
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Immunol., 13 February 2025
Sec. Autoimmune and Autoinflammatory Disorders : Autoimmune Disorders
Volume 16 - 2025 | https://doi.org/10.3389/fimmu.2025.1497695
Objective: This study aimed to investigate the clinical features of neuronal antibodies related to autoimmune cerebellar ataxia (ACA) and to provide guidance for the diagnosis and treatment of this disease.
Methods: Demographic and clinical data were collected from antibody-positive patients with ACA who were admitted to the Department of Neurology, Affiliated Hospital of Hebei University, from January 2018 to February 2023. A retrospective analysis on the clinical manifestations, laboratory examinations, imaging data, treatment, and prognosis was performed.
Results: A total of six patients, including one man and five women, with a median age of 52.5 years, were enrolled in this study. All patients presented with dizziness and gait abnormalities with or without dysarthria. No tumor was found in these patients. Three patients were at the prodromal stage of infection, while one patient exhibited post-ACA fever symptoms and aggravated disease phenotypes. Three patients were positive for anti-glutamate decarboxylase (GAD), while one patient was positive for each of the anti-Tr, anti-mGluR1, and anti-Homer-3 antibodies. The white blood cell (WBC) count and the protein levels of the cerebrospinal fluid (CSF) were increased in four patients, which was in agreement with predominant lymphocytic inflammation. One patient displayed positive signals for CSF-specific oligoclonal proteins. Of the six patients, two were diagnosed with bilateral cerebellar atrophy, and two patients had nonspecific white matter changes. All of the patients received immunotherapy and rehabilitation treatment. Except for the Homer-3-positive patient, the remaining patients showed good prognosis. One patient relapsed.
Conclusion: ACA can be induced or aggravated by infection. The detection of neuronal antibodies is crucial for the precise diagnosis of ACA. Cerebellar system symptoms, such as dizziness, unsteady walking, nystagmus, and dysarthria, are the first and main manifestations of ACA. The head magnetic resonance imaging (MRI) in patients with ACA may be normal or may exhibit abnormalities including cerebellar atrophy and nonspecific white matter changes. Immunotherapy could be effective in most patients with ACA.
Autoimmune cerebellar ataxia (ACA), also known as autoimmune cerebellitis, is a cerebellar disorder mediated by abnormal autoimmune responses. As diagnostic markers of ACA, neuronal antibodies play an important role in its pathogenesis (1). Neuronal antibodies include both neuronal surface antibodies and intracellular antibodies. Neuronal surface antibodies act on specific antigens on synapses or on cell surfaces directly, resulting in nerve conduction dysfunction. When the target antigen is inside the cell, antigen-specific cytotoxic CD8 T cells recognize epitopes from intracellular proteins in the presence of major histocompatibility complex (MHC) class I antigen-presenting molecules, which is considered to be the main factor that leads to neuronal damage (2). There are several etiopathological factors of ACA, including infectious diseases and tumors, which can coexist with autoimmune diseases such as Hashimoto’s thyroiditis. Depending on the diagnosis of malignant tumors, ACA can be categorized into paraneoplastic and non-paraneoplastic types. Patients with certain types of ACA, such as non-paraneoplastic ACA, exhibit better prognosis and treatment outcomes compared with their paraneoplastic counterparts. Therefore, early diagnosis and treatment of patients with ACA are extremely important. ACA is rare and is sometimes difficult to differentiate from neurodegenerative cerebellar ataxia. At the same time, the positive rate of antibody detection in clinical practice is not high. Therefore, at present, ACA is not well understood. In this study, six cases of ACA with positive neuronal antibodies were investigated through analysis of the patients’ demographic and clinical data in order to improve our understanding of this disease.
Six patients with ACA who visited the Department of Neurology of the Affiliated Hospital of Hebei University from January 2018 to February 2023 were enrolled in this study. These patients tested positive for neuronal antibodies and were diagnosed with cerebellar ataxia.
The inclusion criteria were as follows: 1) cerebellar ataxia as the primary symptom; 2) neuronal antibody positivity either in the cerebrospinal fluid (CSF) or in serum samples; 3) patients with complete clinical data in accordance with treatment courses and routine follow-ups.
