- Department of Dermatology, University at Buffalo Jacobs School of Medicine and Biomedical Sciences, Buffalo, NY, United States
Introduction: Cytokines and chemokines direct the inflammatory response and may serve as markers of immune dysregulation in Pemphigus vulgaris (PV), an autoimmune blistering skin disorder. Previous studies on limited numbers of patients and cytokine profiles in PV have produced equivocal results regarding the role these mediators play in disease.
Methods: In this study, we interrogated serum samples from 116 PV patients and 29 healthy controls by multiplexed bead array assays across a comprehensive set of cytokines and chemokines covering several functional categories, including IL-1α, IL-1β, IL-2, IL-4, IL-5, IL-6, IL-8, IL-9, IL-10, IL-12, IL-13, IL-15, IL-17, IL-21, IL-22, IL-23, TNFα, IFNγ, MCP-1, and Eotaxin.
Results: We found that patients with PV generally display an activated cytokine and chemokine immune response compared to controls, but also show remarkable interindividual heterogeneity in terms of cytokine levels, with a limited activation of different T helper cell pathways in different patients. Surprisingly, we also found that healthy individuals that carry the PV susceptibility alleles HLA DR4 (DRB1*0402) and/or DR6 (DQB1*0503) (HLA-matched controls) show an upregulation of cytokine and chemokine levels that are on par with those seen in PV patients for certain pro-inflammatory, Th2, and Th17 mediators and IL-8, while healthy controls that did not carry the PV susceptibility alleles (HLA-unmatched controls) express significantly lower levels of these cytokines and chemokines.
Discussion: Our data suggest the existence of a limited immune activation linked to the presence of key PV associated HLA alleles regardless of disease status. Interestingly, the cytokines IL-10 and IL-15 were found to be significantly downregulated in the HLA-matched control group, suggesting the presence of a possible counter-regulatory function in genetically susceptible but disease-free individuals.
1 Introduction
Pemphigus vulgaris (PV) is a potentially life-threatening autoimmune blistering skin disorder characterized by a well-defined humoral response directed against specific desmosomal proteins involved in cell-cell adhesion and maintenance of epidermal integrity. The hallmark for autoimmunity in PV is the presence of autoantibodies specific for desmoglein (Dsg)-3, and in some cases Dsg-1, both in serum and in lesional skin. The “desmoglein compensation hypothesis” posits that lesion morphology (mucosal vs. mucocutaneous) can be explained by autoantibody patterns (anti-Dsg3 only vs. a combination of anti-Dsg3 and anti-Dsg1, respectively) (1), however, the validity of this theory has been challenged recently (2). While the target organ damage is ultimately mediated by autoantibodies, it is generally accepted that B cell activation requires the participation of T helper cells necessary for immunoglobulin (Ig) production and class switching. T cell recognition of Dsg3 and/or Dsg1 epitopes appears to be the first step in the disease initiation, leading to B-cell activation and production of Dsg3/1 specific immunoreactants (3–6).
The importance of T cell involvement in disease pathogenesis is underscored by the strong genetic association of PV to the HLA DR4 (DRB1*0402) and DR6 (DQB1*0503) haplotypes (7, 8). T cells play a role in the inception and maturation of the humoral immune response, thus bridging the association of PV with MHC and generation of autoantibodies (9). Of course, these haplotypes are also found in healthy individuals. In fact, the vast majority of healthy individuals who express HLA DR4 (DRB1*0402) or DR6 (DQB1*0503) gene sequences do not progress to a disease state. Why healthy individuals who carry these disease risk elements remain healthy remains a mystery.
While the coordination and regulation of the autoimmune response in PV has not been fully elucidated, cytokines and chemokines are likely to orchestrate the interplay between cellular and humoral responses. Cytokines act as mediators for disease activation and maintenance in autoimmune disorders, but also operate as initiators of disease remission and induction of the tolerogenic state (10). Produced by, and acting on various cellular components of the immune cascade, they participate in both effector and regulatory pathways (10).
Limited attempts have been made to define cytokine and chemokine expression patterns in PV. Apart from a recent publication (11), previous studies typically examined only a handful of cytokines in small sample sizes using disparate methods to quantify cytokine concentrations. Thus, perhaps unsurprisingly, reported results are equivocal. In fact, many cytokines in PV were found to be upregulated in one study, only to be shown to have no significant change or even to be downregulated in another (3, 12–59). Table 1 shows a comprehensive literature review of previous serum cytokine studies, particularly which studies show an elevation, decrease, or no significant change in PV patients versus healthy controls, as well as how this data compares to new findings from this study that will be discussed in detail below. Across studies, heterogeneity amongst patients and control subjects in terms of disease phase, clinical phenotype, gender, HLA-association, and treatment may have contributed to ambivalent results, factors that are particularly difficult to account for in small sample sizes.
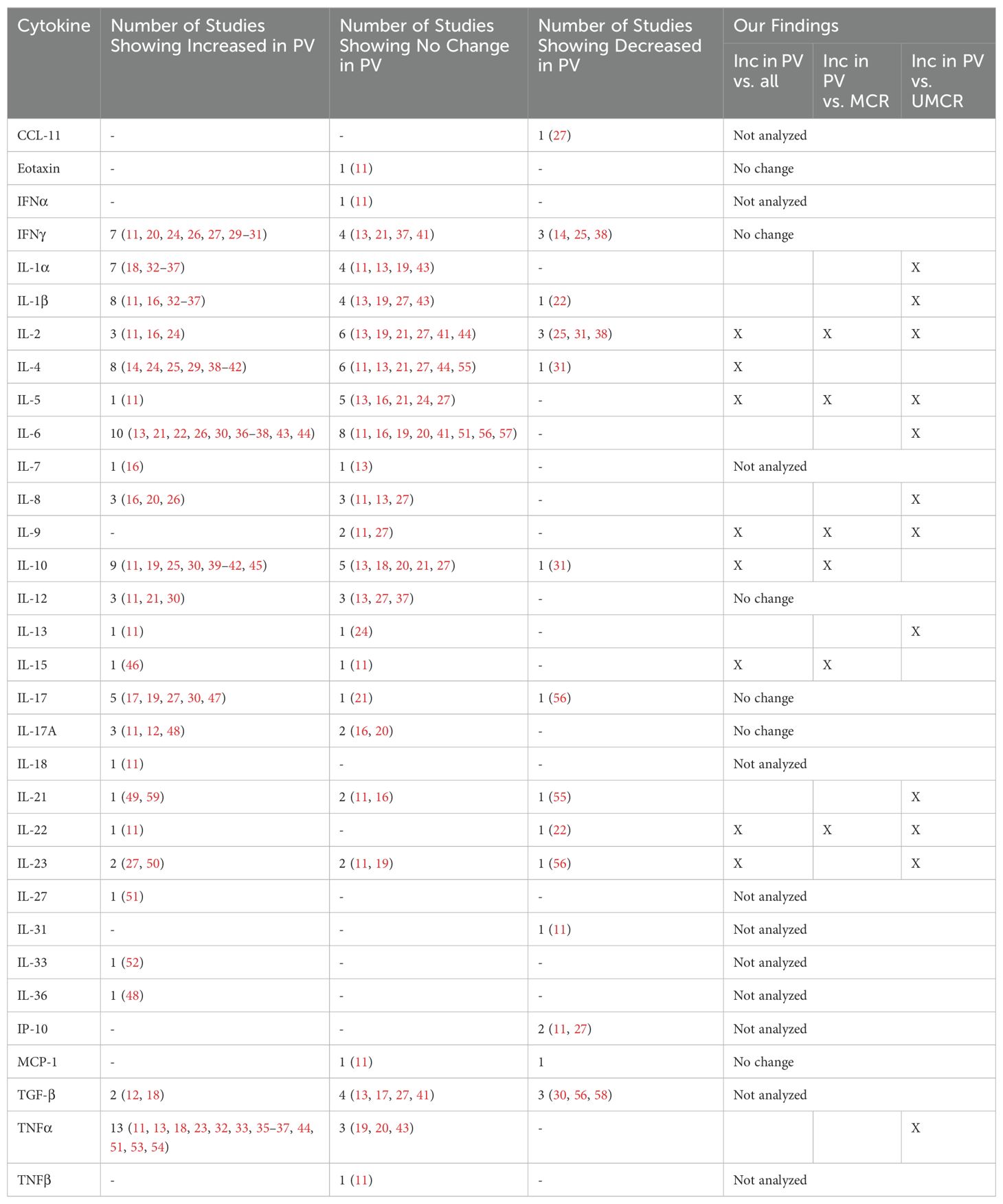
Table 1. Review of literature findings regarding serum cytokine levels in PV patients compared to controls.
To overcome these challenges, we conducted an extensive analysis of serum cytokines/chemokines in PV, both in terms of the range of soluble mediators studied and the number of patient and control samples analyzed. We comprehensively compared multiple serum cytokines in defined clinical categories of PV. Unlike previous studies, we further explored the contribution of HLA haplotype to the cytokine milieu in both patients and controls to pinpoint the role of cytokines in genetically susceptible, but disease-free subjects. We utilized a multiplex bead array platform to evaluate the expression of 20 cytokines and chemokines categorized by function: 1) pro-inflammatory (IL-1α, IL-1β, IL-6, and TNFα), 2) Th1 (IFNγ, IL-2, IL-12), 3) Th2 (IL-4, IL-5, IL-13), 4) Th9 (IL-9), 5) Th17/Th22 (IL-17, IL-21, IL-22, IL-23), 6) Treg (IL-10), 7) NK cell (IL-15), and 8) chemokines (IL-8, MCP-1, Eotaxin) in 130 serum samples obtained from 116 PV patients in different phases of disease activity and remission, along with 15 healthy control subjects that carried the PV-associated HLA alleles DRB1*0402 and/or DQB1*0503 (termed here as HLA-matched controls) and 14 healthy control subjects that did not carry the known PV-associated HLA alleles (HLA-unmatched controls).
