
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
BRIEF RESEARCH REPORT article
Front. Immunol., 17 February 2025
Sec. Vaccines and Molecular Therapeutics
Volume 16 - 2025 | https://doi.org/10.3389/fimmu.2025.1470609
This article is part of the Research TopicSARS-CoV-2 Vaccines Beyond the Pandemic EraView all 25 articles
 Matthew E. Levy1*
Matthew E. Levy1* Vanessa Chilunda1
Vanessa Chilunda1 Phillip R. Heaton2
Phillip R. Heaton2 Deran McKeen3
Deran McKeen3 Jason D. Goldman4,5
Jason D. Goldman4,5 Richard E. Davis6
Richard E. Davis6 Cynthia A. Schandl7
Cynthia A. Schandl7 William B. Glen7
William B. Glen7 Lisa M. McEwen1
Lisa M. McEwen1 Elizabeth T. Cirulli1
Elizabeth T. Cirulli1 Dana Wyman1
Dana Wyman1 Andrew Dei Rossi1
Andrew Dei Rossi1 Hang Dai1
Hang Dai1 Magnus Isaksson1
Magnus Isaksson1 Nicole L. Washington1
Nicole L. Washington1 Tracy Basler1
Tracy Basler1 Kevin Tsan1
Kevin Tsan1 Jason Nguyen1
Jason Nguyen1 Jimmy Ramirez1
Jimmy Ramirez1 Efren Sandoval1
Efren Sandoval1 William Lee1
William Lee1 James Lu1
James Lu1 Shishi Luo1
Shishi Luo1As part of a multi-state viral genomic surveillance program, we conducted a case-only analysis to evaluate the effectiveness of XBB.1.5-adapated mRNA vaccines in preventing severe illness among individuals with medically attended SARS-CoV-2 infection. We compared prior receipt of an XBB.1.5-adapted mRNA vaccine between SARS-CoV-2-infected adults with inpatient or emergency department (ED) visits (as a proxy for severe illness) vs those with outpatient visits (as a proxy for mild illness). Among 6,551 patients between September 2023 and January 2024, 6.1% with inpatient or ED visits vs 12.0% with outpatient visits had received XBB.1.5 vaccination (adjusted odds ratio [aOR]=0.41; 95% confidence interval [CI]: 0.32-0.53). This protective association was weaker among JN.1 (aOR=0.62; 95% CI: 0.40-0.96) vs XBB-lineage (aOR=0.28; 95% CI: 0.18-0.43) variant infections (interaction, p=0.003). XBB.1.5 vaccines protect against severe illness, but protection may be weaker against JN.1 vs XBB-lineage variants. This study highlights the need for COVID-19 vaccines to be routinely updated to align with circulating strains and for individuals to stay up to date with recommended vaccines.
On 11 September 2023, the US Food and Drug Administration (FDA) approved the 2023-2024 BNT162b2 (Pfizer-BioNTech) and mRNA-1273 (Moderna) monovalent mRNA COVID-19 vaccines for individuals aged ≥12 years, with emergency use authorization granted for children aged 6 months to 11 years. These vaccines target the spike protein of the SARS-CoV-2 Omicron variant XBB.1.5, which was predominant in the US from January to May 2023 (1). The US Centers for Disease Control’s (CDC’s) Advisory Committee on Immunization Practices (ACIP) subsequently recommended that individuals aged ≥6 months receive an XBB.1.5-adapted vaccine regardless of their vaccination history, to enhance protection against circulating variants (2).
By September 2023, other XBB variants such as EG.5 and HV.1 (both sublineages of XBB.1.9.2) had surpassed XBB.1.5 in prevalence. By late December 2023, another novel variant, JN.1 (a sublineage of BA.2.86), had become predominant, accounting for 65% of SARS-CoV-2 infections nationwide by 6 January 2024 (1). The rapid rise of JN.1, which possesses more than 30 mutations in the spike protein compared to XBB.1.5 (including the notable L455S mutation), could be attributed to increased immune escape and infectivity (3, 4). In laboratory-based neutralization studies, JN.1 has displayed increased resistance to neutralization by antibodies induced by XBB.1.5-adapted mRNA vaccination (4–6). However, immunogenicity studies suggest that antibody titers are likely to remain effective (7). Initial findings indicate short-term effectiveness of XBB.1.5-adapted mRNA vaccines for protecting against symptomatic SARS-CoV-2 infection and severe COVID-19 outcomes, largely during periods of XBB predominance (8–14).
Case-only studies among SARS-CoV-2-positive individuals have previously been employed to compare the protection offered by vaccines against different variants (13, 15–17). However, variant-specific estimates of XBB.1.5 vaccine protection against severe COVID-19 outcomes, especially those caused by the JN.1 variant, remain limited. In this study, we leverage a multi-state viral genomic surveillance program to conduct a case-only analysis evaluating the association between XBB.1.5-adapted mRNA vaccination and the likelihood of patients requiring inpatient or emergency department (ED) visits (considered severe) vs outpatient visits (considered mild). This association serves as an indicator of the vaccine’s level of protection against severe illness following infection with a particular variant. We have evaluated this association both overall and separately among patients infected with the JN.1 variant and XBB-lineage variants, enabling a direct comparison of protection against these two distinct SARS-CoV-2 lineages.
