- 1Department of Rheumatology, The First Affiliated Hospital of Zhengzhou University, Zhengzhou, China
- 2Department of Clinical Laboratory, The First Affiliated Hospital of Zhengzhou University, Zhengzhou, China
Background: Anti-melanoma differentiation-associated gene 5 antibody-positive dermatomyositis (anti-MDA5+DM) patients are associated with considerable mortality, and opportunistic infections including Pneumocystis jirovecii pneumonia (PJP)is the main cause. This study was to identify clinical characteristics, risk factors, and prognostic factors of PJP diagnosed by bronchoalveolar lavage fluid (BALF) metagenomic next-generation sequencing (mNGS) in anti-MDA5+ DM patients.
Methods: In this retrospective observational study, all patients admitted with suspected pneumonia were detected for mNGS in BALF. The demographics, comorbidities, laboratory parameters, and treatments of the patients were compared and analyzed in both groups to identify the potential risk factors for PJP and death via Logistic regression and Cox proportional hazards regression, respectively.
Results: Overall, 92 patients were included in this study, 46(50.0%) were defined as PJP+ group, and the other 46 (50.0%) as PJP- group, and 31(67.4%) PJP occurred in the first 3 months. Increased neutrophil-lymphocyte ratio (NLR) and CRP were independent risk factors for PJP occurrence, while trimethoprim-sulfamethoxazole (TMP/SMZ) prophylaxis was an independent protective factor (all p<0.05). The three-months mortality rate was higher in the PJP+ group compared to PJP- group (43.5% vs 23.9%, p=0.047). Rapidly progressive interstitial lung disease (RPILD) was a main predictor of mortality in anti-MDA5+DM patients with PJP, whereas glucocorticoid use was a significant protective factor.
Conclusions: PJP has high prevalence and mortality in anti-MDA5+DM, while TMP/SMZ prophylaxis significantly reduces PJP risk. Mortality in PJP+ patients is primarily concentrated within the first 3 months, associated with RPILD. Early intervention with corticosteroids and prophylactic measures are crucial in reducing mortality.
Introduction
Pneumocystis jirovecii pneumonia (PJP) is a common opportunistic infection among immunocompromised individuals, including those with HIV infection, malignancy, and autoimmune diseases, posing significant morbidity and mortality risks (1). Dermatomyositis (DM) is a systemic autoimmune disorder characterized by muscle weakness and skin rash (2). Recent research has revealed an association between DM and increased susceptibility to infections, including PJP (3). Moreover, a longitudinal study spanning 13 years was conducted in patients with five rheumatic diseases, found that PM/DM patients have the highest risk of opportunistic infections, with an incidence rate of 61.3 per 1000 person-years, while, the incidence rate of PJP reaching 1.76 per 1000 person-years (4).
Anti-melanoma differentiation-associated gene 5-positive dermatomyositis (anti-MDA5+DM) is a distinct subtype of DM, typically associated with rapidly progressive interstitial lung disease (RPILD), often leading to a poor prognosis (5). Patients with anti-MDA5+DM typically undergo aggressive immunosuppressive therapy, increasing their vulnerability to opportunistic infections, especially PJP (6, 7). The incidence of infections, including opportunistic ones, in anti-MDA5+ DM patients has attracted attention, underscoring the need for a deeper understanding of infectious complications in this population.
Recently, studies employing metagenomic next-generation sequencing (mNGS) techniques have provided valuable insights into the pathogenesis and epidemiology of infections in organ transplantation, hematologic malignancies, and solid tumors (8, 9). However, their application in anti-MDA5+ DM has been limited (10). Chen et al. found that the mNGS showed a satisfying diagnostic performance with a sensitivity of 100% in detecting PJP (11). In this study, we utilized mNGS technology to identify and compare clinical characteristics, risk factors, and survival status among anti-MDA5+DM patients with PJP infection.
Methods
Patients
In this retrospective study, patients diagnosed with anti-MDA5+ DM who had suspected pneumonia with fever, respiratory symptoms or new radiological changes on chest CT underwent BALF mNGS at the Department of the First Affiliated Hospital of Zhengzhou University from January 2020 to December 2022 were collected. A diagnosis of anti-MDA5+DM was based on the 2017 EULAR/ACR IIM classification criteria or the 2018 EMNC DM criteria (12, 13). This study was approved by the Ethics Committee of the First Affiliated Hospital of Zhengzhou University (2020-KY-522).
Clinical data
Baseline characteristics including demographic, clinical, comorbidities, laboratory data and treatments were acquired from the patients’ electronic medical records. Patients were followed up for at least six months. Anti-MDA5 antibody was determined in the same laboratory using ELISA kits (MBL, Japan). The presence of ILD was evaluated via chest CT. Rapidly progressive ILD (RPILD) was defined as acute progressive dyspnea and hypoxemia within 4 weeks from the onset of respiratory symptoms, accompanied by aggravation of ILD on high-resolution computed tomography (HRCT) (14).Patient treatment parameters included the daily dosage of glucocorticoids (GCs) (recorded as prednisone equivalents), immunosuppressants (IS) including cyclophosphamide (CYC), calcineurin inhibitors (CNI), tofacitinib, and intravenous immunoglobulin (IVIG). PJP prophylaxis was defined as the administration of trimethoprim-sulfamethoxazole (TMP/SMZ) for > 1 month to patients without a PJP diagnosis.
