- 1Department of Periodontology, Qingdao Stomatological Hospital Affiliated to Qingdao University, Qingdao, Shandong, China
- 2Department of Periodontology, Peking University School and Hospital of Stomatology & National Center for Stomatology & National Clinical Research Center for Oral Diseases & National Engineering Research Center of Oral Biomaterials and Digital Medical Devices & Beijing Key Laboratory of Digital Stomatology & NHC Key Laboratory of Digital Stomatology & NMPA Key Laboratory for Dental Materials, Beijing, China
- 3Department of Radiation Oncology, The Affiliated Hospital of Qingdao University, Qingdao, Shandong, China
Periodontitis is a multifactorial disease characterized by chronic destruction of the periodontal supporting tissues and is closely associated with the dysbiosis of the plaque biofilm. It is the leading cause of tooth loss in adults. Bacterial extracellular vesicles (BEVs) are released from bacteria, which range in size from 20 to 400 nm. These vesicles contain various components derived from their parent bacteria, including nucleic acids, proteins, lipids, and other molecules, which facilitate functions such as molecular transfer, metabolic regulation, bacterial interactions, biofilm formation, and immune modulation. BEVs participated in the pathophysiological process of periodontitis. Recently emerging evidence also showed that the contents of EVs in saliva and gingival crevicular fluid (miRNAs, mRNAs, and proteins) could be used as potential biomarkers for periodontitis. While most current research focuses on human-derived components, much less is known about BEVs. Therefore, this review introduces the formation mechanisms and components of BEVs related to periodontitis. Then, this review summarizes the current information about the mechanism, the diagnostic and theraputic value of periodontal pathogen-derived extracellular vesicles in the development of periodontitis. Furthermore, the future challenges of exploring the role of BEVs in periodontitis are also discussed.
1 Introduction
Periodontitis is a common chronic inflammatory condition, primarily characterized by the progressive destruction of the periodontal ligament and alveolar bone. It has a high prevalence, affecting more than 60% of adults worldwide, while the prevalence of severe periodontitis is over 20% (1, 2). This condition imposes a significant economic and health burden on patients, severely impacting their quality of life (3, 4). Periodontitis is recognized as a multifactorial disease, closely linked to dysbiosis within the plaque biofilm. The complex crosstalk between multiple pathogenic microorganisms and the host immune system plays a crucial role in the pathogenesis of periodontitis (5–7). However, the exact mechanisms has yet to be fully elucidated. In the absence of timely intervention and treatment, periodontitis has the potential to induce tooth mobility and even tooth loss. By now, periodontits has become the leading cause of tooth loss in adults and may also trigger systemic inflammatory responses (2, 8). Early-stage periodontitis often remains undiagnosed due to subtle symptoms and limitations in radiographic imaging, and once the disease progresses, periodontal tissue regeneration treatments may yield less-than-ideal outcomes. Thus, addressing the potential pathogenic mechanisms, diagnostic methods, and prevention and treatment strategies for periodontitis remains an urgent challenge in periodontal care.
Extracellular vesicles (EVs) are nanoscale particles enveloped by a lipid bilayer, released by both host and microbial cells, including bacteria and fungi (9). As the concept of the human microbiome in relation to health and disease becomes mature gradually, there is an increasing recognition of microbe-derived EVs, specifically bacterial EVs (BEVs) and their function in facilitating communication between microbes and their hosts (10). BEVs contain various components derived from their parent bacteria, including nucleic acids, proteins, lipids, and other molecules, which facilitate functions such as molecular transfer, metabolic regulation, bacterial interactions, biofilm formation, and immune modulation (11). Pathogenic BEVs play a pivotal role in enhancing pathogenicity due to their small size, structural stability, inclusion of multiple virulence factors, and ability to evade immune detection and facilitate distant dissemination (12, 13). They are considered key novel mediators of bacterial interaction, either between bacteria themselves or with the host.
This review provides an overview of biogenesis and classification of BEVs related to periodontitis and their pathogenic mechanisms in periodontal diseases. Moreover, this review also summarizes the diagnostic and theraputic value in the development of periodontitis. Furthermore, the future challenges of exploring the role of BEVs in periodontitis were also discussed. The review may offer new insights into the role of periodontal pathogen BEVs in disease progression and the development of novel strategies for periodontal diagnosis and therapy.
2 Biogenesis and classification of BEVs
Both Gram-negative and Gram-positive bacteria produce extracellular vesicles (BEVs), which range in size from 20 to 400 nm (14). The composition and mode of BEVs production vary among different bacteria. Gram-positive bacteria typically produce cytoplasmic membrane vesicles (CMVs), while Gram-negative bacteria secrete outer membrane vesicles (OMVs) (15).
2.1 BEVs produced by Gram-negative bacteria
Although a considerable body of research has been carried on the formation of BEVs, the underlying mechanisms remain incompletely understood. Key periodontal pathogens are mostly Gram-negative obligate and facultative anaerobic bacteria, including Porphyromonas gingivalis (P. gingivalis), Fusobacterium nucleatum (F. nucleatum), Aggregatibacter actinomycetemcomitans (A. actinomycetemcomitans), Tannerella forsythia (T. forsythia), and Treponema denticola (T. denticola) (16). The cell walls of Gram-negative bacteria are composed of lipopolysaccharides (LPS) and a thin layer of peptidoglycan, characterized by an outer membrane and an inner membrane separated by the periplasm. The production of Gram-negative bacterial membrane vesicles (MVs) involves mechanisms such as non-lytic vesicle formation (Type B) and explosive cell lysis with subsequent membrane fragment fusion into vesicular structures (Type E) (17). Bacterial outer membrane vesicles (OMVs) represent the primary form of BEVs released by periodontal pathogens. OMVs formation occurs when the expansion of the outer membrane outpaces the peptidoglycan layer, often due to the loss or repositioning of covalent bonds between these two structures (18) (Figure 1). Additionally, peptidoglycan fragments or misfolded proteins may exert expansive pressure on the outer membrane, leading to OMVs formation (19, 20). Specific phospholipids, LPS, and other molecules that accumulate in the bacterial outer membrane can also induce OMVs production by altering membrane curvature (21). For example, OMVs formation in P. gingivalis may be facilitated by the upregulation of certain inner or outer leaflet lipids, linked to the selective incorporation of anionic lipopolysaccharides (A-LPS) and the C-terminal domain (CTD) family of proteins on the bacterial surface (22). Another proposed mechanism involves the VacJ/Yrb ABC phospholipid transporter system, which may regulate OMVs formation (23). OMVs lack DNA and RNA but are enriched in periplasmic proteins and lipids. Conversely, outer-inner MVs contain cytoplasmic components, likely due to the weakening of the bacterial peptidoglycan layer by endolysins (12, 21) (Figure 1). The expression of phage-related endolysins can degrade the peptidoglycan layer, leading to explosive bacterial lysis and the formation of Type E MVs, which include explosive OMVs (EOMVs) and explosive outer-inner MVs (EOIMVs), both containing cytoplasmic contents (24) (Figure 1).
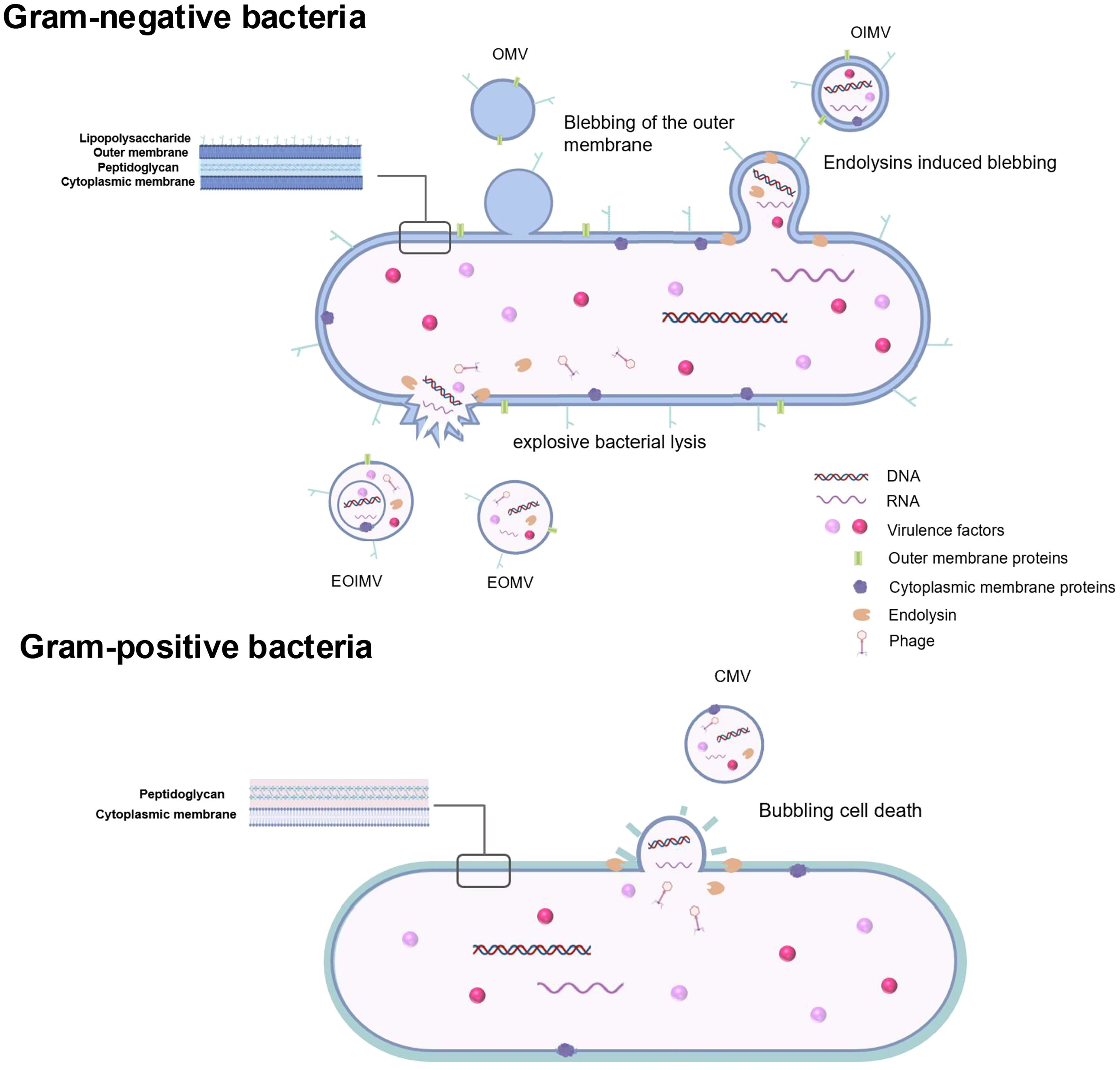
Figure 1. Extracellular vesicles produced by Gram-negative bacteria and Gram-positive bacteria. As for Gram-negative bacteria, blebbing of the outer membranes and explosive bacterial lysis are two main mechanisms in BEVs formation. Typical OMVs generated by Gram-negative periodontopathogens are produced by blebbing of the outer membranes without carrying cytoplasmic components. OIMVs are formed by autolysin and contain cytoplasmic components. EOMVs and EOIMVs are produced by phage-derived endolysin and contain cytoplasmic contents from cells exposive. Gram-positive bacteria which lack an outer membrane generate extracellular vesicles called CMVs mainly by explosive cell lysis. OMVs outer membrane vesicles, OIMVs outer-inner membrane vesicles, EOMVs explosive outer membrane vesicles, explosive outer-inner membrane vesicles, CMVs cytoplasmic membrane vesicles..
