- 1Biotherapy Center & Cancer Center, The First Affiliated Hospital of Zhengzhou University, Zhengzhou, China
- 2State Key Laboratory of Esophageal Cancer Prevention & Treatment, Zhengzhou University, Zhengzhou, China
- 3Department of Radiology, The First Affiliated Hospital of Zhengzhou University, Zhengzhou, Henan, China
- 4Medical Department, Nanjing Bioheng Biotech Co., Ltd., Nanjing, China
- 5Tianjian Laboratory of Advanced Biomedical Sciences, Academy of Medical Sciences, Zhengzhou University, Zhengzhou, Henan, China
- 6School of Life Sciences, Zhengzhou University, Zhengzhou, Henan, China
- 7School of Public Health, Zhengzhou University, Zhengzhou, Henan, China
We reported the pseudoprogression in an elderly patient with advanced gastric cancer after chimeric antigen receptor (CAR)-T cell therapy. The hepatic metastases enlarged 1 month after CAR-T cell infusion and then shrunk the next month as seen through computed tomography scanning. Based on a comprehensive evaluation that includes imaging, pathology, serum tumor markers, and clinical symptoms, we arrived at a diagnosis of pseudoprogression after CAR-T cell therapy, which has not been reported in previous studies. In this report, we provide detailed descriptions of the patient’s clinical presentation, imaging findings, treatment process, and follow-up outcomes. We believe that this case holds important implications for CAR-T cell therapy research and offers valuable insights for clinical practice.
Introduction
Tumor pseudoprogression after immunotherapy in solid tumors is a phenomenon in which tumor lesions are enlarged or newly added to during the course of immunotherapy, followed by tumor shrinkage (1). Pseudoprogression is more common after the use of immune checkpoint inhibitors but has not been reported in chimeric antigen receptor (CAR)-T cell therapy of solid tumors (2, 3).
An elderly patient was diagnosed with advanced gastric cancer and had undergone radical gastrectomy after neoadjuvant chemotherapy, followed by adjuvant chemotherapy. Liver metastases were found 3 months later; therefore, the patient received sequential treatment with chemotherapy and a tyrosine kinase inhibitor. The tumor continued to progress. Subsequently, the patient was enrolled in a clinical trial of Claudin 18.2 CAR-T cell treated with solid tumor. This clinical trial was approved by the Clinical Research Ethics Committee of the First Affiliated Hospital of Zhengzhou University. The intensity (proportion) of Claudin 18.2 expression in the gastric cancer tissue was 3+ (70%), 2+ (20%), and 1+ (5%). Blood mononuclear cells of the patient were collected on day −25 (25 days before CAR-T cell infusion). After preparatory lymphodepletion with cyclophosphamide, fludarabine, and nab-paclitaxel, an infusion of a total of 8.55×108 (1.5×107/kg) Claudin 18.2 CAR-T cells was administered on day 0. Intermittent fever appeared and peaked on day 3. Grade I cytokine release syndrome (CRS) was assessed and tocilizumab was administered. The levels of INF-γ, IL-5, IL-6, and IL-8 in the peripheral blood peaked on day 6 (Figure 1C, middle), the patient’s temperature settled within a week, and the cytokine concentration was also decreased. CAR-T cell expansion in the peripheral blood peaked 9 days after infusion (Figure 1C, left) and other cell subsets were relatively low at 0–6 days (Figure 1C, right). The patient intermittently took an oral chemotherapy drug on the patient’s own initiative from apheresis to 14 days after infusion.