Within a pan-respiratory virus genomic surveillance program, residual clinical samples from patients who tested positive for a respiratory virus (molecular or antigen) were obtained from three health systems spanning five U.S. states (Supplementary Table S1) (18). Samples were initially collected from patients during medical visits and were characterized based on location of collection as inpatient, ED, or outpatient. Patients’ demographic characteristics and COVID-19 vaccination history were extracted from electronic health records (EHRs) and state vaccine registries. Study protocols were reviewed and approved by WIRB CG IRB (Western Institutional Review Board, WIRB-Copernicus Group; approval number 20224919) and the Medical University of South Carolina (MUSC) Institutional Review Board for Human Research (approval number Pro00129083). This study presented minimal risk to participants because there was no interaction or intervention with patients; therefore, the requirement for informed consent was waived.
Viral sequencing was performed by Helix using a hybridization-capture based assay (Twist Biosciences) and short-read genome sequencing technology (Illumina), as previously described (19). SARS-CoV-2 was identified in samples with reads that aligned to the reference genome, and lineages were assigned using pangolin version 4.3.1 (Supplementary Methods; Supplementary Figure S1).
This analysis included SARS-CoV-2-positive samples collected from adults aged ≥18 years between 24 September 2023 and 21 January 2024. SARS-CoV-2 infection was identified from either clinical diagnostic testing (performed/ordered by the health system), viral sequencing (performed by Helix), or both.
The clinical visit type associated with sample collection was a surrogate measure of the severity of illness at time of testing. Inpatient and ED visits represented more severe illness compared to outpatient visits. The reasons for visits and patients’ specific symptoms were not available for analysis.
COVID-19 vaccination status was assigned using the date and type of the most recent dose received prior to the specimen collection date. Patients were considered to be: 1) vaccinated with an XBB.1.5-adapted monovalent mRNA vaccine if their last dose occurred on/after September 12, 2023 and was BNT162b2 or mRNA-1273; 2) vaccinated with a BA.4/BA.5-adapted bivalent mRNA vaccine if their last dose occurred between September 1, 2022 and September 11, 2023 and was BNT162b2 or mRNA-1273; 3) vaccinated with an original wild-type monovalent mRNA or viral vector vaccine if their last dose occurred before September 1, 2022 and was either BNT162b2, mRNA-1273, or Ad26.COV2.S; and 4) unvaccinated if they had received no prior COVID-19 vaccine doses. Patients were excluded if they received any dose 0-6 days before the collection date (n=83) or if their most recent dose was a different (n=20) or unknown (n=124) vaccine type.
In this case-only analysis among adults with medically attended SARS-CoV-2 infection, the odds of prior XBB.1.5 vaccination were compared between inpatient or ED patients and outpatients. We also compared inpatient vs outpatient (excluding ED). Adjusted odds ratios (aORs) and 95% confidence intervals (CIs) for the association between vaccination status and visit type were calculated using multivariable logistic regression, adjusting for age group, sex, race/ethnicity, health system and state of residence, and collection date (natural cubic spline). In multivariable models, effect modification by variant (JN.1 vs XBB-lineage) was assessed using an interaction term with vaccination status. Receipt of an XBB.1.5 vaccine was compared to no receipt of an XBB.1.5 vaccine (irrespective of vaccination history) and to three specific reference groups: 1) BA.4/BA.5 vaccination but no XBB.1.5 vaccine; 2) wild-type vaccination but no BA.4/BA.5 or XBB.1.5 vaccine; and 3) unvaccinated. In a separate analysis, XBB.1.5 vaccine recipients were further categorized based on duration of time since their dose (7-59 vs ≥60 days earlier). In addition, BA.4/BA.5 and wild-type vaccine recipients were compared to unvaccinated. Subgroup analyses were conducted among patients infected with JN.1, XBB-lineage (any), HV.1, and EG.5 variants; among patients aged ≥65 years; and among patients with no other respiratory virus coinfection. P<0.05 was considered statistically significant and analyses were performed using R version 4.2.3.
Among 6,551 adults with medically attended SARS-CoV-2 infection, 1,912 (29.2%) were tested in either an inpatient (1,012; 15.4%) or ED (900; 13.7%) setting, while 4,639 (70.8%) were tested in an outpatient setting. Most SARS-CoV-2 infections were detected through clinical diagnostic testing, with only 8 first identified through viral sequencing. Patient characteristics stratified by visit type are presented in Table 1. Inpatients had a higher median age (73 years; IQR: 61-82) compared to ED patients (55 years; IQR: 35-72) and outpatients (52 years; IQR: 36-68). Lineages were successfully assigned to 4,480 samples (68.4%), with the most prevalent variants being JN.1 (1,084; 24.2%), HV.1 (803; 17.9%), and EG.5 (746; 16.7%). During the most recent 2-week period ending 20 January 2024, JN.1 accounted for 73.1% of sequenced samples, while HV.1 (5.5%) and EG.5 (2.9%) were less prevalent, consistent with national data (1).
