- 1Department of Hepatobiliary Surgery, National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, China
- 2Department of Breast Surgical Oncology, National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, China
- 3Department of Anesthesiology, National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, China
Purpose: Treatment for advanced gallbladder cancer (GBC) remains controversial, with various recommendations regarding the choice and combination of surgery and adjuvant therapy. The present article is targeting for the exploration of optimal treatment models for advanced GBC.
Methods: AJCC (American Joint Committee on Cancer, 8th edition) stage III and stage IV GBC, were defined as advanced GBC. Patients with advanced GBC were identified using the Surveillance, Epidemiology, and End Results (SEER) database and departmental cohort. Because of the most representative, only gallbladder adenocarcinoma (GBAC) patients were selected. Based on their surgical status (No, Non-radical and Radical surgery), chemotherapy status (Chemotherapy, No chemotherapy), and radiotherapy status (Radiotherapy, No radiotherapy), treatment models were categorized. For the purposes of evaluating the treatment outcomes of various treatment models and determining the risk element for cancer-specific survival (CSS), Cox regression analysis was applied. Kaplan-Meier curves were used before and after adjusting for covariates, with log-rank tests used to analyze discrepancies between curves. Immunotherapy was analyzed using clinical data from departmental cohort. Finally, to compensate for the limitations of the database, a review examines the progress in treatment models for advanced GBC.
Results: 5,154 patients aged over 18 years with solitary primary advanced GBC were identified from the SEER database. In advanced GBC patients, the treatment model has emerged as a significant prognostic factor. “Radical surgery + Chemotherapy + Radiotherapy” models maximally improved the CSS of advanced GBC before and after adjusting for covariates, while “No surgery + No chemotherapy + No radiotherapy” model had the lowest CSS. The present conclusions were supported even after subgroup analysis by AJCC stage. The efficacy of immunotherapy was demonstrated in the departmental cohort analysis. Additionally, this article provides a comprehensive overview of recent advancements in various emerging treatment strategies.
Conclusion: Even when optimal treatment model cannot be pursued, providing comprehensive combinations of treatments to advanced GBC patients whenever possible is always beneficial for their survival.
1 Introduction
As the most familiar cancer from biliary tract, GBC ranks 23rd among all tumors and 6th among digestive system tumors (1). The global morbidity and mortality of GBC have been increasing annually, Eastern Asia, South America, and Melanesia were the regions with highest ranking (2, 3), the latest global incidence and mortality data for GBC are visualized in Supplementary Figure 1. Moreover, the etiology of GBC varies across different countries (4). GBC is one of the few cancers that shows a gender difference (5), with the morbidity in females nearly three times that of males (6), and is the only digestive system tumor that is predominantly female (7). As early symptoms are rare and lymph nodes and distant metastases often occur early, three-quarters of GBC patients are diagnosed with advanced stages or metastases, which results in poor prognoses (5, 8). In fact, symptomatic gallstones lead to cholecystectomy in most cases of GBC (9, 10). Additionally, among the biliary tract cancers, GBC has the shortest median survival rate. Despite improvements in diagnosis and treatment over the years, its 5-year survival rate remains lower than 20% (3). Therefore, addressing the treatment of GBC, a highly lethal tumor, is a significant challenge worldwide, and there is still considerable controversy surrounding the treatment of advanced GBC patients. Given that adenocarcinoma is the most prevalent and representative pathological type of GBC (11), and that treatment research has primarily centered on gallbladder adenocarcinoma (GBAC) (12, 13), the present study specifically addresses GBAC to ensure homogeneity, as other histological types exhibit different biological behaviors and treatment responses (14). Currently, among all treatment options for gallbladder cancer (GBC), only surgical intervention has demonstrated clear effectiveness, while other options, including radiotherapy, chemotherapy, and immunotherapy, remain in the exploratory stage (15). In the present article the SEER database, departmental cohort and the PubMed database were utilized to investigate treatment models for patients with advanced GBC, while also summarizing alternative treatment options.
2 Patients and methods
2.1 Patients selection
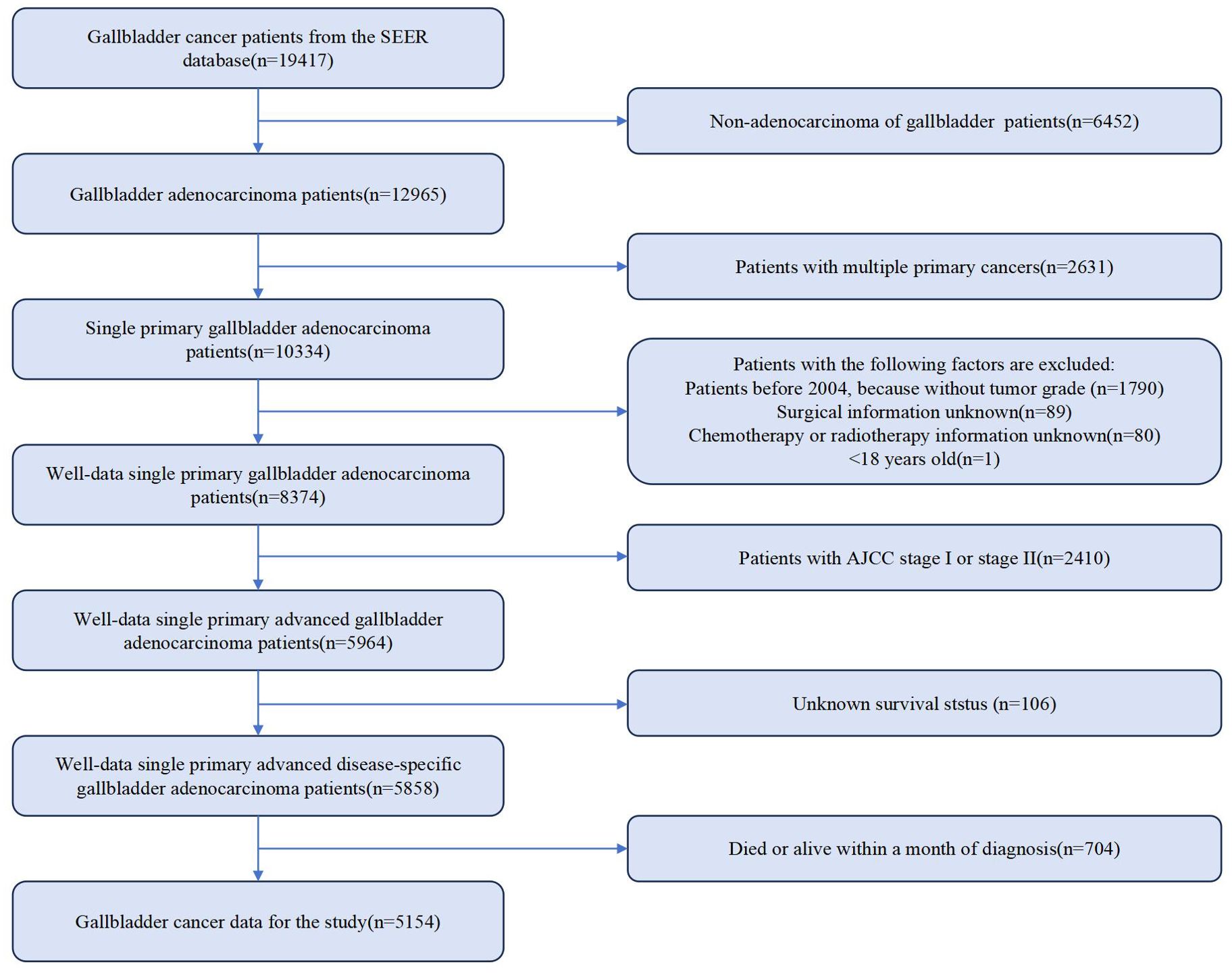
Information on demographic, cancer, treatment and follow-up is provided by the SEER database. To retrospectively collect GBC patient data, SEERStat (version 8.4.3) was used. 19,417 patients diagnosed with GBC from 2000 to 2019 were identified. Inclusion criteria were defined as Site recode ICD-O-3/WHO 2008 registered as gallbladder. Only those with AJCC stage III and stage IV GBC were selected. As per the AJCC, 8th edition, stage IIIA, IIIB, IVA and IVB were defined as “T3 + N0 + M0”, “T1-3 + N1 + M0”, “T4 + N0-1 + M0” and “Any T + N2 + M0” or “Any T + Any N + M1”, respectively. Exclusion criteria were as follows: not adenocarcinoma, not single primary cancer, patients diagnosed before 2004 (without tumor grade), unknown surgical information, without chemotherapy or radiotherapy information, less than 18 years old, AJCC stage I or stage II, unknown survival status, and death or alive within 1 month of diagnosis. Ultimately, 5,154 eligible patients with advanced GBC diagnosis remained. The detailed flowchart is illustrated in Figure 1. Given that immunotherapy is a novel treatment option, we selected only patients who underwent treatment in our department in 2022 for this study, with follow-up completed by July 30, 2023. CSS was recorded. The inclusion and exclusion criteria were identical to those used in SEER database cases. A total of 15 patients with complete data were included in the cohort, with demographic and clinical information presented in Supplementary Table 1.
2.2 Study covariates
The definitions and information on covariates such as age, gender, race, marital status, size, grade, T staging, N staging, M staging, surgical information, radiotherapy information, chemotherapy information and survival status were obtained in the SEER database. Deaths from GBC were taken as events, and survivors were censored based on CSS. For age at diagnosis, according to the WHO elderly classification standards, patients were classified into under 65 years old group and group for 65 years old and above. Gender included female and male. Races of patients were categorized as White, Black, Asian or Pacific Islander, American Indian or Alaska Native, and unknown. On the basis of marital status, patients were classified into groups with partners (Yes), without partners (No), and unknown marital status (Unknown). The without partners group includes single (never married), separated, divorced, and widowed, while for the with partners group, both same-sex and heterosexual couples were included. Tumor grades were classified as well, moderately, poorly and un- differentiated, corresponding to Grades I to IV, respectively, with the remaining patients labeled as Unknown grade. The optimal cutoff points determined by the X-tile software (version 3.6.1) were used to sort the tumors into groups of size <39, 40-60, ≥61, and unknown groups, with measurements in millimeters (mm). A screenshot of the software is presented as Supplementary Figure 2. T, N, and M staging were corrected based on the AJCC, 8th edition, and AJCC stages were generated. In surgical types, besides radical surgery, all other types of surgery were defined as non-radical surgery, with a separate group for no surgery performed. In the radiotherapy status, “None/Unknown” and “Refused” were considered as not received radiotherapy, while the rest were considered as received radiotherapy. In the chemotherapy status, “No/Unknown” was considered as not received chemotherapy, and “yes” was considered as received chemotherapy. Survival status included alive and dead. The data in the departmental cohort were categorized in the same manner.
