- 1Department of Biomedical Sciences, University of Sassari, Sassari, Italy
- 2Discipline of Clinical Pharmacology, College of Medicine and Public Health, Flinders University, Adelaide, SA, Australia
- 3Department of Clinical Pharmacology, Flinders Medical Centre, Southern Adelaide Local Health Network, Adelaide, SA, Australia
There is an ongoing search for novel biomarkers to enhance diagnosing and monitoring patients with rheumatic diseases (RDs). We conducted a systematic review and meta-analysis to investigate the potential role of the soluble cluster of differentiation 40 (sCD40) and sCD40 ligand (sCD40L), involved in humoral and cellular immune response, as candidate biomarkers of RDs. We searched PubMed, Web of Science, and Scopus from inception to 30 June 2024 for studies investigating circulating sCD40 and sCD40L concentrations in RD patients and healthy controls. We assessed the risk of bias using the Joanna Briggs Institute Critical Appraisal Checklist for analytical studies and the certainty of evidence using the Grades of Recommendation, Assessment, Development and Evaluation Working Group system. Compared to controls, RD patients had significantly higher sCD40L (31 studies; standard mean difference, SMD=0.87, 95% CI 0.60 to 1.13, p<0.001; low certainty of evidence) and sCD40 (five studies; SMD=1.32, 95% CI 0.45 to 2.18, p=0.003; very low certainty of evidence) concentrations. In meta-regression and subgroup analysis, the effect size of the between-group differences in sCD40L was significantly associated with sample size, mean RD duration, specific RD, biological matrix assessed, and analytical method used. By contrast, there were no associations with age, sex, C-reactive protein, erythrocyte sedimentation rate, use of disease-modifying antirheumatic drugs or glucocorticoids, or geographical location. There were no significant differences in sCD40L concentrations between RD patients with and without active disease (eight studies; SMD=0.12, 95% CI -0.09 to 0.33, p=0.26; very low certainty). By contrast, sCD40 concentrations were significantly higher in RD patients with active disease (three studies; SMD=0.36, 95% CI 0.08 to 0.84, p=0.013; very low certainty). Our systematic review and meta-analysis suggests the potential role of sCD40 and sCD40L as candidate biomarkers to detect the presence of RDs (sCD40 and sCD40L) and monitor disease activity (sCD40). Large, appropriately designed prospective studies in a wide range of RDs are warranted to investigate whether measuring sCD40 and sCD40L can significantly improve the performance of currently available diagnostic criteria and serological biomarkers. (PROSPERO registration number: CRD42024577430).
Systematic review registration: https://www.crd.york.ac.uk/PROSPERO/view/CRD42024577430, identifier PROSPERO CRD42024577430.
Introduction
Early diagnosis and treatment significantly improve the quality of life and prognosis in patients with rheumatic diseases (RDs), a group of autoimmune (e.g., rheumatoid arthritis, RA), autoimmune-autoinflammatory (e.g., Behcet’s disease, BD), or autoinflammatory (e.g., familial Mediterranean fever, FMF) conditions affecting various organs and systems (1–9). However, diagnosing early, subtle forms of RDs remains challenging, particularly for nonspecialists. This vexing issue has stimulated research to identify novel biomarkers of disease to aid clinical evaluation and management (10–15). Ideally, such biomarkers should adequately reflect alterations of critical pathways regulating immune response and inflammation (16–18).
The cluster of differentiation 40 (CD40)/CD40 ligand (CD40L) dyad is a pivotal regulator of the humoral and cellular immune response (19, 20). CD40 is a membrane glycoprotein that is part of the tumor necrosis factor (TNF) receptor superfamily (21). CD40 is expressed in many cells, including B cells, endothelial cells, epithelial cells, monocytes, macrophages, fibroblasts, and dendritic cells (19–21). CD40L, also a glycoprotein and member of the TNF superfamily, is transiently expressed in activated T cells, mainly the CD4+ T-cell subset, basophils, mast cells, eosinophils, natural killer cells, and platelets (22). The CD40L-mediated activation of CD40 favors the growth and differentiation of B cells, immunoglobulin class switching, and antigen-presenting cell activation by inducing cytokine synthesis (19, 20). The CD40L-mediated activation of CD40 also induces short-term activation and cytokine production in T cells. Following cell activation, CD40L translocates to the cell surface as membrane CD40L (mCD40L). CD40L also exists as a soluble form (sCD40L) that is generated either from enzymatic cleavage of mCD40L or intracellular CD40L. Both mCD40L and sCD40L are biologically active (19, 20). Two forms of CD40 also exist, membrane (mCD40) and soluble (sCD40). sCD40 is formed by alternative splicing in the cytoplasm or following proteolysis of mCD40 following ligation with CD40L. Notably, sCD40 antagonizes the effects of CD40 (19, 20). Therefore, measuring circulating sCD40 and sCD40L may be helpful in characterizing the immune response in different types of RDs, complementing the information provided by clinical assessment and available diagnostic criteria and serological biomarkers.
Therefore, we investigated the potential role of sCD40 and sCD40L as candidate biomarkers by conducting a systematic review and meta-analysis of studies reporting their concentrations in serum or plasma in RD patients and healthy controls. We further investigated possible associations between the effect size of the between-group differences and various study and patient variables, including demographic characteristics, type of RD, mean RD duration, conventional inflammatory markers (i.e., C-reactive protein (CRP) and erythrocyte sedimentation rate (ESR), and use of disease-modifying antirheumatic drugs (DMARDs) and corticosteroids.
Materials and methods
Search strategy, screening, and study selection
We systematically searched PubMed, Web of Science, and Scopus, from inception to 30 June 2024, for relevant articles using the following terms (please refer to Supplementary Table 1 for additional details regarding the search strategy): “soluble cluster of differentiation 40” OR “sCD40” OR “soluble CD40” OR “sCD40L” OR “soluble CD40L” OR “sCD40 ligand” OR “sCD154” AND “rheumatic diseases” OR “rheumatoid arthritis” OR “psoriatic arthritis” OR “ reactive arthritis” OR “ankylosing spondylitis” OR “systemic lupus erythematosus” OR “systemic sclerosis” OR “scleroderma” OR “Sjogren’s syndrome” OR “connective tissue diseases” OR “vasculitis” OR “Behçet’s disease” OR “idiopathic inflammatory myositis” OR “polymyositis” OR “dermatomyositis” OR “gout” OR “pseudogout” OR “ systemic vasculitis” OR “ANCA-associated vasculitis” OR “Takayasu arteritis” OR “polyarteritis nodosa” OR “osteoarthritis” OR “fibromyalgia” OR “granulomatous polyangiitis” OR “Henoch-Schonlein purpura” OR “Wegener’s granulomatosis” OR “familial Mediterranean fever” OR “polymyalgia rheumatica”.
Initially, two investigators independently screened each abstract for relevance. Then, they independently reviewed the full text of each article. The inclusion criteria were: (i) the measurement of circulating sCD40L and/or sCD40 concentrations, (ii) the comparison between RD patients and healthy controls and/or between RD patients with and without active disease (case-control design), (iii) the inclusion of participants aged ≥18 years, (iv) the use of English language, (v) the recruitment of at least ten RD patients and/or controls, and (vi) the availability of the full text of the publication. The exclusion criteria were: (i) in vitro or animal studies, (ii) the inclusion of participants under 18 years, and (iii) the inclusion of less than ten RD patients and/or controls. The references of the retrieved articles were hand-searched to identify additional studies.
