- 1Department of Geriatric Medicine, Affiliated Hospital of Qingdao University, Qingdao, China
- 2Qingdao Medical College, Qingdao University, Qingdao, China
Objective: This study aims to delineate the clinical features underlying the concurrent disease of neuromyelitis optica spectrum disorder (NMOSD) and myasthenia gravis (MG), and to identify efficacious therapeutic strategies.
Background: NMOSD and MG are uncommon autoimmune diseases that infrequently co-exist. Despite previous reports, a consensus on treating NMOSD concurrent with MG is lacking.
Methods: We present the case of a 55-year-old female with both anti-aquaporin-4 (AQP4) antibody-positive NMOSD and anti-acetylcholine receptor (AChR) antibody-positive MG, who achieved stable disease control following treatment with inebilizumab without significant adverse effects. We also conducted a literature review to evaluate the clinical profile of this comorbidity.
Results: Our review identified 85 patients with concurrent NMOSD and MG. In 70 well-documented cases, MG predated NMOSD in 60 (85.8%) cases, with 42 (70%) patients having undergone thymectomy. Six (8.6%) patients were first diagnosed with NMOSD, and then thymectomy was performed in 2 (33.3%) MG patients. For NMOSD treatment, although most patients received steroid hormones and immunosuppressive agents, quite a few patients had persistent severe disability. Additionally, of 44 patients with clear records of disease recurrence, 31 patients(70.5%) experienced frequent relapses of optic neuritis and myelitis, ranging from 1 to 15 attacks, averaging five. The manifestations of MG are mainly included fatigability, diplopia, and blepharoptosis, with symptoms well-controlled in most patients. Our patient treated with inebilizumab for 1 year and no relapse was recorded to date.
Conclusions: Though MG typically precedes NMOSD and thymectomy is frequently performed, it is not a prerequisite for NMOSD development but may represent a potential risk factor. MG generally follows a benign course, in contrast to the more aggressive nature of NMOSD. The utility of biological agents such as inebilizumab for patients with both NMOSD combined with MG warrants further attention.
Introduction
Neuromyelitis optica spectrum disorder (NMOSD) is an uncommon demyelinating disorder of the central nervous system (CNS) caused by the action of the anti-AQP4 antibodies (1). It is marked by recurrent episodes of optic neuritis(ON) and myelitis, which often lead to significant disability if not treated promptly. Myasthenia gravis(MG) is a chronic organ-specific autoimmune disorder caused by antibody-mediated attacks on nicotinic acetylcholine receptors(AChR) at the neuromuscular junction. This interference disrupts neuromuscular transmission, ultimately leading to muscle weakness and fatigue (2). Moreover, the prevalence of MG varies between 0.8 and 20 per 100,000 population (3), and prevalence of NMOSD is reported as ranged from 0.07 to 10 per 100,000 (4). Although both disorders are rare, the coexistence of MG and NMOSD occurs much more common than by chance, with over 100 cases reported to date (5). And a study reported five of 214 reviewed patients with MG (2.3%) who had CNS demyelinating lesion or disease (6).
This co-occurrence can complicate the diagnosis of both conditions, as overlapping symptoms may obscure clinical presentation. Furthermore, the concurrent presence of MG complicates NMOSD treatment and may contribute to poorer outcomes (7). In this report, we described a patient with concurrent NMOSD and MG, highlighting their clinical course and the challenges encountered in managing these overlapping autoimmune disorders. In addition, we provide a comprehensive review of similar cases in the literature to better delineate the clinical features, potential pathogenic mechanisms, and effective therapeutic strategies for patients with these coexisting conditions. Understanding the interplay between NMOSD and MG is crucial for optimizing management, improving outcomes, and guiding future research into these complex autoimmune diseases.
Case report
A 55-year-old female was hospitalized in May 2023 due to dizziness, nausea, vomiting and gait disturbance. She had been diagnosed with MG over 10 years and had received intermittent treatment with traditional Chinese medicine. Ten years ago, she experienced episodes of ptosis and dysarthria, which was improved completely with traditional Chinese medicine. Upon this admission, she exhibited no MG symptoms, such as ptosis, dysarthria, or fatigue.