2.3 Statistical analysis
The quantity (percentage (%)) was used to specify the categorical variables. Pearson’s Chi-squared test and Fisher’s exact test were employed to describe baseline characteristics. Cox proportional hazards models were used to evaluate the impact of covariates on the risk of CSS, and calculate the hazard ratios (HR) and 95% confidence intervals (CI) for advanced GBC. CSS was predicted using Kaplan-Meier curves before and after adjusting for covariates in different models, with log-rank tests used to analyze discrepancies between curves. Cramér’s V analysis was applied to assess the correlation between covariates. Propensity score matching (PSM) was used to match demographic baseline statistical characteristics. P-values < 0.05 is considered statistically significant. By using MSTATA software (https://www.mstata.com/), all analyses were carried out utilizing the R software (version 4.2.2) for statistical computing. Ultimately, through a comprehensive search of the literature database, we supplemented the treatment options not addressed in the SEER database and synthesized these findings to enhance the interpretation of our conclusions.
3 Results
3.1 Demographic and clinical characteristics
Altogether 5,154 eligible GBC patients were involved in the study cohort from 2000 to 2019. Among them, there were 849 cases with “No surgery + No chemotherapy + No radiotherapy” (NSNCNR) model, 1109 cases with “No surgery + Chemotherapy + No radiotherapy” (NSCNR) model, 45 cases with “No surgery + No chemotherapy + Radiotherapy” (NSNCR) model, 132 cases with “No surgery + Chemotherapy + Radiotherapy” (NSCR) model, 1185 cases with “Non-radical surgery + No chemotherapy + No radiotherapy” (NrSNCNR) model, 882 cases with “Non-radical surgery + Chemotherapy + No radiotherapy” (NrSCNR) model, 83 cases with “Non-radical surgery + No chemotherapy + Radiotherapy” (NrSNCR) model, 411 cases with “Non-radical surgery + Chemotherapy + Radiotherapy” (NrSCR) model, 190 cases with “Radical surgery + No chemotherapy + No radiotherapy” (RSNCNR) model, 131 cases with “Radical surgery + Chemotherapy + No radiotherapy” (RSCNR) model, 12 cases with “Radical surgery + No chemotherapy + Radiotherapy” (RSNCR) model, and 125 cases with “Radical surgery + Chemotherapy + Radiotherapy” (RSCR) model. For ease of reading, these treatment models are clearly designated by the letters A to L in the tables and figures. According to Table 1, the overall and different treatment models for GBC patients differ in demographics and clinical characteristics. Most patients were aged 65 or older (61.37%), and the distribution by gender was skewed toward females (70.70%). The racial composition was predominantly White (75.07%), followed by Black (12.44%) and Asian or Pacific Islander (11.08%). Over half of the participants were married (51.96%). The distribution by tumor size varied, with 27.90% having a tumor size of 39 or less, 14.82% between 40-60, and 11.91% greater than or equal to 61, with measurements in millimeters (mm). Grade I, II, III, IV, and grade Unknown accounted for 4.89%, 24.64%, 27.69%, 0.91%, and 41.87%, respectively. For AJCC stage perspective, stage IVB held the highest percentage of participants (56.67%). Various treatment models were administered, with the most common being “NrSNCNR” (23.00%), “NSCNR” (21.52%), “NrSCNR” (17.11%), and “NSNCNR” (16.47%). 81.99% of participants were dead when assessment. The line chart illustrating the selection of various treatment models over time is presented in Supplementary Figure 3.
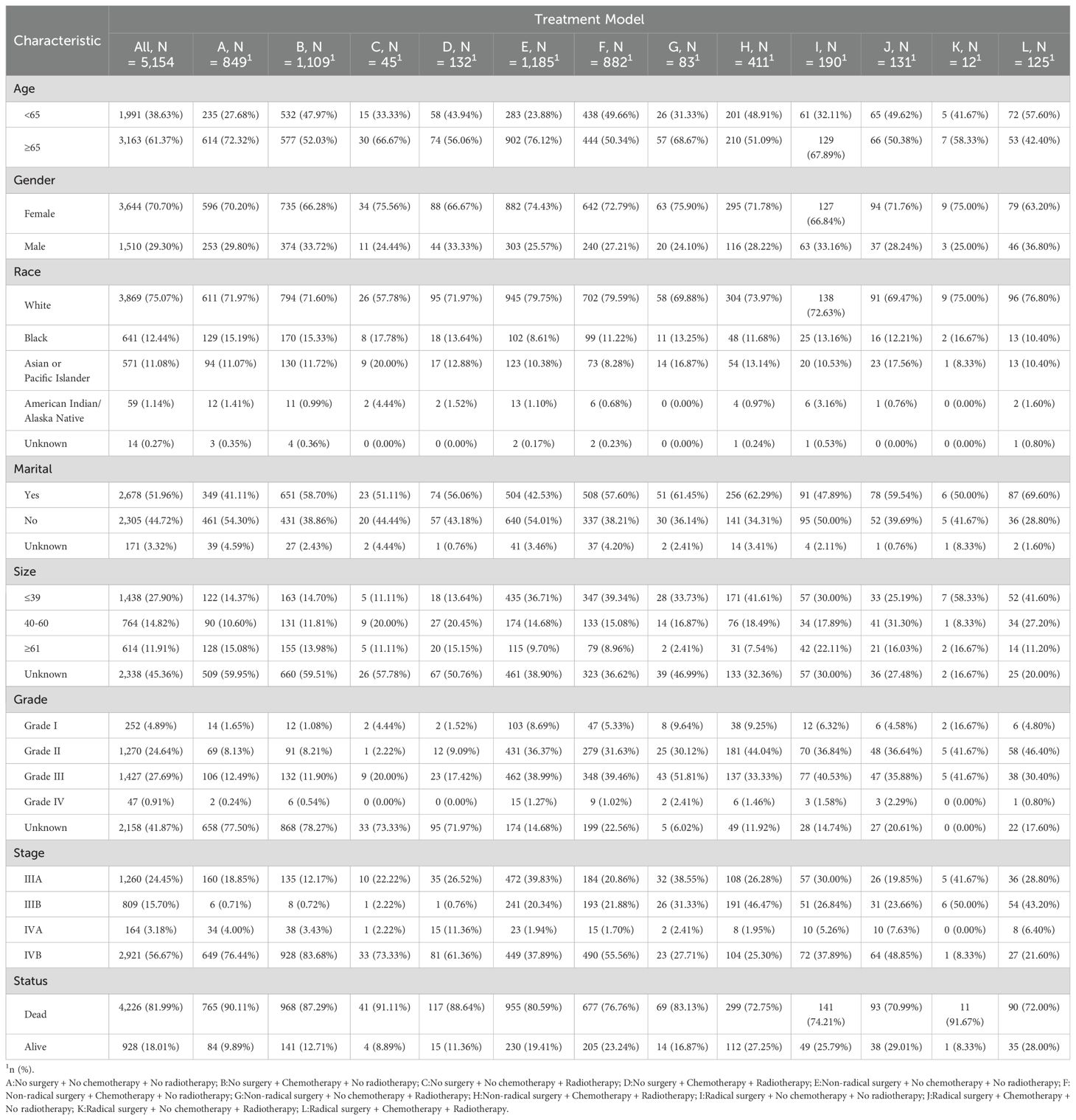
Table 1. Demographic and clinical characteristics of advanced GBC patients receiving twelve treatment models.
3.2 Identification of risk factors
Univariate Cox regression analysis indicated that age, gender, marital status, tumor size, tumor grade, AJCC stage, and treatment model significantly influenced CSS in advanced GBC patients, while race did not show a significant impact. After removing covariates with nonsignificant (p > 0.05) effects on survival time from the univariate Cox regression analysis, Table 2 presents the results for the remaining covariates by multivariate Cox regression analysis. In our analysis, the covariates did not exhibit significant collinearity, as detailed in Supplementary Figure 4.