2.2 BEVs Produced by Gram- positive bacteria
Among periodontal pathogens, Gram-positive bacteria are relatively rare. However, recent oral microbiome studies have highlighted Filifactor alocis (F. alocis), a Gram-positive, obligate anaerobe, as an important periodontal pathogen (25). F. alocis has been frequently detected in chronic periodontitis, aggressive periodontitis and peri-implantitis (21, 26). Gram-positive bacteria, which possess a thick peptidoglycan cell wall, typically release EVs through explosive cell lysis, producing cytoplasmic MVs, a process referred to as “bubbling cell death.” (12, 27) (Figure 1).
3 Composition of periodontal pathogen-derived EVs
The composition of BEVs is influenced by the biogenesis mechanism, bacterial species, growth stage, and environmental conditions. Their cargo plays a critical role in disease progression, biofilm modulation, and immune evasion. However, the precise mechanisms governing cargo selection during BEVs release remain elusive. Periodontal pathogen-derived EVs contain proteins, lipids, nucleic acids and other biomolecules, and carry numerous virulence factors such as toxins, LPS, adhesins, and proteolytic enzymes, which contribute to periodontal diseases.
3.1 Proteins
Proteins found in BEVs originate from the outer membrane, periplasm, and cytoplasm of the parent bacteria (18). Proteomic analyses have revealed a diverse array of proteins in BEVs, including structural proteins, porins, and transporters involved in various biological processes (28–30). Under specific conditions, certain proteins may be preferentially loaded into EVs. Gingipains, lysine-specific (Kgp) and arginine-specific (RgpA) proteases, are major virulence factors in P. gingivalis OM and OMVs (31, 32). The absence of RgpA reduces OMV secretion (33). Studies comparing P. gingivalis OMVs and outer membrane protein cargo have shown that CTD proteins derived from gingipains are concentrated on OMVs and lipoproteins involved in iron acquisition are also selectively sorted into OMVs (34). Under conditions of hemin excess, some moonlighting cytoplasmic proteins, with adhesive potential, are preferentially loaded onto OMVs, promoting P. gingivalis proliferation and co-aggregation with other bacteria in specific environments (35). Peptidylarginine deiminase (PPAD), detected in P. gingivalis OMVs (36), has been shown to be associated with OMV biogenesis through citrullination activity (37). PPAD also facilitates immune evasion and has been implicated in autoimmune diseases, such as rheumatoid arthritis (38).
OMVs produced by A. actinomycetemcomitans are rich in leukotoxin, which selectively kills host immune cells (39, 40). LtxA can be selectively sorted into large OMVs(>300 nm) due to surface-associated DNA driving (41). Additionally, A. actinomycetemcomitans OMVs can deliver cytolethal distending toxin (CDT) to HeLa cells and human gingival fibroblasts (HGFs), causing the characteristic cytolethal distending effect (42). CDT, a genotoxin, induces DNA damage in mammalian cells, leading to G2 cell cycle arrest, progressive cell enlargement, and/or apoptosis (43, 44). CDT toxicity has been linked to GSK-3-dependent cell cycle arrest in gingival keratinocytes (45).
OMVs derived from T. denticola contain adhesins and serine proteases necessary for adhering to and degrading host cells and mammalian matrix proteins (46). OMVs from T. forsythia harbor several virulence factors, including leucine-rich-repeat family virulence factor BspA, a Toll-like receptors 2 (TLR2) agonist, as well as non-TLR2 agonist virulence factors such as sialidase and GroEL (47).
3.2 Lipids and lipopolysaccharides
Lipids are important structural components of BEVs, but their specific composition remains understudied. It has been shown that P. gingivalis can synthesize sphingolipids, which are delivered via OMVs and suppress host immune responses (48, 49). Lipid rafts play a crucial role in OMV-mediated endocytosis by host cells, as seen in both P. gingivalis and A. actinomycetemcomitans OMVs (42, 50).
LPS is the most abundant surface antigen in Gram-negative bacteria and also a critical structural and toxic component of BEVs (51). LPS consists of lipid A, a core oligosaccharide, and an O-antigen polysaccharide chain (52). P. gingivalis expresses two types of LPS: neutral O-LPS and anionic A-LPS, the latter of which is involved in OMV formation (22, 53). In addition, T. forsythia and T. denticola OMVs express low-molecular-weight lipooligosaccharides (54, 55).
3.3 Genetic material
BEVs carry genetic material, including DNA, mRNA, sRNA, and other non-coding RNAs from the parent bacteria, which can mediate horizontal gene transfer (HGT) between species (56). HGT is a crucial driver of gene and genome evolution (57). DNA and RNA have been detected in OMVs from P. gingivalis, T. denticola, and T. forsythia, meanwhile, they can activate TLR7, TLR8, and TLR9 receptors (54). Extracellular DNA (eDNA) has also been identified on OMV surfaces, forming an eDNA/OMV network that may aid in nutrient capture for pathogens residing on the surface of polymicrobial biofilms (54).
A. actinomycetemcomitans-derived OMVs contain extracellular RNA (exRNA), which is protected from enzymatic degradation in body fluids by encapsulation within the EVs (58). exRNA is transferred into host cells via OMVs and may be integrated into the host RNA-induced silencing complex, regulating host target transcripts. A. actinomycetemcomitans OMVs and exRNA influence not only local immune responses but may also cross the blood-brain barrier (59). Furthermore, A. actinomycetemcomitans, T. denticola, and P. gingivalis secrete small RNAs of microRNA size (miRNA-size, small RNAs or msRNAs) via OMVs, which are stably transferred to host cells, modulating immune responses and apoptosis (60, 61).
4 Roles of periodontal pathogen-derived BEVs in the progression of periodontitis
As BEVs serve as media of communication between bacteria and host cells, they can mediate the interaction between bacteria to affect plaque biofilm formation as well as interact with cell receptors or enter cells to exert pathogenic effects. The specific molecular mechanism of BEVs internalization by host cells remains to be further elucidated. Currently, there are several internalization pathways for EVs, including endocytosis, internalization through lipid rafts, membrane fusion, and receptor-mediated signal transduction (62). Endocytosis is the most common internalization pathway for BEVs internalization (63). Moreover, BEVs can also communicate with host cells through signal transduction mediated by toll-like receptors (TLRs) such as TLR2 and TLR4 (15). The internalization of BEVs triggers a series of responses in host cells, including immunomodulation and periodontal tissue destruction.
4.1 Regulation of plaque biofilm
Plaque biofilm formation plays a central role in the onset and progression of periodontitis (64), and bacterial interactions are critical to the formation of subgingival biofilm. Periodontal pathogens can communicate with one another or with other bacteria via BEVs, influencing biofilm formation, bacterial survival, and enhancing their invasive capabilities (65) (Figure 2). A series of studies have shown that OMVs from P. gingivalis, enriched with gingipains or adhesins, significantly promote co-aggregation of various oral pathogens, such as Streptococcus spp., F. nucleatum, Actinomyces naeslundii (A.naeslundii), and Actinomyces viscosus (A. viscosus) (66). Additionally, P. gingivalis OMVs mediate co-aggregation with T. denticola and Lachnoanaerobaculum saburreum (L.saburreum), and enhance the motility of non-motile bacteria, aiding the formation of a multispecies plaque biofilm (67). Moreover, P. gingivalis OMVs enhance T. forsythia adhesion to and invasion of epithelial cells, contributing to its virulence (68). Zhang (69) et al. reported that proteases in P. gingivalis OMVs reduce the expression of adhesion-associated proteins, such as FadA and FomA, on the surface of F. nucleatum, inhibiting its invasion of oral epithelial cells and its auto-aggregation. However, it has no impact on the morphology or proliferation of F. nucleatum. During co-infection with F. nucleatum and P. gingivalis, F. nucleatum paradoxically enhances the invasive capacity of P. gingivalis. By preventing F. nucleatum degradation within cells and maintaining its bioactivity, P. gingivalis promotes deeper infection. Furthermore, periodontal pathogen-derived BEVs have the function of inhibiting and dispersing competitor biofilms. For example, P. gingivalis OMVs appeared to have a negative impact on biofilm formation and the maintenance of Streptococcus gordonii (S. gordonii) in a gingipain-dependent manner, creating a more favorable environment for its own survival (70).
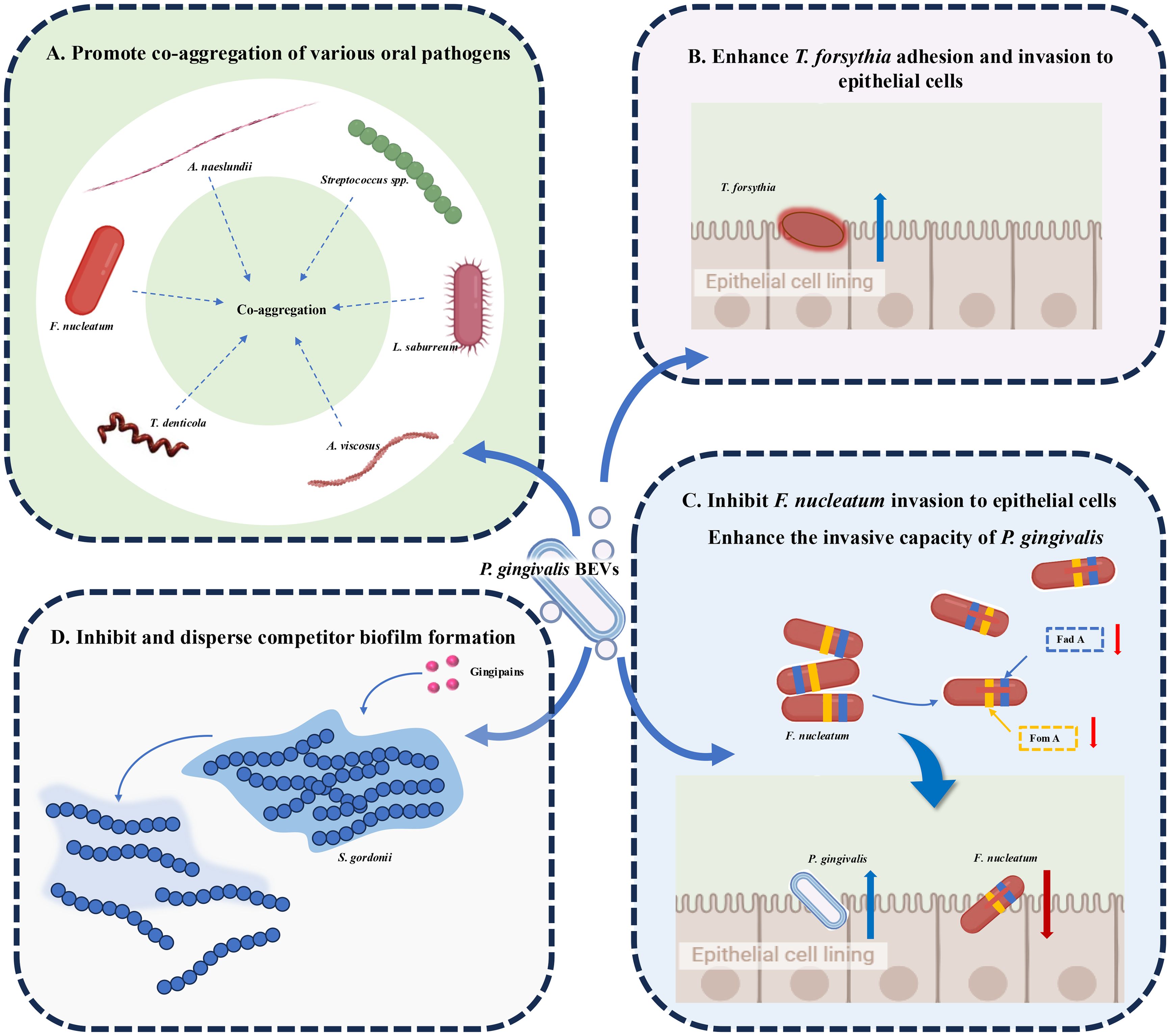
Figure 2. Roles of Periodontopathogen-Derived BEVs in Plaque Biofilm Regulation. (A) P. gingivalis OMVs aggregate Streptococcus spp., F nucleatum, A nucleatum, A viscosus, T. denticola and L. saburreum. (B) P. gingivalis OMVs enhance the ability of T. forsythia to adhere to and invade epithelial cells. (C) P. gingivalis OMVs reduce FadA and FomA on the surface of F nucleatum, inhibiting its invasion of oral epithelial cells and its auto-aggregation. During co-infection, F nucleatum paradoxically enhances the invasive capacity of P. gingivalis. (D) P. gingivalis OMVs inhibit and disperse S. gordonii biofilm formation in a gingipain-dependent manner.