2 Materials and methods
2.1 Patient population and demographics
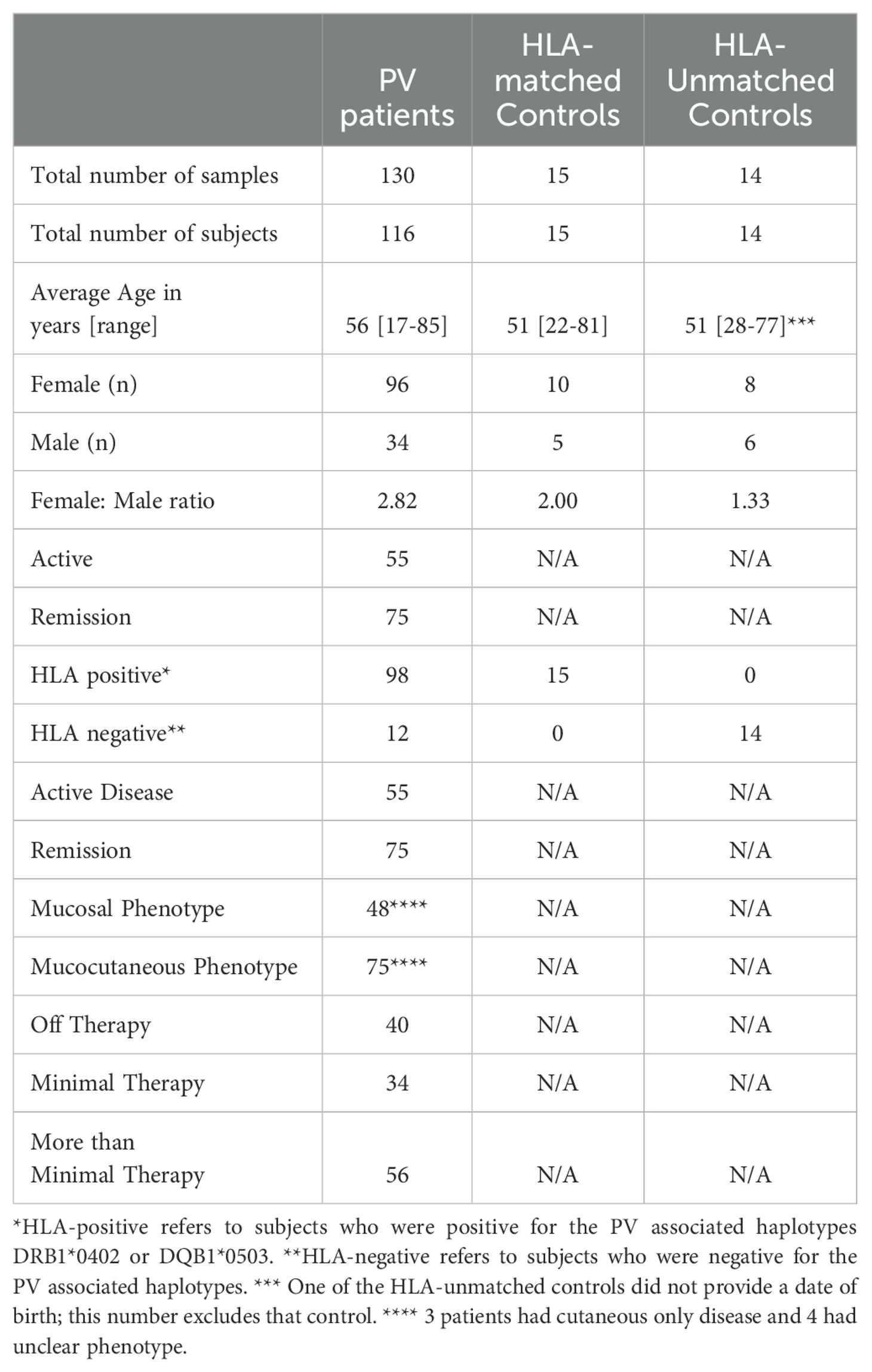
The patients included in this study were recruited from the Dermatology outpatient clinics at Weill-Cornell Medical College (IRB 0998-398), Michigan State University (IRB 05-1034), and the University at Buffalo (IRB 456887), and annual meetings of International Pemphigus and Pemphigoid Foundation (IPPF). Institutional review boards at each of the participating institutions reviewed and approved this study.
After obtaining written informed consent, researchers acquired detailed demographic and clinical information from patients and control subjects. Subsequently, venous blood samples were drawn and each patient’s serum was separated by centrifugation and stored at minus 80°C until use.
The diagnosis of PV was based on established clinical, histopathologic, and/or serologic criteria. Healthy controls did not have a diagnosis or history of PV. All study procedures were identical between healthy controls and PV patients. We included 130 serum samples from 116 patients in different phases of disease (active and remittent) and varying degrees of therapy and 29 samples from 29 control subjects, of which 15 were HLA-matched controls (i.e. carriers of the HLA-susceptibility alleles DRB1*0402 and/or DQB1*0503) [MCR] and 14 were HLA-unmatched controls (i.e. non-carriers of PV susceptibility HLA alleles) [UMCR]. The demographic data for our study population is summarized in Table 2.
We used consensus guidelines developed by the International Pemphigus Committee (60) to determine disease activity in PV patients. Briefly, patients were considered to be active if they had 3 or more non-transient lesions (lasting more than one week) and/or extension of existing lesions. Patients were considered to be remittent if they had no new or established lesions for at least 2 months.
We assigned a therapy status to each patient based on consensus guidelines (60). Patients receiving > 10mg/day of prednisone, IVIg, cyclosporine, dapsone, rituximab, other biologic agents, and/or other adjuvant therapies at full therapeutic doses were defined as “more than minimal” therapy. “Minimal” therapy was defined by prednisone doses of ≤ 10mg/day and/or minimal adjuvant therapy as defined in (60) for at least 2 months. “Off” therapy was reserved for patients that were not receiving any systemic therapy.
2.2 HLA-typing
High resolution HLA-typing of patients and controls was done at Rogosin Institute (New York, NY) and the Tissue Typing Laboratory at Michigan State University (East Lansing, MI). HLA Class II alleles, DRB1 and DQB1, were amplified using sequence specific primers as previously described (61). Patients and controls that expressed the HLA associated alleles (homozygous or heterozygous) were classified as “HLA-positive” or HLA+. Those without were classified as “HLA-negative” or HLA-.
2.3 Multiplex bead assay
Patient and control serum samples were distributed amongst two panels of customized Millipore Multiplex 96-well plates (EMD Millipore, Saint Charles, MO). The first panel included: IL-1α, IL-1β, IL-2, IL-4, IL-5, IL-6, IL-8, IL-9, IL-10, IL-12, IL-13, IL-15, TNFα, IFNγ, MCP-1, and Eotaxin, and the second panel included the Th17 cytokines IL-17, IL-21, IL-22, and IL-23. An aliquot of 50 µl from each PV patient or control serum was added to each well aside internal control samples of known concentrations. Samples were run in duplicates. Standards were serially diluted 1:3 to generate a nine-point standard curve. Analyte capture was carried out according to manufacturer’s instructions. Cytokine concentrations (pg/mL) were measured using the Luminex 200 system with xPONENT version 3.1 software and assessed using BeadView Analysis software. Raw values are compared to standard curves for each cytokine. Cytokines are considered 0 or undetectable when falling within the lower nonlinear portion of the standard curve. We report raw values (pg/mL) as our standard curves do not provide cutoffs for positivity. In addition, we used unbiased hierarchical clustering using Euclidean distance with Morpheus software (https://software.broadinstitute.org/morpheus) to identify any grouping or patterns in cytokine levels.
2.4 Statistical analysis
For certain analyses, PV samples were subclassified according to major clinical parameters of interest and further placed in subgroups established by clinical criteria (in parentheses), i.e. disease phase (active or remission), therapy status (off or minimal or more than minimal), clinical phenotype (mucosal or mucocutaneous), and gender (male or female). Control patients were separated into HLA-matched and HLA-unmatched groups.
Statistical analysis was conducted between clinical subgroups including PV vs. all controls, HLA-matched controls vs. HLA-unmatched controls, and within the PV group between disease activity (active vs. remittent), gender (male vs. female), morphology (mucocutaneous vs. mucosal), and therapy (off therapy vs. minimal therapy, minimal therapy vs. greater than minimal therapy, and greater than minimal therapy vs. off therapy).
Groups were compared by a heteroscedastic T-test. A p-value ≤ 0.05 indicated that the difference between the means, μ1 and μ2, of the populations from which data1 and data2, respectively, were sampled is significantly differently from Δμ=0. The null hypothesis, Δμ=0, was rejected when p-value < 0.05.
3 Results
3.1 Cytokines in PV patients show wide variability and heterogeneity, but are pathway specific and generally elevated compared to healthy controls
We examined 20 cytokines/chemokines in 116 PV patients and 29 healthy controls. For each of the cytokines analyzed, we observed large interindividual differences among serum concentrations ranging from 0 pg/mL (undetectable) to high values such as ~73,000 pg/mL for IL-23, for example. This wide variation was particularly pronounced in the patient population. Interestingly, we saw a remarkable heterogeneity among patients in terms of which cytokines were elevated and also observed that among a given patient cytokine values were typically elevated across just one or two specific pathways, but not for all cytokines analyzed. In order to identify any patterns of cytokine responses (i.e. whether cytokines are regulated in groups), we used an unbiased hierarchical clustering approach. This unbiased clustering approach identified 5 clusters of cytokine responses: (i) Th17: IL17A, IL-21, IL-22, IL-23; (ii) Th2-dominant: IL-2, IL-4, IL-9, IL-10, IL-13, IL-15; (iii) Th1-dominant: IL-1α, IL-2, IFNγ, IL-5; (iv) generally proinflammatory: IL-1β, IL6, IL-8 and TNFα; and (v) the chemokines MCP-1 and eotaxin (Figure 1). Some patients showed an elevation of one or two T helper cell pathways, while others had high levels of proinflammatory cytokines in the absence of T helper pathway cytokines. Conversely, we observed a sizeable number of PV patients that had undetectable levels for the majority of cytokines, suggesting that cytokine responses are remarkably patient-specific and not universally present in all.
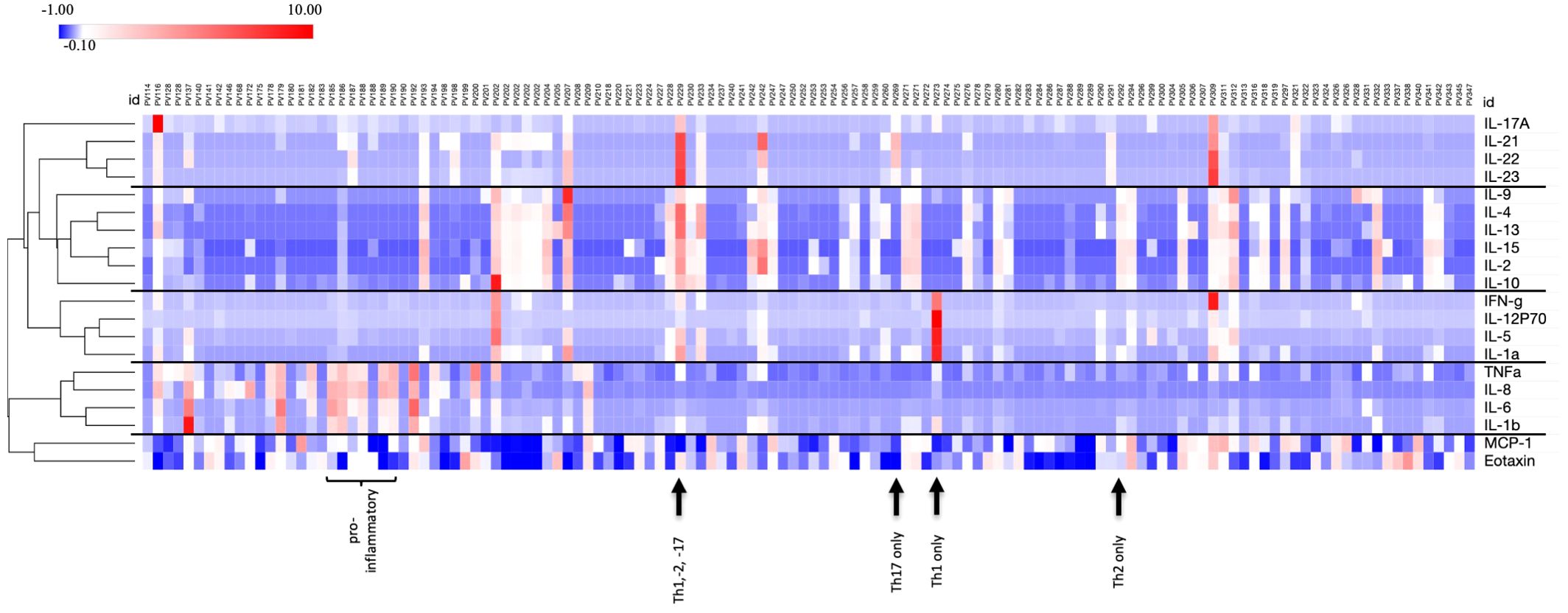
Figure 1. Wide variation and patient specificity for cytokine profiles. Z-score transformed data in heatmap format shows all cytokine concentrations for each PV patient (n=130) simultaneously. Unbiased clustering identifies cytokine subgroups with similar expression levels for individual patients. Examples for a certain cytokine profile are identified as Th2 only (PV292), Th1 only (PV273), Th17 only (PV269), combined Th1, -2, 17 (PV229) and proinflammatory pattern (PV185 to 190). Heatmap was created using Morpheus Software. https://software.broadinstitute.org/morpheus.
