
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Immunol., 09 December 2024
Sec. Cancer Immunity and Immunotherapy
Volume 15 - 2024 | https://doi.org/10.3389/fimmu.2024.1497736
This article is part of the Research TopicInnovative Immunotherapy Strategies for Enhanced Treatment of Hodgkin and Non-Hodgkin LymphomasView all 11 articles
 Xiaodan Luo1†
Xiaodan Luo1† Ao Chen1†
Ao Chen1† Le Qin2
Le Qin2 Robert Weinkove3
Robert Weinkove3 Rong Zhao1
Rong Zhao1 Ting Ye1
Ting Ye1 Sihui Chen1
Sihui Chen1 Jianli Tang1
Jianli Tang1 Jianbo Liu1
Jianbo Liu1 Jiayu Huang1
Jiayu Huang1 Boyun Shi1
Boyun Shi1 Danyun Yuan1
Danyun Yuan1 Huo Tan1
Huo Tan1 Dajiang Qin1
Dajiang Qin1 Zhaoyang Tang4
Zhaoyang Tang4 Peng Li2*
Peng Li2* Runhui Zheng1*
Runhui Zheng1*This study explores a novel therapeutic strategy for relapsed/refractory (R/R) Burkitt lymphoma (BL) by integrating autologous hematopoietic stem cell transplantation (ASCT) with tandem anti-CD19/CD22 chimeric antigen receptor (CAR) T cell therapy. A 20-year-old Asian male with refractory BL, whose lymphoma had not responded to multiple chemoimmunotherapy regimens, received myeloablative ASCT followed three days later by infusion of a novel third-generation CAR T cells engineered with CD28 and CD3ζ signaling domains, along with a TLR2 costimulatory domain. This resulted in sustained complete remission at the 306-day follow-up, without experiencing any severe complications. This case suggests that combining myeloablative ASCT with tandem anti-CD19/CD22 CAR T cell therapy could be an effective approach for R/R BL, warranting further clinical validation.
Burkitt lymphoma (BL) is a highly aggressive B-cell non-Hodgkin lymphoma. Although responses to first-line therapy are high, relapsed or refractory (R/R) BL, carries a dismal prognosis with a median survival < 3 months, and fewer than 5% of patients surviving longer than two years (1). The few durable remissions reported for relapsed Burkitt lymphoma employ chemoimmunotherapy followed by autologous or allogeneic stem cell transplantation (2).
Chimeric antigen receptor (CAR) T-cell therapies directed against the B-cell antigen CD19 have been widely used as a salvage approach for R/R CD19+ acute lymphoblastic leukemia. Patients with BL were excluded from key lymphoma CAR T-cell registration trials, however, likely owing to the challenges that very rapid tumor growth presents to successful CAR T-cell manufacture and delivery. A lack of response to, or relapse following, CD19-directed CAR T-cell therapy for large cell lymphoma is common, due in part to downregulation of CD19 on tumor cells (3, 4). While subsequent treatment with CAR-T cells targeting the alternative B-cell antigen CD22 can result in clinical responses, these responses are often brief (5, 6). The disappointing results of single-targeting CAR-T cells in challenging disease settings have paved the way for the development of CAR-T cell therapies with specificity for two or more antigens (7–9). Liu, et al. sequentially infused CAR-T cells targeting CD19, CD22, and CD20 to 23 children with R/R BL, achieving a complete remission (CR) rate of 91% (10). However, this strategy requires manufacture of multiple CAR T-cell products. Another strategy is to combine multiple antigen specificities within a single CAR construct. We developed third-generation tandem anti-CD19/CD22 CAR T-cells employing CD28 and CD3 zeta (CD3ζ) intracellular signaling domains with a novel TLR2 costimulatory domain. Here, we report a case of successful treatment of an adult with R/R BL using myeloablative auto-HSCT followed by this new CAR T-cell therapy.