The exclusion criteria were: 1) absence of cerebellar ataxia as the primary symptom, despite neuronal antibody positivity in the CSF and/or serum; 2) cerebellar ataxia as the main manifestation, but negative for neuronal antibodies in the CSF and serum; and 3) other causes of cerebellar ataxia, such as genetic, metabolic, toxic, and drug-related etiology, were ruled out.
Serum and CSF antibody panels, including ACA [Tr(DNER)/Homer-3/mGluR1/Zic4/ITPR1/ATP1A3/PCA-2/CARPVIII], autoimmune encephalitis (GAD65/NMDAR/AMPAR1/AMPAR2/GABAR/CASPR2/LGI1/IgLON5/DPPX), and aquaporin protein-4 (AQP-4), were examined using both cell- and tissue-based assays (3, 4). Simultaneously, the paraneoplastic antibody profile (Hu/Yo/Ri/PNMA2/CV2/SOX1/amphiphysin) was also examined.
The following data were collected, analyzed, and summarized: sex, age at disease onset, medical history, neurological symptoms, serum autoantibodies and other immune indicators, routine CSF assays, cytology of the CSF, oligoclonal bands, magnetic resonance imaging (MRI) results, immunotherapy, and prognosis. The modified Rankin scale (mRS) and the Scale for the Assessment and Rating of Ataxia (SARA) were used to evaluate the neurological disabilities of patients at admission, at discharge, and at the last follow-up. An mRS ≤2 at the last follow-up was regarded as a good prognosis (5).
The clinical data of six patients were collected, organized using Excel software, and analyzed with SPSS 22.0 statistical software.
A total of six patients, including one man and five women, were analyzed in this study. The age range was 17–67 years, with a median age of 52.5 years. The disease duration (i.e., the interval between cerebellar symptom onset and diagnosis) ranged from 10 days to 10 months (median, 25 days). In this cohort, one patient was complicated with nephrotic syndrome, and three patients were comorbid with diabetes. None of the patients were accompanied with any systemic autoimmune diseases such as Hashimoto’s thyroiditis and Sjogren’s syndrome. Three patients (case nos. 4, 5, and 6) had definite prodromal symptoms, including fever and cough. One patient (case no. 1) developed post-onset fever with aggravation of cerebellar ataxia. There were no similar cases in the family history of these six patients (Table 1).
Persistent dizziness, limb ataxia, and gait abnormalities with or without dysarthria were the primary neurological symptoms in all the patients. Patient no. 2 had left limb ataxia on admission, who relapsed with bilateral limb ataxia 2 years later. The other five patients had bilateral limb ataxia on admission. Five patients had trunk ataxia (83.33%) and three patients had dysarthria (50%), and all of the patients had central nystagmus (100%). To date, no tumor has been detected in any of these six patients, as described in Table 1. On admission, the mRS scores of these patients ranged from 3 to 4 points, while the SARA scores ranged from 16 to 26 points (Table 2).
Neuronal antibodies were detected in six patients: five patients were positive for both serum and CSF samples, while one patient was positive for the serum sample only due to the absence of a CSF sample. There were one anti-Tr, three anti-GAD65, one anti-mGluR1, and one anti-Homer-3-positive cases in this cohort. However, all of the patients were negative for both AQP-4 and paraneoplastic antibodies.
Examination of the serum immune indicators revealed that all patients were negative for anti-thyroglobulin (TG) antibodies, anti-thyroid peroxidase (TPO) antibodies, anti-neutrophil cytoplasmic antibodies (ANCA), antinuclear antibody (ANA), anti-cardiolipin antibodies (ACA), autoantibodies, and immunoglobulin (Ig) antibodies.
The white blood cell (WBC) count and the CSF protein level were increased in four patients (case nos. 1, 4, 5, and 6), in accordance with predominant lymphocytic inflammation. Only one patient (case no. 4) was positive for CSF-specific oligoclonal bands.
Bilateral cerebellar atrophy was found in two patients: case no. 2 had bilateral cerebellar atrophy symptoms on admission (Figure 1), while case no. 5 was diagnosed with bilateral cerebellar atrophy during follow-up examinations in 2023 (Figure 2). Two patients (case nos. 3 and 4) presented with nonspecific white matter changes. The remaining patients had no obvious abnormalities.