4.2 Immunomodulation
BEVs from periodontal pathogens have significant immunomodulatory effects on host cells including both triggering the activation of the immune system and contributing to immune evasion (Figure 3).
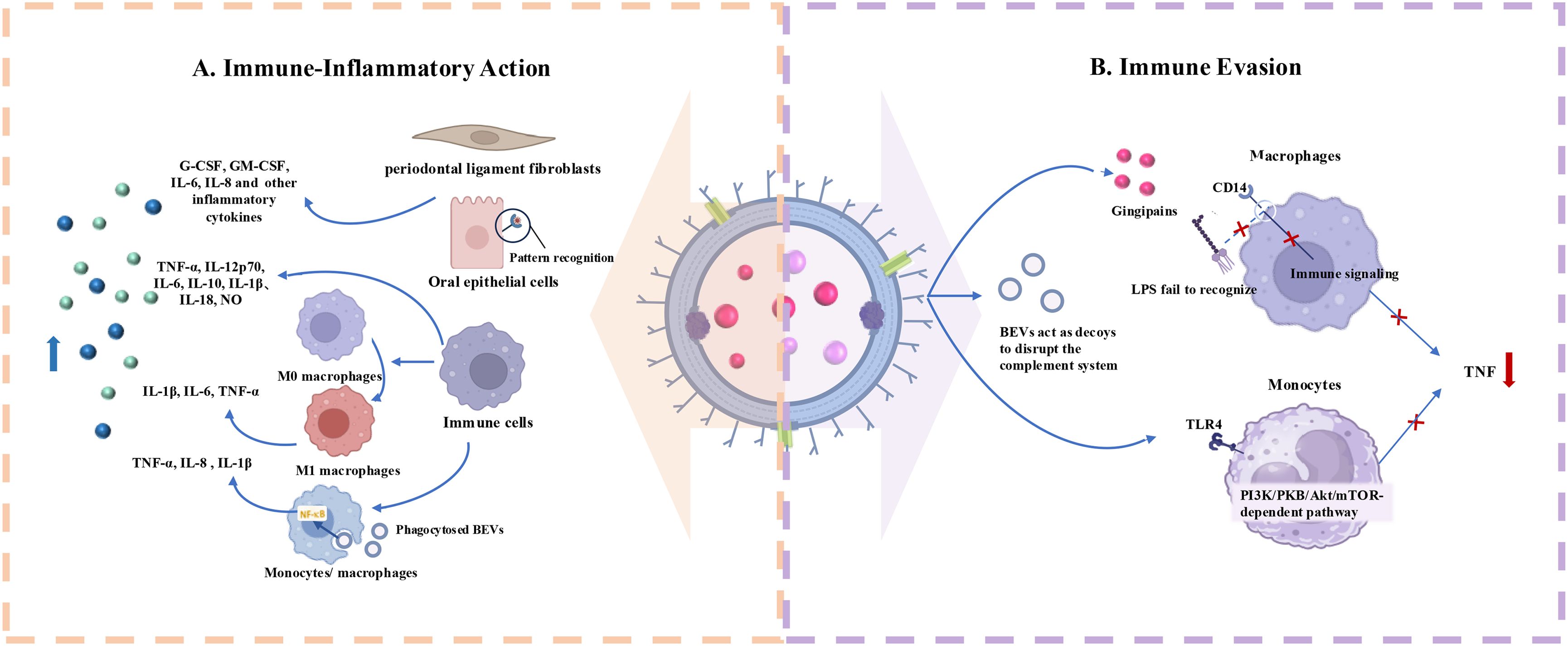
Figure 3. Roles of Periodontopathogen-Derived BEVs in Immunomodulation. (A) Periodontopathogen-Derived BEVs induce immune-inflammatory responses in host cells including immune and non-immune cells. (B) BEVs help periodontal pathogens escape from host immune system by acting on immune cell membrane receptors and serving as decoys to consume complement components.
4.2.1 Induction of immune-inflammatory responses
Periodontitis is an immune-inflammatory reactive disease initiated by plaque biofilm (71). EVs produced by various periodontal pathogens play a pivotal role in inducing host immune-inflammatory responses similar to their parent bacteria, thereby contributing to the pathogenesis of periodontitis (72). Host cells that internalize BEVs and trigger immune responses include both immune and non-immune cells.
Cecil (54) et al. found that EVs produced by periodontal pathogens can activate TLRs and nucleotide-binding oligomerization domain (NOD) pattern recognition receptors (PRRs) in gingival epithelial cells, potentially triggering significant inflammatory responses via multiple signaling pathways. Another study demonstrated that P. gingivalis OMVs activate Erk1/2, JNK, MAPK, STING, and NF-κB signaling pathways, leading to enhanced expression of interleukin (IL)-6 and IL-8 in human gingival epithelial cells (73). F. alocis OMVs have the function of promoting the production of G-CSF, GM-CSF, IL-6, and IL-8 in human oral keratinocytes (HOK-16B cell lines). These bioactive molecules may act as potent immune stimulators, leading to periodontal inflammation (74). Apart from cells in the epithelial layer, BEVs can also act on the cells within the deep connective tissue, thereby triggering inflammatory responses. For example, the OMVs of T. forsythia stimulate the release of IL-6, IL-8, and monocyte chemoattractant protein-1 (MCP-1) from human periodontal ligament fibroblasts (hPDLCs) in a dose-dependent manner, and these pro-inflammatory factors levels are much higher than those induced by T. Forsythia itself (75).
As key effector cells in host defense, macrophages play a crucial role in combating microbial invasion, primarily through phagocytosis (76). Upon external stimulation, macrophages in the M0 basal state can polarize into distinct subtypes (77). M1 macrophages produce pro-inflammatory cytokines and promote osteoclasts formation, exacerbating periodontal inflammation, while M2 macrophages release anti-inflammatory cytokines to counteract the disease (78). OMVs from F. nucleatum promote macrophage polarization towards the pro-inflammatory M1 phenotype, further exacerbating the inflammatory environment and enhancing the toxicity of F. nucleatum OMVs toward mouse gingival fibroblasts (MGFs) (79). Host monocytes, as well as M (naïve) and M (IFNγ)-polarized macrophages, bind and phagocytose periodontal pathogen-derived OMVs, including those from P. gingivalis, T. forsythia, and T. denticola (80). This process activates NF-κB and inflammasome complexes, increasing the expression of inflammatory mediators such as tumor necrosis factor (TNF)-α, IL-8, and IL-1β. In another study, the stimulatory impacts of P. gingivalis EVs on macrophages were further verified. It was found that P. gingivalis OMVs could prompt macrophages to generate significantly higher levels of TNF-α, IL-12p70, IL-6, and IL-10, as well as interferon β (IFNβ) and nitric oxide (NO), compared with P. gingivalis alone. Simultaneously, OMV-stimulated macrophages were effectively able to activate caspase-1, resulting in the production of substantial amounts of IL-1β and IL-18. These macrophages released lactate dehydrogenase and exhibited 7-Aminoactinomycin D (7-AAD) positivity, which are clear indications of pyroptotic cell death (81). OMVs from T. forsythia activated the human monocytic cell line U937, producing inflammatory mediators with more pronounced inflammatory responses than those triggered by T. forsythia cells alone (75). F. alocis OMVs can also significantly increase the expression of cytokines such as C-C motif chemokine (CCL)1, CCL2, macrophage inflammatory protein-1 (MIP-1), CCL5, IL-1β, IL-6, IL-8, and TNF-α in human monocyte-derived THP-1 cells (74).
4.2.2 Promotion of immune evasion
In addition to their pro-inflammatory effects, certain periodontal pathogens also secrete EVs that inhibit inflammation, allowing them to evade host immune defenses. It has been shown that P. gingivalis OMVs promote the loss of the LPS receptor CD14 on macrophages, with gingipains playing a key role in this process (82). This procedure impairs the macrophage response to LPS from Escherichia coli (E. coli) and reduces inflammation. Waller (83) et al. demonstrated that P. gingivalis EVs selectively promoting TNF tolerance via a TLR4- and mTOR-dependent mechanism, blocking host immune responses to the parent cells and facilitating local immune evasion. Moreover, P. gingivalis OMVs can selectively trap and activate neutrophils, initiating degranulation without being destroyed (84). They also degrade antimicrobial granule components, such as antimicrobial peptide LL-37 and myeloperoxidase, thereby protecting bacteria from being killed (84). OMVs from A.actinomycetemcomitans can act as decoys for immune cells, activating the complement system in an LPS-dependent manner and consuming complement components to protect susceptible bacteria in host serum (85). Such function has also been demonstrated for OMVs released by P. gingivalis (86). Choi (60) et al. found that OMVs secreted by major periodontal pathogens (A. actinomycetemcomitans, P. gingivalis, T. denticola) can transfer msRNAs to T cells, suppressing the expression of certain inflammation-related cytokines.
4.3 Periodontal tissue destruction
In addition to their immunomodulatory effects, periodontal pathogen-derived BEVs can also exert the ability to destroy periodontal tissues through various mechanisms. The process of periodontal destruction by BEVs includes direct damage and invasion of the epithelial barrier, inhibition of angiogenesis, and the creation of an immunological microenvironment that induces bone resorption (Figure 4).
