
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Immunol. , 06 February 2025
Sec. T Cell Biology
Volume 16 - 2025 | https://doi.org/10.3389/fimmu.2025.1476123
Preeclampsia (PE) is an obstetrical disorder that occurs after the 20th week of gestation. It is recognized as one of the “Great Obstetrical Syndromes” and principally contributes to maternal morbidity and mortality. PE has been associated with a range of immune disorders, including a preponderance of T helper (Th) 1 over Th2 cells and imbalanced levels of Th17 and T regulatory cells (Tregs). During pregnancy, T cells safeguard the placenta against immune rejection and aid embryo implantation while involved in pregnancy complications, such as PE. Promoting alloantigen-specific Treg cells is a potential preventive and therapeutic strategy for PE. However, ensuring the safety of mothers and infants is of the utmost importance since the risk-benefit ratio of reproductive and obstetric conditions differs significantly from that of immune diseases that pose a life-threatening risk. In this review, we systematically summarize the roles of T-cell immunity in the peripheral blood, reproductive tissues, and at the maternal-fetal interface of PE patients. Furthermore, the recent therapeutic approaches centered on targeting T cell immunity in PE are critically appraised.
Preeclampsia (PE) is a disorder that occurs during pregnancy, with a global incidence rate of 2-8%. Recognized as one of the “Great Obstetrical Syndromes,” it principally contributes to maternal mortality (1). Clinical manifestations, including hypertension (BP ≥ 140/90 mmHg) and proteinuria, manifest after the 20th week of gestation to define this condition (2). Furthermore, PE may be correlated with additional maternal and obstetrical complications, such as impairment of placentation, intrauterine growth restriction, preterm labor, aberrant liver function, acute renal failure, and hematological abnormalities (3, 4). Currently, preventive measures are limited to lifestyle modifications and the use of aspirin, whereas management consists solely of childbirth. Although early pregnancy induction is often required to safeguard the health of the mother in cases of PE, premature birth can have significant adverse effects on neonatal health. For example, respiratory morbidity in neonates has been observed in preterm infants, which exhibited a 4.4-fold increase in comparison to full-term infants (5).
Currently, knowledge holds that PE develops in two stages: an impaired trophoblast invasion and remodeling of spiral arteries, along with changes in the immune system in the early maternal-fetal environment. During the latter phase of pregnancy, systemic inflammation occurs in the maternal body (6). Overall, significant pathogenic factors include the presence of inflammatory processes (7–9), the lack of maternal tolerance towards the fetus (10–12), and cardiovascular maladaptation in the mother (13). Noticeably, PE is associated with a range of immune disorders, including higher activity of neutrophils, monocytes, and natural killer (NK) cells, dysregulated cytokine secretion, a preponderance of T helper (Th) 1 cells relative to Th2 cells, imbalanced levels of Th17 and T regulatory cells (Tregs), and the manifestation of autoimmunity (14–18). Tregs, which have decreased levels of CD127 and increased levels of CD4, CD25, cytotoxic T lymphocyte antigen-4 (CTLA-4), CD45RA, HLA-DR, and forkhead transcription factor 3 (FoxP3), are particularly important in preventing the development of detrimental immune responses and promoting tolerance throughout pregnancy (19).
Over the last two decades, considerable investigation has indicated that T cells exert notable influence on both healthy and unhealthy pregnancies, albeit with the exact characteristics of these influences remaining obscure. Disputing the notion that decidual T cells universally endanger fetal survival due to the “allograft” placenta, distinct T cell subsets contribute to determining pregnancy success or failure. Treg cells safeguard the placenta against immune rejection and aid in embryo implantation. In contrast, others, such as Th1 or Th17 cells, have been reported to be involved in pregnancy complications, such as PE.
In this review, we systematically summarize the roles of T-cell immunity in the peripheral blood, reproductive tissues, and at the maternal-fetal interface of patients diagnosed with PE and further substantiate the notion that T cell regulation may effectively mitigate the detrimental prognosis of PE for both the mother and neonate, ultimately leading to improved pregnancy outcomes.
In the human decidua of the first trimester, T cells comprise a range of 10-20% of the overall leukocyte count, comprised of 30-45% CD4+ and 45-75% CD8+ (20). Afterward, the proportion of decidual T cells increases, eventually encompassing 40-80% of all leukocytes at term (21). The regulation of normal reproductive functions depends on CD4+ T cells and the immune factors they generate. Suitable T cell reactions regulate the fertilization and embryo development, as well as the initial development of the placenta and angiogenesis (22–26). Based mainly on the production of cytokines and surface markers, CD4+ T helper cells are categorized as Th1, Th2, Treg, Th17, and recently Th22 cells (27). A controlled transition to Th1 responses occurs during the peri-implantation phase; these responses are involved in immune surveillance and prevent an overabundance of trophoblast invasion (28). Transitioning towards Th2 cells following placental implantation is imperative to ensure and advance the development of a healthy embryo and placenta by promoting allograft tolerance. It entails the reduction of Th1 and Th17 cells through the secretion of interleukin (IL)-13, IL-4, and IL-17 (28). The Th1/Th2 paradigm during normal pregnancy is that Th1 immune response dominates in the first trimester. However, in the second and third trimesters, the maternal immune system shifts towards Th2 immune response (28–30).
CD8+ T cells are the prevailing immune cells in the decidua during gestation, essential for fostering fetal-maternal tolerance. Effector memory CD8+ T cells (CD8+ EM cells) comprise the majority of decidual CD8+ T cells (dCD8+ T cells); these cells are thought to possess the capability to induce fetal rejection. Naive CD8+ T cells (CD8+ N cells), on the other hand, constitute the main parts of peripheral CD8+ T cells (pCD8+ T cells) (31). In comparison to pCD8+ EM cells, dCD8+ EM cells express greater quantities of interferon-γ (IFN-γ) and IL-4, whereas perforin and granzyme B are less abundantly expressed (31, 32). Programmed cell death-1 (PD-1) was discovered to be highly expressed on Tregs, CD8+ T cells, and NKT-like cells (33–35). A notable up-regulation of programmed death ligand-1 (PD-L1) was observed in immune cells situated at the maternal-fetal interface, as well as extravillous trophoblasts (EVT) and syncytiotrophoblasts (ST) (36–39). Fetal resorption is increased in mouse models when the PD-1/PD-L1 signal is inhibited; this suggests that this pathway is important for maintaining immune tolerance in the decidua (40). Accordingly, immune tolerance to fetal antigens is sustained in pregnancy by regulating the response of decidual CD8+ T cells, although these cells maintain the capability to eliminate virus-infected cells (41).
CD4+CD25+ Treg cells are essential in protecting the fetus from rejection due to their potent ability to suppress immune responses (42). Many Treg subtypes resembling induced type 1 regulatory (Tr1) cells have been detected in the initial and terminal human decidua (43). These subtypes have exhibited the capability to impede the proliferative ability of effector T cells and increase the secretion of IL-10 (44). Healthy pregnancy was related to a rise in CD4+CD25+ Tregs in both rodents (45) and humans (46). There is a rise in the circulating levels of CD4+CD25+ cells in the first trimester, reaching the highest value in mid-pregnancy and then declining after childbirth to levels slightly higher than those before pregnancy (46) (Figure 1). A few weeks prior to delivery, a discernible reduction in CD4+CD25high Treg cells is observed (47). Treg cells are locally enriched in the decidua (48, 49). Researchers observed similar proportions of Tregs in the decidua basalis throughout normal pregnancies, while noting a rise in the decidua parietalis from mid pregnancy to late pregnancy (49).
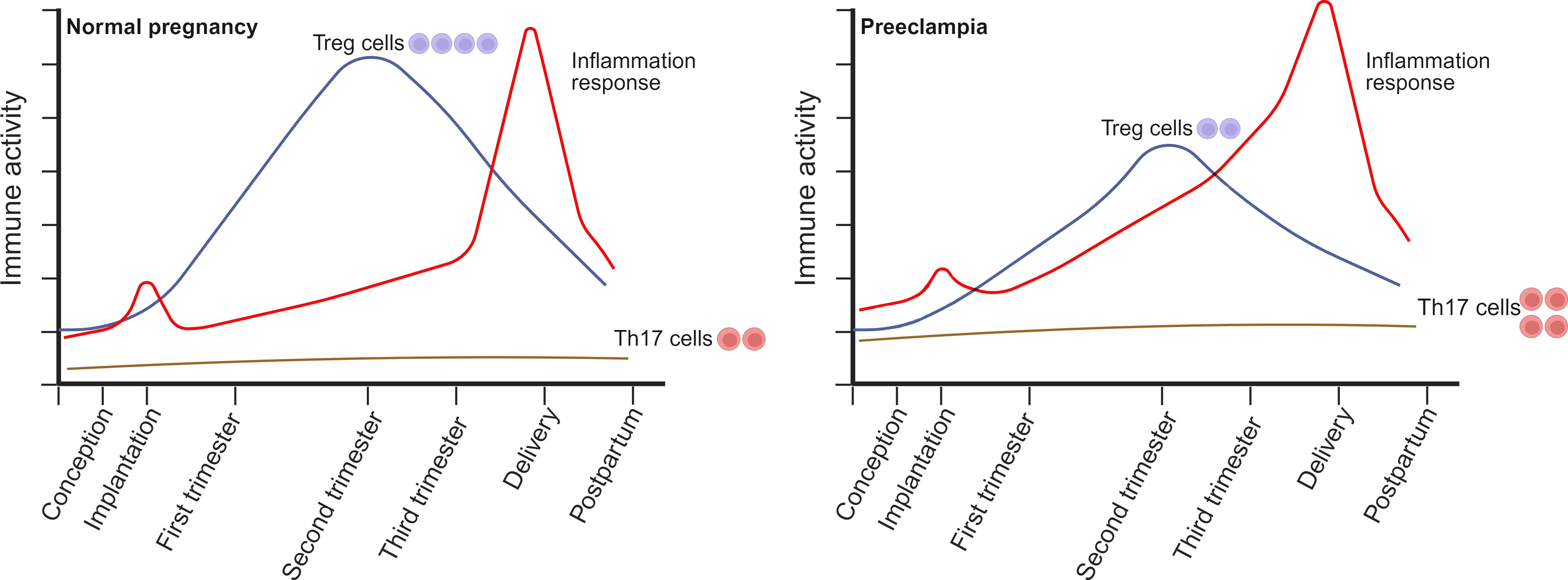
Figure 1. The alterations in the circulating composition of Treg/Th17 cells during normal pregnancy and preeclampsia (PE) occur throughout the peri-conception, various stages of pregnancy, and at delivery. During a healthy pregnancy, Treg cell levels rise during early pregnancy, peak in mid-pregnancy, and then decline progressively after delivery, returning to slightly elevated levels compared to pre-pregnancy levels. In contrast to the non-pregnant state, the quantity of Th17 cells remains comparatively low during the entirety of the pregnancy and does not endure substantial changes. PE is correlated with reduced quantities, compromised suppressive capabilities, or instability of Treg cells. This may be attributed to the limited ability of Treg cells to facilitate normal placentation and regulate the elevated inflammation frequently observed in PE. Furthermore, the quantity of Th17 cells in expectant women with PE could be considerably greater than that of healthy controls. Generated using BioRender.com.
Tregs serve multiple functions, including inhibiting immune system activity, mitigating inflammation, and remodeling of blood vessels to promote successful embryo implantation in the decidua (10, 50). During pregnancy, peripheral Treg cells and uterine Treg cells are generated in reaction to fetal antigens. Peripheral Treg cells inhibit the production of the pro-inflammatory cytokines (IFN-γ, tumor necrosis factor (TNF)-α, IL-2, and IL-12), creating a microenvironment with anti-inflammatory properties (51–53). Uterine Treg cells suppress the expansion of Th1 and Th17 cells, thereby averting their assault on the semi-allogeneic fetus. Inhibiting the immune activity of effector T cells to fetal alloantigens, uterine Tregs have diagnostic features of inhibitory T cells, including elevated expressions of CTLA-4, IL-10, CD25, and transforming growth factor (TGF)-β (54–57). Moreover, Tregs are essential in preventing invariant NKT (iNKT)-induced miscarriage (56). In support of a successful pregnancy, Tregs also aid in the maintenance of a suppressive immune phenotype in other cell types, including macrophages, dendritic cells (DCs), and uterine NK (uNK) cells (50). Conversely, interaction between CD14+ myelomonocytic cells and decidual NK cells initiates a sequence of events that facilitate the production of Treg cells and inhibit the immune response (58).
In contrast, Th17 cells constitute an additional subgroup of CD4+ T cells that contribute to facilitating inflammatory responses (59). Th17 cell-produced IL-22 and IL-17 contribute to the eradication of pathogens and the induction of inflammation in autoimmune disorders, respectively (60, 61). The regulation of Th17 cell development and differentiation is subject to the influence of the retinoic acid-related orphan receptor γt (RORγt), as well as a multitude of positive and negative factors (62, 63). In a healthy pregnancy, CD4+ T cells comprise the majority of IL-17-producing cells in the circulation and decidua (64, 65). Comparatively, the percentage of IL-17+ lymphocytes in the decidua is evidently greater than that in the periphery of first-trimester pregnant women (64). The circulating quantity of Th17 cells exhibited no variation during the entire gestational period (66). Research has documented that pregnant women exhibit reduced quantities of CD4+ IL-17+ T cells and an increased proportion of FoxP3+ Tregs to IL-17+ CD4+ T cells during a healthy pregnancy compared to non-pregnant females (14). IL-17 could augment the invasiveness of JEG-3 cells, which are choriocarcinoma cell lines, and substantially increase progesterone secretion in vitro co-culture models (67, 68). Another study found that IL-17 levels remained in a similar range throughout the whole pregnancy, but the average was higher in the third trimester (69). This suggests that elevated IL-17 levels may contribute to the initiation of labor and/or inflammation. Further research involving humans and in vivo experiments is required to ascertain whether the existence of Th17 cells is a cause or an effect of effective pregnancy establishment.
To summarize, the accumulation of Tregs is critical for maintaining a healthy semi-allogeneic pregnancy during its early stages. The proportion of Tregs increases during the initial and subsequent trimesters of gestation, and through a multitude of mechanisms, Tregs promote the well-being of the fetus. While significantly more Th17 cells are present in the decidua in early pregnancy compared to the second and third trimesters, these cells secrete IL-17, facilitating the invasion and proliferation of trophoblasts.
Women with PE have an increased systemic effector T-cell pool, as demonstrated by either a higher number/proportion or a greater degree of activation (70–73). Patients diagnosed with PE exhibited elevated circulating concentrations of CD4+ T cells compared with those undergoing a healthy pregnancy (74). The elevation in CD4+ T cells could potentially be ascribed to the proliferation of memory T cells since the percentage of this subgroup was greater in women with PE relative to those who conceived successfully; contrarily, the percentage of naive T cells was diminished (72). Aberrant function of CD8+ T cells has also been observed in cases of PE compared to healthy pregnancies (73). A rise in the cytotoxicity of CD8+ T cells was found in preeclamptic women relative to non-preeclamptic women; this may have been due to a reduction in Treg-mediated inhibition (70). In addition to the heightened cytotoxic activity, patients with PE exhibited a significant increase in the proportion of peripheral microparticles originating from cytotoxic T cells compared to controls who were not pregnant (75).
As stated previously, the majority of studies have indicated that women with PE have increased T-cell activity. However, there have been several reports indicating contradictory findings. Compared to women with a normal pregnancy, patients with PE had decreased peripheral T-cell counts (76, 77). According to these reports, while the numbers of CD8+ and CD4+ T cells were comparable between normal and preeclamptic pregnancies, PE patients exhibited significantly lower percentages of CD4+ memory, CD4+ EM, and CD4+ central memory (CM) subgroups than normal pregnancies (78). Reduced T cell populations may indicate a decline in specific subgroups instead of a decline in the T-cell population as a whole. Undoubtedly, pregnancies impacted by PE exhibited a dramatic decrease in the percentage of circulating CD4+ HLA-G+ T cells (79) and diminished levels of soluble HLA-G in maternal plasma (80). This observation holds significance since HLA-G, which is predominantly detected in neonatal tissues (81) and functions to enhance immune tolerance (82, 83), can be detected in a distinct subgroup of T cells that possess immunosuppressive characteristics (84, 85). Hence, specific peripheral T cell subsets may be reduced in the context of PE, which contrasts with the elevated abundance of inflammatory T cells documented in other reports.
Efforts to examine the functionality of effector T cells at the uteroplacental interface have produced contradictory results, potentially due to variations in patient cohorts, experimental approaches, and other specific characteristics. Flow cytometric (86) and immunohistochemical (87) analyses of decidual samples revealed a reduction in the percentage of T cells in PE cases compared to women who experienced preterm delivery or full-term childbirth, respectively. Conversely, an immunohistochemistry method identified a prominent abundance of CD8+ T cells in the decidua of individuals diagnosed with PE compared with those without (88). CD8+ T cells and total T cells were more abundant in placental bed biopsies of women with PE than normal controls (89). Additionally, a greater percentage of CD8+ T cells was identified in placental tissues from pregnancies impacted by PE (90). The modifications in local effector T cells associated with PE may be influenced by distinct aspects: the flow cytometry analyses of early-onset and late-onset PE revealed a decrease in the percentages of CD4+ central-memory (CM) T cells and CD8+ regulatory-(Foxp3+) memory cell (CD45RO+) T cells compared with normal pregnant women, and early-onset PE showed higher proportions of activated CD4+ and CD8+ cells compared to late-onset PE in the decidua parietalis (91). Hence, it is evident that the changes in the local T-cell linked to PE are not solely determined by specific subsets, as early-onset PE is frequently accompanied by more significant immunological changes that may be more severe. The elevated prevalence of decidual effector T cells from women with PE could potentially be attributed to additional regional factors that trigger a secondary immune response.