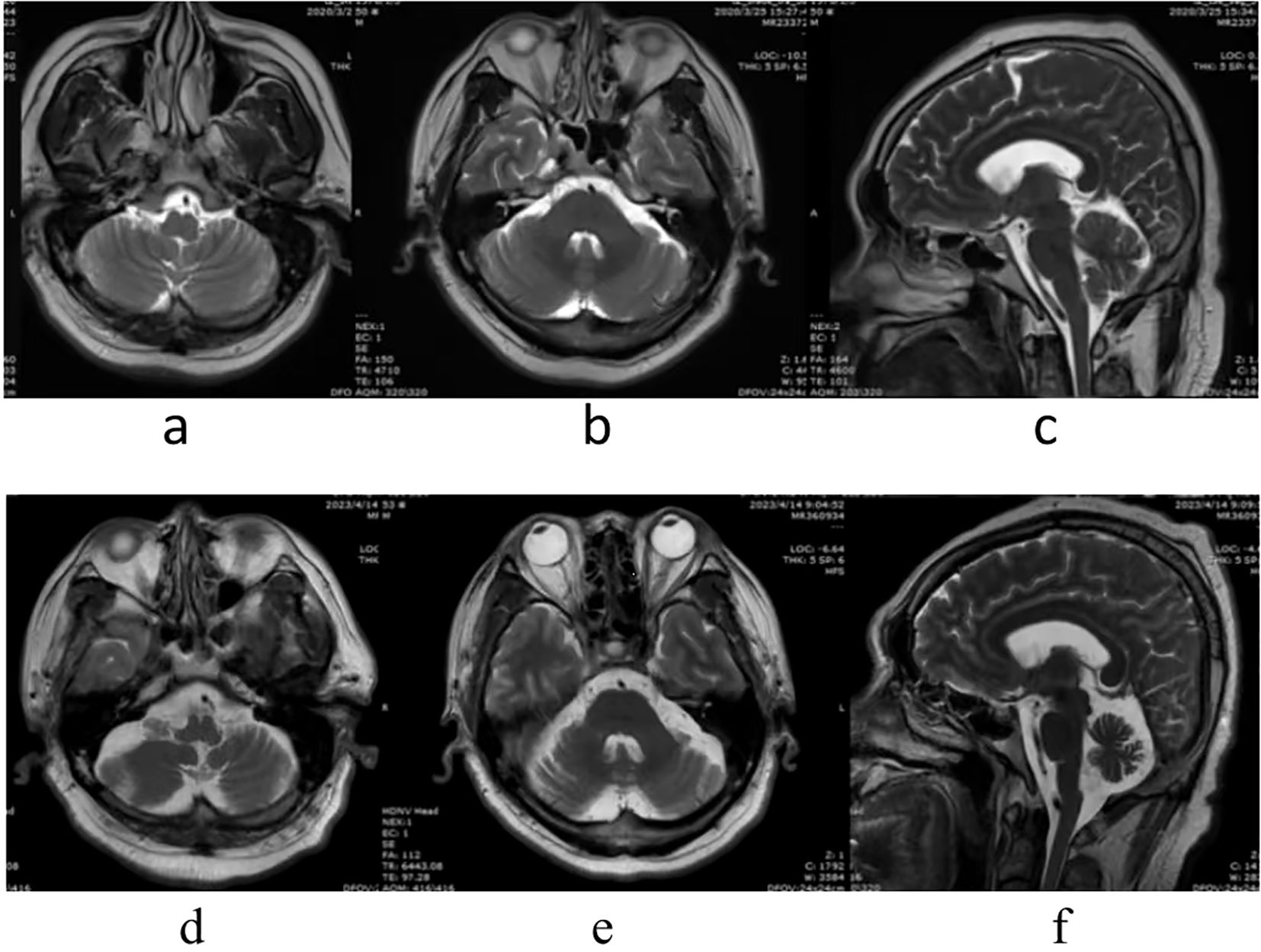
Figure 2. Patient no. 5 had no abnormality on admission, in March 25, 2020 (A–C). However, bilateral cerebellar atrophy was found during follow-up, in April 20, 2023 (D–F).