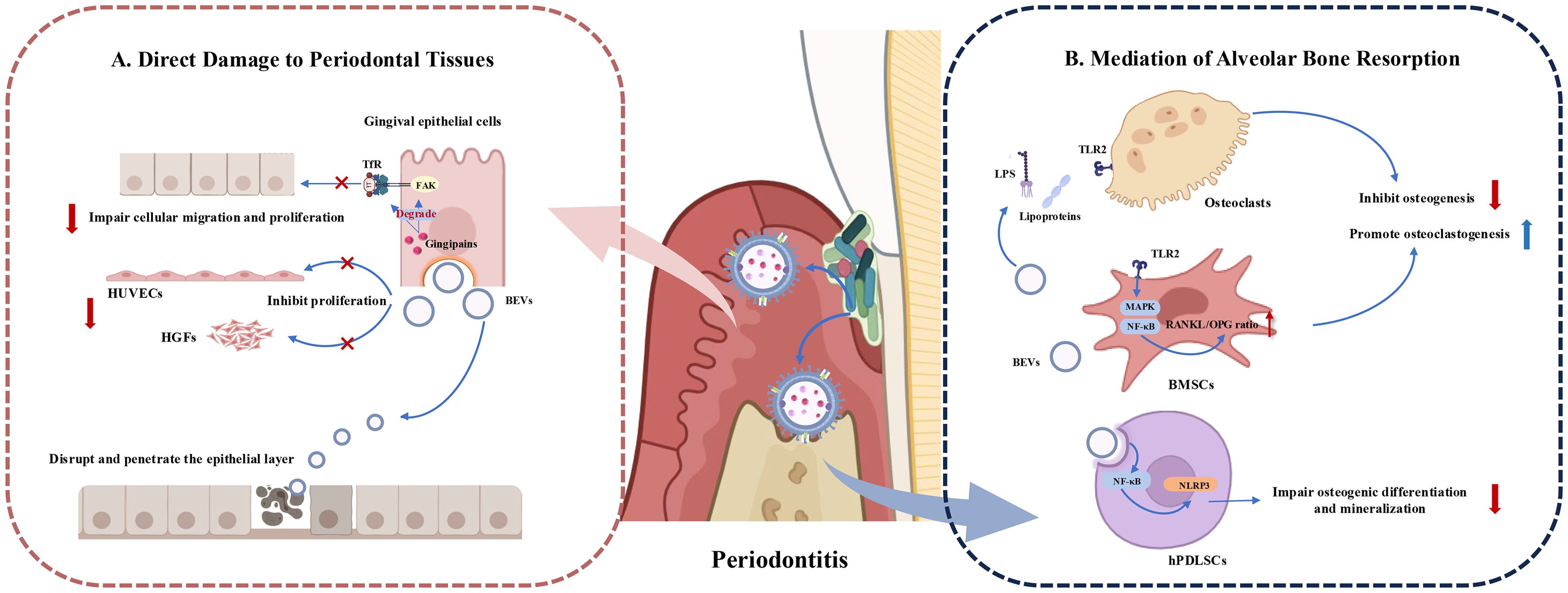
Figure 4. Roles of Periodontopathogen-Derived BEVs in Periodontal Tissue Destruction. (A) BEVs cause periodontal tissue destruction by impairing bioactivity of periodontal tissue cells such as gingival epithelial cells, human gingival fibroblasts (HGFs) and human umbilical vein endothelial cells (HUVECs). BEVs can also disrupt and enter the epithelial layer to play toxic roles. (B) BEVs induce bone destruction by releasing virulence factors and influencing the function of bone marrow stromal cells (BMSCs) and osteoclasts, to promote osteoclastogenesis and inhibit osteogenesis. BEVs can also be endocytosed by human periodontal ligament stem cells (hPDLSCs) and activate the NLRP3 inflammasome via the NF-κB (p65) signaling pathway. influencing osteogenic differentiation and mineralization.
4.3.1 Direct damage to periodontal tissues
EVs produced by Gram-negative bacteria can directly fuse with target cells or be internalized via lipid rafts, micropinocytosis, and clathrin-dependent endocytosis (87). Upon entering host cells, BEVs exhibit multiple virulence factors, exerting toxic effects on periodontal tissue cells, including gingival epithelial cells, vascular endothelial cells, and gingival fibroblasts (31).
Gingival epithelial cell layer is the first barrier to prevent periodontal pathogens from invading deep periodontal tissues (88). The close connection between the gingival epithelial cells ensures the integrity of the gingival epithelial barrier. OMVs from P. gingivalis rapidly enter host epithelial cells, such as HeLa cells and immortalized human gingival epithelial cells, via endocytosis (50). Gingipains associated with OMVs degrade functional molecules like transferrin receptor (TfR) and integrin-related signaling molecules (such as paxillin and focal adhesion kinase (FAK)), inhibiting the migration and proliferation of gingival epithelial cells and leading to cellular impairment (89). T. denticola OMVs can also disrupt and penetrate the epithelial layer (90). OMVs from A. actinomycetemcomitans fuse with lipid rafts on the plasma membranes of HeLa cells and HGFs, releasing cytolethal distending toxin (CDT), which remains biologically active in the nucleus and exerts cytotoxic effects (42).
Additionally, P. gingivalis OMVs dose-dependently inhibit the proliferation of human gingival fibroblasts (HGFs) and human umbilical vein endothelial cells (HUVECs), reducing their capillary formation ability and promoting periodontal disease progression, thus negatively affecting periodontal tissue regeneration (91).
4.3.2 Mediation of alveolar bone resorption and destruction
BEVs from periodontal pathogens can influence osteoblasts and osteoclasts through various mechanisms, leading to an imbalance in periodontal bone homeostasis and exacerbating alveolar bone resorption. Indirectly triggering bone destruction by inducing inflammatory responses is a common mechanism. Studies have shown that EVs from P. gingivalis, T. forsythia, Streptococcus oralis (S. oralis), and F. alocis preferentially activate TLR2 to induce osteoclastogenesis, with aberrant osteoclast activation, consequently cause bone metabolism imbalance and eventual alveolar bone loss (92, 93). Likewise, BEVs produced from Gram-negative periodontal pathogens act on TLR2 primarily through lipoproteins and/or LPS (93). Additionally, F. alocis effectively activates the MAPK and NF-κB signaling pathways downstream of TLR2 and increases the RANKL/OPG ratio in bone-derived mesenchymal stromal cells (BMSCs), thus promote osteoclast differentiation, inhibit osteoblast differentiation, and lead to enhanced bone resorption (94). OMVs from P. gingivalis can also be internalized by human periodontal ligament cells (hPDLCs), inducing apoptosis and promoting alveolar bone resorption, a process regulated by microRNA-sized small RNAs (msRNA) such as sRNA45033 in P. gingivalis OMVs, which modulate DNA methylation (61). F. nucleatum OMVs enter human periodontal ligament stem cells (hPDLSCs) through endocytosis and activate the NLRP3 inflammasome via the NF-κB (p65) signaling pathway. This activation stimulates a series of cascade reactions, which subsequently results in osteogenic differentiation and mineralization impairment of hPDLSCs. Rat periodontitis models also proved F. nucleatum OMVs are important stimulators for alveolar bone loss (72).
5 Applications of BEVs in periodontitis
BEVs embody the dual nature of a double-edged sword. On the one hand, they contribute to the pathogenesis and are implicated in the onset and progression of diseases. On the other hand, they play a role in therapeutics, paving the way for innovative approaches to the diagnosis and treatment of related conditions.
5.1 BEVs for diagnosing periodontitis
Oral biofluids, such as saliva and gingival crevicular fluid (GCF), are rich of biomolecules from both host cells and resident microorganisms, and are commonly used to identify diagnostic markers for periodontitis (95). EVs found in these fluids are emerging as potential biomarkers for periodontal diseases (96–102). While most current research focuses on human-derived components, much less is known about BEVs. Han (102) et al. utilized LPS to label bacterial OMVs in saliva and found a significant increase in the amount of LPS+ OMVs in the saliva of patients with periodontitis compared to healthy individuals. Quantitative PCR analysis of genomic DNA from saliva small EVs (sEVs) indicated a marked increase in four periodontal pathogens (T. denticola, E. corrodens, P. gingivalis, and F. nucleatum) in the periodontitis group, with P. gingivalis and T. denticola being the most sensitive. These findings suggest that LPS+ OMVs, along with OMVs from P. gingivalis and T. denticola, could serve as potential diagnostic biomarkers for periodontitis. However, this study had a small sample size, and pure OMVs from specific periodontal pathogens were not isolated, warranting further investigation.
Other studies have explored the expression of virulence factors in OMVs as a diagnostic tool. For example, monoclonal antibodies have been developed to recognize the conserved P. gingivalis virulence factor RgpA-Kgp complex, forming the basis for a saliva-based diagnostic kit to detect P. gingivalis and its OMVs (103). However, the challenge remains in distinguishing BEVs from human-derived EVs in saliva or GCF due to the lack of specific markers (104). Therefore, separating BEVs for clinical diagnosis remains complex and difficult.
5.2 BEVs in vaccine development for periodontal diseases
BEVs are highly stable under various temperature and treatment conditions, and do not possess the ability to self-replicate, thus providing strong biosafety profiles (105). Due to their nanoscale size, BEVs are preferentially taken up by dendritic cells (DCs) (106). They also contain numerous immunogenic surface- and membrane-associated components of their parent bacterium, which can induce host immune responses (107, 108). Consequently, BEVs hold great promise as immunogenic biological agents that activate the immune system to combat bacterial infections. Moreover, BEVs can be bioengineered to express target antigens with reduced toxicity (109), making them promising candidates for vaccine development (110, 111).
For example, OMVs derived from P. gingivalis maintain the immunodominant epitopes of the bacterium, while animal studies by Nakao et al. (112) have demonstrated that intranasal administration of P. gingivalis OMVs in mice induced the dose-dependent production of salivary IgA, as well as serum IgG and IgA. Previous studies have estabilished that Poly(I:C), a TLR3 agonist, significantly increased antibody production and enhanced the clearance of P. gingivalis (112, 113). Moreover, the study confirmed the safety of low-dose intranasal immunization for adjacent organs and the central nervous system (113). The strong immunogenicity of P. gingivalis OMVs mainly originates from LPS and A-LPS-modified proteins, such as gingipains in the OMVs, while immune reactivity was significantly reduced after serum absorption of LPS (114).
Despite the promising progress, BEVs are still far from being used in clinical settings for the prevention of periodontal diseases. This is primarily due to the high toxicity of BEVs, as well as difficulties in isolating and characterizing them. Therefore, standard methods are urgently needed to isolate and characterize BEVs.
5.3 BEVs for drug delivery
Traditional antibiotic administration delivers drugs systemically via the bloodstream, allowing them to target pathogenic bacteria located in different parts of the body. However, this approach often lacks specificity, requiring higher doses and increased frequency to reach therapeutic concentrations at the infection site, which can lead to side effects and accelerate the development of antibiotic resistance (115). In recent years, the use of nanoparticle-based drug delivery systems has been extensively studied. These systems can enhance drug solubility, modulate drug release, target specific sites, and simultaneously deliver multiple therapeutic agents (116). Compared to synthetic nanoparticle carriers, naturally derived BEVs offer several advantages, including small particle size, stable cargo-carrying capacity, and high biocompatibility (117). BEVs possess immunogenic antigens on their surface, which can elicit robust immune responses against invading pathogens (107).
