
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Immunol., 12 February 2025
Sec. Cancer Immunity and Immunotherapy
Volume 16 - 2025 | https://doi.org/10.3389/fimmu.2025.1476146
This article is part of the Research TopicClinical Implementation of Precision Oncology Data to Direct Individualized and Immunotherapy-Based Treatment StrategiesView all 17 articles
 Biling Gan1†
Biling Gan1† Lei Wu2†
Lei Wu2† Shunan Zhou1†
Shunan Zhou1† Zhihong Chen3
Zhihong Chen3 Fan Wu2
Fan Wu2 Lianqun Xu1
Lianqun Xu1 Zhenrong Chen2
Zhenrong Chen2 Honghui Ma2
Honghui Ma2 Peijia He4
Peijia He4 Dan Fang1*
Dan Fang1* Ning Shi1*
Ning Shi1*Background: Hepatocellular carcinoma (HCC), a prevalent malignancy, is often diagnosed at advanced stages. Recent advances have integrated immunotherapy with targeted therapy, significantly improving treatment outcomes. This study provides a bibliometric overview of these therapeutic combinations, evaluating their development and impact.
Methods: A rigorous selection process was applied to relevant literature from Web of Science, followed by in-depth bibliometric analyses— including timeline visualization, burst detection, and co-occurrence analysis—using CiteSpace and VOSviewer. This approach offered insights into the contributions of countries, institutions, authors, journals, references, and key terms within the field.
Results: A total of 506 studies published between 2014 and 2023 were included, with all articles in English. Mainland China dominated the publication output, contributing 40% (N = 202), followed by significant contributions from the United States and Japan. Kindai University led institutional contributions, accounting for 7.9% of the total (N = 40). The authors Kudo Masatoshi and Hatanaka Takeshi were the most prolific, each with nine publications. The journal Cancers emerged as the top publisher, with 48 relevant articles and an Impact Factor of 5.2 in 2022. A co-citation network analysis traced the evolution of immunotherapy and targeted therapy combinations in HCC treatment. Early research primarily focused on angiogenesis, dendritic cells, and expression markers, while recent trends have shifted towards phase III trials, adverse reactions, and checkpoint inhibitors, underscoring the field’s dynamic progression.
Conclusion: Future research will expand on the pathological mechanisms underlying these therapies and novel interventions and combination strategies. Addressing adverse events and treatment discontinuation will remain central to advancing clinical applications.
Primary liver cancer is the sixth most prevalent cancer worldwide and the fourth leading cause of cancer-related mortality. Hepatocellular carcinoma (HCC) accounts for approximately 75% of all primary liver cancer cases (1). Upon diagnosis, only 30-40% of patients with HCC are candidates for radical surgery, while the majority receive systemic treatment due to the asymptomatic nature of the disease and the lack of specific biomarkers in its early stages (2). Systemic therapies, including chemotherapy, targeted therapy, and immunotherapy, play a pivotal role in managing intermediate and advanced HCC (3). Despite significant progress in chemotherapy and targeted therapies over recent decades, HCC continues to be highly susceptible to drug resistance, recurrence, metastasis, and poor prognosis during treatment (4). With ongoing advancements in immune checkpoint inhibitors (ICIs), the combination of molecular targeted agents and ICIs has shown promising efficacy in HCC treatment, becoming a major area of focus (5, 6).
Recently, an increasing number of studies have been published on the combination of targeted therapy and immunotherapy for HCC. However, no comprehensive literature analysis on this topic has been conducted thus far. Bibliometric analysis, which involves the quantitative examination of knowledge carriers (typically literature) within a particular field using mathematical and statistical methods, helps assess research trends, elucidate the knowledge structure, and forecast potential breakthroughs. Current bibliometric studies predominantly employ tools such as CiteSpace (7) and VOSviewer (8). This approach has been widely applied across various fields (9–12), but no bibliometric study has specifically mapped the knowledge related to the combination of targeted therapy and immunotherapy for liver cancer (13–15). Therefore, the primary objective of this study is to provide an in-depth evaluation of the current state of targeted and immunotherapy combinations for liver cancer and offer researchers clear guidance for future research directions.
The Web of Science (WoS) Core Collection databases were accessed to retrieve relevant English-language articles on the combination of immunotherapy and targeted therapy for HCC published up until December 31, 2023. The search keywords included “primary liver cancer,” “targeted therapy,” and “immunotherapy,” with additional keywords provided in Supplementary File S1. Titles and abstracts of the retrieved articles were manually assessed according to the following inclusion criteria: (1) relevance to immunotherapy and targeted therapy combinations in HCC treatment, (2) publication type (research papers, clinical trials, meta-analyses, or reviews), and (3) English language. Exclusion criteria included: (1) treatment combinations involving chemotherapy, radiotherapy, interventional therapy, radiofrequency ablation, or surgery, (2) single modality targeted therapy or immunotherapy, and (3) inaccessible full texts or abstracts. Relevant information (title, year of publication, authors, country, attribution, journal, keywords, and abstract) from the included literature was downloaded in.txt format.
Data analysis was performed using CiteSpace 6.3.R1, with a time range from January 1, 2014, to December 31, 2023, divided into 10 annual time slices. The analysis employed Pathfinder, pruning sliced networks, and merging network pruning methods. The k-value of the g-index was adjusted based on the data values, while other parameters remained at their default settings. Nodes such as authors, institutions, countries, and keywords were selected for visual analysis. VOSviewer 1.6.20 was utilized to analyze country, institution, author contributions, and research hotspots using default parameters. Data processing was carried out in Microsoft Excel, followed by the establishment of index models.
A total of 2,179 articles focusing on the combination of immunotherapy and targeted therapy in HCC treatment were retrieved from the WoS Core Collection using the described search strategies. After applying stringent inclusion and exclusion criteria, 506 articles were included in the final analysis, as shown in (Figure 1). Publications on this topic have exhibited a significant upward trend from 2014 to 2024, with an estimated 346 articles expected in 2024, based on the fitting curve (Figure 2).
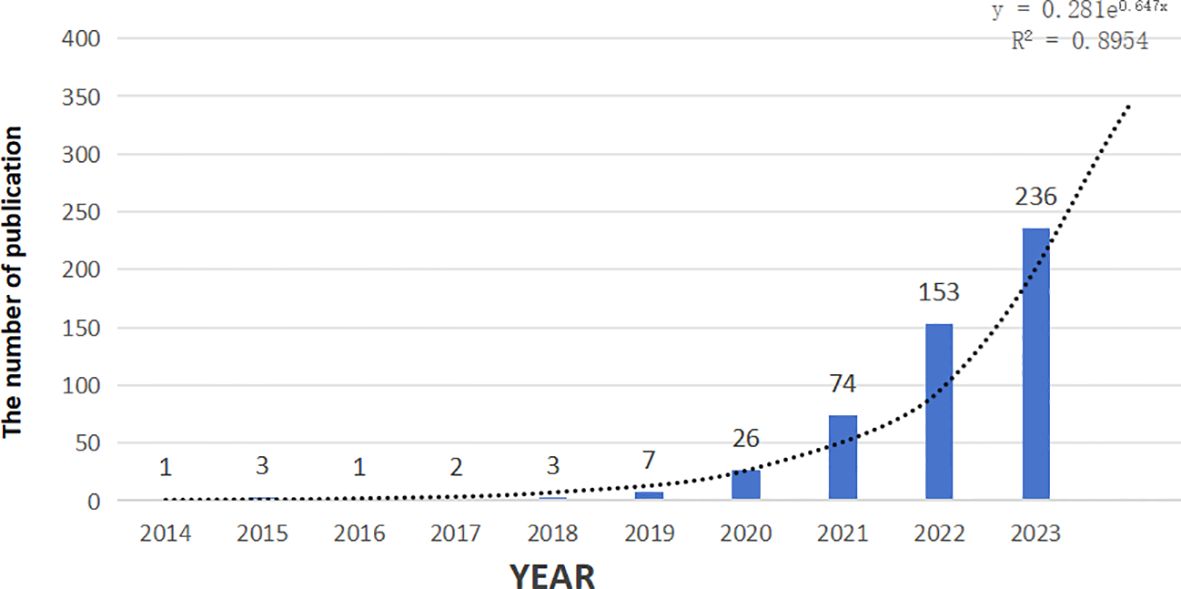
Figure 2. Polynomial curve fitting of publication growth in immunotherapy and targeted therapy combinations for HCC.
The 506 included articles were published by authors from 126 countries and regions (Figure 3). Mainland China led the contributions, publishing 35.4% of the articles (179 publications, centrality = 0.05). Taiwan contributed 23 articles (4%) with a centrality of 0.15. Other leading contributors included Japan (119 articles, 23.5%) and the United States (63 articles, 12.5%). Mainland China also had the highest citation count (N = 204), followed by Japan (N = 153) and the United States (N = 102). France had the highest centrality score of 0.62 (Table 1).
