- 1Department of Orthopedic Trauma, Zhuji People’s Hospital of Zhejiang Province, Zhuji, Zhejiang, China
- 2Department of Emergency and Critical Care, Shanghai Changzheng Hospital, Shanghai, China
Osteosarcoma, a malignant bone tumor primarily affecting adolescents, is highly invasive with a poor prognosis. While surgery and chemotherapy have improved survival for localized cases, pulmonary metastasis significantly reduces survival to approximately 20%, highlighting the need for novel treatments. Immunotherapy, which leverages the immune system to target osteosarcoma cells, shows promise. This review summarizes the biological characteristics of osteosarcoma, mechanisms of pulmonary metastasis, and the tumor immune microenvironment (TME). It involves recent immunotherapy advances, including monoclonal antibodies, tumor vaccines, immune cell therapies, checkpoint inhibitors, and oncolytic viruses, and discusses combining these with standard treatments.
1 Introduction
Osteosarcoma is a heterogeneous malignant tumor affecting bone and soft tissues, primarily in children and adolescents, with high invasiveness and a strong tendency for pulmonary metastasis (1, 2). Surgery alone results in a five-year survival rate of about 20%, but chemotherapy increases this rate to 70% (3). However, the prognosis remains poor once metastasis occurs, particularly to the lungs (4). The biological characteristics of osteosarcoma largely arise from genetic mutations in mesenchymal stem cells (MSCs), such as in P53 and RB1 genes, which increase the risk of MSCs transforming into malignant cells (5, 6). Osteosarcoma cells express Runx2 and Sox9 genes, showing features of osteoblastic and chondrogenic differentiation (7). Ewing sarcoma’s origin remains controversial, with potential sources including neural crest stem cells, embryonic progenitors, or MSCs (8). Osteosarcoma exhibits significant genetic heterogeneity, with around 7-14% of patients harboring actionable mutations, particularly in the IGF signaling pathway (9). Genome-wide studies have identified genes and pathways involved in osteosarcoma progression and metastasis, including WNT/β-catenin, Notch, and CD99, emphasizing the importance of precision medicine in diagnosis and treatment (10).
Immunotherapy has emerged as a promising treatment for cancers (11–13), utilizing immune-mediated cytotoxic effects against tumors (14). The tumor immune microenvironment (TME) includes immune cells, cytokines like IL-6 and TNF-α, and regulatory factors, all of which contribute to tumor progression and metastasis (15). Pulmonary metastasis in osteosarcoma involves the activation of WNT/β-catenin and Notch pathways, high expression of ezrin, and cytokines like TGF-β and IL-6/IL-8, which facilitate cell invasion and migration (16). Immune checkpoint inhibitors and cell therapies have shown potential in osteosarcoma treatment, though challenges remain, including treatment variability, adverse reactions, and high costs (17, 18). This review highlights the biological characteristics, molecular mechanisms of pulmonary metastasis, and progress in immunotherapy, exploring the clinical potential and challenges to inform more effective treatment strategies for osteosarcoma.
2 Classification and biological characteristics of osteosarcoma
Osteosarcoma is a heterogeneous malignancy primarily affecting bone and soft tissues, commonly seen in children and adolescents. Its pathogenesis involves genetic mutations in MSCs, notably in P53 and RB1 genes, which promote malignant transformation (2). Osteosarcoma and chondrosarcoma cells express Runx2 and Sox9 genes, indicating osteogenic and chondrogenic differentiation, respectively (7). Ewing sarcoma’s cellular origin remains debated, with possible derivations from neural crest stem cells, embryonic bone and cartilage progenitors, or MSCs. Fusion proteins in Ewing sarcoma complicate its classification (19). While impaired MSC differentiation is believed to contribute to osteosarcoma and chondrosarcoma, the exact mechanisms are still unclear.
At the molecular level, osteosarcoma shows significant genetic heterogeneity, with about 21% of patients harboring actionable mutations, especially in the IGF signaling pathway. Genome-wide association studies (GWAS) have identified susceptibility loci, including the GRM4 gene (6p21.3) and a gene desert region at 2p25.2 (20). High-grade osteosarcoma samples also show mutations in TP53, RB1, and 82 other genes. The TARGET-OS database has identified 12 survival-related genes, with eight downregulated (e.g., ERCC4, CLUAP1) and four upregulated (e.g., MUC1, JAG2) (21, 22). Recent studies highlight the role of various signaling pathways and genetic alterations in osteosarcoma progression. Weighted gene co-expression network analysis has linked osteosarcoma metastasis to pathways like microtubule formation, Cytochrome P450 drug metabolism, IL-17 signaling, DNA replication, cell adhesion, and heparin binding (23). Whole-transcriptome analysis reveals changes in extracellular matrix degradation and collagen biosynthesis (24). Additionally, CD99 suppresses osteosarcoma malignancy (25). These findings underscore the importance of genomic and transcriptomic analyses for uncovering osteosarcoma’s biological mechanisms and identifying new therapeutic targets for precision medicine.
3 Tumor immune microenvironment of osteosarcoma
TME is a complex system composed of immune cells, cytokines, and regulatory factors surrounding tumor cells. It plays a crucial role in osteosarcoma initiation, progression, and metastasis (26). This section explores the immune cells, regulatory factors, immune suppression and activation mechanisms, and tumor cell strategies to evade immune surveillance within the osteosarcoma TME.
3.1 Immune cells and immune regulatory factors
The osteosarcoma TME includes a variety of immune and non-immune cells, with stromal cells playing a key role in expressing EMT genes, which are linked to immune responses (27, 28). Alaa et al. found that stromal cells secrete cytokines promoting EMT, increasing tumor invasiveness and metastatic potential (29). Osteosarcoma stem cells, due to their chemoresistance, plasticity, and immune modulation abilities, contribute to metastasis and immune evasion (30). Several immune-related genes and cytokines are crucial in the TME (31–34). Liang et al. developed a three-gene risk model (TYROBP, TLR4, and ITGAM), regulating macrophage activation and predicting patient outcomes (35). Lipid metabolism genes were linked with the TME, suggesting their potential as prognostic biomarkers (36–38). Cytokines like IL-6 are pivotal in immune evasion and chemoresistance. Huang et al. identified IL-6’s role in promoting cell proliferation and anti-apoptotic mechanisms via the STAT3 signaling pathway (39). Additionally, mutations in P53 and RB1 within the TME can influence the behavior of immune cells (40). P53 mutations can lead to an immunosuppressive microenvironment by upregulating PD-L1 expression, thereby facilitating immune escape (41). RB1 mutations may enhance the recruitment of myeloid-derived suppressor cells (MDSCs), further contributing to immune evasion and promoting a tumor-friendly environment (42–44). These findings highlight the importance of immune regulatory factors in the osteosarcoma TME and their potential as therapeutic targets.