Additionally, BEVs are involved in signal transduction, facilitating inter-bacterial communication and exchange, and can easily fuse with bacterial membranes (118), delivering bioactive molecules to their parent bacteria and surrounding microbes. Through genetic engineering of parent bacteria, BEVs can be modified with targeting ligands, enhancing drug accumulation at desired sites (119). BEVs themselves can also be surface-modified to improve cellular or site-specific targeting (120). Accordingly, BEVs have been explored as drug delivery vehicles to enhance bacterial uptake of loaded antibiotics. Previous evidence suggests that BEVs are more potent in delivering autolysins and peptidoglycan hydrolases, resulting in higher bacterial killing efficiency compared to gentamicin (121). Another study developed a novel antibiotic delivery system using OMVs isolated from E. coli as a shell and rifampicin-loaded mesoporous silica nanoparticles (MSNs) as the core. In contrast to conventional antibiotics, BEVs could enhance antibiotic uptake and achieve superior antibacterial effects (122).
Currently, the mechanisms of BEV-mediated delivery are not fully understood, and there is a need to improve purification processes and production yields, as well as to standardize the techniques and analyses (123). While the application of BEV-based drug delivery systems in clinical practice remains distant, BEVs hold great promise as novel antibiotic delivery vehicles or potential antimicrobial agents to effectively kill or inhibit periodontal pathogens.
5.4 BEVs inhibitors for inflammation control
Periodontopathic bacteria-derived EVs contain various toxic factors that contribute significantly to periodontal tissue destruction, as discussed previously. Inhibiting the release of these EVs from periodontal pathogens could represent a therapeutic approach to managing periodontitis. Peptidylarginine deiminases (PADs), a group of calcium-activated enzymes, serve as toxic components in P. gingivalis EVs. PADs convert arginine residues into citrulline residues, which lead to the citrullination of host proteins such as fibrinogen and α-enolase. This modification is crucial for various physiological processes, including the biogenesis of OMVs and the initiation of pathological inflammation (124). The use of PAD inhibitors, such as GSK 199, BB-Cl-amidine, Cl-amidine, and AMF30a, has been shown to effectively reduce BEVs production (125, 126). Additionally, cannabidiol (CBD) has been reported to inhibit the release of BEVs from Gram-negative bacteria, potentially reducing antibiotic resistance (127).
Given BEVs’ ability to mediate immune-inflammatory responses and promote periodontitis progression and tissue damage, finding agents to reduce the release of pro-inflammatory factors induced by BEVs opens new therapeutic avenues. Hop bract polyphenol (HBP), for example, has been shown to inhibit the expression of cyclooxygenase (COX)-2, IL-6, IL-8, and matrix metalloproteinases (MMP)-1 and -3 in human gingival epithelial (HGE) cells challenged with P. gingivalis EVs in a dose-dependent manner. This makes HBP a promising inhibitor of the cell inflammatory response induced by P. gingivalis EVs. Key active components of HBP, such as 2-[(2-methylpropanoyl)-phloroglucinol]1-O-β-D-glucopyranoside (MPPG) and kaempferol 3-O-β-glucopyranoside (astragalin), have been identified as effective in mediating these anti-inflammatory effects (128). Curcumin also shows notable efficacy, significantly inhibiting P. gingivalis OMV-stimulated gene expression and protein production of IL-6, IL-1β, and TNF-α in HGE cells, in a dose-dependent manner. Additionally, curcumin attenuates the cytotoxic effects of OMVs on cell migration and reduces OMV adhesion, cell entry, and apoptosis, also in a dose-dependent manner (129).
Probiotic therapy aimed at neutralizing the toxic effects of periodontal pathogen-derived EVs may represent an emerging area of research. Microbial dysbiosis is critical in the progression of periodontitis, typically involving a reduction in probiotic bacteria and/or an increase in periodontal pathogens (130). Lactobacillus reuteri (L. reuteri) is one of the most extensively studied probiotics and shows potential as an adjunctive treatment for chronic periodontitis (131). L. reuteri has been found to downregulate key virulence factors of periodontal pathogens, interfere with interspecies communication among pathogens, inhibit pathogenic adhesion and invasion, and reduce virulence (132). Probiotic-derived EVs also possess anti-infective properties (133). Notwithstanding current evidence on the efficacy of probiotic-derived EVs in periodontal inflammation control is limited, this approach does indicate a promising direction for future research.
6 Conclusion and outlook
Extracellular vesicles (EVs) secreted by key periodontal pathogens play a pivotal role in the interactions between bcteria and between bacteria and the host, thereby influencing periodontal homeostasis and contributing to the progression of periodontitis. This review summarizes the production, composition, and biological characteristics of BEVs from periodontal pathogens and discusses recent advances regarding their involvement in the pathogenesis, diagnosis, treatment, and prevention of periodontitis.
BEVs originate from parent bacterial cells, which carry and transmit a variety of virulence factors. On one hand, BEVs can induce local immune-inflammatory responses, while on the other, they promote immune evasion by suppressing immune surveillance and facilitating the proliferation of parent bacteria. BEVs promote bacterial co-aggregation and play a role in biofilm pathogenicity. In periodontitis, BEVs can directly damage periodontal connective tissues and, through various mechanisms, indirectly mediate alveolar bone resorption and destruction, thus contributing to disease progression.
Given their widespread presence in gingival crevicular fluid and saliva, BEVs have been investigated as potential biomarkers for periodontitis. Moreover, their biological properties suggest they are promising candidates for vaccine development and drug delivery systems. However, current research on the components and pathogenic mechanisms of BEVs remains insufficient. Specific markers for distinguishing and isolating EVs from different sources are lacking, and standardized protocols for the production and purification of BEVs still need to be developed.
In the future, further research on BEVs is expected to deepen our understanding of their role in periodontal diseases. More efforts should be made to clarify the detailed composition and specific pathogenic mechanisms of BEVs. This knowledge could guide the development of targeted therapies that mitigate BEV-mediated inflammation and tissue destruction. Identifying specific molecular markers that are unique to periodontal pathogen-derived BEVs is crucial to enhance diagnostic precision and therapeutic targeting. Developing cost-effective and standardization of BEVs preparation processes will enhance the reliability and reproducibility of their applications as vaccine candidates and drug delivery materials.
Moreover, exploring new strategies to modulate the activity of BEVs may provide novel therapeutic approaches for periodontitis. For example, developing novel drugs that can block the harmful effects of BEVs or enhance their beneficial functions could hold promise. The immunomodulatory potential of probiotic-derived EVs represents an innovative area for managing chronic periodontitis. Future research should assess the anti-inflammatory effects and mechanism of action of probiotic-derived EVs to determine their potential as adjunct therapies in periodontitis treatment. Additionally, combining BEVs with other advanced technologies such as nanotechnology and immunotherapy, may lead to more effective treatment options.
As our knowledge of BEVs continues to expand, their potential applications in periodontal diagnosis, treatment, and prevention will become more prominent, ultimately contributing to better management of periodontal diseases and improve oral health.
Author contributions
RZ: Conceptualization, Investigation, Writing – original draft, Writing – review & editing. GL: Investigation, Software, Writing – review & editing. YW: Methodology, Visualization, Writing – review & editing. XW: Conceptualization, Validation, Writing – review & editing. QL: Conceptualization, Investigation, Supervision, Validation, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. The present study was supported by Qingdao Key Health Discipline Development Fund (2022–2024), Qingdao Clinical Research Center for Oral Diseases (22-3-7-lczx-7-nsh), Shandong Provincial Key Medical and Health Discipline of Oral Medicine (Qingdao University Affiliated Qingdao Stomatological Hospital) (2024–2026).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Trindade D, Carvalho R, MaChado V, Chambrone L, Mendes JJ, Botelho J. Prevalence of periodontitis in dentate people between 2011 and 2020: A systematic review and meta-analysis of epidemiological studies. J Clin Periodontol. (2023) 50:604–26. doi: 10.1111/jcpe.13769
2. Genco RJ, Sanz M. Clinical and public health implications of periodontal and systemic diseases: an overview. Periodontol 2000. (2020) 83:7–13. doi: 10.1111/prd.12344
3. Botelho J, MaChado V, Leira Y, Proença L, Chambrone L, Mendes JJ. Economic burden of periodontitis in the United States and europe: an updated estimation. J Periodontol. (2022) 93:373–9. doi: 10.1002/jper.21-0111
4. Botelho J, MaChado V, Proença L, Bellini DH, Chambrone L, Alcoforado G, et al. The impact of nonsurgical periodontal treatment on oral health-related quality of life: A systematic review and meta-analysis. Clin Oral Investig. (2020) 24:585–96. doi: 10.1007/s00784-019-03188-1