Regarding vaccination status, 675 (10.3%) patients had received XBB.1.5 vaccination (a median of 57 days earlier [IQR: 39-73; range: 7-122]), 1,155 (17.6%) had received BA.4/BA.5 vaccination (but not XBB.1.5 vaccination) (median of 374 days since last dose [IQR: 330-414]), 2,879 (43.9%) had received wild-type vaccination (but not BA.4/BA.5 or XBB.1.5 vaccination) (median of 712 days since last dose [IQR: 625-818]), and 1,842 (28.1%) were unvaccinated. Among XBB.1.5-vaccinated patients, the median time since vaccination was 64 days for JN.1 infections (IQR: 51-80) and 52 days for XBB-lineage infections (IQR: 31-64). In the most recent 14-day period, 19.4% of patients overall were XBB.1.5-vaccinated.
Among all SARS-CoV-2 infections, 6.1% of patients with inpatient/ED visits had received XBB.1.5 vaccination, compared to 12.0% of patients with outpatient visits (Figure 1). In multivariable analysis, XBB.1.5 vaccination a median of 57 days earlier vs no XBB.1.5 vaccination was associated with lower odds of inpatient/ED visits compared to outpatient visits (aOR=0.41; 95% CI: 0.32-0.53). This protective association was significant among any-variant infections regardless of the specific reference group used: vs BA.4/BA.5 vaccination a median of 374 days earlier (aOR=0.60; 95% CI: 0.45-0.79); vs wild-type vaccination a median of 712 days earlier (aOR=0.48; 95% CI: 0.37-0.63); and vs unvaccinated (aOR=0.24; 95% CI: 0.19-0.32).
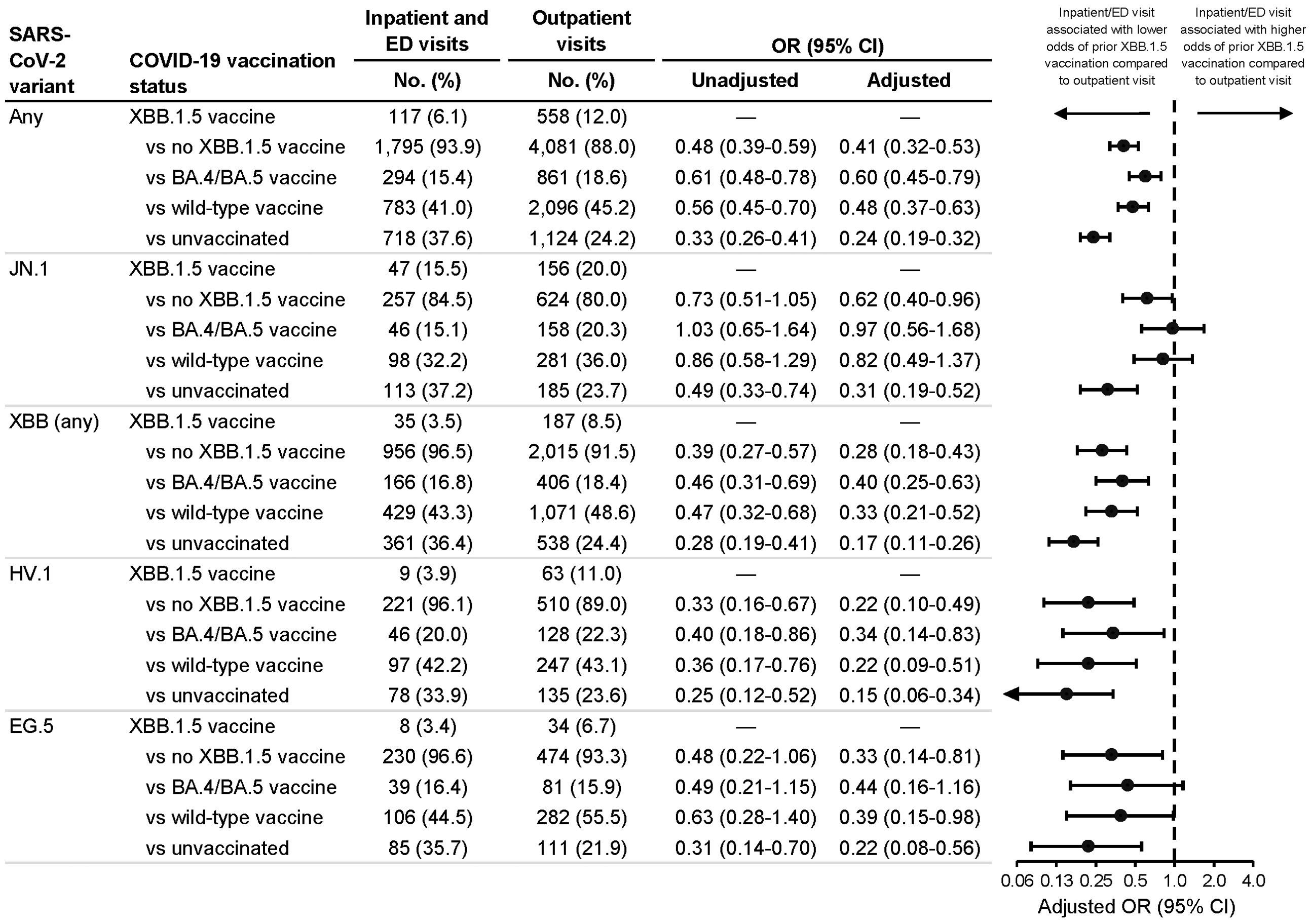
Figure 1. Association between inpatient or emergency department vs outpatient visit type and prior receipt of an XBB.1.5 vaccine. Associations were calculated among all SARS-CoV-2 infections and among JN.1, XBB (any sublineage), HV.1, and EG.5 infections. Odds ratios (ORs) were calculated comparing prior receipt of an XBB.1.5 vaccine to no prior receipt of an XBB.1.5 vaccine (irrespective of previous COVID-19 vaccination history) as well as to each of three specific reference groups: 1) prior receipt of a BA.4/BA.5 vaccine but not an XBB.1.5 vaccine; 2) prior receipt of a wild-type vaccine but not a BA.4/BA.5 or XBB.1.5 vaccine; and 3) unvaccinated. Adjusted ORs were adjusted for age group (18-49, 50-64, 65-74, 75-84, and ≥85 years), sex, race and ethnicity (Asian, non-Hispanic; Black, non-Hispanic; Hispanic; white, non-Hispanic; and other/unknown), health system and state of residence, and collection date (natural cubic spline with 4 degrees of freedom). CI indicates confidence interval; ED, emergency department.