3.2 Interactions between osteosarcoma cells and immune cells
Single-cell RNA sequencing (scRNA-seq) and multi-omics has revealed the complexity of the TME (45–50). Huang et al. identified the diverse spatial distribution and functional states of immune cells in the osteosarcoma TME (51). Chen et al. found that lipid metabolism gene expression is closely linked to the TME, serving as reliable prognostic biomarkers (52). These studies highlight the importance of immune cell distribution and gene expression in developing targeted therapies and improving patient outcomes. Interactions between osteosarcoma cells and immune cells are pivotal in tumor immune evasion and progression. While normal lymphocytes can exert cytotoxic effects on osteosarcoma cells in vitro, osteosarcoma cells can disrupt dendritic cell (DC) function, impairing immune responses (53, 54). Grzegorz et al. showed that osteosarcoma cells secrete IL-10, inhibiting DC maturation and antigen presentation (55). Additionally, osteosarcoma cells interact with host cells and immune responses at multiple levels (56). These interactions provide insights into osteosarcoma pathogenesis and suggest potential targets for immune-based therapies. Audrey et al. found that osteosarcoma cells secrete TGF-β, which suppresses T cell activity and aids immune evasion (57).
3.3 Immune suppression and activation in osteosarcoma
Osteosarcoma is often a “cold tumor” with limited immune cell infiltration, leading to immune suppression through upregulated factors like PD-L1 (58). Despite this, some studies suggest that immune activation is possible using immune checkpoint inhibitors. Park et al. demonstrated that PD-1 inhibitors enhanced T cell cytotoxicity against osteosarcoma cells (17). Additionally, osteosarcoma cells suppress immune responses by modulating CXCL12 (59). Neoadjuvant chemotherapy can transform osteosarcoma into an immunologically “hot” tumor, activating the local immune environment. Myrofora et al. found that chemotherapy increased T cell infiltration in osteosarcoma, suggesting it promotes immune activation, creating potential for immunotherapeutic strategies (60).
3.4 Immune evasion mechanisms
Osteosarcoma cells evade immune responses through extracellular matrix alterations, immune suppressive pathways, and high PD-L1 expression, which inhibit T cell activity (17). Osteosarcoma cells upregulate PD-L1 as a strategic mechanism to evade immune surveillance, thereby facilitating tumor progression and resistance to therapeutic interventions (61). Additionally, TGF-β promotes regulatory T cell (Treg) expansion, further suppressing immunity (62). Targeting immune evasion mechanisms offers promising strategies. Dong et al. showed that inhibiting TGF-β reduced osteosarcoma cell invasiveness (63). Combining PD-L1 and TGF-β inhibitors enhanced immune cell cytotoxicity against osteosarcoma, underscoring the potential of combination therapies to overcome immune escape (64).
4 Immunotherapy strategies for osteosarcoma
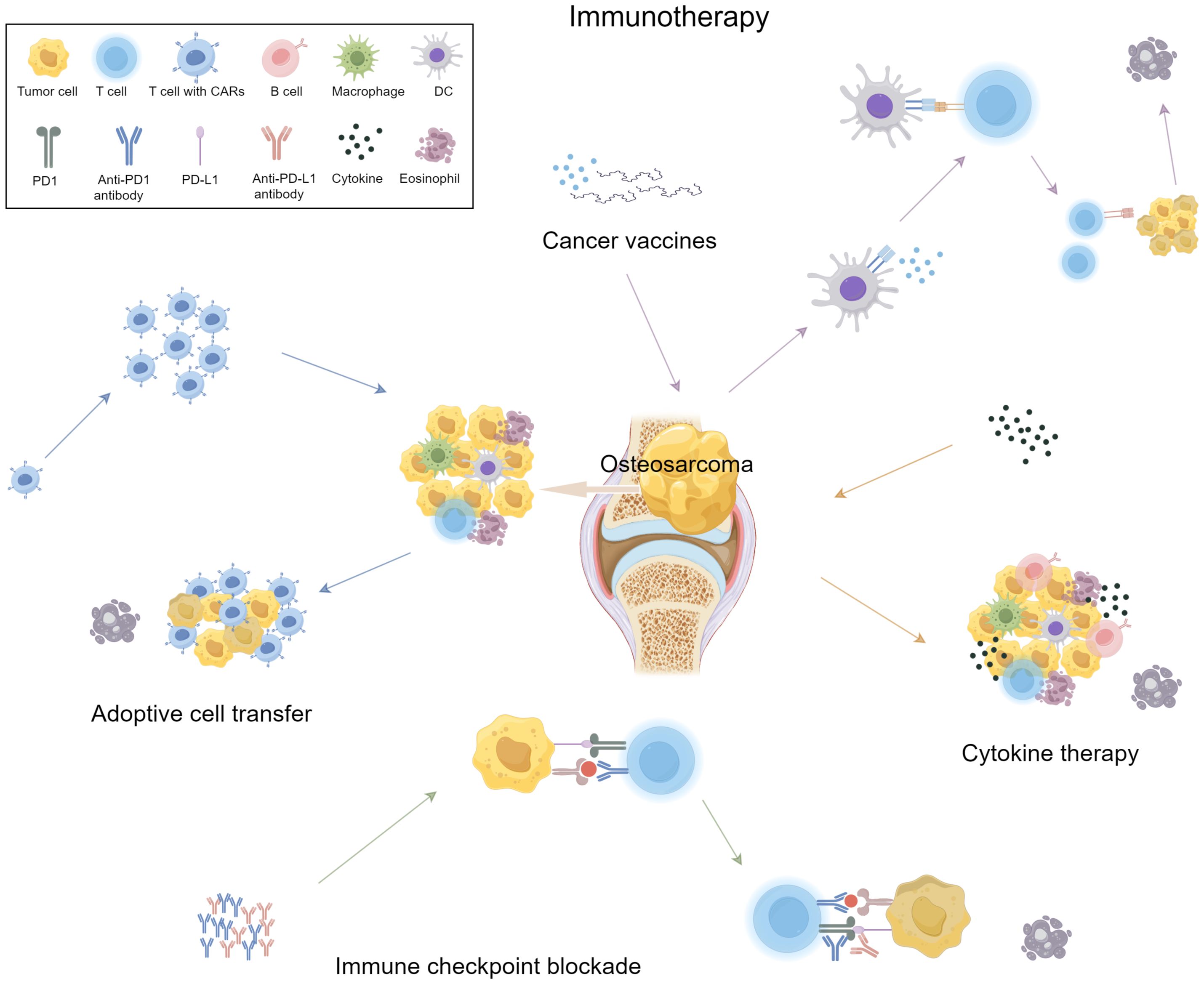
Immunotherapy offers a more targeted approach to cancer treatment compared to traditional chemotherapy, which generally attacks rapidly dividing cells (65). The immune system, through processes like immune surveillance and cell infiltration, plays a crucial role in fighting cancer (66). However, tumor cells can evade immune responses through mechanisms such as immune editing, which includes three phases: elimination, equilibrium, and escape (67). In the elimination phase, immune cells target and destroy cancer cells. In the equilibrium phase, some tumor cells survive and adapt, entering a dormant state. Eventually, these cells may escape immune detection and proliferate (68, 69). Mechanisms of immune escape include loss of tumor antigens, downregulation of HLA expression, recruitment of immune-suppressive cells like Tregs and M2 macrophages, and the upregulation of immune checkpoint receptors such as CTLA-4 and PD-1 (70–72). Immunotherapy seeks to counteract these escape mechanisms by boosting the immune system’s ability to recognize and destroy tumor cells (Figure 1).