5. Yamamoto M, Aizawa R. Maintaining a protective state for human periodontal tissue. Periodontol 2000. (2021) 86:142–56. doi: 10.1111/prd.12367
6. Abdulkareem AA, Al-Taweel FB, Al-Sharqi AJB, Gul SS, Sha A, Chapple ILC. Current concepts in the pathogenesis of periodontitis: from symbiosis to dysbiosis. J Oral Microbiol. (2023) 15:2197779. doi: 10.1080/20002297.2023.2197779
7. Del Pinto R, Ferri C, Giannoni M, Cominelli F, Pizarro TT, Pietropaoli D. Meta-analysis of oral microbiome reveals sex-based diversity in biofilms during periodontitis. JCI Insight. (2024) 9(17):e171311. doi: 10.1172/jci.insight.171311
8. Ravidà A, Qazi M, Rodriguez MV, Galli M, Saleh MHA, Troiano G, et al. The influence of the interaction between staging, grading and extent on tooth loss due to periodontitis. J Clin Periodontol. (2021) 48:648–58. doi: 10.1111/jcpe.13430
9. Kalluri R, LeBleu VS. The biology, function, and biomedical applications of exosomes. Science. (2020) 367(6478):eaau6977. doi: 10.1126/science.aau6977
10. Bittel M, Reichert P, Sarfati I, Dressel A, Leikam S, Uderhardt S, et al. Visualizing transfer of microbial biomolecules by outer membrane vesicles in microbe-host-communication in vivo. J Extracell Vesicles. (2021) 10:e12159. doi: 10.1002/jev2.12159
11. Avila-Calderón ED, Ruiz-Palma MDS, Aguilera-Arreola MG, Velázquez-Guadarrama N, Ruiz EA, Gomez-Lunar Z, et al. Outer membrane vesicles of gram-negative bacteria: an outlook on biogenesis. Front Microbiol. (2021) 12:557902. doi: 10.3389/fmicb.2021.557902
12. Toyofuku M, Nomura N, Eberl L. Types and origins of bacterial membrane vesicles. Nat Rev Microbiol. (2019) 17:13–24. doi: 10.1038/s41579-018-0112-2
13. Zou C, Zhang Y, Liu H, Wu Y, Zhou X. Extracellular vesicles: recent insights into the interaction between host and pathogenic bacteria. Front Immunol. (2022) 13:840550. doi: 10.3389/fimmu.2022.840550
14. Ñahui Palomino RA, Vanpouille C, Costantini PE, Margolis L. Microbiota-host communications: bacterial extracellular vesicles as a common language. PloS Pathog. (2021) 17:e1009508. doi: 10.1371/journal.ppat.1009508
15. Xie J, Haesebrouck F, Van Hoecke L, Vandenbroucke R. Bacterial extracellular vesicles: an emerging avenue to tackle diseases. Trends Microbiol. (2023) 31:1206–24. doi: 10.1016/j.tim.2023.05.010
16. Sedghi L, DiMassa V, Harrington A, Lynch SV, Kapila YL. The oral microbiome: role of key organisms and complex networks in oral health and disease. Periodontol 2000. (2021) 87:107–31. doi: 10.1111/prd.12393
17. Gan Y, Zhao G, Wang Z, Zhang X, Wu M, Lu M. Bacterial membrane vesicles: physiological roles, infection immunology, and applications. Advanced Sci (Weinheim Baden-Wurttemberg Germany). (2023) 10:e2301357. doi: 10.1002/advs.202301357
18. Xie J, Li Q, Haesebrouck F, Van Hoecke L, Vandenbroucke RE. The tremendous biomedical potential of bacterial extracellular vesicles. Trends Biotechnol. (2022) 40:1173–94. doi: 10.1016/j.tibtech.2022.03.005
19. Schwechheimer C, Kulp A, Kuehn MJ. Modulation of bacterial outer membrane vesicle production by envelope structure and content. BMC Microbiol. (2014) 14:324. doi: 10.1186/s12866-014-0324-1
20. Horne JE, Brockwell DJ, Radford SE. Role of the lipid bilayer in outer membrane protein folding in gram-negative bacteria. J Biol Chem. (2020) 295:10340–67. doi: 10.1074/jbc.REV120.011473
21. Toyofuku M, Schild S, Kaparakis-Liaskos M, Eberl L. Composition and functions of bacterial membrane vesicles. Nat Rev Microbiol. (2023) 21:415–30. doi: 10.1038/s41579-023-00875-5
22. Gui MJ, Dashper SG, Slakeski N, Chen YY, Reynolds EC. Spheres of influence: porphyromonas gingivalis outer membrane vesicles. Mol Oral Microbiol. (2016) 31:365–78. doi: 10.1111/omi.12134
23. Zingl FG, Kohl P, Cakar F, Leitner DR, Mitterer F, Bonnington KE, et al. Outer membrane vesiculation facilitates surface exchange and in vivo adaptation of vibrio cholerae. Cell Host Microbe. (2020) 27:225–37.e8. doi: 10.1016/j.chom.2019.12.002
24. Jiang M, Wang Z, Xia F, Wen Z, Chen R, Zhu D, et al. Reductions in bacterial viability stimulate the production of extra-intestinal pathogenic escherichia coli (Expec) cytoplasm-carrying extracellular vesicles (Evs). PloS Pathog. (2022) 18:e1010908. doi: 10.1371/journal.ppat.1010908
25. Griffen AL, Beall CJ, Campbell JH, Firestone ND, Kumar PS, Yang ZK, et al. Distinct and complex bacterial profiles in human periodontitis and health revealed by 16s pyrosequencing. Isme J. (2012) 6:1176–85. doi: 10.1038/ismej.2011.191
26. Aruni W, Chioma O, Fletcher HM. Filifactor alocis: the newly discovered kid on the block with special talents. J Dent Res. (2014) 93:725–32. doi: 10.1177/0022034514538283
27. Nie X, Li Q, Chen X, Onyango S, Xie J, Nie S. Bacterial extracellular vesicles: vital contributors to physiology from bacteria to host. Microbiological Res. (2024) 284:127733. doi: 10.1016/j.micres.2024.127733
28. Lee EY, Choi DS, Kim KP, Gho YS. Proteomics in gram-negative bacterial outer membrane vesicles. Mass Spectrom Rev. (2008) 27:535–55. doi: 10.1002/mas.20175
29. Stentz R, Jones E, Juodeikis R, Wegmann U, Guirro M, Goldson AJ, et al. The proteome of extracellular vesicles produced by the human gut bacteria bacteroides thetaiotaomicron in vivo is influenced by environmental and host-derived factors. Appl Environ Microbiol. (2022) 88:e0053322. doi: 10.1128/aem.00533-22
30. Luo R, Chang Y, Liang H, Zhang W, Song Y, Li G, et al. Interactions between extracellular vesicles and microbiome in human diseases: new therapeutic opportunities. Imeta. (2023) 2:e86. doi: 10.1002/imt2.86
31. Nakao R, Takashiba S, Kosono S, Yoshida M, Watanabe H, Ohnishi M, et al. Effect of porphyromonas gingivalis outer membrane vesicles on gingipain-mediated detachment of cultured oral epithelial cells and immune responses. Microbes Infect. (2014) 16:6–16. doi: 10.1016/j.micinf.2013.10.005
32. Sharaf S, Hijazi K. Modulatory mechanisms of pathogenicity in porphyromonas gingivalis and other periodontal pathobionts. Microorganisms. (2022) 11(1):15. doi: 10.3390/microorganisms11010015
33. Zhang R, Yang J, Wu J, Sun WB, Liu Y. Effect of deletion of the rgpa gene on selected virulence of porphyromonas gingivalis. J Dent Sci. (2016) 11:279–86. doi: 10.1016/j.jds.2016.03.004
34. Veith PD, Chen YY, Gorasia DG, Chen D, Glew MD, O’Brien-Simpson NM, et al. Porphyromonas gingivalis outer membrane vesicles exclusively contain outer membrane and periplasmic proteins and carry a cargo enriched with virulence factors. J Proteome Res. (2014) 13:2420–32. doi: 10.1021/pr401227e
35. Veith PD, Luong C, Tan KH, Dashper SG, Reynolds EC. Outer membrane vesicle proteome of porphyromonas gingivalis is differentially modulated relative to the outer membrane in response to heme availability. J Proteome Res. (2018) 17:2377–89. doi: 10.1021/acs.jproteome.8b00153
36. Gabarrini G, Palma Medina LM, Stobernack T, Prins RC, du Teil Espina M, Kuipers J, et al. There’s no place like om: vesicular sorting and secretion of the peptidylarginine deiminase of porphyromonas gingivalis. Virulence. (2018) 9:456–64. doi: 10.1080/21505594.2017.1421827
37. Vermilyea DM, Moradali MF, Kim HM, Davey ME. Ppad activity promotes outer membrane vesicle biogenesis and surface translocation by porphyromonas gingivalis. J Bacteriol. (2021) 203(4):e00343-20. doi: 10.1128/jb.00343-20
38. Ahmadi P, Mahmoudi M, Kheder RK, Faraj TA, Mollazadeh S, Abdulabbas HS, et al. Impacts of porphyromonas gingivalis periodontitis on rheumatoid arthritis autoimmunity. Int Immunopharmacol. (2023) 118:109936. doi: 10.1016/j.intimp.2023.109936
39. Kieselbach T, Zijnge V, Granström E, Oscarsson J. Proteomics of aggregatibacter actinomycetemcomitans outer membrane vesicles. PloS One. (2015) 10:e0138591. doi: 10.1371/journal.pone.0138591
40. Kato S, Kowashi Y, Demuth DR. Outer membrane-like vesicles secreted by actinobacillus actinomycetemcomitans are enriched in leukotoxin. Microb Pathog. (2002) 32:1–13. doi: 10.1006/mpat.2001.0474
41. Nice JB, Collins SM, Agro SMJ, Sinani A, Moros SD, Pasch LM, et al. Heterogeneity of size and toxin distribution in aggregatibacter actinomycetemcomitans outer membrane vesicles. Toxins (Basel). (2024) 16(3):138. doi: 10.3390/toxins16030138
42. Rompikuntal PK, Thay B, Khan MK, Alanko J, Penttinen AM, Asikainen S, et al. Perinuclear localization of internalized outer membrane vesicles carrying active cytolethal distending toxin from aggregatibacter actinomycetemcomitans. Infect Immun. (2012) 80:31–42. doi: 10.1128/iai.06069-11
43. Guerra L, Cortes-Bratti X, Guidi R, Frisan T. The biology of the cytolethal distending toxins. Toxins (Basel). (2011) 3:172–90. doi: 10.3390/toxins3030172
44. Du L, Song J. Delivery, structure, and function of bacterial genotoxins. Virulence. (2022) 13:1199–215. doi: 10.1080/21505594.2022.2097417
45. Shenker BJ, Walker LP, Zekavat A, Korostoff J, Boesze-Battaglia K. Aggregatibacter actinomycetemcomitans cytolethal distending toxin-induces cell cycle arrest in a glycogen synthase kinase (Gsk)-3-dependent manner in oral keratinocytes. Int J Mol Sci. (2022) 23(19):11831. doi: 10.3390/ijms231911831
46. Rosen G, Naor R, Rahamim E, Yishai R, Sela MN. Proteases of treponema denticola outer sheath and extracellular vesicles. Infect Immun. (1995) 63:3973–9. doi: 10.1128/iai.63.10.3973-3979.1995
47. Lim Y, Kim HY, Han D, Choi BK. Proteome and immune responses of extracellular vesicles derived from macrophages infected with the periodontal pathogen tannerella forsythia. J Extracell Vesicles. (2023) 12:e12381. doi: 10.1002/jev2.12381
48. Rocha FG, Ottenberg G, Eure ZG, Davey ME, Gibson FC 3rd. Sphingolipid-containing outer membrane vesicles serve as a delivery vehicle to limit macrophage immune response to porphyromonas gingivalis. Infect Immun. (2021) 89(4):e00614-20. doi: 10.1128/iai.00614-20