Brain magnetic resonance images (MRI) revealed abnormal lesions around the third ventricle (Figure 1). Wernicke’s encephalopathy was first considered due to the brain MRI lesions, but was then ruled out because of her persistent symptoms without improvement. Cerebrospinal fluid (CSF) examination demonstrated a normal protein level (404mg/L, normal range 120-600mg/L) and white blood cell count of 8/mm3(normal range 0-8/mm3). Thyroid testing indicated anti-thyroglobulin antibody level was 669 IU/mL (normal range <115 IU/ml), and anti-thyroid peroxidase antibody level was 170 IU/mL (normal range <34 IU/ml). Thyroid ultrasound findings were consistent with Hashimoto’s thyroiditis. Serum analysis showed a positive AQP4 antibody titer of 1:1000(cell-based assay). Given the high specificity of AQP4 antibodies, she was diagnosed with NMOSD. Then she received intravenous methylprednisolone (500 mg/day) for 5 days, which partially improved her neurological symptoms. She was discharged on oral prednisone (50 mg/day) and mycophenolate mofetil (MMF) (500 mg twice daily). Five months later, she presented with visual blurring in right eye with visual acuity reduced to 0.12 over about 2 weeks. At this admission, her visual evoked potential response was absent on the right eye. Neurological examination showed grade V muscle strength in all limbs. Her quantitative myasthenia gravis score (QMGS) was 0 and Expanded Disability Status Scale (EDSS) score was 3. Her anti-AChR antibodies were positive (6.36 nmol/L, serum ELISA, normal range <0.45nmol/L),while other autoantibodies including MuSK, LRP4, RyR, Titin, antinuclear antibodies (ANA) profile, anti-neutrophil cytoplasmic antibodies (ANCA), and T-SPOT testing were negative. Chest CT showed no thymus abnormalities, and spinal and orbital MRI revealed no notable findings.
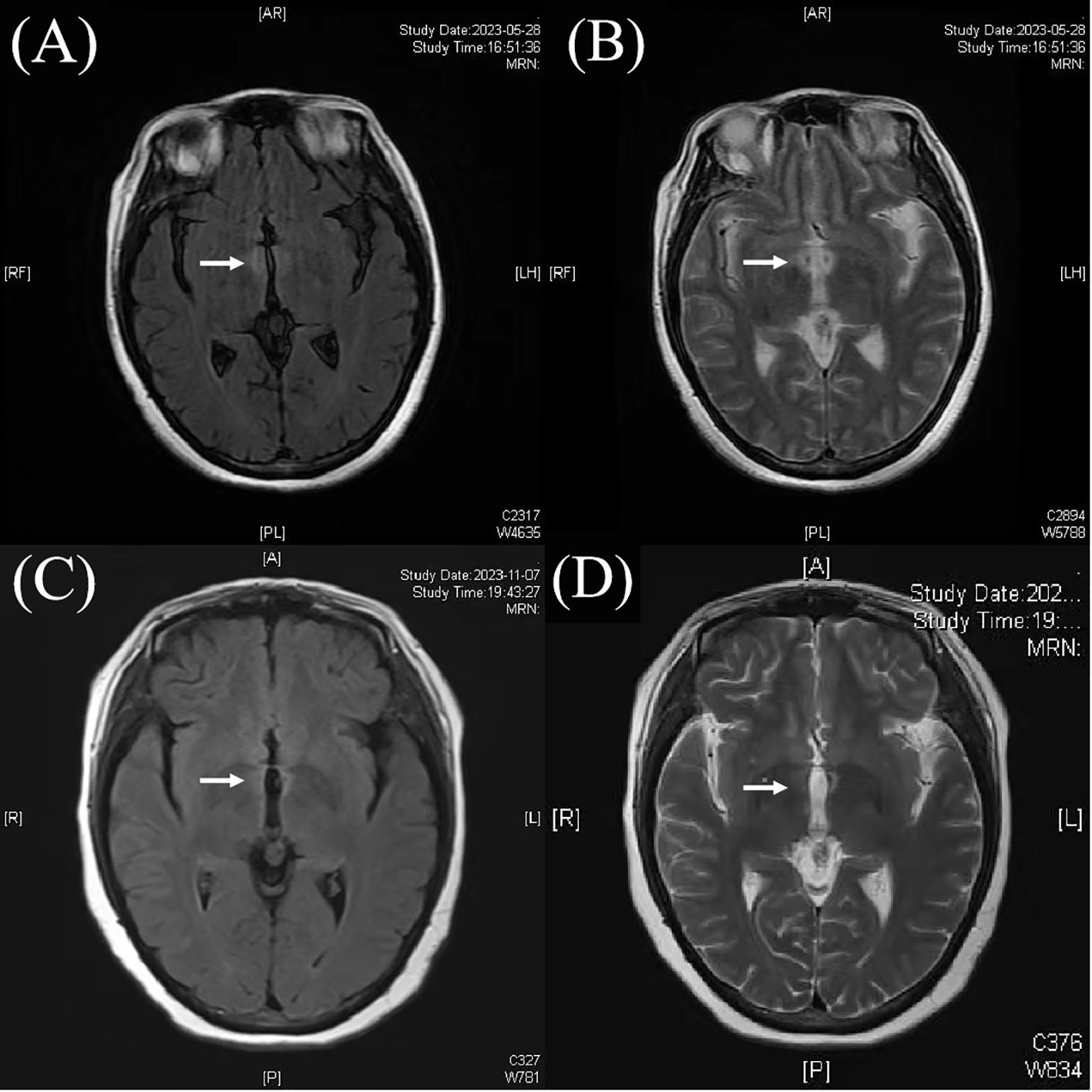
Figure 1. Magnetic resonance images (MRI) showed abnormal lesions around the third ventricle (A, B). After treatment, the abnormal lesions of the third ventricle was weaker than before (C, D).