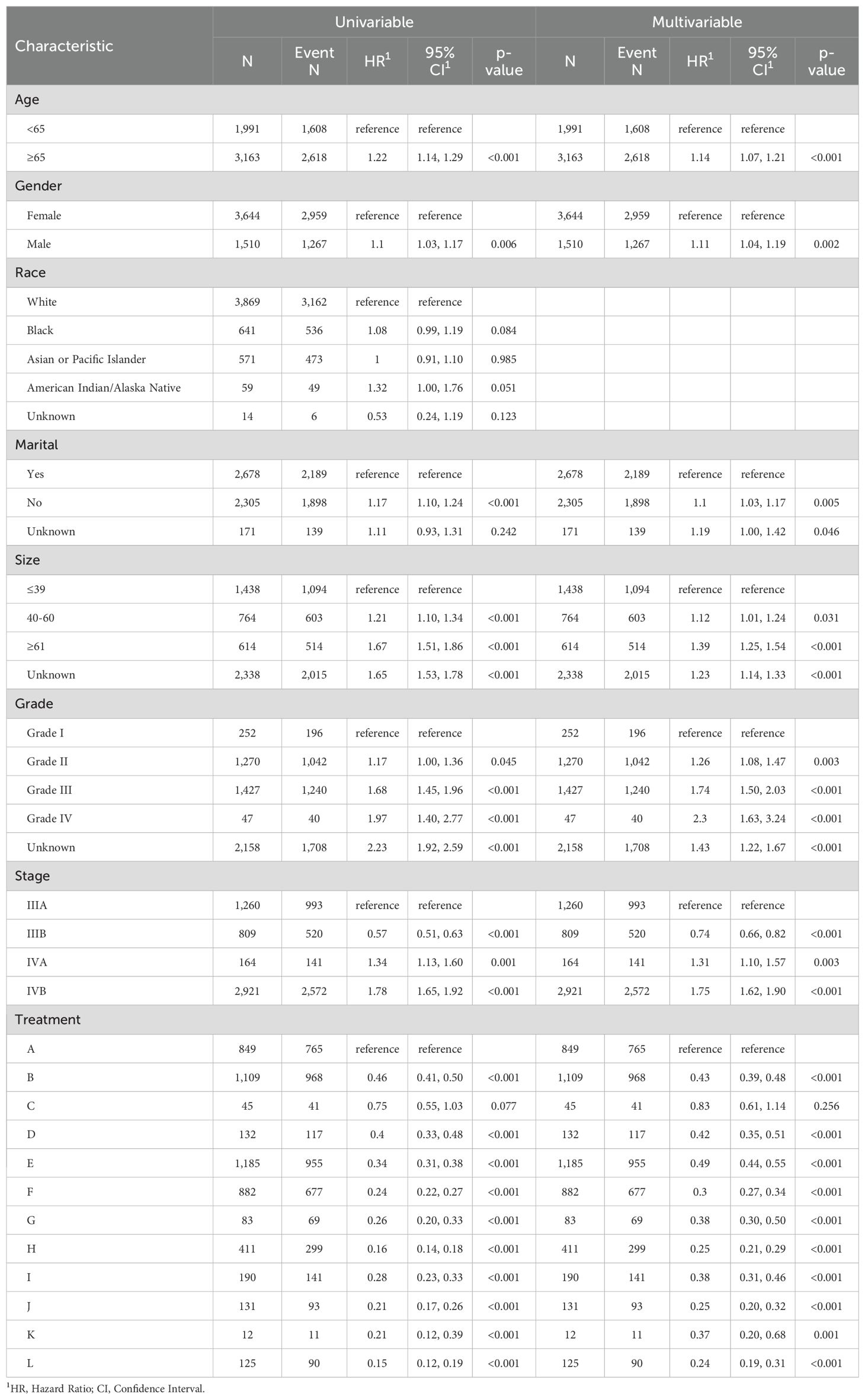
Table 2. Univariate and multivariate Cox proportional hazards models of CSS for advanced GBC patients in twelve treatment models.
In line with the Cox regression analysis, the following treatment models had significantly lower hazard ratios (HR) for CSS than “NSNCNR”: “NSCNR” (HR = 0.43, 95% CI 0.39-0.48, p < 0.001), “NSNCR” (HR = 0.83, 95% CI 0.61-1.14, p = 0.256), “NSCR” (HR = 0.42, 95% CI 0.35-0.51, p < 0.001), “NrSNCNR” (HR = 0.49, 95% CI 0.44-0.55, p < 0.001), “NrSCNR” (HR = 0.30, 95% CI 0.27-0.34, p < 0.001), “NrSNCR” (HR = 0.38, 95% CI 0.30-0.50, p < 0.001), “NrSCR” (HR = 0.25, 95% CI 0.21-0.29, p < 0.001), “RSNCNR” (HR = 0.38, 95% CI 0.31-0.46, p < 0.001), “RSCNR” (HR = 0.25, 95% CI 0.20-0.32, p < 0.001), “RSNCR” (HR = 0.37, 95% CI 0.20-0.68, p = 0.001), and “RSCR” (HR = 0.24, 95% CI 0.19-0.31, p < 0.001). The forest plot was shown in Figure 2. As a supplement, the results of univariate and multivariate analyses for the remaining treatment models, after excluding those with insufficient sample sizes (<1%), have been added to Supplementary Table 2. Additionally, to eliminate baseline differences between groups, PSM was applied based on whether patients received radical or non-radical surgery. The resulting baseline table and the Cox regression analysis have been designated as Supplementary Tables 3, 4, respectively. Due to missing data and a large number of “Unknown” entries, Supplementary Table 5 was created based on patients with complete data. The findings indicated that the results presented in the Supplementary Material showed trends that are nearly identical to those from the original analysis. Therefore, we opted to present the results from the original cohort, which includes a larger number of patients, in the main text.

Figure 2. The forest plot for multivariate Cox proportional hazards models for CSS in twelve treatment models.
3.3 Survival curve of each treatment group
The unadjusted survival curves for advanced GBC patients as a whole and for each treatment model were shown in Figure 3. After adjusting the covariates that were significant in multivariate Cox regression analysis, the CSS curves with advanced GBC and each treatment model were shown in Figure 4. The adjusted survival curves were calculated and plotted using the “conditional” method, which was based on the Cox proportional hazards models (16). This method involves creating multiple copies of the data to balance covariate differences across groups, resulting in a more accurate assessment of the effect of group membership on survival outcomes.
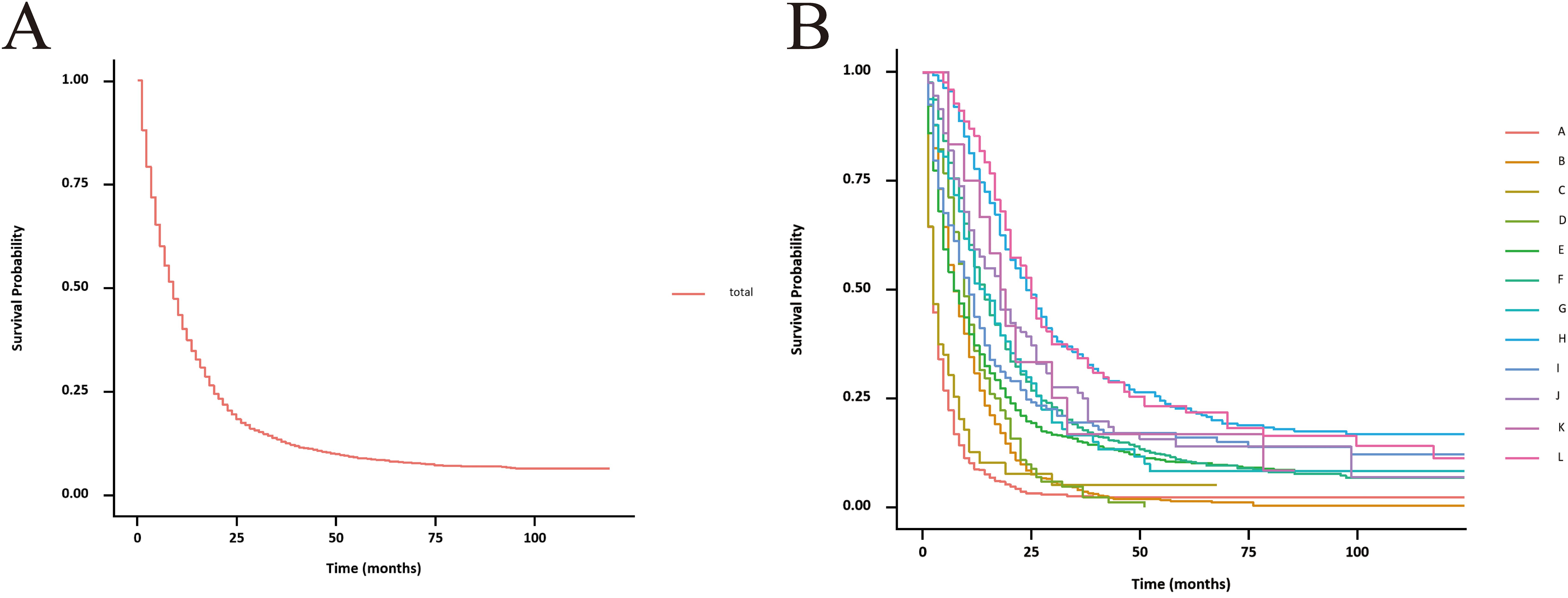
Figure 3. Unadjusted CSS curves for advanced GBC patients. (A), total patients; (B), twelve treatment models.

Figure 4. Adjusted CSS curves for advanced GBC patients. (A), total patients; (B), twelve treatment models.
3.4 Subgroup analysis
To better demonstrate the impact of different treatment models on CSS for advanced GBC patients, we grouped all patients based on AJCC stage in addition to the multivariate Cox regression analysis. It was noticed that for advanced GBC patients, treatment model remained an independent prognostic factor in Table 3 and Figure 5. “RSCR” was regarded as the most effective treatment models for CSS in AJCC stage IIIA, “RSCR” and “RSCNR” were identified as the most effective treatment models for CSS in AJCC stage IIIB, while “RSCNR” was found to be the most effective for AJCC stage IVA, “NrSCR” and “RSCR” were noticed as the most effective for AJCC stage IVB. Based on these speculations, patients were combined into AJCC stage III and stage IV to mitigate differences in patient numbers between different stages, and the results obtained were generally consistent with the previous findings in Table 4 and Figure 6. It was noteworthy that regardless of being in AJCC stage III or stage IV, “RSCR” model exhibited the best HR values, followed by “NrSCR” and “RSCNR”.
