
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Immunol., 04 March 2025
Sec. Cancer Immunity and Immunotherapy
Volume 16 - 2025 | https://doi.org/10.3389/fimmu.2025.1490491
 Xueshuai Ye1
Xueshuai Ye1 Min Ge1
Min Ge1 Mengtian Tan1
Mengtian Tan1 Yongqiang Wu2
Yongqiang Wu2 Haiqiang Zhang3
Haiqiang Zhang3 Zexian Fu4*
Zexian Fu4*CD19-targeted chimeric antigen receptor (CAR) T-cell therapy has achieved marvelous results in the treatment of patients with relapsed and/or refractory B-cell lymphomas, B-cell acute lymphoblastic leukemia, and multiple myeloma. As a new treatment method that has changed the existing treatment paradigm, there has been a short time from its emergence to FDA approval. However, with the increasing number of cases and the passage of time, hidden problems have gradually been exposed. In this review, we summarize the short- and long-term toxicity, such as secondary T-cell tumors and lethal CAR tumors, of patients with hematologic malignancies treated with CD19-CAR-T cells, including cytokine release syndrome (CRS), ICANS, and secondary malignancies with low occurrence rates but high mortality, such as secondary T cell tumors and lethal CAR tumors, which may be related to the gene modification mechanism of viral vectors currently approved for CAR-T cells. We also discuss potential investigational strategies designed to improve the safety of CAR-T-cell therapy.
● CD19-targeted CAR-T-cell therapy is now approved for the treatment of relapsed and/or refractory B-cell lymphomas and B-cell acute lymphoblastic leukemia.
● The most prominent long-term toxicities after treatment include neurotoxicity, thrombocytopenia, and B-cell depletion, along with associated symptoms such as infections and hypogammaglobulinemia.
● Strategies to improve the durability of CAR-T-cell therapy responses include novel CAR designs, such as dual-specific CARs, and modifications to the manufacturing process.
● The preparation process using viral vectors for CAR T cells may result in lethal CAR tumor cells and T-cell-associated secondary malignancies. Potential safety-enhancing strategies involve the use of precise gene editing and nonviral vector tools to avoid the activation of oncogenes in the T-cell genome.
Chimeric antigen receptor (CAR) is an engineered fusion protein that recognizes specific antigens present on tumor cells and activates the first and second signals of T cells. By genetically modifying T cells to express CARs, T cells can target tumor cells and produce antitumor immune responses (1, 2). CD19 is a B-cell-specific antigen that is expressed on both normal and malignant B cells. CAR-T cells targeting CD19 have achieved complete remission rates of 40-54%, 67%, and 69-74% in clinical trials for R/R aggressive B-cell lymphoma, mantle cell lymphoma, and inert B-cell lymphoma, respectively (3–6). Currently, the FDA has approved the launch of six CD19 or BCMA-CAR-T-cell therapies, including KYMRIAH (tisagenlecleucel, a CD19 CAR-T cell), YESCARTA (axicabtagene ciloleucel, a CD19 CAR-T cell), TECARTUS (brexucabtagene autoleucel, a CD19 CAR-T cell), BREYANZI (lisocabtagene maraleucel, a CD19 CAR-T cell), ABECMA (idecabtagene vicleucel, a BCMA CAR-T cell) and CARVYKTI (ciltacabtagene autoleucel, a BCMA CAR-T cell) (7, 8).
The revolutionary therapeutic effects of CAR-T-cell therapy and rapid FDA approval have changed the treatment pattern for hematological malignancies. Currently, approved CAR-T-cell products use second-generation CAR structures, including antigen binding domains, hinge and transmembrane domains, costimulatory domains (derived from CD28 or 4-1BB) (9, 10), and CD3 domains. ζ-T cells activate these domains (11) and express CAR structures through viral transduction of patient-derived T cells. However, as a new type of “living drug”, CD19 CAR-T cells can cause various degrees of cytokine release syndrome (12), immune effector cell-associated neurotoxicity syndrome (ICANS), and various forms of special B-cell deficiency, such as infection and low toxicity (Figure 1) (13–15). At present, relatively complete risk management measures have been established, but the potential long-term adverse events associated with CAR-T-cell therapy are still unknown. We summarize the limitations and risks of CD19 CAR-T-cell therapy and potential solutions to these problems.
After CAR-T-cell infusion, once the interaction between immune cells and tumor cells is too strong, a cytokine storm can occur due to the release of a large amount of cytokines. This is one of the most common and severe side effects, with the severity of CRS classified into 4 levels ranging from mild to severe based on a combination of fever, low blood pressure, and hypoxemia. In clinical trials of CAR-T-cell therapy for B-ALL and large B-cell lymphoma, the incidence of cytokine storms ranged from 57% to 100%, with treatment-related deaths accounting for less than 5%. Cytokine storms typically occur within 1-14 days of CAR-T-cell administration (16, 17). Currently, the occurrence and severity of CRS are assessed by continuously monitoring patient IL6 levels and the biomarker C-reactive protein (CRP) (18). In the past, high doses of corticosteroids were avoided in CAR-T-cell therapy due to the potential risk of treatment failure, so both tocilizumab and corticosteroids were only used to treat severe CRS (19). However, in recent clinical practice, prophylactic use of tocilizumab and corticosteroids in the early stages after CAR-T-cell infusion has been shown to prevent the occurrence of severe CRS (20, 21). To eliminate and limit the cytotoxicity of CAR-T cells, safety switches can be integrated into CAR-T cells to deactivate and eliminate them. Safety switches include suicide genes, such as the FK506-binding protein fusion protein (22) and caspase-9 (iCasp9) (23), which, when integrated into CARs and exposed to a synthetic inducer of dimerization drug, undergo dimerization and ultimately lead to cell apoptosis.
ICANS is defined as the pathological process affecting the central nervous system caused by the activation or involvement of endogenous or exogenous T cells and/or other immune effector cells, including CAR-T cells, resulting from immunotherapy. It is the second most common complication that may occur after CAR-T-cell therapy, with an incidence rate of 20% to 60% and a severe ICANS (≥3 grade) incidence rate of 12% to 30% (24). ICANS typically occurs after cytokine release syndrome (CRS) and often occurs after CRS has resolved. Due to the poor efficacy of tocilizumab, ICANS is considered a separate adverse reaction from CRS (25). The mechanism of ICANS may be as follows: brain mural cells are important components of the blood−brain barrier, and CD19 is specifically expressed in brain mural cells. CAR-T-cell therapy-induced cytokine release syndrome (CRS) can disrupt blood−brain barrier integrity, allowing CAR-T cells to penetrate the blood−brain barrier (26). As brain mural cells express CD19, they become targets of CD19 CAR-T cells, leading to further damage to the blood−brain barrier (Figure 1). This allows a large number of CAR-T cells to enter the central nervous system, causing severe neurotoxicity. The use of tocilizumab, which binds to the interleukin-6 (IL-6) receptor, can increase serum free IL-6 levels, further elevating IL-6 concentrations in the cerebrospinal fluid and potentially exacerbating neurotoxicity (27). Therefore, the use of corticosteroids is more important than the use of tocilizumab in the management of ICANS. Prophylactic use of the IL-1 receptor antagonist anakinra can significantly reduce the incidence of ICANS caused by CD19-targeted CAR-T-cell therapy without affecting the therapeutic effect of CAR-T cells (28). The lymphodepletion regimens prior to CD19-CAR-T reinfusion are mainly used to remove lymphocytes from the patient through cytotoxic chemotherapy. The goal is to remove the original lymphocyte population including T cells in the patient’s body to ensure better implantation and expansion of the reinfused T cells in vivo. This will enhance the survival, persistence, and antitumor activity of reinfused cells by decreasing myeloid suppressor cells and regulatory T cells, increasing homeostatic cytokines such as interleukin (IL)-12 and IL-13, and eliminating resident T cells competing for these trophic cytokines. Phase I/II studies of JCAR014 (late-stage B-cell malignancies) and JCAR017 (pediatric ALL) have shown that pretreating patients with fludarabine and cyclophosphamide to remove lymphocytes before administering CD19-CAR-T cells can enhance the therapeutic effect of CAR-T cells (29). However, in some clinical trials, the use of high doses of fludarabine (>20 mg/h/L) significantly increased the number of deaths from ICANS compared to that in the low-dose group (3 vs 0), suggesting that fludarabine may exacerbate the occurrence of ICANS (30). In addition, in phase I/II studies of ROCKET, the incidence of severe neurotoxic events increased after the addition of fluorouracil to the pretreatment chemotherapy regimen, including the deaths of two patients with treatment-related brain edema (31).
