
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CLINICAL TRIAL article
Front. Immunol. , 20 March 2025
Sec. Vaccines and Molecular Therapeutics
Volume 16 - 2025 | https://doi.org/10.3389/fimmu.2025.1487039
 Denis Y. Logunov1*†
Denis Y. Logunov1*† Inna V. Dolzhikova1†
Inna V. Dolzhikova1† Mamadou Y. Boiro2
Mamadou Y. Boiro2 Anna V. Kovyrshina1
Anna V. Kovyrshina1 Alina S. Dzharullaeva1
Alina S. Dzharullaeva1 Alina S. Erokhova1
Alina S. Erokhova1 Daria M. Grousova1
Daria M. Grousova1 Amir I. Tukhvatulin1
Amir I. Tukhvatulin1 Fatima M. Izhaeva1
Fatima M. Izhaeva1 Yana V. Simakova1
Yana V. Simakova1 Maria K. Ordzhonikidze1
Maria K. Ordzhonikidze1 Nadezhda L. Lubenets1
Nadezhda L. Lubenets1 Olga V. Zubkova1
Olga V. Zubkova1 Dmitrii V. Scheblyakov1
Dmitrii V. Scheblyakov1 Ilias B. Esmagambetov1
Ilias B. Esmagambetov1 Maksim M. Shmarov1
Maksim M. Shmarov1 Alexander S. Semikhin1
Alexander S. Semikhin1 Natalia M. Tukhvatulina1
Natalia M. Tukhvatulina1 Dmitrii N. Shcherbinin1
Dmitrii N. Shcherbinin1 Irina L. Tutykhina1
Irina L. Tutykhina1 Georgiy S. Prokhorov1
Georgiy S. Prokhorov1 Alexander A. Khovaev1
Alexander A. Khovaev1 Tatiana N. Demidova1
Tatiana N. Demidova1 Nikolai A. Malishev1
Nikolai A. Malishev1 Liliya N. Merkulova1‡
Liliya N. Merkulova1‡ Olga L. Voronina1
Olga L. Voronina1 Irina T. Fedyakina1
Irina T. Fedyakina1 Lidiya B. Kisteneva1
Lidiya B. Kisteneva1 Lyudmila V. Kolobukhina1‡
Lyudmila V. Kolobukhina1‡ Dmitry V. Mishin1
Dmitry V. Mishin1 Aleksandr L. Elakov1
Aleksandr L. Elakov1 Ekaterina I. Ermolova1
Ekaterina I. Ermolova1 Kirill G. Krasnoslobodtsev1
Kirill G. Krasnoslobodtsev1 Viktor F. Larichev1
Viktor F. Larichev1 Irina S. Kruzhkova1
Irina S. Kruzhkova1 Egor M. Burmistrov1
Egor M. Burmistrov1 Anna B. Sheremet1
Anna B. Sheremet1 Elizaveta A. Tokarskaya1
Elizaveta A. Tokarskaya1 Alexander V. Gromov1
Alexander V. Gromov1 Dmitrii A. Reshetnikov1
Dmitrii A. Reshetnikov1 Aleksandr I. Fisun3
Aleksandr I. Fisun3 Bogdan N. Kotiv3
Bogdan N. Kotiv3 Dmitrii V. Ovchinnikov3
Dmitrii V. Ovchinnikov3 Evgenii V. Ivchenko3
Evgenii V. Ivchenko3 Konstantin V. Zhdanov3
Konstantin V. Zhdanov3 Sergei M. Zakharenko3
Sergei M. Zakharenko3 Aleksandr N. Solovev3
Aleksandr N. Solovev3 Andrei M. Ivanov3
Andrei M. Ivanov3 Vitalii S. Sukachev3
Vitalii S. Sukachev3 Roman V. Gudkov3
Roman V. Gudkov3 Oleg V. Maltsev3
Oleg V. Maltsev3 Ilnur A. Gabdrakhmanov3
Ilnur A. Gabdrakhmanov3 Anton V. Barsukov3
Anton V. Barsukov3 Vladislav V. Vashchenkov3
Vladislav V. Vashchenkov3 Nikolai I. Demianenko3
Nikolai I. Demianenko3 Sergei B. Ignatev3
Sergei B. Ignatev3 Konstantin V. Asiamov3
Konstantin V. Asiamov3 Nikolai N. Kirichenko3
Nikolai N. Kirichenko3 Andrei V. Liubimov3
Andrei V. Liubimov3 Igor I. Volkov3
Igor I. Volkov3 Evgenii V. Kriukov3
Evgenii V. Kriukov3 Nikolai K. Bazarnov3
Nikolai K. Bazarnov3 Viktoriia A. Kolodiazhnaia3
Viktoriia A. Kolodiazhnaia3 Elena V. Kolomoets4
Elena V. Kolomoets4 Svetlana I. Syromyatnikova5
Svetlana I. Syromyatnikova5 Dmitry E. Chifanov5
Dmitry E. Chifanov5 Alexander F. Andrus5
Alexander F. Andrus5 Dmitry A. Kutaev5
Dmitry A. Kutaev5 Sergei V. Borisevich5
Sergei V. Borisevich5 Boris S. Naroditsky1‡
Boris S. Naroditsky1‡ Alexander L. Gintsburg1,6 on behalf of the GamEvac-Combi trial group
Alexander L. Gintsburg1,6 on behalf of the GamEvac-Combi trial groupBackground: Ebola virus disease (EVD) is one of the most dangerous and lethal diseases affecting humans. There are several licensed vaccines against EVD, but it remains one of the priority diseases for research and development of effective vaccines.
Methods: A double-blind randomized placebo-controlled trial was performed to evaluate safety and immunogenicity of rVSV- and rAd5-vectored vaccine GamEvac-Combi in healthy adults of both sexes between 18 and 60 years. Safety and immunogenicity were assessed during the observation period of 12 months. Immunogenicity was assessed with GP-specific ELISA, IFN-γ ELISA, and plaque pseudoneutralization assay.
Results: Vaccinated participants showed marked GP-specific IFN-γ response at day 28 and neutralizing response at day 42 (GMT = 32.6, seroconversion rate 96.3%). GP-specific IgG antibody levels in vaccinated participants peaked at day 42 (GMT = 9345) and persisted for a year after vaccination (GMT = 650).
Conclusion: The vaccine showed favorable safety profile and induced robust cell-mediated immune response and strong humoral immune response that lasts at least for a year from the start of vaccination.
Clinical trial registration: ClinicalTrials.gov, identifier NCT03072030; Pan African Clinical Trial Registry, identifier PACTR201702002053400.
Since 1976, when the Ebola virus (EBOV) was first detected, all recorded outbreaks of Ebola virus disease (EVD) were mostly confined to rural areas in East and Central Africa. But an outbreak in West Africa in late 2013 had a different epidemiological and geographical profile and evolved into an epidemic with more than 11,000 fatal cases and imported cases outside the African continent (1). Later, in 2018-2020 a large outbreak has occurred in Democratic Rebublic of Congo with more than 3700 reported cases and 66% fatality rate (2).
Ebola virus disease poses the greatest threat to global health due to its epidemic potential and limited availability of specific drugs for therapy and prevention (3). EVD is included in WHO priority diseases list for research and development (4).
Recombinant viral vectors have yet proven as the most effective platform against EVD. To date, 2 vector-based vaccines are licensed for use: rVSV-ZEBOV (Merck) is licensed by EMA, FDA and prequalified by WHO, rAd26+rMVA (Johnson & Johnson) is licensed by EMA. Also, rAd5-based vaccine (CanSinoBio) is registered in China and rVSV+rAd5 vaccine (GamEvac-Combi) is registered in the Russian Federation (5).