Diagnosis of PJP
The diagnosis of PJP was based on comprehensive evaluation by clinical manifestations such as fever or acute dyspnea, characteristic radiographic findings, and etiological evidence. In this study, patients needed to have positive microbiological tests by BALF-mNGS. For these patients, we invited an experienced respiratory physician to confirm the diagnosis of PJP.
Statistical analysis
Statistical analysis was performed with IBM SPSS software (version 26.0, Armonk, NY, USA) and GraphPad Prism software version 9.0 (GraphPad Software, San Diego, California, USA). Continuous variables with normal distributions were presented by mean ± standard deviation and those with non-normal distributions were described by median and quartiles. The independent-sample T-test and Mann-Whitney U test were used for continuous variables, while the Chi-squared test and Fisher’s exact test were used to compare categorical variables. Logistic regression models were performed to identify the independent risk factors of PJP. Covariates with a P<0.05 in the univariate analysis were included in the final model of multivariable logistic regression. Cox proportional hazards regression was used to identify independent risk factors for death. For all analyses, two-sided P-values < 0.05 were considered statistically significant.
Results
Characteristics
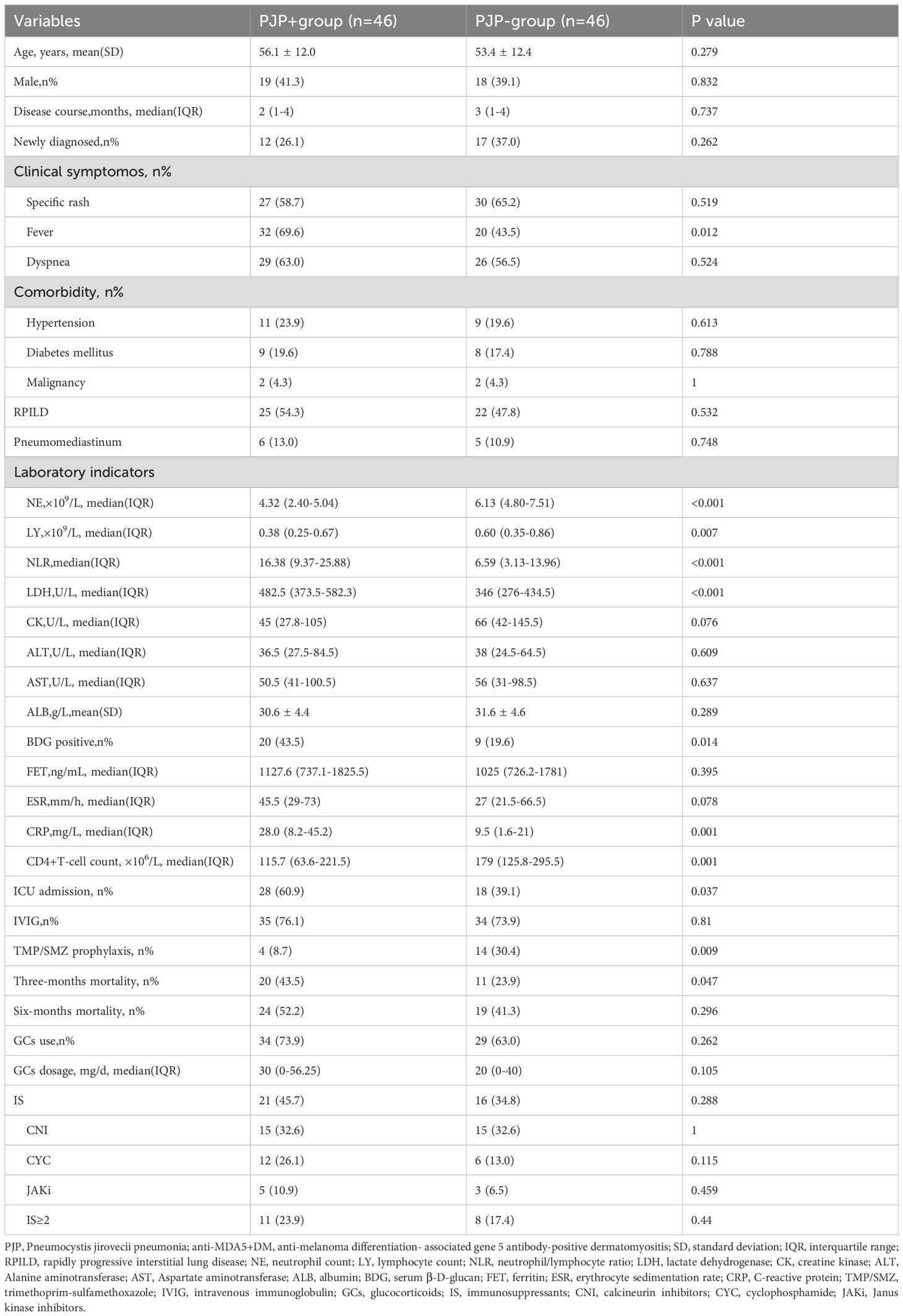
From January 2020 to December 2022, we reviewed 302 cases with anti-MDA5+ DM from the departments of rheumatology, respiratory and ICU at the First Affiliated Hospital of Zhengzhou University. A total of 210 patients without BALF-mNGS were excluded, and 92 patients who had suspected pneumonia with fever, respiratory symptoms or new radiological changes on chest CT underwent mNGS were included in the analysis. Based on the sequencing result, patients were divided into PJP+ group (n=46) and PJP- group(n=46). All of the 92 patients were with ILD. The screening process of the patients were shown in Figure 1. 31.5% of these patients were newly diagnosed. The demographic features, clinical manifestations, comorbidities, laboratory data and treatments between two groups was recorded in Table 1. The two groups had similar ages and gender distributions, with the majority being female. Disease duration, clinical symptoms, comorbidities and treatment strategies don’t have significant difference between the two groups, except for fever, which was more prevalent in PJP+ patients (69.6% vs. 43.5%, p=0.012). Laboratory findings revealed significant differences between the groups. PJP+ patients exhibited higher neutrophil counts, lower lymphocyte counts, elevated neutrophil-to-lymphocyte ratios, and higher levels of lactate dehydrogenase (LDH), C-reactive protein (CRP), and β-D-glucan (BDG) compared to PJP- patients. Additionally, PJP+ patients had a higher rate of intensive care unit (ICU) admissions (60.9% vs. 39.1%) and were less likely to receive trimethoprim/sulfamethoxazole (TMP/SMZ) prophylaxis (8.7% vs. 30.4%). Neither of the groups received methylprednisolone pulse therapy.