Post-CAR-T thrombocytopenia is a common hematological toxicity observed in patients. A predictive model for immune therapy-related hematological toxicity, known as CAR-HEMATOTOX, indicates that 62% of patients experience thrombocytopenia (32). Overall, thrombocytopenia following CAR-T treatment exhibits a biphasic pattern, which may be associated with pretreatment chemotherapy, bone marrow hematopoietic reserves, and levels of inflammation. Some patients may develop isolated thrombocytopenia after CAR-T therapy, with laboratory findings and treatment characteristics meeting the diagnostic criteria for immune thrombocytopenic purpura (ITP); however, the underlying mechanisms remain unclear (33). Additionally, another condition related to CAR-T, termed CAR-T-associated coagulopathy (CARAC), also presents with thrombocytopenia. In the early phase following CAR-T cell infusion—typically within 28 days—most patients experience this condition in association with CRS, leading to bleeding and/or thrombotic events alongside decreased platelet counts and abnormal coagulation parameters (34). Dynamic monitoring, early identification, and graded interventions based on CRS severity are essential for the prevention and management of CARAC (35).
Due to the expression of CD19 on both normal B cells and malignant tumor cells, long-term B-cell depletion, which is the expected “off-tumor” effect of CD19 CAR-T cells, is a common phenomenon following CD19-targeted CAR-T-cell therapy. Maude et al. demonstrated B-cell regeneration impairment in 83% of ALL patients at 6 months after receiving tisagenleucel treatment (36). Studies have shown that 25-38% of patients continue to experience B-cell depletion even years after CAR-T-cell infusion. In some of these patients, detectable CAR-expressing T cells may be lost (37). Immunoglobulin depletion is a result of impaired B-cell and plasma cell activity. Park et al. reported that within one month after CD19 CAR-T-cell therapy, 83% of ALL patients had low IgG levels (38). Long-term follow-up data show that 18-74% of patients treated with CD19-targeted CAR-T cells continue to experience IgG depletion for years after cell infusion. The main treatment for persistent hypogammaglobulinemia following CAR-T-cell therapy is symptomatic intravenous immunoglobulin infusion (IVIG) (39, 40). In patients with available data, 67% of patients had low levels of IgG/IVIG replacement. Locke et al. reported that 44% of patients with sustained remission in the ZUMA-1 study received immunoglobulin injections (IVIGs) (41).
Cancer cells expressing the CD19 antigen targeted by CD19 CAR-T cells exert strong selective pressure (Figure 2A). Due to the nonessential nature of the CD19 antigen for cell survival, downregulation or loss of CD19 expression serves as a natural escape pathway for the target antigen (42). The accurate quantification of relapse rates due to CD19 antigen escape becomes complex because tissue collection is lacking after relapse. It has been reported that the frequency of CD19-negative relapses in patients with leukemia and lymphoma is 27% (Figure 2B) (43). Additionally, as current CD19-CAR T cell therapies typically use mouse-derived single chain variable fragment (scFv), several studies have shown that following the infusion of CAR-T cells into patients, the body generates human anti-mouse antibodies (HAMAs) neutralizing the CD19 scFv, which not only leads to allergic reactions but also results in CAR-T-cell failure (44).
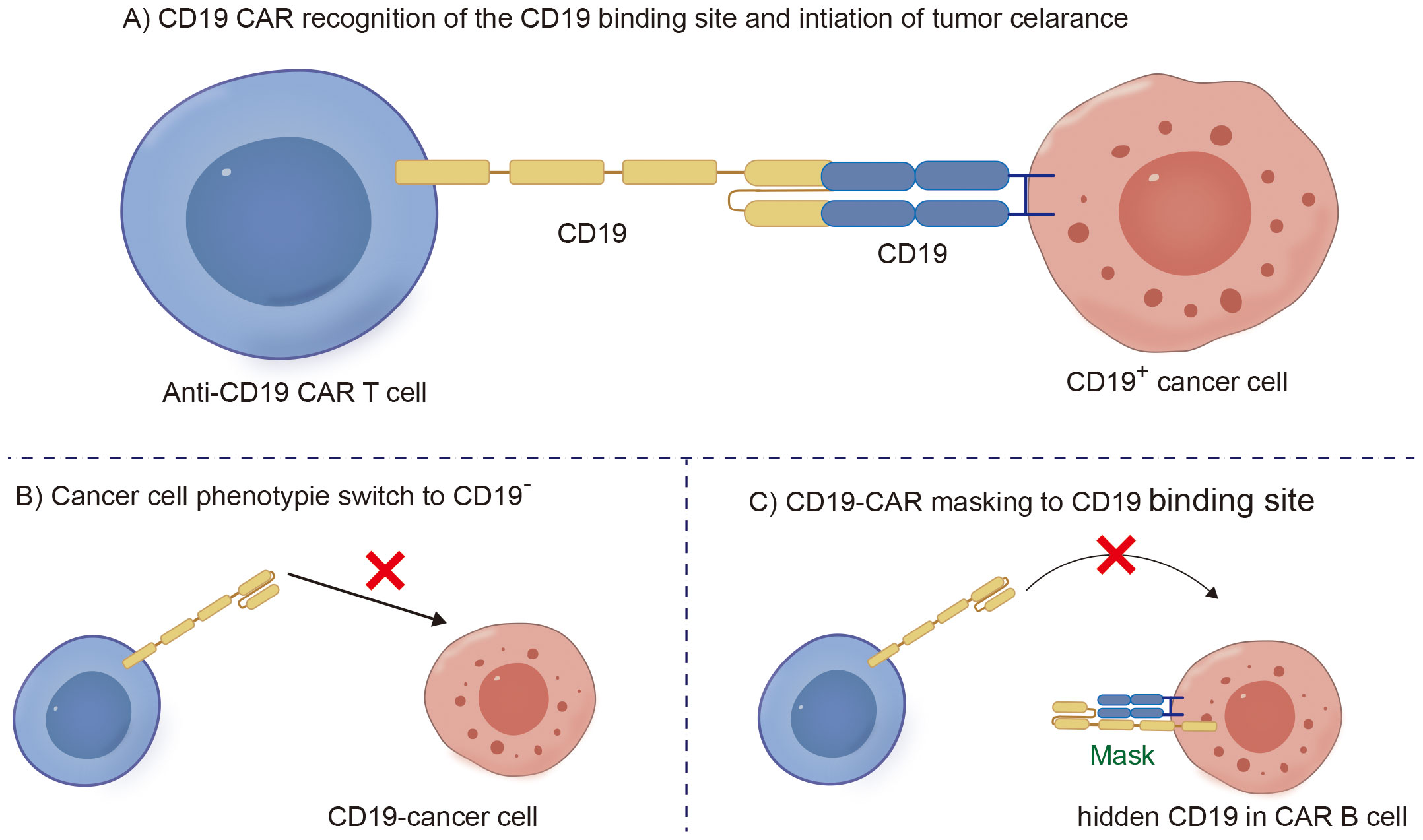
Figure 2. Schematic diagram of CD19-CAR T clearing B malignant cells and B cell CD19 antigen loss mechanism. (A) CD19 CAR recognition of the CD19 binding site and intiation of tumor celarance. (B) Cancer cell phenotypie switch to CD19 negative. (C) CD19-CAR masking to CD19 binding site.