She was then treated with methylprednisolone (1 g/day for 5 days) and initiated with inebilizumab (300 mg at day 0,15, and then every 6 months). After two doses, her CD19 B-cell count decreased to 0.1%, and she tolerated well without adverse effects. Over the following year, the patient remained relapse-free after 3 doses, with a stable QMGS of 0 and EDSS score of 2.
Results: literature review and our case
A total of 27 articles were searched, including a cohort study, a multicentre study (8, 9). Those cases were identified with a PubMed search using the terms “neuromyelitis optica spectrum disorder,” “neuromyelitis optica,” “myelitis,” “optic neuritis,” “multiple sclerosis” and “myasthenia gravis.”
A total of 85 patients were identified with comorbid NMOSD and MG, including our patient (Table 1). Among 70 patients with well-documented reports, MG predated NMOSD in 60 (85.8%) cases, with 54 (94.7%) being female. MG onset occurred between the ages of 8 and 63 years (median 27.5 years). Of these, 46 patients (76.7%) were aged ≤50 years. Forty-two (70%) had undergone thymectomy, with thymoma confirmed in 3 cases. NMOSD occurred first in 6 cases, with 5 patients (83.3%) being female, and one patient (16.7%) being male. NMOSD onset occurred between the ages of 16 and 79 years (median 41.5 years). Two of these patients had undergone thymectomy, one confirmed case of thymoma. Among patients with both MG and NMOSD, 74 patients (91.3%) were positive for anti-AChR antibodies, and anti-AQP4 antibodies were detected in 62 cases (83.8%). Thirteen patients (26%) also had concurrent other autoimmune diseases, such as thyroid disease, systemic lupus erythematosus (SLE), and Sjögren’s syndrome (SS). Additionally, 9 patients (18%) tested positive for other immune-related antibodies, including ANA and double-stranded DNA (dsDNA). These results are summarized in Table 1.
In 50 patients with treatment details, 21 patients started with ON, 16 with myelitis, and 13 were diagnosed with NMOSD in the beginning. Additionally, of 44 patients with clear records of disease recurrence, 31 patients (70.5%) experienced frequent relapses of ON and myelitis, ranging from 1 to 15 attacks, averaging five. Twenty-three patients (63.9%) were treated with intravenous methylprednisolone (IVMP), 6 (16.7%) with plasma exchange (PE), and one (2.7%) with intravenous immunoglobulin (IVIG) during NMOSD acute phases. Regarding the treatment during remission phases, 10 patients (37.0%)were maintained with steroid or prednisolone alone, 14 patients(51.9%) had additional immunosuppressive agents, and 3 patients (11.1%) were supplemented with biological agents, including our patient. One patient passed away in ICU despite IVMP treatment, and 8 patients (28.6%) experienced persistent severe disabilities. Ten patients (35.7%) received completely symptom improvement, and 7 patients (25%) received partially symptoms improvement. Furthermore, most cases responded well to MG therapy, and the prognosis is often favorable. In our review, 13 out of 29 patients (44.8%) exhibited well-controlled symptoms or remained stable, while clinical remission or complete remission was reported in 11 patients (37.9%). However, 3 patients experienced MG crises. Therefore, for individuals with concurrent NMOSD and MG, it is essential to pay attention to the management of MG alongside NMOSD treatment.
Discussion
Our patient was diagnosed with both AQP4 antibody-positive NMOSD and AChR antibody-positive MG. She initially presented with dizziness, nausea, vomiting, gait disturbance, and then vision loss. However, despite receiving standard treatment, her vision improved only slightly. In contrast, her MG symptoms, such as ptosis and dysarthria, were well-controlled prior to the NMOSD onset, with a QMGS score of 0. She had been treated with inebilizumab for one year follow-up, with no relapses reported to date.
