- 1Department of Immunology and Rheumatology, Division of Advanced Preventive Medical Sciences, Nagasaki University Graduate School of Biomedical Sciences, Nagasaki, Japan
- 2Department of Respiratory Medicine, Nagasaki University Graduate School of Biomedical Sciences, Nagasaki, Japan
- 3Rheumatic Disease Centre, Sasebo Chuo Hospital, Sasebo, Japan
Objectives: Little is known about how various treatments impact the progression of interstitial lung disease (ILD) in rheumatoid arthritis (RA) patients. Here, we compared ILD progression in RA patients treated with Janus kinase inhibitors (JAKi) or biological disease-modifying anti-rheumatic drugs (bDMARDs). In vitro experiments were also performed to evaluate the potential effects of the drugs on epithelial–mesenchymal transition (EMT), a key event in pulmonary fibrosis.
Methods: This retrospective study included 93 RA-ILD patients who initiated treatment with JAKi, tumour necrosis factor inhibitors (TNFi), or abatacept between 2017 and 2020. Worsening ILD was quantified by changes in chest computed tomography (CT) scans between baseline and follow-up (mean 14 months, range 6–51 months). Response to treatment was evaluated using Disease Activity Score-28 with erythrocyte sedimentation rate (DAS28-ESR). Expression of the EMT marker N-cadherin in A549 lung cells was assessed by western blotting.
Results and discussion: Worsening ILD was detected in 19.4% (7/36), 16.7% (5/30), and 22.2% (6/27) of patients treated with JAKi, abatacept, and TNFi, respectively. Multivariate analysis identified female gender (P=0.043) and >10% fibrotic lesions (P=0.015) as significant predictors of worsening ILD. DAS28-ESR-based non-responder status was also significantly associated with worsening ILD (P=0.0085). In vitro, combination treatment with methotrexate and baricitinib significantly impeded EMT progression. Worsening ILD was associated with more extensive fibrotic lesions at baseline and female gender in RA patients treated with JAKi or bDMARDs. JAKi and methotrexate co-treatment may prove beneficial in modifying key events underlying the pathogenesis of RA-ILD.
Introduction
Rheumatoid arthritis (RA) is an inflammatory autoimmune disease characterized by chronic joint inflammation, and it is often accompanied by extra-articular effects, such as interstitial lung disease (ILD). RA-associated ILD (RA-ILD) is a significant complication and can affect prognosis (1, 2). Although the advent of biological disease-modifying anti-rheumatic drugs (bDMARDs) and Janus kinase inhibitors (JAKi) has expanded the treatment options for RA, the optimal treatment of RA-ILD remains undefined (3, 4). Notably, several studies have highlighted the need to be aware of the risks of respiratory infection and drug-induced lung injury when using anti-rheumatic drugs in RA patients with ILD (5, 6).
Currently, abatacept, a fusion protein of the extracellular domain of cytotoxic T lymphocyte-associated protein 4 (CTLA4) and the Fc region of human IgG1, is considered to be the most reasonable option for treating RA patients with ILD (7); however, recent reports suggest that the efficacy and safety of JAKi may be comparable to those of abatacept in terms of their impact on the disease behavior of RA-ILD (8, 9). Factors involved in the progression or acute exacerbation of RA-ILD include usual interstitial pneumonia (UIP) pattern, decreased forced vital capacity, cigarette smoking, and high titers of anti-cyclic citrullinated protein antibody (ACPA) (10, 11). Additionally, risk factors associated with new-onset RA-ILD include older age, male gender, cigarette smoking, high titers of rheumatoid factor and ACPA, and poor control of arthritis activity (12–14).
The epithelial–mesenchymal transition (EMT) is a key physiological process during which epithelial cells lose their polarity and change to mesenchymal phenotypes. Downregulation of the epithelial cell marker E-cadherin and upregulation of the mesenchymal marker N-cadherin, also known as cadherin switching, are characteristic features of EMT (15, 16). Although of great physiological importance, EMT is also associated with various pathological states, especially after cell injury and chronic inflammation (17). Indeed, EMT is considered to be one of the key processes involved in the pathogenesis of RA-ILD, similar to the events leading to idiopathic pulmonary fibrosis (18). In vitro studies with human alveolar type II cells have shown that EMT can be induced by treatment with factors such as transforming growth factor-β and interleukin (IL)-6, and it has been reported to be inhibited by blocking the JAK/STAT signaling pathway (19). However, the effects of methotrexate (MTX), an anchor drug for managing RA (20), on EMT remain largely unexplored.
The aim of the present study was to compare the temporal changes in chest computed tomography (CT) images of patients with RA-ILD treated with JAKi or bDMARDs and to identify factors associated with deterioration of RA-ILD on imaging. In addition, we investigated the possible mechanism of action of JAKi and MTX treatment on the fibrotic state of RA-ILD patients by investigating their effects on IL-6-induced EMT in alveolar epithelial cells in vitro. Our results shed light on the potential for JAKi and MTX therapy to inhibit the progression of RA-ILD.
Materials and methods
Study design and participants
This retrospective cohort study focused on RA patients with ILD who started a new treatment program with JAKi (tofacitinib, baricitinib) or bDMARDs (TNFi, abatacept) at Nagasaki University Hospital or Sasebo Chuo Hospital between January 2017 and December 2020. A flow diagram illustrating the selection of research subjects is shown in Figure 1. From the initial pool of 468 included patients, those who discontinued these drugs within 6 months of initiation or whose pre- and post-treatment chest CT images were not available were excluded (n=138). The remaining 330 patients were assessed for ILD using high-resolution CT (HRCT) imaging at the start of treatment. Patients with bronchiolitis or emphysema as the main lesion and those diagnosed with organizing pneumonia were excluded. The final group of 93 patients with confirmed ILD were selected for analysis.
