
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Immunol., 26 November 2024
Sec. Cancer Immunity and Immunotherapy
Volume 15 - 2024 | https://doi.org/10.3389/fimmu.2024.1494138
This article is part of the Research TopicClinical Implementation of Precision Oncology Data to Direct Individualized and Immunotherapy-Based Treatment StrategiesView all 17 articles
 Haijuan Yu1†
Haijuan Yu1† Jie Lin1†
Jie Lin1† Jian Chen1
Jian Chen1 Lijun Chen1
Lijun Chen1 Jianping Zou1
Jianping Zou1 Bin Liu1
Bin Liu1 Dan Hu2
Dan Hu2 Youping Xiao3
Youping Xiao3 Linhao Yu2
Linhao Yu2 Yang Sun1*
Yang Sun1*The outcome of patients with recurrent/metastatic cervical cancer (R/M CC) is poor, with a 5-year survival rate of only 10%–20%. Recent advances in immunotherapy renewed its interest in R/M CC treatment. It has been suggested that cadonilimab, a novel bispecific antibody targeting programmed death 1 (PD-1) and cytotoxic T-lymphocyte antigen-4 (CTLA-4), significantly improved the survival outcomes of the R/M CC. In the present study, we reported a programmed death ligand 1 (PD-L1) and human epidermal growth factor receptor 2 (HER-2) positive CC case at stage IV who was treated with cadonilimab and achieved a surprising radiographic complete response (CR) for 10 months, even in the PD-L1 negative metastatic site. Demographic, clinical, histopathological, laboratory, treatment regime and imaging data were recorded. Unfortunately, the patient progressed rapidly during maintenance therapy when cadonilimab was replaced by sintilimab, the monoclonal antibody against PD-1, indicating the more powerful anti-tumor activity of dual blockade immunotherapy. To conclude, cadonilimab offers a promising and effective therapeutic approach for R/M CC. Notably, HER-2 is also expected to be a new reference target for cadonilimab therapy.
Cervical cancer (CC) is one of the most common cancers in the female reproductive system worldwide (1). with an estimated 661,021 new cases and 348,189 deaths in 2022. China accounts for 22.8% of the worldwide incidence and 16.0% of CC-related mortality (2). Treatment approaches and outcomes for CC patients are highly dependent on the disease stage at diagnosis. The five-year survival rate of patients with early-stage CC is above 90%. However, it dramatically drops to less than 20% in recurrent or metastatic CC (R/M CC) (3, 4), resulting in the median overall survival (OS) of 16.8 months (5–7). Therefore, new therapeutic options for R/M CC patients are desperately needed in the first as well as later lines (8, 9).
Immunotherapy has become a novel treatment option for patients with R/M CC. In the past decade, multiple clinical trials investigated the efficacy of immune checkpoint inhibitors (ICIs) (10), such as pembrolizumab, balstilimab, and nivolumab with objective response rates (ORRs) of 12%∼26% in second line-treatment for R/M CC (11–13). Keynote-826 trial showed pembrolizumab and chemotherapy with or without bevacizumab, as first-line therapy, resulted in significantly longer progression-free survival (PFS) and OS for the programmed death ligand 1 (PD-L1) combined positive score (CPS) ≥1 R/M CC (14, 15). However, the benefits were limited for patients with PD-L1-negative (16). The dual blockade immunotherapy to improve the efficacy of programmed death 1 (PD-1) monotherapy has been widely investigated (17, 18). For example, SHR-1701, a bifunctional antibody composed of the anti-PD-L1 agent and extracellular domain of the transforming growth factor-beta II (TGF-βII) receptor, only achieved an ORR of 15.6% in R/M CC patients based on NCT05179239 trial (19). However, dual blockade immunotherapy has been limited by severe toxicities (20).
Faced with such a dilemma, cadonilimab, a first-in-class bi-specific antibody targeting PD-1 and cytotoxic T lymphocyte antigen 4 (CTLA-4), has emerged to improve the prognosis of patients with R/M CC. Cadonilimab with crystalline fragments not only enhances the antitumor activity but also mutates to eliminate the Fc receptor and complement-mediated cytotoxic effects (21). Results from AK104-201 showed an inspiring ORR of 31.3% (31/99) [ORR PD-L1 positive = 43.8% (28/64); ORR PD-L1 negative = 16.7% (3/18)] (22) in the R/M CC who had failed previous platinum-containing chemotherapy with a low incidence of grade ≥3 immune-related adverse events (irAEs). Based on these results, cadonilimab was approved by China’s National Medical Products Administration for R/M CC as a second-line treatment in June 2022 (23).
Historically, disseminated cancers had a lower probability of a complete response (CR) even with high-intensity comprehensive treatments. However, combining immunotherapy and other comprehensive treatments could bring new hopes and clinical benefits to late-stage and metastatic disease. Our study presented a metastatic CC patient who obtained CR for 10 months with cadonilimab treatment. Furthermore, the toxicities associated with this salvage treatment were tolerable. This case aims to serve as a reference for treating R/M CC patients with similar presentations. Additionally, studies investigating the mechanisms underlying the promising therapeutic regimens need to be conducted in greater depth in the near future.
A 55-year-old postmenopausal treatment-naive female was hospitalized for two-month persistent irregular vaginal bleeding. No genetic, family or psychosocial history was reported. A cervical tumor biopsy was performed and examined by two experienced pathologists. Then, she was diagnosed with cervical adenosquamous carcinoma. Immunohistochemistry (IHC) of the cervical tumor showed PD-L1(28-8) (CPS=10) and human epidermal growth factor receptor 2 (HER-2) (3+). Other positive biomarkers included Ki-67 (70%+), P16 (+), CK5/6 (+/-) (Figure 1A). The positron emission tomography-computed tomography (PET/CT), which was evaluated by two experience radiologists, revealed a cervical mass with multiple lymph node metastases such as bilateral clavicular region, right internal mammary, mediastinum, left hilus, supradiaphragmatic region, abdominal cavity, retroperitoneum, bilateral pelvic cavity, and bilateral groin. Stage IVB was confirmed according to the 2018 International Federation of Gynecology and Obstetrics (FIGO) Cervical Cancer Staging Guidelines. (Figure 2A). To confirm tumor homogeneity, we also conducted a left supraclavicular lymph node biopsy, indicating squamous cell carcinomas (SCC) with PD-L1 (28-8) (CPS=0), and HER-2 (1+). Interestingly, unlike her primary cervical lesions, lymph node metastases did not include any adenocarcinoma component and showed PD-L1 (-) and HER-2 (1+) (Figure 1B).