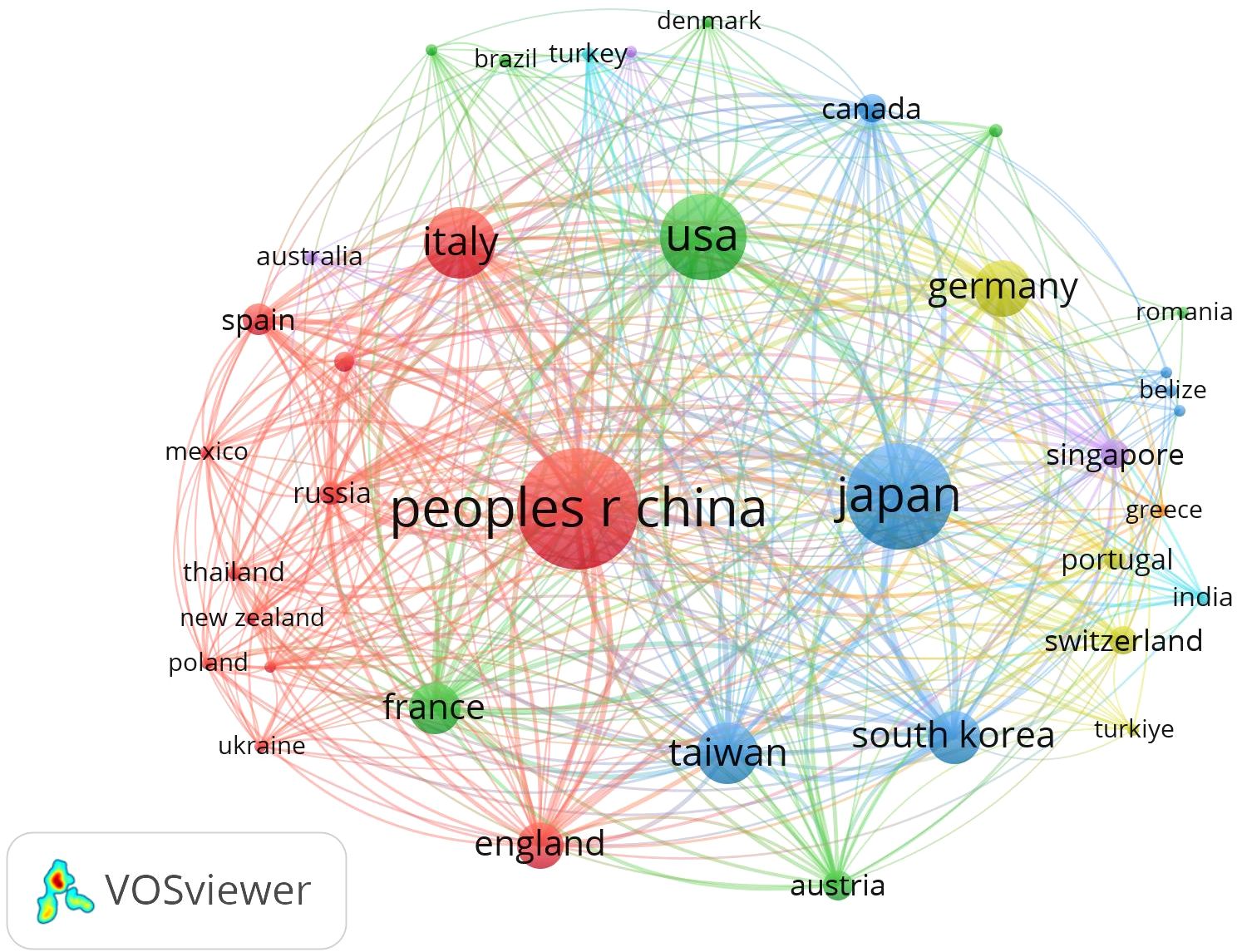
Figure 3. Network map showing countries/regions involved in research on immunotherapy and targeted therapy combinations for HCC.
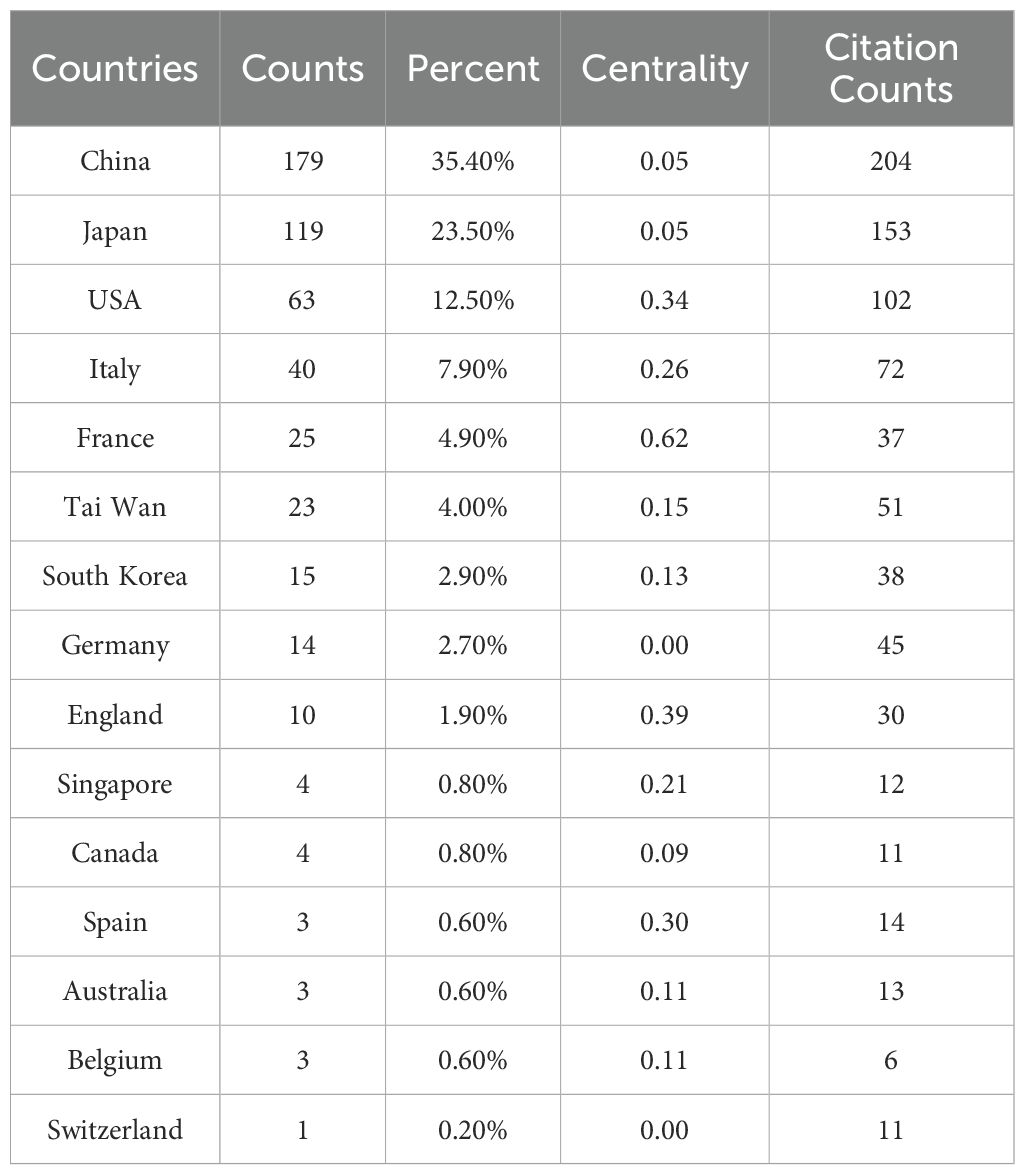
Table 1. Top 15 countries/regions involved in research on immunotherapy and targeted therapy combinations in relation to HCC.
The analysis included data from a total of 2,679 institutions (Figure 4). Kindai University published the most articles (N = 40), while Matsuyama Red Cross Hospital and Kagawa University each contributed 36 articles (Table 2). The top three institutions with the most publications are all based in Japan. Harvard Medical School exhibited the highest centrality score of 0.58 (Table 2). Institutions with higher centrality were predominantly located in the United States and mainland China.