The two investigators independently extracted the following information into separate electronic sheets for further analysis: first author, year of publication, country where the study was conducted, RD type, mean RD duration, number of participants, age, male-to-female ratio, CRP, ESR, use of DMARDs or glucocorticoids, sample matrix assessed (serum or plasma), and analytical method used. Any disagreement was resolved by a third investigator.
We assessed the risk of bias of each article using the Joanna Briggs Institute Critical Appraisal Checklist for analytical studies (23) and the level of the certainty of evidence using the Grades of Recommendation, Assessment, Development and Evaluation (GRADE) Working Group system (24). We wholly adhered to the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) 2020 statement (Supplementary Table 1) (25). We registered the study protocol in the International Prospective Register of Systematic Reviews (PROSPERO registration number: CRD42024577430).
Statistical analysis
We generated forest plots of standardized mean differences (SMDs) and 95% confidence intervals of sCD40L and sCD40 concentrations between RD patients and healthy controls and between RD patients with and without active disease (a p-value <0.05 was considered statistically significant). Medians and interquartile ranges were extracted from graphs using the Graph Data Extractor software (San Diego, CA, USA). Using published methods, we extrapolated the means and standard deviations from the medians and interquartile or full ranges (26). We used the Q statistic (a p-value <0.10 was considered statistically significant) to assess the heterogeneity of SMD across studies. A low, moderate, and high heterogeneity was indicated by I2 values of ≤ 25%, >25% and <75%, and ≥75%, respectively (27, 28). We used a random-effect model based on the inverse-variance method in the presence of high heterogeneity (29). We conducted sensitivity analyses to test the stability of the meta-analysis results and assessed publication bias using standard methods (a p-value <0.05 was considered statistically significant) (30–33). We conducted meta-regression and subgroup analyses to investigate associations between the effect size and year of publication, country where the study was conducted, RD type, mean RD duration, sample size, age, male-to-female ratio, CRP, ESR, use of DMARDs and/or glucocorticoids, sample matrix assessed, and analytical method used. All statistical analyses were performed using Stata 14 (Stata Corp., College Station, TX, USA).
Results
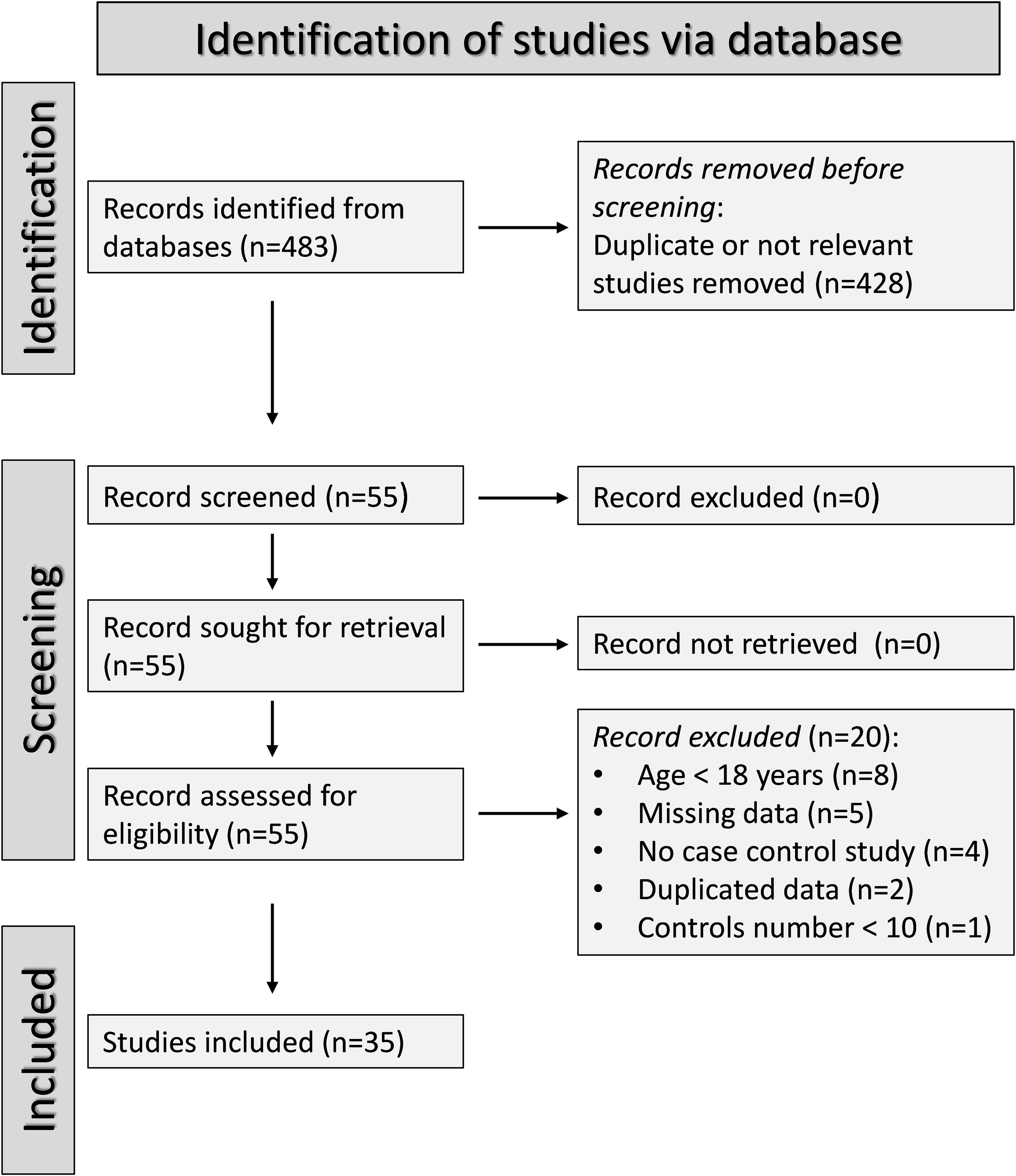
Figure 1 describes the flow chart of the screening process and study selection. We initially identified 483 articles. After the first screening, we excluded 428 articles because they reported duplicate or irrelevant information. After full-text revision of the remaining 55 articles, we excluded eight studies because they enrolled participants under 18 years, five because of missing data, four because of a different study design, two because of duplicate data, and one because the number of controls was less than ten. Thus, we selected 35 studies for analysis (34–68). Their characteristics are described in Table 1. Given the cross-sectional design of the studies identified in our search, we ranked the initial level of the certainty of evidence as low (level 2).

