- 1Diretoria de Ensino e Pesquisa, Fundação de Medicina Tropical Dr Heitor Vieira Dourado, Manaus, AM, Brazil
- 2Programa de Pós-Graduação em Medicina Tropical, Universidade do Estado Do Amazonas, Manaus, AM, Brazil
- 3Programa de Pós-graduação em Biologia da Interação Patógeno Hospedeiro, Instituto Leônidas e Maria Deane (ILMD-FIOCRUZ/AM), Manaus, AM, Brazil
- 4Programa de Pós-graduação em Imunologia Básica e Aplicada, Universidade Federal do Amazonas, Manaus, AM, Brazil
- 5Diretoria, Instituto Todos Pela Saúde, São Paulo, SP, Brazil
- 6Diretoria de Ensino e Pesquisa, Fundação Hospitalar de Hematologia e Hemoterapia do Amazonas, Manaus, AM, Brazil
Background: In SARS-CoV-2 infection, cytokines and laboratory biomarkers play a key role in disease progression and their long-term levels have been associated with the outcome of long COVID-19.
Objectives: I) study the levels of cytokines, hematological and biochemical biomarkers in the acute and post-acute phases of COVID-19 disease; and II) assess the impact of COVID-19 vaccine doses on fatigue symptoms.
Methods: This study is an exploratory cohort nested within a clinical and laboratory follow-up of surviving participants after pre-vaccine acute COVID-19 infection with severe clinical manifestations. We analyzed the inflammatory biomarker profiles of fifty SARS-Cov-2 negative healthy controls from before the COVID-19 pandemic, and eighty patients in the acute phase (Day 1, Day 7 and Day 14), and during 4 months and 2 years after hospitalization.
Results: Four months after hospitalization, 91.3% (73/80) of patients exhibited onset of long COVID symptoms, which persisted in 63.7% (51/80) after 2 years. Comparing the baseline values of the cytokines in the controls versus the follow-up times, the cytokines IL-6, IL-8 and IL-10 were high in the acute phase, declining over time after the individual’s recovery, while IL-1β showed an inverse variation, remaining high after 2 years. IL-1β, IL-10, and TNF increased over time post-acute infection, indicating a long-term inflammatory response. Vaccination with four doses, compared to three doses, showed a slight protective effect against fatigue symptoms in the male population (IRR 0.48, 95% CI 0.22 - 1.02; p=0.054). Neutrophil and leukocyte counts showed a significant reduction 2 years after hospitalization. However, platelet count was the laboratory biomarker that best reflects the prediction of long COVID symptoms up for to 2 years.
Conclusion: Although the frequency of long COVID symptoms declines over time after the acute illness, symptoms continue to persist 2 years after hospital discharge. Vaccination with a fourth dose booster appears to significantly influence reduction of symptoms associated with long COVID fatigue among the males. We further identified important laboratory biomarkers for long COVID. Elevated levels of IL-1β, IL-10, and TNF, along with low levels of IL-6, IL-18, and IL-12p70, also offer new insights into the inflammatory state in long COVID.
1 Introduction
COVID-19 primarily affects the respiratory system; however, months or even years after recovery, it can manifest as a broad spectrum of chronic symptoms impacting various organs and tissues (1). During the acute illness there is an inflammatory response, characterized by high levels of cytokines and chemokines, such as tumor necrosis factor alpha (TNF-α), interleukin-1 beta (IL-1β), interleukin-2 (IL-2), interleukin-6 (IL-6) and interleukin-8 (IL-8), was observed in patients with COVID-19, especially in patients with a severe disease, compared to individuals without severe respiratory disease (2). This inflammatory profile in the acute phase and the development of severe disease may play a crucial role in the frequency of long COVID-19. Globally, it is estimated that at least 65 million individuals present symptoms of long COVID, with an incidence of approximately 10% among survivors (3). Risk factors for developing this condition appear to be female individuals, type 2 diabetes, presence of specific autoantibodies, lower income, inability to rest adequately after hospitalization, or other comorbidities (3). It is a multisystemic condition presenting a wide range of manifestations, including gastrointestinal disturbances, headaches, skin rashes, shortness of breath, chest pain, cognitive impairment, loss of smell and taste, profound fatigue, and musculoskeletal pain (4). Long COVID is a condition defined as the continuation or emergence of new symptoms at least three months after the initial SARS-CoV-2 infection, with symptoms persisting for a minimum of two months (5). The underlying mechanisms of long COVID remain only partially understood. Studies have identified elevated levels of inflammatory cytokines, including interleukins (IL-1β, IL-6, IL-12, IL-18, IL-33), interferons (IFN-α, IFN-γ), tumor necrosis factor (TNF), and growth factors like TGF-β, in patients with long COVID (6). The complement system is also currently considered to play an important role in the pathogenesis of long COVID. Patients with severe disease have significant shedding of C5a and C5b-9 fragments (7) and high levels of MASP-2/C1Inh and C1s/C1Inh in their plasma, indicative of activation of the lectin pathway and the classical pathway, respectively (8). In addition, fragments of C2 molecules, C5bC6/C7 complex, Factor B, von Willebrand factor (vWF) and thrombospondin-1 (TSP1) were observed in a patient with acute COVID-19 and 6 months after hospital discharge, which increases endothelial damage and inflammation in the individual’s blood vessels (9).These circulating cytokine levels, some hematologic abnormalities such as alterations in the coagulation cascade, and molecules involved in the innate and adaptive immune response have already been suggested as valuable diagnostic and prognostic biomarkers of COVID-19 (10–12). Furthermore, the persistence of these pro-inflammatory cytokines has been implicated in the maintenance of chronic inflammation and the formation of fibrinolysis-resistant micro clots, further exacerbating the condition of long COVID (13). This hypothesis is supported by findings showing elevated levels of neutrophil activation proteins and other inflammatory molecules in patients with long COVID (14). Long COVID may develop a persistent inflammatory state. Discovery this profile of Laboratorial Biomarkers and Cytokines would be essential for our understanding of the pathophysiology behind long COVID as well as provide guidance for laboratory diagnostic testing and clinical management. Based on this, we hypothesized that long COVID may be caused by abnormal, sustained, and elevated levels of biomarker inflammation present in the blood after an acute severe SARS-CoV-2 infection has subsided. Thus, to explore this hypothesis we longitudinally characterized the inflammatory profile in long COVID. Furthermore, based on recent studies on the influence of vaccination and gender on long-term symptoms, we sought to stratify and analyze vaccination status to identify any differences between individuals.
2 Methods
2.1 Study design and ethical considerations
This is a clinical and laboratory exploratory longitudinal nested cohort study of surviving COVID-19 participants with severe clinical presentation in Manaus, Amazonas, Brazil. This study consisted of a longitudinal assessment of participants up to 24 months after acute infection. This study was approved by the Brazilian Research Ethics Committee under opinion no. 52378221.7.0000.0005. All participants provided written consent before participation in the study. The collection and use of the negative control group was approved by the Ethics Committee of the Fundação Hospitalar de Hematologia e Hemoterapia do Amazonas (HEMOAM) (CAAE 56413316.9.0000.0009).
2.2 Study participants and data collection
We conducted a 2-year follow-up prospective cohort of a previously published clinical trial (Jeronimo CMP, et al) (15). Results from the four-month clinical follow-up have been previously published by (Barros CMSS, et al) (16). All assessments in this study were performed at the Fundação de Medicina Tropical Dr Heitor Vieira Dourado (FMT-HVD), a reference institution for infectious diseases in the northern region of the country. In this study, clinically stable individuals residing in Manaus-AM of both sexes, aged 18 years or older, who were hospitalized at the Hospital Delphina Rinaldi Abdel Aziz and Emergency Room in Manaus-AM during the first wave of COVID-19 from March to May 2020 and positive for SARS-CoV-2 by RT-qPCR at hospitalization were eligible to participate. Patients were invited by phone to participate in a structured long COVID follow-up multidisciplinary program. Moreover, 50 healthy individuals were included as a control group to compare the cytokine profile at baseline. These control samples were collected from donors at HEMOAM in Manaus, Amazonas, Brazil, before the start of the COVID-19 pandemic. The control samples were negative for SARS-CoV-2 and were only analyzed for baseline comparison. All participants were evaluated during the acute phase (during the hospitalization period), after 4 months, and 2 years after the acute disease. All participants had their data and information collected through a clinical questionnaire consisting of 34 items on self-reported signs and symptoms at the time of visits, aiming to verify the persistence of symptoms after hospitalization. Additionally, they underwent a battery of laboratory tests to evaluate the persistence of inflammatory and metabolic alterations over time. Clinical data with demographic characteristics (age, sex, preexisting comorbidities, signs and symptoms, vaccination status), laboratory test results such as complete blood count, liver function, kidney function, electrolyte tests, medical history, and clinical evolution of patients throughout the course of the disease and recovery were stored in REDCap. For the definition of the severity criteria of acute COVID-19 we used the Coronavirus Disease 2019 (COVID-19) Treatment Guidelines - National Institutes of Health classifying participants into (Moderate illness: Individuals who showed evidence of lower respiratory disease during clinical assessment or imaging and who have an oxygen saturation measured by pulse oximetry (SpO2) ≥94% on admission. Severe illness: Individuals who have an SpO2 30 breaths/min, or lung infiltrates >50% and Critical illness: Individuals who had respiratory failure, septic shock, or multiple organ dysfunction) (17). Long COVID was defined as the presence of at least one sequela symptom after three months based on the WHO Long COVID case definition, confirmed intermittently or continuously lasting for at least 2 months with no other explanation (18).