We developed a novel tandem CAR, termed 192228zT2. This CAR incorporates humanized single-chain variable fragments (scFv) targeting both CD19 and CD22 extracellularly, and intracellularly incorporates a CD28 costimulatory domain, a Toll-like receptor 2 intracellular domain that is known to enhance the antitumor efficacy and migratory capacity of CAR-T cells (11–13), and a CD3ζmotif. The project was approved by the ethics committee of Guangzhou Medical University (GYWY-G2024-02), and the patient gave informed consent. The 192228zT2 cells were manufactured at the Good Manufacturing Practice (GMP) facility of Guangdong Zhaotai Cell Biology Technology Ltd. Briefly, T cells were isolated from peripheral blood mononuclear cells (PBMCs) using CliniMACS CD4 and CD8 reagents (Miltenyi Biotec). These cells were activated with MACS GMP T Cell Transact (Miltenyi Biotec). Subsequently, cells were transduced using a lentiviral vector encoding the 192228zT2 CAR, and the transduction efficiency was measured through protein L staining (Figure 1A). CAR T-cells were expanded in the presence of recombinant human IL-2 and harvested once the cell quantity met dosage requirements. In vitro killing assays demonstrated the efficient lysis of CD19-overexpressing K562 cells (K562-CD19GL) and CD22-overexpressing K562 cells (K562-CD22GL) by 192228zT2 T cells (Figures 1B, C), indicating the function of both anti-CD19 and anti-CD22 single-chain variable fragments (scFvs). Furthermore, 192228zT2 T cells exhibited effective killing of the human B-cell acute lymphoblastic leukemia (B-ALL) cell line, NALM6-GL, which expresses both CD19 and CD22 (Figure 1D).
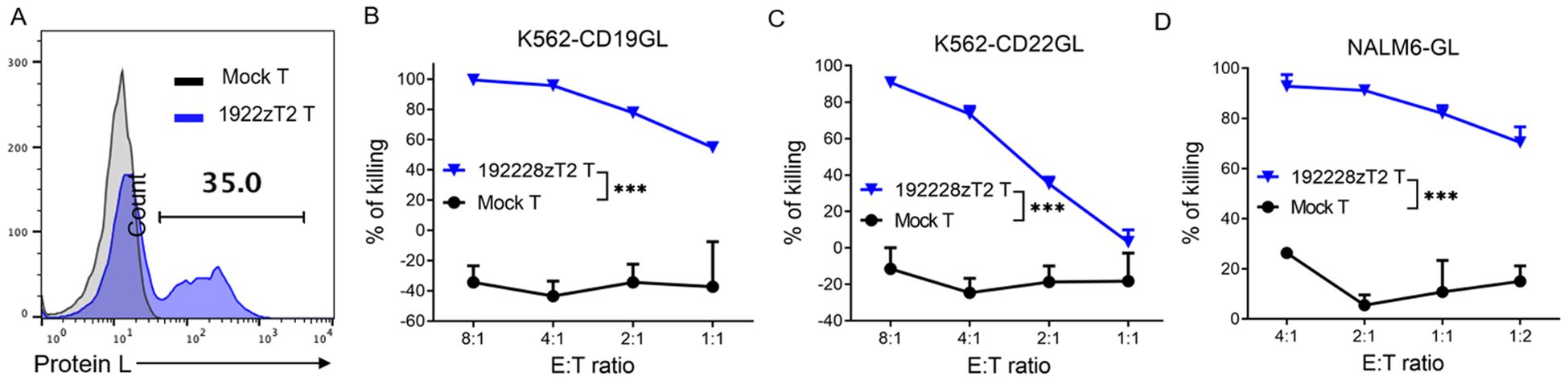
Figure 1. (A) The transduction efficiency of 1922zT2 T cells. (B–D). The cytotoxicity activity of 1922zT2 T and mock T cells against CD19+ K562-CD19GL (B), CD22+ K562-CD22GL (C) and CD19+CD22+ NALM6-GL (D) target cells in vitro. ALL target cells expressed GFP and Luciferase (GL). Data are presented as mean ± SD. p Values of B, C and D were calculated by two-way ANOVA with Tukey’s multiple comparisons test. ***p < 0.001.