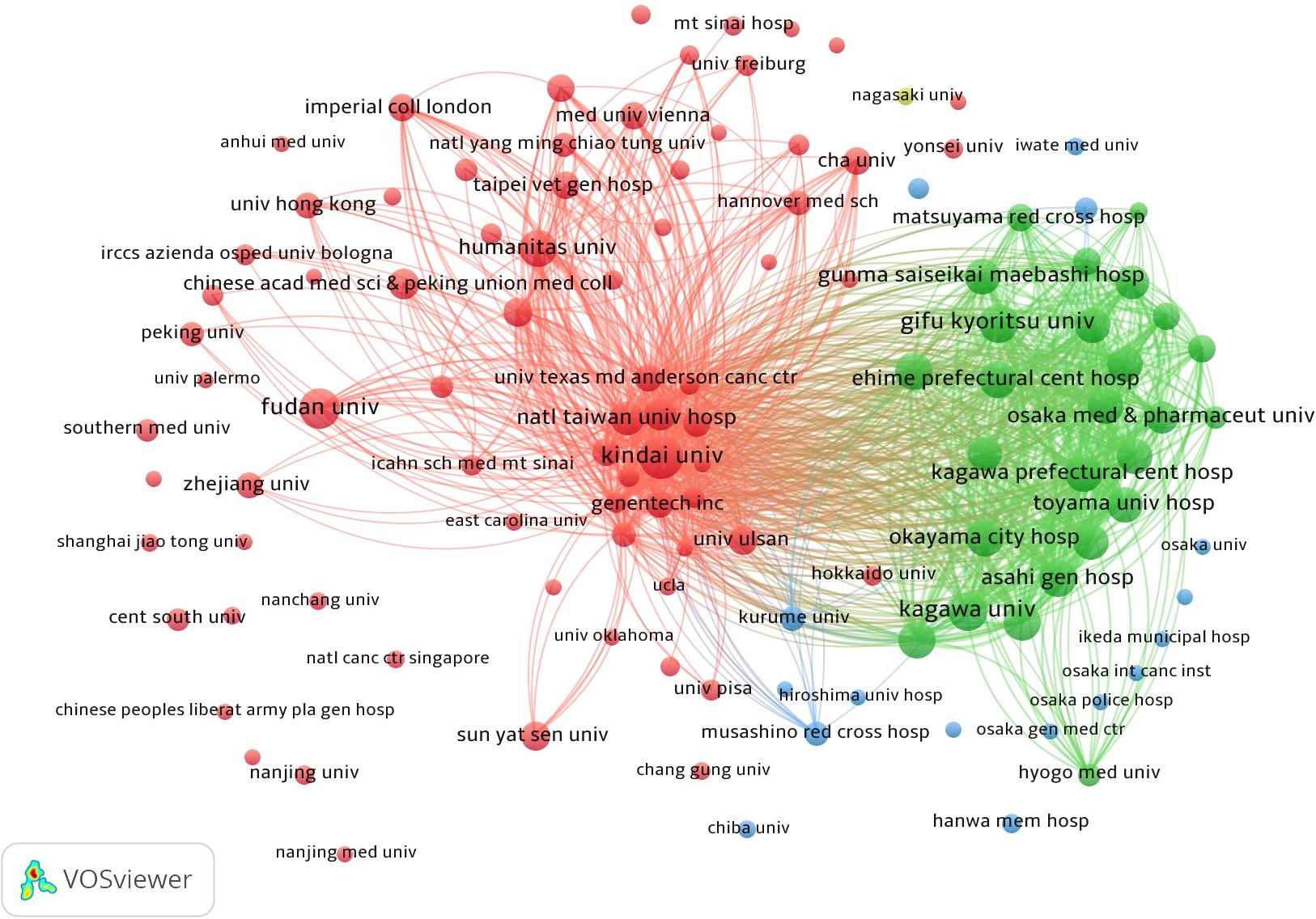
Figure 4. Network map showing institutions involved in research on immunotherapy and targeted therapy combinations for HCC.
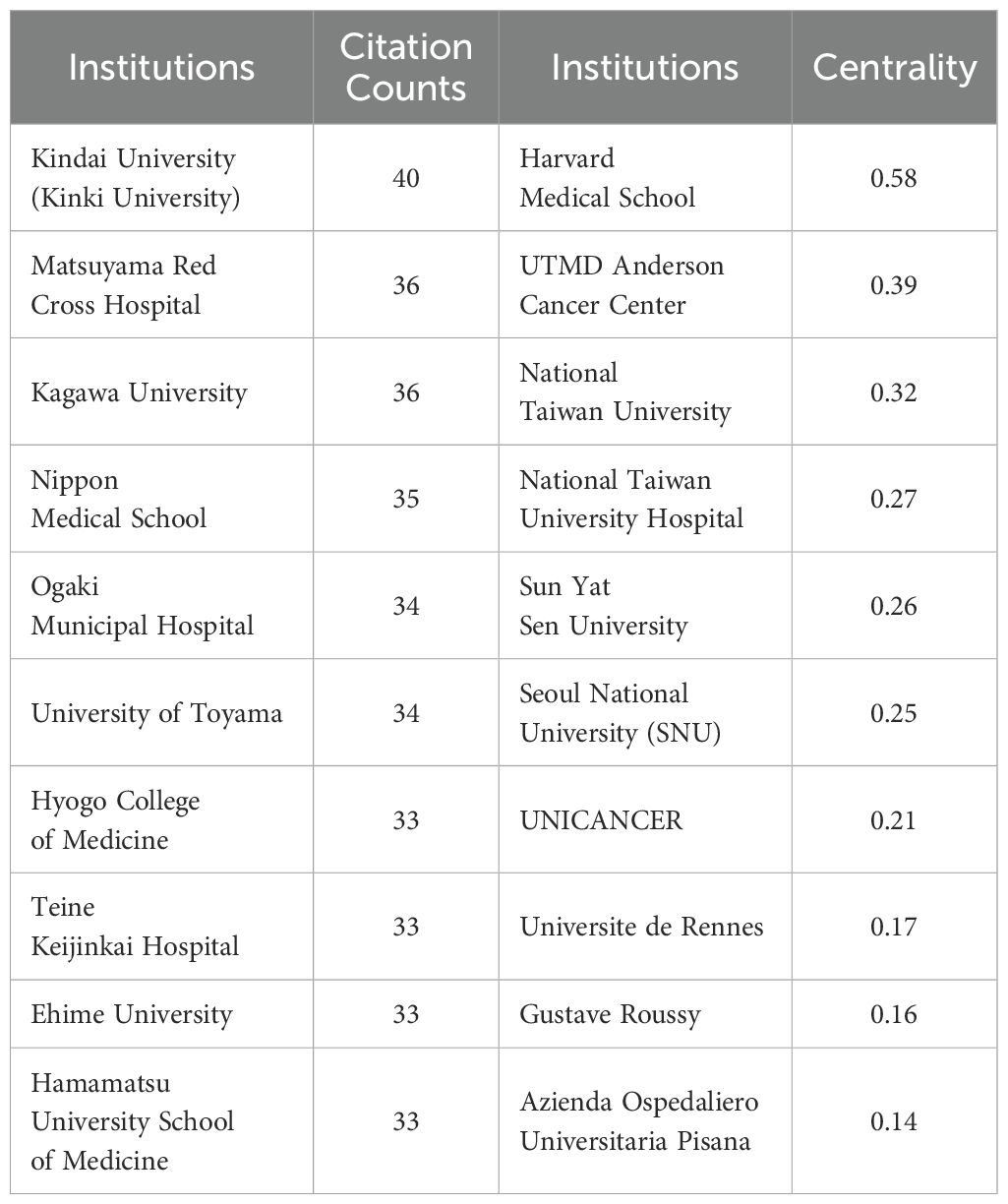
Table 2. Top 10 institutions involved in research on immunotherapy and targeted therapy combinations in relation to HCC.
A total of 3,669 authors contributed to the research in the included articles. Kudo Masatoshi and Hatanaka Takeshi were the most prolific authors, each publishing nine papers, while Tada Toshifumi contributed seven (Table 3). Co-citation analysis revealed that Kudo Masatoshi (N = 44), Tada Toshifumi (N = 38), and Nakamura Shinichiro (N = 38) were the top three authors in terms of co-citations.