2.3 Sample collection
Serum samples were collected from all patients during their hospital stay for routine laboratory tests and stored for subsequent inflammatory profiling. During the convalescent phase, approximately 50ml of peripheral blood samples were collected from participants in vacuum tubes with sodium heparin (BD Vacutainer, Becton Drive, Franklin Lakes, USA) early in the morning. After collection, the samples were transported for hematology and biochemistry tests at the Clinical Analysis Laboratory at FMT-HVD. Aliquots for the inflammatory profile study were centrifuged and stored in a -80°C freezer until analysis.
2.4 Cytokine and laboratorial biomarkers serum levels
The quantification of circulating cytokines in patient and controls serum samples was performed using the Cytometric Bead Array (CBA) technique with the Human Inflammatory Cytokine Kit (BD Biosciences, San Jose, USA), according to the manufacturer’s instructions. The BD™ CBA Kit uses a series of particles (beads) of known size and distinct fluorescence intensity to simultaneously detect various soluble cytokines through a capture surface. Each capture bead in the kit was conjugated with a specific antibody. Samples were run in a FACS CantoII (BD® Biosciences, San Jose, CA, USA) and the FCAP Array software v3 (Soft Flow Inc., USA) was used for data analysis. The quantified cytokines were IL-1β, IL-6, IL-8, IL-10, IL12p70, and TNF, with concentrations in pg/mL and Mean Fluorescence Intensity (MFI) of each cytokine. The hematological and biochemical laboratory markers investigated were hemoglobin, hematocrit, platelets, leukocytes, neutrophils and CK, CK-MB, creatinine, urea, aspartate aminotransferase, alanine aminotransferase, total bilirubin, direct bilirubin, indirect bilirubin, sodium, potassium, ferritin, lactate dehydrogenase, glucose, triglyceride, total cholesterol and HDL.
2.5 Statistical analysis
Descriptive statistics (mean, standard deviation) and analytical statistics (chi-square and Wilcoxon tests) were performed as needed at a 95% significance level after initially being subjected to a Shapiro-Wilk test for normality. A mixed linear random effect model was conducted to evaluate variations in plasma levels of inflammatory markers over time and their relationship with long COVID symptoms. To understand the direct and indirect impact of vaccination on long COVID, we conducted causal mediation analyses, where the treatment was vaccination, and the outcome was the frequency of long COVID symptoms. We adjusted two log-binomial regression models by sex. The most scientifically significant inflammatory and laboratory markers were considered for subsequent network development using the open-source software Cytoscape (version 3.9.1) (Cytoscape Consortium San Diego, CA, USA). In all cases, significance was considered at p-value <0.05. A negative binomial Poisson regression model with a 95% confidence interval was used in the univariate and multivariate analysis with robust variance to analyze laboratory and inflammatory parameters and their effects on long COVID. Statistical analyses were performed using STATA version 16.0, GraphPad Prism version 10.0.1 (GraphPad Software, San Diego, CA, USA), and RStudio 4.0.2.
3 Results
3.1 Clinical and laboratory characteristics of study participants
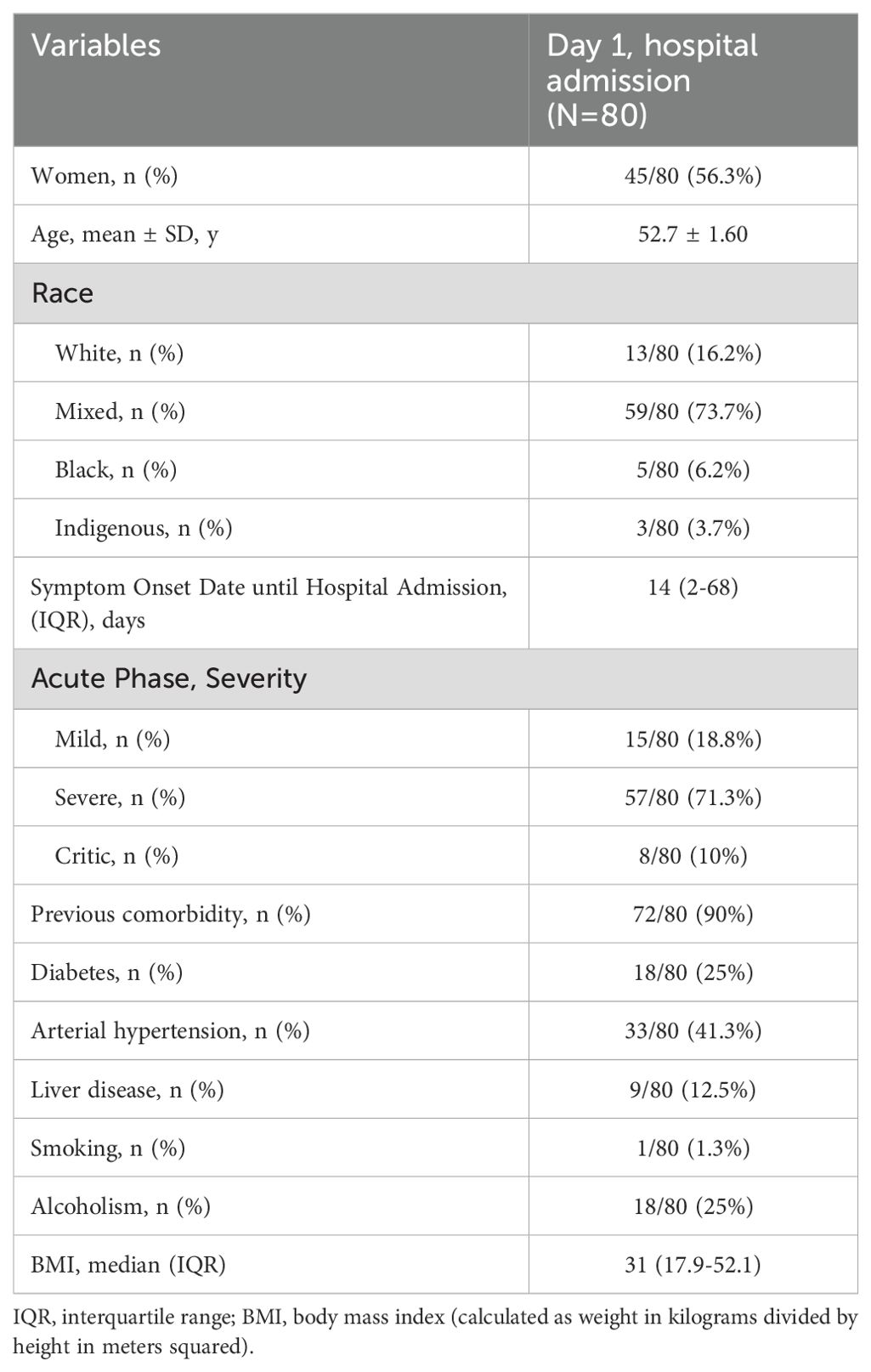
During the study recruitment period, 239 surviving patients were contacted by telephone, and 159 were excluded due to eligibility criteria, refusals to participate, and loss of follow-up. A total of 80 recovered participants were included. The general characteristics of the acute phase are presented in Table 1. During the acute phase, female was the most predominant gender with 56.3% (45/80) of the population. The majority, 71.3% (57/80), were patients with severe infection, 18.8% (15/80) had moderate acute infection and 10% (8/80) had critical illness. 90% (72/80) had pre-existing comorbidities at admission, of which systemic arterial hypertension 33/80 (41.3%) and Diabetes Mellitus 18/80 (25%) were the most frequent comorbidities.
The leukocyte median count was 9.94 mm3 (2.7 – 28.2) in the acute phase, a higher value compared to that after 2-years result similar to the neutrophil count. Platelets had a median value of 304,000 mm³ (8.2 – 649) in the acute phase, slightly higher than after 2 years, 224,000 mm³ (243-424) (Table 2).
3.2 Acute and long COVID clinical presentations
During the acute phase of the disease, fatigue was the most frequently reported symptom upon admission by both sexes, present in all male patients and 86.6% of female patients. Other symptoms also reported in the acute phase, such as dyspnea on exertion 94.2%, fever 88.5%, cough 85.7% and chills 85.7% were more frequent in the male population than in the female population (Figure 1). In the evaluation of persistent symptoms of long COVID, even after 4 months of hospital discharge, 91.3% (73/80) of patients reported having experienced one or more unexplained symptoms related to the acute infection. The most common symptoms reported in this period were myalgia (60%), fatigue (55%), headache (42.5%) and cough (35%). The female population manifested a higher proportion of almost all symptoms. Over the 2-year follow-up, 51/80 (63.7%) of patients still reported persistent long COVID symptoms. The most common symptoms in this period were fatigue (63.7%), hair loss (47.5%), arthralgia (42.5%), palpitation (38.7%), dyspnea on exertion (36.6%) and cough (33.8%). Participants had no reports of nausea, vomiting, history of fever, or dizziness. Women had a higher proportion of fatigue (66%) and hair loss (75.5%) than the male population, while men had a higher proportion of dyspnea on exertion (42.8%) and chest pain (28.5%).