A 20-year-old Asian male had been diagnosed with stage 3B BL, presenting with abdominal and pelvis lymphadenopathy. The patient was HIV-negative at diagnosis, and the tumor tissue was positive for the t (8, 14) IGH/MYC translocation with a proliferation index (Ki67) of 95%. Tumor cells were negative for Epstein-Barr virus-encoded RNA (EBER) and next-generation sequencing (NGS) of lymphoma-related genes indicated a class I mutation of TP53 p.E258fs. A first cycle of R-CODOX-M/R-IVAC (R-CODOX-M: rituximab, cyclophosphamide, vincristine, doxorubicin and methotrexate; R-IVAC: rituximab, ifosfamide, etoposide and cytarabine), resulted in complete metabolic response on (18)F-FDG PET/CT (Deauville 5-point score 2). Following two more cycles, CD34+ cells (10.86 × 106/kg) were harvested in preparation for a future auto-HSCT. However, a repeat PET/CT showed disease progression in the ileocecal mesenteric region. Right hemicolectomy was followed by one cycle of R-GDP (rituximab, gemcitabine, cisplatin and dexamethasone) and a cycle of a combination treatment comprising an anti-PD-1 antibody, demethylating agent, histone deacetylase inhibitor and Bruton tyrosine kinase (BTK) inhibitor, but disease continued to progress, with multiple new lesions in the mesentery and pelvic peritoneum. Re-biopsy of enlarged pericolic lymph nodes confirmed refractory BL and indicated partial CD19 expression (in 50% of tumor cells) and uniform expression of CD22 (in 100% of tumor cells). A decision was made to treat with 192228zT2 CAR T-cell therapy followed by auto-SCT. Time-line of disease evolution and therapeutic interventions were shown in Figure 2A. PET/CT images at different time points were shown in Figure 2B.
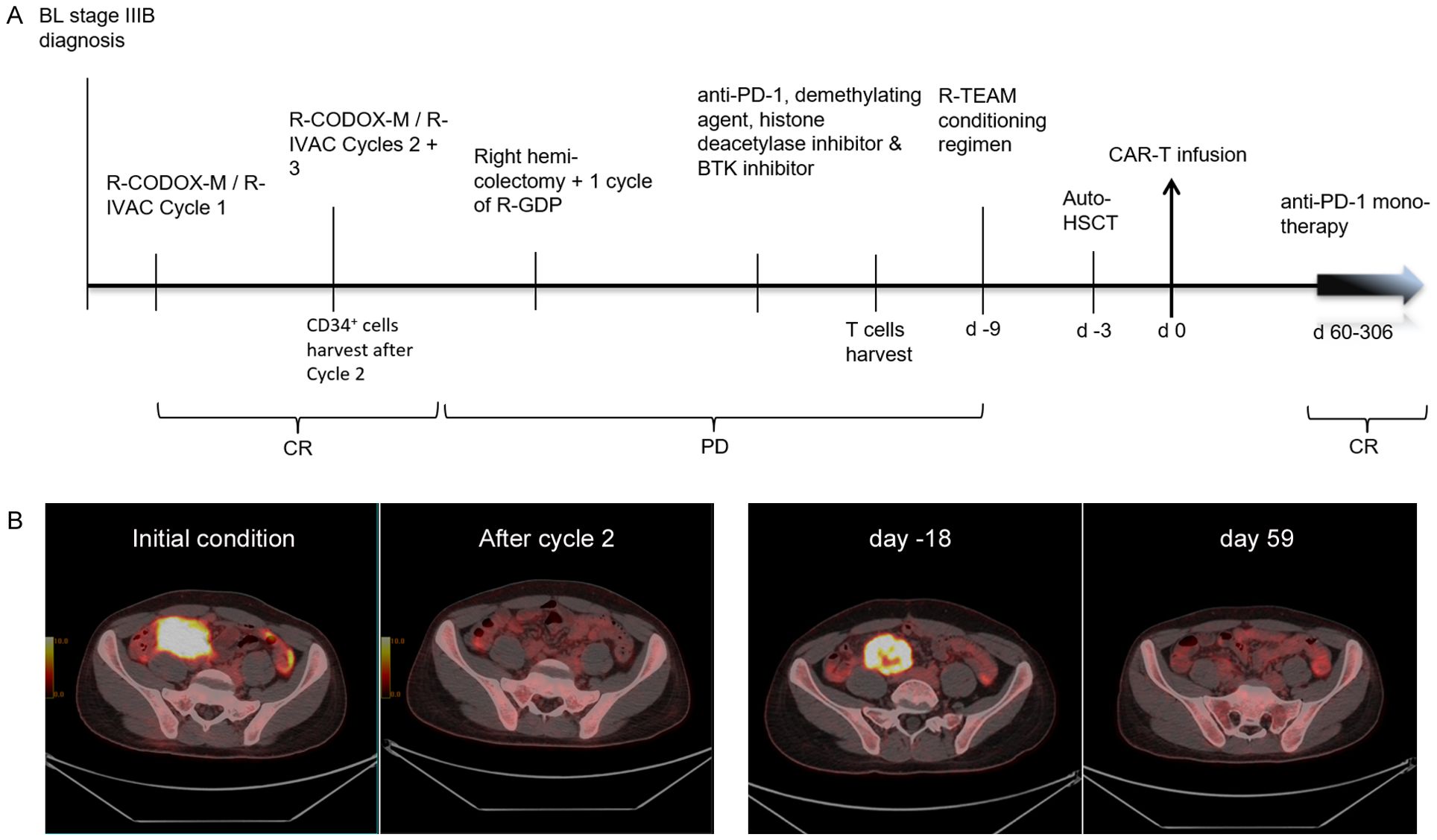