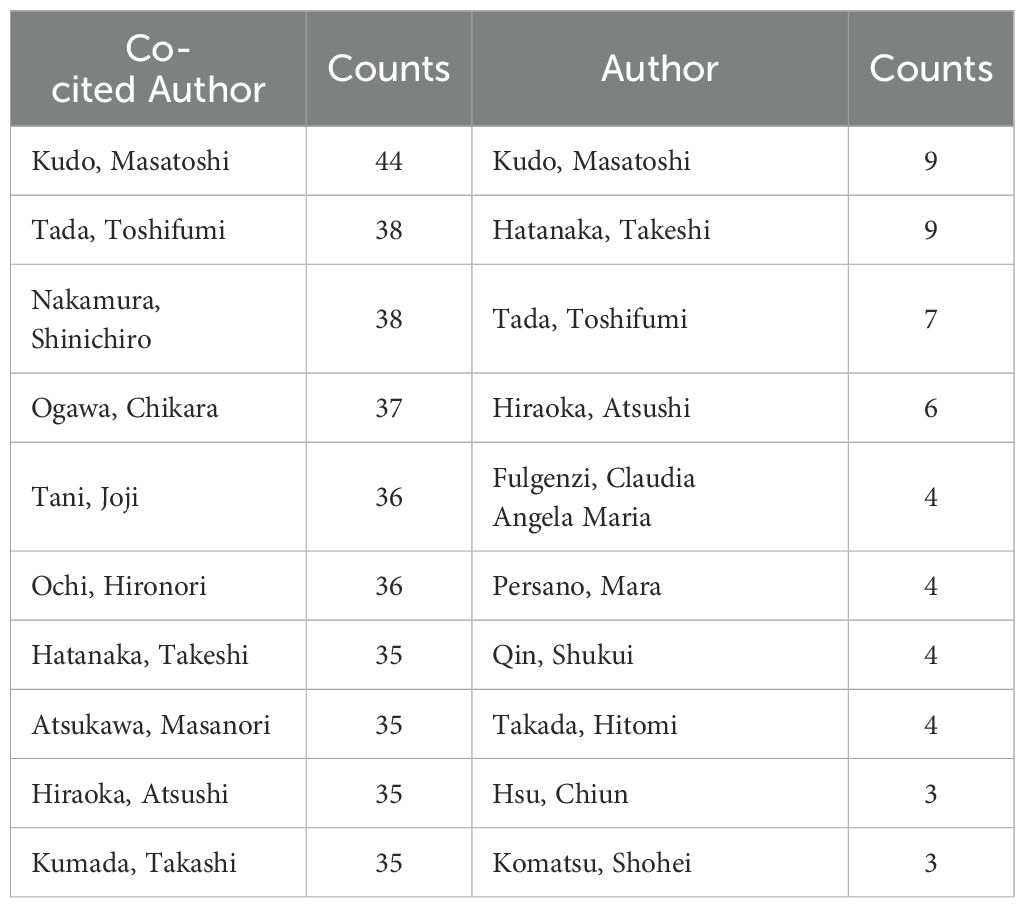
Table 3. Top 10 authors and co-cited authors involved in research on immunotherapy and targeted therapy combinations in relation to HCC.
VOSviewer was used for network analysis of co-authorship and co-citation among the authors of the selected publications (Figure 5). Network nodes represent individual authors, with the size of the circles corresponding to the number of articles published by each author. Co-occurrence relationships are depicted by lines linking the circles. A strong co-occurrence relationship exists between authors and their co-cited counterparts, with authors publishing more frequently generally exhibiting greater co-occurrence relationships with other authors.
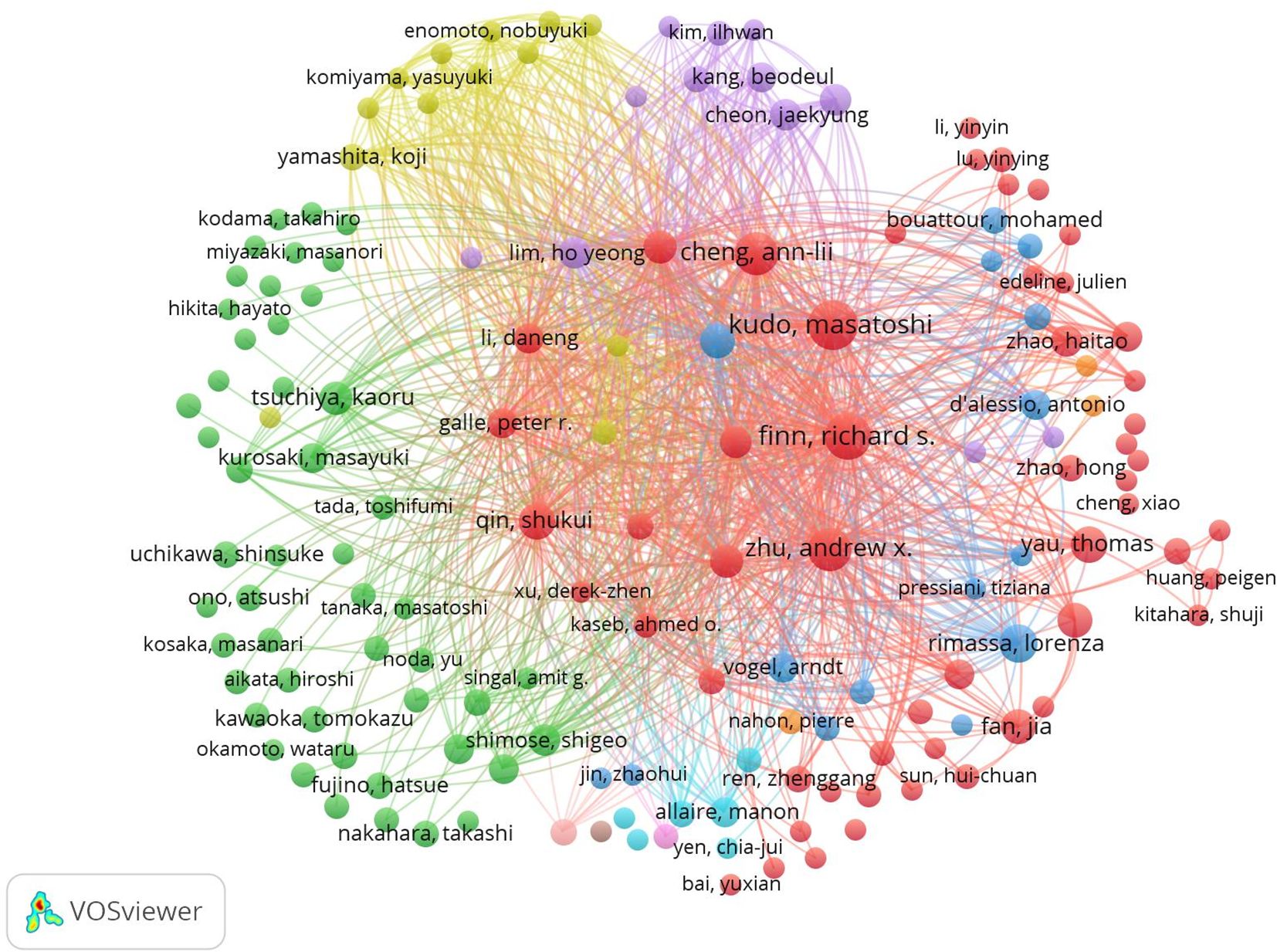
Figure 5. Network map showing authors and co-cited authors involved in research on immunotherapy and targeted therapy combinations for HCC.
A total of 147 academic journals published relevant articles on the combination of immunotherapy and targeted therapy in HCC treatment (Figure 6A). Cancers led with the most publications (N = 48, IF2022 = 5.2), followed by Frontiers in Oncology (N = 25, IF2022 = 4.7) and Liver Cancer (N = 25, IF2022 = 13.8), both ranking second in terms of publication numbers. Among the top 10 journals contributing the most relevant articles, 50% are based in Switzerland, with 30% from the United States. Liver Cancer ranked highest in Impact Factor (IF2022 = 13.8) among journals with over 20 articles published, followed by Frontiers in Immunology (N = 20, IF2022 = 7.3) (Table 4).