Potentially, acute atherosis associated with PE or other placental histological abnormalities may enhance the infiltration of T cells. More T cells were detected in the decidual samples of preeclamptic females with acute atherosis than those without this pathological change (92). The significance of considering possible confounding variables, such as placental lesions, should be emphasized when assessing immune alterations in women with PE.
The reports mentioned above collectively indicate that PE is distinguished by activated T cells in the decidua or placenta and maternal circulation. The prevalence of systemic inflammation and a decrease in the number and functionality of Tregs and immunosuppressive HLA-G+ T cells are probable factors that impact T cell activation. Notably, women who develop PE at an early stage of pregnancy appear to have increased T-cell activities; however, other situations that can induce T-cell-mediated pathogenesis, such as acute atherosis, should also be evaluated.
The hypothesis regarding immune regulation during pregnancy, suggesting the conversion of the maternal immune response from Th1 to Th2, has been widely accepted for a long time (71, 93, 94). This model is built on the observation that the reaction triggering an antigen presented to a Th0 lymphocyte in a non-pregnant woman will be influenced, at least partially, by the cytokine environment surrounding this lymphocyte. For example, a cytokine microenvironment containing higher expressions of IL-12, IL-18, and IFN-γ will support the generation of Th1 cells, which release TNF-α, IL-2 and IFN-γ, and facilitate the stimulation of other cells such as cytotoxic T cells. On the contrary, a milieu characterized by elevated concentrations of IL-10 and IL-4 will induce the secretion of Th2 lymphocytes. Additionally, the response of Th2 is inhibited by the activity of Th1 cytokines.
PE is distinguished by an imbalance of the Th1/Th2 immune systems, with Th1-type immunity more prevalent in the peripheral circulation (15, 71, 95–101) (Table 1). Additionally, cytotoxic T-lymphocytes (CTLs) can be classified as type 1 or 2 subgroups (102, 103). Type 1 cells are distinguished by the inclusion of the IL-18 receptor on the cell surface, whereas type 2 cells exhibit a cell membrane protein resembling IL-1R (104). It has been proposed that the shift towards a Th1 dominance during pregnancy is responsible for the compromised placental function seen in PE. An inquiry observed an increase in the synthesis of IFN-γ, IL-2, and TNF-α in peripheral blood mononuclear cells (PBMCs) from patients with PE. Furthermore, a noteworthy correlation was revealed between the mean blood pressure and Th1 cytokines (105). In another study, 20 PE patients and 20 normotensive counterparts were recruited (106). They discovered that women with PE had a Th1 polarization shift and a Th2 reduction in their peripheral blood profiles. This was linked with elevated concentrations of TNF-α and IFN-γ and lower expressions of TGF-β1 and IL-10, compared with healthy pregnant women (106). These alarmins may cause disease development by modulating CD4+ T cells and encouraging the secretion of pro-inflammatory cytokines, thereby inducing innate and adaptive immune responses (106). An additional study examined the concentrations of Th1/Th2 cytokines in peripheral blood lymphocytes and CD3+ T, CD4+ Th, and CD8+ Tc cells in women with PE (107). When comparing PE to a normal pregnancy, an increased count of CD4+ lymphocytes was observed (107). While the Th1/Th2 shift was not detected in PE involving CD3+ cells, it might be evident in CD4+ and CD3- lymphocytes (107).
In patients with PE, reinforcement of Th1 reactions is evident not only in the periphery but also at the maternal-fetal interface (108). In vitro trophoblast cultures derived from term placentas of preeclamptic patients exhibited a profound reduction in IL-10 expression compared with cultures from normal pregnancies (109, 110). In PE, the placental ratios of TNF-α/IL-10 and IL-2/IL-10 were substantially elevated than in a healthy pregnancy (111). In PE, PD-1 expression was decreased in CD8+ T cells (112, 113), suggesting further activated CD8+ T cells. Compared to normal pregnant women, PE patients exhibited elevated levels of T-bet, Th1 transcription factor and decreased expressions of GATA-3, Th2 transcription factor in circulating and decidual T cells (114, 115).
Numerous studies have investigated the relationship between PE and peripheral blood Treg populations. Some research investigations utilized flow cytometry to examine the populations of Tregs (77, 106, 115–137), whereas others employed qPCR (62, 114, 138, 139). Pregnant women with PE have decreased numbers of circulating Tregs (14, 77, 117, 120, 123–125, 127, 132, 134, 140–144), including CD4+CD25+ FoxP3+ T lymphocytes (125), and CD8+CD25+FoxP3+ cells (138). However, other studies revealed that there was no significant difference in the peripheral proportion of Treg cells between the normal group and the PE group (136, 145, 146). Treg cells might have a diminished suppressive capacity in PE patients (77, 118, 120, 140, 142).
In patients with PE, the proportions of Treg subtypes are distinct from that of healthy expectant women. In comparison to normal pregnant females, the frequency of fully functional effector Tregs (CD4+ FoxP3+ CD45RA−) was lower in patients with PE. Conversely, naive Tregs (CD4+ FoxP3+ CD45RA+) were unchanged (116). In one study, a distinct subset of Treg cells that express HLA-G was characterized (147). These cells, which are hypo-proliferative and thymic-derived, do not show expression of FoxP3 and CD25 molecules. These entities are identified in instances of multiple sclerosis, HIV-1 infection, and transplantation (148). Compared to normal pregnant women, the proportions of peripheral CD4+HLA-G+, CD8+HLA-G+, and CD4+CD25+CD127low cells are diminished in PE patients (77). Likewise, an additional investigation demonstrated an evident reduction in the circulating CD4+HLA-G+ T cells in individuals with PE compared to healthy pregnant women (79). Moreover, healthy pregnant women have a significantly higher percentage of circulating CD4+CD127lowCD25+, CD4+FoxP3+, and CD4+CD25high cells compared to patients with PE (14). Furthermore, an increased Th17/Treg ratio was identified in PE by researchers via an examination of the proportions of CD4+CD25+CD127+ and CD4+ IL-17+ cells (122). In addition, the quantity of CD45RA+CD31+ recent thymic emigrant Tregs are diminished in patients with PE (119).
In PE cases, the inhibitory function of Treg cells diminishes in tandem with their diminished quantity (Table 2). It has been discovered that CD4+CD25+CD127low/neg Treg cells isolated from PE women via magnetic sorting showed a diminished suppressive activity in comparison to Treg cells isolated from healthy expectant individuals (140). In addition, CD4+CD25+ Treg cells obtained from PE patients via magnetic activated cell sorting (MACS) failed to impede the growth of CD4+CD25− responder cells (120). Nevertheless, contradictory studies have been reported that the inhibitory capacity of the Treg cell subset was comparable between PE patients and healthy pregnant controls (14, 126).
Several studies, however, failed to identify any distinctions between the populations of Treg cells in preeclamptic patients and those of healthy pregnancies. However, these studies used frozen PBMCs with a small sample size (145) and investigated CD4+CD25+ T lymphocytes without specific Treg markers (146). Minimal variation was observed in the quantity and ratio of Treg cells between women with normal pregnancy and PE (146). Additionally, an independent investigation revealed no distinction in the population of activated and resting Treg cells among women with severe and early-onset PE, pregnant women who did not have PE, or non-pregnant females (149). However, an analysis of functional and migratory Treg markers (CCR4 and CTLA-4) revealed that untreated preeclamptic individuals had higher percentages of CTLA-4+ and CCR4+ resting and inactivated Treg populations than healthy pregnant controls. It is worth noting that ten of eighteen PE patients in this study (149) were treated with glucocorticoids, which may affect the experimental results. It has been reported that glucocorticoids could affect the number and phenotype of Treg cells (150–152). Therefore, it is necessary to select PE patients who have not received glucocorticoids for further research.
The divergent results can likely be attributed primarily to the various definitions of Treg cells. A notable concern pertains to the expression of the critical phenotypic markers, namely CD25 and FoxP3, upon stimulation of conventional CD4+ T cells. Determining the precise quantity of “authentic” Treg cells in conditions marked by systemic T-cell stimulation, such as PE, is thus complicated. Diverse flow cytometric methods and markers have been devised to prevent the inclusion of non-suppressive activated Treg cells, which could potentially generate erroneous outcomes. These encompass the identification of suppressive Treg cells characterized by reduced CD4 expression (CD4dim) relative to conventional CD4 cells (153), as well as diminished or nonexistent expression of the IL-7 alpha receptor subunit CD127 (154). Additionally, the use of CD45RA helps distinguish between suppressive resting Treg cells (FoxP3dimCD45RA+) and non-suppressive, activated FoxP3 expressing T helper cells (FoxP3dimCD45RA-) (155).
The dysregulation linked to PE has been illustrated by modifications in transcription factors of T-cells. RORγt and FoxP3 are transcription factors that contribute to the development of Th17 and Treg cells, respectively (122). Ribeiro et al. (115) revealed that early-onset PE women had a greater proportion of CD4+ T cells expressing the RORc transcription factor and a consequential decline in the percentage of Treg cells expressing FoxP3, indicating more severe early-onset PE. Additionally, the mRNA expressions of FoxP3 and RORγt in peripheral T cells of patients with PE were higher and lower, respectively, in comparison to healthy expectant women (114, 122).
Altered concentrations of circulating cytokines may influence the equilibrium between Th17 and Treg cells, thus potentially contributing to the pathogenesis of PE. Pro-inflammatory responses result from the stimulation of chemokines (CXCL1, CXCL2, CCL20), cytokines (IL-6 and TNF-α), and inflammatory factors (the complement system and acute-phase proteins) by IL-17 (156). Consequently, IL-17 could facilitate the proliferation of Th17 cells. Circulating IL-17 concentrations are significantly elevated in PE women relative to normal pregnant females and non-pregnant controls (156). Furthermore, IL-17 concentrations in the plasma of pregnant women with severe PE are higher than those of healthy pregnant women (123). Another group, nevertheless, failed to identify a statistically significant difference in serum IL-17 concentrations between pregnant women with PE and those without complications (157). Furthermore, considering the capacity of IL-6 and IL-1β to stimulate the transformation of Treg cells into Th17 cells (158, 159), it is conceivable that heightened concentrations of these cytokines in PE could trigger the transformation from Treg to Th17 cells (160). It has been found that PE induces a rise in IL-6 and IL-1β production (161). IL-6 expression was found to be elevated in chorionic villus sampling (CVS) tissues of women who subsequently develop PE accompanied by fetal growth restriction (FGR) (162). Increased levels of IL-6 expression promote Th17 generation and undermine the stability of Tregs, while concurrently reducing the quantity of alternatively activated M2 macrophages and T cell markers (163).
Variations in the expression of apoptotic molecules contribute to the premature deletion of Tregs, which may account in part for the diminished quantity of Tregs observed in patients with PE (117). Compared with normal pregnancies, the percentage of Tregs expressing the anti-apoptotic molecule Bcl-2 was considerably diminished in PE patients. Furthermore, there was a significant rise in the expression of pro-apoptotic molecule, Bax, in Tregs of PE patients (117). These findings indicate that the presence of PE may increase the vulnerability of Tregs to apoptotic cell death, which is consistent with the reduced Treg counts observed in this specific clinical scenario. Indeed, the signatures of Tregs and effector T cells were found to be differently regulated in women with PE (164). A consistent reduction in STAT5 signaling in Th1 cells was observed in individuals who subsequently experienced PE (164). IL-2/STAT5 signal contributes to the differentiation of T helper (165) and Treg (166) cells, and it also potentially inhibits Th17 differentiation (167). Furthermore, an increase in the p38 signaling pathway, which is essential for Tregs to exert their suppressive function (168), was observed in Tregs from women who were carrying a healthy pregnancy, but not in those who were diagnosed with PE (164). These results suggest the potential utilization of specific signal pathways in maternal blood for the evaluation of PE.
Various studies have revealed decreased Treg cells and increased Th1 and Th17 immune responses in women with PE using various modalities (Table 3). There have been numerous hypotheses regarding the decrease in Tregs observed in women with PE. An initial factor to consider is the potential reduction in decidual Treg differentiation (169). The evidence that peripheral DCs from pregnant women with PE have a stronger ability to stimulate Th1/Th17-like T-cell responses than those from normotensive controls supports this view (170). In addition, alterations in circulating DCs were related to lower peripheral Treg levels in patients with PE (134) and elevated Th17 cell levels in women with early-onset PE (171). Furthermore, the insufficient maturation of decidual lymphatic vessels in PE might impede the penetration of immune cells into this region (172). In fact, a correlation between the number of Tregs and the density of lymphatic vessels in the decidua was established, suggesting that in cases of PE, the entry of circulating Tregs into the decidua may be obstructed (172). Lastly, a study investigating the T-cell receptor (TCR) repertoires of Tregs in the decidua discovered that the proportion of clonally-expanded Tregs decreased obviously in PE pregnancies (173), indicating that the inability of decidual effector Treg cell populations to undergo clonal expansion may be associated with the onset of PE.
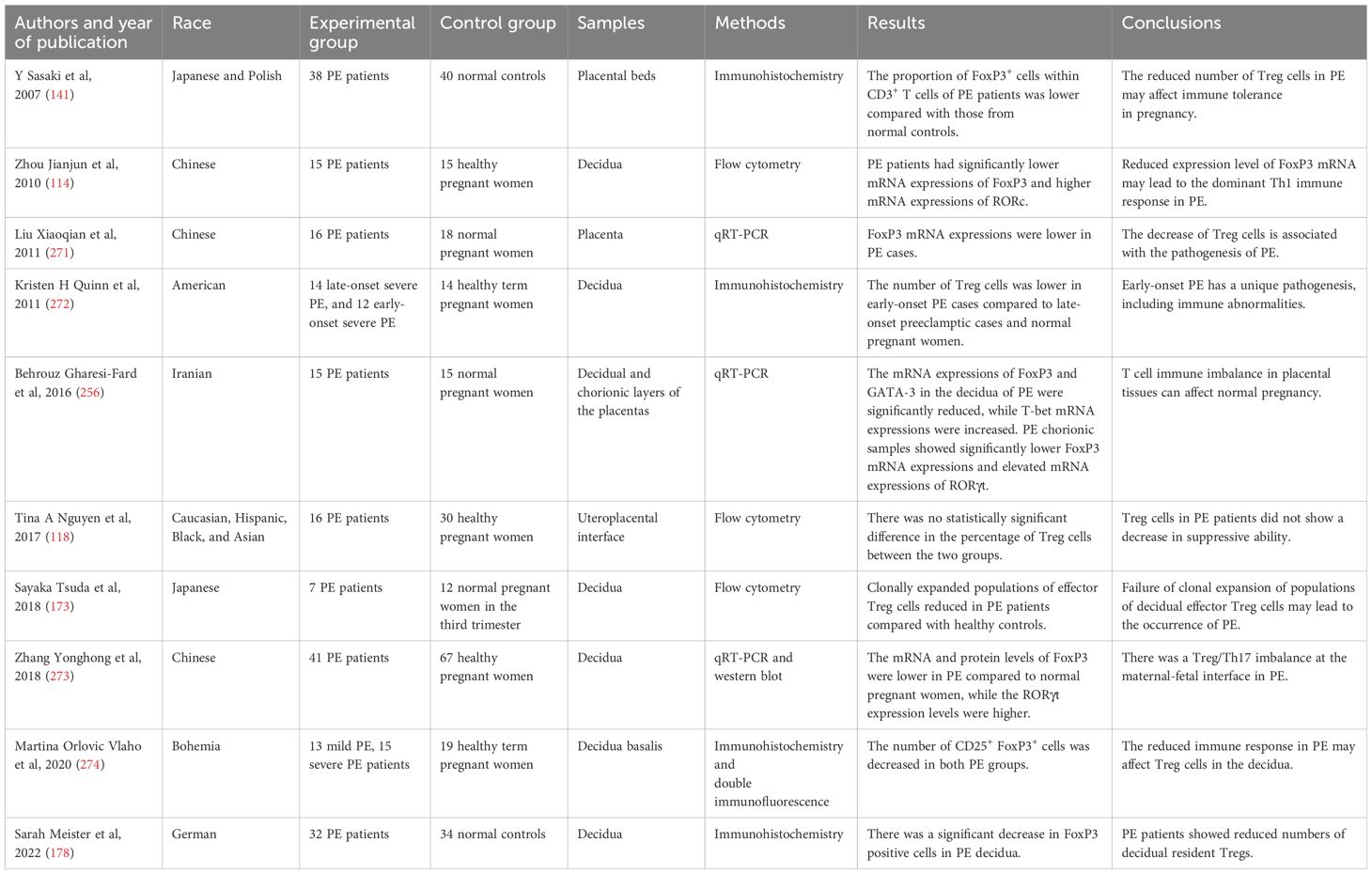
Table 3. Studies about the Treg/Th17 imbalance at the maternal-fetal interface in preeclampsia (PE) patients.