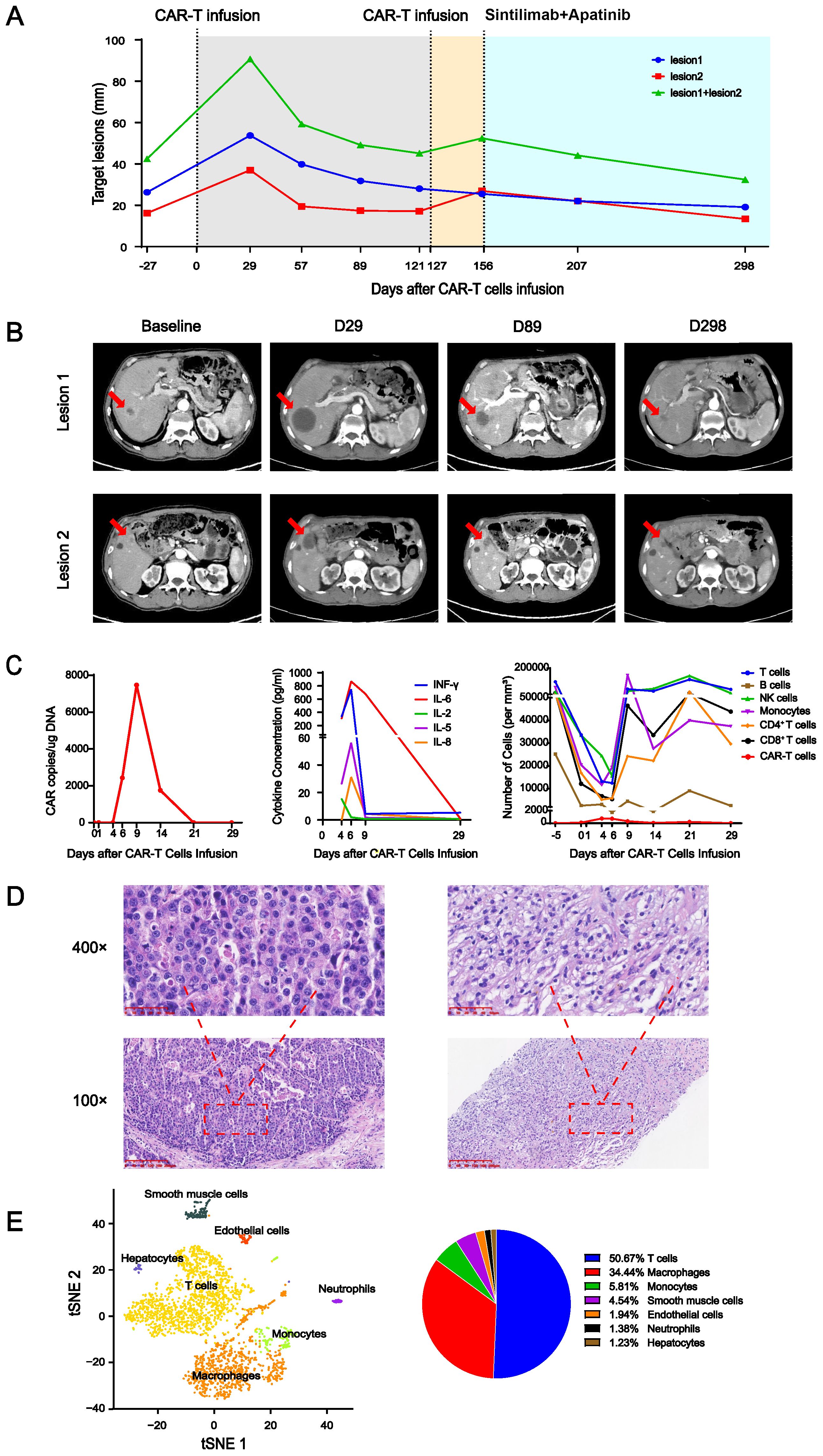
Figure 1. (A) Changes in the target lesion on enhanced CT. The gray area indicates the period after the first CAR-T cell infusion, yellow indicates the period after the second CAR-T cell infusion, and blue indicates the period of sintilimab+apatinib treatment. (B) Several enhanced CT images of selected key points. Red arrow indicates lesions. (C) Peripheral blood parameters. Left panel: CAR copy number over time. Middle panel: dynamics of cytokine levels over time. Right panel: immune cell counts over time. (D) Hematoxylin and eosin (H&E) staining of the surgical specimen of gastric cancer (left) was magnified at 100× and 400×. H&E staining of the liver metastasis specimen taken 100 days after CAR-T cell infusion (right) was magnified at 100× and 400×. (E) The single-cell sequencing (left) of the liver metastasis specimen taken 100 days after CAR-T cell infusion and its statistical pie chart (right).
