
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Immunol. , 26 November 2024
Sec. Inflammation
Volume 15 - 2024 | https://doi.org/10.3389/fimmu.2024.1496390
Lactate has been traditionally regarded as a mere byproduct of glycolysis or metabolic waste. However, an increasing body of literature suggests its critical role in regulating various physiological and pathological processes. Lactate is generally associated with hypoxia, inflammation, viral infections, and tumors. It performs complex physiological roles by activating monocarboxylate transporter (MCT) or the G protein-coupled receptor GPR81 across the cell membrane. Lactate exerts immunosuppressive effects by regulating the functions of various immune cells (such as natural killer cells, T cells, dendritic cells, and monocytes) and its role in macrophage polarization and myeloid-derived suppressor cell (MDSC) differentiation in the tumor microenvironment. Lactic acid has also recently been found to increase the density of CD8+ T cells, thereby enhancing the antitumor immune response. Acute or chronic inflammatory diseases have opposite immune states in the inflammatory disease microenvironment. Factors such as cell types, transcriptional regulators, ionic mediators, and the microenvironment all contribute to the diverse functions lactate exhibits. Herein, we reviewed the pleiotropic effects of lactate on the regulation of various functions of immune cells in the tumor microenvironment and under inflammatory conditions, which may help to provide new insights and potential targets for the diagnosis and treatment of inflammatory diseases and malignancies.
Lactate is a metabolic end product of glycolysis and is mainly produced in the brain, skeletal muscle, intestine, and red blood cells. Under stress conditions, lactate is also produced in white blood cells, lungs, and viscera (1). The human body predominantly produces the L-isomer of lactate, facilitated by lactate dehydrogenase (LDH), an enzyme involved in both glycolysis and potential posttranscriptional gene regulation (2). Lactate connecting glycolysis to mitochondrial respiration in anaerobic conditions (3). In 1927, Otto Warburg noted that glycolysis in cancer cells under aerobic conditions can replace the normal aerobic cycle, resulting in a marked increase in lactate production, referred to as the “Warburg effect” (4). Tumor cells specifically consume glucose through Warburg metabolism, and tumor-infiltrating immune cells also rely on glucose. The altered metabolism of these immune cells within the tumor microenvironment contributes to the tumor cells’ ability to achieve immune escape (5). At the same time, the harsh metabolic microenvironment of tumors can also promote the Warburg effect in cells (6).
Now Lactate is understood to be produced and utilized under aerobic conditions as a signaling molecule. In the tumor microenvironment, lactate serves as a regulator of metabolic pathways, immune responses, and intercellular communication. Its significance extends beyond energy metabolism, making it an important target for understanding and potentially therapeutic intervention in various diseases, including cancer (7). At the systemic level, lactate metabolism is considered vital for at least three reasons (1): lactate serves as the primary energy source; (2) L-lactate is the primary isomer produced in humans; and (3) lactate is a signal molecule exhibiting autocrine, paracrine, and endocrine-like functions (3). The concepts of the “cell-to-cell lactate shuttle” and “intracellular lactate shuttle” describe the role of lactate in the transfer of oxidation and gluconogenic substrates, as well as in cell signaling.
Lactate triggers a series of intracellular signals that regulate various inflammatory responses in inflammatory diseases. Lactate levels were first identified as a marker of clinical outcome in patients with undifferentiated shock in 1964 (8). Since then, high lactate levels have been associated with ischemia, shock, diabetic ketoacidosis, trauma, liver dysfunction, and sepsis (8). In addition, lactate levels are associated with the level of inflammation. Given that the site of inflammation is in a hypoxic state (1% oxygen), the lactate level is often higher than normal (9). It has been established that the lactate concentration can increase to 15-17 mM in rheumatic synovial fluid (10), and in gingival sulcus liquid of patients with periodontitis, the levels can be as high as 19.69 ± 9.76 mM (11). In addition, Shapiro et al. reported that increased mortality in infected patients (such as pneumonia, severe sepsis or septic shock, bacteremia, etc.) was associated with increased lactate levels (12). Collectively, these results suggest that changes in lactic acid levels may significantly affect the inflammatory diseases. Lactate is excreted from tumor cells via monocarboxylate transporters (MCTs) after glucose metabolism, resulting in lactate accumulation in the tumor microenvironment (TME) and a decrease in pH (13). Under normal physiological conditions, the lactate concentration is approximately 1.5-3.0 mM, while the lactate concentration in the TME can reach 10-30 mM, and the pH can reach 6.0-6.5 (14–16). Interestingly, the tumor microenvironment creates an ecological environment that supports tumor growth rather than favoring antitumor immune monitoring, which is promoted to a certain extent by the accelerated metabolism of tumor cells and cancer-related fibroblasts (17). Increased metabolites in the TME, especially lactate, contribute to an immunosuppressive environment conducive to cancer cell growth and escape from the immune system (18). There is a growing consensus that lactate is not only an end product of glycolysis but also a key regulator of several signaling pathways in normal and tumor cells (3). For example, lactate has been shown to delay LPS-induced signaling pathways (19), while affecting several MAP kinases, NF-κB signaling, and the PI3K/AKT pathway (20–22). In this review, we focus on the immunomodulatory effects of lactate on tumors and inflammation, aiming to elucidate the mechanisms underlying the pathogenesis of diseases with similar acidic microenvironments and to provide novel insights and prospective targets for the treatment of related diseases.
The microenvironment of cancerous tissues is immunosuppressive and protumorigenic, whereas the microenvironment of tissues affected by chronic inflammatory diseases is proinflammatory and antitumor. Despite these opposing immune states, the metabolic states in the tissue microenvironments of cancer and inflammatory diseases are similar: both are hypoxic, with elevated levels of lactate and other metabolic byproducts and reduced levels of nutrients [7]. The TME comprises the surrounding mesenchymal and immune cells, the extracellular matrix, and metabolites and signaling molecules in the intercellular space (23). Current evidence suggests that the TME plays an active role in regulating key features of cancer, including energy metabolism, protumor growth, angiogenesis, invasion and metastasis, and immune evasion (24). Many cells reportedly contribute to the establishment of the TME (25). In this environment, the balance of normal cells and tissues is disrupted, resulting in the production of pro-/antitumor growth factors, extracellular vesicles, cytokines, extracellular matrix (ECM) proteins, and ECM remodeling enzymes that trigger a shift of surrounding cells to a more tumor-friendly immune response (24). In addition, the TME has anoxic or semianoxic properties (26).
Tumor cell metabolism produces a large amount of lactate, Lactate is co-transported by monocarboxylate transporters (MCTs), along with protons (H+), resulting in the accumulation of lactate and acidification of the tumor microenvironment (27). New research has revealed that the elevated activity of glycolytic and glutamine enzymatic pathways in cancer cells is the foremost cause of lactate accumulation in the TME. Additionally, lactate buildup in the TME impedes the immune system’s ability to fight against tumors (4, 28–30). More importantly, lactate is a major disruptor of TME immune function. On the one hand, it can directly mediate immunosuppressive effects on other cells (NK cells and T cells) by blocking the function of cytotoxic, motility, or transcription factors of immune cells. On the other hand, it can indirectly exert its immunosuppressive function by inducing immunosuppressive cells such as Tregs, TAMs, and MDSCs (31) (Figure 1).
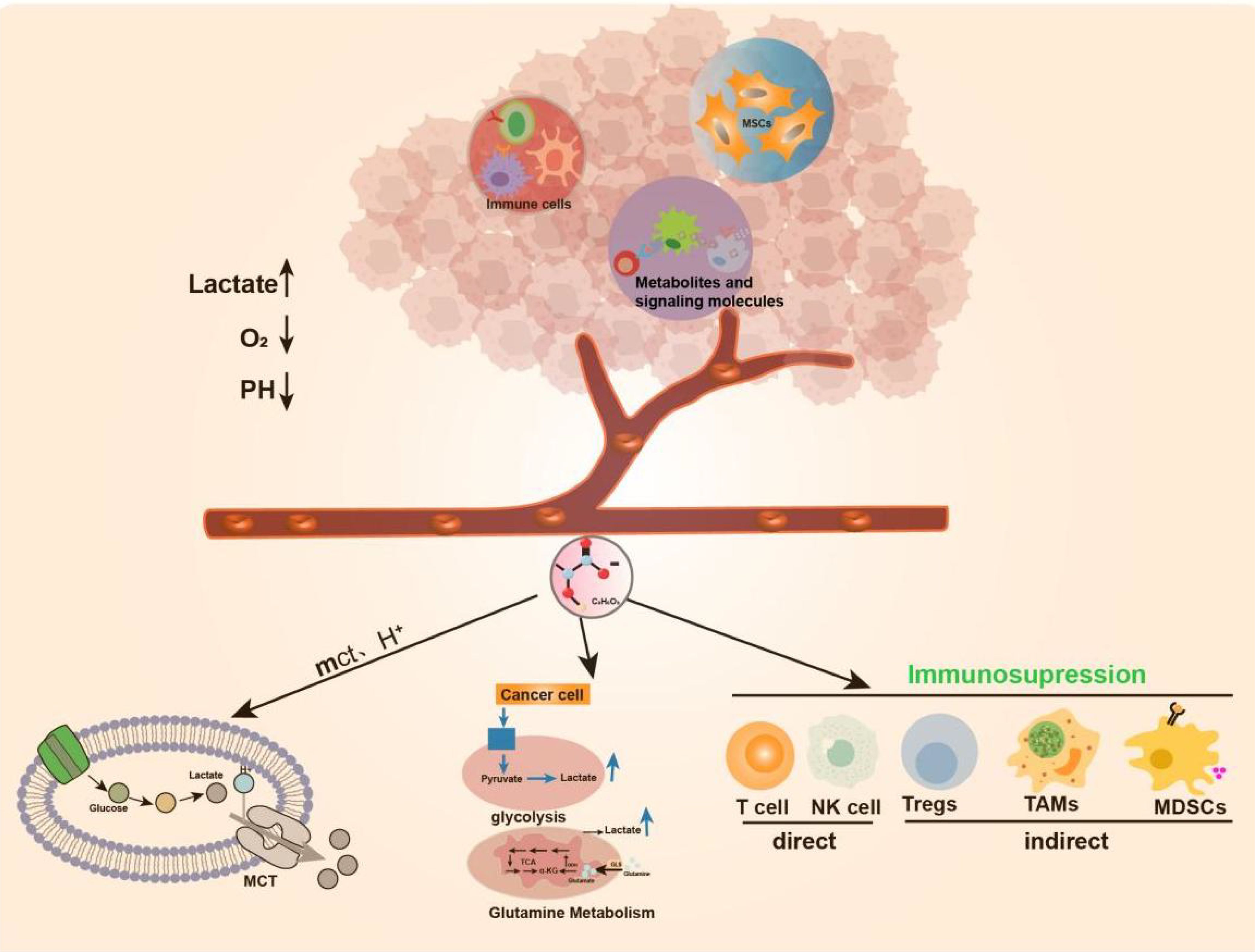
Figure 1. Production of Lactate in TME. TME includes surrounding mesenchymal and immune cells, extracellular matrix, metabolites and signaling molecules in the intercellular space.TME has anoxic or semi-anoxic properties, and tumor cells metabolize glucose or glutamine to produce a large amount of lactic acid, Lactate is co-transported by monocarboxylate transporters (MCTs), along with protons (H+), resulting in the accumulation of lactic acid and acidification of the tumor microenvironment.