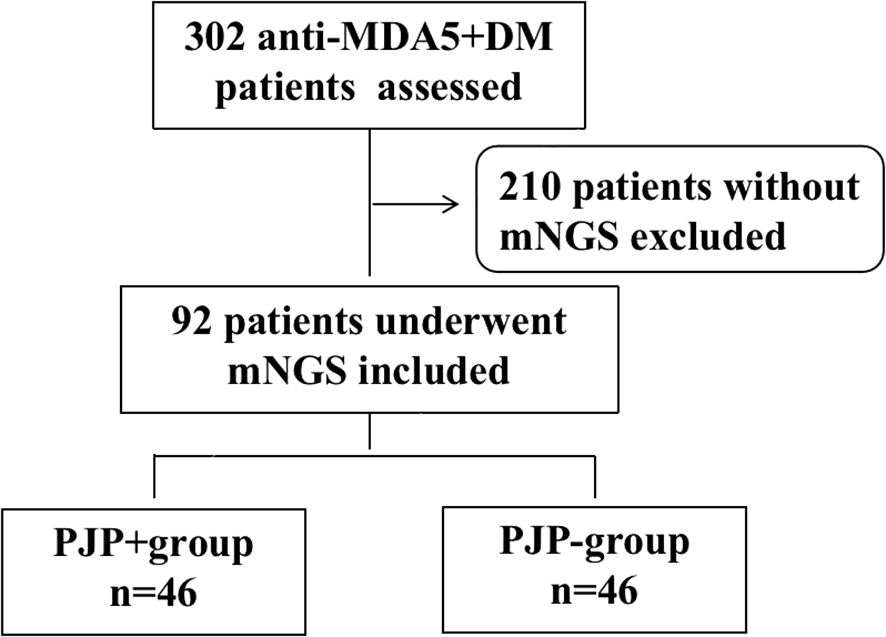
Figure 1. Flow chart of analyzed patients. anti-MDA5+DM:anti-melanoma differentiation-associated gene 5 antibody-positive dermatomyositis; mNGS: metagenomic next-generation sequencing.
Risk factors for PJP in anti-MDA5+ DM patients
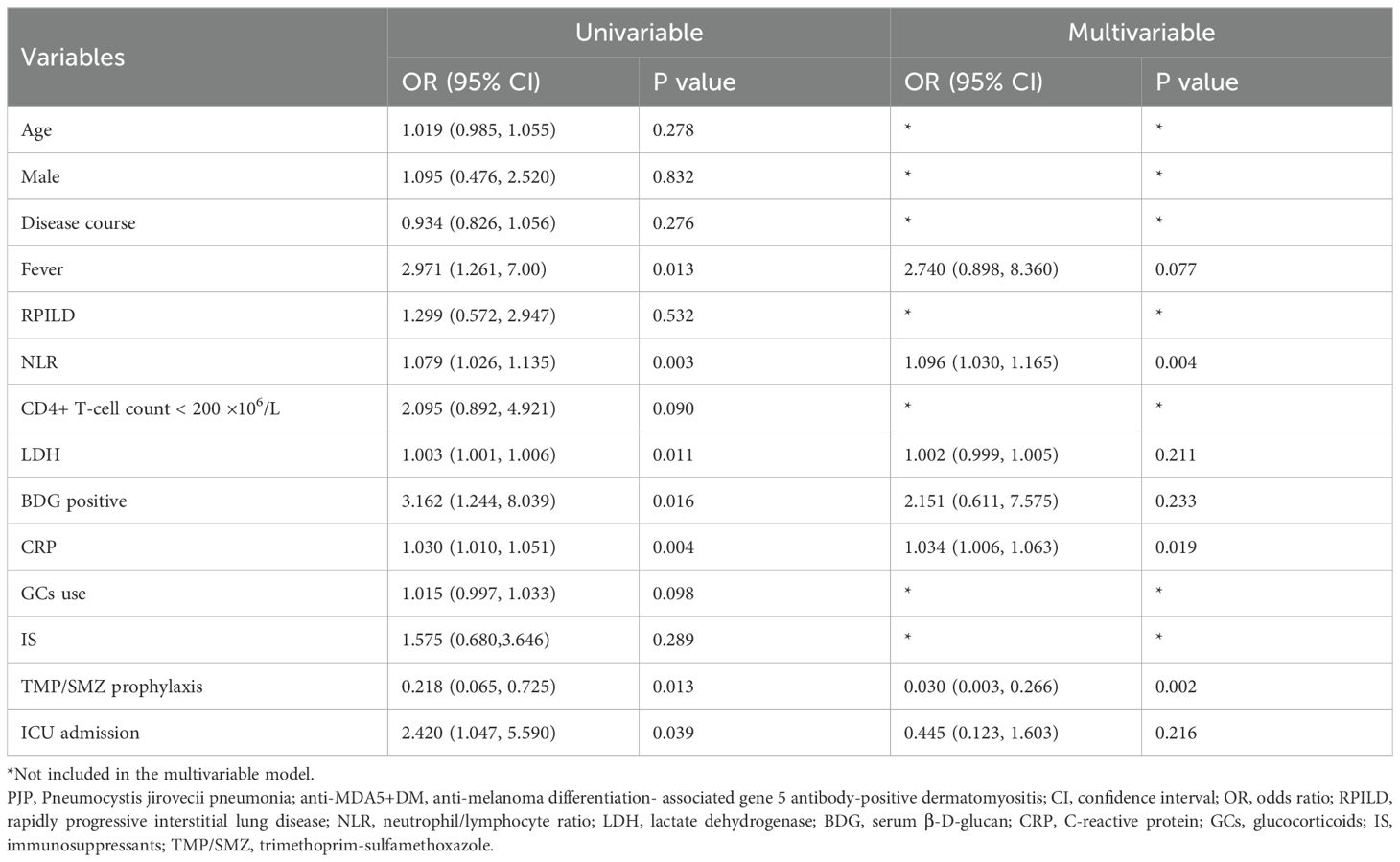
Table 2 presents the risk factors for PJP occurrence in anti-MDA5+DM patients analyzed through both univariable and multivariable models. In the univariable analysis, age, male gender, disease course, RPILD, CD4+ T-cell count, LDH, and IS showed no significant association with PJP occurrence (p>0.05). Significant risk factors included fever (OR=2.971, 95% CI: 1.261-7.00, p=0.013), NLR (OR= 1.079, 95% CI: 1.026-1.135, p=0.003), BDG positivity (OR=3.162, 95% CI: 1.244-8.039, p =0.016), CRP (OR=1.030, 95% CI: 1.010-1.051, p=0.004), and ICU admissions (OR=2.420, 95% CI: 1.047-5.590, p=0.039). TMP/SMZ prophylaxis was associated with a reduced risk of PJP (OR=0.218, 95% CI: 0.065-0.725, p=0.013). In the multivariable analysis, NLR (OR=1.096, 95% CI: 1.030-1.165, p=0.004), CRP (OR=1.034, 95% CI: 1.006-1.063, p=0.019), and TMP/SMZ prophylaxis (OR=0.030, 95% CI: 0.003-0.266, p=0.002) remained significant, while fever, BDG positivity, and ICU admissions demonstrated borderline significance.
PJP mortality in anti-MDA5+ DM patients
The 3 or 6 month mortality was calculated from the time of onset of symptoms. Notably, we found that in the first 3 months, the mortality of PJP+ group was significant higher compared to the PJP- group (43.5% vs 23.9%, p=0.047). Kaplan-Meier curves between PJP+ and PJP- groups showed that patients with PJP had a higher mortality rate (Figure 2A), the difference approaching statistical significance. We then used a Cox regression model to identify risk factors for mortality in patients with anti-MDA5+DM and PJP within 3 months (Table 3); we identified RPILD (HR: 27.602, 95%CI: 3.671-207.526), LDH (HR: 1.003, 95%CI: 1.001-1.006), and ICU (HR: 18.382, 95%CI: 2.454-137.714) as risk factors, GC use (HR: 0.141, 95%CI: 0.056-0.351) and IS (HR: 0.290, 95%CI: 0.105-0.799) as protective factors by univariate analysis. In the multivariate analysis, RPILD (HR: 14.628, 95%CI: 1.723-124.204) were ultimately confirmed as an independent risk factor, and GC use (HR:0.164, 95%CI: 0.038-0.703) were an independent protective factor.