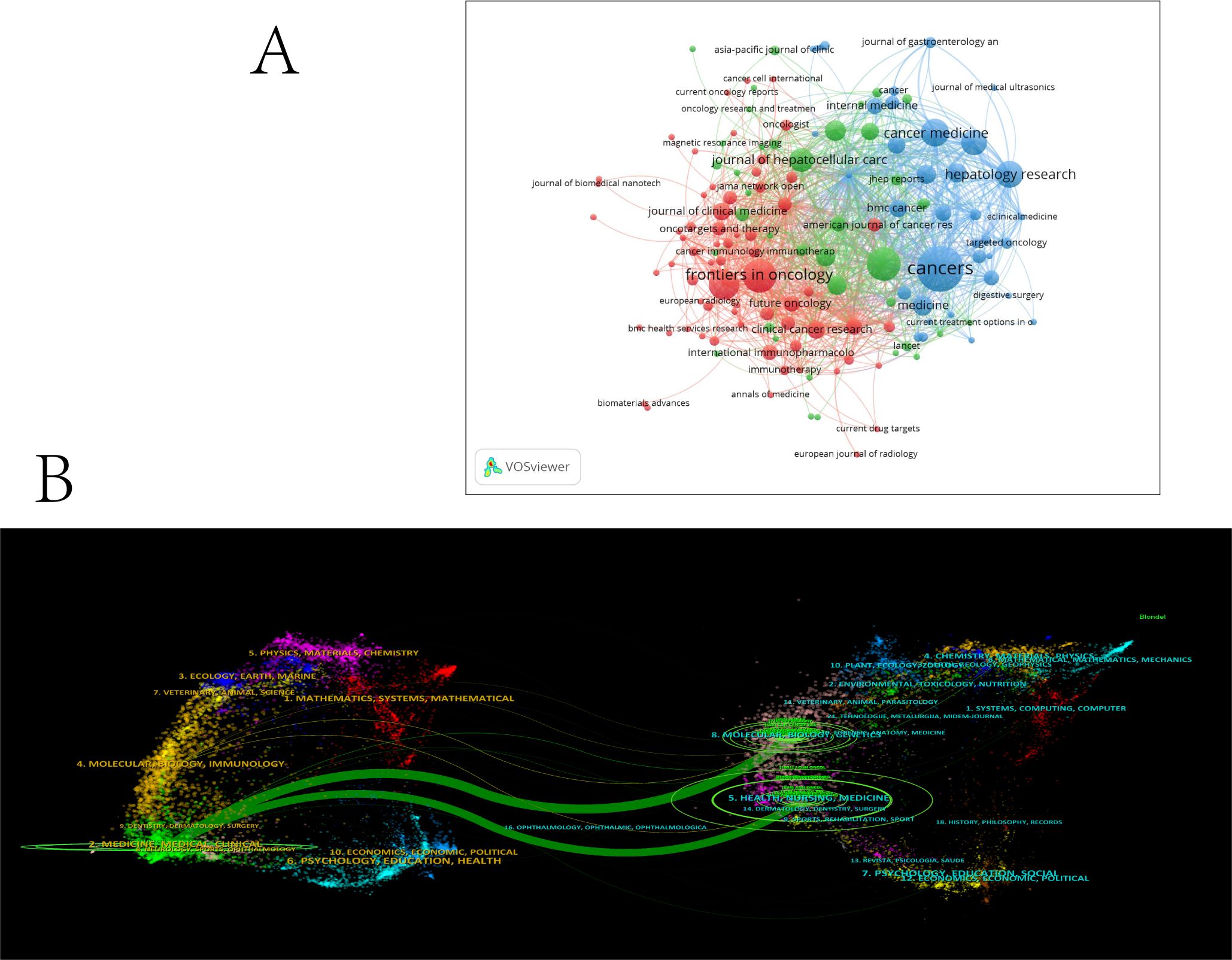
Figure 6. (A) Network map showing academic journals involved in research on immunotherapy and targeted therapy combinations for HCC. (B) A dual-map overlay of journals related in research on immunotherapy and targeted therapy combinations for HCC.
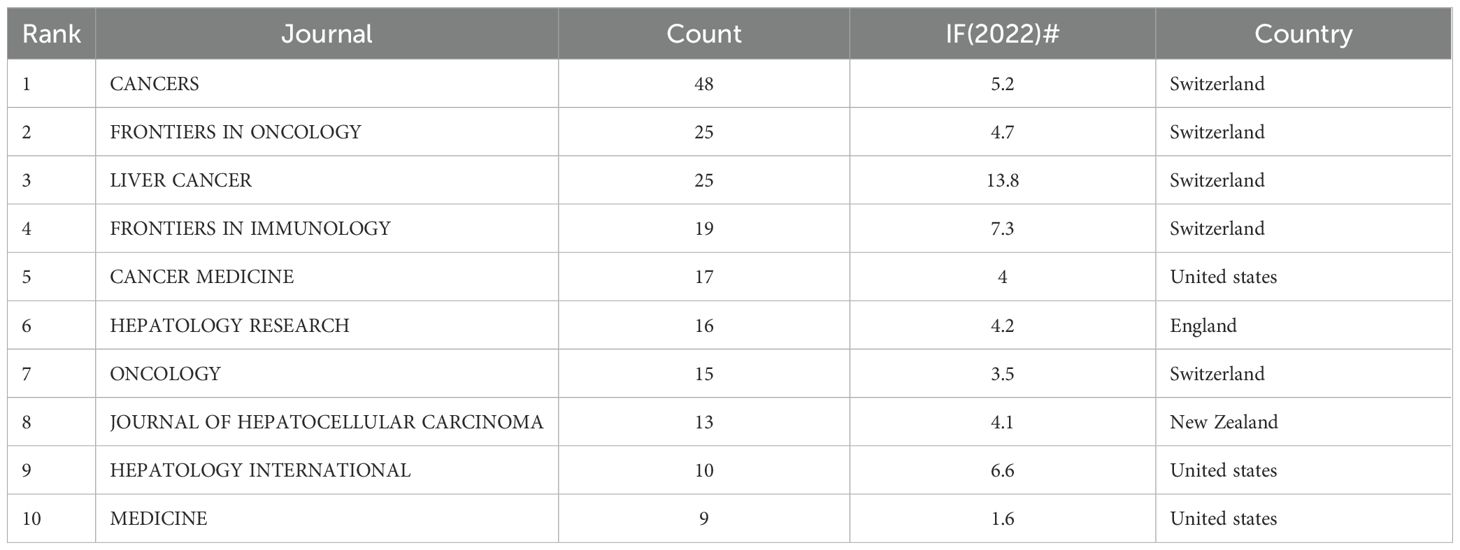
Table 4. Top 10 academic journals involved in research on immunotherapy and targeted therapy combinations in relation to HCC.
The dual-map overlay in Figure 6B illustrates the distribution of topics within the journals. The citing journals are located on the left, and the cited journals are on the right, with labels representing the disciplines covered by each journal. Colored lines represent citation paths from left to right. The vertical axis indicates the number of papers published, and the horizontal axis denotes the number of authors. Two green pathways highlight frequent citations between journals in the molecular/biology/genetics and health/nursing/medicine domains, pointing to their influence on clinical/medical journals.
Table 5 lists the top 10 most-cited papers, with the most citations stemming from Atezolizumab plus Bevacizumab in Unresectable Hepatocellular Carcinoma by Richard S. Finn et al. (N = 391). All top 10 papers have been cited more than 100 times.
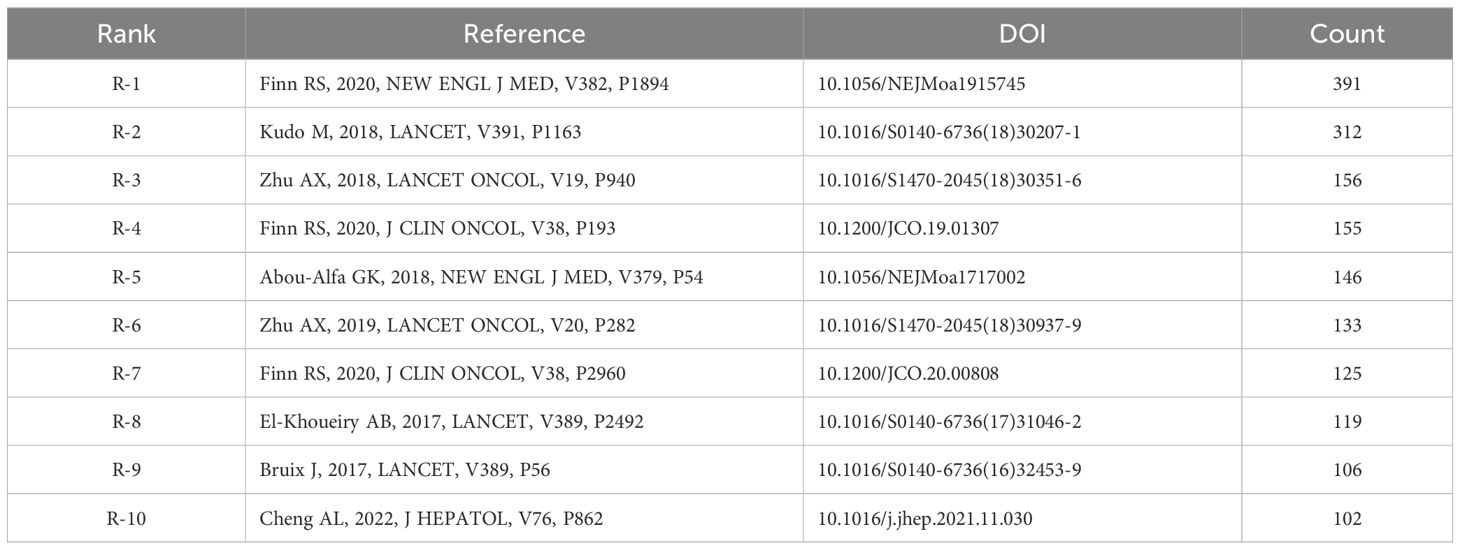
Table 5. Top 10 co-cited references involved in research on immunotherapy and targeted therapy combinations in relation to HCC.