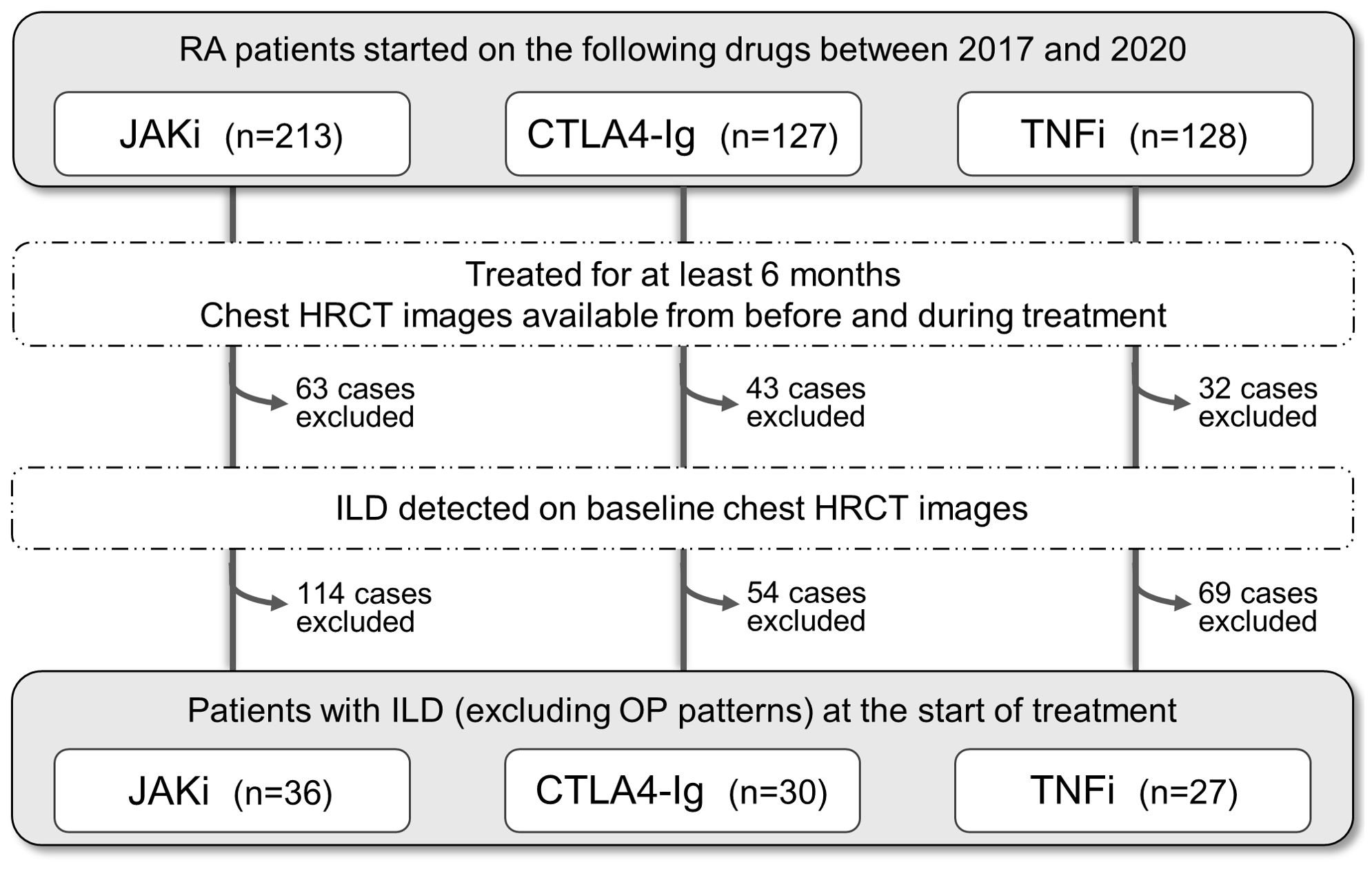
Figure 1. Selection of rheumatoid arthritis patients with interstitial lung disease for this study. Flowchart of the selection process. The initial pool comprised total 468 patients with rheumatoid arthritis (RA) who started treatment with JAK inhibitors (JAKi), T cell co-stimulation inhibitor (CTLA4-Ig), or TNF inhibitors (TNFi) between 2017 and 2020. Patients were excluded if (i) treatment duration was <6 months, (ii) chest high-resolution computed tomography (HRCT) images were unavailable at either baseline or follow-up, (iii) interstitial lung disease was not noted on baseline HRCT images, (iv) bronchiolitis or emphysema was the main lesion, and (v) organizing pneumonia (OP) pattern was diagnosed.
Ethics statement
This study was approved by the Institutional Review Board of Nagasaki University (approval number 11032819), and informed consent was obtained from each patient.
Chest CT image evaluation and scoring
The classification of HRCT patterns of RA-ILD was performed by experienced pulmonologists affiliated with the Japanese Respiratory Society (RM and TT), and by board-certified members (DO, HY, HI, TK, and NS) according to the joint statement of the American Thoracic Society/European Respiratory Society/Japanese Respiratory Society/Latin American Thoracic Society (21). The patterns were evaluated by two pulmonologists. In case of disagreement, one of the board-certified pulmonologists acted as a third reviewer and made the final assessment. The presence of ground-glass attenuation, reticular shadows, consolidation, traction bronchiectasis/bronchiolectasis and honeycombing, and the proportion of each lesion in the total lung field were assessed on a case-by-case basis by two pulmonologists independently. Ground-glass attenuation, reticular shadows, and consolidation were defined as inflammatory lesions; and traction bronchiectasis/bronchiolectasis and honeycombing were defined as fibrotic lesions. A CT score was calculated using the formula described by Ichikado et al. (22).
CT score = (1 × percentage of lung area with normal attenuation) + (2 × percentage of lung area with ground-glass attenuation or reticular shadows) + (3 × percentage of lung area with consolidation) + (4 × percentage of lung area with ground-glass attenuation or reticular shadows accompanied by traction bronchiectasis/bronchiolectasis) + (5 × percentage of lung area with consolidation accompanied by traction bronchiectasis/bronchiolectasis) + (6 × percentage of lung area with honeycombing).
In this formula, the percentage represents the proportion of the total lung area affected. For example, if the whole lung was normal, the score would be 1 × 100 = 100 points. If 80% of the lung was normal and 20% was affected by honeycombing, the score would be calculated as (1 × 80) + (6 × 20) = 200 points. The final score was obtained by averaging the scores of the two evaluators. Cases were deemed “worsened ILD” if the scores assigned by both evaluators were higher post-treatment compared with pre-treatment.
Disease activity and improvement assessment
Disease activity status was evaluated using the 4-variable Disease Activity Score in 28 joints with erythrocyte sedimentation rate (DAS28-ESR). The definitions were: high disease activity (HDA) >5.1, moderate disease activity (MDA) ≥3.2 to ≤5.1, low disease activity (LDA) ≥2.6 to <3.2, and remission <2.6 (23). Patients were assigned to responder groups using the European League Against Rheumatism (EULAR) response criteria based on DAS28-ESR scores: good responders (improvement >1.2 and current score ≤3.2), moderate responders (improvement >0.6 to ≤1.2 and current score ≤5.1 or improvement >1.2 and current score >3.2), and non-responders (improvement ≤0.6 regardless of current score or improvement >0.6 to ≤1.2 and current score >5.1) (24).
Cell culture
A549 cells (human type II alveolar epithelial cell line) were purchased from the European Collection of Authenticated Cell Cultures (ECACC, #86012804) and cultured in Kaighn’s modified Ham’s F12 medium (F12K Medium, Life Technologies, Tokyo, Japan) supplemented with 10% fetal bovine serum and 1% penicillin/streptomycin. Cells were maintained at 37°C in a 5% CO2 environment. For experiments, cells were plated into 6-well plates at 2.0 × 105 cells/well and treated with MTX (FUJIFILM Wako, Osaka, Japan) and/or baricitinib (Chemscene, Monmouth Junction, NJ, USA) at the indicated concentrations for 48 h and 3 h, respectively. EMT was induced by addition of IL-6 (Sigma-Aldrich, St. Louis, MO, USA) at 50 ng/mL for 24 h (25) and the cells were harvested for analysis.
Western blot analysis
Proteins were extracted from A549 cells using RIPA Buffer (FUJIFILM Wako) supplemented with protease inhibitors (Takara Bio, Shiga, Japan) and phosphatase inhibitors (Thermo Fisher Scientific, Waltham, MA, USA). Lysates were centrifuged at 15,000 rpm for 15 min at 4°C and the supernatants were collected. N-cadherin and β-actin levels were quantified using an automated Wes Simple Western system (ProteinSimple, San Jose, CA, USA) according to the manufacturer’s instructions. The primary antibodies were anti-N-cadherin (#GTX127345, GeneTex, Irvine, CA, USA; 1:100 dilution) and anti-β-actin (#GTX109639, GeneTex; 1:500 dilution).
Statistical analysis
Continuous variables are presented as medians with interquartile ranges (IQR), and categorical variables as numbers and percentages. Statistical analyses were performed using JMP Pro 17 software (SAS Institute, Cary, NC, USA). Proportions of binary data were compared using Fisher’s exact test. Continuous variables were compared using either Student’s t-test or the Mann–Whitney U test. Predictive factors for ILD progression on CT images were identified using Cox regression analysis, with variable selection based on existing knowledge, clinical experience, and the results of univariate analyses. Statistical significance was defined as a two-tailed P value of <0.05. This study was exploratory and no adjustment for multiplicity was planned.