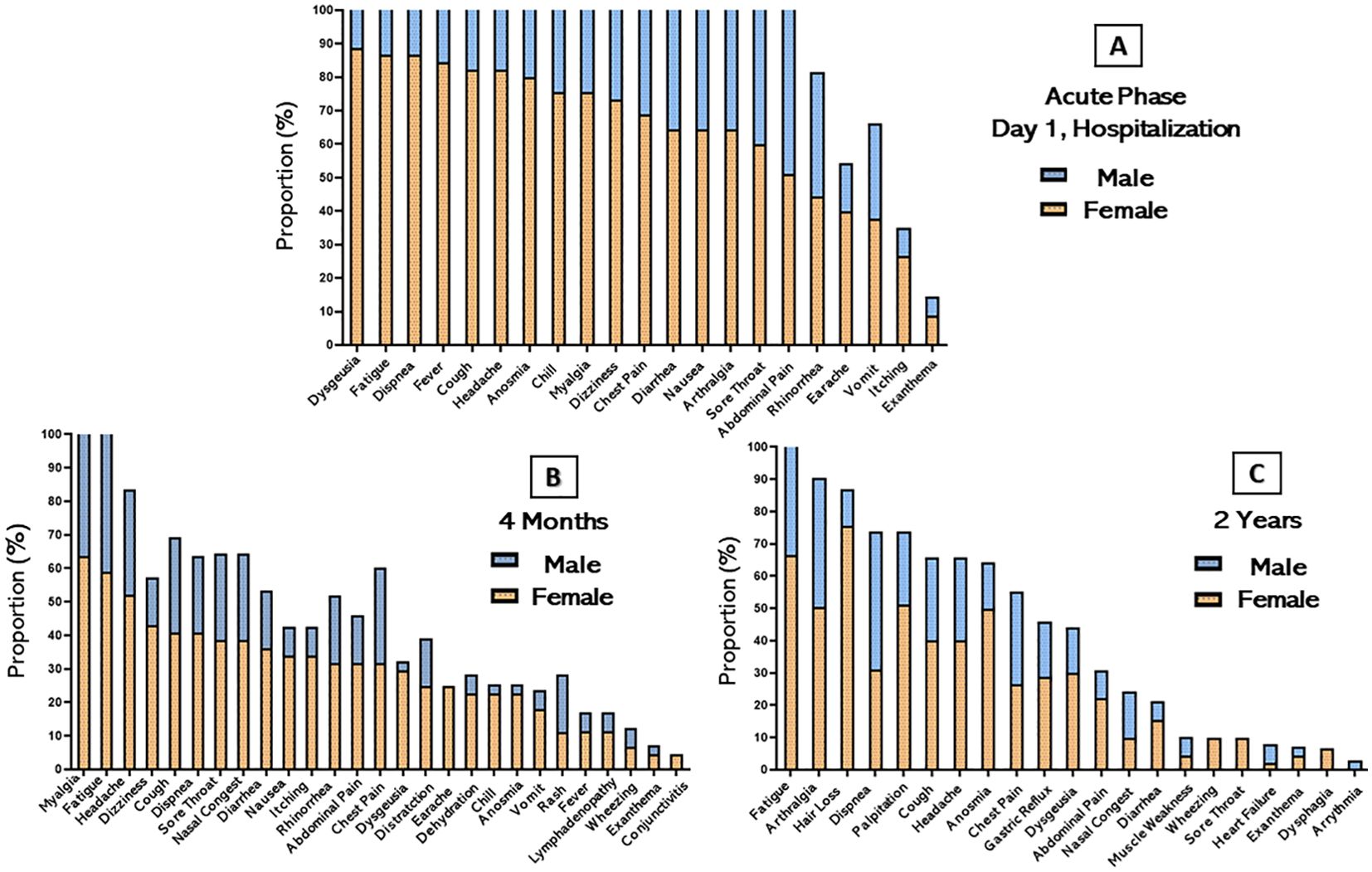
Figure 1. Distribution of persistent symptoms or signs after acute COVID-19 stratified by sex. The bars represent the percentage of participants in their respective categories who presented and/or continued to present each symptom over time. (A) Acute phase of hospitalization (B) 4 months after hospital discharge and (C) 2 years after hospital discharge.
3.3 Long COVID and vaccination status
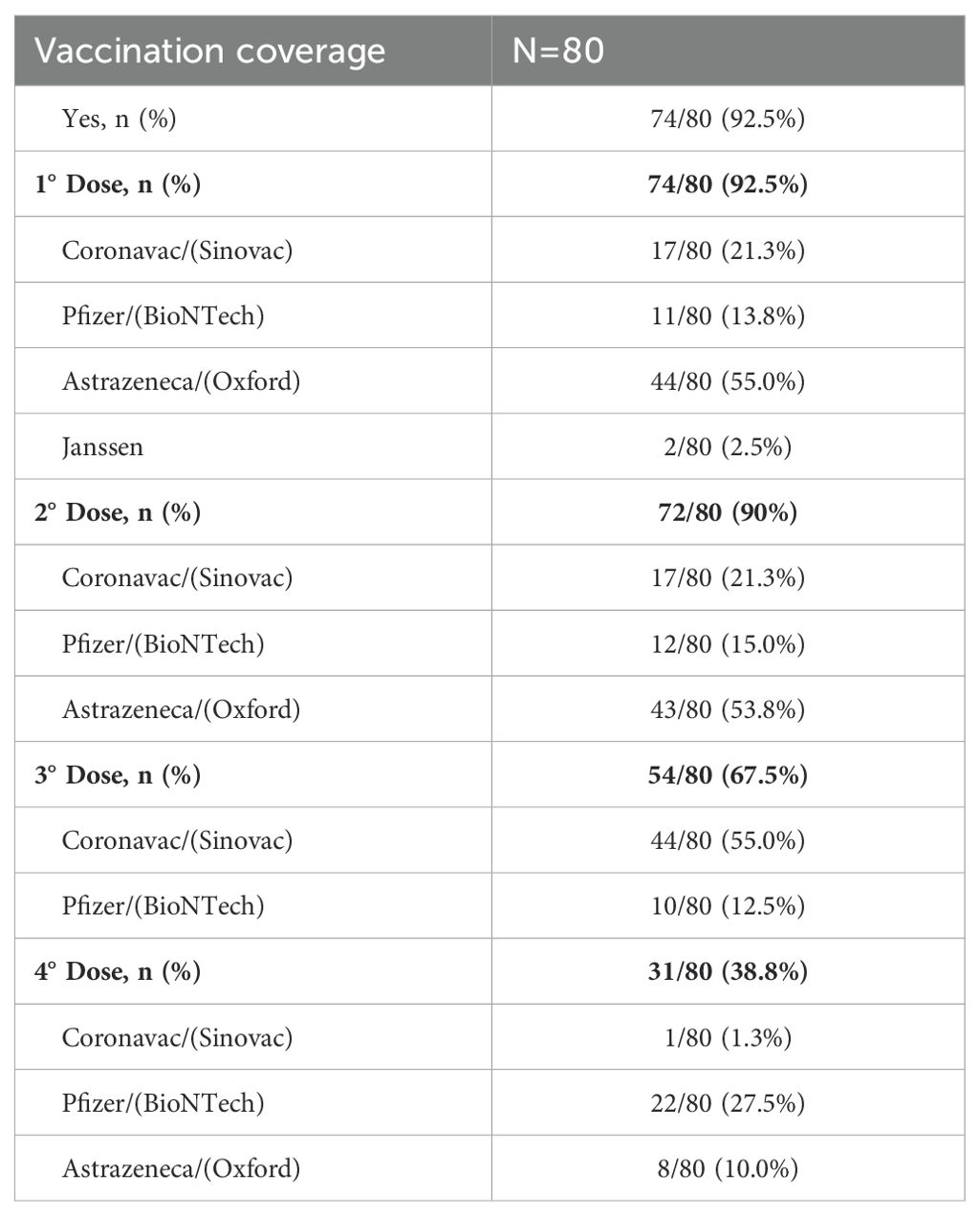
We recorded the number and type of vaccines of SARS-CoV-2 administered until October 2022, when all participants had completed the 2-year follow-up assessments. The vaccination and booster were first applied in Amazonas to the elderly and health care professionals. In the study population, 92.5% (74/80) of participants were vaccinated with at least 1 dose of the SARS-CoV-2 vaccine. They had an average age of 52.8 years and had comorbidities such as diabetes and hypertension. Regarding the number of doses received by vaccinated participants, 2.5% (2/80) of patients took only the 1st dose; 22.5% (18/80) 2 doses, 18.8% (23/80) 3 doses, and 39.9% (31/80) 4 doses. The mean interval between the 1st and 2nd dose was 77 days ± 4.5 (95% CI: 68.6 - 86.8); and between the 1st and 4th dose was 438 days ± 9 (95% CI: 419.6 - 456.4) (Table 3). In our adjusted investigation, a four-dose regimen was associated with a greater protective effect and appeared to decrease the frequency of fatigue symptoms in the male population (Figure 2) (IRR 0.48, 95%CI: 0.22 - 1.02; p=0.054). When considering the remaining symptoms of long COVID, no significant differences in benefit were found between the dose regimens in females and the general population.