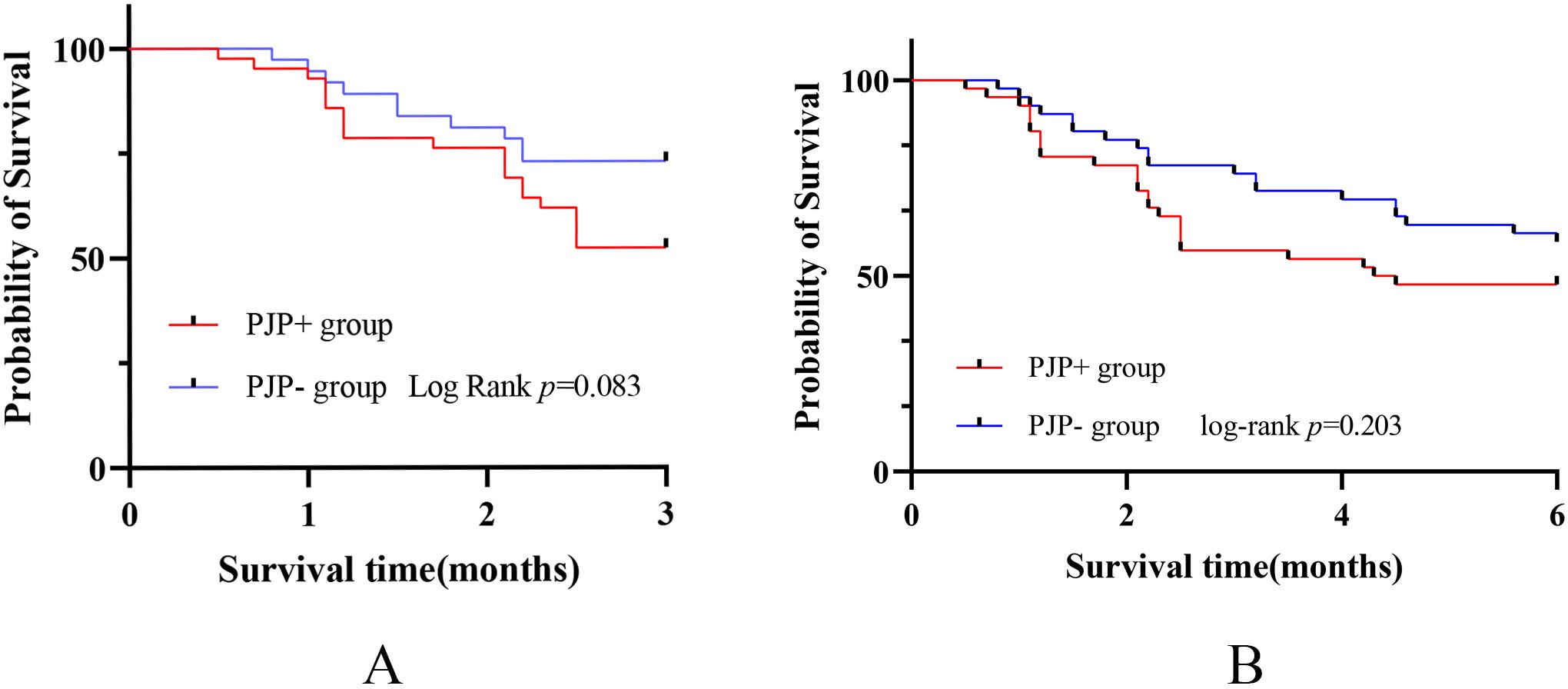
Figure 2. Analysis of mortality associated with PJP in anti-MDA5+ DM patients. (A) Kaplan-Meier curves between PJP+ and PJP- groups showed that patients with PJP had a higher mortality rate in the first 3 months. (B)Kaplan-Meier curves showed no statistically significant difference in mortality between the two groups within 6 months.

Table 3. Cox regression analysis of risk factors for three-months mortality in anti-MDA5+ DM patients with PJP.
However, when the follow-up period was extended to 6 months, there was no statistically significant difference in mortality between the two groups(52.2% vs 41.3%, P=0.296)(Figure 2B). Univariate analysis showed GC use (HR:0.968, 95%CI: 0.949-0.988)) and IS (HR:0.282, 95%CI: 0.101-0.786)) were main protective factors. In the multivariate analysis, GC use (HR: 0.161, 95%CI: 0.042-0.611) was still an independent protective factor (Table 4).

Table 4. Cox regression analysis of risk factors for six-months mortality in anti-MDA5+ DM patients with PJP.
Discussion
Although guidelines have been published for the use of PJP prophylaxis in patients with HIV and cancer and transplant patients, there is no consensus concerning whether patients with rheumatic diseases should receive PJP prophylaxis. 2022 EULAR recommendations provide some general guidance on PJP prophylaxis in patients with autoimmune inflammatory rheumatic diseases. Daily doses >15-30mg of prednisolone or equivalent for more than 2-4 weeks, concomitant immunosuppressants, older age, underlying lung disease and persistent lymphopenia were recognized as risk factors of PJP infection, which was quite common in MDA5+DM patients (15). In this study, we offer valuable insights into the characteristics, risk factors, and mortality rates associated with PJP in patients with anti-MDA5+ DM.
PJP+ group has more fever, lower lymphocyte counts, and higher levels of CRP, and further multivariable logistic analysis suggested elevated NLR and CRP levels were independent risk factors for PJP infection, but TMP/SMZ prophylaxis was an independent protective role. However, in our study, only a small proportion of patients received TMP/SMZ prophylaxis in anti-MDA5+DM patients. The limited use of TMP/SMZ prophylaxis was manly attributed to nearly one-third of these patients being newly diagnosed. While, definitive guidelines are lacking to aid clinicians in appropriately administering prophylactic treatment. There is an urgent need for evidence-based guidelines to help healthcare providers identify specific risk factors for PJP development and initiate timely PJP prophylaxis, thus minimizing the occurrence of PJP (16).
