- 1Dermatologic Unit, Department of Systems Medicine, University of Rome Tor Vergata, Rome, Italy
- 2Department of Experimental Medicine, University of Rome “Tor Vergata”, Rome, Italy
- 3Genomic Medicine Laboratory UILDM, IRCCS Santa Lucia Foundation, Rome, Italy
- 4Department of Biostatistics, Tor Vergata University of Rome, Rome, Italy
Introduction: Fungi, including Candida, may be a trigger or exacerbate psoriasis, especially in difficult to treat (DTT) areas, through the activation of IL-17/23 axis.
Methods: In this study, seventy patients with DDT psoriasis were enrolled to evaluate Candida species and/or other opportunistic fungi colonization rate at baseline (T0) and the impact of apremilast on fungal load, clinical outcome, serum cytokine levels and biochemical serum profile of patients after 16, 24 and 52 weeks of treatment.
Results: In our population, 33 (47%) patients were colonized by Candida spp. at baseline. In 24 (34%) individuals Candida was detected in the oral cavity while in the remaining 9 (13%) individuals the fungus was isolated from stool samples. Twenty subjects were colonized by only the species C. albicans, whereas in the remaining 13 a combination of two or more species (C. albicans plus non-albicans strains) was found in the oral cavity. Moreover, 27 (39%) patients were affected by onychomycosis. At 52 weeks, apremilast treatment induced a full recovery from Candida colonization in 83% of patients colonized with a single species of Candida (C. albicans); while in those co-infected by two or more Candida spp. induced a significant reduction (colony counts >10 CFU/mL) in fungal load was observed in comparison to baseline. Among patients with onychomycosis, 78% (21/27) of them presented a complete clinical resolution of nail psoriasis and concomitant nail infections. Finally, improvements in clinical scores i.e., PASI, NAPSI, DLQI, itch VAS, PAIN VAS, scPGA and sPGA-G and biochemical serum profile, as well as a significant decrease in serum IL-17A, TGF-β 1 and IL-10 levels (from 8.51 to 4.16 pg/mL; from 66.10 to 48.70 ng/mL and from 20.05 to 14 pg/mL, respectively) were observed in all patients.
Conclusions: Fungi may play a role in the psoriasis pathogenesis. Apremilast has been shown to ameliorate psoriasis signs and symptoms and counteract fungal overgrowth, probably by dampening inflammation, triggered by the fungal infections themselves. Thus, apremilast may represent an effective therapeutic approach in the treatment of DTT psoriasis and modulate the fungal colonization.
1 Introduction
Psoriasis (PsO) is a complex chronic, autoimmune-inflammatory disorder, affecting approximately 2% of the general population, mainly involving the skin, although it is considered a systemic disease, which can be coupled with psoriatic arthritis (PsA) (1–3). The trunk and extremities are the most commonly affected areas in plaque psoriasis, but nails, scalp and intertriginous areas, including groins, abdominal skin folds, inframammary folds, and interdigital spaces can be involved (4). The involvement of these areas has a significant impact on quality of life (QoL) with severe negative physical, psychological and psychosocial effects for psoriatic patients (5). Psoriasis that affects these areas of the skin is often referred to as “difficult-to-treat” (6) and it is usually more resistant to traditional therapies than other psoriatic clinical variants, and normally these clinical features are present simultaneously (6–8). Dysregulation of the immune system, as well as abnormal keratinocyte proliferation and differentiation, seem to play an important role in PsO pathogenesis (9). In terms of the implications for patients and clinicians, PsO is associated with an increased risk of serious infections, especially respiratory and soft‐tissue/skin infections, underlining the burden of infective comorbidities in psoriatic patients (10). Moreover, several evidence have indicated that infections are an environmental trigger for PsO and may play multiple roles in its maintenance, as evidenced by the frequent association between guttate psoriasis onset and acute streptococcal infection (11). Various bacterial, viral and fungal pathogens may play a role as superantigens that trigger the immune cells to produce inflammatory cytokines, leading to the onset or exacerbation of psoriasis (10). Moreover, over the past several years, many studies confirmed the association between gut and/or skin dysbiosis and psoriasis (12–14). In this regard, increasing evidence suggests that gut microbiota plays a central role in the maintenance of host immune homeostasis. Alterations in microbiota composition and diversity have been proposed to contribute to PsO pathogenicity in a susceptible host with sub-clinical inflammation by triggering an exaggerated immune response (15–21). Notably, Candida species overgrowth in gut dysbiosis may play an important role in the pathogenesis of psoriasis. Several molecular pathways associated with psoriasis and other inflammatory diseases, such as the interleukin (IL)-23/IL-17 axis, are also involved in host defense mechanisms against fungal pathogens (22). The Interleukin-17 family plays a key role in host defenses against certain pathogenic fungi including Candida. Elevated serum levels of the proinflammatory IL-17 have been documented in psoriatic patients and a positive correlation was found between serum concentration of IL-17 and psoriasis severity, suggesting its implication in psoriasis pathogenicity (23). It is assumed that high colonization level of C. albicans in the gut leads to the release of Candida superantigens, contributing to non-specific T-cell activation and excessive production of pro-inflammatory cytokines, which can act as trigger factors of psoriatic disease (24). This may create a “vicious cycle” in which inflammation promotes Candida colonization, and fungal colonization further improves inflammation. Significant levels of C. albicans detected in saliva and stool samples of psoriatic patients, reinforce the hypothesis that C. albicans may trigger both exacerbation and persistence of psoriasis (15). Even though increasing evidence suggests the implication of Candida in the complex pathophysiology of psoriasis, there is no knowledge of the prevalence of Candida in patients with concomitant nail, scalp and inverse psoriasis. Further, the association between Candida colonization rates and psoriasis severity still remains controversial (25, 26). Additionally, despite significant advances in knowledge of psoriasis pathogenesis have been made over the years, there is not yet a complete recovery of psoriasis, especially in DTT psoriasis and multifailure patients (27). A major challenge addresses DTT areas, especially in those individuals with the absence of significant body surface involvement (26). Moreover, recent data indicate that psoriasis is associated with an increased risk of cardiovascular disease and related comorbidities such as diabetes mellitus, metabolic syndrome, dyslipidaemia, and obesity (28). The common factor is a low-grade chronic inflammation and the increases in systemic oxidation that favor a vicious circle. Although psoriasis is considered a systemic disease (3), topical treatments are commonly used to treat mild-to-moderate psoriasis and have shown efficacy and tolerability in randomized clinical trials (29, 30). However, recent guidelines suggest systemic treatments for patients with mild-to-moderate skin involvement who experience high disease burden or inadequate disease control with topical therapies, as well as with PsO in special areas (31). On the other hand, topical corticosteroid can cause contact allergy, especially in sensible body area, and favor an increase in fungal infections as mucocutaneous candidiasis, when applied on genital area (32–34). Among systemic therapies, IL-17 inhibitors, have shown therapeutic benefits in treating moderate/severe plaque psoriasis (7, 35). Notably, ixekizumab, an inhibitor of interleukin-17A, has shown to be effective and safe in the treatment of moderate-to-severe genital PsO (7, 35). Likewise genital and scalp PsO is a major issue to be researched, as it is not only a physical burden, but at the same time inflicts significant psychological stress on the patient, disproportional to the body area affected (36). Nevertheless, due to the protective role of IL-17 against fungal infections, these drugs have been associated with an increased risk of mucocutaneous candidiasis in PsO patients (37–39). Besides IL-17 inhibitors, recent reports documented the efficacy of apremilast, a selective phosphodiesterase 4 (PDE4) inhibitor, in treating psoriasis (40, 41). In 2023, a double-blinded placebo-controlled trial demonstrated the effectiveness of apremilast in the treatment of genital PsO (42), other than special areas, such as scalp, nails, and palmoplantar areas (43). Based on this evidence, the present study aimed at evaluating: i) the prevalence of Candida species and other opportunistic fungi in psoriatic patients with DTT and the impact of apremilast on Candida colonization rates; ii) the clinical efficacy of apremilast in the study population iii) the effect of apremilast on serum cytokine and biochemical profiles to provide new insights into the beneficial effects of this drug in psoriatic disease.
2 Material and methods
2.1 Patient enrolment and study design
A pivotal, prospective, single-center study was performed to evaluate the prevalence of fungal colonization in DTT psoriatic patients treated with apremilast (30 mg bid, orally). Seventy patients were enrolled in this study and followed for 52 weeks. Candida colonization rates were documented by cultural examination and expressed as colony-forming units (CFU). Patients with active fungal infection (regardless of baseline presence or development during the study) were not excluded but received an antifungal treatment as in common clinical practice. We regularly follow international guidelines, which include the following treatments: for mild disease, clotrimazole, ciclopirox or miconazole; for moderate to severe disease, oral fluconazole (20). Psoriasis Area Severity Index (PASI), Nail Psoriasis Severity Index (NAPSI), Pain VAS, Itch VAS, Dermatology Life Quality Index (DLQI), and Scalp Physician Global Assessment (scPGA) and Genital PGA (sPGA-G) scores were collected for each patient at baseline (T0), 16, 24 and 52-weeks (W). Moreover, all the patients were evaluated for serum cytokine levels, as described below. This study was conducted following the Declaration of Helsinki and approved by the Institutional Review Board’s (IRB) Independent Ethical Committee Tor Vergata University Hospital (R.S. 133.19).
2.2 Inclusion and exclusion criteria
Inclusion criteria comprised patients who have undergone cultural examination for fungal infections within the last 15 days before the enrolment; patients ≥18 years of age and affected by psoriasis with concomitant nail, scalp and intertriginous disease; patients who were consecutively enrolled after having received apremilast, and accordingly had to meet all the requested conditions to be treated with apremilast; signed and dated written informed consent; appropriate wash out from previous systemic and biological therapies from at least 3 months for psoriasis and fungal infections. Exclusion criteria comprised pregnancy, breast-feeding, or female who planned to become pregnant while in the trial; underage; galactose-intolerant; diagnosed anorexia; currently enrolled in another investigational device or drug study, or less than 30 days since ending another investigational device or drug study(s) or receiving other investigational treatment; previous treatment with IL-17 inhibitors.
2.3 Primary objective
The primary objective of this study was to investigate the skin, oral mucosa and gut Candida spp. prevalence in patients affected by nail, scalp and inverse psoriasis at baseline (T0) and the effect of apremilast on Candida colonization at 16 (16W), 24 (24W) and 52 (52W) weeks of treatment.
2.4 Secondary objectives
The secondary objective was to evaluate the efficacy, safety and tolerability of apremilast in patients with simultaneous involvement of DTT areas (nail, scalp and folds) by evaluating changes in PASI, NAPSI, DLQI, itch VAS, PAIN VAS, scPGA and sPGA-G scores at week 0, 16, 24 and 52. Moreover, we explored whether Candida colonization negatively impacted the patient’s outcomes. The safety and tolerability of apremilast were evaluated throughout the study (including any adverse events (AEs), as reported by patients during follow-up examinations at 16, 24 and 52 weeks of treatment.