4.1 Antibody-based therapies targeting cell surface proteins
Osteosarcoma cells express specific surface antigens that are potential targets for immunotherapy (17). Monoclonal antibodies can bind to these antigens, activating NK cells and macrophages to release cytotoxic substances, leading to tumor destruction via antibody-dependent cellular cytotoxicity (ADCC) (73, 74). For instance, Persaud et al. (75) demonstrated the efficacy of antibody therapy in neuroblastoma, suggesting similar potential in osteosarcoma. Bispecific T-cell engagers (BiTEs), which bind both T cell CD3 receptors and tumor antigens, enhance T cell activation and cancer cell lysis (76). Holzmayer et al. showed that bispecific antibodies boosted T cell-mediated osteosarcoma cell killing (77). Additionally, antibody-drug conjugates (ADCs) link antibodies to cytotoxic agents like vedotin, targeting cancer cells with higher specificity and efficacy (78). Antibody-based therapies can cause infusion reactions (fever, chills, allergies), cardiotoxicity, neurotoxicity, infections, requiring careful monitoring and supportive care (79).
4.2 Tumor vaccines
Tumor vaccines function by exposing or administering tumor antigens to induce tumor-specific immune responses, thereby enabling the immune system to recognize and attack tumor cells. These vaccines come in various forms, including whole tumor cells, lysates, proteins, DNA, RNA, and peptides (80). DCs are pivotal antigen-presenting cells capable of activating T cells and stimulating the proliferation of cytotoxic T lymphocytes (CTLs) (81). For instance, Lu et al. (3) developed DC vaccines by combining tumor cell lysates with immunostimulatory adjuvants, significantly enhancing immune-mediated cytotoxicity against osteosarcoma in patients. Moreover, the development of personalized tumor vaccines, such as those based on patient-specific tumor mutations, is emerging as a critical component of precision medicine (82). These vaccines offer new avenues for osteosarcoma immunotherapy by providing tailored immune responses against unique tumor antigens. The integration of personalized vaccines into clinical practice holds promise for improving treatment outcomes and patient survival rates. Adverse reactions to tumor vaccines are generally mild, including injection site inflammation and systemic symptoms, though rare severe immune-mediated events may occur (83). Ongoing research aims to mitigate these effects through enhanced vaccine design and delivery.
4.3 Immune cell therapy
Immune cell therapy is a promising approach for metastatic and recurrent osteosarcoma, particularly when combined with neoadjuvant chemotherapy. Neoadjuvant chemotherapy activates the local immune milieu, transforming osteosarcoma into an immunologically “hot” tumor, thereby enhancing the efficacy of subsequent immunotherapies (59). Wang et al. observed increased T cell infiltration in the tumor microenvironment post-chemotherapy, improving immune responses (84). Mifamurtide has shown clinical efficacy as adjuvant therapy for non-metastatic osteosarcoma, indicating that immune checkpoint inhibitors may significantly improve prognosis (85). Additionally, immune-related gene expression diagnostics could support personalized treatments (86). Challenges include patient selection and managing immune-related adverse effects (87). Phase I/II trials are addressing these to enhance safety and efficacy while elucidating the tumor microenvironment’s role in osteosarcoma pathogenesis (88). Integration of immunotherapies, such as mifamurtide and checkpoint inhibitors, holds substantial potential for better outcomes (89). Adoptive cell therapy, including Chimeric antigen receptor T-cell (CAR-T) therapy targeting HER2 (90), NK cells and tumor-infiltrating lymphocytes (TILs) enhances anti-tumor immunity by overcoming immune escape (91, 92). These findings highlight the potential of adoptive cell therapy in osteosarcoma treatment, though clinical application requires further optimization. However, immune cell therapies may induce cytokine release syndrome, neurotoxicity, and autoimmunity (93), which can be mitigated through monitoring, safety switches, and supportive care.
4.4 Checkpoint inhibitors
Immune checkpoint inhibitors block inhibitory signals between tumor and immune cells, reactivating T cell-mediated anti-tumor responses. Osteosarcoma cells often upregulate immune checkpoint molecules like PD-L1, which suppress T cell activity and facilitate immune escape (15). Studies show that anti-PD-1 and anti-PD-L1 antibodies significantly improve survival in osteosarcoma mouse models and reduce pulmonary metastasis (94). Zheng et al. found that anti-PD-1 antibody treatment effectively controlled lung metastases in osteosarcoma models (95). Combining checkpoint inhibitors with chemotherapeutic agents (e.g., doxorubicin) is an effective strategy, as chemotherapy can reverse the tumor’s immunosuppressive state, enhancing inhibitor efficacy (96). Additionally, combining checkpoint inhibitors with radiotherapy shows potential, though more clinical evidence is needed. Overall, immune checkpoint inhibitors offer substantial promise for osteosarcoma treatment, particularly in prolonging survival and addressing pulmonary metastasis. However, optimizing and personalizing their use remains an important area for future research. Checkpoint inhibitors, while effective, induce immune-related adverse events across multiple organs (97), necessitating immunosuppression and careful monitoring.
4.5 Oncolytic virus therapy
Oncolytic virus therapy employs genetically engineered viruses that selectively replicate within and lyse malignant cells, representing an innovative immunotherapeutic approach. These viruses not only exert specific cytotoxic effects on tumor cells but also promote an inflammatory response within the tumor microenvironment, enhancing antigen presentation and the maturation of antigen-presenting cells, thereby boosting the immune system’s ability to recognize and attack tumors (98, 99). Recently, several genetically modified oncolytic viruses have shown promise in preclinical and clinical trials for osteosarcoma. Herpes simplex virus (HSV), a complex double-stranded DNA virus, has been genetically modified (e.g., G207 and NV1020) to enhance its selective oncolytic activity against tumor cells while minimizing damage to normal cells (100). Neeti et al. reported that G207 exhibited significant oncolytic activity in osteosarcoma cell lines and effectively inhibited tumor growth in animal models (101). Similarly, adenoviruses (e.g., VCN-01) and modified Delta-24-RGD oncolytic adenoviruses have demonstrated potent anti-tumor effects, capable of suppressing primary osteosarcoma growth and preventing metastasis (102, 103). Additionally, vaccinia virus (VV), known for its efficient replication and large genome capacity, has shown considerable potential in tumor therapy (104). Morales et al. found that genetically modified VV exhibited significant anti-tumor efficacy in osteosarcoma models, further validating its feasibility as an emerging immunotherapeutic agent (105). In summary, oncolytic virus therapy, as an innovative immunotherapeutic approach, has demonstrated favorable safety and efficacy profiles in osteosarcoma treatment, supporting its further clinical application and research. Adverse reactions to oncolytic virus therapy include flu-like symptoms, injection site reactions, rare systemic inflammation (e.g., myocarditis, cytokine release syndrome), and potential viral transmission, necessitating strict biosafety protocols (106).