Table 1. Characteristics of studies investigating sCD40L and sCD40 in patients with rheumatic diseases and healthy controls.
sCD40L
Presence of RDs
Thirty-one studies, including 37 group comparators, investigated sCD40L concentrations in 2,414 RD patients (mean age 44.2 years, 87.8% females) and 1,384 healthy controls (mean age 42.3 years, 83.4% females) (34–51, 53–59, 61, 62, 64, 65, 67, 68). Thirteen studies were conducted in Europe (37, 38, 40, 43, 47, 49, 53, 57, 58, 62, 64, 65, 68), 11 in Asia (34, 36, 41, 42, 46, 48, 50, 51, 54, 55, 67), six in America (35, 39, 44, 56, 59, 61), and one in Africa (45). Systemic lupus erythematosus (SLE) patients were investigated in 15 study groups (34, 35, 38–40, 44, 45, 47, 51, 55, 57, 59, 64, 67), RA patients in eight (36, 38, 42, 47, 51, 61, 62, 68), BD patients in three (49, 53, 56), systemic sclerosis (SSc) patients in three (37, 41, 54), ankylosing spondylitis (AS) patients in three (48, 50, 58), primary Sjogren syndrome (pSS) patients in two (38, 47), psoriatic arthritis (PsA) patients in two (46, 65), and connective tissue disease (CTD) patients in one (43). sCD40L was measured in serum in 19 studies (35, 38–40, 42, 44–46, 48, 50, 51, 53, 54, 57, 58, 62, 65, 67, 68) and plasma in 11 (34, 36, 37, 41, 43, 47, 49, 55, 56, 61, 64). One study did not provide relevant information regarding the biological matrix used (59). An enzyme-linked immunosorbent assay (ELISA) was used in 25 studies (34–51, 53, 55, 56, 58, 61, 64, 68) and a platform for multi-analyte profiling in the remaining six (54, 57, 59, 62, 65, 67). Nineteen studies reported the mean RD duration, which ranged between 1.34 and 16.9 years (37, 39, 42, 43, 45, 47, 49, 51, 53, 55–57, 59, 61, 62, 64, 65, 67, 68). The risk of bias was low in 19 studies (36, 37, 39, 42, 43, 46–49, 51, 55–59, 62, 64, 65, 68), moderate in ten (34, 35, 38, 40, 45, 50, 53, 54, 61, 67), and high in two (41, 44) (Supplementary Table 2).
The forest plot showed that sCD40L concentrations were significantly higher in RD patients than in controls (SMD=0.87, 95% CI 0.60 to 1.13, p<0.001; I2 = 91.7%, p<0.001; Figure 2). The meta-analysis results were stable in sensitivity analysis, with the corresponding pooled SMD values ranging between 0.82 and 0.91 (Figure 3).

Figure 2. Forest plot of sCD40L concentrations in patients with rheumatic diseases and healthy controls.
We observed a significant publication bias with Begg’s (p=0.002) and Egger’s (p<0.001) tests. The “trim-and-fill” method identified 15 missing studies to be added to the left side of the funnel plot to ensure symmetry (Figure 4). The resulting pooled SMD was significantly decreased and not significant (SMD=0.25, 95% CI -0.03 to 0.54, p=0.08).

Figure 4. Funnel plot of the association between sCD40L and rheumatic diseases after “trimming-and-filling”. The enclosed circles and free circles represent dummy studies and genuine studies, respectively.
We did not observe significant associations in meta-regression analysis between the effect size and age (t=-0.93, p=0.36), male-to-female ratio (t=0.01, p=0.99), CRP (t=-0.23, p=0.82), ESR (t=-0.78, p=0.45), and use of DMARDs (t=-0.63, p=0.54) or glucocorticoids (t=0.18, p=0.86). By contrast, there was a significant negative association with sample size (t=-2.49, p=0.018; Figure 5A) and a positive association with the mean RD duration (t=2.09, p=0.049; Figure 5B).

Figure 5. Bubble plot of the univariate meta-regression analysis between effect size and sample size (A) and mean disease duration (B).
In subgroup analysis, the pooled SMD was statistically significant in studies in SLE (SMD=0.91, 95% CI 0.40 to 1.43, p=0.001; I2 = 95.2%, p<0.001), RA (SMD=0.53, 95% CI 0.19 to 0.86, p=0.002; I2 = 78.5%, p<0.001), BD (SMD=1.58, 95% CI 1.26 to 1.90, p<0.001; I2 = 0.0%, p=0.51), SSc (SMD=0.79, 95% CI 0.05 to 1.54 p=0.036; I2 = 83.7%, p=0.002), and pSS patients (SMD=0.55, 95% CI 0.03 to 1.07 p=0.036; I2 = 46.9%, p=0.17), but not in AS (SMD=0.58, 95% CI -0.04 to 1.21, p=0.066; I2 = 82.3%, p=0.003) or PsA patients (SMD=1.03, 95% CI -0.28 to 2.34, p=0.12; I2 = 87.7%, p=0.004; Figure 6). In addition, the effect size in studies performed in BD patients was significantly larger than that in studies in RA (p=0.01), AS (p=0.047), and pSS patients (p=0.030), with a reduction of between-study variance in the BD (I2 = 0.0%) and pSS (I2 = 46.9%) subgroups. The pooled SMD was significant regardless of whether the studies were conducted in Europe (SMD=1.06, 95% CI 0.68 to 1.43, p<0.001; I2 = 88.9%, p<0.001), Asia (SMD=0.47, 95% CI 0.14 to 0.80, p=0.005; I2 = 84.8%, p<0.001), or America (SMD=1.03, 95% CI 0.14 to 1.93, p=0.024; I2 = 96.9%, p<0.001; Figure 7). There was a non-significant trend (p=0.06) toward a greater effect size in European studies compared to those conducted in Asia. The pooled SMD was significantly higher (p=0.013) in studies investigating plasma (SMD=1.30, 95% CI 0.95 to 1.65, p<0.001; I2 = 80.0%, p<0.001) compared to those in serum (SMD=0.60, 95% CI 0.26 to 0.95, p<0.001; I2 = 93.0%, p<0.001; Figure 8). Furthermore, the pooled SMD was significant in studies using ELISA (SMD=0.93, 95% CI 0.64 to 1.22, p<0.001; I2 = 91.8%, p<0.001) but not in those using a platform for multi-analyte profiling (SMD=0.61, 95% CI -0.03 to 1.24, p=0.061; I2 = 92.4%, p<0.001; Figure 9).

Figure 6. Forest plot of sCD40L concentrations in patients with rheumatic diseases and healthy controls according to the type of rheumatic disease.

Figure 7. Forest plot of sCD40L concentrations in patients with rheumatic diseases and healthy controls according to the geographical area where the study was conducted.

Figure 8. Forest plot of sCD40L concentrations in patients with rheumatic diseases and healthy controls according to the sample matrix assessed (serum or plasma).

Figure 9. Forest plot of sCD40L concentrations in patients with rheumatic diseases and healthy controls according to the analytical method used.
The overall level of the certainty of evidence remained low (level 2) after considering the low-moderate risk of bias in most studies (no change), the extreme but partially explainable heterogeneity (no change), the lack of indirectness (no change), the large effect size (SMD=0.87, upgrade one level) (69), and the presence of publication bias which was not addressed using the “trim-and-fill” method (downgrade one level).
Disease activity
Eight studies investigated sCD40L concentrations in 192 RD patients with active disease and 172 without (35, 40, 42, 45, 50, 53, 56, 61). Three focused on patients with SLE (35, 40, 45), two with RA (42, 61), two with BD (53, 56), and one with AS (50). The risk of bias was low in two studies (42, 56) and moderate in the remaining six (35, 40, 45, 50, 53, 61) (Supplementary Table 2).