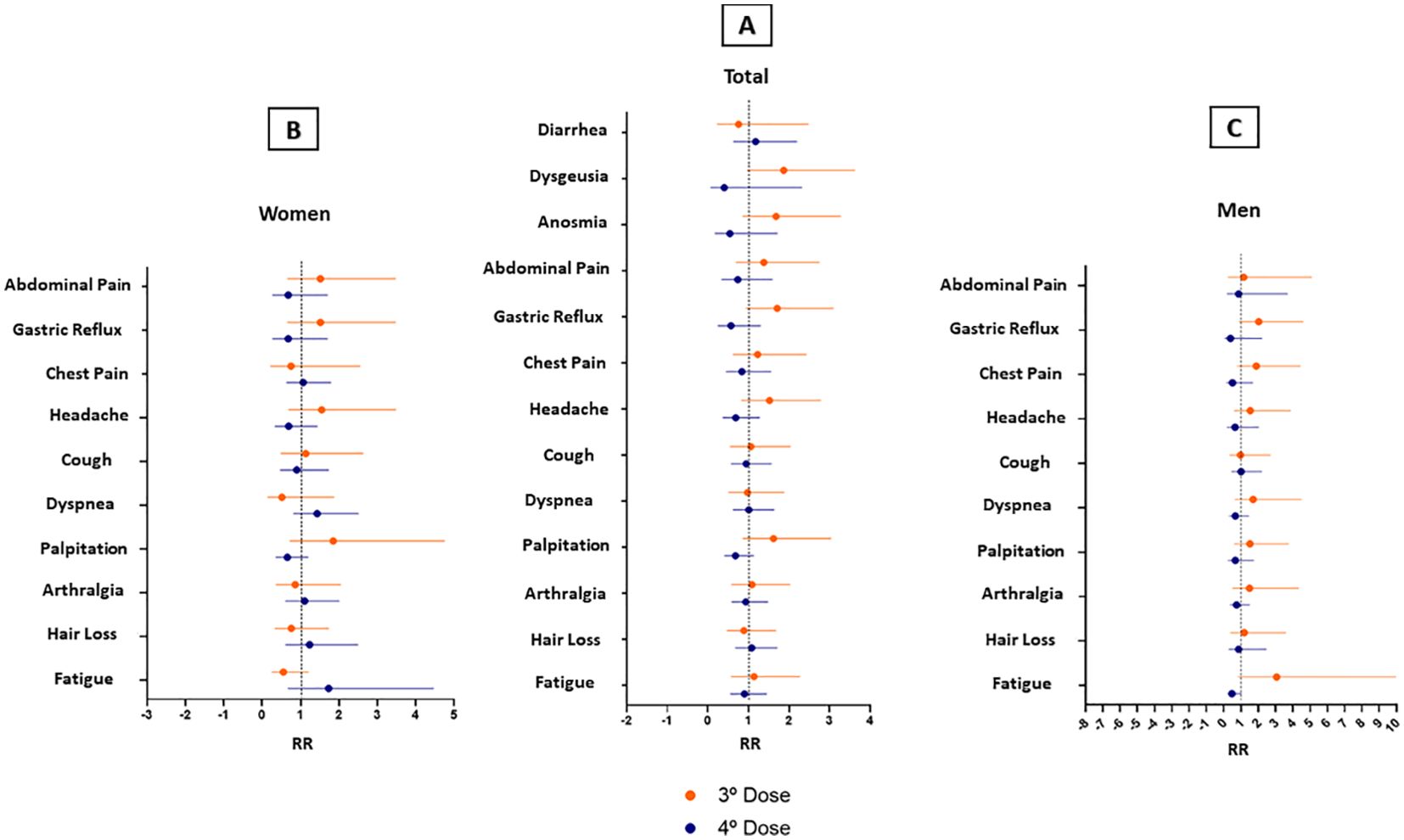
Figure 2. Long Covid symptoms in participants vaccinated in a 3-dose schedule versus a 4-dose schedule. (A) Total Patients (B) Women (C) Men. Error bars represent 95% CI.
3.4 Inflammatory profile and long COVID
When comparing the results of baseline cytokine values in controls versus follow-up times, as seen in Figure 3, four cytokines were significantly elevated. During the acute phase IL-6 (D1, D7 and D14), IL-8 (D1, D7 and D14) and IL-10 (D1 and D7) remained high compared to the control group, declining over time after the individual’s recovery, while IL-1β showed an inverse variation, remaining high after 2 years. A mixed linear regression model that explores the relationship between cytokine levels and different variables, including time (on different days after hospitalization), sex, and their interactions was also done (Figure 4). The Figure 4 was created by combining the cytokines IL-1β, IL6, IL8, IL10, IL12p-70, and TNF, without distinction of sex over time. It is possible to see that the cytokines IL-1β, IL-6, and TNF intersect at some point in the graph, highlighting the proximity of this information at some period of the study, an inverse effect observed with IL-8, IL-10, and IL-12p70 despite the proximity of their confidence intervals. It is also possible to highlight the trend and variation of IL-6 and IL-8 among the other cytokines, which did not vary as much among themselves over time. To assess whether there was a trend of change in the levels of each cytokine (Supplementary Table S1), an estimation was performed for each time unit (Day 1, Day 7, Day 14) and (4 Months, 2 Years). Regarding IL-1β, the estimation coefficient at 4 months is 0.03 (95%CI: 0.00-0.06), which, on average, is expected to be an increase of 0.03 in units after 4 months, compared to day 1 of the acute phase. This effect is statistically significant at the 4-month and 2-year follow-up (p=0.035 and p<0.001, respectively), indicating that IL-1β levels increase over time after acute infection, unlike IL-10, which had a negative coefficient of - 0.05 (95%CI: 0.08-0.02; p=0.004). To verify a possible influence of the participants’ sex among the different cytokines over time in Figure 4B, the figures draw a smoothed line that shows the trend of the data and a shadow that represents the 95% confidence interval. When observing these variations comparing the sexes, the analysis indicates an expected difference between the cytokine units between the sexes and a statistical effect, given that we obtained a significant result in IL-6 with a coefficient (0.12, p<0.001). The interactions between time and sex (D7, D14, 4 Months and 2 Years x Sex), explore whether the relationship between time and cytokine levels differs between the sexes after Day 1 of hospitalization. This variation is observed in both IL-6 and IL-1β, over the 2-year follow-up. Random effects include the residual variance (σ2) and the variance between subjects (τ00 id). The ICC (Intraclass Correlation Coefficient) indicates the variation in cytokine levels. The model includes the number of subjects (N id) and observations. The marginal R² indicates how much the variability in cytokine levels is explained by the model variables. The conditional R² indicates whether the variability is explained by the full model, including random effects (Supplementary Table S1).
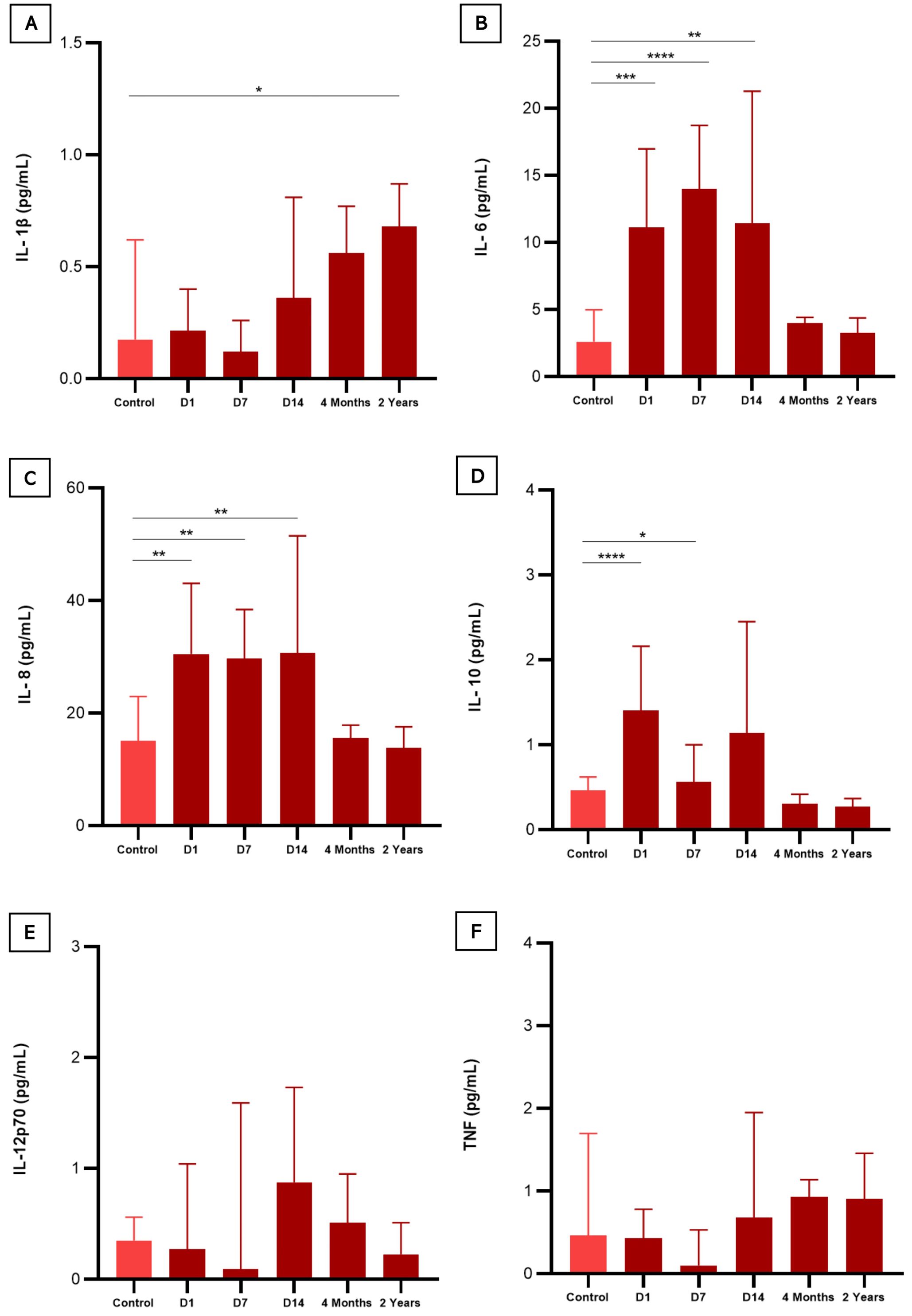
Figure 3. Serum levels of cytokines in COVID-19 infected patients compared to healthy participants (negative controls). (A) (IL-1β) interleukin 1-beta; (B) (IL-6) interleukin 6; (C) (IL-8) interleukin 8; (D) (IL-10) interleukin 10; (E) (IL-12p70) interleukin 12p70; and (F) (TNF) Tumor Necrosis Factor. Statistical difference was calculated by Mann-Whitney test. *p < 0,05, **p < 0,01, ***p < 0,001 e ****p < 0,0001.