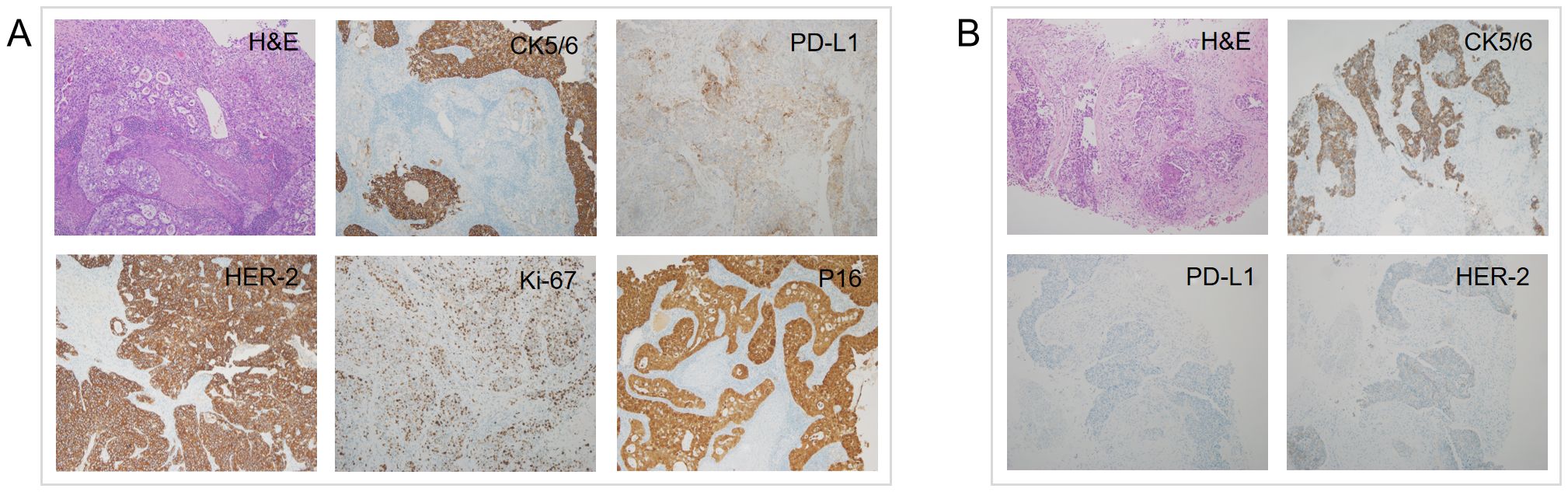
Figure 1. H&E and IHC of the patient’s cervix (A) and left supraclavicular lymph node (B) biopsy pathology (H&E staining, ×100; IHC, ×100). H&E, Hematoxylin and eosin stain; IHC, immunohistochemistry.
Considering that the patient had a cervical tumor with PD-L1 (+) and lymph node metastases with PD-L1(-), cadonilimab was chosen as the best treatment option. Hence, the patient was recommended the following regimen: taxol (175 mg/m²) on day 1, cisplatin (60 mg/m²) on day 1, and cadonilimab (10 mg/kg) on day 2, with an interval of 21 days for six cycles. Then, bevacizumab (15mg/kg) was added at the second cycle. Additionally, local radical radiotherapy was also performed during the treatment. The detailed radiation plan showed below: 1) The 47.25 Gy/27F for volumetric modulated arc therapy (VMAT) in the whole uterus, the vagina, the part of the parametrium, the pelvic lymph node, and the retroperitoneal lymph node; 2) The 45.90 Gy/27F for bilateral inguinal lymphatic area; 3) The 47.60 Gy/28F for additional irradiation of retroperitoneal lymph nodes; 4) Four fractions brachytherapy (27.5165 Gy). The patient’s abdomen magnetic resonance imaging (MRI) and chest computed tomography (CT), which were performed one month after radiotherapy, indicated CR without any signs of tumor. After chemotherapy and radiotherapy were all finished, maintenance therapies with bevacizumab and cadonilimab were continued for five cycles. It is worth mentioning that the patient did not experience any adverse events (AEs) (24) of grade 3-4 during this salvage treatment. The trend of mass change and the fluctuation of SCC are shown in Figure 2B, Figures 3A, B, respectively.
