
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Immunol. , 29 November 2024
Sec. Cancer Immunity and Immunotherapy
Volume 15 - 2024 | https://doi.org/10.3389/fimmu.2024.1495233
 Chiara Bernardi1,2*
Chiara Bernardi1,2* Yan Beauverd1
Yan Beauverd1 Thien An Tran1
Thien An Tran1 Marie Maulini1
Marie Maulini1 Maria Mappoura1
Maria Mappoura1 Sarah Morin1
Sarah Morin1 Federico Simonetta1,2
Federico Simonetta1,2 Anne Cairoli3
Anne Cairoli3 Holger W. Auner3
Holger W. Auner3 Kaveh Samii1
Kaveh Samii1 Yves Chalandon1
Yves Chalandon1 Carmen de Ramon Ortiz1*
Carmen de Ramon Ortiz1*Plasma cell leukemia (PCL) is an aggressive and high-risk variant of multiple myeloma (MM) with a very poor prognosis. Given its rarity and aggressiveness, there is a lack of clinical trials testing the efficacity of novel therapies in these patients. New immune approaches such as B-cell maturation antigen (BCMA) and G protein-coupled receptor, family C, group 5, member D (GPRC5D) -targeting agents, including chimeric antigen receptor (CAR) T-cells and bispecific antibodies could play a role in PCL treatment. However, PCL patients were excluded from recent pivotal clinical trials testing those agents and only some case reports have been published. We present here the clinical course of a patient with relapsed/refractory (R/R) primary (p) PCL who was treated with anti-BCMA and anti-GPRC5D bispecific antibodies at our center.
Plasma cell leukemia (PCL) is a rare and aggressive disease defined by the presence of 5% or more circulating plasma cells in peripheral blood smears in patients otherwise diagnosed with multiple myeloma (MM) (1). PCL is further classified as primary (pPCL; 60% of cases) when occurring de novo or secondary (sPCL; 40% of cases) when it represents a transformation from a previously known plasma cell disorder (1). Patients with pPCL are younger than MM patients and often have a higher tumor burden, extramedullary involvement, high levels of LDH, cytopenias and poor-risk cytogenetic abnormalities (2). Given the rarity and aggressiveness of the disease, there is a lack of clinical trials testing the efficacity of novel therapies in these patients. Only some trials, such as the GMMG-CONCEPT, include patients with pPCL (3). Currently, there is no established standard of care. The survival of patients with pPCL has only mildly improved in the last decade with the use of novel agents, such as immunomodulators, proteasome inhibitors, monoclonal antibodies targeting CD38 in conjunction with stem cell transplantation but its prognosis remains very poor (1, 4, 5). New immune approaches such as chimeric antigen receptor (CAR) T-cells and bispecific antibodies could play a role in PCL treatment. However, PCL patients were excluded from recent pivotal clinical trials testing those agents and only some case reports have been published (6, 7). A phase 1 study shows a potential benefit of CAR-T cell in patients with pPCL with a short duration of response (6). However, given the time needed for CAR-T cell manufacturing, the disease often progresses too quickly to allow for CAR-T cells delivery. Bispecific antibodies have the advantage of being off-the-shelf, potentially representing a more interesting treatment option for these patients. Here we present the clinical course of a patient with pPCL who was treated with BCMA and GPRC5D bispecific antibodies.
