
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Immunol. , 23 January 2025
Sec. Cancer Immunity and Immunotherapy
Volume 15 - 2024 | https://doi.org/10.3389/fimmu.2024.1510145
 Fei Yang1†
Fei Yang1† Min Dan1†
Min Dan1† Jindan Shi1
Jindan Shi1 Ling Fan1
Ling Fan1 Haoluo Zhang1
Haoluo Zhang1 Tiantian Jian1
Tiantian Jian1 Kelu Lei1
Kelu Lei1 Yue Wang1
Yue Wang1 Juan Xin1
Juan Xin1 Zhigang Yu1*
Zhigang Yu1* Wei Chen2*
Wei Chen2*Background: PD-1 inhibitors have shown promising efficacy in enhancing OS and AEs as second-line therapies for patients with advanced esophageal squamous cell carcinoma (ESCC). However, there remains no clear consensus on which PD-1 inhibitor provides the best balance between efficacy and safety. To address this key issue in the second-line treatment of ESCC, we conducted a network meta-analysis (NMA) with a focus on OS benefits, particularly in patients with different levels of PD-L1 expression.
Methods: A systematic search of relevant literature was conducted in Web of Science, Embase, PubMed, and Cochrane Library, covering publications from the inception of these database to June 2024. The evaluated endpoints included OS, progression-free survival (PFS), objective response rate (ORR), AEs, and Grade ≥ 3 adverse events (Grade ≥ 3 AEs). A systematic review and Bayesian network meta-analysis were performed to assess the efficacy and safety of various immunotherapy regimens in patients with advanced ESCC. To ensure transparency, novelty, and reliability, this study was prospectively registered in the systematic review registry (CRD42024540581).
Results: Five randomized controlled trials (RCTs), encompassing 2,078 patients and six treatment regimens, were included in this study. Among advanced ESCC patients not selected based on PD-L1 expression, Sintilimab demonstrated the greatest OS benefit (HR = 0.70, 95% CI: 0.50-0.98). Camrelizumab showed the most favorable improvement in PFS compared to chemotherapy (HR = 0.64, 95% CI: 0.47-0.87) and also achieved the best ORR benefit (OR = 3.72, 95% CI: 1.98-6.99). In terms of safety, Nivolumab (OR = 0.10, 95% CI: 0.05-0.19) and Tislelizumab (OR = 0.18, 95% CI: 0.10-0.33) exhibited significant safety advantages over chemotherapy concerning AEs. Moreover, Nivolumab (OR = 0.13, 95% CI: 0.08-0.20) was associated with a markedly lower risk of Grade ≥ 3 AEs compared to chemotherapy. Subgroup analysis based on PD-L1 expression revealed that Tislelizumab (HR = 0.53, 95% CI: 0.37-0.76) offered the greatest OS benefit for patients with PD-L1 ≥ 10%, while Camrelizumab (HR = 0.71, 95% CI: 0.57-0.89) was the most likely regimen to provide an OS advantage for patients with PD-L1 < 10%.
Conclusion: Compared to chemotherapy, PD-1 inhibitors may provide improved survival outcomes for patients with advanced ESCC. Among patients not selected based on PD-L1 expression, Sintilimab is most likely to deliver the best survival benefit. For patients with PD-L1 expression ≥ 10%, Tislelizumab is expected to offer the greatest efficacy, while Camrelizumab appears to be the most effective for those with PD-L1 < 10%.
Systematic Review Registration: https://www.crd.york.ac.uk/PROSPERO/, identifier CRD42024540581.
Esophageal cancer is the 11th most prevalent cancer globally and the 7th leading cause of cancer-related deaths (1). Esophageal cancer poses an enormous healthcare burden, with an average of more than 400,000 deaths per year, while at the same time approximately 470,000+ new cases are created each year (2). ESCC is the most common histological subtype, particularly in populations from Asia and East Africa, representing 90% of esophageal cancer cases (3). More than half of global ESCC cases occur in China (4). The asymptomatic nature of ESCC in its early stages often leads to diagnosis at a locally advanced stage. The current 5-year survival rate for patients with locally advanced ESCC is about 10-20% (5). Furthermore, the majority of patients experience early recurrence or metastasis, leading to a poor prognosis for those with advanced ESCC (6–8).
Second-line treatments, including monotherapy with paclitaxel, docetaxel, or irinotecan, are commonly used after the failure of first-line therapies in advanced or metastatic ESCC. As second-line treatment usually implies disease progression in patients, accompanied by a decline in function, worsening of symptoms and quality of life (9), the use of chemotherapy induces DNA damage with the risk of secondary malignancy. At the same time chemotherapy-induced side effects of nausea, vomiting, anemia and increased risk of infections make the efficacy obtained with second-line treatment with chemotherapy unsatisfactory, with overall survival typically less than 10 months (10, 11). Therefore, there is a critical need to explore innovative therapies that can improve the prognosis for patients with advanced ESCC.
Tumor immunotherapy is a treatment modality that controls and kills tumor cells by repairing and enhancing the function of the body’s immune system (12). Since the FDA approval of immune checkpoint inhibitor antibodies targeting CTLA-4 in 2011, there has been a renewed interest in the immune system for the treatment of a wide range of malignancies. Over the next few years, more antibodies targeting immune checkpoint inhibitors such as PD-1, PD-L1, and LAG-3 entered clinical practice (13), offering renewed hope to patients (14). These inhibitors work by attaching to protein receptors on the surface of T cells, which activates T cell-mediated immune responses, blocking the PD-L1/PD-1 inhibitory signaling pathway, and enhancing the body’s anti-tumor immune response (15, 16). The inflammatory microenvironment of squamous esophageal cancer is capable of enriching immunosuppressive T regulatory cells, which makes immunotherapy potentially surprisingly effective in treating squamous esophageal cancer (17). Programmed cell death protein 1 (PD-1) is the most prevalent immune checkpoint in ESCC, and its inhibition by monoclonal antibodies has shown therapeutic efficacy (18). Results from the ATTRACTION-3 (19), KEYNOTE-181 (20), and ESCORT (21) studies demonstrate that PD-1 inhibitors, In second-line treatment regimens, PD-1 inhibitors are associated with longer overall survival and higher overall remission rates compared to standard chemotherapy. Also PD-1 inhibitors have a more manageable safety profile with a lower incidence of treatment-related adverse events (22–24). Currently in network meta-analyses, only cost-effectiveness analyses of PD-1 inhibitors as second-line treatment for advanced squamous esophageal cancer have been performed (25, 26), however, no consensus has been reached on the optimal choice of PD-1 inhibitors for second-line treatment of patients with advanced ESCC in terms of efficacy and safety.
With the increasing use of PD-1 inhibitors in ESCC, most RCTs have directly compared the outcomes of PD-1 inhibitors with chemotherapy (27, 28). As a result, there is an urgent need to develop strategies for optimizing PD-1 inhibitor treatment regimens to inform the design of future head-to-head clinical trials.
This study aims to evaluate the efficacy and safety of all currently available second-line PD-1 inhibitors in patients with advanced ESCC. Utilizing a Bayesian framework for comparisons of the effects of all second-line PD-1 inhibitors on survival in patients with advanced ESCC and to rank the use of these treatments in patients (29). Additionally, a systematic review and meta-analysis will be conducted to identify the optimal treatment strategy based on varying levels of PD-L1 expression.
This network meta-analysis (NMA) adheres to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) extension guidelines for network meta-analyses (Supplementary Table 1) (30). In the absence of randomized controlled trials directly comparing different PD-1 inhibitors, the Bayesian methods indirectly compare and predict the probability of efficacy and safety of different PD-1 inhibitors given the probability distributions of the model parameters of the observational data and the reference values of the prior beliefs of the external information (31). The study protocol has been registered on the PROSPERO platform to guarantee transparency, reliability, and innovation (Registration No: CRD42024540581) (32).
A thorough search of the literature was performed across the Web of Science, Embase, PubMed, and Cochrane Library databases. Search terms included advanced squamous cell esophageal cancer, immune checkpoint inhibitors, PD-1 receptor, PD-1 inhibitors, and randomized controlled trials. To ensure thoroughness, we also reviewed references from relevant published studies and review articles to address any gaps in the keyword search. The search period spanned from the inception of the databases to December 2024, utilizing a combination of free-text and subject heading search strategies. The specifics of the database search strategy can be found in Supplementary Table 2.
1. Randomized controlled trials of locally advanced or metastatic ESCC that are refractory or intolerant to first-line treatment;
2. Treatment groups receiving PD-1 inhibitors must utilize a single PD-1 inhibitor, while the chemotherapy group must use standard second-line chemotherapy agents for ESCC;
3. Trials must report at least three of the following outcomes: OS, PFS, ORR, and the incidence of treatment-related AEs or Grade ≥ 3 AEs as outcome measures. This ensures a comprehensive assessment of the efficacy and safety of PD-1 inhibitors.
4. Correlation data between PD-L1 expression and clinicopathologic parameters are available.
5. Sufficient survival data are available to estimate prognosis, such as minimum follow-up time.
1. Exclude reviews or case reports;
2. Exclude duplicate randomized controlled trials;
3. Exclude non-randomized controlled trials and animal studies;
4. Exclude randomized controlled trials with unclear outcome measures.
All included randomized controlled trials were independently reviewed and verified by two reviewers to ensure the data were up to date. When clinical trial results were published in different journals or in different years, the article with the most complete data was selected. Any discrepancies were resolved through group discussion.
Two investigators (FY and MD) independently extracted data from the RCTs in compliance with PRISMA guidelines, with any disagreements resolved through consensus with a third investigator (WC). The data extracted from each article included trial name, publication source, design, randomization ratio, trial phase, publication year, sample size, clinical trial registration number, treatment regimens for both experimental and control groups, patient age, sex distribution, histological type, PD-L1 expression, patient ethnicity, ECOG status, and disease state. The key outcome measures were summarized, including hazard ratios (HR) with their respective 95% confidence intervals (95% CI) for OS and PFS, along with odds ratios (OR) with 95% CI for ORR, the incidence of any AEs, and Grade ≥ 3 AEs.