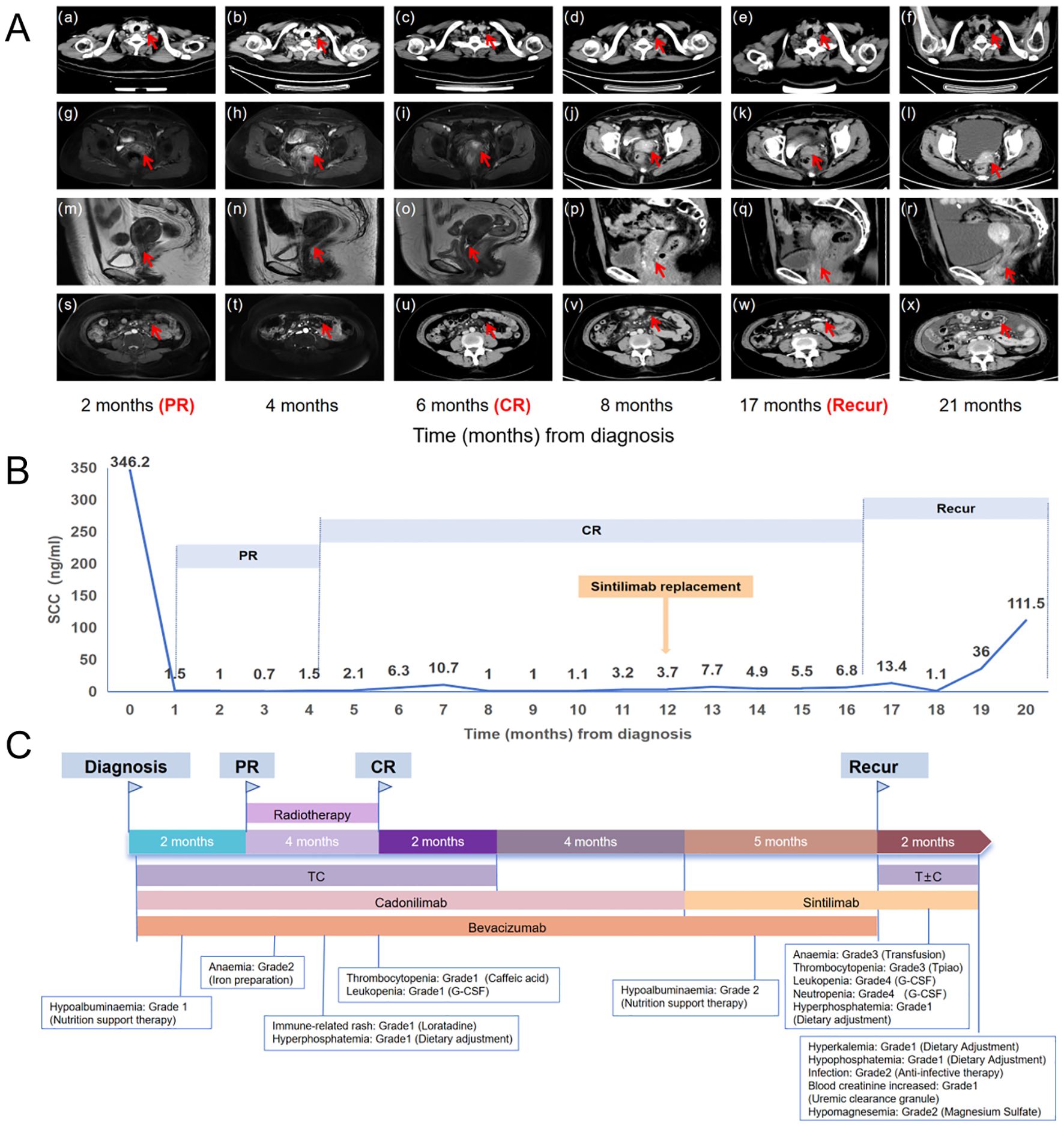
Figure 3. (A) The trend of lesion changes during comprehensive treatment. (a-f) The red arrow in the chest CT indicates the left supraclavicular lymph node. (g-i, m-o) The arrow in the MRI indicates the cervical mass. (j-l, p-r) The arrow in the CT indicates the cervical mass. (s, t) The arrow in the MRI indicates the celiac lymph nodes. (u-x) The arrow in the CT indicates the celiac lymph nodes. (B) SCC antigen levels during treatment (reference range: 0–1.5 ng/ml). (C) The treatment timeline for the patient. T, taxol; C, cisplatin; PR, partial response; CR, complete remission.
After five cycles of maintenance therapy, cadonilimab was replaced by sintilimab due to patient’s financial constraints. Unfortunately, we observed a recurrence in abdomen CT with large ascites, enlarged abdominal lymph nodes, and thickened peritoneum after 5 cycles of maintenance therapy with sintilimab. Whereupon the patient received taxol + carboplatin (TC) + sintilimab for just one cycle which was suspended by the occurrence of grade four bone marrow suppression and multiple times of infections. The treatment timeline for the patient and AEs were shown in Figure 3C and Table 1.
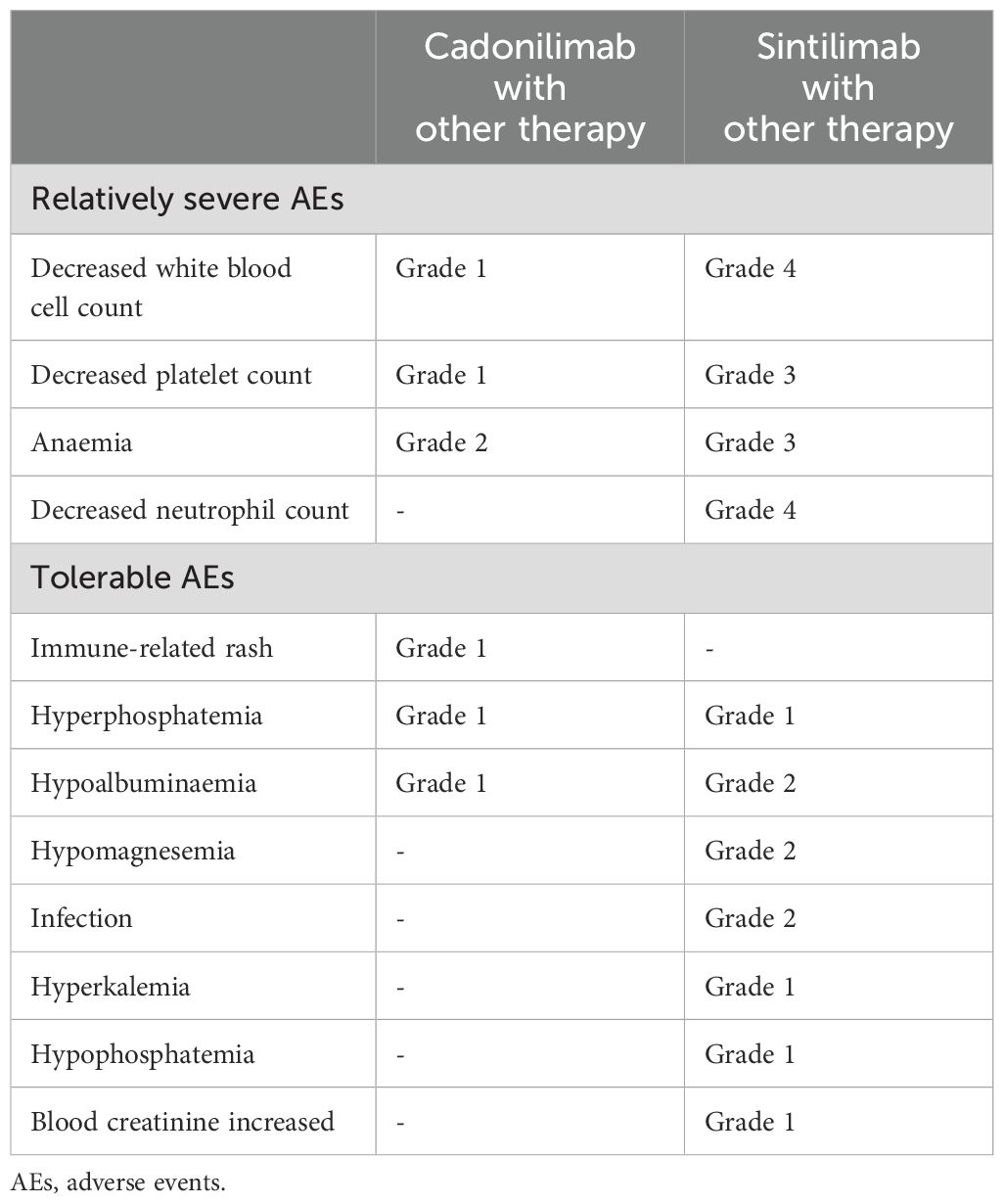
Table 1. AEs during treatment evaluated by the Common Terminology Criteria for Adverse Events (CTCAE) version 5.0.
We reported a R/M CC patient who maintained unexpected CR for 10 months with safety profiles treated with cadonilimab. Surprisingly, metastasis with PD-1 negative also disappeared. As far as we know, this case achieved CR in the shortest cadonilimab treatment period and maintained CR longer than other cases in the literature (25, 26).
In preclinical and clinical studies, the combination of ICIs targeting PD-1 and CTLA-4 has exhibited synergistic antitumor activity (16, 27). In this case, the disseminated CC patients presented CR after being treated with cadonilimab and other combined therapies. However, the patient progressed rapidly after replacing cadonilimab with sintilimab, indicating dual blockade immunotherapy offers substantial advantages over ICI monotherapy (17, 18), which were consistent with the previous studies on the comparison of cadonilimab and pembrolizumab [ORR cadonilimab = 33% (33/100) vs ORR pembrolizumab = 12.2% (12/98)] (28, 29). Such enhanced anti-tumor efficacy of cadonilimab was also observed in lung cancer, rectal cancer, nasopharyngeal cancer, and so on (30–36). The remarkable anticancer mechanism of cadonilimab