A 40-year-old male presented with bone pain. Initial laboratory investigations (Oct-21) showed severe anemia with hemoglobin at 7.6 g/dL and thrombopenia at 16 G/l. White blood cells count was at 15.1 G/l with 47% circulating plasma cells, hypercalcemia, elevated LDH and β2-microglobulin levels. Serum protein electrophoresis showed a γ- and - β migrating paraprotein quantified at 26.6 g/l with a monoclonal IgA lambda at serum protein immunofixation (IgA level of 31.6 g/l and free lambda chains (FLC) at 6730 mg/l). Bone marrow (BM) aspirate showed an infiltration by plasma cells constituting 80%. Chromosomal karyotyping revealed high-risk cytogenetics with complex karyotype and loss of 17p13.1 region. FISH analysis revealed t(14;16). PET-CT imaging showed increased gastric and BM activity. Histology of a gastric biopsy confirmed gastric infiltration by monoclonal plasma cells. Lumbar punction was negative. The patient was diagnosed with pPCL. He received induction therapy consisting of 4 cycles of KRD therapy (carfilzomib; lenalidomide and dexamethasone) (8), with addition of weekly subcutaneous (SC) 1800mg Daratumumab to the cycle 3 and 4, after approval of insurance reimbursement (5). After cycle 1, the patient achieved a very good partial response (VGPR) (9), but he quickly progressed after 4 cycles (Figure 1). Second line therapy with VP-DPACE was started (Feb-22) (dexamethasone, cisplatin, doxorubicin, cyclophosphamide, etoposide; bortezomib and pomalidomide) (10), resulting in VGPR after 2 cycles, followed by tandem autologous hematopoietic stem cell transplant (HSCT) after a melphalan based-regimen (200mg/m2) and maintenance therapy with Pomalidomide from day +30. At day 100 post-second autologous HSCT (Dec-22), he experimented a biochemical progression. He received one cycle of PVD (OPTIMISMM protocol: bortezomib, dexamethasone and pomalidomide) (11), but was refractory with rapidly progressive disease. Fourth line treatment with Elranatamab, an anti-BCMA bispecific antibody, was started (Feb-23) (SC Elranatamab 76mg once weekly in a 28-d cycle). After two step-up priming doses of 12mg and 32mg given on day 1 and day 4 (12), he presented a grade 2 CRS (cytokine release syndrome) and grade 4 neutropenia, requiring treatment with IV Tocilizumab 8mg/kg. grade 1 CRS and hematologic toxicity grade 4 was observed after the second step-up dose. CR was achieved after 1 cycle of treatment of Elranatamab and a total of 5 cycles were administered. Patient had an ECOG of 0 and travelled regularly. Monthly intravenous immunoglobulin replacement and antibiotic prophylaxis with trimetoprim-sulphametoxazol and valaciclovir 500mg bid were administered throughout treatment. After cycle 5 (Jul-23), a biochemical progression was observed, with a significant rise in FLC and a BM aspirate showing a 20% infiltration with monoclonal plasma cells. A 5th line was started with a GPRC5D bispecific antibody, Talquetamab (Sept-23) (13). During the first cycle (SC, ramp up doses of 0.01 mg/kg at day 1, 0.06 mg/kg at day 3, 0.4 mg/kg at day 5 and 7) patient presented grade 1 CRS. Because of persistent fever, a dose of IV Tocilizumab 8mg/kg was administered at day 8. After 2 cycles of Talquetamab (Oct-23), the patient presented an explosive relapse (Figure 2), with a rapid rise in FLC and clinical and radiologic relapse with multiple bone lesions on PET-CT. Palliative care was offered to the patient and he died 3 weeks later.