2.5 Tertiary objectives
The tertiary objective of this study was to investigate the impact of apremilast on serum cytokine levels (IL-17A, IL-10 and TGF-β 1) and biochemical serum profile in our population.
2.6 Sampling and isolation of Candida species and other fungi
The presence of Candida spp. in the skin folds (axilla, intergluteal, infra-mammary, and genitocrural fold), scalp, oral cavity and stools was assessed by cultural examination at baseline and 16, 24 and 52 weeks after treatment with apremilast. Swabs (eSwab™; Copan, Italy) were sampled from psoriatic lesions and the oral cavity and collected in sterile polystyrene tubes (Copan spa, Brescia, Italy). One stool sample and three nail clipping were also collected from each patient. Specifically, Candida gut colonization was achieved by diluting 100 µL of stool sample (approx. 100 µg) in 900 µL of PBS, plating 100 µL on Sabouraud Dextrose Agar (SDA) supplemented with chloramphenicol (Difco Laboratories, Detroit, MI, USA); Candida oral colonization was achieved by plating 100 µL of Amies transport liquid medium after swab vortexing on SDA with chloramphenicol (Oxoid spa, Milan, Italy). The nail fragments were plated directly into SDA with chloramphenicol and in SDA supplemented with chloramphenicol and cycloheximide (Oxoid DTM spa, Milan, Italy). The culture plates were maintained at a temperature of 25°C for a period of at least three days and inspected daily and 30 days at 28°C in case of DTM for dermatophytes. Fungi isolated from nail samples were firstly identified by macroscopic features of colonies and morphological analyses by using lactophenol blue mount. The microscopic results were confirmed by proteomic analyses by applying the Matrix-assisted laser desorption ionization time-of-flight mass spectrometry (MALDI-TOF MS) (Bruker Daltonics, MS, USA) method. Captured spectra were analyzed using the MALDI Biotyper software package (version 3.4) containing the Filamentous Fungi Library 4.0 (Bruker Daltonics, Bremen, Germany). Candida species were identified by color, texture and microscopical morphology. The fungal growth was expressed as the number of colony-forming units per millilitre (CFU/mL). All samples with a colony count of >10 CFU/mL were considered positive for Candida spp. (20). For further differentiation, yeasts were grown on the brilliance™ Candida Agar (Oxoid Chromogenic Candida Agar), a selective differential medium used for the rapid identification (within 48 hr) of the clinically important Candida species. Green colonies are interpreted as C. albicans, blue colonies are defined as C. tropicalis, and light white to purple colonies are defined as C. glabrata; purple to pink colonies are defined as C. krusei; and pale colonies are referred to C. parapsilosis.
2.7 Candida genus identification by Oxford Nanopore’s MinION sequencing device
The nucleic acids of cultured samples were extracted utilizing Molgen Universal Extraction kit (Adaltis s.r.l. via Durini, 27, 20122 Milano, Italy) following the producer protocol. Candida species were further characterized by sequencing using a CE_IVD (Microbiome plus panel long kit _ 4bases, Manno, Switzerland) kit for the molecular identification of human microbiome communities, on the Nanopore platform. This kit contains all reagents necessary for the amplification and sequencing of both bacterial and fungal genes. Only the part relating to the fungal genes (18S, ITS1, 5.8S and ITS2) was used in our case. The data obtained (Q-fast) by sequencing were analyzed using a software analysis (One Codex database).
2.8 Cytokine detection
The serum levels of the cytokines IL-17A, IL-10 and TGF-β 1 were assessed at 0, 16, 24 and 52 weeks. Blood samples were collected from patients in serum-separating tubes and centrifuged for 15 minutes at 1000 rpm/min. Then, serum samples were subdivided into small aliquots and stored at −80°C. Cytokine levels were detected using a commercial Enzyme-linked Immunosorbent Assay (ELISA) (Tecan s.r.l., Milan, Italy), according to the manufacturer’s instructions. The detection limits (picogram per milliliter) of the assays were 1.6 for IL-17A, 0.390 for IL-10 and 22.000 for TGF-β 1. All tests were conducted using a pipetting robot and an automated ELISA analyzer (ThunderBolt®, Tecan s.r.l., Milan, Italy).
2.9 Statistical analyses
All data were initially entered into an Excel database (Microsoft, Version 2406, Redmond, Washington – United States) and the analysis was performed using IBM Corp.2017. IBM SPSS Statistics for Windows, vers.25.0. Armonk, NY: IBM Corp. The descriptive statistics consisted of the mean ± standard deviation (mean ± SD) for the parameters with normal distributions (after confirmation with histograms and Kolgomorov-Smirnov test), and median and range (min.; max.) for variables with non-normal distributions, while for the occurrences or frequencies the values were expressed as a percentage (%). Error Bar graphs report data as means and 95% confidence interval (mean and 95%CI). Comparison among groups was performed with the Anova one-way, 2factor Anova or Anova for repeated measures, for normal variables or the Chi-Square test or Fisher’s exact test (if cells <5) for frequency variables. A p<0.05 was considered statistically significant (Supplementary Material).
3 Results
3.1 Demographic characteristics of enrolled patients
The clinical and demographic characteristics of the 70 patients (mean age 52.7 ± 14.8 years) recorded at baseline (T0) are shown in Table 1. In detail, 29 were females and 41 males, with 25 being smokers and 14 consumed alcohols more than 1 time a day. The mean age of disease onset was 27 years old. In the entire study population, there was a statistically significant decrease in weight during the 52 weeks of evaluation, ranging from an average value of 77.60 to 72.20 Kg, and it is completely comparable to the decrease in the average BMI in the population, from 26.02 ± 4.74 to 24.28 ± 4.75 Kg/m2. As expected between gender groups, males are statistically heavier (81.70 ± 13.80 Kg) and taller (176.60 ± 7.60 cm) (p<0.05, one-way ANOVA), while the study population resulted homogeneous for age and BMI. Patients were affected by moderate-to-severe plaque-type psoriasis with a mean PASI score of 12.17 (range 2–50), a mean DLQI score of 6.85 (range 0–30), a mean NAPSI scores of 28.02 (1-120), itch scores of 7.42 (2-10), PAIN VAS scores of 19.78 (0-100), scPGA moderate (3) 18.6% and severe (4) 2.9% and sPGA-G moderate 21.4% (3) and severe (4) 4.3%. Patients showed a body mass index (BMI) mean value of 26.02 (pre-obesity status), whereas 22 of 70 enrolled patients were overweight (BMI 26–29.99) and 6 were obese with a BMI> 30 Kg/m2 (range 30-39). Furthermore, eight patients (11.4%) had concomitant psoriatic arthritis (PsA). In addition, patient stratification was carried out for previous treatments: 15.7% (11/70) of subjects started therapy with apremilast as their first treatment; 71.43% (50/70) had previously received only topical treatment (corticosteroids) and 9 (12.9%) both topical and systemic treatments. Eleven patients had one or more systemic treatments, specifically 10% (7/70) cyclosporine, 4.3% (3/70) methotrexate and 10% (7/70) anti TNF-α. Clinical features and previous anti-psoriatic treatments are reported in Table 1. Cyclosporine administration was more used by male than female patients (4 vs 1 patients; p<0.05, Chi-Square test). Patient comorbidities were also recorded, with cardiovascular comorbidities (39%), mainly blood hypertension (35.7%), and followed by psoriatic arthritis (PsA) (11.4%), diabetes (10%), infections (11.4%), hypercholesterolemia (7.1%) and hypo-HDL-cholesterolemia (14.3%), inflammatory bowel diseases (1.5%), whereas 3.7% were affected by other pathologies. Twenty-three patients did not declare any comorbidities. Regarding the safety profile of the drug, during the 52 weeks of treatment, 21% of the patients presented diarrhea during the first two weeks of treatment with spontaneous resolution, 10% reported nausea and 9% insomnia, consistent with the known safety profile of apremilast. No serious AEs were reported. Only four of them discontinued the treatment primary due to inefficacy, while 6 were lost during follow-up. All the patients that did not complete 52 weeks of observation were excluded from the statistical analyses. Female patients dropped-out of the trial more than males (7 vs 2 patients; p<0.05; Chi-Square test). In the present study, 86% of patients reached treatment at W52.
3.2 Prevalence of Candida spp. colonization in psoriatic patients
In our population 33 out of 70 patients (47%) were colonized by Candida spp., detected in the oral cavity of 24 (34%) individuals and in stool samples of 9 (13%) individuals. The most common species was C. albicans, isolated in all 33 colonized patients. Notably, in 20 subjects only one species (C. albicans) was isolated, while in the remaining 13 a combination of two or more species (C. albicans plus non-albicans strains) was found in the oral cavity. With regard to non-albicans species, culture and molecular methods revealed that the most prevalent strains isolated from the oral cavity was C. glabrata, detected in 9/13 (69%) patients, followed by C. parapsilosis, detected in 4/13 (31%) patients. Other species, to a lesser extent, such as C. krusei, C. africana and C. auris were also found. All the data are deposited on BioProject NIH, number PRJNA1170201. A full list of the strains isolated from Candida co-infected patients is shown in Table 2. No significant Candida load (>10 CFU/mL) was found in DTT cutaneous lesions of psoriatic subjects. Moreover, the results indicated that Candida colonization was influenced by the gender as well as by comorbidities. In fact, the colonization rate in the oral cavity was higher in females than in males (19 vs 14 patients; p<0.05; one-way ANOVA) and, among females, the elderly were more infected than the younger ones (54.7 vs 45.8 years; p<0.05, 2-factors ANOVA). In addition, a high prevalence of Candida spp. was observed in patients with neoplasms (26 vs 19 patients; p<0.05; Chi-Square test), cardiovascular comorbidities and a high BMI (>25 Kg/m2) in comparison to those with no comorbidities (Supplementary Material). Interestingly, despite the high colonization rate of Candida spp. in our study population, the patients did not have any clinical signs or symptoms of oral candidiasis or even received antifungal treatments.