was worth exploring. Cell binding assays showed that cadonilimab blocked PD-1 binding to PD-L1 and PD-L2 and CTLA-4 binding to B7-1 and B7-2 simultaneously and crosslinked cells expressing PD-1 and CTLA-4. In addition, cadonilimab had a higher affinity for tumor infiltrating lymphocytes in the tumor microenvironment compared with surrounding tissues (23, 37). PD-1 inhibitors and CTLA-4 inhibitors exhibited no cross-resistance and assisted in reshaping immune memory, resulting in a long-term immune response (17).
Moreover, this case might give us two clues for CR possibility after cadonilimab treatment. First, as shown in this case, both the PD-L1-positive cervical primary lesion and the PD-L1-negative left clavicular metastases achieved a CR after treatment, which was consistent with findings of the COMPASSION-13 study (ORR PD-L1 positive = 77.8% (21/27); ORR PD-L1 negative = 70.6% (12/17)), suggesting that cadonilimab could provide clinical benefits for patients with PD-L1-negative CC (30, 38). Second, it was likely that comprehensive treatmentwas the important reason for the remarkable efficacy. Despite patients with PD-L1 CPS < 1 also demonstrated the possibility of an impressive response rate with the treatment of cadonilimab, CR patients still remain a minority. Cadonilimab combined with chemotherapy, radiotherapy, and anti-angiogenic therapy might be an explanation for this CR case. When it came to chemotherapy, many studies showed that chemotherapy not only stimulated tumor antigen release and presentation, resulting in increased activation of tumor-infiltrating lymphocytes, but also synergized with PD-1 pathway blockade to prolong the efficacy of immunotherapy (39, 40). Of note, radiotherapy in the combined treatment of the role also can not be underestimated. Combination treatments with radiotherapy and anti-PD-1 antibodies or anti-CTLA-4 antibodies could activate tumor-specific T cells in the tumor microenvironment (TME), increase the infiltration of CD8-positive T cells, and reduce the accumulation of myeloid-derived suppressor cells (MDSCs) and regulatory T cells, thereby improving anti-tumor immunity (41–44). Besides, the restoration of immune responsiveness induced by anti-PD-1 antibodies or anti-CTLA-4 antibodies and inhibition of angiogenesis after anti-angiogenic therapy could revert the effect resulting in hypoxia on the TME and restore the reciprocal efficacy of the two treatments (45). In conclusion, all these factors might contribute to CR status of the patient.
