- 1The School of Basic Medicine, Binzhou Medical University, Yantai, Shandong, China
- 2Translational Biology and Engineering Program, Ted Rogers Centre for Heart Research, Toronto, ON, Canada
- 3Department of Gynecology, the Affiliated Yantai Yuhuangding Hospital of Qingdao University, Yantai, Shandong, China
Immunotherapy for breast cancer is now being considered clinically, and more recently, the number of investigations aimed specifically at nano-biomaterials-assisted immunotherapy for breast cancer treatment is growing. Alterations of the breast cancer micro-environment can play a critical role in anti-tumor immunity and cancer development, progression and metastasis. The improvement and rearrangement of tumor micro-environment (TME) may enhance the permeability of anti-tumor drugs. Therefore, targeting the TME is also an ideal and promising option during the selection of effective nano-biomaterial-based immuno-therapeutic strategies excepted for targeting intrinsic resistant mechanisms of the breast tumor. Although nano-biomaterials designed to specifically release loaded anti-tumor drugs in response to tumor hypoxia and low pH conditions have shown promises and the diversity of the TME components also supports a broad targeting potential for anti-tumor drug designs, yet the applications of nano-biomaterials for targeting immunosuppressive cells/immune cells in the TME for improving the breast cancer treating outcomes, have scarcely been addressed in a scientific review. This review provides a thorough discussion for the application of the different forms of nano-biomaterials, as carrier vehicles for breast cancer immunotherapy, targeting specific types of immune cells in the breast tumor microenvironment. In parallel, the paper provides a critical analysis of current advances/challenges with leading nano-biomaterial-mediated breast cancer immunotherapeutic strategies. The current review is timely and important to the cancer research field and will provide a critical tool for nano-biomaterial design and research groups pushing the clinical translation of new nano-biomaterial-based immuno-strategies targeting breast cancer TME, to further open new avenues for the understanding, prevention, diagnosis and treatment of breast cancer, as well as other cancer types.
1 Introduction of breast cancer and immunotherapy
Breast cancer, which is referred to as a complex and heterogeneous disease, is one of the most common malignant tumors in women with a high mortality rate (1, 2). Both genetic and external risk factors can contribute to its incidence (1, 2). The main risk factors for breast cancer include sex, age, genetic factors, hormone therapies, lifestyles and dietary habits etc. (3). The conventional treatment options for breast cancer include surgery, chemotherapy and radiotherapy in clinical practices (4, 5). However, in some instances, recurrence is inevitable due to the undetected or residual breast cancer tissues (4, 5). In addition, therapeutic resistance is also a significant obstacle for the effective treatment of breast cancer, due in part to the distinctive molecular profiles and biological characteristics of the different breast cancer subtypes (6).
Generally, based on the presence or absence of specific receptors on the cell surface, breast cancer can be classified into three main subtypes, estrogen receptor (ER) and/or progesterone receptor (PR) positive (Luminal A/B, based on Ki-67 expression), human epidermal growth factor receptor 2 (HER2) positive, and triple negative breast cancer (TNBC) (7). Therefore, different therapeutic approaches have been introduced to treat the distinctive breast cancer subtypes to prolong the progression-free survival and overall survival of the patients. Normally, endocrine therapy is used for breast cancer types that express hormone receptors. In addition, chemotherapy and inhibitor treatments, targeting the cell cycle or specific receptor-related signaling pathways, are also common for patients with hormone receptor-positive breast cancer (7). Monoclonal antibodies and chemotherapy are commonly used in HER2 positive breast cancer patients (8). However, TNBC, which accounts for 10%-20% of all breast cancer cases, has been considered to be the most aggressive subtype and the most challenging one to treat because of its deficiency of ER, PR and HER2 (9). Since TNBC cells lack the expression of druggable cell-surface target receptors, specific targeted therapy for TNBC cannot be proposed, instead, TNBC patients are often treated with neoplastic drugs and chemotherapies (10).
The immune system plays a vital role during the progression and regression of breast cancer (11). While the traditional radiotherapy and chemotherapy focuses on directly killing the tumor cells, immunotherapy works by activating the patients’ own immune systems to recognize and combat breast cancer cells (including the abscopal metastatic tumors) (12, 13). In addition, immunotherapy is also endowed with the ability to impede tumor metastasis and recurrence (12, 13). Recently, the application of immunotherapy exhibited the potential for improved clinical outcomes in cancer treatment (14, 15). As the most invasive type of breast cancer, TNBC was perceived as the most immunogenic type due to its high genome instability, increased mutation rates, enhanced tumor antigen production and frequent lymphocytic infiltration (4, 16). Programmed death-ligand 1 (PD-L1), which is a negative regulator in the immune system, has been shown to be highly expressed in TNBC (17, 18), indicating the feasibility of immunotherapy for TNBC.
The current approaches for cancer immunotherapy include adoptive cell therapy (e.g., tumor-infiltrating lymphocyte (TIL), chimeric antigen receptor (CAR) T-cell, T-cell receptor-engineered (TCR) T-cell, chimeric antigen receptor-natural killer cell (CAR-NK) therapies), and immune checkpoint blockade and cancer vaccine therapies (7, 12). Specifically, the current immunotherapies utilized in breast cancer treatment comprise of tumor-targeting antibodies, adoptive T cell therapy, cancer vaccines and immune checkpoint blockade (4). Among those different strategies, monoclonal antibodies, such as trastuzumab and pertuzumab targeting HER2, have shown efficacy, and been widely applied clinically (19). In addition, cancer vaccines including DNA and peptide vaccines have been reported to exhibit their efficacy by activating the immune system in breast cancer therapeutic regimens (20–22). Although the latter breast cancer immunotherapies have shown promise, limitations and challenges have also been identified. For example, the frequent and unpredictable mutations in breast tumor cells may decrease the drug response and targeting efficiency of monoclonal antibodies (4). Moreover, the lack of appropriate tumor antigens and the immunosuppressive tumor microenvironment can limit the application of tumor vaccines (4). To enhance the therapeutic efficacy of breast cancer treatments, the combination of multiple immunotherapies may offer an alternative option.
Immune checkpoint inhibition, such as the monotherapy targeting the negative immune checkpoint molecules, programmed cell death protein 1 (PD-1) or cytotoxic T-lymphocyte-associated protein 4 (CTLA-4), have great efficacy in the treatment of solid tumors (23). CTLA-4 is a specific target in antibody-based immunotherapy due to its competition with CD28 (on T cells) for the binding with CD80/B7-1 and CD86/B7-2 on the antigen-presenting cells (APCs), inhibiting the T-cell function (24–27). Additionally, the PD-1/PD-L1 axis also plays an inhibitory role in T-cells’ activation. The antibodies targeting this axis could enhance T-cells’ anti-tumor activity (28). In a recent phase II clinical trial for breast cancer, simultaneous inhibition of PD-1 and CTLA-4 by durvalumab and tremelimumab respectively, exhibited clinical beneficial effects in 71% of the enrolled TNBC patients (28). Further, the combination of immune checkpoint inhibition and conventional cancer therapeutic approaches provide another option to boost the treatment effects. For instance, the combination of atezolizumab (anti-PD-L1 antibody) and nab-paclitaxel (chemotherapeutic agent) demonstrated better effects in treating metastatic TNBC vs. antibody treatment or chemotherapy alone in clinical trials (23). This combinatorial treatment has also recently been approved by the U.S. Food and Drug Administration (FDA) for locally advanced or metastatic PD-L1+ TNBC (23). Similarly, pembrolizumab (anti-PD-1 antibody) plus chemotherapy was tested for treating locally recurrent, unresectable, or metastatic TNBC. The latter was also approved by the FDA (23). Further, the combined application of trastuzumab (monoclonal antibody targeting HER2) and HER2 vaccine showed better effects in treating HER2+ breast cancer patients vs. trastuzumab or HER2 vaccine treatment alone (29). However, it has been noted that the systemic long-term use of monoclonal antibodies targeting immune checkpoint inhibition can cause immune-related adverse effects in a large number of patients, reducing the immune therapy efficacy (30). Consequently, the adequate delivery and the stable release of the drugs are key factors to consider for efficiently activating the anti-tumor immune responses in the breast cancer microenvironment.
Currently, there are review articles that have discussed the drug-delivery technologies designed to improve the effectiveness of in situ vaccine-based anti-breast cancer therapies (31). In addition, review papers have also covered the application of nano-biomaterials that could respond to physiological stimuli within breast tissue, and how they can be loaded with both drugs and gene products (e.g., siRNAs, RNAi and noncoding RNAs) and have controlled release at the tumor target site to improve the conventional strategies for treating breast cancer (32). However, the existing review papers have not yet discussed the important role that breast cancer tumor-micro-environment (TME), which contains both breast tissue cells and immune cells that reside within the local breast tissue niche (e.g., macrophages, cancer-associated fibroblasts, myeloid-derived suppressor cells, dendritic cells, natural killer cells and T lymphocytes) and the growth factors/cytokines produced/released, could have played in regulating breast cancer progression and metastasis. The lack of appreciation of the importance of targeting breast cancer TME and the specific immune cell types within the different subtypes of breast cancer could significantly hinder the effectiveness of current anti-breast cancer therapeutics. As a result, this manuscript thoroughly discusses the mechanisms underlying how breast cancer TME, as well as the different immune cells can modulate breast tumor progression/metastasis. Additionally, some of the most advanced progress regarding the use of innovative biomaterials for targeting the immunosuppressive cells and immune cells in the TME, to improve the breast cancer treating outcomes are thoroughly examined. Further, the manuscript critically evaluates the limitations of the current biomaterial-based immunotherapy targeting breast cancer and the potential future research directions of the field have been pointed out.
This current paper would significantly enhance the field’s understanding of how breast cancer TME (tissue cells, immune cells, growth factors/cytokines/chemokines) could lead to the development of the various breast cancer subtypes and modulate the breast cancer progression characteristics, as well as provide great insights into innovative biomaterial and drug designs to precisely target specific cell types to boost immunotherapy efficiency.
2 The tumor micro-environment (TME)
TME is referred to as the special ecosystem that surrounds a tumor inside the body, which includes the resident and recruited host cells (such as cancer-associated fibroblasts (CAFs) and immune cells), cell products (such as pro-inflammatory cytokines and chemokines), growth factors, extracellular matrix (ECM), stromal cells, blood vessels, lymphatic vessels and other cells or biomolecules closely associated with the tumor cells (33–35). The complex cellular and non-cellular components in TME not only participate in the initiation, progression and metastasis of cancers, but also respond to the specific therapeutic regimen (36). For the different cell types within the TME, inflammatory cells and fibroblasts have been found to participate in key processes such as angiogenesis and ECM remodeling, resulting in the uncontrolled growth and metastasis of tumor cells (37, 38). Stromal cells, immune cells and non-stromal factors, could promote the resistant phenotype and affect the efficacy of chemotherapy during chemotherapy for breast cancer (35). Alterations of the breast cancer micro-environment can play a critical role in antitumor immunity and cancer development, progression and metastasis (39). And the improvement and rearrangement of TME may enhance the permeability of anti-tumor drugs (40). Therefore, targeting the TME is also an ideal and promising option during the selection of effective therapeutic strategies excepted for targeting intrinsic resistant mechanisms of the breast tumor (41).
In recent years, nanotechnology has opened up a new avenue for breast cancer treatment (42). Specifically, biomaterials-based nanoparticles (NPs) that exhibit both drug delivery and stimulative functions hold great promise for breast cancer therapeutics and there are some nano-platforms that have been approved by the FDA (6, 43). Many NP-based cancer medications have been evaluated or applied in clinical trials, for example, NP albumin-bound-paclitaxel is one of the most widely used nano-medication in cancer treatment (44). Some properties of NPs such as small sizes, large surface area, high surface-volume ratio, high surface reactivity, unique physicochemical properties, enhanced permeability and retention (EPR) effects and superior reactivity over their bulk counterparts enable their application potential in the early diagnosis and improved treatment of breast cancer (6, 43). Since NPs could increase tumor immunogenicity by generating free radicals, NP-based therapeutics delivery systems can enhance the immunotherapy efficacy of breast cancer (45, 46). NPs can also be designed to have good biocompatibility, minimal cytotoxicity, targeted accumulation in solid tumors and enhanced drug delivery efficiency (13, 47–49). Specifically, the anti-breast cancer drug and the assisted NP delivery system could be designed based on the tumor tissue pathological characteristics (50, 51). The distinct physical, chemical and biological properties between the normal and tumor tissues, such as pH of the tissue micro-environment, oxidation-reduction (redox) state, temperature, oxygen level and the differently expressed genes and proteins, endow the possibility of the targeted delivery of biomaterial-carried anti-tumor drugs (50, 51).
Biomaterials, designed to specifically release loaded anti-tumor drugs in response to tumor hypoxia and low pH conditions, have emerged in recent years (52). What is more, the diversity of TME components also supports a broad targeting potential for anti-tumor drug designs (53, 54). Encouragingly, TME-targeting therapeutic strategies, enabled with specific biomaterials, could potentially exhibit advantages such as precise targeted delivery for anti-tumor drugs or biomacromolecules, and reduced risks of side effects from the treatments, paving a new way for more effective cancer treatments when compared to the traditional chemotherapy or radiotherapy. Consequently, biomaterial-assisted immunotherapies have been considered as an alternative strategy to effectively treat cancer patients and need to be extensively explored. Some current progress regarding the use biomaterials for targeting immunosuppressive cells and immune cells in the TME, for improving the breast cancer treating outcomes are discussed in Sections 3&4.
3 Biomaterials targeting immunosuppressive cells in breast cancer TME
The solid TME is often very complex due to the presence of immunosuppressive cells and factors (55, 56). The immunosuppressive cells in TME, such as tumor-associated macrophage (TAMs), CAFs, regulatory T cells (Tregs), myeloid-derived suppressor cells (MDSCs) could influence the immunotherapy effects in the tumors (57). The application of biomaterials toward specifically targeting those immunosuppressive cells or factors in the TME could be a powerful strategy to augment the breast cancer treatment outcomes. For example, biomaterials can be designed to be effective cargo carriers to deliver specific drugs to enhance immunogenicity, improve antigen presentation or T cell infiltration, to eventually contribute to the remodeling of the tumor TME. In this section, how biomaterials have been designed to target the different immunosuppressive cells of the breast cancer TME, toward boosting the therapeutic effects, are discussed in greater detail.
3.1 M2-like tumor-associated macrophages (TAMs)
Macrophages are distributed in all tissues across the human body and play a vital role in innate immunity (58, 59). Macrophages originate from monocytic precursors in blood and differentiate in the presence of cytokines and growth factors in tissues (58, 59). Macrophage abnormalities can result in disease progression such as tissue fibrosis, diabetes, and different cancers (60). In tumors, the TME influences the recruitment and polarization of macrophages and determines the pro-tumorigenic outcomes (58, 60, 61). During breast tumor progression, macrophages can cause inflammatory responses, promote tumor cell growth, angiogenesis, migration and invasion of breast cancer cells, ultimately progressing to malignancy (58, 60, 61).
3.1.1 M2-like TAMs in breast cancer TME
Macrophages can change their phenotypes in response to tumor microenvironmental cues and become TAMs (61). As a major cellular component of TME, TAMs have recently been found to be present in multiple malignant tumor types (60, 62, 63). Generally speaking, M1 macrophages play an anti-tumor role, while the TAMs are more of a M2 polarization phenotype and have tumor-promoting properties (64). Therefore, M2 TAMs were regarded as an attractive therapeutic target for cancer immunotherapy, due to their pivotal role in cancer progression phases including angiogenesis, immune suppression, hypoxia induction, invasion, and metastasis (58, 65, 66).
Tissues isolated from TNBC patients have demonstrated a high infiltration of M2-like TAMs, and those patients typically have poor prognosis (35, 66). Considering the relationship between TAMs’ abundance and breast cancer patients’ prognosis, removal of the M2-like TAMs, restoration of TAMs’ immunostimulatory phenotype or inhibition of TAMs’ tumor-promoting functions might be effective breast tumor targeting therapies (6, 60). However, it should be noted that the extensive distribution of macrophages and the multifaceted roles that those cells play in different tissues across the body increases the difficulty for the effective delivery of therapeutic drugs specifically to the tumor-promoting M2-like TAMs (64).
3.1.2 Biomaterial-assisted immunotherapeutic approaches targeting M2-like TAMs
Some biomaterial-assisted immunotherapeutic approaches have been developed to specifically block the survival of M2-like TAMs, switch the cells’ phenotypes from M2- to M1-like, or directly kill the M2-like TAMs, to achieve better breast tumor control outcomes. More specifically, the switch of TAMs’ polarization states (M2 to M1) can be achieved by modulating the signaling pathways associated with macrophage activation (67, 68).
The binding of macrophage colony stimulating factor (MCSF) secreted by cancer cells with colony stimulating factor 1 receptor (CSF1-R, a class III tyrosine receptor) expressed on macrophages can promote the M2 polarization of TAMs (67). Ramesh et al. reported a stable supramolecular nano-assembly formed by CSF1-R, Src homology region 2 (SH2) domain-phosphatase (SHP2) inhibitors and co-lipid based phospholipid-polymer conjugates (1, 2-distearoyl-sn-glycero-3-phosphoethanolamine-poly(ethylene-glycol) (DSPE-PEG) and phosphatidyl choline) (67). The self-assembled dual-inhibitor-loaded NPs (DNPs) could target M2 TAMs to inhibit the MCSF-CSF1-R and CD47-signal regulatory protein α (SIRPα) pathways simultaneously, resulting in their repolarization to anti-tumorigenic M1 phenotypes, and enhancing their phagocytic function (67). What is more, DNPs showed high anti-tumor efficacy, minimal normal tissue toxicity in breast cancer mouse models, indicating their future promise for applications in breast cancer immunotherapy (67).
Additionally, the mitogen-activated protein kinase (MAPK) signaling pathway, a downstream signaling pathway of CSF1R, also plays a vital role in modulating the proliferation, survival and functional activity of M2 macrophages (68). Ramesh et al. also achieved the concurrent inhibition of CSF-1R and MAPK signaling pathways by synthesizing NPs using supramolecular self-assembly, to further improve the anti-breast cancer effects of macrophage-based immunotherapy (68). The NPs were synthesized in the presence of phosphatidyl choline and DSPE-PEG2000-amine with amphiphilic dual-kinase inhibitors targeting CSF1R and MEK (68). These supramolecular NPs could accumulate in TAMs at a high concentration and reprogrammed the polarization of immunosuppressive M2-like macrophages to M1-like phenotype in a highly aggressive 4T1 breast cancer model (Figure 1) (68). The design of those NPs carrying inhibitors, targeting specific signaling pathways that can modulate M2-like TAMs’ phenotypic switches, showed effectiveness in remodeling of the immunosuppressive breast cancer TME (68).