Cancer is a multifaceted disease characterized by the buildup of mutated genetic material, constant apoptosis of healthy cells, and uncontrolled growth and spread of cancerous cells (32). The “immune escape” of tumors is considered one of the primary reasons behind their development. Rich literature substantiates that the metabolism of tumors is tightly correlated with the body’s immunity. Tumor cells take up glucose under hypoxic or “Warburg effect” conditions and release lactate, an essential metabolic byproduct, to alter their microenvironment. The massive accumulation of lactate exerts tumor immunosuppressive effects by regulating and affecting the functions of various immune cells and the release of cytokines. There is growing evidence that immune surveillance deficiencies promote immune escape from tumors, thus favoring tumor cell development and survival (33, 34). First, lactate affects immune cell cytotoxicity, motility, and transcription factor expression, leading to inhibition of the function of T-cells and NK cells. According to Brand et al., high lactate levels serve as strong inhibitors of T-cell and NK cell function and survival, thus facilitating the immune escape of tumors (24). For example, lactate can exert a protumor immune escape effect by directly inhibiting the lysis capacity of natural killer (NK) cells or inducing their apoptosis (35–37). In T cells, lactate can regulate CD4+ T-cell polarization, inhibit antitumor Th1 cells, promote Treg differentiation, and maintain the progression of prostate cancer (PC) through the TLR8/miR21 axis (38). Lactate produced by highly glucose-dependent tumors can also impair the infiltration and function of cytotoxic CD8+ T cells, thereby preventing immune surveillance (39, 40). Furthermore, studies have shown that the production of type I interferon (IFN-γ) in both T cells and NK cells is inhibited by lactate, thereby limiting immune cell activation (24). Therefore, lactate served as a key metabolite of glycolysis-mediated inhibition of RIG-I like receptors (RLR) signaling, can reduce host defense against viral infection and cancer immunosurveillance by inhibiting the production of IFN-γ (41). These findings suggest that lactate can play an essential role in tumor development by altering the behavior of immune cells (e.g., T cells and NK cells) in theTME (42).
Lactate is also involved in immune escape by altering several immune infiltrating cells. Like macrophages in normal tissues and organs, TAMs play a critical role in maintaining tumor growth and homeostasis (43). Macrophages can be classified as M1/M2-type macrophages depending on their source of energy (glycolysis or oxidative metabolism) (44). Zhang et al. documented a novel epistatic modification mechanism that stimulates histone lactonization by lactate produced during hypoxia (45). By increasing histone lactonization, lactate can trigger the polarization of macrophages from the M1 phenotype, which is cytotoxic and inflammatory, to the M2 phenotype, which is more tumor friendly, thereby suppressing the immune response within the TME (44, 46). Moreover, Colegio et al. reported that lactate produced by cancer cells induces increased expression of arginase 1 (Arg1) and vascular endothelial growth factor (VEGF) derived from tumor-associated macrophages (TAMs), thereby supporting tumor growth (47). In addition, lactate immunosuppression mechanisms include driving immune escape by increasing the infiltration of suppressor cells in the tumor environment, such as myeloid-derived suppressor cells (MDSCs) (48). Tumor-derived lactate can inhibit NK cell function by increasing the level of MDSCs, thus playing an immunosuppressive role in suppressing the natural immune response against tumor development (49) (Figure 2).
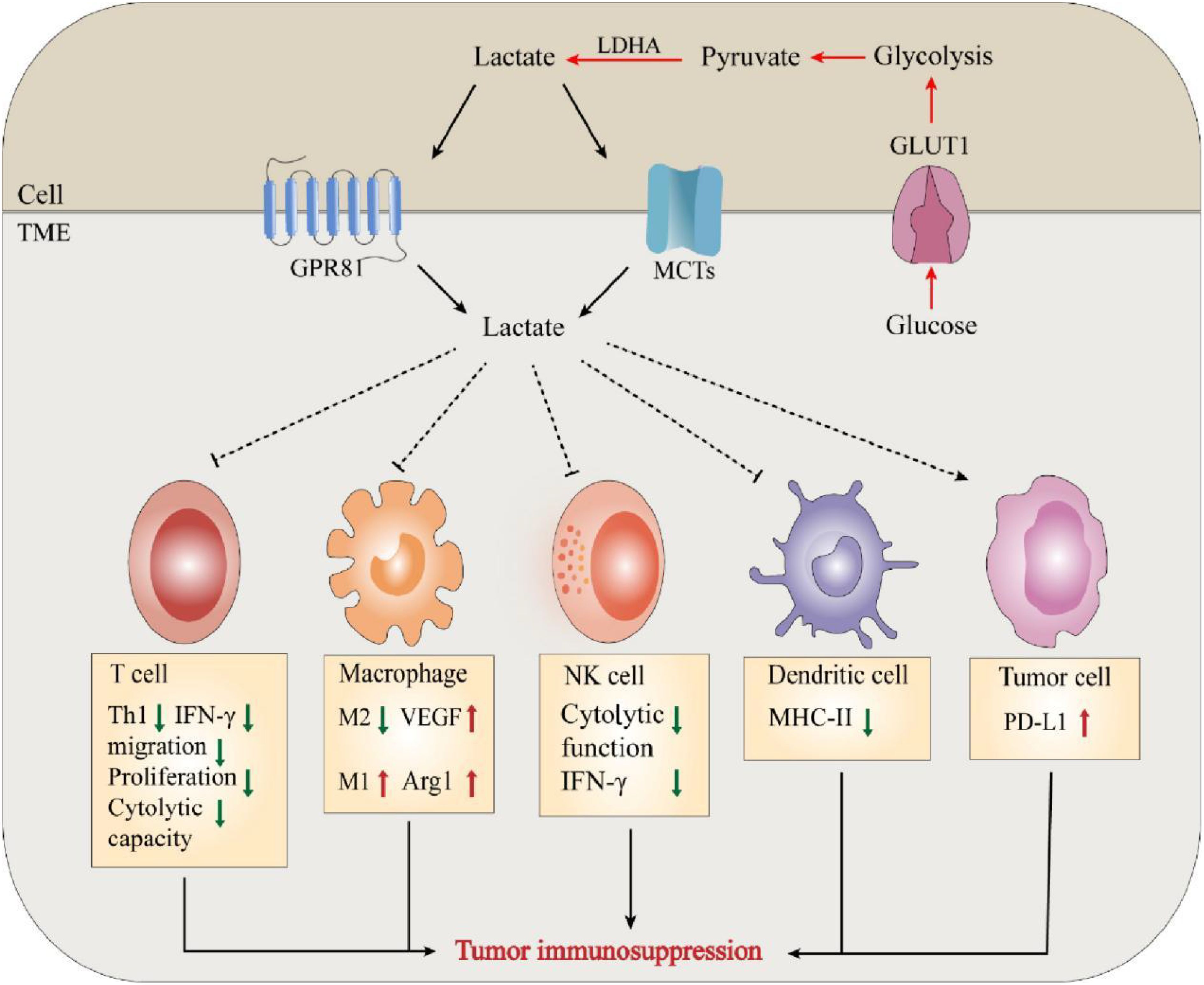
Figure 2. Lactate mediates tumor immunosuppression in TME. The massive accumulation of lactate exerts tumor immunosuppressive effects by regulating and affecting the functions of various immune cells and the release of cytokines.
The exchange of lactate between tumor cells or mesenchymal cells and the TME is mediated by the corresponding lactate transporters. Monocarboxylate transporters (MCTs) belong to the SLC16 family of the solute carrier (SLC) superfamily, and the SLC superfamily consists of over 400 protein membrane transporters, four allozymes (MCT1-4) are involved in lactate transport (50, 51). In this regard, cytotoxic T cells (CTLs) are the main effector cells of antitumor immunity, and the pathway by which lactate is produced by glycolysis in the TME is usually mediated by MCTs (52, 53). However, high lactate levels in the TME inhibit this process, and consequently, intracellular lactate inhibits the proliferation of CTLs and the production of cytokines, perforin, and granzyme B, resulting in a decrease in cytotoxic activity of 50% (54). Lactate exerts its protumor effects by binding to the cell surface receptor GPR81. Activation of GPR81 ultimately leads to the promotion of vasculogenesis, evasion of the immune system, and resistance to chemotherapy (55). Recent studies have shown that lactate activates GPR81 in tumor cells, which results in elevated PD-L1 production and reduced IFN-γ expression, highlighting the role of autocrine mechanisms (56). In addition, the above autocrine mechanism was complemented by the paracrine mechanism reported by Brown et al. in a mouse model of constitutive breast cancer (MMTV-PyMT-Tg) (46). They showed that GPR81 is upregulated in breast cancer and can exert an autocrine effect through tumor cell-derived lactate, thereby promoting tumor growth. Tumor cell-derived lactate can also exert protumorigenic effects by activating GPR81 in dendritic cells and blocking the presentation of MHCII cell surface tumor-specific antigens. These findings suggest that lactate promotes tumor activity through the GPR81 signaling pathway during tumor development through autocrine or paracrine secretion (57).
Neovascularization is an essential biological process in tumor growth, infiltration, and metastasis. Many studies have substantiated the positive role of lactate in promoting angiogenesis, reducing muscle atrophy, and accelerating wound healing during trauma healing (58, 59). Kolev et al. retrospectively analyzed 152 patients with different stages of gastric cancer and found a significant correlation between LDH5 overexpression and increased levels of HIF-1α and VEGF, indirectly suggesting that lactate metabolism contributes to angiogenesis (60). Moreover, lactate can promote angiogenesis in tumor cells by affecting endothelial cells (61, 62). Sonveaux et al. found that lactate excreted by tumor cells into the tissue interstitium could be taken up by endothelial cells with the help of MCT1, which inhibited the degradation of HIF-1α in nonhypoxic endothelial cells and significantly upregulated endothelial cell production of VEGF and fibroblast growth factor (FGF), thus promoting angiogenesis (61) (Figure 3). In contrast, the application of MCT1 inhibitors reversed these effects, further confirming the proangiogenic effect of lactate. These studies highlight the need to further investigate the association between lactate metabolism and angiogenesis and its underlying mechanisms.