Currently, there are some innovative methodologies attempting to explore the molecular mechanisms underlying the onset of PE, including single-cell RNA sequencing (scRNA-seq). In one study, researchers conducted scRNA-seq on the placenta and decidual tissues of individuals with late-onset PE as well as those in a healthy pregnancy (174). It was discovered that the cells in the decidua that do not express extracellular matrix (ECM) are primarily immune cells, which consist of two subtypes of T cells (T1 and T2) (174). T2 cells show the expression of Treg marker genes, and there is a decrease in the down-regulation of the GO term “regulatory T cell differentiation” in T2 cells within the PE group. The pathway for “positive regulation of cytokine production” and several pathways for interleukin production show decreased activity in T cells, suggesting a significant impairment of their regulatory functions in late-onset PE (174). Another study examined transcriptomic changes specific to different cell types through unbiased scRNA-seq of placental samples, including two individuals with PE and two normotensive pregnant women (175). This comprehensive analysis involved 29,008 cells across 11 distinct cell types, encompassing trophoblasts and immune cells (175). They found that compared with normal pregnancies, there are significant differences in the gene expressions of GZMA, CD3E1, and CD3G in T cells from PE placental tissues, suggesting that these genes may be involved in the pathogenesis of PE (175). These findings provide a novel molecular theoretical foundation for the treatment of PE.
Treg cell dysfunction or a deficiency in quantity within the decidua has been linked to diminished invasion of EVT and ineffectual remodeling of spiral arteries. These factors collectively disrupt placental growth and ultimately result in placentation disturbance (176, 177). Therefore, it is not difficult to hypothesize that a deficiency of Treg cells during pre- and peri-conception could act as an initial catalyst for a cascade of events culminating in impaired placental development and shallow placentation, which ultimately manifest as conspicuous symptoms of PE later in pregnancy. Furthermore, in PE, Tregs undergo apoptosis at the feto-maternal interface, and galectin-2 (Gal-2) appears capable of inhibiting this process (178). Moreover, modifications in the activity of uNK cells may result in a potential dysregulation between Th17 and Treg cells. It has been shown that uNK cells communicate with CD14+ monocytic cells to induce the development of Treg cells and that IFN-γ produced by decidual NK cells can impede Th17 cells; these effects result in reduced inflammation and increased maternal-fetal tolerance (58, 179).
Treg cells possess a unique combination of anti-inflammatory and immune-regulatory properties that endow them with formidable capabilities to support placental development, facilitate the adaptation of maternal blood vessels, suppress inflammation, and preserve maternal acceptance of the fetus. As a result, enhancing the functionality of Tregs and augmenting their quantity in order to regulate the immune response may represent a viable therapeutic strategy for these pregnancy-related complications, including PE. However, in the context of human reproduction, any experimental evaluation of a method to stimulate Tregs must be conducted extremely cautiously and in strict adherence to robust principles of clinical trial design. Ensuring the safety of mothers and infants is of the utmost importance, and it is critical to recognize that the risk-benefit ratio of reproductive and obstetric conditions differs significantly from that of immune diseases that pose a life-threatening risk. Consideration should be given to the potential adverse effects of artificially increasing maternal Treg cells, such as a diminished capacity to combat pathogens (180) or immune surveillance for cancer (181). Notwithstanding the necessity for a comprehensive assessment of various approaches and identification of appropriate patient cohorts, the advanced immune therapy must take precedence to control over reducing adverse health outcomes and mortality rates associated with PE.
Memory T cells are a distinct subgroup of T cells that develop subsequent to a previous encounter with an antigen (182). Cytokines including IL-23, IL-7, and IL-15 influence the survival, function, and cytokine production of memory T-cells (183–185). CD4+ and CD8+ cell lineages both demonstrate the ability to recognize CM and EM cells (182, 186–188). Research on memory T cells in PE has been scant and inconsistent to date. Two investigations identified elevated circulating numbers of the general CD4+ memory T cells in PE cases compared with normal pregnant women (72, 144). However, whether the observed differences pertained to the EM or CM cell subset was not specified. An alternative investigation revealed a significant elevation in the quantities of DRlow+CD45RA- Tregs and DRhigh+CD45RA- Tregs among pregnant women diagnosed with PE compared to the pregnant women with normal blood pressure (140). Nevertheless, DRlow+CD45RA- and DRhigh+CD45RA- Tregs were not classified as memory Tregs in this investigation, notwithstanding the widespread usage of CD45RA- as a memory marker. In addition, the percentages of mature naive Tregs and circulating recent thymic immigrant (RTE) were lower in women with PE. Conversely, the proportion of memory Treg cells increased, suggesting a possible disruption in the differentiation of peripheral Treg cells (119, 189). PE patients had lower percentages of circulating CD4+ memory, CD4+ EM, and CD4+ CM subgroups than normal pregnancies (78). Furthermore, comparing healthy pregnant females and PE women, the levels of CD4+ EM cells in peripheral blood and lymphocytes isolated from intrauterine biopsies obtained during cesarean section were identical (118). However, caution should be exercised in interpreting these findings due to the inability to ascertain the precise cell origin extracted from the biopsy. Furthermore, clonally expanded CD8+ EM cells in the deciduae of PE women exhibit decreased PD-1 expression compared with normal pregnancies (112), indicating that the existence of local expansion of effector T cells may be due to a decrease in their suppressive function in PE patients.
Th22 cells are known for their production of IL-22. An in vitro experiment has shown that IL-22 could prevent premature birth caused by inflammation (190). RORγt and T-bet have opposing effects on the differentiation of Th22 cells from naive Th cells; RORγt promotes it while T-bet inhibits it. On the other hand, Th22 cells have the potential to differentiate into either Th1 or Th2 cells. The Th22 cells showed significant flexibility in generating IFN-γ under conditions that promote Th1 immunity or in an inflammatory environment rich in IFN-γ in living organisms (191). Th22 cells exhibit the ability to enhance IL-13 production when exposed to a Th2 environment (191). The presence of decidual IL-22+ Th17/Th2 and Th17/Th0 subsets in normal pregnancy suggests that these cells are important for embryo implantation (192). In patients with severe PE, there was a notable rise in the circulating levels of Th22 cells and IL-22 compared with healthy pregnant individuals (123).
The proportion of Treg cells in cord blood from normal pregnancies has been examined in three studies; the percentages are 2.63-8.94% (193), 4.0-10.0% (194), and 2-3% (195). The normal ranges for T lymphocyte subgroups in cord blood from healthy full-term neonates have been reported: 15.40% to 70.06% for Th cells (CD3+/CD4+); 9.65% to 34.28% for cytotoxic T cells (CD3+/CD8+). The reference interval for Treg cells spanned from 0.35% to 9.07%, in contrast to the reference interval for adult peripheral blood (1.64% to 6.45%) (196).
Several investigations have explored the modifications in umbilical cord T cells of women with PE. A shift in the percentages of Th1/Th2 and Th17/Treg in the direction of inflammation with unchanged Th1 and Th17 cells and reduced Th2 and Treg cells was noticed (197). Furthermore, an obvious decrease in the proportion of CD4+ T cells was observed in the umbilical cord blood of neonates delivered by mothers with PE (198). Compared to healthy pregnant individuals, this decline was accompanied by a substantial decrease in the proportion of FoxP3+ Treg, specifically within the FoxP3lo populations (198). Additionally, the proportions of CD4+CD25highFoxP3+ and CD4+FoxP3+ Treg were evidently lower, whereas the proportion of CD4+CD25low was prominently increased in the cord blood of neonates born from preeclamptic women (199). Hence, it suggests that PE is associated with aberrant fetal immunity, manifesting as a reduction in Treg levels within the cord blood.
IL-17 is important to the body’s response to bacterial infections and inflammation. In addition to compromised tolerance in conditions such as PE, it has been associated with contact dermatitis, autoimmune disorders, and organ rejection following transplantation. Secukinumab, a monoclonal antibody that specifically targets IL-17, has been employed in treating contact dermatitis (NCT02778711) and discoid lupus erythematosus (NCT03866317) to correct the Th17 imbalance. Tibulizumab (LY3090106) is a tetravalent antibody currently under development for the treatment of Sjögren’s disease (NCT04563195). It functions as a dual antagonist against B cell activating factor and IL-17. The long-term use of recombinant mouse IL-17 receptor C (IL-17RC), a soluble receptor that inhibits IL-17, reduced uterine perfusion pressure (RUPP) in rats as well as reduced levels of circulating IL-17 and placental Th17 cells (200). Thus, the prospective efficacy of IL-17RC in the treatment of pro-inflammatory effects mediated by IL-17 in PE was demonstrated (Figure 2).
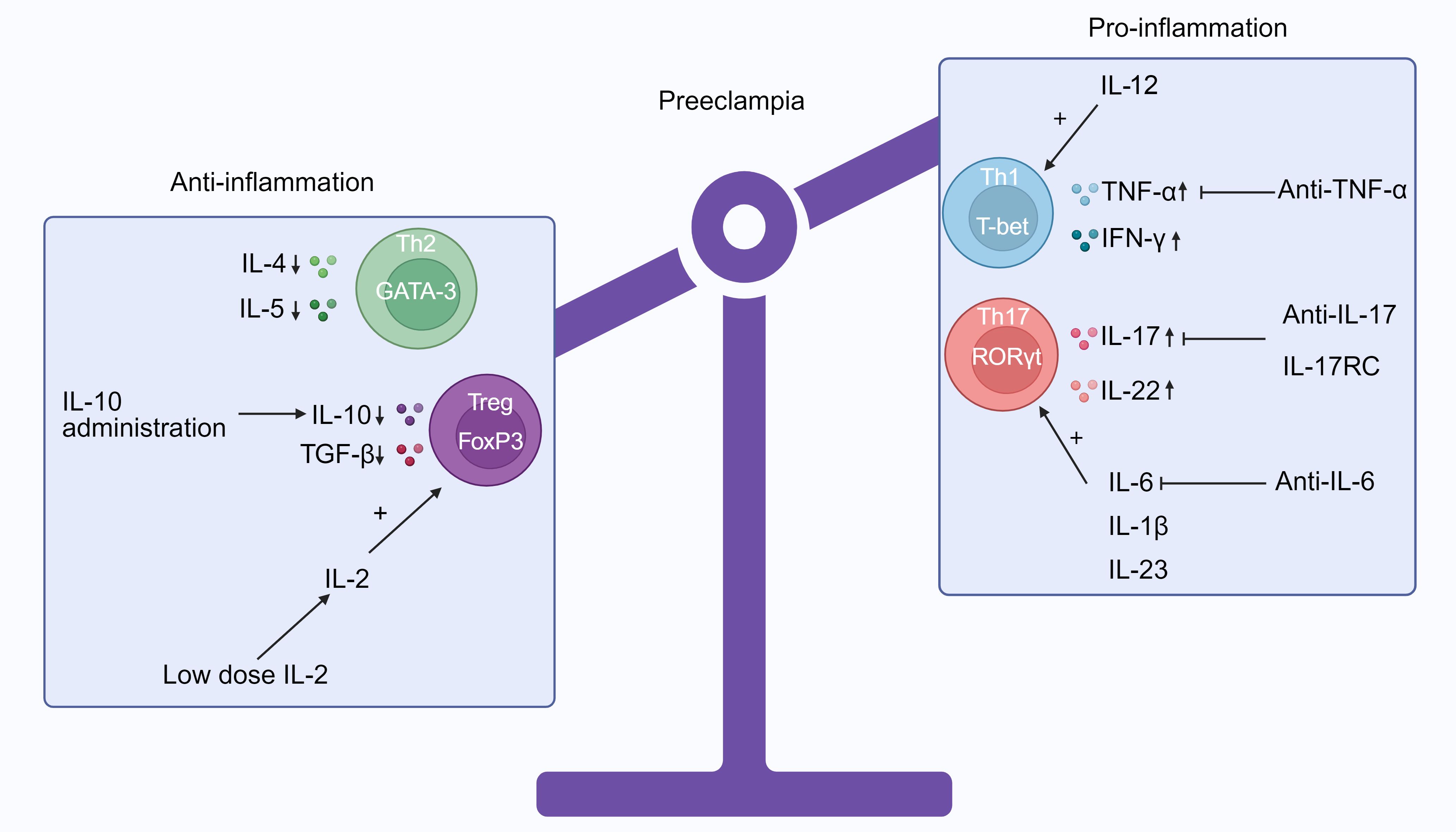
Figure 2. The immune imbalance between Th1/Th2 and Treg/Th17 in preeclampsia (PE) and potential treatments that target CD4+ T cells. Patients with PE have a propensity to Th1 and Th17 phenotypes, as indicated by the cytokine profiles in their peripheral blood. These phenotypes are distinguished by elevated concentrations of TNF-α, IFN-γ, IL-17, and IL-22 and reduced expressions of IL-4, IL-5, IL-10, and TGF-β. Additionally, IL-6, IL-1β, and IL-23 can induce the differentiation of Treg cells into Th17 cells. Increased levels of IL-12 will support the generation of Th1 cells. Potentially, regulating the proportion of pro- to anti-inflammatory CD4+ T cells could be implemented to prevent PE by promoting healthy placental development and maintaining a viable pregnancy. It is also possible to repress particular cytokines, such as IL-6 via anti-IL-6, IL-17 via IL-17RC and anti-IL-7, and TNF-α via anti-TNF-α. It is known that IL-2 directly regulates Treg homeostasis by enhancing their survival, proliferation, and function. Consequently, low-dose IL-2 can augment the quantity and durability of Treg cells. In addition, IL-10 administration, which increases Treg cell differentiation and Th2 immune response and significantly decreases circulating Th1 and Th17 cells, might be a potential treatment option (Produced with BioRender.com).
In addition to inhibiting immunosuppressive Tregs and Th2 cells, IL-6 enhances the differentiation of Th17 and cytotoxic T cells, thereby leading to inflammation in PE (201). IL-6 inhibitors, such as anti-IL-6 receptor mAbs (sarilumab and tocilizumab) and anti-IL-6 mAbs (siltuximab), have the potential to drive the development of inexperienced CD4+ T cells away from inflammatory Th1 and Th17 subsets (202). Clinically, anti-IL-6 therapy has been used to treat atherosclerosis (203) and rheumatoid arthritis (202). Incorporating strategies to decrease IL-6 for the management of PE may potentially promote the transformation of naive CD4+ T cells into Treg and Th2 cells, as opposed to Th1 and Th17 cells.
In the RUPP model, IL-10 administration via infusion through an osmotic mini-pump implanted intraperitoneally results in modest reduction in blood pressure as well as a substantial reduction in the levels of circulating Th1 and Th17 cells, an up-regulation of Treg cell differentiation, and the restoration of TNF-α expressions to normal (204). Culturing naive CD4+ T cells with IL-10-producing DCs could enhance the differentiation of Treg cells (204), a process that potentially alleviates PE. This study (204) indicate that IL-10 may function as an innovative treatment for PE.
TNF-α, a potential target for modulating CD4+ T cells, is produced by Th1 and Th17 cells. TNF-α expressions in PE could be considerably increased, reaching 2-3 times higher than in pregnancies with normal blood pressure (176, 205). These elevated levels have been linked to complications such as gestational hypertension, compromised endothelial function, and unfavorable obstetric outcomes (206). In addition to its essential function in facilitating successful implantation and placental development, TNF-α also functions as an inhibitor of CD4+ T cell proliferation via a mechanism dependent on IL-10 (11). By inhibiting effector T cells and repressing the activity of TNF-α, anti-TNF-α neutralizing mAbs promote the development of Tregs (207). The British Association of Dermatologists suggests that TNF-α inhibitors may be used during pregnancy, when large cohort studies that are well-designed, with a follow-up of over 10 years are performed in the future (208). According to the European Crohn’s and Colitis Organization, it is acceptable to utilize TNF-α inhibitors during the third trimester of pregnancy (208). Considering its perceived safety, anti-TNF-α could potentially function as a treatment option for PE by increasing the quantity of Treg cells and restoring the Th1/Th2 and Th17/Treg ratios.
17-hydroxyprogesterone caproate (17-OHPC), a progesterone derivative, is deemed secure for application in obstetrics. In a PE rat model, 17-OHPC induced an elevation in infant weight and a reduction in uterine artery resistance index (UARI) (209). Implementing an intervention such as 17-OHPC to enhance the current treatment for PE patients may have positive effects on both the mother and child. Furthermore, by activating glucocorticoid receptors, progesterone induces the proliferation of Treg cells and advances apoptosis in conventional T cells (210). Consequently, the use of the orally-administered progestogen or dydrogesterone may have the potential to alleviate the adverse pregnancy outcomes in women with PE (211–213).
IVIg is employed in the treatment of various immune-regulated disorders, organ transplantation, and systemic inflammatory conditions by virtue of its capacity to inhibit the proliferation of DCs, increase concentrations of anti-inflammatory IL-10, and diminish the functionality of pro-inflammatory T cells (214). Moreover, IVIg improves pregnancy outcomes by stimulating Tregs’ responses (215). IVIg therapy may, therefore, be contemplated as a prospective therapeutic intervention for PE. In women with a history of recurrent pregnancy loss (RPL) and unexplained infertility, the prevalence of PE was comparable with normal pregnant women when they were treated with prednisone and IVIg (216).
VD has the ability to affect many cell types, such as immune cells, specifically CD4+ T cells (217). The circulating concentrations of VD were considerably diminished in pregnant women with PE in comparison to those without (218–220). Furthermore, in comparison to pregnant women with VD levels above 20 ng/mL, those with VD deficiency (25(OH) D < 20 ng/mL) have a five-fold increased risk of developing PE (220). Patients with PE who received VD supplementation exhibited decreased levels of IFN-γ, TNF-α, IL-17, IL-6, and IL-23, whereas IL-10 and TGF-β levels elevated (218, 219). VD exerts its immunomodulatory effects through multifaceted mechanisms, including inhibition of Th1 and Th17 cells, up-regulation of Th2 cell expression, and facilitation of Treg cell proliferation (221–225). Therefore, VD could potentially be suggested as a strategy for regulating the systemic inflammatory response in PE. Moreover, exposure to sunlight (226) and exercise (227) contribute to the maintenance of Treg homeostasis. Nevertheless, the precise mechanisms of VD in human Treg cells remain obscure.