We developed a GamEvac-Combi vaccine against EVD based on recombinant vectors rVSV and rAd5. Preclinical studies demonstrated the high efficacy of the vaccine in non-human primates - vaccine protected 100% of the animals from a lethal EBOV challenge both 4 weeks after vaccination and 5 months after the start of vaccination (6). GamEvac-Combi, demonstrated a good safety and immunogenicity profile in phase 1-2 clinical trial conducted in Russia which allowed to license the vaccine in the Russian Federation (6).
In this study, we present final results of a GamEvac-Combi clinical trial in the Republic of Guinea and in Russia conducted in 2017-2019.
The study was reviewed and approved by the appropriate national and local competent authorities, including the Ethics Committee of the Ministry of Health of the Russian Federation (#119 16/02/2016, additional #155 19/09/2017, #203 19/11/2019) and Ethics Committee of the Ministry of Health of Republic of Guinea (76/CNERS/16 20/06/2016, № 064/CNERS/17 07/05/2017, № 041/CNERS/18 09/03/2018). The study was carried out under the constant supervision of the National Agency for Health Safety of the Republic of Guinea, interim reports on the safety of the studied drug were regularly provided to the supervisor. All participants provided signed informed consent before enrollment in the study.
This study is a double-blind randomized placebo-controlled study of safety and immunogenicity of the GamEvac-Combi vaccine against EVD. The study was conducted in two countries: Republic of Guinea («Centre de recherche en épidémiologie, microbiologie et de soins médicaux (CREMS) de Pastoria à Kindia», Kindia) and Russian Federation (Infectious Disease Clinical Hospital No. 1 of the Moscow Healthcare Department, Moscow). The study received the following identifiers: ClinicalTrials.gov Identifier: NCT03072030, Pan African Clinical Trial Registry PACTR201702002053400. Volunteers for the study were selected based on inclusion and exclusion criteria (Table 1).
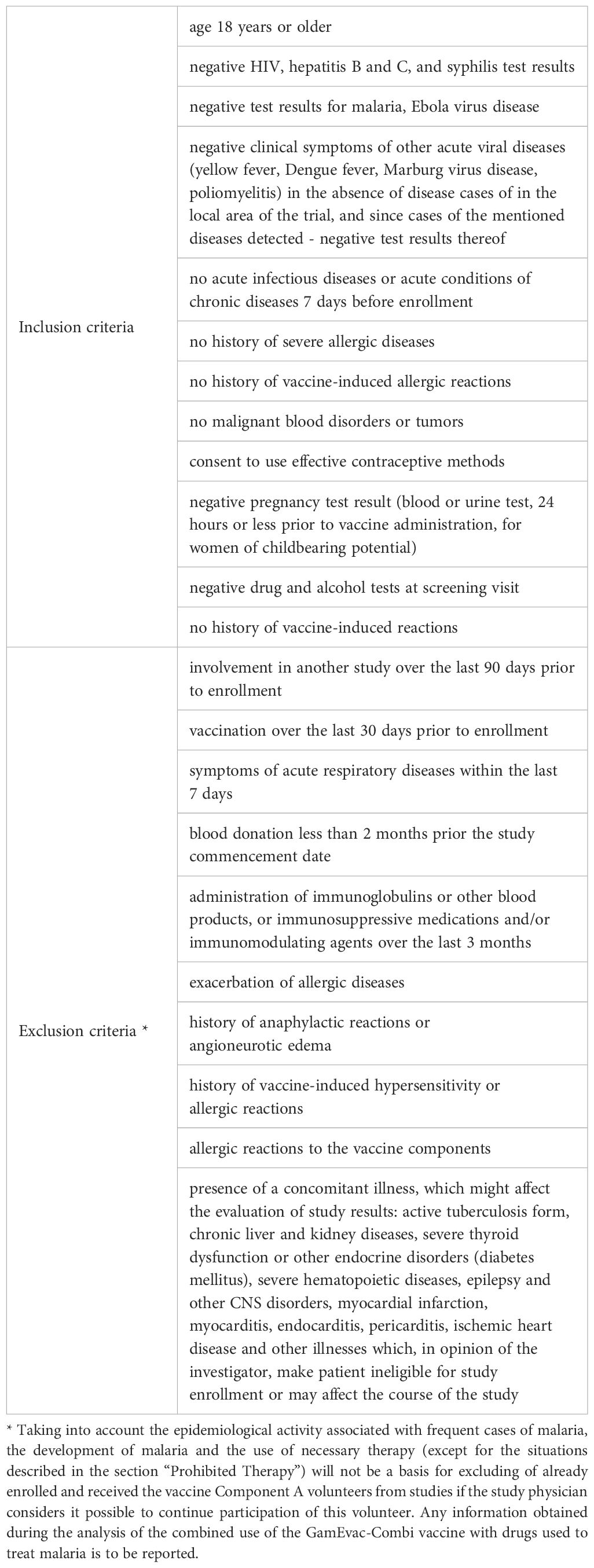
Table 1. Inclusion and exclusion criteria for volunteers for double-blind randomized placebo-controlled trial to evaluate safety and immunogenicity of GamEvac-Combi vaccine against EVD.
Enrolled participants were assigned to two study groups using stratified randomization in a ratio of 19:1 to the vaccine group or the placebo group. Study participants were assigned unique randomization numbers that remained unchanged throughout the study. The statistician generated a sequence, according to which the drug was labelled. A list of drug randomization codes was compiled based on the sequence of random numbers using a computer program that generates sequence of random numbers using the Mersenne vortex method, based on properties of Mersenne prime numbers and providing fast generation pseudorandom numbers of high quality. This method does not have the disadvantages inherent to other simple random number generators, such as short period, predictability, easily detectable statistical dependence. Generated by Mersenne vortex pseudorandom numbers successfully pass the DIEHARD tests (a set of statistical tests to determine the qualities of random numbers). The distribution of patients into groups was carried out using a random envelope method. The drug and placebo were outwardly indistinguishable (packaging, label, and content). Investigators, participants, and all study staff were masked to group assignment.
All participants who consented to participate attended a screening visit and were tested for eligibility according to eligibility criteria. Testing included physical examination, checks of vital signs, blood tests for infections, tests for drugs and alcohol, pregnancy test (in female participants) etc.
Participants, who passed screening procedures, were accommodated in the hospital on the evening before vaccination. In the morning before vaccination, venous blood samples were collected for subsequent immunogenicity analyzes (day 0 and 21). After vaccination, the participants stayed in the hospital under the supervision of medical personnel over the next two days.
The vaccine GamEvac-Combi is based on two recombinant viral vectors: rVSV-GP and rAd5-GP, both expressing Zaire Ebolavirus glycoprotein. Full doses (1 ml/dose) are 2.5 × 107 plaque forming units of recombinant vesicular stomatitis virus (component A) and 2.5 × 1011 viral particles of recombinant human adenovirus 5 serotype (component B). The placebo consists of the vaccine buffer compositions (buffers A and B, accordingly) but without the recombinant viral vectors, made up to equal the vaccine volume. Both vaccine components and placebo were developed, manufactured, and stored by Gamaleya National Research Center of Epidemiology and Microbiology (Moscow, Russia) according to GMP standards.
The vaccine (rVSV-GP on day 0, rAd5-GP on day 21) or placebo were administered intramuscularly into the deltoid muscle with a 21-day interval between doses. An outpatient visit took place on the day 7 after the administration of rVSV-GP. Subsequent visits were planned on day 28, day 42, 3 months, 6 months and 12 months. Systemic and local reactions were monitored via examination and tests at visits, through volunteer’s anamnesis and regular diary signs during the whole period of the study.
Blood samples to obtain sera for antibody analysis were collected on days 0, 21, 28, 42; 3, 6 and 12 months. Blood samples for PBMC isolation and subsequent IFN-γ response analyzes were collected at days 0 and 28.