Figure 2. (A) Time-line of disease evolution and therapeutic interventions. (B). PET/CT images at different time points: baseline, after 2 cycles of chemotherapy (CR), on day -18, and on day +59 following CAR T-cell therapy.
After successful 192228zT2 T manufacture, the patient received a myeloablative R-TEAM (rituximab, thiotepa, etoposide, cytarabine and melphalan) conditioning regimen, which replaced the lymphodepletion (LD) regimen, followed by auto-HSCT. Three days after auto-HSCT, 1.2 × 106 CAR T cells per kilogram 192228zT2 CAR T-cells were administered. Grade 1 cytokine release syndrome (CRS) was diagnosed, and tocilizumab (8 mg/kg, administered every day to every 8 hours) was delivered for persistent fevers, which resolved by day 7. The patient achieved neutrophil engraftment on day 19 and platelet engraftment on day 22, No infectious complications were observed. PET/CT at day 59 revealed complete metabolic remission (Deauville 5-point score 3) (Figure 2B). Following this, the patient continued maintenance monotherapy with Tislelizumab (anti-PD-1 monoclonal antibody, BeiGene) every 28 days. We detected the proportion of 192228zT2 T in peripheral blood after injection. Gating strategy for detecting CAR-T cells is shown in Figure 3A. The result showed that 192228zT cell began to expand on day 7 (3.66%) and persist for an extended period (Figure 3B). Total CD3+ cells and Protein L+ cells per milliliter of blood at all time points (Figure 3C). As at day 306, the patient is in ongoing complete remission.
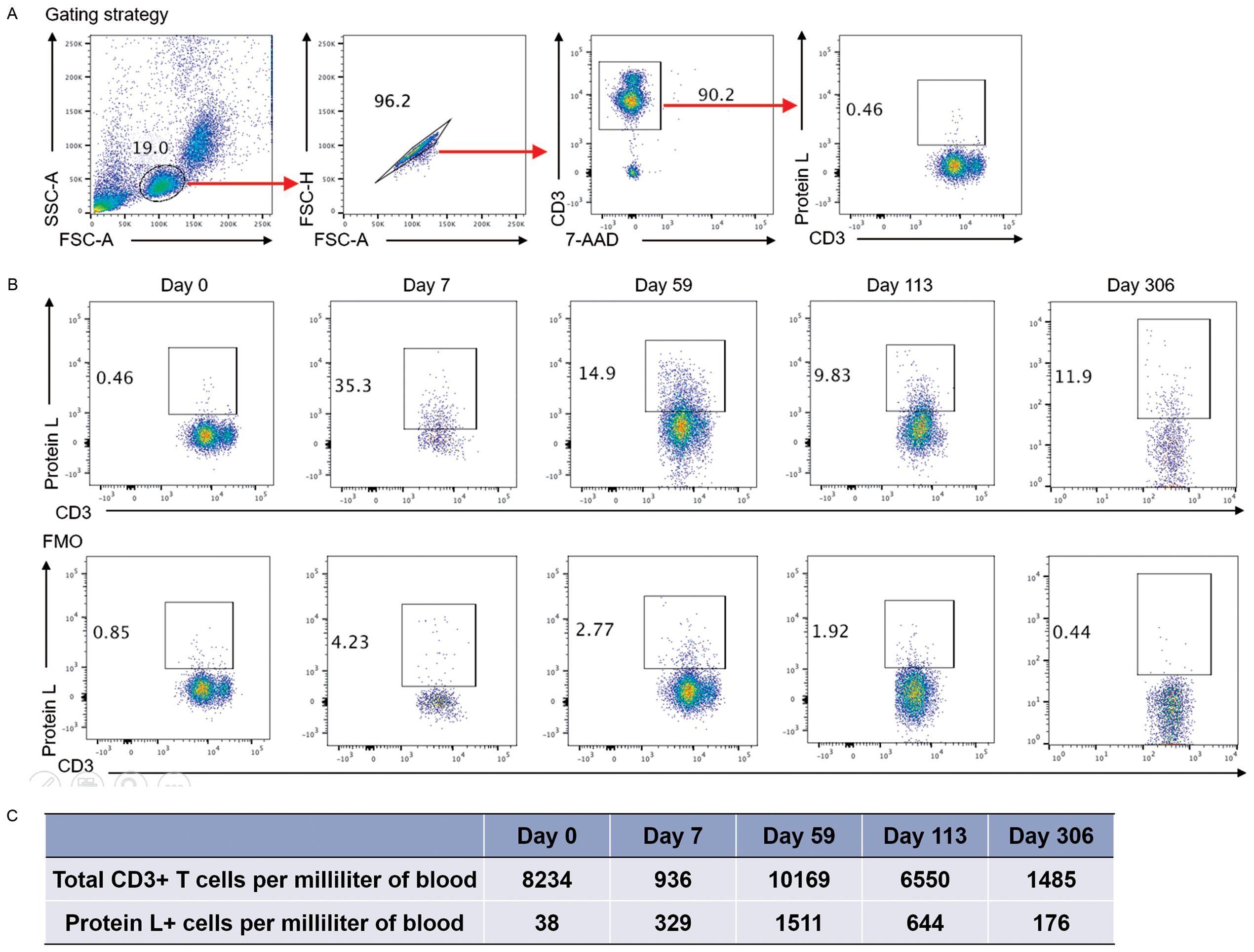
Figure 3. (A) Gating strategy for detecting CAR-T cells. The flow cytometry staining panel includes CD3-PE-Cy, 7-AAD, Protein L-Biotin, and either APC-streptavidin or PE-streptavidin. (B). Flow Cytometry of Peripheral Blood: Blood samples were collected on day 7, 14, 28, 59, 113 and 306 days, and the presence of CAR T-cells