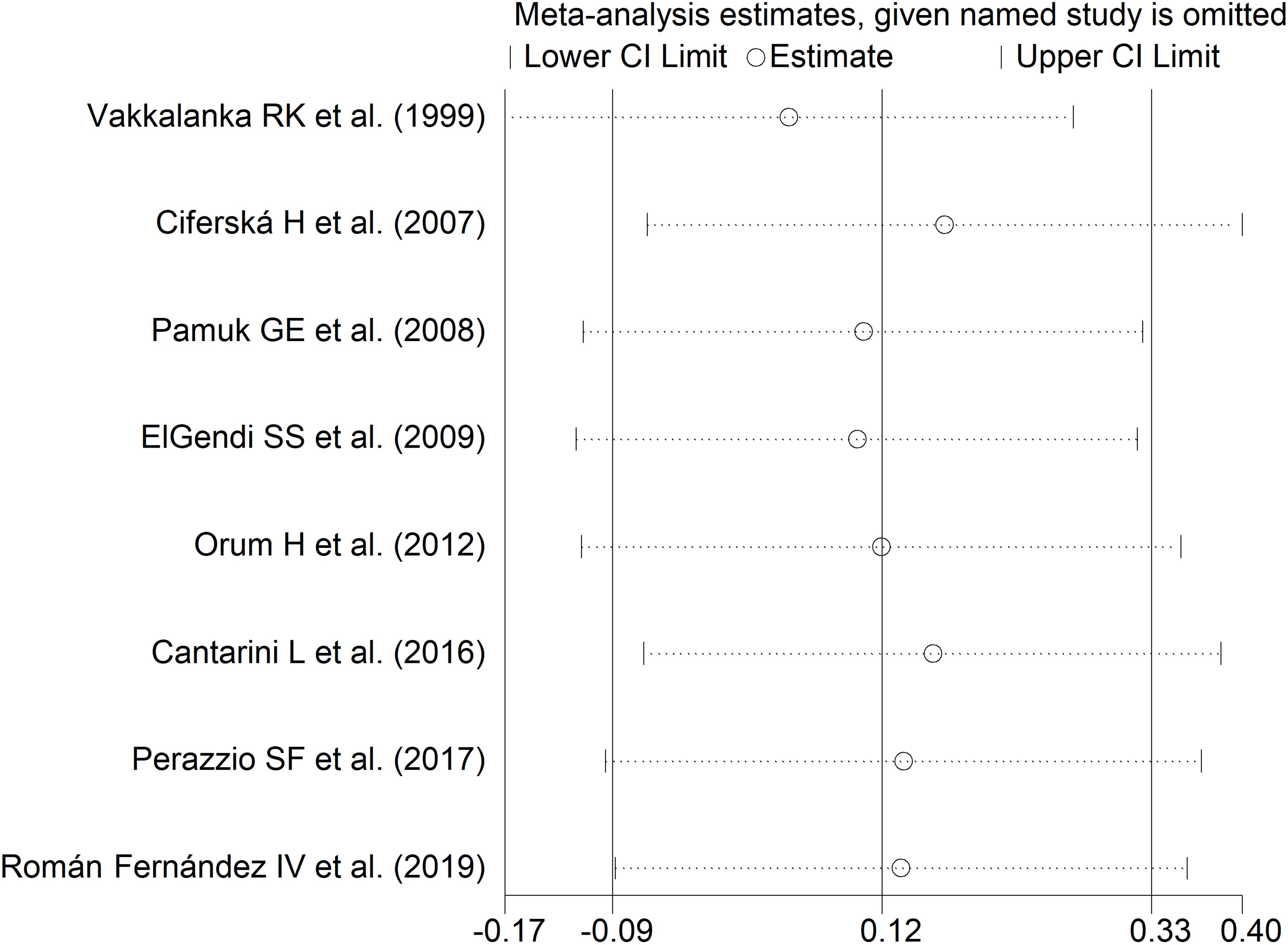
The forest plot showed no significant difference in sCD40L concentrations between RD patients with and without active disease (SMD=0.12, 95% CI -0.09 to 0.33, p=0.26; I2 = 0.0%, p=0.52; Figure 10). The results were stable in sensitivity analysis, with pooled SMD values ranging between 0.05 and 0.17 (Figure 11). The overall level of the certainty of evidence was downgraded to very low (level 1) as the relatively small number of studies prevented the assessment of publication bias and the conduct of meta-regression and subgroup analysis.

Figure 10. Forest plot of sCD40L concentrations in patients with rheumatic disease with and without active disease.
sCD40
Presence of RDs
Five studies investigated sCD40 concentrations in 711 RD patients and 589 healthy controls (52, 60, 61, 63, 66). Two studies were conducted in Asia (52, 66), two in America (61, 63), and one in Africa (60). Three studies included patients with SLE (52, 60, 63), one with RA (61), and one with BD (66). An ELISA was used in all studies. Three studies measured serum (60, 63, 66) and the remaining two plasma (52, 61). The risk of bias was low in one study (66) and moderate in the remaining four (52, 60, 61, 63) (Supplementary Table 2).
The forest plot showed that sCD40 concentrations were significantly higher in RD patients than in controls (SMD=1.32, 95% CI 0.45 to 2.18, p=0.003; I2 = 97.5%, p<0.001; Figure 12).

Figure 12. Forest plot of sCD40 concentrations in patients with rheumatic diseases and healthy controls.
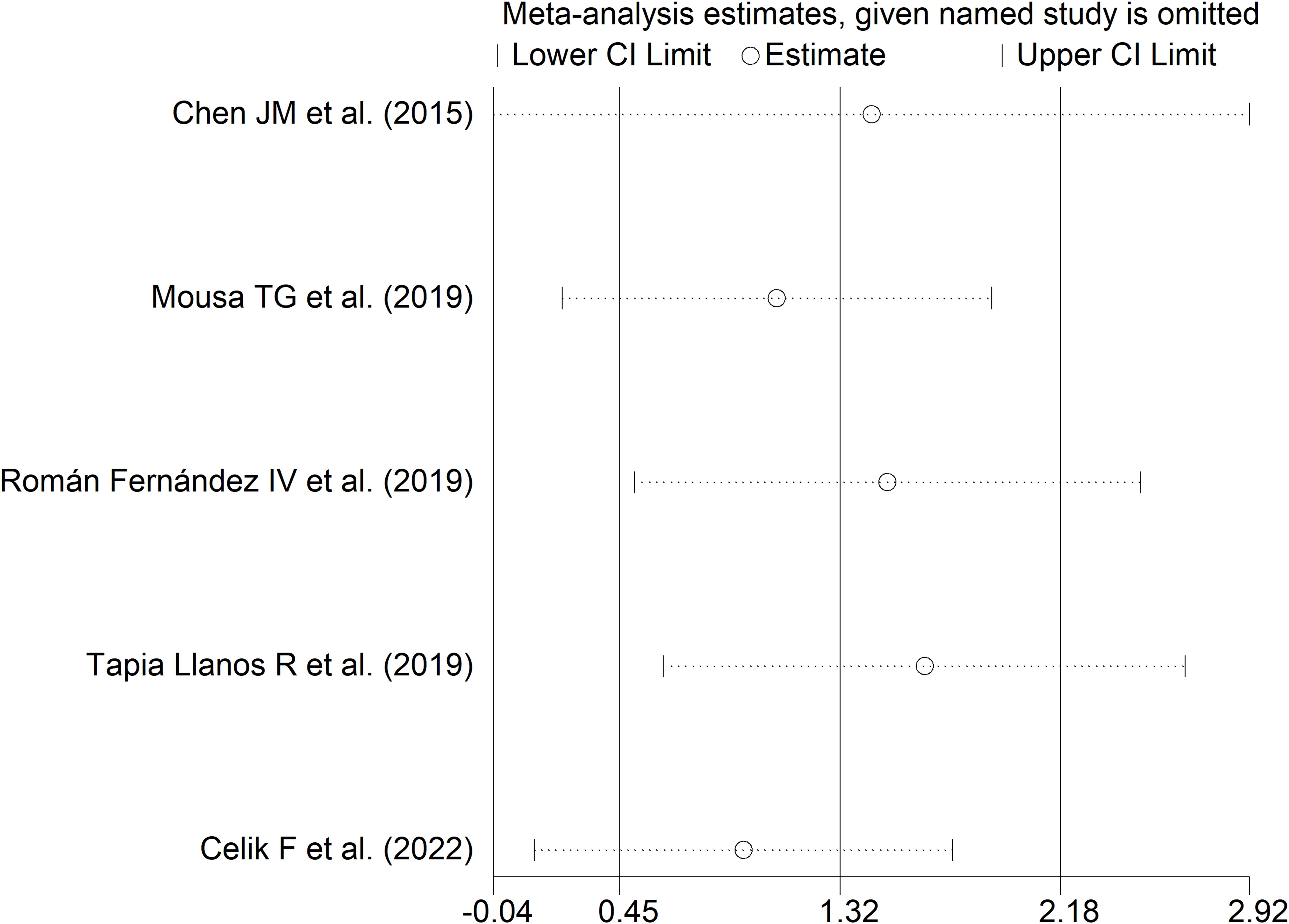
Sensitivity analysis (SMD ranging between 0.94 and 0.65; Figure 13) showed that the effect size was not significant after excluding the study by Chen JM et al. (SMD=1.44; 95% CI -0.04 to 2.92; p=0.057; I2 = 98.0%, p<0.001) (52).