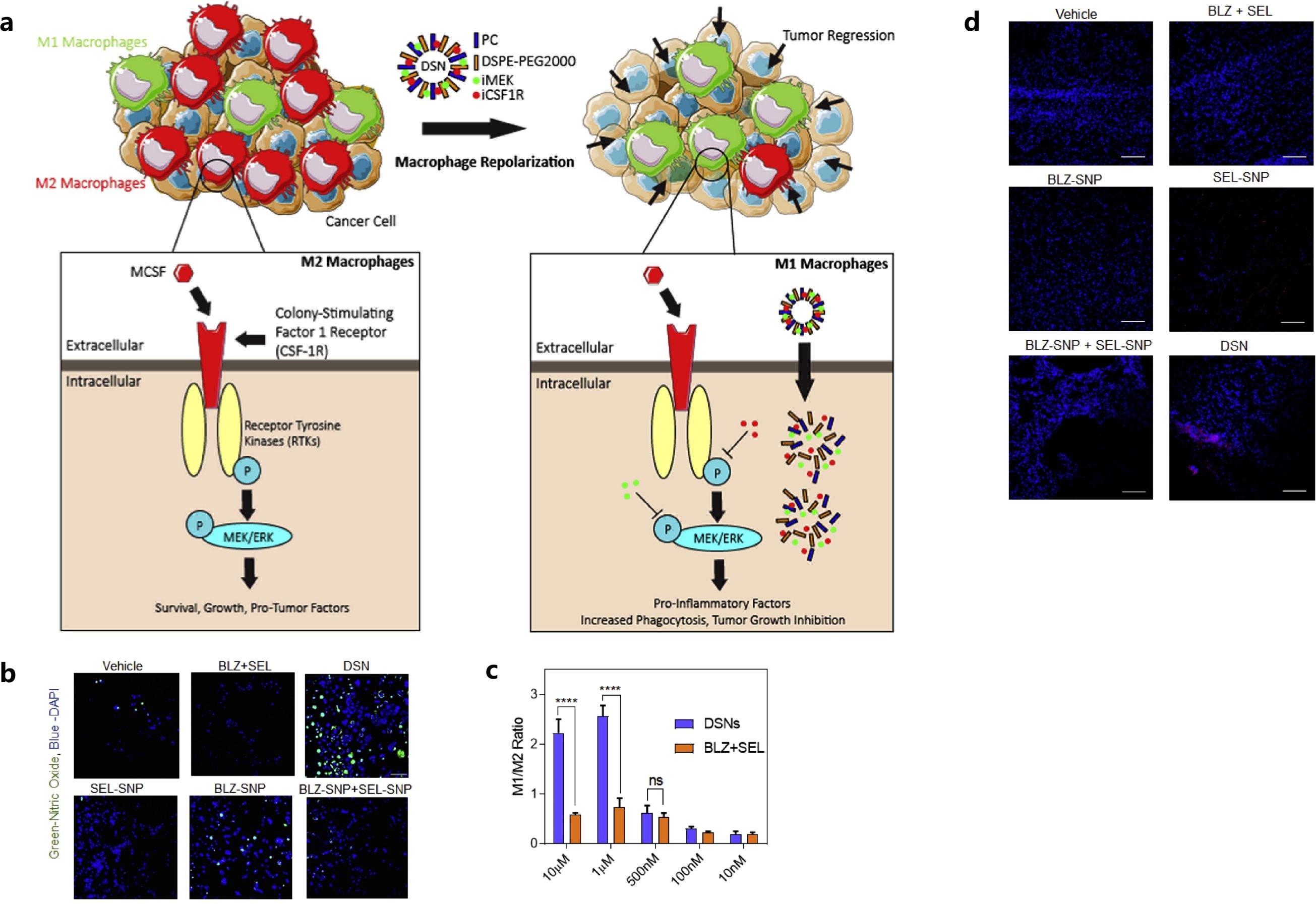
Figure 1. Mechanism of the action of dual-kinase inhibitor-loaded supramolecular nanoparticles (DSN) targeting CSF1R and MEK in macrophages (A), confocal images (B) and flow cytometry plots (C) showed the repolarization of M2 to M1 phenotype of the macrophages, tumor cell deaths in DSN treatment by confocal analysis (D). Reprinted with permission from Ramesh A, Brouillard A, Kumar S, et al. (68). Copyright 2019, Elsevier. ns means not significant, ****p<0.0001 (one-way ANOVA).
The intratumoral injection of stimulators of interferon gene (STING) agonists could have some benefits for inhibiting breast cancer progression, however, the uneven diffusion and distribution of agonists limited the treatment efficiency (69). It can be seen that liposomal NPs can improve the diffusion and distribution of loaded drugs in tumors for better treating outcomes. Since cyclic guanosine monophosphate–adenosine monophosphate (cyclic GMP-AMP, cGAMP) is an intracellular second messenger that can activate the innate immune STING pathway, Cheng et al. designed liposomal NPs to deliver cGAMP for STING receptors in TNBC (70). Liposomal NP-delivered cGAMP reprogrammed the M2-like macrophages into M1-like macrophages in the TME, which was also accompanied with increased major histocompatibility complex (MHC) expression and CD4+ and CD8+ T cell infiltration for enhanced anti-breast tumor immunity (70). This NP-assisted delivery strategy exhibited more effectively activated STING than direct loading of cGAMP, without NP assistance in the treatment of PD-L1 insensitive TNBC.
In addition to the liposomal NPs, a hydrogel was also developed to improve the TME. Huo et al. reported the development of a CaCO3 biomineralized silk fibroin hydrogel-based dendritic cell vaccine, to further improve the immunogenicity and remodel the immunosuppressive TME for efficient TNBC immunotherapy (71). This silk fibroin-based dendritic cell vaccine was generated by fixing the 4T1 cells-DCs fusion cells’ (FPs’) membrane proteins into biomineralized CaCO3 silk fibroin hydrogel (71). The CaCO3 dendritic cell vaccine presented defined tumor-associated antigens and achieved sustained membrane protein release, which enhanced the immunogenicity and promoted the maturation and activation of dendritic cells and T cells within the TME (71). Additionally, the effective incorporation of CaCO3 in the hydrogel vaccine, promoted the polarization of M2-like macrophages to M1-like macrophages, increased pH of TME, reversed the immune-inhibitory state of the TME, and reduced the immunosuppression of T cells (Figure 2) (71).
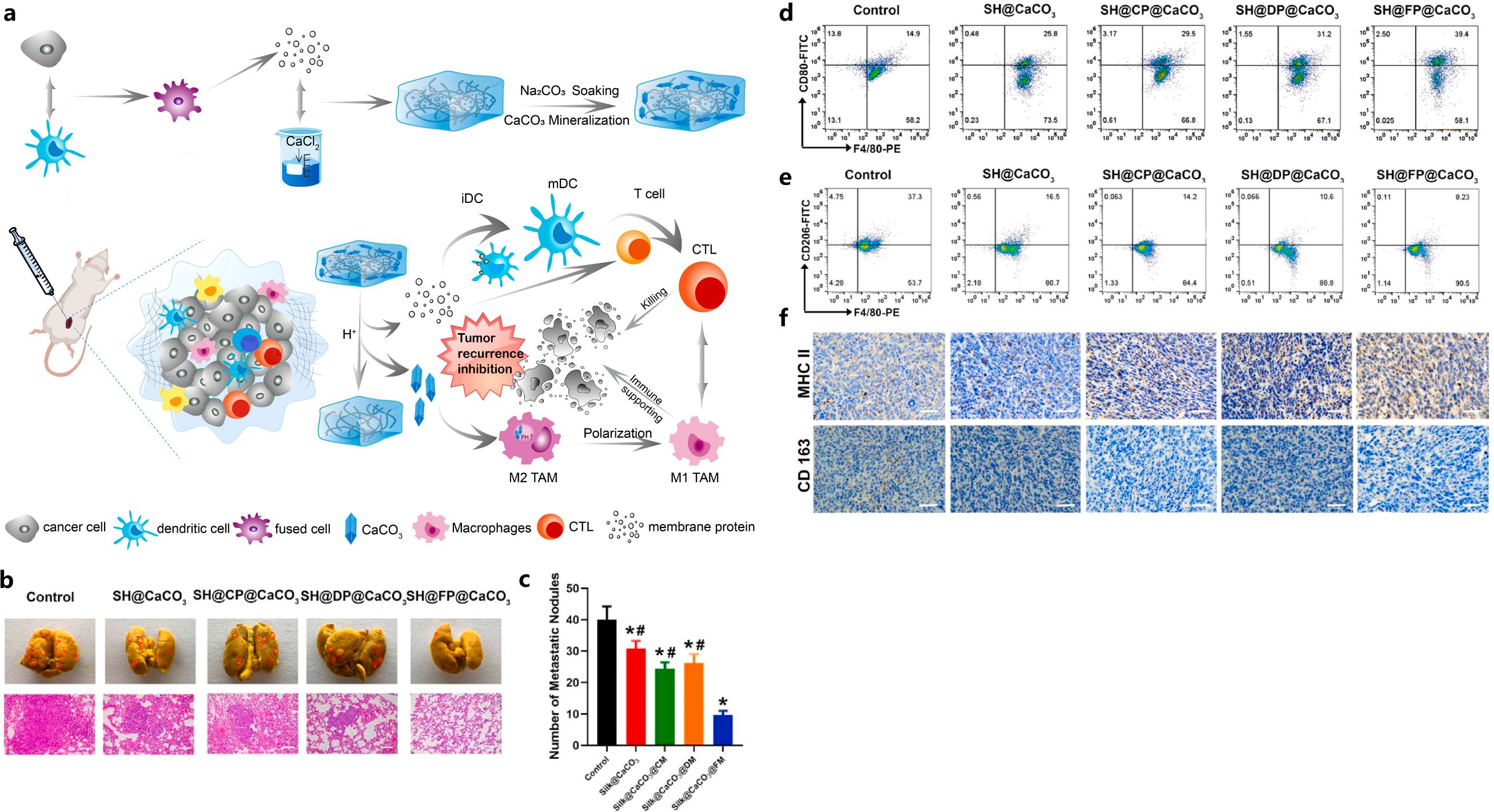
Figure 2. Illustration of FP-containing biomineralized hydrogel vaccine (SH@FP@CaCO3) that inhibited tumor recurrence (A), effects of SH@FP@CaCO3 on tumor metastasis (B, C), reduction of M2-type macrophages with SH@FP@CaCO3 treatment confirmed by flow cytometry (D, E) and IHC (F). Reprinted with permission from Huo W, Yang X, Wang B, et al. (71). Copyright 2022, Elsevier. *p<0.05 vs. control, #p<0.05 vs. SH@FP@CaCO3.
On the other hand, strategies for deleting M2-like TAMs in the TME were also explored in other cancer types. For example, M2-like TAM dual-targeting NPs (M2NPs), which were biocompatible fusion peptide-functionalized lipid NPs, were fabricated to specifically inhibit the survival of M2-like TAMs, and eventually delete them (64). Specifically, anti-CSF1-R siRNA (siCD115) was loaded on M2NPs, which structure and function were controlled by α-peptide (a scavenger receptor B type 1 (SR-B1) targeting peptide) linked with M2pep (an M2 macrophage binding peptide) (64). M2NPs efficiently delivered siCD115 into M2-like TAMs, which blocked the survival signaling pathway of M2-like TAMs and eliminated them (64). Further, M2NP-siCD115 exhibited the ability to reprogram the cytokine profile in the breast cancer TME (inhibited immunosuppressive IL-10 and transforming growth factor beta (TGF-β) production and boosted the immunostimulatory IL-12 and IFN-γ expression) and restored the IFN-γ secretion of tumor-infiltrated CD8+ T cells (64). Similarly, Jin et al. developed a melittin-RADA32-doxorubicin (DOX) hydrogel (MRD hydrogel) that exhibited interweaving nanofiber structures and provided good biocompatibility to actively regulate TME (72). The MRD hydrogel offered a melittin and DOX-based direct effect to specifically delete M2-like TAMs, activate dendritic cells of the draining lymph nodes, and produce more active cytotoxic T cells within the immunosuppressive TME (72). Due to its effects on tumor inhibition or ablation, the MRD hydrogel has shown great potential to be applied as a powerful immunotherapy in the prevention of local tumor recurrences after surgical resections in breast cancer.
Moreover, except the reverse of immunosuppressive TME, Song et al. reported the significance of adjusting the lymph nodes’ immunosuppressive microenvironment for effective anti-breast cancer immunotherapy (73). The researchers designed albumin NP, Nano-PI, containing phosphatidylinositol 3-kinase γ (PI3Kγ) inhibitors IPI-549 and paclitaxel (PTX) (73). Nano-PI achieved immunomodulator delivery to macrophages in both tumor sites and the lymph nodes, with the combination of α-PD1. M2 to M1 macrophage repolarization occurred in the microenvironment of both the tumor tissue and the lymph nodes (73). At the same time, increased infiltration of CD4+ and CD8+ T cells, B cells, and dendritic cells, and T cell exhaustion prevention, was demonstrated. The combination of Nano-PI and α-PD1 achieved the modulation of the immune microenvironment in both tumors and lymph nodes (73). Aimed at breast cancer bone metastases, biomaterials have represented a promising approach. For instance, a multi-functional CePO4/chitosan (CS)/graphene oxide (GO) scaffold was formed by hydrated CePO4 nanorods, bioactive CS and GO NPs, which promoted the switch of M2 macrophages to M1 phenotype by releasing Ce3+ ions (74). As a result, this CePO4/CS/GO scaffold-based treating method induced the apoptosis of breast cancer cells, inhibited angiogenesis, bone metastases and promoted tissue with osteo-inductive capabilities (74).
Finally, NP according to the specific biological properties of macrophages, to enhance treating performance for lung metastasis of breast cancer, was designed. Isolated macrophage membranes were coated on emtansine liposome, conferring the biomimetic functions of macrophage. This system promoted the targeting specificity to tumor metastatic sites, and improved the delivery efficiency (43).
Overall, TAMs play a significant role in immuno-regulation and breast tumor developmental phases (75, 76). Different biomaterial-based strategies could be considered according to the specific characteristics of TAMs in a given breast cancer TME. For example, repolarizing M2 TAMs into M1 phenotypes, blocking M2 TAMs tumor-promoting activities or directly killing the M2 TAMs can be used alone or together for treating different breast cancer patients. The application of multifarious biomaterials, such as different liposomal NPs, biocompatible hydrogels (e.g., silk fibroin), graphene-modified nanorods combined with biomolecules specifically targeting M2 TAMs in breast cancer TME, holds great potential for treating breast cancer clinically in the future. The representative studies that explored the use of biomaterials targeting M2 TAMs for breast cancer treatments are summarized in Table 1.
3.2 Cancer-associated fibroblasts (CAFs)
CAFs possess a myofibroblastic phenotype and could be found both in primary and metastatic tumors, occupying a great proportion in the tumor stromal cell population (77–79). In breast cancer, CAFs could originate from breast tissue resident fibroblasts, bone marrow-derived fibroblasts, adipocytes, mesenchymal stem cells or pericytes (80, 81). Generally speaking, CAFs can be activated by tumor cell-derived TGF-β and their activation could be sustained by more TGF-β released from the activated CAFs in the whole population (36, 41). The activated CAFs could further secrete a series of growth factors and cytokines/chemokines to modulate the tumor cells and TME, including hepatocyte growth factor (HGF), epidermal growth factor (EGF), TGF-β, interleukins (such as IL-1, IL-4, IL-6, IL-8, IL-10), chemokines (such as CXCL1, CXCL12, CXCL14, CCL2, CCL5, CCL7) (80–82).
3.2.1 CAFs’ interactions with other cell types within breast cancer TME
CAFs could participate in ECM remodeling by producing ECM proteins or matrix metalloproteinases (MMPs) such as several types of collagens, fibronectin, MMP2, MMP9 (41, 80). This indicates the enabling role of CAF-mediated ECM to remodel in tumor cell migration, invasion and metastasis (41, 80). The complex interactions between CAFs and components in the tumor TME, including cancer cells, T cells, myeloid cells, and endothelial cells, has enabled their critical role in the progression of cancer, ECM remodeling, and metastasis, and has also made them an important potential targeting point for cancer immunotherapy (80).
What is more relevant, is that it has been found that CAFs and the immune cells in the TME can regulate each other’s functions via paracrine signaling pathways (41). Therefore, strategies targeting CAFs to enhance the infiltration and activation of T-cells could be effective to reverse the immunosuppressive TME and achieve good treatment outcomes for cancer patients. At present, the CAF-targeting therapeutic strategies in breast cancer could be achieved via multiple pathways, including blocking the activation of CAFs via TGF-β and Hedgehog signaling pathway inhibition, applying fibroblast-activation protein (FAP) vaccine, FAP-CART cells and inhibitors to target the activated CAFs, and targeting the different growth factors, chemokines, cytokines secreted by CAFs (83).
Overall, the remodeling of the immunosuppressive TME, by ablating the CAFs, reprogramming the activated CAFs, and targeting CAFs’ growth factor/cytokine production and secretion networks, hold great promise for effective breast cancer immunotherapy.
3.2.2 Different biomarkers expressed by CAFs
The activated CAFs express some biomarkers at a significantly different level when compared to normal fibroblasts, such as α-SMA, vimentin, fibroblast-specific protein 1 (FSP1), FAP, caveolin-1, desmin, discoidin domain-containing receptor 2 (DDR2), and platelet-derived growth factor receptors alpha and beta (PDGFRα/β) (41, 84, 85). CAFs expressing different markers participate in different pathways (84, 85). For example, the FAP+/Vimentin+ CAFs could secret MMP1, collagen and TGF-β could modulate the ECM remodeling and tumor cell metastasis, while α-SMA+/FAP+/CD29+/PDPN+/PDGFRβ+ CAFs could secrete TGF-β and CXCL12 to affect tumor cell proliferation, migration and epithelial-mesenchymal transition (EMT) (83).