Results
Characteristics of participants
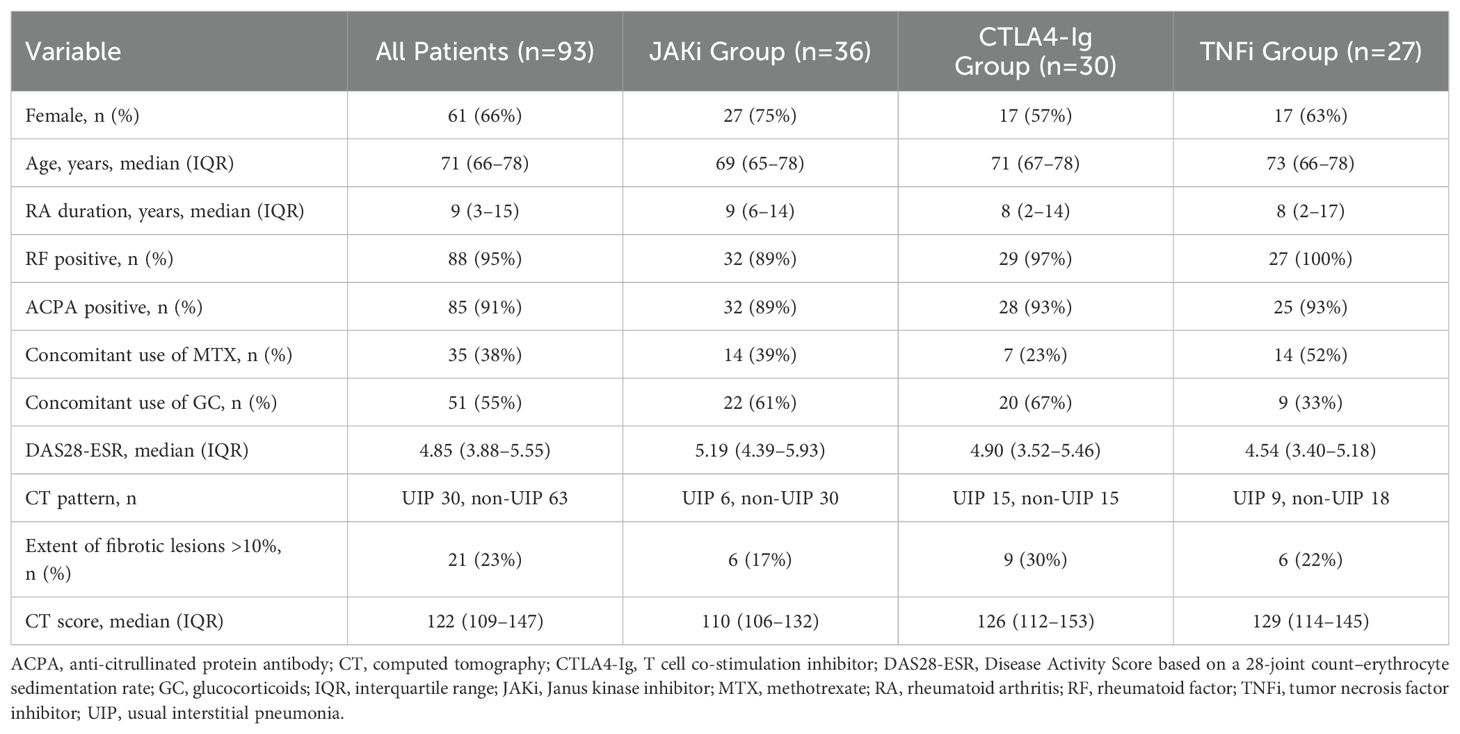
Patient characteristics at baseline are summarized in Table 1. This study included 36 patients treated with JAKi (29 with tofacitinib, 7 with baricitinib), 30 patients treated with the T cell co-stimulation inhibitor CTLA4-Ig (abatacept), and 27 patients treated with TNFi (14 with golimumab, 6 with etanercept, 4 with adalimumab, and 3 with certolizumab pegol) (Table 2). A total of 61 of the 93 patients (66%) were female. The median age was 71 years (IQR 66–78 years) and the median duration of RA was 9 years (range 3–15 years). The rheumatoid factor positivity rate was 95% and the ACPA positivity rate was 91%. The proportion of patients taking concomitant MTX and glucocorticoids (GC) was 38% and 55%, respectively. The median baseline DAS28-ESR was 4.85 (IQR 3.88–5.55). HRCT imaging assessment classified 30 patients (32%) as having definite UIP pattern and the remaining 63 patients (68%) as having non-UIP pattern, which included probable UIP, indeterminant for UIP, and alternative diagnosis pattern (21). Baseline RA disease activity was highest in the JAKi-treated group; the proportion of UIP pattern was highest in the CTLA4-Ig-treated group; and the proportion of concomitant treatment with MTX was highest in the TNFi-treated group.
CT score evaluation of chest CT images
CT scores were calculated at baseline and follow-up (average, 14 months; range, 6–51 months). An increase in CT score from baseline to follow-up (indicating worsening lung lesions) was observed in 18 of the 93 patients (19.4%); which included 19.4% (7/36) of the JAKi group, 16.7% (5/30) of the CTLA4-Ig group, and 22.2% (6/27) of the TNFi group (Table 2).
Association between disease activity and worsening CT score
The relation between disease activity and worsening CT score was investigated in a total of 79 patients with continuously assessed DAS28-ESR. At baseline, 40%, 48%, 6%, and 6% of patients had HDA, MDA, LDA, or were in remission, respectively; at follow-up, these proportions were 8%, 49%, 25%, and 18%, respectively. The percentage of patients with worsening CT scores was 17.7% (6/34) of patients with LDA or in remission compared with 22.2% (10/45) of patients with HDA or MDA (P=0.78). Based on the EULAR response criteria, non-responders had a significantly higher rate of CT score worsening (42.9%, 9/21) compared to the good/moderate responders (12.1%, 7/58) (P=0.0085) at follow-up. When the changes in inflammatory lesions and fibrotic lesions were examined separately, both were found to be significantly worse in the non-responders compared with the good/moderate responders (P=0.0050 for inflammatory lesions, P=0.0024 for fibrotic lesions) (Figure 2).