We found that the mortality rate in the PJP+ group was significantly higher in the first 3 months compared to the PJP- group among anti-MDA5+DM patients. However, the overall mortality rate was similar at 6 months. This suggests that the primary mortality risk for PJP+ patients is concentrated in the first 3 months, which was is generally consistent with the previous report (6, 16). Therefore, for anti-MDA5+ patients, it is crucial to actively prevent PJP infection in the early stages of the disease, especially within the first 3 months. Our multivariable analysis identified elevated NLR and CRP levels as independent risk factors for PJP infection. Conversely, effective TMP/SMZ prophylaxis was shown to significantly reduce the incidence of PJP (1). Therefore, for anti-MDA5+DM patients with markedly decreased lymphocyte counts and significantly elevated inflammatory markers, early implementation of TMP/SMZ prophylaxis is recommended. Once anti-MDA5+DM patients developed PJP infection in the first 3 months, the concomitant RPILD appears to become the primary risk factor for mortality, given its significantly elevated HR. Similarity, Yoshida et al. reported that baseline lung involvement was a possible factor leading to fatal interstitial pneumonia in PJP+ rheumatoid arthritis patients (17). It is thought that the presence of underlying lung disease might create a conducive environment for opportunistic pathogens, as studies have indicated a disproportionate impact of PJP on patients with non-rheumatic pulmonary conditions (7, 18).
However, once the disease course extends beyond 3 months, the mortality risk for PJP+ patients become comparable to that of PJP- patients, and RPILD was no longer an independent risk factor for mortality, which could be linked to the stabilization of lung conditions following intensive treatment. It is worth noting that the use of corticosteroids (mean value: 30mg) can reduce the mortality rate of anti-MDA5+ patients with PJP+ at both 3 and 6 months. The debate over the use of GCs in PJP began early in the HIV epidemic (19). In 1990, several RCTs demonstrated a benefit when GCs were used in HIV patients with moderate to severe PJP (20, 21). These studies reported significant reductions in respiratory failure and death in the early stages of the disease. In non-HIV patients, the data on GCs is less clear, although some guidelines have recommended the use of GCs in PJP infection (22, 23). Retrospective studies have contradictory outcomes (24, 25); however, our results supported the use of GCs in anti-MDA5+DM patients with PJP infection. Theoretically, GCs could reduce the inflammatory response associated with PJP cell death. Some in-vivo studies also showed GCs had been shown to decrease cytokine release from alveolar macrophages, thereby reducing lung inflammation (26). Also, as RPILD was identified to be an independent risk factor of mortality in the MDA5+DM patients with PJP, the potential observed benefit with GCs might be contributed by therapeutic effect on RPILD.
Data concerning the effects of immunosuppressant therapy on PJP development in patients with rheumatic diseases are limited. Some IS have been reported as risk factors for PJP in some rheumatic diseases (27, 28). However, our results suggested IS were neither a risk factor for PJP infection nor do they affect the mortality rate in anti-MDA5+ DM patients with PJP infection. Recent studies have shown that early aggressive treatment is often needed, and multiagent immunosuppression is potentially more effective than traditional treatment (GC plus a single IS) (5, 29). Therefore, we suggested that the urgency of treating the primary disease should be balanced against the risk of PJP infection. When intensified treatment for the primary disease was urgently required, IS can be used without undue concern about PJP infection. Nonetheless, further large-scale cohort studies are necessary to elucidate the impact of IS on PJP infection more comprehensively.
Our study has several limitations. Firstly, it was a single-center study conducted in one hospital with a focus on a specific population, thus single-center effects cannot be ruled out. Conducting a multicenter experiment is necessary to further validate our results. Secondly, the sample size of our cohort was relatively small, which might have restricted the detection of certain potential positive outcomes. Thirdly, we only revealed risk factors for PJP occurrence in anti-MDA5+ DM patients only at one time. This is far from enough, we are conducting prospective study to explore the occurrence of PJP, and analyze the effect of PJP on anti-MDA5+ DM patients. Lastly, the high overall mortality of these patients might be attributed to more serious condition and high proportion of ICU admission. Further prospective studies with larger cohorts are warranted to confirm our findings.
Conclusions
In this retrospective cohort study, we found that there was a high incidence and mortality in anti-MDA5+DM patients with PJP, while TMP/SMZ prophylaxis significantly reduces PJP risk. Mortality in PJP+ patients is primarily concentrated within the first 3 months, associated with RPILD. Early intervention with corticosteroids and prophylactic measures are crucial in reducing mortality.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by the Ethics Committee of the First Affiliated Hospital of Zhengzhou University 2020-KY-522). The studies were conducted in accordance with the local legislation and institutional requirements. The human samples used in this study were acquired from the human samples used in this study were acquired from medical records. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements.
Author contributions
CG: Conceptualization, Formal Analysis, Investigation, Methodology, Validation, Visualization, Writing – original draft, Writing – review & editing. GW: Formal Analysis, Visualization, Writing – review & editing. CZ: Formal Analysis, Methodology, Writing – review & editing. CW: Data curation, Formal Analysis, Writing – review & editing. CL: Investigation, Writing – review & editing. RL: Investigation, Writing – review & editing. ZS: Investigation, Writing – review & editing. ZZ: Funding acquisition, Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was supported by The Key R & D Special Project of Henan Province in China (grant number LHGJ20220309), Henan Provincial Science and Technology Research Project (grant number SBGJ202302043) and The Provincial Science Foundation of Henan (grant number 242300421267).