Primary outcome was to determine immunogenicity and safety of GamEvac-Combi in healthy adult participants. Safety was assessed by monitoring of adverse events in vaccinated and placebo groups, Systemic and local post-vaccination reactions in participants were registered throughout the study (up to 12 months after vaccination). Immunogenicity study in vaccinated and placebo groups included evaluation of GP-specific antibody titers on days 21, 28, 42 and 3, 6, 12 months after the vaccination, evaluation of GP-specific T-cell IFN-γ response on day 28. Given the fact that immune response to Plasmodium falciparum antigens may significantly influence the effectiveness of subsequent vaccination, statistical analysis of the subgroup of participants who received antimalarial treatment between the administration of components A and B or after vaccination during follow-up were carried out separately.
Sera samples for antibody response were collected in all enrolled participants, who attended corresponding visits on days 21, 28, 42 and 3, 6, 12 months. Titers of glycoprotein-specific antibodies in sera samples of participants were evaluated by enzyme-linked immunosorbent assay (ELISA) as described before (6). Briefly, ELISA plates were coated overnight with a recombinant EBOV GP subtype Zaire, strain H.sapiens-wt/GIN/2014/Kissidougou-C15 (Sino Biological, SB40442), washed with phosphate buffer saline containing 0.1% Tween-20 (PBST) and blocked. Diluted sera samples were added in the plates and incubated for 2h, the plates were washed and HRP-conjugated anti-human IgG were added. After the wash, TMB was added, the reaction was stopped by adding H2SO4 and OD was detected at 450 nm (baseline 620 nm). The sample (day 21-360) was evaluated as positive if its optical density was ≥2 times greater than the average optical density of pre-vaccination (day 0) sample of the volunteer within the same dilution. The titer was considered as reciprocal value of maximal dilution which meets the above criteria. Initial dilution of samples was 1:50, negative samples were assigned the titer of 12.5 for statistical analysis. Relative amounts of glycoprotein-specific antibodies are reported as geometric mean end-point titers (GMT) with 95% confidence intervals.
Neutralizing antibody titers were determined on days 0 and 42 by plaque pseudoneutralization assay with rVSV-GP. Briefly, Vero E6 cells were seeded in 24-well plates on the day prior to assay to yield monolayer on the day of the assay. Sera samples were heat-inactivated (56°C 30 min) and diluted in DMEM with 2% heat-inactivated fetal bovine serum (Capricorn). Then 100 PFU of rVSV-GP was added to diluted samples (or to equal volume of DMEM for viral dose control), mixed, incubated at 37°C for 1 hour and transferred to the plates with cell monolayer. The plates were incubated for 1 hour (37°C, 5% CO2), then medium was discarded and cells were immediately coated with DMEM-CMC pre-heated to 37°C. After 2 days the plates were washed with sterile isotonic saline solution, fixed and stained with 2.5% Crystal Violet solution (PanReac AppliChem) containing ethanol and formaldehyde (PanReac AppliChem). Neutralization titers were defined as a reciprocal value of the highest serum dilution that resulted in >50% reduction in the number of virus plaques. Initial dilution of samples was 1:10, negative samples were assigned the titer of 2.5 for statistical analysis.
Cell-mediated immune response was assessed in 190 vaccinated and 10 placebo participants on days 0 and 28. Concentration of interferon gamma (IFN-γ) was evaluated by ELISA (Human IFN-gamma Platinum ELISA, BMS228CE, eBioscience) as described before (6). For this, peripheral blood mononuclear cells (PBMCs) were isolated on day 0 before vaccination and day 28 using Ficoll 1.077 (Paneco) and scattered into 96-well plates. Then recombinant EBOV GP subtype Zaire, strain H.sapiens-wt/GIN/2014/Kissidougou-C15 (Sino Biological, 40442-V08B1) was added to stimulate PBMC proliferation. As a positive control, PHA was added to the cells. The culture medium of samples was analyzed after 48 hours incubation to determine IFN-γ concentration. Results are reported as increase in IFN-γ concentration in PBMC samples upon exposure to EBOV GP on day 28 compared to day 0 (baseline IFN-γ concentration in GP-stimulated PBMC sample of the participant).
The statistical analysis was performed using GraphPad 10 software (v. 10.2.3. GraphPad, USA). The normality of data distribution was analyzed using the Shapiro-Wilks test. Depending on the normality, t-test or the Wilcoxon test was used for analysis of paired values, t-test or the Mann–Whitney test – for unpaired values. Differences were considered significant at p <0.05. Antibody response is reported as geometric mean titers with 95% confidence intervals (CI), cell-mediated response is reported as median with 95% confidence intervals. To compare the frequency indicators between groups, the χ² test and, if necessary, Fisher’s exact test were used (if the expected frequency in any of the cells was <5).
In the Republic of Guinea between August 2017 and December 2018, we recruited and screened 4137 healthy adults for compliance with the criteria, of whom 2000 were randomly assigned to receive vaccine (n=1900) or placebo (n=100) (Figure 1). The vaccination was started on August 6, 2017 and completed in December 2018: 1894 participants received first dose of the vaccine and were included in safety analysis, 100 participants received first dose of placebo and were included in safety analysis; 1805 participants were administered both doses of the vaccine (rVSV-GP + rAd5-GP). In Russia 21 healthy adults were screened, 10 volunteers were enrolled and received two doses of the vaccine. Safety set included all participants who received first dose of vaccine/placebo: 1904 vaccinated and 100 placebo participants. Analysis of antibody response included all participants, who attended corresponding visits, IFN-γ response and neutralization analyses included 190 vaccinated participants and 10 participants from placebo group.
Among the participants who received at least one dose, the mean age was 24.5 years (SD 6.8) in the vaccine group and 24.0 years (SD 6.5) in the placebo group; the distribution by sex was similar between the two groups (Table 2).
Clinical monitoring of adverse events was conducted during the entire study up to 12 months after vaccination. Collected data shows that the frequency and nature of adverse events recorded after the vaccine administration corresponds to the available information on the safety of the drug indicated in the Researcher’s Brochure and official instructions for the use of the vaccine.
During the whole period of the trial 2494 adverse events (AE) were reported in 1001 subjects. Of the 2494 AEs, only 1054 AEs in 561 subjects were attributable to vaccine or placebo administration; however, for 294 AEs, the category of causality was marked as unasessable. During the period of day 21 after the second vaccine/placebo administration to final visit, 1342 adverse events were reported in 616 subjects. Of the 1342 AEs, only 389 AEs in 245 subjects were caused by vaccine or placebo administration; however, for 131 AEs, the category of causality was marked by the study physicians as unasessable.
The majority of AEs associated with vaccine/placebo use (AAE) were mild: 96.97% and 96.94% after 1st and 2nd doses, respectively. At the same time, no AAEs with severity grades 3-5 (Severe, Life-threatening or disabling, Death related) were reported (Supplementary Table 1). Most of the AEs resolved without sequelae. To relieve 60,1% of AEs, it was necessary to use medications or other types of therapy.
In the structure of frequent adverse events, systemic reactions are represented by: an increase in body temperature between 37.2°C and 38.0°C, fever, headache, muscle pain, joint pain, chills, weakness; local reactions – pain and hyperemia at the injection site (Table 3). During the whole period of observation, we registered 194 cases of malaria in 172 participants: 183 cases in 162 vaccinated participants and 11 cases in 10 participants in placebo group. No significant difference of frequency of occurrence of adverse events in participants with and without malaria treatment was reported. There were no cases requiring emergency medical care due to administration of GamEvac-Combi vaccine. No deaths or life-threatening or disabling, or severe adverse events, or adverse events resulting in withdrawal from the study related to the vaccine administration were reported (Supplementary Table 2).