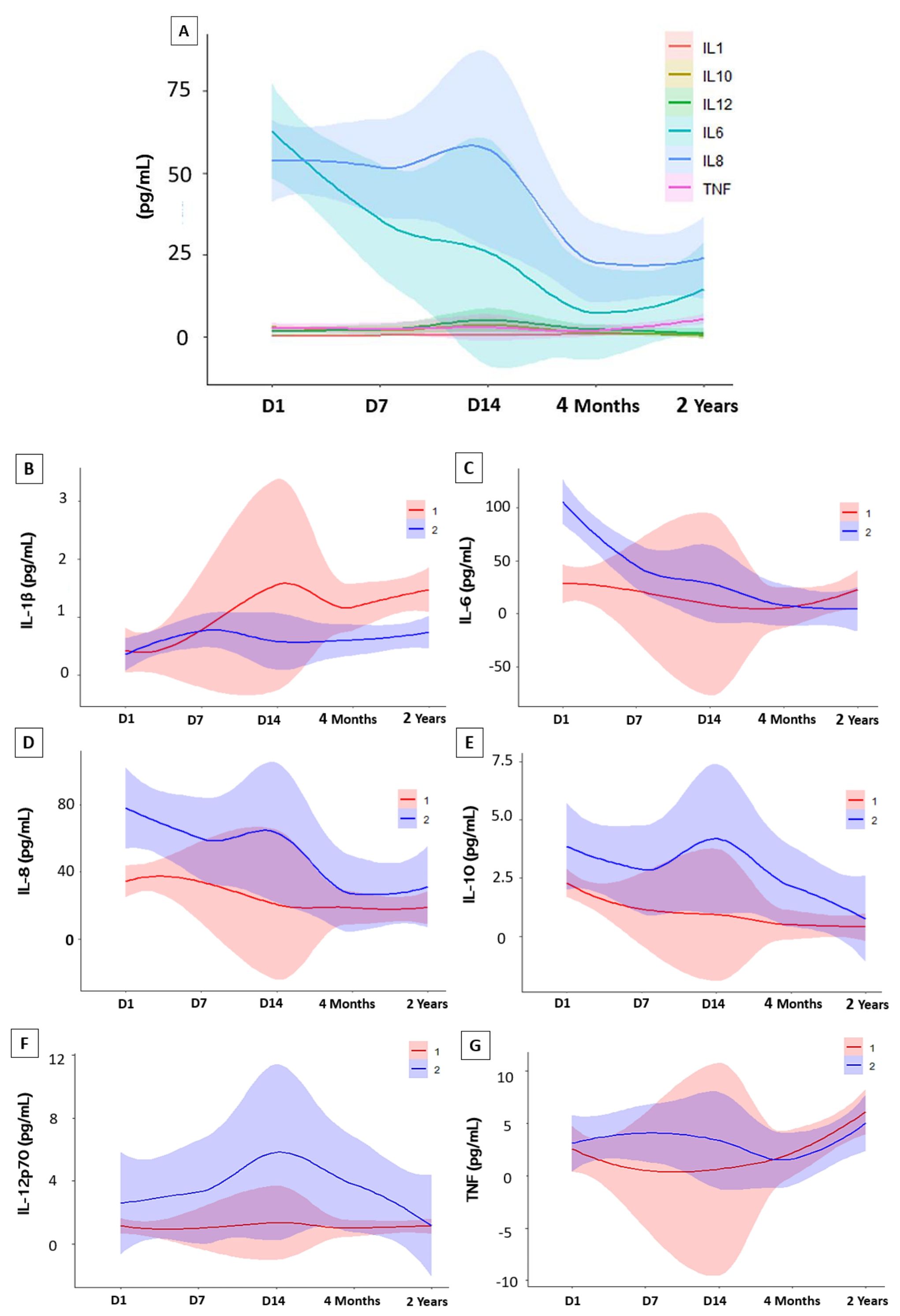
Figure 4. Variations in cytokine levels during follow-up. Statistical analysis were performed using interactions in a linear mixed-effects model. (A) Cytokine Trend Model of study participants in the Acute Phase (Day 1, Day 7 and Day 14) and Convalescent Phase (4 months and 2 years). Analysis of interactions between sex. (B) (IL-1β) interleukin 1-beta; (C) (IL-6) interleukin 6; (D) (IL-8) interleukin 8 (E) (IL-10) interleukin 10; (F) (IL-12p70) interleukin 12p70 and (G) (TNF) Tumor Necrosis Factor. Abbreviations: (1) Women; (2) Men.
In Figure 5A, we present the heatmaps that represent the profile of laboratory and inflammatory parameters after 4 months and 2 years. The heatmaps show the hierarchical clustering of these markers in individuals with persistent symptoms of long COVID. Patients had some significant interaction networks as shown in Figure 5B, when we investigated whether there was a correlation between cytokine values and laboratory parameters, with the number of long COVID symptoms. In the 4-month evaluation, patients showed a moderate positive correlation between Platelet values (R = 0.408, p<0.001), and negative for Urea (R = - 0.375, p=0.002). Regarding the cytokines evaluated at this time point, only IL-6 (R = 0.313, p=0.011) was positively correlated with the number of long COVID symptoms. After 2 years of evaluation, platelet values maintained a moderate positive correlation (R=0.398, p<0.001) together with Creatinine, with a negative correlation (R= - 0.410, p<0.001). These associations highlight the importance of these markers as possible indicators of the evolution of long COVID symptoms over time.
