. Cevostamab monotherapy continues to show clinically meaningful activity and manageable safety in patients with heavily pre-treated relapsed/refractory multiple myeloma (RRMM): updated results from an ongoing phase I study. Blood. (2021) 138:157. doi: 10.1182/blood-2021-147983
47. Hsi ED, Steinle R, Balasa B, Szmania S, Draksharapu A, Shum BP, et al. CS1, a potential new therapeutic antibody target for the treatment of multiple myeloma. Clin Cancer Res. (2008) 14:2775–84. doi: 10.1158/1078-0432.CCR-07-4246
48. Wang X, Walter M, Urak R, Weng L, Huynh C, Lim L, et al. Lenalidomide enhances the function of CS1 chimeric antigen receptor–redirected T cells against multiple myeloma. Clin Cancer Res. (2018) 24:106–19. doi: 10.1158/1078-0432.CCR-17-0344
49. Gogishvili T, Danhof S, Prommersberger S, Rydzek J, Schreder M, Brede C, et al. SLAMF7-CAR T cells eliminate myeloma and confer selective fratricide of SLAMF7(+) normal lymphocytes. Blood. (2017) 130:2838–47. doi: 10.1182/blood-2017-04-778423
50. Prommersberger S, Reiser M, Beckmann J, Danhof S, Amberger M, Quade-Lyssy P, et al. CARAMBA: a first-in-human clinical trial with SLAMF7 CAR-T cells prepared by virus-free Sleeping Beauty gene transfer to treat multiple myeloma. Gene Ther. (2021) 28:560–71. doi: 10.1038/s41434-021-00254-w
51. Hosen N. Multiple myeloma and integrins. [Rinsho ketsueki] Japanese J Clin hematology. (2020) 61:827–31. doi: 10.1186/s41232-020-00113-y
52. Hosen N, Yoshihara S, Takamatsu H, Ri M, Nagata Y, Kosugi H, et al. Expression of activated integrin β7 in multiple myeloma patients. Int J Hematology. (2021) 114:3–7. doi: 10.1007/s12185-021-03162-2
53. Hosen N, Matsunaga Y, Hasegawa K, Matsuno H, Nakamura Y, Makita M, et al. The activated conformation of integrin β7 is a novel multiple myeloma–specific target for CAR T cell therapy. Nat Med. (2017) 23:1436–43. doi: 10.1038/nm.4431
54. Barber A, Meehan KR, Sentman CL. Treatment of multiple myeloma with adoptively transferred chimeric NKG2D receptor-expressing T cells. Gene Ther. (2011) 18:509–16. doi: 10.1038/gt.2010.174
55. Barber A, Rynda A, Sentman CL. Chimeric NKG2D expressing T cells eliminate immunosuppression and activate immunity within the ovarian tumor microenvironment. J Immunol (Baltimore Md: 1950). (2009) 183:6939–47. doi: 10.4049/jimmunol.0902000
56. Barber A, Zhang T, Megli CJ, Wu J, Meehan KR, Sentman CL. Chimeric NKG2D receptor-expressing T cells as an immunotherapy for multiple myeloma. Exp hematology. (2008) 36:1318–28. doi: 10.1016/j.exphem.2008.04.010
57. Baumeister SH, Murad J, Werner L, Daley H, Trebeden-Negre H, Gicobi JK, et al. Phase I trial of autologous CAR T cells targeting NKG2D ligands in patients with AML/MDS and multiple myeloma. Cancer Immunol Res. (2019) 7:100–12. doi: 10.1158/2326-6066.CIR-18-0307
58. Costello CL, Cohen AD, Patel KK, Ali SS, Berdeja JG, Shah N, et al. Phase 1/2 study of the safety and response of P-BCMA-101 CAR-T cells in patients with relapsed/refractory (r/r) multiple myeloma (MM) (PRIME) with novel therapeutic strategies. Blood. (2020) 136:29–30. doi: 10.1182/blood-2020-142695
59. Van Oekelen O, Aleman A, Upadhyaya B, Schnakenberg S, Madduri D, Gavane S, et al. Neurocognitive and hypokinetic movement disorder with features of parkinsonism after BCMA-targeting CAR-T cell therapy. Nat Med. (2021) 27:2099–103. doi: 10.1038/s41591-021-01564-7