The risk of bias in network meta-analysis (RoB NMA) tool project aims to develop the first tool to assess risk of bias in a review with network meta-analyses (NMAs) (33). This study utilized the Cochrane tool as the primary method to assess both the quality and potential risk of bias. The evaluation focused on five core areas: bias linked to the randomization process, deviations from planned interventions, incomplete reporting of outcomes, biases in outcome measurement, and selective result reporting. Two reviewers independently carried out the assessments, while a third reviewer mediated and resolved any conflicts. After the final review, the randomized controlled trials (RCTs) were grouped into three risk levels: low risk, high risk, and moderate concerns. All included RCT studies had a relatively low risk (34). Two independent authors evaluated the included articles, and another author made the final decision on the controversial parts.
The primary outcomes were OS, PFS, and ORR. Secondary outcomes included AEs and Grade ≥ 3 AEs. HR with 95% CI were used to measure effect sizes for OS and PFS, while OR with 95% CI were used for ORR, AEs, and Grade ≥ 3 AEs.
NMA was performed using a Bayesian framework with the ‘rjags’ and ‘gemtc’ packages in R software (35). A fixed-effect model was applied, utilizing three independent Markov chains, each running 10,000 burn-in iterations followed by 30,000 sample iterations. HR and OR were utilized as effect size metrics to rank the efficacy and safety of various treatment regimens, based on the iterations of the Markov chains, with the results displayed visually.
In the absence of direct head-to-head studies among the various PD-1 inhibitor groups, this study employed NMA for indirect comparisons. To ensure the accuracy of the NMA’s indirect comparisons, pairwise meta-analyses based on frequentist methods were conducted for the available head-to-head studies, and the results were compared with the corresponding Bayesian framework summaries (Supplementary Table 3).
This study employed RevMan 5.3 software to conduct pairwise meta-analyses using frequentist methods, comparing the efficacy and safety of second-line immunotherapy to standard chemotherapy (36). Heterogeneity was assessed using the Q test and the I² statistic, with I² ≤ 50% or P ≥ 0.1 indicating low heterogeneity and I² > 50% or P < 0.1 indicating high heterogeneity (37). For studies with low heterogeneity, a fixed-effect model was employed, whereas a random-effects model was utilized for those with high heterogeneity. Sensitivity analyses were also conducted for studies with high heterogeneity by sequentially excluding studies that had a significant impact on heterogeneity. This approach allowed for the comparison of efficacy and safety both before and after exclusions, along with statistical significance assessments. Funnel plots were used to evaluate potential publication bias.
In the initial literature search for this systematic review, 1,401 records were retrieved from the databases. After screening abstracts to eliminate duplicates and irrelevant articles, 138 records met the criteria for full-text review. Ultimately, five studies fulfilled our eligibility criteria (Figure 1), involving a total of 2,078 patients who received the following second-line treatments: Sintilimab, Tislelizumab, Pembrolizumab, Nivolumab, Camrelizumab, and chemotherapy. Notably, the study involving Pembrolizumab included two types of esophageal cancer; we extracted data specifically for patients with advanced ESCC. Of these, Sintilimab and Camrelizumab were performed in China, and the other three were performed globally. Pembrolizumab and Tislelizumab used the Composite Positivity Score (CPS), which assesses PD-L1 expression by dividing the number of PD-L1-positive cells by the total number of tumor cells, and Camrelizumab uses the Tumor Proportion Score (TPS), which assesses PD-L1 expression by the fraction of positive tumor cells, and Tislelizumab uses the Tumor Area Positivity Score (TAP), which assesses PD-L1 expression by the area of PD-L1-stained tumor cells and immune cells as a percentage of the area of all tumors (38). The primary characteristics of these studies are detailed in Tables 1, 2, and Supplementary Table 4, while the risk of bias assessment is shown in Supplementary Figure 1.
All five studies reported data on OS, PFS, and ORR. For OS, there was no statistically significant heterogeneity among the studies (P > 0.1, I² = 0). We therefore performed Meta-analysis using a fixed-effects model capable of assuming a true effect size for each comparison experiment. Patients with ESCC who did not have selected PD-L1 expression and were treated with PD-1 inhibitors demonstrated improved OS (HR = 0.73, 95% CI: 0.66-0.81) compared to those receiving standard chemotherapy. The results indicate that all PD-1 inhibitors significantly prolong OS compared to chemotherapy. Details are provided in Figure 2.
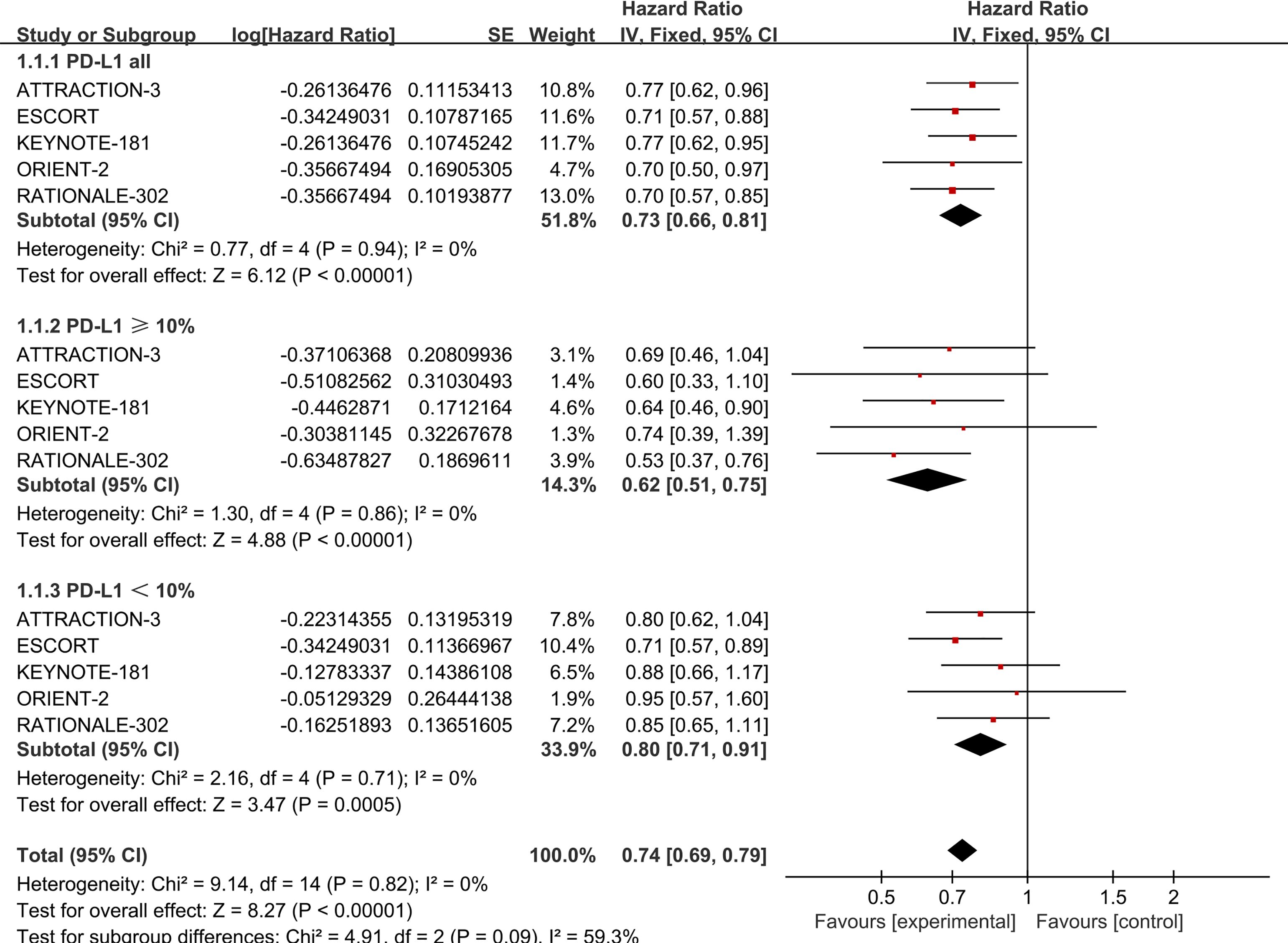
Figure 2. Forest plot comparing OS in ESCC patients with unselected PD-L1 expression (P=0.94, I2=0), PD-L1 ≥ 10% (P=0.86, I2=0), and PD-L1 < 10% (P=0.71, I2=0) receiving PD-1 inhibitors versus standard chemotherapy.
A random-effects model was applied to account for the possibility of varying true effect sizes across studies, as significant heterogeneity was observed among the five included studies (heterogeneity for PFS:P > 0.1, I² = 57%; heterogeneity for ORR: P > 0.1, I² = 71%), The results demonstrated that, in terms of PFS, the PD-1 inhibitor treatment group (HR = 0.91, 95% CI: 0.76-1.08) did not exhibit a significant improvement in PFS. Details are provided in Figure 3A.