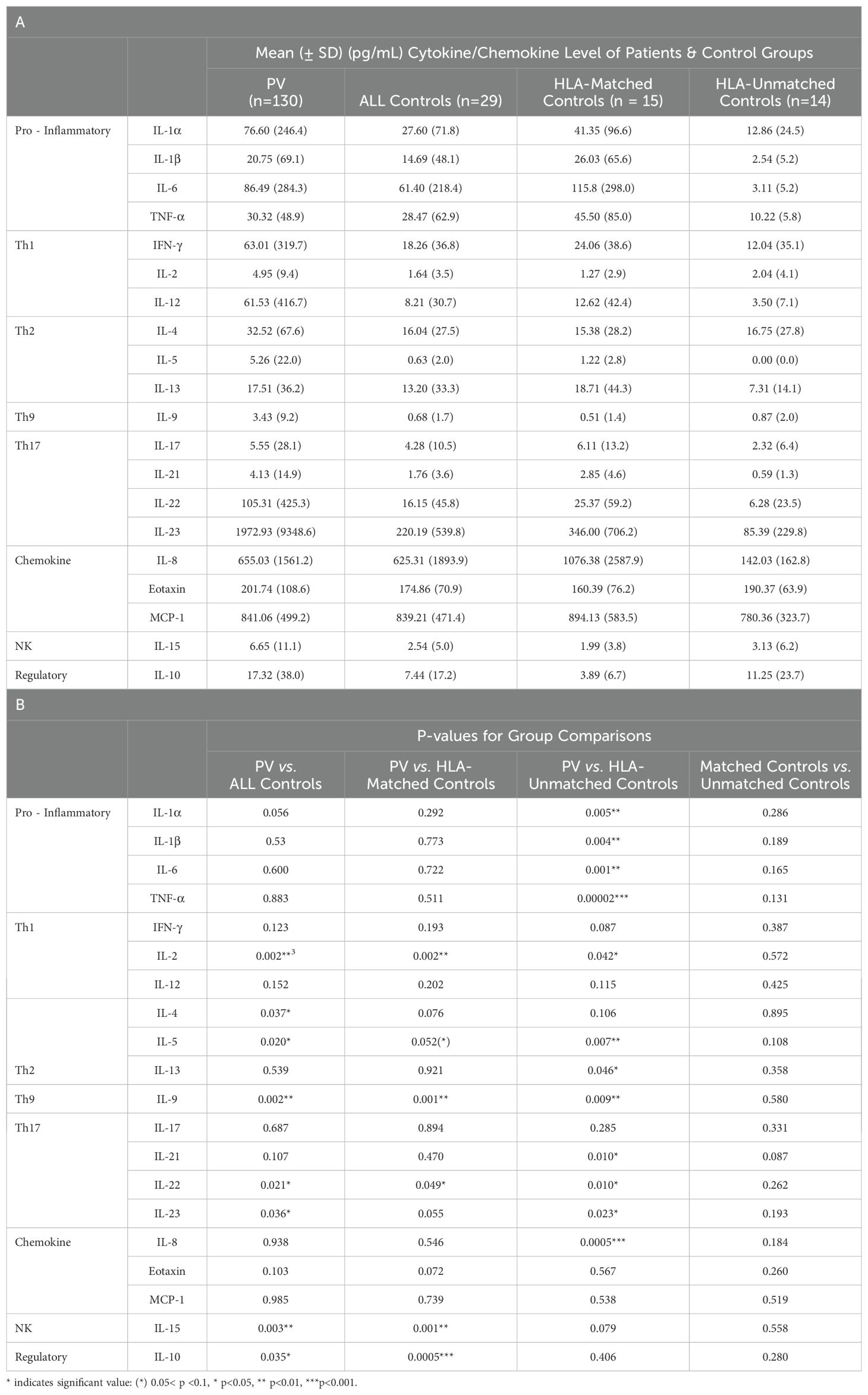
Despite this variation among patients, all of the cytokines and chemokines analyzed had higher mean levels in PV patients compared to all healthy controls (Table 3A), with IL-2, IL-4, IL-5, IL-9, IL-10, IL-15, IL-22, and IL-23 reaching statistical significance (p-value <0.05) (Table 3B), indicating a general immune activation in patients compared to controls.
3.2 Healthy controls carrying PV-associated HLA susceptibility alleles exhibit a heightened cytokine activity within pro-inflammatory, Th2-, Th17-, and IL-8 pathways
We noticed some heterogeneity regarding cytokine levels within the control population similar to what we had observed in the patient population. To investigate whether cytokines differed based on HLA association, we divided the control population into those that expressed the known PV-associated HLA-alleles DRB1*0402 and DQB1*0503 (“HLA-matched controls”, n=15) and those that did not express any of the known PV associated HLA alleles (“HLA-unmatched controls”, n=14) and then analyzed which cytokines differentiated PV from both control groups or either one of the control groups alone.
We observed that the higher serum levels of multiple cytokines persisted in a more limited set of cytokines when compared to HLA-matched or HLA-unmatched control groups separately. Of the cytokines that were significantly elevated in PV compared to all controls, only IL-2, IL-9, and IL-22 displayed significantly higher mean levels than both HLA-matched controls or HLA-unmatched controls. IL-5, though not significant, was highly trending towards significance (Figure 2A and Tables 3A, B).
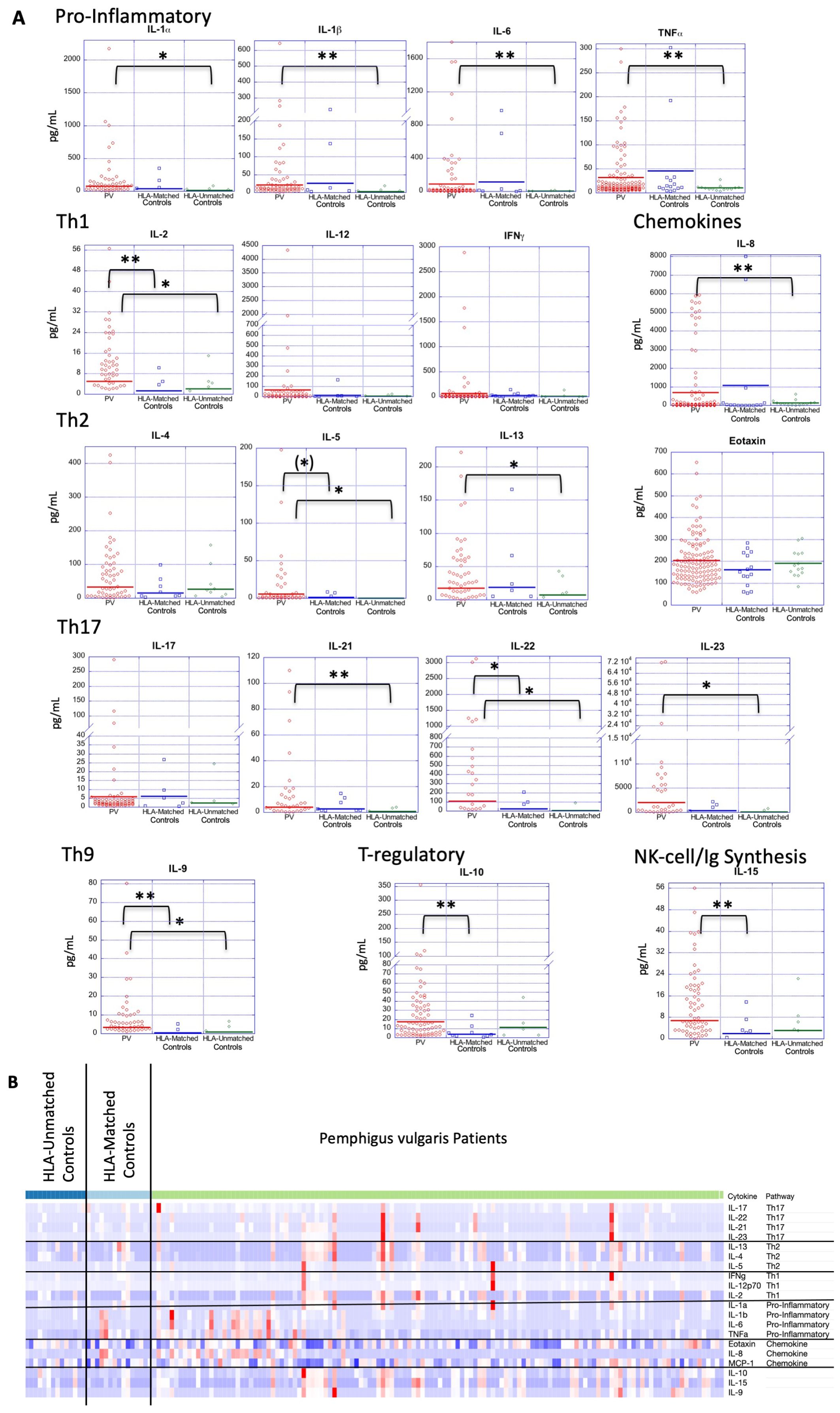
Figure 2. Cytokine expression profiles in PV patients and controls, both HLA-matched and HLA-unmatched. (A) Dot plots depict cytokine levels in PV patients and controls (HLA-matched and HLA-unmatched), with each dot representing an individual sample. The horizontal bar in each category represents the mean. P-values ≤ 0.05 are indicated with *; p-values ≤ 0.005 are indicated with **. (B) Z-score transformed data in heatmap format shows all cytokine concentrations for each PV patient (n=130) an HLA-matched (n=15) and HLA-unmatched controls (n=14) simultaneously. Cytokines are listed in order of functional subgroup. Heatmap was created using Morpheus Software. https://software.broadinstitute.org/morpheus.
Another group of cytokines retained or achieved significance only when patients were compared to “HLA-unmatched” healthy controls, i.e. those that do not carry PV-associated genetic susceptibility elements. PV patients had significantly elevated levels of the Th2 cytokine IL-13, the pro-inflammatory cytokines IL-1α, IL-1β, IL-6, TNFα and the chemokine IL-8 in this comparison (Figure 2A and Tables 3A, B). No significant differences were found between PV patients and “HLA-matched” controls for these cytokines. “HLA-unmatched” controls expressed very low levels of several cytokines (IL-1α: 12.01 ± 23.9 pg/mL, IL-1β: 2.55 ± 5.2 pg/mL, IL-6: 3.12 ± 5.2 pg/mL, TNFα: 10.22 ± 5.8 pg/mL, IL-8: 142.21 ± 162.9 pg/mL, and IL-13: 7.31 ± 14.1 pg/mL, respectively), while “HLA-matched” controls expressed levels on par or greater than those found in PV patients for numerous cytokines (IL-1α: 41.35 ± 96.6 pg/mL, IL-1β: 26.03 ± 65.7 pg/mL, IL-6: 115.8 ± 297.9 pg/mL, TNFα: 45.51 ± 85.0 pg/mL, IL-8: 1076.47 ± 2587.9 pg/mL, and IL-13: 18.7 ± 44.4 pg/mL, respectively) (Figure 2A and Tables 3A, B).
These findings are also reflected when looking at cytokine concentrations in a heatmap format, with highest expression found in PV patients, followed by HLA-matched controls, with visibly lower values for HLA-unmatched controls (Figure 2B). Our data indicate that healthy controls that carry PV-associated HLA alleles exhibit a limited immunological activation similar to PV patients in terms of the pro-inflammatory cytokines IL-1α, IL-1β, IL-6, TNF-α, the Th2 cytokine IL-13, the Th17 cytokines IL-21 and IL-23, and the chemokine IL-8.