was detected by protein L labeling within the CD3+ T-cell population. Fluorescence Minus One (FMO) control were applied at each time point to validate the threshold and ensure accurate detection. (C). Total CD3+ cells and Protein L+ cells per milliliter of blood at all time points.
BL disease progression after multi-line chemotherapy is a catastrophic event lacking a defined effective treatment approach. The expected therapeutic efficacy of HSCT is not optimistic, since on one hand, disease progression indicates high chemo-resistance, which diminishes the potential benefits from heavy conditioning regimen before HSCT. On the other hand, HSCT showed poor outcome in BL patients, and it is unclear whether there is a strong graft-versus-lymphoma effect following allo-SCT (14, 15). The Center for International Bone and Marrow Transplantation Research (CIBMTR) reported the outcomes of 241 patients undergoing HSCT for BL, and those with R/R disease had a 5-year PFS and OS of 27% and 31% for auto HSCT, and only 19% and 20% for allogenic HSCT, respectively (14).
While CAR T-cell therapy is a promising modality of treatment, patients with R/R BL are liable to experience disease progression during CAR T-cell manufacture, LD chemotherapy and/or before CAR T-cells can proliferate and exert effector activity in vivo. There’s an urgent need for new treatment strategies. Here, we describe a novel approach that combines three strategies: (1) the use of myeloablative chemotherapy followed by auto-HSCT to both debulk the BL and provide lymphodepletion; (2) the administration of CAR T-cells shortly after auto-HSCT, before engraftment; (3) use of a new third-generation tandem CD19/CD22 CAR T-cell product to maximize antitumor efficacy, and (4) maintenance anti-PD1 therapy in an effort to prevent CAR T-cell exhaustion.