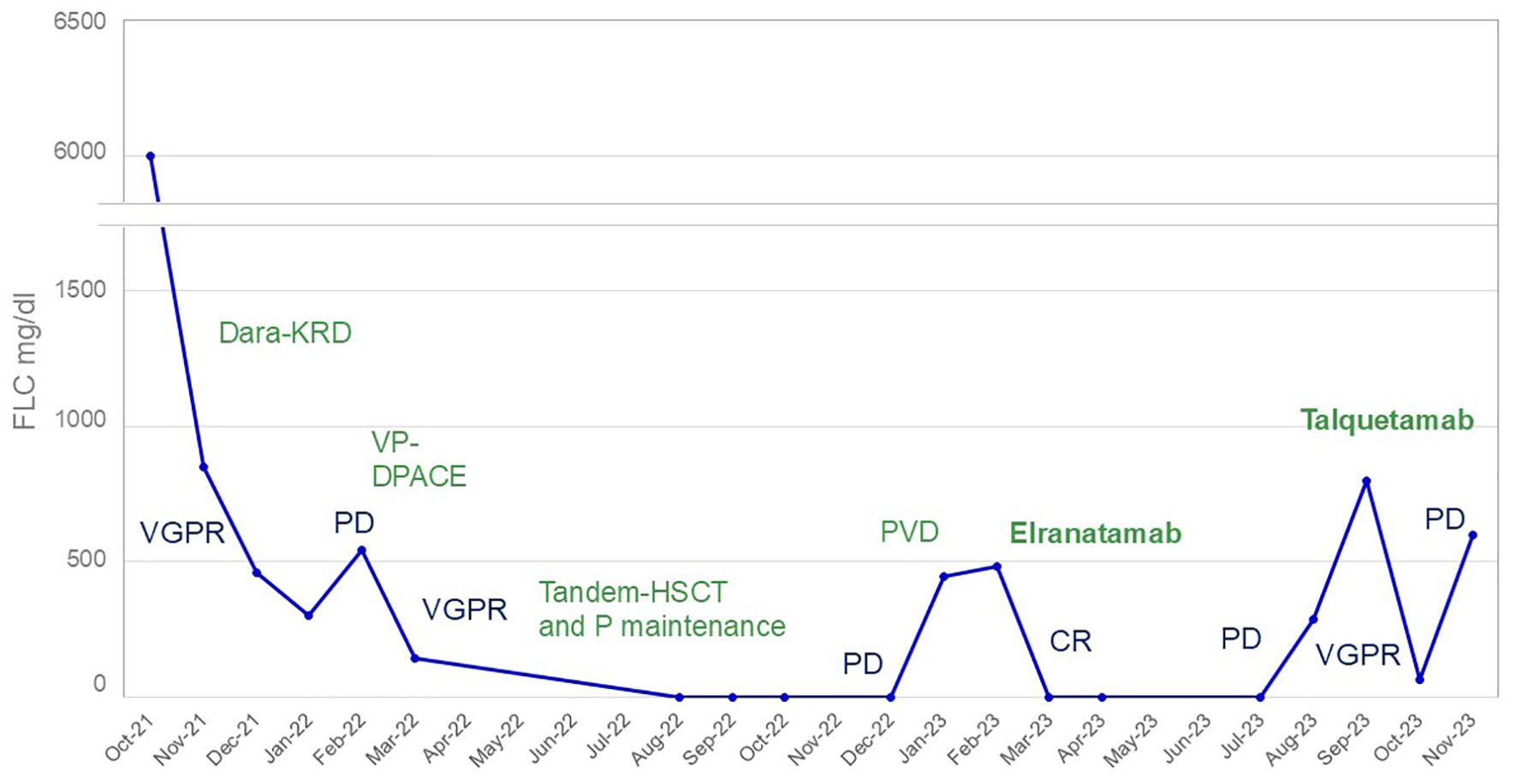
Figure 1. Free lambda light chain evolution during treatment. In blue: response to treatment; in green: treatment. Dara-KRD, daratumumab, carfilzomib, lenalidomide and dexamethasone; HSCT, hematopoietic stem cell transplantation; P, pomalidomide; PVD, pomalidomide, bortezomib, dexamethasone; VP-DPACE, dexamethasone, pomalidomide, cisplatin, doxorubicin, cyclophosphamide, bortezomib and etoposide; CR, complete response; PD, progressive disease; VGPR, very good partial response.