Receiving low-dose aspirin from 11-14 weeks reduced the occurrence of preterm PE in high-risk women compared to those who received a placebo (228). This discovery indicates that giving aspirin early on may decrease the likelihood of PE, potentially by enhancing placental development. A study done on mice revealed that aspirin greatly increased the percentage of functional CD4+CD25+FoxP3+ Treg cells (229). Patients with RPL associated with antiphospholipid syndrome (APS) showed elevated levels of serum cytokines, T cell phenotypes, and transcription factor gene expressions indicative of Th1 responses, while those indicative of Th2 responses were decreased (230). The imbalance between Th1 and Th2 could be restored in patients who responded well to the combination of low-molecular weight heparin (LMWH) and aspirin treatment (230). Moreover, aspirin functions through the production of aspirin-triggered lipoxin, which inhibits the effects of antiphospholipid antibodies on the migration of human trophoblasts and their interactions with endothelial cells (231). In other diseases, aspirin can exert therapeutic effects by regulating T cell function. One example is the potential for aspirin to mitigate the progression of atherosclerosis through restoring balance to the Th17/Treg axis (232). Additionally, aspirin could potentially suppress the autoimmune reaction in atherosclerosis by promoting the expansion of CD4+CD25+FOXP3+ Treg cells (233). In studies on experimental autoimmune encephalomyelitis (EAE), aspirin could enhance Tregs while reducing Th1 and Th17 responses (234, 235). This includes an increase in the levels of FoxP3 and IL-4 in T cells, as well as inhibiting the differentiation of naive T cells into Th17 and Th1 cells (234, 235). The above findings indicate that aspirin may play a role in the treatment of PE by targeting T cells.
Elevated levels of indicators for oxidative stress and reduced levels of antioxidants (such as vitamin E, vitamin C, and lycopene) in women with PE indicate that oxidative stress markers are significantly involved in the development of PE (236–238). AC-11 (AC-11®, hot-water extract of U. tomentosa) has a potential antioxidant effect (239). The administration of AC-11 resulted in a significant decrease in blood pressure and a significant down-regulation of CD8+ T cells and CD8/CD4 ratio in PE mice models induced by angiotensin II, compared with healthy pregnant animals (239). A survey of existing research suggests that the products of the kynurenine (Kyn) pathway have the ability to promote T cell differentiation into Treg cells and trigger the apoptosis of Th1 cells (240). Therefore, the Kyn pathway may be a potential therapeutic target for PE due to the antioxidant effect of its metabolites (240). Moreover, the well-established powerful antioxidant pentoxifylline can mitigate oxidative stress and enhance placentation (241). Its function is to maintain the balance between Th1 and Th2 immunity, reduce Th1-type immune responses, and promote a shift towards Th2 immune reactions (242). A different type of antioxidant, known as heme oxygenase-1 (HO-1), has the ability to stimulate the release of Th2 cytokines (243). Moreover, one study has shown that severe PE is linked to elevated T-cell-endothelium adhesion, and effective antioxidants can prevent this impact (244). Clinically achievable concentrations of antioxidants in the circulation can be attained through oral intake of vitamins E and C, or by intravenous administration of N-acetylcysteine, as demonstrated by this study (244). Accordingly, supplementing with antioxidants for PE patients may be a simple and effective treatment.
The promotion of alloantigen-specific Treg cells is a potential preventive and therapeutic strategy for PE. This could be achieved by increasing exposure to paternal antigen in high-risk population. In addition, medications that enhance the activity of Treg cells by targeting immune checkpoints, such as CTLA-4Ig (245), may represent viable alternatives for the treatment of PE. To develop therapeutics that target immune checkpoints, it is imperative to investigate costimulatory and inhibitory molecules that are unique to antigen-specific Treg cells. Additional investigation about Treg cells at the single-cell level may yield valuable knowledge regarding the progression of immunotherapeutic approach for PE.
In rodent models of PE, proof-of-concept trials have already demonstrated that PD-L1 Fc (246), CD28 superligand (247), and low dose IL-2 (248, 249) were efficacious biological agents for increasing the percentage and stability of Treg cells. In light of the swift advancements in Treg cell immunology, including the development of numerous intervention therapies and the application of flow cytometry for peripheral blood diagnosis, it is important to investigate Treg cell therapy in high-risk women. Prior to or in the early phases of pregnancy, it is essential to develop diagnostic tools and treatment modailities that can halt the progression of disease and prevent fetal or placental damage. Regarding the “window of opportunity” for timely and effective PE treatment, since Treg cells play an essential role in implantation and placentation during early pregnancy, initiating treatments before conception or as soon as pregnancy is identified is the optimal choice (10).
There is a limitation in this review due to the insufficient distinction between early-onset and late-onset PE T cell profiles, which arises from the lack of specific studies. In conclusion, a decreased maternal tolerance to paternal antigens is associated with preeclamptic pregnancy. This decrease can be attributed to several factors, including compromised placentation, inadequate oxygen supply to the placenta, heightened activation of the immune system both locally and systematically, a bias to inflammatory T cell response, and increased inflammation. Notwithstanding the copious body of evidence demonstrating the substantial impact of T cell immunity in PE, insufficient endeavors persist to implement these discoveries and improve the standard of care for women afflicted with this obstetric syndrome. As a result, subsequent investigations ought to strive to generate outcomes and frameworks with pragmatic implications, thereby advancing PE prevention, detection, and management.
XP: Data curation, Investigation, Project administration, Writing – original draft. ICO-A: Data curation, Investigation, Project administration, Writing – original draft. JK-K: Supervision, Validation, Writing – review & editing. XHY: Conceptualization, Data curation, Investigation, Project administration, Validation, Writing – original draft.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Brosens I, Puttemans P, Benagiano G. Placental bed research: I. The placental bed: from spiral arteries remodeling to the great obstetrical syndromes. Am J Obstet Gynecol. (2019) 221:437–56. doi: 10.1016/j.ajog.2019.05.044
2. Gestational hypertension and preeclampsia: ACOG practice bulletin, number 222. Obstet Gynecol. (2020) 135:e237–e60. doi: 10.1097/AOG.0000000000003891
3. Tranquilli AL, Dekker G, Magee L, Roberts J, Sibai BM, Steyn W, et al. The classification, diagnosis and management of the hypertensive disorders of pregnancy: A revised statement from the ISSHP. Pregnancy Hypertens. (2014) 4:97–104. doi: 10.1016/j.preghy.2014.02.001
4. Mol BWJ, Roberts CT, Thangaratinam S, Magee LA, de Groot CJM, Hofmeyr GJ. Pre-eclampsia. Lancet. (2016) 387:999–1011. doi: 10.1016/S0140-6736(15)00070-7
5. Khashu M, Narayanan M, Bhargava S, Osiovich H. Perinatal outcomes associated with preterm birth at 33 to 36 weeks’ gestation: a population-based cohort study. Pediatrics. (2009) 123:109–13. doi: 10.1542/peds.2007-3743
6. Matthiesen L, Berg G, Ernerudh J, Ekerfelt C, Jonsson Y, Sharma S. Immunology of preeclampsia. Chem Immunol Allergy. (2005) 89:49–61.
7. Apicella C, Ruano CSM, Mehats C, Miralles F, Vaiman D. The role of epigenetics in placental development and the etiology of preeclampsia. Int J Mol Sci. (2019) 20. doi: 10.3390/ijms20112837
8. Chakraborty D, Cui W, Rosario GX, Scott RL, Dhakal P, Renaud SJ, et al. HIF-KDM3A-MMP12 regulatory circuit ensures trophoblast plasticity and placental adaptations to hypoxia. Proc Natl Acad Sci U S A. (2016) 113:E7212–E21. doi: 10.1073/pnas.1612626113
9. Deng Z, Zhang L, Tang Q, Xu Y, Liu S, Li H. Circulating levels of IFN-γ, IL-1, IL-17 and IL-22 in pre-eclampsia: A systematic review and meta-analysis. Eur J Obstet Gynecol Reprod Biol. (2020) 248:211–21. doi: 10.1016/j.ejogrb.2020.03.039
10. Robertson SA, Green ES, Care AS, Moldenhauer LM, Prins JR, Hull ML, et al. Therapeutic potential of regulatory T cells in preeclampsia-opportunities and challenges. Front Immunol. (2019) 10:478. doi: 10.3389/fimmu.2019.00478
11. Alijotas-Reig J, Esteve-Valverde E, Ferrer-Oliveras R, Llurba E, Gris JM. Tumor necrosis factor-alpha and pregnancy: focus on biologics. Updated Compr Review. Clin Rev Allergy Immunol. (2017) 53:40–53. doi: 10.1007/s12016-016-8596-x
12. Scholz C, Toth B, Santoso L, Kuhn C, Franz M, Mayr D, et al. Distribution and maturity of dendritic cells in diseases of insufficient placentation. Am J Reprod Immunol. (2008) 60:238–45. doi: 10.1111/j.1600-0897.2008.00619.x
13. Ushida T, Macdonald-Goodfellow SK, Quadri A, Tse MY, Winn LM, Pang SC, et al. Persistence of risk factors associated with maternal cardiovascular disease following aberrant inflammation in rat pregnancy. Biol Reprod. (2017) 97:143–52. doi: 10.1093/biolre/iox072
14. Santner-Nanan B, Peek MJ, Khanam R, Richarts L, Zhu E, Fazekas de St Groth B, et al. Systemic increase in the ratio between Foxp3+ and IL-17-producing CD4+ T cells in healthy pregnancy but not in preeclampsia. J Immunol. (2009) 183:7023–30. doi: 10.4049/jimmunol.0901154
15. Azizieh F, Raghupathy R, Makhseed M. Maternal cytokine production patterns in women with pre-eclampsia. Am J Reprod Immunol. (2005) 54:30–7. doi: 10.1111/j.1600-0897.2005.00278.x
16. Germain SJ, Sacks GP, Sooranna SR, Sargent IL, Redman CW. Systemic inflammatory priming in normal pregnancy and preeclampsia: the role of circulating syncytiotrophoblast microparticles. J Immunol. (2007) 178:5949–56. doi: 10.4049/jimmunol.178.9.5949
17. Chen Z, Zhou X, Qu H, Zhang X, Kwak-Kim J, Wang W. Characteristics and functions of memory regulatory T cells in normal pregnancy cycle and pregnancy complications. J Reprod Immunol. (2024) 163:104235. doi: 10.1016/j.jri.2024.104235
18. Xia Y, Zhou CC, Ramin SM, Kellems RE. Angiotensin receptors, autoimmunity, and preeclampsia. J Immunol. (2007) 179:3391–5. doi: 10.4049/jimmunol.179.6.3391
19. Green S, Politis M, Rallis KS, Saenz de Villaverde Cortabarria A, Efthymiou A, Mureanu N, et al. Regulatory T cells in pregnancy adverse outcomes: A systematic review and meta-analysis. Front Immunol. (2021) 12:737862. doi: 10.3389/fimmu.2021.737862
20. Nancy P, Erlebacher A. T cell behavior at the maternal-fetal interface. Int J Dev Biol. (2014) 58:189–98. doi: 10.1387/ijdb.140054ae
21. Tilburgs T, Claas FH, Scherjon SA. Elsevier Trophoblast Research Award Lecture: Unique properties of decidual T cells and their role in immune regulation during human pregnancy. Placenta. (2010) 31 Suppl:S82–6. doi: 10.1016/j.placenta.2010.01.007
22. Trowsdale J, Betz AG. Mother’s little helpers: mechanisms of maternal-fetal tolerance. Nat Immunol. (2006) 7:241–6. doi: 10.1038/ni1317
23. Ingman WV, Jones RL. Cytokine knockouts in reproduction: the use of gene ablation to dissect roles of cytokines in reproductive biology. Hum Reprod Update. (2008) 14:179–92. doi: 10.1093/humupd/dmm042
24. Howerton CL, Bale TL. Prenatal programing: at the intersection of maternal stress and immune activation. Horm Behav. (2012) 62:237–42. doi: 10.1016/j.yhbeh.2012.03.007
25. Krishnan L, Nguyen T, McComb S. From mice to women: the conundrum of immunity to infection during pregnancy. J Reprod Immunol. (2013) 97:62–73. doi: 10.1016/j.jri.2012.10.015
26. Gohner C, Plosch T, Faas MM. Immune-modulatory effects of syncytiotrophoblast extracellular vesicles in pregnancy and preeclampsia. Placenta. (2017) 60 Suppl 1:S41–51. doi: 10.1016/j.placenta.2017.06.004
27. Fujita H. The role of IL-22 and Th22 cells in human skin diseases. J Dermatol Sci. (2013) 72:3–8. doi: 10.1016/j.jdermsci.2013.04.028
28. Wang W, Sung N, Gilman-Sachs A, Kwak-Kim J. T helper (Th) cell profiles in pregnancy and recurrent pregnancy losses: th1/th2/th9/th17/th22/tfh cells. Front Immunol. (2020) 11:2025. doi: 10.3389/fimmu.2020.02025
29. Danaii S, Ghorbani F, Ahmadi M, Abbaszadeh H, Koushaeian L, Soltani-Zangbar MS, et al. IL-10-producing B cells play important role in the pathogenesis of recurrent pregnancy loss. Int Immunopharmacol. (2020) 87:106806. doi: 10.1016/j.intimp.2020.106806
30. Azizi R, Soltani-Zangbar MS, Sheikhansari G, Pourmoghadam Z, Mehdizadeh A, Mahdipour M, et al. Metabolic syndrome mediates inflammatory and oxidative stress responses in patients with recurrent pregnancy loss. J Reprod Immunol. (2019) 133:18–26. doi: 10.1016/j.jri.2019.05.001
31. Tilburgs T, Schonkeren D, Eikmans M, Nagtzaam NM, Datema G, Swings GM, et al. Human decidual tissue contains differentiated CD8+ effector-memory T cells with unique properties. J Immunol. (2010) 185:4470–7. doi: 10.4049/jimmunol.0903597
32. Powell RM, Lissauer D, Tamblyn J, Beggs A, Cox P, Moss P, et al. Decidual T cells exhibit a highly differentiated phenotype and demonstrate potential fetal specificity and a strong transcriptional response to IFN. J Immunol. (2017) 199:3406–17. doi: 10.4049/jimmunol.1700114
33. Wang SC, Li YH, Piao HL, Hong XW, Zhang D, Xu YY, et al. PD-1 and Tim-3 pathways are associated with regulatory CD8+ T-cell function in decidua and maintenance of normal pregnancy. Cell Death Dis. (2015) 6:e1738. doi: 10.1038/cddis.2015.112
34. Taglauer ES, Trikhacheva AS, Slusser JG, Petroff MG. Expression and function of PDCD1 at the human maternal-fetal interface. Biol Reprod. (2008) 79:562–9. doi: 10.1095/biolreprod.107.066324
35. Meggyes M, Miko E, Szigeti B, Farkas N, Szereday L. The importance of the PD-1/PD-L1 pathway at the maternal-fetal interface. BMC Pregnancy Childbirth. (2019) 19:74. doi: 10.1186/s12884-019-2218-6
36. Petroff MG, Chen L, Phillips TA, Azzola D, Sedlmayr P, Hunt JS. B7 family molecules are favorably positioned at the human maternal-fetal interface. Biol Reprod. (2003) 68:1496–504. doi: 10.1095/biolreprod.102.010058
37. Guleria I, Khosroshahi A, Ansari MJ, Habicht A, Azuma M, Yagita H, et al. A critical role for the programmed death ligand 1 in fetomaternal tolerance. J Exp Med. (2005) 202:231–7. doi: 10.1084/jem.20050019
38. Nagamatsu T, Schust DJ, Sugimoto J, Barrier BF. Human decidual stromal cells suppress cytokine secretion by allogenic CD4+ T cells via PD-1 ligand interactions. Hum Reprod. (2009) 24:3160–71. doi: 10.1093/humrep/dep308
39. Arck PC, Hecher K. Fetomaternal immune cross-talk and its consequences for maternal and offspring’s health. Nat Med. (2013) 19:548–56. doi: 10.1038/nm.3160
40. D’Addio F, Riella LV, Mfarrej BG, Chabtini L, Adams LT, Yeung M, et al. The link between the PDL1 costimulatory pathway and Th17 in fetomaternal tolerance. J Immunol. (2011) 187:4530–41. doi: 10.4049/jimmunol.1002031
41. Tilburgs T, Strominger JL. CD8+ effector T cells at the fetal-maternal interface, balancing fetal tolerance and antiviral immunity. Am J Reprod Immunol. (2013) 69:395–407. doi: 10.1111/aji.2013.69.issue-4
42. Guerin LR, Prins JR, Robertson SA. Regulatory T-cells and immune tolerance in pregnancy: a new target for infertility treatment? Hum Reprod Update. (2009) 15:517–35. doi: 10.1093/humupd/dmp004
43. Gagliani N, Magnani CF, Huber S, Gianolini ME, Pala M, Licona-Limon P, et al. Coexpression of CD49b and LAG-3 identifies human and mouse T regulatory type 1 cells. Nat Med. (2013) 19:739–46. doi: 10.1038/nm.3179
44. Salvany-Celades M, van der Zwan A, Benner M, Setrajcic-Dragos V, Bougleux Gomes HA, Iyer V, et al. Three types of functional regulatory T cells control T cell responses at the human maternal-fetal interface. Cell Rep. (2019) 27:2537–47.e5. doi: 10.1016/j.celrep.2019.04.109
45. Aluvihare VR, Kallikourdis M, Betz AG. Regulatory T cells mediate maternal tolerance to the fetus. Nat Immunol. (2004) 5:266–71. doi: 10.1038/ni1037
46. Jameel S, Bhuwalka R, Begum M, Bonu R, Jahan P. Circulating levels of cytokines (IL-6, IL-10 and TGF- β) and CD4(+)CD25(+)FOXP3(+)Treg cell population in recurrent pregnancy loss. Reprod Biol. (2024) 24:100842. doi: 10.1016/j.repbio.2023.100842
47. Xiong YH, Yuan Z, He L. Effects of estrogen on CD4(+) CD25(+) regulatory T cell in peripheral blood during pregnancy. Asian Pac J Trop Med. (2013) 6:748–52. doi: 10.1016/S1995-7645(13)60131-5
48. Dimova T, Nagaeva O, Stenqvist AC, Hedlund M, Kjellberg L, Strand M, et al. Maternal Foxp3 expressing CD4+ CD25+ and CD4+ CD25- regulatory T-cell populations are enriched in human early normal pregnancy decidua: a phenotypic study of paired decidual and peripheral blood samples. Am J Reprod Immunol. (2011) 66 Suppl 1:44–56. doi: 10.1111/j.1600-0897.2011.01046.x
49. Tilburgs T, Roelen DL, van der Mast BJ, van Schip JJ, Kleijburg C, de-Groot-Swings GM, et al. Differential distribution of CD4(+)CD25(bright) and CD8(+)CD28(-) T-cells in decidua and maternal blood during human pregnancy. Placenta. (2006) 27 Suppl A:S47–53. doi: 10.1016/j.placenta.2005.11.008
50. Robertson SA, Care AS, Moldenhauer LM. Regulatory T cells in embryo implantation and the immune response to pregnancy. J Clin Invest. (2018) 128:4224–35. doi: 10.1172/JCI122182
51. Luo L, Zeng X, Huang Z, Luo S, Qin L, Li S. Reduced frequency and functional defects of CD4(+)CD25(high)CD127(low/-) regulatory T cells in patients with unexplained recurrent spontaneous abortion. Reprod Biol Endocrinol. (2020) 18:62. doi: 10.1186/s12958-020-00619-7
52. Polgar K, Hill JA. Identification of the white blood cell populations responsible for Th1 immunity to trophoblast and the timing of the response in women with recurrent pregnancy loss. Gynecol Obstet Invest. (2002) 53:59–64. doi: 10.1159/000049413
53. Hill JA 3rd, Choi BC. Immunodystrophism: evidence for a novel alloimmune hypothesis for recurrent pregnancy loss involving Th1-type immunity to trophoblast. Semin Reprod Med. (2000) 18:401–5. doi: 10.1055/s-2000-13730
54. Mjosberg J, Berg G, Jenmalm MC, Ernerudh J. FOXP3+ regulatory T cells and T helper 1, T helper 2, and T helper 17 cells in human early pregnancy decidua. Biol Reprod. (2010) 82:698–705. doi: 10.1095/biolreprod.109.081208
55. Samstein RM, Josefowicz SZ, Arvey A, Treuting PM, Rudensky AY. Extrathymic generation of regulatory T cells in placental mammals mitigates maternal-fetal conflict. Cell. (2012) 150:29–38. doi: 10.1016/j.cell.2012.05.031
56. Li L, Tu J, Jiang Y, Zhou J, Schust DJ. Regulatory T cells decrease invariant natural killer T cell-mediated pregnancy loss in mice. Mucosal Immunol. (2017) 10:613–23. doi: 10.1038/mi.2016.84
57. Zhang YH, Sun HX. Immune checkpoint molecules in pregnancy: Focus on regulatory T cells. Eur J Immunol. (2020) 50:160–9. doi: 10.1002/eji.201948382
58. Vacca P, Cantoni C, Vitale M, Prato C, Canegallo F, Fenoglio D, et al. Crosstalk between decidual NK and CD14+ myelomonocytic cells results in induction of Tregs and immunosuppression. Proc Natl Acad Sci U S A. (2010) 107:11918–23. doi: 10.1073/pnas.1001749107
59. Kimura A, Kishimoto T. IL-6: regulator of Treg/Th17 balance. Eur J Immunol. (2010) 40:1830–5. doi: 10.1002/eji.201040391
60. Anvari F, Dabagh-Gorjani F, Soltani-Zangbar MS, Kamali-Sarvestani E, Malek-Hosseini Z, Gharesi-Fard B. Investigating the association of IL-17A and IL-17F with susceptibility to pre-eclampsia in Iranian women. Iran J Immunol. (2015) 12:117–28.