A co-citation reference network was constructed using CiteSpace, revealing 9 clusters (Figure 7A). The clustering analysis identified the most frequently cited keywords, with #0 COMBINING ANTI-VEGF THERAPY as the leading cluster label, followed by #1 COMBINATION THERAPY in second place. A timeline for co-citation references (Figure 7B) visually represents the distribution of topics over time. The timeline merges clustering and time-slicing techniques to illustrate research themes’ evolution. Nodes on the timeline, marked by color, represent different years; those on the left are older, and those on the right are more recent. Horizontal lines at the same level indicate the collection of all citations in each cluster, with the labels positioned to the far right. The clusters most adjacent in the timeline include #1 COMBINATION THERAPY, #6 HEPATOCELLULAR CARCINOMA, #7 BEVACIZUMAB TREATMENT, and #8 ADVANCED HEPATOCELLULAR CARCINOMA.

Figure 7. Co-citation analysis of the literature in CiteSpace. (A) Cluster analysis of the co-cited literature network. (B) Time-axis view of co-citation literature analysis data. (C) High-reference outbreak analysis of clustering data.
To assess the references with high citation bursts, CiteSpace was used to identify significant bursts in citation frequency. A citation burst indicates a sudden surge in the number of citations, suggesting the research’s growing influence. Among the 15 papers with the strongest citation bursts, El-Khoueiry AB, 2017, LANCET, V389, P2492, DOI 10.1016/S0140-6736 (17)31046-2 (2019-2020, burst = 22.57) and Bruix J, 2017, LANCET, V389, P56, DOI 10.1016/S0140-6736 (16)32453-9 (2019-2020, burst = 20.32) were notable for their high citation numbers (Figure 7C).
Keyword timeline and clustering analyses serve as effective tools for identifying prominent research themes within a given field. In this study, CiteSpace was employed for clustering keyword data related to immunotherapy in HCC, with cluster numbers based on size, the largest being designated as #0. A total of 13 clusters were identified, which were further analyzed using CiteSpace’s timeline view. These clusters include #0 second-line therapy, #1 potential synergistic anti-tumor activity, #2 controlled trial, #3 programmed death-ligand, #4 treatment perspective, #5 PD-1 blockade, #6 muscle volume loss, #7 non-viral unresectable hepatocellular carcinoma, #8 co-delivery of MEK inhibitors, #9 hepatocellular carcinoma patient, #10 adverse event, #11 dual programmed death receptor-1, and #12 hepatocellular carcinoma. The timeline derived from cluster analysis is shown in Figure 8. Four key research areas emerged from co-occurrence analysis: adverse reactions (#6, #10), liver cancer (#7, #9, #12), tumor-targeted immune responses (#1, #3, #5, #8, #11), and the integration of immunotherapy and targeted therapy (#0, #2, #4). Notably, interest in clusters #2, #3, #4, and #5 has grown significantly in recent years and remains high, signaling sustained attention on the combination of targeted therapy and immunotherapy in HCC treatment. As efforts to refine treatment strategies and improve patient outcomes continue, this upward trend is likely to persist.
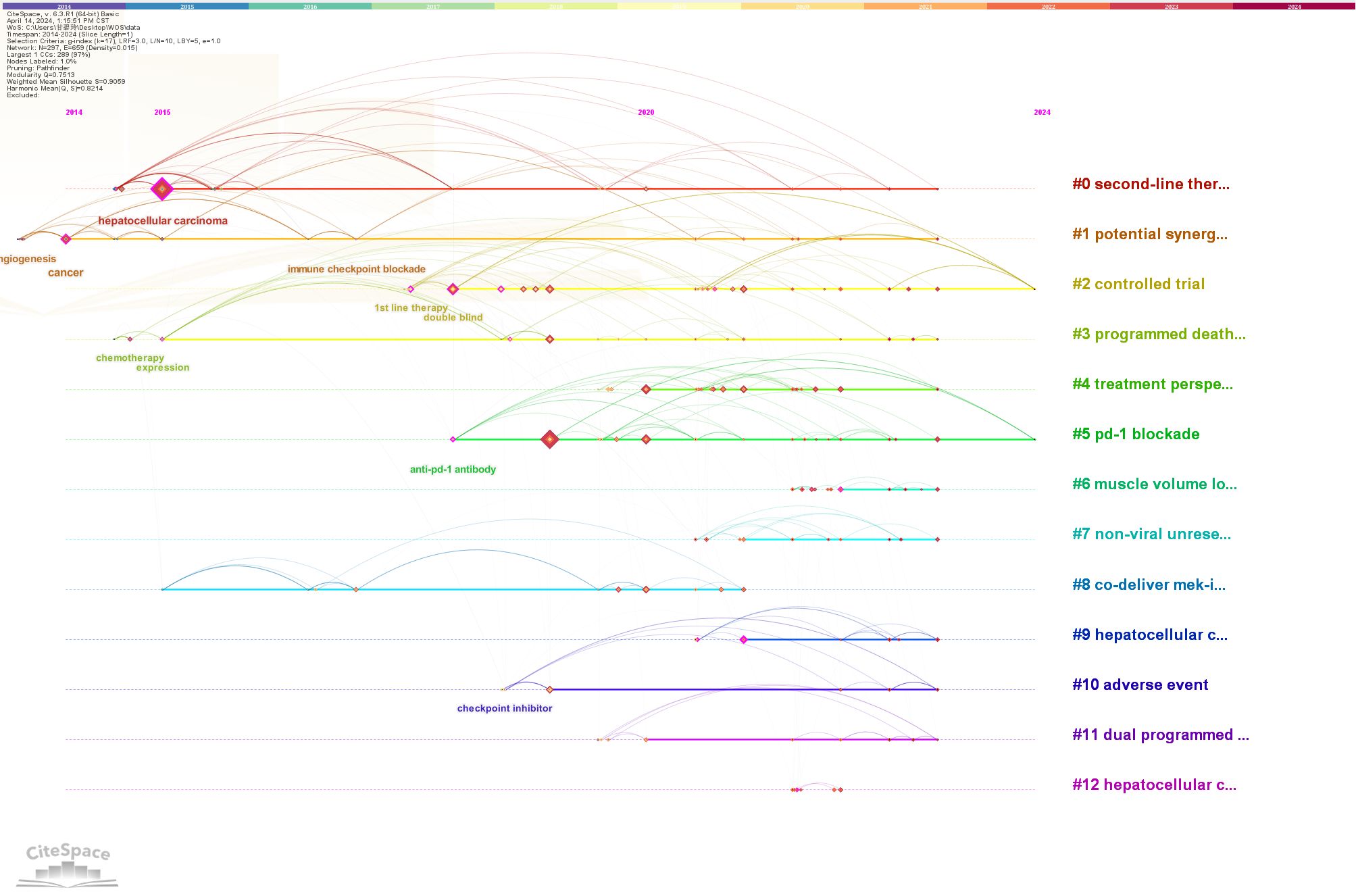
Figure 8. Cluster analysis of keywords in research on immunotherapy and targeted therapy combinations for HCC.
A keyword network diagram, constructed with VOSviewer (Figure 9), visualizes these findings. The top 22 keywords exhibiting citation bursts were identified using CiteSpace, ranked by duration, start time, and burst intensity (Figure 10). Periods spanning 2014 to 2023 are represented by green lines, with burst cycles marked by red lines.