60. Ali SA, Shi V, Maric I, Wang M, Stroncek DF, Rose JJ, et al. T cells expressing an anti-B-cell maturation antigen chimeric antigen receptor cause remissions of multiple myeloma. Blood. (2016) 128:1688–700. doi: 10.1182/blood-2016-04-711903
61. Mailankody S, Ghosh A, Staehr M. Clinical responses and pharmacokinetics of MCARH171, a human-derived Bcma targeted CAR T cell therapy in relapsed/refractory multiple myeloma: final results of a phase I clinical trial. Blood. (2018) 132. doi: 10.1182/blood-2018-99-119717
62. Raje N, Berdeja J, Lin Y, Siegel D, Jagannath S, Madduri D, et al. Anti-BCMA CAR T-cell therapy bb2121 in relapsed or refractory multiple myeloma. N Engl J Med. (2019) 380:1726–37. doi: 10.1056/NEJMoa1817226
63. Popat R, Zweegman S, Cavet J, Yong K, Lee L, Faulkner J, et al. Phase 1 first-in-human study of AUTO2, the first chimeric antigen receptor (CAR) T cell targeting APRIL for patients with relapsed/refractory multiple myeloma (RRMM). Blood. (2019) 134:3112. doi: 10.1182/blood-2019-126689
64. Wang X, He H, Zhang M, Li C, Jia C. Case report: multiple vertebral compression fractures in 14-year-old children with multiple myeloma. Front Oncol. (2021) 11:662169. doi: 10.3389/fonc.2021.662169
65. Heczey A, Courtney AN, Montalbano A, Robinson S, Liu K, Li M, et al. Anti-GD2 CAR-NKT cells in patients with relapsed or refractory neuroblastoma: an interim analysis. Nat Med. (2020) 26:1686–90. doi: 10.1038/s41591-020-1074-2
66. Wang S, Yang Y, Ma P, Zha Y, Zhang J, Lei A, et al. CAR-macrophage: An extensive immune enhancer to fight cancer. EBioMedicine. (2022) 76:103873. doi: 10.1016/j.ebiom.2022.103873
67. Chang Y, Syahirah R, Wang X, Jin G, Torregrosa-Allen S, Elzey BD, et al. Engineering chimeric antigen receptor neutrophils from human pluripotent stem cells for targeted cancer immunotherapy. Cell Rep. (2022) 40:111128. doi: 10.1016/j.celrep.2022.111128
68. Baulu E, Gardet C, Chuvin N, Depil S. TCR-engineered T cell therapy in solid tumors: State of the art and perspectives. Sci Adv. (2023) 9:eadf3700. doi: 10.1126/sciadv.adf3700
69. Hwang MS, Miller MS, Thirawatananond P, Douglass J, Wright KM, Hsiue EH, et al. Structural engineering of chimeric antigen receptors targeting HLA-restricted neoantigens. Nat Commun. (2021) 12:5271. doi: 10.1038/s41467-021-25605-4
70. Shah NN, Fry TJ. Mechanisms of resistance to CAR T cell therapy. Nat Rev Clin Oncol. (2019) 16:372–85. doi: 10.1038/s41571-019-0184-6
71. Steiner N, Gunsilius E. CAR-T cells in multiple myeloma: current status. memo - Magazine Eur Med Oncol. (2020) 13:43–9. doi: 10.1007/s12254-020-00571-7
72. Laurent SA, Hoffmann FS, Kuhn P-H, Cheng Q, Chu Y, Schmidt-Supprian M, et al. [amp]]gamma;-secretase directly sheds the survival receptor BCMA from plasma cells. Nat Commun. (2015) 6:7333. doi: 10.1038/ncomms8333
73. Roex G, Timmers M, Wouters K, Campillo-Davo D, Flumens D, Schroyens W, et al. Safety and clinical efficacy of BCMA CAR-T-cell therapy in multiple myeloma. J Hematol Oncol. (2020) 13:164. doi: 10.1186/s13045-020-01001-1
74. Lee DW, Gardner R, Porter DL, Louis CU, Ahmed N, Jensen M, et al. Current concepts in the diagnosis and management of cytokine release syndrome. Blood. (2014) 124:188–95. doi: 10.1182/blood-2014-05-552729