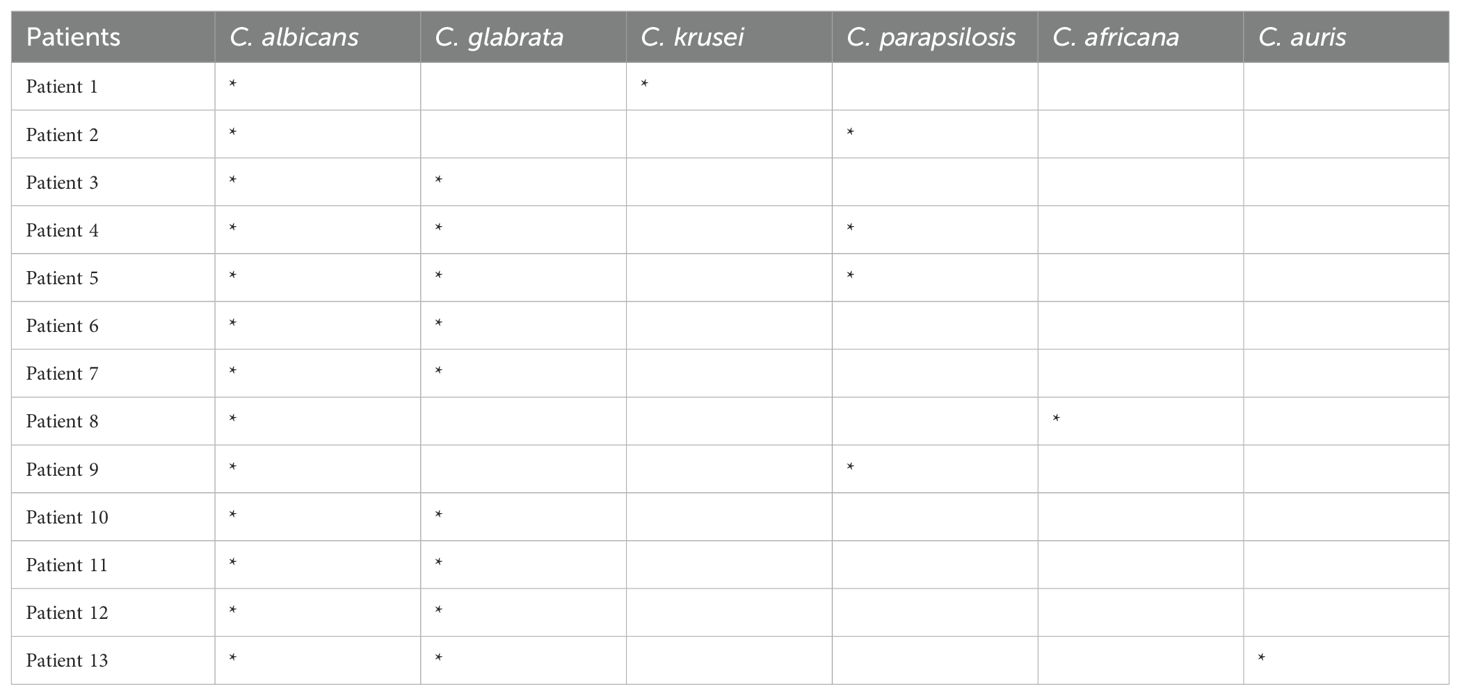
Table 2. Patients simultaneously colonized in the oral cavity by C. albicans and non-albicans Candida species.
3.3 Prevalence of onychomycosis in the study population
In this study, we also evaluated the prevalence of onychomycosis in psoriatic patients. The results show that 27 (39%) patients were affected by onychomycosis. In detail, the prevalence of dermatophytes (T. mentagrophytes complex; T. rubrum species complex; M. gypseum) and non-dermatophytic mold (NDM; Penicillium spp; Scopulariopsis brevicaulis; Aspergillus spp., Fusarium spp. Alternaria spp.) isolated from nail clinical samples was similar (44.4%), followed by yeasts, including Candida spp. and Trichosporon spp., detected in 11.2% of the samples. In Figure 1, the microscopic images of various mold isolated from nail samples of psoriatic patients at baseline have been reported.

Figure 1. Microscopic examination of positive fungal cultures from nail clipping of psoriasis patients shows the presence of (A) Aspergillus spp. (B) Microsporum canis (C) Alternaria alternata and (D) Trichophyton mentagrophytes complex. Scale bar 50 µm.
3.4 Effect of apremilast on the prevalence of Candida species and other fungal pathogens
To evaluate the impact of apremilast on the prevalence of Candida spp. in psoriasis patients, the CFU quantification from oral cavity swabs and stool samples was performed at baseline (T0), 16W, 24W and 52W. Interestingly, as reported in Figure 2A, the treatment with apremilast induced a full recovery from Candida colonization after 52 weeks in the majority of patients (83%) colonized with a single species of Candida (C. albicans); whereas in those co-infected by two or more Candida spp. apremilast failed to completely eliminate the fungus (colony counts >10 CFU/mL), although a significant reduction in Candida burden (40.52 vs 26.69 CFU/mL; one-way ANOVA; p<0.05) was observed in comparison to baseline. As shown in Figures 2B, C, apremilast exhibited higher effectiveness against C. albicans than non-albicans Candida species by inducing a reduction by 84% and 30% of Candida CFU, respectively, after 52 weeks of treatment.
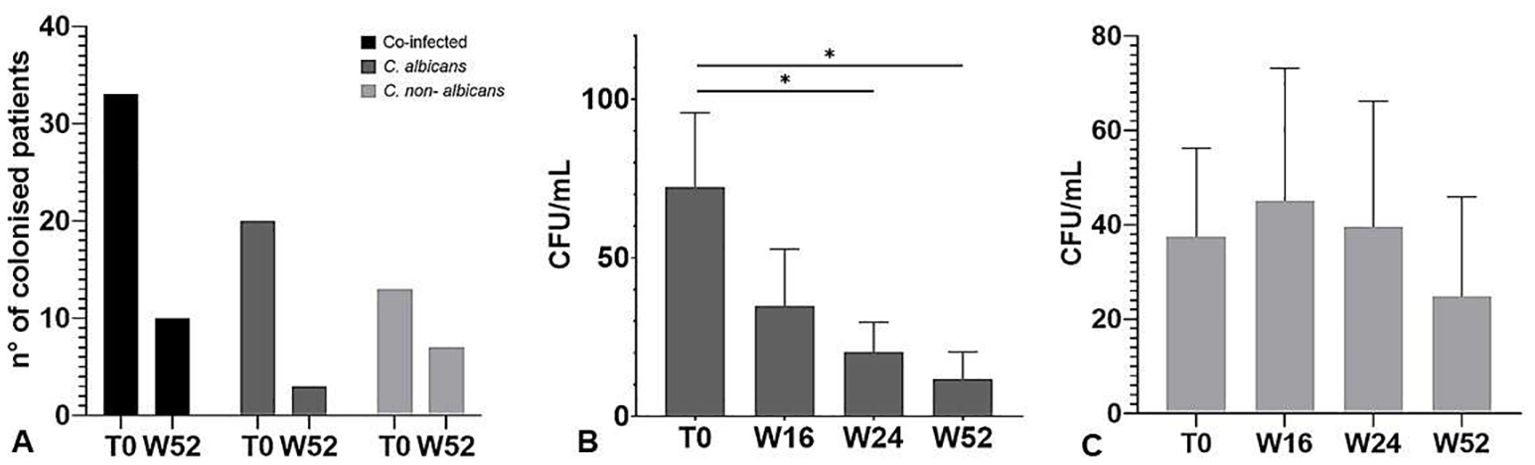
Figure 2. (A) Number of patients with positive culture for Candida species at baseline (T0) and 52 weeks of treatment. (B) CFU counts of Candida albicans and (C) non-albicans species at baseline (T0) and at all-time points considered. The graph shows the mean ± SD. *p<0.05 (One-way ANOVA).
Regarding onychomycosis, after 52 weeks of treatment, 78% (21/27) of the patients presented a complete clinical resolution of nail psoriasis and concomitant onychomycosis (Figure 3) with negative fungal cultures. The results are summarized in Table 3.
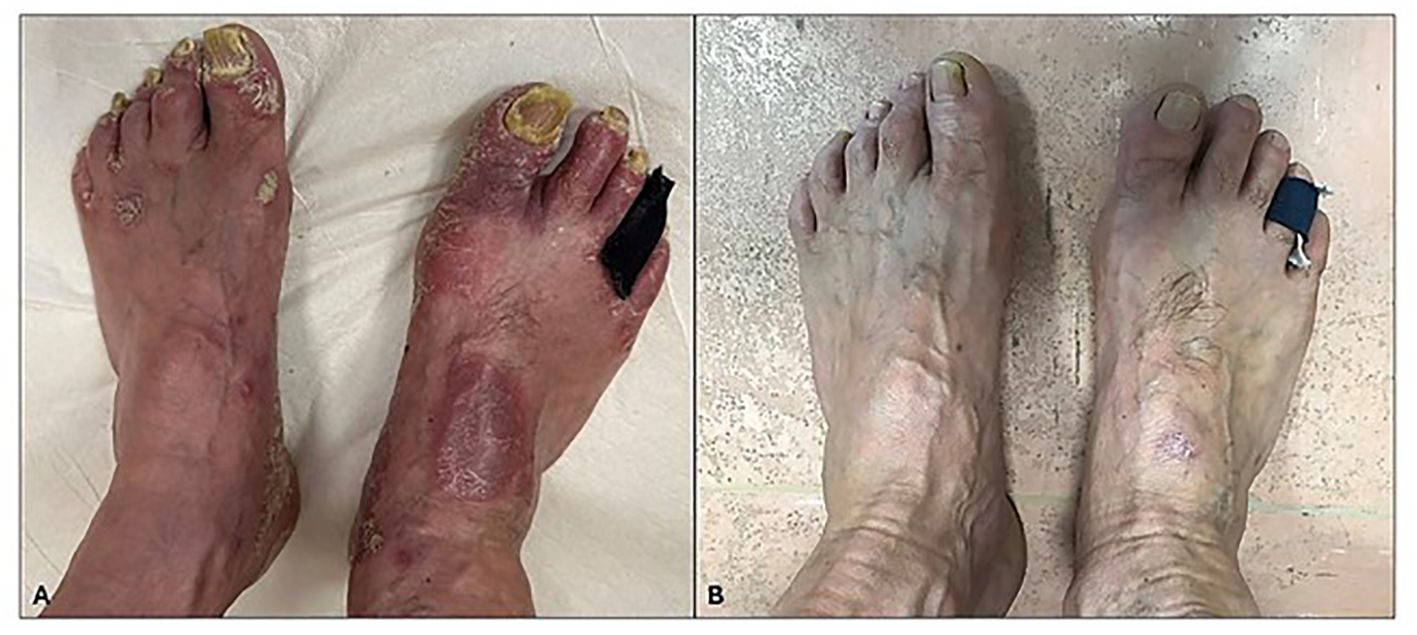
Figure 3. (A) Severe 10-nail and nail fold psoriasis before apremilast treatment. (B) Improvement in nail psoriasis after 52 weeks of treatment. Complete remission of psoriasis and concomitant onychomycosis.