Interestingly, we observed that mean IL-10 and IL-15 concentrations were lower in both the “HLA-matched” and “HLA-unmatched” control groups than the levels found in PV patients (Figure 2A). However, mean IL-10 and IL-15 levels in “HLA-matched” controls (means: 3.89 ± 3.8 pg/mL and 1.99 ± 6.7 pg/mL, respectively) were found to be even lower than that seen in “HLA-unmatched” controls (means: 11.25 ± 6.2 pg/mL and 3.13 ±23.7 pg/mL, respectively) (Table 3A). In fact, when compared to PV patients, IL-10 and IL-15 cytokine levels were significantly lower only in “HLA-matched” controls (p-value: 0.0007 and 0.001, respectively) but not in unmatched controls (Figure 2 and Table 3B). The differences noted above raise the intriguing possibility that the downregulation of these two cytokines may contribute to mechanisms that protect genetically susceptible individuals from developing Pemphigus vulgaris.
3.3 PV patients do not differ in cytokine expression based on their level of disease activity, apart from a limited number of pro-inflammatory cytokines which are elevated in remission
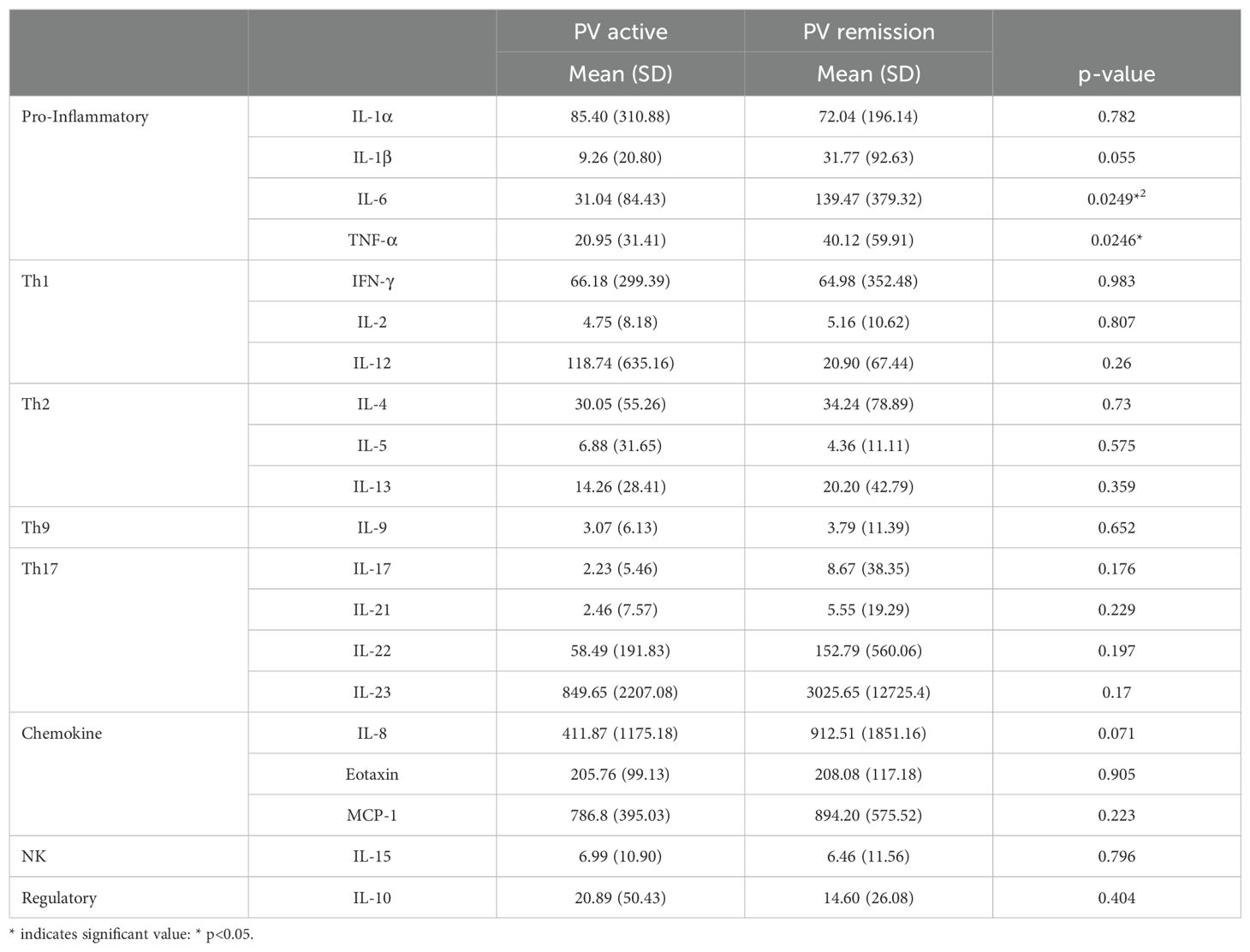
In order to assess the extent to which the level of disease activity influences serum cytokine levels in PV, we divided patients into those who were in active disease (n=55) vs remission (n=75) (Figure 3). Surprisingly, we found no significant differences for most of the cytokines analyzed, with both the active and remittent groups continuing to show remarkable heterogeneity of cytokine levels. Even more surprisingly, TNFα and IL-6 were significantly higher in remission versus active disease and the pro-inflammatory cytokine IL-1β and chemokine IL-8 had increased expression in remission vs. active disease which trended toward significance (Figure 3 and Table 4). These data indicate that active disease is not associated with an across-the-board activated cytokine profile. If anything, a limited number of pro-inflammatory cytokines increase as the disease goes into remission. One speculation for this surprising finding is that a persistent proinflammatory milieu does not preclude or prevent the critical (and thus far unknown) factors responsible for limiting lesional activity.
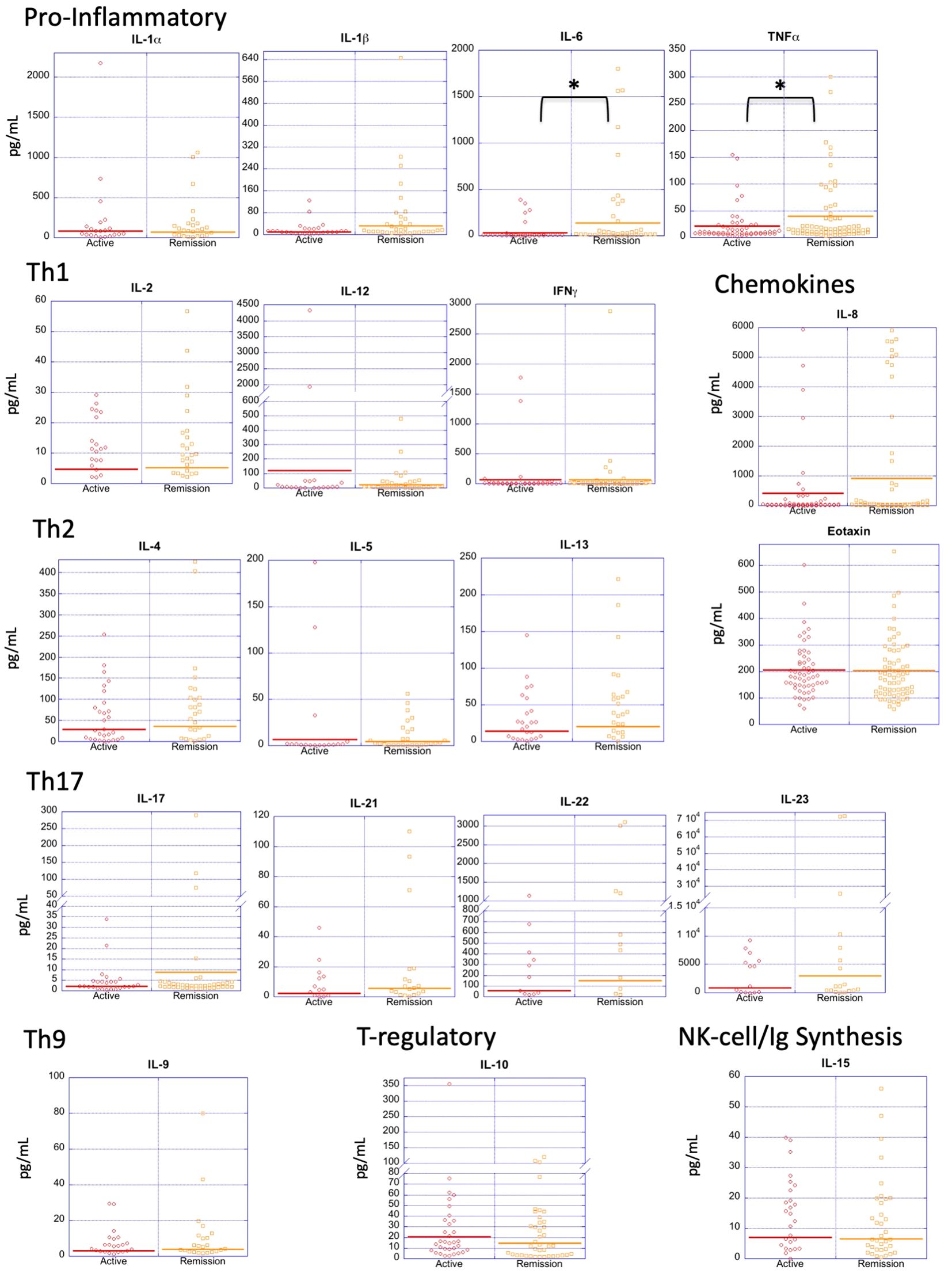
Figure 3. Cytokine expression profiles by disease activity. Dot plots depict cytokine levels in PV patients in active disease and remission, with each dot representing an individual sample. Only IL-6 and TNF-α were significantly different between patients with active PV vs. remittent PV. The horizontal bar in each category represents the mean. P-values ≤ 0.05 are indicated with *.
Interestingly, when patients were subdivided by disease activity and these subgroups were compared individually to HLA-matched and unmatched controls as done in section 3.1, the significant differences found for all patients largely remained (Supplementary Table 1), suggesting again that cytokine elevations in patients are independent of disease activity. The only exception was the group of Th17 cytokines which lost most of the significant differences in the subgroup analysis, likely due to the low number of patients expression elevated levels of Th17 cytokines to begin with.
A possible confounding factor is that most of our data points are devised from individual patients, with only a few examples of patients (n=3) with longitudinal data in both active and remittent phases of disease. Of those patients sampled in different disease phases, only one showed considerable cytokine elevation across the board (PV202, Supplementary Table 2). For this individual in particular, active disease was generally associated with higher cytokine levels than remission. For the other two patients, general cytokine concentration tended to be lower overall with one patient being higher in active disease and the other in remission (Supplementary Table 2).
3.4 Therapy does not produce appreciable differences in PV cytokine levels
To evaluate the extent to which therapy influences cytokine levels, we divided patients into 3 groups: 1) off therapy, 2) on minimal therapy, and 3) receiving greater than minimal therapy. No significant differences were found between the various therapy groups when including the entire data set (data not shown). Despite the fact that therapy status does not seem to affect cytokine expression overall in PV patients, we nevertheless directly analyzed cytokine levels in active and remittent PV patients who were completely off therapy (n=11, n=27, respectively). Only MCP-1 was found to be significantly elevated in active PV off therapy compared to remittent PV patients off therapy (p-value 0.0298) (Supplementary Figure 1). Additionally, when patients were subdivided by therapy and these subgroups were compared individually to HLA-matched and unmatched controls as done in section 3.1, the significant differences found for all patients vs. the control group largely remained, again suggesting that cytokine elevations are largely independent of therapy status (Supplementary Table 3).