In subgroup analysis, the pooled SMD was significant in studies measuring plasma (SMD=0.86, 95% CI 0.66 to 1.06, p<0.001; I2 = 1.2%, p=0.31) but not serum (SMD=1.74, 95% CI -0.15 to 3.63, p=0.072; I2 = 98.7%, p<0.001; Figure 14), with a virtually absent heterogeneity in the plasma subgroup.

Figure 14. Forest plot of sCD40 concentrations in patients with rheumatic diseases and healthy controls according to the sample matrix assessed (serum or plasma).
Because of the small number of studies, assessment of publication bias and meta-regression could not be performed. Consequently, the overall level of the certainty of evidence was downgraded to very low (level 1).
Disease activity
Three studies investigated sCD40 concentrations in 135 RD patients with active disease and 82 without (61, 63, 66). Two studies were conducted in America (61, 63) and the remaining one in Asia (66). One study focused on RA patients (61), another on SLE patients (63), and the third on BD patients (66). An ELISA was used in all studies. The risk of bias was low in one study (66) and moderate in the other two (61, 63) (Supplementary Table 2).
The forest plot showed that sCD40 concentrations were significantly higher in RD patients with active disease than in those with inactive disease (SMD=0.36, 95% CI 0.08 to 0.84, p=0.013; I2 = 12.4%, p=0.32; Figure 15).

Figure 15. Forest plot of sCD40 concentrations in patients with rheumatic disease with and without active disease.
The small number of studies prevented sensitivity analysis and the conduct of meta-regression and subgroup analysis, consequently downgrading the final level of the certainty of evidence to very low (level 1).
Discussion
In this systematic review and meta-analysis, we observed significant RD-associated alterations in circulating sCD40 and sCD40L, critical humoral and cellular immune response regulators. Specifically, RD patients had significantly higher sCD40L concentrations when compared to healthy controls. However, the results require confirmation in further studies because of the observed publication bias and the absence of significant between-group differences after using the “trim-and-fill method”. In meta-regression, we did not observe significant associations between the effect size of the between-group differences in sCD40L concentrations and various demographic and clinical characteristics, particularly CRP, ESR, and use of DMARDs or glucocorticoids. However, there was a significant inverse association with the study sample size and a positive association with the mean RD duration. In subgroup analysis, the elevations in sCD40L concentrations were consistent across different types of RD (SLE, RA, BD, SSc, pSS, AS, and PsA), although they were not statistically significant in patients with AS and PsA. Furthermore, such elevations were observed in studies conducted in different geographical locations. Significant differences in the effect size were observed according to the biological matrix and the analytical method used. By contrast, we did not observe any between-group difference in circulating sCD40L between RD patients with and without active disease. In further analyses, RD patients had significant elevations in circulating sCD40 concentrations compared to controls, although the observed differences were primarily driven by one study in sensitivity analysis (52). Active disease was also associated with significant elevations in circulating sCD40. Albeit the limitations described warrant some caution, our study suggests that measuring sCD40L and sCD40 is worthy of further investigation to determine their role as candidate biomarkers of RDs.
One potential advantage of measuring sCD40L over conventional biomarkers of inflammation (e.g., CRP and ESR) is its capacity to reflect alterations in immune response in the context of autoimmune and autoinflammatory disorders (19, 20). The sCD40L-mediated CD40 intracellular signaling is initiated by members of the TNF receptor-associated factor (TRAF), which activates the canonical and non-canonical nuclear factor (NF)-κB pathway (70). This, in turn, leads to the nuclear translocation of p50/p65, p65/p65 and p52/RelB dimers and their DNA binding. Additional downstream pathways activated by the CD40-TRAF interaction include the mitogen-activated protein kinase, phosphoinositide-3-kinase-protein kinase B, and Janus kinase 3-signal transducer and activator of transcription pathways (71–73). The absence of significant associations in meta-regression between the effect size of sCD40L and CRP and ESR supports the proposition that measuring sCD40L may provide complementary information to conventional biomarkers of inflammation.
The observed elevations in circulating sCD40 in RD patients and in those with active disease are counterintuitive, given that sCD40 inhibits the interaction between CD40L and mCD40 and can be considered a negative control feedback mechanism to prevent excess activation of mCD40 (19, 20). However, an additional element of complexity is related to the role of a disintegrin and metalloprotease 17 (ADAM17), involved in various functions, including CD40 ectodomain shedding and the release of sCD40 in B cells and endothelial cells (74, 75). Notably, some studies have reported an anti-inflammatory effect of ADAM17 by shedding adhesion molecules and the TNF receptor (76–78), whereas other studies suggest a proinflammatory effect (79, 80). Further research is therefore required to investigate whether sCD40 can exert opposing effects on immune and inflammatory pathways in patients with RDs, including those with active disease.
While our analyses suggest a potential role of sCD40 and sCD40L as biomarkers of different types of RDs, further studies are required to confirm these findings and justify their utility in routine clinical practice. Larger, accurately designed prospective studies should investigate the diagnostic performance in a wider range of autoimmune, mixed autoimmune-autoinflammatory, and autoinflammatory RDs (1–4). Such performance should be compared to existing diagnostic criteria, serological biomarkers, and non-specific markers of inflammation in individual RDs to determine whether measuring circulating sCD40 and sCD40L significantly enhances diagnosis over and above available tools.
Our systematic review and meta-analysis has several strengths, including the comprehensive assessment of sCD40 and sCD40L in different RDs, the evaluation of the level of the certainty of evidence for each studied endpoint (presence of RD and active disease), and the study of possible associations between the effect size and various study and patient characteristics. Significant limitations are the relatively low number of studies investigating sCD40 and the cross-sectional design of the selected studies, which did not allow for the investigation of a cause-effect relationship between sCD40 and sCD40L and RDs and active disease.