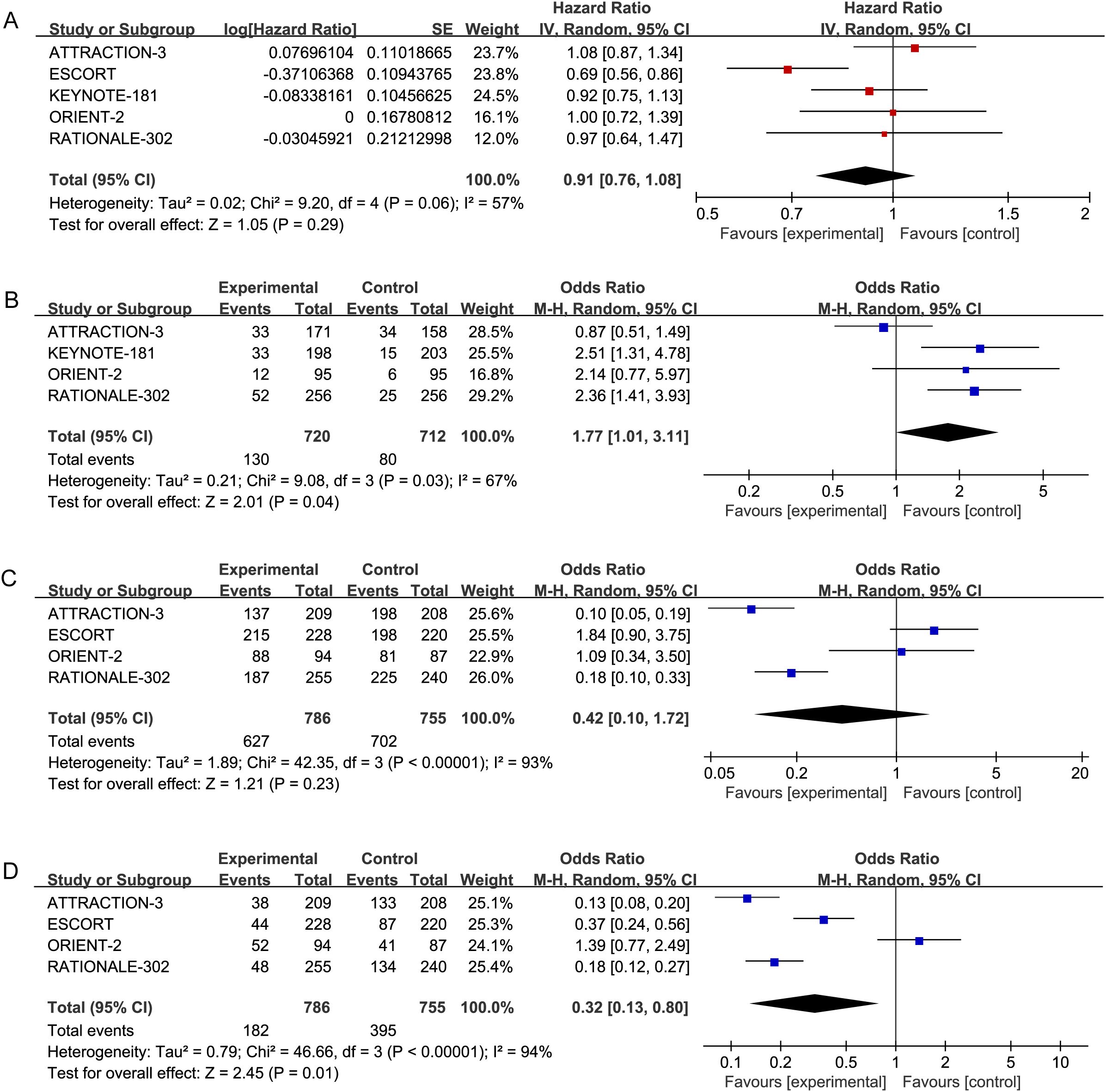
Figure 3. Forest plots: (A) PFS (P=0.06,I2=57%) in advanced ESCC patients receiving PD-1 inhibitors versus standard chemotherapy. (B) ORR (P=0.008, I2=71%) in advanced ESCC patients receiving PD-1 inhibitors versus standard chemotherapy. (C) Incidence of AEs (P<0.00001, I2=94%) in advanced ESCC patients receiving PD-L1inhibitors versus standard chemotherapy. (D) Incidence of Grade ≥AEs (P<0.00001, I2=92%) in advanced ESCC patients receiving PD-L1 inhibitors versus standard chemotherapy.
Regarding ORR, Patients with ESCC receiving PD-1 inhibitors (OR = 2.07, 95% CI: 1.22-3.52) were more likely to experience an improvement in ORR compared to those receiving standard chemotherapy. These results indicate that PD-1 inhibitors significantly enhance ORR compared to chemotherapy. These results indicate that PD-1 inhibitors significantly enhance ORR compared to chemotherapy alone. Details are provided in Figure 3B.
The incidence of AEs and Grade ≥ 3 AEs was used to assess the safety and toxicity of PD-1 inhibitors. Five studies reported on AEs (P > 0.1, I² = 93%) and Grade ≥ 3 AEs (P > 0.1, I² = 94%), necessitating the use of a random-effects model for the meta-analysis. The results indicated no significant difference in the incidence of AEs among advanced ESCC patients receiving PD-1 inhibitors (OR = 0.42, 95% CI: 0.10-1.72). The incidence of Grade ≥ 3 AEs, however, was lower in patients treated with PD-1 inhibitors compared to those receiving standard chemotherapy (OR = 0.32, 95% CI: 0.13-0.80). Further details are presented in Figures 3C, D.
The study examined the OS outcomes of five PD-1 inhibitors across varying levels of PD-L1 expression. All five studies reported OS data for subgroups with PD-L1 ≥10% and PD-L1 <10%. Therefore, a subgroup analysis was performed on these two datasets.
No statistical heterogeneity was found among the studies (P > 0.1, I² = 0), supporting the use of a fixed-effect model in the meta-analysis. The results showed that ESCC patients with PD-L1 ≥ 10% (HR = 0.62, 95% CI: 0.51-0.75) and those with PD-L1 < 10% (HR = 0.80, 95% CI: 0.71-0.91) experienced significantly longer OS with PD-1 inhibitors compared to standard chemotherapy. Further details are provided in Figure 2.
The primary outcomes of this study—OS, PFS, and ORR—were used to evaluate treatment efficacy. The NMA included six treatment regimens for advanced ESCC patients, analyzing their OS, PFS, and ORR (Figure 4A).
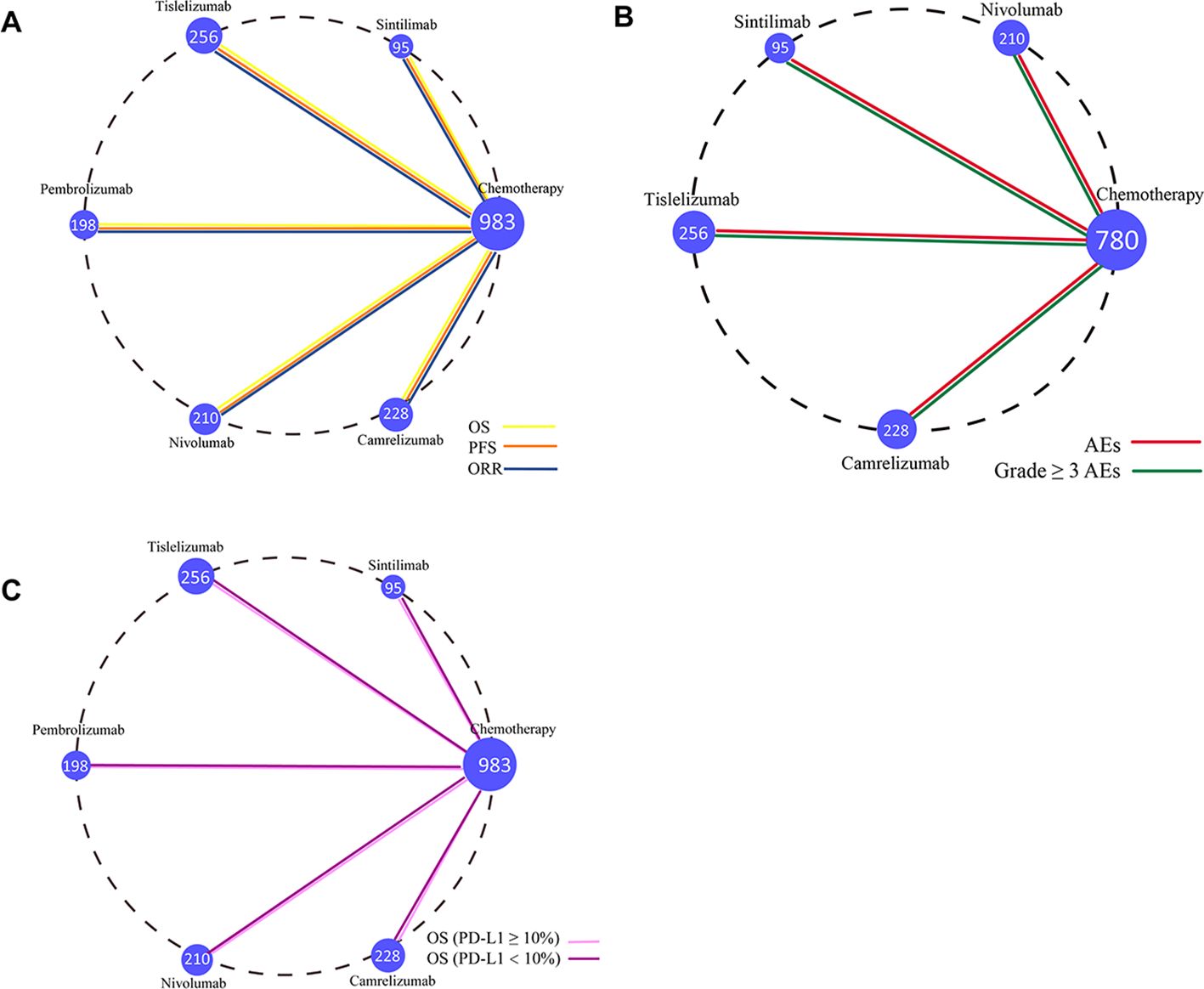
Figure 4. Network diagram comparing treatment outcomes in ESCC patients across different treatment groups. (A) Comparison of overall survival, progression-free survival, and objective response rate in ESCC patients. (B) Comparison of adverse event rates and Grade ≥ 3 AEs in ESCC patients. (C) Comparison of OS in ESCC patients with PD-L1 ≥ 10% and PD-L1 < 10%. Each node represents a treatment type, with the size of the node corresponding to the number of patients included. Two treatment regimens directly compared in a clinical trial are connected by a straight line.
Regarding OS (Figure 5A), patients treated with PD-1 inhibitors were more likely to experience improved OS compared to those receiving chemotherapy. Among the PD-1 inhibitors, Sintilimab showed the greatest OS benefit (HR = 0.70, 95% CI: 0.50-0.98). Tislelizumab (HR = 0.70, 95% CI: 0.57-0.85) and Camrelizumab (HR = 0.71, 95% CI: 0.57-0.88) provided comparable OS benefits. Additionally, Pembrolizumab (HR = 0.77, 95% CI: 0.62-0.95) and Nivolumab (HR = 0.77, 95% CI: 0.62-0.96) also demonstrated similar OS advantages.