On day 28, enhanced computed tomography (CT) scanning showed an increase in the total diameter of the two liver metastases from 42.47 mm at baseline to 90.73 mm (Figures 1A, B), which was assessed as disease progression according to Response Evaluation Criteria in Solid Tumors (RECIST) 1.1. The patient’s Karnofsky Performance Status score gradually improved, multiple tumor markers decreased in the peripheral blood, and no deterioration of liver function was observed. CT reexamination showed that the total diameters of the two target lesions were 59.27 mm on day 56 and 49.15 mm on day 89 after infusion. On day 100, pathological biopsy of liver metastasis showed a large amount of immune cell infiltration, but no tumor cells compared to the primary tumor (Figure 1D), and single-cell sequencing showed a mass of immune cell infiltration dominated by T lymphocytes (Figure 1E). This was analogous to the pseudoprogression observed in immune checkpoint inhibitor therapy (1). The total diameter of the target lesions on day 121 was smaller than before, but a new lesion appeared. At this time, no CAR-T cells were detected in the peripheral blood; therefore, after preparatory lymphodepletion, a total of 1×109 (2×107/kg) Claudin 18.2 CAR-T cells were infused on day 127. No any-grade CRS or immune effector cell-associated neurotoxicity syndrome (ICANS) occurred. The CAR-T cell copy number in peripheral blood peaked at day 14 after reinfusion at 4,858.59 copies/μg DNA. On day 156, after reinfusion for 29 days, the target lesions were enlarged again with no CAR-T cells detected in peripheral blood, at which time the treatment regimen of sintilimab (an anti-PD-1 antibody) and apatinib (a tyrosine kinase inhibitors) was started. The therapy was continued for seven cycles, and the tumor continued to shrink at the last follow-up CT imaging on day 298. At the most recent long-term survival follow-up, the patient remained alive at 619 days after CAR-T cell reinfusion.
In this case, the total diameter of the target lesions increased by more than double rapidly after the infusion of CAR-T cell. According to RECIST, it would be assessed as disease progression (4). However, it allows for more time to observe and confirm whether it is true disease progression or potentially an immune-related event according to iRECIST (5). Subsequent observations revealed that the total diameters of the tumor target lesions gradually decreased. Combined with the patient’s clinical symptoms, tumor markers in serum, and pathology, we believed this to be pseudoprogression (6). Interestingly, the lesion of the patient temporarily enlarged again after reinfusion of the CAR-T cell. This case indicates that iRECIST may be more suitable for evaluating the efficacy of CAR-T cell therapy in solid tumors than RECIST (7, 8). After CAR-T cell infusion combined with anti-PD-1 antibody treatment, the application of this combined immunotherapy resulted in sustained disease remission, indicating the feasibility of combined immunotherapy (9, 10).
In conclusion, this case describes the phenomenon of pseudoprogression after CAR-T cell therapy in solid tumors for the first time. This case excluded the possibility of hyperprogression after treatment through biopsy and emphasizes the importance of using iRECIST in evaluating the disease status during CAR-T cell therapy for solid tumors. Additionally, this case demonstrates the feasibility and potential benefits of CAR-T cell infusion combined with anti-PD-1 antibody for the treatment of solid tumors.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving humans were approved by the Clinical Research Ethics Committee of the First Affiliated Hospital of Zhengzhou University. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
XZ: Funding acquisition, Writing – review & editing, Conceptualization, Investigation, Methodology, Writing – original draft. YL: Formal analysis, Methodology, Writing – original draft. GQ: Investigation, Methodology, Validation, Writing – original draft. YG: Data curation, Software, Writing – original draft. QL: Data curation, Software, Validation, Writing – original draft. XC: Data curation, Investigation, Writing – original draft. XT: Data curation, Investigation, Writing – original draft. YY: Data curation, Investigation, Writing – review & editing. JR: Investigation, Writing – original draft. YZ: Funding acquisition, Supervision, Validation, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This study was supported by the National Key R&D Program: Intergovernmental International Science and Technology Innovation Cooperation Project (Grant no. 2022YFE0141000) and the Healthy Talents Project of Henan Province (YXKC2021035). The funding sources played a role in providing financial support for the research and also participated in date collection. We would like to clarify that we have not been paid to write this article by any pharmaceutical company or other agency.