4.6 Combination therapy strategies
Combination therapies are increasingly recognized as essential for osteosarcoma treatment, integrating traditional approaches such as chemotherapy, radiotherapy, and surgery with emerging immunotherapies (107). This integrative strategy enhances therapeutic efficacy, reduces recurrence, and improves survival. For instance, a recent phase II clinical trial combined PD-L1 inhibitors with doxorubicin-based chemotherapy, demonstrating a synergistic effect that improved response rates and overcame chemoresistance in osteosarcoma patients (108). Another study combined CTLA-4 inhibitors with targeted therapies against the IGF signaling pathway, resulting in enhanced tumor regression and reduced metastatic spread (109). These examples illustrate how combination therapies can effectively address the complex resistance mechanisms inherent in osteosarcoma. Additionally, combining PD-L1 antibodies with chemotherapy agents can reverse chemotherapy-induced immunosuppression, boosting the immune system’s ability to target tumor cells (110). Additionally, trials involving the combination of immune checkpoint inhibitors with PARP inhibitors have shown promising results in preclinical models, suggesting potential for overcoming DNA repair-related resistance in osteosarcoma. Clinical trials by Zhang et al. demonstrated improved response rates with this combination (111). Demethylation pretreatment combined with immunotherapy also shows promise, with Wang et al. finding that demethylating agents enhance immune recognition and treatment efficacy (112). Furthermore, integrating oncolytic virus therapy with checkpoint blockade has enhanced antigen presentation and T cell infiltration, overcoming the immunosuppressive tumor microenvironment (113). Other novel therapies, including inhalation therapy, targeted radiotherapy, and antibody therapy, improved survival when combining targeted radiotherapy with antibody therapy (114).
Multimodal approaches incorporating surgery and radiotherapy have shown significant benefits, such as reducing tumor recurrence and enhancing survival rates in jaw osteosarcoma (114). Furthermore, the presence of P53 and RB1 mutations may influence the efficacy of combination therapies. For instance, tumors harboring P53 mutations may exhibit resistance to certain chemotherapeutic agents, necessitating the inclusion of targeted immunotherapies to overcome this resistance (115). Similarly, RB1 mutations may enhance the metastatic potential of osteosarcoma cells, making it imperative to integrate therapies that specifically address metastatic pathways alongside conventional treatments (116). Overall, combination therapy offers more comprehensive, personalized treatment options, improving therapeutic outcomes by integrating traditional and novel approaches and significantly reducing recurrence and mortality rates. However, combination therapies may exacerbate adverse reactions, including myelosuppression and organ toxicities (117, 118). This underscores the imperative for meticulous monitoring and the development of individualized treatment protocols to mitigate such risks effectively.
5 Conclusion
Immunotherapy strategies for osteosarcoma encompass a range of approaches, including antibody-based therapies, tumor vaccines, immune cell therapies, adoptive cell therapies, checkpoint inhibitors, and oncolytic virus therapies. By thoroughly understanding the distribution and roles of immune cells within the tumor immune microenvironment, the mechanisms of immune regulation, and the strategies employed by tumor cells to evade immune responses, researchers can develop more precise and effective immunotherapeutic protocols. Although immunotherapy has shown substantial promise in the treatment of osteosarcoma, several challenges remain, such as the realization of personalized treatment, management of immune-related adverse effects, and control of treatment costs. Future research should focus on optimizing immunotherapy strategies, exploring the best combinations for multimodal therapy, and validating their safety and efficacy through clinical trials. These efforts are essential to advancing immunotherapy for osteosarcoma, ultimately improving clinical outcomes and the quality of life for patients.
Author contributions
CL: Writing – original draft. XM: Writing – original draft. DZ: Writing – review & editing, Writing – original draft.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that no competing financial interests or commercial relationships have influenced the research presented herein.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Brown HK, Tellez-Gabriel M, Heymann D. Cancer stem cells in osteosarcoma. Cancer Lett. (2017) 386:189–95. doi: 10.1016/j.canlet.2016.11.019
2. Ritter J, Bielack SS. Osteosarcoma. Ann Oncol. (2010) 21 Suppl 7:vii320–325. doi: 10.1093/annonc/mdq276
3. Lu Y, Zhang J, Chen Y, Kang Y, Liao Z, He Y, et al. Novel immunotherapies for osteosarcoma. Front Oncol. (2022) 12:830546. doi: 10.3389/fonc.2022.830546
4. Odri GA, Tchicaya-Bouanga J, Yoon DJY, Modrowski D. Metastatic progression of osteosarcomas: A review of current knowledge of environmental versus oncogenic drivers. Cancers (Basel). (2022) 14. doi: 10.3390/cancers14020360
5. Rohilla R, Shafiq N, Malhotra S. Efficacy and safety of aspirin as an adjunctive therapy in tubercular meningitis: A systematic review and meta-analysis. EClinicalMedicine. (2021) 34:100819. doi: 10.1016/j.eclinm.2021.100819
6. Urlic I, Jovicic MS, Ostojic K, Ivkovic A. Cellular and genetic background of osteosarcoma. Curr Issues Mol Biol. (2023) 45:4344–58. doi: 10.3390/cimb45050276
7. Li N, Luo D, Hu X, Luo W, Lei G, Wang Q, et al. RUNX2 and osteosarcoma. Anticancer Agents Med Chem. (2015) 15:881–7. doi: 10.2174/1871520615666150304151228
8. Monument MJ, Bernthal NM, Randall RL. Salient features of mesenchymal stem cells-implications for Ewing sarcoma modeling. Front Oncol. (2013) 3:24. doi: 10.3389/fonc.2013.00024
9. Liu W, Cheng H, Huang Z, Li Y, Zhang Y, Yang Y, et al. The correlation between clinical outcomes and genomic analysis with high risk factors for the progression of osteosarcoma. Mol Oncol. (2024) 18:939–55. doi: 10.1002/1878-0261.13526
10. Perkins RS, Murray G, Suthon S, Davis L, Perkins NB 3rd, Fletcher L, et al. WNT5B drives osteosarcoma stemness, chemoresistance and metastasis. Clin Transl Med. (2024) 14:e1670. doi: 10.1002/ctm2.v14.5
11. Xia Z, Chen S, He M, Li B, Deng Y, Yi L, et al. Editorial: Targeting metabolism to activate T cells and enhance the efficacy of checkpoint blockade immunotherapy in solid tumors. Front Immunol. (2023) 14:1247178. doi: 10.3389/fimmu.2023.1247178