It is noteworthy that the patient experienced only mild AEs during treatment with cadonilimab, which was in line with COMPASSION-01/03/06/13 and AK104-201, where the incidence of AEs≥grade 3 was only 12% to 26% (30, 31, 35, 46). Although subsequent adverse reactions in the patient might be attributed to toxicity accumulation from previous treatment, the fragment (Fc)-null design of cadonilimab could suggest lower toxicity in this case. The crystallizable fragment (Fc)-null design could eliminate binding to FcγRs and C1q and to minimize lymphocyte loss and antibody-dependent cytokine release from macrophages. What’s more, cadonilimab showed no affinity for FcγRIa, FcγRIIa_H131, FcγRIIIa_V158, FcγRIIIa_F158 or C1q and did not elicit antibody-mediated cell-dependent cytotoxicity, complementary-dependent cell-mediated cytotoxicity or antibody-dependent cellular phagocytosis activity and interleukin-6 (IL-6)/interleukin-8 (IL-8) release. These features all likely contribute to significantly lower toxicities of cadonilimab observed in the clinic (21, 37).
Notably, IHC showed that the cervical tumor was strongly positive for HER-2, indicating HER-2 might play an important role in CR of R/M CC with cadonilimab. Similar findings have been reported in other cancers. Peng J et al (47) reported a patient with HER-2 positive advanced gastroesophageal junction (GEJ) cancer received cadonilimab combined with chemotherapy and achieved CR. The KEYNOTE-811 trial also demonstrated that 15 gastric cancer patients with HER-2 positive obtained CR status by adding pembrolizumab and trastuzumab to chemotherapy (48), which was consistent with our findings. There was the clear evidence of crosstalk between HER-2 and PD-L1 pathways (49, 50). PD-1 inhibitors increased the proportion of intratumoral CD8+ T cells, which could enhance the therapeutic efficacy of HER2-targeted antibody-drug conjugates and reduce tumor growth (51). At the same time, HER2-targeted antibody-drug conjugates could also upregulate PD-L1 expression by upregulating IFN-γlevel (52). In addition, there was a cross-linking between HER-2 positive tumor cells and PD-1 positive T cells in immune synapses. Then, PD-1/HER-2 bispecific antibodies could eliminate tumor cells without antigen recognition (53) Hence, the enhanced antitumor effect of PD-1 inhibitors might be more evident in HER2-overexpressing tumors. However, there is much more to know regarding the underlying interactions pertaining to these two targets. The role of HER-2 in R/M CC patients treated with cadonilimab is worth exploring.
The high expense of specific cancer treatments might influence clinical practice and treatment choices. Although the patient was satisfied with cadonilimab value for its potential for robust and durable responses, she was discouraged by the expense of cadonilimab. To enable the wide usage of immunotherapy, such as cadonilimab and pembrolizumab, much effort and action will needed in order to benefit more patients, such as lower medicine prices and broaden insurance coverage.
There are some limitations that needed to be acknowledged. First, some medical information was incomplete, such as infrequent imaging evaluations and a lack of pathological confirmation for CR status. Second, the safety comparison between cadonilimab and sintilimab might be influenced by several other factors, such as the accumulation of anti-cancer drug toxicity and the side effects of other treatments. Third, more comprehensive and focused studies are required to thoroughly characterize the immunological mechanism of CR status. Overall, we presented a CR case in R/M CC patients treated with cadonilimab, which had excellent performance in anti-cancer effects. This case report will provide valuable information for the future application of cadonilimab in the treatment of R/M CC.
With the approval of an increasing number of immunotherapeutic agents, the treatment of malignant tumors has entered the era of immunotherapy. ICIs have provided significant benefits for late-stage CC patients, such as increasing the probability of obtaining CR. Cadonilimab offers a promising and effective therapeutic approach for R/M CC. Further investigations are merited to explore its potential mechanisms.
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author/s.
The studies involving humans were approved by This retrospective study was approved by the Ethics Committee of Fujian Medical Cancer Hospital (K2023-102-01) and adhered to the Helsinki Declaration. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
HY: Data curation, Formal analysis, Methodology, Project administration, Visualization, Writing – original draft, Investigation, Supervision, Writing – review & editing. JL: Data curation, Formal analysis, Methodology, Project administration, Visualization, Writing – original draft, Supervision, Writing – review & editing. JC: Data curation, Investigation, Writing – original draft. LC: Data curation, Investigation, Writing – original draft. JZ: Data curation, Investigation, Writing – original draft. BL: Data curation, Investigation, Writing – original draft. DH: Data curation, Investigation, Writing – original draft. YX: Data curation, Investigation, Writing – original draft. LY: Data curation, Investigation, Writing – original draft. YS: Conceptualization, Formal analysis, Resources, Writing – review & editing, Writing – original draft.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by the Major Scientific Research Program for Young and Middle-aged Health Professionals of Fujian Province, China (Grant No. 2022ZQNZD008), the High-level Talents Training Project of Fujian Cancer Hospital (Grant No.2022YNG04) and the Joint Funds for the Innovation of Science and Technology, Fujian Province (Grant No.2023Y9404).
We thank the patient, her family, and the dedicated support of the members from each department at Fujian Cancer Hospital.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
CC, Cervical Cancer; R/M CC, Recurrent/Metastatic Cervical Cancer; PD-1, Programmed Death 1; CTLA-4, Cytotoxic T-lymphocyte Antigen-4; PD-L1, Programmed Death Ligand 1; HER-2, Human Epidermal Growth Factor Receptor 2; CR, Complete Response; OS, Overall Survival; ICIs, Immune Checkpoint Inhibitors; ORRs, Objective Response Rates; PFS, Progression-Free Survival; CPS, Combined Positive Score; TGF, Transforming Growth Factor; IrAEs, Immune-Related Adverse Events; IHC, Immunohistochemistry; PET/CT, Positron Emission Tomography-Computed Tomography; FIGO, International Federation of Gynecology and Obstetrics; SCC, Squamous Cell Carcinomas; VMAT, Volumetric Modulated Arc Therapy; MRI, Magnetic Resonance Imaging; CT, Computed Tomography; T, Taxol; C, Cisplatin; PR, Partial Response; AEs, Adverse Events; CTCAE, Common Terminology Criteria for Adverse Events; TME, Tumor Microenvironment; MDSCs, Myeloid-Derived Suppressor Cells; IL-6, Interleukin-6; IL-8, Interleukin-8; GEJ, Gastroesophageal Junction.