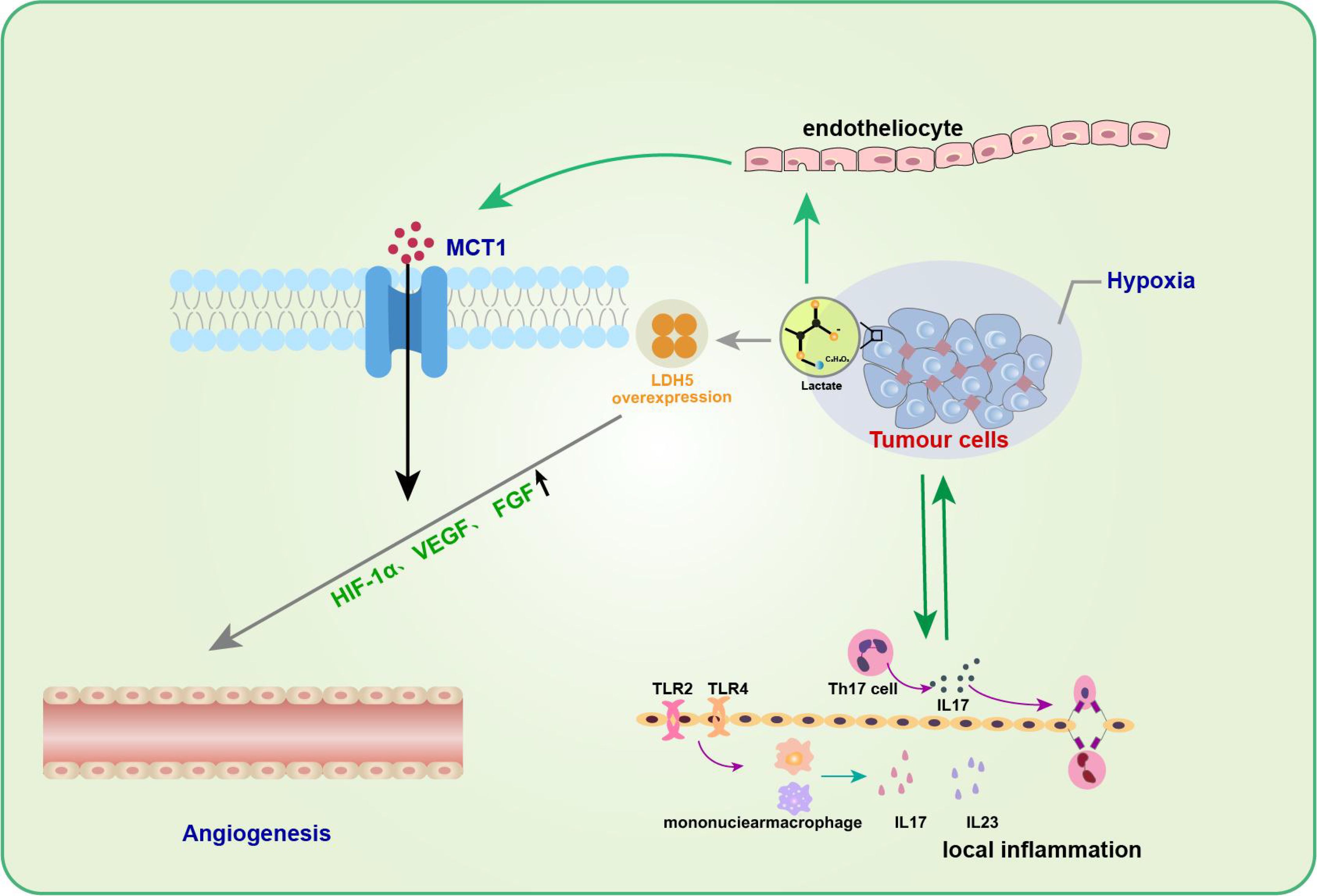
Figure 3. Lactate in tumor inflammation and angiogenesis. Lactic acid promotes angiogenesis of tumor cells by affecting endothelial cells. The entry of lactic acid into the tissue stroma by tumor cells can be facilitated by endothelial cells via MCT1 to inhibit HIF-1α degradation of non-hypoxic endothelial cells and significantly up-regulate endothelial cell production of VEGF and fibroblast growth factor (FGF), thus promoting angiogenesis. In addition to promoting neovascularization, the role of lactic acid in promoting tumor development is often associated with inflammatory responses.Th17 cells in the tumor microenvironment gradually increase and promote the secretion of cytokine IL-17 during tumor development, which can up-regulate the production of a variety of pro-inflammatory cytokines and pro-angiogenic factors, and promote tumor development.
In addition to facilitating neovascularization, the role of lactate in promoting tumor development is often associated with an inflammatory response. Heinemeyer et al. showed that Th17 cells in theTME gradually increase and promote the secretion of the cytokine IL-17 during tumor development (63), which can upregulate the production of various proinflammatory cytokines and proangiogenic factors to promote tumor development (64, 65). IL-23, a proinflammatory cytokine, has also been shown to be expressed excessively in and around tumor cells, thereby inducing topical inflammation and promoting tumor growth. Shime et al. further found that in TLR2 and TLR4 agonist-stimulated monocytes/macrophages and tumor-infiltrating immune cells, tumor cells could secrete lactate to activate the IL-23/IL-17 pathway and promote tumorigenesis and growth by promoting angiogenesis and triggering a local inflammatory response (66).
Numerous studies on lactate in different types of tumors, including colorectal cancer (35), cancer of the cervix (67), head and neck cancer (68, 69), and stomach cancer (70), have confirmed that lactate levels strongly correlate with tumor invasion and metastasis. Harmon et al. showed that lactate-mediated activation of the TME could be induced by liver-resident NK cell apoptosis, which leads to the survival of metastatic cancer cells in the liver (35). Further research has shown that lactate produced by CRLM (Coloretal Liver Metabases) can lower the pH in the tumor microenvironment, causing liver NK cells migrating to the tumor to lose their ability to regulate intracellular pH. This, in turn, results in mitochondrial stress and apoptosis in NK cells, ultimately benefiting the survival of metastatic cancer cells and promoting tumor metastasis. Rizwan et al. also reported that LDHA knockdown delayed tumor metastasis and improved overall survival in a mouse model of breast cancer (71). Interestingly, Hirschhaeuser et al. noted that tumor metastasis is promoted by lactate-induced secretion of hyaluronic acid by tumor-associated fibroblasts, which creates an environment conducive to migration and thus promotes tumor metastasis (72). Notably, evidence from mouse cancer models suggests that tumor metastasis can be inhibited by neutralizing acidity with oral buffers (73, 74). These studies suggested that lactate is closely associated with tumor invasion and metastasis.
However, the exact mechanism by which lactate is involved in tumor metastasis is not fully understood. Baumann et al. discovered that lactate could stimulate transforming growth factor-B2 (TGFB2) expression in glioma cells. TGFB2 is a critical regulator of glioma cell migration and is likely one of the significant mechanisms underlying glioma metastasis (75). In addition, the overexpression of HIF-1α in tumor cells leads to metabolic reprogramming, resulting in the Warburg effect, which results in the production of large amounts of lactate (76). HIF-1α has also been associated with breast cancer growth and metastasis and poor prognosis and aggressiveness. These findings offer a new approach for preventing breast cancer metastasis (77, 78). Notably, cells in a high-lactate environment exhibit rapid increases in the mRNA and protein expression of the transporter protein MCT1 (79). In addition, high levels of MCT1 expression have been associated with cancer invasion in non-small cell lung cancer and melanoma (80, 81). Similarly, Zhao et al. proposed that the molecular mechanism underlying osteosarcoma cell migration involves enhanced NF-κB signaling and is facilitated by MCT1 (82). Similar conclusions were obtained in a single test on cervical and mammary cancer cells (83). To conclude, future research should investigate the precise mechanisms through which lactate regulates tumor invasion and metastasis in various types of tumors due to the heterogeneity and complexity of tumor metabolism.
Lactate plays dual roles as a fuel for metabolism and a signaling molecule in tumors (4). In addition, lactate is necessary for the growth and development of tumor cells (84). Therefore, targeting the aberrant production of lactate in tumor cells, a key factor in cancer genesis and progression, has become a promising approach for cancer therapy (85) (Table 1).
Lactate metabolism has become an attractive target for enhancing cancer immunotherapy (86, 87). PI3K-Akt-mTOR-HIF axis was firstly used to inhibit lactate production by indirect targeting of glycolysis, and Akt inhibitors are being explored in patients with advanced or metastatic solid tumors in combination with a PARP inhibitor and anti-PD-L1 (NCT03772561) (88). Recent studies showed that targeting HIF-1α could increase CD8+T cell infiltration and support anti-PD-1/PD-L1 treatment as effectively as CTLA-4 blockade (89, 90). Inhibiting or eliminating the m6A demethylase ALK-BH5 leads to a notable decrease in lactate levels and the recruitment of Treg cells and MDSCs within the TME during anti-PD-1 therapy in mouse models of melanoma and colorectal cancer (91).
Lactate and lactic acid on the effectiveness of tumor vaccines were also studied. Feng et al. found that PC7A nano-tumor vaccine in lactate solution could significantly improve anti-tumor efficacy using an MC38 mouse tumor model (40). Lactic acid can augment the immunogenicity of whole UV-irradiated tumor cell vaccines by promoting dendritic cell (DC) maturation, aggregation and phagocytosis in mouse xenograft models (92). Furthermore, the injection of lactic acid-stimulated tumor vaccines could significantly reduce the number of CD11b+Gr1+MDSCs in tumor tissues, which plays a crucial role in immune evasion, tumor occurrence, and development (93). In the latest research, NaHCo3NPs nanoparticles could alleviate immune suppression and pyroptosis induced immune activation through acid neutralization, exhibiting enhanced anti-tumor immune efficiency by inhibiting primary/distal tumor growth and metastasis (94).
It is known that lactate dehydrogenases play a crucial role in lactate production. LDH enzymes are a subset of metabolic enzymes that facilitate the change from pyruvate to lactate and are equally crucial in cancer metabolism (95). Hence, targeting lactate production has emerged as a promising strategy for cancer therapy, and inhibitors targeting LDH enzymes are being extensively studied for their effectiveness in cancer treatment (96, 97) (Table 1). For example, GNE-140, a small molecule inhibitor of LDHA, has been shown to effectively inhibit the development of B16 melanoma in mice and human adenocarcinoma and pancreatic cancer in vitro, and its drug activity depends on cell metabolic activity (98, 99). Fang A et al. tested a prospective new compound (compound 11) in the MG-63 osteosarcoma cell line (100). The results showed that compound 11 could inhibit LDHA by reducing lactate production and causing extrinsic metabolic acidification (99). Given the complexity of lactate metabolism in the development of different tumors, despite the numerous effective LDH enzyme inhibitors reported, very few are currently available for clinical use. Therefore, further research is needed to optimize existing compounds and develop new lactate dehydrogenase inhibitors to improve cancer therapy.