Some researchers have explored CAFs-targeted therapeutic strategies according to the specific biomarkers expressed on the CAFs (81, 86, 87). FAP, a membrane-bound serine protease, can act as a tumor targeting antigen, with relevant clinical drugs targeting FAP (81, 86, 87). However, since FAPs are extensively expressed in both tumor tissues and normal tissues [e.g., embryo (88), bone marrow (89) and placenta (90)], serious side effects or even death can be caused by systematic FAP-targeted therapy. As a result, biomaterial-based strategies targeting biomarkers expressed by CAFs have been explored for more effective cancer treatments, as compared with systematic biomarker-targeted therapies.
3.2.3 Biomaterial-based strategies targeting CAFs for breast cancer treatments
Both biomaterial-based NPs and hydrogels have been explored to target CAFs for more effective breast cancer treatments. For instance, in order to selectively kill CAFs without leading to systemin toxicity, Zhen et al. developed a NP-based, FAP-targeted photo-immunotherapy that could selectively kill CAFs in breast tumors (81). Specifically, ZnF16Pc (a photosensitizer) was physically entrapped within ferritin (FRT, a compact nanoparticle protein cage), and a FAP-specific single chain variable fragment (scFv) was conjugated on the surface of FRT (81). This nanoparticle-based photoimmunotherapy (nano-PIT) approach could efficiently target and eliminate CAFs in breast tumors but exhibited little damage to healthy breast tissues. Further, CXCL12 secretion and ECM deposition was suppressed (81), and the tumor suppressive environment was reversed with enhanced cytotoxic T cell infiltration (81). Therefore, this photo-immunotherapy supplied a new approach for selectively targeting CAFs toward enhancing immunity and modulating the breast cancer TME to better treat breast cancer patients. Additionally, Sitia et al. engineered H-ferritin nanocages, loaded with navitoclax (Nav, a Bcl-2 inhibitor pro-apoptotic drug), could specifically bind to FAP+ CAFs in a mouse model of TNBC by conjugating the FAP antibody fragments on H-ferritin nanocages (91). Nav-loaded nanocages achieved efficient targeted delivery and yielded controlled drug release profile in FAP+ CAFs toward improving the breast cancer TME (91).
Moreover, CAF-targeted drug delivery nano-systems were also investigated in a xenograft mouse model of MCF-7 breast cancer (87). A cleavable amphiphilic peptide (CAP) containing a TGPA sequence that can be digested by FAP-α was designed to specifically respond to FAP-α expressed on the CAFs’ surfaces in tumor (87). CAP could self-assemble into fiber-like nanostructures in solution, while the self-assemblies can be transformed into stable drug-loaded spherical NPs (CAP-NPs), in the presence of hydrophobic chemotherapeutic drugs such as DOX, irinotecan (Iri) and paclitaxel (Tax) (87). It was found that cleavage enabled by FAP-α being expressed on the surface of CAFs, can rapidly disassemble the NPs to yield the efficient release of the encapsulated drugs specifically targeting at breast cancer sites. Such a transformer-based system can significantly increase local drug accumulation by disrupting the stromal barrier (87). Since FAP-α is commonly expressed and activated in most human tumors (92, 93), this FAP-α-targeted drug delivery nano-system has great application potential.
Aside from NPs, hydrogel was also explored for regulating CAFs in 4T1 breast tumors. The peptide C16-GNNQQNYKD-OH (C16-N), which can self-assemble into supramolecular long filaments with hydrophobic cores, was co-assembled with losartan to form a hydrogel (94). This injectable peptide hydrogel inhibited the activity of CAFs, reduced the growth factor secretion of CAFs, and perturbed collagen synthesis. Further, it improved the efficacy of PEGylated DOX-loaded liposomes (Dox-L) toward inhibiting breast tumor growth, demonstrating the potential of an injectable hydrogel in regulating CAFs toward enhancing the chemotherapy effectiveness for breast cancer (94). In addition, puerarin nano-emulsion (nanoPue), with the ability of CAFs targeting by modifying with aminoethyl anis amide (AEAA) was developed. The AEAA-modified NPs were able to accumulate in CAFs and down-regulate reactive oxygen species (ROS) production to deactivate CAFs and improve the stromal microenvironment in murine TNBC model (with approximately a 6-fold reduction of CAFs in the nanoPue treatment group vs. control group), which also significantly enhanced the chemotherapy efficacy of paclitaxel (95). It was demonstrated that nanoPue effectively removed the intra-tumoral physical barrier formed by the dense ECM components produced by CAFs. As well, it increased the intra-tumoral infiltration of cytotoxic T cells and the activated immune activities in TME that further enabled nanoPue to synergize PD-L1 blockade therapy (Figure 3) (95).
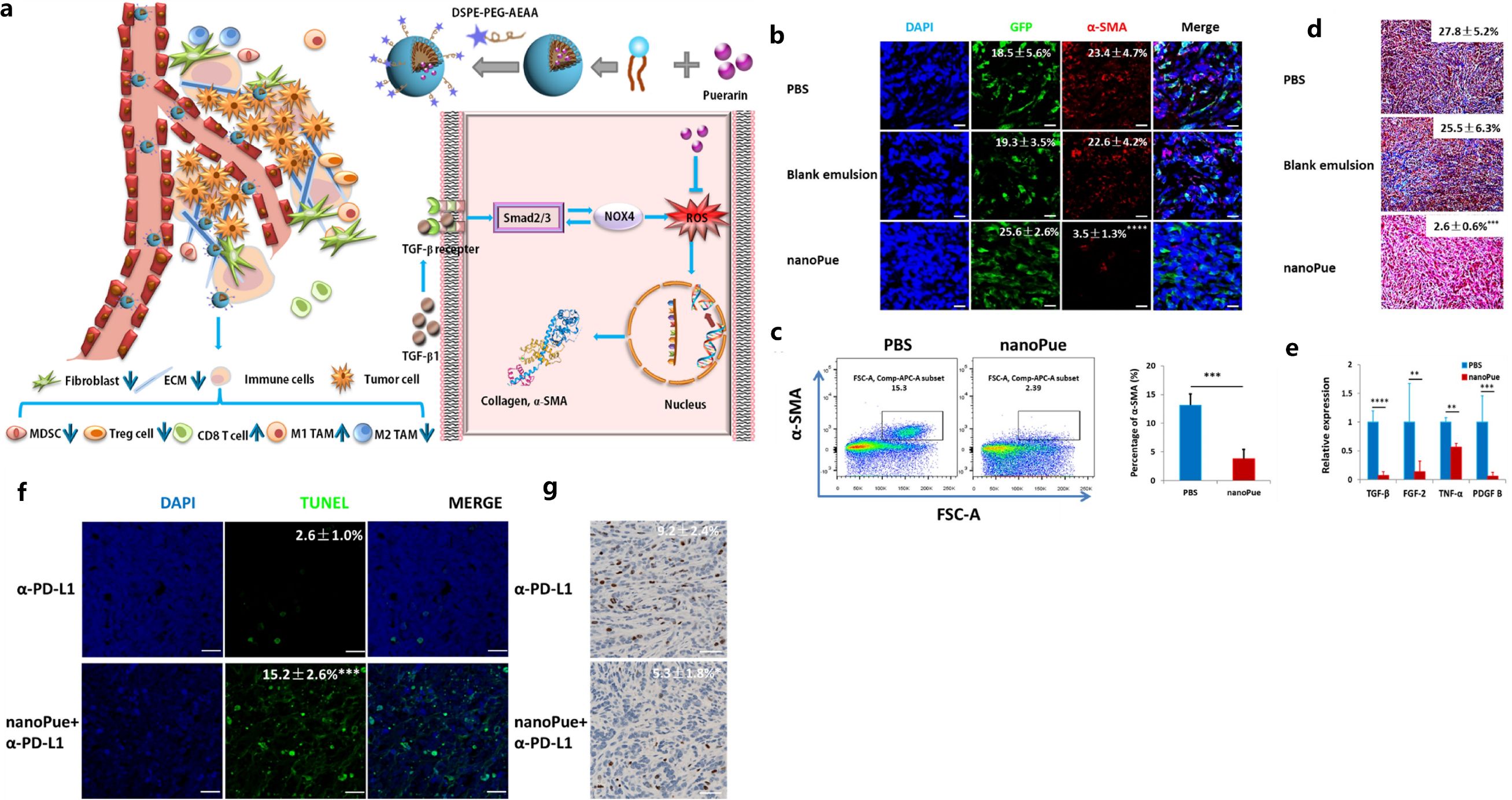
Figure 3. Illustration of TME remodulation by targeted puerarin delivery (A), reduction of α-SMA positive TAFs in tumors by immunofluorescence staining (B) and flow cytometry analysis (C), reduction of collagen by Masson’s trichrome staining (D) and TGF-β by RT-PCR (E), higher apoptosis (F) and down-regulated Ki67 expression (G) by the nanoPue in combination with α-PD-L1. Reprinted with permission from Xu H, Hu M, Liu M, et al. (95). Copyright 2020, Elsevier. **p<0.01, ***p<0.001, ****p <0.0001.
Further, site-specific release of anti-breast cancer drugs, triggered by special proteins expressed in TME, has been investigated to remodel the immunosuppressive TME and improve the breast cancer immunotherapy efficiency. For instance, considering the high MMP2 expression level in breast cancer TME, Zhang et al. developed MMP2-sensitive lipid layer constructs, and demonstrated controlled release of LY3200882 (a TGFβ inhibitor) in MMP2-abundant TME (96). In addition, the combined release of LY3200882 and PD-L1 siRNA was achieved by the site-specific liposome-based delivery system to further enhance the anti-TNBC immunotherapy efficacy (96). The PD-L1 expression on CAFs decreased with the PD-L1 siRNA treatment, and LY3200882 caused reduced production of breast cancer ECM which promoted the nanomedicine and immune effector cells’ penetration into the tumor tissues (96). The dual inhibition of TGF-β and PD-L1 for both breast cancer cells and CAFs can effectively reverse the immunosuppressive TME for TNBC, suppress breast cancer cell proliferation and metastasis with minimal side effects, paving a new therapeutic strategy targeting breast cancer in the future (97).
Although some biomaterial-based strategies targeting CAFs for breast cancer treatments have shown some promise, there are still limitations. One of the significant challenges for targeting CAFs is their heterogeneity in the breast cancer TME and the lack of in-depth understanding of the specific biomarkers expressed, as well as signaling pathways triggered by the different CAF subtypes (41). As a result, clinical therapies targeting CAFs or the related signaling pathways sometimes do not show desirable treatment efficacy due to the presence of complicated tumor-restraining CAF subtypes that exhibit a suppression role in tumor progression (98–100). Thus, distinct therapeutic regimens could be considered for targeting the different subtypes of CAFs in the future breast cancer drug designs (80). Several biomarkers that have been regarded as being “specific” to CAFs, and utilized in previous anti-cancer drug designs, have recently been shown to be expressed on other cell types (36, 80). For example, FAPα is not only expressed on CAFs, but is also highly expressed in mesodermal cells (80). Similarly, α-SMA is not specific to CAFs either, but is abundantly present on normal fibroblasts, pericytes and smooth muscle cells (80). Those findings implied that anti-breast cancer therapies targeting those unspecific biomarkers can result in limited drug efficacy and future studies need to spend more efforts to identify the unique proteins or signaling molecules associated with the different CAF subtypes (36). Representative studies which have explored the use of biomaterials targeting CAFs for breast cancer treatments are summarized in Table 2.
3.3 Myeloid-derived suppressor cells (MDSCs)
MDSCs are a class of bone marrow-derived inflammatory progenitor cells that play immunosuppressive roles in tumors. They are incapable of differentiating into dendritic cells, granulocytes, or macrophages (101). MDSCs could impair the normal function of effector immune cells to influence the TME (102).
3.3.1 Interactions between MDSCs and breast tumor cells within TME
Release of breast tumor-derived inflammatory factors and chemokines within the TME can significantly stimulate MDSCs’ proliferation and infiltration in cancer tissues (103, 104). A series of proinflammatory cytokines such as interferon-γ (IFN-γ), IL-1β, IL-4, IL-13, PGE2, as well as STAT1, STAT6, nuclear factor-κB (NF-κB) signaling pathways all participate in MDSC activation (105). Once the MDSCs are activated, they could accelerate breast tumor growth and metastasis by generating more ROS, nitric oxide (NO) and immunosuppressive factors such as IL-10, impairing the anti-tumor immunity via deleting arginine and inhibiting CD8+ T cells (96, 106–108). Studies have shown the correlation between the MDSCs levels in peripheral blood/primary tumor tissues and the progression/metastasis status of breast cancer patients (109, 110). As a result, considering the MDSCs’ immunosuppressive characteristics, therapies targeting MDSCs’ recruitment, proliferation, and differentiation could be effective in terms of treating breast cancer patients.
3.3.2 Biomaterial-based strategies targeting MDSCs for breast cancer treatments
Clinically, MDSCs-targeted strategies involved depletion, differentiation, modulation, and immunosuppressive activity inhibition of the MDSCs (105). In order to increase breast cancer immunotherapy efficacy, some researchers have developed biomaterials to improve the immunosuppressive TME via targeting MDSCs. Considering the fact that inflammatory reactions are often inevitable after surgical resection, Lu et al. developed hyaluronic acid (HA)-coated chitosan oligosaccharide-all-trans-retinoic-acid (COS-ATRA) micellar NPs, loaded with DOX (HA@CA/DOX) to modulate TME and alleviate the inflammatory responses after resection in 4T1 breast cancer model (104). In the HA@CA/DOX NP system, it was demonstrated that the hydrophilic COS and hydrophobic ATRA blocked the NF-κB pathway in breast cancer cells/MDSCs to reduce inflammation after resection, while HA shielded the excessive positive charge and enhanced breast cancer cell targeting via CD44 expressed on the cell surfaces (104). Additionally, ATRA eliminated the MDSCs not only in the breast tumor tissues, but also in the lung tissues after metastasis, thus inhibiting the formation of a pre-metastatic niche (PMN) (104). Moreover, to improve the MDSC deletion efficiency, they also developed NPs using low molecular-weight-heparin-all-trans-retinoic-acid (LMWH-ATRA) modified by a tumor targeting c(RGDfk) peptide and loaded with DOX, and immune adjuvant (α-galactosylceramide, αGC) (103). Such RLA/DOX/αGC NPs improved the immunosuppressive breast cancer TME, with αGC significantly enhancing the anti-tumor immunity in the breast and lung tissues (103). Specifically, it was found that the hydrophilic segment LMWH could tightly bind with P-selectin expressed on vascular endothelial cells (VECs), which inhibited MDSC recruitment, while the hydrophobic ATRA induced MDSC differentiation and depletion (103).
Indoleamine 2,3-dioxygenase (IDO), which is a rate-limiting enzyme of tryptophan catabolism, was revealed to be unregulated in tumor-infiltrating MDSCs and could hinder the anti-tumor immunity (109). Qiao et al. (111) developed a folated pH-degradable poly (vinyl alcohol) (PVA)-based nanogel (FA-NG) to simultaneously deliver docetaxel (DTX) and IDO1 inhibitor NLG919 (N9) to modulate the MDSCs’ infiltration in breast cancer tissues (111). For the FA-NG, PVA was modified with vinyl ether acrylate (VEA) groups for UV-crosslinking and degradation in response to low pH in TME (111). The study showed that FA-NG could be efficiently taken up by breast cancer cells followed by endo/lysosomal pH-triggered intracellular drug release, in which DTX induced cytotoxicity effects and immunogenic cell death (ICD), while N9 inhibited the IDO1-mediated immunosuppression and led to enhanced infiltration of CD8+ T cells/NK cells and reduced infiltration of MDSCs (111). This PVA-based degradable nanogel system provided a potential avenue for combining chemotherapy and immunotherapy to re-activate anti-tumor immunity in breast cancer treatment.
Aiming at reducing MDSC infiltration in breast cancer and pulmonary metastasis, Luo et al. designed a cathepsin B/pH dual-sensitive block copolymer conjugated with DOX, which was then loaded with nifuroxazide (NFX) to self-assemble into co-prodrug-loaded micelles (CLM) (112). This micelle could be delivered to the tumor site, and DOX was released upon pH/enzyme stimuli, which caused tumor cell apoptosis, reduced expression of MMPs, and decreased MDSC infiltration (Figure 4) (112). DTX@VTX NPs (DTX: Docetaxel and VTX: VTX-2337 or Motolimod) with a core/shell structure were developed to address the immunosuppressive character of the breast cancer TME by depleting MDSCs and repolarizing the macrophages from M2-like phenotype to M1-like phenotype (113). Interestingly, it was found that the simultaneous application of DTX@VTX NPs with BMS-1 (a small-molecule PD-1/PD-L1 nano-inhibitor) NPs achieved synergistic chemo-immunotherapeutic anti-breast cancer effects when compared to the use of any single type of NPs (113).