Due to the multifaceted mechanisms of malignant tumors, under the same carcinogenic factors, primary malignant tumors in different systems may appear at different times, leading to a greater risk of secondary malignancies in patients with hematologic malignancies than in the general population (45). We conducted a statistical analysis of the occurrence of secondary malignancies in 273 patients treated with CD19 CAR-T-cell therapy across 5 clinical trials. These secondary malignancies were mainly concentrated in patients with primary MDS and nonhematologic malignancies, including lung cancer, prostate cancer, and ovarian cancer (41, 46–50). For example, in the CARTITUDE-1 clinical trial, 10% (10/97) of patients were observed to develop myeloid malignancies, including myelodysplastic syndrome (MDS), acute myeloid leukemia (AML), or MDS progressing to AML after receiving treatment with idecabtagene vicleucel.
Notably, in addition to the aforementioned secondary malignancies, as the number of patients receiving CD19 CAR-T-cell therapy increases, attention is gradually being given to patients with a lower incidence of secondary tumors. As of December 2023, the FDA reported 22 cases of T-cell cancers occurring after treatment with CAR-T-cell products. These cancers include T-cell lymphoma, T-cell large granular lymphocytic leukemia, peripheral T-cell lymphoma, and cutaneous T-cell lymphoma. In 14 patients with sufficient data, cancer occurred within 2 years after CAR-T-cell therapy (1 to 19 months). In three cases where genetic sequencing was performed, CAR transgenes were detected in the malignant clones, indicating the potential involvement of CAR T cell-cells in the development of T-cell cancers (51). Mechanistically, since currently available CD19 CAR-T-cell therapies use viral vectors for gene delivery and modification, the random insertion of the gene encoding CAR into the infected T-cell genome may pose a potential oncogenic risk (Figures 2C, 3, 4) (52).
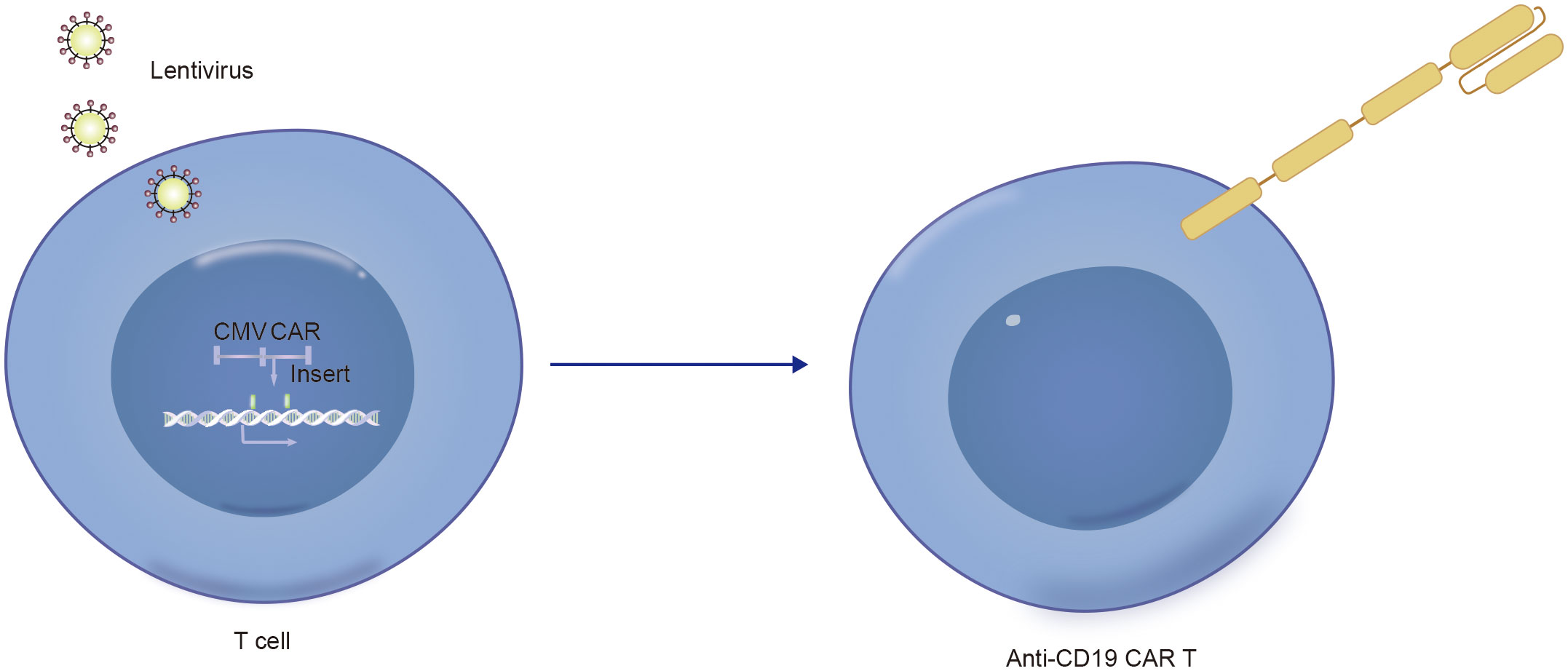
Figure 3. Technical schematic diagram for preparing CD19-CAR T by infecting T cells with lentivirus vector.
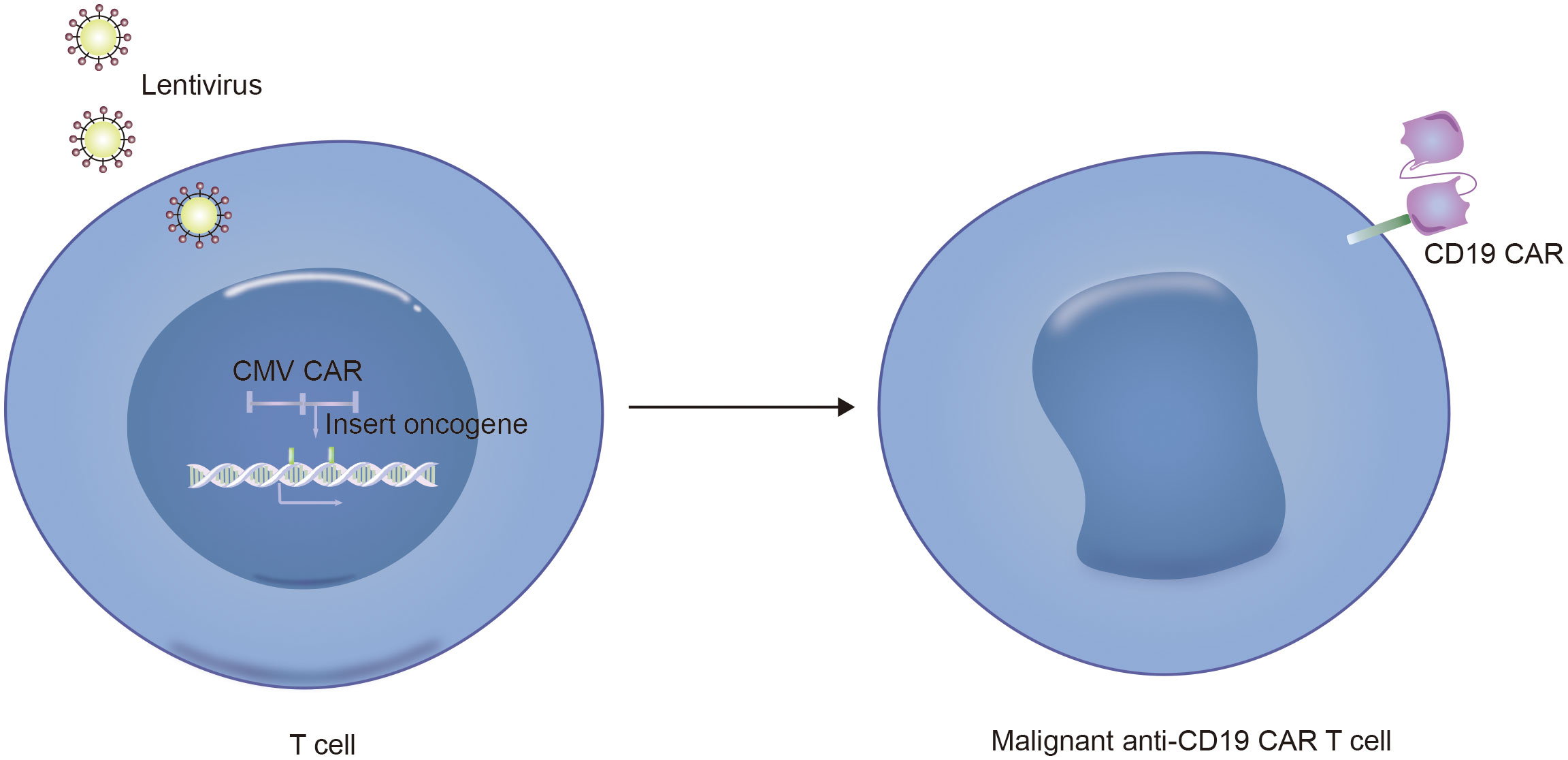
Figure 4. Technical schematic diagram for preparing CD19-CAR T malignant cells by infecting T cells with lentivirus vector.