In conclusion, our study has shown that patients with RDs have significantly elevated circulating concentrations of sCD40 and sCD40L when compared to healthy controls. Such alterations likely reflect a dysregulated humoral and cellular immune response and are not associated with elevations in conventional inflammatory biomarkers, i.e., CRP and ERS. Further prospective studies in a broader range of RDs are required to establish whether measuring sCD40 and sCD40L can be helpful in the clinical evaluation and monitoring of RDs.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
AZ: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Writing – original draft, Writing – review & editing. AM: Data curation, Methodology, Supervision, Validation, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2025.1479904/full#supplementary-material
References
1. McGonagle D, McDermott MF. A proposed classification of the immunological diseases. PLoS Med. (2006) 3:e297. doi: 10.1371/journal.pmed.0030297
2. Moutsopoulos HM. Autoimmune rheumatic diseases: One or many diseases? J Transl Autoimmun. (2021) 4:100129. doi: 10.1016/j.jtauto.2021.100129
3. Szekanecz Z, McInnes IB, Schett G, Szamosi S, Benko S, Szucs G. Autoinflammation and autoimmunity across rheumatic and musculoskeletal diseases. Nat Rev Rheumatol. (2021) 17:585–95. doi: 10.1038/s41584-021-00652-9
4. Hedrich CM. Shaping the spectrum - From autoinflammation to autoimmunity. Clin Immunol. (2016) 165:21–8. doi: 10.1016/j.clim.2016.03.002
5. Peckham D, Scambler T, Savic S, McDermott MF. The burgeoning field of innate immune-mediated disease and autoinflammation. J Pathol. (2017) 241:123–39. doi: 10.1002/path.4812
6. Rachid B, El Zorkany B, Youseif E, Tikly M. Early diagnosis and treatment of ankylosing spondylitis in Africa and the Middle East. Clin Rheumatol. (2012) 31:1633–9. doi: 10.1007/s10067-012-2058-5
7. Clarke AE, Weinstein A, Piscitello A, Heer A, Chandra T, Doshi S, et al. Evaluation of the economic benefit of earlier systemic lupus erythematosus (SLE) diagnosis using a multivariate assay panel (MAP). ACR Open Rheumatol. (2020) 2:629–39. doi: 10.1002/acr2.11177
8. Monti S, Montecucco C, Bugatti S, Caporali R. Rheumatoid arthritis treatment: the earlier the better to prevent joint damage. RMD Open. (2015) 1:e000057. doi: 10.1136/rmdopen-2015-000057
9. Hioki T, Komine M, Ohtsuki M. Diagnosis and intervention in early psoriatic arthritis. J Clin Med. (2022) 11(7):2051. doi: 10.3390/jcm11072051
10. Mallen CD, Helliwell T, Scott IC. How can primary care physicians enhance the early diagnosis of rheumatic diseases? Expert Rev Clin Immunol. (2018) 14:171–3. doi: 10.1080/1744666X.2018.1429919
11. Mohan C, Assassi S. Biomarkers in rheumatic diseases: how can they facilitate diagnosis and assessment of disease activity? BMJ. (2015) 351:h5079. doi: 10.1136/bmj.h5079
12. Guma M, Tiziani S, Firestein GS. Metabolomics in rheumatic diseases: desperately seeking biomarkers. Nat Rev Rheumatol. (2016) 12:269–81. doi: 10.1038/nrrheum.2016.1
13. Cui S, Qian J. Future biomarkers for infection and inflammation in rheumatoid arthritis. J Inflammation Res. (2023) 16:2719–26. doi: 10.2147/JIR.S413579
14. Fenton KA, Pedersen HL. Advanced methods and novel biomarkers in autoimmune diseases − a review of the recent years progress in systemic lupus erythematosus. Front Med (Lausanne). (2023) 10:1183535. doi: 10.3389/fmed.2023.1183535
15. Pritzker KPH. Blood-based biomarkers of chronic inflammation. Expert Rev Mol Diagn. (2023) 23:495–504. doi: 10.1080/14737159.2023.2215928
16. Theofilopoulos AN, Kono DH, Baccala R. The multiple pathways to autoimmunity. Nat Immunol. (2017) 18:716–24. doi: 10.1038/ni.3731
17. Pisetsky DS. Pathogenesis of autoimmune disease. Nat Rev Nephrol. (2023) 19:509–24. doi: 10.1038/s41581-023-00720-1
18. Xiang Y, Zhang M, Jiang D, Su Q, Shi J. The role of inflammation in autoimmune disease: a therapeutic target. Front Immunol. (2023) 14:1267091. doi: 10.3389/fimmu.2023.1267091
19. Chand Dakal T, Dhabhai B, Agarwal D, Gupta R, Nagda G, Meena AR, et al. Mechanistic basis of co-stimulatory CD40-CD40L ligation mediated regulation of immune responses in cancer and autoimmune disorders. Immunobiology. (2020) 225:151899. doi: 10.1016/j.imbio.2019.151899
20. Tang T, Cheng X, Truong B, Sun L, Yang X, Wang H. Molecular basis and therapeutic implications of CD40/CD40L immune checkpoint. Pharmacol Ther. (2021) 219:107709. doi: 10.1016/j.pharmthera.2020.107709
21. Ledbetter JA, Shu G, Gallagher M, Clark EA. Augmentation of normal and Malignant B cell proliferation by monoclonal antibody to the B cell-specific antigen BP50 (CDW40). J Immunol. (1987) 138:788–94. doi: 10.4049/jimmunol.138.3.788
22. Armitage RJ, Fanslow WC, Strockbine L, Sato TA, Clifford KN, Macduff BM, et al. Molecular and biological characterization of a murine ligand for CD40. Nature. (1992) 357:80–2. doi: 10.1038/357080a0
23. Moola S, Munn Z, Tufanaru C, Aromataris E, Sears K, Sfetcu R, et al. Systematic reviews of etiology and risk. In: Aromataris E, Munn Z, editors. Joanna Briggs Institute Reviewer’s Manual. Johanna Briggs Institute, Adelaide, Australia (2017).
24. Balshem H, Helfand M, Schunemann HJ, Oxman AD, Kunz R, Brozek J, et al. GRADE guidelines: 3. Rating Qual Evid J Clin Epidemiol. (2011) 64:401–6. doi: 10.1016/j.jclinepi.2010.07.015
25. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. (2021) 372:n71. doi: 10.1136/bmj.n71
26. Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. (2014) 14:135. doi: 10.1186/1471-2288-14-135
27. Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. (2002) 21:1539–58. doi: 10.1002/sim.1186
28. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. (2003) 327:557–60. doi: 10.1136/bmj.327.7414.557
29. Dettori JR, Norvell DC, Chapman JR. Fixed-effect vs random-effects models for meta-analysis: 3 points to consider. Global Spine J. (2022) 12:1624–6. doi: 10.1177/21925682221110527
30. Tobias A. Assessing the influence of a single study in the meta-analysis estimate. Stata Tech Bull. (1999) 47:15–7.
31. Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. (1994) 50:1088–101.
32. Sterne JA, Egger M. Funnel plots for detecting bias in meta-analysis: guidelines on choice of axis. J Clin Epidemiol. (2001) 54:1046–55. doi: 10.1016/s0895-4356(01)00377-8
33. Duval S, Tweedie R. Trim and fill: A simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. (2000) 56:455–63. doi: 10.1111/j.0006-341x.2000.00455.x
34. Kato K, Santana-Sahagun E, Rassenti LZ, Weisman MH, Tamura N, Kobayashi S, et al. The soluble CD40 ligand sCD154 in systemic lupus erythematosus. J Clin Invest.. (1999) 104:947–55. doi: 10.1172/JCI7014
35. Vakkalanka RK, Woo C, Kirou KA, Koshy M, Berger D, Crow MK. Elevated levels and functional capacity of soluble CD40 ligand in systemic lupus erythematosus sera. Arthritis Rheumatol. (1999) 42:871–81. doi: 10.1002/1529-0131(199905)42:5<871::AID-ANR5>3.0.CO;2-J
36. Tamura N, Kobayashi S, Kato K, Bando H, Haruta K, Oyanagi M, et al. Soluble CD154 in rheumatoid arthritis: elevated plasma levels in cases with vasculitis. J Rheumatol. (2001) 28:2583–90.