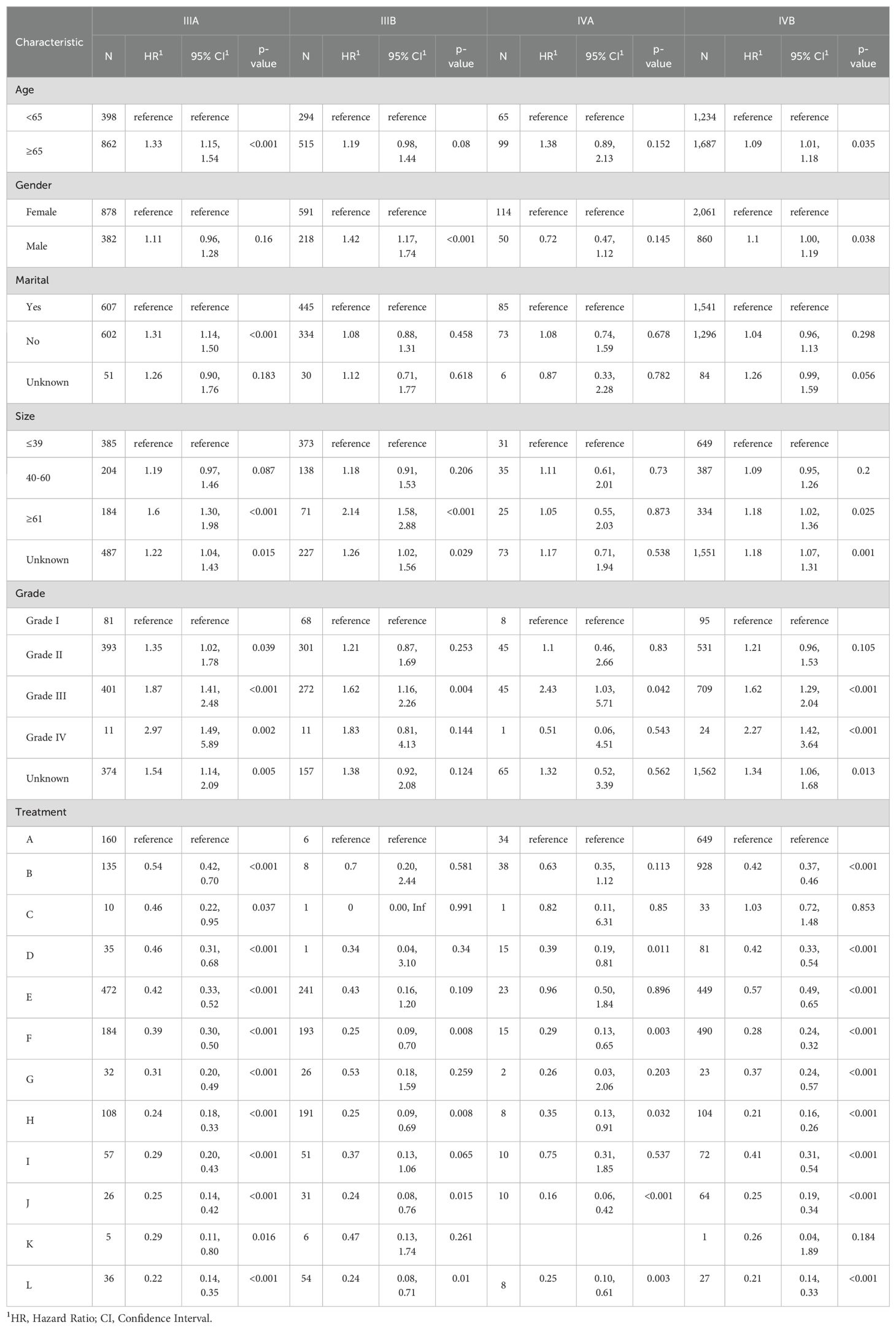
Table 3. Multivariate Cox proportional hazards models of CSS for advanced GBC in twelve treatment models at different pathological stages, reported separately for stage IIIA, IIIB, IVA and IVB.
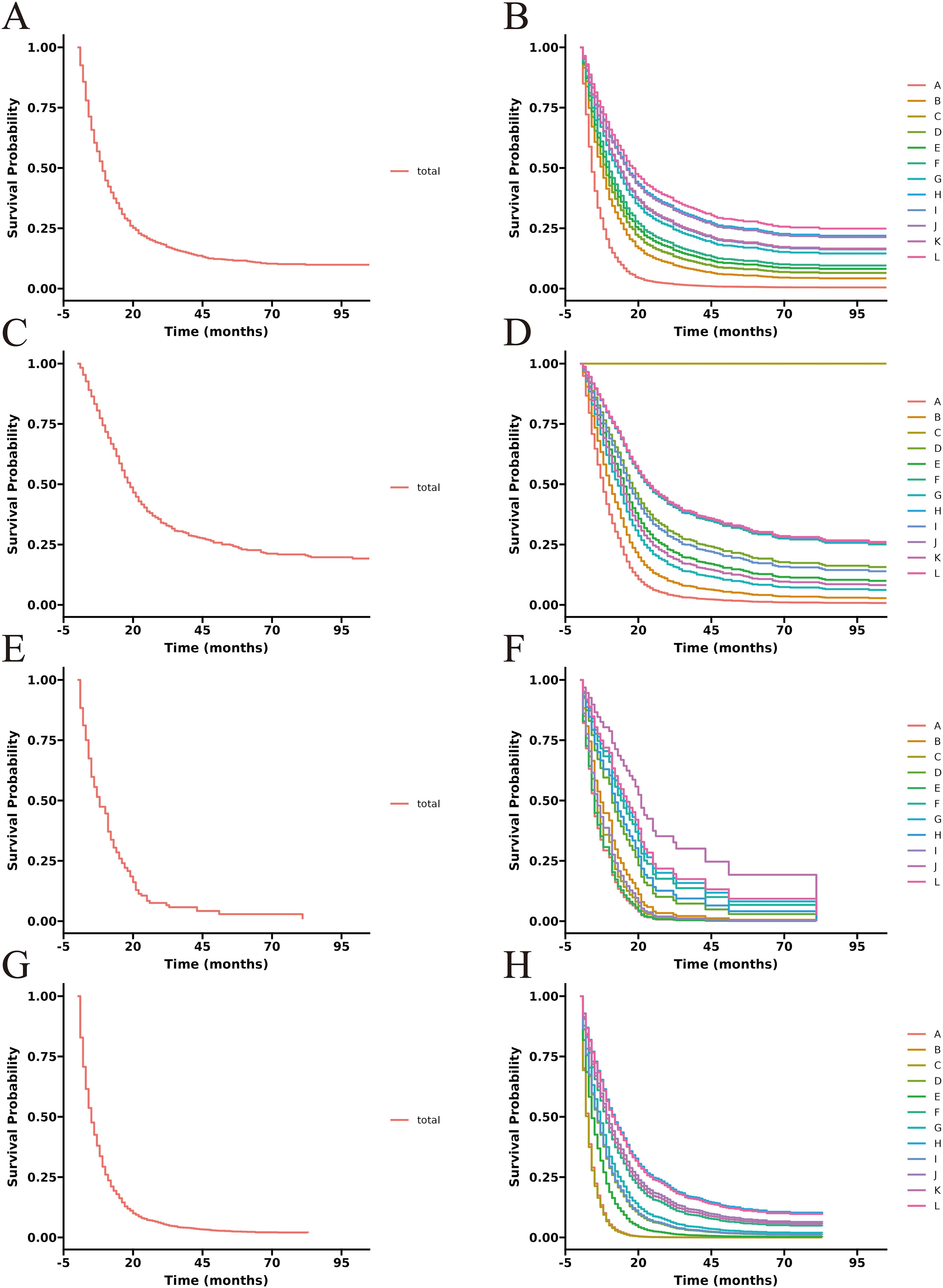
Figure 5. Adjusted CSS curves for advanced GBC patients at different pathological stages. Stage IIIA: (A), total patients; (B), twelve treatment models. Stage IIIB: (C), total patients; (D), twelve treatment models. Stage IVA: (E), total patients; (F), twelve treatment models. Stage IVB: (G), total patients; (H), twelve treatment models.
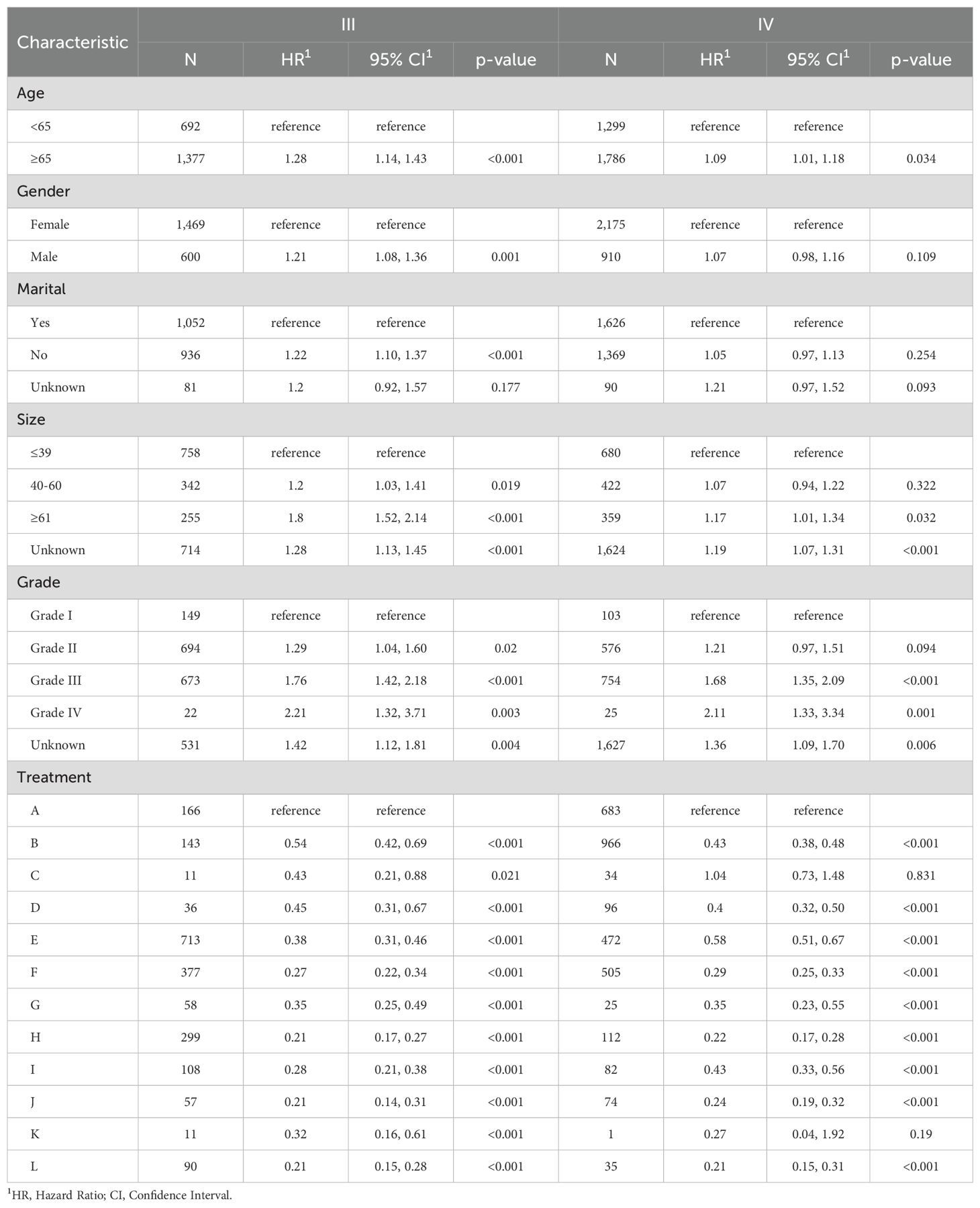
Table 4. Multivariate Cox proportional hazards models of CSS for advanced GBC in twelve treatment models at different pathological stages, reported separately for stage III and IV.