Furthermore, another possible disadvantage of viral vectors is the preparation of autologous CD19 CAR-T cells from patient-derived sources. During the collection of raw T cells, contamination by malignant cells may occur (53). Although the majority of malignant cells are eliminated during subsequent cell culture and CAR-T-cell preparation processes, if CD19-CAR is expressed in tumor cells, it can artificially block the CD19 antigen in tumor cells, preventing recognition and clearance by CD19-CAR-T cells and resulting in fatal CAR Tumor cells (Figures 5, 6) (54).
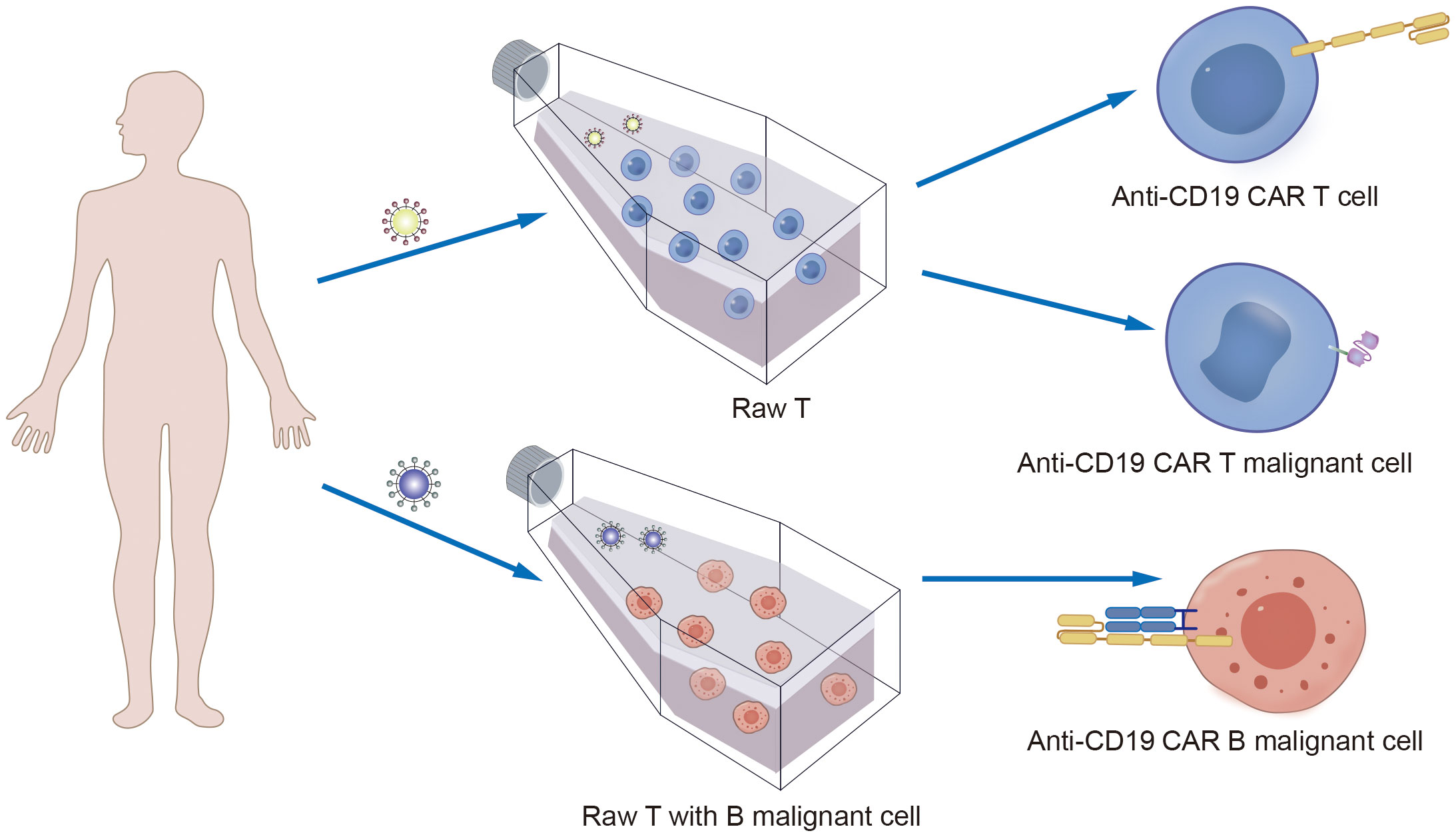
Figure 5. The mechanism of CAR T cells production process involves the contamination of raw T cells by B malignant cells, leading to the fatal of CAR B production.
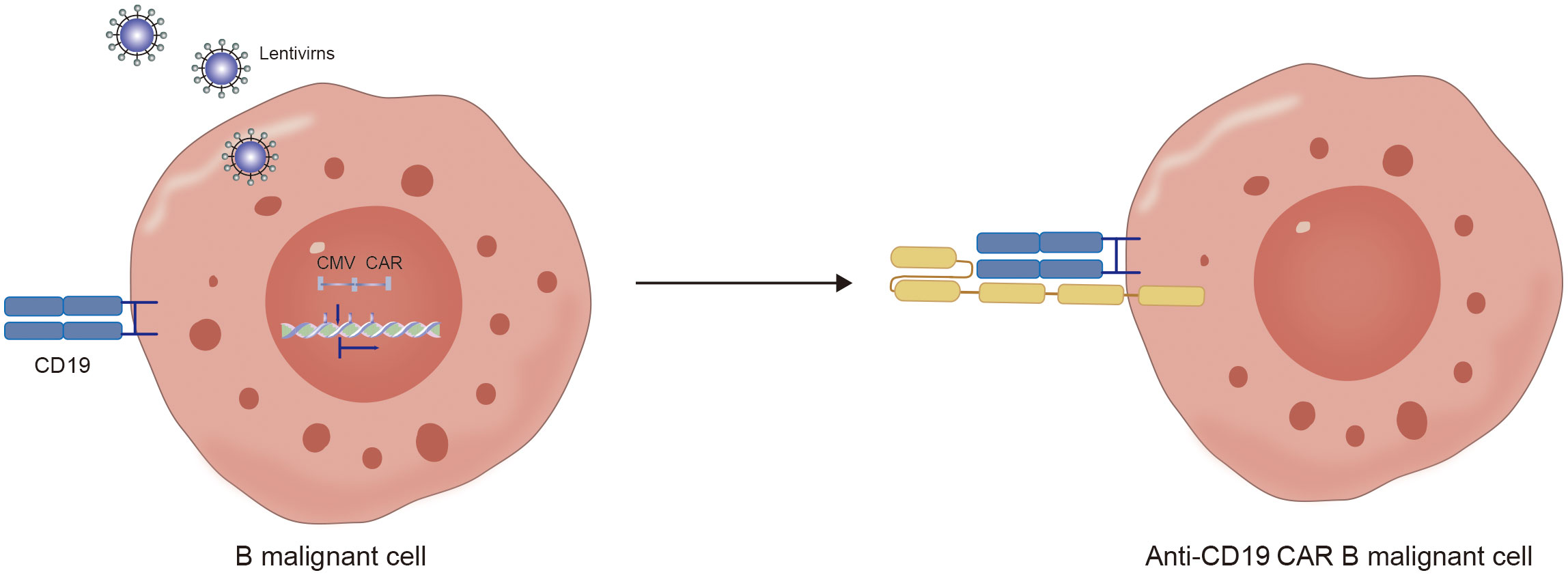
Figure 6. The mechanism of hidden CD19 antigen by CD19 CAR receptor expressed in lentivirus infected malignant B cells.