37. Allanore Y, Borderie D, Meune C, Lemaréchal H, Ekindjian O, Kahan A. Increased plasma soluble CD40 ligand concentrations in systemic sclerosis and association with pulmonary arterial hypertension and digital ulcers. Ann Rheum Dis. (2005) 63:109–. doi: 10.1136/ard.2003.020040
38. Goules A, Tzioufas AG, Manousakis MN, Kirou KA, Crow MK, Routsias JG. Elevated levels of soluble CD40 ligand (sCD40L) in serum of patients with systemic autoimmune diseases. J Autoimmun. (2006) 26:165–71. doi: 10.1016/j.jaut.2006.02.002
39. Von Feldt JM, Scalzi LV, Cucchiara AJ, Morthala S, Kealey C, Flagg SD. Homocysteine levels and disease duration independently correlate with coronary artery calcification in patients with systemic lupus erythematosus. Arthritis Rheumatol. (2006) 54:2220–7. doi: 10.1002/art.21967
40. Ciferská H, Horák P, Heřmanová Z, Ordeltová M, Zadražil J, Tichý T, et al. The levels of sCD30 and of sCD40L in a group of patients with systemic lupus erythematodes and their diagnostic value. Clin Rheumatol. (2007) 26:723–8. doi: 10.1007/s10067-006-0389-9
41. Nomura S, Inami N, Ozaki Y, Kagawa H, Fukuhara S. Significance of microparticles in progressive systemic sclerosis with interstitial pneumonia. Platelets. (2008) 19:192–8. doi: 10.1080/09537100701882038
42. Pamuk GE, Vural Ö, Turgut B, Demır M, Pamuk ÖN, Çakir N. Increased platelet activation markers in rheumatoid arthritis: Are they related with subclinical atherosclerosis? Platelets. (2008) 19:146–54. doi: 10.1080/09537100701210057
43. Cella G, Vianello F, Cozzi F, Marotta H, Tona F, Saggiorato G, et al. Effect of bosentan on plasma markers of endothelial cell activity in patients with secondary pulmonary hypertension related to connective tissue diseases. J Rheumatol. (2009) 36:760–7. doi: 10.3899/jrheum.080542
44. de Sanctis JB, Garmendia JV, Chaurio R, Zabaleta M, Rivas L. Total and biologically active CD154 in patients with SLE. Autoimmunity. (2009) 42:263–5. doi: 10.1080/08916930902827942
45. ElGendi SS, El-Sherif WT. Anti-C1q antibodies, sCD40L, TWEAK and CD4/CD8 ratio in systemic lupus erythematosus and their relations to disease activity and renal involvement. Egypt J Immunol. (2009) 16:135–48.
46. Pamuk GE, Nuri Pamuk O, Orum H, Arican O, Turgut B, Demir M. Elevated platelet-monocyte complexes in patients with psoriatic arthritis. Platelets. (2009) 20:493–7. doi: 10.3109/09537100903165174
47. Sellam J, Proulle V, Jüngel A, Ittah M, Richard CM, Gottenberg JE, et al. Increased levels of circulating microparticles in primary Sjogren’s syndrome, systemic lupus erythematosus and rheumatoid arthritis and relation with disease activity. Arthritis Res Ther. (2009) 11(5):R156. doi: 10.1186/ar2833
48. Sari I, Alacacioglu A, Kebapcilar L, Taylan A, Bilgir O, Yildiz Y, et al. Assessment of soluble cell adhesion molecules and soluble CD40 ligand levels in ankylosing spondylitis. Joint Bone Spine.. (2010) 77:85–7. doi: 10.1016/j.jbspin.2009.07.005
49. Fernández Bello I, Álvarez MT, López-Longo FJ, Arias-Salgado EG, Martín M, Jiménez-Yuste V, et al. Platelet soluble CD40L and matrix metalloproteinase 9 activity are proinflammatory mediators in Behçet disease patients. Thromb Haemost.. (2012) 107:88–98. doi: 10.1160/th11-08-0556
50. Örüm H, Pamuk GE, Pamuk ÖN, Demir M, Turgut B. Does anti-tnf therapy cause any change in platelet activation in ankylosing spondylitis patients? J Thromb Thrombolys. (2012) 33:154–9. doi: 10.1007/s11239-011-0663-9
51. Pamuk ON, Tozkir H, Uyanik MS, Gurkan H, Saritas F, Duymaz J, et al. PECAM-1 gene polymorphisms and soluble PECAM-1 level in rheumatoid arthritis and systemic lupus erythematosus patients: any link with clinical atherosclerotic events? Clin Rheumatol. (2014) 33:1737–43. doi: 10.1007/s10067-014-2771-3
52. Chen JM, Guo J, Wei CD, Wang CF, Luo HC, Wei YS, et al. The association of CD40 polymorphisms with CD40 serum levels and risk of systemic lupus erythematosus. BMC Genet. (2015) 16:121. doi: 10.1186/s12863-015-0279-8
53. Cantarini L, Pucino V, Vitale A, Talarico R, Lucherini OM, Magnotti F, et al. Immunometabolic biomarkers of inflammation in Behcet’s disease: relationship with epidemiological profile, disease activity and therapeutic regimens. Clin Exp Immunol. (2016) 184:197–207. doi: 10.1111/cei.12768
54. Yalcinkaya Y, Cinar S, Artim-Esen B, Kamali S, Ocal L, Deniz G, et al. The relationship between vascular biomarkers and disease characteristics in systemic sclerosis: elevated MCP-1 is predominantly associated with fibrotic manifestations. Clin Exp Rheumatol. (2016) 34:110–4.
55. Kim KJ, Baek IW, Yoon CH, Kim WU, Cho CS. Elevated levels of soluble CD40 ligand are associated with antiphospholipid antibodies in patients with systemic lupus erythematosus. Clin Exp Rheumatol. (2017) 35:823–30.