In addition, lactate exchange between various tumor cells, mesenchymal cells, and the TME, is one of the necessary pathways for tumor cell nutrient depletion. Therefore, targeting the key molecules associated with lactate transport is an effective therapeutic approach for treating tumors. Among them, targeting MCTs may significantly impact intercellular lactate exchange (101, 102). Inhibition of MCT1 specifically impairs the influx of intercellular lactate, forcing metabolic conversion of tumor cells to aerobic glycolysis. This indirectly leads to the death of anoxic cancer cells due to glucose deprivation (103, 104). Common MCT inhibitors include AstraZeneca’s AZ3965 compound, as well as AR-C155858 and SR1380 [95,96]. These inhibitors specifically or nonspecifically target MCT1 and/or MCT2, leading to impairment of cancer cell survival and proliferation and ultimately exerting antitumor effects (105). In addition to MCT1, blocking lactate transport between diverse cell groups by downregulating the MCT4 gene may provide an efficient treatment strategy (106, 107). For example, A recent study has shown that inhibiting MCT4 could enhance the therapeutic efficacy of anti-PD-1 therapy and the combination treatment of MCT4 inhibitors and anti-PD-L1 therapy exhibited beneficial effects in 3D colorectal cancer sphere models (108). AstraZeneca’s MCT4 inhibitor AZ93 can not only target MCT4 but also reduce the propagation of various cancer cell lineages in which MCT1 is repressed (109).
Interestingly, clinical and retrospective analysis revealed that MCT4 could play a compensatory role when MCT1 is inhibited (101, 105). Therefore, it is highly conceivable that under hypoxic conditions, simultaneously inhibiting MCT1 and MCT4 can disrupt tumor growth. Dual inhibitors targeting both MCT1 and MCT4 have exhibited promising results as potential antitumor agents (110–112). Pilon-Thoma et al. reported that neutralizing the tumor pH with bicarbonate inhibited tumor growth in mice (113). In conclusion, the future of antitumor therapy may be more dedicated to combining various antitumor tools, such as chemotherapy, radiotherapy, and inhibitors, to observe their additional or synergistic effects to achieve better efficacy in oncology treatment.
In recent years, studies reported that elevated lactate levels in inflammatory diseases due to impaired production or clearance of lactate, which affects immune cell function (114). The cellular entry of lactate is dependent on MCT-1 and SLC5A12 (41, 115). Once lactate and its associated H+ ions enter the cell, they typically function as negative feedback regulators of glycolytic ATP production (114). In general, glycolysis supplies fuel to inflammatory cells, while oxidative phosphorylation is the origin of energy for anti-inflammatory regulatory cells (116), suggesting that lactate-mediated glycolysis may inhibit the function of inflammatory cells and promote regulatory function (Figure 4). Below, we discuss how lactate affects immune cells and thus suppresses inflammation.
Specific receptor signaling cascades in monocytes and macrophages have been demonstrated to be suppressed by lactate (114). For example, lactate delays the phosphorylation of protein kinase B (AKT), inhibits the degradation of IκB-α inhibitors, and promotes the accumulation of NF-κB in the nucleus, thereby inhibiting LPS receptor signaling (19, 117). Lactate can also stimulate GPR81-induced inhibitory signals that impair TLR-4-mediated inflammasome assembly, among other effects (41, 117). In addition, lactate also suppresses the activation of dendritic cells and reduces the production of cytokines induced by LPS (118, 119). Lactate can activate the GPR81 receptor and enhance Th2 activation on dendritic cells, leading to a decrease in antigen presentation and cytokine production and a reduction in cAMP activation (46, 120). This finding suggested that lactate inhibits the activation of mononuclear cells and suppresses cell-mediated inflammation.
Lactate can inhibit inflammation mediated by mast cells. On the one hand, lactate can inhibit IL-33-induced mast cell activation both in vitro and in vivo (115). Abebayehu et al. pointed out that lactate can enhance the expression of HIF1-α in a MCT-1 and pH dependent manner (121). After activating IL-33 and LPS, lactate can amplify inflammatory signals by inhibiting the expression of miR-155-5p (122, 123). On the contrary, mimetics of miR-155-5p can reverse the inhibitory effect of lactate, indicating that lactate may maintain the negative feedback pathway by inhibiting the expression of miR-155-5p (123). On the other hand, lactate can affect the function of mast cells by inhibiting calcium mobilization, degranulation, and release of cytokines and chemokines under the influence of MAS-related GPR coupled receptor X2 (mrgprx2) (124).
Like mast cells, lactate also inhibits regulatory T cells. Studies have shown that regulatory T cells can absorb and metabolize lactate to maintain their suppressive function under high lactate conditions (125). Several studies have reported that lactate reduces the IL-1β-induced transcription of key proinflammatory cytokines, such as IL-1β, IL-6, CCL2, and Pghs2, via GPR81 (2). Lactate produced during labor can also eliminate or reduce inflammation in a negative feedback manner via GPR81 in the uterus (126). Interestingly, lactate reportedly suppresses proinflammatory signaling pathways via a mechanism that does not involve GPR81 (127). The transcription of the proinflammatory genes IL-1B, IL-6, IL-12B, and CD40 was downregulated after lactate treatment in the bone marrow mesenchymal stem cells (BMMS) of GPR81-deficient mice (127). Furthermore, lactate has been shown to impair metabolic reprogramming following macrophage activation in a GPR81-independent manner (127), which has also been associated with inhibiting the response to proinflammatory cytokines (128). These findings suggest that GPR81 may play an essential role in the depression of inflammation by lactate, but whether this anti-inflammatory effect depends on GPR81 in different pathophysiological environments remains to be further explored.
Unlike acute inflammation, the most dominant feature of chronic inflammation is the infiltration of mononuclear cells such as lymphocytes, macrophages, and plasma cells (129). Lactate promotes immune cell inflammation during chronic inflammation (Figure 3). Below, we discuss the impacts of lactate on immune cells during chronic inflammation.
Lactate has been found to inhibit the cytolytic function of NK cells and reduce cell proliferation, motility, cytolytic activity, and the secretion of degranulation and inflammatory mediators in CD8+ T cells (54, 130, 131). These effects of lactate are attributed to its dependence on MCT-1, which inhibits the activity of protein kinases (130, 132). Interestingly, the effects of lactate on CD8+ T cells are not solely dependent on the inhibition of glycolysis. Lactate inhibits the locomotion of CD8+ T cells while decreasing cytolytic capacity by increasing inflammatory cytokine production, prolonging chronic inflammation (132, 133). Furthermore, CD8+ T cells reportedly contribute to rheumatoid arthritis (RA) by releasing proinflammatory and cytolytic mediators in the synovial membrane (SM) microenvironment lacking oxygen and nutrients (134).
Lactate and lactic acid have distinct effects on CD4+ T cells. Lactate inhibits the movement of CD4+ T cells, while lactic acid promotes the differentiation of naive CD4+ T cells into proinflammatory Th17 cells (114). Unlike CD8+ T cells, CD4+ T cells rely on the expression of SLC5A12 rather than MCT-1. This difference in transporter dependence may explain the opposing effects of lactate and lactic acid on immune cells (132). By acting through SLC5A12, lactate not only hinders the production of glycolytic energy but also boosts oxidative stress, thereby promoting the translocation of pyruvate kinase to the nucleus. In the nucleus, pyruvate kinase phosphorylates signal-transducing nuclear transduction activators (STAT3/1) and triggers RORγt-dependent IL-17 transcription (133). Pucino et al. reported that lactate driven by SLC5A12 leads to the reprogramming of cellular metabolism and facilitates IL-17 production via PKM2/STAT3 signaling, thereby promoting the inflammatory response of CD4+ T cells in patients with rheumatoid arthritis (133). These findings have significant implications for understanding the role of lactate in inflammatory disorders such as synovial membrane infection in rheumatoid arthritis patients. Interestingly, unlike that of CD8+ T cells, the inhibition of CD4+ T-cell motility by sodium lactate is dependent on interference with glycolysis. These findings suggest a complex mechanism by which lactate or sodium lactate modulates T-cell motility, and further studies are needed to investigate its pathological significance and molecular mechanism. Current evidence suggests that sodium lactate induces the conversion of CD4+ T cells into a Th17 subpopulation that generates large quantities of the proinflammatory cytokine IL-17 [69]. IL-17, a proinflammatory cytokine secreted by Th17 cells, also plays a key role in the pathogenesis of periodontitis (135). One study revealed that the concentrations of lactate and lactate dehydrogenase in the gingival crevicular fluid of chronic periodontitis patients were also elevated due to the hypoxic periodontal microenvironment. After undergoing nonsurgical treatment, patients with periodontitis exhibit reductions in lactate levels, IL-17 concentrations, and Th17 cell counts [11]. This observation raises the question of whether there is a link between increased lactate levels and elevated IL-17 levels in periodontitis, although further research is needed to confirm this association.
In addition, sodium lactate could increase the secretion of LPS-stimulated matrix metalloproteinases (MMP) -1, IL-1β, and IL-6 of U937 histiocytes by enhancing AP-1 and NF-κB transcriptional activities. Lactate could boost TLR4 signaling and NF-κB mediated gene transcription in macrophages via MCTs (136, 137). It has also been reported that monocyte differentiation is capable of increasing inflammatory (M1) and regulatory (M2) mediators when lactate is present in conjunction with granulocyte-macrophage colony-stimulating factor or in adenocarcinoma-conditioned media (elevated lactate and many other mediators), consistent with a TAM phenotype (138, 139). Further experiments revealed that the combination of GM-CSF and lactate drives the production and depletion of IL-6-dependent macrophage colony-stimulating factor (M-CSF), promoting an inflammatory feedforward loop that exacerbates inflammation.
In the previous section, we discussed that the differential effects of lactate on CD4+ T cells and CD8+ T cells could be attributed to the selective expression of these cells to SCL5A12 and MCT-1, respectively. Lactate can inhibit the proliferation, degranulation, motility, cytolytic activity, and secretion of inflammatory mediators by CD8+ T cells. Lactate mediated the switch from CD4+ T cells to Th17 cells, promoting inflammation. It is highly conceivable that differences in acidity and concentration play a role in this mechanism. Several studies have reported that the effect of lactate is pH dependent (140). The role of acidity was maintained in dendritic cells, where its inhibitory effect was reversed by modulating the pH to 7.4 at a lactate concentration of 10 mM but was less effective at lactate concentrations above 10 mM (118). This finding suggested that the lactate concentration can also alter the inhibitory effect of the same transporter. Another mechanism may involve the effect of lactate on transcriptional regulators such as HIF-1α and HIF-2α. Lactate promotes HIF-1α activity while inhibiting glycolysis and the production of inflammatory cytokines (47, 141). Lactate has been shown to selectively induce HIF-1α-dependent transcription of VEGF while also promoting Th17 polarization. It is widely thought that lactate controls HIFs through multiple levels of regulation, including transcriptional and posttranslational regulation. This allows lactate and associated H+ to influence the function of these important transcription factors in the regulation of inflammation and angiogenesis (141–143). This phenomenon may be attributed to the fact that lactate can enhance the biological responses of Th17, Th2, and M2 cells in distinct inflammatory microenvironments (133). Although the exact mechanism remains unclear, lactate has been demonstrated to stimulate Th17 differentiation and induce IL-23 production. As we mentioned earlier, Th17 and IL-23 are significant in inflammation. These proposed mechanisms suggest that future research should elucidate how lactate affects various types of immune cells.