12. Zhang X, Zhang P, Cong A, Feng Y, Chi H, Xia Z, et al. Unraveling molecular networks in thymic epithelial tumors: deciphering the unique signatures. Front Immunol. (2023) 14:1264325. doi: 10.3389/fimmu.2023.1264325
13. Wang Y, Ma L, He J, Gu H, Zhu H. Identification of cancer stem cell-related genes through single cells and machine learning for predicting prostate cancer prognosis and immunotherapy. Front Immunol. (2024) 15:1464698. doi: 10.3389/fimmu.2024.1464698
14. Xie H, Xi X, Lei T, Liu H, Xia Z. CD8(+) T cell exhaustion in the tumor microenvironment of breast cancer. Front Immunol. (2024) 15:1507283. doi: 10.3389/fimmu.2024.1507283
15. Orrapin S, Moonmuang S, Udomruk S, Yongpitakwattana P, Pruksakorn D, Chaiyawat P. Unlocking the tumor-immune microenvironment in osteosarcoma: insights into the immune landscape and mechanisms. Front Immunol. (2024) 15:1394284. doi: 10.3389/fimmu.2024.1394284
16. Nirala BK, Yamamichi T, Yustein JT. Deciphering the signaling mechanisms of osteosarcoma tumorigenesis. Int J Mol Sci. (2023) 24. doi: 10.3390/ijms241411367
17. Park JA, Cheung NV. Promise and challenges of T cell immunotherapy for osteosarcoma. Int J Mol Sci. (2023) 24. doi: 10.3390/ijms241512520
18. Wang K, Qin X, Ran T, Pan Y, Hong Y, Wang J, et al. Causal link between gut microbiota and four types of pancreatitis: a genetic association and bidirectional Mendelian randomization study. Front Microbiol. (2023) 14:1290202. doi: 10.3389/fmicb.2023.1290202
19. Todorova R. Ewing's sarcoma cancer stem cell targeted therapy. Curr Stem Cell Res Ther. (2014) 9:46–62. doi: 10.2174/1574888x08666131203123125
20. Fan TM, Khanna C. Comparative aspects of osteosarcoma pathogenesis in humans and dogs. Vet Sci. (2015) 2:210–30. doi: 10.3390/vetsci2030210
21. Urban W, Krzystanska D, Piekarz M, Nazar J, Jankowska A. Osteosarcoma's genetic landscape painted by genes' mutations. Acta Biochim Pol. (2023) 70:671–8. doi: 10.18388/abp.2020_6869
22. Rothzerg E, Xu J, Wood D, Koks S. 12 Survival-related differentially expressed genes based on the TARGET-osteosarcoma database. Exp Biol Med (Maywood). (2021) 246:2072–81. doi: 10.1177/15353702211007410
23. Wang JS, Wang YG, Zhong YS, Li XD, Du SX, Xie P, et al. Identification of co-expression modules and pathways correlated with osteosarcoma and its metastasis. World J Surg Oncol. (2019) 17:46. doi: 10.1186/s12957-019-1587-7
24. Ho XD, Phung P, Le QV, HN V, Reimann E, Prans E, et al. Whole transcriptome analysis identifies differentially regulated networks between osteosarcoma and normal bone samples. Exp Biol Med (Maywood). (2017) 242:1802–11. doi: 10.1177/1535370217736512
25. Manara MC, Pasello M, Scotlandi K. CD99: A cell surface protein with an oncojanus role in tumors. Genes (Basel). (2018) 9. doi: 10.3390/genes9030159
26. Biray Avci C, Goker Bagca B, Nikanfar M, Takanlou LS, Takanlou MS, Nourazarian A. Tumor microenvironment and cancer metastasis: molecular mechanisms and therapeutic implications. Front Pharmacol. (2024) 15:1442888. doi: 10.3389/fphar.2024.1442888
27. Li MP, Long SP, Liu WC, Long K, Gao XH. EMT-related gene classifications predict the prognosis, immune infiltration, and therapeutic response of osteosarcoma. Front Pharmacol. (2024) 15:1419040. doi: 10.3389/fphar.2024.1419040
28. Cascini C, Chiodoni C. The immune landscape of osteosarcoma: implications for prognosis and treatment response. Cells. (2021) 10. doi: 10.3390/cells10071668
29. El Alaa RSA, Al-Mannai W, Darwish N, Al-Mansoori L. Adipose-derived stromal cells and cancer-associated fibroblasts: interactions and implications in tumor progression. Int J Mol Sci. (2024) 25. doi: 10.3390/ijms252111558
30. Della Sala G, Pacelli C, Agriesti F, Laurenzana I, Tucci F, Tamma M, et al. Unveiling metabolic vulnerability and plasticity of human osteosarcoma stem and differentiated cells to improve cancer therapy. Biomedicines. (2021) 10. doi: 10.3390/biomedicines10010028
31. Li C, Wirth U, Schardey J, Ehrlich-Treuenstatt VV, Bazhin AV, Werner J, et al. An immune-related gene prognostic index for predicting prognosis in patients with colorectal cancer. Front Immunol. (2023) 14:1156488. doi: 10.3389/fimmu.2023.1156488
32. Pang ZQ, Wang JS, Wang JF, Wang YX, Ji B, Xu YD, et al. JAM3: A prognostic biomarker for bladder cancer via epithelial-mesenchymal transition regulation. Biomol BioMed. (2024) 24:897–911. doi: 10.17305/bb.2024.9979
33. Zhang H, Xia T, Xia Z, Zhou H, Li Z, Wang W, et al. KIF18A inactivates hepatic stellate cells and alleviates liver fibrosis through the TTC3/Akt/mTOR pathway. Cell Mol Life Sci. (2024) 81:96. doi: 10.1007/s00018-024-05114-5
34. Liu T, Li C, Zhang J, Hu H, Li C. Unveiling efferocytosis-related signatures through the integration of single-cell analysis and machine learning: a predictive framework for prognosis and immunotherapy response in hepatocellular carcinoma. Front Immunol. (2023) 14:1237350. doi: 10.3389/fimmu.2023.1237350
35. Liang T, Chen J, Xu G, Zhang Z, Xue J, Zeng H, et al. TYROBP, TLR4 and ITGAM regulated macrophages polarization and immune checkpoints expression in osteosarcoma. Sci Rep. (2021) 11:19315. doi: 10.1038/s41598-021-98637-x
36. Zhu M, Zeng Q, Fan T, Lei Y, Wang F, Zheng S, et al. Clinical significance and immunometabolism landscapes of a novel recurrence-Associated lipid metabolism signature in early-Stage lung adenocarcinoma: A comprehensive analysis. Front Immunol. (2022) 13:783495. doi: 10.3389/fimmu.2022.783495
37. Xiao J, Lin H, Liu B, Xia Z, Zhang J, Jin J. Decreased S1P and SPHK2 are involved in pancreatic acinar cell injury. biomark Med. (2019) 13:627–37. doi: 10.2217/bmm-2018-0404
38. Wang K, Wang S, Qin X, Chen Y, Chen Y, Wang J, et al. The causal relationship between gut microbiota and biliary tract cancer: comprehensive bidirectional Mendelian randomization analysis. Front Cell Infect Microbiol. (2024) 14:1308742. doi: 10.3389/fcimb.2024.1308742
39. Huang B, Lang X, Li X. The role of IL-6/JAK2/STAT3 signaling pathway in cancers. Front Oncol. (2022) 12:1023177. doi: 10.3389/fonc.2022.1023177
40. Hientz K, Mohr A, Bhakta-Guha D, Efferth T. The role of p53 in cancer drug resistance and targeted chemotherapy. Oncotarget. (2017) 8:8921–46. doi: 10.18632/oncotarget.13475
41. Wang C, Tan JYM, Chitkara N, Bhatt S. TP53 mutation-mediated immune evasion in cancer: mechanisms and therapeutic implications. Cancers (Basel). (2024) 16. doi: 10.3390/cancers16173069