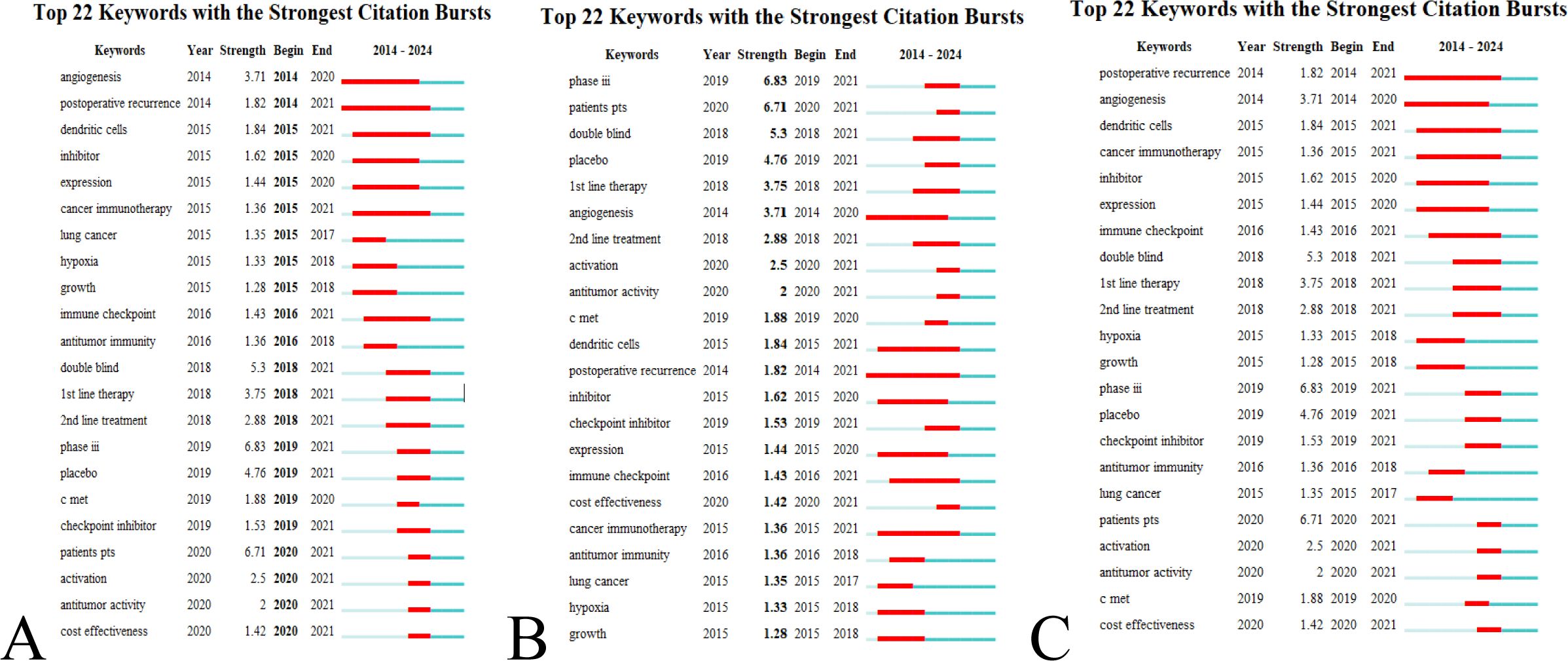
Figure 10. Burst keywords in research on immunotherapy and targeted therapy combinations for HCC. (A) Ranking by beginning. (B) Ranking by strength. (C) Ranking by duration.
Angiogenesis emerged as the earliest burst keyword in 2014 (Figure 10A), reflecting early and sustained interest in its role in immunotherapy and targeted therapy for HCC.
The keyword “Phase III” showed the most intense burst, highlighting a significant focus on this stage of clinical trials (Figure 10B).
“Postoperative recurrence” demonstrated the longest period of sustained citation bursts (Figure 10C), indicating ongoing research interest in recurrence after surgical intervention.
The 22 burst keywords were classified into two primary categories: body immunity relevant to HCC and immunotherapy for HCC. Between 2018 and 2021, the concentration of burst keywords increased, suggesting that research on targeted immunotherapy for HCC is becoming more specialized, as illustrated by the keyword timeline (Figure 8).
Researchers from mainland China lead global publications on immunotherapy and targeted therapy combinations for HCC treatment, contributing 40% of the total output. Several factors underpin this dominance. First, China bears a significant liver cancer burden, with around 55% of global cases attributed to hepatitis B infections (1), compounded by widespread aflatoxin contamination (16). Additionally, regional, economic, and socio-cultural factors, including dietary habits and historical medical practices—such as the reuse of syringes during vaccination campaigns in the 1990s—further intensify this burden. Second, the Chinese government has actively promoted immunotherapy research, establishing vital infrastructure and providing financial support to advance the field, particularly targeting hepatitis B and its liver cancer risks. Japanese researchers rank second in publication volume, facing similar hepatitis B infection challenges (17). Substantial government investment in health research, along with dietary habits such as the predilection of raw food, contributes to Japan’s research output. However, both nations display low centrality, indicating limited academic visibility and influence, highlighting the need for improved publication quality and increased international collaboration.
Among the top 10 publishing countries, France exhibits the highest centrality, reflecting its substantial academic influence and extensive global collaboration in liver cancer research.
American scholars rank third in both publication volume and centrality in the field of immunotherapy and targeted therapy combinations for HCC. While the incidence of liver cancer in the United States remains relatively low compared to countries with higher rates, this is likely due to the country’s advanced healthcare system, comprehensive health education, and robust medical security infrastructure. Nevertheless, the large population base in the U.S. results in a substantial number of patients with liver cancer. As lifestyle changes and the aging population contribute to rising incidence rates, the prevalence of liver cancer in the U.S. is expected to increase. Key factors driving the rise of HCC include obesity, diabetes, and the growing incidence of non-alcoholic fatty liver disease (NAFLD). Additionally, disparities in healthcare access, preventive measures like hepatitis vaccinations, and early screening disproportionately impact vulnerable populations. Racial and ethnic minorities, such as Blacks and Native Americans, face a higher risk of liver cancer compared to Whites, with these disparities potentially linked to genetic, environmental, and socioeconomic factors.
The increasing number of publications on immunotherapy and targeted therapy combinations reflects a growing interest in this approach as a promising treatment for liver cancer. This upward trend not only facilitates the accumulation and dissemination of knowledge but also promotes international collaboration and the exchange of insights, driving further exploration and innovation. The expanding body of research signals a positive outlook for the field, offering more precise treatment options for clinical practice, improving survival rates for patients with HCC, and providing valuable theoretical guidance for clinical management.
Recent advancements in liver cancer treatment have been marked by significant breakthroughs through the combination of molecular targeted therapy and immune checkpoint inhibitors. Clinical trial outcomes have demonstrated sustained clinical benefits, with manageable efficacy and safety profiles (18). Moreover, the development of new drugs is expected to lead to the emergence of more innovative combination regimens in the future (19, 20). In addition to the established combination of targeted therapy and immunotherapy, clinical practice has gradually incorporated other combinations, such as targeted therapy combined with local treatments (21, 22). These regimens, which pair targeted drugs with immunotherapy, offer more precise approaches to liver cancer treatment. This combination strategy can more effectively reduce tumor size and number, thereby increasing patient survival rates and improving quality of life. As new targeted drugs and immunotherapies continue to evolve, treatment outcomes are anticipated to further improve.
With ongoing technological advancements and deeper research, the future of targeted immunotherapy for liver cancer holds promising prospects. Future research can leverage bibliometric analysis to identify existing knowledge gaps and steer subsequent investigations. As understanding of targeted immunotherapy for HCC deepens, the development of more effective, tailored treatment approaches is expected to improve the prognosis and quality of life for individuals diagnosed with liver cancer.
The systemic treatment of HCC has transitioned from single-agent targeted therapies to dual immunotherapies or combined targeted and immunotherapy regimens. Among these, the combination of PD-1/PD-L1 inhibitors with MEK inhibitors has become a major focus of research. Multiple Phase III studies, including the prospective randomized controlled STORM study (23), the international multicenter IMbrave050 study, which met its primary endpoint in a pre-specified interim analysis (24), and ongoing Phase III trials such as JUPITER-04, EMERALD-2, and KEYNOTE-937, suggest that “clinical research” will remain a dominant topic in this field. However, as Phase III studies progress, concerns have emerged regarding the cumulative toxicity of combined targeted and immunotherapy regimens, which has increased the incidence of severe treatment-related adverse events (25, 26). This trend underscores the need for careful monitoring and potential treatment interruptions due to adverse events.
This study has several limitations. Firstly, while the current research status is adequately described, the analysis relies solely on data from the WOS database, introducing potential bias due to the use of a single data source. Secondly, the inclusion criteria limited studies to English-language publications, which may result in language bias by excluding relevant research published in other languages. Additionally, disparities in national wealth and population size may contribute to research bias, as these factors influence a country’s investment in health research. Finally, bibliometric analyses often suffer from temporal biases, as publications with lower citation counts in their early stages may be undervalued despite their high quality. Thus, ongoing attention to emerging studies and publications in diverse languages is crucial for capturing current, valuable insights.
It is anticipated that more research on immunotherapy and targeted therapy combinations for HCC treatment will be published in the coming years, reflecting the rapid development in this field. Future research hotspots are likely to focus on the pathological mechanisms of liver cancer, the development of new drugs, and the design of novel combination regimens. In conclusion, the combination of immunotherapy and targeted therapy represents a promising and emerging treatment modality for liver cancer, with significant potential to improve patient prognosis and quality of life.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
BG: Writing – original draft, Data curation, Investigation, Project administration. LW: Data curation, Writing – original draft, Investigation, Supervision. SZ: Data curation, Writing – review & editing, Supervision, Formal analysis. ZhC: Writing – review & editing, Conceptualization. FW: Writing – review & editing, Formal analysis. LX: Formal analysis, Writing – review & editing. ZrC: Formal analysis, Writing – review & editing. HM: Data curation, Writing – review & editing. PH: Formal analysis, Writing – review & editing. DF: Supervision, Writing – review & editing. NS: Formal analysis, Methodology, Supervision, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by grants from the National Key Clinical Specialty Construction Project (Grant Number: 2022YW030009).