Herein, we investigated the role of lactate in tumors and inflammation. On the one hand, lactate can act as a metabolite that promotes tumor growth and development; on the other hand, the immunoregulatory effect of lactate has dual roles in acute and chronic inflammation.
Lactate promotes tumor growth, immune escape, tumor inflammation, angiogenesis, and tumor metastasis by regulating the function of various immune cells. We discussed the dual role of lactate as both a metabolic fuel and a signaling molecule. Currently, cancer continues to be a disease with a high mortality rate. The presence of lactate can promote tumor growth and metastasis and plays an essential role in tumor progression. However, the currently available drugs are far from meeting our needs. We should dedicate more resources to the development of therapeutic drugs in the future, as inhibiting the tumor-promoting effect of lactate holds promise for the recovery of cancer patients.
Lactate plays a beneficial role during acute inflammation by acting as a negative feedback regulator that inhibits the function of immune cells, thus reducing inflammation. However, elevated lactate levels can prolong the course of inflammation during chronic inflammation, indicating a different role for lactate in this context. However, the specific effects of cells, transcriptional regulators, H+ mediators, and the microenvironment on lactate remain largely unclear and warrant further study.
In summary, although it has been established that lactate can have both positive and negative effects on our bodies, more research is warranted. In this respect, future studies should focus on two areas: 1) developing effective pharmaceuticals to inhibit lactate and its tumor-promoting effects and 2) investigating how lactate influences the development of different cell types and the role of the microenvironment in shaping these responses.
HL: Conceptualization, Funding acquisition, Supervision, Writing – original draft, Writing – review & editing. MP: Conceptualization, Writing – original draft. ML: Writing – review & editing. ZZ: Visualization, Writing – review & editing. YL: Visualization, Writing – review & editing. ZH: Visualization, Writing – review & editing. CG: Writing – original draft, Writing – review & editing. HW: Conceptualization, Supervision, Visualization, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work is supported by the Science and Technology Research Project of Henan Province (232102310129, 242102310337).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Reddy AJ, Lam SW, Bauer SR, Guzman JA. Lactic acidosis: Clinical implications and management strategies. Cleve Clin J Med. (2015) 82:615–24. doi: 10.3949/ccjm.82a.14098
2. Andersen LW, Mackenhauer J, Roberts JC, Berg KM, Cocchi MN, Donnino MW. Etiology and therapeutic approach to elevated lactate levels. Mayo Clin Proc. (2013) 88:1127–40. doi: 10.1016/j.mayocp.2013.06.012
3. Brooks GA. The science and translation of lactate shuttle theory. Cell Metab. (2018) 27:757–85. doi: 10.1016/j.cmet.2018.03.008
4. Perez-Tomas R, Perez-Guillen I. Lactate in the tumor microenvironment: an essential molecule in cancer progression and treatment. Cancers (Basel). (2020) 12:3244. doi: 10.3390/cancers12113244
5. Bradley I R, Matthew Z M, Melissa M W, Anna C, Jackie E B, Andrew R P, et al. Cell-programmed nutrient partitioning in the tumour microenvironment. Nature. (2021) 593:282–8. doi: 10.1038/s41586-021-03442-1
6. Mehdi D, Jeffrey W, Mark R-T, Liping X, Meghan C F-F, Paul A S, et al. The harsh microenvironment in early breast cancer selects for a Warburg phenotype. Proc Natl Acad Sci U.S.A. (2021) 118:e2011342118. doi: 10.1073/pnas.2011342118
7. Ippolito L, Morandi A, Giannoni E, Chiarugi P. Lactate: A metabolic driver in the tumour landscape. Trends Biochem Sci. (2019) 44:153–66. doi: 10.1016/j.tibs.2018.10.011
8. Cassim S, Pouyssegur J. Tumor microenvironment: A metabolic player that shapes the immune response. Int J Mol Sci. (2019) 21:157. doi: 10.3390/ijms21010157
9. Robb CT, Regan KH, Dorward DA, Rossi AG. Key mechanisms governing resolution of lung inflammation. Semin Immunopathol. (2016) 38:425–48. doi: 10.1007/s00281-016-0560-6
10. Arthur RE, Stern M, Galeazzi M, Baldassare AR, Weiss TD, Rogers JR, et al. Synovial fluid lactic acid in septic and nonseptic arthritis. Arthritis Rheum. (1983) 26:1499–505. doi: 10.1002/art.1780261212
11. Qiqiang L, Huanxin M, Xuejun G. Longitudinal study of volatile fatty acids in the gingival crevicular fluid of patients with periodontitis before and after nonsurgical therapy. J Periodontal Res. (2012) 47:740–9. doi: 10.1111/j.1600-0765.2012.01489.x
12. Shapiro NI, Howell MD, Talmor D, Nathanson LA, Lisbon A, Wolfe RE, et al. Serum lactate as a predictor of mortality in emergency department patients with infection. Ann Emerg Med. (2005) 45:524–8. doi: 10.1016/j.annemergmed.2004.12.006
13. Halestrap AP. The monocarboxylate transporter family–Structure and functional characterization. IUBMB Life. (2012) 64:1–9. doi: 10.1002/iub.573
14. Kompanje EJ, Jansen TC, van der Hoven B, Bakker J. The first demonstration of lactic acid in human blood in shock by Johann Joseph Scherer (1814-1869) in January 1843. Intensive Care Med. (2007) 33:1967–71. doi: 10.1007/s00134-007-0788-7
15. Walenta S, Wetterling M, Lehrke M, Schwickert G, Sundfor K, Rofstad EK, et al. High lactate levels predict likelihood of metastases, tumor recurrence, and restricted patient survival in human cervical cancers. Cancer Res. (2000) 60:916–21.
16. Goetze K, Walenta S, Ksiazkiewicz M, Kunz-Schughart LA, Mueller-Klieser W. Lactate enhances motility of tumor cells and inhibits monocyte migration and cytokine release. Int J Oncol. (2011) 39:453–63. doi: 10.3892/ijo.2011.1055
17. Certo M, Tsai CH, Pucino V, Ho PC, Mauro C. Lactate modulation of immune responses in inflammatory versus tumour microenvironments. Nat Rev Immunol. (2021) 21:151–61. doi: 10.1038/s41577-020-0406-2
18. Ngwa VM, Edwards DN, Philip M, Chen J. Microenvironmental metabolism regulates antitumor immunity. Cancer Res. (2019) 79:4003–8. doi: 10.1158/0008-5472.CAN-19-0617
19. Peter K, Rehli M, Singer K, Renner-Sattler K, Kreutz M. Lactic acid delays the inflammatory response of human monocytes. Biochem Biophys Res Commun. (2015) 457:412–8. doi: 10.1016/j.bbrc.2015.01.005
20. Kellum JA, Song M, Li J. Lactic and hydrochloric acids induce different patterns of inflammatory response in LPS-stimulated RAW 264. 7 Cells Am J Physiol Regul Integr Comp Physiol. (2004) 286:R686–92. doi: 10.1152/ajpregu.00564.2003
21. Klug M, Heinz S, Gebhard C, Schwarzfischer L, Krause SW, Andreesen R, et al. Active DNA demethylation in human postmitotic cells correlates with activating histone modifications, but not transcription levels. Genome Biol. (2010) 11:R63. doi: 10.1186/gb-2010-11-6-r63
22. Li X, Jiang S, Tapping RI. Toll-like receptor signaling in cell proliferation and survival. Cytokine. (2010) 49:1–9. doi: 10.1016/j.cyto.2009.08.010
23. Lyssiotis CA, Kimmelman AC. Metabolic interactions in the tumor microenvironment. Trends Cell Biol. (2017) 27:863–75. doi: 10.1016/j.tcb.2017.06.003
24. Brand A, Singer K, Koehl GE, Kolitzus M, Schoenhammer G, Thiel A, et al. LDHA-associated lactic acid production blunts tumor immunosurveillance by T and NK cells. Cell Metab. (2016) 24:657–71. doi: 10.1016/j.cmet.2016.08.011
25. Buck MD, O'Sullivan D, Pearce EL. T cell metabolism drives immunity. J Exp Med. (2015) 212:1345–60. doi: 10.1084/jem.20151159
26. Tatum JL, Kelloff GJ, Gillies RJ, Arbeit JM, Brown JM, Chao KS, et al. Hypoxia: importance in tumor biology, noninvasive measurement by imaging, and value of its measurement in the management of cancer therapy. Int J Radiat Biol. (2006) 82:699–757. doi: 10.1080/09553000601002324
27. Rawat D, Chhonker SK, Naik RA, Mehrotra A, Trigun SK, Koiri RK. Lactate as a signaling molecule: Journey from dead end product of glycolysis to tumor survival. Front Biosci (Landmark Ed). (2019) 24:366–81. doi: 10.2741/4723
28. Damiani C, Colombo R, Gaglio D, Mastroianni F, Pescini D, Westerhoff HV, et al. A metabolic core model elucidates how enhanced utilization of glucose and glutamine, with enhanced glutamine-dependent lactate production, promotes cancer cell growth: The WarburQ effect. PLoS Comput Biol. (2017) 13:e1005758. doi: 10.1371/journal.pcbi.1005758
29. Webb BA, Chimenti M, Jacobson MP, Barber DL. Dysregulated pH: a perfect storm for cancer progression. Nat Rev Cancer. (2011) 11:671–7. doi: 10.1038/nrc3110
30. Schaaf MB, Garg AD, Agostinis P. Defining the role of the tumor vasculature in antitumor immunity and immunotherapy. Cell Death Dis. (2018) 9:115. doi: 10.1038/s41419-017-0061-0