42. Youn JI, Kumar V, Collazo M, Nefedova Y, Condamine T, Cheng P, et al. Epigenetic silencing of retinoblastoma gene regulates pathologic differentiation of myeloid cells in cancer. Nat Immunol. (2013) 14:211–20. doi: 10.1038/ni.2526
43. Deng Y, Shi M, Yi L, Naveed Khan M, Xia Z, Li X. Eliminating a barrier: Aiming at VISTA, reversing MDSC-mediated T cell suppression in the tumor microenvironment. Heliyon. (2024) 10:e37060. doi: 10.1016/j.heliyon.2024.e37060
44. Hu Y, Wang K, Chen Y, Jin Y, Guo Q, Tang H. Causal relationship between immune cell phenotypes and risk of biliary tract cancer: evidence from Mendelian randomization analysis. Front Immunol. (2024) 15:1430551. doi: 10.3389/fimmu.2024.1430551
45. Sun Z, Wang J, Fan Z, Yang Y, Meng X, Ma Z, et al. Investigating the prognostic role of lncRNAs associated with disulfidptosis-related genes in clear cell renal cell carcinoma. J Gene Med. (2024) 26:e3608. doi: 10.1002/jgm.v26.1
46. Wang Y, Wang J, He J, Ji B, Pang Z, Wang J, et al. Comprehensive analysis of PRPF19 immune infiltrates, DNA methylation, senescence-associated secretory phenotype and ceRNA network in bladder cancer. Front Immunol. (2023) 14:1289198. doi: 10.3389/fimmu.2023.1289198
47. Wang J, Zuo Z, Yu Z, Chen Z, Tran LJ, Zhang J, et al. Collaborating single-cell and bulk RNA sequencing for comprehensive characterization of the intratumor heterogeneity and prognostic model development for bladder cancer. Aging (Albany NY). (2023) 15:12104–19. doi: 10.18632/aging.205166
48. Wang Y, He J, Zhao Q, Bo J, Zhou Y, Sun H, et al. Evaluating the predictive value of angiogenesis-related genes for prognosis and immunotherapy response in prostate adenocarcinoma using machine learning and experimental approaches. Front Immunol. (2024) 15:1416914. doi: 10.3389/fimmu.2024.1416914
49. Zhang J, Peng G, Chi H, Yang J, Xie X, Song G, et al. CD8 + T-cell marker genes reveal different immune subtypes of oral lichen planus by integrating single-cell RNA-seq and bulk RNA-sequencing. BMC Oral Health. (2023) 23:464. doi: 10.1186/s12903-023-03138-0
50. Wang Y, Wang J, Liu Y, Wang X, Ren M. Multidimensional pan-cancer analysis of HSPA5 and its validation in the prognostic value of bladder cancer. Heliyon. (2024) 10:e27184. doi: 10.1016/j.heliyon.2024.e27184
51. Huang R, Wang X, Yin X, Zhou Y, Sun J, Yin Z, et al. Combining bulk RNA-sequencing and single-cell RNA-sequencing data to reveal the immune microenvironment and metabolic pattern of osteosarcoma. Front Genet. (2022) 13:976990. doi: 10.3389/fgene.2022.976990
52. Chen W, Zhao Z, Zhou H, Dong S, Li X, Hu S, et al. Development of prognostic signatures and risk index related to lipid metabolism in ccRCC. Front Oncol. (2024) 14:1378095. doi: 10.3389/fonc.2024.1378095
53. Casanova JM, Almeida JS, Reith JD, Sousa LM, Fonseca R, Freitas-Tavares P, et al. Tumor-infiltrating lymphocytes and cancer markers in osteosarcoma: influence on patient survival. Cancers (Basel). (2021) 13. doi: 10.3390/cancers13236075
54. Gupta YH, Khanom A, Acton SE. Control of dendritic cell function within the tumour microenvironment. Front Immunol. (2022) 13:733800. doi: 10.3389/fimmu.2022.733800
55. Woszczek G, Chen LY, Nagineni S, Shelhamer JH. IL-10 inhibits cysteinyl leukotriene-induced activation of human monocytes and monocyte-derived dendritic cells. J Immunol. (2008) 180:7597–603. doi: 10.4049/jimmunol.180.11.7597
56. Fan Q, Wang Y, Cheng J, Pan B, Zang X, Liu R, et al. Single-cell RNA-seq reveals T cell exhaustion and immune response landscape in osteosarcoma. Front Immunol. (2024) 15:1362970. doi: 10.3389/fimmu.2024.1362970
57. Lamora A, Talbot J, Mullard M, Brounais-Le Royer B, Redini F, Verrecchia F. TGF-beta signaling in bone remodeling and osteosarcoma progression. J Clin Med. (2016) 5. doi: 10.3390/jcm5110096
58. Toda Y, Kohashi K, Yamada Y, Yoshimoto M, Ishihara S, Ito Y, et al. PD-L1 and IDO1 expression and tumor-infiltrating lymphocytes in osteosarcoma patients: comparative study of primary and metastatic lesions. J Cancer Res Clin Oncol. (2020) 146:2607–20. doi: 10.1007/s00432-020-03242-6
59. Cheng S, Wang H, Kang X, Zhang H. Immunotherapy innovations in the fight against osteosarcoma: emerging strategies and promising progress. Pharmaceutics. (2024) 16. doi: 10.3390/pharmaceutics16020251
60. Panagi M, Mpekris F, Voutouri C, Hadjigeorgiou AG, Symeonidou C, Porfyriou E, et al. Stabilizing tumor-Resident mast cells restores T-Cell infiltration and sensitizes sarcomas to PD-L1 inhibition. Clin Cancer Res. (2024) 30:2582–97. doi: 10.1158/1078-0432.CCR-24-0246
61. Zhang J, Song Y, Ahn AR, Park HS, Park SH, Moon YJ, et al. PAK4 is involved in the stabilization of PD-L1 and the resistance to doxorubicin in osteosarcoma and predicts the survival of diagnosed patients. Cells. (2024) 13. doi: 10.3390/cells13171444
62. Dahmani A, Delisle JS. TGF-beta in T cell biology: implications for cancer immunotherapy. Cancers (Basel). (2018) 10. doi: 10.3390/cancers10060194
63. Dong F, Liu T, Jin H, Wang W. Chimaphilin inhibits human osteosarcoma cell invasion and metastasis through suppressing the TGF-beta1-induced epithelial-to-mesenchymal transition markers via PI-3K/Akt, ERK1/2, and Smad signaling pathways. Can J Physiol Pharmacol. (2018) 96:1–7. doi: 10.1139/cjpp-2016-0522
64. Yi M, Li T, Niu M, Wu Y, Zhao Z, Wu K. TGF-beta: A novel predictor and target for anti-PD-1/PD-L1 therapy. Front Immunol. (2022) 13:1061394. doi: 10.3389/fimmu.2022.1061394
65. Garg P, Pareek S, Kulkarni P, Horne D, Salgia R, Singhal SS. Next-generation immunotherapy: advancing clinical applications in cancer treatment. J Clin Med. (2024) 13. doi: 10.3390/jcm13216537
66. Burgos-Molina AM, Tellez Santana T, Redondo M, Bravo Romero MJ. The crucial role of inflammation and the immune system in colorectal cancer carcinogenesis: A comprehensive perspective. Int J Mol Sci. (2024) 25. doi: 10.3390/ijms25116188
67. Mundhara N, Sadhukhan P. Cracking the codes behind cancer cells' Immune evasion. Int J Mol Sci. (2024) 25. doi: 10.3390/ijms25168899
68. Teng MW, Swann JB, Koebel CM, Schreiber RD, Smyth MJ. Immune-mediated dormancy: an equilibrium with cancer. J Leukoc Biol. (2008) 84:988–93. doi: 10.1189/jlb.1107774
69. Wilczynski JR, Nowak M. Cancer immunoediting: elimination, equilibrium, and immune escape in solid tumors. Exp Suppl. (2022) 113:1–57. doi: 10.1007/978-3-030-91311-3_1
70. Tang Q, Chen Y, Li X, Long S, Shi Y, Yu Y, et al. The role of PD-1/PD-L1 and application of immune-checkpoint inhibitors in human cancers. Front Immunol. (2022) 13:964442. doi: 10.3389/fimmu.2022.964442