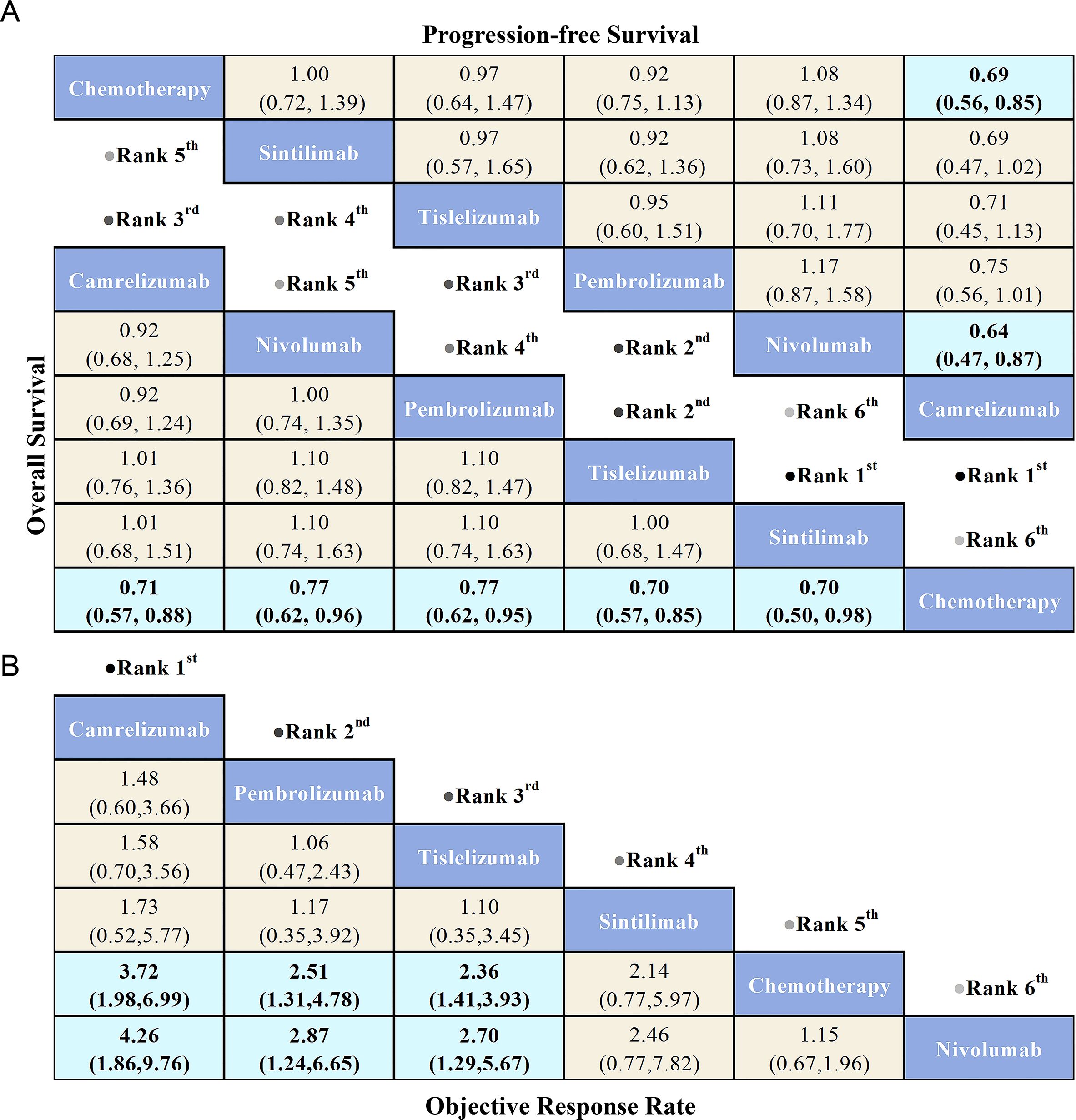
Figure 5. League table comparing the efficacy of various PD-1 inhibitors and chemotherapy in advanced ESCC patients based on Bayesian network meta-analysis. (A) The bottom-left triangular region shows the HRs and 95% CIs for overall survival, while the top-right triangular region shows HRs and 95% CIs for progression-free survival. An HR < 1.00 indicates better survival benefits. (B) The OR and 95% CIs for objective response rate, where an OR > 1.00 indicates better treatment benefits.
Regarding PFS (Figure 5A), PD-1 inhibitors did not show significant PFS benefits compared to chemotherapy, with the exception of Camrelizumab, which provided the greatest PFS advantage (HR = 0.69, 95% CI: 0.56-0.85). Notably, Nivolumab exhibited the poorest PFS among all treatment options, while Camrelizumab offered superior PFS compared to Nivolumab (HR = 0.64, 95% CI: 0.47-0.87).
Regarding ORR (Figure 5B), PD-1 inhibitors demonstrated superior ORR compared to chemotherapy, with the exception of Nivolumab, which showed the lowest ORR among all treatments. Camrelizumab provided the greatest ORR benefit relative to chemotherapy (OR = 3.72, 95% CI: 1.98-6.99), followed by Pembrolizumab (OR = 2.51, 95% CI: 1.31-4.78) and Tislelizumab (OR = 2.36, 95% CI: 1.41-3.93), both showing significant improvements in ORR. Additionally, Camrelizumab demonstrated a better ORR compared to Nivolumab (OR = 4.26, 95% CI: 1.86-9.76), with Pembrolizumab (OR = 2.87, 95% CI: 1.24-6.65) and Tislelizumab (OR = 2.70, 95% CI: 1.29-5.67) following closely.
The secondary outcomes of this study were AEs and Grade ≥ 3 AEs, which were used to assess safety and toxicity. To better interpret the ranking of adverse event occurrence rates, the results were reverse-ordered, meaning a higher ranking corresponds to a lower incidence of AEs. The NMA included five treatment regimens for advanced ESCC, focusing on AEs and Grade ≥ 3 AEs (Figure 4B).
Regarding AEs (Figure 6B), the Nivolumab group (OR = 0.10, 95% CI: 0.05-0.19) and Tislelizumab group (OR = 0.18, 95% CI: 0.10-0.33) demonstrated a significant safety advantage compared to chemotherapy. In contrast, Sintilimab (OR = 0.92, 95% CI: 0.29-2.97) and Camrelizumab (OR = 0.54, 95% CI: 0.27-1.11) did not show a significant safety benefit over chemotherapy. Notably, Nivolumab exhibited greater safety benefits compared to both Sintilimab (OR = 0.09, 95% CI: 0.02-0.35) and Camrelizumab (OR = 0.05, 95% CI: 0.02-0.14).
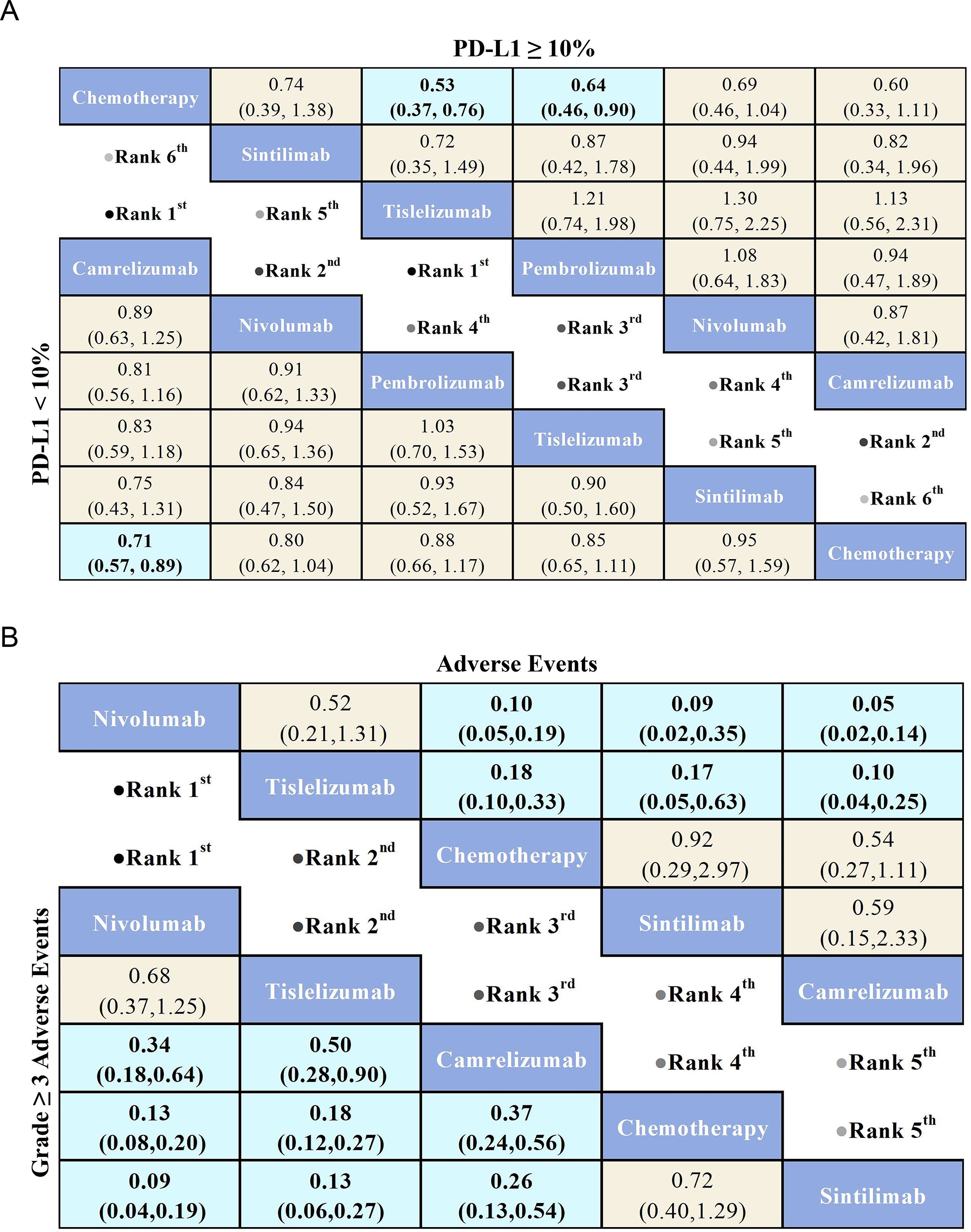
Figure 6. League table comparing the efficacy and safety of PD-1 inhibitors versus chemotherapy in advanced ESCC patients based on Bayesian network meta-analysis. (A) The top-right triangular region shows HRs and 95% CIs for overall survival in patients with PD-L1 ≥ 10%, while the bottom-left triangular region shows HRs and 95% CIs for those with PD-L1 < 10%. An HR < 1.00 indicates better survival benefits. (B) The top-right triangular region presents ORs and 95% CIs for adverse events, while the bottom-left triangular region shows ORs and 95% CIs for Grade ≥ 3 AEs. An OR < 1.00 indicates better safety.