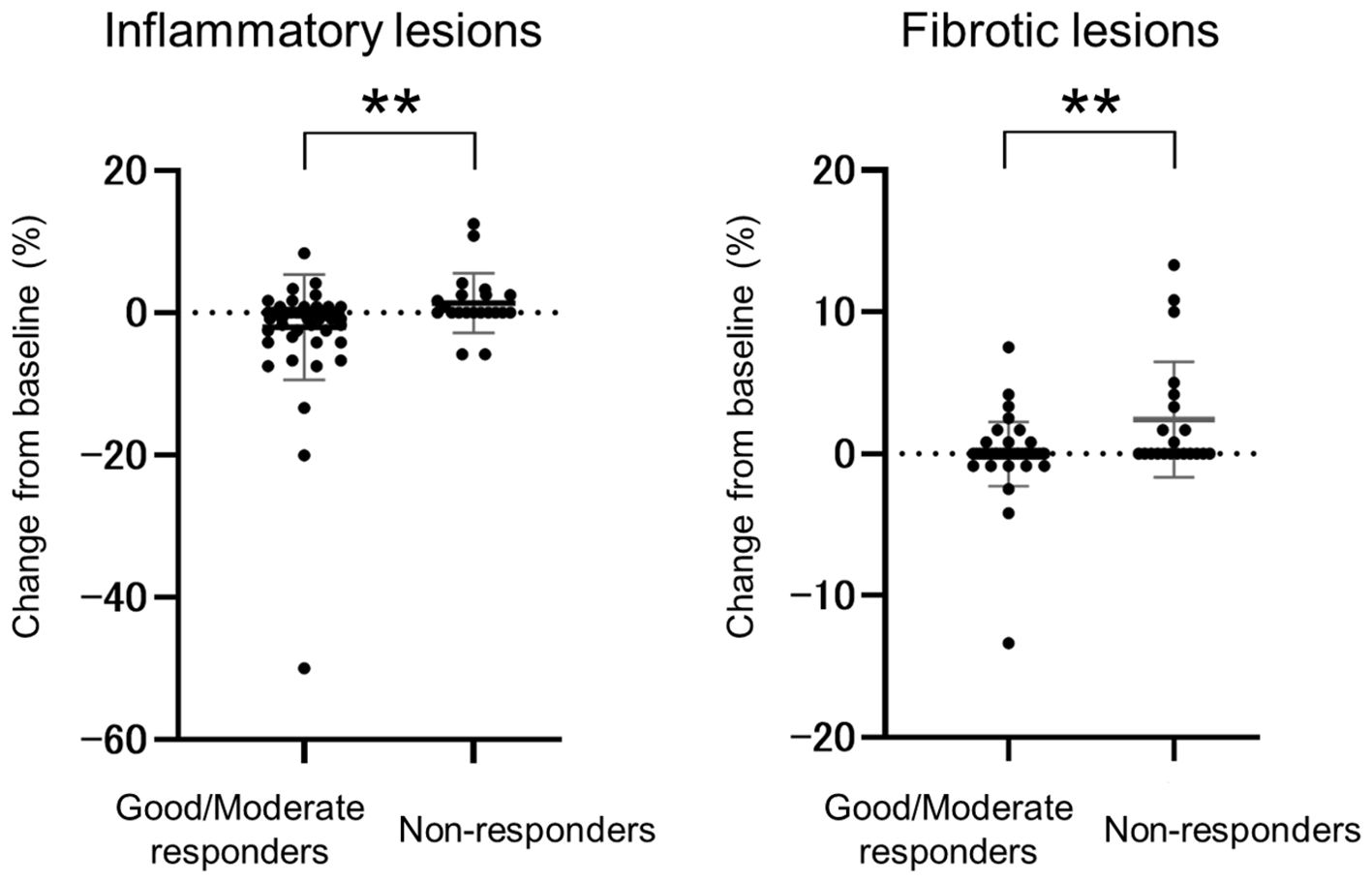
Figure 2. Comparison of inflammatory and fibrotic lesion changes between good/moderate responders and non-responders. Change from baseline to follow-up in the good/moderate responder group (n=58) and non-responder group (n=21) based on EULAR response criteria and DAS28-ESR scores. Data are presented as the mean and standard deviation; circles represent individual patients. ** P <0.01.
Analysis of predictive factors for CT score worsening
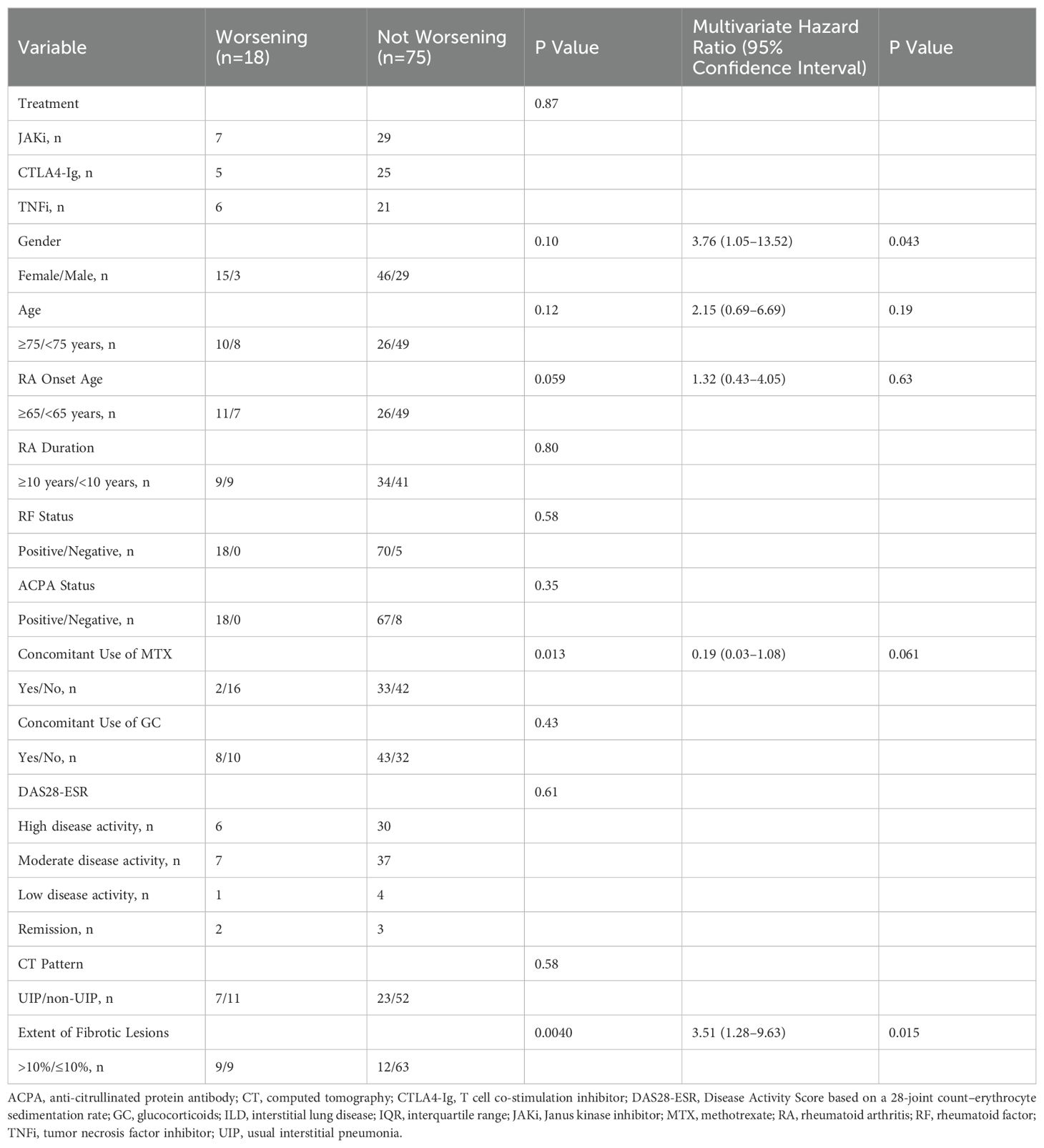
Table 2 presents the results of Cox multivariate analysis for the 93 patients according to worsening (n=18) or not worsening (n=75) CT scores at follow-up. Explanatory variables were selected based on their potential for involvement in the progression of RA-ILD as assessed by existing knowledge, clinical experience, and the results of the univariate analysis. Multivariate analysis identified “extent of fibrotic lesions >10%” (HR 3.51, 95% CI 1.28–9.63, P=0.015) and “female gender” (HR 3.76, 95% CI 1.05–13.52, P=0.043) as factors significantly associated with an increased risk of worsening CT score. Co-administration of MTX showed a trend towards a reduced risk; however, the difference narrowly missed reaching statistical significance (HR 0.19, 95% CI 0.03–1.08, P=0.061).