71. Yang H, Zou X, Yang S, Zhang A, Li N, Ma Z. Identification of lactylation related model to predict prognostic, tumor infiltrating immunocytes and response of immunotherapy in gastric cancer. Front Immunol. (2023) 14:1149989. doi: 10.3389/fimmu.2023.1149989
72. Zhai X, Zhang H, Xia Z, Liu M, Du G, Jiang Z, et al. Oxytocin alleviates liver fibrosis via hepatic macrophages. JHEP Rep. (2024) 6:101032. doi: 10.1016/j.jhepr.2024.101032
73. Dixon KJ, Wu J, Walcheck B. Engineering anti-tumor monoclonal antibodies and fc receptors to enhance ADCC by human NK cells. Cancers (Basel). (2021) 13. doi: 10.3390/cancers13020312
74. Wang K, Wang S, Chen Y, Lu X, Wang D, Zhang Y, et al. Causal relationship between gut microbiota and risk of gastroesophageal reflux disease: a genetic correlation and bidirectional Mendelian randomization study. Front Immunol. (2024) 15:1327503. doi: 10.3389/fimmu.2024.1327503
75. Persaud NV, Park JA, Cheung NKV. High-risk neuroblastoma challenges and opportunities for antibody-based cellular immunotherapy. J Clin Med. (2024) 13. doi: 10.3390/jcm13164765
76. Einsele H, Borghaei H, Orlowski RZ, Subklewe M, Roboz GJ, Zugmaier G, et al. The BiTE (bispecific T-cell engager) platform: Development and future potential of a targeted immuno-oncology therapy across tumor types. Cancer. (2020) 126:3192–201. doi: 10.1002/cncr.v126.14
77. Holzmayer SJ, Liebel K, Hagelstein I, Salih HR, Marklin M. The bispecific B7H3xCD3 antibody CC-3 induces T cell immunity against bone and soft tissue sarcomas. Front Immunol. (2024) 15:1391954. doi: 10.3389/fimmu.2024.1391954
78. Gogia P, Ashraf H, Bhasin S, Xu Y. Antibody-drug conjugates: A review of approved drugs and their clinical level of evidence. Cancers (Basel). (2023) 15. doi: 10.3390/cancers15153886
79. Crombie JL, Graff T, Falchi L, Karimi YH, Bannerji R, Nastoupil L, et al. Consensus recommendations on the management of toxicity associated with CD3xCD20 bispecific antibody therapy. Blood. (2024) 143:1565–75. doi: 10.1182/blood.2023022432
80. Sellars MC, Wu CJ, Fritsch EF. Cancer vaccines: Building a bridge over troubled waters. Cell. (2022) 185:2770–88. doi: 10.1016/j.cell.2022.06.035
81. Mazzoccoli L, Liu B. Dendritic cells in shaping anti-tumor T cell response. Cancers (Basel). (2024) 16. doi: 10.3390/cancers16122211
82. Xie N, Shen G, Gao W, Huang Z, Huang C, Fu L. Neoantigens: promising targets for cancer therapy. Signal Transduct Target Ther. (2023) 8:9. doi: 10.1038/s41392-022-01270-x
83. Schirrmacher V. Cancer vaccines and oncolytic viruses exert profoundly lower side effects in cancer patients than other systemic therapies: A comparative analysis. Biomedicines. (2020) 8:61. doi: 10.3390/biomedicines8030061
84. Wang Z, Li B, Ren Y, Ye Z. T-cell-based immunotherapy for osteosarcoma: challenges and opportunities. Front Immunol. (2016) 7:353. doi: 10.3389/fimmu.2016.00353
85. Kokkali S, Kotsantis I, Magou E, Sophia T, Kormas T, Diakoumis G, et al. The addition of the immunomodulator mifamurtide to adjuvant chemotherapy for early osteosarcoma: a retrospective analysis. Invest New Drugs. (2022) 40:668–75. doi: 10.1007/s10637-022-01225-7
86. Cortiana V, Abbas RH, Chorya H, Gambill J, Mahendru D, Park CH, et al. Personalized medicine in pancreatic cancer: the promise of biomarkers and molecular targeting with dr. Michael J. Pishvaian. Cancers (Basel). (2024) 16. doi: 10.3390/cancers16132329
87. Shemesh CS, Hsu JC, Hosseini I, Shen BQ, Rotte A, Twomey P, et al. Personalized cancer vaccines: clinical landscape, challenges, and opportunities. Mol Ther. (2021) 29:555–70. doi: 10.1016/j.ymthe.2020.09.038
88. Spreafico A, Couselo EM, Irmisch A, Bessa J, Au-Yeung G, Bechter O, et al. Phase 1, first-in-human study of TYRP1-TCB (RO7293583), a novel TYRP1-targeting CD3 T-cell engager, in metastatic melanoma: active drug monitoring to assess the impact of immune response on drug exposure. Front Oncol. (2024) 14:1346502. doi: 10.3389/fonc.2024.1346502
89. Kager L, Potschger U, Bielack S. Review of mifamurtide in the treatment of patients with osteosarcoma. Ther Clin Risk Manag. (2010) 6:279–86. doi: 10.2147/TCRM.S5688
90. Lin Z, Wu Z, Luo W. Chimeric antigen receptor T-cell therapy: the light of day for osteosarcoma. Cancers (Basel). (2021) 13. doi: 10.3390/cancers13174469
91. Cozar B, Greppi M, Carpentier S, Narni-Mancinelli E, Chiossone L, Vivier E. Tumor-infiltrating natural killer cells. Cancer Discovery. (2021) 11:34–44. doi: 10.1158/2159-8290.CD-20-0655
92. Zhang Y, Zhang Z. The history and advances in cancer immunotherapy: understanding the characteristics of tumor-infiltrating immune cells and their therapeutic implications. Cell Mol Immunol. (2020) 17:807–21. doi: 10.1038/s41423-020-0488-6
93. Siegler EL, Kenderian SS. Neurotoxicity and cytokine release syndrome after chimeric antigen receptor T cell therapy: insights into mechanisms and novel therapies. Front Immunol. (2020) 11:1973. doi: 10.3389/fimmu.2020.01973
94. Kawano M, Itonaga I, Iwasaki T, Tsumura H. Enhancement of antitumor immunity by combining anti-cytotoxic T lymphocyte antigen-4 antibodies and cryotreated tumor lysate-pulsed dendritic cells in murine osteosarcoma. Oncol Rep. (2013) 29:1001–6. doi: 10.3892/or.2013.2224
95. Zheng B, Ren T, Huang Y, Sun K, Wang S, Bao X, et al. PD-1 axis expression in musculoskeletal tumors and antitumor effect of nivolumab in osteosarcoma model of humanized mouse. J Hematol Oncol. (2018) 11:16. doi: 10.1186/s13045-018-0560-1
96. Dong S, Guo X, Han F, He Z, Wang Y. Emerging role of natural products in cancer immunotherapy. Acta Pharm Sin B. (2022) 12:1163–85. doi: 10.1016/j.apsb.2021.08.020
97. Ramos-Casals M, Brahmer JR, Callahan MK, Flores-Chavez A, Keegan N, Khamashta MA, et al. Immune-related adverse events of checkpoint inhibitors. Nat Rev Dis Primers. (2020) 6:38. doi: 10.1038/s41572-020-0160-6
98. Mardi A, Shirokova AV, Mohammed RN, Keshavarz A, Zekiy AO, Thangavelu L, et al. Biological causes of immunogenic cancer cell death (ICD) and anti-tumor therapy; Combination of Oncolytic virus-based immunotherapy and CAR T-cell therapy for ICD induction. Cancer Cell Int. (2022) 22:168. doi: 10.1186/s12935-022-02585-z
99. Kajiwara Y, Tazawa H, Yamada M, Kanaya N, Fushimi T, Kikuchi S, et al. Oncolytic virus-mediated reducing of myeloid-derived suppressor cells enhances the efficacy of PD-L1 blockade in gemcitabine-resistant pancreatic cancer. Cancer Immunol Immunother. (2023) 72:1285–300. doi: 10.1007/s00262-022-03334-x
100. Cinatl J Jr., Cinatl J, Michaelis M, Kabickova H, Kotchetkov R, Vogel JU, et al. Potent oncolytic activity of multimutated herpes simplex virus G207 in combination with vincristine against human rhabdomyosarcoma. Cancer Res. (2003) 63:1508–14.