Essentially, ICANS, B-cell deficiency, and infections are manifestations of on-target, off-tumor effects. For ICANS, CD19-CAR-T cells attack brain mural cells. B-cell deficiency and infection are caused by CD19 CAR-T cells targeting normal B cells. Therefore, the main challenge is to improve the antigen recognition and specificity of CAR-T cells. Since the CD19 antigen on tumor cells and normal B cells, as well as brain mural cells, may have mutations, it is possible to avoid attack on normal cells by using scFv for specific recognition of CD19 (55). Another strategy is to reduce the attack on normal tissues by adjusting the affinity between scFv and CD19, utilizing the difference in CD19 expression levels on tumor cells and normal cells (56). In some clinical trials, the risk of developing ICANS significantly increased in patients treated with fludarabine during lymphocyte depletion pretreatment. Therefore, selecting the appropriate chemotherapy pretreatment is highly important for regulating the migration and homing of T cells in the body (29, 31). Prophylactic use of the IL-1 receptor antagonist anakinra can significantly reduce the incidence of ICANS caused by CD19-targeted CAR-T-cell therapy without affecting the therapeutic effect of CAR-T cells. For allergies, using variable domain of heavy chain of heavy-chain antibody (VHH) as a replacement for traditional scFv can achieve specific recognition of CD19 without causing allergic reactions and can to some extent address the issue of short-term relapse after treatment with mouse-derived CAR-T cells (57, 58). Another strategy to address relapse due to loss of the CD19 antigen is to use CD19/CD20 bispecific CAR-T cells, which can prevent loss of a single antigen (59–61).
There are several strategies to enhance the safety of CAR-T cells in response to the potential oncogenic risk of using viral vectors and fatal CAR Tumor. One approach is to use CRISPR technology for precise gene editing of T cells, allowing the gene encoding CAR to be inserted at specific loci in the genome to avoid activation of oncogenes (62). However, due to the limitations of gene editing efficiency and off-target effects of gene editing tools, continuous clinical experiments are needed to determine their safety. Another strategy is to use nonviral vectors such as mRNA or plasmids and minicircle DNA vectors to deliver the vector into T cells through LNPs, liposomes or electroporation, achieving transient expression of CARs (63–66). Although CAR-T cells prepared in this way cannot express CAR for a long time, limiting the sustainability of their antitumor effects, they do not modify the T-cell genome, thus avoiding potential oncogenic risks and sustained CD19 antigen blockade. Notably, by introducing S/MAR self-replicating sequences into minicircle DNA vectors, it is possible to achieve long-term retention of minicircle DNA vectors in T cells, increasing the sustainability of the antitumor effects of gene-modified T cells (67).
By optimizing the costimulatory signal structure, the proliferation and persistence of CAR-T cells can be enhanced. Integrating one or more costimulatory domains into the CAR structure can affect its effector function. CAR-T cells stimulated with 4-1BB are known to persist longer, while CD28 costimulation enhances proliferation and tumor clearance (68). CD28 and 4-1BB are widely used, but ICOS, OX40, CD27, and others are still under investigation (69, 70). Building upon the traditional second-generation CAR structure, CARs can be redesigned to express a structure driven by transcription factors that induce gene expression in response to signals, known as Universal CAR S/MAR. For example, transgenic T cells carrying the IL-7 receptor (C7R) can be integrated into the CAR structure. When encountering antigens, this promotes constant signal transduction, activating intracellular STAT5 signal transduction, a key IL-7 signaling node that supports antitumor activity (71). Another synthetic biology approach is chimeric switch receptor (CSR), which converts inhibitory signals transmitted by inhibitory molecules received by T cells into activation signals. For instance, Liang et al. designed CD19-targeting CAR-T cells expressing a PD-1 CSR to treat patients with CD19 CAR-T-cell failure by inhibiting PD-1/PD-L1-mediated T-cell exhaustion. In clinical trials, three out of six patients achieved complete remission (72).
Sorting raw T cells to obtain T cells with 100% purity can prevent the generation of CAR Tumor cells. However, the existing CAR-T-cell preparation process using flow cytometry or FACS for sorting cannot guarantee 100% cell purity. The persistent expression of CAR in tumor cells implies sustained blockade of the CD19 antigen; therefore, modifying T cells to transiently express CAR using nonintegrating gene vectors such as mRNA and minicircle DNA vectors can be utilized (73, 74). Even if tumor cells are mixed with raw T cells, gene-modified tumor cells will not permanently block CD19 after modification. However, transient CAR expression means that the retention time of CAR-T cells in the body may affect the longevity of CAR-T-cell therapy. This can be addressed by administering multiple doses of CAR-T cells to prolong their effectiveness (74).
CAR-T-cell therapy is an effective treatment option for patients with hematologic malignancies, with long-term data demonstrating strong efficacy and overall low levels of toxicity. The highly durable remissions observed in patients with B-cell-related malignancies treated with CD19-targeted CAR-T-cell therapy demonstrate the potential for inducing long-lasting cures with this treatment approach. Currently, the indications for CD19-CAR-T cells are expanding, serving not only as a crucial bridge for B-ALL patients undergoing allogeneic hematopoietic stem cell transplantation but also for providing long-term remission for patients with multiple myeloma. However, as the number of treated patients increases, some potential risks are emerging in the process of CD19 CAR-T-cell therapy, including aspects such as autologous cell collection and selection, gene vector selection, lymphodepletion regimens, posttreatment infection prophylaxis, and sequential bone marrow transplantation. Various targeted strategies are being researched and demonstrated for their safety in clinical trials to address these risks. Nevertheless, given the existence of multiple risks, a comprehensive approach will be needed in the future to modify CD19 CAR-T cells to mitigate these risks.
XY: Conceptualization, Funding acquisition, Investigation, Software, Visualization, Writing – original draft, Writing – review & editing. MG: Data curation, Conceptualization, Methodology, Writing – original draft. MT: Conceptualization, Resources, Writing – original draft. YW: Conceptualization, Data curation, Resources, Validation, Writing – original draft. HZ: Investigation, Software, Validation, Writing – original draft. ZF: Writing – review & editing, Funding acquisition, Project administration.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This research was supported by Hebei University of Engineering’s high-level talents for scientific research initiation (SJ2401002069) and Hebei Natural Science Foundation (Grant No. H2024402005 and C2022402032).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
CAR T, Chimeric antigen receptor; FDA, food and drug administration; CRS, cytokine release syndrome; ICANS, Immune Effector Cell-Associated Neurotoxicity Syndrome; BCMA, B-cell maturation antigen; Il-6, Interleukin-6; Il-7, Interleukin-7; LNP, lipid nanoparticles; S/MAR, Scaffold/matrix attachment regions; ALL, acute lymphoblastic leukemia; MDS, myelodysplastic syndrome; AML, acute myeloid leukemia; HAMAs, human anti-mouse antibodies; IVIG, intravenous immunoglobulin infusion; CSR, chimeric switch receptor; PD-1, programmed death-1; PD-L1, programmed death-ligand 1; CRISPR, Clustered Regularly Interspaced Short Palindromic Repeats.