Regarding Grade ≥ 3 AEs (Figure 6B), Nivolumab (OR = 0.13, 95% CI: 0.08-0.20) demonstrated a significant safety advantage over chemotherapy, with Tislelizumab (OR = 0.18, 95% CI: 0.12-0.27) and Camrelizumab (OR = 0.37, 95% CI: 0.24-0.56) also showing notable safety benefits compared to chemotherapy. In contrast, Sintilimab (OR = 0.72, 95% CI: 0.40-1.29) did not exhibit a significant safety advantage over chemotherapy. Additionally, Nivolumab (OR = 0.09, 95% CI: 0.04-0.19), Tislelizumab (OR = 0.13, 95% CI: 0.06-0.27), and Camrelizumab (OR = 0.26, 95% CI: 0.13-0.54) demonstrated greater safety benefits compared to Sintilimab. Commonly reported treatment-related adverse events for PD-1 inhibitors included anemia, nausea, decreased appetite, and reductions in white blood cell and neutrophil counts (Figure 7). Each PD-1 inhibitor exhibited varying rates of adverse events: The most common adverse events associated with Sintilimab were hypothyroidism, pneumonitis, anemia, and decreased white blood cell count. The most frequent severe adverse event was pneumonitis, with one fatal case each reported for upper gastrointestinal bleeding, pneumonia, and pulmonary infection.
The most common adverse events associated with Tislelizumab were elevated aspartate aminotransferase levels, anemia, and hypothyroidism. Fatal cases included one patient each for hemoptysis, pulmonary hypertension, upper respiratory tract infection, pneumonia, and thrombocytopenia, as well as three fatalities due to septic shock. The most common adverse events associated with Nivolumab were rash, diarrhea, and decreased appetite. The most frequent severe adverse events were fever and interstitial lung disease, with one fatal case each caused by interstitial lung disease and pneumonia, and the most common severe adverse events associated with Camrelizumab were anemia, liver dysfunction, and diarrhea. Fatal cases included one patient each due to colitis, liver dysfunction, pneumonia, and myocarditis. (Supplementary Table S5).
In the subgroup analysis, OS was used as the primary outcome measure. The NMA included six treatment regimens for advanced ESCC patients with varying PD-L1 expression levels (Figure 4C) to evaluate the efficacy of these treatments in patients with positive PD-L1 expression.
For OS in advanced ESCC patients with PD-L1 ≥ 10% (Figure 6A), five PD-1 inhibitors were included in the subgroup analysis. All treatment regimens provided an OS benefit compared to chemotherapy, with Tislelizumab (HR = 0.53, 95% CI: 0.37-0.76) being the most likely to offer the greatest OS benefit. Additionally, Pembrolizumab (HR = 0.64, 95% CI: 0.46-0.90) and Camrelizumab (HR=0.60,95%CI:0.33-1.11) also demonstrated a significant OS advantage over chemotherapy.
For OS in advanced ESCC patients with PD-L1 < 10% (Figure 6A), the results showed that PD-1 inhibitor treatments provided a clear OS benefit over standard chemotherapy. Among the treatments, Camrelizumab (HR = 0.71, 95% CI: 0.57-0.89) was the most likely to offer the greatest OS benefit. Additionally, Nivolumab (HR=0.80,95%CI:0.62-1.04) and Tislelizumab (HR=0.85,95%CI:0.65-1.11) also demonstrated a significant OS advantage over chemotherapy.
Bayesian ranking curve analysis (Figures 8, 9, Supplementary Tables S6–Supplementary Table S10) was performed to assess the ranking probabilities of the six treatment regimens included in the study. For patients without selected PD-L1 expression, Sintilimab ranked first for OS, with a probability of 35.19%. Among patients with PD-L1 ≥ 10%, Tislelizumab ranked first for OS (probability of 43.56%), followed by Camrelizumab in second place (probability of 28.28%). For patients with PD-L1 < 10%, Camrelizumab ranked first for OS, with a probability of 55.63%.
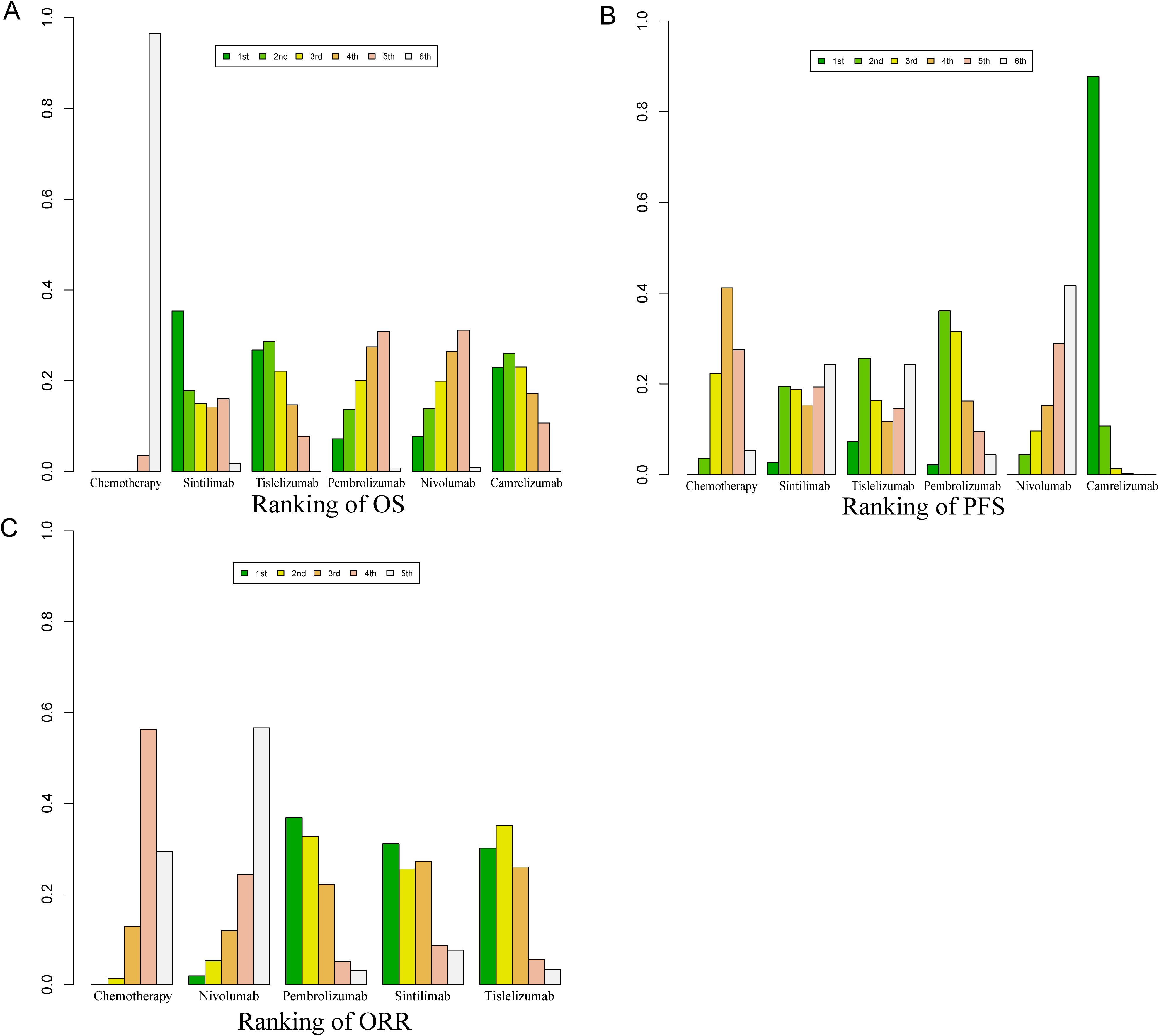
Figure 8. Bayesian ranking profile for the efficacy of various PD-1 inhibitor treatment regimens in advanced ESCC patients. (A) Ranking of OS. (B) Ranking of PFS. (C) Ranking of ORR.
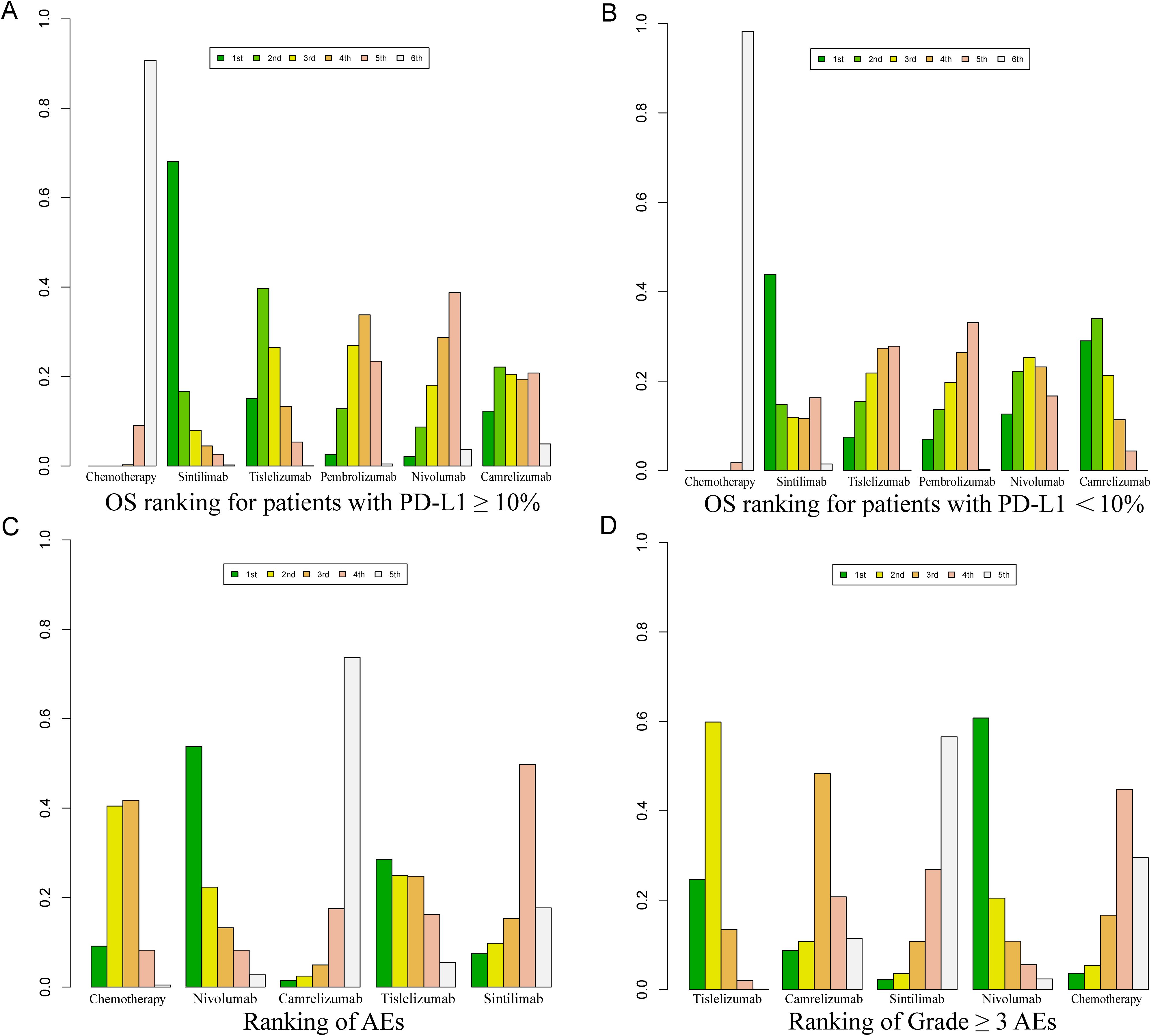
Figure 9. Bayesian ranking profile for the efficacy and safety of various PD-1 inhibitor treatment regimens in advanced ESCC patients. (A) OS ranking for patients with PD-L1 ≥ 10%. (B) OS ranking for patients with PD-L1 < 10%. (C) Ranking of AEs. (D) Ranking of Grade ≥ 3 AEs.