3.5 Interleukin 9 and IL-13 distinguish mucocutaneous PV from mucosal PV
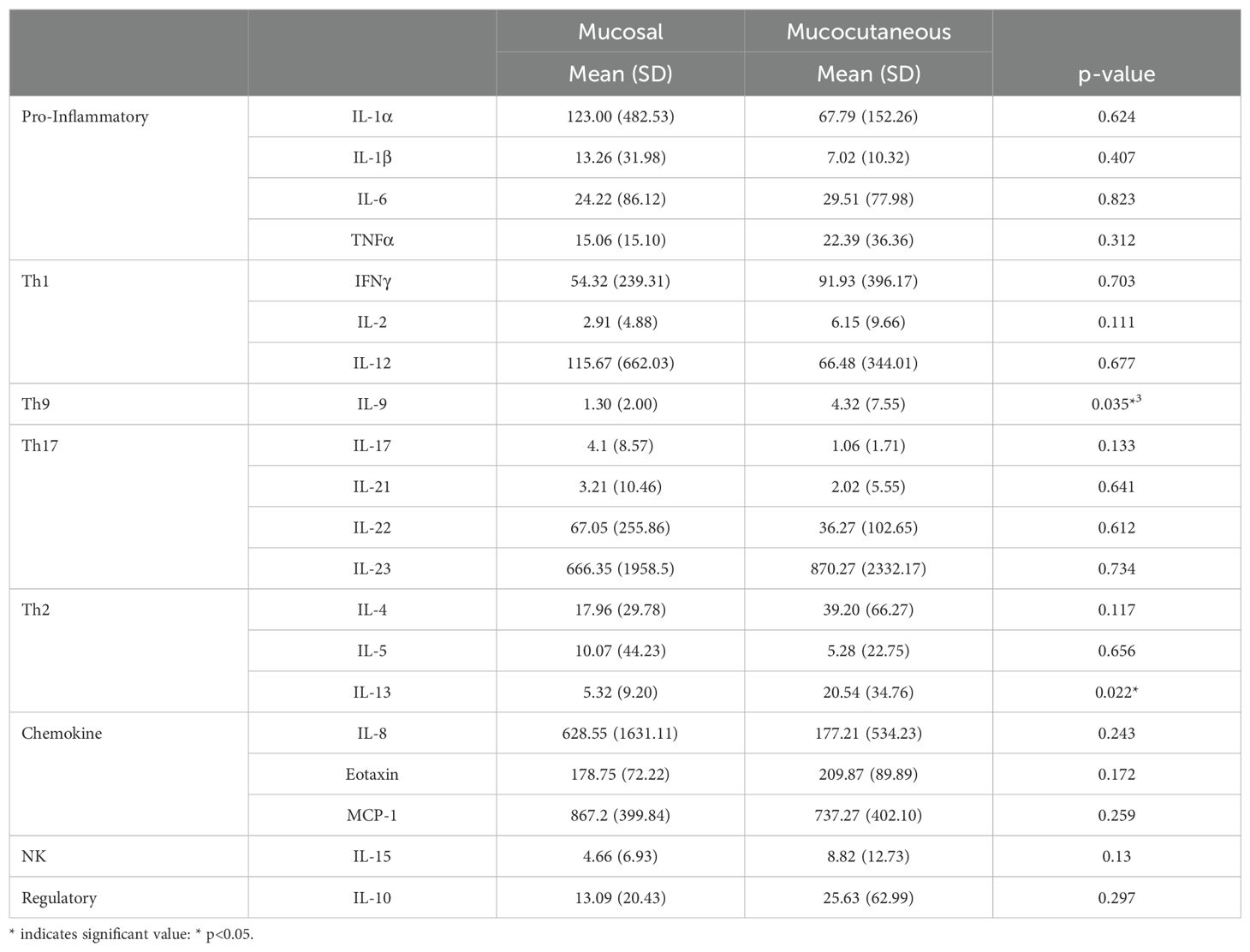
It has been previously suggested that mucocutaneous PV differs from mucosal PV immunologically (62, 63). In order to assess whether cytokine levels correlate with disease morphology, we analyzed 55 patients that were in active disease, 33 of whom had mucocutaneous lesions, and 20 had mucosal only lesions. Patients with exclusively cutaneous lesions (n=2) were not included in this analysis due to limited numbers. We found the only significant differences between morphological groups to be for Th9 cytokine IL-9 and the Th2 cytokine IL-13, which were significantly elevated in those who had active mucocutaneous PV compared to those who had active mucosal only PV (p-value: 0.035 and 0.022, respectively) (Figure 4A and Table 5).
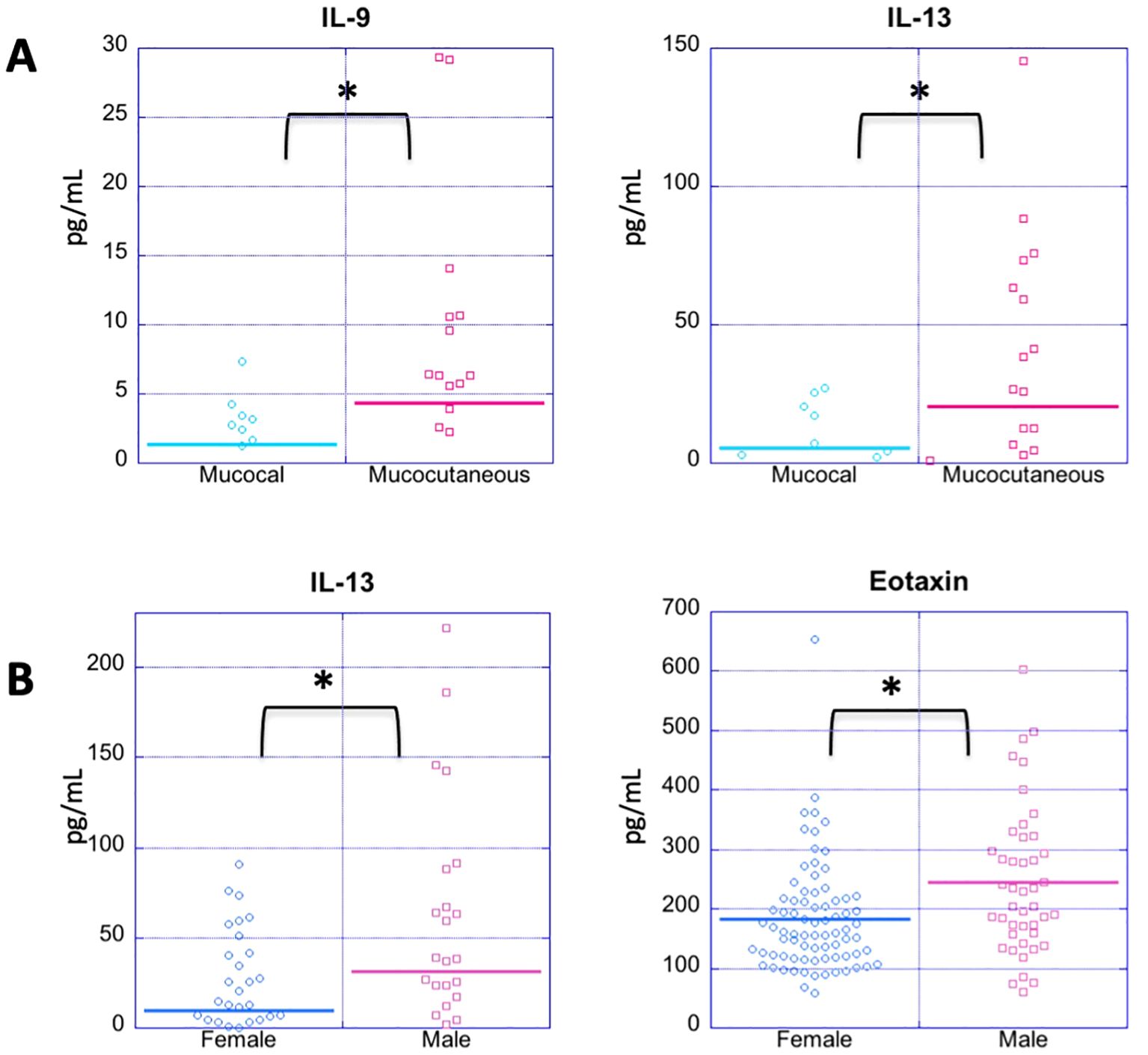
Figure 4. Cytokine expression profiles by disease morphology and gender. (A) Dot plots depict cytokine levels in PV patients in patients in active disease with either mucosal or mucocutaneous morphology (A) and female vs. male PV patients regardless of disease status (B), with each dot representing an individual sample. (A) PV patients with active mucocutaneous lesions have significantly elevated levels of IL-9 and IL-13 compared to the patients with active mucosal lesions. (B) Males have significantly higher levels of IL-13 and Eotaxin levels than females with PV. Cytokines not showing significant differences for these comparisons are not shown. The horizontal bar in each category represents the mean. P-values ≤ 0.05 are indicated with *.
3.6 Interleukin-13 and eotaxin expression distinguish females from males with PV
There is a clear female predominance in PV (64). We analyzed samples from a total of 96 women and 34 men with PV regardless of disease activity. For the most part, we did not observe any significant differences in cytokine/chemokine levels between men and women with PV except for IL-13 and eotaxin (Figure 4B). Male patients had higher mean levels of both IL-13 and eotaxin than female patients (male: 31.48 ± 53.08 pg/mL, 244.43 ± 123.55 pg/ml, respectively, and female: 9.91 ± 20.64 pg/mL, 183.79 ± 92.24 pg/ml, respectively) (p-value: 0.0125 and 0.0059, respectively) (Figure 4B).
4 Discussion
A comprehensive view of the immune mechanisms leading to tissue damage in Pemphigus vulgaris has not been elucidated. Although the presence of B cell-produced autoantibodies directed against specific cell adhesion molecules including Dsg3 and Dsg1 (and perhaps other non-Dsg targets) is critical to lesional development, T cells are certain to play a central role in the pathogenesis of disease. While the rules that govern the coordinated T and B cell response in PV remains unclear, cytokines and chemokines are likely key modulators of the effector and regulatory pathways at play in disease.
It is unlikely that changes in a single cytokine is sufficient to cause the pathological phenomena in any given autoimmune disease (28). Accumulating literature suggests a model in which the action of multiple cytokines ultimately promotes immune-mediated tissue damage (15, 32, 33, 38, 39, 65). A recent study from China found generally elevated levels of Th1, Th2 and Th17 cytokines in PV patients (11). However, it remains clear that a more in-depth knowledge of cytokine profiles in patients is still required for a better understanding of disease mechanisms, and to facilitate the discovery of novel targets for future therapies. To address the mechanisms that underlie the variable and heterogeneous disease expression in PV, we undertook a non-reductionist strategy to comprehensively survey global changes in multiple cytokines/chemokines.
We unexpectedly found that PV patients show a wide variation of cytokine levels across all cytokines tested with levels ranging from undetectable to highly elevated for each individual cytokine regardless of disease phase or therapy status. Interestingly, patients tended to be either ‘high-expressors’ or ‘low-expressors’ of cytokines with ‘high expressors’ exhibiting high serum cytokine concentrations across numerous cytokines (albeit not necessarily all), while ‘low-expressors’ tended to have uniformly lower cytokine levels. Thus, cytokine expression appears to operate relatively, at differing scales dependent on the person. Intriguingly, our data show that if a patient displays elevated cytokine levels, they typically span multiple cytokines within a given pathway (e.g. one or two of the T helper pathways analyzed or generally pro-inflammatory cytokines), but not the entire set of cytokines analyzed. The reasons for this remarkable cytokine pathway heterogeneity among PV patients are intriguing but remain opaque and in need of further exploration, including the investigation of genetic factors such as HLA. Nevertheless, our data have implications for understanding the immunologic basis of clinical heterogeneity, disease operative mechanisms, and have the potential for guiding treatment decisions for patients.