Acknowledgments
Thanks to all patients and site staff of the local hospital who participated in this study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Chen X, Shu X, He L, Yang H, Lu X, Wang G, et al. High prevalence and mortality of Pneumocystis jirovecii pneumonia in anti-MDA5 antibody-positive dermatomyositis. Rheumatol (Oxford). (2023) 62:3302–9. doi: 10.1093/rheumatology/kead063
2. Didona D, Solimani F, Caposiena Caro RD, Sequeira Santos AM, Hinterseher J, Kussini J, et al. Dermatomyositis: a comprehensive review of clinical manifestations, serological features, and therapeutic approaches. Ital J Dermatol Venerol. (2023) 158:84–98. doi: 10.23736/S2784-8671.23.07458-3
3. Hsu HC, Chang YS, Hou TY, Chen LF, Hu LF, Lin TM, et al. Pneumocystis jirovecii pneumonia in autoimmune rheumatic diseases: a nationwide population-based study. Clin Rheumatol. (2021) 40:3755–63. doi: 10.1007/s10067-021-05660-4
4. Hsu CY, Ko CH, Wang JL, Hsu TC, Lin CY. Comparing the burdens of opportunistic infections among patients with systemic rheumatic diseases: a nationally representative cohort study. Arthritis Res Ther. (2019) 21:211. doi: 10.1186/s13075-019-1997-5
5. Tsuji H, Nakashima R, Hosono Y, Imura Y, Yagita M, Yoshifuji H, et al. Multicenter prospective study of the efficacy and safety of combined immunosuppressive therapy with high-dose glucocorticoid, tacrolimus, and cyclophosphamide in interstitial lung diseases accompanied by anti-melanoma differentiation-associated gene 5-positive dermatomyositis. Arthritis Rheumatol. (2020) 72:488–98. doi: 10.1002/art.41105
6. Huang L, Fu Q, Ye Y, Lin Y, Yan Q, Chen S. High incidence and mortality of Pneumocystis jirovecii infection in anti-MDA5-antibody-positive dermatomyositis: experience from a single center. Arthritis Res Ther. (2021) 23:232. doi: 10.1186/s13075-021-02606-8
7. Sabbagh SE, Neely J, Chow A, DeGuzman M, Lai J, Lvovich S, et al. Risk factors associated with Pneumocystis jirovecii pneumonia in juvenile myositis in North America. Rheumatol (Oxford). (2021) 60:829–36. doi: 10.1093/rheumatology/keaa436
8. Jiang J, Bai L, Yang W, Peng W, An J, Wu Y, et al. Metagenomic next-generation sequencing for the diagnosis of pneumocystis jirovecii pneumonia in non-HIV-infected patients: A retrospective study. Infect Dis Ther. (2021) 10:1733–45. doi: 10.1007/s40121-021-00482-y
9. Sun H, Wang F, Zhang M, Xu X, Li M, Gao W, et al. Diagnostic value of bronchoalveolar lavage fluid metagenomic next-generation sequencing in pneumocystis jirovecii pneumonia in non-HIV immunosuppressed patients. Front Cell Infect Microbiol. (2022) 12:872813. doi: 10.3389/fcimb.2022.872813
10. Huang W, Ren F, Chen D, Wang Z, Tang L. The value of metagenomic next-generation sequencing in bronchoalveolar lavage fluid samples of anti-MDA5-positive dermatomyositis patients. Rheumatol (Oxford). (2023) 62:e36–e8. doi: 10.1093/rheumatology/keac475
11. Chen H, Liang Y, Wang R, Wu Y, Zhang X, Huang H, et al. Metagenomic next-generation sequencing for the diagnosis of Pneumocystis jirovecii Pneumonia in critically pediatric patients. Ann Clin Microbiol Antimicrob. (2023) 22:6. doi: 10.1186/s12941-023-00555-5
12. Lundberg IE, Tjärnlund A, Bottai M, Werth VP, Pilkington C, de Visser M, et al. 2017 European league against rheumatism/American college of rheumatology classification criteria for adult and juvenile idiopathic inflammatory myopathies and their major subgroups. Arthritis Rheumatol. (2017) 69:2271–82. doi: 10.1002/art.40320
13. Mammen AL, Allenbach Y, Stenzel W, Benveniste O. 239th ENMC international workshop: classification of dermatomyositis, amsterdam, the Netherlands, 14-16 december 2018. Neuromuscul Disord. (2020) 30:70–92. doi: 10.1016/j.nmd.2019.10.005
14. Sato S, Hirakata M, Kuwana M, Suwa A, Inada S, Mimori T, et al. Autoantibodies to a 140-kd polypeptide, CADM-140, in Japanese patients with clinically amyopathic dermatomyositis. Arthritis Rheum. (2005) 52:1571–6. doi: 10.1002/art.21023
15. Fragoulis GE, Nikiphorou E, Dey M, Zhao SS, Courvoisier DS, Arnaud L, et al. 2022 EULAR recommendations for screening and prophylaxis of chronic and opportunistic infections in adults with autoimmune inflammatory rheumatic diseases. Ann Rheum Dis. (2023) 82:742–53. doi: 10.1136/ard-2022-223335
16. Li J, Wang S, Zheng J, Li Q, Li J, Lu L. Clinical characteristics of and risk factors for Pneumocystis jirovecii pneumonia in anti-melanoma differentiation-associated gene 5 (Anti-MDA5) antibody-positive dermatomyositis patients: a single-center retrospective study. Clin Rheumatol. (2023) 42:453–62. doi: 10.1007/s10067-022-06403-9
17. Yoshida Y, Takahashi Y, Minemura N, Ueda Y, Yamashita H, Kaneko H, et al. Prognosis of pneumocystis pneumonia complicated in patients with rheumatoid arthritis (RA) and non-RA rheumatic diseases. Mod Rheumatol. (2012) 22:509–14. doi: 10.3109/s10165-011-0523-7
18. Kadoya A, Okada J, Iikuni Y, Kondo H. Risk factors for Pneumocystis carinii pneumonia in patients with polymyositis/dermatomyositis or systemic lupus erythematosus. J Rheumatol. (1996) 23:1186–8.