When stratifying by variant, patients with XBB.1.5 vaccination (vs. no XBB.1.5 vaccination) had lower odds of inpatient/ED visits among JN.1 infections (aOR=0.62; 95% CI: 0.40-0.96) and among XBB-lineage infections (aOR=0.28; 95% CI: 0.18-0.43) (Figure 1), but this association was weaker among JN.1 vs XBB-lineage infections (vaccination-variant interaction, p=0.003). This interaction between vaccination and JN.1 vs XBB-lineage variant remained significant when comparing XBB.1.5-vaccinated patients to BA.4/BA.5-vaccinated (p=0.009), wild-type-vaccinated (p=0.003), and unvaccinated (p=0.035) patients. Regarding specific vaccination reference groups, protective associations for XBB.1.5 vaccination were strongest when comparing XBB.1.5-vaccinated to unvaccinated patients.
In additional analyses, findings were similar when only including adults aged ≥65 years (Supplementary Figure S2; vaccination-variant interaction, p=0.043), when comparing only inpatient vs outpatient visits (Supplementary Figure S3; interaction, p=0.013), and when excluding patients with respiratory virus coinfections (Supplementary Figure S4; interaction, p=0.005). Among JN.1 and XBB-lineage infections, similar protective associations were detected for patients XBB.1.5-vaccinated 7-59 days earlier and ≥60 days earlier (Supplementary Figure S5). BA.4/BA.5 vaccination and wild-type vaccination were also associated with lower odds of inpatient/ED visits compared to unvaccinated (Supplementary Figure S6).
In this multi-state study of adults with medically attended SARS-CoV-2 infection between September 2023 and January 2024, XBB.1.5 mRNA-vaccinated individuals had an overall 59% lower odds of having an inpatient or ED visit vs outpatient visit. Unlike numerous other observational studies examining XBB.1.5 vaccination and severe COVID-19 outcomes (8, 9, 11, 14), our study provides novel data on XBB.1.5 vaccine-associated protection against specific contemporary circulating variants. This was made possible through the linkage of viral sequencing and patient-level clinical data, which is a key component of genomic surveillance programs that enhances their capacity to evaluate vaccine effectiveness against specific SARS-CoV-2 variants (20).
While findings provide evidence that XBB.1.5 vaccines protected against severe illness associated with both JN.1 and XBB-lineage variants, protection against JN.1 was significantly lower. Among JN.1-infected patients, XBB.1.5 vaccination was associated with 38% lower odds of inpatient/ED vs outpatient visits, compared to the 72% lower odds observed among XBB-lineage-infected patients. Other studies have similarly reported lower estimates for vaccine effectiveness against JN lineages compared to XBB lineages, even when comparing similar intervals since vaccination (10, 13, 21, 22). JN.1 has more than 30 spike protein mutations compared to the XBB.1.5 spike protein, including the L455S mutation which is hypothesized to contribute to increased immune evasion (3). In contrast, XBB lineages such as EG.5 and HV.1 are more genetically similar to XBB.1.5, which could explain why XBB.1.5-adapted vaccines may be less effective against the JN.1 variant than against XBB-lineage variants. This key finding emphasizes the need for COVID-19 vaccines to be routinely updated to align with circulating strains and for individuals to stay up to date with recommended vaccines. This may be particularly evident when saltational evolution occurs, as happened to produce BA.2.86. With the addition of L455S, the JN.1 variant has displaced XBB variants.