For PFS, Camrelizumab ranked first with a probability of 87.74%, and for ORR, it also ranked first with a probability of 40.40%. Regarding AEs of any grade, Nivolumab ranked first with a probability of 91.70%, and for Grade ≥ 3 AEs, Nivolumab again ranked first with a probability of 89.3%.
Tislelizumab achieved a balance between efficacy and safety. It ranked second for OS in patients without selected PD-L1 expression (probability of 28.40%), first for OS in patients with PD-L1 ≥ 10% (probability of 43.56%), and third for OS in patients with PD-L1 < 10% (probability of 26.27%). In PFS, Tislelizumab ranked third (probability of 16.35%), in ORR it ranked third (probability of 41%), second for AEs (probability of 91.30%), and second for Grade ≥ 3 AEs (probability of 88.3%).
The pairwise meta-analysis results using the frequentist approach aligned with the corresponding summaries from the Bayesian framework (Supplementary Table 3). Heterogeneity was assessed using the Q test and I² statistic, with results indicating high heterogeneity (I² > 50%) (Figures 2–4). Sequential exclusion of individual studies did not significantly reduce the heterogeneity. All RCTs involved comparisons between a single PD-1 inhibitor and chemotherapy, eliminating the possibility of inconsistency. Therefore, an inconsistency check was not performed (39). A funnel plot analysis was performed to evaluate publication bias, with OS as the outcome measure. The symmetrical distribution of scatter points, with no outliers detected, indicates a low probability of publication bias in this study (Supplementary Figure 1).
To the best of our knowledge, this is the most comprehensive systematic review and NMA to date, comparing the efficacy and safety of second-line PD-1 inhibitors. It also includes an evaluation of OS outcomes in both PD-L1 ≥ 10% and PD-L1 < 10% populations. This study offers evidence-based insights to inform clinical practice and highlights the following key findings:
1. All immunotherapy regimens demonstrated superior OS and ORR compared to standard chemotherapy.
2. PD-1 inhibitors did not show a significant advantage in PFS over chemotherapy, with the exception of Camrelizumab, which provided a distinct PFS benefit.
3. For advanced ESCC patients with PD-L1 ≥ 10%, Tislelizumab was associated with the most pronounced OS benefit, whereas Camrelizumab was most likely to yield the greatest OS improvement for patients with PD-L1 <10%. (4) The safety profiles of different PD-1 inhibitors varied significantly compared to standard chemotherapy. Nivolumab (OR = 0.10, 95% CI: 0.05-0.19) and Tislelizumab (OR = 0.18, 95% CI: 0.10-0.33) demonstrated better safety in terms of AEs, while Nivolumab (OR = 0.10, 95% CI: 0.05-0.19) showed superior safety in terms of Grade ≥ 3 AEs compared to chemotherapy.
The PD-L1/PD-1 pathway acts as a synergistic inhibitory signaling mechanism, in which PD-L1 binds to its receptor PD-1 on activated T cells, preserving peripheral T cell tolerance and maintaining immune homeostasis to prevent overactivation and autoimmune disorders (40). In malignancies, the interaction between PD-L1 and PD-1 suppresses T cell activation, enabling tumors to evade anti-tumor immunity (41). Additionally, PD-L1 is frequently overexpressed in cancer cells, with its production triggered by various cytokines within the tumor microenvironment (42). Consequently, PD-1 inhibitors demonstrate anti-tumor activity by blocking the PD-L1/PD-1 pathway and reactivating T cell immune function (16, 43). In contrast, chemotherapy agents elicit specific immune responses against tumors through the induction of immunogenic cell death (44, 45). Taxane-based therapies are widely utilized as second-line treatments following first-line failure, with OS typically ranging from 8 to 10 months (46). With the remarkable success of immunotherapy clinical trials, numerous studies have investigated the use of immunotherapy in advanced ESCC. Notably, several phase III trials, including ESCORT-1 (47, 48), have successfully established PD-1 inhibitors as key treatments for advanced ESCC.
This study conducted a statistical analysis of AEs and Grade ≥ 3 AEs, with no new safety events identified. The occurrence of AEs with different PD-1 inhibitors was consistent with the findings of Wang’s meta-analysis (49). However, unlike Wang’s study, our analysis provides additional data on the specific probabilities of adverse events associated with each PD-1 inhibitor.
Notably, compared to chemotherapy, PD-1 inhibitors significantly improved OS and ORR in second-line treatment for ESCC, although the difference in PFS was less pronounced. This may be attributed to the longer duration of immunotherapy.
Challenges in early diagnosis and accurate prognosis for ESCC patients have hindered effective treatment of this disease. Identifying biomarkers for clinical diagnosis and prognosis of ESCC remains an urgent priority in this field (50, 51). PD-L1 expression levels are used as a biomarker to predict the clinical efficacy of immunotherapy, a meta-analysis indicated an association between PD-L1 expression and OS in ESCC (52). The meta-analyses by Wang (53) and Zhu (28) focused solely on comparing the OS benefits of individual PD-1 inhibitors between patients with high and low PD-L1 expression and chemotherapy. However, they did not conduct an in-depth analysis of PD-1 inhibitor treatment in PD-L1-positive advanced ESCC patients. This study stratified patients based on PD-L1 expression levels and found that, except for the Sintilimab group, advanced ESCC patients with PD-L1 ≥10% showed greater OS benefits compared to those without PD-L1 expression selection. Additionally, the OS benefits of different PD-1 inhibitors were compared between the PD-L1 ≥10% and PD-L1 <10% subgroups, identifying the optimal immunotherapy regimens for each PD-L1 expression subgroup.
A comprehensive meta-analysis of the most robust randomized controlled trial data to evaluate the efficacy and safety of second-line PD-1 inhibitors, stratified by PD-L1 expression levels, offering valuable insights for clinical decision-making. Considering both clinical efficacy and safety, Tislelizumab was identified as a promising second-line immunotherapy option for advanced ESCC patients without selected PD-L1 expression. Additionally, the findings show that patients with PD-L1 ≥ 10% experience improved survival outcomes with Tislelizumab. This could contribute to refining clinical guidelines regarding the selection of PD-1 inhibitors for second-line immunotherapy in advanced ESCC patients. Specifically, for advanced ESCC patients with PD-L1 expression levels ≥10%, we recommend considering Tislelizumab as the preferred treatment option.
While this study draws several important conclusions, it also has the following limitations. First, PD-L1 expression is anticipated to serve as a crucial biomarker in the development of immune checkpoint inhibitors, aiding in the precise selection of treatment strategies and providing prognostic information (54). However, there are significant differences in how PD-L1 is evaluated across various PD-1 inhibitors. For instance, Pembrolizumab and Nivolumab use the Combined Positive Score (CPS) to assess PD-L1 expression in advanced ESCC patients, while Camrelizumab uses the Tumor Proportion Score (TPS). In March 2024, Tislelizumab was approved by the FDA for the treatment of advanced ESCC and employs the Tumor Area Positive Score (TAP) to evaluate PD-L1 expression in these patients. The differences in PD-L1 expression can be attributed to variations in detection methods, resulting in discrepancies in PD-L1 staining outcomes. Additionally, PD-L1 expression may fluctuate over time and across tumor sites, further influencing evaluators’ assessments and contributing to intergroup heterogeneity in PD-L1 expression. Additionally, the clinical utility of biomarkers such as CPS and TPS remains uncertain, which may introduce bias when comparing PD-L1 expression outcomes across different PD-1 inhibitor trials in the second-line treatment of locally advanced ESCC. Among the five PD-1 inhibitors, only Camrelizumab and Nivolumab explored PD-L1 expression levels beyond the PD-L1 ≥10% and PD-L1 <10% subgroups, including PD-L1 ≥1%, PD-L1 <1%, PD-L1 ≥5%, and PD-L1 <5%. Consequently, our analysis of OS outcomes in patients with different PD-L1 expression levels is limited to the PD-L1 ≥10% and PD-L1 <10% subgroups. This limitation may also affect the evaluation of different PD-1 inhibitors in treating advanced ESCC patients with varying PD-L1 expression levels.
Second, ESCC was found to exhibits significant regional heterogeneity, with notable biological and clinical differences between Eastern and Western patients, which presents challenges for clinical research (55). For instance, the KEYNOTE-181 trial conducted a subgroup analysis of advanced ESCC patients, dividing them into an Asian subgroup and a Western subgroup. The analysis revealed that the Asian subgroup experienced greater benefits, suggesting that patients from different regions may derive varying levels of benefit. Consequently, the applicability of these findings to other ethnic groups remains uncertain and warrants further discussion. This regional heterogeneity may also influence our analysis of the benefits of PD-1 inhibitors in the second-line treatment of advanced ESCC.