31. Hayes C, Donohoe CL, Davern M, Donlon NE. The oncogenic and clinical implications of lactate induced immunosuppression in the tumour microenvironment. Cancer Lett. (2021) 500:75–86. doi: 10.1016/j.canlet.2020.12.021
32. Tian T, Olson S, Whitacre JM, Harding A. The origins of cancer robustness and evolvability. Integr Biol (Camb). (2011) 3:17–30. doi: 10.1039/c0ib00046a
33. Swann JB, Smyth MJ. Immune surveillance of tumors. J Clin Invest. (2007) 117:1137–46. doi: 10.1172/JCI31405
34. Dunn GP, Bruce AT, Ikeda H, Old LJ, Schreiber RD. Cancer immunoediting: from immunosurveillance to tumor escape. Nat Immunol. (2002) 3:991–8. doi: 10.1038/ni1102-991
35. Harmon C, Robinson MW, Hand F, Almuaili D, Mentor K, Houlihan DD, et al. Lactate-mediated acidification of tumor microenvironment induces apoptosis of liver-resident NK cells in colorectal liver metastasis. Cancer Immunol Res. (2019) 7:335–46. doi: 10.1158/2326-6066.CIR-18-0481
36. Kumar A, Pyaram K, Yarosz EL, Hong H, Lyssiotis CA, Giri S, et al. Enhanced oxidative phosphorylation in NKT cells is essential for their survival and function. Proc Natl Acad Sci U.S.A. (2019) 116:7439–48. doi: 10.1073/pnas.1901376116
37. Husain Z, Huang Y, Seth P, Sukhatme VP. Tumor-derived lactate modifies antitumor immune response: effect on myeloid-derived suppressor cells and NK cells. J Immunol. (2013) 191:1486–95. doi: 10.4049/jimmunol.1202702
38. Comito G, Iscaro A, Bacci M, Morandi A, Ippolito L, Parri M, et al. Lactate modulates CD4(+) T-cell polarization and induces an immunosuppressive environment, which sustains prostate carcinoma progression via TLR8/miR21 axis. Oncogene. (2019) 38:3681–95. doi: 10.1038/s41388-019-0688-7
39. Scott KE, Cleveland JL. Lactate wreaks havoc on tumor-infiltrating T and NK cells. Cell Metab. (2016) 24:649–50. doi: 10.1016/j.cmet.2016.10.015
40. Feng Q, Liu Z, Yu X, Huang T, Chen J, Wang J, et al. Lactate increases stemness of CD8 + T cells to augment anti-tumor immunity. Nat Commun. (2022) 13:4981. doi: 10.1038/s41467-022-32521-8
41. Zhang W, Wang G, Xu ZG, Tu H, Hu F, Dai J, et al. Lactate is a natural suppressor of RLR signaling by targeting MAVS. Cell. (2019) 178:176–189 e15. doi: 10.1016/j.cell.2019.05.003
42. Rostamian H, Khakpoor-Koosheh M, Jafarzadeh L, Masoumi E, Fallah-Mehrjardi K, Tavassolifar MJ, et al. Restricting tumor lactic acid metabolism using dichloroacetate improves T cell functions. BMC Cancer. (2022) 22:39. doi: 10.1186/s12885-021-09151-2
43. Qian BZ, Pollard JW. Macrophage diversity enhances tumor progression and metastasis. Cell. (2010) 141:39–51. doi: 10.1016/j.cell.2010.03.014
44. Galvan-Pena S, O'Neill LA. Metabolic reprograming in macrophage polarization. Front Immunol. (2014) 5:420. doi: 10.3389/fimmu.2014.00420
45. Zhang D, Tang Z, Huang H, Zhou G, Cui C, Weng Y, et al. Metabolic regulation of gene expression by histone lactylation. Nature. (2019) 574:575–80. doi: 10.1038/s41586-019-1678-1
46. Brown TP, Bhattacharjee P, Ramachandran S, Sivaprakasam S, Ristic B, Sikder MOF, et al. The lactate receptor GPR81 promotes breast cancer growth via a paracrine mechanism involving antigen-presenting cells in the tumor microenvironment. Oncogene. (2020) 39:3292–304. doi: 10.1038/s41388-020-1216-5
47. Colegio OR, Chu NQ, Szabo AL, Chu T, Rhebergen AM, Jairam V, et al. Functional polarization of tumour-associated macrophages by tumour-derived lactic acid. Nature. (2014) 513:559–63. doi: 10.1038/nature13490
48. Huber V, Camisaschi C, Berzi A, Ferro S, Lugini L, Triulzi T, et al. Cancer acidity: An ultimate frontier of tumor immune escape and a novel target of immunomodulation. Semin Cancer Biol. (2017) 43:74–89. doi: 10.1016/j.semcancer.2017.03.001
49. Veglia F, Perego M, Gabrilovich D. Myeloid-derived suppressor cells coming of age. Nat Immunol. (2018) 19:108–19. doi: 10.1038/s41590-017-0022-x
50. Spencer TL, Lehninger AL. L-lactate transport in Ehrlich ascites-tumour cells. Biochem J. (1976) 154:405–14. doi: 10.1042/bj1540405
51. Halestrap AP. Monocarboxylic acid transport. Compr Physiol. (2013) 3:1611–43. doi: 10.1002/cphy.c130008
52. Blasberg RG, Gelovani-Tjuvajev J. In vivo molecular-genetic imaging. J Cell Biochem Suppl. (2002) 39:172–83. doi: 10.1002/jcb.10433
53. Gallagher FA, Kettunen MI, Day SE, Hu DE, Ardenkjaer-Larsen JH, Zandt R, et al. Magnetic resonance imaging of pH in vivo using hyperpolarized 13C-labelled bicarbonate. Nature. (2008) 453:940–3. doi: 10.1038/nature07017
54. Fischer K, Hoffmann P, Voelkl S, Meidenbauer N, Ammer J, Edinger M, et al. Inhibitory effect of tumor cell-derived lactic acid on human T cells. Blood. (2007) 109:3812–9. doi: 10.1182/blood-2006-07-035972
55. Brown TP, Ganapathy V. Lactate/GPR81 signaling and proton motive force in cancer: Role in angiogenesis, immune escape, nutrition, and Warburg phenomenon. Pharmacol Ther. (2020) 206:107451. doi: 10.1016/j.pharmthera.2019.107451
56. Feng J, Yang H, Zhang Y, Wei H, Zhu Z, Zhu B, et al. Tumor cell-derived lactate induces TAZ-dependent upregulation of PD-L1 through GPR81 in human lung cancer cells. Oncogene. (2017) 36:5829–39. doi: 10.1038/onc.2017.188
57. Roland CL, Arumugam T, Deng D, Liu SH, Philip B, Gomez S, et al. Cell surface lactate receptor GPR81 is crucial for cancer cell survival. Cancer Res. (2014) 74:5301–10. doi: 10.1158/0008-5472.CAN-14-0319
58. Polet F, Feron O. Endothelial cell metabolism and tumour angiogenesis: glucose and glutamine as essential fuels and lactate as the driving force. J Intern Med. (2013) 273:156–65. doi: 10.1111/joim.12016
59. Porporato PE, Payen VL, De Saedeleer CJ, Preat V, Thissen JP, Feron O, et al. Lactate stimulates angiogenesis and accelerates the healing of superficial and ischemic wounds in mice. Angiogenesis. (2012) 15:581–92. doi: 10.1007/s10456-012-9282-0
60. Kolev Y, Uetake H, Takagi Y, Sugihara K. Lactate dehydrogenase-5 (LDH-5) expression in human gastric cancer: association with hypoxia-inducible factor (HIF-1alpha) pathway, angiogenic factors production and poor prognosis. Ann Surg Oncol. (2008) 15:2336–44. doi: 10.1245/s10434-008-9955-5
61. Sonveaux P, Copetti T, De Saedeleer CJ, Vegran F, Verrax J, Kennedy KM, et al. Targeting the lactate transporter MCT1 in endothelial cells inhibits lactate-induced HIF-1 activation and tumor angiogenesis. PLoS One. (2012) 7:e33418. doi: 10.1371/journal.pone.0033418
62. Vegran F, Boidot R, Michiels C, Sonveaux P, Feron O. Lactate influx through the endothelial cell monocarboxylate transporter MCT1 supports an NF-kappaB/IL-8 pathway that drives tumor angiogenesis. Cancer Res. (2011) 71:2550–60. doi: 10.1158/0008-5472.CAN-10-2828
63. Heinemeyer T, Wingender E, Reuter I, Hermjakob H, Kel AE, Kel OV, et al. Databases on transcriptional regulation: TRANSFAC, TRRD and COMPEL. Nucleic Acids Res. (1998) 26:362–7. doi: 10.1093/nar/26.1.362
64. Karin M, Lawrence T, Nizet V. Innate immunity gone awry: linking microbial infections to chronic inflammation and cancer. Cell. (2006) 124:823–35. doi: 10.1016/j.cell.2006.02.016
65. Kim JW, Dang CV. Cancer's molecular sweet tooth and the Warburg effect. Cancer Res. (2006) 66:8927–30. doi: 10.1158/0008-5472.CAN-06-1501
66. Shime H, Yabu M, Akazawa T, Kodama K, Matsumoto M, Seya T, et al. Tumor-secreted lactic acid promotes IL-23/IL-17 proinflammatory pathway. J Immunol. (2008) 180:7175–83. doi: 10.4049/jimmunol.180.11.7175
67. Schwickert G, Walenta S, Sundfor K, Rofstad EK, Mueller-Klieser W. Correlation of high lactate levels in human cervical cancer with incidence of metastasis. Cancer Res. (1995) 55:4757–9. doi: 10.1007/BF01517220
68. Walenta S, Salameh A, Lyng H, Evensen JF, Mitze M, Rofstad EK, et al. Correlation of high lactate levels in head and neck tumors with incidence of metastasis. Am J Pathol. (1997) 150:409–15. doi: 10.1006/brln.1997.1857
69. Brizel DM, Schroeder T, Scher RL, Walenta S, Clough RW, Dewhirst MW, et al. Elevated tumor lactate concentrations predict for an increased risk of metastases in head-and-neck cancer. Int J Radiat Oncol Biol Phys. (2001) 51:349–53. doi: 10.1016/s0360-3016(01)01630-3
70. Hur H, Xuan Y, Kim YB, Lee G, Shim W, Yun J, et al. Expression of pyruvate dehydrogenase kinase-1 in gastric cancer as a potential therapeutic target. Int J Oncol. (2013) 42:44–54. doi: 10.3892/ijo.2012.1687
71. Rizwan A, Serganova I, Khanin R, Karabeber H, Ni X, Thakur S, et al. lactate, and metastases in 4T1 breast tumors. Clin Cancer Res. (2013) 19:5158–69. doi: 10.1158/1078-0432.CCR-12-3300
72. Hirschhaeuser F, Sattler UG, Mueller-Klieser W. Lactate: a metabolic key player in cancer. Cancer Res. (2011) 71:6921–5. doi: 10.1158/0008-5472.CAN-11-1457