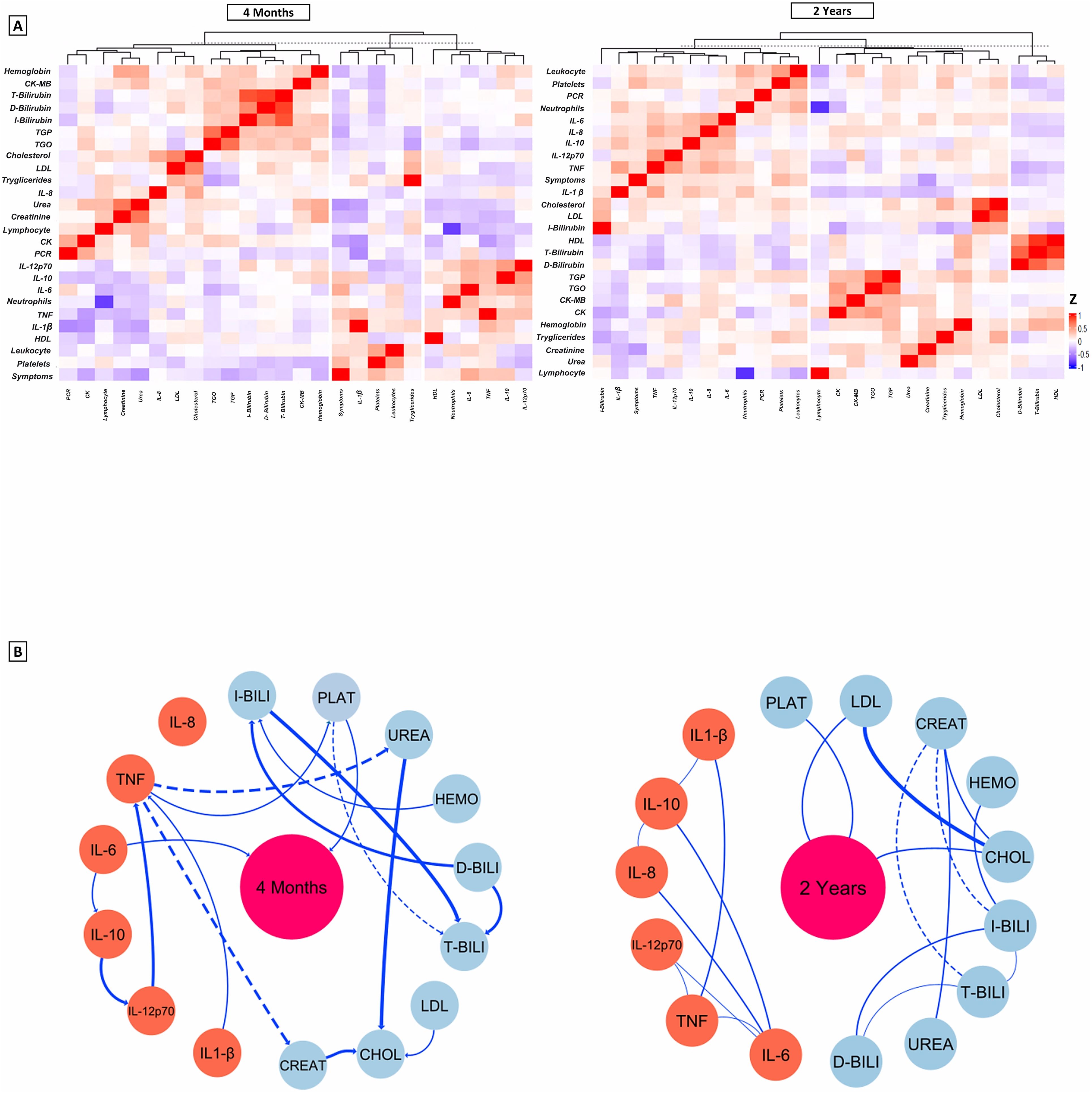
Figure 5. (A) Representative average heat map of the main functional and laboratory parameters at 4 months and 2 years. The settings configure the display of lines grouped by similarity based on Spearman Correlation, the colors represent the values of the variables in relation to the number of symptoms. The graphs emphasize the hierarchization of rows that group variables with similar patterns across observations, while the hierarchization of columns groups observations with similar patterns across observations. (B) Interaction networks between inflammatory and laboratory parameters and the number of Long Covid symptoms. Dashed lines between molecules show a negative correlation, while solid lines indicate a positive correlation. The thickness of these indicates the strength of the shine. The radiance index (r) used to categorize the strength of clarity as weak (r ≤ 0.35), moderate (r≥0.36 to r ≤ 0.67) or strong (r≥0.68). IQR, interquartile range; BMI, body mass index (calculated as weight in kilograms divided by height in meters squared) PLAT, Platelets; LDL, Low density lipoprotein; CREAT, Creatinine; HEMO, Hemoglobin; CHOL, Total Cholesterol; I-BILI, Indirect bilirubin; T-BILI, Total bilirubin; D-BILI, Direct bilirubin; IL-6, interleukin 6; IL-8, interleukin 8; TNF, Tumor Necrosis Factor; IL-12p70, interleukin 12p70; IL-10, interleukin 10; IL-1β, interleukin 1-beta.
Regression analysis shows the influence of hematological, biochemical and inflammatory laboratory parameters on the multiplicative effect of the number of long COVID symptoms. In the analysis described in Table 4 the cytokines IL-10 (IRR 1.770, p=0.007) and TNF, (IRR 1.025, p=0.053) were significantly and positively associated with the sum score of long COVID symptoms after 4 months of recovery. IL-12p70 was the cytokine most negatively associated with the number of symptoms. The other pro-inflammatory cytokines such as IL-1β and IL-6 were not associated with a multiplicative effect on symptoms. In the evaluation 2 years after hospital discharge, parameters such as Platelets and IL-12p70 were positively associated with a multiplier effect on symptoms, while Creatinine (IRR 0.387, p=0.001) was associated with a protective effect on the number of symptoms. The results highlight the importance of several laboratory and inflammatory parameters in the evolution of long COVID symptoms. They suggest that the inflammatory response and possible organ dysfunctions, such as renal and hepatic, may play a significant role in the persistence and severity of symptoms over time.
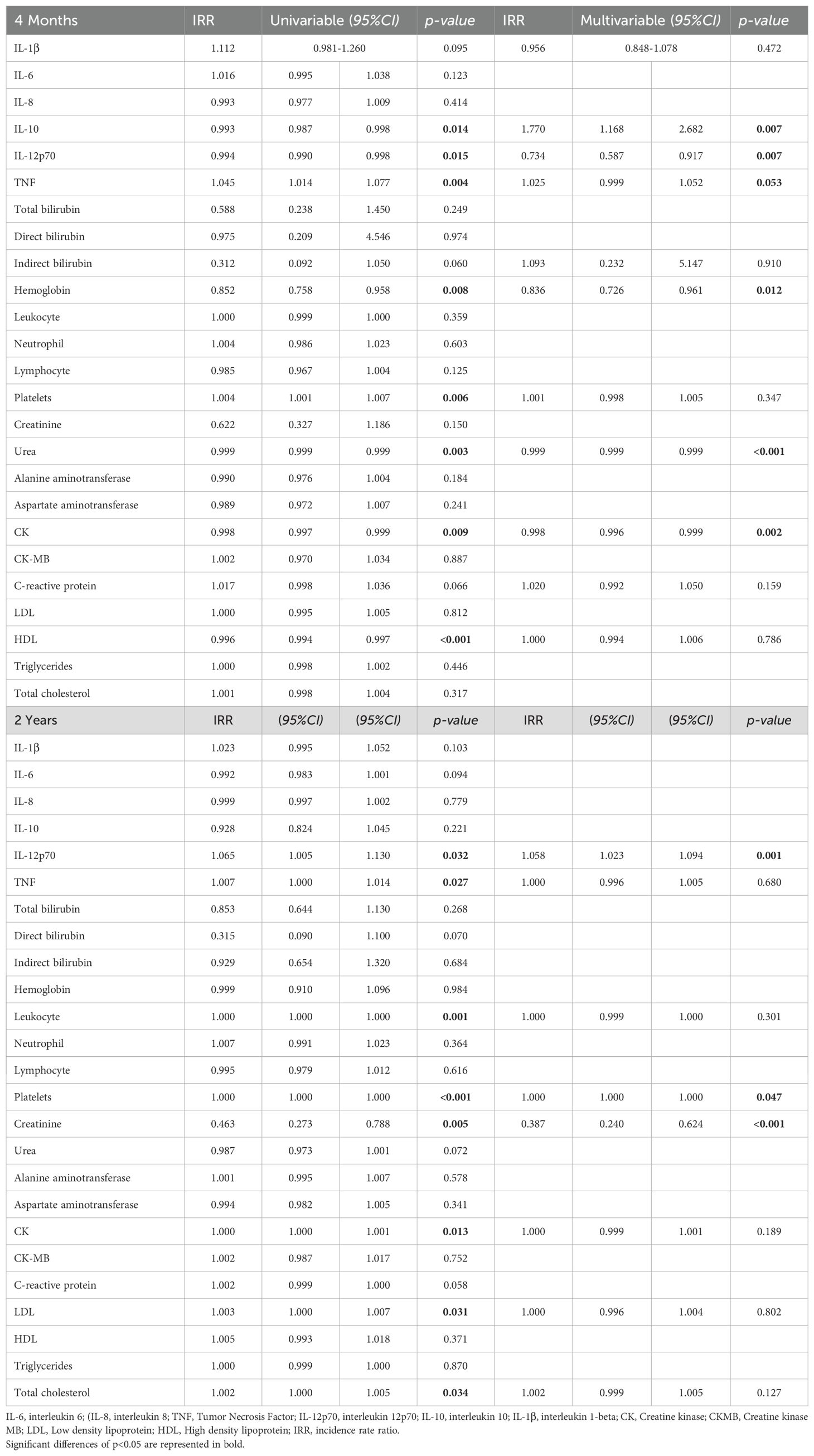
Table 4. Univariate and multivariate analysis of participants’ laboratory and inflammatory parameters, and their influence on the multiplicative probability of Long Covid symptoms at 4 months and 2 years after hospitalization.