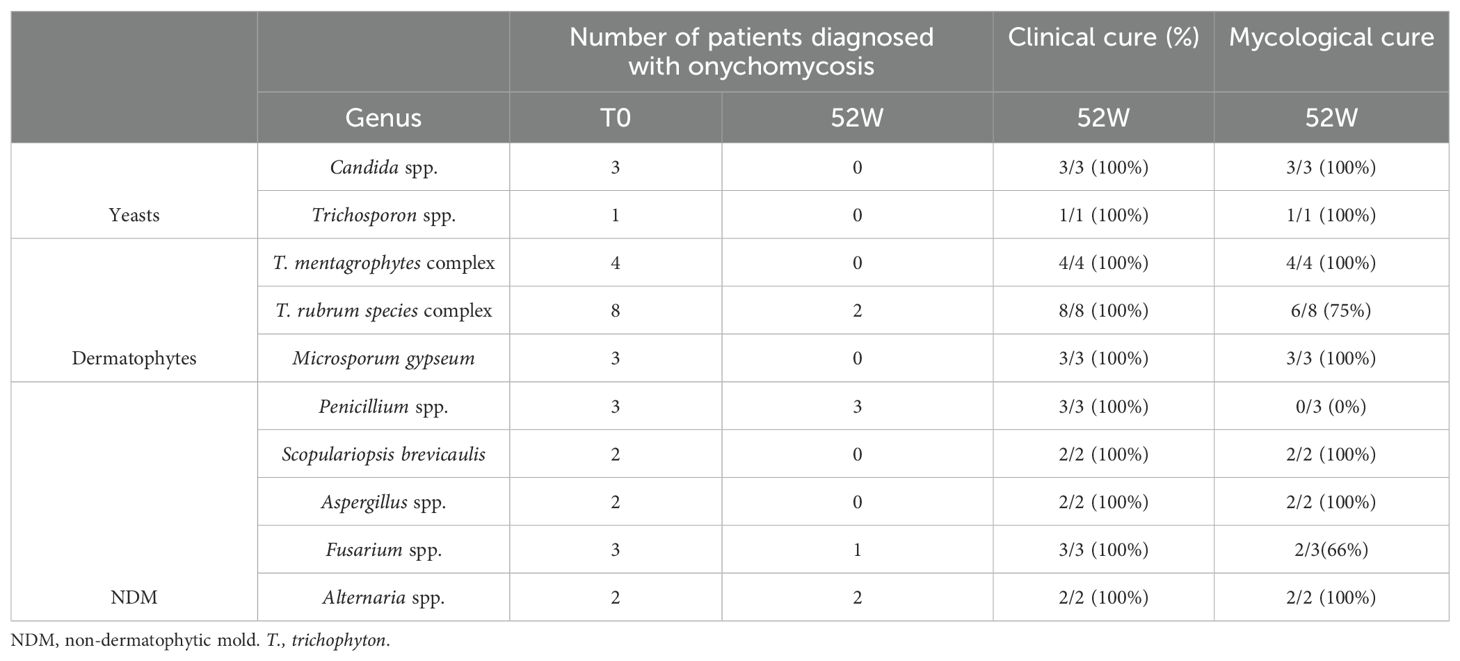
Table 3. Fungi identified from nail samples and mycological and clinical cure rates at the end of the study.
3.5 Evaluation of PASI, NAPSI, DLQI, ITCH VAS, scPGA and sPGA-G indexes
To investigate the efficacy of apremilast in DTT psoriasis, we evaluated PASI, NAPSI, DLQI, itch VAS, PAIN VAS, scPGA and sPGA-G scores at baseline and at different time points (week 16, 24, 52). Statistical analysis showed a significant reduction of PASI, from 12.17 to 1.57 after 52 weeks of treatment (P<0.0001; Repeated measures ANOVA). The mean NAPSI score decreased from 28,02 at T0 to 6.53 at week 52 (p<0.0001; Repeated measures ANOVA). In addition, to the improvement in terms of QoL, we also found a statistically significant reduction after 52 weeks of treatment (p<0.0001; Repeated measures ANOVA), from 6.85 to 0.58 (Repeated measures ANOVA test; p<0.05), itch VAS from 7.42 to 0.93 (p<0.0001; Repeated measures ANOVA test) and pain VAS from 19.78 to 3.97 (p<0.0001; Repeated measures ANOVA test) (Figures 4, 5). The decrease of the above-mentioned parameters was statistically significant from the 4th month of treatment. In this study we also examined a possible correlation between the prevalence of isolated Candida spp. and severity of psoriasis. For this purpose, we stratified the patient population into two groups: Candida colonized (>10 CFU/mL) and Candida non-colonized patients at baseline and at week 52. Despite slightly higher baseline values for patients colonized by Candida spp., no statistically significant differences were found in PASI, NAPSI, ITCH, PAIN, ScPGA and PGA-G scores between the two subgroups in line with previous works (22,23), except in the 4 week, where patients with comorbidities and colonized by Candida showed a slow reduction in mean PASI score, compared to patients without comorbidities (p<0.05, one-way ANOVA), suggesting a delayed drug response in patients colonized by Candida spp.

Figure 4. PASI, NAPSI, DLQI, ITCH VAS, ScPGA and sPGA-G scores variation during treatment. The graph shows the mean ± 95% CI. ****p<0.001 (Repeated measures ANOVA).
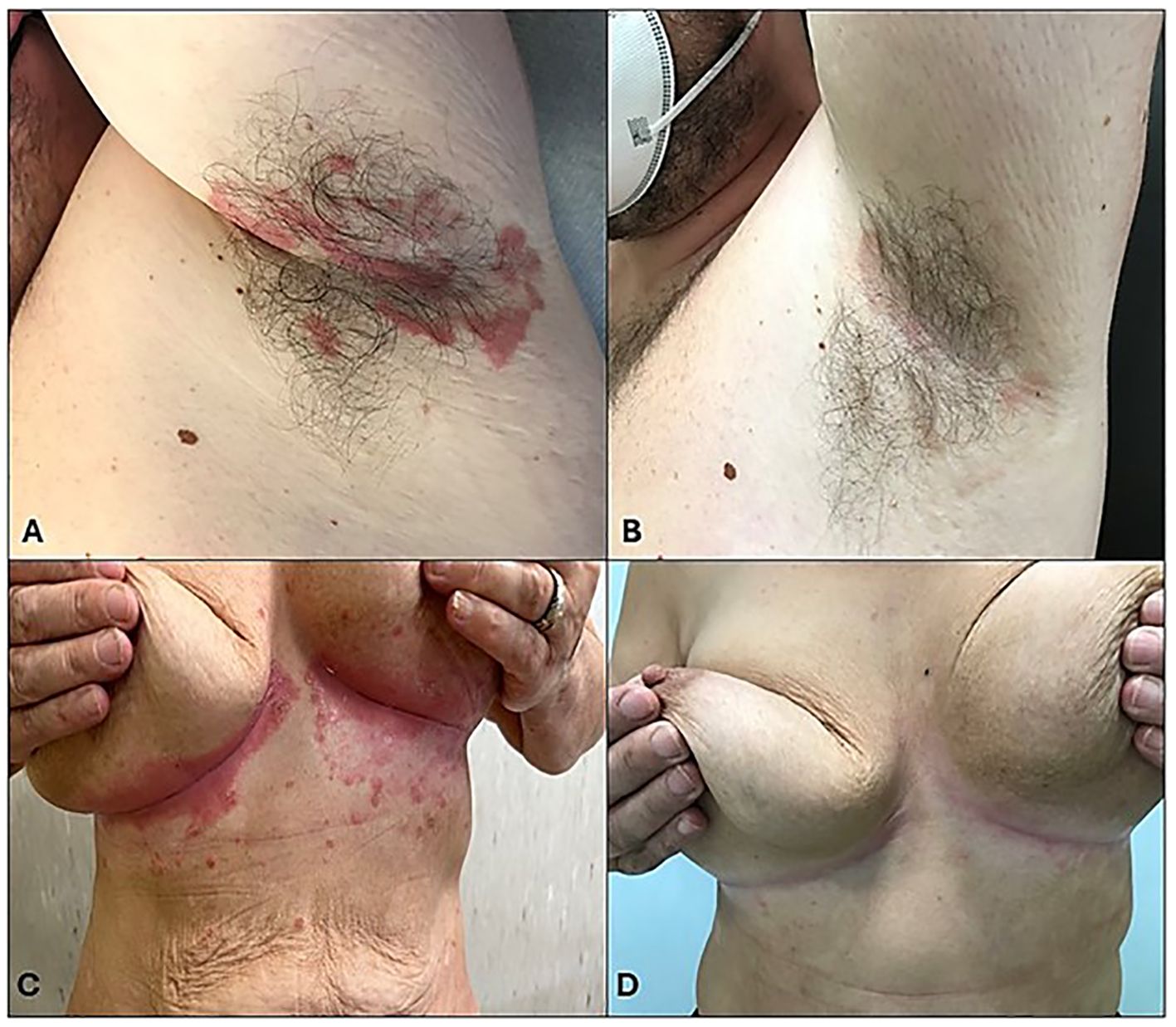
Figure 5. Clinical evaluation at T0 and after 52 weeks of treatment. (A–C) Clinical examination revealed inverse psoriasis in the context of metabolic syndrome, (B–D) resolved after 52 weeks of treatment.
3.6 Effect of apremilast on biochemical blood values
Psoriasis patients are at increased risk of developing metabolic diseases. Proinflammatory cytokines such as IL-17, IL-6, IL-1 and TNF-α are increased in psoriasis and numerous studies suggest that they play a pivotal role in developing type 2 diabetes, hypertension, dyslipidaemia, obesity, insulin resistance and their complications (40). In this study the impact of apremilast on metabolic parameter was also evaluated. As shown in Figure 6, in our patients the drug significantly reduced the serum levels of glucose and total cholesterol after 52 weeks of treatment. Further, a significant decrease in circulating levels of C reactive Protein (CRP), triglycerides and LDL cholesterol was also induced by apremilast in comparison to baseline. The plasmatic glucose level showed a decreasing trend in the first 4 months of therapy that stabilized in the range between 91-93 mg/L at week 52, in comparison to the mean value of 99.32 mg/L at baseline. Interestingly, with regard to the patients with hypo-HLD cholesterolemia, a slight increase in HDL from baseline to week 52, ranging from 29.70 to 37.50 mg/dL was observed. In light of these evidences our results underline the beneficial role of apremilast in psoriasis patients with dysregulation of lipid pathway.