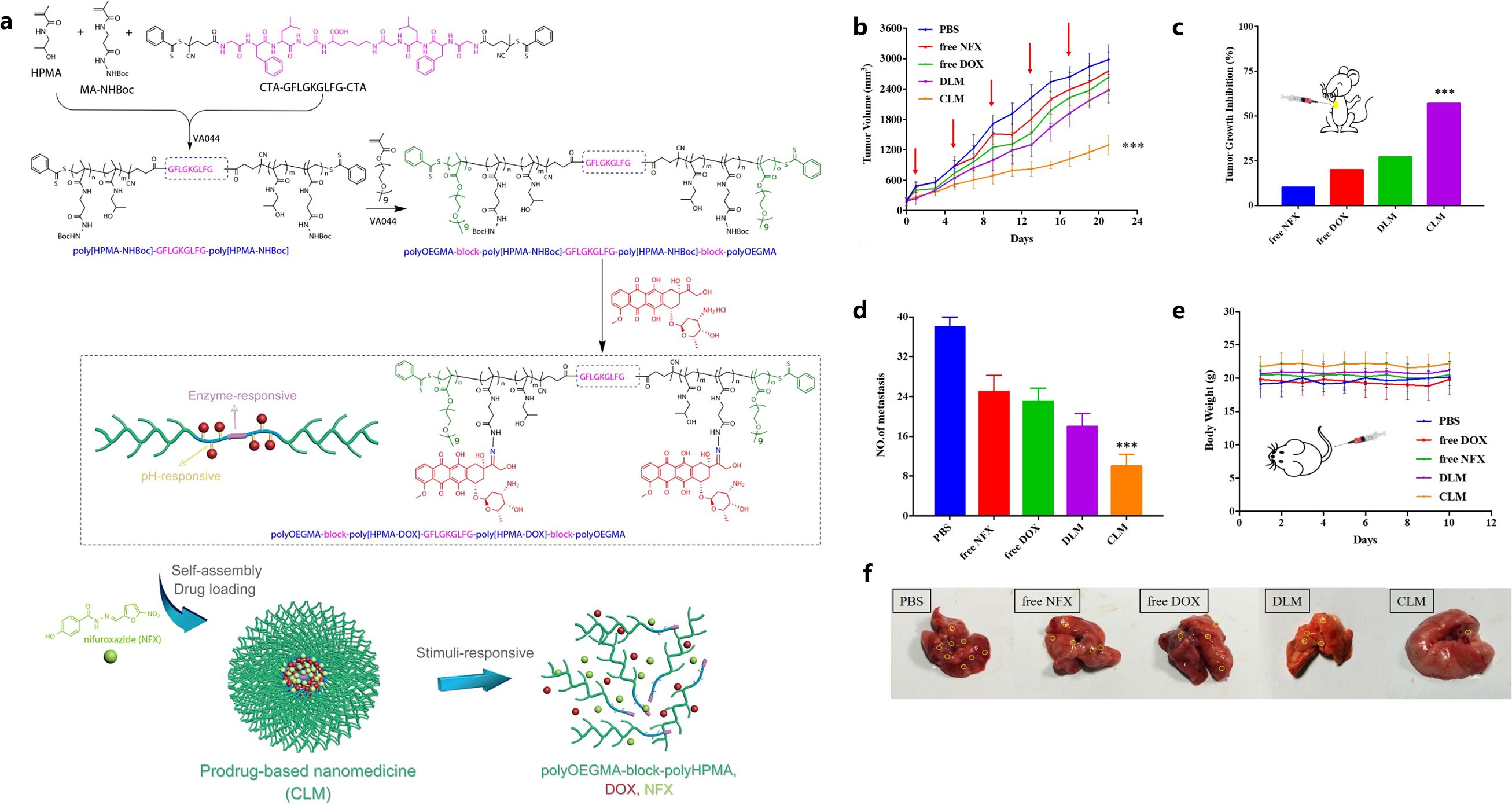
Figure 4. Illustration of CLM formation and stimuli-responsive drug release and degradation (A), tumor growth curves (B), tumor growth inhibition rate (C), mean lung metastasis nodule numbers (D), body weight changes (E), visualized lung metastatic nodules (F). Reprinted with permission from Luo L, Xu F, Peng H, et al. (112), Copyright 2019, Elsevier. ***p< 0.001.
Chemokine CCL2 is positively associated with increased numbers of M2-like macrophages and MDSCs (114). Liu et al. fabricated a targeted lipid-protamine-DNA (LPD) NP to deliver the plasmid DNA encoding CCL2 trap into TME, which can significantly reduce the numbers of M2-like macrophages and MDSCs in TNBC by blocking the CCL2/CCR2 signaling pathway (114). The LDP NPs was constructed by cationic protamine with plasmid DNA (pDNA) coated with DOTAP (1,2-dioleoyl-3- trimethylammonium propane chloride salt) liposome, and polyethylene glycol (PEG) was coated on the surface of LPD NPs, DSPE–PEG and the target ligand DSPE–PEG–AEAA were grafted onto the surface of liposomes. Specifically, the CCL2 trap could bind to CCL2 with a high affinity and specificity, which led to a significant reduction of CCL2 levels within breast cancer tissues, and increased infiltration of cytotoxic T cells (114).
Finally, it has been observed that complicated growth factor/cytokine/chemokine networks are responsible for regulating the proliferation, differentiation and activation of MDSCs, and that the latter cells are oftentimes heterogeneous populations that could modulate the TME via different mechanisms (115, 116). For example, in TNBC, monocytic-MDSCs (HLA-DR−CD33+CD14+) occupied predominant subsets when compared to granulocytic-MDSCs (HLA-DR−CD33+CD15+) (117). Therefore, different strategies based on MDSCs’ heterogeneity in distinct breast cancer subtypes could be considered in the future biomaterial-based immunotherapies targeting MDSCs. The representative studies that explored the use of biomaterials targeting MDSCs for breast cancer treatments are summarized in Table 3.
4 Biomaterials targeting other immune cells in TME to improve breast cancer immunotherapy
The number and composition of infiltrated innate and adaptive immune cells varies significantly in the TME of different breast cancer subtypes, and the infiltrated immune cell ratios could influence the anti-cancer immunotherapy outcomes (118, 119). Breast tumor infiltrating lymphocytes (TILs), such as helper T cells (CD3+/CD4+), cytotoxic T lymphocytes (CTL, CD3+/CD8+) and Tregs cells, were observed in breast tumors, and they were key indicators for breast tumor immunogenicity (107, 120). It was shown that high CD8+ T cells/Tregs ratios indicated favorable prognosis in primary breast tumors (121). Currently, facilitating the infiltration of beneficial immune cells to improve the immunosuppressive TME to achieve better breast cancer treatment efficiency remains a challenge. In the following, biomaterial strategies targeting other beneficial immune cells, including dendritic cells, natural killer (NK) cells, and T lymphocytic cells in the TME to improve breast cancer immunotherapies are discussed.
4.1 Dendritic cells (DCs)
Robust activation of tumor-specific antigen-presenting cells (APCs) warrants the efficiency of cancer immunotherapy (122, 123). DCs are representative APCs that are capable of capturing, processing, and presenting tumor antigens, and are crucial for inducing and regulating the innate and adaptive immune responses by activating T cells (124, 125). Thus, DCs typically play a positive role in inducing cancer immunity, and they could be classified into conventional DCs (cDCs), plasmacytoid DCs (pDCs) and monocyte-derived DCs (MoDCs), according to their ontogeny, phenotype and anatomical location (126). In TNBC, the ratio of cDCs:pDCs was higher when compared with other breast cancer subtypes (127) and it has been revealed that high levels of pDCs and cDCs are related with better survival outcomes for breast cancer patients (127, 128). It has also been reported that the abundancy of DCs is related to the sensitivity of chemotherapy drugs in breast cancer (129).
4.1.1 DCs in breast cancer immunotherapy
It is known that DCs are vital targets in breast cancer immunotherapy and DCs-based vaccines have been developed as one of the main strategies to boost the anti-tumor immunity (130, 131). Currently, DC-based cancer vaccines mainly include MoDCs generated in vitro, DCs derived from CD34+ hematopoietic precursors, DCs isolated from the circulating blood, as well as allogeneic pDCs and pDC-derived exosomes (132). At present, DC-based immunotherapies used to potentiate host effector and memory CD8+ T cell responses have been widely studied and started to be applied clinically as DC vaccines (133). However, those different sources of DCs and DC-derived exosomes have shown limited anti-breast cancer efficacy in clinical applications due to DC population heterogeneity, high complexity of the breast cancer TME (133) and low DC targeting specificity for different subtypes of breast cancer tissues (132).
4.1.2 Biomaterial-based DC strategies for breast cancer treatments
Recently, biomaterials have been explored to enhance the anti-breast-cancer effects of DCs. For example, Poly-lactide-co-glycolic acid (PLGA) is a biodegradable and relatively biocompatible material approved by FDA with great potential in vaccine delivery. PLGA NPs can enhance breast cancer antigen presentation (134). PLGA NPs that encapsulated tumor lysate antigens, derived from breast cancer tissues, can significantly enhance DC maturation and T cell immune response activation, with high targeting specificity, controlled release of antigen and improved antigen stability (135). At present, the main strategy for efficient and specific delivery of PLGA NPs to DCs within the TME include the coupling of NPs with monoclonal antibodies against DC specific receptors or natural ligands associated with DC endocytic receptors (e.g., mannan, a ligand for mannose receptor (MR) of DCs) (134). Mannan-incorporation of PLGA NPs resulted in their increased uptake by DCs vs. the non-modified control (134). Further, in breast tumor-bearing mice, Sanaz et al. investigated the effects of mannan-coupled PLGA NPs that contained both breast tumor cell lysate and poly-riboinosinic polyribocytidylic acid (poly I:C), which is a synthetic analog of dsRNA that can stimulate DC activation (124). It was found that simultaneous use of breast cancer cell lysate and DC-stimulating adjuvant in this PLGA NP system achieved efficient activation of DCs, with a large quantity of concomitant tumor associated antigens (TAAs) and C-type lectin receptor (CLR) ligands, causing elevated T cell responses and improved the breast cancer TME (124).
Antigen-based strategies targeting MRs of DCs showed some effectiveness in boosting the immunological effects of DCs in treating breast cancer. As well, other therapeutic avenues for DCs modulation were also exploited for breast cancer treatment. For instance, Wu et al. prepared the N-alkyl-PEI2k-LAC/SPIO nanocomposites that aided the transfection of siRNA targeting indoleamine 2, 3-dioxygenase (IDO) and enhanced MRI labeling efficiency in DCs (133). It was found that this N-alkyl-PEI2k-LAC/SPIO system minimized the degradability of exogenous siRNAs in vivo by loading and protecting those gene fragments, achieving the anti-tumor function by modulating DC vaccines (133). Additionally, chitosan-shelled nanobubbles (NBs) were functionalized with anti-CD1a antibodies to target DCs in breast cancer TME (136). The antiCD1a-functionalized NBs induced DC activation and enhanced their immune responses to inhibit tumor growth, showing great potential for breast cancer treatment (136). The representative studies that explored the use of biomaterials targeting DCs for breast cancer treatments are summarized in Table 4.
4.2 Natural killer (NK) cells
NK cells, which belong to the innate lymphoid cell family (145), are derived from the multipotent CD34+ hematopoietic progenitors in bone marrow and they mature in the bone marrow and lymphoid organs (146). As a type of natural cytotoxic lymphocyte, they can recognize and kill MHC I-low or MHC I-negative breast cancer cells that have escaped from CD8+ cells with minimal damage to healthy tissues due to their expression of specific stimulatory and inhibitory receptors (145, 147). Therefore, NK cells are important anti-tumor agents to be considered in cancer immunotherapy (145, 148).
Human tissues mainly consist of two NK subpopulations (CD56bright and CD56dim) with different functional and metabolic characteristics (149). 80%-90% of CD56dim NK cells are present in human blood, bone marrow, lung and spleen, and only less than 20% of them exist in the lymph nodes (149). Allogeneic NK cells have great potential for cancer immunotherapy because they can be easily accessed from peripheral blood, umbilical cord blood or post-partum placenta (150). In addition, it was found that gap junctions, membrane nanotubes and exosomes are key mediators enabling the effective intercellular communications between NK cells and cancer cells, which are critical for the accumulation of ions, proteins and cytokines in cancer cells, enabling the induction of their apoptosis or necrosis (151).
4.2.1 Interactions between NK cells and other immune cells within the TME
Moreover, cross-talk between NK cells and other immune cells enables the cooperation between them in order to regulate their anti-tumor immunity (152–154). Some factors produced by NK cells, such as IFN-γ, CCL5, XC-chemokine ligand 1 (XCL1) and FMS-related tyrosine kinase 3 ligand (FLT3L) could interact with T cells and DCs in the TME to enhance the T cell responses, and NK cells could also directly kill the inhibitory MDSCs (152–154). Immunosuppressive TME played a negative role for NK cell activation and limited their anti-tumor effects by disturbing their energy consumption and metabolism patterns (148). Some soluble factors in the TME, such as TGF-β, prostaglandin E2, L-kynurenine and picolinic acid, could reduce NK cells’ proliferation, activation and cytotoxicity effects (150). What’s more, Tregs can also inhibit NK cell function via TGF-β-related signaling pathways (155, 156). Therefore, modulating the abundance and activation of NK cells at tumor sites is critical for effective anti-tumor treatments. It has been well-acknowledged that the NKs’ anti-tumor activity can be modulated via altering the balance of stimulatory and inhibitory signals in the TME, including cytokines/receptors’ expression patterns (157).
4.2.2 Biomaterial-based NK strategies for breast cancer treatments
Since the hypoxic and nutrient-lacking TME could suppress NK cell metabolism and their cytotoxic function (158), improving TME hypoxia with the help of biomaterials has been investigated to enhance the potency of NK cells. For instance, PLGA-encapsulated-MnO2 NPs were designed to sustain high oxygen level in the core of cancer spheroids, in order to boost NK cell cytotoxicity (137). Specifically, the MnO2 NPs were used to catalyze the degradation of tumor-produced H2O2, producing oxygen in the TME. It was found that PLGA encapsulation endowed MnO2 NPs with improved biocompatibility, enabled a first-order oxygen production profile and sustained high oxygen tension when compared to bare MnO2 NPs, in the presence of H2O2 (137).
To enhance the expansion and activation of NK cells in tumor sites, degradable biomaterials were explored to deliver immune stimulating molecules and improve the anti-breast tumor immune responses. For example, acetylated dextran (Ace-DEX) microparticles (MPs) were designed to deliver pathogen associated molecular patterns (PAMPs) to induce immune response (138). Cyclic GMP-AMP (cGAMP) MPs exhibited the most effective anti-tumor efficacy, causing an increase in NK cell number in the TME. These MPs activated NKs and T cells-dependent anti-tumor immune responses and exhibited significant tumor inhibition effects in both melanoma and TNBC models (138). In other work, an immuno-NP made of 1,2-dioleoyl-sn-glycero-3-phosphocholine (DOPC), 1,2-dipalmitoyl-sn-glycero-3-phosphocholine (DPPC) and methoxy-poly (ethylene glycol)-1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N (mPEG2000-DSPE) was investigated for treating breast cancer (139). Cyclic diguanylate monophosphate (cdGMP, an agonist of the STING pathway) and mono-phosphoryl lipid A (MPLA, a toll-like receptor 4 agonist) were both encapsulated into the DOPC-DPPC-mPEG2000-DSPE NPs, which significantly upregulated the expression of IFN-β and boosted the activity of APCs and NK cells in blood, and tumor tissues in the TNBC model (139). When considering the high expression pattern of PD-L1 and CD155 in TNBC cells, Chen et al. developed the PD-L1 and CD155 asynchronous blockade NP system (140). Specifically, CD155 siRNA (siCD155) was loaded in mPEG-PLGA-PLL (PEAL) NPs coated with PD-L1 blocking antibodies (P/PEALsiCD155), which modulated CD155-mediated immune surveillance in the immune escape TNBC model, causing increased NK cell number, reduced percentage of Tregs, significant inhibition of breast cancer cell growth and metastasis and induction of ICD (140). The representative studies that explored the use of biomaterials targeting NKs for breast cancer treatments are summarized in Table 4.
4.3 T lymphocyte cells
T lymphocyte cells refer to the immune cells that are derived from bone marrow and mature in thymus. These cells could specifically recognize antigens via the TCR on their cell surface in order to exhibit robust lethality for infected cells (159). The infiltration of CD8+ T cells is associated with breast cancer patient survival (160), while CD4+ T helper cells also play a critical role in anti-tumor immune response (161). Immune checkpoints and receptors, which exhibit their suppressive role to inhibit or exhaust T lymphocyte cells and prevent the potential damage from excessive immune responses, are promising immunotherapeutic targets in cancer treatment (101, 162). These include PD-1 and its ligand PD-L1, cytotoxic T lymphocyte antigen 4 (CTLA-4), T cell immunoglobulin and mucin domain-containing 3 (TIM3), IDO, lymphocyte activation gene 3 (LAG3) and V domain Ig suppressor of T cell activation (VISTA) (101, 162). PD-1, which acted as a suppressive factor in TME, is a potent therapeutic target in breast tumor immune therapy (163). The interactions between PD-1 (expressed on tumor-infiltrating T cells surface) and PD-L1 (expressed on the cancer cells or antigen-presenting cells), negatively regulate the activation of cytotoxic T cells and can lead to T cell exhaustion and suppress the immune responses against tumor cells (164). Therefore, targeting PD-1 or PD-L1 could effectively improve the immunosuppressive TME. The antibodies that bind to the above inhibitory receptors or ligands to enable T cell activation could enhance the anti-tumor activity.
Currently, several immune checkpoint-targeting antibodies have been approved by the FDA, such as monoclonal antibodies pembrolizumab (KEYTRUDA) and nivolumab (OPDIVO, anti-PD-1), atezolizumab, avelumab, durvalumab (anti-PD-L1) and ipilimumab (anti-CTLA-4), which could increase overall survival of patients for different cancers (162, 165). However, the different immune status of patients and their specific immune checkpoint blocker expression levels can affect the treatment efficacy for immune checkpoint targeting antibodies (166).
In addition to the traditional approaches that indirectly promote the activation of T cells, biomaterial-based breast cancer immunotherapies which stimulate proliferation, differentiation, activation and infiltration of T cells and induce the tumor-specific T cell responses have been explored.
4.3.1 Biomaterial-based strategies targeting T cells for breast cancer treatments
At present, biomaterials have been used to design some antibody-free molecules targeting for PD-1/PD-L1 blockade and T cell-mediated anti-tumor immunity, for enhancing the effectiveness of breast cancer treatments. For example, a liposome system was designed to co-deliver chidamide (CHI, a novel subtype-selective histone deacetylase inhibitor) and BMS-202 (a small-molecule PD-L1 inhibitor) in order to enhance T-cell recognition for treating TNBC (141). CHI, the epigenetic modulator, increased the expression level of PD-L1, MHC I and MHC II on cancer cells and improved the immune cell infiltration by inducing tumor cell apoptosis and ICD (141). The steady release of BMS-202 in tumors induced the dimerization of PD-L1 to inhibit the interaction of PD-1/PD-L1 and boosted the T cell-mediated anti-tumor immunity. This combined treatment of epigenetic regulation and immune checkpoint blockade (ICB) for TNBC exhibited effective inhibition of breast tumor growth and metastasis (141). Although ICB is an efficient treatment strategy for cancer, inflammatory side effects, which are termed immune-related adverse events including vitiligo, encephalitis, pneumonitis and colitis, can often occur clinically (165). Additionally, some chemotherapeutic agents have been reported to cause the overexpression of PD-L1 on cancer cells and inhibit the activation of CD8+ T cells at tumor sites, which can assist the cancer cells to evade the T cell immune surveillance, since the deletion of PD-L1 can activate the function of CD8+ T cells, indicating the combination of chemotherapy and ICB could exhibit promising treating outcome for cancer patients (167).