73. Lardner A. The effects of extracellular pH on immune function. J Leukoc Biol. (2001) 69:522–30. doi: 10.1189/jlb.69.4.522
74. Li Z, Dong L, Dean E, Yang LV. Acidosis decreases c-Myc oncogene expression in human lymphoma cells: a role for the proton-sensing G protein-coupled receptor TDAG8. Int J Mol Sci. (2013) 14:20236–55. doi: 10.3390/ijms141020236
75. Baumann F, Leukel P, Doerfelt A, Beier CP, Dettmer K, Oefner PJ, et al. Lactate promotes glioma migration by TGF-beta2-dependent regulation of matrix metalloproteinase-2. Neuro Oncol. (2009) 11:368–80. doi: 10.1215/15228517-2008-106
76. Nagao A, Kobayashi M, Koyasu S, Chow CCT, Harada H. HIF-1-dependent reprogramming of glucose metabolic pathway of cancer cells and its therapeutic significance. Int J Mol Sci. (2019) 20:238. doi: 10.3390/ijms20020238
77. San-Millan I, Julian CG, Matarazzo C, Martinez J, Brooks GA. Is lactate an oncometabolite? Evidence supporting a role for lactate in the regulation of transcriptional activity of cancer-related genes in MCF7 breast cancer cells. Front Oncol. (2019) 9:1536. doi: 10.3389/fonc.2019.01536
78. Gilkes DM, Semenza GL. Role of hypoxia-inducible factors in breast cancer metastasis. Future Oncol. (2013) 9:1623–36. doi: 10.2217/fon.13.92
79. Hashimoto T, Hussien R, Oommen S, Gohil K, Brooks GA. Lactate sensitive transcription factor network in L6 cells: activation of MCT1 and mitochondrial biogenesis. FASEB J. (2007) 21:2602–12. doi: 10.1096/fj.07-8174com
80. Lee GH, Kim DS, Chung MJ, Chae SW, Kim HR, Chae HJ. Lysyl oxidase-like-1 enhances lung metastasis when lactate accumulation and monocarboxylate transporter expression are involved. Oncol Lett. (2011) 2:831–8. doi: 10.3892/ol.2011.353
81. Pinheiro C, Miranda-Goncalves V, Longatto-Filho A, Vicente AL, Berardinelli GN, Scapulatempo-Neto C, et al. The metabolic microenvironment of melanomas: Prognostic value of MCT1 and MCT4. Cell Cycle. (2016) 15:1462–70. doi: 10.1080/15384101.2016.1175258
82. Zhao Z, Wu MS, Zou C, Tang Q, Lu J, Liu D, et al. Downregulation of MCT1 inhibits tumor growth, metastasis and enhances chemotherapeutic efficacy in osteosarcoma through regulation of the NF-kappaB pathway. Cancer Lett. (2014) 342:150–8. doi: 10.1016/j.canlet.2013.08.042
83. Payen VL, Hsu MY, Radecke KS, Wyart E, Vazeille T, Bouzin C, et al. Monocarboxylate transporter MCT1 promotes tumor metastasis independently of its activity as a lactate transporter. Cancer Res. (2017) 77:5591–601. doi: 10.1158/0008-5472.CAN-17-0764
84. Le A, Cooper CR, Gouw AM, Dinavahi R, Maitra A, Deck LM, et al. Inhibition of lactate dehydrogenase A induces oxidative stress and inhibits tumor progression. Proc Natl Acad Sci U.S.A. (2010) 107:2037–42. doi: 10.1073/pnas.0914433107
85. Doherty JR, Cleveland JL. Targeting lactate metabolism for cancer therapeutics. J Clin Invest. (2013) 123:3685–92. doi: 10.1172/JCI69741
86. Christoph H, Kathrin R, Marina K, Luca G. Targeting lactate metabolism for cancer immunotherapy - a matter of precision. Semin Cancer Biol. (2022) 88:32–45. doi: 10.1016/j.semcancer.2022.12.001
87. Jinhui Z, Junan Z, Jingfen L, Guangcheng Z, Mengzhan H, Weiming G, et al. A review of lactate-lactylation in Malignancy: its potential in immunotherapy. Front Immunol. (2024) 15:1384948. doi: 10.3389/fimmu.2024.1384948
88. Yasir H I, Celina G-G, Violeta S, Lei H, Kristine T-L, Aleix P, et al. PI3K inhibition impairs BRCA1/2 expression and sensitizes BRCA-proficient triple-negative breast cancer to PARP inhibition. Cancer Discovery. (2012) 2:1036–47. doi: 10.1158/2159-8290.cd-11-0348
89. Salman S, Meyers DJ, Wicks EE, Lee SN, Datan E, Thomas AM, Anders NM, et al. HIF inhibitor 32-134D eradicates murine hepatocellular carcinoma in combination with anti-PD1 therapy. J Clin Invest. (2022) 132:e156774. doi: 10.1172/jci156774
90. Christopher M B, Yan L, Mingyue L, Xuexiang D, Martin D, Pan Z, et al. Targeting HIF-1α abrogates PD-L1-mediated immune evasion in tumor microenvironment but promotes tolerance in normal tissues. J Clin Invest. (2022) 132:e150846. doi: 10.1172/jci150846
91. Na L, Yuqi K, Lingling W, Sarah H, Rachel T, Hui H, et al. ALKBH5 regulates anti-PD-1 therapy response by modulating lactate and suppressive immune cell accumulation in tumor microenvironment. Proc Natl Acad Sci U.S.A. (2020) 117:20159–70. doi: 10.1073/pnas.1918986117
92. Jiayun Y, Bin S, Min L, Wei D, Wen N, Jingyun Y, et al. Irradiated lactic acid-stimulated tumour cells promote the antitumour immunity as a therapeutic vaccine. Cancer Lett. (2019) 469:367–79. doi: 10.1016/j.canlet.2019.11.018
93. Xin L, James W H, Erin P, Xiaoying S, Patricia T, Pingna D, et al. Erratum: Effective combinatorial immunotherapy for castration-resistant prostate cancer. Nature. (2017) 545:116. doi: 10.1038/nature22348
94. Binbin D, Pan Z, Jia T, Hao C, Qi M, Jing L, et al. Sodium bicarbonate nanoparticles for amplified cancer immunotherapy by inducing pyroptosis and regulating lactic acid metabolism. Angew Chem Int Ed Engl. (2023) 62:e202307706. doi: 10.1002/anie.202307706
95. Valvona CJ, Fillmore HL, Nunn PB, Pilkington GJ. The regulation and function of lactate dehydrogenase A: therapeutic potential in brain tumor. Brain Pathol. (2016) 26:3–17. doi: 10.1111/bpa.12299
96. Farabegoli F, Vettraino M, Manerba M, Fiume L, Roberti M, Di Stefano G. Galloflavin, a new lactate dehydrogenase inhibitor, induces the death of human breast cancer cells with different glycolytic attitude by affecting distinct signaling pathways. Eur J Pharm Sci. (2012) 47:729–38. doi: 10.1016/j.ejps.2012.08.012
97. Lea MA, Guzman Y, Desbordes C. Inhibition of growth by combined treatment with inhibitors of lactate dehydrogenase and either phenformin or inhibitors of 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase 3. Anticancer Res. (2016) 36:1479–88.
98. Zdralevic M, Brand A, Di Ianni L, Dettmer K, Reinders J, Singer K, et al. Double genetic disruption of lactate dehydrogenases A and B is required to ablate the "Warburg effect" restricting tumor growth to oxidative metabolism. J Biol Chem. (2018) 293:15947–61. doi: 10.1074/jbc.RA118.004180
99. Boudreau A, Purkey HE, Hitz A, Robarge K, Peterson D, Labadie S, et al. Metabolic plasticity underpins innate and acquired resistance to LDHA inhibition. Nat Chem Biol. (2016) 12:779–86. doi: 10.1038/nchembio.2143
100. Fang A, Zhang Q, Fan H, Zhou Y, Yao Y, Zhang Y, et al. Discovery of human lactate dehydrogenase A (LDHA) inhibitors as anticancer agents to inhibit the proliferation of MG-63 osteosarcoma cells. Medchemcomm. (2017) 8:1720–6. doi: 10.1039/c7md00222j
101. Doherty JR, Yang C, Scott KE, Cameron MD, Fallahi M, Li W, et al. Blocking lactate export by inhibiting the Myc target MCT1 Disables glycolysis and glutathione synthesis. Cancer Res. (2014) 74:908–20. doi: 10.1158/0008-5472.CAN-13-2034
102. Guan X, Rodriguez-Cruz V, Morris ME. Cellular uptake of MCT1 inhibitors AR-C155858 and AZD3965 and their effects on MCT-mediated transport of L-lactate in murine 4T1 breast tumor cancer cells. AAPS J. (2019) 21:13. doi: 10.1208/s12248-018-0279-5
103. Spugnini EP, Sonveaux P, Stock C, Perez-Sayans M, De Milito A, Avnet S, et al. Proton channels and exchangers in cancer. Biochim Biophys Acta. (2015) 1848:2715–26. doi: 10.1016/j.bbamem.2014.10.015
104. Sonveaux P, Vegran F, Schroeder T, Wergin MC, Verrax J, Rabbani ZN, et al. Targeting lactate-fueled respiration selectively kills hypoxic tumor cells in mice. J Clin Invest. (2008) 118:3930–42. doi: 10.1172/JCI36843
105. Polanski R, Hodgkinson CL, Fusi A, Nonaka D, Priest L, Kelly P, et al. Activity of the monocarboxylate transporter 1 inhibitor AZD3965 in small cell lung cancer. Clin Cancer Res. (2014) 20:926–37. doi: 10.1158/1078-0432.CCR-13-2270
106. Sasaki S, Futagi Y, Ideno M, Kobayashi M, Narumi K, Furugen A, et al. Effect of diclofenac on SLC16A3/MCT4 by the Caco-2 cell line. Drug Metab Pharmacokinet. (2016) 31:218–23. doi: 10.1016/j.dmpk.2016.03.004
107. Futagi Y, Kobayashi M, Narumi K, Furugen A, Iseki K. Identification of a selective inhibitor of human monocarboxylate transporter 4. Biochem Biophys Res Commun. (2018) 495:427–32. doi: 10.1016/j.bbrc.2017.10.025
108. Tian L, Shangcong H, Yu Y, Guiming Z. Role of human monocarboxylate transporter 1 (hMCT1) and 4 (hMCT4) in tumor cells and the tumor microenvironment. Cancer Manag Res. (2023) 15:957–75. doi: 10.2147/cmar.s421771
109. Marchiq I, Pouyssegur J. Hypoxia, cancer metabolism and the therapeutic benefit of targeting lactate/H(+) symporters. J Mol Med (Berl). (2016) 94:155–71. doi: 10.1007/s00109-015-1307-x