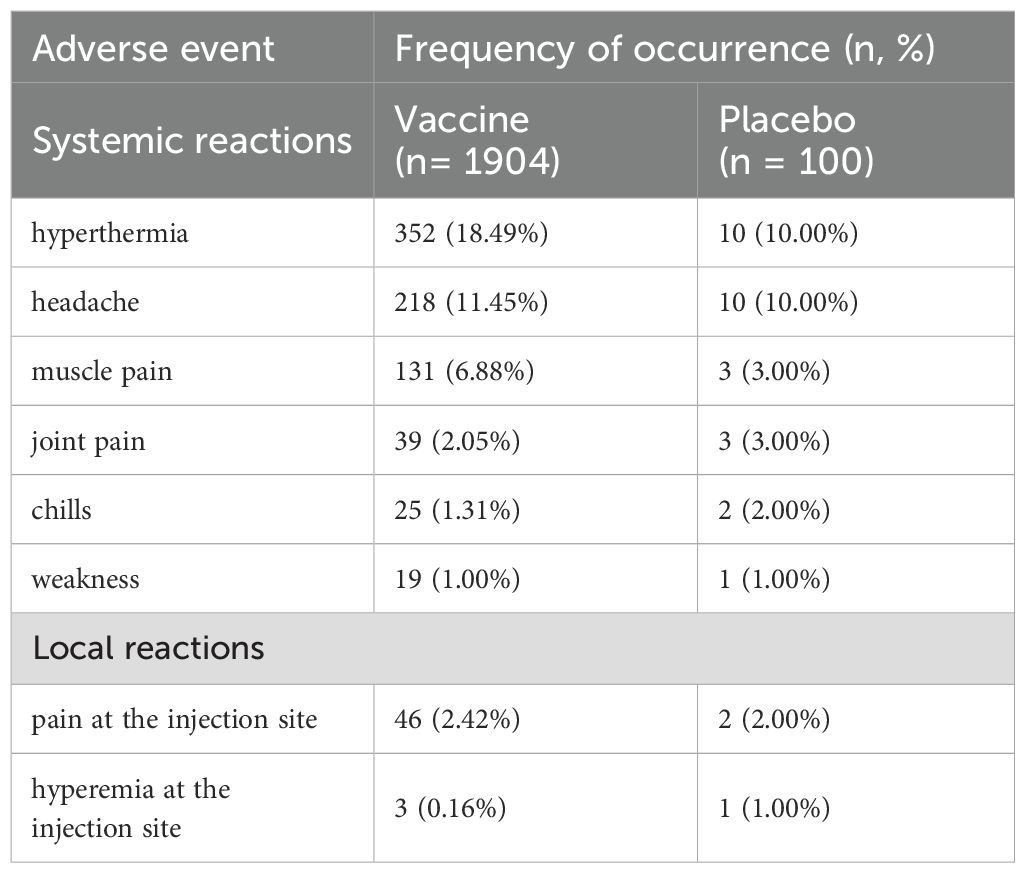
Table 3. Frequent adverse events associated with vaccine/placebo administration (AAE). AAEs reported during the whole study are shown in the table as a n (%) of subjects with reported AE.
To evaluate post-vaccination antibody immune response, we analyzed GP-specific antibody titers by ELISA on days 21, 28, 42 and 3, 6, 12 months (Figure 2). Sample sets included all participants, who attended corresponding visits: day 21 (n = 1805), day 28 (n = 1775), day 42 (n = 1756), 3 months (n = 1722), 6 months (n = 1686), 12 months (n = 1743). Geometric mean titers (GMT) of GP-specific antibodies and 95% CI were: 21 day – 144.9 (132.6-158.4), 28 day – 9192 (95% CI 8586-9840), 42 day – 9345 (95% CI 8759-9971), 3 months – 3364 (95% CI 3149-3593), 6 months – 1003 (95% CI 941.0-1070), 12 months – 650.0 (95% CI 607.0-696.1). All vaccinated participants showed significant increases in titers on day 28 (7 days after rAd5-GP boost vaccination) compared to day 21 (p < 0.0001). GP-specific antibody levels in vaccinated participants peaked on days 28-42 and gradually decreased over the whole observation period, but stayed significantly higher up to 12 months after vaccination than before booster vaccination (p < 0.0001). Analysis of GP-specific IgG in the group of vaccinated volunteers showed that on day 21 of the study, seroconversion rate (4+ folds increase) was 67.9%, day 28 – 97.7%, day 42 – 97.9%, day 90 – 97.3%, day 180 – 96.3%, day 365 – 94.5%.
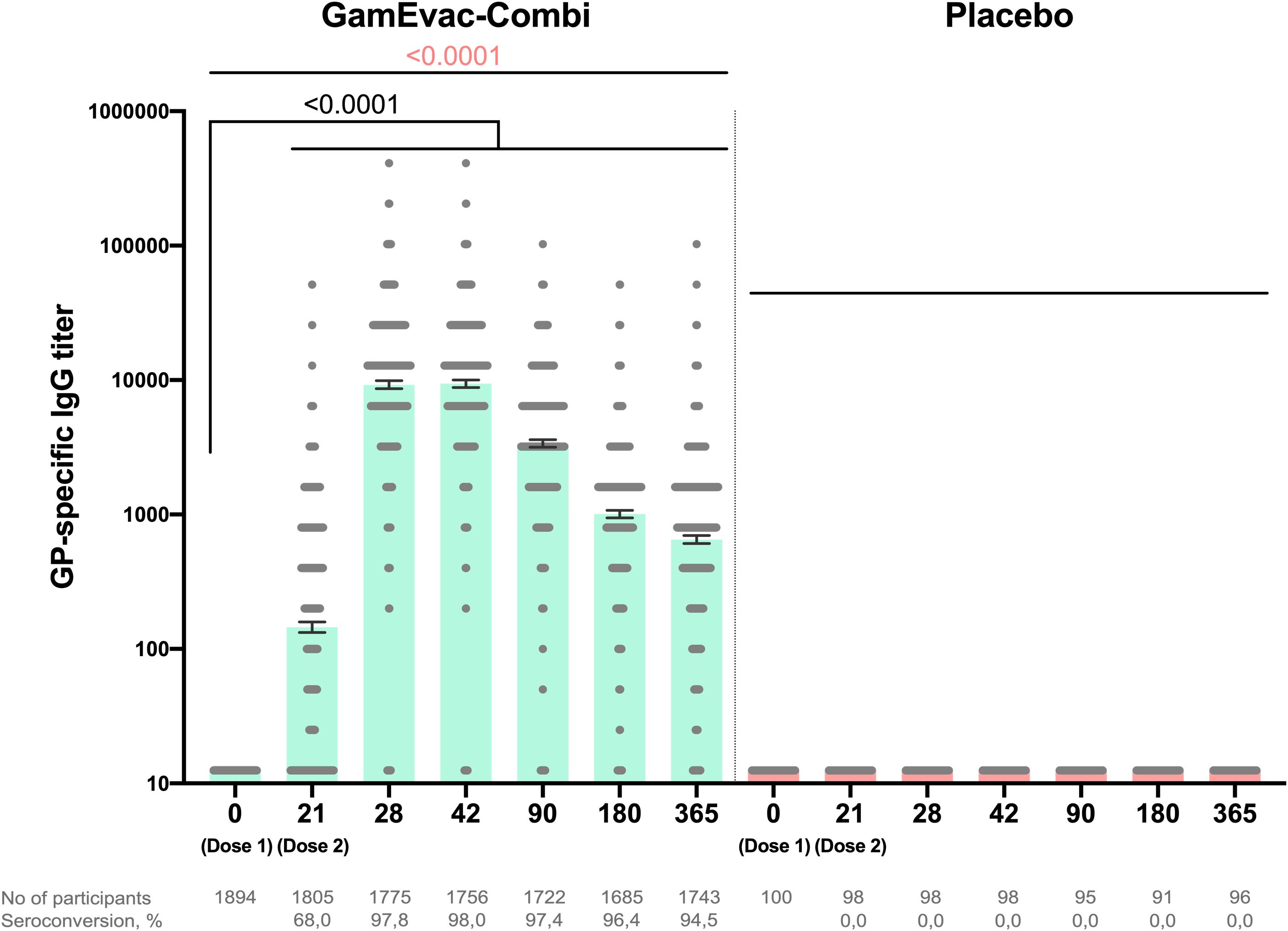
Figure 2. Humoral immune response in vaccinated participants. GP-specific antibody titers at days 0, 21, 28, 42, 90, 180 and 365, as measured by ELISA are shown. Bars show GMT, 95% CI are marked by whiskers. Visits on 3, 6, 12 months are marked as day 90, 180, 365 correspondingly. Differences between IgG titers at days 21 and 28, 42, 90, 180, 365 in comparison with day 0 were calculated with Wilcoxon test (black color). Differences within groups were calculated by ANOVA test (pink color).