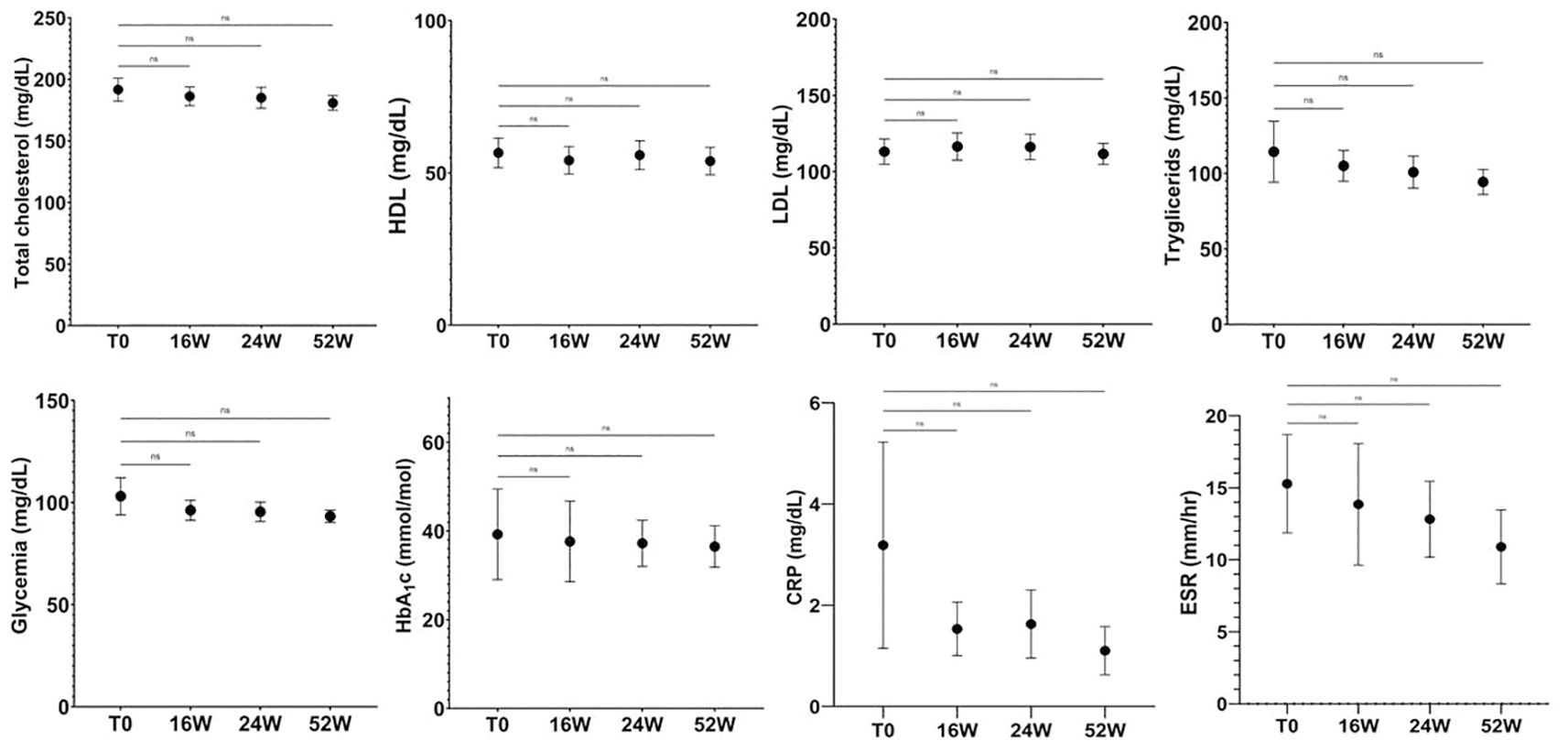
Figure 6. Plasma glucose level value over study period. At week 52, glucose levels decreased from 103.10 to 93.30 mg/dL. In parallel, total cholesterol decreased, from 191.70 to 180.90 mg/dL without diet modification in the population. Ns, not significant; repeated measures ANOVA. Error bars represent 95% CI. CI, Confidence interval.
3.7 Effect of apremilast on serum cytokine levels
Herein, we evaluated the impact of apremilast on circulating levels of IL-17A, TGF-β 1 and IL-10 at baseline and after 16, 24 and 52 weeks of apremilast treatment. Our results (Figure 7) revealed a significant decrease in serum IL-17A, TGF-β 1 and IL-10 levels up 52 weeks of apremilast administration compared with baseline (from 8.51 to 4.16 pg/mL; from 66.10 to 48.70 ng/mL and from 20.05 to 14 pg/mL, respectively). Moreover, no statistically significant differences in serum cytokine concentrations were observed between Candida colonized and non-colonized patients, as well as between patients colonized by a single Candida species (C. albicans) and those co-infected by more than one Candida species. In addition, no correlation was found between the serum cytokines and gender in both Candida spp. colonized and non-colonized groups.
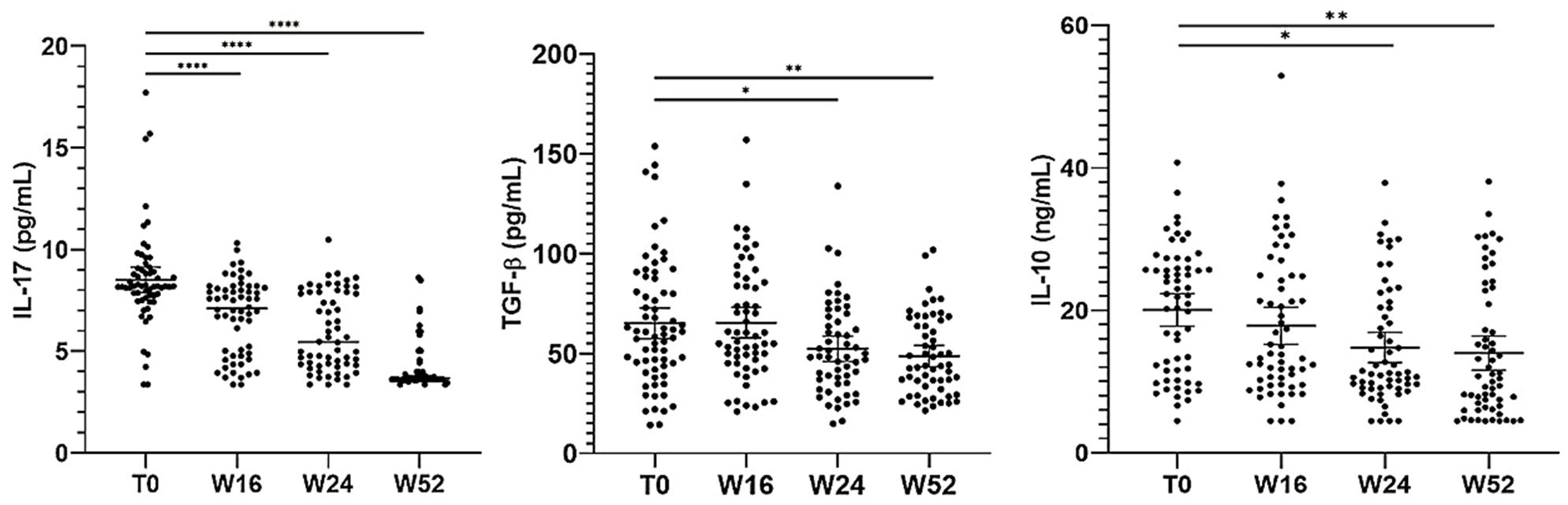
Figure 7. Serum cytokine levels at different time points of the study. The graph shows the mean ± 95% CI. *p<0.05, **p<0.01, ****p<0.0001 (one-way ANOVA).
4 Discussion
Psoriasis is a chronic inflammatory disease caused by a complex interaction between genetic and immunologic factors in a predisposing environment. Although it was considered primarily a disease of keratinocyte dysfunction, dysregulation of the immune system, potentially correlated with gut and/or skin dysbiosis among others, is now recognized as a key event in the pathogenesis of this disease (43). Restoring the gut microbiome is considered nowadays a promising preventive and therapeutic option for psoriasis. In this regard, several reports suggest that high levels of Candida spp. colonization in the gut and/or skin may play a crucial role in initiating, exacerbating, and maintaining chronic inflammation in psoriasis (44–46). Waldman et al. (15) detected a high Candida load in saliva and stool samples of psoriatic patients, although no correlation was found between Candida load and PsO severity. In another study conducted by Lesan et al. (25) on 70 psoriatic patients, Candida was isolated from the oral cavity of 20% subjects versus 2.8% of healthy controls, but none of them had clinical features of oral candidiasis. Moreover, Picciani et al. (26) found that 26% of psoriatic patients were positive for oral candidiasis, in comparison to 0 of 140 healthy controls, and candidiasis was more common in patients with severe psoriasis than in those with mild or moderate disease. Based on these evidences, we investigated the prevalence of Candida spp. colonization in psoriatic patient with DTT PsO. In line with the previous studies, our results confirm a high Candida spp. colonization rate in the oral cavity and gut of psoriatic patients. In fact, Candida was detected in 33 (47%) out of 70 patients, with C. albicans being the most frequent species. Notably, 20 individuals were positive for only one Candida species (C. albicans), while the remaining 13 were positive for two or more Candida spp. In the latter, C. glabrata was the most common non-albicans species associated with C. albicans. Other authors have shown that C. albicans and C. glabrata are frequently co-isolated in oropharyngeal candidiasis. C. albicans adhesins, and in particular agglutinin-like sequence (Asl) 3, in fact, contribute not only to the ability of the fungus to adhere and colonize host tissues, but also serve as binding moieties for bacteria i.e., Streptococcus gordonii, Pseudomonas aeruginosa and Staphylococcus aureus, as well as other non-hyphal forming Candida species such as C. glabrata (47). Moreover, our results reveal that Candida colonization was strongly influenced by the gender and comorbidities, resulting to be higher in female than in male patients and a high colonization rate was also observed in subjects with neoplasms, cardiovascular comorbidities and overweight. However, despite the high prevalence of Candida among patients with psoriasis, according to other authors (15, 25), we did not observe any correlation between Candida colonization and clinical severity of psoriasis (PASI, NAPSI, scPGA, sPGA-G). To our best knowledge, this is the first clinical study demonstrating a high prevalence of oral/gut Candida spp. in DTT psoriatic patients. Notably, this clinical phenotype of PsO could be initially responsive to systemic first line drugs (cyclosporine, methotrexate, retinoids) although during treatment relapses may occur. Moreover, biological treatments, such as IL-17 inhibitors, have shown to be more effective than traditional drugs, favoring a rapid healing of psoriasis but in some patients, such treatments could increase the risk of mycotic infections. In addition to Candida, other fungi may play a role in psoriasis pathogenesis. In our study population, 27 (39%) patients presented a concomitant onychomycosis caused by dermatophytes, NDM and yeasts. Interestingly, after 52 weeks of treatment, 78% of the patients presented a complete clinical resolution of nail psoriasis and onychomycosis and in 70% mycological culture tests were negative. It should be noted that in our patients all the concomitant onychomycoses have been identified before apremilast treatment as clinical routine practice and no new nail infections were observe in the study period. Nail psoriasis and onychomycosis are the two most frequent diseases affecting the nail unit (48) and their coexistence could not be incidental. Ghosal et al. (49) found that nail onychomycosis was more frequent in psoriatic patients with skin Koebner phenomenon respect to patients without traumatism in anamnesis. These observations support the hypothesis that Koebner phenomenon induced by the fungal invasion may worsen nail psoriasis. Although over the years significant advances have been made in understanding the pathogenic mechanisms of psoriasis, leading to the development of various therapeutic options, the treatment of this disease remains challenging in clinical practice. A major challenge in addressing DTT, mainly in patients without significant body surface involvement (28), is a tailored treatment for this clinical psoriasis phenotype (50). Recently, IL-17 inhibitor drugs represent the most effective therapeutic option for patients with moderate/severe plaque psoriasis, also in those with DTT involvement (35–37). However, randomized clinical trials (RCT) and real-life studies demonstrated an increase in fungal and bacterial infections in patients treated with IL-23 or IL-17 inhibitors (38, 49, 51). In 2023, in a double-blinded placebo-controlled trial apremilast demonstrated the clinical efficacy in the treatment of psoriasis affecting genital areas (41) as well as other specific sites, such as the scalp, nails, and palmoplantar areas (42). In line with these clinical studies, here we demonstrated the therapeutic efficacy and safety of apremilast in the treatment of DTT psoriatic patients, achieving improvement in itching and relative QoL, in both Candida colonized and Candida non-colonized subjects. In terms of clinical efficacy, no significant differences were found in our population between the two groups of psoriatic patients. The beneficial effects of apremilast on clinimetric scores correlated in our study population with a significant decrease in serum levels of IL-17A, and TGF-β 1, which have proven to be higher in individuals with psoriasis in comparison to healthy controls (52, 53), although no statistical correlation has been found between IL-17 concentrations and PASI/NAPSI scores (54, 55). A significant reduction in IL-10 serum levels was also observed in our patients after apremilast treatment. This result seems to be in contrast with the immunoregulatory properties of IL-10. However, it should be pointed out that the role of this cytokine in psoriasis remains unclear. Immunohistochemical investigations suggested a low IL-10 protein expression in psoriatic lesional skin as compared to healthy skin (56). On the other hand, no significant differences were reported in the literature in the baseline serum levels between patients with psoriasis and healthy individuals (57–60). Moreover, in a previous report Wakiya et al. (61) investigated the therapeutic efficacy of apremilast and its impact on serum cytokine levels in Behçet’s disease patients. The authors found no significant changes in IL-10 concentrations after three months of apremilast initiation. Conversely, previous in vitro studies have demonstrated that apremilast was able to increase IL-10 production by B cells (62). Thus, conflicting data are reported in the literature on the impact of apremilast on IL-10 serum levels. Further studies are needed on a large cohort of psoriatic patients to better clarify this aspect. The ability of apremilast to modulate the serum levels of the inflammatory cytokine IL-17 could also explain, at least in part, the negative impact of such drug on Candida colonization in our psoriatic population. In fact, in this study we demonstrated for the first time the effectiveness of apremilast in inhibiting the fungal growth in the majority of patients (83%) colonized by only one Candida species. It is assumed that the intestinal barrier dysfunction and the consequent increase of intestinal permeability that occurs in gut dysbiosis may cause microbial translocation, leading to systemic, low-grade inflammation. These effects may create a “vicious cycle” in which low-level inflammation may promote fungal colonization, which in turn, can promote further inflammation (63, 64). In this scenario it is likely that apremilast, due to its own anti-inflammatory properties, could restore gut homeostasis in psoriatic patients, thus counteracting Candida overgrowth. In the inflamed tissues, overexpression of PDE4 isoforms and defective cAMP-mediating pathway were identified for the first time in chronic ulcerative colitis (UC) patients. Therapeutic inhibition of PDE4 by apremilast ameliorated the clinical symptoms in patients with chronic UC, as evidenced by improvements on mucosal ulcerations, tissue fibrosis, and inflammatory infiltrations (65). Consistent with these findings, in a murine model of chronic UC apremilast was proven to play a role in the maintenance of intestinal barrier integrity, reestablishing the mucosal immune homeostasis, by interfering with the crosstalk between human epithelial barrier and immune cells. In addition, by modulating the gut microbiota composition this drug might exert regulatory effects on antimicrobial responses (66) Comorbidities like diabetes, obesity, dyslipidaemia, metabolic syndrome, and non-alcoholic fatty liver disease, have been consistently found to be associated with chronic plaque psoriasis (67). Notably, metabolic syndrome is a condition of low-grade chronic inflammation where gut dysbiosis plays a crucial role. Besides intestinal bacteria, opportunistic fungi such as C. albicans, Aspergillus spp. and Meyerozyma spp. may also contribute to the onset of metabolic diseases by activating the immune system and/or producing harmful metabolites (68). Yeter et al. (69) found that oral Candida colonization represented a risk factor for chronic inflammation, atherosclerosis and coronary artery disease in hemodialysis patients. Consistently, in our study a high Candida colonization rate was found in patients with cardiometabolic comorbidities and overweighed. A beneficial effect of apremilast, via inhibiting PDE4, has been previously associated with weight loss and other cardiometabolic benefits in psoriatic patients (70, 71), including reduced glycated hemoglobin A1c (HbA1c) and serum glucose levels, thus suggesting potential novel therapeutic uses of PDE4 inhibitors (72–76). Interestingly, in line with the literature data, our results demonstrated that apremilast affected the body weight, BMI and metabolic profile in our population, favoring a reduction in serum levels of total cholesterol, triglycerides and blood glucose. The mechanism behind the potential role of apremilast on metabolic and cardiovascular benefits is still not fully understood. However, Ikonomidis et al. (77) demonstrated that apremilast confers a greater improvement of endothelial glycocalyx integrity, microvascular perfusion, arterial elasticity and left ventricular myocardial function compared with etanercept or cyclosporine treatment, suggesting a favorable profile of PDE4 inhibition on cardiovascular function (74–79). In addition, Wang et al. (78) also demonstrated a significant efficacy of apremilast on ischemic stroke outcomes (79, 80). Thus, those findings could pave the way for the potential use of PDE4 inhibitors in the treatment of cardiometabolic comorbidities and oxidative stress in the future. Despite the promising results, this study presents some limitations. First of all, it is a real-life study with a high percentage of patients who dropped out (14%) and did not complete the planned treatment. Moreover, a longer follow-up time to evaluate drug survival and maintenance of clinical response should be performed. It should be also pointed out that, although the sample collection procedures have been standardized in all patients, oral and cutaneous swabs collection are operator-dependent, thus representing a limitation of the study. Finally, we evaluated the levels of IL-10, IL-17 and TGF-β 1 only in the serum. Nevertheless, the cytokine analysis in the psoriatic skin lesions based on the expression quantification of cytokine mRNA or immunohistochemistry analyses would be more useful to explain the contribution of inflammatory/regulatory cytokines in the immunopathogenesis of this disease.
5 Conclusion
Overall, our results suggest that although psoriasis is considered a multifactorial disease, Candida may play a possible role as a trigger factor in the pathogenic process of this inflammatory disease. In this context, apremilast has been shown to ameliorate psoriasis symptoms and counteract Candida overgrowth. We hypothesized that apremilast, by dampening inflammation, due its ability to modulate the serum levels of the inflammatory cytokine IL-17, might arrest the “vicious cycle”, triggered by Candida itself, thus restoring the gut homeostasis in psoriatic patients. Thus, considering the crucial role of IL-17 in protecting against Candida infections, apremilast by modulating this cytokine does not increase the risk of fungal infections, exerting a protective effect against mucosal candidiasis. Given the high rate of Candida colonization in psoriatic patients and the potential role of Candida in psoriasis onset, the use of PDE-4 inhibitors may represent an effective therapeutic approach for better management of sub-clinical risk of candidiasis in those patients. Moreover, apremilast may offer a unique opportunity to control systemic inflammation by improving the metabolic profile in psoriatic subjects. Finally, this study confirms the safety profile and therapeutic efficacy of apremilast in treating DTT psoriasis, regardless of age, sex, disease onset, BMI, cardiovascular comorbidities and neoplastic conditions. Despite the promising results, further studies are needed to better understand the relationship between gut homeostasis, obesity and psoriasis and the pleiotropic role of PDE4 inhibitors in the management of psoriasis as a complex disease.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary Material.
Ethics statement
The studies involving humans were approved by the Institutional Review Board’s (IRB) Independent Ethical Committee Tor Vergata University Hospital (R.S. 133.19). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
EC: Writing – original draft, Writing – review & editing, Conceptualization, Funding acquisition, Project administration, Resources, Supervision, Validation, Visualization. TC: Writing – original draft, Writing – review & editing, Formal analysis, Methodology. EP: Methodology, Validation, Writing – review & editing. FA: Investigation, Writing – review & editing. RGS: Investigation, Writing – review & editing. CB: Investigation, Writing – review & editing. AR: Writing – review & editing. VC: Investigation, Writing – review & editing. MF: Investigation, Writing – review & editing. RS: Formal analysis, Investigation, Methodology, Software, Validation, Writing – original draft. FP: Investigation, Methodology, Validation, Writing – review & editing. LB: Writing – review & editing. RG: Writing – original draft, Conceptualization, Formal analysis, Methodology, Validation.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. The article has been published with the unconditional contribution of Amgen S.p.A., research pursuant to Ministerial Decree 17/12/2004.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2024.1508489/full#supplementary-material
References
1. Campanati A, Marani A, Martina E, Diotallevi F, Radi. G, Offidani A. Psoriasis as an immune-mediated and inflammatory systemic disease: from pathophysiology to novel therapeutic approaches. Biomedicines. (2021) 9:1511. doi: 10.3390/biomedicines9111511
2. Caputo V, Strafella C, Termine A, Dattola A, Mazzilli S, Caterina Lanna C, et al. Overview of the molecular determinants contributing to the expression of Psoriasis and Psoriatic Arthritis phenotypes. J Cell Mol Med. (2020) 24:13554–63. doi: 10.1111/jcmm.15742
3. Korman NJ. Management of psoriasis as a systemic disease: what is the evidence? Br J Dermatol. (2020) 182:840–8. doi: 10.1111/bjd.18245
4. Dopytalska K, Sobolewski P, Błaszczak A, Szymańska E, Walecka I. Psoriasis in special localizations. Reumatologia. (2018) 56:392–8. doi: 10.5114/reum.2018.80718
5. Merola JF, Li T, Li WQ, Cho E, Qureshi AA. Prevalence of psoriasis phenotypes among men and women in the USA. Clin Exp Dermatol. (2016) 41:486–9. doi: 10.1111/ced.1280547
6. Sánchez-Regaña M, Aldunce Soto MJ, Belinchón Romero I, Ribera Pibernat M, Lafuente-Urrez RF, Carrascosa Carrillo JM, et al. Evidence-based guidelines of the spanish psoriasis group on the use of biologic therapy in patients with psoriasis in difficult-to-treat sites (nails, scalp, palms, and soles). Actas Dermosifiliogr. (2014) 105:923–34. doi: 10.1016/j.ad.2014.02.015
7. Nast A, Smith C, Spuls PI, Avila Valle G, Bata-Csörgö Z, Boonen H, et al. EuroGuiDerm Guideline on the systemic treatment of Psoriasis vulgaris - Part 1: treatment and monitoring recommendations. J Eur Acad Dermatol Venereol. (2020) 34:2461–98. doi: 10.1111/jdv.16915
8. Licata G, Tancredi V, Giuffrida G, Bernardini N, Zichichi L, Campione E. The challenges of managing psoriasis on the ear. J Clin aesthetic Dermatol. (2024) 17:18.