Computed tomography (CT) examinations of the chest, abdomen, and pelvic regions and screening of tumor markers were completed during the hospital stay of all patients. At the follow-up, tumor screening, including chest, abdominal, and pelvic CT, tumor marker screening, thyroid and lymph node ultrasonography, breast ultrasonography or mammography for the female patients, and urogenital ultrasonography for the male patient, was performed once every 6 months, with gastrointestinal endoscopy once a year. No tumor has been detected in the patients to date (Table 3).
All patients were treated with intravenous immunoglobulin (IVIg) doses at 0.4 g kg−1 day−1 for five consecutive days, combined with pulse corticosteroid therapy (methylprednisolone 1,000 mg/day for 3 days, followed by 500 mg/day for 3 days, 250 mg/day for 3 days, and 125 mg/day for 3 days). After completion of the treatment, the symptoms of cerebellar ataxia were improved in five patients, except for case no. 6. After pulse corticosteroid therapy, all of the six patients were tapered to oral prednisone (1 mg kg−1 day−1), the dose of which was reduced by 5 mg every 2 weeks (the total course of treatment was approximately 6 months). Mycophenolate mofetil (MMF) was prescribed to five patients as long-term immunotherapy. Case no. 2 was not treated with long-term immunotherapy drugs; instead, this patient was administered a repeated course of a combined regimen of IVIg and methylprednisolone plus MMF orally due to a relapse after 2 years of the first course. The condition of the patient improved after the second course. Except for case no. 6, all of the other patients achieved good prognosis (mRS ≤2) within 6 months (Table 2).
ACA is a common cause of sporadic cerebellar ataxia, which can occur in both children and adults (6). Compared with hereditary ataxia, ACA is more treatable with proper and timely diagnosis and treatments. Depending on the etiopathology, the clinical presentation, and the expression of autoantibodies, ACA can be divided into the following subtypes: primary ACA (PACA), paraneoplastic cerebellar degeneration (PCD), autoimmune encephalitis-associated ACA, anti-glutamate decarboxylase (GAD)-associated cerebellar ataxia, opsoclonus–myoclonus syndrome (OMS), and Miller Fisher syndrome (MFS). Among these, PACA and PCD are the most common pathologies (5, 7). Hongzhi Guan’s team found that, with the development of novel anti-cerebellar Purkinje cell antibodies and the application of tissue-based immune assays in major neural immunology study centers, the technology of neuronal antibody detection has been dramatically improved. In the new version of the diagnostic criteria for PACA in 2022, the importance of neuronal antibody detection was emphasized, and the simultaneous applications of cell- and tissue-based assays were recommended in order to compare and validate the results, which can further improve the accuracy and sensitivity of the tests (5).
The neuronal antibody panel includes both neuronal surface antibodies and intracellular antibodies, which have different pathomechanistic implications (2, 8). In this study, the anti-Tr, anti-GAD65, and anti-Homer-3 antibodies were the intracellular antibodies, while the anti-mGluR1 antibody served as a neuronal surface marker antibody. In general, different neuronal antibodies correspond to specific neurological syndromes. For example, in PCD samples, the anti-Tr antibody mainly binds to the extracellular segment of the transmembrane protein DNER in the cytoplasmic and dendritic regions of Purkinje cells. Hence, patients positive for anti-Tr antibodies often present with simple cerebellar ataxia, although some patients exhibit sensory peripheral neuropathies or limbic encephalitis (9). The anti-GAD65 antibody has a direct pathogenic effect on the onset of ataxia by interfering with the release of GABA and by influencing the regulation of excitability of Purkinje cells. The pathogenicity of anti-GAD65 antibodies is related to the recognized antigenic sites, as different epitopes are associated with different diseases, such as stiff person syndrome, cerebellar ataxia, type I diabetes, and thyroiditis (10). However, the PACA, anti-Homer-3, and anti-mGluR1 antibodies are relatively rare, and their direct roles in the pathogenicity of ACA remain to be investigated further (1). Homer-3, encoded by the HOMER3 gene, is mainly expressed on the axons of Purkinje cells and is involved in the regulation of Ca2+ metabolism through interaction with mGluR1 and IP3R. The anti-Homer-3 antibody can act like an anti-Purkinje cell antibody; therefore, the main disease manifestation of related cases positive for this antibody is cerebellar ataxia (11). As a metabolic glutamate receptor, mGluR1 is highly expressed in the dendrites of cerebrocortical Purkinje cells. mGluR1 plays an important role in the rapid signaling of