Although the mechanisms underlying co-occurrence of NMOSD and MG remain unclear, it is hypothesized that the two diseases share common immune pathological mechanisms. In NMOSD, B cells contribute to pathogenesis through the production of pathogenic AQP4-IgG antibodies by plasmablasts (PBs) and plasma cells (PCs) (33), secretion of pro-inflammatory cytokines, and antigen presentation that activates autoreactive T cells (34). MG is due to the action of pathogenic antibodies secreted by PCs at the neuromuscular junction, leading to neuromuscular dysfunction. Both diseases have highly specific autoantibodies, which secreted by PBs and PCs differentiated from B cells. Moreover, genetic predispositions also contribute to the co-occurrence of NMOSD and MG. Human Leukocyte Antigen (HLA) -DPB1*05:01 in both southern Han Chinese and Japanese populations are linked to an increased risk of developing NMOSD (35). HLA-C07:01:01 is a well-characterized risk factor for MG. Additionally, HLA-DRB1*03:01 and HLA-DRB1*15:01 have been emerged as an independent risk allele for both disease (36). Certain HLA types have been pinpointed that correlate with the susceptibility to developing NMOSD and MG (37). When appropriate, HLA genotyping should be considered.
In patients with both conditions, MG typically precedes NMOSD and is often associated with thymectomy. A previous study reported that more than 50% of MG patients have their thymus removed (38). Our literature review showed that approximately 70% patients with both NMOSD and MG had undergone thymectomy. It is a higher proportion than in MG patients without NMOSD. However, it has been reported that NMOSD can develop in MG patients without thymectomy (8), as observed in our case. This indicates that thymectomy is not a necessary factor for the occurrence of NMOSD. One possible explanation is that AQP4, expressed at the peripheral neuromuscular junction, may act as a shared target for both diseases (39). The degeneration of the postsynaptic membrane induced by AChR antibodies may trigger AQP4 sensitization within the inflammatory environment of MG, consequently leading to autoimmunity against AQP4 (7). This explains why the MG patients without thymectomy would develop NMOSD. Therefore, thymectomy is not prerequisite for NMOSD onset in MG patients. In contrast, for patients who develop NMOSD after thymectomy, the expression of AQP4 in the thymus gland may play a role. The abnormal thymus associated with MG could generate anti-AQP4 antibodies (6). In some cases, an immune response against AQP4 on thymoma cells may trigger NMOSD. And there is another viewpoint that regulatory T cells in the adult thymus play a role in preventing the emergence of autoimmune diseases by keeping autoreactive cells in check. A reduction in regulatory T cells after thymectomy may contribute to the development of NMOSD (40).
In the literature review, many patients were found to have concurrent autoimmune conditions or other immune-related antibodies, such as ANA and dsDNA. Evidence suggests that over 25% of patients with autoimmune disorders are likely to develop another autoimmune condition, which can be either organ-specific or systemic-specific (40). Common co-occurring conditions include thyroid disease, SLE, SS, rheumatoid arthritis, antiphospholipid syndrome, ulcerative colitis and sarcoidosis, et al. Therefore, after the diagnosis of an autoimmune disease, screening for antibodies related to other autoimmune disorders is recommended. A similar situation is observed in patients with both NMOSD and MG. In most cases, MG precedes NMOSD by more than 10 years. Additionally, AQP4-Abs have been detected in some MG patients years even in the absence of clinical manifestations of NMOSD (8). Based on this, we recommend routine evaluation of AQP4-Abs in MG patients, as well as thyroid antibodies, ANA, and dsDNA et al. Moreover, clinical symptoms and signs are essential for determining whether MG coexists with NMOSD or only antibodies are present without active disease. It is also recognized that NMOSD can occur in MG patients even in the absence of AQP4-Abs. This highlights the importance of screening for NMOSD in MG patients, especially when clinical features overlap, regardless of antibody status. A thorough clinical evaluation and the use of advanced diagnostic techniques are crucial for accurately identifying coexisting autoimmune conditions.