Third, immune checkpoint inhibitor monotherapy was observed to be better tolerated, with a lower incidence of AEs and significantly fewer Grade ≥ 3 AEs compared to chemotherapy (27, 53). However, due to their distinct mechanism of action,immune checkpoint inhibitors are associated with specific adverse events, predominantly affecting the endocrine system, gastrointestinal tract, lungs, and skin (56, 57). It is important to note that serious immune-related adverse events (IRAEs) may develop in a small subset of patients, and these reactions can lead to severe outcomes, such as high mortality rates from cardiovascular complications induced by immune checkpoint inhibitor therapy. Unfortunately, most studies involving immune checkpoint inhibitors do not clearly differentiate between IRAEs and common AEs. This limits our ability to comprehensively assess the safety profile of immune checkpoint inhibitors in the treatment of advanced ESCC.
Fourth, as our NMA included only five RCTs with a relatively small number of trials and participants, this may impact the validity of the Bayesian analysis results. Additionally, since the ROB 2.0 tool lacks specific evaluation criteria related to data bias, we remain concerned about the reliability of the bias assessment outcomes (58). In the future, more refined literature evaluation tools will be needed to study the efficacy and safety of PD-1 inhibitors in treating advanced ESCC patients, providing better guidance for clinical decision-making.
Fifth, as second-line treatment with PD-1 inhibitors exclusively involves single-agent regimens, our discussion focuses solely on the efficacy and safety of single-agent PD-1 inhibitors for ESCC as second-line therapy, without addressing combination immunotherapy. While PD-1 inhibitors demonstrate better efficacy and safety compared to chemotherapy, they still have limitations, including immune-related adverse events and poor tumor tissue penetration. Future efforts could focus on developing PD-L1 small-molecule drugs to reduce attacks on normal cells and minimize immune-related adverse events. Alternatively, combining PD-1 inhibitors with targeted therapies could enhance drug delivery directly to tumor tissues, achieving more effective treatment outcomes (53).
Despite these limitations, this study offers a thorough summary of randomized controlled trials evaluating second-line immunotherapy for patients with advanced ESCC. This also provides a valuable reference for the treatment of advanced ESCC patients with varying PD-L1 expression levels using PD-1 inhibitors.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
FY: Conceptualization, Data curation, Investigation, Methodology, Writing – original draft. MD: Conceptualization, Data curation, Formal Analysis, Writing – original draft. JS: Data curation, Funding acquisition, Software, Writing – original draft. LF: Formal Analysis, Supervision, Writing – review & editing. HZ: Methodology, Software, Writing – review & editing. TJ: Data curation, Writing – review & editing. KL: Methodology, Writing – review & editing. YW: Software, Writing – review & editing. JX: Formal Analysis, Writing – review & editing. ZY: Data curation, Investigation, Methodology, Software, Supervision, Writing – review & editing. WC: Conceptualization, Data curation, Formal Analysis, Methodology, Resources, Software, Writing – review & editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Our gratitude extends to the research team for their daily contributions to designing the study, collecting data, analyzing data, interpreting results, and writing the manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declare that no Generative AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2024.1510145/full#supplementary-material
1. Bray F, Laversanne M, Sung H, Ferlay J, Siegel RL, Soerjomataram I, et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: A Cancer J Clin. (2024) 74:229–63. doi: 10.3322/caac.21834
2. Nathaniel D, Christopher MJ, Kohei Y, Jaffer AA, Wayne LH. Advances in diagnosis and management of cancer of the esophagus. BMJ. (2024) 385. doi: 10.1136/bmj-2023-074962
3. Abnet CC, Arnold M, Wei W-Q. Epidemiology of esophageal squamous cell carcinoma. Gastroenterology. (2018) 154:360–73. doi: 10.1053/j.gastro.2017.08.023
4. Zhou M, Wang H, Zeng X, Yin P, Zhu J, Chen W, et al. Mortality, morbidity, and risk factors in China and its provinces, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. (2019) 394:1145–58. doi: 10.1016/S0140-6736(19)30427-1
5. Testa U, Castelli G, Pelosi E. The molecular characterization of genetic abnormalities in esophageal squamous cell carcinoma may foster the development of targeted therapies. Curr Oncol. (2023) 30:610–40. doi: 10.3390/curroncol30010048
6. Zhang Y, Li Z, Huang Y, Xu Y, Zou B. Advancements in immunotherapy for advanced esophageal squamous cell carcinoma: a comprehensive review of current strategies and future directions. Expert Rev Clin Immunol. (2024) 20:971–84. doi: 10.1080/1744666X.2024.2368194
7. Ku GY. Systemic therapy for esophageal cancer: chemotherapy. Chin Clin Oncol. (2017) 6:49–9. doi: 10.21037/cco.2017.07.06
8. Njei B, McCarty TR, Birk JW. Trends in esophageal cancer survival in United States adults from 1973 to 2009: A SEER database analysis. J Gastroenterol Hepatology. (2016) 31:1141–6. doi: 10.1111/jgh.2016.31.issue-6
9. van Kleef JJ, ter Veer E, van den Boorn HG, Schokker S, Ngai LL, Prins MJ, et al. Quality of life during palliative systemic therapy for esophagogastric cancer: systematic review and meta-analysis. JNCI: J Natl Cancer Institute. (2019) 11:0. doi: 10.1093/jnci/djz133
10. Muro K, Hamaguchi T, Ohtsu A, Boku N, Chin K, Hyodo I, et al. A phase II study of single-agent docetaxel in patients with metastatic esophageal cancer. Ann Oncol. (2004) 15:955–9. doi: 10.1093/annonc/mdh231
11. Ford HER, Marshall A, Bridgewater JA, Janowitz T, Coxon FY, Wadsley J, et al. Docetaxel versus active symptom control for refractory oesophagogastric adenocarcinoma (COUGAR-02): an open-label, phase 3 randomised controlled trial. Lancet Oncol. (2014) 15:78–86. doi: 10.1016/S1470-2045(13)70549-7
12. Cortinovis D, Abbate M, Bidoli P, Capici S, Canova S. Targeted therapies and immunotherapy in non-small-cell lung cancer. ecancermedicalscience. (2016) 10:648. doi: 10.3332/ecancer.2016.648
13. Dmitriy S, Anish T, Beverly A. Teicher. Cancer treatments: Past, present, and future. Cancer Genetics. (2024) 286–287. doi: 10.1016/j.cancergen.2024.06.002
14. Kitagawa Y, Ishihara R, Ishikawa H, Ito Y, Oyama T, Oyama T, et al. Esophageal cancer practice guidelines 2022 edited by the Japan esophageal society: part 1. Esophagus. (2023) 20:343–72. doi: 10.1007/s10388-023-00993-2
15. Zhang J, Dang F, Ren J, Wei W. Biochemical aspects of PD-L1 regulation in cancer immunotherapy. Trends Biochem Sci. (2018) 43:1014–32. doi: 10.1016/j.tibs.2018.09.004
16. Boussiotis VA, Longo DL. Molecular and biochemical aspects of the PD-1 checkpoint pathway. New Engl J Med. (2016) 375:1767–78. doi: 10.1056/NEJMra1514296
17. Liao G, Dai N, Xiong T, Wang L, Diao X, Xu Z, et al. Single-cell transcriptomics provides insights into the origin and microenvironment of human oesophageal high-grade intraepithelial neoplasia. Clin Trans Med. (2022) 12:5. doi: 10.1002/ctm2.v12.5
18. Liu J, Chen Z, Li Y, Zhao W, Wu J, Zhang Z. PD-1/PD-L1 checkpoint inhibitors in tumor immunotherapy. Front Pharmacol. (2021) 12doi: 10.3389/fphar.2021.731798
19. Kato K, Cho BC, Takahashi M, Okada M, Lin C-Y, Chin K, et al. Nivolumab versus chemotherapy in patients with advanced oesophageal squamous cell carcinoma refractory or intolerant to previous chemotherapy (ATTRACTION-3): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. (2019) 20:1506–17. doi: 10.1016/S1470-2045(19)30626-6
20. Kojima T, Shah MA, Muro K, Francois E, Adenis A, Hsu C-H, et al. Randomized phase III KEYNOTE-181 study of pembrolizumab versus chemotherapy in advanced esophageal cancer. J Clin Oncol. (2020) 38:4138–48. doi: 10.1200/JCO.20.01888
21. Huang J, Xu J, Chen Y, Zhuang W, Zhang Y, Chen Z, et al. Camrelizumab versus investigator’s choice of chemotherapy as second-line therapy for advanced or metastatic oesophageal squamous cell carcinoma (ESCORT): a multicentre, randomised, open-label, phase 3 study. Lancet Oncol. (2020) 21:832–42. doi: 10.1016/S1470-2045(20)30110-8
22. Shen L, Kato K, Kim S-B, Ajani JA, Zhao K, He Z, et al. Tislelizumab versus chemotherapy as second-line treatment for advanced or metastatic esophageal squamous cell carcinoma (RATIONALE-302): A randomized phase III study. J Clin Oncol. (2022) 40:3065–76. doi: 10.1200/JCO.21.01926
23. Ajani J, El Hajbi F, Cunningham D, Alsina M, Thuss-Patience P, Scagliotti GV, et al. Tislelizumab versus chemotherapy as second-line treatment for European and North American patients with advanced or metastatic esophageal squamous cell carcinoma: a subgroup analysis of the randomized phase III RATIONALE-302 study. ESMO Open. (2024) 9:1. doi: 10.1016/j.esmoop.2023.102202