9. Ortiz-Lopez LI, Choudhary V, Bollag WB. Updated perspectives on keratinocytes and psoriasis: keratinocytes are more than innocent bystanders. Psoriasis (Auckl). (2022) 12:73–87. doi: 10.2147/PTT.S327310
10. Zaredar N, Mahmoudi H, Soori T, Teimourpour A, Balighi K, Farid AS, et al. Infections in hospitalized patients with psoriasis in a skin referral hospital. Dermatol Pract Concept. (2023) 13:e2023027. doi: 10.5826/dpc.1301a27
11. Zhou S, Yao Z. Roles of infection in psoriasis. Int J Mol Sci. (2022) 23:6955. doi: 10.3390/ijms23136955
12. Hidalgo-Cantabrana C, Gómez J, Delgado S, Requena-López S, Queiro-Silva R, Margolles A, et al. Gut microbiota dysbiosis in a cohort of patients with psoriasis. Br J Dermatol. (2019) 181:1287–95. doi: 10.1111/bjd.17931
13. Chang HW, Yan D, Singh R, Liu J, Lu X, Ucmak D, et al. Alteration of the cutaneous microbiome in psoriasis and potential role in Th17 polarization. Microbiome. (2018) 6:154. doi: 10.1186/s40168-018-0533-1
14. Radaschin DS, Iancu AV, Ionescu AM, Gurau G, Niculet E, Bujoreanu FC, et al. Comparative analysis of the cutaneous microbiome in psoriasis patients and healthy individuals—Insights into microbial dysbiosis. Final Results Int J Mol Sci. (2024) 25:10583. doi: 10.3390/ijms251910583
15. Waldman A, Gilhar A, Duek L, Berdicevsky I. Incidence of Candida in psoriasis–a study on the fungal flora of psoriatic patients. Mycoses. (2001) 44:77–81. doi: 10.1046/j.1439-0507.2001.00608.x
16. Nemoto Y, Kanai T, Takahara M, Oshima S, Okamoto R, Tsuchiya K, et al. Th1/Th17-mediated interstitial pneumonia in chronic colitis mice independent of intestinal microbiota. J Immunol. (2013) 190:6616–25. doi: 10.4049/jimmunol.1202930
17. Geem D, Medina-Contreras O, McBride M, Newberry RD, Koni PA, Denning TL. Specific microbiota-induced intestinal Th17 differentiation requires MHC class II but not GALT and mesenteric lymph nodes. J Immunol. (2014) 193:431–8. doi: 10.4049/jimmunol.1303167
18. Cicerone C, Nenna R, Pontone S. Th17, intestinal microbiota and the abnormal immune response in the pathogenesis of celiac disease. Gastroenterol Hepatol Bed Bench. (2015) 8:117–22.
19. Belkaid Y, Segre JA. Dialogue between skin microbiota and immunity. Science. (2014) 346:954–9. doi: 10.1126/science.1260144
20. Armstrong AW, Bukhalo M, Blauvelt AA. Clinician’s guide to the diagnosis and treatment of candidiasis in patients with psoriasis. Am J Clin Dermatol. (2016) 17:329–36. doi: 10.1007/s40257-016-0206-4
21. Crestez AM, Nechita A, Daineanu MP, Busila C, Tatu AL, Ionescu MA, et al. Oral cavity microbiome impact on respiratory infections among children. Pediatr health Med Ther. (2024) 15:311–23. doi: 10.2147/PHMT.S471588
22. Campione E, Cosio T, Lanna C, Ventura A, Dika E, Gaziano R, et al. Predictive role of vitamin A serum concentration in psoriatic patients treated with IL-17 inhibitors to prevent skin and systemic fungal infections. J Pharmacol Sci. (2020) 144:52–6. doi: 10.1016/j.jphs.2020.06.003
23. Baliwag J, Barnes DH, Johnston A. Cytokines in psoriasis. Cytokine. (2015) 73:342–50. doi: 10.1016/j.cyto.2014.12.014
24. Teng Y, Xie W, Tao X, Liu N, Yu Y, Huang Y, et al. Infection-provoked psoriasis: Induced or aggravated (Review). Exp Ther Med. (2021) 21:567. doi: 10.3892/etm.2021.9999
25. Lesan S, Toosi R, Aliakbarzadeh R, Daneshpazhooh M, Mahmoudi L, Tavakolpour S, et al. Oral Candida colonization and plaque type psoriasis: Is there any relationship? J Investig Clin Dent. (2018) 9:e12335. doi: 10.1111/jicd.12335
26. Picciani BL, Michalski-Santos B, Carneiro S, Sampaio AL, Avelleira JC, Azulay DR, et al. Oral candidiasis in patients with psoriasis: correlation of oral examination and cytopathological evaluation with psoriasis disease severity and treatment. J Am Acad Dermatol. (2013) 68:986–91. doi: 10.1016/j.jaad.2012.11.033
27. Viola R, Mastorino L, Megna M, Damiani G, Gisondi P, Argenziano G, et al. Multi-failure psoriasis patients: characterization of the patients and response to biological therapy in a multicenter Italian cohort. Int J Dermatol. (2024) 63:351–8. doi: 10.1111/ijd.17005
28. Nicolescu AC, Ionescu MA, Constantin MM, Ancuta I, Ionescu S, Niculet E, et al. Psoriasis management challenges regarding difficult-to-treat areas: therapeutic decision and effectiveness. Life (Basel). (2022) 12:2050. doi: 10.3390/life12122050
29. Masson W, Lobo M, Molinero G. Psoriasis and cardiovascular risk: A comprehensive review. Adv Ther. (2020) 37:2017–33. doi: 10.1007/s12325-020-01346-6
30. Fargnoli MC, De Simone C, Gisondi P, Pellacani G, Calzavara-Pinton P. Topical treatment for the management of mild-to-moderate psoriasis: A critical appraisal of the current literature. Dermatol Ther (Heidelb). (2023) 13:2527–47. doi: 10.1007/s13555-023-01024-9
31. Wu W, Gao N, Han J, Zhang Y, Fang X. Efficacy and safety of newer topical therapies in psoriasis: A systematic review and network meta-analysis. Dermatology. (2024) 240:1–12. doi: 10.1159/000535056
32. Kądziela M, Kutwin M, Karp P, Woźniacka A. Role of cytokines and chemokines in vitiligo and their therapeutic implications. J Clin Med. (2024) 13:4919. doi: 10.3390/jcm13164919
33. Tatu AL, Ionescu MA, Nwabudike LC. Contact allergy to topical mometasone furoate confirmed by rechallenge and patch test. Am J Ther. (2018) 25:e497–8. doi: 10.1097/MJT.0000000000000581
34. Farhan MA, Moharram AM, Salah T, Shaaban OM. Types of yeasts that cause vulvovaginal candidiasis in chronic users of corticosteroids. Med mycology. (2019) 57:681–7. doi: 10.1093/mmy/myy117
35. AlMutairi N, Eassa BI. Comparing the efficacy and safety of IL-17 inhibitors for treatment of moderate-to-severe psoriasis: a randomized double blind pilot study with a review of literature. Postepy Dermatol Alergol. (2021) 38:281–8. doi: 10.5114/ada.2019.91496
36. Papadimitriou I, Bakirtzi K, Katoulis A, Ioannides D. Scalp psoriasis and biologic agents: A review. Skin Appendage Disord. (2021) 7:439–48. doi: 10.1159/000517806
37. Canavan TN, Elmets CA, Cantrell WL, Evans JM, Elewski BE. Anti-IL-17 medications used in the treatment of plaque psoriasis and psoriatic arthritis: A comprehensive review. Am J Clin Dermatol. (2016) 17:33–47. doi: 10.1007/s40257-015-0162-4
38. Davidson L, van den Reek JMPA, Bruno M, van Hunsel F, Herings RMC, Matzaraki V, et al. Risk of candidiasis associated with interleukin-17 inhibitors: A real-world observational study of multiple independent sources. Lancet Reg Health Eur. (2021) 13:100266. doi: 10.1016/j.lanepe.2021.100266
39. Mengesha BG, Conti HR. The role of IL-17 in protection against mucosal candida infections. J Fungi (Basel). (2017) 3:52. doi: 10.3390/jof3040052
40. Papp K, Reich K, Leonardi CL, Kircik L, Chimenti S, Langley RG, et al. Apremilast, an oral phosphodiesterase 4 (PDE4) inhibitor, in patients with moderate to severe plaque psoriasis: Results of a phase III, randomized, controlled trial (Efficacy and Safety Trial Evaluating the Effects of Apremilast in Psoriasis [ESTEEM] 1). J Am Acad Dermatol. (2015) 73:37–49. doi: 10.1016/j.jaad.2015.03.049
41. Carrascosa JM, Del-Alcazar E. Apremilast for psoriasis treatment. G Ital Dermatol Venereol. (2020) 155:421–33. doi: 10.23736/S0392-0488.20.06684-5
42. Merola JF, Parish LC, Guenther L, Lynde C, Lacour JP, Staubach P, et al. Efficacy and safety of apremilast in patients with moderate-to-severe genital psoriasis: Results from DISCREET, a phase 3 randomized, double-blind, placebo-controlled trial. J Am Acad Dermatol. (2024) 90:485–93. doi: 10.1016/j.jaad.2023.10.020
43. Maguire M, Maguire G. The role of microbiota, and probiotics and prebiotics in skin health. Arch Dermato.l Res. (2017) 309:411–21. doi: 10.1007/s00403-017-1750-3
44. Pietrzak A, Grywalska E, Socha M, Roliński J, Franciszkiewicz-Pietrzak K, Rudnicka L, et al. Prevalence and possible role of candida species in patients with psoriasis: A systematic review and meta-analysis. Mediators Inflamm. (2018) 2018:9602362. doi: 10.1155/2018/9602362
45. Devore-Carter D, Kar S, Vellucci V, Bhattacherjee V, Domanski P, Hostetter MK, et al. Superantigen-like effects of a Candida albicans polypeptide. J Infect Dis. (2008) 197:981–9. doi: 10.1086/529203
46. Kashem SW, Kaplan DH. Skin immunity to candida albicans. Trends Immunol. (2016) 37:440–50. doi: 10.1016/j.it.2016.04.007
47. Tati S, Davidow P, McCall A, Hwang-Wong E, Rojas IG, Cormack B, et al. Candida glabrata Binding to Candida albicans Hyphae Enables Its Development in Oropharyngeal Candidiasis. PloS Pathog. (2016) 12:e1005522. doi: 10.1371/journal.ppat.1005522
48. Rigopoulos D, Papanagiotou V, Daniel R 3rd, Piraccini BM. Onychomycosis in patients with nail psoriasis: a point to point discussion. Mycoses. (2017) 60:6–10. doi: 10.1111/myc.12542
49. Ghosal A, Gangopadhyay DN, Chanda M, Das NK. Study of nail changes in psoriasis. Indian J Dermatol. (2004) 49:18–21. doi: 10.3329/kyamcj.v8i2.35699
50. Zhang H, Yang Z, Tang K, Sun Q, Jin H. Stigmatization in patients with psoriasis: A mini review. Front Immunol. (2021) 12:715839. doi: 10.3389/fimmu.2021.715839
51. Mills KHG. IL-17 and IL-17-producing cells in protection versus pathology. Nat Rev Immunol. (2023) 23:38–54. doi: 10.1038/s41577-022-00746-9
52. Boehncke WH. Systemic inflammation and cardiovascular comorbidity in psoriasis patients: causes and consequences. Front Immunol. (2018) 9:579. doi: 10.3389/fimmu.2018.00579
53. Mosca M, Hong J, Hadeler E, Hakimi M, Liao W, Bhutani T. The role of IL-17 cytokines in psoriasis. Immunotargets Ther. (2021) 10:409–18. doi: 10.2147/ITT.S240891
54. Kyriakou A, Patsatsi A, Vyzantiadis TA, Sotiriadis D. Serum levels of TNF- α, IL-12/23 p40, and IL-17 in psoriatic patients with and without nail psoriasis: a cross-sectional study. ScientificWorldJournal. (2014) 2014:508178. doi: 10.1155/2014/508178
55. de Oliveira PS, Cardoso PR, Lima EV, Pereira MC, Duarte AL, Pitta IR, et al. IL-17A, IL-22, IL-6, and IL-21 serum levels in plaque-type psoriasis in Brazilian patients. Mediators Inflamm. (2015) 2015:819149. doi: 10.1155/2015/819149
56. Mussi A, Bonifati C, Carducci M, Viola M, Tomaselli R, Sacerdoti G, et al. IL-10 levels are decreased in psoriatic lesional skin as compared to the psoriatic lesion-free and normal skin suction blister fluids. J Biol Regul Homeost Agents. (1994) 8:117–20.