We also analyzed antibody response in participants with malaria (since taking antimalarial drugs may affect the formation of a post-vaccination immune response) and no significant difference in antibody responses in participants with and without malaria treatment was reported on days 28-180 post vaccination (Supplementary Figure 1).
Cell-mediated response was evaluated in 190 vaccinated participants and 10 placebo participants by measuring IFN-γ production in PBMC samples by ELISA on days 0 and 28 and reported as increase in IFN-γ concentration in EBOV GP-stimulated PBMCs on day 28 against day 0 in vaccine group (Figure 3). Median IFN-γ concentration in vaccinated volunteers was 0 (interquartile range [IQR] 0–2.0) on day 0 and 26.8 (IQR 4.8– 126.8) on day 28. This indicates formation of GP-specific T-cell response on day 7 after boost vaccination. IFN-γ response was not detected in placebo group.
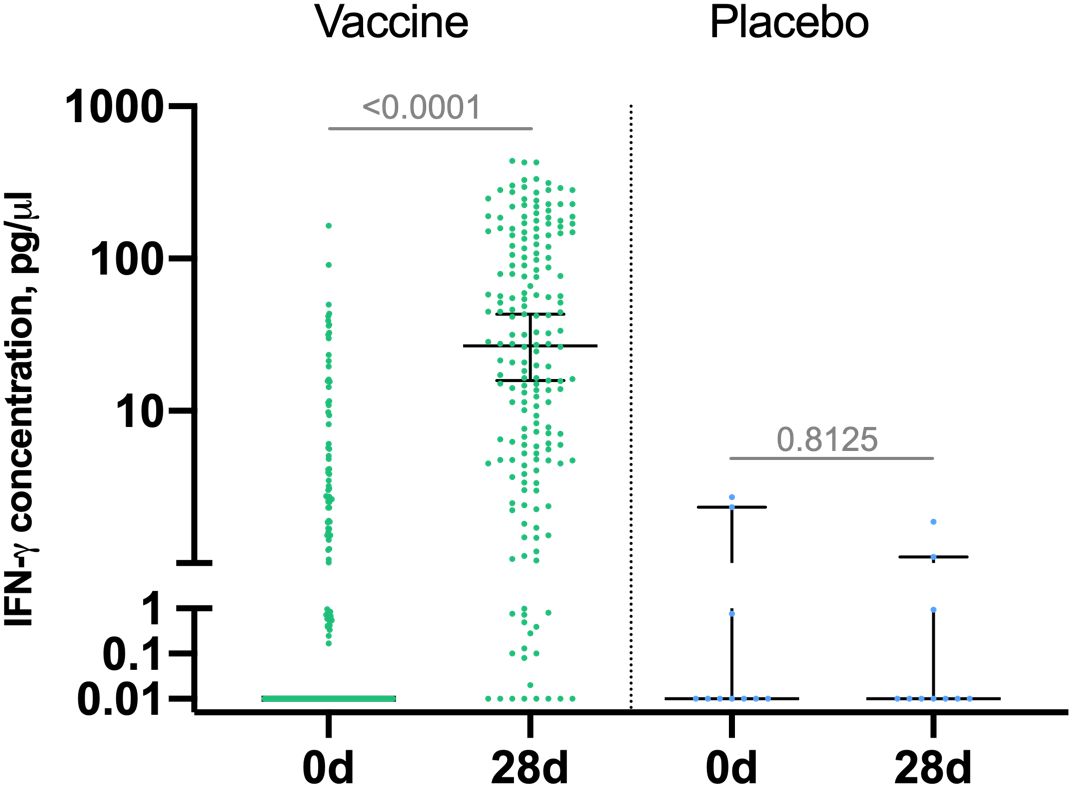
Figure 3. IFN-γ response to Zaire Ebolavirus glycoprotein (GP) in participants. Peripheral blood mononuclear cells were isolated from blood samples of vaccinated participants (n = 190) at days 0 and 28 and stimulated with EBOV-GP. IFN -γ concentration in samples was measured by ELISA. Increase in IFN-γ production (pg/µl) by GP-stimulated PBMCs compared to intact PBMCs at days 0 and 28 is shown on a logarithmic scale. Median is marked with bars, IQR is marked with whiskers. Difference between IFN -γ concentration at days 0 and 28 was calculated with Wilcoxon test.
Additionally, neutralizing immune response was evaluated in 190 vaccinated participants and 10 placebo participants. Neutralizing antibody titers were determined in plaque pseudoneutralization assay. A statistically significant increase in neutralizing antibodies (NtAb) titers was demonstrated in vaccinated participants on day 42 (p<0,0001) (Figure 4): geometric mean titer of NtAb in vaccinated individuals was 32.61, seroconversion rate – 96.3%.