Despite these inter-individual differences, our analysis uncovers a pronounced dysregulation of multiple cytokine immune pathways in PV compared to healthy controls, namely with elevation of the Th2 cytokines IL-4 and IL-5, the Th17 cytokines IL-22 and IL-23, as well as IL-2, IL-9, IL-10 and IL-15. However, a more composite picture emerges when we divide our healthy control population into those controls that carry PV-associated HLA-susceptibility alleles (“HLA-matched”) vs. those that do not (“HLA-unmatched”). It is well established that PV-associated HLA alleles such as DRB1*0402 are necessary for T cells to recognize Dsg3 (40), and that Dsg-reactive Th2 cells can be detected in patients with PV (5). Accordingly, PV has been considered a Th2 driven disease (66). Although disease-free, we found that HLA-matched controls express the pro-inflammatory cytokines IL-1α, IL-1β, IL-6, and TNFα, and Th2 cytokine IL-13, and chemokine IL-8 at similar concentrations to that seen in PV patients. These cytokines, however, are significantly higher in PV when compared to HLA-unmatched controls, suggesting that healthy controls carrying the PV associated HLA-haplotypes have a heightened ability to mount a pro-inflammatory and Th2 cytokine response similar to PV patients, while those lacking expression of the PV-associated HLA alleles do not. These findings echo other work in our lab using CyTOF technology to interrogate immune cell distribution in PV patients vs. healthy controls. Using CyTOF, we found an increase in Th2 cells in PV patients compared to “HLA-unmatched” controls, while “HLA-matched” controls exhibited similar numbers of Th2 cells to those found in PV patients (manuscript in preparation). Our CyTOF studies also showed an increase in Th17 cells in PV patients as compared to HLA-matched controls and HLA-unmatched controls which is supported by our observation of higher levels of the Th17 cytokines IL-21, IL-22 and IL-23 in this study. Table 1 summarizes differences in cytokine concentration between PV patients and controls that carry PV susceptibility alleles (“HLA-matched”) and those who do not carry HLA susceptibility alleles (“HLA-unmatched”).
Looking more broadly at the genetic basis of autoimmune development, it is striking to note that the pattern of HLA-linked partial activation of autoimmune-linked pathways is not limited only to cytokine expression as revealed in this study, but extends to also gene expression (67), auto-antibody profiles (68), and anti-oxidant status (69) as revealed in published work from our group. These parallel patterns across multiple datasets exploring intersecting biological levels promote a new hypotheses regarding the immunological currents that either promote or prevent autoimmune development (67). Specifically, there may be HLA-independent pathways required for patients to progress to the disease state in PV, but also protective counter-regulatory mechanisms in genetically susceptible (HLA-matched) controls, restraining them from disease despite their genetic susceptibility.
Along these lines, we found that HLA-matched controls had significantly lower levels of IL-10 and IL-15 than PV patients, while HLA-unmatched controls did not, setting up the intriguing hypothesis that the downregulation of these cytokines affords those healthy people that carry genetic susceptibility a certain protection. IL-10, produced by T regulatory cells (amongst others), is thought to be a predominantly inhibitory cytokine. It is known to inhibit production of IFNγ, and activation of macrophages, NK cells, and neutrophils (10, 70). IL-10 plays a critical role in slowing the progression of autoimmune diseases (70). Nevertheless, multiple studies found elevated levels of IL-10 in serum, blister fluid, and supernatants of T cell and B regulatory cell cultures from PV patients compared to controls (25, 40, 45, 71, 72). Also, recent studies suggest that IL-10 is ineffective at accomplishing its downstream regulatory effects in PV (41, 72, 73). Without reaching its downstream target, IL-10 concentration may increase because feedback inhibition does not occur. Perhaps this seemingly contradictory data on IL-10 in PV could be explained by other immune-promoting functions of IL-10, such as induction of B-cell maturation, survival, and differentiation, as well as immunoglobulin production (74–76). Kowalski et al. suggested IL-10 may be higher in PV due this effect on B-cell production of IgG autoantibodies (19). Further, IL-10 can inhibit Th1 cytokines, and thus shift Th1/Th2 balance and promote Th2 cells in the context of PV (25).
Similarly to IL-10, we find that for IL-15, HLA-matched controls show significantly lower serum levels than PV patients, while HLA-unmatched controls do not. IL-15, produced by epithelial cells, fibroblasts, and peripheral blood mononuclear (monocyte enriched) cells promotes immunoglobulin switching, memory immunoglobulins, and NK cell differentiation and proliferation (77, 78). In support of these cytokine data, CyTOF analysis found that the balance of early vs. late NK cells was shifted towards late NK cells in PV patients when compared to all controls, as well as when compared to HLA-matched controls (manuscript in preparation). Downregulation of IL-15 in matched controls, as shown in this work, could explain why we also see a shift towards early NK cells in these same controls when compared to patients. Also, it is conceivable that a specific downregulation of IL-15 could lead to a reduction in immunoglobulin switching and memory immunoglobulin production and thus mediate disease protection in healthy individuals carrying PV susceptibility alleles.
Surprisingly, we do not see an appreciable difference in cytokine levels between different phases of disease activity, neither in patients regardless of therapy status nor when looking at the subgroup completely off therapy. It is possible that differences between groups in this study may be obscured by the wide variations in cytokine levels across patients. Thus, it will be important in future work to analyze samples taken from patients in a longitudinal study design to truly appreciate if disease activity is paralleled by changed in cytokine levels. In our study, we had three patients with longitudinal samples. Of these three, two exhibited generally higher cytokine levels in active disease, while one had higher levels in remission. Follow up studies are needed to include more patients followed longitudinally across different phases of disease to discern meaningful differences. Similarly, we observed only minimal differences based on disease morphology (mucocutaneous vs. mucosal) and based on sex (male vs. female). Meaningful differences between these subgroups may again be obscured by the wide variation of cytokine expression between patients.
Taken together, our study reveals that compared to healthy controls (regardless of HLA association), PV patients harbor elevated cytokine expression levels in generally pro-inflammatory- and more specifically Th2 and Th17 pathways, albeit at a very patient-specific, individualized level. This sets up the intriguing future management paradigm that based on an individual patient’s cytokine pattern, cytokine-targeted therapies could be aligned to be maximally effective. Cytokine profiling of individual patients could be leveraged in the future to personalize treatment plans.
Currently, the mainstay of treatment of autoimmune disease remains dependent on potent immunosuppressants that inhibit multiple immune pathways. In 2018, Rituximab was the first biologic to be FDA-approved for treatment of PV. Rituximab is now considered first line for treatment of this disease, although not without side effects. While achieving disease control in a majority of cases, these treatments also place the patient at risk for opportunistic infections and untoward side effects. Front runners of anti-cytokine biologic therapies for various autoimmune diseases include drugs targeting TNFα, IL-1, IL-6, IL-12/IL-23, and IL-17 (79). In the past years, several therapies that target specific cytokines, eg. etanercept (TNFα receptor), adalimumab (anti-TNFα), ustekinumab (anti-IL-23/IL-12), have been developed, with many more in the pipeline. To date, there is limited literature on anti-cytokine therapy in PV. However, multiple recent case reports show Dupilumab, an inhibitor of IL-4 and IL-13 to be effective in treating refractory or severe cases of disease (80–82). Larger studies and clinical trials are necessary to elucidate the true efficacy of dupilumab in PV, as well as explore additional anti-cytokine therapies. Our finding of high cytokine concentrations across one or two T-helper cell pathways may be relevant to predicting which PV patients are likely responders to dupilumab.
While this study emphasizes the importance of cytokines in disease pathogenesis, naturally occurring anti-cytokine autoantibodies are becoming an increasingly prevalent topic in autoimmunity. Though anti-cytokine autoantibodies can be found at low levels in disease-free, healthy subjects, they are found at higher titers in certain autoimmune diseases and immunodeficiencies (83). There are limited reports on the presence of these anti-cytokine autoantibodies in pemphigus and pemphigoid diseases (84, 85). Interestingly, rituximab has been shown to decrease levels of anti-IL-8 autoantibodies in rheumatoid arthritis (86). Given the significance of cytokines in PV pathogenesis, and increasing use of rituximab in PV, further exploration of anti-cytokine autoantibodies is warranted.
Our data support the role of the Th2 pathway in PV, while also lending credence to the increasing literature on the importance of the Th17 pathway. Thus, multiple cytokine pathways appear to be operative in the development of PV. However, in any given patient, not all pathways are simultaneously active; some patients are more Th2 and/orTh17 dominant, while others lean more towards pro-inflammatory or Th1 pathways. We propose a paradigm shift in our understanding of the pathogenesis of PV, specifically that there seem to be different ways individuals can reach disease, either through a majority Th2 and Th17 response, a pro-inflammatory response, or a Th1 response or a combination thereof. The precise molecular means by which patients are directed to different paths leading to PV are unknown, but perhaps there is a genetic basis for which HLA, especially, might play a role. Future mechanistic studies are needed to gain insight into disease risk and prognosis. For now, stratifying patients based on cytokine profiles has the potential to change our approach to therapy. By matching cytokine profiles to more targeted anti-cytokine/anti-pathway treatment, we can envision personalizing care to improve outcomes based on patients’ specific inflammatory profile.
Overall, our studies shed light on the complexities of critical cytokine networks impacting PV on a personalized level. This work can be expected to deepen our understanding of underlying pathogenetic mechanisms relevant to autoimmunity, help to resolve the biologic underpinnings of clinical heterogeneity that confound our ability to prognose and manage patients, and ultimately serve to advance efforts to align appropriately targeted therapies in select patients while reducing side effects associated with current approaches reliant largely on general immunosuppression.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by the institutional review boards of Weill-Cornell Medical College (IRB 0998-398), Michigan State University (IRB 05-1034), and the University at Buffalo (IRB 456887). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
RS: Data curation, Formal Analysis, Visualization, Writing – original draft, Writing – review & editing. KS-S: Conceptualization, Data curation, Methodology, Writing – original draft, Writing – review & editing. AS: Conceptualization, Investigation, Methodology, Resources, Supervision, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
We thank Canting Guo for help performing the initial experiment.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2024.1500231/full#supplementary-material
References
1. Yang M, Wu H, Zhao M, Chang C, Lu Q. The pathogenesis of bullous skin diseases. J Transl Autoimmun. (2019) 2:100014. doi: 10.1016/j.jtauto.2019.100014
2. Sielski L, Baker J, DePasquale MC, Attwood K, Seiffert-Sinha K, Sinha AA. Desmoglein compensation hypothesis fidelity assessment in Pemphigus. Front Immunol. (2022) 13:969278. doi: 10.3389/fimmu.2022.969278
3. Hertl M, Riechers R. Analysis of the T cells that are potentially involved in autoantibody production in pemphigus vulgaris. J Dermatol. (1999) 26:748–52. doi: 10.1111/j.1346-8138.1999.tb02086.x
4. Sinha AA, Lopez MT, McDevitt HO. Autoimmune diseases: the failure of self tolerance. Science. (1990) 248:1380–8. doi: 10.1126/science.1972595
5. Veldman C, Stauber A, Wassmuth R, Uter W, Schuler G, Hertl M. Dichotomy of autoreactive Th1 and Th2 cell responses to desmoglein 3 in patients with pemphigus vulgaris (PV) and healthy carriers of PV-associated HLA class II alleles. J Immunol. (2003) 170:635–42. doi: 10.4049/jimmunol.170.1.635
6. Veldman CM, Gebhard KL, Uter W, Wassmuth R, Grotzinger J, Schultz E, et al. T cell recognition of desmoglein 3 peptides in patients with pemphigus vulgaris and healthy individuals. J Immunol. (2004) 172:3883–92. doi: 10.4049/jimmunol.172.6.3883
7. Sinha AA, Brautbar C, Szafer F, Friedmann A, Tzfoni E, Todd JA, et al. A newly characterized HLA DQ beta allele associated with pemphigus vulgaris. Science. (1988) 239:1026–9. doi: 10.1126/science.2894075
8. Baker J, Seiffert-Sinha K, Sinha AA. Patient genetics shape the autoimmune response in the blistering skin disease pemphigus vulgaris. Front Immunol. (2022) 13:1064073. doi: 10.3389/fimmu.2022.1064073
9. Chow S, Rizzo C, Ravitskiy L, Sinha AA. The role of T cells in cutaneous autoimmune disease. Autoimmunity. (2005) 38:303–17. doi: 10.1080/08916930500124429
10. Mayer G. Microbiology and immunology. Hunt R, editor. Charleston, SC: The Board of Trustees of the University of South Carolina (2010).