57. Sobhan MR, Farshchian M, Hoseinzadeh A, Ghasemibasir HR, Solgi G. Serum levels of IL-10 and IL-22 cytokines in patients with psoriasis. Iran J Immunol. (2016) 13:317–23.
58. Krueger JG, Ohtsuki M, Garcet S, Correa J, Gonzales J, Li X, et al. Apremilast reduces IL-17F, IL-17A, IL-22, and TNF- plasma protein levels in patients with moderate to severe plaque psoriasis: Pharmacodynamic and correlative results from phase 2/3 studies. J Am Acad Dermatol. (2017) 76:AB47. doi: 10.1016/j.jaad.2017.04.201
59. Dowlatshahi EA, van der Voort EA, Arends LR, Nijsten T. Markers of systemic inflammation in psoriasis: a systematic review and meta-analysis. Br J Dermatol. (2013) 169:266–82. doi: 10.1111/bjd.12355
60. Bai F, Zheng W, Dong Y, Wang J, Garstka MA, Li R, et al. Serum levels of adipokines and cytokines in psoriasis patients: a systematic review and meta analysis. Oncotarget. (2017) 9:1266–78. doi: 10.18632/oncotarget.22260
61. Wakiya R, Ushio Y, Ueeda K, Kameda T, Shimada H, Nakashima S, et al. Efficacy and safety of apremilast and its impact on serum cytokine levels in patients with Behçet’s disease. Dermatol Ther. (2022) 35:e15616. doi: 10.1111/dth.15616
62. Traupe H. Psoriasis and the interleukin-10 family: evidence for a protective genetic effect, but not an easy target as a drug. Br J Dermatol. (2017) 176:1438–9. doi: 10.1111/bjd.15158
63. Kumamoto CA, Gresnigt MS, Hube B. The gut, the bad and the harmless: Candida albicans as a commensal and opportunistic pathogen in the intestine. Curr Opin Microbiol. (2020) 56:7–15. doi: 10.1016/j.mib.2020.05.006
64. Zeng MY, Inohara N, Nuñez G. Mechanisms of inflammation-driven bacterial dysbiosis in the gut. Mucosal Immunol. (2017) 10:18–26. doi: 10.1038/mi.2016.75
65. Danese S, Neurath MF, Kopoń A, Zakko SF, Simmons TC, Fogel R, et al. Effects of apremilast, an oral inhibitor of phosphodiesterase 4, in a randomized trial of patients with active ulcerative colitis. Clin Gastroenterol Hepatol. (2020) 18:2526–2534.e9. doi: 10.1016/j.cgh.2019.12.032
66. Li H, Zhang Y, Liu M, Fan C, Feng C, Lu Q, et al. Targeting PDE4 as a promising therapeutic strategy in chronic ulcerative colitis through modulating mucosal homeostasis. Acta Pharm Sin B. (2022) 12:228–45. doi: 10.1016/j.apsb.2021.04.007
67. Mrowietz U, Barker J, Conrad C, Jullien D, Gisondi P, Flower A, et al. Efficacy and safety of apremilast in patients with limited skin involvement, plaque psoriasis in special areas and impaired quality of life: Results from the EMBRACE randomized trial. J Eur Acad Dermatol Venereol. (2023) 37:348–55. doi: 10.1111/jdv.18689
68. Wang L, Zhang K, Zeng Y, Luo Y, Peng J, Zhang J, et al. Gut mycobiome and metabolic diseases: The known, the unknown, and the future. Pharmacol Res. (2023) 193:106807. doi: 10.1016/j.phrs.2023.106807
69. Yeter HH, Erten Y, Sevmez. H, Korucu B, Kalkanci A, Elbeg S, et al. Oral candida colonization as a risk factor for chronic inflammation and atherosclerosis in hemodialysis patients. Ther Aphe.r Dial. (2019) 23:542–9. doi: 10.1111/1744-9987.12803
70. Ferguson LD, Cathcart S, Rimmer D, Semple G, Brooksbank K, Paterson C, et al. Effect of the phosphodiesterase 4 inhibitor apremilast on cardiometabolic outcomes in psoriatic disease-results of the Immune Metabolic Associations in Psoriatic Arthritis study. Rheumatol (Oxford). (2022) 61:1026–34. doi: 10.1093/rheumatology/keab474
71. Barbarroja N, López-Medina C, Escudero-Contreras A, Arias-de la Rosa I. Clinical and molecular insights into cardiovascular disease in psoriatic patients and the potential protective role of apremilast. Front Immunol. (2024) 15:1459185. doi: 10.3389/fimmu.2024.1459185
72. Lugnier C. The complexity and multiplicity of the specific cAMP phosphodiesterase family: PDE4, open new adapted therapeutic approaches. Int J Mol Sci. (2022) 23:10616. doi: 10.3390/ijms231810616
73. Mazzilli S, Lanna C, Chiaramonte C, Cesaroni GM, Zangrilli A, Palumbo V, et al. Real life experience of apremilast in psoriasis and arthritis psoriatic patients: Preliminary results on metabolic biomarkers. J Dermatol. (2020) 47:578–82. doi: 10.1111/1346-8138.15293
74. Gualtierotti R, De Lucia O. Efficacy and metabolic effect on serum lipids of apremilast in psoriatic arthritis: A case report. J Clin Med. (2019) 8:398. doi: 10.3390/jcm8030398
75. Ikonomidis I, Pavlidis G, Lambadiari V, Rafouli-Stergiou P, Makavos G, Thymis J, et al. Endothelial glycocalyx and microvascular perfusion are associated with carotid intima-media thickness and impaired myocardial deformation in psoriatic disease. J Hum Hypertens. (2022) 36:1113–20. doi: 10.1038/s41371-021-00640-2
76. Campione E, Zarabian N, Cosio T, Borselli C, Artosi F, Cont R, et al. Apremilast as a potential targeted therapy for metabolic syndrome in patients with psoriasis: an observational analysis. Pharm (Basel). (2024) 17:989. doi: 10.3390/ph17080989
77. Ikonomidis I, Pavlidis G, Kadoglou N, Makavos G, Katogiannis K, Kountouri A, et al. Apremilast improves endothelial glycocalyx integrity, vascular and left ventricular myocardial function in psoriasis. Pharm (Basel). (2022) 15:172. doi: 10.3390/ph15020172
78. Wang M, Meng X, Cheng Z. Apremilast exerts protective effects on stroke outcomes and blood-brain barrier (BBB) dysfunction through regulating Rho-associated protein kinase 2 expression. Brain Behav. (2022) 12:e2677. doi: 10.1002/brb3.2677
79. Lee JH, Zheng Y, von Bornstadt D, Wei Y, Balcioglu A, Daneshmand A, et al. Selective ROCK2 inhibition in focal cerebral ischemia. Ann Clin Transl Neurol. (2014) 1:2–14. doi: 10.1002/acn3.19
Keywords: apremilast, difficult-to-treat psoriasis areas, Candida species, fungal infections, cytokines, IL-17
Citation: Campione E, Cosio T, Pistoia ES, Artosi F, Shumack RG, Borselli C, Rivieccio A, Caputo V, Favaro M, Sorge R, Pica F, Bianchi L and Gaziano R (2024) Prevalence of fungal colonization among patients with psoriasis in difficult-to-treat areas: impact of apremilast on mycotic burden and clinical outcomes. Front. Immunol. 15:1508489. doi: 10.3389/fimmu.2024.1508489
Received: 09 October 2024; Accepted: 25 November 2024;
Published: 10 December 2024.
Edited by:
Panagiotis Skendros, Democritus University of Thrace, GreeceReviewed by:
Theocharis Konstantinidis, Democritus University of Thrace, GreeceAlin Laurentiu Tatu, Dunarea de Jos University, Romania
Copyright © 2024 Campione, Cosio, Pistoia, Artosi, Shumack, Borselli, Rivieccio, Caputo, Favaro, Sorge, Pica, Bianchi and Gaziano. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Elena Campione, ZWxlbmEuY2FtcGlvbmVAdW5pcm9tYTIuaXQ=; Terenzio Cosio, dGVyZW56aW9jb3Npb0BnbWFpbC5jb20=
 Elena Campione
Elena Campione Terenzio Cosio
Terenzio Cosio Enrico Salvatore Pistoia2
Enrico Salvatore Pistoia2 Fabio Artosi
Fabio Artosi Valerio Caputo
Valerio Caputo Francesca Pica
Francesca Pica Luca Bianchi
Luca Bianchi Roberta Gaziano
Roberta Gaziano