61. Yang J, Sundrud MS, Skepner J, Yamagata T. Targeting Th17 cells in autoimmune diseases. Trends Pharmacol Sci. (2014) 35:493–500. doi: 10.1016/j.tips.2014.07.006
62. Cao W, Wang X, Chen T, Zhu H, Xu W, Zhao S, et al. The expression of notch/notch ligand, IL-35, IL-17, and th17/treg in preeclampsia. Dis Markers. (2015) 2015:316182. doi: 10.1155/2015/316182
63. Mathur AN, Chang HC, Zisoulis DG, Stritesky GL, Yu Q, O’Malley JT, et al. Stat3 and Stat4 direct development of IL-17-secreting Th cells. J Immunol. (2007) 178:4901–7. doi: 10.4049/jimmunol.178.8.4901
64. Nakashima A, Ito M, Yoneda S, Shiozaki A, Hidaka T, Saito S. Circulating and decidual Th17 cell levels in healthy pregnancy. Am J Reprod Immunol. (2010) 63:104–9. doi: 10.1111/j.1600-0897.2009.00771.x
65. Ito M, Nakashima A, Hidaka T, Okabe M, Bac ND, Ina S, et al. A role for IL-17 in induction of an inflammation at the fetomaternal interface in preterm labour. J Reprod Immunol. (2010) 84:75–85. doi: 10.1016/j.jri.2009.09.005
66. Braga A, Neves E, Guimarães J, Braga J, Vasconcelos C. Th17/Regulatory T cells ratio evolution: A prospective study in a group of healthy pregnant women. J Reprod Immunol. (2022) 149:103468. doi: 10.1016/j.jri.2021.103468
67. Pongcharoen S, Supalap K. Interleukin-17 increased progesterone secretion by JEG-3 human choriocarcinoma cells. Am J Reprod Immunol. (2009) 61:261–4. doi: 10.1111/j.1600-0897.2009.00693.x
68. Pongcharoen S, Niumsup P, Sanguansermsri D, Supalap K, Butkhamchot P. The effect of interleukin-17 on the proliferation and invasion of JEG-3 human choriocarcinoma cells. Am J Reprod Immunol. (2006) 55:291–300. doi: 10.1111/j.1600-0897.2006.00366.x
69. Martinez-Garcia EA, Chavez-Robles B, Sanchez-Hernandez PE, Nunez-Atahualpa L, Martin-Maquez BT, Munoz-Gomez A, et al. IL-17 increased in the third trimester in healthy women with term labor. Am J Reprod Immunol. (2011) 65:99–103. doi: 10.1111/j.1600-0897.2010.00893.x
70. de Groot CJ, van der Mast BJ, Visser W, De Kuiper P, Weimar W, Van Besouw NM. Preeclampsia is associated with increased cytotoxic T-cell capacity to paternal antigens. Am J Obstet Gynecol. (2010) 203:496.e1–6. doi: 10.1016/j.ajog.2010.06.047
71. Saito S, Sakai M, Sasaki Y, Tanebe K, Tsuda H, Michimata T. Quantitative analysis of peripheral blood Th0, Th1, Th2 and the Th1:Th2 cell ratio during normal human pregnancy and preeclampsia. Clin Exp Immunol. (1999) 117:550–5. doi: 10.1046/j.1365-2249.1999.00997.x
72. Chaiworapongsa T, Gervasi MT, Refuerzo J, Espinoza J, Yoshimatsu J, Berman S, et al. Maternal lymphocyte subpopulations (CD45RA+ and CD45RO+) in preeclampsia. Am J Obstet Gynecol. (2002) 187:889–93. doi: 10.1067/mob.2002.127309
73. Molvarec A, Shiozaki A, Ito M, Toldi G, Stenczer B, Szarka A, et al. Increased prevalence of peripheral blood granulysin-producing cytotoxic T lymphocytes in preeclampsia. J Reprod Immunol. (2011) 91:56–63. doi: 10.1016/j.jri.2011.03.012
74. Wallace K, Cornelius DC, Scott J, Heath J, Moseley J, Chatman K, et al. CD4+ T cells are important mediators of oxidative stress that cause hypertension in response to placental ischemia. Hypertension. (2014) 64:1151–8. doi: 10.1161/HYPERTENSIONAHA.114.03590
75. Lok CA, Jebbink J, Nieuwland R, Faas MM, Boer K, Sturk A, et al. Leukocyte activation and circulating leukocyte-derived microparticles in preeclampsia. Am J Reprod Immunol. (2009) 61:346–59. doi: 10.1111/j.1600-0897.2009.00701.x
76. Shen C, Song Y, Fan W, Guo X, Li J, Zhao R. Changes in expression levels of immune cells and inflammatory cytokines in pre-eclampsia patients before and after delivery. J Reprod Immunol. (2023) 156:103812. doi: 10.1016/j.jri.2023.103812
77. Zare M, Namavar Jahromi B, Gharesi-Fard B. Analysis of the frequencies and functions of CD4(+)CD25(+)CD127(low/neg), CD4(+)HLA-G(+), and CD8(+)HLA-G(+) regulatory T cells in pre-eclampsia. J Reprod Immunol. (2019) 133:43–51. doi: 10.1016/j.jri.2019.06.002
78. Kieffer TEC, Scherjon SA, Faas MM, Prins JR. Lower activation of CD4(+) memory T cells in preeclampsia compared to healthy pregnancies persists postpartum. J Reprod Immunol. (2019) 136:102613. doi: 10.1016/j.jri.2019.102613
79. Hsu P, Santner-Nanan B, Joung S, Peek MJ, Nanan R. Expansion of CD4(+) HLA-G(+) T Cell in human pregnancy is impaired in pre-eclampsia. Am J Reprod Immunol. (2014) 71:217–28. doi: 10.1111/aji.2014.71.issue-3
80. Jacobsen DP, Lekva T, Moe K, Fjeldstad HES, Johnsen GM, Sugulle M, et al. Pregnancy and postpartum levels of circulating maternal sHLA-G in preeclampsia. J Reprod Immunol. (2021) 143:103249. doi: 10.1016/j.jri.2020.103249
81. Wedenoja S, Yoshihara M, Teder H, Sariola H, Gissler M, Katayama S, et al. Fetal HLA-G mediated immune tolerance and interferon response in preeclampsia. EBioMedicine. (2020) 59:102872. doi: 10.1016/j.ebiom.2020.102872
82. Lombardelli L, Aguerre-Girr M, Logiodice F, Kullolli O, Casart Y, Polgar B, et al. HLA-G5 induces IL-4 secretion critical for successful pregnancy through differential expression of ILT2 receptor on decidual CD4+ T cells and macrophages. J Immunol. (2013) 191:3651–62. doi: 10.4049/jimmunol.1300567
83. Du MR, Guo PF, Piao HL, Wang SC, Sun C, Jin LP, et al. Embryonic trophoblasts induce decidual regulatory T cell differentiation and maternal-fetal tolerance through thymic stromal lymphopoietin instructing dendritic cells. J Immunol. (2014) 192:1502–11. doi: 10.4049/jimmunol.1203425
84. Amodio G, Mugione A, Sanchez AM, Vigano P, Candiani M, Somigliana E, et al. HLA-G expressing DC-10 and CD4(+) T cells accumulate in human decidua during pregnancy. Hum Immunol. (2013) 74:406–11. doi: 10.1016/j.humimm.2012.11.031
85. Huang YH, Zozulya AL, Weidenfeller C, Schwab N, Wiendl H. T cell suppression by naturally occurring HLA-G-expressing regulatory CD4+ T cells is IL-10-dependent and reversible. J Leukoc Biol. (2009) 86:273–81. doi: 10.1189/jlb.1008649
86. Rieger L, Segerer S, Bernar T, Kapp M, Majic M, Morr AK, et al. Specific subsets of immune cells in human decidua differ between normal pregnancy and preeclampsia–a prospective observational study. Reprod Biol Endocrinol. (2009) 7:132. doi: 10.1186/1477-7827-7-132
87. Williams PJ, Bulmer JN, Searle RF, Innes BA, Robson SC. Altered decidual leucocyte populations in the placental bed in pre-eclampsia and foetal growth restriction: a comparison with late normal pregnancy. Reproduction. (2009) 138:177–84. doi: 10.1530/REP-09-0007
88. Stallmach T, Hebisch G, Orban P, Lü X. Aberrant positioning of trophoblast and lymphocytes in the feto-maternal interface with pre-eclampsia. Virchows Arch. (1999) 434:207–11. doi: 10.1007/s004280050329
89. Milosevic-Stevanovic J, Krstic M, Stefanovic M, Zivadinovic R, Vukomanovic P, Trajkovic-Dinic SP, et al. T lymphocytes in the third trimester decidua in preeclampsia. Hypertens Pregnancy. (2019) 38:52–7. doi: 10.1080/10641955.2019.1575393
90. Lager S, Sovio U, Eddershaw E, van der Linden MW, Yazar C, Cook E, et al. Abnormal placental CD8(+) T-cell infiltration is a feature of fetal growth restriction and pre-eclampsia. J Physiol. (2020) 598:5555–71. doi: 10.1113/tjp.v598.23
91. Kieffer TEC, Laskewitz A, Vledder A, Scherjon SA, Faas MM, Prins JR. Decidual memory T-cell subsets and memory T-cell stimulatory cytokines in early- and late-onset preeclampsia. Am J Reprod Immunol. (2020) 84:e13293. doi: 10.1111/aji.13293
92. Johnsen GM, Storvold GL, Alnaes-Katjavivi PH, Roald B, Golic M, Dechend R, et al. Lymphocyte characterization of decidua basalis spiral arteries with acute atherosis in preeclamptic and normotensive pregnancies. J Reprod Immunol. (2019) 132:42–8. doi: 10.1016/j.jri.2019.03.003
93. Lin H, Mosmann TR, Guilbert L, Tuntipopipat S, Wegmann TG. Synthesis of T helper 2-type cytokines at the maternal-fetal interface. J Immunol. (1993) 151:4562–73. doi: 10.4049/jimmunol.151.9.4562
94. Tsuda H, Michimata T, Sakai M, Nagata K, Nakamura M, Saito S. A novel surface molecule of Th2- and Tc2-type cells, CRTH2 expression on human peripheral and decidual CD4+ and CD8+ T cells during the early stage of pregnancy. Clin Exp Immunol. (2001) 123:105–11. doi: 10.1046/j.1365-2249.2001.01422.x
95. Darmochwal-Kolarz D, Rolinski J, Leszczynska-Goarzelak B, Oleszczuk J. The expressions of intracellular cytokines in the lymphocytes of preeclamptic patients. Am J Reprod Immunol. (2002) 48:381–6. doi: 10.1034/j.1600-0897.2002.01089.x
96. Kuwajima T, Suzuki S, Yoneyama Y, Sawa R, Asakura H, Araki T. Relation between plasma endothelin 1 levels and T helper 1: T helper 2 cell immunity in women with preeclampsia. Gynecol Obstet Invest. (2001) 52:260–3. doi: 10.1159/000052987
97. Rein DT, Schondorf T, Gohring UJ, Kurbacher CM, Pinto I, Breidenbach M, et al. Cytokine expression in peripheral blood lymphocytes indicates a switch to T(HELPER) cells in patients with preeclampsia. J Reprod Immunol. (2002) 54:133–42. doi: 10.1016/S0165-0378(01)00128-0
98. Darmochwal-Kolarz D, Leszczynska-Gorzelak B, Rolinski J, Oleszczuk J. T helper 1- and T helper 2-type cytokine imbalance in pregnant women with pre-eclampsia. Eur J Obstet Gynecol Reprod Biol. (1999) 86:165–70. doi: 10.1016/S0301-2115(99)00065-2
99. Daniel Y, Kupferminc MJ, Baram A, Jaffa AJ, Fait G, Wolman I, et al. Plasma interleukin-12 is elevated in patients with preeclampsia. Am J Reprod Immunol. (1998) 39:376–80. doi: 10.1111/j.1600-0897.1998.tb00372.x
100. Sakai M, Tsuda H, Tanebe K, Sasaki Y, Saito S. Interleukin-12 secretion by peripheral blood mononuclear cells is decreased in normal pregnant subjects and increased in preeclamptic patients. Am J Reprod Immunol. (2002) 47:91–7. doi: 10.1034/j.1600-0897.2002.1o020.x
101. Ohkuchi A, Minakami H, Aoya T, Haga T, Kimura H, Suzuki M, et al. Expansion of the fraction of Th1 cells in women with preeclampsia: inverse correlation between the percentage of Th1 cells and the plasma level of PAI-2. Am J Reprod Immunol. (2001) 46:252–9. doi: 10.1034/j.1600-0897.2001.d01-10.x
102. Cooper MA, Fehniger TA, Caligiuri MA. The biology of human natural killer-cell subsets. Trends Immunol. (2001) 22:633–40. doi: 10.1016/S1471-4906(01)02060-9
103. Kadowaki N, Antonenko S, Ho S, Rissoan MC, Soumelis V, Porcelli SA, et al. Distinct cytokine profiles of neonatal natural killer T cells after expansion with subsets of dendritic cells. J Exp Med. (2001) 193:1221–6. doi: 10.1084/jem.193.10.1221
104. Chan WL, Pejnovic N, Lee CA, Al-Ali NA. Human IL-18 receptor and ST2L are stable and selective markers for the respective type 1 and type 2 circulating lymphocytes. J Immunol. (2001) 167:1238–44. doi: 10.4049/jimmunol.167.3.1238
105. Saito S, Umekage H, Sakamoto Y, Sakai M, Tanebe K, Sasaki Y, et al. Increased T-helper-1-type immunity and decreased T-helper-2-type immunity in patients with preeclampsia. Am J Reprod Immunol. (1999) 41:297–306. doi: 10.1111/j.1600-0897.1999.tb00442.x
106. Romao-Veiga M, Ribeiro VR, Matias ML, Nunes PR, Romagnoli GG, Peracoli JC, et al. DAMPs are able to skew CD4(+) T cell subsets and increase the inflammatory profile in pregnant women with preeclampsia. J Reprod Immunol. (2022) 149:103470. doi: 10.1016/j.jri.2021.103470
107. Dos Santos Fagundes I, Brendler EP, Nunes Erthal I, Eder Ribeiro RJ, Caron-Lienert RS, MaChado DC, et al. Total Th1/Th2 cytokines profile from peripheral blood lymphocytes in normal pregnancy and preeclampsia syndrome. Hypertens Pregnancy. (2022) 41:15–22. doi: 10.1080/10641955.2021.2008424
108. Wilczyński JR, Tchórzewski H, Głowacka E, Banasik M, Lewkowicz P, Szpakowski M, et al. Cytokine secretion by decidual lymphocytes in transient hypertension of pregnancy and pre-eclampsia. Mediators Inflamm. (2002) 11:105–11. doi: 10.1080/09629350220131962
109. Hennessy A, Pilmore HL, Simmons LA. Painter DM. A deficiency placental IL-10 preeclampsia. J Immunol. (1999) 163:3491–5. doi: 10.4049/jimmunol.163.6.3491