Given that associations among JN.1-infected individuals were similar irrespective of time since XBB.1.5 vaccination, differences in protection by variant were not attributed to waning effectiveness over the approximately four months of available data. Within the sample of JN.1-infected individuals, this finding was based on a comparison of receipt of vaccination either 7-59 days earlier or 60-122 days earlier, which could have masked differences within or beyond the time frames examined. In other studies of XBB.1.5 vaccine effectiveness, there has been mixed evidence on waning effectiveness, likely related to the durations of follow-up and/or whether studies classified infections based on variant lineage. Several short-term studies found little evidence of waning effectiveness through 3-5 months post-vaccination (10, 22–26). Other studies found that effectiveness waned over time, yet individual-level sequencing data were often unavailable, and temporal differences in variant circulation may have confounded results (21, 27, 28). These findings underscore the importance of continued genomic surveillance and longer-term follow-up to better understand the durability of protection and to inform future vaccine formulation strategies.
The inverse association between XBB.1.5 vaccination (administered a median of 57 days earlier) and inpatient/ED visit type was consistently observed regardless of the specific reference group used, including prior receipt of BA.4/BA.5 vaccination approximately one year earlier, on average, without subsequent XBB.1.5 vaccination. Thus, the protection associated with XBB.1.5 vaccination was enhanced beyond the remaining immunity conferred by prior BA.4/BA.5 vaccination administered during the previous respiratory illness season. However, because vaccination subgroups varied in both vaccine type and time since last vaccination, we cannot conclusively determine whether the observed protection is primarily attributable to the vaccine type or to declining neutralizing antibody titers over time. It is also important to note that results were obtained within the context of widespread natural immunity from prior infection, which could have resulted in attenuated associations if individuals who had not received XBB.1.5 vaccination were more likely to have infection-induced immunity. Thus, estimated vaccine protection represents the incremental benefit of XBB.1.5 vaccination in a population with high levels of infection-induced and/or vaccine-induced immunity.
In this case-only study of SARS-CoV-2-positive patients with sequenced samples, we compared the vaccination status of inpatients or ED patients with that of outpatients. This measure of association provides an indicator of the vaccine’s level of protection against severe vs mild illness and facilitates a comparison across circulating variants. Other studies of XBB.1.5-adapated vaccines, either cohort studies or test-negative case-control studies, have evaluated protection against both severe and mild illness (although not for specific variants), and had similar conclusions regarding the relative protection against severe vs mild illness. In one study, effectiveness was 60% against hospitalization compared to 33% against any medically attended COVID-19, representing 82% greater protection against severe illness (29). In another study, effectiveness was 77% against hospitalization compared to 65% against symptomatic infection, representing 16% greater protection against severe illness (30). Similar patterns were also reported in studies of earlier COVID-19 vaccines. In a study of BA.4/BA.5-adapted bivalent mRNA vaccination, effectiveness was 61% against hospitalization, 46% against ED or urgent care visits, and 35% against outpatient visits (31). In a study of the original wild-type vaccines, the odds that hospitalized patients had moderate or severe illness (vs ‘mild’ illness, i.e., hospitalized for ≤24 hours) was 63%-68% lower in vaccinated vs unvaccinated individuals (32).
This study has several limitations. First, although all patients had laboratory-confirmed SARS-CoV-2 infection at a medical visit, we lacked data on symptoms and reasons for visits. Second, visit type was assessed upon sample collection for SARS-CoV-2 testing and it is unknown whether outpatients later had ED or inpatient visits, potentially contributing to misclassification. Third, we lacked data on social factors (e.g., insurance type) and underlying medical conditions, which could have resulted in residual confounding. Fourth, misclassification of vaccination status is possible if vaccine doses documented in EHRs and registries were incomplete. Vaccination status was defined based on the most recent vaccine dose administered and XBB.1.5-vaccinated individuals were not able to be further classified based on the receipt and timing of earlier booster doses. Taken together, these limitations may have introduced biases but are unlikely to account for observed differences by variant, particularly given that the infecting variant was not yet known at the time of medically attended testing. Finally, data availability for the current study only allowed for an evaluation of vaccine protection through a maximum interval of approximately four months post-vaccination; thus, we were unable to analyze durability over a longer time frame.
This study provides evidence of the effectiveness of XBB.1.5-adapted mRNA vaccines in protecting against severe illness requiring inpatient or ED visits among adults infected with JN.1 or XBB-lineage variants. These results support the prior recommendation that all adults should, irrespective of their previous COVID-19 vaccination history, receive the 2023-2024 COVID-19 vaccine to enhance their protection. However, findings also suggest that XBB.1.5 vaccines provide comparatively less protection against the JN.1 variant than they do against earlier XBB-lineage variants. Future research should continue to confirm the degree of COVID-19 vaccine protection against severe illness associated with infection with emerging SARS-CoV-2 variants and to accordingly evaluate the potential for waning effectiveness over longer durations of follow-up post-vaccination.
The datasets presented in this article are not readily available because data sharing agreements between Helix and partner institutions prohibit Helix from making this dataset publicly available. Requests to access the datasets should be directed to ML (bWF0dC5sZXZ5QGhlbGl4LmNvbQ==) and SL (c2hpc2hpLmx1b0BoZWxpeC5jb20=).
The studies involving humans were approved by WIRB CG IRB (Western Institutional Review Board, WIRB-Copernicus Group) and the Medical University of South Carolina (MUSC) Institutional Review Board for Human Research. The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants’ legal guardians/next of kin because this study presented minimal risk to participants as there was no interaction or intervention with patients.