49. Rocha FG, Moye ZD, Ottenberg G, Tang P, Campopiano DJ, Gibson FC 3rd, et al. Porphyromonas gingivalis sphingolipid synthesis limits the host inflammatory response. J Dent Res. (2020) 99:568–76. doi: 10.1177/0022034520908784
50. Furuta N, Tsuda K, Omori H, Yoshimori T, Yoshimura F, Amano A. Porphyromonas gingivalis outer membrane vesicles enter human epithelial cells via an endocytic pathway and are sorted to lysosomal compartments. Infect Immun. (2009) 77:4187–96. doi: 10.1128/iai.00009-09
51. Pin C, David L, Oswald E. Modulation of autophagy and cell death by bacterial outer-membrane vesicles. Toxins (Basel). (2023) 15(8):502. doi: 10.3390/toxins15080502
52. Raetz CR, Whitfield C. Lipopolysaccharide endotoxins. Annu Rev Biochem. (2002) 71:635–700. doi: 10.1146/annurev.biochem.71.110601.135414
53. Naskar A, Cho H, Lee S, Kim KS. Biomimetic nanoparticles coated with bacterial outer membrane vesicles as a new-generation platform for biomedical applications. Pharmaceutics. (2021) 13(11):1887. doi: 10.3390/pharmaceutics13111887
54. Cecil JD, O’Brien-Simpson NM, Lenzo JC, Holden JA, Chen YY, Singleton W, et al. Differential responses of pattern recognition receptors to outer membrane vesicles of three periodontal pathogens. PloS One. (2016) 11:e0151967. doi: 10.1371/journal.pone.0151967
55. Hager-Mair FF, Bloch S, Schäffer C. Glycolanguage of the oral microbiota. Mol Oral Microbiol. (2024) 39:291–320. doi: 10.1111/omi.12456
56. Faddetta T, Vassallo A, Del Duca S, Gallo G, Fani R, Puglia AM. Unravelling the DNA sequences carried by streptomyces coelicolor membrane vesicles. Sci Rep. (2022) 12:16651. doi: 10.1038/s41598-022-21002-z
57. Sun D. Pull in and push out: mechanisms of horizontal gene transfer in bacteria. Front Microbiol. (2018) 9:2154. doi: 10.3389/fmicb.2018.02154
58. Wang J, Liu C, Cutler J, Ivanovski S, Lee RS, Han P. Microbial- and host immune cell-derived extracellular vesicles in the pathogenesis and therapy of periodontitis: A narrative review. J Periodontal Res. (2024) 59(6):1115–29. doi: 10.1111/jre.13283
59. Han EC, Choi SY, Lee Y, Park JW, Hong SH, Lee HJ. Extracellular rnas in periodontopathogenic outer membrane vesicles promote tnf-A Production in human macrophages and cross the blood-brain barrier in mice. FASEB J. (2019) 33:13412–22. doi: 10.1096/fj.201901575R
60. Choi JW, Kim SC, Hong SH, Lee HJ. Secretable small rnas via outer membrane vesicles in periodontal pathogens. J Dent Res. (2017) 96:458–66. doi: 10.1177/0022034516685071
61. Fan R, Zhou Y, Chen X, Zhong X, He F, Peng W, et al. Porphyromonas gingivalis outer membrane vesicles promote apoptosis via msrna-regulated DNA methylation in periodontitis. Microbiol Spectr. (2023) 11:e0328822. doi: 10.1128/spectrum.03288-22
62. Doré E, Boilard E. Bacterial extracellular vesicles and their interplay with the immune system. Pharmacol Ther. (2023) 247:108443. doi: 10.1016/j.pharmthera.2023.108443
63. Ho MY, Liu S, Xing B. Bacteria extracellular vesicle as nanopharmaceuticals for versatile biomedical potential. Nano Converg. (2024) 11:28. doi: 10.1186/s40580-024-00434-5
64. Hajishengallis G, Darveau RP, Curtis MA. The keystone-pathogen hypothesis. Nat Rev Microbiol. (2012) 10:717–25. doi: 10.1038/nrmicro2873
65. Jiang Y, Chen Y, Ge L, Wang L, Wang L, Pathak JL. Multi-prospects of bacterial extracellular vesicles in immune modulation, inflammation regulation, and periodontitis treatment. Nano Today. (2024) 55:102210. doi: 10.1016/j.nantod.2024.102210
66. Kamaguchi A, Nakayama K, Ichiyama S, Nakamura R, Watanabe T, Ohta M, et al. Effect of porphyromonas gingivalis vesicles on coaggregation of staphylococcus aureus to oral microorganisms. Curr Microbiol. (2003) 47:485–91. doi: 10.1007/s00284-003-4069-6
67. Grenier D. Porphyromonas gingivalis outer membrane vesicles mediate coaggregation and piggybacking of treponema denticola and lachnoanaerobaculum saburreum. Int J Dent. (2013) 2013:305476. doi: 10.1155/2013/305476
68. Inagaki S, Onishi S, Kuramitsu HK, Sharma A. Porphyromonas gingivalis vesicles enhance attachment, and the leucine-rich repeat bspa protein is required for invasion of epithelial cells by “Tannerella forsythia. Infect Immun. (2006) 74:5023–8. doi: 10.1128/iai.00062-06
69. Zhang Z, Liu S, Zhang S, Li Y, Shi X, Liu D, et al. Porphyromonas gingivalis outer membrane vesicles inhibit the invasion of fusobacterium nucleatum into oral epithelial cells by downregulating fada and foma. J Periodontol. (2022) 93:515–25. doi: 10.1002/jper.21-0144
70. Ho MH, Chen CH, Goodwin JS, Wang BY, Xie H. Functional advantages of porphyromonas gingivalis vesicles. PloS One. (2015) 10:e0123448. doi: 10.1371/journal.pone.0123448
71. Villoria GEM, Fischer RG, Tinoco EMB, Meyle J, Loos BG. Periodontal disease: A systemic condition. Periodontol 2000. (2024) 96:7–19. doi: 10.1111/prd.12616
72. Zhang L, Zhang D, Liu C, Tang B, Cui Y, Guo D, et al. Outer membrane vesicles derived from fusobacterium nucleatum trigger periodontitis through host overimmunity. Adv Sci (Weinh). (2024):e2400882. doi: 10.1002/advs.202400882
73. Uemura Y, Hiroshima Y, Tada A, Murakami K, Yoshida K, Inagaki Y, et al. Porphyromonas gingivalis outer membrane vesicles stimulate gingival epithelial cells to induce pro-inflammatory cytokines via the mapk and sting pathways. Biomedicines. (2022) 10(10):2643. doi: 10.3390/biomedicines10102643
74. Kim HY, Lim Y, An SJ, Choi BK. Characterization and immunostimulatory activity of extracellular vesicles from filifactor alocis. Mol Oral Microbiol. (2020) 35:1–9. doi: 10.1111/omi.12272
75. Friedrich V, Gruber C, Nimeth I, Pabinger S, Sekot G, Posch G, et al. Outer membrane vesicles of tannerella forsythia: biogenesis, composition, and virulence. Mol Oral Microbiol. (2015) 30:451–73. doi: 10.1111/omi.12104
76. Locati M, Curtale G, Mantovani A. Diversity, mechanisms, and significance of macrophage plasticity. Annu Rev Pathol. (2020) 15:123–47. doi: 10.1146/annurev-pathmechdis-012418-012718
77. Russell DG, Huang L, VanderVen BC. Immunometabolism at the interface between macrophages and pathogens. Nat Rev Immunol. (2019) 19:291–304. doi: 10.1038/s41577-019-0124-9
78. Zhang B, Yang Y, Yi J, Zhao Z, Ye R. Hyperglycemia modulates M1/M2 macrophage polarization via reactive oxygen species overproduction in ligature-induced periodontitis. J periodontal Res. (2021) 56:991–1005. doi: 10.1111/jre.12912
79. Chen G, Sun Q, Cai Q, Zhou H. Outer membrane vesicles from fusobacterium nucleatum switch M0-like macrophages toward the M1 phenotype to destroy periodontal tissues in mice. Front Microbiol. (2022) 13:815638. doi: 10.3389/fmicb.2022.815638
80. Cecil JD, O’Brien-Simpson NM, Lenzo JC, Holden JA, Singleton W, Perez-Gonzalez A, et al. Outer membrane vesicles prime and activate macrophage inflammasomes and cytokine secretion in vitro and in vivo. Front Immunol. (2017) 8:1017. doi: 10.3389/fimmu.2017.01017
81. Fleetwood AJ, Lee MKS, Singleton W, Achuthan A, Lee MC, O’Brien-Simpson NM, et al. Metabolic remodeling, inflammasome activation, and pyroptosis in macrophages stimulated by porphyromonas gingivalis and its outer membrane vesicles. Front Cell Infect Microbiol. (2017) 7:351. doi: 10.3389/fcimb.2017.00351
82. Duncan L, Yoshioka M, Chandad F, Grenier D. Loss of lipopolysaccharide receptor cd14 from the surface of human macrophage-like cells mediated by porphyromonas gingivalis outer membrane vesicles. Microb Pathog. (2004) 36:319–25. doi: 10.1016/j.micpath.2004.02.004
83. Waller T, Kesper L, Hirschfeld J, Dommisch H, Kölpin J, Oldenburg J, et al. Porphyromonas gingivalis outer membrane vesicles induce selective tumor necrosis factor tolerance in a toll-like receptor 4- and mtor-dependent manner. Infect Immun. (2016) 84:1194–204. doi: 10.1128/iai.01390-15
84. du Teil Espina M, Fu Y, van der Horst D, Hirschfeld C, López-Álvarez M, Mulder LM, et al. Coating and corruption of human neutrophils by bacterial outer membrane vesicles. Microbiol Spectr. (2022) 10:e0075322. doi: 10.1128/spectrum.00753-22
85. Lindholm M, Metsäniitty M, Granström E, Oscarsson J. Outer membrane vesicle-mediated serum protection in aggregatibacter actinomycetemcomitans. J Oral Microbiol. (2020) 12:1747857. doi: 10.1080/20002297.2020.1747857
86. Grenier D, Bélanger M. Protective effect of porphyromonas gingivalis outer membrane vesicles against bactericidal activity of human serum. Infect Immun. (1991) 59:3004–8. doi: 10.1128/iai.59.9.3004-3008.1991
87. Cai R, Wang L, Zhang W, Liu B, Wu Y, Pang J, et al. The role of extracellular vesicles in periodontitis: pathogenesis, diagnosis, and therapy. Front Immunol. (2023) 14:1151322. doi: 10.3389/fimmu.2023.1151322
88. Vitkov L, Singh J, Schauer C, Minnich B, Krunić J, Oberthaler H, et al. Breaking the gingival barrier in periodontitis. Int J Mol Sci. (2023) 24(5):4544. doi: 10.3390/ijms24054544
89. Furuta N, Takeuchi H, Amano A. Entry of porphyromonas gingivalis outer membrane vesicles into epithelial cells causes cellular functional impairment. Infect Immun. (2009) 77:4761–70. doi: 10.1128/iai.00841-09
90. Chi B, Qi M, Kuramitsu HK. Role of dentilisin in treponema denticola epithelial cell layer penetration. Res Microbiol. (2003) 154:637–43. doi: 10.1016/j.resmic.2003.08.001
91. Bartruff JB, Yukna RA, Layman DL. Outer membrane vesicles from porphyromonas gingivalis affect the growth and function of cultured human gingival fibroblasts and umbilical vein endothelial cells. J Periodontol. (2005) 76:972–9. doi: 10.1902/jop.2005.76.6.972
92. Kim HY, Song MK, Gho YS, Kim HH, Choi BK. Extracellular vesicles derived from the periodontal pathogen filifactor alocis induce systemic bone loss through toll-like receptor 2. J Extracell Vesicles. (2021) 10:e12157. doi: 10.1002/jev2.12157