101. Bharatan NS, Currier MA, Cripe TP. Differential susceptibility of pediatric sarcoma cells to oncolysis by conditionally replication-competent herpes simplex viruses. J Pediatr Hematol Oncol. (2002) 24:447–53. doi: 10.1097/00043426-200208000-00008
102. Martinez-Velez N, Xipell E, Vera B, Acanda de la Rocha A, Zalacain M, Marrodan L, et al. The oncolytic adenovirus VCN-01 as therapeutic approach against pediatric osteosarcoma. Clin Cancer Res. (2016) 22:2217–25. doi: 10.1158/1078-0432.CCR-15-1899
103. Lang FF, Conrad C, Gomez-Manzano C, Yung WKA, Sawaya R, Weinberg JS, et al. Phase I study of DNX-2401 (Delta-24-RGD) oncolytic adenovirus: replication and immunotherapeutic effects in recurrent Malignant glioma. J Clin Oncol. (2018) 36:1419–27. doi: 10.1200/JCO.2017.75.8219
104. Guo ZS, Lu B, Guo Z, Giehl E, Feist M, Dai E, et al. Vaccinia virus-mediated cancer immunotherapy: cancer vaccines and oncolytics. J Immunother Cancer. (2019) 7:6. doi: 10.1186/s40425-018-0495-7
105. Morales-Molina A, Gambera S, Leo A, Garcia-Castro J. Combination immunotherapy using G-CSF and oncolytic virotherapy reduces tumor growth in osteosarcoma. J Immunother Cancer. (2021) 9. doi: 10.1136/jitc-2020-001703
106. Zhang J, He Q, Mao D, Wang C, Huang L, Wang M, et al. Efficacy and adverse reaction management of oncolytic viral intervention combined with chemotherapy in patients with liver metastasis of gastrointestinal Malignancy. Front Oncol. (2023) 13:1159802. doi: 10.3389/fonc.2023.1159802
107. Zheng S, Wang F, Huang J, Zhou Y, Yang Q, Qian G, et al. Case report: sequential chemotherapy and immunotherapy produce sustained response in osteosarcoma with high tumor mutational burden. Front Endocrinol (Lausanne). (2021) 12:625226. doi: 10.3389/fendo.2021.625226
108. Pandey P, Khan F, Qari HA, Upadhyay TK, Alkhateeb AF, Oves M. Revolutionization in cancer therapeutics via targeting major immune checkpoints PD-1, PD-L1 and CTLA-4. Pharm (Basel). (2022) 15. doi: 10.3390/ph15030335
109. Anderson KG, Stromnes IM, Greenberg PD. Obstacles posed by the tumor microenvironment to T cell activity: A case for synergistic therapies. Cancer Cell. (2017) 31:311–25. doi: 10.1016/j.ccell.2017.02.008
110. Wells K, Liu T, Zhu L, Yang L. Immunomodulatory nanoparticles activate cytotoxic T cells for enhancement of the effect of cancer immunotherapy. Nanoscale. (2024) 16:17699–722. doi: 10.1039/D4NR01780C
111. Zhang L, Zhou C, Zhang S, Chen X, Liu J, Xu F, et al. Chemotherapy reinforces anti-tumor immune response and enhances clinical efficacy of immune checkpoint inhibitors. Front Oncol. (2022) 12:939249. doi: 10.3389/fonc.2022.939249
112. Wang Z, Wang Z, Li S, Li B, Sun L, Li H, et al. Decitabine enhances vgamma9Vdelta2 T cell-mediated cytotoxic effects on osteosarcoma cells via the NKG2DL-NKG2D axis. Front Immunol. (2018) 9:1239. doi: 10.3389/fimmu.2018.01239
113. Shi T, Song X, Wang Y, Liu F, Wei J. Combining oncolytic viruses with cancer immunotherapy: establishing a new generation of cancer treatment. Front Immunol. (2020) 11:683. doi: 10.3389/fimmu.2020.00683
114. Fernandes R, Nikitakis NG, Pazoki A, Ord RA. Osteogenic sarcoma of the jaw: a 10-year experience. J Oral Maxillofac Surg. (2007) 65:1286–91. doi: 10.1016/j.joms.2006.10.030
115. Synoradzki KJ, Bartnik E, Czarnecka AM, Fiedorowicz M, Firlej W, Brodziak A, et al. TP53 in biology and treatment of osteosarcoma. Cancers (Basel). (2021) 13. doi: 10.3390/cancers13174284
116. Du X, Wei H, Zhang B, Wang B, Li Z, Pang LK, et al. Molecular mechanisms of osteosarcoma metastasis and possible treatment opportunities. Front Oncol. (2023) 13:1117867. doi: 10.3389/fonc.2023.1117867
117. Wang X, Niu X, An N, Sun Y, Chen Z. Comparative efficacy and safety of immunotherapy alone and in combination with chemotherapy for advanced non-small cell lung cancer. Front Oncol. (2021) 11:611012. doi: 10.3389/fonc.2021.611012
Keywords: osteosarcoma, immune microenvironment, immunotherapy, immune evasion, combination therapy
Citation: Luo C, Min X and Zhang D (2025) New insights into the mechanisms of the immune microenvironment and immunotherapy in osteosarcoma. Front. Immunol. 15:1539696. doi: 10.3389/fimmu.2024.1539696
Received: 04 December 2024; Accepted: 30 December 2024;
Published: 17 January 2025.
Edited by:
Minghua Ren, First Affiliated Hospital of Harbin Medical University, ChinaReviewed by:
Kui Wang, Shandong University, ChinaCopyright © 2025 Luo, Min and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Danying Zhang, emhhbmdkYW55aW5nMTEyNkAxMjYuY29t
 Cong Luo
Cong Luo Xingxing Min1
Xingxing Min1 Danying Zhang
Danying Zhang