ML: Conceptualization, Data curation, Formal Analysis, Investigation, Writing – original draft, Writing – review & editing. VC: Conceptualization, Data curation, Investigation, Writing – review & editing. PH: Investigation, Project administration, Writing – review & editing. DM: Investigation, Writing – review & editing. JG: Investigation, Project administration, Writing – review & editing. RD: Investigation, Writing – review & editing. CS: Investigation, Project administration, Writing – review & editing. WG: Investigation, Writing – review & editing. LM: Data curation, Investigation, Writing – review & editing. EC: Project administration, Writing – review & editing. DW: Data curation, Investigation, Writing – review & editing. AR: Data curation, Investigation, Writing – review & editing. HD: Data curation, Investigation, Writing – review & editing. MI: Data curation, Investigation, Writing – review & editing. NW: Data curation, Investigation, Project administration, Writing – review & editing. TB: Investigation, Writing – review & editing. KT: Investigation, Writing – review & editing. JN: Investigation, Writing – review & editing. JR: Investigation, Writing – review & editing. ES: Investigation, Writing – review & editing. WL: Project administration, Writing – review & editing. JL: Project administration, Writing – review & editing. SL: Conceptualization, Data curation, Investigation, Project administration, Writing – review & editing.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article.
The authors thank the Helix Clinical Informatics and Bioinformatics teams for their contributions to electronic health record data and viral sequencing pipelines. They also thank Catherine Clinton for her oversight and guidance in ensuring research compliance. They thank the investigators and staff at HealthPartners, Providence Health, and the Medical University of South Carolina who contributed to the ViEW Network™. This manuscript was previously published as a preprint at: https://www.medrxiv.org/content/10.1101/2024.03.05.24303796v1.
The authors declare the following commercial or financial relationships that could be construed as potential conflicts of interest: ML, VC, LM, EC, DW, AR, HD, MI, NW, TB, KT, JN, JR, ES, WL, JL, and SL are employees of Helix, Inc. ML, MI, and SL report contracted research from Pfizer. ML, MI, WL, and SL report contracted research from the Centers for Disease Control and Prevention CDC. ML reports contracted research and travel support from Novavax. PH reports contracted research from Seegene USA and Helix, Inc. JG reports contracted research from Helix, Gilead, Eli Lilly, and Regeneron, grants from Merck BARDA and Gilead, speaking honoraria and personal fees from Gilead Sciences, Inc, and Eli Lilly & Co, and collaborative services agreements with Adaptive Biotechnologies, Monogram Biosciences, and LabCorp, and serving as a speaker or advisory board member for Gilead and Eli Lilly. CS reports giving educational lectures sponsored by Eli Lilly.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
This study received funding from Helix, Inc. The funder had the following involvement with the study: study design, data collection and analysis, interpretation of data, preparation of the manuscript, and decision to submit it for publication.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2025.1470609/full#supplementary-material
1. Centers for Disease Control and Prevention. COVID data tracker: variant proportions (2024). Available online at: https://covid.cdc.gov/covid-data-tracker/variant-proportions (Accessed March 4, 2024).
2. Regan JJ, Moulia DL, Link-Gelles R, Godfrey M, Mak J, Najdowski M, et al. Use of updated COVID-19 vaccines 2023–2024 formula for persons aged ≥6 months: recommendations of the Advisory Committee on Immunization Practices — United States, September 2023. Morb Mortal Wkly Rep. (2023) 72:1140–6. doi: 10.15585/mmwr.mm7242e1
3. Yang S, Yu Y, Xu Y, Jian F, Song W, Yisimayi A, et al. Fast evolution of SARS-CoV-2 BA.2.86 to JN.1 under heavy immune pressure. Lancet Infect Dis. (2024) 24:e70–2. doi: 10.1016/S1473-3099(23)00744-2
4. Planas D, Staropoli I, Michel V, Lemoine F, Donati F, Prot M, et al. Distinct evolution of SARS-CoV-2 Omicron XBB and BA.2.86/JN.1 lineages combining increased fitness and antibody evasion. Nat Commun. (2024) 15:2254. doi: 10.1038/s41467-024-46490-7
5. Kaku Y, Okumura K, Padilla-Blanco M, Kosugi Y, Uriu K, Hinay AA Jr, et al. Virological characteristics of the SARS-CoV-2 JN.1 variant. Lancet Infect Dis. (2024) 24:e82. doi: 10.1016/S1473-3099(23)00813-7
6. Wang Q, Guo Y, Bowen A, Mellis IA, Valdez R, Gherasim C, et al. XBB.1.5 monovalent mRNA vaccine booster elicits robust neutralizing antibodies against XBB subvariants and JN.1. Cell Host Microbe. (2024) 32:315–21. doi: 10.1016/j.chom.2024.01.014
7. Chalkias S, McGhee N, Whatley JL, Essink B, Brosz A, Tomassini JE, et al. Interim report of the reactogenicity and immunogenicity of SARS-CoV-2 XBB-containing vaccines. J Infect Dis. (2024) 230:e279–86. doi: 10.1093/infdis/jiae067