The treatment strategies for NMOSD and MG have advanced rapidly in recent years, with novel therapeutic biologics targeting diverse mechanisms emerging. For NMOSD, three biologics have been approved by the U.S. Food and Drug Administration (FDA) for patients with NMOSD: eculizumab, satralizumab, and inebilizumab (41–43). Furthermore, rituximab (RTX) has class I evidence supporting its use in AQP4-IgG positive NMOSD (44). For MG, eculizumab is FDA-approved for refractory cases with efficacy supported by phase III trial data. Efgartigimod has also been approved for the treatment of generalized MG (45). Interleukin-6 inhibitors like satralizumab are under active evaluation (46). RTX has demonstrated promising results in MG, with studies reporting reductions in autoantibody levels and improvements in clinical symptoms (47).
MG tends to be a milder condition in patients with comorbid NMOSD and MG, and its relapse was rare once NMOSD developed. NMOSD appears to be more aggressive and tends to have recurrent attacks. In the case of our patient, MG remained stable, so a special focus on the treatment of NMOSD. NMOSD and MG are both B cell-mediated autoimmune diseases, making B cell-depleting therapies a logical therapeutic approach. Inebilizumab is FDA-approved for NMOSD. And evidence supports inebilizumab is effective for MG and may even outperform other anti-CD20 therapies such as ocrelizumab, ofatumumab, and obinutuzumab in this context (48). Additionally, inebilizumab has demonstrated potential in other humoral immune-mediated autoimmune diseases. Studies have indicated that it reduces the risk of flares in IgG4-related disease and increases the likelihood of achieving flare-free complete remission within one year (49). Phase I clinical trials of inebilizumab for the treatment of multiple sclerosis (NCT01585766) and systemic sclerosis (NCT00946699) have been completed, showing signals of clinical effectiveness (50). Phase III clinical trials for the treatment of systemic sclerosis (NCT05198557) and N-methyl-D-aspartate receptor encephalitis (NCT04372615)are currently underway (51).
It is important to acknowledge the limitations of our study to provide a balanced perspective on the findings. Firstly, the duration of the follow-up period was relatively short, limited to one year, which restricts conclusions about the long-term sustainability of the observed effects. Secondly, other than monitoring the quantity and functionality of immune cells such as T cells and B cells, glial fibrillary acidic protein(GFAP), neurofilament light chain(NfL) and AQP4 antibody level is crucial for comprehensively evaluating treatment efficacy and predicting prognosis. In light of these limitations, we encourage future research to address these gaps through studies with extended follow-up periods and more robust assessments in patients with NMOSD and MG.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
XS: Writing – original draft. JC: Writing – review & editing. CJ: Writing – original draft. YP: Writing – original draft. YS: Writing – original draft. XZ: Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by the Key Project of Shandong Province Medical and Health Science and Technology Project (number, 202403070867).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that Generative AI was used in the creation of this manuscript. During the preparation of this work the authors used ChatGPT in order to polish. After using this tool, the authors reviewed and edited the content as needed and take full responsibility for the content of the publication.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Chatterton S, Parratt JDE, Ng K. Eculizumab for acute relapse of neuromyelitis optica spectrum disorder: Case report. Front Neurol. (2022) 13:951423. doi: 10.3389/fneur.2022.951423
2. Uzawa A, Mori M, Iwai Y, Kobayashi M, Hayakawa S, Kawaguchi N, et al. Association of anti-aquaporin-4 antibody-positive neuromyelitis optica with myasthenia gravis. J Neurol Sci. (2009) 287:105–7. doi: 10.1016/j.jns.2009.08.040