The change in CT score (ΔCT score) for each patient is displayed using cumulative probability plots in Figure 3. The beneficial effect of MTX co-treatment was evident even among patients with fibrotic lesions in less than 10% of the lung field. Only 3% (1/33) of patients in the MTX group had worsening CT scores compared with 21% (8/39) of patients in the non-MTX group (P=0.033). Non-responders (EULAR criteria) were also significantly less frequent in the MTX group (12%, 3/25) compared with the non-MTX group (38%, 13/34) (P=0.038).
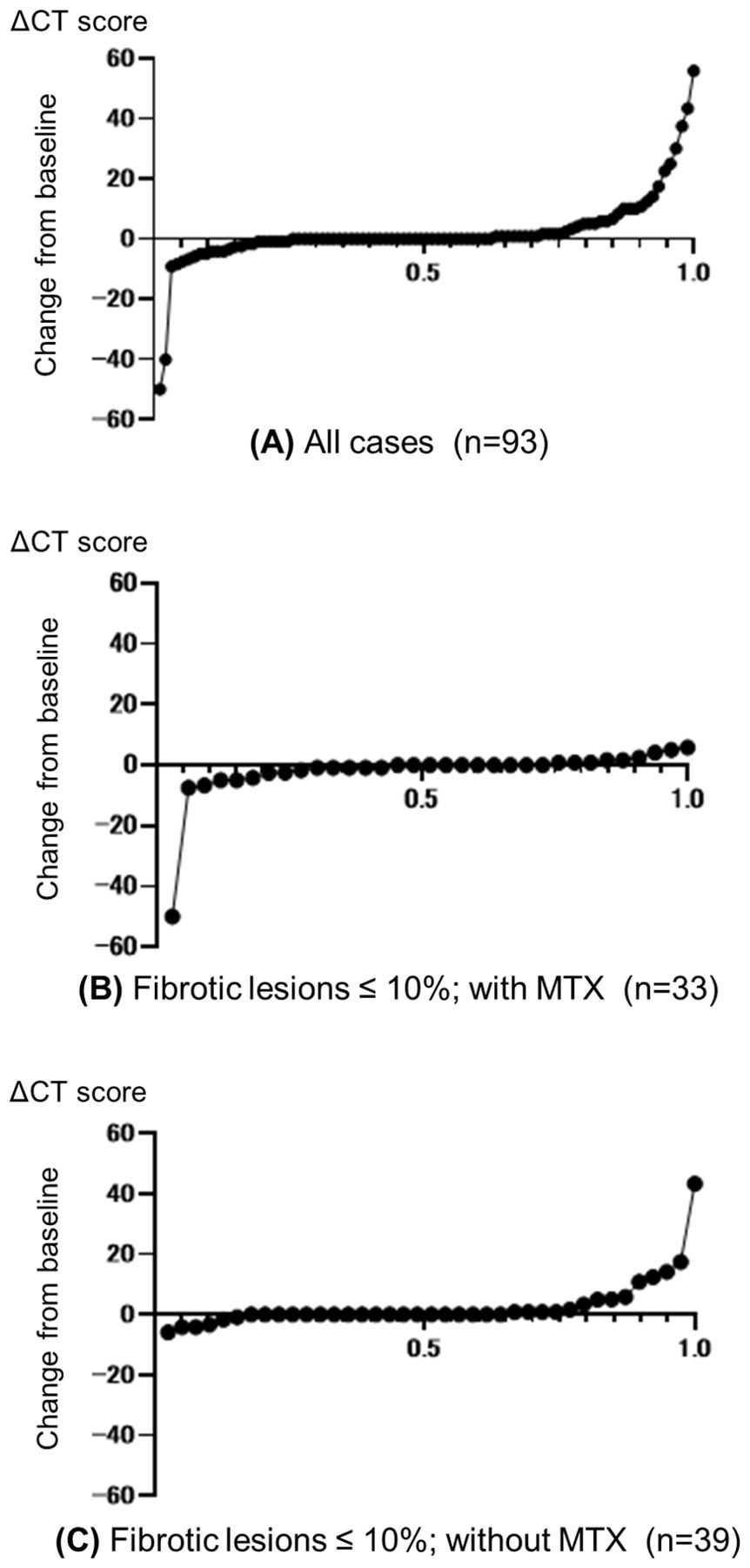
Figure 3. Cumulative probability plots of change from baseline in CT scores. Change from baseline to follow-up (ΔCT score) for (A) all patients and (B, C) patients with pulmonary fibrosis lesions in ≤10% of the total lung field who were treated with (B) or without (C) concomitant methotrexate (MTX). Circles represent individual patients.
In vitro effects of MTX and baricitinib on EMT of A549 cells
Because the EMT is a crucial mechanism in the pathogenesis of RA-ILD fibrosis, we assessed the potential effects of MTX and the JAKi baricitinib on the EMT in vitro. To this end, western blot analysis was performed to quantify expression of the mesenchymal phenotypic marker N-cadherin in human A549 cells pre-treated with the drugs and then treated with IL-6 to induce EMT. Pre-treatment of A549 cells with either MTX for 48 h (Figure 4A) or baricitinib for 3 h (Figure 4B) inhibited the IL-6-induced increase in N-cadherin expression. However, combined pre-treatment with MTX at 0.3 µM and baricitinib at 0.2 µM, which are concentrations comparable to their maximal serum drug concentrations in clinical practice, resulted in a significant reduction in IL-6-induced N-cadherin levels that was superior to the reductions achieved by treatment with baricitinib alone (Figure 4C). This result suggests that MTX and baricitinib have an additive effect in modulating progression through the EMT, which is a key pathway in fibrosis pathologies.