11. You S, Ouyang J, Wu Q, Zhang Y, Gao J, Luo X, et al. Comparison of serum cytokines and chemokines levels and clinical significance in patients with pemphigus vulgaris-A retrospective study. Exp Dermatol. (2024) 33:e15173. doi: 10.1111/exd.15173
12. Asothai R, Anand V, Das D, Antil PS, Khandpur S, Sharma VK, et al. Distinctive Treg associated CCR4-CCL22 expression profile with altered frequency of Th17/Treg cell in the immunopathogenesis of Pemphigus Vulgaris. Immunobiology. (2015) 220:1129–35. doi: 10.1016/j.imbio.2015.06.008
13. D'Auria L, Bonifati C, Mussi A, D'Agosto G, De Simone C, Giacalone B, et al. Cytokines in the sera of patients with pemphigus vulgaris: interleukin-6 and tumour necrosis factor-alpha levels are significantly increased as compared to healthy subjects and correlate with disease activity. Eur Cytokine Netw. (1997) 8:383–7.
14. El-Darouti MA, Hegazy RA, Abdel Hay RM, El Hawary MS, Tawdy AM, Fawzy MM, et al. Study of T helper 1 and T helper 2 responses in pemphigus vulgaris patients receiving interferon alpha 2a injections in addition to a standard protocol therapy: a randomized controlled trial. Arch Dermatol Res. (2015) 307:299–307. doi: 10.1007/s00403-014-1522-2
15. Hertl M, Amagai M, Sundaram H, Stanley J, Ishii K, Katz SI. Recognition of desmoglein 3 by autoreactive T cells in pemphigus vulgaris patients and normals. J Invest Dermatol. (1998) 110:62–6. doi: 10.1046/j.1523-1747.1998.00086.x
16. Huang S, Mao J, Zhou L, Xiong X, Deng Y. The imbalance of gut microbiota and its correlation with plasma inflammatory cytokines in pemphigus vulgaris patients. Scand J Immunol. (2019) 90:e12799. doi: 10.1111/sji.2019.90.issue-3
17. Joshi N, Minz RW, Anand S, Parmar NV, Kanwar AJ. Vitamin D deficiency and lower TGF-beta/IL-17 ratio in a North Indian cohort of pemphigus vulgaris. BMC Res Notes. (2014) 7:536. doi: 10.1186/1756-0500-7-536
18. Khozeimeh F, Savabi O, Esnaashari M. Evaluation of interleukin-1alpha, interleukin-10, tumor necrosis factor-alpha and transforming growth factor-beta in the serum of patients with pemphigus vulgaris. J Contemp Dent Pract. (2014) 15:746–9. doi: 10.5005/jp-journals-10024-1610
19. Kowalski EH, Kneibner D, Kridin K, Amber KT. Serum and blister fluid levels of cytokines and chemokines in pemphigus and bullous pemphigoid. Autoimmun Rev. (2019) 18:526–34. doi: 10.1016/j.autrev.2019.03.009
20. Lee SH, Hong WJ, Kim SC. Analysis of serum cytokine profile in pemphigus. Ann Dermatol. (2017) 29:438–45. doi: 10.5021/ad.2017.29.4.438
21. Masjedi M, Esmaeil N, Saffaei A, Abtahi-Naeini B, Pourazizi M, Haghjooy Javanmard S, et al. Cytokine indexes in pemphigus vulgaris: perception of its immunpathogenesis and hopes for non-steroidal treatment. Iran J Pharm Res. (2017) 16:1223–9.
22. Mortazavi H, Esmaili N, Khezri S, Khamesipour A, Vasheghani Farahani I, Daneshpazhooh M, et al. The effect of conventional immunosuppressive therapy on cytokine serum levels in pemphigus vulgaris patients. Iran J Allergy Asthma Immunol. (2014) 13:174–83.
23. Ragab N, Abdallah M, El-Gohary E, Elewa R. Stress and serum TNF-alpha levels may predict disease outcome in patients with pemphigus: a preliminary study. Cutis. (2011) 87:189–94.
24. Rico MJ, Benning C, Weingart ES, Streilein RD, Hall RP 3rd. Characterization of skin cytokines in bullous pemphigoid and pemphigus vulgaris. Br J Dermatol. (1999) 140:1079–86. doi: 10.1046/j.1365-2133.1999.02907.x
25. Satyam A, Khandpur S, Sharma VK, Sharma A. Involvement of T(H)1/T(H)2 cytokines in the pathogenesis of autoimmune skin disease-Pemphigus vulgaris. Immunol Invest. (2009) 38:498–509. doi: 10.1080/08820130902943097
26. Stern JN, Keskin DB, Barteneva N, Zuniga J, Yunis EJ, Ahmed AR. Possible role of natural killer cells in pemphigus vulgaris - preliminary observations. Clin Exp Immunol. (2008) 152:472–81. doi: 10.1111/j.1365-2249.2008.03638.x
27. Timoteo RP, Silva MV, Miguel CB, Silva DA, Catarino JD, Junior Rodrigues V, et al. Th1/th17-related cytokines and chemokines and their implications in the pathogenesis of pemphigus vulgaris. Mediators Inflamm 2017. (2017) p:7151285. doi: 10.1155/2017/7151285
28. Giordano CN, Sinha AA. Cytokine networks in Pemphigus vulgaris: An integrated viewpoint. Autoimmunity. (2012) 45:427–39. doi: 10.3109/08916934.2012.697593
29. Das D, Anand V, Khandpur S, Sharma VK, Sharma A. T helper type 1 polarizing gammadelta T cells and Scavenger receptors contribute to the pathogenesis of Pemphigus vulgaris. Immunology. (2018) 153:97–104. doi: 10.1111/imm.2018.153.issue-1
30. Singh PK, Das S, Rai G, Ansari MA, Dar SA, Singh T, et al. A snapshot of T cell subset cytokines in pemphigus vulgaris: A cross-sectional study. Cureus. (2022) 14:e29890. doi: 10.7759/cureus.29890
31. Hertl M. Humoral and cellular autoimmunity in autoimmune bullous skin disorders. Int Arch Allergy Immunol. (2000) 122:91–100. doi: 10.1159/000024364
32. Feliciani C, Toto P, Amerio P, Pour SM, Coscione G, Shivji G, et al. In vitro and in vivo expression of interleukin-1alpha and tumor necrosis factor-alpha mRNA in pemphigus vulgaris: interleukin-1alpha and tumor necrosis factor-alpha are involved in acantholysis. J Invest Dermatol. (2000) 114:71–7. doi: 10.1046/j.1523-1747.2000.00835.x
33. Feliciani C, Toto P, Wang B, Sauder DN, Amerio P, Tulli A. Urokinase plasminogen activator mRNA is induced by IL-1alpha and TNF-alpha in in vitro acantholysis. Exp Dermatol. (2003) 12:466–71. doi: 10.1034/j.1600-0625.2002.120415.x
34. Bhol KC, Desai A, Kumari S, Colon JE, Ahmed AR. Pemphigus vulgaris: the role of IL-1 and IL-1 receptor antagonist in pathogenesis and effects of intravenous immunoglobulin on their production. Clin Immunol. (2001) 100:172–80. doi: 10.1006/clim.2001.5061
35. Feliciani C, Toto P, Amerio P. In vitro C3 mRNA expression in Pemphigus vulgaris: complement activation is increased by IL-1alpha and TNF-alpha. J Cutan Med Surg. (1999) 3:140–4. doi: 10.1177/120347549900300306
36. Ludwig RJ, Schmidt E. Cytokines in autoimmune bullous skin diseases. Epiphenomena or contribution to pathogenesis? G Ital Dermatol Venereol. (2009) 144:339–49.
37. Keskin DB, Stern JNHS, Fridkis-Hareli M, Ahmed Razzaque A. Cytokine profiles in pemphigus vulgaris patients treated with intravenous immunoglobulins as compared to conventional immunosuppressive therapy. Cytokine. (2008) 41:315–21. doi: 10.1016/j.cyto.2007.12.007
38. Lin MS, Swartz SJ, Lopez A, Ding X, Fernandez-Vina MA, Stastny P, et al. Development and characterization of desmoglein-3 specific T cells from patients with pemphigus vulgaris. J Clin Invest. (1997) 99:31–40. doi: 10.1172/JCI119130
39. Rizzo C, Fotino M, Zhang Y, Chow S, Spizuoco A, Sinha AA. Direct characterization of human T cells in pemphigus vulgaris reveals elevated autoantigen-specific Th2 activity in association with active disease. Clin Exp Dermatol. (2005) 30:535–40. doi: 10.1111/j.1365-2230.2005.01836.x
40. Wucherpfennig KW, Yu B, Bhol K, Monos DS, Argyris E, Karr RW, et al. Structural basis for major histocompatibility complex (MHC)-linked susceptibility to autoimmunity: charged residues of a single MHC binding pocket confer selective presentation of self-peptides in pemphigus vulgaris. Proc Natl Acad Sci U S A. (1995) 92:11935–9. doi: 10.1073/pnas.92.25.11935
41. Takahashi H, Amagai M, Tanikawa A, Suzuki S, Ikeda Y, Nishikawa T, et al. T helper type 2-biased natural killer cell phenotype in patients with pemphigus vulgaris. J Invest Dermatol. (2007) 127:324–30. doi: 10.1038/sj.jid.5700527
42. Yokoyama T, Amagai M. Immune dysregulation of pemphigus in humans and mice. J Dermatol. (2010) 37:205–13. doi: 10.1111/j.1346-8138.2009.00797.x
43. Narbutt J, Lukamowicz J, Bogaczewicz J, Sysa-Jedrzejowska A, Torzecka JD, Lesiak A. Serum concentration of interleukin-6 is increased both in active and remission stages of pemphigus vulgaris. Mediators Inflamm 2008. (2008) p:875394. doi: 10.1155/2008/875394
44. Eid HE-G. Basiouny, Cytokine profiles in the sera of Egyptian patients with oral pemphigus vulgaris. Braz J Oral Sci. (2011) 10:83–7.
45. Bhol KC, Rojas AI, Khan IU, Ahmed AR. Presence of interleukin 10 in the serum and blister fluid of patients with pemphigus vulgaris and pemphigoid. Cytokine. (2000) 12:1076–83. doi: 10.1006/cyto.1999.0642
46. Kheirodin M, Tehranchinia Z, Ketabi Y, Tavakolpour S, Dadkhahfar S, Faghankhani M, et al. Elevated serum levels of interleukin-15 in pemphigus vulgaris patients: a potential therapeutic target. Dermatol Pract Concept. (2022) 12:e2022118. doi: 10.5826/dpc.1203a118
47. Arakawa M, Dainichi T, Yasumoto S, Hashimoto T. Lesional Th17 cells in pemphigus vulgaris and pemphigus foliaceus. J Dermatol Sci. (2009) 53:228–31. doi: 10.1016/j.jdermsci.2008.09.008
48. Zebrowska A, Wozniacka A, Juczynska K, Ociepa K, Waszczykowska E, Szymczak I, et al. Correlation between IL36alpha and IL17 and activity of the disease in selected autoimmune blistering diseases. Mediators Inflamm 2017. (2017) p:8980534. doi: 10.1155/2017/8980534
49. Morsy H, Zedan H, Kassim M, Mwafey IM, Negm D. Serum IL-21 level in patients with pemphigus: Before and after treatment. Dermatol Ther. (2020) 33:e14482. doi: 10.1111/dth.14482
50. Pouralibaba F, Babaloo Z, Pakdel F, Abdollahian T, Pourzar S. Elevated levels of interleukin-23 in sera of patients with pemphigus vulgaris. Iran J Immunol. (2012) 9:261–5.