110. Rein DT, Breidenbach M, Hönscheid B, Friebe-Hoffmann U, Engel H, Göhring UJ, et al. Preeclamptic women are deficient of interleukin-10 as assessed by cytokine release of trophoblast cells in vitro. Cytokine. (2003) 23:119–25. doi: 10.1016/S1043-4666(03)00220-5
111. Dong M, He J, Wang Z, Xie X, Wang H. Placental imbalance of Th1- and Th2-type cytokines in preeclampsia. Acta Obstet Gynecol Scand. (2005) 84:788–93. doi: 10.1111/j.0001-6349.2005.00714.x
112. Morita K, Tsuda S, Kobayashi E, Hamana H, Tsuda K, Shima T, et al. Analysis of TCR repertoire and PD-1 expression in decidual and peripheral CD8(+) T cells reveals distinct immune mechanisms in miscarriage and preeclampsia. Front Immunol. (2020) 11:1082. doi: 10.3389/fimmu.2020.01082
113. Zhao Y, Zhang X, Du N, Sun H, Chen L, Bao H, et al. Immune checkpoint molecules on T cell subsets of pregnancies with preeclampsia and gestational diabetes mellitus. J Reprod Immunol. (2020) 142:103208. doi: 10.1016/j.jri.2020.103208
114. Jianjun Z, Yali H, Zhiqun W, Mingming Z, Xia Z. Imbalance of T-cell transcription factors contributes to the Th1 type immunity predominant in pre-eclampsia. Am J Reprod Immunol. (2010) 63:38–45. doi: 10.1111/j.1600-0897.2009.00763.x
115. Ribeiro VR, Romao-Veiga M, Romagnoli GG, Matias ML, Nunes PR, Borges VTM, et al. Association between cytokine profile and transcription factors produced by T-cell subsets in early- and late-onset pre-eclampsia. Immunology. (2017) 152:163–73. doi: 10.1111/imm.2017.152.issue-1
116. Toldi G, Vásárhelyi ZE, Rigó J Jr., Orbán C, Tamássy Z, Bajnok A, et al. Prevalence of regulatory T-cell subtypes in preeclampsia. Am J Reprod Immunol. (2015) 74:110–5. doi: 10.1111/aji.2015.74.issue-2
117. Darmochwal-Kolarz D, Saito S, Tabarkiewicz J, Kolarz B, Rolinski J, Leszczynska-Gorzelak B, et al. Apoptosis signaling is altered in CD4+CD25+FoxP3+ T regulatory lymphocytes in pre-eclampsia. Int J Mol Sci. (2012) 13:6548–60. doi: 10.3390/ijms13066548
118. Nguyen TA, Kahn DA, Loewendorf AI. Maternal-Fetal rejection reactions are unconstrained in preeclamptic women. PloS One. (2017) 12:e0188250. doi: 10.1371/journal.pone.0188250
119. Wagner MI, Jöst M, Spratte J, Schaier M, Mahnke K, Meuer S, et al. Differentiation of ICOS+ and ICOS- recent thymic emigrant regulatory T cells (RTE T regs) during normal pregnancy, pre-eclampsia and HELLP syndrome. Clin Exp Immunol. (2016) 183:129–42. doi: 10.1111/cei.12693
120. Darmochwal-Kolarz D, Kludka-Sternik M, Tabarkiewicz J, Kolarz B, Rolinski J, Leszczynska-Gorzelak B, et al. The predominance of Th17 lymphocytes and decreased number and function of Treg cells in preeclampsia. J Reprod Immunol. (2012) 93:75–81. doi: 10.1016/j.jri.2012.01.006
121. Toldi G, Rigó J Jr., Stenczer B, Vásárhelyi B, Molvarec A. Increased prevalence of IL-17-producing peripheral blood lymphocytes in pre-eclampsia. Am J Reprod Immunol. (2011) 66:223–9. doi: 10.1111/j.1600-0897.2011.00987.x
122. Eghbal-Fard S, Yousefi M, Heydarlou H, Ahmadi M, Taghavi S, Movasaghpour A, et al. The imbalance of Th17/Treg axis involved in the pathogenesis of preeclampsia. J Cell Physiol. (2019) 234:5106–16. doi: 10.1002/jcp.v234.4
123. Zhang Z, Liu H, Shi Y, Xu N, Wang Y, Li A, et al. Increased circulating Th22 cells correlated with Th17 cells in patients with severe preeclampsia. Hypertens Pregnancy. (2017) 36:100–7. doi: 10.1080/10641955.2016.1239737
124. Salazar Garcia MD, Mobley Y, Henson J, Davies M, Skariah A, Dambaeva S, et al. Early pregnancy immune biomarkers in peripheral blood may predict preeclampsia. J Reprod Immunol. (2018) 125:25–31. doi: 10.1016/j.jri.2017.10.048
125. Toldi G, Saito S, Shima T, Halmos A, Veresh Z, Vásárhelyi B, et al. The frequency of peripheral blood CD4+ CD25high FoxP3+ and CD4+ CD25- FoxP3+ regulatory T cells in normal pregnancy and pre-eclampsia. Am J Reprod Immunol. (2012) 68:175–80. doi: 10.1111/j.1600-0897.2012.01145.x
126. Zeng B, Kwak-Kim J, Liu Y, Liao AH. Treg cells are negatively correlated with increased memory B cells in pre-eclampsia while maintaining suppressive function on autologous B-cell proliferation. Am J Reprod Immunol. (2013) 70:454–63. doi: 10.1111/aji.2013.70.issue-6
127. Moreno-Eutimio MA, Tovar-Rodríguez JM, Vargas-Avila K, Nieto-Velázquez NG, Frías-De-León MG, Sierra-Martinez M, et al. Increased serum levels of inflammatory mediators and low frequency of regulatory T cells in the peripheral blood of preeclamptic Mexican women. BioMed Res Int. (2014) 2014:413249. doi: 10.1155/2014/413249
128. Hu M, Eviston D, Hsu P, Mariño E, Chidgey A, Santner-Nanan B, et al. Decreased maternal serum acetate and impaired fetal thymic and regulatory T cell development in preeclampsia. Nat Commun. (2019) 10:3031. doi: 10.1038/s41467-019-10703-1
129. Daraei N, Ghafourian M, Ghadiri A, Amari A, Najafian M, Rokhafrooz S. Evaluation of exhausted regulatory T cells in preeclampsia. Iran J Immunol. (2019) 16:163–9. doi: 10.22034/IJI.2019.80259
130. Ding H, Dai Y, Lei Y, Wang Z, Liu D, Li R, et al. Upregulation of CD81 in trophoblasts induces an imbalance of Treg/Th17 cells by promoting IL-6 expression in preeclampsia. Cell Mol Immunol. (2019) 16:302–12. doi: 10.1038/s41423-018-0186-9
131. Chen J, Zhao L, Wang D, Xu Y, Gao H, Tan W, et al. Contribution of regulatory T cells to immune tolerance and association of microRNA−210 and Foxp3 in preeclampsia. Mol Med Rep. (2019) 19:1150–8. doi: 10.3892/mmr.2018.9733
132. Zare M, Doroudchi M, Gharesi-Fard B. Altered frequencies of CD4+ CD25+ Foxp3+ and CD8+ CD25+ Foxp3+ Regulatory T cells in pre-eclampsia. Iran J Allergy Asthma Immunol. (2018) 17:540–7.
133. Wang Y, Liu Y, Shu C, Wan J, Shan Y, Zhi X, et al. Inhibition of pregnancy-associated granulocytic myeloid-derived suppressor cell expansion and arginase-1 production in preeclampsia. J Reprod Immunol. (2018) 127:48–54. doi: 10.1016/j.jri.2018.05.002
134. Li J, Huang L, Wang S, Zhang Z. The prevalence of regulatory T and dendritic cells is altered in peripheral blood of women with pre-eclampsia. Pregnancy Hypertens. (2019) 17:233–40. doi: 10.1016/j.preghy.2019.07.003
135. Meggyes M, Miko E, Lajko A, Csiszar B, Sandor B, Matrai P, et al. Involvement of the PD-1/PD-L1 co-inhibitory pathway in the pathogenesis of the inflammatory stage of early-onset preeclampsia. Int J Mol Sci. (2019) 20. doi: 10.3390/ijms20030583
136. Nagayama S, Ohkuchi A, Shirasuna K, Takahashi K, Suzuki H, Hirashima C, et al. The frequency of peripheral blood CD4(+)FoxP3(+) regulatory T cells in women with pre-eclampsia and those with high-risk factors for pre-eclampsia. Hypertens Pregnancy. (2015) 34:443–55. doi: 10.3109/10641955.2015.1065884
137. Vianna P, Mondadori AG, Bauer ME, Dornfeld D, Chies JA. HLA-G and CD8+ regulatory T cells in the inflammatory environment of pre-eclampsia. Reproduction. (2016) 152:741–51. doi: 10.1530/REP-15-0608
138. Yu J, Qian L, Wu F, Li M, Chen W, Wang H. Decreased frequency of peripheral blood CD8(+)CD25(+)FoxP3(+)regulatory T cells correlates with IL-33 levels in pre-eclampsia. Hypertens Pregnancy. (2017) 36:217–25. doi: 10.1080/10641955.2017.1302470
139. Wang J, Wen ZQ, Cheng XY, Mei TY, Chen ZF, Su LX. siRNA−mediated knockdown of T−bet and RORγt contributes to decreased inflammation in pre−eclampsia. Mol Med Rep. (2017) 16:6368–75. doi: 10.3892/mmr.2017.7348
140. Steinborn A, Schmitt E, Kisielewicz A, Rechenberg S, Seissler N, Mahnke K, et al. Pregnancy-associated diseases are characterized by the composition of the systemic regulatory T cell (Treg) pool with distinct subsets of Tregs. Clin Exp Immunol. (2012) 167:84–98. doi: 10.1111/j.1365-2249.2011.04493.x
141. Sasaki Y, Darmochwal-Kolarz D, Suzuki D, Sakai M, Ito M, Shima T, et al. Proportion of peripheral blood and decidual CD4(+) CD25(bright) regulatory T cells in pre-eclampsia. Clin Exp Immunol. (2007) 149:139–45. doi: 10.1111/j.1365-2249.2007.03397.x
142. Steinborn A, Haensch GM, Mahnke K, Schmitt E, Toermer A, Meuer S, et al. Distinct subsets of regulatory T cells during pregnancy: is the imbalance of these subsets involved in the pathogenesis of preeclampsia? Clin Immunol. (2008) 129:401–12. doi: 10.1016/j.clim.2008.07.032
143. Prins JR, Boelens HM, Heimweg J, van der Heide S, Dubois AE, Van Oosterhout AJ, et al. Preeclampsia is associated with lower percentages of regulatory T cells in maternal blood. Hypertens Pregnancy. (2009) 28:300–11. doi: 10.1080/10641950802601237
144. Darmochwal-Kolarz D, Saito S, Rolinski J, Tabarkiewicz J, Kolarz B, Leszczynska-Gorzelak B, et al. Activated T lymphocytes in pre-eclampsia. Am J Reprod Immunol. (2007) 58:39–45. doi: 10.1111/j.1600-0897.2007.00489.x
145. Paeschke S, Chen F, Horn N, Fotopoulou C, Zambon-Bertoja A, Sollwedel A, et al. Pre-eclampsia is not associated with changes in the levels of regulatory T cells in peripheral blood. Am J Reprod Immunol. (2005) 54:384–9. doi: 10.1111/j.1600-0897.2005.00334.x
146. Hu D, Chen Y, Zhang W, Wang H, Wang Z, Dong M. Alteration of peripheral CD4+CD25+ regulatory T lymphocytes in pregnancy and pre-eclampsia. Acta Obstet Gynecol Scand. (2008) 87:190–4. doi: 10.1080/00016340701823991
147. Feger U, Tolosa E, Huang YH, Waschbisch A, Biedermann T, Melms A, et al. HLA-G expression defines a novel regulatory T-cell subset present in human peripheral blood and sites of inflammation. Blood. (2007) 110:568–77. doi: 10.1182/blood-2006-11-057125
148. Pankratz S, Bittner S, Herrmann AM, Schuhmann MK, Ruck T, Meuth SG, et al. Human CD4+ HLA-G+ regulatory T cells are potent suppressors of graft-versus-host disease in vivo. FASEB J. (2014) 28:3435–45. doi: 10.1096/fj.14-251074
149. Boij R, Mjösberg J, Svensson-Arvelund J, Hjorth M, Berg G, Matthiesen L, et al. Regulatory T-cell subpopulations in severe or early-onset preeclampsia. Am J Reprod Immunol. (2015) 74:368–78. doi: 10.1111/aji.2015.74.issue-4
150. Dimeloe S, Nanzer A, Ryanna K, Hawrylowicz C. Regulatory T cells, inflammation and the allergic response-The role of glucocorticoids and Vitamin D. J Steroid Biochem Mol Biol. (2010) 120:86–95. doi: 10.1016/j.jsbmb.2010.02.029
151. Karagiannidis C, Akdis M, Holopainen P, Woolley NJ, Hense G, Rückert B, et al. Glucocorticoids upregulate FOXP3 expression and regulatory T cells in asthma. J Allergy Clin Immunol. (2004) 114:1425–33. doi: 10.1016/j.jaci.2004.07.014
152. Navarro J, Aristimuño C, Sánchez-Ramón S, Vigil D, Martínez-Ginés ML, Fernández-Cruz E, et al. Circulating dendritic cells subsets and regulatory T-cells at multiple sclerosis relapse: differential short-term changes on corticosteroids therapy. J Neuroimmunol. (2006) 176:153–61. doi: 10.1016/j.jneuroim.2006.03.022
153. Mjosberg J, Svensson J, Johansson E, Hellstrom L, Casas R, Jenmalm MC, et al. Systemic reduction of functionally suppressive CD4dimCD25highFoxp3+ Tregs in human second trimester pregnancy is induced by progesterone and 17beta-estradiol. J Immunol. (2009) 183:759–69. doi: 10.4049/jimmunol.0803654
154. Liu W, Putnam AL, Xu-Yu Z, Szot GL, Lee MR, Zhu S, et al. CD127 expression inversely correlates with FoxP3 and suppressive function of human CD4+ T reg cells. J Exp Med. (2006) 203:1701–11. doi: 10.1084/jem.20060772
155. Miyara M, Yoshioka Y, Kitoh A, Shima T, Wing K, Niwa A, et al. Functional delineation and differentiation dynamics of human CD4+ T cells expressing the FoxP3 transcription factor. Immunity. (2009) 30:899–911. doi: 10.1016/j.immuni.2009.03.019
156. Molvarec A, Czegle I, Szijártó J, Rigó J Jr. Increased circulating interleukin-17 levels in preeclampsia. J Reprod Immunol. (2015) 112:53–7. doi: 10.1016/j.jri.2015.05.007
157. Batebi A, Namavar-Jahromi B, Ali-Hassanzadeh M, Ahmadi M, Hosseini MS, Gharesi-Fard B. Evaluation of IL-17 and IL-35 serum levels in patients with preeclampsia. J Reprod Infertil. (2019) 20:237–43.