75. Morgan RA, Yang JC, Kitano M, Dudley ME, Laurencot CM, Rosenberg SA. Case report of a serious adverse event following the administration of t cells transduced with a chimeric antigen receptor recognizing ERBB2. Mol Ther. (2010) 18:843–51. doi: 10.1038/mt.2010.24
76. Smith EL, Harrington K, Staehr M, Masakayan R, Jones J, Long TJ, et al. GPRC5D is a target for the immunotherapy of multiple myeloma with rationally designed CAR T cells. (2019) 11:. doi: 10.1126/scitranslmed.aau7746
77. Wehrli M, Gallagher K, Chen YB, Leick MB, McAfee SL, El-Jawahri AR, et al. Single-center experience using anakinra for steroid-refractory immune effector cell-associated neurotoxicity syndrome (ICANS). J immunotherapy Cancer. (2022) 10. doi: 10.1136/jitc-2021-003847
78. Bonifant CL, Jackson HJ, Brentjens RJ, Curran KJ. Toxicity and management in CAR T-cell therapy. Mol Ther oncolytics. (2016) 3:16011. doi: 10.1038/mto.2016.11
79. Gust J, Hay KA, Hanafi LA, Li D, Myerson D, Gonzalez-Cuyar LF, et al. Endothelial activation and blood-brain barrier disruption in neurotoxicity after adoptive immunotherapy with CD19 CAR-T cells. Cancer discovery. (2017) 7:1404–19. doi: 10.1158/2159-8290.CD-17-0698
80. Manier S, Ingegnere T, Escure G, Prodhomme C, Nudel M, Mitra S, et al. Current state and next-generation CAR-T cells in multiple myeloma. Blood Rev. (2022) 54:100929. doi: 10.1016/j.blre.2022.100929
81. Desantis V, Savino FD, Scaringella A, Potenza MA, Nacci C, Frassanito MA, et al. The leading role of the immune microenvironment in multiple myeloma: A new target with a great prognostic and clinical value. J Clin Med. (2022) 11. doi: 10.3390/jcm11092513
82. Holthof LC, Mutis T. Challenges for immunotherapy in multiple myeloma: bone marrow microenvironment-mediated immune suppression and immune resistance. Cancers. (2020) 12. doi: 10.3390/cancers12040988
83. Chang C-H, Qiu J, O’Sullivan D, Buck Michael D, Noguchi T, Curtis Jonathan D, et al. Metabolic competition in the tumor microenvironment is a driver of cancer progression. Cell. (2015) 162:1229–41. doi: 10.1016/j.cell.2015.08.016
84. Eil R, Vodnala SK, Clever D, Klebanoff CA, Sukumar M, Pan JH, et al. Ionic immune suppression within the tumour microenvironment limits T cell effector function. Nature. (2016) 537:539–43. doi: 10.1038/nature19364
85. Das RK, Vernau L, Grupp SA, Barrett DM. Naïve T-cell Deficits at Diagnosis and after Chemotherapy Impair Cell Therapy Potential in Pediatric Cancers. Cancer discovery. (2019) 9:492–9. doi: 10.1158/2159-8290.CD-18-1314
86. Xu J, Chen LJ, Yang SS, Sun Y, Wu W, Liu YF, et al. Exploratory trial of a biepitopic CAR T-targeting B cell maturation antigen in relapsed/refractory multiple myeloma. Proc Natl Acad Sci U S A. (2019) 116:9543–51. doi: 10.1073/pnas.1819745116
87. Lam N, Trinklein ND, Buelow B, Patterson GH, Ojha N, Kochenderfer JN. Anti-BCMA chimeric antigen receptors with fully human heavy-chain-only antigen recognition domains. Nat Commun. (2020) 11:283. doi: 10.1038/s41467-019-14119-9
88. Kang L, Zhang J, Li M, Xu N, Qi W, Tan J, et al. Characterization of novel dual tandem CD19/BCMA chimeric antigen receptor T cells to potentially treat multiple myeloma. biomark Res. (2020) 8:14. doi: 10.1186/s40364-020-00192-6