Hu et al. developed a ROS-responsive synergistic delivery system (pep-PAPM@PTX) that combined immunotherapy (ICB therapy) and chemotherapy in a TNBC model (142). This micelle integrated physically-encapsulated paclitaxel and surface-modified anti-PD-L1 peptide, in which the PD-L1-targeting D-peptide on the micelle surface could multivalently bind the PD-L1 on breast cancer cell surface and downregulate PD-L1 by driving it into lysosomal degradation, and then alleviate its immunosuppression to cytotoxic T cells (142). What’s more, the micelle could respond to elevated ROS levels by releasing PTX, and this micelle-mediated combined therapy exhibited significant efficacy in TNBC model, by improving the immune-microenvironment with the enhanced T cell infiltration and increased release of tumor immune activation factors (Figure 5) (142).
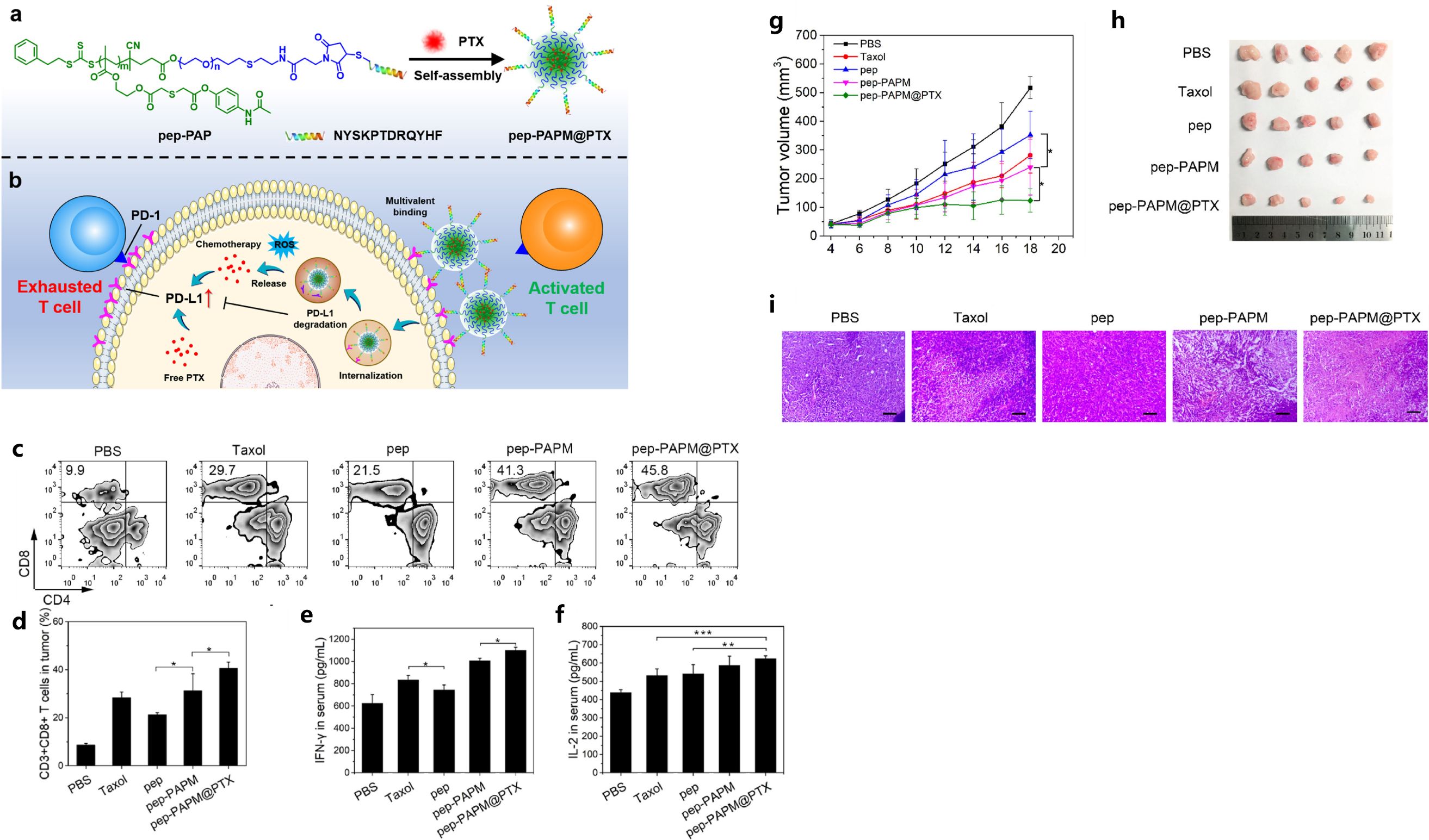
Figure 5. The illustration of pep-PAPM@PTX micelle (A), schematic illustration of the ROS-responsive synergistic delivery system (B), pep-PAPM@PTX micelle increased CTLs’ infiltration and contents of inflammatory cytokines (C-F), tumor volume changes (G) and pictures of resected tumors (H), H&E staining of tumors (I) in different groups. Reprinted with permission from Hu D, Zhang W, Xiang J, et al. (142). Copyright 2022, Elsevier. *p<0.05, **p<0.01, ***p<0.001.
In addition, conventional biomaterial-based NPs such as ZrC NPs were used in photothermal and radiotherapies for breast cancer treatment (143). Firstly, ZrC NPs were modified by bovine serum albumin (BSA) and folic acid (FA) to improve the overall biocompatibility of the particles, as well as their tumor-targeting capability. The engineered ZrC NPs possessed radiosensitizer and photosensitizer potential without obvious systemic toxicity, and endowed more curative efficiency for radiation therapy (RT) and photothermal therapy (PTT) in breast cancer treatment (143). This strategy resulted in improved immune responses by raising the tumor-infiltrating cytotoxic T cells and helper T cells, and also achieved lower dose PTT and RT for TNBC treatment and minimized the associated side effects (143).
Furthermore, strategies for increasing the proliferation and anti-tumor activity of T cells were also investigated. A2aR is one of the adenosine receptors and its inhibition could improve the T cell anti-tumor activity (168, 169). SPION (superparamagnetic iron oxide)-chitosan lactate (CL)-TAT NPs loaded with PD-1/adenosine-A2A receptor (A2aR) specific siRNA combined with DC vaccines were used for breast cancer treatment (144). This combined therapy efficiently delivered the siRNA to tumor-derived T cells at the breast tumor site and then suppressed PD-1 and A2aR expression. On the other hand, the DC vaccination significantly increased the anti-tumor responses in A2aR−/− and PD-1−/− T cells (144). It was also noted that IL-17 and IFN-γ secretion, T cell proliferation, and anti-tumor activities were enhanced, while the breast tumor growth, metastasis and angiogenesis were inhibited (144). The representative studies that explored the use of biomaterials targeting T cells for breast cancer treatments are summarized in Table 4.
5 Discussion and future perspectives
Breast cancer is one of the most prevalent malignancy worldwide and its distal metastasis (such as lymph nodes, lung, bone, liver) can seriously threaten the health and life quality of patients and significantly lower their survival rate (170). Although there are various subtypes of breast cancer, TNBC has been identified to be the most aggressive form with limited treatment options due to its unique molecular characteristics (171). The main purpose of breast cancer therapy is to eradicate the primary tumor tissue, inhibit the growth and metastasis of cancer cells, and avoid the risks of breast cancer recurrence (172). Traditional therapies for breast cancers, such as chemotherapy, radiotherapy and photodynamic therapy could directly kill cancer cells accompanied with the cancer-associated ICD and lead to the release of antigens to initiate immune responses (57, 141, 173). However, these traditional strategies also have some unavoidable drawbacks such as the non-specific killing of normal proliferating cells within the breast tissue or in the neighboring tissues and patients who are diagnosed at an advanced stage appear to be resistant to the traditional strategies (173). As a result, a more immunogenic TME needs to be induced to precisely attack the tumor cells, appreciating that the immune-suppressive TME is a major barrier for effective breast cancer therapy (174, 175). Recently, it has been noted that delivering effective amounts of immunostimulatory agents/tumor antigens into immune cells is critical for inducing robust immune response against breast cancer. However, insufficient transfer of breast tumor antigens and adjuvants into desired regions (e.g., lymph nodes, APCs), as well as enzymatic degradation of those molecules are significant obstacles of typical breast cancer immunotherapies.
Over the most recent few years, biomaterials have been designed and applied for mainly improving breast cancer immunotherapy efficacy and minimizing the anti-cancer side effects (12). Biomaterial-based NPs have been designed as anti-breast cancer drug carriers due to their biocompatibility, lower toxicity and the relatively small sizes that allow them to be easily taken up by the breast cancer cells (124). In addition to being anti-cancer drug carriers, since NPs can be designed to be selectively accumulate in breast tumor tissues, they can also act as imaging agents as well (176). Targeting breast cancer TME has become a promising strategy for breast cancer treatments. The inhibitory factors and inflammatory cells in the TME can promote breast tumor growth by reducing anti-tumor responses (144).
Multiple studies have indicated that biomaterials could play important roles in breast cancer treatments, including modulating the M2 TAMs, CAFs, MDSCs and activating DCs, NKs, and multiple T cell types, as discussed in Sections 3&4. Although biomaterial-based strategies targeting those cell types have shown some promise, there are still limitations. For example, one of the significant challenges for targeting M2-TAMs and CAFs is their significant heterogeneity in the breast cancer TME (84, 177) and the lack of in-depth understanding of the specific biomarkers expressed, and signaling pathways triggered by the M2-TAMs and different CAF molecular subtypes (41). Specifically, the biomarkers for M2-TAMs and CAFs may lack specificity and sensitivity due to their population heterogeneity (84, 177). As a result, clinical therapies targeting M2-TAMs and CAFs, or their related signaling pathways sometimes do not show desirable treatment efficacy due to the presence of complicated tumor-restraining M2-TAMs and CAF subtypes (98–100). Future biomaterial-based therapeutic strategies need to be designed to target specific biomarkers expressed by the M2-TAMs and different CAF subpopulations in the breast cancer tissues (80, 178, 179) in order to advance more effective treating outcomes.
Additionally, it has been observed that complicated growth factor/cytokine/chemokine networks are responsible for regulating the proliferation, differentiation, and activation of MDSCs, and the latter are oftentimes heterogeneous populations in different cancer subtypes and could modulate the TME via different mechanisms (105, 116). For example, in TNBC, monocytic-MDSCs (HLA-DR−CD33+CD14+) occupied the predominant subsets compared to granulocytic-MDSCs (HLA-DR−CD33+CD15+) (117). Therefore, different strategies based on MDSCs’ heterogeneity in distinct breast cancer subtypes could be considered in future biomaterial-based immunotherapies targeting MDSCs.
Further, within the breast cancer TME, it was also found that the DCs play a vital role in initiating and regulating antigen-specific immune responses by acting as the sentinels of the immune system (129). Recently, Zhao et al. revealed new biomarkers of DCs for the breast cancer treatment and prognosis, including BCL9 (B-cell lymphoma 9), TPR (tetra-tripeptide repeat), and RBBP5 (Retinoblastoma-binding protein 5) (129). For the first time, the group found that HNRNPU and PEX19 were closely related with the prognosis of DCs (129). Future studies can consider those biomarkers as potential targets in designing biomaterial-based drug delivery systems for breast cancer immunotherapy. Additionally, biomaterial-based strategies targeting NKs/multiple T cell types showed some promises, however, there is still lack of deep understanding for the molecular signaling pathways underlying the interactions between NKs, or different types of T cells, with the breast tumor cells and other types of tissue cells within the breast tumor niche environment. Therefore, future studies need to spend more effort on revealing the detailed interaction networks involving NKs, various T cell types, breast tumor cells, cancer stem cells, epithelial cells and fibroblasts etc.
Recently, it was also found that tumor-associated adipocytes can play a vital role in the TME of TNBC tissues and promote breast tumor growth and metastasis (114). NPs have been designed to inhibit tumor-associated adipocytes to remodel the TME of TNBC (114). However, studies using NPs to target tumor-associated adipocytes for TNBC treatment are still very limited. Future studies are needed to more deeply investigate the mechanisms involved in the remodeling of the breast cancer TME by the tumor-associated adipocytes, and to enable more effective biomaterial-based strategies targeting tumor-associated adipocytes.
Biomaterial-based immunotherapies targeting breast cancer TME have shown some effectiveness in treating breast cancer patients. However, complete eradication of breast cancer, especially the TNBC, still remains a great challenge. As multiple cell types (e.g., breast cancer cells, epithelial cells, M2 TAMs, CAFs, MDSCs, DCs, NKs and multiple T cell types) are involved in the breast cancer TME and they share intertwining signaling mechanisms controlling the breast cancer development and progression, future biomaterial-based strategies need to consider those different cell types and their interaction mechanisms, to enable more effective treating outcomes. Several of the biomaterial-assisted treatment therapies discussed above are depicted in Figure 6.
Since suppressive TME hinders effectiveness of anti-breast cancer immunotherapy, nanomaterials have been designed to be used in combination with anti-breast cancer drugs (e.g., mRNA drugs) to activate immune cells within the breast cancer TME to achieve enhanced biocompatibility and targeting specificity. However, efficient permeation and accumulation of cargo-loaded-NPs in breast cancer TME are still challenging to accomplish. Both physical and chemical properties need to be well-considered for the nanomaterials designed for anti-breast cancer applications. Specifically, in terms of physical properties of the nano-biomaterials, the shape and structure of the NPs (Spiky, Spherical DNA or RNA, polyhedral, sphere-nanosheet shape transitions etc.), size of NPs (100-1000 nm), surface charge of NPs (cationic, anionic, zwitterionic), mechanical strength of NPs (such as elastic modulus) as well as the hydrophobicity/hydrophilicity of the NPs can all directly or indirectly affect the functions of immune cells and immune responses within the breast cancer TME (180). Additionally, the chemical properties of nano-biomaterials such as possession of specific functional groups or bioactive ingredients can influence the biomaterials’ biocompatibility, biodegradability, specific interactions with immune cell types and endocytosis efficiency (181). Therefore, future biomaterial designs for anti-breast cancer immunotherapies need to put great emphasis on developing biomaterials with both appropriate physical and chemical properties that can be biocompatible with the drugs (e.g., mRNA) and the breast cancer TME, and more importantly, can efficiently penetrate into the desired immune cells (e.g., T cells, dendritic cells, NK cells) to load the immunostimulatory agents/tumor antigens/anti-breast cancer drugs (e.g., mRNA), in order to activate the immune cells for enhanced anti-breast cancer activities.
In addition, novel receptors that are overexpressed in the different subtypes of breast cancer need to be identified in future research and new nano-biomaterials can be designed to have surface modifications according to the unique expression pattern of those novel receptors to achieve specific and efficient interactions between cargo-loaded nano-biomaterials and breast cancer cells to boost the effectiveness of the anti-breast cancer treatment. For instance, a recent study designed a self-assembled nanoplatform named GENP by using amphiphilic molecule of stearic acid-modified EGFR-targeting peptide GE11 with DOX and GENP exhibited high loading efficiency and sustainable DOX release (182). GENP itself suppressed TNBC cell proliferation via EGFR-downstream PI3K/AKT signaling pathways, contributing to the synergistic treatment with its drug release (182). In addition to design nano-biomaterials that can target specific receptors expressed within breast cancer tissues, biomaterials that are responsive to the specific physiological stimuli (pH, enzymes and redox potential) of the breast cancer TME and exogenous energetic stimuli (light, magnetics and ultrasound) could be designed (183) for breast cancer treatments.
Liposomes could exhibit adjuvant effects for breast cancer treatments by regulating the accumulation and release of cargos to elicit host immunity (184). Previous study has demonstrated that both cationic and anionic liposomes with good biocompatibility could exhibit highly-efficient adjuvant effect in colorectal cancer treatment (185). Those liposomes functioned by mobilizing DCs via the MyD88-TRAF6 pathways, which could further activate T helper cells and CD8+ T cells and enhance host immunity by regulating Th1, Th2 and Tregs (185). In addition, a library of antigens and adjuvant-free liposomes with variable surface charges (via changing the composition of cationic, anionic and zwitterionic lipids) has been developed (185). With those interesting findings, it can be seen that an alternative strategy that the field can pursue for future anti-breast cancer treatments is utilizing liposomes (e.g., anionic, anionic) to enhance DC maturation and mobilize the immune responses in the TME.
Further, with in-depth research of the literature, it was found that more anti-breast cancer drugs/biosimilars have been developed recently and they have been approved to work alone or in combination with others to target different breast cancer subtypes (Table 5). For example, ENHERTU (fam-trastuzumab deruxtecan-nxki) has been approved to be used in patients with unresectable or metastatic HER2- breast cancer, while the combination of Abemaciclib with endocrine drugs can be used for adjuvant treatment of HR+HER2- breast cancer. Further, it was found that the triple combination of Inavolisib, palbociclib and fulvestrant could treat HR+HER2-, locally advanced or metastatic breast cancer. With the success of using multiple anti-breast cancer drugs simultaneously, it can be expected that future studies can design nano-biomaterials that can efficiently incorporate multiple types of anti-breast cancer drugs to further enhance targeted delivery to the breast cancer TME and boost immunotherapeutic treatment outcomes.
6 Conclusion
Overall, it can be stated that biomaterial-based immunotherapeutic strategies have shown great promise in breast cancer treatments, however, more investigations are needed to better design the biomaterials to achieve more precise treatment regimens for breast cancer patients possessing unique characteristics.