CAR-T cell treatment failure have been reported for lymphomas compared with ALL patients. The mechanism may include rapid lymphoma progression, antigen escape, immunosuppressive tumor microenvironment (TME) and CAR-T cell exhaustion (3, 16–18). By combining a myeloablative auto-HSCT with CAR T-cell therapy, the opportunities for rapid lymphoma progression were diminished. Noting the partial CD19 expression on the BL cells at the time of lymphoma progression, 1922zT2 T cells used in this study, incorporating humanized scFvs targeting both CD19 and CD22, may have reduced the risk of antigen escape. Incorporation of a TLR2-derived intracellular domain has the potential to promote specific activation and expansion of T cells, showing improved TME and antitumor efficacy of CARs (11, 12, 19). T-cell exhaustion and an immunosuppressive TME may contribute to CAR T-cell failure, and PD-1 blockade has been reported to improve the antitumor activity of CAR-T cells (20–22). In the phase 1b PORTIA study (NCT03630159), the ORR of patients with R/R DLBCL treated with tisagenlecleucel in combination with pembrolizumab was 50% and the CR rate was 33.3% (23). A phase 1/2a trial (NCT02650999), evaluating pembrolizumab for B-cell lymphomas relapsing after or refractory to CD19-directed CAR T-cell therapy, showed a best ORR of 25%. In this trial, CAR T-cell profiling before and after pembrolizumab treatment was analyzed and high levels of inhibitory receptors such as LAG-3, Tim-3 and CTLA-4 before permbrolizumab were found to be decreased after PD-1 blockade (21). Therefore, the pre-emptive use of anti-PD1 therapy in this case may have improve the CAR T-cell activity.
With auto-HSCT alone, it is difficult for patients to achieve CR, while with CAR T-cell therapy alone, a high tumor burden increases the risk of severe cytokine release syndrome (CRS) and affects hematopoiesis. Combining myeloablative auto-HSCT with CAR T-cell therapy could effectively reduce tumor burden, diminish the immunosuppressive microenvironment, and therefore enhance CAR T-cell function, promote engraftment, and support immune reconstitution (19, 24, 25). Despite the potential risks of CRS or Immune Effector Cell-Associated Neurotoxicity Syndrome (ICANS) caused by an inflammatory environment, we did not observe severe CRS or ICANS, nor was hematopoietic reconstitution delayed in this case.
In conclusion, the combination of auto-HSCT followed by 192228zT2 therapy offers a promising new approach for treating R/R BL.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving humans were approved by the ethics committee of Guangzhou Medical University. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
XL: Conceptualization, Writing – original draft. AC: Conceptualization, Formal analysis, Writing – review & editing. LQ: Methodology, Writing – review & editing. RW: Data curation, Writing – review & editing. RoZ: Investigation, Writing – review & editing. TY: Investigation, Writing – review & editing. SC: Data curation, Writing – review & editing. JT: Data curation, Writing – review & editing. JL: Data curation, Writing – review & editing. JH: Data curation, Writing – review & editing. BS: Data curation, Writing – review & editing. DY: Data curation, Writing – review & editing. HT: Validation, Writing – review & editing. DQ: Validation, Writing – review & editing. ZT: Supervision, Writing – review & editing. PL: Methodology, Supervision, Writing – review & editing. RuZ: Methodology, Supervision, Writing – review & editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Short NJ, Kantarjian HM, Ko H, Khoury JD, Ravandi F, Thomas DA, et al. Outcomes of adults with relapsed or refractory Burkitt and high-grade B-cell leukemia/lymphoma. Am J Hematol. (2017) 92:E114–E7. doi: 10.1002/ajh.24720