110. Kim EY, Chung TW, Han CW, Park SY, Park KH, Jang SB, et al. A novel lactate dehydrogenase inhibitor, 1-(Phenylseleno)-4-(Trifluoromethyl) benzene, suppresses tumor growth through apoptotic cell death. Sci Rep. (2019) 9:3969. doi: 10.1038/s41598-019-40617-3
111. Benjamin D, Robay D, Hindupur SK, Pohlmann J, Colombi M, El-Shemerly MY, et al. Dual inhibition of the lactate transporters MCT1 and MCT4 is synthetic lethal with metformin due to NAD+ Depletion in cancer cells. Cell Rep. (2018) 25:3047–3058.e4. doi: 10.1016/j.celrep.2018.11.043
112. Nancolas B, Guo L, Zhou R, Nath K, Nelson DS, Leeper DB, et al. The anti-tumour agent lonidamine is a potent inhibitor of the mitochondrial pyruvate carrier and plasma membrane monocarboxylate transporters. Biochem J. (2016) 473:929–36. doi: 10.1042/BJ20151120
113. Pilon-Thomas S, Kodumudi KN, El-Kenawi AE, Russell S, Weber AM, Luddy K, et al. Neutralization of tumor acidity improves antitumor responses to immunotherapy. Cancer Res. (2016) 76:1381–90. doi: 10.1158/0008-5472.CAN-15-1743
114. Caslin HL, Abebayehu D, Pinette JA, Ryan JJ. Lactate is a metabolic mediator that shapes immune cell fate and function. Front Physiol. (2021) 12:688485. doi: 10.3389/fphys.2021.688485
115. Caslin HL, Abebayehu D, Abdul Qayum A, Haque TT, Taruselli MT, Paez PA, et al. Lactic acid inhibits lipopolysaccharide-induced mast cell function by limiting glycolysis and ATP availability. J Immunol. (2019) 203:453–64. doi: 10.4049/jimmunol.1801005
116. O'Neill LA, Kishton RJ, Rathmell J. A guide to immunometabolism for immunologists. Nat Rev Immunol. (2016) 16:553–65. doi: 10.1038/nri.2016.70
117. Hoque R, Farooq A, Ghani A, Gorelick F, Mehal WZ. Lactate reduces liver and pancreatic injury in Toll-like receptor- and inflammasome-mediated inflammation via GPR81-mediated suppression of innate immunity. Gastroenterology. (2014) 146:1763–74. doi: 10.1053/j.gastro.2014.03.014
118. Gottfried E, Kunz-Schughart LA, Ebner S, Mueller-Klieser W, Hoves S, Andreesen R, et al. Tumor-derived lactic acid modulates dendritic cell activation and antigen expression. Blood. (2006) 107:2013–21. doi: 10.1182/blood-2005-05-1795
119. Nasi A, Fekete T, Krishnamurthy A, Snowden S, Rajnavolgyi E, Catrina AI, et al. Dendritic cell reprogramming by endogenously produced lactic acid. J Immunol. (2013) 191:3090–9. doi: 10.4049/jimmunol.1300772
120. Selleri S, Bifsha P, Civini S, Pacelli C, Dieng MM, Lemieux W, et al. Human mesenchymal stromal cell-secreted lactate induces M2-macrophage differentiation by metabolic reprogramming. Oncotarget. (2016) 7:30193–210. doi: 10.18632/oncotarget.8623
121. Abebayehu D, Spence AJ, Caslin H, Taruselli M, Haque TT, Kiwanuka KN, et al. Lactic acid suppresses IgE-mediated mast cell function in vitro and in vivo. Cell Immunol. (2019) 341:103918. doi: 10.1016/j.cellimm.2019.04.006
122. Huffaker TB, O'Connell RM. miR-155-SOCS1 as a functional axis: satisfying the burden of proof. Immunity. (2015) 43:3–4. doi: 10.1016/j.immuni.2015.06.020
123. Lind EF, Millar DG, Dissanayake D, Savage JC, Grimshaw NK, Kerr WG, et al. miR-155 upregulation in dendritic cells is sufficient to break tolerance in vivo by negatively regulating SHIP1. J Immunol. (2015) 195:4632–40. doi: 10.4049/jimmunol.1302941
124. Syed M, Kammala AK, Callahan B, Oskeritzian CA, Subramanian H. Lactic acid suppresses MRGPRX2 mediated mast cell responses. Cell Immunol. (2021) 368:104422. doi: 10.1016/j.cellimm.2021.104422
125. Watson MJ, Vignali PDA, Mullett SJ, Overacre-Delgoffe AE, Peralta RM, Grebinoski S, et al. Metabolic support of tumour-infiltrating regulatory T cells by lactic acid. Nature. (2021) 591:645–51. doi: 10.1038/s41586-020-03045-2
126. Madaan A, Nadeau-Vallee M, Rivera JC, Obari D, Hou X, Sierra EM, et al. Lactate produced during labor modulates uterine inflammation via GPR81 (HCA1). Am J Obstet Gynecol. (2017) 216:60 e1–60 e17. doi: 10.1016/j.ajog.2016.09.072
127. Errea A, Cayet D, Marchetti P, Tang C, Kluza J, Offermanns S, et al. Lactate inhibits the pro-inflammatory response and metabolic reprogramming in murine macrophages in a GPR81-independent manner. PLoS One. (2016) 11:e0163694. doi: 10.1371/journal.pone.0163694
128. Jha AK, Huang SC, Sergushichev A, Lampropoulou V, Ivanova Y, Loginicheva E, et al. Network integration of parallel metabolic and transcriptional data reveals metabolic modules that regulate macrophage polarization. Immunity. (2015) 42:419–30. doi: 10.1016/j.immuni.2015.02.005
129. Rajendran P, Chen YF, Chen YF, Chung LC, Tamilselvi S, Shen CY, et al. The multifaceted link between inflammation and human diseases. J Cell Physiol. (2018) 233:6458–71. doi: 10.1002/jcp.26479
130. Mendler AN, Hu B, Prinz PU, Kreutz M, Gottfried E, Noessner E. Tumor lactic acidosis suppresses CTL function by inhibition of p38 and JNK/c-Jun activation. Int J Cancer. (2012) 131:633–40. doi: 10.1002/ijc.26410
131. Fischbeck AJ, Ruehland S, Ettinger A, Paetzold K, Masouris I, Noessner E, et al. Tumor lactic acidosis: protecting tumor by inhibiting cytotoxic activity through motility arrest and bioenergetic silencing. Front Oncol. (2020) 10:589434. doi: 10.3389/fonc.2020.589434
132. Haas R, Smith J, Rocher-Ros V, Nadkarni S, Montero-Melendez T, D'Acquisto F, et al. Lactate regulates metabolic and pro-inflammatory circuits in control of T cell migration and effector functions. PLoS Biol. (2015) 13:e1002202. doi: 10.1371/journal.pbio.1002202
133. Pucino V, Certo M, Bulusu V, Cucchi D, Goldmann K, Pontarini E, et al. Lactate buildup at the site of chronic inflammation promotes disease by inducing CD4(+) T cell metabolic rewiring. Cell Metab. (2019) 30:1055–1074.e8. doi: 10.1016/j.cmet.2019.10.004
134. Souto-Carneiro MM, Klika KD, Abreu MT, Meyer AP, Saffrich R, Sandhoff R, et al. Effect of increased lactate dehydrogenase A activity and aerobic glycolysis on the proinflammatory profile of autoimmune CD8+ T cells in rheumatoid arthritis. Arthritis Rheumatol. (2020) 72:2050–64. doi: 10.1002/art.41420
135. Cheng WC, Hughes FJ, Taams LS. The presence, function and regulation of IL-17 and Th17 cells in periodontitis. J Clin Periodontol. (2014) 41:541–9. doi: 10.1111/jcpe.12238
136. Nareika A, He L, Game BA, Slate EH, Sanders JJ, London SD, et al. Sodium lactate increases LPS-stimulated MMP and cytokine expression in U937 histiocytes by enhancing AP-1 and NF-kappaB transcriptional activities. Am J Physiol Endocrinol Metab. (2005) 289:E534–42. doi: 10.1152/ajpendo.00462.2004
137. Samuvel DJ, Sundararaj KP, Nareika A, Lopes-Virella MF, Huang Y. Lactate boosts TLR4 signaling and NF-kappaB pathway-mediated gene transcription in macrophages via monocarboxylate transporters and MD-2 up-regulation. J Immunol. (2009) 182:2476–84. doi: 10.4049/jimmunol.0802059
138. Penny HL, Sieow JL, Adriani G, Yeap WH, See Chi Ee P, San Luis B, et al. Warburg metabolism in tumor-conditioned macrophages promotes metastasis in human pancreatic ductal adenocarcinoma. Oncoimmunology. (2016) 5:e1191731. doi: 10.1080/2162402X.2016.1191731
139. Paolini L, Adam C, Beauvillain C, Preisser L, Blanchard S, Pignon P, et al. Lactic acidosis together with GM-CSF and M-CSF induces human macrophages toward an inflammatory protumor phenotype. Cancer Immunol Res. (2020) 8:383–95. doi: 10.1158/2326-6066.CIR-18-0749
140. Dietl K, Renner K, Dettmer K, Timischl B, Eberhart K, Dorn C, et al. Lactic acid and acidification inhibit TNF secretion and glycolysis of human monocytes. J Immunol. (2010) 184:1200–9. doi: 10.4049/jimmunol.0902584
141. Abebayehu D, Spence AJ, Qayum AA, Taruselli MT, McLeod JJ, Caslin HL, et al. Lactic Acid Suppresses IL-33-Mediated Mast Cell Inflammatory Responses via Hypoxia-Inducible Factor-1alpha-Dependent miR-155 Suppression. J Immunol. (2016) 197:2909–17. doi: 10.4049/jimmunol.1600651
142. Imtiyaz HZ, Simon MC. Hypoxia-inducible factors as essential regulators of inflammation. Curr Top Microbiol Immunol. (2010) 345:105–20. doi: 10.1007/82_2010_74
Keywords: lactate, tumor, inflammatory, diseases, therapy
Citation: Liu H, Pan M, Liu M, Zeng L, Li Y, Huang Z, Guo C and Wang H (2024) Lactate: a rising star in tumors and inflammation. Front. Immunol. 15:1496390. doi: 10.3389/fimmu.2024.1496390
Received: 14 September 2024; Accepted: 04 November 2024;
Published: 26 November 2024.
Edited by:
Eva Reali, University of Ferrara, ItalyReviewed by:
Khalid Omer Alfarouk, Alfarouk Biomedical Research LLC, United StatesCopyright © 2024 Liu, Pan, Liu, Zeng, Li, Huang, Guo and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hui Wang, d2FuZ2h1aUB4eG11LmVkdS5jbg==; Chunlei Guo, Y2h1bmxlaWVyQDE2My5jb20=
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.