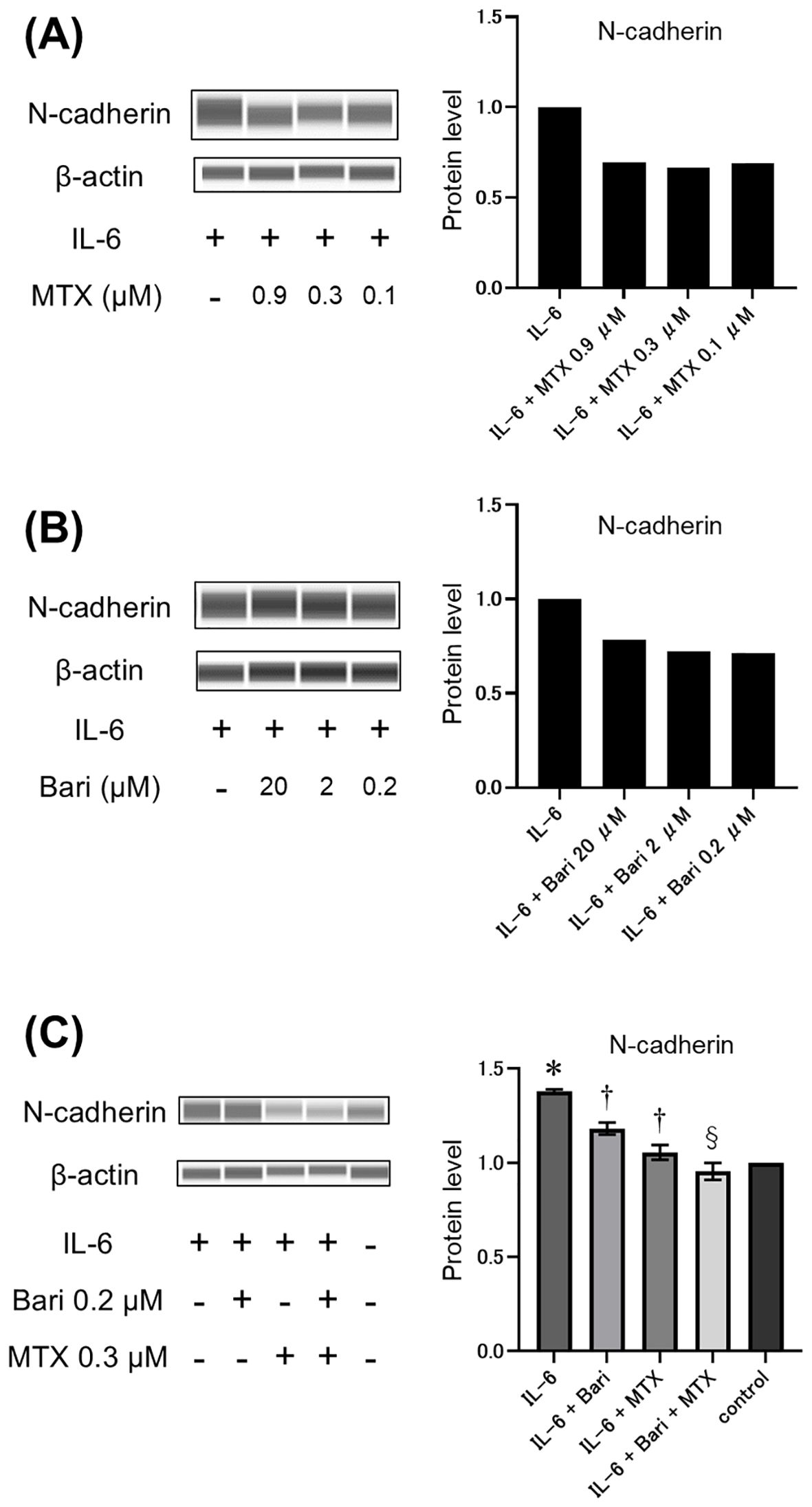
Figure 4. Effects of MTX and baricitinib treatment on IL-6-induced N-cadherin expression in A549 cells. (A–C) Western blot analysis (left panels) and relative quantification (right panels) of N-cadherin expression in A549 cells pre-treated with various concentrations of methotrexate (MTX) for 48 h (A), baricitinib (Bari) for 3 h (B), or 0.2 μM Bari and/or 0.3 μM MTX for 48 h and 3 h, respectively (C) and then stimulated with 50 ng/mL IL-6 for 24 h Data are expressed as the mean of duplicates (a and b) or the mean ± standard error of quadruplicates (C). *P<0.001 compared to the control group, †P<0.01 compared to the IL-6 group, and §P<0.05 compared to the IL-6+Bari group.
Discussion
In this study, we identified factors associated with worsening of pre-existing ILD in RA patients treated with JAKi or bDMARDs and, further, examined the impact of JAKi and MTX pre-treatment on IL-6-induced EMT in A549 cells in vitro, thereby providing new insights into therapeutic strategies for patients with RA-ILD.
We quantified changes in ILD by assigning a CT score on chest HRCT images. Overall, 19.4% of the 93 patients had worsening CT scores, which included 19.4% of the JAKi group, 16.7% of the CTLA4-Ig group, and 22.2% of the TNFi group. It should be noted that the clinical characteristics of the patients in the present study were not homogeneous between groups, which limits our ability to directly compare the effects of these drugs on RA-ILD. Abatacept is currently considered the optimal bDMARD for RA patients with ILD (7, 26); however, recent studies reported that the efficacy and safety of JAKi are comparable to those of abatacept in RA-ILD (8, 9). The proportions of patients with worsening of RA-ILD in our study were similar to those reported in previous studies, even though the HRCT assessment methods differed between studies (8, 9, 27).
In our patient population, multivariate analysis identified “extent of fibrotic lesions >10%” and “female gender” as factors significantly associated with worsening RA-ILD. Lesions with traction bronchiectasis/bronchiolectasis and honeycombing were defined as fibrotic lesions in our study. Although UIP pattern has been reported to be an exacerbating factor in RA-ILD (11), it is difficult to clearly distinguish between UIP and non-specific interstitial pneumonia on HRCT. Therefore, it is important to evaluate both the presence and the extent of fibrotic lesions to predict disease behavior in RA-ILD (10, 28). Fibrosing lung disease affecting more than 10% of the lung volume on HRCT, as used in the inclusion criteria of the INBUILD trial (29), suggests a progression-prone phenotype, serving as an indicator for the use of anti-fibrotic drugs.
We found that patients with poor improvement in DAS28-ESR (non-responders based on EULAR response criteria) showed a higher tendency for worsening CT score than good or moderate responders. This result is consistent with previous findings that high RA disease activity significantly affects RA-ILD progression (30, 31), suggesting that reducing systemic inflammation with anti-rheumatic drugs could modify the natural course of RA-ILD. Although male gender is generally reported as a risk factor for worsening RA-ILD (11), our study identified female gender as a predictor of worsening ILD based on HRCT imaging. One possible explanation for this discrepancy is that, in our cohort, a higher proportion of women than men were non-responders (32.1% vs. 15.4%). It is also possible that the results could be driven by specific characteristics of our study population, such as age, smoking status, complications, or the severity of RA at baseline.
Patients co-administered MTX exhibited a lower rate of worsening ILD in this study, which is intriguing. Consistent with guidance that MTX is contraindicated in patients with severe respiratory disorders or severe pulmonary fibrosis, as judged by chest radiographs (32), most patients receiving MTX in our cohort had ≤10% fibrotic lesions. Notably, the MTX group had better outcomes than the non-MTX group even after accounting for potential bias related to the severity of lung lesions. Moreover, the MTX group contained fewer non-responders (based on EULAR response criteria) than did the non-MTX group, suggesting that a reduction in disease activity might have contributed to modifying the natural course of RA-ILD.
The local effects of anti-rheumatic drugs in lung tissues are not well understood. Recent findings from a retrospective study of RA patients treated with adalimumab, abatacept, tocilizumab, rituximab, or tofacitinib showed that the incidence of new-onset RA-ILD was lowest in patients treated with tofacitinib (33). Furthermore, it has been reported that MTX may lead to delayed onset and a lower incidence of RA-ILD in early RA inception cohorts (34). The mechanisms by which tofacitinib and MTX inhibit the development of RA-ILD are not fully understood. We conducted in vitro experiments with A549 cells to determine the effect of baricitinib and MTX on EMT, a key mechanism in lung fibrosis. Our finding that baricitinib attenuated IL-6-induced EMT in A549 cells corroborates the findings of a previous study (25). However, in contrast to reports that MTX induces EMT-like changes in A549 cells (35), we found that MTX inhibited IL-6-induced EMT. Interestingly, the effect on EMT of baricitinib and MTX in combination was greater than that of baricitinib alone, suggesting that combining JAKi and MTX may prove beneficial in modifying the pathogenesis of lung fibrosis. Similar results were obtained in the RA-BEGIN trial, which showed that the baricitinib and MTX combination therapy group had the lowest rate of progression of structural joint damage on X-ray (36). A meta-analysis of JAKi and MTX therapy also indicated that combination MTX and JAKi therapy was superior to JAKi monotherapy in achieving LDA or remission in RA patients, although it also carried a higher risk of adverse events (37). The beneficial effect of JAKi and MTX on RA disease activity may also aid in slowing the progression of ILD. Further studies will be required to elucidate the detailed mechanisms involved; however, our findings suggest the possibility that JAKi and MTX may have a combined effect on fibrosis in local lung tissue.