1. Wang Z, Wu Z, Liu Y, Han W. New development in CAR-T cell therapy. J Hematol Oncol. (2017) 10(1):53. doi: 10.1186/s13045-017-0423-1
2. Sterner RC, Sterner RM. CAR-T cell therapy: current limitations and potential strategies. Blood Cancer J. (2021) 11(4):69. doi: 10.1038/s41408-021-00459-7
3. Frey NV, Shaw PA, Hexner EO, Pequignot E, Gill S, Luger SM, et al. Optimizing chimeric antigen receptor T-cell therapy for adults with acute lymphoblastic leukemia. J Clin Oncol. (2020) 38(5):415–22. doi: 10.1200/JCO.19.01892
4. Westin JR, Kersten MJ, Salles G, Abramson JS, Schuster SJ, Locke FL, et al. Efficacy and safety of CD19-directed CAR-T cell therapies in patients with relapsed/refractory aggressive B-cell lymphomas: Observations from the JULIET, ZUMA-1, and TRANSCEND trials. Am J Hematol. (2021) 96(10):1295–312. doi: 10.1002/ajh.26301
5. Nie EH, Su YJ, Baird JH, Agarwal N, Bharadwaj S, Weng WK, et al. Clinical features of neurotoxicity after CD19 CAR T-cell therapy in mantle cell lymphoma. Blood Adv. (2024) 8(6):1474–86. doi: 10.1182/bloodadvances.2023011896
6. Boardman AP, Salles G. CAR T-cell therapy in large B cell lymphoma. Hematol Oncol. (2023) 41 Suppl 1(Suppl 1):112–8. doi: 10.1002/hon.3153
7. Munshi NC, Anderson LD Jr, Shah N, Madduri D, Berdeja J, Lonial S, et al. Idecabtagene vicleucel in relapsed and refractory multiple myeloma. N Engl J Med. (2021) 384(8):705–16. doi: 10.1056/NEJMoa2024850
8. Khan AN, Chowdhury A, Karulkar A, Jaiswal AK, Banik A, Asija S, et al. Immunogenicity of CAR-T cell therapeutics: Evidence, mechanism and mitigation. Front Immunol. (2022) 13:886546. doi: 10.3389/fimmu.2022.886546
9. Katsarou A, Sjöstrand M, Naik J, Mansilla-Soto J, Kefala D, Kladis G, et al. Combining a CAR and a chimeric costimulatory receptor enhances T cell sensitivity to low antigen density and promotes persistence. Sci Transl Med. (2021) 13(623):eabh1962. doi: 10.1126/scitranslmed.abh1962
10. Long AH, Haso WM, Shern JF, Wanhainen KM, Murgai M, Ingaramo M, et al. 4-1BB costimulation ameliorates T cell exhaustion induced by tonic signaling of chimeric antigen receptors. Nat Med. (2015) 21(6):581–90. doi: 10.1038/nm.3838
11. Kath J, Franke C, Drosdek V, Du W, Glaser V, Fuster-Garcia C, et al. Integration of ζ-deficient CARs into the CD3ζ gene conveys potent cytotoxicity in t and NK cells. Blood. (2024) 143(25):2599–611. doi: 10.1182/blood.2023020973
12. Yoo JW. Management of adverse events in young adults and children with acute B-cell lymphoblastic leukemia receiving anti-CD19 chimeric antigen receptor (CAR) T-cell therapy. Blood Res. (2023) 58(S1):S20–8. doi: 10.5045/br.2023.2023026
13. Haubner S, Mansilla-Soto J, Nataraj S, Kogel F, Chang Q, de Stanchina E, et al. Cooperative CAR targeting to selectively eliminate AML and minimize escape. Cancer Cell. (2023) 41(11):1871–1891.e6. doi: 10.1016/j.ccell.2023.09.010
14. Qin H, Dong Z, Wang X, Cheng WA, Wen F, Xue W, et al. CAR T cells targeting BAFF-r can overcome CD19 antigen loss in B cell malignancies. Sci Transl Med. (2019) 11(511):eaaw9414. doi: 10.1126/scitranslmed.aaw9414
15. Qi Y, Liu H, Li X, Shi Y, Mu J, Li J, et al. Blinatumomab as salvage therapy in patients with relapsed/refractory b-ALL who have failed/progressed after anti-CD19-CAR t therapy. Ann Med. (2023) 55(1):2230888. doi: 10.1080/07853890.2023.2230888
16. Brudno JN, Kochenderfer JN. Recent advances in CAR T-cell toxicity: Mechanisms, manifestations and management. Blood Rev. (2019) 34:45–55. doi: 10.1016/j.blre.2018.11.002
17. Si X, Gu T, Liu L, Huang Y, Han Y, Qian P, et al. Hematologic cytopenia post CAR T cell therapy: Etiology, potential mechanisms and perspective. Cancer Lett. (2022) 550:215920. doi: 10.1016/j.canlet.2022.215920
18. Pennisi M, Sanchez-Escamilla M, Flynn JR, Shouval R, Alarcon Tomas A, Silverberg ML, et al. Modified EASIX predicts severe cytokine release syndrome and neurotoxicity after chimeric antigen receptor T cells. Blood Adv. (2021) 5(17):3397–406. doi: 10.1182/bloodadvances.2020003885
19. Dufranc E, Del Bello A, Belliere J, Kamar N, Faguer S. TAIDI (Toulouse acquired immune deficiency and infection) study group. IL6-r blocking with tocilizumab in critically ill patients with hemophagocytic syndrome. Crit Care. (2020) 24(1):166. doi: 10.1186/s13054-020-02878-7
20. Freyer CW, Porter DL. Cytokine release syndrome and neurotoxicity following CAR T-cell therapy for hematologic malignancies. J Allergy Clin Immunol. (2020) 146(5):940–8. doi: 10.1016/j.jaci.2020.07.025
21. Shima H, Ishikawa T, Ito J, Emoto K, Kurosawa T, Keino D, et al. Severe bloody diarrhea due to cytokine release syndrome after chimeric antigen receptor T cell therapy for refractory acute lymphoblastic leukemia. Blood Cell Ther. (2021) 5(1):31–4. doi: 10.31547/bct-2021-009
22. Zhang B, Napoleon JV, Liu X, Luo Q, Srinivasarao M, Low PS. Sensitive manipulation of CAR T cell activity using a chimeric endocytosing receptor. J Immunother Cancer. (2020) 8(2):e000756. doi: 10.1136/jitc-2020-000756
23. Warda W, Da Rocha MN, Trad R, Haderbache R, Salma Y, Bouquet L, et al. Overcoming target epitope masking resistance that can occur on low-antigen-expresser AML blasts after IL-1RAP chimeric antigen receptor T cell therapy using the inducible caspase 9 suicide gene safety switch. Cancer Gene Ther. (2021) 28(12):1365–75. doi: 10.1038/s41417-020-00284-3
24. Schubert ML, Schmitt M, Wang L, Ramos CA, Jordan K, Müller-Tidow C, et al. Side-effect management of chimeric antigen receptor (CAR) T-cell therapy. Ann Oncol. (2021) 32(1):34–48. doi: 10.1016/j.annonc.2020.10.478
25. Sheth VS, Gauthier J. Taming the beast: CRS and ICANS after CAR T-cell therapy for ALL. Bone Marrow Transplant. (2021) 56(3):552–66. doi: 10.1038/s41409-020-01134-4
26. Parker KR, Migliorini D, Perkey E, Yost KE, Bhaduri A, Bagga P, et al. Single-cell analyses identify brain mural cells expressing CD19 as potential off-tumor targets for CAR-t immunotherapies. Cell. (2020) 183(1):126–142.e17. doi: 10.1016/j.cell.2020.08.022