3. Luzanova E, Stepanova S, Nadtochiy N, Kryukova E, Karpova M. Cross-syndrome: myasthenia gravis and the demyelinating diseases of the central nervous system combination. Systematic literature review and case reports. Acta Neurol Belg. (2023) 123:367–74. doi: 10.1007/s13760-022-01926-z
4. Bagherieh S, Afshari-Safavi A, Vaheb S, Kinai M, Ghaffary EM, Barzegar M, et al. Worldwide prevalence of neuromyelitis optica spectrum disorder (NMOSD) and neuromyelitis optica (NMO): a systematic review and meta-analysis. Neurol Sci. (2023) 44:1905–15. doi: 10.1007/s10072-023-06617-y
5. Bong JB, Lee MA, Kang HG. Newly diagnosed multiple sclerosis in a patient with ocular myasthenia gravis: A case report. Med (Baltimore). (2022) 101:e28887. doi: 10.1097/MD.0000000000028887
6. Zhu Y, Wang B, Hao Y, Zhu R. Clinical features of myasthenia gravis with neurological and systemic autoimmune diseases. Front Immunol. (2023) 14:1223322. doi: 10.3389/fimmu.2023.1223322
7. Bibic VC, Brust TB, Burton JM. Neuromyelitis optica spectrum disorder presenting with concurrent autoimmune diseases. Mult Scler Relat Disord. (2019) 28:125–8. doi: 10.1016/j.msard.2018.12.028
8. Leite MI, Coutinho E, Lana-Peixoto M, Apostolos S, Waters P, Sato D, et al. Myasthenia gravis and neuromyelitis optica spectrum disorder: a multicenter study of 16 patients. NEUROLOGY. (2012) 78:1601–7. doi: 10.1212/WNL.0b013e31825644ff
9. Vaknin-Dembinsky A, Abramsky O, Petrou P, Ben-Hur T, Gotkine M, Brill L, et al. Myasthenia gravis-associated neuromyelitis optica-like disease: an immunological link between the central nervous system and muscle? Arch Neurol. (2011) 68:1557–61. doi: 10.1001/archneurol.2011.200
10. Isbister CM, Mackenzie PJ, Anderson D, Wade NK, Oger J. Co-occurrence of multiple sclerosis and myasthenia gravis in british columbia. Mult Scler (2003) 9:550–3. doi: 10.1191/1352458503ms964oa
11. Kay CS, Scola RH, Lorenzoni PJ, Jarius S, Arruda WO, Werneck LC. NMO-IgG positive neuromyelitis optica in a patient with myasthenia gravis but no thymectomy. J Neurol Sci (2008) 275:148–50. doi: 10.1016/j.jns.2008.06.038
12. Furukawa Y, Yoshikawa H, Yachie A, Yamada M. Neuromyelitis optica associated with myasthenia gravis: characteristic phenotype in japanese population. Eur J Neurol (2006) 13:655–8. doi: 10.1111/j.1468-1331.2006.01392.x
13. Kister I, Gulati S, Boz C, Bergamaschi R, Piccolo G, Oger J, et al. Neuromyelitis optica in patients with myasthenia gravis who underwent thymectomy. Arch Neurol (2006) 63:851–6. doi: 10.1001/archneur.63.6.851
14. Nakamura M, Nakashima I, Sato S, Miyazawa I, Fujihara K, Itoyama Y. Clinical and laboratory features of neuromyelitis optica with oligoclonal IgG bands. Mult Scler (2007) 13:332–5. doi: 10.1177/13524585070130030201
15. Bichuetti DB, Barros TM, Oliveira EM, Annes M, Gabbai AA. Demyelinating disease in patients with myasthenia gravis. Arq Neuro. (2008) 66:5–7. doi: 10.1590/s0004-282x2008000100002
16. Kohsaka M, Tanaka M, Tahara M, Araki Y, Mori S, Konishi T. [A case of subacute myelitis with anti-aquaporin 4 antibody after thymectomy for myasthenia gravis: review of autoimmune diseases after thymectomy]. Rinsho Shinkeigaku. (2010) 50:111–3. doi: 10.5692/clinicalneurol.50.111
17. Ogaki K, Hirayama T, Chijiiwa K, Fukae J, Furuya T, Noda K, et al. Anti-aquaporin-4 antibody-positive definite neuromyelitis optica in a patient with thymectomy for myasthenia gravis. Neurologist. (2012) 18:76–9. doi: 10.1097/NRL.0b013e318247bc91
18. Hironishi M, Ishimoto S, Sawanishi T, Miwa H, Kawachi I, Kondo T. [Neuromyelitis optica following thymectomy with severe spinal cord atrophy after frequent relapses for 30 years]. Brain Nerve. (2012) 64:951–5. doi: 10.11477/mf.1416101273
19. Jarius S, Paul F, Franciotta D, de Seze J, Münch C, Salvetti M, et al. Neuromyelitis optica spectrum disorders in patients with myasthenia gravis: ten new aquaporin-4 antibody positive cases and a review of the literature. Mult Scler (2012) 18:1135–43. doi: 10.1177/1352458511431728