8. Hansen CH, Moustsen-Helms IR, Rasmussen M, Søborg B, Ullum H, Valentiner-Branth P. Short-term effectiveness of the XBB.1.5 updated COVID-19 vaccine against hospitalisation in Denmark: a national cohort study. Lancet Infect Dis. (2024) 24:e73–4. doi: 10.1016/S1473-3099(23)00746-6
9. van Werkhoven CH, Valk A-W, Smagge B, de Melker HE, Knol MJ, Hahné SJ, et al. Early COVID-19 vaccine effectiveness of XBB.1.5 vaccine against hospitalisation and admission to intensive care, the Netherlands, 9 October to 5 December 2023. Euro Surveill. (2024) 29:2300703. doi: 10.2807/1560-7917.ES.2024.29.1.2300703
10. Link-Gelles R, Ciesla AA, Mak J, Miller JD, Silk BJ, Lambrou AS, et al. Early estimates of updated 2023-2024 (monovalent XBB.1.5) COVID-19 vaccine effectiveness against symptomatic SARS-CoV-2 infection attributable to co-circulating Omicron variants among immunocompetent adults - Increasing Community Access to Testing Program, United States, September 2023-January 2024. Morb Mortal Wkly Rep. (2024) 73:77–83. doi: 10.15585/mmwr.mm7304a2
11. Tartof SY, Slezak JM, Frankland TB, Puzniak L, Hong V, Ackerson BK, et al. Estimated effectiveness of the BNT162b2 XBB vaccine against COVID-19. JAMA Intern Med. (2024) 184:932–40. doi: 10.1001/jamainternmed.2024.1640
12. Skowronski DM, Zhan Y, Kaweski SE, Sabaiduc S, Khalid A, Olsha R, et al. 2023/24 mid-season influenza and Omicron XBB.1.5 vaccine effectiveness estimates from the Canadian Sentinel Practitioner Surveillance Network (SPSN). Euro Surveill. (2024) 29:2400076. doi: 10.2807/1560-7917.ES.2024.29.7.2400076
13. Huiberts AJ, Hoeve CE, de Gier B, Cremer J, van der Veer B, de Melker HE, et al. Effectiveness of Omicron XBB.1.5 vaccine against infection with SARS-CoV-2 Omicron XBB and JN.1 variants, prospective cohort study, the Netherlands, October 2023 to January 2024. Euro Surveill. (2024) 29:2400109. doi: 10.2807/1560-7917.ES.2024.29.10.2400109
14. DeCuir J, Payne AB, Self WH, Rowley EAK, Dascomb K, DeSilva MB, et al. Interim effectiveness of updated 2023–2024 (monovalent XBB.1.5) COVID-19 vaccines against COVID-19–associated emergency department and urgent care encounters and hospitalization among immunocompetent adults Aged ≥18 years — VISION and IVY Networks, September 2023–January 2024. Morb Mortal Wkly Rep. (2024) 73:180–8. doi: 10.15585/mmwr.mm7308a5
15. Moustsen-Helms IR, Bager P, Graakjær Larsen T, Møller FT, Skafte Vestergaard L, Rasmussen M, et al. Relative vaccine protection, disease severity and symptoms associated with the SARS-CoV-2 omicron subvariant BA.2.86 and descendent JN.1 in Denmark: a nationwide observational study. Lancet Infect Dis. (2024) 24:964–73. doi: 10.1016/S1473-3099(24)00220-2
16. Andeweg SP, de Gier B, Vennema H, van Walle I, van Maarseveen N, Kusters NE, et al. Higher risk of SARS-CoV-2 Omicron BA.4/5 infection than of BA.2 infection after previous BA.1 infection, the Netherlands, 2 May to 24 July 2022. Euro Surveill. (2023) 28:2200724. doi: 10.2807/1560-7917.ES.2023.28.7.2200724
17. Kustin T, Harel N, Finkel U, Perchik S, Harari S, Tahor M, et al. Evidence for increased breakthrough rates of SARS-CoV-2 variants of concern in BNT162b2-mRNA-vaccinated individuals. Nat Med. (2021) 27:1379–84. doi: 10.1038/s41591-021-01413-7
18. Levy ME, Chilunda V, Davis RE, Heaton PR, Pawloski PA, Goldman JD, et al. Reduced likelihood of hospitalization with the JN.1 or HV.1 severe acute respiratory syndrome coronavirus 2 variants compared with the EG.5 variant. J Infect Dis. (2024) 230:1197–201. doi: 10.1093/infdis/jiaa720
19. Bolze A, Basler T, White S, Dei Rossi A, Wyman D, Dai H, et al. Evidence for SARS-CoV-2 Delta and Omicron co-infections and recombination. Med. (2022) 3:848–59.e4. doi: 10.1016/j.medj.2022.10.002
20. Struelens MJ, Ludden C, Werner G, Sintchenko V, Jokelainen P, Ip M, et al. Real-time genomic surveillance for enhanced control of infectious diseases and antimicrobial resistance. Front Sci. (2024) 2:1298248. doi: 10.3389/fsci.2024.1298248
21. Kirsebom FC, Stowe J, Bernal JL, Allen A, Andrews N. Effectiveness of autumn 2023 COVID-19 vaccination and residual protection of prior doses against hospitalisation in England, estimated using a test-negative case-control study. J Infect. (2024) 89:106177. doi: 10.1016/j.jinf.2023.12.001
22. Tartof SY, Slezak JM, Puzniak L, Frankland TB, Ackerson BK, Jodar L, et al. Effectiveness of BNT162b2 XBB vaccine against XBB and JN.1 sublineages. Open Forum Infect Dis. (2024) 11:ofae370. doi: 10.1093/ofid/ofae370
23. Nguyen JL, Mitratza M, Volkman HR, de Munter L, Tran TM, Marques C, et al. Effectiveness of the BNT162b2 XBB.1.5-adapted vaccine against COVID-19 hospitalization related to the JN.1 variant in Europe: a test-negative case-control study using the id. DRIVE platform. EClinicalMedicine. (2025) 79:101090. doi: 10.1016/j.eclinm.2024.101090
24. Andersson NW, Thiesson EM, Pihlström N, Perälä J, Faksová K, Gram MA, et al. Comparative effectiveness of monovalent XBB. 1.5 containing covid-19 mRNA vaccines in Denmark, Finland, and Sweden: target trial emulation based on registry data. BMJ Med. (2024) 3(1):e001074.