2. Gardenswartz A, Mehta B, El-Mallawany NK, van de Ven C, Hochberg J, Flower A, et al. Safety and efficacy of combinatorial therapy utilizing myeloablative conditioning and autologous stem cell transplantation, targeted immunotherapy, and reduced intensity conditioning and allogeneic stem cell transplantation in children, adolescents, and young adults with relapsed/refractory mature B-cell non-Hodgkin lymphoma. Leuk Lymphoma. (2023) 64:234–7. doi: 10.1080/10428194.2022.2133542
3. Neelapu SS, Locke FL, Bartlett NL, Lekakis LJ, Miklos DB, Jacobson CA, et al. Axicabtagene ciloleucel CAR T-cell therapy in refractory large B-cell lymphoma. N Engl J Med. (2017) 377:2531–44. doi: 10.1056/NEJMoa1707447
4. Schuster SJ, Bishop MR, Tam CS, Waller EK, Borchmann P, McGuirk JP, et al. Tisagenlecleucel in adult relapsed or refractory diffuse large B-cell lymphoma. N Engl J Med. (2019) 380:45–56. doi: 10.1056/NEJMoa1804980
5. Fry TJ, Shah NN, Orentas RJ, Stetler-Stevenson M, Yuan CM, Ramakrishna S, et al. CD22-targeted CAR T cells induce remission in B-ALL that is naive or resistant to CD19-targeted CAR immunotherapy. Nat Med. (2018) 24:20–8. doi: 10.1038/nm.4441
6. Shah NN, Highfill SL, Shalabi H, Yates B, Jin J, Wolters PL, et al. CD4/CD8 T-cell selection affects chimeric antigen receptor (CAR) T-cell potency and toxicity: updated results from a phase I anti-CD22 CAR T-cell trial. J Clin Oncol. (2020) 38:1938–50. doi: 10.1200/JCO.19.03279
7. Furqan F, Shah NN. Bispecific CAR T-cells for B-cell Malignancies. Expert Opin Biol Ther. (2022) 22:1005–15. doi: 10.1080/14712598.2022.2086043
8. Roddie C, Lekakis LJ, Marzolini MAV, Ramakrishnan A, Zhang Y, Hu Y, et al. Dual targeting of CD19 and CD22 with bicistronic CAR-T cells in patients with relapsed/refractory large B-cell lymphoma. Blood. (2023) 141:2470–82. doi: 10.1182/blood.2022018598
9. Du J, Zhang Y. Sequential anti-CD19, 22, and 20 autologous chimeric antigen receptor T-cell (CAR-T) treatments of a child with relapsed refractory Burkitt lymphoma: a case report and literature review. J Cancer Res Clin Oncol. (2020) 146:1575–82. doi: 10.1007/s00432-020-03198-7
10. Liu Y, Deng B, Hu B, Zhang W, Zhu Q, Liu Y, et al. Sequential different B-cell antigen-targeted CAR T-cell therapy for pediatric refractory/relapsed Burkitt lymphoma. Blood Adv. (2022) 6:717–30. doi: 10.1182/bloodadvances.2021004557
11. Lai Y, Weng J, Wei X, Qin L, Lai P, Zhao R, et al. Toll-like receptor 2 costimulation potentiates the antitumor efficacy of CAR T Cells. Leukemia. (2018) 32:801–8. doi: 10.1038/leu.2017.249
12. Weng J, Lai P, Qin L, Lai Y, Jiang Z, Luo C, et al. A novel generation 1928zT2 CAR T cells induce remission in extramedullary relapse of acute lymphoblastic leukemia. J Hematol Oncol. (2018) 11:25. doi: 10.1186/s13045-018-0572-x
13. Qin L, Zhao R, Chen D, Wei X, Wu Q, Long Y, et al. Chimeric antigen receptor T cells targeting PD-L1 suppress tumor growth. biomark Res. (2020) 8:19. doi: 10.1186/s40364-020-00198-0
14. Maramattom LV, Hari PN, Burns LJ, Carreras J, Arcese W, Cairo MS, et al. Autologous and allogeneic transplantation for burkitt lymphoma outcomes and changes in utilization: a report from the center for international blood and marrow transplant research. Biol Blood Marrow Transplant. (2013) 19:173–9. doi: 10.1016/j.bbmt.2012.11.016