20. Spillane J, Christofi G, Sidle KC, Kullmann DM, Howard RS. Myasthenia gravis and neuromyelitis opica: A causal link. Mult Scler Relat Disord (2013) 2:233–7. doi: 10.1016/j.msard.2013.01.003
21. Ikeguchi R, Shimizu Y, Suzuki S, Shimizu S, Kabasawa C, Hashimoto S, et al. Japanese cases of neuromyelitis optica spectrum disorder associated with myasthenia gravis and a review of the literature. Clin Neurol Neurosurg (2014) 125:217–21. doi: 10.1016/j.clineuro.2014.07.036
22. Balarabe SA, Adamu MD, Watila MM, Jiya N. Neuromyelitis optica and myasthenia gravis in a young nigerian girl. BMJ Case Rep (2015). doi: 10.1136/bcr-2014-207362
23. O'Riordan JI, Gallagher HL, Thompson AJ, Howard RS, Kingsley DP, Thompson EJ, et al. Clinical, CSF, and MRI findings in devic's neuromyelitis optica. J Neurol Neurosurg Psychiatry (1996) 60:382–7. doi: 10.1136/jnnp.60.4.382
24. Kimura K, Okada Y, Fujii C, Komatsu K, Takahashi R, Matsumoto S, et al. Clinical characteristics of autoimmune disorders in the central nervous system associated with myasthenia gravis. J Neurol (2019) 266:2743–51. doi: 10.1007/s00415-019-09461-3
25. Bates M, Chisholm J, Miller E, Avasarala J, Guduru Z. Anti-MOG and anti-AQP4 positive neuromyelitis optica spectrum disorder in a patient with myasthenia gravis. Mult Scler Relat Disord (2020) 44:102205. doi: 10.1016/j.msard.2020.102205
26. Gotkine M, Fellig Y, Abramsky O. Occurrence of CNS demyelinating disease in patients with myasthenia gravis. Neurology. (2006) 67:881–3. doi: 10.1212/01.wnl.0000234142.41728.a0
27. Tola MR, Casetta I, Granieri E, Caniatti LM, Monetti VC, Pascarella R. Systemic lupus erythematosus related recurrent transverse myelitis in a patient with myasthenia gravis and multiple sclerosis. Eur Neurol (1996) 36:327–8. doi: 10.1159/000117285
28. Piñar Morales R, Todorova Petrova M, Barrero Hernández FJ. Copresence of myasthenia gravis and neuromyelitis optica: 2 case reports. Neurologia (2021) 36:174–6. doi: 10.1016/j.nrl.2020.02.008
29. Etemadifar M, Abtahi SH, Dehghani A, Abtahi MA, Akbari M, Tabrizi N, et al. Myasthenia gravis during the course of neuromyelitis optica. Case Rep Neurol (2011) 3:268–73. doi: 10.1159/000334128
30. Yau GS, Lee JW, Chan TT, Yuen CY. Neuromyelitis optica spectrum disorder in a chinese woman with ocular myasthenia gravis: First reported case in the chinese population. Neuroophthalmology. (2014) 38:140–4. doi: 10.3109/01658107.2013.879903
31. Bonner K, Aboul Nour H, Memon AB. Overlapping autoimmune neurological syndrome: A case report of triple-positive antibody. Cureus. (2022) 14:e29379. doi: 10.7759/cureus.29379
32. Antoine JC, Camdessanché JP, Absi L, Lassablière F, Féasson L. Devic disease and thymoma with anti-central nervous system and antithymus antibodies. Neurology. (2004) 62:978–80. doi: 10.1212/01.wnl.0000115168.73299.88
33. Jasiak-Zatonska M, Kalinowska-Lyszczarz A, Michalak S, Kozubski W. The immunology of neuromyelitis optica-current knowledge, clinical implications, controversies and future perspectives. Int J Mol Sci. (2016) 17:273. doi: 10.3390/ijms17030273
34. Bennett JL, Aktas O, Rees WA, Smith MA, Gunsior M, Li Y, et al. Association between B-cell depletion and attack risk in neuromyelitis optica spectrum disorder: An exploratory analysis from N-MOmentum, a double-blind, randomised, placebo-controlled, multicentre phase 2/3 trial. EBioMedicine. (2022) 86:104321. doi: 10.1016/j.ebiom.2022.104321
35. Jarius S, Aktas O, Ayzenberg I, Bellmann-Strobl J, Berthele A, Giglhuber K, et al. Update on the diagnosis and treatment of neuromyelits optica spectrum disorders (NMOSD) - revised recommendations of the Neuromyelitis Optica Study Group (NEMOS). Part I: Diagnosis and differential diagnosis. J Neurol. (2023) 270:3341–68. doi: 10.1007/s00415-023-11634-0