Conflict of interest
YY and JR were employees of Nanjing Bioheng Biotech Co., Ltd.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Jia W, Gao Q, Han A, Zhu H, Yu J. The potential mechanism, recognition and clinical significance of tumor pseudoprogression after immunotherapy. Cancer Biol Med. (2019) 16:655–70. doi: 10.20892/j.issn.2095-3941.2019.0144
2. Szeto GL, Finley SD. Integrative approaches to cancer immunotherapy. Trends Cancer. (2019) 5:400–10. doi: 10.1016/j.trecan.2019.05.010
3. Danylesko I, Shouval R, Shem-Tov N, Yerushalmi R, Jacoby E, Besser M, et al. Immune imitation of tumor progression after anti-CD19 chimeric antigen receptor T cells treatment in aggressive B-cell lymphoma. Bone Marrow Transplant. (2021) 56:1134–43. doi: 10.1038/s41409-020-01156-y
4. Litière S, Collette S, de Vries EG, Seymour L, Bogaerts J. RECIST - learning from the past to build the future. Nat Rev Clin Oncol. (2017) 14:187–92. doi: 10.1038/nrclinonc.2016.195
5. Ferté C, Marabelle A. iRECIST: A clarification of tumour response assessment in the immunotherapy era. Eur J Cancer. (2017) 77:165–7. doi: 10.1016/j.ejca.2017.02.015
6. Nishino M, Hatabu H, Hodi FS. Imaging of cancer immunotherapy: current approaches and future directions. Radiology. (2019) 290:9–22. doi: 10.1148/radiol.2018181349
7. Park HJ, Kim GH, Kim KW, Lee CW, Yoon S, Chae YK, et al. Comparison of RECIST 1.1 and iRECIST in patients treated with immune checkpoint inhibitors: A systematic review and meta-analysis. Cancers (Basel). (2021) 3(1):120. doi: 10.3390/cancers13010120
8. Seymour L, Bogaerts J, Perrone A, Ford R, Schwartz H, Mandrekar S, et al. iRECIST: guidelines for response criteria for use in trials testing immunotherapeutics. Lancet Oncol. (2017) 18:e143–52. doi: 10.1016/S1470-2045(17)30074-8
9. Adusumilli PS, Zauderer MG, Rivière I, Solomon SB, Rusch VW, O'Cearbhaill RE, et al. A phase I trial of regional mesothelin-targeted CAR T-cell therapy in patients with Malignant pleural disease, in combination with the anti-PD-1 agent pembrolizumab. Cancer Discovery. (2021) 11:2748–63. doi: 10.1158/2159-8290.CD-21-0407
Keywords: pseudoprogression, CAR-T cell therapy, gastric cancer, evaluation of therapeutic efficacy, cellular immunotherapy
Citation: Zhao X, Liu Y, Qin G, Ge Y, Li Q, Chen X, Tian X, Yu Y, Ren J and Zhang Y (2025) Pseudoprogression in CAR-T cell therapy for solid tumor: Case report. Front. Immunol. 15:1504104. doi: 10.3389/fimmu.2024.1504104
Received: 30 September 2024; Accepted: 13 December 2024;
Published: 08 January 2025.
Edited by:
Paulo Rodrigues-Santos, University of Coimbra, PortugalReviewed by:
Yangmin Zhu, Guangdong Second Provincial General Hospital, ChinaYufei Wang, Dana–Farber Cancer Institute, United States
Copyright © 2025 Zhao, Liu, Qin, Ge, Li, Chen, Tian, Yu, Ren and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yi Zhang, eWl6aGFuZ0B6enUuZWR1LmNu; Xuan Zhao, emhhb3h1YW45MjlAZm94bWFpbC5jb20=
†These authors have contributed equally to this work
 Xuan Zhao
Xuan Zhao Yanfen Liu1,2†
Yanfen Liu1,2† Guohui Qin
Guohui Qin Xinfeng Chen
Xinfeng Chen Yi Zhang
Yi Zhang