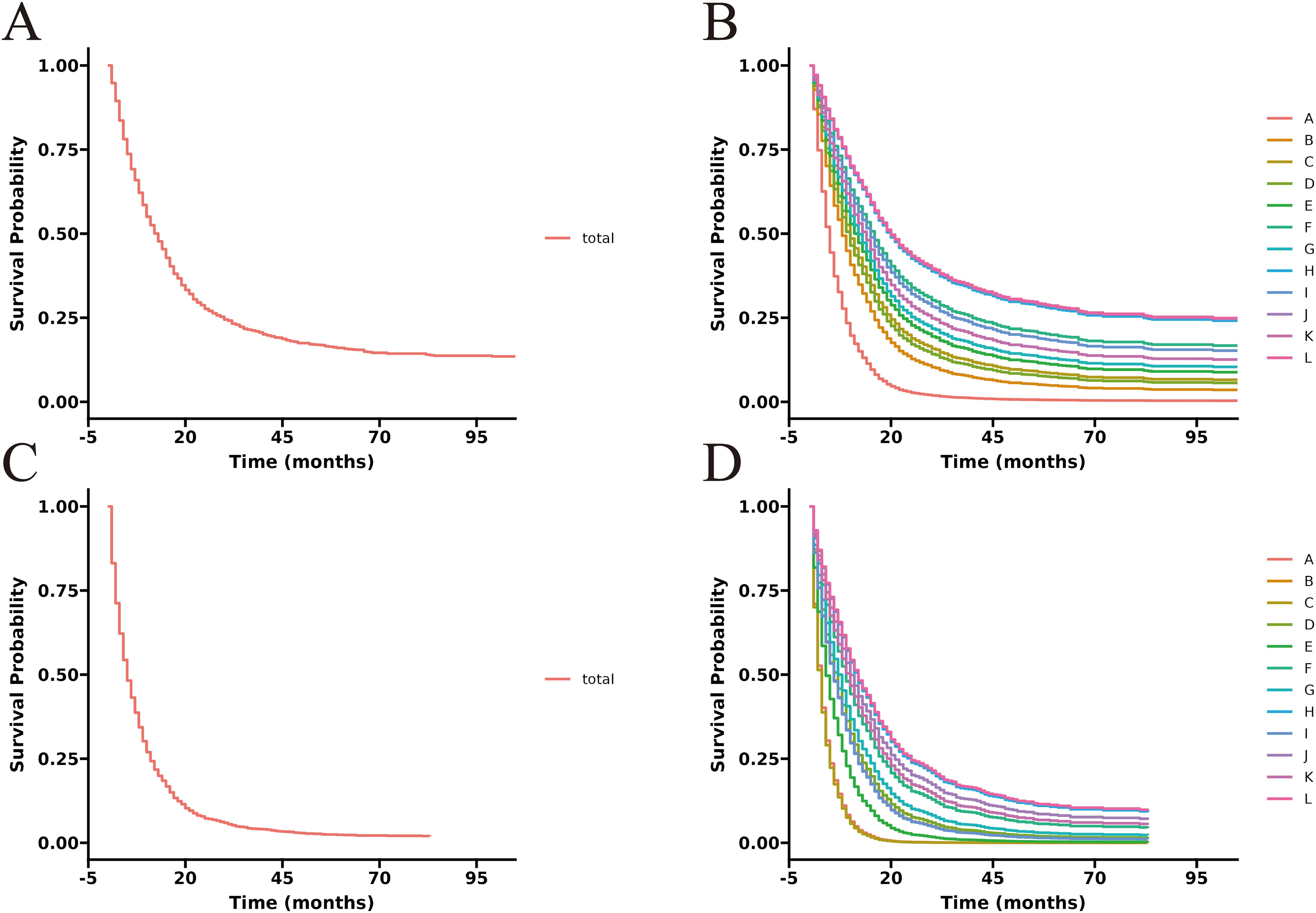
Figure 6. Adjusted CSS curves for advanced GBC patients at different pathological stages. Stage III: (A), total patients; (B), twelve treatment models. Stage IV: (C), total patients; (D), twelve treatment models.
3.5 Departmental cohort analysis
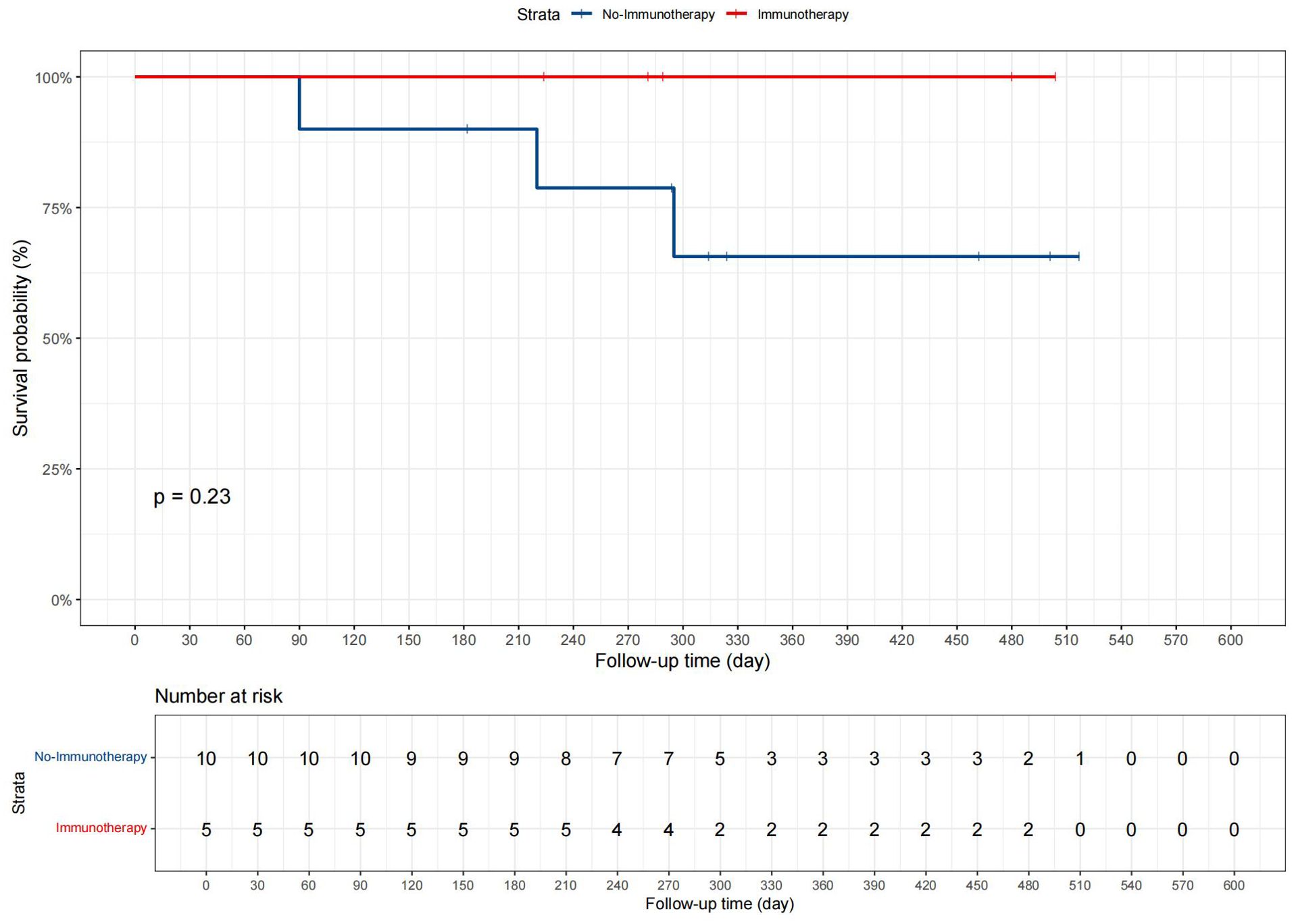
Patients were divided into two groups based on whether they received immunotherapy: 5 in the “Immunotherapy” group and 10 in the “No immunotherapy” group. Data such as age, gender, and tumor size were included in the analysis, and the demographic and clinical characteristics are displayed in Supplementary Table 6. Statistical analysis revealed no significant differences between the groups for any measured variables, with all p-values greater than 0.05. Additionally, in the present study, we conducted Kaplan-Meier survival analysis, as shown in Figure 7, to compare CSS between patients who received immunotherapy and those who did not. The survival curves suggested a trend toward improved prognosis in the immunotherapy group, but the p-value from the log-rank test was 0.23.