51. Hennerici T, Pollmann R, Schmidt T, Seipelt M, Tackenberg B, Mobs C, et al. Increased frequency of T follicular helper cells and elevated interleukin-27 plasma levels in patients with pemphigus. PLoS One. (2016) 11:e0148919. doi: 10.1371/journal.pone.0148919
52. Bakr RM, Sayed DS, Abd-Elkader AS, Kamel AA, Badran AY. Does interleukin-33 level correlate with the activity of Pemphigus vulgaris?: A case-control study. Dermatol Ther. (2021) 34:e14605. doi: 10.1111/dth.14605
53. Alecu M, Alecu S, Coman G, Gălăţescu E, Ursaciuc C. ICAM-1, ELAM-1, TNF-alpha and IL-6 in serum and blister liquid of pemphigus vulgaris patients. Roumanian Arch Microbiol Immunol. (1999) 58:121–30.
54. Lopez-Robles E, Avalos-Diaz E, Vega-Memije E, Hojyo-Tomoka T, Villalobos R, Fraire S, et al. TNFalpha and IL-6 are mediators in the blistering process of pemphigus. Int J Dermatol. (2001) 40:185–8.doi: 10.1046/j.1365-4362.2001.01083.x
55. Shahbazian P, Izad M, Daneshpazhooh M, Mortazavi H, Salehi Z, Behruzifar S, et al. Decreased serum levels of interleukin-4 and interleukin-21 in new pemphigus vulgaris patients, but not chronic patients with inactive disease compared to healthy controls. Dermatol Pract Concept. (2021) 11:e2021035. doi: 10.5826/dpc.1102a35
56. Gholibeigian Z, Izad M, Daneshpazhooh M, Mortazavi H, Salehi Z, Behruzifar S, et al. Decreased serum levels of interleukin-17, interleukin-23, TGF-beta in pemphigus vulgaris patients, and their association with disease phase. Dermatol Ther. (2020) 33:e14071. doi: 10.1111/dth.14071
57. Metwally D, Fawzy M, ElKalioby M, Hegazy R, Hay RA, Abd-Elreheem H, et al. Assessment of the quality of life, prevalence of depression, and the level of interleukin 6 in patients with pemphigus vulgaris. Acta Dermatovenerol Croat. (2020) 28:57–62.
58. Sugiyama H, Matsue H, Nagasaka A, Nakamura Y, Tsukamoto K, Shibagaki N, et al. CD4+CD25high regulatory T cells are markedly decreased in blood of patients with pemphigus vulgaris. Dermatology. (2007) 214:210–20. doi: 10.1159/000099585
59. Akman Karakas A, Ergun E, Ekinci Sayin N, Toylu A, Uzun S, Alpsoy E. The role of T follicular helper cells in clinical remission and relapse in patients with pemphigus treated with rituximab. Acta Dermatovenerol Croat. (2023) 31:72–9.
60. Murrell DF, Dick S, Ahmed AR, Amagai M, Barnadas MA, Borradori L, et al. Consensus statement on definitions of disease, end points, and therapeutic response for pemphigus. J Am Acad Dermatol. (2008) 58:1043–6. doi: 10.1016/j.jaad.2008.01.012
61. Olerup O, Zetterquist H. HLA-DR typing by PCR amplification with sequence-specific primers (PCR-SSP) in 2 hours: an alternative to serological DR typing in clinical practice including donor-recipient matching in cadaveric transplantation. Tissue Antigens. (1992) 39:225–35. doi: 10.1111/j.1399-0039.1992.tb01940.x
62. Gonzalez A, Espana A, Lopez-Zabalza MJ, Pelacho B, Sanchez-Carpintero I, Santiago E, et al. Correlation between profile of circulating mononuclear cells and clinical manifestations in patients with pemphigus vulgaris. Autoimmunity. (2000) 32:115–28. doi: 10.3109/08916930008994081
63. Kavusi S, Daneshpazhooh M, Farahani F, Abedini R, Lajevardi V, Chams-Davatchi C. Outcome of pemphigus vulgaris. J Eur Acad Dermatol Venereol. (2008) 22:580–4. doi: 10.1111/j.1468-3083.2007.02537.x
64. Gupta VK, Kelbel TE, Nguyen D, Melonakos KC, Murrell DF, Xie Y, et al. A globally available internet-based patient survey of pemphigus vulgaris: epidemiology and disease characteristics. Dermatol Clin. (2011) 29:393–404. doi: 10.1016/j.det.2011.03.016
65. Bhol K, Natarajan K, Nagarwalla N, Mohimen VAoki A, Ahmed AR. Correlation of peptide specificity and IgG subclass with pathogenic and nonpathogenic autoantibodies in pemphigus vulgaris: a model for autoimmunity. Proc Natl Acad Sci U S A. (1995) 92:5239–43. doi: 10.1073/pnas.92.11.5239
66. Geng RSQ, Wilken B, Sood S, Sibbald RG, Sibbald C. Biomarkers in pemphigus vulgaris: A systematic review. J Cutan Med Surg. (2024) 28(5):12034754241266136. doi: 10.1177/12034754241266136
67. Dey-Rao R, Seiffert-Sinha K, Sinha AA. Genome-wide expression analysis suggests unique disease-promoting and disease-preventing signatures in Pemphigus vulgaris. Genes Immun. (2013) 14:487–99. doi: 10.1038/gene.2013.44
68. Sajda T, Hazelton J, Patel M, Seiffert-Sinha K, Steinman L, Robinson W, et al. Multiplexed autoantigen microarrays identify HLA as a key driver of anti-desmoglein and -non-desmoglein reactivities in pemphigus. Proc Natl Acad Sci U S A. (2016) 113:1859–64. doi: 10.1073/pnas.1525448113
69. Shah AA, Dey-Rao R, Seiffert-Sinha K, Sinha AA. Increased oxidative stress in pemphigus vulgaris is related to disease activity and HLA-association. Autoimmunity. (2016) 49:248–57. doi: 10.3109/08916934.2016.1145675
70. Ries S, Hilgenberg E, Lampropoulou V, Shen P, Dang VD, Wilantri S, et al. B-type suppression: a role played by "regulatory B cells" or "regulatory plasma cells"? Eur J Immunol. (2014) 44:1251–7. doi: 10.1002/eji.201343683
71. Baroni A, Perfetto B, Ruocco E, Greco R, Criscuolo D, Ruocco V. Cytokine pattern in blister fluid and sera of patients with pemphigus. Dermatology. (2002) 205:116–21. doi: 10.1159/000063895
72. Xu RC, Zhu HQ, Li WP, Zhao XQ, Yuan HJ, Zheng J, et al. The imbalance of Th17 and regulatory T cells in pemphigus patients. Eur J Dermatol. (2013) 23:795–802. doi: 10.1684/ejd.2013.2177
73. Zakka LR, Shetty SS, Ahmed AR. Rituximab in the treatment of pemphigus vulgaris. Dermatol Ther (Heidelb). (2012) 2:17. doi: 10.1007/s13555-012-0017-3
74. Rousset F, Garcia E, Defrance T, Peronne C, Vezzio N, Hsu DH, et al. Interleukin 10 is a potent growth and differentiation factor for activated human B lymphocytes. Proc Natl Acad Sci U S A. (1992) 89:1890–3. doi: 10.1073/pnas.89.5.1890
75. Levy Y, Brouet JC. Interleukin-10 prevents spontaneous death of germinal center B cells by induction of the bcl-2 protein. J Clin Invest. (1994) 93:424–8. doi: 10.1172/JCI116977
76. Rousset F, Peyrol S, Garcia E, Vezzio N, Andujar M, Grimaud JA, et al. Long-term cultured CD40-activated B lymphocytes differentiate into plasma cells in response to IL-10 but not IL-4. Int Immunol. (1995) 7:1243–53. doi: 10.1093/intimm/7.8.1243
77. Grabstein KH, Eisenman J, Shanebeck K, Rauch C, Srinivasan S, Fung V, et al. Cloning of a T cell growth factor that interacts with the beta chain of the interleukin-2 receptor. Science. (1994) 264:965–8. doi: 10.1126/science.8178155
78. Huntington ND. The unconventional expression of IL-15 and its role in NK cell homeostasis. Immunol Cell Biol. (2014) 92:210–3. doi: 10.1038/icb.2014.1
79. Rider P, Carmi Y, Cohen I. Biologics for targeting inflammatory cytokines, clinical uses, and limitations. Int J Cell Biol 2016. (2016) p:9259646. doi: 10.1155/2016/9259646
80. Jiang C, Adjei S, Santiago S, Lu J, Duran M, Tyring S. Novel use of dupilumab in pemphigus vulgaris and pemphigus foliaceus. JAAD Case Rep. (2023) 42:12–5. doi: 10.1016/j.jdcr.2023.09.018
81. Moore AY, Hurley K. Dupilumab monotherapy suppresses recalcitrant pemphigus vulgaris. JAAD Case Rep. (2023) 31:16–8. doi: 10.1016/j.jdcr.2022.10.035
82. Chen S, Zhan S, Hua C, Tang Y, Cheng H. A novel combined use of dupilumab for treatment of aggressive refractory pemphigus vulgaris complicated with pulmonary tuberculosis: A case report and the RNA-seq analysis. Front Immunol. (2022) 13:825796. doi: 10.3389/fimmu.2022.825796
83. Meager A, Wadhwa M. Detection of anti-cytokine antibodies and their clinical relevance. Expert Rev Clin Immunol. (2014) 10:1029–47. doi: 10.1586/1744666X.2014.918848
84. Mizutani H, Ohmoto Y, Kupper TS, Shimizu M. Endogenous neutralizing anti-IL-1 alpha autoantibodies in inflammatory skin diseases: possible natural inhibitor for over expressed epidermal IL-1. J Dermatol Sci. (1998) 20:63–71. doi: 10.1016/s0923-1811(98)00074-7
85. Prummer O, Zillikens D, Porzsolt F. High-titer interferon-alpha antibodies in a patient with pemphigus foliaceus. Exp Dermatol. (1996) 5:213–7. doi: 10.1111/j.1600-0625.1996.tb00119.x
Keywords: Pemphigus, HLA, cytokine, autoimmunity, Th17, Th2
Citation: Schwartz RR, Seiffert-Sinha K and Sinha AA (2024) Cytokine profiling reveals HLA-linked Th2 and Th17 driven immune activation in pemphigus vulgaris patients and genetically susceptible healthy controls. Front. Immunol. 15:1500231. doi: 10.3389/fimmu.2024.1500231
Received: 23 September 2024; Accepted: 08 November 2024;
Published: 04 December 2024.
Edited by:
Ming Yu Lien, China Medical University Hospital, TaiwanReviewed by:
Chiara Colarusso, University of Salerno, ItalyAna Maria Roselino, University of São Paulo, Brazil
Copyright © 2024 Schwartz, Seiffert-Sinha and Sinha. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Animesh A. Sinha, YWFzaW5oYUBidWZmYWxvLmVkdQ==
 Rebekah R. Schwartz
Rebekah R. Schwartz Kristina Seiffert-Sinha
Kristina Seiffert-Sinha Animesh A. Sinha
Animesh A. Sinha