36. Vakrakou A, Chatzistamatiou T, Koros C, Karathanasis D, Tentolouris-Piperas V, Tzanetakos D, et al. HLA-genotyping by next-generation-sequencing reveals shared and unique HLA alleles in two patients with coexisting neuromyelitis optica spectrum disorder and thymectomized myasthenia gravis: Immunological implications for mutual aetiopathogenesis? Mult Scler Relat Disord. (2022) 63:103858. doi: 10.1016/j.msard.2022.103858
37. Saruhan-Direskeneli G, Hughes T, Yilmaz V, Durmus H, Adler A, Alahgholi-Hajibehzad M, et al. Genetic heterogeneity within the HLA region in three distinct clinical subgroups of myasthenia gravis. Clin Immunol. (2016) 166-167:81–8. doi: 10.1016/j.clim.2016.05.003
38. Taioli E, Paschal PK, Liu B, Kaufman AJ, Flores RM. Comparison of conservative treatment and thymectomy on myasthenia gravis outcome. Ann Thorac Surg. (2016) 102:1805–13. doi: 10.1016/j.athoracsur.2016.08.052
39. Wakayama Y. Aquaporin expression in normal and pathological skeletal muscles: a brief review with focus on AQP4. J BioMed Biotechnol. (2010) 2010:731569. doi: 10.1155/2010/731569
40. Castro-Suarez S, Guevara-Silva E, Caparó-Zamalloa C, Cortez J, Meza-Vega M. Neuromyelitis optica in patients with myasthenia gravis: Two case-reports. Mult Scler Relat Disord. (2020) 43:102173. doi: 10.1016/j.msard.2020.102173
41. Frampton JE. Inebilizumab: first approval. Drugs. (2020) 80:1259–64. doi: 10.1007/s40265-020-01370-4
42. Pittock SJ, Berthele A, Fujihara K, Kim HJ, Levy M, Palace J, et al. Eculizumab in aquaporin-4-positive neuromyelitis optica spectrum disorder. N Engl J Med. (2019) 381:614–25. doi: 10.1056/NEJMoa1900866
43. Yamamura T, Kleiter I, Fujihara K, Palace J, Greenberg B, Zakrzewska-Pniewska B, et al. Trial of satralizumab in neuromyelitis optica spectrum disorder. N Engl J Med. (2019) 381:2114–24. doi: 10.1056/NEJMoa1901747
44. Redenbaugh V, Flanagan EP. Monoclonal antibody therapies beyond complement for NMOSD and MOGAD. Neurotherapeutics. (2022) 19:808–22. doi: 10.1007/s13311-022-01206-x
46. Menon D, Bril V. Pharmacotherapy of generalized myasthenia gravis with special emphasis on newer biologicals. Drugs. (2022) 82:865–87. doi: 10.1007/s40265-022-01726-y
47. Carnero Contentti E, Correale J. Neuromyelitis optica spectrum disorders: from pathophysiology to therapeutic strategies. J Neuroinflamm. (2021) 18:208. doi: 10.1186/s12974-021-02249-1
48. Furman MJ, Meuth SG, Albrecht P, Dietrich M, Blum H, Mares J, et al. B cell targeted therapies in inflammatory autoimmune disease of the central nervous system. Front Immunol. (2023) 14:1129906. doi: 10.3389/fimmu.2023.1129906
49. Stone JH, Khosroshahi A, Zhang W, Della Torre E, Okazaki K, Tanaka Y, et al. Inebilizumab for treatment of igG4-related disease. N Engl J Med. (2024) NEJMoa2409712. doi: 10.1056/NEJMoa2409712
50. Chen D, Gallagher S, Monson NL, et al. Inebilizumab, a B cell-depleting anti-CD19 antibody for the treatment of autoimmune neurological diseases: insights from preclinical studies. J Clin Med. (2016) 5:107. doi: 10.3390/jcm5120107
Keywords: neuromyelitis optica spectrum disorder, myasthenia gravis, inebilizumab, anti-AQP4 antibodies, anti-acetylcholine receptors antibodies
Citation: Song X, Chen J, Jin C, Peng Y, Sun Y and Zheng X (2025) Inebilizumab treatment in a patient with co-occurring AQP4-IgG positive neuromyelitis optica spectrum disorder and myasthenia gravis: a case report and literature review. Front. Immunol. 15:1528989. doi: 10.3389/fimmu.2024.1528989
Received: 15 November 2024; Accepted: 18 December 2024;
Published: 13 January 2025.
Edited by:
Michael A Firer, Ariel University, IsraelReviewed by:
Ennio Polilli, Azienda USL di Pescara, ItalyPing Li, General Hospital of Northern Theater Command, China
Copyright © 2025 Song, Chen, Jin, Peng, Sun and Zheng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xueping Zheng, c2ltcGxleHVlcGluZ0AxNjMuY29t
 Xiaoqian Song
Xiaoqian Song Jingjiao Chen1
Jingjiao Chen1 Chenyang Jin
Chenyang Jin Xueping Zheng
Xueping Zheng