4 Discussion
In this prospective cohort that followed up hospitalized individuals who survived acute COVID-19 in a pre-vaccination era, we sought to characterize and investigate the longitudinal relationship between inflammation markers and the development of long COVID. We found notable elevations in plasma inflammatory cytokine levels among individuals with long COVID. Furthermore, fatigue, dyspnea, fever and cough were the most common symptoms during the acute phase. Our findings show that even after recovery, a large number of long-term symptoms of musculoskeletal, cardiovascular and gastrointestinal origin still remained in the subjects even after 2 years, despite a considerable decline after 4 months, regardless of disease severity in the acute phase. These findings are similar to those found by Kim, et al., 2023 (19) who also found a high incidence of persistent symptoms of musculoskeletal and gastrointestinal origin. Overall, the prevalence of long COVID symptoms was 91.3% at 4 months and 63.7% at 2 years after diagnosis. This result is similar to that described by Geng, et al (20) in a cohort with 2,469 patients who reported the prevalence of long COVID in 65% of patients after 2 years. Few authors reported sex-disaggregated data, but overall, female patients were more likely to experience prolonged COVID-19 syndrome than their male counterparts, a trend observed in several studies (21). Other studies have reported that the risk of long-term symptoms is higher among previously hospitalized female patients than among males, with the female sex being a risk factor for chronic fatigue (22). These results suggest that the persistence of sequelae after SARS-CoV-2 infection is prolonged even among individuals with different acute disease profiles and has a greater impact on the female population, especially during the recovery phase. In addition to this gender difference in the influence of long COVID, several variants emerged after the first wave of the disease with different levels of transmission and virulence, such as the B.1.1.7 or Alpha SARS-CoV-2 variant, first isolated in the United Kingdom in September 2020. However, the impact of the variants on vaccination and long-term symptoms, as far as we know, was somewhat related compared to this association (23). Fernández-de-las-Peñas, et al. (24) showed that individuals infected with the historical Wuhan variant exhibited a greater number of post-COVID symptoms, than those patients infected with the Alpha or Delta variant. Therefore, it is important to consider the introduction of variants when determining the effect of symptoms in long COVID. Our study showed a small and significant protective effect of vaccination with 4 doses versus a 3-dose regimen in reducing long-term fatigue in men. Azzolini, et al. (25) found that the number of vaccine doses was associated with a lower prevalence of long COVID: 41.8% (95% CI, 37.0% - 46.7%) in unvaccinated patients and 16.0% (95% CI, 11.8% - 21.0%) with 3 doses. On the other hand, Wynberg, et al. (26) reported that most patients reported feeling that symptoms had not changed within a month after the first vaccination, regardless of the initial severity of COVID-19. Even with these new findings from studies reporting improvement in persistent symptoms after vaccination, the results are divergent. The inflammatory profile of our patients revealed higher levels of the inflammatory cytokines IL-1β and TNF after 2 years of hospital discharge, with the female population having a higher profile of IL-1β. The cytokines IL-1β, IL-10 and TNF showed a slight upward trend over the 4-month and 2-year follow-ups when compared with the baseline levels of the acute phase. Although IL-6 represents a key inflammatory factor in the acute phase, it did not show this same upward trend in the long term (27). The elevation of IL-1β and TNF may be associated with a possible acute pro-inflammatory reprogramming of long-lived lung macrophages or their precursors, which may result in a vicious cycle of production of these cytokines (28). Our regression also showed that TNF was positively associated with a multiplicative effect on the sum of long COVID symptoms at the 4-month follow-up. In a longitudinal analysis of the cytokine profile and its specific phenotype relationship in respiratory symptoms, it was shown that participants with persistent respiratory symptoms had significantly higher levels of IL-1β and TNF after 2 years. These results suggest that long COVID has evidence of being associated with residual inflammation, as reported by Moreno Perez, et al. (29). Patients with long COVID also present increased activation of the complement system. The complement system is a defense pathway against pathogens, including viruses, and triggers one or more activation pathways resulting from interaction with pathogens. Carlo Cervia-Hasler et al. (9) reported high levels of C2, C5bC6, Factor B molecules during severe acute disease and up to 6 months after recovery in a cohort with 39 healthy controls and 113 patients with COVID-19 for up to 1 year after initial confirmation of acute infection, and Afzali, et al., 2022. who notes that patients with severe covid have high levels of C5a and fragments of the C5b-9 terminals (7), these results indicate a possible activation of the classical or lectin pathway. Since complement activation involves hemolysis; induction of thromboinflammatory responses, including platelet and endothelial activation, an increase in serum levels of von Willebrand factor (vWF) and thrombospondin-1 (TSP-1) was also observed, in addition to high levels of MASP-2/C1Inh and C1s/C1Inh described by Lynch, et al. (8) which may explain the persistent inflammatory profile in this population. This study has some limitations, which should be considered. For instance, long COVID symptoms were self-reported by patients. It is possible that using different scales that assess different symptoms (fatigue or dyspnea) could reveal potential differences between groups. The lack of inclusion of uninfected controls, non-hospitalized or asymptomatic patients limits the ability to assess a direct association of SARS-CoV-2 infection with general and specific long COVID symptoms after 2 years. Our findings included patients discharged from the hospital in 2020 and therefore would not include those infected with other variants different from those circulating in Manaus, Brazil, by the time of acute infection.
5 Conclusions
Although the frequency of long COVID symptoms declines over time after the acute illness, symptoms persist for more than 2 years after hospital discharge. Vaccination with a fourth dose booster appears to significantly influence the male population in reducing symptoms of fatigue associated with long COVID. Furthermore, elevated serum levels of IL-1β, IL-10, and TNF, as well as low levels of IL-6 and IL-8, IL-12p70, appear to constitute a cytokine long COVID profile. In our study, we demonstrated that it was possible to identify important positive correlations between platelet counts and the number of long COVID symptoms, even after 2 years. As the components of the complete blood count are readily available, we recommend the routine use of this biomarker in the monitoring of long COVID. Therefore, we suggest continued research on long COVID to better understand the potential long-term health consequences of COVID-19, including symptom persistence, impact on quality of life and the efficacy of interventions such as vaccination.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Comitê de Ética em Pesquisa. Fundação de Medicina Tropical do Amazonas - FMT/IMT/AM. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
AM: Conceptualization, Investigation, Methodology, Project administration, Supervision, Writing – original draft, Writing – review & editing. AP: Investigation, Methodology, Supervision, Writing – review & editing. BM: Data curation, Formal analysis, Methodology, Software, Writing – review & editing. CG: Data curation, Formal analysis, Methodology, Supervision, Writing – review & editing, Validation. LS: Methodology, Writing – review & editing. AC: Methodology, Writing – review & editing. GM: Investigation, Methodology, Writing – review & editing. CS: Methodology, Writing – review & editing. JV: Investigation, Methodology, Validation, Writing – review & editing. VM: Writing – review & editing, Data curation, Methodology, Validation. GM: Funding acquisition, Resources, Validation, Writing – review & editing. WM: Funding acquisition, Resources, Validation, Writing – review & editing. ML: Funding acquisition, Resources, Validation, Writing – review & editing. GA: Funding acquisition, Resources, Validation, Writing – review & editing. VS: Funding acquisition, Resources, Validation, Writing – review & editing. AC: Funding acquisition, Resources, Validation, Writing – review & editing. FA-V: Funding acquisition, Resources, Validation, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. Financial support was provided in the form of grants from Fundaç;ão de Amparo à Pesquisa do Estado do Amazonas (FAPEAM) (Pró-Estado Program - #002/2008, #007/2018, #005/2019, POSGRAD #002/2024, PCTI-EMERGESAUDE II - #006/2020), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) and Coordenação de Aperfeiçoamento de Pessoal de Nı́vel Superior (CAPES) (PDPG-CONSOLIDACAO-3-4 Program #88887.707248/2022-00). AM, BM, LS, GSM, CS, and JV have fellowships from FAPEAM, CAPES and CNPq (Master and PhD student fellowships). AGC and VS are research fellows from CNPq. The funders did not contribute to the study’s design, data collection and analysis, the decision to publish, or the preparation of the manuscript. Support was also acquired by CNPq (CNPq/MCTI/CT-Saude Call – Research, development and innovation in long COVID), notice n°53/2022 (RECLAIM Brazil Study).
Acknowledgments
The authors thank the HEMOAM research platform for the use of their facilities. We are also grateful to the patients and controls who participated in the study, as well as their parents or legal guardians for allowing participation. We thank also the staff of the Delphina Rinaldi Abdel Aziz Hospital and Emergency Room, FMT-HVD as well as many members of the partner research groups.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2024.1520193/full#supplementary-material.
References
1. Hu B, Guo H, Zhou P, Shi ZL. Characteristics of SARS-CoV-2 and COVID-19. Nat Rev Microbiol. (2021) 19:141–54. doi: 10.1038/s41579-020-00459-7
2. Ghazavi A, Ganji A, Keshavarzian N, Rabiemajd S, Mosayebi G. Cytokine profile and disease severity in patients with COVID-19. Cytokine. (2021) 137:155323. doi: 10.1016/j.cyto.2020.155323
3. Davis HE, McCorkell L, Vogel JM, Topol EJ. Long COVID: major findings, mechanisms and recommendations. Nat Rev Microbiol. (2023) 21:133–46. doi: 10.1038/s41579-022-00846-2
4. Greenhalgh T, Sivan M, Perlowski A, Nikolich JŽ. Long COVID: a clinical update. Lancet. (2024) 404:707–24. doi: 10.1016/S0140-6736(24)01136-X
5. World Health Organization. Post COVID-19 condition (Long COVID) (2021). Available online at: https://www.who.int/europe/news-room/fact-sheets/item/post-covid-19-condition (Accessed August 15, 2024).