56. Perazzio SF, Soeiro-Pereira PV, dos Santos VC, de Brito MV, Salu B, Oliva MLV, et al. Soluble CD40L is associated with increased oxidative burst and neutrophil extracellular trap release in Behcet’s disease. Arthritis Res Ther. (2017) 19(1):235. doi: 10.1186/s13075-017-1443-5
57. Petrackova A, Smrzova A, Gajdos P, Schubertova M, Schneiderova P, Kromer P, et al. Serum protein pattern associated with organ damage and lupus nephritis in systemic lupus erythematosus revealed by PEA immunoassay. Clin Proteom. (2017) 14:32. doi: 10.1186/s12014-017-9167-8
58. Stanek A, Cholewka A, Wielkoszynski T, Romuk E, Sieron K, Sieron A. Increased levels of oxidative stress markers, soluble CD40 ligand, and carotid intima-media thickness reflect acceleration of atherosclerosis in male patients with ankylosing spondylitis in active phase and without the classical cardiovascular risk factors. Oxid Med Cell Longev. (2017) 2017:1–8. doi: 10.1155/2017/9712536
59. Willis R, Smikle M, DeCeulaer K, Romay-Penabad Z, Papalardo E, Jajoria P, et al. Clinical associations of proinflammatory cytokines, oxidative biomarkers and vitamin D levels in systemic lupus erythematosus. Lupus. (2017) 26:1517–27. doi: 10.1177/0961203317706557
60. Mousa TG, Omar HH, Emad R, Salama MI, Omar W, Fawzy M, et al. The association of CD40 polymorphism (rs1883832C/T) and soluble CD40 with the risk of systemic lupus erythematosus among Egyptian patients. Clin Rheumatol. (2018) 38:777–84. doi: 10.1007/s10067-018-4349-y
61. Román-Fernández IV, García-Chagollán M, Cerpa-Cruz S, Jave-Suárez LF, Palafox-Sánchez CA, García-Arellano S, et al. Assessment of CD40 and CD40L expression in rheumatoid arthritis patients, association with clinical features and DAS28. Clin Exp Med. (2019) 19:427–37. doi: 10.1007/s10238-019-00568-5
62. Södergren A, Karp K, Bengtsson C, Möller B, Rantapää-Dahlqvist S, Wållberg-Jonsson S. Biomarkers associated with cardiovascular disease in patients with early rheumatoid arthritis. PLoS One. (2019) 14(8):e0220531. doi: 10.1371/journal.pone.0220531
63. Tapia-Llanos R, Muñoz-Valle JF, Román-Fernández IV, Marín-Rosales M, Salazar-Camarena DC, Cruz A, et al. Association of soluble CD40 levels with -1 C > T CD40 polymorphism and chronic kidney disease in systemic lupus erythematosus. Mol Genet Genom Med. (2019) 7(12):e1014. doi: 10.1002/mgg3.1014
64. Zamora C, Toniolo E, Diaz-Torne C, Canto E, Magallares B, Ortiz MA, et al. Association of platelet binding to lymphocytes with B cell abnormalities and clinical manifestations in systemic lupus erythematosus. Mediators Inflamm. (2019) 2019:2473164. doi: 10.1155/2019/2473164
65. Venerito V, Natuzzi D, Bizzoca R, Lacarpia N, Cacciapaglia F, Lopalco G, et al. Serum sCD40L levels are increased in patients with psoriatic arthritis and are associated with clinical response to apremilast. Clin Exp Immunol. (2020) 201:200–4. doi: 10.1111/cei.13451
66. Celik F, Coteli E, Gul FC, Ozsoy E, Kobat SG, Karagoz ZK, et al. Interleukin 18, soluble cluster of differentiation 40, platelet factor 4 variant 1, and neutrophil gelatinase-associated lipocalin can be used as biomarkers to aid activity and diagnosis in ocular Behcet’s disease. Int Ophthalmol. (2022) 42:3321–31. doi: 10.1007/s10792-022-02331-4
67. Hoang TTT, Ichinose K, Morimoto S, Furukawa K, Le LHT, Kawakami A. Measurement of anti-suprabasin antibodies, multiple cytokines and chemokines as potential predictive biomarkers for neuropsychiatric systemic lupus erythematosus. Clin Immunol. (2022) 237:108980. doi: 10.1016/j.clim.2022.108980
68. Gerasimova EV, Popkova TV, Gerasimova DA, Markina YV, Kirichenko TV. Subclinical carotid atherosclerosis in patients with rheumatoid arthritis at low cardiovascular risk. Biomedicines. (2023) 11(3):974. doi: 10.3390/biomedicines11030974
69. Cohen J. Statistical power analysis. Curr Dir Psychol Sci. (1992) 1:98–101. doi: 10.1111/1467-8721.ep10768783
70. Moschonas A, Ioannou M, Eliopoulos AG. CD40 stimulates a “feed-forward” NF-kappaB-driven molecular pathway that regulates IFN-beta expression in carcinoma cells. J Immunol. (2012) 188:5521–7. doi: 10.4049/jimmunol.1200133
71. Revy P, Hivroz C, Andreu G, Graber P, Martinache C, Fischer A, et al. Activation of the Janus kinase 3-STAT5a pathway after CD40 triggering of human monocytes but not of resting B cells. J Immunol. (1999) 163:787–93. doi: 10.4049/jimmunol.163.2.787
72. Pearson LL, Castle BE, Kehry MR. CD40-mediated signaling in monocytic cells: up-regulation of tumor necrosis factor receptor-associated factor mRNAs and activation of mitogen-activated protein kinase signaling pathways. Int Immunol. (2001) 13:273–83. doi: 10.1093/intimm/13.3.273
73. Elgueta R, Benson MJ, de Vries VC, Wasiuk A, Guo Y, Noelle RJ. Molecular mechanism and function of CD40/CD40L engagement in the immune system. Immunol Rev. (2009) 229:152–72. doi: 10.1111/j.1600-065X.2009.00782.x
74. Contin C, Pitard V, Itai T, Nagata S, Moreau JF, Dechanet-Merville J. Membrane-anchored CD40 is processed by the tumor necrosis factor-alpha-converting enzyme. Implications for CD40 signaling. J Biol Chem. (2003) 278:32801–9. doi: 10.1074/jbc.M209993200
75. Klersy A, Meyer S, Leuschner F, Kessler T, Hecker M, Wagner AH. Ectodomain shedding by ADAM17 increases the release of soluble CD40 from human endothelial cells under pro-inflammatory conditions. Cells. (2023) 12(15):1926. doi: 10.3390/cells12151926
76. Garton KJ, Gough PJ, Philalay J, Wille PT, Blobel CP, Whitehead RH, et al. Stimulated shedding of vascular cell adhesion molecule 1 (VCAM-1) is mediated by tumor necrosis factor-alpha-converting enzyme (ADAM 17). J Biol Chem. (2003) 278:37459–64. doi: 10.1074/jbc.M305877200
77. Tsakadze NL, Sithu SD, Sen U, English WR, Murphy G, D’Souza SE. Tumor necrosis factor-alpha-converting enzyme (TACE/ADAM-17) mediates the ectodomain cleavage of intercellular adhesion molecule-1 (ICAM-1). J Biol Chem. (2006) 281:3157–64. doi: 10.1074/jbc.M510797200
78. Nicolaou A, Zhao Z, Northoff BH, Sass K, Herbst A, Kohlmaier A, et al. Adam17 deficiency promotes atherosclerosis by enhanced TNFR2 signaling in mice. Arterioscler Thromb Vasc Biol. (2017) 37:247–57. doi: 10.1161/ATVBAHA.116.308682
79. Stohr R, Cavalera M, Menini S, Mavilio M, Casagrande V, Rossi C, et al. Loss of TIMP3 exacerbates atherosclerosis in ApoE null mice. Atherosclerosis. (2014) 235:438–43. doi: 10.1016/j.atherosclerosis.2014.05.946
Keywords: sCD40, sCD40L, B cells, T cells, rheumatic diseases, inflammation, autoimmunity, disease activity
Citation: Zinellu A and Mangoni AA (2025) sCD40 and sCD40L as candidate biomarkers of rheumatic diseases: a systematic review and meta-analysis with meta-regression. Front. Immunol. 16:1479904. doi: 10.3389/fimmu.2025.1479904
Received: 13 August 2024; Accepted: 28 February 2025;
Published: 19 March 2025.
Edited by:
Xiaoming Zhang, Chinese Academy of Sciences (CAS), ChinaReviewed by:
Andreas H. Wagner, Heidelberg University, GermanyAditya Yashwant Sarode, Columbia University, United States
Ming Yu Lien, China Medical University Hospital, Taiwan
Copyright © 2025 Zinellu and Mangoni. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Arduino A. Mangoni, YXJkdWluby5tYW5nb25pQGZsaW5kZXJzLmVkdS5hdQ==
 Angelo Zinellu
Angelo Zinellu Arduino A. Mangoni
Arduino A. Mangoni