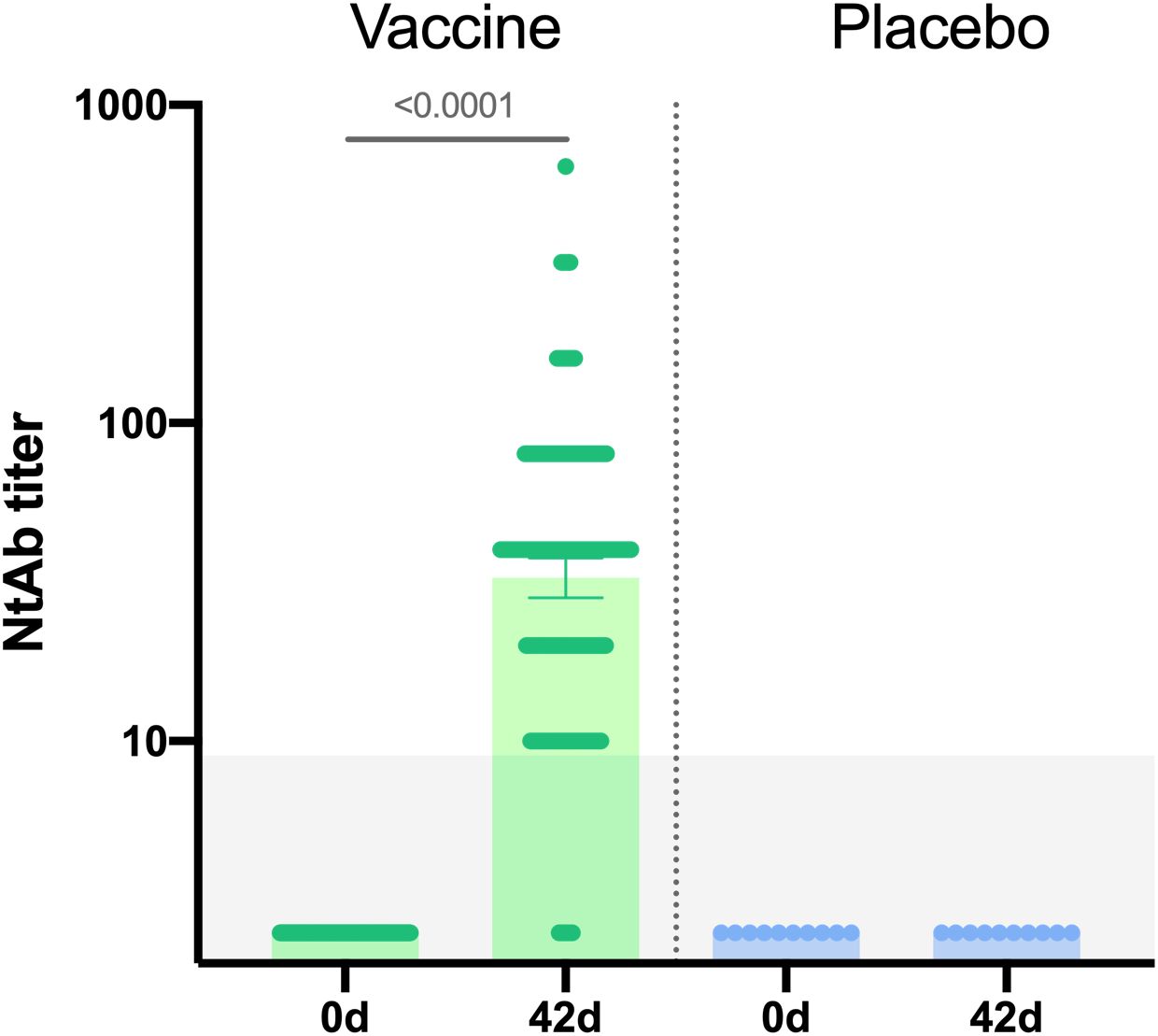
Figure 4. Neutralizing antibodies in sera samples of participants from vaccine group (n = 190) and placebo group (n = 10) at days 0 and 42. Neutralizing antibody (NtAb) titers were determined in plaque pseudoneutralization assay with rVSV-GP. Bars show GMT, 95% CI are marked by whiskers. Difference between NtAb titers at days 0 and 42 was calculated with Wilcoxon test (p < 0.0001).
In December 2015, GamEvac-Combi – a combined EVD vaccine based on the recombinant viral vectors rVSV and rAd5 - was registered in the Russian Federation after successful completion of phase 1-2 clinical trial (6). In August 2017 – December 2019 double-blind randomized placebo-controlled clinical trial of the vaccine was conducted in the Republic of Guinea and in Russia. The primary outcomes of the study were to evaluate safety and immunogenicity of the GamEvac-Combi vaccine in healthy adults. To evaluate vaccine safety, systemic and local reactions were monitored during all study in 1900 vaccinated participants and 100 participants, who received placebo, in the Republic of Guinea and in 10 vaccinated participants in Russia. Antigen-specific humoral immune response was studied in all attended participants from vaccinated and placebo groups up to 12 months after vaccination, IFN-γ responses on day 28 were analyzed in 190 participants from vaccine group and 10 participants from placebo group.
Current study presents the final results of clinical trial of rVSV and rAd5 vaccine against Ebola virus disease. The vaccine showed a favorable safety profile, which is in line with phase 1-2 clinical trial results (6). No severe, life-threatening or disabling, or death related adverse events, associated with vaccination were reported. Most of the adverse events, associated with the vaccine administration, were mild. All registered adverse events were solicited, occurred within the first days after vaccination and resolved within the next 1-2 days. No vaccine-related deaths were reported. When comparing the immunogenicity data obtained in the present study and in the phase 1-2 study, we noticed that in general, the level of immune response in the population of the Republic of Guinea was higher: for example, on day 42, the geometric mean titer of GP-specific antibodies in phase 1-2 clinical trial was 3277 (95% CI 2401-4473) (6), while in this study the antibody level at day 42 was 9349 (95% CI 8762-9975).
According to heterologous prime-boost approach, component A (rVSV) was administrated on day 0 and component B (rAd5) – on day 21, which resulted in significant boost of antibody levels on day 28, prominent peak of GP-specific antibodies on day 42 and a year-long persistence of GP-specific IgG antibody response. Vaccination with rVSV and rAd5 also induced significant IFN-γ response 7 days after the boost. A part of vaccinees prior to vaccination showed elevated levels of IFN-γ secretion upon antigen stimulation that could be attributed to contribution of other immune cells present in PBMC fraction (NK, eosinophils) besides T cells (7, 8). Similar effect was also seen in phase 1-2 clinical trial conducted in Russia (6). The cellular immune response plays a significant role in protecting against Ebola virus disease (9). GamEvac-Combi vaccine is administered using a heterologous vaccination schedule, and such vaccines have been shown in other studies to stimulate a more pronounced cellular immune response. In a comparative study of the cellular response in different vaccination regimens, it was shown that the total level of cytokines produced by EBOV-specific CD4+ or CD8+ T cells (IFN-γ ± IL-2 ± TNF ± MIP-1β) was significantly higher for the group with the heterologous Ad26.ZEBOV/MVA-BN-Filo vaccination regimen than for the group vaccinated once or twice with rVSVΔG-ZEBOV-GP (10).
An important advantage of the GamEvac-Combi vaccine is the use of a heterologous prime-boost vaccination approach. The kinetics of the immune response in volunteers is adequate: after vaccination, a peak response is detected on days 28-42, and then a systematic decrease in the level of antibodies at 6-12 months. A year after vaccination, specific antibodies were detected in the blood serum of more than 94% of volunteers. The seroconversion rate is significantly higher than with a single vaccination with registered vaccines based on recombinant chimpanzee adenovirus (ChAd3-EBO-Z) and recombinant vesicular stomatitis virus (rVSVΔG-ZEBOV-GP): studies in Liberia showed that one year after vaccination % of volunteers with IgG response was 63.5% in the ChAd3-EBO-Z group and 79.5% in the rVSVΔG-ZEBOV-GP group (11). Thus, vaccination with GamEvac-Combi allows to form a long-term intense immune response.
Correlates of protection for Ebola virus were studied in NHPs. According to different studies, protection of vaccinated laboratory animals against subsequent EBOV challenge correlated with total level of GP-specific antibodies (12), level of neutralizing antibodies (13), antibody specificity and Fc-mediated effects (14). Therefore, it appears that there is a complex interplay between the factors of specific antifiloviral immune response, that contribute to protection. As for humans, it was shown that survival during a natural human filovirus infection correlated with the level of the filovirus binding antibodies and neutralizing antibody response. Accordingly, convalescent plasma treatment during the EVD epidemic in west Africa did not show clear efficacy, as it was based on measuring only of GP-specific IgG antibody level, but not neutralizing antibodies (15). Approach of immunobridging allows to translate human immunogenicity data into the likelihood of protection (12). But the magnitude of protective responses in humans is yet to be established. Clinical trial of an rVSV-ZEBOV vaccine, that established its efficacy against EVD included survival data, but not IgG or neutralizing response data (16).
The efficacy of the rVSVΔG-ZEBOV-GP vaccine during the ring vaccination in the Republic of Guinea in 2014-2016 with 8334 participants was 100% (95% CI 68.9–100.0, p=0.0045) (16). At the same time, studies of the immunogenicity of the rVSVΔG-ZEBOV-GP vaccine, used in the same administration regimen (2×107 PFU once), demonstrate that the peak of the humoral immune response was detected on the 28th day after vaccination (GMT anti-GP IgG – 4079), while the presence of antibodies persisted for six months (17). Our study of the immunogenicity of the GamEvac-Combi vaccine demonstrates a peak IgG-response at day 42 (GMT anti-GP IgG – 9345), and the duration of the response is at least one year. Unfortunately, due to the absence of an outbreak of Ebola virus disease in the Republic of Guinea during the period of our clinical studies (2017-2019), we cannot draw conclusions about the epidemiological efficacy of GamEvac-Combi vaccine.