Author contributions
YY: Conceptualization, Writing – original draft. JPS: Conceptualization, Writing – original draft, Funding acquisition. ZD: Writing – original draft. WW: Conceptualization, Writing – review & editing. XZ: Conceptualization, Funding acquisition, Project administration, Supervision, Writing – original draft.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was funded by a Taishan scholars-young experts grant #tsqn202103111, a Natural Science Foundation of Shandong Province-Youth Foundation grant #ZR2021QC034 and National Foreign Experts Programs #G2021023010L and #G2021023012L.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Glossary
TNBC: triple negative breast cancer
ER: estrogen receptor
PR: progesterone receptor
HER2: human epidermal growth factor receptor 2
PD-L1: programmed death-ligand 1
TIL: tumor-infiltrating lymphocyte
CAR: chimeric antigen receptor
TCR: T-cell receptor
PD-1: programmed cell death protein 1
CTLA-4: cytotoxic T-lymphocyte-associated protein 4
APCs: antigen-presenting cells
FDA: U.S. Food and Drug Administration
TME: tumor micro-environment
CAFs: cancer-associated fibroblasts
ECM: extracellular matrix
NPs: nanoparticles
EPR: enhanced permeability and retention
Redox: oxidation-reduction
TAMs: tumor-associated macrophages
Tregs: regulatory T cells
MDSCs: myeloid-derived suppressor cells
MCSF: macrophage colony stimulating factor
CSF1-R: colony stimulating factor 1 receptor
SH2: Src homology region 2
DSPE-PEG: 1, 2-distearoyl-sn-glycero-3-phosphoethanolamine-poly(ethylene-glycol)
SIRPα: signal regulatory protein α
MAPK: mitogen-activated protein kinase
STING: stimulators of interferon gene
cyclic GMP-AMP: cyclic guanosine monophosphate–adenosine monophosphate
MHC: major histocompatibility complex
M2NPs: M2-like TAM dual-targeting NPs
SR-B1: scavenger receptor B type 1
M2pep: M2 macrophage binding peptide
TGF-β: transforming growth factor beta
DOX: doxorubicin
MRD: melittin-RADA32-doxorubicin
PI3Kγ: phosphatidylinositol 3-kinase γ
PTX: paclitaxel
CS: chitosan
GO: graphene oxide
HGF: hepatocyte growth factor
EGF: epidermal growth factor
MMPs: matrix metalloproteinases
FAP: fibroblast-activation protein
FSP: fibroblast-specific protein 1
DDR2: discoidin domain-containing receptor 2
PDGFRα/β: platelet-derived growth factor receptors alpha and beta
EMT: epithelial-mesenchymal transition
scFv: FAP-specific single chain variable fragment
CAP: cleavable amphiphilic peptide
Iri: irinotecan
C16-N: peptide C16-GNNQQNYKD-OH
Dox-L: DOX-loaded liposomes
nanoPue: puerarin nano-emulsion
AEAA: aminoethyl anis amide
ROS: reactive oxygen species
IFN-γ: interferon-γ
NF-κB: nuclear factor-κB
NO: nitric oxide
HA: hyaluronic acid
COS-ATRA: chitosan oligosaccharide-all-trans-retinoic-acid
PMN: pre-metastatic niche
LMWH-ATRA: low molecular-weight-heparin-all-trans-retinoic-acid
αGC: α-galactosylceramide
VECs: vascular endothelial cells
IDO: Indoleamine 2,3-dioxygenase
PVA: poly(vinyl alcohol)
NG: nanogel
DTX: docetaxel
VEA: vinyl ether acrylate
ICD: immunogenic cell death
NFX: nifuroxazide
CLM: co-prodrug-loaded micelles
LPD: lipid-protamine-DNA
pDNA: plasmid DNA
DOTAP: 1,2-dioleoyl-3-trimethylammonium propane chloride salt
PEG: polyethylene glycol
NK: natural killer cells
DCs: Dendritic cells
cDCs: conventional DCs
pDCs: plasmacytoid DCs
MoDCs: monocyte-derived DCs
PLGA: Poly-lactide-co-glycolic acid
MR: mannose receptor
TAAs: tumor associated antigens
CLR: C-type lectin receptor
NBs: nanobubbles
XCL1: XC-chemokine ligand 1
FLT3L: FMS-related tyrosine kinase 3 ligand
Ace-DEX: acetylated dextran
MPs: microparticles
PAMPs: pathogen associated molecular patterns
DOPC: 1,2-dioleoyl-sn-glycero-3-phosphocholine
DPPC: 1,2-dipalmitoyl-sn-glycero-3-phosphocholine
mPEG2000-DSPE: methoxy-poly (ethylene glycol)-1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N
cdGMP: Cyclic diguanylate monophosphate
MPLA: mono-phosphoryl lipid A
TIM3: T cell immunoglobulin and mucin domain-containing 3
LAG3: lymphocyte activation gene 3
VISTA: V domain Ig suppressor of T cell activation
CHI: chidamide
ICB: immune checkpoint blockade
BSA: bovine serum albumin
FA: folic acid
RT: radiation therapy
PTT: photothermal therapy
CL: chitosan lactate
SPION: superparamagnetic iron oxide
EGFR: epidermal growth factor receptor
References
1. Anemoulis M, Vlastos A, Kachtsidis V, Karras SN. Intermittent fasting in breast cancer: A systematic review and critical update of available studies. Nutrients. (2023) 15:532. doi: 10.3390/nu15030532
2. Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin. (2022) 72:7–33. doi: 10.3322/caac.21708
3. Kashyap D, Pal D, Sharma R, Garg VK, Goel N, Koundal D, et al. Global increase in breast cancer incidence: risk factors and preventive measures. BioMed Res Int. (2022) 2022:9605439. doi: 10.1155/2022/9605439
4. Barzaman K, Moradi-Kalbolandi S, Hosseinzadeh A, Kazemi MH, Khorramdelazad H, Safari E, et al. Breast cancer immunotherapy: Current and novel approaches. Int Immunopharmacol. (2021) 98:107886. doi: 10.1016/j.intimp.2021.107886
5. Abe O, Abe R, Enomoto K, Kikuchi K, Koyama H, Masuda H, et al. Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: an overview of the randomised trials. Lancet. (2005) 366:2087–106. doi: 10.1016/S0140-6736(05)67887-7
6. Barzaman K, Karami J, Zarei Z, Hosseinzadeh A, Kazemi MH, Moradi-Kalbolandi S, et al. Breast cancer: Biology, biomarkers, and treatments. Int Immunopharmacol. (2020) 84:106535. doi: 10.1016/j.intimp.2020.106535
7. Gautam N, Elleson KM, Ramamoorthi G, Czerniecki BJ. Current state of cell therapies for breast cancer. Cancer J. (2022) 28:301–9. doi: 10.1097/PPO.0000000000000607
8. Swain SM, Shastry M, Hamilton E. Targeting HER2-positive breast cancer: advances and future directions. Nat Rev Drug Discovery. (2023) 22:101–26. doi: 10.1038/s41573-022-00579-0
9. Chen Z, Zeng Z, Wan Q, Liu X, Qi J, Zu Y. Targeted immunotherapy of triple-negative breast cancer by aptamer-engineered NK cells. Biomaterials. (2022) 280:121259. doi: 10.1016/j.biomaterials.2021.121259
10. Lyons TG. Targeted therapies for triple-negative breast cancer. Curr Treat Options Oncol. (2019) 20:82. doi: 10.1007/s11864-019-0682-x
11. Emens LA, Loi S. Immunotherapy approaches for breast cancer patients in 2023. Cold Spring Harb Perspect Med. (2023) 13:a041332. doi: 10.1101/cshperspect.a041332
12. Yan S, Luo Z, Li Z, Wang Y, Tao J, Gong C, et al. Improving cancer immunotherapy outcomes using biomaterials. Angew Chem Int Ed Engl. (2020) 59:17332–43. doi: 10.1002/anie.202002780
13. Bahreyni A, Mohamud Y, Luo H. Emerging nanomedicines for effective breast cancer immunotherapy. J Nanobiotechnol. (2020) 18:180. doi: 10.1186/s12951-020-00741-z
14. Gridelli C, Peters S, Mok T, Garassino M, Paz-Ares L, Attili I, et al. Face to face among different chemo-immunotherapy combinations in the first line treatment of patients with advanced non-small cell lung cancer: Results of an international expert panel meeting by the italian association of thoracic oncology (AIOT). Lung Cancer. (2024) 187:107441. doi: 10.1016/j.lungcan.2023.107441
15. Rastin F, Javid H, Oryani MA, Rezagholinejad N, Afshari AR, Karimi-Shahri M. Immunotherapy for colorectal cancer: Rational strategies and novel therapeutic progress. Int Immunopharmacol. (2024) 126:111055. doi: 10.1016/j.intimp.2023.111055
16. Jia H, Truica CI, Wang B, Wang Y, Ren X, Harvey HA, et al. Immunotherapy for triple-negative breast cancer: Existing challenges and exciting prospects. Drug Resist Update. (2017) 32:1–15. doi: 10.1016/j.drup.2017.07.002
17. Ali HR, Glont SE, Blows FM, Provenzano E, Dawson SJ, Liu B, et al. PD-L1 protein expression in breast cancer is rare, enriched in basal-like tumours and associated with infiltrating lymphocytes. Ann Oncol. (2015) 26:1488–93. doi: 10.1093/annonc/mdv192
18. Mittendorf EA, Philips AV, Meric-Bernstam F, Qiao N, Wu Y, Harrington S, et al. PD-L1 expression in triple-negative breast cancer. Cancer Immunol Res. (2014) 2:361–70. doi: 10.1158/2326-6066.CIR-13-0127
19. Moradi-Kalbolandi S, Hosseinzade A, Salehi M, Merikhian P, Farahmand L. Monoclonal antibody-based therapeutics, targeting the epidermal growth factor receptor family: from herceptin to Pan HER. J Pharm Pharmacol. (2018) 70:841–54. doi: 10.1111/jphp.12911
20. Aurisicchio L, Pallocca M, Ciliberto G, Palombo F. The perfect personalized cancer therapy: cancer vaccines against neoantigens. J Exp Clin Cancer Res. (2018) 37:86. doi: 10.1186/s13046-018-0751-1
21. Li X, Bu X. Progress in vaccine therapies for breast cancer. Adv Exp Med Biol. (2017) 1026:315–30. doi: 10.1007/978-981-10-6020-5_15
22. Aldous AR, Dong JZ. Personalized neoantigen vaccines: A new approach to cancer immunotherapy. Bioorg Med Chem. (2018) 26:2842–9. doi: 10.1016/j.bmc.2017.10.021
23. Mediratta K, El-Sahli S, D’Costa V, Wang L. Current progresses and challenges of immunotherapy in triple-negative breast cancer. Cancers (Basel). (2020) 12:3529. doi: 10.3390/cancers12123529
24. Sasidharan Nair V, Elkord E. Immune checkpoint inhibitors in cancer therapy: a focus on T-regulatory cells. Immunol Cell Biol. (2018) 96:21–33. doi: 10.1111/imcb.2018.96.issue-1
25. Walunas TL, Lenschow DJ, Bakker CY, Linsley PS, Freeman GJ, Green JM, et al. Pillars article: CTLA-4 can function as a negative regulator of T cell activation. Immunity. 1994. 1: 405-413. J Immunol. (2011) 187:3466–74.
26. Buchbinder EI, Desai A. CTLA-4 and PD-1 pathways: similarities, differences, and implications of their inhibition. Am J Clin Oncol. (2016) 39:98–106. doi: 10.1097/COC.0000000000000239
27. Krummey SM, Hartigan CR, Liu D, Ford ML. CD28-dependent CTLA-4 expression fine-tunes the activation of human th17 cells. iScience. (2020) 23:100912. doi: 10.1016/j.isci.2020.100912
28. Santa-Maria CA, Kato T, Park JH, Flaum LE, Jain S, Tellez C, et al. Durvalumab and tremelimumab in metastatic breast cancer (MBC): Immunotherapy and immunopharmacogenomic dynamics. J Clin Oncol. (2017) 35:3052. doi: 10.1200/JCO.2017.35.15_suppl.3052
29. Solinas C, Aiello M, Migliori E, Willard-Gallo K, Emens LA. Breast cancer vaccines: Heeding the lessons of the past to guide a path forward. Cancer Treat Rev. (2020) 84:101947. doi: 10.1016/j.ctrv.2019.101947
30. Blum SM, Rouhani SJ, Sullivan RJ. Effects of immune-related adverse events (irAEs) and their treatment on antitumor immune responses. Immunol Rev. (2023) 318:167–78. doi: 10.1111/imr.v318.1
31. Gong N, Alameh MG, El-Mayta R, Xue L, Weissman D, Mitchell MJ. Enhancing in situ cancer vaccines using delivery technologies. Nat Rev Drug Discovery. (2024) 23:607–25. doi: 10.1038/s41573-024-00974-9
32. Ashrafizadeh M, Zarrabi A, Bigham A, Taheriazam A, Saghari Y, Mirzaei S, et al. (Nano)platforms in breast cancer therapy: Drug/gene delivery, advanced nanocarriers and immunotherapy. Med Res Rev. (2023) 43:2115–76. doi: 10.1002/med.21971
33. Li Y, Wang C, Huang T, Yu X, Tian B. The role of cancer-associated fibroblasts in breast cancer metastasis. Front Oncol. (2023) 13:1194835. doi: 10.3389/fonc.2023.1194835
34. Deepak KGK, Vempati R, Nagaraju GP, Dasari VR, Nagini S, Rao DN, et al. Tumor microenvironment: Challenges and opportunities in targeting metastasis of triple negative breast cancer. Pharmacol Res. (2020) 153:104683. doi: 10.1016/j.phrs.2020.104683
35. Mehraj U, Dar AH, Wani NA, Mir MA. Tumor microenvironment promotes breast cancer chemoresistance. Cancer Chemother Pharmacol. (2021) 87:147–58. doi: 10.1007/s00280-020-04222-w
36. Wang M, Zhao J, Zhang L, Wei F, Lian Y, Wu Y, et al. Role of tumor microenvironment in tumorigenesis. J Cancer. (2017) 8:761–73. doi: 10.7150/jca.17648
37. Avagliano A, Fiume G, Ruocco MR, Martucci N, Vecchio E, Insabato L, et al. Influence of fibroblasts on mammary gland development, breast cancer microenvironment remodeling, and cancer cell dissemination. Cancers (Basel). (2020) 12:1697. doi: 10.3390/cancers12061697
38. Cui X, Morales RT, Qian W, Wang H, Gagner JP, Dolgalev I, et al. Hacking macrophage-associated immunosuppression for regulating glioblastoma angiogenesis. Biomaterials. (2018) 161:164–78. doi: 10.1016/j.biomaterials.2018.01.053
39. Soysal SD, Tzankov A, Muenst SE. Role of the tumor microenvironment in breast cancer. Pathobiology. (2015) 82:142–52. doi: 10.1159/000430499
40. Truffi M, Mazzucchelli S, Bonizzi A, Sorrentino L, Allevi R, Vanna R, et al. Nano-strategies to target breast cancer-associated fibroblasts: rearranging the tumor microenvironment to achieve antitumor efficacy. Int J Mol Sci. (2019) 20:1263. doi: 10.3390/ijms20061263
41. Houthuijzen JM, Jonkers J. Cancer-associated fibroblasts as key regulators of the breast cancer tumor microenvironment. Cancer Metastasis Rev. (2018) 37:577–97. doi: 10.1007/s10555-018-9768-3
42. Madamsetty VS, Tavakol S, Moghassemi S, Dadashzadeh A, Schneible JD, Fatemi I, et al. Chitosan: A versatile bio-platform for breast cancer theranostics. J Control Release. (2022) 341:733–52. doi: 10.1016/j.jconrel.2021.12.012
43. Cao H, Dan Z, He X, Zhang Z, Yu H, Yin Q, et al. Liposomes coated with isolated macrophage membrane can target lung metastasis of breast cancer. ACS Nano. (2016) 10:7738–48. doi: 10.1021/acsnano.6b03148
44. Ma P, Wang G, Men K, Li C, Gao N, Li L. Advances in clinical application of nanoparticle-based therapy for cancer treatment: A systematic review. Nano TransMed. (2024) 3:100036. doi: 10.1016/j.ntm.2024.100036
45. Dos Santos MSC, Gouvêa AL, de Moura LD, Paterno LG, de Souza PEN, Bastos AP, et al. Nanographene oxide-methylene blue as phototherapies platform for breast tumor ablation and metastasis prevention in a syngeneic orthotopic murine model. J Nanobiotechnol. (2018) 16:9. doi: 10.1186/s12951-018-0333-6
46. Liu TI, Lu TY, Yang YC, Chang SH, Chen HH, Lu IL, et al. New combination treatment from ROS-Induced sensitized radiotherapy with nanophototherapeutics to fully eradicate orthotopic breast cancer and inhibit metastasis. Biomaterials. (2020) 257:120229. doi: 10.1016/j.biomaterials.2020.120229
47. Gurunathan S, Kang MH, Qasim M, Kim JH. Nanoparticle-mediated combination therapy: two-in-one approach for cancer. Int J Mol Sci. (2018) 19:3264. doi: 10.3390/ijms19103264
48. Shi J, Kantoff PW, Wooster R, Farokhzad OC. Cancer nanomedicine: progress, challenges and opportunities. Nat Rev Cancer. (2017) 17:20–37. doi: 10.1038/nrc.2016.108
49. Wang J, Chen HJ, Hang T, Yu Y, Liu G, He G, et al. Physical activation of innate immunity by spiky particles. Nat Nanotechnol. (2018) 13:1078–86. doi: 10.1038/s41565-018-0274-0
50. Ren M, Zheng X, Gao H, Jiang A, Yao Y, He W. Nanomedicines targeting metabolism in the tumor microenvironment. Front Bioeng Biotechnol. (2022) 10:943906. doi: 10.3389/fbioe.2022.943906
51. Tong R, Langer R. Nanomedicines targeting the tumor microenvironment. Cancer J. (2015) 21:314–21. doi: 10.1097/PPO.0000000000000123
52. Zhang K, Ma Y, Wang D, Liu J, An J, Li Y, et al. In vivo activation of T-cell proliferation by regulating cell surface receptor clustering using a pH-driven interlocked DNA nano-spring. Nano Lett. (2022) 22:1937–45. doi: 10.1021/acs.nanolett.1c04562
53. Fridman WH, Miller I, Sautès-Fridman C, Byrne AT. Therapeutic targeting of the colorectal tumor stroma. Gastroenterology. (2020) 158:303–21. doi: 10.1053/j.gastro.2019.09.045