This study has several limitations. First, the retrospective design limits the ability to infer causal relationships between treatments and observed outcomes; thus, prospective studies are warranted to confirm these findings. Second, the small sample size of 93 cases did not allow us to apply propensity score matching to adjust for imbalances in clinical characteristics or to perform subgroup analyses according to specific drug types. Third, the lack of information on smoking status and diabetes diagnosis prevented us from analyzing the impact of these risk factors on RA-ILD progression. Fourth, it was not possible to analyze biomarkers such as Krebs von den Lungen-6, which indicates disease activity in ILD. Finally, the interpretation of HRCT images, which is critical for the classification of fibrosis patterns, was dependent on clinician assessment and thus subject to inter-observer variability, potentially introducing bias in the evaluation of ILD progression.
In conclusion, we found that extensive fibrotic lesions, female gender, and poor improvement in disease activity were significantly associated with worsening ILD in RA patients treated with JAKi and bDMARDs, and we also showed the potential for co-administered MTX to modify the course of RA-ILD in these patients. Additionally, our in vitro results suggest that the combination of MTX and JAKi may have an additive effect in attenuating EMT, a key mechanism in lung fibrosis. Further research will be necessary to fully understand these interactions and to refine treatment strategies for RA-ILD.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by the Institutional Review Board of Nagasaki University. The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants’ legal guardians/next of kin because The methods and content of this epidemiological study do not require written informed consent. Ethical approval was not required for the studies on animals in accordance with the local legislation and institutional requirements because only commercially available established cell lines were used.
Author contributions
SK: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Writing – original draft, Writing – review & editing. ToK: Conceptualization, Funding acquisition, Methodology, Project administration, Supervision, Validation, Writing – review & editing. MU: Supervision, Validation, Writing – review & editing. NI: Data curation, Writing – review & editing. RM: Investigation, Writing – review & editing. TT: Investigation, Writing – review & editing. DO: Investigation, Writing – review & editing. HY: Investigation, Writing – review & editing. HI: Investigation, Writing – review & editing. TaK: Investigation, Methodology, Project administration, Supervision, Writing – review & editing. NS: Investigation, Methodology, Project administration, Supervision, Writing – review & editing. YU: Data curation, Writing – review & editing. HM: Supervision, Writing – review & editing. AK: Conceptualization, Funding acquisition, Methodology, Project administration, Supervision, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by the Nagasaki University “Doctoral Program for Worldleading Innovative and Smart Education” for Global Health, KYOIKU KENKYU SHIEN KEIHI (to SK, TK, AK) and the Japan Society for the Promotion of Science (JSPS) KAKENHI (grant number 22K08544 to TK).
Acknowledgments
We thank Anne M. O’Rourke, PhD, from Edanz (https://jp.edanz.com/ac) for editing a draft of this manuscript.
Conflict of interest
TK and AK received honoraria for speaking engagements from Eli Lilly, Japan.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be constructed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Bongartz T, Nannini C, Medina-Velasquez YF, Achenbach SJ, Crowson CS, Ryu JH, et al. Incidence and mortality of interstitial lung disease in rheumatoid arthritis: a population-based study. Arthritis Rheumatol. (2010) 62:1583–91. doi: 10.1002/art.27405
2. Hyldgaard C, Hilberg O, Pedersen AB, Ulrichsen SP, Løkke A, Bendstrup E, et al. A population-based cohort study of rheumatoid arthritis-associated interstitial lung disease: comorbidity and mortality. Ann Rheum Dis. (2017) 76:1700–6. doi: 10.1136/annrheumdis-2017-211138
3. Dai Y, Wang W, Yu Y, Hu S. Rheumatoid arthritis-associated interstitial lung disease: an overview of epidemiology, pathogenesis and management. Clin Rheumatol. (2021) 40:1211–20. doi: 10.1007/s10067-020-05320-z
4. Cassone G, Manfredi A, Vacchi C, Luppi F, Coppi F, Salvarani C, et al. Treatment of rheumatoid arthritis-associated interstitial lung disease: lights and shadows. J Clin Med. (2020) 9:1082. doi: 10.3390/jcm9041082
5. Zamora-Legoff JA, Krause ML, Crowson CS, Ryu JH, Matteson EL. Risk of serious infection in patients with rheumatoid arthritis-associated interstitial lung disease. Clin Rheumatol. (2016) 35:2585–9. doi: 10.1007/s10067-016-3357-z
6. Roubille C, Haraoui B. Interstitial lung diseases induced or exacerbated by DMARDS and biologic agents in rheumatoid arthritis: a systematic literature review. Semin Arthritis Rheumatol. (2014) 43:613–26. doi: 10.1016/j.semarthrit.2013.09.005
7. Vicente-Rabaneda EF, Atienza-Mateo B, Blanco R, Cavagna L, Ancochea J, Castañeda S, et al. Efficacy and safety of abatacept in interstitial lung disease of rheumatoid arthritis: A systematic literature review. Autoimmun Rev. (2021) 20:102830. doi: 10.1016/j.autrev.2021.102830
8. Mochizuki T, Yano K, Ikari K, Okazaki K. Radiological evaluation of interstitial lung disease in patients with rheumatoid arthritis treated with abatacept or JAK inhibitors for 1 year. Respir Investig. (2023) 61:359–63. doi: 10.1016/j.resinv.2023.02.007
9. Tardella M, Di Carlo M, Carotti M, Ceccarelli L, Giovagnoni A, Salaffi F. A retrospective study of the efficacy of JAK inhibitors or abatacept on rheumatoid arthritis-interstitial lung disease. Inflammopharmacology. (2022) 30:705–12. doi: 10.1007/s10787-022-00936-w
10. Yamakawa H, Ogura T, Kameda H, Kishaba T, Iwasawa T, Takemura T, et al. Decision-making strategy for the treatment of rheumatoid arthritis-associated interstitial lung disease (RA-ILD). J Clin Med. (2021) 10:3806. doi: 10.3390/jcm10173806
11. Xie M, Zhu C, Ye Y. Incidence, risk factors, and prognosis of acute exacerbation of rheumatoid arthritis-associated interstitial lung disease: a systematic review and meta-analysis. BMC Pulm Med. (2023) 23:255. doi: 10.1186/s12890-023-02532-2
12. Akiyama M, Kaneko Y. Pathogenesis, clinical features, and treatment strategy for rheumatoid arthritis-associated interstitial lung disease. Autoimmun Rev. (2022) 21:103056. doi: 10.1016/j.autrev.2022.103056
13. Wang HF, Wang YY, Li ZY, He PJ, Liu S, Li QS. The prevalence and risk factors of rheumatoid arthritis-associated interstitial lung disease: a systematic review and meta-analysis. Ann Med. (2024) 56:2332406. doi: 10.1080/07853890.2024.2332406
14. Zhang M, Yin J, Zhang X. Factors associated with interstitial lung disease in patients with rheumatoid arthritis: A systematic review and meta-analysis. PLoS One. (2023) 18:e0286191. doi: 10.1371/journal.pone.0286191