1. Bray F, Laversanne M, Sung H, Ferlay J, Siegel RL, Soerjomataram I, et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: A Cancer J Clin. (2024) 74:229–63. doi: 10.3322/caac.21834
2. Singh D, Vignat J, Lorenzoni V, Eslahi M, Ginsburg O, Lauby-Secretan V, et al. Global estimates of incidence and mortality of cervical cancer in 2020: a baseline analysis of the WHO Global Cervical Cancer Elimination Initiative. Lancet Global Health. (2023) 11:e197–206. doi: 10.1016/S2214-109X(22)00501-0
3. Ferrall L, Lin KY, Roden RBS, Hung CF, Wu TC. Cervical cancer immunotherapy: facts and hopes. Clin Cancer Res. (2021) 27:4953–73. doi: 10.1158/1078-0432.CCR-20-2833
4. Li H, Wu X, Cheng X. Advances in diagnosis and treatment of metastatic cervical cancer. J Gynecologic Oncol. (2016) 27(4):e43. doi: 10.3802/jgo.2016.27.e43
5. Monk BJ, Colombo N, Tewari KS, Dubot C, Caceres MV, Hasegawa K, et al. First-line pembrolizumab + Chemotherapy versus placebo + Chemotherapy for persistent, recurrent, or metastatic cervical cancer: final overall survival results of KEYNOTE-826. J Clin Oncol. (2023) 41:5505–11. doi: 10.1200/JCO.23.00914
6. Jiang S, Cui Z, Zheng J, Wu Q, Yu H, You Y, et al. Significance of immunogenic cell death-related prognostic gene signature in cervical cancer prognosis and anti-tumor immunity. J Inflammation Res. (2023) 16:2189–207. doi: 10.2147/JIR.S410140
7. Liu L, Lin J, Deng S, Yu H, Xie N, Sun Y. A novel nomogram and risk stratification for early metastasis in cervical cancer after radical radiotherapy. Cancer Med. (2023) 12:21798–806. doi: 10.1002/cam4.v12.24
8. Gennigens C, Jerusalem G, Lapaille L, De Cuypere M, Streel S, Kridelka F, et al. Recurrent or primary metastatic cervical cancer: current and future treatments. ESMO Open. (2022) 7(5):100579. doi: 10.1016/j.esmoop.2022.100579
9. Tewari KS, Sill MW, Penson RT, Huang H, Ramondetta LM, Landrum LM, et al. Bevacizumab for advanced cervical cancer: final overall survival and adverse event analysis of a randomised, controlled, open-label, phase 3 trial (Gynecologic Oncology Group 240). Lancet. (2017) 390:1654–63. doi: 10.1016/S0140-6736(17)31607-0
10. Lee S-M, Lee S, Cho H-W, Min KJ, Hong JH, Song JY, et al. Application of immune checkpoint inhibitors in gynecological cancers: what do gynecologists need to know before using immune checkpoint inhibitors? Int J Mol Sci. (2023) 24(2):974. doi: 10.3390/ijms24020974
11. Chung HC, Ros W, Delord J-P, Perets R, Italiano A, Shapira-Frommer R, et al. Efficacy and safety of pembrolizumab in previously treated advanced cervical cancer: results from the phase II KEYNOTE-158 study. J Clin Oncol. (2019) 37:1470–8. doi: 10.1200/JCO.18.01265
12. O’malley DM, Oaknin A, Monk BJ, Selle F, Rojas C, Gladieff L, et al. Phase II study of the safety and efficacy of the anti-PD-1 antibody balstilimab in patients with recurrent and/or metastatic cervical cancer. Gynecologic Oncol. (2021) 163:274–80. doi: 10.1016/j.ygyno.2021.08.018
13. Naumann RW, Hollebecque A, Meyer T, Devlin MJ, Oaknin A, Kerger JT, et al. Safety and efficacy of nivolumab monotherapy in recurrent or metastatic cervical, vaginal, or vulvar carcinoma: results from the phase I/II checkMate 358 trial. J Clin Oncol. (2019) 37:2825–34. doi: 10.1200/JCO.19.00739
14. Monk BJ, Colombo N, Tewari KS, Dubot C. Final overall survival results from a randomized, double-blinded phase 3 study of pembrolizumab + chemotherapy vs placebo + chemotherapy for first-line treatment of persistent, recurrent, or metastatic cervical cancer. J Clin Oncol. (2023) 41(Suppl):5500. doi: 10.1136/ijgc-2023-ESGO.886
15. Qu X, Wang Y, Jiang Q, Ren T, Guo C, Hua K, et al. Interactions of Indoleamine 2,3-dioxygenase-expressing LAMP3+ dendritic cells with CD4+ regulatory T cells and CD8+ exhausted T cells: synergistically remodeling of the immunosuppressive microenvironment in cervical cancer and therapeutic implications. Cancer Commun. (2023) 43:1207–28. doi: 10.1002/cac2.v43.11
16. Ge Y, Zhang Y, Zhao K-N, Zhu H. Emerging therapeutic strategies of different immunotherapy approaches combined with PD-1/PD-L1 blockade in cervical cancer. Drug Design Dev Ther. (2022) 16:3055–70. doi: 10.2147/DDDT.S374672
17. Cheng W, Kang K, Zhao A, Wu Y. Dual blockade immunotherapy targeting PD-1/PD-L1 and CTLA-4 in lung cancer. J Hematol Oncol. (2024) 17(1):54. doi: 10.1186/s13045-024-01581-2
18. Sorkhabi AD, Sarkesh A, Fotouhi A, Saeedi H, Aghebati-Maleki L. Cancer combination therapies by silencing of CTLA-4, PD-L1, and TIM3 in osteosarcoma. IUBMB Life. (2022) 74:908–17. doi: 10.1002/iub.v74.9