4 Discussion
Currently, treatment options for advanced GBC patients remain uncertain. Surgery continues to be the preferred treatment option for advanced GBC. Radical surgical is the only potential curative method for advanced GBC patients. Unfortunately, due to lymph node and distant metastases in advanced GBC, which limit the opportunity for radical surgery, non-radical surgery, as an alternative, including procedures aimed at relieving jaundice or pain, simple cholecystectomy, and tumor debulking surgeries, are often adopted. However, the efficacy of single surgical treatment is unsatisfactory. Therefore, adjuvant therapies, including chemotherapy and radiotherapy, as well as more advanced immunotherapy and targeted therapy, are also frequently used in the comprehensive management of advanced GBC patients to improve their survival time (17).
There is currently a controversy regarding whether radical surgery should be performed for advanced GBC patients. The scope of radical surgery for advanced GBC typically includes the removal of the gallbladder, adjacent liver tissue, lymph node(s), and affected organ(s). More aggressive procedures involve pancreaticoduodenectomy (PD) and hepatopancreaticoduodenectomy (HPD) (18, 19). Some scholars argue that aggressive surgery is beneficial for patient survival (10, 20–24). A multicenter cohort study analyzed the effects of extended resection surgery on locally advanced GBC patients, confirming the role of radical resection, resulting in some patients achieving a survival time of over two years (25). Even in AJCC stage IV GBC patients, the survival rate after radical surgery has been shown to be significantly higher than that of no surgery (26, 27). Conversely, some physicians question the benefits of radical surgery, considering routine or prophylactic extended surgery to have no significant survival advantage, and non-radical surgery is more recommended (18, 28). In one report, although HPD could eradicate locally advanced GBC, it did not show superiority over non-radical surgery in terms of overall survival, complication morbidity, and mortality (28–30). Therefore, in light of the above perspectives and the findings of this study, as an alternative, non-radical surgery is adopted, as it has also been proven effective for survival in advanced GBC (19, 28, 29).
Chemotherapy is widely used in gastrointestinal tumors, and although the progression-free survival of advanced GBC is relatively short due to its particularity, it still has a positive impact (31). Currently, the gemcitabine and cisplatin (GS) regimen is the most widely accepted chemotherapy regimen for GBC (32–35). With the emergence of neoadjuvant chemotherapy, it has also created opportunities for radical surgery and longer survival time (36–40). Recently, with the use of new drugs and the conduct of more clinical trials, more chemotherapy regimens have emerged, such as FOLFOX, modified FOLFIRINOX (mFOLFIRINOX), and GEMOX regimens (35, 41). Additionally, hepatic arterial infusion chemotherapy (HAIC) is also a choice (36, 42). It is worth mentioning that even without surgery, palliative chemotherapy is beneficial for the survival of advanced GBC (43, 44).
Radiotherapy, as a treatment option, is not widely used in advanced GBC, but still has a positive role (45). Currently, radiotherapy mainly includes four forms: preoperative radiotherapy (neoadjuvant radiotherapy), intraoperative radiotherapy, postoperative radiotherapy (adjuvant radiotherapy), and palliative radiotherapy, with doses mostly concentrated in the range of 45-54 Gy (46, 47). Some scholars have pointed out that after surgery for advanced GBC, not using radiotherapy or chemoradiotherapy can increase the risk of local recurrence (47, 48). In advanced GBC, radiotherapy is usually jointly used with chemotherapy for effect enhancement, including adjuvant chemoradiotherapy and neoadjuvant chemoradiotherapy (49, 50). It is worth mentioning that for unresectable advanced GBC, both radiotherapy and chemoradiotherapy can bring survival benefits to patients and create opportunities for radical surgery (50, 51).
Immunotherapy and targeted therapy also boost the survival of advanced GBC patients (34, 52). The term immunotherapy refers mainly to immune checkpoint inhibitors, such as PD-1, PD-L1, TMB-H, and MSI/MMRd (53–56). In the phase III TOPAZ-1 trial in 2022 and the KEYNOTE-966 trial in 2023, the combination of durvalumab or pembrolizumab with gemcitabine-cisplatin demonstrated improved survival compared to treatment with gemcitabine-cisplatin alone (57–59). Additionally, several phase II clinical trials have provided evidence supporting the effectiveness of other immunotherapy regimens, such as stereotactic body radiotherapy (SBRT) combined with nivolumab and ipilimumab, camrelizumab plus FOLFOX4 or GEMOX, and nab-paclitaxel combined with sintilimab (60–64). Targeted drugs include trastuzumab, erdafitinib, lenvatinib, and so on, mainly targeting specific molecular pathways such as Hedgehog, PI3K/AKT/mTOR, Notch, ErbB, MAPK/ERK, and Angiogenesis (53, 65–67). Additionally, patient-derived tumor organoid and patient-derived tumor xenograft models can facilitate personalized treatment for patients with advanced GBC (68).
The SEER database was utilized to compare treatment models for advanced GBC patients in this study. Among all patients, through Cox regression analyses, it was observed that the “RSCR” model exhibited the most significant improvement in CSS compared to the “NSNCNR” model, with other models also showing varying degrees of improvement. After excluding treatment models with insufficient sample sizes and analyzing the post-PSM data, or after conducting analysis using only cases with complete data, the results remained robust. These results consistent with the current understanding. After subgroup analysis by AJCC stage, the efficacy of the “RSCR” model remained significant. Some discordant results observed in subgroup analyses may stem from variations in the underlying patient profiles at each AJCC stage, or from the relatively small sample sizes in certain treatment models and stage categories. It is noteworthy that the extension effect of the “NrSCR” model on CSS was also considerable, often ranking second only to the “RSCR” model. Considering the difficulty of achieving radical surgery in advanced GBC, non-radical surgery is also a treatment option for patients who cannot undergo radical surgery, of course, in conjunction with other treatment options as much as possible. Additionally, it was found that the use of radical or non-radical surgery, chemotherapy, and radiotherapy provided significant benefits to patients compared to no surgery, no chemotherapy, and no radiotherapy, and the survival time was prolonged to varying degrees after the combination of treatment options.
In our departmental cohort analysis, the absence of significant differences in clinical and demographic characteristics between the two groups (p > 0.05) indicates that the patients were comparable in terms of baseline factors at the time of treatment assignment. This comparability is crucial as it reduces the likelihood of confounding bias, allowing any observed differences in survival outcomes to be more confidently attributed to the effect of immunotherapy rather than baseline imbalances. In the KM analysis, the lack of statistical significance (p = 0.23) may be attributed to the limited sample size in our cohort, particularly with only 5 patients receiving immunotherapy. Small sample sizes often reduce the power of statistical tests, making it more difficult to detect significant differences even if a true effect exists. While the trend observed in the Kaplan-Meier curves suggests a potential benefit of immunotherapy, further studies with larger patient cohorts are needed to confirm this finding and achieve adequate statistical power. It is believed that these results can provide new insights to clinicians, indicating that when conditions permit, comprehensive treatment including surgery, chemotherapy, radiotherapy, as well as immunotherapy and targeted therapy should be provided to patients with advanced GBC, rather than limiting treatment to one or two options.
However, several limitations still exist in this innovative study. Firstly, the SEER database is a retrospective database, and data such as vital signs, nutritional status, underlying diseases of patients are not reflected in the database. Secondly, the specific location of the tumor, tumor burden, and surgical procedures were not mentioned. Additionally, the specific modes, doses, and durations of chemotherapy and radiotherapy, as well as the sequence and intervals of surgery, chemotherapy, and radiotherapy, were not within the scope of the study, and treatment options such as targeted therapy and immunotherapy were not embraced. Despite these limitations, the SEER database is still useful in terms of providing the most comprehensive data on treatment patterns and survival status in the United States to date. Additionally, to address the limitations of the database, we conducted a review of our departmental cohort and literature from the PubMed database to enhance our understanding of immunotherapy and related treatments. Based on our knowledge, a comprehensive study of all twelve treatment models selected from the SEER database is being conducted for the first time, while several studies have utilized the database to analyze GBC, none have combined such a broad range of treatment models and ranked them accordingly (69). Additionally, this study is one of the few that focuses on advanced GBC. Previous researchers have often overlooked this patient group, as advanced GBC has traditionally been considered unsuitable for surgical intervention (70). Given that the SEER database analysis is retrospective in nature, future research should incorporate more detailed clinical data, including specific surgical approaches, chemotherapy regimens, radiation doses, and the sequencing of various treatment options. Moreover, larger departmental cohorts should be established to enhance the reliability of the findings. Furthermore, we recommend that prospective studies explore these aspects in greater depth and assess their applications in clinical practice.
5 Conclusion
For the SEER database, the “Radical surgery + Chemotherapy + Radiotherapy” models provide the greatest survival benefit for advanced GBC patients. At the same time, the departmental cohort analysis suggests that incorporating immunotherapy may offer further advantages to patients. Providing patients with the most comprehensive treatment possible, even if the optimal treatment effect is not achieved, is a way to extend the survival of patients. As long as treatment options are taken, it is always beneficial for patient survival. This innovative finding requires more comprehensive data and prospective studies for validation.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving humans were approved by Ethics Committee of National Cancer Center/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements.