27. Amidi Y, Eckhardt CA, Quadri SA, Malik P, Firme MS, Jones DK, et al. Forecasting immune effector cell-associated neurotoxicity syndrome after chimeric antigen receptor T-cell therapy. J Immunother Cancer. (2022) 10(11):e005459. doi: 10.1136/jitc-2022-005459
28. Park JH, Nath K, Devlin SM, Sauter CS, Palomba ML, Shah G, et al. CD19 CAR T-cell therapy and prophylactic anakinra in relapsed or refractory lymphoma: phase 2 trial interim results. Nat Med. (2023) 29(7):1710–7. doi: 10.1038/s41591-023-02404-6
29. Hirayama AV, Kimble EL, Wright JH, Fiorenza S, Gauthier J, Voutsinas JM, et al. Timing of anti-PD-L1 antibody initiation affects efficacy/toxicity of CD19 CAR T-cell therapy for large B-cell lymphoma. Blood Adv. (2024) 8(2):453–67. doi: 10.1182/bloodadvances.2023011287
30. Ong SY, Pak S, Mei M, Wang Y, Popplewell L, Baird JH, et al. Bendamustine lymphodepletion is a well-tolerated alternative to fludarabine and cyclophosphamide lymphodepletion for axicabtagene ciloleucel therapy for aggressive B-cell lymphoma. Am J Hematol. (2023) 98(11):1751–61. doi: 10.1002/ajh.27069
31. Lowe KL, Mackall CL, Norry E, Amado R, Jakobsen BK, Binder G. Fludarabine and neurotoxicity in engineered T-cell therapy. Gene Ther. (2018) 25(3):176–91. doi: 10.1038/s41434-018-0019-6
32. Rejeski K, Perez A, Iacoboni G, Penack O, Bücklein V, Jentzsch L, et al. The CAR-HEMATOTOX risk-stratifies patients for severe infections and disease progression after CD19 CAR-t in R/R LBCL. J Immunother Cancer. (2022) 10(5):e004475. doi: 10.1136/jitc-2021-004475
33. Mei H, Li C, Jiang H, Zhao X, Huang Z, Jin D, et al. A bispecific CAR-T cell therapy targeting BCMA and CD38 in relapsed or refractory multiple myeloma. J Hematol Oncol. (2021) 14(1):161. doi: 10.1186/s13045-021-01170-7
34. Jiang H, Liu L, Guo T, Wu Y, Ai L, Deng J, et al. Improving the safety of CAR-T cell therapy by controlling CRS-related coagulopathy. Ann Hematol. (2019) 98(7):1721–32. doi: 10.1007/s00277-019-03685-z
35. Mingot-Castellano ME, Reguera-Ortega JL, Zafra Torres D, Hernani R, Lopez-Godino O, Guerreiro M, et al. Use of eltrombopag to improve thrombocytopenia and tranfusion requirement in anti-CD19 CAR-T cell-treated patients. J Clin Med. (2024) 13(17):5117. doi: 10.3390/jcm13175117
36. Pfeiffer A, Thalheimer FB, Hartmann S, Frank AM, Bender RR, Danisch S, et al. In vivo generation of human CD19-CAR T cells results in B-cell depletion and signs of cytokine release syndrome. EMBO Mol Med. (2018) 10(11):e9158. doi: 10.15252/emmm.201809158
37. Kansal R, Richardson N, Neeli I, Khawaja S, Chamberlain D, Ghani M, et al. Sustained B cell depletion by CD19-targeted CAR T cells is a highly effective treatment for murine lupus. Sci Transl Med. (2019) 11(482):eaav1648. doi: 10.1126/scitranslmed.aav1648
38. Otani IM, Lehman HK, Jongco AM, Tsao LR, Azar AE, Tarrant TK, et al. Practical guidance for the diagnosis and management of secondary hypogammaglobulinemia: A work group report of the AAAAI primary immunodeficiency and altered immune response committees. J Allergy Clin Immunol. (2022) 149(5):1525–60. doi: 10.1016/j.jaci.2022.01.025
39. Wat J, Barmettler S. Hypogammaglobulinemia after chimeric antigen receptor (CAR) T-cell therapy: Characteristics, management, and future directions. J Allergy Clin Immunol Pract. (2022) 10(2):460–6. doi: 10.1016/j.jaip.2021.10.037
40. Hill JA, Giralt S, Torgerson TR, Lazarus HM. CAR-t - and a side order of IgG, to go? - immunoglobulin replacement in patients receiving CAR-T cell therapy. Blood Rev. (2019) 38:100596. doi: 10.1016/j.blre.2019.100596
41. Locke FL, Ghobadi A, Jacobson CA, Miklos DB, Lekakis LJ, Oluwole OO, et al. Long-term safety and activity of axicabtagene ciloleucel in refractory large B-cell lymphoma (ZUMA-1): a single-arm, multicentre, phase 1-2 trial. Lancet Oncol. (2019) 20(1):31–42. doi: 10.1016/S1470-2045(18)30864-7
42. Duell J, Leipold AM, Appenzeller S, Fuhr V, Rauert-Wunderlich H, Da Via M, et al. Sequential antigen loss and branching evolution in lymphoma after CD19- and CD20-targeted T-cell-redirecting therapy. Blood. (2024) 143(8):685–96. doi: 10.1182/blood.2023021672
43. Locatelli F, Shah B, Thomas T, Velasco K, Adedokun B, Aldoss I, et al. Incidence of CD19-negative relapse after CD19-targeted immunotherapy in R/R BCP acute lymphoblastic leukemia: a review. Leuk Lymphoma. (2023) 64(10):1615–33. doi: 10.1080/10428194.2023.2232496
44. Mukherjee S, Ayanambakkam A, Ibrahimi S, Schmidt S, Charkrabarty JH, Khawandanah M. Ibritumomab tiuxetan (Zevalin) and elevated serum human anti-murine antibody (HAMA). Hematol Oncol Stem Cell Ther. (2018) 11(3):187–8. doi: 10.1016/j.hemonc.2017.12.004
45. Paillassa J, Cornet E, Noel S, Tomowiak C, Lepretre S, Vaudaux S, et al. Analysis of a cohort of 279 patients with hairy-cell leukemia (HCL): 10 years of follow-up. Blood Cancer J. (2020) 10(5):62. doi: 10.1038/s41408-020-0328-z
46. Neelapu SS, Dickinson M, Munoz J, Ulrickson ML, Thieblemont C, Oluwole OO, et al. Axicabtagene ciloleucel as first-line therapy in high-risk large B-cell lymphoma: the phase 2 ZUMA-12 trial. Nat Med. (2022) 28(4):735–42. doi: 10.1038/s41591-022-01731-4
47. Zhao WH, Wang BY, Chen LJ, Fu WJ, Xu J, Liu J, et al. Four-year follow-up of LCAR-B38M in relapsed or refractory multiple myeloma: a phase 1, single-arm, open-label, multicenter study in china (LEGEND-2). J Hematol Oncol. (2022) 15(1):86. doi: 10.1186/s13045-022-01301-8
48. Cappell KM, Sherry RM, Yang JC, Goff SL, Vanasse DA, McIntyre L, et al. Long-term follow-up of anti-CD19 chimeric antigen receptor T-cell therapy. J Clin Oncol. (2020) 38(32):3805–15. doi: 10.1200/JCO.20.01467
49. Chong EA, Ruella M, Schuster SJ, Lymphoma Program Investigators at the University of Pennsylvania. Five-year outcomes for refractory B-cell lymphomas with CAR T-cell therapy. N Engl J Med. (2021) 384(7):673–4. doi: 10.1056/NEJMc2030164
50. Locke FL, Miklos DB, Jacobson CA, Perales MA, Kersten MJ, Oluwole OO, et al. Axicabtagene ciloleucel as second-line therapy for large B-cell lymphoma. N Engl J Med. (2022) 386(7):640–54. doi: 10.1056/NEJMoa2116133