15. Malfona F, Testi AM, Chiaretti S, Moleti ML. Refractory burkitt lymphoma: diagnosis and interventional strategies. Blood Lymphat Cancer. (2024) 14:1–15. doi: 10.2147/BLCTT.S407804
16. Chong EA, Ruella M, Schuster SJ. Lymphoma program investigators at the university of P. Five-year outcomes for refractory B-cell lymphomas with CAR T-cell therapy. N Engl J Med. (2021) 384:673–4. doi: 10.1056/NEJMc2030164
17. Laetsch TW, Maude SL, Rives S, Hiramatsu H, Bittencourt H, Bader P, et al. Three-year update of tisagenlecleucel in pediatric and young adult patients with relapsed/refractory acute lymphoblastic leukemia in the ELIANA trial. J Clin Oncol. (2023) 41:1664–9. doi: 10.1200/JCO.22.00642
18. Shah BD, Ghobadi A, Oluwole OO, Logan AC, Boissel N, Cassaday RD, et al. KTE-X19 for relapsed or refractory adult B-cell acute lymphoblastic leukaemia: phase 2 results of the single-arm, open-label, multicentre ZUMA-3 study. Lancet. (2021) 398:491–502. doi: 10.1016/S0140-6736(21)01222-8
19. Lin H, Deng T, Jiang L, Meng F, Cao Y, Zhang Y, et al. Adverse reactions in relapsed/refractory B-cell lymphoma administered with chimeric antigen receptor T cell alone or in combination with autologous stem cell transplantation. Cancers (Basel). (2024) 16(9):1722. doi: 10.3390/cancers16091722
20. Xin X, Zhu X, Yang Y, Wang N, Wang J, Xu J, et al. Efficacy of programmed cell death 1 inhibitor maintenance after chimeric antigen receptor T cells in patients with relapsed/refractory B-cell non-Hodgkin-lymphoma. Cell Oncol (Dordr). (2024) 47(4):1425–40. doi: 10.1007/s13402-024-00940-y
21. Chong EA, Alanio C, Svoboda J, Nasta SD, Landsburg DJ, Lacey SF, et al. Pembrolizumab for B-cell lymphomas relapsing after or refractory to CD19-directed CAR T-cell therapy. Blood. (2022) 139:1026–38. doi: 10.1182/blood.2021012634
22. Lee YH, Lee HJ, Kim HC, Lee Y, Nam SK, Hupperetz C, et al. PD-1 and TIGIT downregulation distinctly affect the effector and early memory phenotypes of CD19-targeting CAR T cells. Mol Ther. (2022) 30:579–92. doi: 10.1016/j.ymthe.2021.10.004
23. Jaeger U, Worel N, McGuirk JP, Riedell PA, Fleury I, Du Y, et al. Safety and efficacy of tisagenlecleucel plus pembrolizumab in patients with r/r DLBCL: phase 1b PORTIA study results. Blood Adv. (2023) 7:2283–6. doi: 10.1182/bloodadvances.2022007779
24. Liu W, Liu W, Zou H, Chen L, Huang W, Lv R, et al. Combinational therapy of CAR T-cell and HDT/ASCT demonstrates impressive clinical efficacy and improved CAR T-cell behavior in relapsed/refractory large B-cell lymphoma. J Immunother Cancer. (2024) 12(4):e008857. doi: 10.1136/jitc-2024-008857
Keywords: relapsed/refractory Burkitt lymphoma, CAR T-cell therapy, autologous hematopoietic stem cell transplantation, CD19/CD22 dual target, immunotherapy
Citation: Luo X, Chen A, Qin L, Weinkove R, Zhao R, Ye T, Chen S, Tang J, Liu J, Huang J, Shi B, Yuan D, Tan H, Qin D, Tang Z, Li P and Zheng R (2024) Case report: A novel third-generation anti-CD19/CD22 CAR T-cells combined with auto-HSCT for relapsed Burkitt lymphoma. Front. Immunol. 15:1497736. doi: 10.3389/fimmu.2024.1497736
Received: 17 September 2024; Accepted: 20 November 2024;
Published: 09 December 2024.
Edited by:
Penny Fang, University of Texas MD Anderson Cancer Center, United StatesReviewed by:
Jichang Han, Washington University in St. Louis, United StatesCopyright © 2024 Luo, Chen, Qin, Weinkove, Zhao, Ye, Chen, Tang, Liu, Huang, Shi, Yuan, Tan, Qin, Tang, Li and Zheng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Runhui Zheng, emhlbmdydW5odWlAZ3pobXUuZWR1LmNu; Peng Li, bGlfcGVuZ0BnaWJoLmFjLmNu
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.