Author contributions
RL: Conceptualization, Formal analysis, Investigation, Methodology, Software, Writing – original draft, Writing – review & editing. XC: Conceptualization, Investigation, Methodology, Writing – review & editing. BW: Conceptualization, Formal analysis, Software, Writing – review & editing. BA: Conceptualization, Formal analysis, Investigation, Writing – review & editing. FM: Conceptualization, Writing – review & editing. DC: Supervision, Validation, Writing – review & editing. JZ: Supervision, Validation, Writing – review & editing. TY: Supervision, Validation, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
We extend our sincere gratitude to all participants involved in this study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2024.1500091/full#supplementary-material
References
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: Cancer J Clin. (2021) 71:209–49. doi: 10.3322/caac.21660
2. Vuthaluru S, Sharma P, Chowdhury S, Are C. Global epidemiological trends and variations in the burden of gallbladder cancer. J Surg Oncol. (2023) 128:980–8. doi: 10.1002/jso.27450
3. Huang J, Patel HK, Boakye D, Chandrasekar VT, Koulaouzidis A, Lucero-Prisno Iii DE, et al. Worldwide distribution, associated factors, and trends of gallbladder cancer: A global country-level analysis. Cancer Lett. (2021) 521:238–51. doi: 10.1016/j.canlet.2021.09.004
4. Narayan RR, Creasy JM, Goldman DA, Gönen M, Kandoth C, Kundra R, et al. Regional differences in gallbladder cancer pathogenesis: Insights from a multi-institutional comparison of tumor mutations. Cancer. (2019) 125:575–85. doi: 10.1002/cncr.31850
5. Schmidt MA, Marcano-Bonilla L, Roberts LR. Gallbladder cancer: epidemiology and genetic risk associations. Chin Clin Oncol. (2019) 8:31. doi: 10.21037/cco.2019.08.13
6. Randi G, Franceschi S, La Vecchia C. Gallbladder cancer worldwide: geographical distribution and risk factors. Int J Cancer. (2006) 118:1591–602. doi: 10.1002/ijc.21683
7. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: Cancer J Clin. (2018) 68:394–424. doi: 10.3322/caac.21492
8. Rawla P, Sunkara T, Thandra KC, Barsouk A. Epidemiology of gallbladder cancer. Clin Exp Hepatol. (2019) 5:93–102. doi: 10.5114/ceh.2019.85166
9. Piehler JM, Crichlow RW. Primary carcinoma of the gallbladder. Surgery gynecology obstetrics. (1978) 147:929–42.
10. de Savornin Lohman E, de Bitter T, Verhoeven R, van der Geest L, Hagendoorn J, Haj Mohammad N, et al. Trends in treatment and survival of gallbladder cancer in the Netherlands; identifying gaps and opportunities from a nation-wide cohort. Cancers. (2020) 12. doi: 10.3390/cancers12040918
11. Wernberg JA, Lucarelli DD. Gallbladder cancer. Surg Clinics North America. (2014) 94:343–60. doi: 10.1016/j.suc.2014.01.009
12. Wang Y, Kong Y, Yang Q, Zhong C, Zhou D, Wang W. Survival benefit of adjuvant chemotherapy in patients with resected gallbladder adenocarcinoma: An updated retrospective cohort analysis. Eur J Surg Oncol. (2024) 50:108047. doi: 10.1016/j.ejso.2024.108047
13. Jh L RA, Lw G, Tr H. Immunotherapy in biliary tract cancers: current standard-of-care and emerging strategies. Cancers. (2023) 15. doi: 10.3390/cancers15133312
14. Ayabe RI, Wach MM, Ruff SM, Diggs LP, Martin SP, Wiemken T, et al. Gallbladder squamous cell carcinoma: An analysis of 1084 cases from the National Cancer Database. J Surg Oncol. (2020) 122:716–22. doi: 10.1002/jso.26066
15. Roa JC, García P, Kapoor VK, Maithel SK, Javle M, Koshiol J. Gallbladder cancer. Nat Rev Dis Primers. (2022) 8:69. doi: 10.1038/s41572-022-00398-y
16. Terry M, Crowson CS, Atkinson EJ. “Adjusted survival curves”. The R Project for Statistical Computing. (2015). Available at: https://cran.r-project.org/web/packages/survival//vignettes/adjcurve.pdf.
17. Zhou Y, Yuan K, Yang Y, Ji Z, Zhou D, Ouyang J, et al. Gallbladder cancer: current and future treatment options. Front Pharmacol. (2023) 14:1183619. doi: 10.3389/fphar.2023.1183619
18. Krell RW, Wei AC. Gallbladder cancer: surgical management. Chin Clin Oncol. (2019) 8:36. doi: 10.21037/cco.2019.06.06
19. Todoroki T, Takahashi H, Koike N, Kawamoto T, Kondo T, Yoshida S, et al. Outcomes of aggressive treatment of stage IV gallbladder cancer and predictors of survival. Hepato-gastroenterology. (1999) 46:2114–21.
20. Feo CF, Ginesu GC, Fancellu A, Perra T, Ninniri C, Deiana G, et al. Current management of incidental gallbladder cancer: A review. Int J Surg (London England). (2022) 98:106234. doi: 10.1016/j.ijsu.2022.106234
21. Chan SY, Poon RT, Lo CM, Ng KK, Fan ST. Management of carcinoma of the gallbladder: a single-institution experience in 16 years. J Surg Oncol. (2008) 97:156–64. doi: 10.1002/jso.20885
22. Patkar S, Patel S, Kazi M, Goel M. Radical surgery for stage IV gallbladder cancers: Treatment strategies in patients with limited metastatic burden. Ann hepato-biliary-pancreatic Surg. (2023) 27:180–8. doi: 10.14701/ahbps.22-111
23. Coburn NG, Cleary SP, Tan JC, Law CH. Surgery for gallbladder cancer: a population-based analysis. J Am Coll Surgeons. (2008) 207:371–82. doi: 10.1016/j.jamcollsurg.2008.02.031
24. Chen C, Wang L, Zhang R, Li Q, Zhao YL, Zhang GJ, et al. Who benefits from R0 resection? A single-center analysis of patients with stage IV gallbladder cancer. Chronic Dis Trans Med. (2019) 5:188–96. doi: 10.1016/j.cdtm.2019.08.004
25. Kuipers H, de Savornin Lohman EAJ, van Dooren M, Braat AE, Daams F, van Dam R, et al. Extended resections for advanced gallbladder cancer: results from a nationwide cohort study. Ann Surg Oncol. (2021) 28:835–43. doi: 10.1245/s10434-020-08858-z
26. Kang MJ, Song Y, Jang JY, Han IW, Kim SW. Role of radical surgery in patients with stage IV gallbladder cancer. HPB : Off J Int Hepato Pancreato Biliary Assoc. (2012) 14:805–11. doi: 10.1111/j.1477-2574.2012.00544.x
27. Nekarakanti P KS, Nag H. Surgery versus no surgery in stage IV gallbladder carcinoma: A propensity score-matched analysis. Turkish J Surg. (2023) 39:153–61. doi: 10.47717/turkjsurg.2023.5975
28. Mizuno T, Ebata T, Yokoyama Y, Igami T, Yamaguchi J, Onoe S, et al. Major hepatectomy with or without pancreatoduodenectomy for advanced gallbladder cancer. Br J Surg. (2019) 106:626–35. doi: 10.1002/bjs.11088
29. Chang Y, Li Q, Wu Q, Chi L, Bi X, Zeng Q, et al. Impact of surgical strategies on the survival of gallbladder cancer patients: analysis of 715 cases. World J Surg Oncol. (2020) 18:142. doi: 10.1186/s12957-020-01915-7
30. Higuchi R, Ota T, Araida T, Kajiyama H, Yazawa T, Furukawa T, et al. Surgical approaches to advanced gallbladder cancer : a 40-year single-institution study of prognostic factors and resectability. Ann Surg Oncol. (2014) 21:4308–16. doi: 10.1245/s10434-014-3885-1
31. Azizi AA, Lamarca A, McNamara MG, Valle JW. Chemotherapy for advanced gallbladder cancer (GBC): A systematic review and meta-analysis. Crit Rev oncology/hematology. (2021) 163:103328. doi: 10.1016/j.critrevonc.2021.103328
32. Valle J, Wasan H, Palmer DH, Cunningham D, Anthoney A, Maraveyas A, et al. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. New Engl J Med. (2010) 362:1273–81. doi: 10.1056/NEJMoa0908721
33. Ramaswamy A, Ostwal V, Pinninti R, Kannan S, Bhargava P, Nashikkar C, et al. Gemcitabine-cisplatin versus gemcitabine-oxaliplatin doublet chemotherapy in advanced gallbladder cancers: a match pair analysis. J hepato-biliary-pancreatic Sci. (2017) 24:262–7. doi: 10.1002/jhbp.439
34. Lamarca A, Edeline J, Goyal L. How I treat biliary tract cancer. ESMO Open. (2022) 7:100378. doi: 10.1016/j.esmoop.2021.100378
35. Javle M, Zhao H, Abou-Alfa GK. Systemic therapy for gallbladder cancer. Chin Clin Oncol. (2019) 8:44. doi: 10.21037/cco.2019.08.14
36. Singh S, Goel S, Aggarwal A, Iqbal A, Hazarika D, Talwar V. Combination of portal vein embolization and neoadjuvant chemotherapy for locally advanced gallbladder cancer requiring extended hepatectomy - A novel approach. Indian J gastroenterology : Off J Indian Soc Gastroenterol. (2021) 40:580–9. doi: 10.1007/s12664-021-01182-8
37. Inoue M, Hakoda K, Sawada H, Hotta R, Ohmori I, Miyamoto K, et al. Locally advanced gallbladder cancer treated with effective chemotherapy and subsequent curative resection: a case report. J Med Case Rep. (2022) 16:30. doi: 10.1186/s13256-021-03248-9
38. Sirohi B, Mitra A, Jagannath P, Singh A, Ramadvar M, Kulkarni S, et al. Neoadjuvant chemotherapy in patients with locally advanced gallbladder cancer. Future Oncol (London England). (2015) 11:1501–9. doi: 10.2217/fon.14.308
39. Ozer M, Goksu SY, Sanford NN, Porembka M, Khurshid H, Ahn C, et al. A propensity score analysis of chemotherapy use in patients with resectable gallbladder cancer. JAMA network Open. (2022) 5:e2146912. doi: 10.1001/jamanetworkopen.2021.46912