158. Weaver CT, Hatton RD. Interplay between the TH17 and TReg cell lineages: a (co-)evolutionary perspective. Nat Rev Immunol. (2009) 9:883–9. doi: 10.1038/nri2660
159. Afzali B, Mitchell P, Lechler RI, John S, Lombardi G. Translational mini-review series on Th17 cells: induction of interleukin-17 production by regulatory T cells. Clin Exp Immunol. (2010) 159:120–30. doi: 10.1111/j.1365-2249.2009.04038.x
160. Rahimzadeh M, Norouzian M, Arabpour F, Naderi N. Regulatory T-cells and preeclampsia: an overview of literature. Expert Rev Clin Immunol. (2016) 12:209–27. doi: 10.1586/1744666X.2016.1105740
161. Casart YC, Tarrazzi K, Camejo MI. Serum levels of interleukin-6, interleukin-1beta and human chorionic gonadotropin in pre-eclamptic and normal pregnancy. Gynecol Endocrinol. (2007) 23:300–3. doi: 10.1080/09513590701327638
162. Prins JR, Gomez-Lopez N, Robertson SA. Interleukin-6 in pregnancy and gestational disorders. J Reprod Immunol. (2012) 95:1–14. doi: 10.1016/j.jri.2012.05.004
163. Prins JR, Faas MM, Melgert BN, Huitema S, Timmer A, Hylkema MN, et al. Altered expression of immune-associated genes in first-trimester human decidua of pregnancies later complicated with hypertension or foetal growth restriction. Placenta. (2012) 33:453–5. doi: 10.1016/j.placenta.2012.02.010
164. Han X, Ghaemi MS, Ando K, Peterson LS, Ganio EA, Tsai AS, et al. Differential dynamics of the maternal immune system in healthy pregnancy and preeclampsia. Front Immunol. (2019) 10:1305. doi: 10.3389/fimmu.2019.01305
165. Liao W, Lin JX, Wang L, Li P, Leonard WJ. Modulation of cytokine receptors by IL-2 broadly regulates differentiation into helper T cell lineages. Nat Immunol. (2011) 12:551–9. doi: 10.1038/ni.2030
166. Cohen AC, Nadeau KC, Tu W, Hwa V, Dionis K, Bezrodnik L, et al. Cutting edge: Decreased accumulation and regulatory function of CD4+ CD25(high) T cells in human STAT5b deficiency. J Immunol. (2006) 177:2770–4. doi: 10.4049/jimmunol.177.5.2770
167. Laurence A, Tato CM, Davidson TS, Kanno Y, Chen Z, Yao Z, et al. Interleukin-2 signaling via STAT5 constrains T helper 17 cell generation. Immunity. (2007) 26:371–81. doi: 10.1016/j.immuni.2007.02.009
168. Adler HS, Kubsch S, Graulich E, Ludwig S, Knop J, Steinbrink K. Activation of MAP kinase p38 is critical for the cell-cycle-controlled suppressor function of regulatory T cells. Blood. (2007) 109:4351–9. doi: 10.1182/blood-2006-09-047563
169. Hsu P, Santner-Nanan B, Dahlstrom JE, Fadia M, Chandra A, Peek M, et al. Altered decidual DC-SIGN+ antigen-presenting cells and impaired regulatory T-cell induction in preeclampsia. Am J Pathol. (2012) 181:2149–60. doi: 10.1016/j.ajpath.2012.08.032
170. Wang J, Tao YM, Cheng XY, Zhu TF, Chen ZF, Yao H, et al. Dendritic cells derived from preeclampsia patients influence Th1/Th17 cell differentiation in vitro. Int J Clin Exp Med. (2014) 7:5303–9.
171. Nagayama S, Shirasuna K, Nagayama M, Nishimura S, Takahashi M, Matsubara S, et al. Decreased circulating levels of plasmacytoid dendritic cells in women with early-onset preeclampsia. J Reprod Immunol. (2020) 141:103170. doi: 10.1016/j.jri.2020.103170
172. Jung YJ, Park Y, Kim HS, Lee HJ, Kim YN, Lee J, et al. Abnormal lymphatic vessel development is associated with decreased decidual regulatory T cells in severe preeclampsia. Am J Reprod Immunol. (2018) 80:e12970. doi: 10.1111/aji.2018.80.issue-1
173. Tsuda S, Zhang X, Hamana H, Shima T, Ushijima A, Tsuda K, et al. Clonally expanded decidual effector regulatory T cells increase in late gestation of normal pregnancy, but not in preeclampsia, in humans. Front Immunol. (2018) 9:1934. doi: 10.3389/fimmu.2018.01934
174. Yang J, Gong L, Liu Q, Zhao H, Wang Z, Li X, et al. Single-cell RNA-seq reveals developmental deficiencies in both the placentation and the decidualization in women with late-onset preeclampsia. Front Immunol. (2023) 14:1142273. doi: 10.3389/fimmu.2023.1142273
175. Zhou W, Wang H, Yang Y, Guo F, Yu B, Su Z. Trophoblast cell subtypes and dysfunction in the placenta of individuals with preeclampsia revealed by single−Cell RNA sequencing. Mol Cells. (2022) 45:317–28. doi: 10.14348/molcells.2021.0211
176. Harmon AC, Cornelius DC, Amaral LM, Faulkner JL, Cunningham MW Jr., Wallace K, et al. The role of inflammation in the pathology of preeclampsia. Clin Sci (Lond). (2016) 130:409–19. doi: 10.1042/CS20150702
177. Nevers T, Kalkunte S, Sharma S. Uterine Regulatory T cells, IL-10 and hypertension. Am J Reprod Immunol. (2011) 66 Suppl 1:88–92. doi: 10.1111/j.1600-0897.2011.01040.x
178. Meister S, Hahn L, Beyer S, Mannewitz M, Perleberg C, Schnell K, et al. Regulatory T cell apoptosis during preeclampsia may be prevented by gal-2. Int J Mol Sci. (2022) 23. doi: 10.3390/ijms23031880
179. Acar N, Ustunel I, Demir R. Uterine natural killer (uNK) cells and their missions during pregnancy: a review. Acta Histochem. (2011) 113:82–91. doi: 10.1016/j.acthis.2009.12.001
180. Rowe JH, Ertelt JM, Xin L, Way SS. Regulatory T cells and the immune pathogenesis of prenatal infection. Reproduction. (2013) 146:R191–203. doi: 10.1530/REP-13-0262
181. Tanaka A, Sakaguchi S. Regulatory T cells in cancer immunotherapy. Cell Res. (2017) 27:109–18. doi: 10.1038/cr.2016.151
182. Sallusto F, Lenig D, Förster R, Lipp M, Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. (1999) 401:708–12. doi: 10.1038/44385
183. Li Y, Wang H, Lu H, Hua S. Regulation of memory T cells by interleukin-23. Int Arch Allergy Immunol. (2016) 169:157–62. doi: 10.1159/000445834
184. Kondrack RM, Harbertson J, Tan JT, McBreen ME, Surh CD, Bradley LM. Interleukin 7 regulates the survival and generation of memory CD4 cells. J Exp Med. (2003) 198:1797–806. doi: 10.1084/jem.20030735
185. Richer MJ, Pewe LL, Hancox LS, Hartwig SM, Varga SM, Harty JT. Inflammatory IL-15 is required for optimal memory T cell responses. J Clin Invest. (2015) 125:3477–90. doi: 10.1172/JCI81261
186. Sallusto F, Kremmer E, Palermo B, Hoy A, Ponath P, Qin S, et al. Switch in chemokine receptor expression upon TCR stimulation reveals novel homing potential for recently activated T cells. Eur J Immunol. (1999) 29:2037–45. doi: 10.1002/(SICI)1521-4141(199906)29:06<2037::AID-IMMU2037>3.0.CO;2-V
187. Sallusto F, Geginat J, Lanzavecchia A. Central memory and effector memory T cell subsets: function, generation, and maintenance. Annu Rev Immunol. (2004) 22:745–63. doi: 10.1146/annurev.immunol.22.012703.104702
188. Förster R, Schubel A, Breitfeld D, Kremmer E, Renner-Müller I, Wolf E, et al. CCR7 coordinates the primary immune response by establishing functional microenvironments in secondary lymphoid organs. Cell. (1999) 99:23–33. doi: 10.1016/S0092-8674(00)80059-8
189. Wagner MI, Mai C, Schmitt E, Mahnke K, Meuer S, Eckstein V, et al. The role of recent thymic emigrant-regulatory T-cell (RTE-Treg) differentiation during pregnancy. Immunol Cell Biol. (2015) 93:858–67. doi: 10.1038/icb.2015.51
190. Dambaeva S, Schneiderman S, Jaiswal MK, Agrawal V, Katara GK, Gilman-Sachs A, et al. Interleukin 22 prevents lipopolysaccharide- induced preterm labor in mice. Biol Reprod. (2018) 98:299–308. doi: 10.1093/biolre/iox182
191. Plank MW, Kaiko GE, Maltby S, Weaver J, Tay HL, Shen W, et al. Th22 cells form a distinct th lineage from th17 cells in vitro with unique transcriptional properties and tbet-dependent th1 plasticity. J Immunol. (2017) 198:2182–90. doi: 10.4049/jimmunol.1601480
192. Logiodice F, Lombardelli L, Kullolli O, Haller H, Maggi E, Rukavina D, et al. Decidual interleukin-22-producing CD4+ T cells (Th17/th0/IL-22+ and th17/th2/IL-22+, th2/IL-22+, th0/IL-22+), which also produce IL-4, are involved in the success of pregnancy. Int J Mol Sci. (2019) 20. doi: 10.3390/ijms20020428
193. Takahata Y, Nomura A, Takada H, Ohga S, Furuno K, Hikino S, et al. CD25+CD4+ T cells in human cord blood: an immunoregulatory subset with naive phenotype and specific expression of forkhead box p3 (Foxp3) gene. Exp Hematol. (2004) 32:622–9. doi: 10.1016/j.exphem.2004.03.012
194. Sakaguchi S, Miyara M, Costantino CM, Hafler DA. FOXP3+ regulatory T cells in the human immune system. Nat Rev Immunol. (2010) 10:490–500. doi: 10.1038/nri2785
195. Mackroth MS, Malhotra I, Mungai P, Koech D, Muchiri E, King CL. Human cord blood CD4+CD25hi regulatory T cells suppress prenatally acquired T cell responses to Plasmodium falciparum antigens. J Immunol. (2011) 186:2780–91. doi: 10.4049/jimmunol.1001188
196. Kim H, Moon HW, Hur M, Park CM, Yun YM, Hwang HS, et al. Distribution of CD4+ CD25 high FoxP3+ regulatory T-cells in umbilical cord blood. J Matern Fetal Neonatal Med. (2012) 25:2058–61. doi: 10.3109/14767058.2012.666591
197. Vargas-Rojas MI, Solleiro-Villavicencio H, Soto-Vega E. Th1, Th2, Th17 and Treg levels in umbilical cord blood in preeclampsia. J Matern Fetal Neonatal Med. (2016) 29:1642–5. doi: 10.3109/14767058.2015.1057811
198. Loewendorf AI, Nguyen TA, Yesayan MN, Kahn DA. Preeclampsia is characterized by fetal NK cell activation and a reduction in regulatory T cells. Am J Reprod Immunol. (2015) 74:258–67. doi: 10.1111/aji.2015.74.issue-3
199. El-Chennawi F, Rageh IM, Mansour AI, Darwish MI, Elghzaly AA, Sakr BES, et al. Comparison of the percentages of CD4(+) CD25(high) FOXP3(+), CD4(+) CD25(low) FOXP3(+), and CD4(+) FOXP3(+) Tregs, in the umbilical cord blood of babies born to mothers with and without preeclampsia. Am J Reprod Immunol. (2017) 78. doi: 10.1111/aji.12761
200. Travis OK, White D, Baik C, Giachelli C, Thompson W, Stubbs C, et al. Interleukin-17 signaling mediates cytolytic natural killer cell activation in response to placental ischemia. Am J Physiol Regul Integr Comp Physiol. (2020) 318:R1036–r46. doi: 10.1152/ajpregu.00285.2019
201. Redman CW, Sacks GP, Sargent IL. Preeclampsia: an excessive maternal inflammatory response to pregnancy. Am J Obstet Gynecol. (1999) 180:499–506. doi: 10.1016/S0002-9378(99)70239-5
202. Narazaki M, Tanaka T, Kishimoto T. The role and therapeutic targeting of IL-6 in rheumatoid arthritis. Expert Rev Clin Immunol. (2017) 13:535–51. doi: 10.1080/1744666X.2017.1295850
203. Tousoulis D, Oikonomou E, Economou EK, Crea F, Kaski JC. Inflammatory cytokines in atherosclerosis: current therapeutic approaches. Eur Heart J. (2016) 37:1723–32. doi: 10.1093/eurheartj/ehv759
204. Harmon A, Cornelius D, Amaral L, Paige A, Herse F, Ibrahim T, et al. IL-10 supplementation increases Tregs and decreases hypertension in the RUPP rat model of preeclampsia. Hypertens Pregnancy. (2015) 34:291–306. doi: 10.3109/10641955.2015.1032054
205. Rambaldi MP, Weiner E, Mecacci F, Bar J, Petraglia F. Immunomodulation and preeclampsia. Best Pract Res Clin Obstet Gynaecol. (2019) 60:87–96. doi: 10.1016/j.bpobgyn.2019.06.005
206. Small HY, Nosalski R, Morgan H, Beattie E, Guzik TJ, Graham D, et al. Role of tumor necrosis factor-α and natural killer cells in uterine artery function and pregnancy outcome in the stroke-prone spontaneously hypertensive rat. Hypertension. (2016) 68:1298–307. doi: 10.1161/HYPERTENSIONAHA.116.07933
207. Xiao Q, Li X, Li Y, Wu Z, Xu C, Chen Z, et al. Biological drug and drug delivery-mediated immunotherapy. Acta Pharm Sin B. (2021) 11:941–60. doi: 10.1016/j.apsb.2020.12.018
208. Johansen CB, Jimenez-Solem E, Haerskjold A, Sand FL, Thomsen SF. The use and safety of TNF inhibitors during pregnancy in women with psoriasis: A review. Int J Mol Sci. (2018) 19. doi: 10.3390/ijms19051349
209. Elfarra JT, Cottrell JN, Cornelius DC, Cunningham MW Jr., Faulkner JL, Ibrahim T, et al. 17-Hydroxyprogesterone caproate improves T cells and NK cells in response to placental ischemia; new mechanisms of action for an old drug. Pregnancy Hypertens. (2020) 19:226–32. doi: 10.1016/j.preghy.2019.11.005
210. Engler JB, Kursawe N, Solano ME, Patas K, Wehrmann S, Heckmann N, et al. Glucocorticoid receptor in T cells mediates protection from autoimmunity in pregnancy. Proc Natl Acad Sci U S A. (2017) 114:E181–E90. doi: 10.1073/pnas.1617115114
211. Raghupathy R, Szekeres-Bartho J. Progesterone: A unique hormone with immunomodulatory roles in pregnancy. Int J Mol Sci. (2022) 23. doi: 10.3390/ijms23031333
212. Schindler AE. New data about preeclampsia: some possibilities of prevention. Gynecol Endocrinol. (2018) 34:636–7. doi: 10.1080/09513590.2018.1441401
213. Tskhay V, Schindler A, Shestakova M, Klimova O, Narkevich А. The role of progestogen supplementation (dydrogesterone) in the prevention of preeclampsia. Gynecol Endocrinol. (2020) 36:698–701. doi: 10.1080/09513590.2019.1706085
214. Yang X, Meng T. Is there a role of intravenous immunoglobulin in immunologic recurrent pregnancy loss? J Immunol Res. (2020) 2020:6672865. doi: 10.1155/2020/6672865
215. Kim DJ, Lee SK, Kim JY, Na BJ, Hur SE, Lee M, et al. Intravenous immunoglobulin G modulates peripheral blood Th17 and Foxp3(+) regulatory T cells in pregnant women with recurrent pregnancy loss. Am J Reprod Immunol. (2014) 71:441–50. doi: 10.1111/aji.2014.71.issue-5
216. Han AR, Ahn H, Vu P, Park JC, Gilman-Sachs A, Beaman K, et al. Obstetrical outcome of anti-inflammatory and anticoagulation therapy in women with recurrent pregnancy loss or unexplained infertility. Am J Reprod Immunol. (2012) 68:418–27. doi: 10.1111/j.1600-0897.2012.01178.x
217. Zhao H, Wei X, Yang X. A novel update on vitamin D in recurrent pregnancy loss (Review). Mol Med Rep. (2021) 23. doi: 10.3892/mmr.2021.12021
218. Ribeiro VR, Romao-Veiga M, Nunes PR, de Oliveira LRC, Romagnoli GG, Peracoli JC, et al. Immunomodulatory effect of vitamin D on the STATs and transcription factors of CD4(+) T cell subsets in pregnant women with preeclampsia. Clin Immunol. (2022) 234:108917. doi: 10.1016/j.clim.2021.108917
219. Ribeiro VR, Romao-Veiga M, Nunes PR, Matias ML, Peracoli JC, Peracoli MTS. Vitamin D modulates the transcription factors of T cell subsets to anti-inflammatory and regulatory profiles in preeclampsia. Int Immunopharmacol. (2021) 101:108366. doi: 10.1016/j.intimp.2021.108366
220. Muyayalo KP, Huang XB, Qian Z, Li ZH, Mor G, Liao AH. Low circulating levels of vitamin D may contribute to the occurrence of preeclampsia through deregulation of Treg/Th17 cell ratio. Am J Reprod Immunol. (2019) 82:e13168. doi: 10.1111/aji.13168