We thank Bullet Edits Limited for the linguistic editing and proofreading of the manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2025.1476146/full#supplementary-material
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. Ca: A Cancer J Clin. (2021) 71:209–49. doi: 10.3322/caac.21660
2. Moon H, Choi JE, Lee IJ, Kim TH, Kim SH, Ko YH, et al. All-treatment array of hepatocellular carcinoma from initial diagnosis to death: observation of cumulative treatments. J Cancer Res Clin. (2017) 143:2327–39. doi: 10.1007/s00432-017-2480-9
3. Zhou J, Sun H, Wang Z, Cong W, Zeng M, Zhou W, et al. Guidelines for the diagnosis and treatment of primary liver cancer (2022 edition). Liver Cancer. (2023) 12:405–44. doi: 10.1159/000530495
4. Qin Y, Han S, Yu Y, Qi D, Ran M, Yang M, et al. Lenvatinib in hepatocellular carcinoma: resistance mechanisms and strategies for improved efficacy. Liver International: Off J Int Assoc Study Liver. (2024) 44:1808–31. doi: 10.1111/liv.15953
5. Xu J, Zhang Y, Jia R, Yue C, Chang L, Liu R, et al. Anti-pd-1 antibody shr-1210 combined with apatinib for advanced hepatocellular carcinoma, gastric, or esophagogastric junction cancer: an open-label, dose escalation and expansion study. Clin Cancer Research: Off J Am Assoc Cancer Res. (2019) 25:515–23. doi: 10.1158/1078-0432.CCR-18-2484
6. Mei K, Qin S, Chen Z, Liu Y, Wang L, Zou J. Camrelizumab in combination with apatinib in second-line or above therapy for advanced primary liver cancer: cohort a report in a multicenter phase ib/ii trial. J Immunother Cancer. (2021) 9. doi: 10.1136/jitc-2020-002191
7. Synnestvedt MB, Chen C, Holmes JH. Citespace ii: visualization and knowledge discovery in bibliographic databases. Amia Symposium. (2005) 2005:724–28.
8. van Eck NJ, Waltman L. Citation-based clustering of publications using citnetexplorer and vosviewer. Scientometrics. (2017) 111:1053–70. doi: 10.1007/s11192-017-2300-7
9. Fijačko N, Creber RM, Abella BS, Kocbek P, Metličar ŠChecktae, Greif R, et al. Using generative artificial intelligence in bibliometric analysis: 10 years of research trends from the european resuscitation congresses. Resuscitation Plus. (2024) 18:100584. doi: 10.1016/j.resplu.2024.100584
10. Qu F, Wang G, Wen P, Liu X, Zeng X. Knowledge mapping of immunotherapy for breast cancer: a bibliometric analysis from 2013 to 2022. Hum Vacc Immunother. (2024) 20:2335728. doi: 10.1080/21645515.2024.2335728
11. Hernández-Contreras M, Cruz JC, Gurrola MP, Pamplona Solis B, Vega-Azamar RE. Application of nanosilica in the construction industry: a bibliometric analysis using methodi ordinatio. Methodsx. (2024) 12:102642. doi: 10.1016/j.mex.2024.102642
12. Yusoff ZM, Ismail N, Nordin SA. Dataset for five recent years (2019 - 2023) agarwood essential oil research trends: a bibliometric analysis. Data Brief. (2024) 54:110310. doi: 10.1016/j.dib.2024.110310
13. Yang X, Jin R, Zhang L, Ying D. Global trends of targeted therapy for hepatocellular carcinoma: a bibliometric and visualized study from 2008 to 2022. Med (Baltimore). (2023) 102:e34932. doi: 10.1097/MD.0000000000034932
14. Shen J, Shen H, Ke L, Chen J, Dang X, Liu B, et al. Knowledge mapping of immunotherapy for hepatocellular carcinoma: a bibliometric study. Front Immunol. (2022) 13:815575. doi: 10.3389/fimmu.2022.815575
15. Li Z, Zhang Y, Zhang B, Guo R, He M, Liu Z, et al. Bibliometric study of immunotherapy for hepatocellular carcinoma. Front Immunol. (2023) 14:1210802. doi: 10.3389/fimmu.2023.1210802
16. Global prevalence, cascade of care, and prophylaxis coverage of hepatitis b in 2022: a modelling study. Lancet Gastroenterol Hepatol. (2023) 8:879–907. doi: 10.1016/S2468-1253(23)00197-8
17. Umemura T, Wattanakamolkul K, Nakayama Y, Takahashi Y, Sbarigia U, KyungHwa L, et al. Real-world epidemiology, clinical and economic burden of chronic hepatitis b in Japan: a retrospective study using jmdc claims database. Infect Dis Ther. (2023) 12:1337–49. doi: 10.1007/s40121-023-00795-0
18. Tada T, Kumada T, Hiraoka A, Hirooka M, Kariyama K, Tani J, et al. Outcomes of patients with hepatocellular carcinoma treated with atezolizumab plus bevacizumab in real-world clinical practice who met or did not meet the inclusion criteria for the phase 3 imbrave150 trial. Aliment Pharm Ther. (2024) 60:233–45. doi: 10.1111/apt.18037
19. Zhang Y, Zhang H, Xu H, Wang Y, Feng L, Yi F. Efficacy and safety of hepatic arterial infusion chemotherapy combined with lenvatinib and pd-1 inhibitors for advanced hepatocellular carcinoma with macrovascular invasion. World J Surg Oncol. (2024) 22:122. doi: 10.1186/s12957-024-03396-4
20. Feng J, Zhao Y, Zhai L, Zhou J. Efficacy and safety of transarterial chemoembolization combined with targeted therapy and immunotherapy versus with targeted monotherapy in unresectable hepatocellular carcinoma: a systematic review and meta-analysis. Medicine. (2024) 103:e38037. doi: 10.1097/MD.0000000000038037
21. Wang L, Lin L, Zhou W. Efficacy and safety of transarterial chemoembolization combined with lenvatinib and pd-1 inhibitor in the treatment of advanced hepatocellular carcinoma: a meta-analysis. Pharmacol Therapeut. (2024) 257:108634. doi: 10.1016/j.pharmthera.2024.108634
22. Fu S, Xu Y, Mao Y, He M, Chen Z, Huang S, et al. Hepatic arterial infusion chemotherapy, lenvatinib plus programmed cell death protein-1 inhibitors: a promising treatment approach for high-burden hepatocellular carcinoma. Cancer Med-Us. (2024) 13:e7105. doi: 10.1002/cam4.7105
23. Bruix J, Takayama T, Mazzaferro V, Chau G, Yang J, Kudo M, et al. Adjuvant sorafenib for hepatocellular carcinoma after resection or ablation (storm): a phase 3, randomised, double-blind, placebo-controlled trial. Lancet Oncol. (2015) 16:1344–54. doi: 10.1016/S1470-2045(15)00198-9
24. Ad hoc announcement pursuant to art. 53 lr. roche’s tecentriq plus avastin is the first treatment combination to reduce the risk of cancer returning in people with certain types of early-stage liver cancer in a phase iii trial. Available online at: https://www.roche.com/media/releases/med-cor-2023-01-19.
25. Abou-Alfa GK, Chan SL, Kudo M, Lau G, Kelley RK, Furuse J, et al. Phase 3 randomized, open-label, multicenter study of tremelimumab (t) and durvalumab (d) as first-line therapy in patients (pts) with unresectable hepatocellular carcinoma (uhcc): himalaya. J Clin Oncol. (2022) 40:379. doi: 10.1200/JCO.2022.40.4_suppl.379
Keywords: hepatocellular carcinoma, immunotherapy, targeted therapy, combination therapy, bibliometric study, VOSviewer, CiteSpace
Citation: Gan B, Wu L, Zhou S, Chen Z, Wu F, Xu L, Chen Z, Ma H, He P, Fang D and Shi N (2025) Comprehensive analysis of publications concerning combinations of immunotherapy and targeted therapies for hepatocellular carcinoma: a bibliometric study. Front. Immunol. 16:1476146. doi: 10.3389/fimmu.2025.1476146
Received: 05 August 2024; Accepted: 23 January 2025;
Published: 12 February 2025.
Edited by:
Emanuel Petricoin, George Mason University, United StatesReviewed by:
Sweta Sikder, National Institutes of Health (NIH), United StatesCopyright © 2025 Gan, Wu, Zhou, Chen, Wu, Xu, Chen, Ma, He, Fang and Shi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Dan Fang, MzQyODU4ODQyQHFxLmNvbQ==; Ning Shi, c2hpbmluZ19kb2NAMTYzLmNvbQ==
†These authors share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.