40. Yang Z, Wu Z, Xiong Y, Liu S, Cai C, Shao Z, et al. Successful conversion surgery for locally advanced gallbladder cancer after gemcitabine and nab-paclitaxel chemotherapy. Front Oncol. (2022) 12:977963. doi: 10.3389/fonc.2022.977963
41. Cui XY, Li XC, Cui JJ, Wu XS, Zou L, Song XL, et al. Modified FOLFIRINOX for unresectable locally advanced or metastatic gallbladder cancer, a comparison with GEMOX regimen. Hepatobiliary Surg Nutr. (2021) 10:498–506. doi: 10.21037/hbsn-20-846
42. Zheng K, Wang X, Cao G, Xu L, Zhu X, Fu L, et al. Hepatic arterial infusion chemotherapy with oxaliplatin and 5-fluorouracil for advanced gallbladder cancer. Cardiovasc interventional Radiol. (2021) 44:271–80. doi: 10.1007/s00270-020-02661-9
43. Bravo-Soto GA, Brañes R, Peña J, Nervi B. Palliative chemotherapy for advanced gallbladder cancer. Medwave. (2021) 21:e8045. doi: 10.5867/medwave.2021.03.8046
44. Dierks J, Gaspersz MP, Belkouz A, van Vugt JLA, Coelen RJS, de Groot JWB, et al. Translating the ABC-02 trial into daily practice: outcome of palliative treatment in patients with unresectable biliary tract cancer treated with gemcitabine and cisplatin. Acta Oncol (Stockholm Sweden). (2018) 57:807–12. doi: 10.1080/0284186x.2017.1418532
45. Kamarajah SK, Al-Rawashdeh W, White SA, Abu Hilal M, Salti GI, Dahdaleh FS. Adjuvant radiotherapy improves long-term survival after resection for gallbladder cancer A population-based cohort study. Eur J Surg oncology : J Eur Soc Surg Oncol Br Assoc Surg Oncol. (2022) 48:425–34. doi: 10.1016/j.ejso.2021.09.002
46. Alam MN, Agrawal S, Rastogi N, Maria Das KJ. Consolidation chemoradiation (cCTRT) improves survival in responders to first-line chemotherapy (CT) in locally advanced gallbladder cancer (LA-GBC): A new standard of care? Indian J Cancer. (2022) 59:577–83. doi: 10.4103/ijc.IJC_1145_20
47. Verma V, Crane CH. Contemporary perspectives on the use of radiation therapy for locally advanced gallbladder cancer. Chin Clin Oncol. (2019) 8:41. doi: 10.21037/cco.2019.08.12
48. Kim TG. Patterns of initial failure after resection for gallbladder cancer: implications for adjuvant radiotherapy. Radiat Oncol J. (2017) 35:359–67. doi: 10.3857/roj.2017.00388
49. Engineer R, Patkar S, Lewis SC, Sharma AD, Shetty N, Ostwal V, et al. A phase III randomised clinical trial of perioperative therapy (neoadjuvant chemotherapy versus chemoradiotherapy) in locally advanced gallbladder cancers (POLCAGB): study protocol. BMJ Open. (2019) 9:e028147. doi: 10.1136/bmjopen-2018-028147
50. Sinha S, Engineer R, Ostwal V, Ramaswamy A, Chopra S, Shetty N. Radiotherapy for locally advanced unresectable gallbladder cancer - A way forward: Comparative study of chemotherapy versus chemoradiotherapy. J Cancer Res Ther. (2022) 18:147–51. doi: 10.4103/jcrt.JCRT_1568_20
51. Song J, Kang X, Di Y, Ren G, Wang Y. Associations between external beam radiotherapy and overall survival in patients with gallbladder cancer: A population-based study. Front Public Health. (2022) 10:1012142. doi: 10.3389/fpubh.2022.1012142
52. Harding JJ, Khalil DN, Fabris L, Abou-Alfa GK. Rational development of combination therapies for biliary tract cancers. J Hepatol. (2023) 78:217–28. doi: 10.1016/j.jhep.2022.09.004
53. Wu T, Pu C, Wang Q, Zhang K. Comparison of efficacy and safety of anti-programmed cell death-1 antibody plus lenvatinib and chemotherapy as first-line therapy for patients with stage IV gallbladder cancer: A real-world study in a chinese population. Biomedicines. (2023) 11. doi: 10.3390/biomedicines11112933
54. Sturm N, Schuhbaur JS, Hüttner F, Perkhofer L, Ettrich TJ. Gallbladder cancer: current multimodality treatment concepts and future directions. Cancers. (2022) 14. doi: 10.3390/cancers14225580
55. Frega G, Cossio FP, Banales JM, Cardinale V, Macias RIR, Braconi C, et al. Lacking immunotherapy biomarkers for biliary tract cancer: A comprehensive systematic literature review and meta-analysis. Cells. (2023) 12. doi: 10.3390/cells12162098
56. Zuo B, Yang X, Yang X, Bian J, Long J, Wang D, et al. A real-world study of the efficacy and safety of anti-PD-1 antibodies plus lenvatinib in patients with advanced gallbladder cancer. Cancer immunology immunotherapy : CII. (2022) 71:1889–96. doi: 10.1007/s00262-021-03121-0
57. Oh D-Y, Ruth He A, Qin S, Chen L-T, Okusaka T, Vogel A, et al. Durvalumab plus gemcitabine and cisplatin in advanced biliary tract cancer. NEJM Evid. (2022) 1:EVIDoa2200015. doi: 10.1056/EVIDoa2200015
58. Oh D-Y, He AR, Bouattour M, Okusaka T, Qin S, Chen L-T, et al. Durvalumab or placebo plus gemcitabine and cisplatin in participants with advanced biliary tract cancer (TOPAZ-1): updated overall survival from a randomised phase 3 study. Lancet Gastroenterol Hepatol. (2024) 9:694–704. doi: 10.1016/S2468-1253(24)00095-5
59. Kelley RK, Ueno M, Yoo C, Finn RS, Furuse J, Ren Z, et al. Pembrolizumab in combination with gemcitabine and cisplatin compared with gemcitabine and cisplatin alone for patients with advanced biliary tract cancer (KEYNOTE-966): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. (2023) 401:1853–65. doi: 10.1016/S0140-6736(23)00727-4
60. Klein O, Kee D, Nagrial A, Markman B, Underhill C, Michael M, et al. Evaluation of combination nivolumab and ipilimumab immunotherapy in patients with advanced biliary tract cancers: subgroup analysis of a phase 2 nonrandomized clinical trial. JAMA Oncol. (2020) 6:1405–9. doi: 10.1001/jamaoncol.2020.2814
61. Sahai V, Griffith KA, Beg MS, Shaib WL, Mahalingam D, Zhen DB, et al. A randomized phase 2 trial of nivolumab, gemcitabine, and cisplatin or nivolumab and ipilimumab in previously untreated advanced biliary cancer: BilT-01. Cancer. (2022) 128:3523–30. doi: 10.1002/cncr.34394
62. Markussen A, Johansen JS, Larsen FO, Theile S, Hasselby JP, Willemoe GL, et al. Nivolumab with or without ipilimumab combined with stereotactic body radiotherapy in patients with metastatic biliary tract cancer: A randomized phase 2 study. Clin Cancer Res. (2024) 30:3428–37. doi: 10.1158/1078-0432.CCR-24-0286
63. Chen X, Qin S, Gu S, Ren Z, Chen Z, Xiong J, et al. Camrelizumab plus oxaliplatin-based chemotherapy as first-line therapy for advanced biliary tract cancer: A multicenter, phase 2 trial. Int J Cancer. (2021) 149:1944–54. doi: 10.1002/ijc.33751
64. Li X, Zhou N, Yang Y, Lu Z, Gou H. Efficacy and biomarker analysis of second-line nab-paclitaxel plus sintilimab in patients with advanced biliary tract cancer. Cancer Sci. (2024) 115:2371–83. doi: 10.1111/cas.16179
65. Bizama C, García P, Espinoza JA, Weber H, Leal P, Nervi B, et al. Targeting specific molecular pathways holds promise for advanced gallbladder cancer therapy. Cancer Treat Rev. (2015) 41:222–34. doi: 10.1016/j.ctrv.2015.01.003
66. Lee CK, Chon HJ, Cheon J, Lee MA, Im HS, Jang JS, et al. Trastuzumab plus FOLFOX for HER2-positive biliary tract cancer refractory to gemcitabine and cisplatin: a multi-institutional phase 2 trial of the Korean Cancer Study Group (KCSG-HB19-14). Lancet Gastroenterol Hepatol. (2023) 8:56–65. doi: 10.1016/s2468-1253(22)00335-1
67. Feng Y, Su W, Oh D, Shen L, Kim L, Liu X, et al. Updated analysis with longer follow up of a phase 2a study evaluating erdafitinib in Asian patients (pts) with advanced cholangiocarcinoma (CCA) and fibroblast growth factor receptor (FGFR) alterations. J Clin Oncol. (2022) 40:430–0. doi: 10.1200/JCO.2022.40.4_suppl.430
68. Tan D, An J, Gong M, Wang H, Li H, Meng H, et al. Screening of an individualized treatment strategy for an advanced gallbladder cancer using patient-derived tumor xenograft and organoid models. Front Oncol. (2022) 12:1043479. doi: 10.3389/fonc.2022.1043479
69. Mao W, Deng F, Wang D, Gao L, Shi X. Treatment of advanced gallbladder cancer: A SEER-based study. Cancer Med. (2020) 9:141–50. doi: 10.1002/cam4.2679
Keywords: advanced gallbladder cancer, surgery, chemotherapy, radiotherapy, immunotherapy, SEER, cancer-specific survival
Citation: Li R, Chen X, Wang B, Ai B, Min F, Cao D, Zhou J and Yan T (2024) Comparison of treatment models for single primary advanced gallbladder cancer. Front. Immunol. 15:1500091. doi: 10.3389/fimmu.2024.1500091
Received: 22 September 2024; Accepted: 21 October 2024;
Published: 13 November 2024.
Edited by:
Xiaosheng Tan, Rutgers, United StatesReviewed by:
Chengshuo Zhang, China Medical University, ChinaZilu Chen, Rutgers, The State University of New Jersey, United States
Chunliang Liu, Indiana University Indianapolis, United States
Yu Li, Duke University, United States
Copyright © 2024 Li, Chen, Wang, Ai, Min, Cao, Zhou and Yan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tao Yan, YmxpenphcmR5dEAxNjMuY29t; Jianguo Zhou, empndHlkb2N0QDE2My5jb20=; Dayong Cao, Y2FvZGF5b25nZG9jdHR5QHRvbS5jb20=
†These authors have contributed equally to this work and share first authorship
 Rongxuan Li1†
Rongxuan Li1† Bolun Ai
Bolun Ai Jianguo Zhou
Jianguo Zhou Tao Yan
Tao Yan