25. Caffrey AR, Appaneal HJ, Lopes VV, Puzniak L, Zasowski EJ, Jodar L, et al. Effectiveness of BNT162b2 XBB vaccine in the US veterans affairs healthcare system. Nat Commun. (2024) 15:9490. doi: 10.1038/s41467-024-19940-0
26. Nunes B, Humphreys J, Nicolay N, Braeye T, Van Evercooren I, Holm Hansen C, et al. Monovalent XBB.1.5 COVID-19 vaccine effectiveness against hospitalisations and deaths during the Omicron BA.2.86/JN.1 period among older adults in seven European countries: A VEBIS-EHR network study. Expert Rev Vaccines. (2024) 23:1085–90. doi: 10.1080/14760584.2024.1983451
27. Lin DY, Du Y, Xu Y, Paritala S, Donahue M, Maloney P. Durability of XBB.1.5 vaccines against Omicron subvariants. N Engl J Med. (2024) 390:2124–7. doi: 10.1056/NEJMoa2400567
28. Antunes L, Mazagatos C, Martínez-Baz I, Naesens R, Borg ML, Petrović G, et al. Early COVID-19 XBB.1.5 vaccine effectiveness against hospitalisation among adults targeted for vaccination, VEBIS hospital network, Europe, October 2023–January 2024. Influenza Other Respir Viruses. (2024) 18:e13360. doi: 10.1111/irv.13360
29. Kopel H, Araujo AB, Bogdanov A, Zeng N, Winer I, Winer-Jones JP, et al. Effectiveness of the 2023–2024 Omicron XBB.1.5-containing mRNA COVID-19 vaccine (mRNA-1273.815) in preventing COVID-19–related hospitalizations and medical encounters among adults in the United States. Open Forum Infect Dis. (2024) 11:ofae695. doi: 10.1093/ofid/ofae695
30. Lee JA, Jang H, Ahn SM, Seong JE, Kim YK, Sohn Y, et al. Estimates of vaccine effectiveness of the updated monovalent XBB.1.5 COVID-19 vaccine against symptomatic SARS-CoV-2 infection, hospitalization, and receipt of oxygen therapy in South Korea, October 26 to December 31, 2023. Int J Infect Dis. (2024) 148:107249. doi: 10.1016/j.ijid.2024.07.022
31. Tartof SY, Slezak JM, Puzniak L, Hong V, Frankland TB, Ackerson BK, et al. Effectiveness of BNT162b2 BA.4/5 bivalent mRNA vaccine against a range of COVID-19 outcomes in a large health system in the USA: A test-negative case–control study. Lancet Respir Med. (2023) 11:1089–100. doi: 10.1016/S2213-2600(23)30359-4
Keywords: COVID-19 vaccines, SARS-COV-2 variants, hospitalization, emergency room visits, epidemiology
Citation: Levy ME, Chilunda V, Heaton PR, McKeen D, Goldman JD, Davis RE, Schandl CA, Glen WB, McEwen LM, Cirulli ET, Wyman D, Dei Rossi A, Dai H, Isaksson M, Washington NL, Basler T, Tsan K, Nguyen J, Ramirez J, Sandoval E, Lee W, Lu J and Luo S (2025) XBB.1.5 mRNA COVID-19 vaccine protection against inpatient or emergency department visits among adults infected with SARS-CoV-2 JN.1 and XBB-lineage variants. Front. Immunol. 16:1470609. doi: 10.3389/fimmu.2025.1470609
Received: 25 July 2024; Accepted: 03 February 2025;
Published: 17 February 2025.
Edited by:
Farid Rahimi, Australian National University, AustraliaCopyright © 2025 Levy, Chilunda, Heaton, McKeen, Goldman, Davis, Schandl, Glen, McEwen, Cirulli, Wyman, Dei Rossi, Dai, Isaksson, Washington, Basler, Tsan, Nguyen, Ramirez, Sandoval, Lee, Lu and Luo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Matthew E. Levy, bWF0dC5sZXZ5QGhlbGl4LmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.