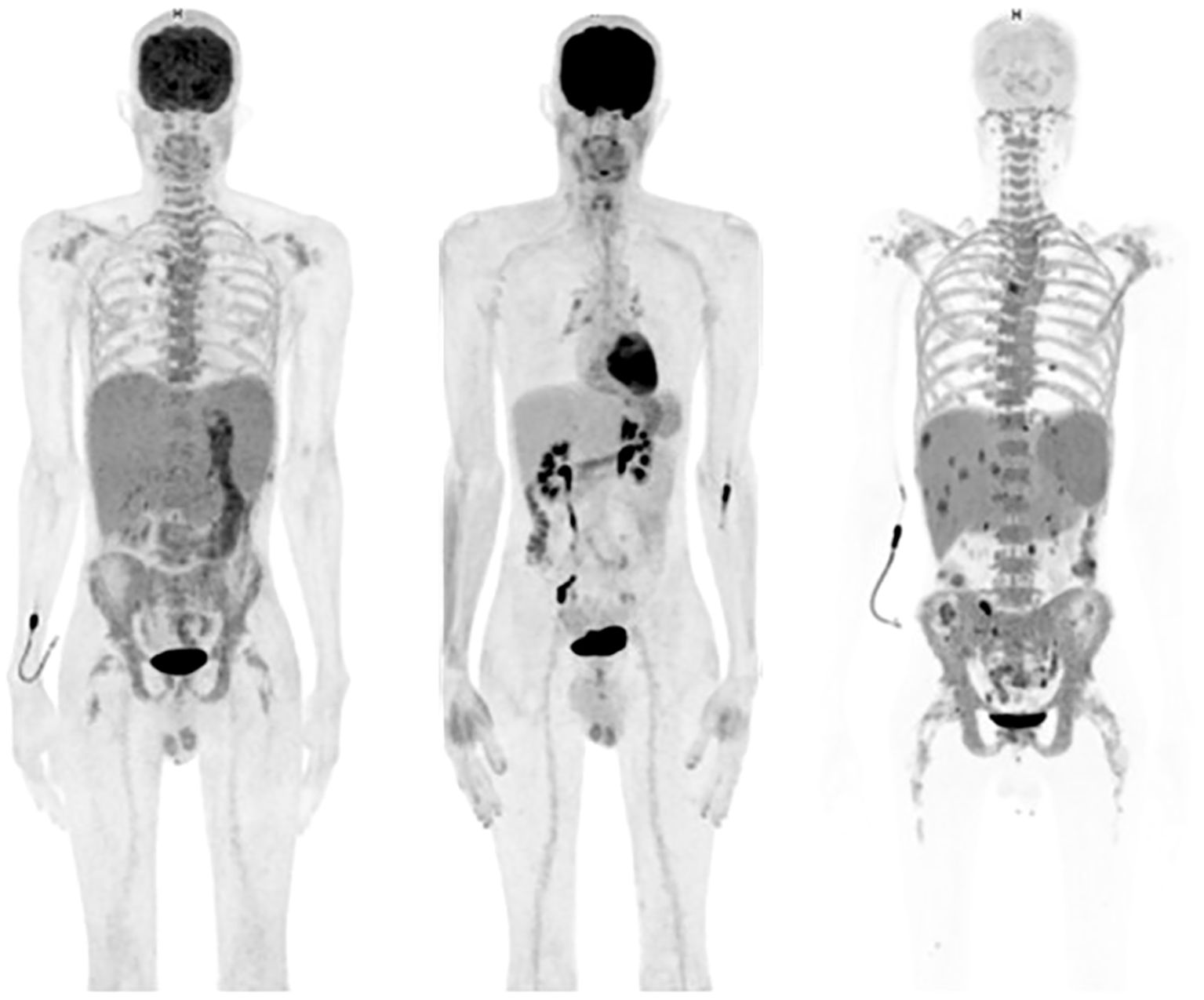
Figure 2. Left: initial positron emission tomography/computed tomography (PET/CT) imaging showing hepatosplenomegaly, a diffuse hypermetabolism of the bone marrow and the stomach; middle: PET/CT at day 100 after tandem HSCT showing no pathological hypermetabolism; right (patient in hypoglycemia): PET/CT at the moment of the last progression after Talquetamab showing an explosive disease with hypermetabolic hepatosplenomegaly and multiple hypermetabolic lesions in the liver and spleen and hypermetabolism of the axial skeleton.