Purkinje cell dendrites. Anti-mGluR1 antibodies can affect cerebellar function by preventing Purkinje cells from inducing synaptic actions. Recent studies have shown that anti-mGluR1 antibodies can specifically lead to a significant reduction in the total and synaptic mGluR1 levels in cultured neurons without affecting the density of other synaptic proteins, such as postsynaptic dense protein 95 (12). ACA could also have overlapping antibodies. The cohort study by Hongzhi Guan’s team revealed that 12.5% of patients with ACA present autoimmune encephalitis mediated by neuronal surface antibodies, such as anti-NMDAR, anti-CasPR2, anti-DPPX, and anti-GABAR (5). Notably, no such overlapping antibodies were found in any of the patients in this cohort, which might be related to the small number of enrolled cases. Highly specific neuronal antibodies, such as anti-Tr, anti-Yo, anti-Hu, anti-Ri, and anti-MA2 antibodies, in the serum and CSF are the key diagnostic biomarkers of PCD (13). In this study, case no. 1, who was positive for the anti-Tr antibody, was not diagnosed with malignant tumors such as lymphoma even after 6 years of follow-up. In the other five patients, the tumor screening also resulted negative. As PCD might appear earlier than the tumor for months or even years, it is necessary to continuously monitor the occurrence of a tumor in patients with positive PCD antibody (14).
In this study, the initial symptom in all patients was cerebellar ataxia, which presented as persistent dizziness (100%), nystagmus (100%), and limb ataxia (100%). The vestibulocerebellar system is an important part of the balanced triad (vision, proprioception, and the vestibulocerebellar system). The vestibulocerebellum receives nerve impulses from the vestibular organ with regard to the spatial position of the head and head movement, and damage to this structure can lead to balance disorders such as dizziness and gait instability, as well as eye movement disorders and central nystagmus. It has been reported that spontaneous downbeat nystagmus (sDBN) can be caused by a variety of lesions involving the cerebellar flocculus or para-flocculus, including paraneoplastic syndrome and encephalitis, among others. In the early stage of these diseases, sDBN and oscillopsia may be the main symptoms and signs of some patients even in a longer period of time. Electronystagmus can be used to assess vestibulocerebellar system function in patients with inconspicuous nystagmus or those with no obvious lesions on imaging. In this cohort, the limb ataxia was bilateral in most cases, except for case no. 2, who had left limb ataxia on admission and who relapsed with bilateral limb ataxia 2 years later. According to the literature review, ACA cases manifested by unilateral ataxia are rare, and the underlying mechanism is unknown. The clinical characteristics of the cases in this study were related to the inclusion criteria, and it should be noted that cerebellar ataxia is not the only phenotype of ACA. The study by Hongzhi Guan’s team was based on 127 patients with ACA and found that many PACA patients had diverse neurological phenotypes, including cerebellar ataxia as the core symptom and other manifestations such as pyramidal tract sign and peripheral nerve radiculopathy as secondary complications (5).
In this study, all patients were positive for serum neuronal antibodies. Except for case no. 5, whose CSF sample was absent, the other five patients were all positive for corresponding antibodies in their CSF samples. The CSF-specific oligoclonal bands indicate the synthesis of intrathecal Ig, which was positive (16.67%) only in case no. 4. The relationship between the oligoclonal bands and neuronal antibodies in the CSF remains unclear. Liu et al. observed that the positive rate of neuronal antibodies in the CSF was higher in ACA patients with oligoclonal bands, but suggested that the study sample size needs to be expanded for further confirmation (15).
Three patients (case nos. 4, 5, and 6) had prodromal infection symptoms, while case no. 1 developed a fever on day 3 of admission and later showed aggravated cerebellar ataxia symptoms. The WBC count and the CSF protein level were increased in four patients (case nos. 1, 4, 5, and 6). These results suggest that immune-associated cerebellitis may be secondary to infection, but the specific mechanism remains unknown. It has been shown that post-infectious cerebellitis (PIC) is mediated by an abnormal autoimmune process triggered by infection (most commonly chicken pox), which is more common in young children (7). Patients with ACA without definite infections occasionally develop fever and other infection-like prodromal symptoms, similar to patients with anti-mGluR2 and anti-Tr (16, 17). However, it remains unclear how the prodromal symptoms indicate infection and immune activation in patients with ACA (18).