19. Feng J, Tang D, Wang J, Zhou Q, Peng J, Lou H, et al. SHR-1701, a bifunctional fusion protein targeting PD-L1 and TGFbeta, for recurrent or metastatic cervical cancer: A clinical expansion cohort of a phase I study. Clin Cancer Res. (2022) 28:5297–305. doi: 10.1158/1078-0432.CCR-22-0346
20. Wojtukiewicz MZ, Rek MM, Karpowicz K, Górska M, Polityńska B, Wojtukiewicz AM, et al. Inhibitors of immune checkpoints—PD-1, PD-L1, CTLA-4—new opportunities for cancer patients and a new challenge for internists and general practitioners. Cancer Metastasis Rev. (2021) 40:949–82. doi: 10.1007/s10555-021-09976-0
21. Pang X, Huang Z, Zhong T, Zhang P, Wang ZM, Xia M, et al. Cadonilimab, a tetravalent PD-1/CTLA-4 bispecific antibody with trans-binding and enhanced target binding avidity. mAbs. (2023) 15(1):2180794. doi: 10.1080/19420862.2023.2180794
22. Wu Xh, Ji J, Lou Hm, Li YX, Feng M, Xu N, et al. Efficacy and safety of cadonilimab, an anti-PD-1/CTLA4 bi-specifc antibody, in previously treated recurrent or metastatic (R/M) cervical cancer: A multicenter, open-label, single-arm, phase II trial. Soc Gynecologic Oncol (SGO) Meeting. (2022) 166(Supplement 1):S47–S48. doi: 10.1016/S0090-8258(22)01293-8
24. Freites-Martinez A, Santana N, Arias-Santiago S, Viera A, Arias-Santiago S, et al. CTCAE versión 5.0. Evaluación de la gravedad de los eventos adversos dermatológicos de las terapias antineoplásicas. Actas Dermo-Sifiliográficas. (2021) 112:90–2. doi: 10.1016/j.ad.2019.05.009
25. Yu X, Dong S, Wang W, Sun X, Wang Y, Yu F. Case report: A case of recurrent cervical cancer with bronchial and esophageal metastases presenting with hemoptysis and dysphagia. Front Oncol. (2024) 14. doi: 10.3389/fonc.2024.1375035
26. Zhu R, Chen T-Z, Sun M-T, Zhu CR. Advanced cervix cancer patient with chemotherapy-induced grade IV myelosuppression achieved complete remission with cadonilimab: A case report. World J Clin cases. (2024) 12:1510–6. doi: 10.12998/wjcc.v12.i8.1510
27. Wang Y, Zhang H, Liu C, Wang Z, Wu W, Zhang N, et al. Immune checkpoint modulators in cancer immunotherapy: recent advances and emerging concepts. J Hematol Oncol. (2022) 15(1):111. doi: 10.1186/s13045-022-01325-0
28. Li C, Cang W, Gu Y, Chen L, Xiang Y. The anti-PD-1 era of cervical cancer: achievement, opportunity, and challenge. Front Immunol. (2023) 14. doi: 10.3389/fimmu.2023.1195476
29. Colombo N, Dubot C, Lorusso D, Caceres MV, Hasegawa K, Shapira-Frommer R, et al. Pembrolizumab for persistent, recurrent, or metastatic cervical cancer. New Engl J Med. (2021) 385:1856–67. doi: 10.1056/NEJMoa2112435
30. Gao X, Xu N, Li Z, Shen L, Ji K, Zheng Z, et al. Safety and antitumour activity of cadonilimab, an anti-PD-1/CTLA-4 bispecific antibody, for patients with advanced solid tumours (COMPASSION-03): a multicentre, open-label, phase 1b/2 trial. Lancet Oncol. (2023) 24:1134–46. doi: 10.1016/S1470-2045(23)00411-4
31. Zhao Y, Ma Y, Fan Y, Zhou J, Yang N, Yu Q, et al. A multicenter, open-label phase Ib/II study of cadonilimab (anti PD-1 and CTLA-4 bispecific antibody) monotherapy in previously treated advanced non–small-cell lung cancer (AK104-202 study). Lung Cancer. (2023) 184:107355. doi: 10.1016/j.lungcan.2023.107355
32. Qiao Q, Han C, Ye S, Li J, Shao G, Bai Y, et al. The efficacy and safety of cadonilimab combined with lenvatinib for first-line treatment of advanced hepatocellular carcinoma (COMPASSION-08): a phase Ib/II single-arm clinical trial. Front Immunol. (2023) 14:1238667. doi: 10.3389/fimmu.2023.1238667
33. Gao X, Ji K, Jia Y, Shan F, Chen Y, Xu N, et al. Cadonilimab with chemotherapy in HER2-negative gastric or gastroesophageal junction adenocarcinoma: the phase 1b/2 COMPASSION-04 trial. Nat Med. (2024) 30:1943–51. doi: 10.1038/s41591-024-03007-5
34. Chen B, Yao W, Li X, Lin G, Chu Q, Liu H, et al. A phase Ib/II study of cadonilimab (PD-1/CTLA-4 bispecific antibody) plus anlotinib as first-line treatment in patients with advanced non-small cell lung cancer. Br J Cancer. (2023) 130:450–6. doi: 10.1038/s41416-023-02519-0
35. Chen Q-Y, Guo S-S, Luo Y, Qu S, Wu DH, Chen XZ, et al. Efficacy and safety of cadonilimab in previously treated recurrent or metastatic nasopharyngeal carcinoma(COMPASSION-06): A phase II multicenter study. Oral Oncol. (2024) 151:106723. doi: 10.1016/j.oraloncology.2024.106723
36. Xu T, Feng L, Zhang W, Li H, Ma H, Abulimiti M, et al. The efficacy and safety of short-course radiotherapy followed by sequential chemotherapy and Cadonilimab for locally advanced rectal cancer: a protocol of a phase II study. BMC Cancer. (2024) 24(1):501. doi: 10.1186/s12885-024-12254-1