24. Xu J, Li Y, Fan Q, Shu Y, Yang L, Cui T, et al. Clinical and biomarker analyses of sintilimab versus chemotherapy as second-line therapy for advanced or metastatic esophageal squamous cell carcinoma: a randomized, open-label phase 2 study (ORIENT-2). Nat Commun. (2022) 13:1. doi: 10.1038/s41467-022-28408-3
25. Liu S, Zhao L, Shi F, Kuai L, Liu R, Tang J. Cost-effectiveness analysis of PD-1 inhibitors as second-line therapy for advanced or metastatic esophageal squamous cell carcinoma in China: an economic evaluation based on network meta-analysis. Int J Clin Pharmacy. (2024) 46:675–83. doi: 10.1007/s11096-023-01696-5
26. Yang X, Zheng X, Hu S, Huang J, Zhang M, Huang P, et al. Immune checkpoint inhibitors as the second-line treatment for advanced esophageal squamous cell carcinoma: a cost-effectiveness analysis based on network meta-analysis. BMC Cancer. (2024) 24:1. doi: 10.1186/s12885-024-12423-2
27. Zhu X, Shanzhou Q, Li D, Pang X, Ma D. PD-1 inhibitors versus chemotherapy as second-line treatment for advanced esophageal squamous cell carcinoma: a meta-analysis. BMC Cancer. (2021) 21:1. doi: 10.1186/s12885-021-08958-3
28. Zhu K, Chen H, Xu C, Chen D, Jin Z, Ren S, et al. Efficacy and safety of immune checkpoint inhibitors versus chemotherapy in the second-line treatment of advanced esophageal squamous cell carcinoma: a meta-analysis and systematic review. J Thorac Disease. (2023) 15:1186–95. doi: 10.21037/jtd-22-1169
29. Doosti-Irani A, Mansournia MA, Cheraghi Z, Rahimi-Foroushani A, Haddad P, Holakouie-Naieni K. Network meta-analysis of palliative treatments in patients with esophageal cancer. Crit Rev Oncology/Hematology. (2021) 168. doi: 10.1016/j.critrevonc.2021.103506
30. Hutton B, Salanti G, Caldwell DM, Chaimani A, Schmid CH, Cameron C, et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann Internal Med. (2015) 162:777–84. doi: 10.7326/M14-2385
31. Sadeghirad B, Foroutan F, Zoratti MJ, Busse JW, Brignardello-Petersen R, Guyatt G, et al. Theory and practice of Bayesian and frequentist frameworks for network meta-analysis. BMJ Evidence-Based Med. (2023) 28:204–9. doi: 10.1136/bmjebm-2022-111928
32. Rethlefsen ML, Kirtley S, Waffenschmidt S, Ayala AP, Moher D, Page MJ, et al. PRISMA-S: an extension to the PRISMA statement for reporting literature searches in systematic reviews. Systematic Rev. (2021) 10:1. doi: 10.1186/13643-020-01542-z
33. Lunny C, Veroniki AA, Hutton B, White I, Higgins JPT, Wright JM, et al. Knowledge user survey and Delphi process to inform development of a new risk of bias tool to assess systematic reviews with network meta-analysis (RoB NMA tool). BMJ Evidence-Based Med. (2023) 28:58–67. doi: 10.1136/bmjebm-2022-111944
34. Higgins JPT, Altman DG, Gotzsche PC, Juni P, Moher D, Oxman AD, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. Bmj. (2011) 343:d5928–d. doi: 10.1136/bmj.d5928
35. Dias S, Sutton AJ, Ades AE, Welton NJ. Evidence synthesis for decision making 2. Med Decision Making. (2012) 33:607–17. doi: 10.1177/0272989X12458724
36. van Valkenhoef G, Lu G, de Brock B, Hillege H, Ades AE, Welton NJ. Automating network meta-analysis. Res Synthesis Methods. (2012) 3:285–99. doi: 10.1002/jrsm.1054
37. Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. (2003) 327:7414. doi: 10.1136/bmj.327.7414.557
38. Baba Y, Nomoto D, Okadome K, Ishimoto T, Iwatsuki M, Miyamoto Y, et al. Tumor immune microenvironment and immune checkpoint inhibitors in esophageal squamous cell carcinoma. Cancer Science. (2020) 111:3132–41. doi: 10.1111/cas.v111.9
39. Janjua S, Mathioudakis AG, Fortescue R, Walker RAE, Sharif S, Threapleton CJD, et al. Prophylactic antibiotics for adults with chronic obstructive pulmonary disease: a network meta-analysis. Cochrane Database Systematic Rev. (2021) 1:1. doi: 10.1002/14651858.CD013198.pub2
40. Janjigian YY, Bendell J, Calvo E, Kim JW, Ascierto PA, Sharma P, et al. CheckMate-032 study: efficacy and safety of nivolumab and nivolumab plus ipilimumab in patients with metastatic esophagogastric cancer. J Clin Oncol. (2018) 36:2836–44. doi: 10.1200/JCO.2017.76.6212
41. Joanna DK. The role of the immune system in non-small cell lung carcinoma and potential for therapeutic intervention. Transl Lung Cancer Res. (2015) 4:0. doi: 10.3978/j.issn.2218-6751.2015.01.11
42. Sharpe AH, Pauken KE. The diverse functions of the PD1 inhibitory pathway. Nat Rev Immunol. (2017) 18:153–67. doi: 10.1038/nri.2017.108
43. Huang J, Xu B, Mo H, Zhang W, Chen X, Wu D, et al. Safety, activity, and biomarkers of SHR-1210, an anti-PD-1 antibody, for patients with advanced esophageal carcinoma. Clin Cancer Res. (2018) 24:1296–304. doi: 10.1158/1078-0432.CCR-17-2439
44. Wong DYQ, Ong WWF, Ang WH. Induction of immunogenic cell death by chemotherapeutic platinum complexes. Angewandte Chemie Int Edition. (2015) 54:6483–7. doi: 10.1002/anie.201500934
45. Demaria S, Guha C, Schoenfeld J, Morris Z, Monjazeb A, Sikora A, et al. Radiation dose and fraction in immunotherapy: one-size regimen does not fit all settings, so how does one choose. J ImmunoTherapy Cancer. (2021) 9:4. doi: 10.1136/jitc-2020-002038
46. Puhr HC, Prager GW, Ilhan-Mutlu A. How we treat esophageal squamous cell carcinoma. ESMO Open. (2023) 8:1. doi: 10.1016/j.esmoop.2023.100789
47. Song Y, Zhang B, Xin D, Kou X, Tan Z, Zhang S, et al. First-line serplulimab or placebo plus chemotherapy in PD-L1-positive esophageal squamous cell carcinoma: a randomized, double-blind phase 3 trial. Nat Med. (2023) 29:473–82. doi: 10.1038/s41591-022-02179-2
48. Luo H, Lu J, Bai Y, Mao T, Wang J, Fan Q, et al. Effect of camrelizumab vs placebo added to chemotherapy on survival and progression-free survival in patients with advanced or metastatic esophageal squamous cell carcinoma. JAMA. (2021) 326:10. doi: 10.1001/jama.2021.12836
49. Wang R, Liu S, Chen B, Xi M. Recent advances in combination of immunotherapy and chemoradiotherapy for locally advanced esophageal squamous cell carcinoma. Cancers. (2022) 14:20. doi: 10.3390/cancers14205168
50. Li Y, Liu H. Clinical powers of Aminoacyl tRNA Synthetase Complex Interacting Multifunctional Protein 1 (AIMP1) for head-neck squamous cell carcinoma. Cancer Biomarkers. (2022) 34:359–74. doi: 10.3233/CBM-210340
51. Liu H, Li Y. Potential roles of Cornichon Family AMPA Receptor Auxiliary Protein 4 (CNIH4) in head and neck squamous cell carcinoma. Cancer Biomarkers. (2022) 35:439–50. doi: 10.3233/CBM-220143
52. Liu Z, Sun L, Cai L, Guo M, Xu G, Liu S, et al. Clinicopathological and prognostic values of PD-L1 expression in oesophageal squamous cell carcinoma: a meta-analysis of 31 studies with 5368 patients. Postgraduate Med J. (1166) 2022:948–57:98. doi: 10.1136/postgradmedj-2021-140029
53. Wang Y, Yang Q, Liu J, Shen X, Tai G, Gu H, et al. Comprehensive curative effect of targeting PD-1 or traditional single-agent chemotherapy in second-line therapy for terminal or metastatic esophageal cancer: A systematic review and meta-analysis. J Oncol. (2022) 4033863:1–12. doi: 10.1155/2022/4033863
54. Nagata Y, Yamamoto S, Kato K. Immune checkpoint inhibitors in esophageal cancer: Clinical development and perspectives. Hum Vaccines Immunotherapeutics. (2022) 18:6. doi: 10.1080/21645515.2022.2143177
55. Sun M. Advancements in the research of immune checkpoint inhibitors for the treatment of advanced esophageal squamous cell carcinoma. Am J Cancer Res. (2024) 14:1981–98. doi: 10.62347/XUWC6412
56. Khoja L, Day D, Wei-Wu Chen T, Siu LL, Hansen AR. Tumour- and class-specific patterns of immune-related adverse events of immune checkpoint inhibitors: a systematic review. Ann Oncol. (2017) 28:2377–85. doi: 10.1093/annonc/mdx286
57. Wang Y, Zhou S, Yang F, Qi X, Wang X, Guan X, et al. Treatment-related adverse events of PD-1 and PD-L1 inhibitors in clinical trials. JAMA Oncol. (2019) 5:7. doi: 10.1001/jamaoncol.2019.0393
58. Gao Z, Huang S, Wang S, Tang D, Xu W, Zeng R, et al. Efficacy and safety of immunochemotherapy, immunotherapy, chemotherapy, and targeted therapy as first-line treatment for advanced and metastatic esophageal cancer: a systematic review and network meta-analysis. Lancet Regional Health - Western Pacific. (2023) 38. doi: 10.1016/j.lanwpc.2023.100841
Keywords: esophageal squamous cell carcinoma (ESCC), immune checkpoint inhibitors (ICIs), network meta-analysis, efficacy, safety
Citation: Yang F, Dan M, Shi J, Fan L, Zhang H, Jian T, Lei K, Wang Y, Xin J, Yu Z and Chen W (2025) Efficacy and safety of PD-1 inhibitors as second-line treatment for advanced squamous esophageal cancer: a systematic review and network meta-analysis with a focus on PD-L1 expression levels. Front. Immunol. 15:1510145. doi: 10.3389/fimmu.2024.1510145
Received: 12 October 2024; Accepted: 27 December 2024;
Published: 23 January 2025.
Edited by:
Hailin Tang, Sun Yat-sen University Cancer Center (SYSUCC), ChinaReviewed by:
Hengrui Liu, University of Cambridge, United KingdomCopyright © 2025 Yang, Dan, Shi, Fan, Zhang, Jian, Lei, Wang, Xin, Yu and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhigang Yu, bWFvbWFvY2FvMjAxMkAxNjMuY29t; Wei Chen, Y3dqenp5eUAxNjMuY29t
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.