It has been noted that cerebellar atrophy can be observed on the brain MRI in patients with cerebellar ataxia, and the severity of atrophy depends on the duration of the disease (19). Of the six cases, two patients (case nos. 1 and 6) displayed no significant abnormalities in MRI examinations. Case no. 2 had bilateral cerebellar atrophy on admission, and the course of the disease had been 10 months at that time. Case no. 5 developed cerebellar atrophy in the third year of the course of disease. However, the symptoms of both patients (case nos. 2 and 5) were significantly improved after immunotherapy and rehabilitation treatments. Patient no. 6 had normal MRI, but poor treatment outcomes, suggesting that the imaging findings were not parallel to the severity of the clinical symptoms and that dynamic observations are required. The other two patients (case nos. 3 and 4) had nonspecific white matter changes, suggesting that MRI in patients with ACA could show abnormalities other than cerebellar atrophy (20).
Similar to other cerebellar ataxia, the common pathological outcome of ACA is irreversible loss of Purkinje cells. However, Mitoma et al. (19) used physiological methods to monitor cerebellar functional survival indicators, and the results showed that ACA is reversible and treatable in the early stage, suggesting the importance of early diagnosis and treatment. There is no widely accepted definition of relapse in ACA currently; therefore, we referred to that in autoimmune encephalitis as exacerbation of cerebellar ataxia after clinical improvement or stabilization for at least 2 months (21). Relapse is not uncommon in ACA, which can present with an initial relapsing course, often occurring after corticosteroid discontinuation or IVIg treatment, especially in patients with PCD (16, 22). Of the six patients, only patient no. 2 relapsed after the first admission, but improved after treatment with the second course of first-line immunotherapy plus MMF oral doses. It was speculated that the relapse might have been caused by the failure of the patient to tolerate long-term immunotherapy at the first visit. Therefore, based on first-line immunotherapy, a timely and effective long-term immunotherapy is extremely important. Although patient no. 6, who was positive for the anti-Homer3 antibody, had timely treatment and routinely received medications, the outcome of immunotherapy was poor, which might be related to the type of antibody involved. Thus far, only more than 10 cases of ACA with the anti-Homer-3 antibody have been reported worldwide. A number of researchers have summarized these reports and found that, after immunotherapy, only some of the patients’ conditions improved and that even some of the conditions worsened, for which the specific mechanism is still unclear (23).
This study has certain limitations. Firstly, this is a single-center study with a small number of patients aged over 14 years, which could have led to bias in the results. In the future, we will increase the number of study cases and conduct multicenter prospective cohort studies to better describe the clinical features of ACA-associated neuronal antibodies.
In summary, ACA is a common cause of sporadic cerebellar ataxia and has a certain degree of curability. ACA can be induced or aggravated by infectious diseases. Neuronal antibodies play an important role in the pathogenesis of ACA, and the detection of cerebellar autoantibodies can help in the diagnosis and in guiding the screening of malignant tumors in these patients. Cerebellar symptoms such as persistent dizziness, unsteady walking, central nystagmus, and dysarthria are the initial and major manifestations of ACA. The relationship between CSF-specific oligoclonal bands and the expression of neuronal antibodies in the CSF needs to be further studied with larger samples. In patients with ACA, MRI may be normal or may show certain abnormalities such as cerebellar atrophy and nonspecific white matter changes. Dynamic observations of the imaging findings are required due to these not being parallel to the severity of the clinical symptoms. Early diagnosis and implementation of first-line immunotherapy (often with glucocorticoids and/or IVIg) can improve the neurological symptoms in patients with ACA. Standardized long-term immunotherapy can stabilize the disease and reduce its recurrence. At the same time, the type of antibody may affect the prognosis of patients.
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
The studies involving humans were approved by the institutional ethics committee of Affiliated Hospital of Hebei University. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin. Written informed consent was obtained from the individual(s), and minor(s)’ legal guardian/next of kin, for the publication of any potentially identifiable images or data included in this article.