37. Huang Zl, Pang X, Zhong Tt, Chen N, He X, Xia D, et al. Cadonilimab, an antiPD1/CTLA4 bi-specifc antibody with Fc efector null backbone. J Immunother Cancer. (2021) 9:A313–314. doi: 10.1136/jitc-2021-SITC2021.289
38. Lou H, Cai H, Huang X, Li G, Wang L, Liu F, et al. Cadonilimab combined with chemotherapy with or without bevacizumab as first-line treatment in recurrent or metastatic cervical cancer (COMPASSION-13): A phase 2 study. Clin Cancer Res. (2024) 30:1501–8. doi: 10.1158/1078-0432.CCR-23-3162
39. Wu M, Huang Q, Xie Y, Wu X, Ma H, Zhang Y, et al. Improvement of the anticancer efficacy of PD-1/PD-L1 blockade via combination therapy and PD-L1 regulation. J Hematol Oncol. (2022) 15(1):24. doi: 10.1186/s13045-022-01242-2
40. Mariniello A, Nasti TH, Chang DY, Hashimoto M, Malik S, McManus DT, et al. Platinum-based chemotherapy attenuates the effector response of CD8 T cells to concomitant PD-1 blockade. Clin Cancer Res. (2024) 30:1833–45. doi: 10.1158/1078-0432.CCR-23-1316
41. Gong X, Li X, Jiang T, Xie H, Zhu Z, Zhou F, et al. Combined radiotherapy and anti–PD-L1 antibody synergistically enhances antitumor effect in non–small cell lung cancer. J Thorac Oncol. (2017) 12:1085–97. doi: 10.1016/j.jtho.2017.04.014
42. Kim S, Sanders PD, Weihe E, Purcell T, Kato S, Patel S, et al. Analysis of immune correlates using anti-PD-1 checkpoint blockade immunotherapy combined with stereotactic body radiation therapy. Int J Radiat OncologyBiologyPhysics. (2017) 99:E602–3. doi: 10.1016/j.ijrobp.2017.06.2051
43. Formenti SC, Rudqvist N-P, Golden E, Cooper B, Wennerberg E, Lhuillier C, et al. Radiotherapy induces responses of lung cancer to CTLA-4 blockade. Nat Med. (2018) 24:1845–51. doi: 10.1038/s41591-018-0232-2
44. Wang L, Lynch C, Pitroda SP, Piffkó A, Yang K, Huser AK, et al. Radiotherapy and immunology. J Exp Med. (2024) 221(7):e20232101. doi: 10.1084/jem.20232101
45. Maiorano BA, Parisi A, Maiello E, Ciardiello D. The interplay between anti-angiogenics and immunotherapy in colorectal cancer. Life. (2022) 12(10):1552. doi: 10.3390/life12101552
46. Frentzas S, Gan HK, Cosman R, Coward J, Tran B, Millward M, et al. A phase 1a/1b first-in-human study (COMPASSION-01) evaluating cadonilimab in patients with advanced solid tumors. Cell Rep Med. (2023) 4(11):101242. doi: 10.1016/j.xcrm.2023.101242
47. Peng J, Zhu Q, Peng Z, Chen Z, Liu Y, Liu B. Patients with positive HER-2 amplification advanced gastroesophageal junction cancer achieved complete response with combined chemotherapy of AK104/cadonilimab (PD-1/CTLA-4 bispecific): A case report. Front Immunol. (2022) 13:1049518. doi: 10.3389/fimmu.2022.1049518
48. Janjigian YY, Kawazoe A, Yañez P, Li N, Lonardi S, Kolesnik O, et al. The KEYNOTE-811 trial of dual PD-1 and HER2 blockade in HER2-positive gastric cancer. Nature. (2021) 600:727–30. doi: 10.1038/s41586-021-04161-3
49. Vranic S, Cyprian FS, Gatalica Z, Palazzo J. PD-L1 status in breast cancer: Current view and perspectives. Semin Cancer Biol. (2021) 72:146–54. doi: 10.1016/j.semcancer.2019.12.003
50. Triulzi T, Forte L, Regondi V, Di Modica M, Ghirelli C, Carcangiu ML, et al. HER2 signaling regulates the tumor immune microenvironment and trastuzumab efficacy. OncoImmunology. (2018) 8(1):e1512942. doi: 10.1080/2162402X.2018.1512942
51. Yamashita K, Iwatsuki M, Yasuda-Yoshihara N, Morinaga T, Nakao Y, Harada K, et al. Trastuzumab upregulates programmed death ligand-1 expression through interaction with NK cells in gastric cancer. Br J Cancer. (2021) 124:595–603. doi: 10.1038/s41416-020-01138-3
52. Zheng G, Guo Z, Li W, Xi W, Zuo B, Zhang R, et al. Interaction between HLA-G and NK cell receptor KIR2DL4 orchestrates HER2-positive breast cancer resistance to trastuzumab. Signal Transduct Target Ther. (2021) 6:236. doi: 10.1038/s41392-021-00629-w
Keywords: cadonilimab, recurrent and/or metastatic cervical cancer, complete response, HER-2, case report
Citation: Yu H, Lin J, Chen J, Chen L, Zou J, Liu B, Hu D, Xiao Y, Yu L and Sun Y (2024) A surprising complete response to cadonilimab in a primary metastatic cervical cancer: a case report. Front. Immunol. 15:1494138. doi: 10.3389/fimmu.2024.1494138
Received: 10 September 2024; Accepted: 11 November 2024;
Published: 26 November 2024.
Edited by:
Nelson Shu-Sang Yee, Penn State Milton S. Hershey Medical Center, United StatesReviewed by:
Christopher Hillyar, University of Oxford, United KingdomCopyright © 2024 Yu, Lin, Chen, Chen, Zou, Liu, Hu, Xiao, Yu and Sun. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yang Sun, c3VueWFuZ2ZqemxAc2luYS5jb20=
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.