During the last few years, the outcome of MM patients has dramatically improved with the addition of novel agents. However, this is not the case for pPCL, and early relapses are still observed and prognosis remains dismal (2). Novel T cell-engaging immunotherapies, such as bispecific antibodies and CAR T-cell have not yet been tested in PCL patients. Our experience evidences the potential feasibility of bispecific antibodies use in this disease with no limiting toxicities and a clear advantage of off-the-shelf in this rapidly progressive and aggressive disease. Our case experienced a very quick achievement of response, after only 1 cycle, and allowed the patient an improved quality of life. However, duration of response was short, 5 months on Elranatamab and 2 months on Talquetamab. Potential strategies to increase duration of response could be to use bispecific antibodies at earlier lines of therapies or as a maintenance therapy after HSCT. Bispecific antibodies could also represent an interesting bridging therapy before anti-BCMA CAR T cells, especially Talquetamab given its different antigen target. Given the extremely poor prognosis of PCL and the lack of data in this setting, prospective clinical trials for PCL patients are needed.
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
Written informed consent was obtained from the individual for the publication of any potentially identifiable images or data included in this article.
CB: Writing – original draft, Writing – review & editing. YB: Writing – review & editing. TT: Writing – review & editing. MM: Writing – review & editing. MM: Writing – review & editing. SM: Writing – review & editing. FS: Writing – review & editing. AC: Writing – review & editing. HA: Writing – review & editing. KS: Writing – review & editing. YC: Writing – review & editing. CD: Writing – original draft, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. Open access funding by University of Geneva.
FS: institutional consulting fees from BMS/Celgene, Incyte, Kite/Gilead; speaker fees from Kite/Gilead, Incyte; travel support from Kite/Gilead, Novartis, AstraZeneca, Neovii, Janssen; research funding from Kite/Gilead, Novartis, BMS/Celgene. CB: research Grant from BMS/Celgene. HA received speaker fees from Pfizer and BMS/Celgene. YC: YC has received instituional consulting fees for advisory board from MSD, Novartis, Incyte, BMS, Pfizer, Abbvie, Roche, Jazz, Gilead, Amgen, Astra-Zeneca, Servier, Takeda, Pierre Fabre, Medac; Travel support from MSD, Roche, Novartis, Pfizer, BMS, Gilead, Amgen, Incyte, Abbvie, Janssen, Astra-Zeneca, Jazz, Pierre Fabre, Sanofi all via the institution.
The remaining author(s) declare(s) that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Fernández De Larrea C, Kyle R, Rosiñol L, Paiva B, Engelhardt M, Usmani S, et al. Primary plasma cell leukemia: consensus definition by the International Myeloma Working Group according to peripheral blood plasma cell percentage. Blood Cancer J. (2021) 11:192. doi: 10.1038/s41408-021-00587-0
2. Van De Donk NWCJ. How we manage newly diagnosed multiple myeloma with circulating tumor cells. J Clin Oncol. (2023) 41:1342–9. doi: 10.1200/JCO.22.02114
3. Leypoldt LB, Besemer B, Asemissen AM, Hänel M, Blau IW, Görner M, et al. Isatuximab, carfilzomib, lenalidomide, and dexamethasone (Isa-KRd) in front-line treatment of high-risk multiple myeloma: interim analysis of the GMMG-CONCEPT trial. Leukemia. (2022) 36:885–8. doi: 10.1038/s41375-021-01431-x