YC: Conceptualization, Data curation, Funding acquisition, Investigation, Writing – original draft. ZH: Conceptualization, Investigation, Methodology, Writing – original draft. YNC: Investigation, Methodology, Writing – review & editing. XC: Investigation, Methodology, Writing – review & editing. NL: Formal analysis, Methodology, Project administration, Writing – review & editing. TL: Formal analysis, Methodology, Project administration, Writing – review & editing. QZ: Formal analysis, Methodology, Project administration, Writing – review & editing. JL: Resources, Supervision, Validation, Writing – review & editing. WD: Funding acquisition, Methodology, Supervision, Validation, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was supported by grants from the Government-funded Clinical Medicine Excellence Training Program in 2022 (no. 361007), Medical Science Foundation of Hebei University in 2023 (no. 2023A03), and Foundation Project of Affiliated Hospital of Hebei University in 2021 (no. 2021Z012).
We express our gratitude to all the patients who contributed to this study.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Hadjivassiliou M, Graus F, Honnorat J, Jarius S, Titulaer M, Manto M, et al. Diagnostic criteria for primary autoimmune cerebellar ataxia-guidelines from an intemational task force on immune-mediated cerebellar ataxias. Cerebellum. (2020) 19:605–10. doi: 10.1007/s12311-020-01132-8
2. Yshii L, Bost C, Liblau R. Immunological bases of paraneoplastic cerebellar degeneration and therapeutic implications. Front Immunol. (2020) 11:991. doi: 10.3389/fimmu.2020.00991
3. Mange L, Haitao R, Lixin Z, Siyuan F, Jing W, Hongzhi G. Cerebellar ataxia and myeloradiculopathy associated with AP3B2 antibody: a case report and literature review. J Neurol. (2021) 268:4163–69. doi: 10.1007/s00415-021-10496-8
4. Ren H, Zhao D, Xu X, Yang Y, Fan S, Li W, et al. Paraneoplastic cerebellar degeneration associated with anti-protein kinase Cgamma antibodies in a Chinese patient. J Neuroimmunol. (2020) 350:577408. doi: 10.1016/j.jneuroim.2020.577408
5. Liu M, Ren H, Zhu Y, Fan S, Bai L, Wang J, et al. Autoimmune cerebellar ataxia: etiology and clinical characteristics of a case series from China. Cerebellum. (2023) 22:379–85. doi: 10.1007/s12311-022-01412-5
6. Hadjivassiliou M, Martindale J, Shanmugarajah P, Grünewald RA, Sarrigiannis PG, Beauchamp N, et al. Causes of progressive cerebellar ataxia: prospective evaluation of 1500 patients. J Neurol Neurosurg Psychiatry. (2017) 88:301–9. doi: 10.1136/jnnp-2016-314863
7. Mitoma H, Manto M, Hampe CS. Immune-mediated cerebellar ataxias: practical guidelines and therapeutic challenges. Curr Neuropharmacol. (2019) 17:33–58. doi: 10.2174/1570159X16666180917105033
8. Nanri K, Yoshikura N, Kimura A, Nakayama S, Otomo T, Shimohata T, et al. Cerebellar ataxia and autoantibodies. Brain Nerve. (2018) 70:371–82. doi: 10.11477/mf.1416201010
9. Jarius S, Wildemann B. ‘Medusa head ataxia’: the expanding spectrum of Purkinje cell antibodies in autoimmune cerebellar ataxia. Part 3: Anti-Yo/CDR2, anti-Nb/AP3B2, PCA-2, anti-Tr/DNER, other antibodies, diagnostic pitfalls, summary and outlook. J Neuroinflamm. (2015) 12:168. doi: 10.1186/s12974-015-0358-9
10. McKeon A, Tracy JA. GAD65 neurological autoimmunity. Muscle Nerve. (2017) 56:15–27. doi: 10.1002/mus.25565
11. Liu M, Ren H, Fan S, Zhang W, Xu Y, Zhao W, et al. Neurological autoimmunity associated with Homer-3 antibody: a case series From China. Neurol Neuroimmunol Neuroinfamm. (2021) 8:e1077. doi: 10.1212/NXI.0000000000001077
12. Spatola M, Petit Pedrol M, Maudes E, Simabukuro M, Muñiz-Castrillo S, Pinto AL, et al. Clinical features, prognostic factors, and antibody effects in anti-mGluR1 encephalitis. Neurology. (2020) 95:e3012–25. doi: 10.1212/WNL.0000000000010854
13. Nanri K, Okuma M, Sato S, Yoneda M, Taguchi T, Mitoma H, et al. Prevalence of autoantibodies and the emcacy of immunotherapy for autoimmune cerebellar ataxia. Intern Med. (2016) 55:449–54. doi: 10.2169/internalmedicine.55.5156
14. Damato V, Papi C, Spagni G, Evoli A, Silvestri G, Masi G, et al. Clinical features and outcome of patients with autoimmune cerebellar ataxia evaluated with the scale for the assessment and rating of ataxia. Eur J Neurol. (2022) 29:564–72. doi: 10.1111/ene.15161
15. Jing L, Chunqiu F, Aihua L, Dongju Y, Fang C, Ningning H. Analysis of 15 cases anti-neuronal antibodies related autoimmune cerebellar ataxia. J Brain Nervous Dis. (2023) 31:336–42.