Our study has some limitations. First, predominantly male participants and mainly of young age. Second, the lack of laboratory correlates of protection makes it difficult to interpret the clinical significance of changes in antibody levels. Thirdly, within the framework of this study, it was not possible to assess the epidemiological effectiveness of the vaccine, since no cases of EVD were recorded in the region during the study.
Heterologous prime-boost approach was used in development of Sputnik V (Gam-COVID-Vac) vaccine against COVD-19. Gam-COVID-Vac consists of two doses: rAd26-based vector is used for the prime and rAd5-based vector is used for the boost 21 days later. This approach has proven to effectively induce antigen-specific immune response in multiple clinical studies (18, 19). GamEvac-Combi uses the same approach with rVSV for priming and rAd5 for boosting immune response. Recombinant replication-competent VSV induces rapid innate immunity activation, which was shown to contribute to early protection against lethal EBOV infection in primate studies (20). The vaccine rVSV-ZEBOV has shown high efficacy in clinical studies and to date is the only vaccine with reported efficacy in EVD outbreaks (16, 21). The findings from this trial might contribute to data on vaccine-induced immune responses against Ebola virus disease.
According to the results of a double-blind randomized placebo-controlled trial of safety and immunogenicity of GamEvac-Combi vaccine in healthy adults, the vaccine showed favorable safety profile and induced robust cell-mediated immune response and strong humoral immune response that lasts at least for a year from the start of vaccination.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
The studies involving humans were approved by The Ethics Committee of the Ministry of Health of the Russian Federation (#119 16/02/2016, additional #155 19/09/2017, #203 19/11/2019) and Ethics Committee of the Ministry of Health of Republic of Guinea (76/CNERS/16 20/06/2016, № 064/CNERS/17 07/05/2017, № 041/CNERS/18 09/03/2018). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
DL: Conceptualization, Methodology, Project administration, Writing – review & editing. ID: Formal analysis, Investigation, Methodology, Visualization, Writing – original draft, Writing – review & editing. MB: Resources, Writing – review & editing. AVK: Formal analysis, Investigation, Visualization, Writing – original draft. AD: Formal analysis, Investigation, Methodology, Writing – review & editing. ASE: Investigation, Writing – review & editing. DG: Investigation, Writing – review & editing. AT: Formal analysis, Investigation, Methodology, Writing – review & editing. FI: Investigation, Writing – review & editing. YS: Methodology, Project administration, Writing – review & editing. MO: Formal analysis, Visualization, Writing – review & editing. NL: Resources, Writing – review & editing. OZ: Investigation, Methodology, Writing – review & editing. DVS: Formal analysis, Investigation, Methodology, Writing – review & editing. IE: Formal analysis, Investigation, Writing – review & editing. MS: Formal analysis, Investigation, Writing – review & editing. ASS: Resources, Writing – review & editing. NT: Investigation, Writing – review & editing. DNS: Investigation, Writing – review & editing. IT: Investigation, Writing – review & editing. GP: Data curation, Writing – review & editing. AAK: Data curation, Writing – review & editing. TD: Data curation, Writing – review & editing. NM: Data curation, Writing – review & editing. LM: Data curation, Writing – review & editing. OLV: Data curation, Writing – review & editing. IF: Data curation, Writing – review & editing. LBK: Data curation, Writing – review & editing. LVK: Data curation, Writing – review & editing. DM: Data curation, Writing – review & editing. ALE: Data curation, Writing – review & editing. EE: Data curation, Writing – review & editing. KK: Data curation, Writing – review & editing. VL: Data curation, Writing – review & editing. IK: Data curation, Writing – review & editing. EB: Data curation, Writing – review & editing. ABS: Data curation, Writing – review & editing. ET: Data curation, Writing – review & editing. AG: Formal analysis, Writing – review & editing. DR: Investigation, Writing – review & editing. FI: Resources, Writing – review & editing. KN: Resources, Writing – review & editing. ODV: Resources, Writing – review & editing. IV: Resources, Writing – review & editing. ZM: Resources, Writing – review & editing. ZM: Resources, Writing – review & editing. SAN: Resources, Writing – review & editing. IM: Resources, Writing – review & editing. SS: Resources, Writing – review & editing. GV: Data curation, Writing – review & editing. MV: Data curation, Writing – review & editing. GA: Data curation, Writing – review & editing. BV: Data curation, Writing – review & editing. VV: Data curation, Writing – review & editing. DI: Data curation, Writing – review & editing. IB: Data curation, Writing – review & editing. AV: Data curation, Writing – review & editing. KN: Data curation, Writing – review & editing. LV: Data curation, Writing – review & editing. VI: Data curation, Writing – review & editing. KV: Data curation, Resources, Writing – review & editing. BK: Data curation, Writing – review & editing. KA: Data curation, Writing – review & editing. EK: Resources, Writing – review & editing. SS: Investigation, Writing – review & editing. DC: Investigation, Writing – review & editing. AA: Investigation, Writing – review & editing. DK: Investigation, Writing – review & editing. SB: Conceptualization, Supervision, Writing – review & editing. BN: Conceptualization, Supervision, Writing – review & editing. AG: Conceptualization, Funding acquisition, Writing – review & editing.
The author(s) declare that financial support was received for the research and/or publication of this article. This research was funded by Ministry of Health of Russian Federation. Funding agencies had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all data, and had final responsibility in the decision to publish.
We thank the people in Kindia for their participation, the entire staff of Medical service CBK RUSAL, Research Center for Epidemiology, Microbiology and Medical Care.
DL, MS, OZ, DVS, ID, AD, NT, AT, SS, SB, BN and AG report patent for an immunobiological agent and method of its use for induction of specific immunity against Ebolavirus.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2025.1487039/full#supplementary-material
1. Baseler L, Chertow DS, Johnson KM, Feldmann H, Morens DM. The pathogenesis of ebola virus disease. Annu Rev Pathol. (2017) 24:387–418. doi: 10.1146/annurev-pathol-052016-100506
2. Centers for Disease Control and Prevention. History of ebola outbreaks (2024). Available online at: https://www.cdc.gov/vhf/ebola/history/chronology.html (Accessed July 15, 2024).
3. Fausther-Bovendo H, Kobinger G. The road to effective and accessible antibody therapies against Ebola virus. Curr Opin Virol. (2022) 54:101210. doi: 10.1016/j.coviro.2022.101210
4. World Health Organization. Prioritizing diseases for research and development in emergency contexts (2017). Available online at: https://www.who.int/activities/prioritizing-diseases-for-research-and-development-in-emergency-contexts (Accessed July 15, 2024).