4. Gonsalves WI, Rajkumar SV, Go RS, Dispenzieri A, Gupta V, Singh PP, et al. Trends in survival of patients with primary plasma cell leukemia: a population-based analysis. Blood. (2014) 124:907–12. doi: 10.1182/blood-2014-03-565051
5. Parrondo R, Alhaj Moustafa M, Reeder CB, Sher T, Roy V, Warsame RM, et al. Efficacy of daratumumab (Dara)-based regimens for the treatment of plasma cell leukemia (PCL). Blood. (2020) 136:29–30. doi: 10.1182/blood-2020-139559
6. Li C, Cao W, Que Y, Wang Q, Xiao Y, Gu C, et al. A phase I study of anti-BCMA CAR T cell therapy in relapsed/refractory multiple myeloma and plasma cell leukemia. Clin Transl Med. (2021) 11:e346. doi: 10.1002/ctm2.v11.3
7. Deng J, Lin Y, Zhao D, Tong C, Chang AH, Chen W, et al. Case report: Plasma cell leukemia secondary to multiple myeloma successfully treated with anti-BCMA CAR-T cell therapy. Front Oncol. (2022) 12:901266. doi: 10.3389/fonc.2022.901266
8. Van De Donk NWCJ, van der Holt B, Schjesvold FH, Wu KL, Spada S, Broyl A, et al. Treatment of primary plasma cell leukemia with carfilzomib and lenalidomide-based therapy: results of the first interim analysis of the phase 2 EMN12/HOVON129 study. Blood. (2019) 134:693–3. doi: 10.1182/blood-2019-125120
9. Kumar S, Paiva B, Anderson KC, Durie B, Landgren O, Moreau P, et al. International Myeloma Working Group consensus criteria for response and minimal residual disease assessment in multiple myeloma. Lancet Oncol. (2016) 17:e328–46. doi: 10.1016/S1470-2045(16)30206-6
10. Ghandili S, Alihodzic D, Wiessner C, Bokemeyer C, Weisel K, Leypoldt LB. VTd-PACE and VTd-PACE-like regimens are effective salvage therapies in difficult-to-treat relapsed/refractory multiple myeloma: a single-center experience. Ann Hematol. (2023) 102:117–24. doi: 10.1007/s00277-022-05027-y
11. Richardson PG, Oriol A, Beksac M, Liberati AM, Galli M, Schjesvold F, et al. Pomalidomide, bortezomib, and dexamethasone for patients with relapsed or refractory multiple myeloma previously treated with lenalidomide (OPTIMISMM): a randomised, open-label, phase 3 trial. Lancet Oncol. (2019) 20:781–94. doi: 10.1016/S1470-2045(19)30152-4
12. Lesokhin AM, Tomasson MH, Arnulf B, Bahlis NJ, Miles Prince H, Niesvizky R, et al. Elranatamab in relapsed or refractory multiple myeloma: phase 2 MagnetisMM-3 trial results. Nat Med. (2023) 29:2259–67. doi: 10.1038/s41591-023-02528-9
Keywords: bispecific Ab, plasma cell leukemia (PCL), BCMA, GPRC5D, elranatamab, talquetamab
Citation: Bernardi C, Beauverd Y, Tran TA, Maulini M, Mappoura M, Morin S, Simonetta F, Cairoli A, Auner HW, Samii K, Chalandon Y and de Ramon Ortiz C (2024) Anti-BCMA and GPRC5D bispecific antibodies in relapsed/refractory primary plasma cell leukemia: a case report. Front. Immunol. 15:1495233. doi: 10.3389/fimmu.2024.1495233
Received: 12 September 2024; Accepted: 14 November 2024;
Published: 29 November 2024.
Edited by:
Nicola Giuliani, University of Parma, ItalyReviewed by:
Catarina Geraldes, Centro Hospitalar e Universitário de Coimbra, PortugalCopyright © 2024 Bernardi, Beauverd, Tran, Maulini, Mappoura, Morin, Simonetta, Cairoli, Auner, Samii, Chalandon and de Ramon Ortiz. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chiara Bernardi, Y2hpYXJhLmJlcm5hcmRpQGhjdWdlLmNo; Carmen de Ramon Ortiz, Q2FybWVuSnVsaWEuZGVSYW1vbk9ydGl6QGhjdWdlLmNo
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.