54. Petitprez F, Vano YA, Becht E, Giraldo NA, de Reyniès A, Sautès-Fridman C, et al. Transcriptomic analysis of the tumor microenvironment to guide prognosis and immunotherapies. Cancer Immunol Immunother. (2018) 67:981–8. doi: 10.1007/s00262-017-2058-z
55. Anderson NM, Simon MC. The tumor microenvironment. Curr Biol. (2020) 30:R921–r5. doi: 10.1016/j.cub.2020.06.081
56. Goenka A, Khan F, Verma B, Sinha P, Dmello CC, Jogalekar MP, et al. Tumor microenvironment signaling and therapeutics in cancer progression. Cancer Commun (Lond). (2023) 43:525–61. doi: 10.1002/cac2.12416
57. Yang J, Zhang C. Regulation of cancer-immunity cycle and tumor microenvironment by nanobiomaterials to enhance tumor immunotherapy. Wiley Interdiscip Rev Nanomed Nanobiotechnol. (2020) 12:e1612. doi: 10.1002/wnan.v12.4
58. Boutilier AJ, Elsawa SF. Macrophage polarization states in the tumor microenvironment. Int J Mol Sci. (2021) 22:6995. doi: 10.3390/ijms22136995
59. Okabe Y, Medzhitov R. Tissue-specific signals control reversible program of localization and functional polarization of macrophages. Cell. (2014) 157:832–44. doi: 10.1016/j.cell.2014.04.016
60. Ngambenjawong C, Gustafson HH, Pun SH. Progress in tumor-associated macrophage (TAM)-targeted therapeutics. Adv Drug Delivery Rev. (2017) 114:206–21. doi: 10.1016/j.addr.2017.04.010
61. Qian BZ, Pollard JW. Macrophage diversity enhances tumor progression and metastasis. Cell. (2010) 141:39–51. doi: 10.1016/j.cell.2010.03.014
62. Fan Y, He S. The characteristics of tumor microenvironment in triple negative breast cancer. Cancer Manag Res. (2022) 14:1–17. doi: 10.2147/CMAR.S316700
63. Pan Y, Yu Y, Wang X, Zhang T. Corrigendum: Tumor-associated macrophages in tumor immunity. Front Immunol. (2020) 11:583084. doi: 10.3389/fimmu.2020.583084
64. Qian Y, Qiao S, Dai Y, Xu G, Dai B, Lu L, et al. Molecular-targeted immunotherapeutic strategy for melanoma via dual-targeting nanoparticles delivering small interfering RNA to tumor-associated macrophages. ACS Nano. (2017) 11:9536–49. doi: 10.1021/acsnano.7b05465
65. Petitprez F, Meylan M, de Reyniès A, Sautès-Fridman C, Fridman WH. The tumor microenvironment in the response to immune checkpoint blockade therapies. Front Immunol. (2020) 11:784. doi: 10.3389/fimmu.2020.00784
66. De Palma M, Lewis CE. Macrophage regulation of tumor responses to anticancer therapies. Cancer Cell. (2013) 23:277–86. doi: 10.1016/j.ccr.2013.02.013
67. Ramesh A, Kumar S, Nandi D, Kulkarni A. CSF1R- and SHP2-inhibitor-loaded nanoparticles enhance cytotoxic activity and phagocytosis in tumor-associated macrophages. Adv Mater. (2019) 31:e1904364. doi: 10.1002/adma.201904364
68. Ramesh A, Brouillard A, Kumar S, Nandi D, Kulkarni A. Dual inhibition of CSF1R and MAPK pathways using supramolecular nanoparticles enhances macrophage immunotherapy. Biomaterials. (2020) 227:119559. doi: 10.1016/j.biomaterials.2019.119559
69. Wu YT, Fang Y, Wei Q, Shi H, Tan H, Deng Y, et al. Tumor-targeted delivery of a STING agonist improvescancer immunotherapy. Proc Natl Acad Sci U.S.A. (2022) 119:e2214278119. doi: 10.1073/pnas.2214278119
70. Cheng N, Watkins-Schulz R, Junkins RD, David CN, Johnson BM, Montgomery SA, et al. A nanoparticle-incorporated STING activator enhances antitumor immunity in PD-L1-insensitive models of triple-negative breast cancer. JCI Insight. (2018) 3:e120638. doi: 10.1172/jci.insight.120638
71. Huo W, Yang X, Wang B, Cao L, Fang Z, Li Z, et al. Biomineralized hydrogel DC vaccine for cancer immunotherapy: A boosting strategy via improving immunogenicity and reversing immune-inhibitory microenvironment. Biomaterials. (2022) 288:121722. doi: 10.1016/j.biomaterials.2022.121722
72. Jin H, Wan C, Zou Z, Zhao G, Zhang L, Geng Y, et al. Tumor ablation and therapeutic immunity induction by an injectable peptide hydrogel. ACS Nano. (2018) 12:3295–310. doi: 10.1021/acsnano.7b08148
73. Song Y, Bugada L, Li R, Hu H, Zhang L, Li C, et al. Albumin nanoparticle containing a PI3Kγ inhibitor and paclitaxel in combination with α-PD1 induces tumor remission of breast cancer in mice. Sci Transl Med. (2022) 14:eabl3649. doi: 10.1126/scitranslmed.abl3649
74. Ge YW, Liu XL, Yu DG, Zhu ZA, Ke QF, Mao YQ, et al. Graphene-modified CePO4 nanorods effectively treat breast cancer-induced bone metastases and regulate macrophage polarization to improve osteo-inductive ability. J Nanobiotechnol. (2021) 19:11. doi: 10.1186/s12951-020-00753-9
75. Zhang L, Cui S, Ding N, Zhang J, Cui E, Xiang Q, et al. Tumor-associated macrophages regulating a polymer nanoplatform for synergistic treatment of breast tumors. ACS Appl Mater Interfaces. (2023) 15:34527–39. doi: 10.1021/acsami.3c05497
76. Gunnarsdottir FB, Briem O, Lindgren AY, Källberg E, Andersen C, Grenthe R, et al. Breast cancer associated CD169(+) macrophages possess broad immunosuppressive functions but enhance antibody secretion by activated B cells. Front Immunol. (2023) 14:1180209. doi: 10.3389/fimmu.2023.1180209
77. Singh S, Singh AP, Mitra R. Cancer-associated fibroblasts: major co-conspirators in tumor development. Cancers (Basel). (2024) 16:211. doi: 10.3390/cancers16010211
78. Kennel KB, Bozlar M, De Valk AF, Greten FR. Cancer-associated fibroblasts in inflammation and antitumor immunity. Clin Cancer Res. (2023) 29:1009–16. doi: 10.1158/1078-0432.CCR-22-1031
79. Ma C, Yang C, Peng A, Sun T, Ji X, Mi J, et al. Pan-cancer spatially resolved single-cell analysis reveals the crosstalk between cancer-associated fibroblasts and tumor microenvironment. Mol Cancer. (2023) 22:170. doi: 10.1186/s12943-023-01876-x
80. Chen Y, McAndrews KM, Kalluri R. Clinical and therapeutic relevance of cancer-associated fibroblasts. Nat Rev Clin Oncol. (2021) 18:792–804. doi: 10.1038/s41571-021-00546-5
81. Zhen Z, Tang W, Wang M, Zhou S, Wang H, Wu Z, et al. Protein nanocage mediated fibroblast-activation protein targeted photoimmunotherapy to enhance cytotoxic T cell infiltration and tumor control. Nano Lett. (2017) 17:862–9. doi: 10.1021/acs.nanolett.6b04150
82. Luo X, Zhang Q, Chen H, Hou K, Zeng N, Wu Y. Smart nanoparticles for breast cancer treatment based on the tumor microenvironment. Front Oncol. (2022) 12:907684. doi: 10.3389/fonc.2022.907684
83. Hu D, Li Z, Zheng B, Lin X, Pan Y, Gong P, et al. Cancer-associated fibroblasts in breast cancer: Challenges and opportunities. Cancer Commun (Lond). (2022) 42:401–34. doi: 10.1002/cac2.12291
84. Nurmik M, Ullmann P, Rodriguez F, Haan S, Letellier E. In search of definitions: Cancer-associated fibroblasts and their markers. Int J Cancer. (2020) 146:895–905. doi: 10.1002/ijc.v146.4
85. Yamamoto Y, Kasashima H, Fukui Y, Tsujio G, Yashiro M, Maeda K. The heterogeneity of cancer-associated fibroblast subpopulations: Their origins, biomarkers, and roles in the tumor microenvironment. Cancer Sci. (2023) 114:16–24. doi: 10.1111/cas.v114.1
86. Hofheinz RD, al-Batran SE, Hartmann F, Hartung G, Jäger D, Renner C, et al. Stromal antigen targeting by a humanised monoclonal antibody: an early phase II trial of sibrotuzumab in patients with metastatic colorectal cancer. Onkologie. (2003) 26:44–8. doi: 10.1159/000069863
87. Ji T, Zhao Y, Ding Y, Wang J, Zhao R, Lang J, et al. Transformable peptide nanocarriers for expeditious drug release and effective cancer therapy via cancer-associated fibroblast activation. Angew Chem Int Ed Engl. (2016) 55:1050–5. doi: 10.1002/anie.201506262
88. Niedermeyer J, Garin-Chesa P, Kriz M, Hilberg F, Mueller E, Bamberger U, et al. Expression of the fibroblast activation protein during mouse embryo development. Int J Dev Biol. (2001) 45:445–7.
89. Kraman M, Bambrough PJ, Arnold JN, Roberts EW, Magiera L, Jones JO, et al. Suppression of antitumor immunity by stromal cells expressing fibroblast activation protein-alpha. Science. (2010) 330:827–30. doi: 10.1126/science.1195300
90. Dolznig H, Schweifer N, Puri C, Kraut N, Rettig WJ, Kerjaschki D, et al. Characterization of cancer stroma markers: in silico analysis of an mRNA expression database for fibroblast activation protein and endosialin. Cancer Immun. (2005) 5:10.
91. Sitia L, Bonizzi A, Mazzucchelli S, Negri S, Sottani C, Grignani E, et al. Selective targeting of cancer-associated fibroblasts by engineered H-ferritin nanocages loaded with navitoclax. Cells. (2021) 10:328. doi: 10.3390/cells10020328
92. An J, Hou D, Wang L, Wang L, Yang Y, Wang H. Fibroblast activation protein-alpha knockdown suppresses prostate cancer cell invasion and proliferation. Histol Histopathol. (2022) 37:597–607. doi: 10.14670/HH-18-430
93. Dzobo K, Dandara C. Broadening drug design and targets to tumor microenvironment? Cancer-associated fibroblast marker expression in cancers and relevance for survival outcomes. Omics. (2020) 24:340–51. doi: 10.1089/omi.2020.0042
94. Hu C, Liu X, Ran W, Meng J, Zhai Y, Zhang P, et al. Regulating cancer associated fibroblasts with losartan-loaded injectable peptide hydrogel to potentiate chemotherapy in inhibiting growth and lung metastasis of triple negative breast cancer. Biomaterials. (2017) 144:60–72. doi: 10.1016/j.biomaterials.2017.08.009
95. Xu H, Hu M, Liu M, An S, Guan K, Wang M, et al. Nano-puerarin regulates tumor microenvironment and facilitates chemo- and immunotherapy in murine triple negative breast cancer model. Biomaterials. (2020) 235:119769. doi: 10.1016/j.biomaterials.2020.119769
96. Zhang P, Qin C, Liu N, Zhou X, Chu X, Lv F, et al. The programmed site-specific delivery of LY3200882 and PD-L1 siRNA boosts immunotherapy for triple-negative breast cancer by remodeling tumor microenvironment. Biomaterials. (2022) 284:121518. doi: 10.1016/j.biomaterials.2022.121518
97. Mueller KL, Madden JM, Zoratti GL, Kuperwasser C, List K, Boerner JL. Fibroblast-secreted hepatocyte growth factor mediates epidermal growth factor receptor tyrosine kinase inhibitor resistance in triple-negative breast cancers through paracrine activation of Met. Breast Cancer Res. (2012) 14:R104. doi: 10.1186/bcr3224
98. Ramanathan RK, McDonough SL, Philip PA, Hingorani SR, Lacy J, Kortmansky JS, et al. Phase IB/II randomized study of FOLFIRINOX plus pegylated recombinant human hyaluronidase versus FOLFIRINOX alone in patients with metastatic pancreatic adenocarcinoma: SWOG S1313. J Clin Oncol. (2019) 37:1062–9. doi: 10.1200/JCO.18.01295
99. Van Cutsem E, Tempero MA, Sigal D, Oh DY, Fazio N, MacArulla T, et al. Randomized phase III trial of pegvorhyaluronidase alfa with nab-paclitaxel plus gemcitabine for patients with hyaluronan-high metastatic pancreatic adenocarcinoma. J Clin Oncol. (2020) 38:3185–94. doi: 10.1200/JCO.20.00590
100. Catenacci DV, Junttila MR, Karrison T, Bahary N, Horiba MN, Nattam SR, et al. Randomized phase ib/II study of gemcitabine plus placebo or vismodegib, a hedgehog pathway inhibitor, in patients with metastatic pancreatic cancer. J Clin Oncol. (2015) 33:4284–92. doi: 10.1200/JCO.2015.62.8719
101. Barrueto L, Caminero F, Cash L, Makris C, Lamichhane P, Deshmukh RR. Resistance to checkpoint inhibition in cancer immunotherapy. Transl Oncol. (2020) 13:100738. doi: 10.1016/j.tranon.2019.12.010
102. Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. (2009) 9:162–74. doi: 10.1038/nri2506
103. Lu Z, Liu H, Ma L, Ren K, He Z, Li M, et al. Micellar nanoparticles inhibit breast cancer and pulmonary metastasis by modulating the recruitment and depletion of myeloid-derived suppressor cells. Nanoscale. (2022) 14:17315–30. doi: 10.1039/D2NR03880C
104. Lu Z, Ma L, Mei L, Ren K, Li M, Zhang L, et al. Micellar nanoparticles inhibit the postoperative inflammation, recurrence and pulmonary metastasis of 4T1 breast cancer by blocking NF-κB pathway and promoting MDSCs depletion. Int J Pharm. (2022) 628:122303. doi: 10.1016/j.ijpharm.2022.122303
105. Wu Y, Yi M, Niu M, Mei Q, Wu K. Myeloid-derived suppressor cells: an emerging target for anticancer immunotherapy. Mol Cancer. (2022) 21:184. doi: 10.1186/s12943-022-01657-y
106. Wculek SK, Malanchi I. Neutrophils support lung colonization of metastasis-initiating breast cancer cells. Nature. (2015) 528:413–7. doi: 10.1038/nature16140
107. de la Cruz-Merino L, Chiesa M, Caballero R, Rojo F, Palazón N, Carrasco FH, Sánchez-Margalet V. Breast cancer immunology and immunotherapy: current status and future perspectives. Int Rev Cell Mol Biol. (2017) 331:1–53. doi: 10.1016/bs.ircmb.2016.09.008
108. Lu Z, Long Y, Li J, Ren K, Zhao W, Wang X, et al. Simultaneous inhibition of breast cancer and its liver and lung metastasis by blocking inflammatory feed-forward loops. J Control Release. (2021) 338:662–79. doi: 10.1016/j.jconrel.2021.08.047
109. Yu J, Du W, Yan F, Wang Y, Li H, Cao S, et al. Myeloid-derived suppressor cells suppress antitumor immune responses through IDO expression and correlate with lymph node metastasis in patients with breast cancer. J Immunol. (2013) 190:3783–97. doi: 10.4049/jimmunol.1201449
110. Gonda K, Shibata M, Ohtake T, Matsumoto Y, Tachibana K, Abe N, et al. Myeloid-derived suppressor cells are increased and correlated with type 2 immune responses, malnutrition, inflammation, and poor prognosis in patients with breast cancer. Oncol Lett. (2017) 14:1766–74. doi: 10.3892/ol.2017.6305
111. Qiao H, Chen X, Chen E, Zhang J, Huang D, Yang D, et al. Folated pH-degradable nanogels for the simultaneous delivery of docetaxel and an IDO1-inhibitor in enhancing cancer chemo-immunotherapy. Biomater Sci. (2019) 7:2749–58. doi: 10.1039/C9BM00324J
112. Luo L, Xu F, Peng H, Luo Y, Tian X, Battaglia G, et al. Stimuli-responsive polymeric prodrug-based nanomedicine delivering nifuroxazide and doxorubicin against primary breast cancer and pulmonary metastasis. J Control Release. (2020) 318:124–35. doi: 10.1016/j.jconrel.2019.12.017
113. Zhang R, Wan Y, Lv H, Li F, Lee CS. DTX@VTX NPs synergy PD-L1 immune checkpoint nanoinhibitor to reshape immunosuppressive tumor microenvironment for enhancing chemo-immunotherapy. J Mater Chem B. (2021) 9:7544–56. doi: 10.1039/D1TB00269D
114. Liu Y, Tiruthani K, Wang M, Zhou X, Qiu N, Xiong Y, et al. Tumor-targeted gene therapy with lipid nanoparticles inhibits tumor-associated adipocytes and remodels the immunosuppressive tumor microenvironment in triple-negative breast cancer. Nanoscale Horiz. (2021) 6:319–29. doi: 10.1039/D0NH00588F
115. Weber R, Groth C, Lasser S, Arkhypov I, Petrova V, Altevogt P, et al. IL-6 as a major regulator of MDSC activity and possible target for cancer immunotherapy. Cell Immunol. (2021) 359:104254. doi: 10.1016/j.cellimm.2020.104254
116. Tcyganov E, Mastio J, Chen E, Gabrilovich DI. Plasticity of myeloid-derived suppressor cells in cancer. Curr Opin Immunol. (2018) 51:76–82. doi: 10.1016/j.coi.2018.03.009
117. De Cicco P, Ercolano G, Ianaro A. The new era of cancer immunotherapy: targeting myeloid-derived suppressor cells to overcome immune evasion. Front Immunol. (2020) 11:1680. doi: 10.3389/fimmu.2020.01680
118. Adams S, Gatti-Mays ME, Kalinsky K, Korde LA, Sharon E, Amiri-Kordestani L, et al. Current landscape of immunotherapy in breast cancer: A review. JAMA Oncol. (2019) 5:1205–14. doi: 10.1001/jamaoncol.2018.7147
119. Monnot GC, Romero P. Rationale for immunological approaches to breast cancer therapy. Breast. (2018) 37:187–95. doi: 10.1016/j.breast.2017.06.009