15. Lamouille S, Xu J, Derynck R. Molecular mechanisms of epithelial-mesenchymal transition. Nat Rev Mol Cell Biol. (2014) 15:178–96. doi: 10.1038/nrm3758
16. Wheelock MJ, Shintani Y, Maeda M, Fukumoto Y, Johnson KR. Cadherin switching. J Cell Sci. (2008) 121:727–35. doi: 10.1242/jcs.000455
17. Zhang C, Wang S, Lau J, Roden AC, Matteson EL, Sun J, et al. IL-23 amplifies the epithelial-mesenchymal transition of mechanically conditioned alveolar epithelial cells in rheumatoid arthritis-associated interstitial lung disease through mTOR/S6 signaling. Am J Physiol Lung Cell Mol Physiol. (2021) 321:L1006–l22. doi: 10.1152/ajplung.00292.2021
18. Kim Y, Yang HI, Kim KS. Etiology and pathogenesis of rheumatoid arthritis-interstitial lung disease. Int J Mol Sci. (2023) 24:14509. doi: 10.3390/ijms241914509
19. Milara J, Hernandez G, Ballester B, Morell A, Roger I, Montero P, et al. The JAK2 pathway is activated in idiopathic pulmonary fibrosis. Respir Res. (2018) 19:24. doi: 10.1186/s12931-018-0728-9
20. Smolen JS, Landewé RBM, Bergstra SA, Kerschbaumer A, Sepriano A, Aletaha D, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2022 update. Ann Rheum Dis. (2023) 82:3–18. doi: 10.1136/ard-2022-223356
21. Raghu G, Remy-Jardin M, Richeldi L, Thomson CC, Inoue Y, Johkoh T, et al. Idiopathic pulmonary fibrosis (an update) and progressive pulmonary fibrosis in adults: an official ATS/ERS/JRS/ALAT clinical practice guideline. Am J Respir Crit Care Med. (2022) 205:e18–47. doi: 10.1164/rccm.202202-0399ST
22. Ichikado K, Suga M, Müller NL, Taniguchi H, Kondoh Y, Akira M, et al. Acute interstitial pneumonia: comparison of high-resolution computed tomography findings between survivors and nonsurvivors. Am J Respir Crit Care Med. (2002) 165:1551–6. doi: 10.1164/rccm.2106157
23. Anderson J, Caplan L, Yazdany J, Robbins ML, Neogi T, Michaud K, et al. Rheumatoid arthritis disease activity measures: American College of Rheumatology recommendations for use in clinical practice. Arthritis Care Res (Hoboken). (2012) 64:640–7. doi: 10.1002/acr.21649
24. Wells G, Becker JC, Teng J, Dougados M, Schiff M, Smolen J, et al. Validation of the 28-joint Disease Activity Score (DAS28) and European League Against Rheumatism response criteria based on C-reactive protein against disease progression in patients with rheumatoid arthritis, and comparison with the DAS28 based on erythrocyte sedimentation rate. Ann Rheum Dis. (2009) 68:954–60. doi: 10.1136/ard.2007.084459
25. Liu Y, Hu M, Fan G, Xing N, Zhang R. Effect of Baricitinib on the epithelial-mesenchymal transition of alveolar epithelial cells induced by IL-6. Int Immunopharmacol. (2022) 110:109044. doi: 10.1016/j.intimp.2022.109044
26. Holroyd CR, Seth R, Bukhari M, Malaviya A, Holmes C, Curtis E, et al. The British Society for Rheumatology biologic DMARD safety guidelines in inflammatory arthritis. Rheumatol (Oxford). (2019) 58:e3–e42. doi: 10.1093/rheumatology/key208
27. Fernández-Díaz C, Castañeda S, Melero-González RB, Ortiz-Sanjuán F, Juan-Mas A, Carrasco-Cubero C, et al. Abatacept in interstitial lung disease associated with rheumatoid arthritis: national multicenter study of 263 patients. Rheumatol (Oxford). (2020) 59:3906–16. doi: 10.1093/rheumatology/keaa621
28. Ito Y, Arita M, Kumagai S, Takei R, Noyama M, Tokioka F, et al. Radiological fibrosis score is strongly associated with worse survival in rheumatoid arthritis-related interstitial lung disease. Mod Rheumatol. (2019) 29:98–104. doi: 10.1080/14397595.2018.1442170
29. Flaherty KR, Wells AU, Cottin V, Devaraj A, Walsh SLF, Inoue Y, et al. Nintedanib in progressive fibrosing interstitial lung diseases. N Engl J Med. (2019) 381:1718–27. doi: 10.1056/NEJMoa1908681
30. Sparks JA, He X, Huang J, Fletcher EA, Zaccardelli A, Friedlander HM, et al. Rheumatoid arthritis disease activity predicting incident clinically apparent rheumatoid arthritis-associated interstitial lung disease: A prospective cohort study. Arthritis Rheumatol. (2019) 71:1472–82. doi: 10.1002/art.40904
31. Akiyama M, Kaneko Y, Yamaoka K, Kondo H, Takeuchi T. Association of disease activity with acute exacerbation of interstitial lung disease during tocilizumab treatment in patients with rheumatoid arthritis: a retrospective, case-control study. Rheumatol Int. (2016) 36:881–9. doi: 10.1007/s00296-016-3478-3
32. Kameda H, Yamaoka K, Yamanishi Y, Tada M, Koike R, Nakajima A, et al. Japan College of Rheumatology guidance for the use of methotrexate in patients with rheumatoid arthritis: Secondary publication. Mod Rheumatol. (2023) 34:1–10. doi: 10.1093/mr/road098
33. Baker MC, Liu Y, Lu R, Lin J, Melehani J, Robinson WH. Incidence of interstitial lung disease in patients with rheumatoid arthritis treated with biologic and targeted synthetic disease-modifying antirheumatic drugs. JAMA Netw Open. (2023) 6:e233640. doi: 10.1001/jamanetworkopen.2023.3640
34. Kiely P, Busby AD, Nikiphorou E, Sullivan K, Walsh DA, Creamer P, et al. Is incident rheumatoid arthritis interstitial lung disease associated with methotrexate treatment? Results from a multivariate analysis in the ERAS and ERAN inception cohorts. BMJ Open. (2019) 9:e028466. doi: 10.1136/bmjopen-2018-028466
35. Kawami M, Harabayashi R, Miyamoto M, Harada R, Yumoto R, Takano M. Methotrexate-induced epithelial-mesenchymal transition in the alveolar epithelial cell line A549. Lung. (2016) 194:923–30. doi: 10.1007/s00408-016-9935-7
36. Fleischmann R, Schiff M, van der Heijde D, Ramos-Remus C, Spindler A, Stanislav M, et al. Baricitinib, methotrexate, or combination in patients with rheumatoid arthritis and no or limited prior disease-modifying antirheumatic drug treatment. Arthritis Rheumatol. (2017) 69:506–17. doi: 10.1002/art.39953
Keywords: rheumatoid arthritis, interstitial lung disease, RA-ILD, JAK inhibitors, methotrexate, epithelial-mesenchymal transition
Citation: Kurushima S, Koga T, Umeda M, Iwamoto N, Miyashita R, Tokito T, Okuno D, Yura H, Ishimoto H, Kido T, Sakamoto N, Ueki Y, Mukae H and Kawakami A (2024) Impact of Janus kinase inhibitors and methotrexate on interstitial lung disease in rheumatoid arthritis patients. Front. Immunol. 15:1501146. doi: 10.3389/fimmu.2024.1501146
Received: 24 September 2024; Accepted: 02 December 2024;
Published: 16 December 2024.
Edited by:
Ciro Romano, University of Campania Luigi Vanvitelli, ItalyReviewed by:
Jing Luo, Second Hospital of Shanxi Medical University, ChinaSelvi Enrico, Siena University Hospital, Italy
Copyright © 2024 Kurushima, Koga, Umeda, Iwamoto, Miyashita, Tokito, Okuno, Yura, Ishimoto, Kido, Sakamoto, Ueki, Mukae and Kawakami. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tomohiro Koga, dGtvZ2FAbmFnYXNha2ktdS5hYy5qcA==
 Shota Kurushima
Shota Kurushima Tomohiro Koga
Tomohiro Koga Masataka Umeda
Masataka Umeda Naoki Iwamoto
Naoki Iwamoto Ritsuko Miyashita2
Ritsuko Miyashita2 Daisuke Okuno
Daisuke Okuno Noriho Sakamoto
Noriho Sakamoto Atsushi Kawakami
Atsushi Kawakami