93. Kim HY, Song MK, Lim Y, Jang JS, An SJ, Kim HH, et al. Effects of extracellular vesicles derived from oral bacteria on osteoclast differentiation and activation. Sci Rep. (2022) 12:14239. doi: 10.1038/s41598-022-18412-4
94. Song MK, Kim HY, Choi BK, Kim HH. Filifactor alocis-derived extracellular vesicles inhibit osteogenesis through tlr2 signaling. Mol Oral Microbiol. (2020) 35:202–10. doi: 10.1111/omi.12307
95. Dawes C, Wong DTW. Role of saliva and salivary diagnostics in the advancement of oral health. J Dent Res. (2019) 98:133–41. doi: 10.1177/0022034518816961
96. Yu J, Lin Y, Xiong X, Li K, Yao Z, Dong H, et al. Detection of exosomal pd-L1 rna in saliva of patients with periodontitis. Front Genet. (2019) 10:202. doi: 10.3389/fgene.2019.00202
97. Han P, Bartold PM, Salomon C, Ivanovski S. Salivary small extracellular vesicles associated mirnas in periodontal status-a pilot study. Int J Mol Sci. (2020) 21(8):2809. doi: 10.3390/ijms21082809
98. Chaparro Padilla A, Weber Aracena L, Realini Fuentes O, Albers Busquetts D, Hernández Ríos M, Ramírez Lobos V, et al. Molecular signatures of extracellular vesicles in oral fluids of periodontitis patients. Oral Dis. (2020) 26:1318–25. doi: 10.1111/odi.13338
99. Fatima T, Khurshid Z, Rehman A, Imran E, Srivastava KC, Shrivastava D. Gingival crevicular fluid (Gcf): A diagnostic tool for the detection of periodontal health and diseases. Molecules. (2021) 26(5):1208. doi: 10.3390/molecules26051208
100. Huang X, Hu X, Zhao M, Zhang Q. Analysis of salivary exosomal proteins in young adults with severe periodontitis. Oral Dis. (2020) 26:173–81. doi: 10.1111/odi.13217
101. Nik Mohamed Kamal NNS, Awang RAR, Mohamad S, Shahidan WNS. Plasma- and saliva exosome profile reveals a distinct microrna signature in chronic periodontitis. Front Physiol. (2020) 11:587381. doi: 10.3389/fphys.2020.587381
102. Han P, Bartold PM, Salomon C, Ivanovski S. Salivary outer membrane vesicles and DNA methylation of small extracellular vesicles as biomarkers for periodontal status: A pilot study. Int J Mol Sci. (2021) 22(5):2423. doi: 10.3390/ijms22052423
103. O’Brien-Simpson NM, Burgess K, Brammar GC, Darby IB, Reynolds EC. Development and evaluation of a saliva-based chair-side diagnostic for the detection of porphyromonas gingivalis. J Oral Microbiol. (2015) 7:29129. doi: 10.3402/jom.v7.29129
104. Han P, Bartold PM, Ivanovski S. The emerging role of small extracellular vesicles in saliva and gingival crevicular fluid as diagnostics for periodontitis. J Periodontal Res. (2022) 57:219–31. doi: 10.1111/jre.12950
105. Kaparakis-Liaskos M, Ferrero RL. Immune modulation by bacterial outer membrane vesicles. Nat Rev Immunol. (2015) 15:375–87. doi: 10.1038/nri3837
106. Xiang SD, Scholzen A, Minigo G, David C, Apostolopoulos V, Mottram PL, et al. Pathogen recognition and development of particulate vaccines: does size matter? Methods. (2006) 40:1–9. doi: 10.1016/j.ymeth.2006.05.016
107. Peregrino ES, Castañeda-Casimiro J, Vázquez-Flores L, Estrada-Parra S, Wong-Baeza C, Serafín-López J, et al. The role of bacterial extracellular vesicles in the immune response to pathogens, and therapeutic opportunities. Int J Mol Sci. (2024) 25(11):6210. doi: 10.3390/ijms25116210
108. Krishnan N, Kubiatowicz LJ, Holay M, Zhou J, Fang RH, Zhang L. Bacterial membrane vesicles for vaccine applications. Adv Drug Delivery Rev. (2022) 185:114294. doi: 10.1016/j.addr.2022.114294
109. Gerritzen MJH, Martens DE, Wijffels RH, van der Pol L, Stork M. Bioengineering bacterial outer membrane vesicles as vaccine platform. Biotechnol Adv. (2017) 35:565–74. doi: 10.1016/j.bioteChadv.2017.05.003
110. Cai W, Kesavan DK, Wan J, Abdelaziz MH, Su Z, Xu H. Bacterial outer membrane vesicles, a potential vaccine candidate in interactions with host cells based. Diagn Pathol. (2018) 13:95. doi: 10.1186/s13000-018-0768-y
111. Puca V, Marinacci B, Pellegrini B, Campanile F, Santagati M, Grande R. Biofilm and bacterial membrane vesicles: recent advances. Expert Opin Ther Pat. (2024) 34:475–91. doi: 10.1080/13543776.2024.2338101
112. Nakao R, Hasegawa H, Ochiai K, Takashiba S, Ainai A, Ohnishi M, et al. Outer membrane vesicles of porphyromonas gingivalis elicit a mucosal immune response. PloS One. (2011) 6:e26163. doi: 10.1371/journal.pone.0026163
113. Nakao R, Hasegawa H, Dongying B, Ohnishi M, Senpuku H. Assessment of outer membrane vesicles of periodontopathic bacterium porphyromonas gingivalis as possible mucosal immunogen. Vaccine. (2016) 34:4626–34. doi: 10.1016/j.vaccine.2016.06.016
114. Bai D, Nakao R, Ito A, Uematsu H, Senpuku H. Immunoreactive antigens recognized in serum samples from mice intranasally immunized with porphyromonas gingivalis outer membrane vesicles. Pathog Dis. (2015) 733(3):ftu006. doi: 10.1093/femspd/ftu006
115. Nazli A, He DL, Liao D, Khan MZI, Huang C, He Y. Strategies and progresses for enhancing targeted antibiotic delivery. Adv Drug Delivery Rev. (2022) 189:114502. doi: 10.1016/j.addr.2022.114502
116. Gao W, Thamphiwatana S, Angsantikul P, Zhang L. Nanoparticle approaches against bacterial infections. Wiley Interdiscip Rev Nanomed Nanobiotechnol. (2014) 6:532–47. doi: 10.1002/wnan.1282
117. Wang S, Gao J, Wang Z. Outer membrane vesicles for vaccination and targeted drug delivery. Wiley Interdiscip Rev Nanomed Nanobiotechnol. (2019) 11:e1523. doi: 10.1002/wnan.1523
118. Sartorio MG, Pardue EJ, Feldman MF, Haurat MF. Bacterial outer membrane vesicles: from discovery to applications. Annu Rev Microbiol. (2021) 75:609–30. doi: 10.1146/annurev-micro-052821-031444
119. Liu H, Zhang H, Wang S, Cui J, Weng W, Liu X, et al. Bone-targeted bioengineered bacterial extracellular vesicles delivering sirna to ameliorate osteoporosis. Composites Part B: Eng. (2023) 255:110610. doi: 10.1016/j.compositesb.2023.110610
120. Chen Q, Bai H, Wu W, Huang G, Li Y, Wu M, et al. Bioengineering bacterial vesicle-coated polymeric nanomedicine for enhanced cancer immunotherapy and metastasis prevention. Nano Lett. (2020) 20:11–21. doi: 10.1021/acs.nanolett.9b02182
121. Kadurugamuwa JL, Beveridge TJ. Bacteriolytic effect of membrane vesicles from pseudomonas aeruginosa on other bacteria including pathogens: conceptually new antibiotics. J Bacteriol. (1996) 178:2767–74. doi: 10.1128/jb.178.10.2767-2774.1996
122. Wu S, Huang Y, Yan J, Li Y, Wang J, Yang YY, et al. Bacterial outer membrane-coated mesoporous silica nanoparticles for targeted delivery of antibiotic rifampicin against gram-negative bacterial infection in vivo. Advanced Funct Materials. (2021) 31:2103442. doi: 10.1002/adfm.202103442
123. Collins SM, Brown AC. Bacterial outer membrane vesicles as antibiotic delivery vehicles. Front Immunol. (2021) 12:733064. doi: 10.3389/fimmu.2021.733064
124. Chow YC, Yam HC, Gunasekaran B, Lai WY, Wo WY, Agarwal T, et al. Implications of porphyromonas gingivalis peptidyl arginine deiminase and gingipain R in human health and diseases. Front Cell Infect Microbiol. (2022) 12:987683. doi: 10.3389/fcimb.2022.987683
125. Chen J, Zhang H, Wang S, Du Y, Wei B, Wu Q, et al. Inhibitors of bacterial extracellular vesicles. Front Microbiol. (2022) 13:835058. doi: 10.3389/fmicb.2022.835058
126. Kosgodage US, Matewele P, Mastroianni G, Kraev I, Brotherton D, Awamaria B, et al. Peptidylarginine deiminase inhibitors reduce bacterial membrane vesicle release and sensitize bacteria to antibiotic treatment. Front Cell Infect Microbiol. (2019) 9:227. doi: 10.3389/fcimb.2019.00227
127. Kosgodage US, Mould R, Henley AB, Nunn AV, Guy GW, Thomas EL, et al. Cannabidiol (Cbd) is a novel inhibitor for exosome and microvesicle (Emv) release in cancer. Front Pharmacol. (2018) 9:889. doi: 10.3389/fphar.2018.00889
128. Kou Y, Inaba H, Kato T, Tagashira M, Honma D, Kanda T, et al. Inflammatory responses of gingival epithelial cells stimulated with porphyromonas gingivalis vesicles are inhibited by hop-associated polyphenols. J Periodontol. (2008) 79:174–80. doi: 10.1902/jop.2008.070364
129. Izui S, Sekine S, Murai H, Takeuchi H, Amano A. Inhibitory effects of curcumin against cytotoxicity of porphyromonas gingivalis outer membrane vesicles. Arch Oral Biol. (2021) 124:105058. doi: 10.1016/j.archoralbio.2021.105058
130. Zidar A, Kristl J, Kocbek P, Zupančič Š. Treatment challenges and delivery systems in immunomodulation and probiotic therapies for periodontitis. Expert Opin Drug Delivery. (2021) 18:1229–44. doi: 10.1080/17425247.2021.1908260
131. Ochôa C, Castro F, Bulhosa JF, Manso C, Fernandes JCH, Fernandes GVO. Influence of the probiotic L. Reuteri on periodontal clinical parameters after nonsurgical treatment: A systematic review. Microorganisms. (2023) 11(6):1449. doi: 10.3390/microorganisms11061449
132. Zhou K, Xie J, Su Y, Fang J. Lactobacillus reuteri for chronic periodontitis: focus on underlying mechanisms and future perspectives. Biotechnol Genet Eng Rev. (2024) 40:381–408. doi: 10.1080/02648725.2023.2183617
Keywords: periodontal pathogen, periodontitis, bacterial extracellular vesicles, outer membrane vesicles, periodontitis pathogenesis, BEVs application
Citation: Zhang R, Li G, Wu Y, Wang X and Luan Q (2024) Pathogenic mechanisms and potential applications of extracellular vesicles from periodontal pathogens in periodontitis. Front. Immunol. 15:1513983. doi: 10.3389/fimmu.2024.1513983
Received: 19 October 2024; Accepted: 03 December 2024;
Published: 20 December 2024.
Edited by:
Yuzhou Li, Chongqing Medical University, ChinaReviewed by:
Roberta Gaziano, University of Rome Tor Vergata, ItalyMi Du, Shandong University, China
Yaqian Chen, Zhejiang University, China
Copyright © 2024 Zhang, Li, Wu, Wang and Luan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qingxian Luan, a3FsdWFucXhAMTI2LmNvbQ==; Xiaoxuan Wang, NTIwNHh4c2FyYWhAMTYzLmNvbQ==
 Ruiqing Zhang
Ruiqing Zhang Guoliang Li
Guoliang Li Yingtao Wu1
Yingtao Wu1 Qingxian Luan
Qingxian Luan