16. Ruiz-García R, Martínez-Hernández E, Joubert B, Petit-Pedrol M, Pajarón-Boix E, Fernández V, et al. Paraneoplastic cerebellar ataxia and antibodies to metabotropic glutamate receptor 2. Neurol Neuroimmunol Neuroinfamm. (2020) 7:e658. doi: 10.1212/NXI.0000000000000658
17. Weihua Z, Haitao R, Fang F, Xunzhe Y, Jing W, Hongzhi G. Neurochondrin antibody serum positivity in three cases of autoimmune cerebellar ataxia. Cerebellum. (2019) 18:1137–42. doi: 10.1007/s12311-019-01048-y
18. Newrick L, Hoggard N, Hadjivassiliou M. Recognition and management of rapid-onset glutenataxias: case series. Cerebellum Ataxias. (2021) 8:16. doi: 10.1186/s40673-021-00139-z
19. Mitoma H, Adhikari K, Aeschlimann D, Chattopadhyay P, Hadjivassiliou M, Hampe CS, et al. Consensus paper: neuroimmune mechanisms of cerebellar ataxias. Cerebellum. (2016) 15:213–32. doi: 10.1007/s12311-015-0664-x
20. Muñiz-Castrillo S, Vogrig A, Ciano-Petersen NL, Villagrán-García M, Joubert B, Honnorat J. Novelties in autoimmune and paraneoplastic cerebellar ataxias: twenty years of progresses. Cerebellum. (2022) 21:573–91. doi: 10.1007/s12311-021-01363-3
21. Xu X, Lu Q, Huang Y, Fan S, Zhou L, Yuan J, et al. Anti-NMDAR encephalitis: a single-center, longitudinal study in China. Neurol Neuroimmunol Neuroinfamm. (2019) 7:e633. doi: 10.1212/NXI.0000000000000633
22. Mitoma H, Hadjivassiliou M, Honnorat J. Guidelines for treatment of immune-mediated cerebellar ataxias. Cerebellum Ataxias. (2015) 2:14. doi: 10.1186/s40673-015-0034-y
Keywords: autoimmune cerebellar ataxia, neuronal antibody, immunotherapy, cerebellar atrophy, prognosis
Citation: Cai Y, Hua Z, Chen Y, Chen X, Liu N, Liu T, Zhou Q, Li J and Di W (2025) Clinical features of autoimmune cerebellar ataxia related to neuronal antibodies. Front. Immunol. 16:1497695. doi: 10.3389/fimmu.2025.1497695
Received: 29 September 2024; Accepted: 27 January 2025;
Published: 13 February 2025.
Edited by:
Harry Alexopoulos, National and Kapodistrian University of Athens, GreeceReviewed by:
Jinming Han, Capital Medical University, ChinaCopyright © 2025 Cai, Hua, Chen, Chen, Liu, Liu, Zhou, Li and Di. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Weiying Di, ZGl3ZWl5aW5nQDEyNi5jb20=; Jinghua Li, bGlqaW5naHVhNTY3ODlAc2luYS5jb20=
†These authors have contributed equally to this work and share first authorship
‡These authors have contributed equally to this work and share last authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.