5. Malik S, Kishore S, Nag S, Dhasmana A, Preetam S, Mitra O, et al. Ebola virus disease vaccines: development, current perspectives & Challenges. Vaccines (Basel). (2023) 11:268. doi: 10.3390/vaccines11020268
6. Dolzhikova IV, Zubkova OV, Tukhvatulin AI, Dzharullaeva AS, Tukhvatulina NM, Shcheblyakov DV, et al. Safety and immunogenicity of GamEvac-Combi, a heterologous VSV- and Ad5-vectored Ebola vaccine: An open phase I/II trial in healthy adults in Russia. Hum Vaccin Immunother. (2017) 13:613–20. doi: 10.1080/21645515.2016.1238535
7. Wagstaffe HR, Clutterbuck EA, Bockstal V, Stoop JN, Luhn K, Douoguih M, et al. Antibody-dependent natural killer cell activation after ebola vaccination. J Infect Diseases. (2021) 223:1171–82. doi: 10.1093/infdis/jiz657
8. Carmo LAS, Bonjour K, Spencer LA, Weller PF, Melo RCN. Single-cell analyses of human eosinophils at high resolution to understand compartmentalization and vesicular trafficking of interferon-gamma. Front Immunol. (2018) 9:15428. doi: 10.3389/fimmu.2018.015428
9. Escudero-Pérez B, Lawrence P, Castillo-Olivares J. Immune correlates of protection for SARS-CoV-2, Ebola and Nipah virus infection. Front Immunol. (2023) 14:1156758. doi: 10.3389/fimmu.2023.1156758
10. Wiedemann A, Lhomme E, Huchon M, Foucat E, Bérerd-Camara M, Guillaumat L, et al. Long-term cellular immunity of vaccines for Zaire Ebola Virus Diseases. Nat Commun. (2024) 15:7666. doi: 10.1038/s41467-024-51453-z
11. Kennedy SB, Bolay F, Kieh M, Grandits G, Badio M, Ballou R, et al. Phase 2 placebo-controlled trial of two vaccines to prevent ebola in Liberia. N Engl J Med. (2017) 377:1438–47. doi: 10.1056/NEJMoa1614067
12. McLean C, Dijkman K, Gaddah A, Keshinro B, Katwere M, Douoguih M, et al. Persistence of immunological memory as a potential correlate of long-term, vaccine-induced protection against Ebola virus disease in humans. Front Immunol. (2023) 14:1215302. doi: 10.3389/fimmu.2023.1215302
13. Roozendaal R, Hendriks J, van Effelterre T, Spiessens B, Dekking L, Solforosi L, et al. Nonhuman primate to human immunobridging to infer the protective effect of an Ebola virus vaccine candidate. NPJ Vaccines. (2020) 5:112. doi: 10.1038/s41541-020-00261-9
14. Meyer M, Gunn BM, Malherbe DC, Gangavarapu K, Yoshida A, Pietzsch C, et al. Ebola vaccine-induced protection in nonhuman primates correlates with antibody specificity and Fc-mediated effects. Sci Transl Med. (2021) 13:eabg6128. doi: 10.1126/scitranslmed.abg6128
15. Ilinykh PA, Bukreyev A. Antibody responses to filovirus infections in humans: protective or not? Lancet Infect Dis. (2021) 21:e348–55. doi: 10.1016/S1473-3099(21)00006-2
16. Henao-Restrepo AM, Camacho A, Longini IM, Watson CH, Edmunds WJ, Egger M, et al. Efficacy and effectiveness of an rVSV-vectored vaccine in preventing Ebola virus disease: final results from the Guinea ring vaccination, open-label, cluster-randomised trial (Ebola Ça Suffit)! Lancet. (2017) 389:505–18. doi: 10.1016/S0140-6736(16)32621-6
17. Regules JA, Beigel JH, Paolino KM, Voell J, Castellano AR, Hu Z, et al. A recombinant Vesicular Stomatitis virus Ebola vaccine. N Engl J Med. (2017) 376:330–41. doi: 10.1056/NEJMoa1414216
18. Logunov DY, Dolzhikova IV, Shcheblyakov DV, Tukhvatulin AI, Zubkova OV, Dzharullaeva AS, et al. Safety and efficacy of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine: an interim analysis of a randomised controlled phase 3 trial in Russia. Lancet. (2021) 397:671–81. doi: 10.1016/S0140-6736(21)00234-8
19. Toubasi AA, Al-Sayegh TN, Obaid YY, Al-Harasis SM, AlRyalat SAS. Efficacy and safety of COVID-19 vaccines: A network meta-analysis. J Evid Based Med. (2022) 15:245–62. doi: 10.1111/jebm.12492
20. Pinski AN, Messaoudi I. Therapeutic vaccination strategies against EBOV by rVSV-EBOV-GP: the role of innate immunity. Curr Opin Virol. (2021) 51:179–89. doi: 10.1016/j.coviro.2021.10.007
21. Coulborn RM, Bastard M, Peyraud N, Gignoux E, Luquero F, Guai B, et al. Case fatality risk among individuals vaccinated with rVSVΔG-ZEBOV-GP: a retrospective cohort analysis of patients with confirmed Ebola virus disease in the Democratic Republic of the Congo. Lancet Infect Dis. (2024) 24:602–10. doi: 10.1016/S1473-3099(23)00819-8
Keywords: clinical trials, vector vaccine, EVD, Ebola vaccine, prime-boost, rVSV, rAd5
Citation: Logunov DY, Dolzhikova IV, Boiro MY, Kovyrshina AV, Dzharullaeva AS, Erokhova AS, Grousova DM, Tukhvatulin AI, Izhaeva FM, Simakova YV, Ordzhonikidze MK, Lubenets NL, Zubkova OV, Scheblyakov DV, Esmagambetov IB, Shmarov MM, Semikhin AS, Tukhvatulina NM, Shcherbinin DN, Tutykhina IL, Prokhorov GS, Khovaev AA, Demidova TN, Malishev NA, Merkulova LN, Voronina OL, Fedyakina IT, Kisteneva LB, Kolobukhina LV, Mishin DV, Elakov AL, Ermolova EI, Krasnoslobodtsev KG, Larichev VF, Kruzhkova IS, Burmistrov EM, Sheremet AB, Tokarskaya EA, Gromov AV, Reshetnikov DA, Fisun AI, Kotiv BN, Ovchinnikov DV, Ivchenko EV, Zhdanov KV, Zakharenko SM, Solovev AN, Ivanov AM, Sukachev VS, Gudkov RV, Maltsev OV, Gabdrakhmanov IA, Barsukov AV, Vashchenkov VV, Demianenko NI, Ignatev SB, Asiamov KV, Kirichenko NN, Liubimov AV, Volkov II, Kriukov EV, Bazarnov NK, Kolodiazhnaia VA, Kolomoets EV, Syromyatnikova SI, Chifanov DE, Andrus AF, Kutaev DA, Borisevich SV, Naroditsky BS and Gintsburg AL (2025) Safety and immunogenicity of GamEvac-Combi, a heterologous rVSV- and rAd5-vectored Ebola vaccine: a randomized controlled multicenter clinical trial in the Republic of Guinea and Russia. Front. Immunol. 16:1487039. doi: 10.3389/fimmu.2025.1487039
Received: 27 August 2024; Accepted: 19 February 2025;
Published: 20 March 2025.
Edited by:
Rajko Reljic, University of London, United KingdomReviewed by:
Jiaming Lan, Chinese Academy of Sciences (CAS), ChinaCopyright © 2025 Logunov, Dolzhikova, Boiro, Kovyrshina, Dzharullaeva, Erokhova, Grousova, Tukhvatulin, Izhaeva, Simakova, Ordzhonikidze, Lubenets, Zubkova, Scheblyakov, Esmagambetov, Shmarov, Semikhin, Tukhvatulina, Shcherbinin, Tutykhina, Prokhorov, Khovaev, Demidova, Malishev, Merkulova, Voronina, Fedyakina, Kisteneva, Kolobukhina, Mishin, Elakov, Ermolova, Krasnoslobodtsev, Larichev, Kruzhkova, Burmistrov, Sheremet, Tokarskaya, Gromov, Reshetnikov, Fisun, Kotiv, Ovchinnikov, Ivchenko, Zhdanov, Zakharenko, Solovev, Ivanov, Sukachev, Gudkov, Maltsev, Gabdrakhmanov, Barsukov, Vashchenkov, Demianenko, Ignatev, Asiamov, Kirichenko, Liubimov, Volkov, Kriukov, Bazarnov, Kolodiazhnaia, Kolomoets, Syromyatnikova, Chifanov, Andrus, Kutaev, Borisevich, Naroditsky and Gintsburg. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Denis Y. Logunov, bG9ndW5vdkBnYW1hbGV5YS5vcmc=
†These authors have contributed equally to this work
‡Deceased
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.