89. Roddie C, Lekakis LJ, Marzolini MAV, Ramakrishnan A, Zhang Y, Hu Y, et al. Dual targeting of CD19 and CD22 with bicistronic CAR-T cells in patients with relapsed/refractory large B-cell lymphoma. Blood. (2023) 141:2470–82. doi: 10.1182/blood.2022018598
90. Dos Santos MH, MaChado MP, Kumaresan PR, da Silva TA. Modification of hinge/transmembrane and signal transduction domains improves the expression and signaling threshold of GXMR-CAR specific to cryptococcus spp. Cells. (2022) 11(21):1–20. doi: 10.3390/cells11213386
91. Cronk RJ, Zurko J, Shah NN. Bispecific chimeric antigen receptor T cell therapy for B cell Malignancies and multiple myeloma. Cancers. (2020) 12. doi: 10.3390/cancers12092523
92. Zhang H, Gao L, Liu L, Wang J, Wang S, Gao L, et al. A bcma and CD19 bispecific CAR-T for relapsed and refractory multiple myeloma. Blood. (2019) 134:3147. doi: 10.1182/blood-2019-131056
93. Yan Z, Cao J, Cheng H, Qiao J, Zhang H, Wang Y, et al. A combination of humanised anti-CD19 and anti-BCMA CAR T cells in patients with relapsed or refractory multiple myeloma: a single-arm, phase 2 trial. Lancet Haematology. (2019) 6:e521–e9. doi: 10.1016/S2352-3026(19)30115-2
94. Ying Z, Huang XF, Xiang X, Liu Y, Kang X, Song Y, et al. A safe and potent anti-CD19 CAR T cell therapy. Nat Med. (2019) 25:947–53. doi: 10.1038/s41591-019-0421-7
95. Kagoya Y, Tanaka S, Guo T, Anczurowski M, Wang C-H, Saso K, et al. A novel chimeric antigen receptor containing a JAK–STAT signaling domain mediates superior antitumor effects. Nat Med. (2018) 24:352–9. doi: 10.1038/nm.4478
96. Arcangeli S, Bove C, Mezzanotte C, Camisa B, Falcone L, Manfredi F, et al. CAR T cell manufacturing from naive/stem memory T lymphocytes enhances antitumor responses while curtailing cytokine release syndrome. J Clin Invest. (2022) 132(12). doi: 10.1172/JCI150807
97. Galustian C, Meyer B, Labarthe M-C, Dredge K, Klaschka D, Henry J, et al. The anti-cancer agents lenalidomide and pomalidomide inhibit the proliferation and function of T regulatory cells. Cancer Immunology Immunother. (2009) 58:1033–45. doi: 10.1007/s00262-008-0620-4
98. Chim CS, Kumar SK, Orlowski RZ, Cook G, Richardson PG, Gertz MA, et al. Management of relapsed and refractory multiple myeloma: novel agents, antibodies, immunotherapies and beyond. Leukemia. (2018) 32:252–62. doi: 10.1038/leu.2017.329
99. Ludwig H, Boccadoro M, Moreau P, San-Miguel J, Cavo M, Pawlyn C, et al. Recommendations for vaccination in multiple myeloma: a consensus of the European Myeloma Network. Leukemia. (2021) 35:31–44. doi: 10.1038/s41375-020-01016-0
Keywords: multiple myeloma, CAR, T cell, immunotherapy, CAR-T
Citation: Yu T, Jiao J-H and Wu M-F (2025) CAR‐T cells in the treatment of multiple myeloma: an encouraging cell therapy. Front. Immunol. 16:1499590. doi: 10.3389/fimmu.2025.1499590
Received: 21 September 2024; Accepted: 06 February 2025;
Published: 26 February 2025.
Edited by:
Srinivas Devarakonda, The Ohio State University, United StatesReviewed by:
Irfan Naseem Bandey, University of Texas MD Anderson Cancer Center, United StatesJoselle Cook, Mayo Clinic, United States
Copyright © 2025 Yu, Jiao and Wu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Min-Fei Wu, d3VtZkBqbHUuZWR1LmNu; Jian-Hang Jiao, amlhbmhhbmdAamx1LmVkdS5jbg==
 Tong Yu
Tong Yu Jian-Hang Jiao
Jian-Hang Jiao Min-Fei Wu
Min-Fei Wu