19. Weyant RB, Kabbani D, Doucette K, Lau C, Cervera C. Pneumocystis jirovecii: a review with a focus on prevention and treatment. Expert Opin Pharmacother. (2021) 22:1579–92. doi: 10.1080/14656566.2021.1915989
20. Bozzette SA, Sattler FR, Chiu J, Wu AW, Gluckstein D, Kemper C, et al. A controlled trial of early adjunctive treatment with corticosteroids for Pneumocystis carinii pneumonia in the acquired immunodeficiency syndrome. California Collaborative Treatment Group. N Engl J Med. (1990) 323:1451–7. doi: 10.1056/NEJM199011223232104
21. Gagnon S, Boota AM, Fischl MA, Baier H, Kirksey OW, La Voie L. Corticosteroids as adjunctive therapy for severe Pneumocystis carinii pneumonia in the acquired immunodeficiency syndrome. A double-blind, placebo-controlled trial. N Engl J Med. (1990) 323:1444–50. doi: 10.1056/NEJM199011223232103
22. Kasiske BL, Zeier MG, Chapman JR, Craig JC, Ekberg H, Garvey CA, et al. KDIGO clinical practice guideline for the care of kidney transplant recipients: a summary. Kidney Int. (2010) 77:299–311. doi: 10.1038/ki.2009.377
23. Fishman JA, Gans H. Pneumocystis jiroveci in solid organ transplantation: Guidelines from the American Society of Transplantation Infectious Diseases Community of Practice. Clin Transplant. (2019) 33:e13587. doi: 10.1111/ctr.13587
24. Fujikura Y, Manabe T, Kawana A, Kohno S. Adjunctive corticosteroids for pneumocystis jirovecii pneumonia in non-HIV-infected patients: A systematic review and meta-analysis of observational studies. Arch Bronconeumol. (2017) 53:55–61. doi: 10.1016/j.arbres.2016.06.016
25. Ding L, Huang H, Wang H, He H. Adjunctive corticosteroids may be associated with better outcome for non-HIV Pneumocystis pneumonia with respiratory failure: a systemic review and meta-analysis of observational studies. Ann Intensive Care. (2020) 10:34. doi: 10.1186/s13613-020-00649-9
26. Huang ZB, Eden E. Effect of corticosteroids on IL1 beta and TNF alpha release by alveolar macrophages from patients with AIDS and Pneumocystis carinii pneumonia. Chest. (1993) 104:751–5. doi: 10.1378/chest.104.3.751
27. Schmajuk G, Jafri K, Evans M, Shiboski S, GianFrancesco M, Izadi Z, et al. Pneumocystis jirovecii pneumonia (PJP) prophylaxis patterns among patients with rheumatic diseases receiving high-risk immunosuppressant drugs. Semin Arthritis Rheum. (2019) 48:1087–92. doi: 10.1016/j.semarthrit.2018.10.018
28. Marie I, Ménard JF, Hachulla E, Chérin P, Benveniste O, Tiev K, et al. Infectious complications in polymyositis and dermatomyositis: a series of 279 patients. Semin Arthritis Rheum. (2011) 41:48–60. doi: 10.1016/j.semarthrit.2010.08.003
Keywords: pneumocystis jirovecii pneumonia, anti-melanoma differentiation-related gene 5, dermatomyositis, risk factor, mortality
Citation: Gao C, Wei G, Zhang C, Wang C, Li C, Li R, Su Z and Zheng Z (2024) Pneumocystis jirovecii pneumonia increases the 3-months mortality of anti-MDA5-antibody-positive dermatomyositis patients. Front. Immunol. 15:1504380. doi: 10.3389/fimmu.2024.1504380
Received: 30 September 2024; Accepted: 13 November 2024;
Published: 28 November 2024.
Edited by:
Dana P. Ascherman, University of Pittsburgh, United StatesReviewed by:
Antonella Notarnicola, Karolinska Institutet (KI), SwedenNaveen Ravichandran, Royal Wolverhampton Hospitals NHS Trust, United Kingdom
Copyright © 2024 Gao, Wei, Zhang, Wang, Li, Li, Su and Zheng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhaohui Zheng, ZmNjemhlbmd6aEB6enUuZWR1LmNu
†ORCID: Zhaohui Zheng, orcid.org/0000-0003-3817-0171
Congcong Gao, orcid.org/0000-0003-1771-8847
 Congcong Gao
Congcong Gao Gaohui Wei2
Gaohui Wei2 Zhaohui Zheng
Zhaohui Zheng