51. Verdun N, Marks P. Secondary cancers after chimeric antigen receptor T-cell therapy. N Engl J Med. (2024) 390(7):584–6. doi: 10.1056/NEJMp2400209
52. Fraietta JA, Nobles CL, Sammons MA, Lundh S, Carty SA, Reich TJ, et al. Disruption of TET2 promotes the therapeutic efficacy of CD19-targeted T cells. Nature. (2018) 558(7709):307–12. doi: 10.1038/s41586-018-0178-z
53. Vormittag P, Gunn R, Ghorashian S, Veraitch FS. A guide to manufacturing CAR T cell therapies. Curr Opin Biotechnol. (2018) 53:164–81. doi: 10.1016/j.copbio.2018.01.025
54. Ruella M, Xu J, Barrett DM, Fraietta JA, Reich TJ, Ambrose DE, et al. Induction of resistance to chimeric antigen receptor T cell therapy by transduction of a single leukemic B cell. Nat Med. (2018) 24(10):1499–503. doi: 10.1038/s41591-018-0201-9
55. Zhang Z, Chen X, Tian Y, Li F, Zhao X, Liu J, et al. Point mutation in CD19 facilitates immune escape of B cell lymphoma from CAR-T cell therapy. J Immunother Cancer. (2020) 8(2):e001150. doi: 10.1136/jitc-2020-001150
56. Alabanza L, Pegues M, Geldres C, Shi V, Wiltzius JJW, Sievers SA, et al. Function of novel anti-CD19 chimeric antigen receptors with human variable regions is affected by hinge and transmembrane domains. Mol Ther. (2017) 25(11):2452–65. doi: 10.1016/j.ymthe.2017.07.013
57. Kalinin RS, Ukrainskaya VM, Chumakov SP, Moysenovich AM, Tereshchuk VM, Volkov DV, et al. Engineered removal of PD-1 from the surface of CD19 CAR-T cells results in increased activation and diminished survival. Front Mol Biosci. (2021) 8:745286. doi: 10.3389/fmolb.2021.745286
58. Ren Q, Zu Y, Su H, Lu Q, Xiang B, Luo Y, et al. Single VHH-directed BCMA CAR-NK cells for multiple myeloma. Exp Hematol Oncol. (2023) 12(1):98. doi: 10.1186/s40164-023-00461-8
59. Zah E, Lin MY, Silva-Benedict A, Jensen MC, Chen YY. T cells expressing CD19/CD20 bispecific chimeric antigen receptors prevent antigen escape by malignant B cells. Cancer Immunol Res. (2016) 4(6):498–508. doi: 10.1158/2326-6066.CIR-15-0231
60. Martyniszyn A, Krahl AC, André MC, Hombach AA, Abken H. CD20-CD19 bispecific CAR T cells for the treatment of B-cell malignancies. Hum Gene Ther. (2017) 28(12):1147–57. doi: 10.1089/hum.2017.126
61. Larson SM, Walthers CM, Ji B, Ghafouri SN, Naparstek J, Trent J, et al. CD19/CD20 bispecific chimeric antigen receptor (CAR) in Naive/Memory T cells for the treatment of relapsed or refractory non-hodgkin lymphoma. Cancer Discovery. (2023) 13(3):580–97. doi: 10.1158/2159-8290.CD-22-0964
62. Cuciniello R, Filosa S, Crispi S. Novel approaches in cancer treatment: preclinical and clinical development of small non-coding RNA therapeutics. J Exp Clin Cancer Res. (2021) 40(1):383. doi: 10.1186/s13046-021-02193-1
63. Kaštánková I, Štach M, Žižková H, Ptáčková P, Šmilauerová K, Mucha M, et al. Enzymatically produced piggyBac transposon vectors for efficient non-viral manufacturing of CD19-specific CAR T cells. Mol Ther Methods Clin Dev. (2021) 23:119–27. doi: 10.1016/j.omtm.2021.08.006
64. Álvarez-Benedicto E, Tian Z, Chatterjee S, Orlando D, Kim M, Guerrero ED, et al. Spleen SORT LNP generated in situ CAR T cells extend survival in a mouse model of lymphoreplete B cell lymphoma. Angew Chem Int Ed Engl. (2023) 62(44):e202310395. doi: 10.1002/anie.202310395
65. Zhang J, Hu Y, Yang J, Li W, Zhang M, Wang Q, et al. Non-viral, specifically targeted CAR-T cells achieve high safety and efficacy in b-NHL. Nature. (2022) 609(7926):369–74. doi: 10.1038/s41586-022-05140-y
66. Wang H, Ye X, Ju Y, Cai Z, Wang X, Du P, et al. Minicircle DNA-mediated CAR T cells targeting CD44 suppressed hepatocellular carcinoma both in vitro and in vivo. Onco Targets Ther. (2020) 13:3703–16. doi: 10.2147/OTT.S247836
67. Jin C, Fotaki G, Ramachandran M, Nilsson B, Essand M, Yu D. Safe engineering of CAR T cells for adoptive cell therapy of cancer using long-term episomal gene transfer. EMBO Mol Med. (2016) 8(7):702–11. doi: 10.15252/emmm.201505869
68. Jin X, Xu Q, Pu C, Zhu K, Lu C, Jiang Y, et al. Therapeutic efficacy of anti-CD19 CAR-T cells in a mouse model of systemic lupus erythematosus. Cell Mol Immunol. (2021) 18(8):1896–903. doi: 10.1038/s41423-020-0472-1
69. Liu G, Luo P. Targeting CD137 (4-1BB) towards improved safety and efficacy for cancer immunotherapy. Front Immunol. (2023) 14:1208788. doi: 10.3389/fimmu.2023.1208788
70. Honikel MM, Olejniczak SH. Co-stimulatory receptor signaling in CAR-T cells. Biomolecules. (2022) 12(9):1303. doi: 10.3390/biom12091303
71. Zhao Z, Li Y, Liu W, Li X. Engineered IL-7 receptor enhances the therapeutic effect of AXL-CAR-T cells on triple-negative breast cancer. BioMed Res Int. (2020) 2020:4795171. doi: 10.1155/2020/4795171
72. Chen C, Gu YM, Zhang F, Zhang ZC, Zhang YT, He YD, et al. Construction of PD1/CD28 chimeric-switch receptor enhances anti-tumor ability of c-met CAR-t in gastric cancer. Oncoimmunology. (2021) 10(1):1901434. doi: 10.1080/2162402X.2021.1901434
73. Billingsley MM, Singh N, Ravikumar P, Zhang R, June CH, Mitchell MJ. Ionizable lipid nanoparticle-mediated mRNA delivery for human CAR T cell engineering. Nano Lett. (2020) 20(3):1578–89. doi: 10.1021/acs.nanolett.9b04246
Keywords: CD19, CAR T cancer therapy, cytokine release syndrome (CRS), immune effector cell-associated neurotoxicity syndrome (ICANS), secondary malignancies
Citation: Ye X, Ge M, Tan M, Wu Y, Zhang H and Fu Z (2025) CD19 -targeted CAR T therapy treating hematologic malignancies: hidden danger is the next neighbor to security? Front. Immunol. 16:1490491. doi: 10.3389/fimmu.2025.1490491
Received: 03 September 2024; Accepted: 18 February 2025;
Published: 04 March 2025.
Edited by:
Thomas Luft, Heidelberg University Hospital, GermanyReviewed by:
Irfan Naseem Bandey, University of Texas MD Anderson Cancer Center, United StatesCopyright © 2025 Ye, Ge, Tan, Wu, Zhang and Fu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zexian Fu, ZnV6ZXhpYW5AaGViZXUuZWR1LmNu
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.