6. Dennis A, Wamil M, Alberts J, Oben J, Cuthbertson DJ, Wootton D, et al. Multiorgan impairment in low-risk individuals with post-COVID-19 syndrome: a prospective, community-based study. BMJ Open. (2021) 11:e048391. doi: 10.1136/bmjopen-2020-048391
7. Afzali B, Noris M, Lambrecht BN, Claudia K. The state of complement in COVID-19. Nat Rev Immunol. (2022) 22:77–84. doi: 10.1038/s41577-021-00665-1
8. Lynch NJ, Chan ACY, Ali YM, Khatri P, Bamigbola IE, Demopulos G, et al. Inhibition of the lectin pathway of complement ameliorates hypocomplementemia and restores serum bactericidal activity in patients with severe COVID-19. Clin Transl Med. (2022) 12:e980. doi: 10.1002/ctm2.980
9. Cervia-Hasler C, Brüningk SC, Hoch T, Fan B, Muzio G, Thompson RC, et al. Persistent complement dysregulation with signs of thromboinflammation in active Long Covid. Science. (2024) 383:eadg7942. doi: 10.1126/science.adg7942
10. Sabbatino F, Conti V, Franci G, Sellitto C, Manzo V, Pagliano P, et al. PD-L1 dysregulation in COVID-19 patients. Front Immunol. (2021) 12:695242. doi: 10.3389/fimmu.2021.695242
11. Chen CH, Lin SW, Shen CF, Hsieh KS, Cheng CM. Biomarkers during COVID-19: mechanisms of change and implications for patient outcomes. Diagn (Basel). (2022) 12:509. doi: 10.3390/diagnostics12020509
12. Papadopoulou G, Manoloudi E, Repousi N, Skoura L, Hurst T, Karamitros T. Molecular and clinical prognostic biomarkers of COVID-19 severity and persistence. Pathogens. (2022) 11:311. doi: 10.3390/pathogens11030311
13. Turner S, Naidoo CA, Usher TJ, Kruger A, Venter C, Laubscher GJ, et al. Increased levels of inflammatory molecules in blood of Long COVID patients point to thrombotic endotheliitis. Semin Thromb Hemost. (2024) 50:288–94. doi: 10.1055/s-0043-1769014
14. Shrivastava S, Chelluboina S, Jedge P, Doke P, Palkar S, Mishra AC, et al. Elevated levels of neutrophil activated proteins, alpha-defensins (DEFA1), calprotectin (S100A8/A9) and myeloperoxidase (MPO) are associated with disease severity in COVID-19 patients. Front Cell Infect Microbiol. (2021) 11:751232. doi: 10.3389/fcimb.2021.751232
15. Jeronimo CMP, Farias MEL, Val FFA, Sampaio VS, Alexandre MAA, Melo GC, et al. Methylprednisolone as adjunctive therapy for patients hospitalized with coronavirus disease 2019 (COVID-19; metcovid): A randomized, double-blind, phase IIb, placebo-controlled trial. Clin Infect Dis. (2021) 72:e373–81. doi: 10.1093/cid/ciaa1177
16. Barros CMSS, Freire RS, Frota E, Rezende Santos AG, Farias MEL, Rodrigues MGA, et al. Short-course of methylprednisolone improves respiratory functional parameters after 120 days in hospitalized COVID-19 patients (Metcovid trial): A randomized clinical trial. Front Med. (2021) 8:758405. doi: 10.3389/fmed.2021.758405
17. National Institutes of Health. Information on COVID-19 treatment, prevention and research. COVID-19 treatment guidelines, in: COVID-19 treatment guidelines (2019). Available online at: https://files.covid19treatmentguidelines.nih.gov/guidelines/covid19treatmentguidelines.pdf (Accessed August 15, 2024).
18. World Health OrganizationA clinical case definition of post COVID-19 condition by a Delphi consensus (2021). Available online at: https://www.who.int/publications/i/item/WHO-2019-nCoV-Post_COVID-19_condition-Clinical_case_definition-2021.1 (Accessed August 15, 2024).
19. Kim Y, Bae S, Chang HH, Kim SW. Long COVID prevalence and impact on quality of life 2 years after acute COVID-19. Sci Rep. (2023) 13:11207. doi: 10.1038/s41598-023-36995-4
20. Geng YJ, Wei ZY, Qian HY, Huang J, Lodato R, Castriotta RJ. Pathophysiological characteristics and therapeutic approaches for pulmonary injury and cardiovascular complications of coronavirus disease 2019. Cardiovasc Pathol. (2020) 47:107228. doi: 10.1016/j.carpath.2020.107228
21. Pan F, Yang L, Liang B, Ye T, Li L, Li L, et al. Chest CT patterns from diagnosis to 1 year of follow-up in patients with COVID-19. Radiology. (2022) 302:709–19. doi: 10.1148/radiol.2021211199
22. Munblit D, Bobkova P, Spiridonova E, Shikhaleva A, Gamirova A, Blyuss O, et al. Incidence and risk factors for persistent symptoms in adults previously hospitalized for COVID-19. Clin Exp Allergy. (2021) 51:1107–20. doi: 10.1111/cea.13997
23. Davies NG, Jarvis CI, Edmunds WJ, Jewell NP, Diaz-Ordaz K, Keogh RH, et al. Increased mortality in community-tested cases of SARS-CoV-2 lineage B.1.1.7. Nature. (2021) 593:270–4. doi: 10.1038/s41586-021-03426-1
24. Fernández-de-las-Peñas C, Cancela-Cilleruelo I, Rodríguez-Jiménez J, Gómez-Mayordomo V, Pellicer-Valero OJ, Martín-Guerrero JD, et al. Associated-onset symptoms and post-COVID-19 symptoms in hospitalized COVID-19 survivors infected with Wuhan, Alpha or Delta SARS-CoV-2 variant. Pathogens. (2022) 11:725. doi: 10.3390/pathogens11070725
25. Azzolini E, Levi R, Sarti R, Pozzi C, Mollura M, Mantovani A, et al. Association between BNT162b2 vaccination and long COVID after infections not requiring hospitalization in health care workers. JAMA. (2022) 328:676–8. doi: 10.1001/jama.2022.11691
26. Wynberg E, Han AX, Boyd A, van Willigen HDG, Verveen A, Lebbink R, et al. RECoVERED Study Group. The effect of SARS-CoV-2 vaccination on post-acute sequelae of COVID-19 (PASC): A prospective cohort study. Vaccine. (2022) 40:4424–31. doi: 10.1016/j.vaccine.2022.05.090
27. Del Valle DM, Kim-Schulze S, Huang HH, Beckmann ND, Nirenberg S, Wang B, et al. An inflammatory cytokine signature predicts COVID-19 severity and survival. Nat Med. (2020) 26:1636–43. doi: 10.1038/s41591-020-1051-9
28. Theobald SJ, Simonis A, Georgomanolis T, Kreer C, Zehner M, Eisfeld HS, et al. Long-lived macrophge reprogramming drives spike protein-mediated inflammasome activation in COVID-19. EMBO Mol Med. (2021) 13:e14150. doi: 10.15252/emmm.202114150
Keywords: long COVID, cytokines, SARS-CoV-2, inflammation, biomarkers
Citation: Maciel ABS, Pinto AS, Maia Silva B, Goulart CL, Silva LFA, Chaves AS, Mouta GS, Sato CMS, Valente J, Mwangi VI, de Melo GC, Monteiro W, Lacerda M, Arêas GPT, Sampaio VS, Costa AG and Almeida-Val F (2024) Inflammatory discoveries two years after acute severe COVID-19: a longitudinal biomarker profile assessment in long COVID individuals in the Brazilian Amazon. Front. Immunol. 15:1520193. doi: 10.3389/fimmu.2024.1520193
Received: 30 October 2024; Accepted: 09 December 2024;
Published: 23 December 2024.
Edited by:
Beate E. Kehrel, University Hospital Münster, GermanyReviewed by:
Youssif M. Ali, University of Cambridge, United KingdomAndreu Comas-Garcia, Universidad Cuauhtémoc San Luis Potosí, Mexico
Copyright © 2024 Maciel, Pinto, Maia Silva, Goulart, Silva, Chaves, Mouta, Sato, Valente, Mwangi, de Melo, Monteiro, Lacerda, Arêas, Sampaio, Costa and Almeida-Val. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Fernando Almeida-Val, ZmZhdmFsQGdtYWlsLmNvbQ==
 Alex Bezerra Silva Maciel
Alex Bezerra Silva Maciel Arlene Santos Pinto
Arlene Santos Pinto Bernardo Maia Silva
Bernardo Maia Silva Cassia Luz Goulart
Cassia Luz Goulart Luis Felipe Alho Silva
Luis Felipe Alho Silva Amanda Silva Chaves
Amanda Silva Chaves Gabriel Santos Mouta
Gabriel Santos Mouta Camila Miriam Suemi Sato
Camila Miriam Suemi Sato Jefferson Valente
Jefferson Valente Victor Irungu Mwangi
Victor Irungu Mwangi Gisely Cardoso de Melo
Gisely Cardoso de Melo Wuelton Monteiro
Wuelton Monteiro Marcus Lacerda
Marcus Lacerda Guilherme Peixoto Tinoco Arêas
Guilherme Peixoto Tinoco Arêas Vanderson Souza Sampaio
Vanderson Souza Sampaio Allyson Guimaraes Costa
Allyson Guimaraes Costa Fernando Almeida-Val
Fernando Almeida-Val