221. Pichler J, Gerstmayr M, Szépfalusi Z, Urbanek R, Peterlik M, Willheim M. 1 alpha,25(OH)2D3 inhibits not only Th1 but also Th2 differentiation in human cord blood T cells. Pediatr Res. (2002) 52:12–8. doi: 10.1203/00006450-200207000-00005
222. Staeva-Vieira TP, Freedman LP. 1,25-dihydroxyvitamin D3 inhibits IFN-gamma and IL-4 levels during in vitro polarization of primary murine CD4+ T cells. J Immunol. (2002) 168:1181–9. doi: 10.4049/jimmunol.168.3.1181
223. Korf H, Wenes M, Stijlemans B, Takiishi T, Robert S, Miani M, et al. 1,25-Dihydroxyvitamin D3 curtails the inflammatory and T cell stimulatory capacity of macrophages through an IL-10-dependent mechanism. Immunobiology. (2012) 217:1292–300. doi: 10.1016/j.imbio.2012.07.018
224. Fawaz L, Mrad MF, Kazan JM, Sayegh S, Akika R, Khoury SJ. Comparative effect of 25(OH)D3 and 1,25(OH)2D3 on Th17 cell differentiation. Clin Immunol. (2016) 166-167:59–71. doi: 10.1016/j.clim.2016.02.011
225. Sassi F, Tamone C, D’Amelio P. Vitamin D: nutrient, hormone, and immunomodulator. Nutrients. (2018) 10. doi: 10.3390/nu10111656
226. Yamazaki S, Nishioka A, Kasuya S, Ohkura N, Hemmi H, Kaisho T, et al. Homeostasis of thymus-derived Foxp3+ regulatory T cells is controlled by ultraviolet B exposure in the skin. J Immunol. (2014) 193:5488–97. doi: 10.4049/jimmunol.1400985
227. Burzyn D, Kuswanto W, Kolodin D, Shadrach JL, Cerletti M, Jang Y, et al. A special population of regulatory T cells potentiates muscle repair. Cell. (2013) 155:1282–95. doi: 10.1016/j.cell.2013.10.054
228. Rolnik DL, Wright D, Poon LC, O’Gorman N, Syngelaki A, de Paco Matallana C, et al. Aspirin versus Placebo in Pregnancies at High Risk for Preterm Preeclampsia. N Engl J Med. (2017) 377:613–22. doi: 10.1056/NEJMoa1704559
229. Javeed A, Zhang B, Qu Y, Zhang A, Sun C, Zhang L, et al. The significantly enhanced frequency of functional CD4+CD25+Foxp3+ T regulatory cells in therapeutic dose aspirin-treated mice. Transpl Immunol. (2009) 20:253–60. doi: 10.1016/j.trim.2008.12.001
230. Wang M, Zhang P, Yu S, Zhou G, Lv J, Nallapothula D, et al. Heparin and aspirin combination therapy restores T-cell phenotype in pregnant patients with antiphospholipid syndrome-related recurrent pregnancy loss. Clin Immunol. (2019) 208:108259. doi: 10.1016/j.clim.2019.108259
231. Alvarez AM, Mulla MJ, Chamley LW, Cadavid AP, Abrahams VM. Aspirin-triggered lipoxin prevents antiphospholipid antibody effects on human trophoblast migration and endothelial cell interactions. Arthritis Rheumatol. (2015) 67:488–97. doi: 10.1002/art.38934
232. Bai Z, Liu Y, Zhao Y, Yan R, Yang L, Ma H, et al. Aspirin ameliorates atherosclerotic immuno-inflammation through regulating the Treg/Th17 axis and CD39-CD73 adenosine signaling via remodeling the gut microbiota in ApoE(-/-) mice. Int Immunopharmacol. (2023) 120:110296. doi: 10.1016/j.intimp.2023.110296
233. Hussain M, Javeed A, Ashraf M, Riaz A, Mushtaq MH. Aspirin may do wonders by the induction of immunological self-tolerance against autoimmune atherosclerosis. Med Hypotheses. (2012) 78:171–3. doi: 10.1016/j.mehy.2011.10.019
234. Pahan S, Pahan K. Mode of action of aspirin in experimental autoimmune encephalomyelitis. DNA Cell Biol. (2019) 38:593–6. doi: 10.1089/dna.2019.4814
235. Mondal S, Jana M, Dasarathi S, Roy A, Pahan K. Aspirin ameliorates experimental autoimmune encephalomyelitis through interleukin-11-mediated protection of regulatory T cells. Sci Signal. (2018) 11. doi: 10.1126/scisignal.aar8278
236. Sharma JB, Sharma A, Bahadur A, Vimala N, Satyam A, Mittal S. Oxidative stress markers and antioxidant levels in normal pregnancy and pre-eclampsia. Int J Gynaecol Obstet. (2006) 94:23–7. doi: 10.1016/j.ijgo.2006.03.025
237. Wang YP, Walsh SW, Guo JD, Zhang JY. The imbalance between thromboxane and prostacyclin in preeclampsia is associated with an imbalance between lipid peroxides and vitamin E in maternal blood. Am J Obstet Gynecol. (1991) 165:1695–700. doi: 10.1016/0002-9378(91)90017-L
238. Chappell LC, Seed PT, Briley A, Kelly FJ, Hunt BJ, Charnock-Jones DS, et al. A longitudinal study of biochemical variables in women at risk of preeclampsia. Am J Obstet Gynecol. (2002) 187:127–36. doi: 10.1067/mob.2002.122969
239. Oogaki Y, Ozawa R, Seshima K, Shinoda R, Torii Y, Takahashi H, et al. Uncaria tomentosa extract (AC-11) improves pregnancy hypertension together with suppression of sFlt-1 and sEng. Pregnancy Hypertens. (2021) 26:127–32. doi: 10.1016/j.preghy.2021.10.013
240. Worton SA, Greenwood SL, Wareing M, Heazell AE, Myers J. The kynurenine pathway; A new target for treating maternal features of preeclampsia? Placenta. (2019) 84:44–9. doi: 10.1016/j.placenta.2019.04.007
241. Lauterbach R, Rytlewski K, Pawlik D, Hurkała J, Wójtowicz A, Bręborowicz G, et al. Effect of pentoxifylline, administered in preterm labour, on the foetal-placental circulation and neonatal outcome: a randomized, prospective pilot study. Basic Clin Pharmacol Toxicol. (2012) 110:342–6. doi: 10.1111/j.1742-7843.2011.00809.x
242. Azimi A, Ziaee SM, Farhadi P, Sagheb MM. Hypothesis: Pentoxifylline explores new horizons in treatment of preeclampsia. Med Hypotheses. (2015) 85:468–74. doi: 10.1016/j.mehy.2015.06.031
243. Zenclussen AC, Sollwedel A, Bertoja AZ, Gerlof K, Zenclussen ML, Woiciechowsky C, et al. Heme oxygenase as a therapeutic target in immunological pregnancy complications. Int Immunopharmacol. (2005) 5:41–51. doi: 10.1016/j.intimp.2004.09.011
244. Ryu S, Huppmann AR, Sambangi N, Takacs P, Kauma SW. Increased leukocyte adhesion to vascular endothelium in preeclampsia is inhibited by antioxidants. Am J Obstet Gynecol. (2007) 196:400.e1–7. doi: 10.1016/j.ajog.2006.12.023
245. Tsuda S, Nakashima A, Morita K, Shima T, Yoneda S, Kishi H, et al. The role of decidual regulatory T cells in the induction and maintenance of fetal antigen-specific tolerance: Imbalance between regulatory and cytotoxic T cells in pregnancy complications. Hum Immunol. (2021) 82:346–52. doi: 10.1016/j.humimm.2021.01.019
246. Tian M, Zhang Y, Liu Z, Sun G, Mor G, Liao A. The PD-1/PD-L1 inhibitory pathway is altered in pre-eclampsia and regulates T cell responses in pre-eclamptic rats. Sci Rep. (2016) 6:27683. doi: 10.1038/srep27683
247. Przybyl L, Ibrahim T, Haase N, Golic M, Rugor J, Luft FC, et al. Regulatory T cells ameliorate intrauterine growth retardation in a transgenic rat model for preeclampsia. Hypertension. (2015) 65:1298–306. doi: 10.1161/HYPERTENSIONAHA.114.04892
248. Chen T, Darrasse-Jeze G, Bergot AS, Courau T, Churlaud G, Valdivia K, et al. Self-specific memory regulatory T cells protect embryos at implantation in mice. J Immunol. (2013) 191:2273–81. doi: 10.4049/jimmunol.1202413
249. Sharabi A, Tsokos MG, Ding Y, Malek TR, Klatzmann D, Tsokos GC. Regulatory T cells in the treatment of disease. Nat Rev Drug Discovery. (2018) 17:823–44. doi: 10.1038/nrd.2018.148
250. Kupferminc MJ, Peaceman AM, Wigton TR, Rehnberg KA, Socol ML. Tumor necrosis factor-alpha is elevated in plasma and amniotic fluid of patients with severe preeclampsia. Am J Obstet Gynecol. (1994) 170:1752–7. doi: 10.1016/S0002-9378(12)91845-1
251. Anim-Nyame N, Gamble J, Sooranna SR, Johnson MR, Steer PJ. Microvascular permeability is related to circulating levels of tumour necrosis factor-alpha in pre-eclampsia. Cardiovasc Res. (2003) 58:162–9. doi: 10.1016/s0008-6363(02)00844-1
252. Sharma A, Satyam A, Sharma JB. Leptin, IL-10 and inflammatory markers (TNF-alpha, IL-6 and IL-8) in pre-eclamptic, normotensive pregnant and healthy non-pregnant women. Am J Reprod Immunol. (2007) 58:21–30. doi: 10.1111/j.1600-0897.2007.00486.x
253. Szarka A, Rigó J Jr., Lázár L, Beko G, Molvarec A. Circulating cytokines, chemokines and adhesion molecules in normal pregnancy and preeclampsia determined by multiplex suspension array. BMC Immunol. (2010) 11:59. doi: 10.1186/1471-2172-11-59
254. Tosun M, Celik H, Avci B, Yavuz E, Alper T, Malatyalioğlu E. Maternal and umbilical serum levels of interleukin-6, interleukin-8, and tumor necrosis factor-alpha in normal pregnancies and in pregnancies complicated by preeclampsia. J Matern Fetal Neonatal Med. (2010) 23:880–6. doi: 10.3109/14767051003774942
255. Cristofalo R, Bannwart-Castro CF, Magalhães CG, Borges VT, Peraçoli JC, Witkin SS, et al. Silibinin attenuates oxidative metabolism and cytokine production by monocytes from preeclamptic women. Free Radic Res. (2013) 47:268–75. doi: 10.3109/10715762.2013.765951
256. Gharesi-Fard B, Mobasher-Nejad F, Nasri F. The expression of T-helper associated transcription factors and cytokine genes in pre-eclampsia. Iran J Immunol. (2016) 13:296–308.
257. Xu J, Gu Y, Sun J, Zhu H, Lewis DF, Wang Y. Reduced CD200 expression is associated with altered Th1/Th2 cytokine production in placental trophoblasts from preeclampsia. Am J Reprod Immunol. (2018) 79. doi: 10.1111/aji.2018.79.issue-1
258. Hao H, He M, Li J, Zhou Y, Dang J, Li F, et al. Upregulation of the Tim-3/Gal-9 pathway and correlation with the development of preeclampsia. Eur J Obstet Gynecol Reprod Biol. (2015) 194:85–91. doi: 10.1016/j.ejogrb.2015.08.022
259. Wilczyński JR, Tchórzewski H, Banasik M, Głowacka E, Wieczorek A, Lewkowicz P, et al. Lymphocyte subset distribution and cytokine secretion in third trimester decidua in normal pregnancy and preeclampsia. Eur J Obstet Gynecol Reprod Biol. (2003) 109:8–15. doi: 10.1016/S0301-2115(02)00350-0
260. Banerjee S, Smallwood A, Moorhead J, Chambers AE, Papageorghiou A, Campbell S, et al. Placental expression of interferon-gamma (IFN-gamma) and its receptor IFN-gamma R2 fail to switch from early hypoxic to late normotensive development in preeclampsia. J Clin Endocrinol Metab. (2005) 90:944–52. doi: 10.1210/jc.2004-1113
261. Cemgil Arikan D, Aral M, Coskun A, Ozer A. Plasma IL-4, IL-8, IL-12, interferon-γ and CRP levels in pregnant women with preeclampsia, and their relation with severity of disease and fetal birth weight. J Matern Fetal Neonatal Med. (2012) 25:1569–73. doi: 10.3109/14767058.2011.648233
262. Madazli R, Aydin S, Uludag S, Vildan O, Tolun N. Maternal plasma levels of cytokines in normal and preeclamptic pregnancies and their relationship with diastolic blood pressure and fibronectin levels. Acta Obstet Gynecol Scand. (2003) 82:797–802. doi: 10.1034/j.1600-0412.2003.00206.x
263. Freeman DJ, McManus F, Brown EA, Cherry L, Norrie J, Ramsay JE, et al. Short- and long-term changes in plasma inflammatory markers associated with preeclampsia. Hypertension. (2004) 44:708–14. doi: 10.1161/01.HYP.0000143849.67254.ca
264. Jonsson Y, Rubèr M, Matthiesen L, Berg G, Nieminen K, Sharma S, et al. Cytokine mapping of sera from women with preeclampsia and normal pregnancies. J Reprod Immunol. (2006) 70:83–91. doi: 10.1016/j.jri.2005.10.007
265. Zhao S, Gu Y, Dong Q, Fan R, Wang Y. Altered interleukin-6 receptor, IL-6R and gp130, production and expression and decreased SOCS-3 expression in placentas from women with pre-eclampsia. Placenta. (2008) 29:1024–8. doi: 10.1016/j.placenta.2008.09.011
266. Sakai M, Shiozaki A, Sasaki Y, Yoneda S, Saito S. The ratio of interleukin (IL)-18 to IL-12 secreted by peripheral blood mononuclear cells is increased in normal pregnant subjects and decreased in pre-eclamptic patients. J Reprod Immunol. (2004) 61:133–43. doi: 10.1016/j.jri.2004.01.001
267. Huang X, Huang H, Dong M, Yao Q, Wang H. Serum and placental interleukin-18 are elevated in preeclampsia. J Reprod Immunol. (2005) 65:77–87. doi: 10.1016/j.jri.2004.09.003
268. Ozkan ZS, Simsek M, Ilhan F, Deveci D, Godekmerdan A, Sapmaz E. Plasma IL-17, IL-35, interferon-γ, SOCS3 and TGF-β levels in pregnant women with preeclampsia, and their relation with severity of disease. J Matern Fetal Neonatal Med. (2014) 27:1513–7. doi: 10.3109/14767058.2013.861415
269. Jonsson Y, Matthiesen L, Berg G, Ernerudh J, Nieminen K, Ekerfelt C. Indications of an altered immune balance in preeclampsia: a decrease in in vitro secretion of IL-5 and IL-10 from blood mononuclear cells and in blood basophil counts compared with normal pregnancy. J Reprod Immunol. (2005) 66:69–84. doi: 10.1016/j.jri.2005.02.002
270. Borekci B, Aksoy H, Al RA, Demircan B, Kadanali S. Maternal serum interleukin-10, interleukin-2 and interleukin-6 in pre-eclampsia and eclampsia. Am J Reprod Immunol. (2007) 58:56–64. doi: 10.1111/j.1600-0897.2007.00491.x
271. Liu X, Liu Y, Ding M, Wang X. Reduced expression of indoleamine 2,3-dioxygenase participates in pathogenesis of preeclampsia via regulatory T cells. Mol Med Rep. (2011) 4:53–8. doi: 10.3892/mmr.2010.395
272. Quinn KH, Lacoursiere DY, Cui L, Bui J, Parast MM. The unique pathophysiology of early-onset severe preeclampsia: role of decidual T regulatory cells. J Reprod Immunol. (2011) 91:76–82. doi: 10.1016/j.jri.2011.05.006
273. Zhang Y, Liu Z, Tian M, Hu X, Wang L, Ji J, et al. The altered PD-1/PD-L1 pathway delivers the ‘one-two punch’ effects to promote the Treg/Th17 imbalance in pre-eclampsia. Cell Mol Immunol. (2018) 15:710–23. doi: 10.1038/cmi.2017.70
274. Orlovic Vlaho M, Tomic V, Vukojevic K, Vasilj A, Pejic R, Lesko J, et al. CD25(+) FOXP3(+) and CD4(+) CD25(+) cells distribution in decidual departments of women with severe and mild pre-eclampsia: Comparison with healthy pregnancies. Am J Reprod Immunol. (2020) 84:e13281. doi: 10.1111/aji.13281
Keywords: pregnancy, preeclampsia, T cells, Treg cells, Th17 cells
Citation: Peng X, Chinwe Oluchi-Amaka I, Kwak-Kim J and Yang X (2025) A comprehensive review of the roles of T-cell immunity in preeclampsia. Front. Immunol. 16:1476123. doi: 10.3389/fimmu.2025.1476123
Received: 05 August 2024; Accepted: 22 January 2025;
Published: 06 February 2025.
Edited by:
Jochen Mattner, University of Erlangen Nuremberg, GermanyReviewed by:
Panicos Shangaris, King’s College London, United KingdomCopyright © 2025 Peng, Chinwe Oluchi-Amaka, Kwak-Kim and Yang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Joanne Kwak-Kim, am9hbm5lLmt3YWtraW1Acm9zYWxpbmRmcmFua2xpbi5lZHU=; Xiuhua Yang, eGh5YW5nQGNtdS5lZHUuY24=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.