120. Li Z, Qiu Y, Lu W, Jiang Y, Wang J. Immunotherapeutic interventions of triple negative breast cancer. J Transl Med. (2018) 16:147. doi: 10.1186/s12967-018-1514-7
121. Cimino-Mathews A, Ye X, Meeker A, Argani P, Emens LA. Metastatic triple-negative breast cancers at first relapse have fewer tumor-infiltrating lymphocytes than their matched primary breast tumors: a pilot study. Hum Pathol. (2013) 44:2055–63. doi: 10.1016/j.humpath.2013.03.010
122. Wang C, Barnoud C, Cenerenti M, Sun M, Caffa I, Kizil B, et al. Dendritic cells direct circadian anti-tumour immune responses. Nature. (2023) 614:136–43. doi: 10.1038/s41586-022-05605-0
123. Linde MH, Fan AC, Köhnke T, Trotman-Grant AC, Gurev SF, Phan P, et al. Reprogramming cancer into antigen-presenting cells as a novel immunotherapy. Cancer Discovery. (2023) 13:1164–85. doi: 10.1158/2159-8290.CD-21-0502
124. Sheikhzadeh S, Delirezh N, Hobbenaghi R. Mannosylated polylactic-co-glycolic acid (MN-PLGA) nanoparticles induce potent anti-tumor immunity in murine model of breast cancer. BioMed Pharmacother. (2021) 142:111962. doi: 10.1016/j.biopha.2021.111962
125. Lee YS, Radford KJ. The role of dendritic cells in cancer. Int Rev Cell Mol Biol. (2019) 348:123–78. doi: 10.1016/bs.ircmb.2019.07.006
126. Bordon Y. Dendritic cells: Sorting, sorted! Nat Rev Immunol. (2016) 16:657. doi: 10.1038/nri.2016.115
127. Oshi M, Newman S, Tokumaru Y, Yan L, Matsuyama R, Kalinski P, et al. Plasmacytoid dendritic cell (pDC) infiltration correlate with tumor infiltrating lymphocytes, cancer immunity, and better survival in triple negative breast cancer (TNBC) more strongly than conventional dendritic cell (cDC). Cancers (Basel). (2020) 12:3342. doi: 10.3390/cancers12113342
128. Tian S, Yan L, Fu L, Zhang Z, Zhang J, Meng G, et al. A comprehensive investigation to reveal the relationship between plasmacytoid dendritic cells and breast cancer by multiomics data analysis. Front Cell Dev Biol. (2021) 9:640476. doi: 10.3389/fcell.2021.640476
129. Zhao F, Yan F, Liu H. New biomarkers based on dendritic cells for breast cancer treatment and prognosis diagnosis. Int J Mol Sci. (2023) 24:4058. doi: 10.3390/ijms24044058
130. Gelao L, Criscitiello C, Esposito A, Laurentiis MD, Fumagalli L, Locatelli MA, et al. Dendritic cell-based vaccines: clinical applications in breast cancer. Immunotherapy. (2014) 6:349–60. doi: 10.2217/imt.13.169
131. Qian D, Li J, Huang M, Cui Q, Liu X, Sun K. Dendritic cell vaccines in breast cancer: Immune modulation and immunotherapy. BioMed Pharmacother. (2023) 162:114685. doi: 10.1016/j.biopha.2023.114685
132. Fu C, Zhou L, Mi QS, Jiang A. DC-based vaccines for cancer immunotherapy. Vaccines (Basel). (2020) 8:706. doi: 10.3390/vaccines8040706
133. Wu C, Zhu W, Jin R, Ai H, Xu Y. The MRI-visible nanocomposite facilitates the delivery and tracking of siRNA loaded DC vaccine in the breast cancer model. Front Oncol. (2020) 10:621642. doi: 10.3389/fonc.2020.621642
134. Ghotbi Z, Haddadi A, Hamdy S, Hung RW, Samuel J, Lavasanifar A. Active targeting of dendritic cells with mannan-decorated PLGA nanoparticles. J Drug Target. (2011) 19:281–92. doi: 10.3109/1061186X.2010.499463
135. Iranpour S, Nejati V, Delirezh N, Biparva P, Shirian S. Enhanced stimulation of anti-breast cancer T cells responses by dendritic cells loaded with poly lactic-co-glycolic acid (PLGA) nanoparticle encapsulated tumor antigens. J Exp Clin Cancer Res. (2016) 35:168. doi: 10.1186/s13046-016-0444-6
136. Argenziano M, Occhipinti S, Scomparin A, Angelini C, Novelli F, Soster M, et al. Exploring chitosan-shelled nanobubbles to improve HER2 + immunotherapy via dendritic cell targeting. Drug Delivery Transl Res. (2022) 12:2007–18. doi: 10.1007/s13346-022-01185-8
137. Murphy DA, Cheng H, Yang T, Yan X, Adjei IM. Reversing hypoxia with PLGA-encapsulated manganese dioxide nanoparticles improves natural killer cell response to tumor spheroids. Mol Pharm. (2021) 18:2935–46. doi: 10.1021/acs.molpharmaceut.1c00085
138. Watkins-Schulz R, Tiet P, Gallovic MD, Junkins RD, Batty C, Bachelder EM, et al. A microparticle platform for STING-targeted immunotherapy enhances natural killer cell- and CD8(+) T cell-mediated anti-tumor immunity. Biomaterials. (2019) 205:94–105. doi: 10.1016/j.biomaterials.2019.03.011
139. Atukorale PU, Raghunathan SP, Raguveer V, Moon TJ, Zheng C, Bielecki PA, et al. Nanoparticle encapsulation of synergistic immune agonists enables systemic codelivery to tumor sites and IFNβ-driven antitumor immunity. Cancer Res. (2019) 79:5394–406. doi: 10.1158/0008-5472.CAN-19-0381
140. Chen C, Guo Q, Fu H, Yu J, Wang L, Sun Y, et al. Asynchronous blockade of PD-L1 and CD155 by polymeric nanoparticles inhibits triple-negative breast cancer progression and metastasis. Biomaterials. (2021) 275:120988. doi: 10.1016/j.biomaterials.2021.120988
141. Tu K, Yu Y, Wang Y, Yang T, Hu Q, Qin X, et al. Combination of chidamide-mediated epigenetic modulation with immunotherapy: boosting tumor immunogenicity and response to PD-1/PD-L1 blockade. ACS Appl Mater Interfaces. (2021) 13:39003–17. doi: 10.1021/acsami.1c08290
142. Hu D, Zhang W, Xiang J, Li D, Chen Y, Yuan P, et al. A ROS-responsive synergistic delivery system for combined immunotherapy and chemotherapy. Mater Today Bio. (2022) 14:100284. doi: 10.1016/j.mtbio.2022.100284
143. Jiang S, Liu Z, Tian Y, Zhuang M, Piao S, Gao Y, et al. A comprehensive evaluation of zrC nanoparticle in combined photothermal and radiation therapy for treatment of triple-negative breast cancer. Front Oncol. (2021) 11:801352. doi: 10.3389/fonc.2021.801352
144. Karoon Kiani F, Izadi S, Ansari Dezfouli E, Ebrahimi F, Mohammadi M, Chalajour H, et al. Simultaneous silencing of the A2aR and PD-1 immune checkpoints by siRNA-loaded nanoparticles enhances the immunotherapeutic potential of dendritic cell vaccine in tumor experimental models. Life Sci. (2022) 288:120166. doi: 10.1016/j.lfs.2021.120166
145. Chiossone L, Dumas PY, Vienne M, Vivier E. Natural killer cells and other innate lymphoid cells in cancer. Nat Rev Immunol. (2018) 18:671–88. doi: 10.1038/s41577-018-0061-z
146. Yu J, Freud AG, Caligiuri MA. Location and cellular stages of natural killer cell development. Trends Immunol. (2013) 34:573–82. doi: 10.1016/j.it.2013.07.005
147. Bubeník J. MHC class I down-regulation: tumour escape from immune surveillance? (review). Int J Oncol. (2004) 25:487–91. doi: 10.3892/ijo.25.2.487
148. Choi C, Finlay DK. Optimising NK cell metabolism to increase the efficacy of cancer immunotherapy. Stem Cell Res Ther. (2021) 12:320. doi: 10.1186/s13287-021-02377-8
149. Crinier A, Narni-Mancinelli E, Ugolini S, Vivier E. SnapShot: natural killer cells. Cell. (2020) 180:1280–.e1. doi: 10.1016/j.cell.2020.02.029
150. Shimasaki N, Jain A, Campana D. NK cells for cancer immunotherapy. Nat Rev Drug Discovery. (2020) 19:200–18. doi: 10.1038/s41573-019-0052-1
151. Zhou Y, Cheng L, Liu L, Li X. NK cells are never alone: crosstalk and communication in tumour microenvironments. Mol Cancer. (2023) 22:34. doi: 10.1186/s12943-023-01737-7
152. Böttcher JP, Bonavita E, Chakravarty P, Blees H, Cabeza-Cabrerizo M, Sammicheli S, et al. NK Cells Stimulate Recruitment of cDC1 into the Tumor Microenvironment Promoting Cancer Immune Control. Cell. (2018) 172:1022–37.e14. doi: 10.1016/j.cell.2018.01.004
153. Barry KC, Hsu J, Broz ML, Cueto FJ, Binnewies M, Combes AJ, et al. A natural killer-dendritic cell axis defines checkpoint therapy-responsive tumor microenvironments. Nat Med. (2018) 24:1178–91. doi: 10.1038/s41591-018-0085-8
154. Parihar R, Rivas C, Huynh M, Omer B, Lapteva N, Metelitsa LS, et al. NK cells expressing a chimeric activating receptor eliminate MDSCs and rescue impaired CAR-T cell activity against solid tumors. Cancer Immunol Res. (2019) 7:363–75. doi: 10.1158/2326-6066.CIR-18-0572
155. Bozward AG, Warricker F, Oo YH, Khakoo SI. Natural killer cells and regulatory T cells cross talk in hepatocellular carcinoma: exploring therapeutic options for the next decade. Front Immunol. (2021) 12:643310. doi: 10.3389/fimmu.2021.643310
156. Liu W, Wei X, Li L, Wu X, Yan J, Yang H, et al. CCR4 mediated chemotaxis of regulatory T cells suppress the activation of T cells and NK cells via TGF-β pathway in human non-small cell lung cancer. Biochem Biophys Res Commun. (2017) 488:196–203. doi: 10.1016/j.bbrc.2017.05.034
157. Raulet DH, Gasser S, Gowen BG, Deng W, Jung H. Regulation of ligands for the NKG2D activating receptor. Annu Rev Immunol. (2013) 31:413–41. doi: 10.1146/annurev-immunol-032712-095951
158. O’Brien KL, Finlay DK. Immunometabolism and natural killer cell responses. Nat Rev Immunol. (2019) 19:282–90. doi: 10.1038/s41577-019-0139-2
159. Sun L, Su Y, Jiao A, Wang X, Zhang B. T cells in health and disease. Signal Transduct Target Ther. (2023) 8:235. doi: 10.1038/s41392-023-01471-y
160. Castoldi A, Lee J, de Siqueira Carvalho D, Souto FO. CD8(+) T cell metabolic changes in breast cancer. Biochim Biophys Acta Mol Basis Dis. (2023) 1869:166565. doi: 10.1016/j.bbadis.2022.166565
161. Kreiter S, Vormehr M, van de Roemer N, Diken M, Löwer M, Diekmann J, et al. Mutant MHC class II epitopes drive therapeutic immune responses to cancer. Nature. (2015) 520:692–6. doi: 10.1038/nature14426
162. Blair AB, Zheng L. Rational combinations of immunotherapy for pancreatic ductal adenocarcinoma. Chin Clin Oncol. (2017) 6:31. doi: 10.21037/cco.2017.06.04
163. Bastaki S, Irandoust M, Ahmadi A, Hojjat-Farsangi M, Ambrose P, Hallaj S, et al. PD-L1/PD-1 axis as a potent therapeutic target in breast cancer. Life Sci. (2020) 247:117437. doi: 10.1016/j.lfs.2020.117437
164. Webster RM. The immune checkpoint inhibitors: where are we now? Nat Rev Drug Discovery. (2014) 13:883–4. doi: 10.1038/nrd4476
165. Postow MA, Sidlow R, Hellmann MD. Immune-related adverse events associated with immune checkpoint blockade. N Engl J Med. (2018) 378:158–68. doi: 10.1056/NEJMra1703481
166. Broos K, Lecocq Q, Raes G, Devoogdt N, Keyaerts M, Breckpot K. Noninvasive imaging of the PD-1:PD-L1 immune checkpoint: Embracing nuclear medicine for the benefit of personalized immunotherapy. Theranostics. (2018) 8:3559–70. doi: 10.7150/thno.24762
167. Peng J, Hamanishi J, Matsumura N, Abiko K, Murat K, Baba T, et al. Chemotherapy induces programmed cell death-ligand 1 overexpression via the nuclear factor-κB to foster an immunosuppressive tumor microenvironment in ovarian cancer. Cancer Res. (2015) 75:5034–45. doi: 10.1158/0008-5472.CAN-14-3098
168. Sun C, Wang B, Hao S. Adenosine-A2A receptor pathway in cancer immunotherapy. Front Immunol. (2022) 13:837230. doi: 10.3389/fimmu.2022.837230
169. Ye H, Zhao J, Xu X, Zhang D, Shen H, Wang S. Role of adenosine A2a receptor in cancers and autoimmune diseases. Immun Inflammation Dis. (2023) 11:e826. doi: 10.1002/iid3.v11.4
170. Luo W, Ali YF, Liu C, Wang Y, Liu C, Jin X, et al. Particle therapy for breast cancer: benefits and challenges. Front Oncol. (2021) 11:662826. doi: 10.3389/fonc.2021.662826
171. So JY, Ohm J, Lipkowitz S, Yang L. Triple negative breast cancer (TNBC): Non-genetic tumor heterogeneity and immune microenvironment: Emerging treatment options. Pharmacol Ther. (2022) 237:108253. doi: 10.1016/j.pharmthera.2022.108253
172. Hwang J, An EK, Zhang W, Park HB, Kim SJ, Yadav D, et al. Recombinant programmed cell death protein 1 functions as an immune check point blockade and enhances anti-cancer immunity. Biomaterials. (2022) 285:121550. doi: 10.1016/j.biomaterials.2022.121550
173. Yuan J, Yuan X, Wu K, Gao J, Li L. A local and low-dose chemotherapy/autophagy-enhancing regimen treatment markedly inhibited the growth of established solid tumors through a systemic antitumor immune response. Front Oncol. (2021) 11:658254. doi: 10.3389/fonc.2021.658254
174. Singh A, Mishra R, Mazumder A. Breast cancer and its therapeutic targets: A comprehensive review. Chem Biol Drug Des. (2024) 103:e14384. doi: 10.1111/cbdd.v103.1
175. Akinpelu A, Akinsipe T, Avila LA, Arnold RD, Mistriotis P. The impact of tumor microenvironment: unraveling the role of physical cues in breast cancer progression. Cancer Metastasis Rev. (2024) 43(2):823–44. doi: 10.1007/s10555-024-10166-x
176. Li L, Zhou S, Lv N, Zhen Z, Liu T, Gao S, et al. Photosensitizer-encapsulated ferritins mediate photodynamic therapy against cancer-associated fibroblasts and improve tumor accumulation of nanoparticles. Mol Pharm. (2018) 15:3595–9. doi: 10.1021/acs.molpharmaceut.8b00419
177. Han C, Liu T, Yin R. Biomarkers for cancer-associated fibroblasts. biomark Res. (2020) 8:64. doi: 10.1186/s40364-020-00245-w
178. Tchou J, Kossenkov AV, Chang L, Satija C, Herlyn M, Showe LC, et al. Human breast cancer associated fibroblasts exhibit subtype specific gene expression profiles. BMC Med Genomics. (2012) 5:39. doi: 10.1186/1755-8794-5-39
179. Sebastian A, Hum NR, Martin KA, Gilmore SF, Peran I, Byers SW, et al. Single-cell transcriptomic analysis of tumor-derived fibroblasts and normal tissue-resident fibroblasts reveals fibroblast heterogeneity in breast cancer. Cancers (Basel). (2020) 12:1307. doi: 10.3390/cancers12051307
180. Jan N, Madni A, Khan S, Shah H, Akram F, Khan A, et al. Biomimetic cell membrane-coated poly(lactic-co-glycolic acid) nanoparticles for biomedical applications. Bioeng Transl Med. (2023) 8:e10441. doi: 10.1002/btm2.10441
181. Voronovic E, Skripka A, Jarockyte G, Ger M, Kuciauskas D, Kaupinis A, et al. Uptake of upconverting nanoparticles by breast cancer cells: surface coating versus the protein corona. ACS Appl Mater Interfaces. (2021) 13:39076–87. doi: 10.1021/acsami.1c10618
182. Li D, Ma S, Xu D, Meng X, Lei N, Liu C, et al. Peptide-functionalized therapeutic nanoplatform for treatment orthotopic triple negative breast cancer and bone metastasis. Nanomedicine. (2023) 50:102669. doi: 10.1016/j.nano.2023.102669
183. Xue L, Thatte AS, Mai D, Haley RM, Gong N, Han X, et al. Responsive biomaterials: optimizing control of cancer immunotherapy. Nat Rev Materials. (2024) 9:100–18. doi: 10.1038/s41578-023-00617-2
184. Wang N, Chen M, Wang T. Liposomes used as a vaccine adjuvant-delivery system: From basics to clinical immunization. J Control Release. (2019) 303:130–50. doi: 10.1016/j.jconrel.2019.04.025
Keywords: breast cancer, immunotherapy, biomaterials, tumor micro-environment, nanoparticles, immune cells
Citation: Santerre JP, Yang Y, Du Z, Wang W and Zhang X (2024) Biomaterials’ enhancement of immunotherapy for breast cancer by targeting functional cells in the tumor micro-environment. Front. Immunol. 15:1492323. doi: 10.3389/fimmu.2024.1492323
Received: 06 September 2024; Accepted: 21 October 2024;
Published: 12 November 2024.
Edited by:
Xuefeng Wang, Soochow University, ChinaReviewed by:
Lulu Xue, University of Pennsylvania, United StatesFazhan Wang, Zhengzhou University, China
Copyright © 2024 Santerre, Yang, Du, Wang and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wenshuang Wang, amVzdXNsb3ZleW91YW5kbWVAMTYzLmNvbQ==; Xiaoqing Zhang, eHF6QGJ6bWMuZWR1LmNu
†These authors have contributed equally to this work and share first authorship
 J. Paul Santerre
J. Paul Santerre Yangyang Yang1†
Yangyang Yang1† Xiaoqing Zhang
Xiaoqing Zhang