
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Immunol. , 11 November 2024
Sec. Cancer Immunity and Immunotherapy
Volume 15 - 2024 | https://doi.org/10.3389/fimmu.2024.1487353
This article is part of the Research Topic Hepatocellular Carcinoma: Novel Treatment Strategies - Volume III View all 17 articles
Background: The five-year recurrence rate for patients with hepatocellular carcinoma (HCC) is as high as 70%. Patients with high-risk recurrence factors experience significantly poorer prognosis. Local regional therapies, including transarterial chemoembolisation (TACE), hepatic arterial infusion chemotherapy (HAIC), radiotherapy, and emerging immunotherapy, are commonly used adjuvant treatment options. We conducted an indirect comparison of these adjuvant therapies for such patients.
Methods: We conducted a systematic search in public databases for relevant studies and assessed the efficacy and safety of the corresponding therapies by consolidating disease-free survival (DFS), overall survival (OS), and adverse events (AEs).
Results: A total of eight randomised controlled trials were ultimately included. The Gelman-Rubin plot and kernel density estimation indicate that the stability of the combined model is satisfactory.
Conclusion: immunotherapy is not inferior to local regional therapies in delaying tumour recurrence, however, the higher incidence of AEs remains a significant concern. Adjuvant radiotherapy demonstrated superior efficacy in delaying tumour recurrence compared to adjuvant TACE, although further support from phase III clinical trial evidence is required.
Systematic review registration: https://www.crd.york.ac.uk/prospero/, identifier CRD42024576316.
Hepatocellular carcinoma (HCC) is one of the top three causes of cancer mortality in 46 countries and ranks among the top five causes of cancer death in 90 countries globally. It is projected that by 2024, the incidence and mortality of liver cancer will increase by over 50% (1). To date, radical surgery remains the primary curative treatment for patients with early-stage tumours; however, its five-year recurrence rate still reaches 40% to 70% (2). Patients with high-risk recurrence factors, including vascular invasion, tumour diameter ≥5 cm, multifocal tumours, satellite nodules, poorly differentiated tumours (corresponding to Edmonson-Steiner grades III-IV), and non-capsulated tumours, exhibit significantly higher early and late recurrence rates, which pose a substantial challenge to patient prognosis (3–5). Therefore, it is essential to develop appropriate adjuvant therapies to reduce the postoperative recurrence rate in HCC patients with high-risk recurrence factors.
In China, adjuvant TACE is recommended to reduce recurrence rates (2), and its efficacy has been validated by phase III clinical trials (6–8). In addition, other adjuvant therapies, including hepatic arterial infusion chemotherapy (HAIC) and radiotherapy (RT), have also been implemented in clinical practice and are supported by high-quality randomized controlled trials (9–11).
With the advent of the era of immunotherapy, immune checkpoint inhibitors have shown surprising efficacy in this patient population. With the advent of the era of immunotherapy, immune checkpoint inhibitors have demonstrated surprising efficacy in this patient population (12, 13) and have significantly prolonged the recurrence-free survival of these patients in randomized controlled trials (14, 15).
With the emergence of various therapies, there is a need for a deeper understanding of their efficacy. In the absence of head-to-head clinical trials, we conducted a network meta-analysis to indirectly compare the efficacy of endovascular therapy, RT, and immune checkpoint inhibitors.
This systematic review and network meta-analysis complied with the Preferred Reporting Items for Systematic Review and Meta-Analyses (PRISMA) statement (Supplementary Table S1) (16). The protocol for this meta-analysis was registered with PROSPERO (ID: CRD42024576316).
We conducted a comprehensive search of articles and references in PubMed, Embase, Web of Science, Cochrane Library and Scoups databases. Literature searches were conducted from the time the database was created until October 3, 2024 with the language restricted to English. The detailed search strategies are provided in Supplementary Table S2. The studies were independently selected by two researchers who screened the titles and abstracts to identify relevant articles and read the full texts for inclusion. Any disagreements regarding eligibility for inclusion in the analysis were resolved through discussion with the team of researchers.
Published studies that met the following criteria were included:
1. Patients with hepatocellular carcinoma (HCC) who have undergone radical surgery and are assessed preoperatively/intraoperatively as having a moderate to high risk of recurrence include those with the following criteria: multiple lesions ≥ 3, a single lesion with a diameter ≥ 5 cm, microvascular invasion or portal/hepatic vein invasion, and poor differentiation (Edmonson-Steiner grade III/IV);
2. The trial reported one or more of the following clinical outcomes: disease-free survival (DFS), defined as the time from the start of the study to the patient’s first tumour progression or death; 1-year DFS (1-DFS), defined as the proportion of patients in the cohort who have not experienced tumor progression or death within one year after the initiation of the study; overall survival (OS), defined as the time from the start of the study to the onset of death; ≥ 3 grade adverse events (≥ 3 AEs), defined as the incidence of level 3 or higher adverse events as defined by the National Cancer Institute Common Terminology Criteria for Adverse events (CTCAE).
3. The interventions of interest include endovascular therapies such as TACE and HAIC, RT, targeted therapies, and immunotherapy;
4. A randomised controlled trial.
In addition, abstracts, reviews, conference papers, case reports, and animal or in vitro studies were excluded.
The research team members prepared a standardised Excel spreadsheet in advance for data extraction. The extracted data included the basic characteristics of the studies, which encompassed the first author, year of publication, country, duration of the study, study phase, clinical trial registration number, inclusion criteria, and sample size; the basic characteristics of the patient cohort, including median age, sex ratio, HBV infection rate, proportion of multiple tumours, proportion of vascular invasion, Child-Pugh liver function classification, and interventions; as well as the outcome data of the studies, including DFS, 1-DFS, OS, and ≥3 AEs.
Two researchers (JiahaoL and YL) independently used the Cochrane tool (RoB2) to assess the methodological quality of included studies. Five domains of the original study were assessed: bias during randomisation, bias in deviation from established interventions, bias in missing outcome data, bias in outcome measurement, and bias in selective reporting. Any disagreements were resolved by consensus through discussions among the team researchers.
The primary outcomes were DFS and 1-DFS. The secondary outcomes were OS and ≥3 AEs. We assessed the efficacy of the different treatment regimens by combining the odds ratios (ORs) of the 1-DFS outcomes and the hazard ratios (HRs) of the two survival outcomes with their 95% confidence intervals (95% CIs) and chose whether to use either a random-effects model or a fixed-effects model based on the I2 value.
We constructed network plots of various treatment regimens to visually display the direct and indirect comparisons among different treatment options. The gemtc package in R version 4.4.1 was used to fit a consistency model, and we assessed the convergence of the Markov chains using the Gelman-Rubin diagnostic and kernel density estimation plots. In addition, we performed heterogeneity tests. Subsequently, subgroup analysis was conducted for the primary outcome measure, DFS, and meta-regression analysis was performed to explore possible factors influencing the analysis results. All tests were two-sided, and a p-value of <0.05 was considered statistically significant.
A total of 12010 articles were retrieved from PubMed, Cochrane Library, Embase, Web of Science and Scoups databases. After removing duplicate records and excluding reviews, abstracts, editorials, conference articles, and studies not relevant to the research content through reading titles and abstracts, a total of 13 articles were subjected to full-text review. Ultimately, 8 studies were included in this meta-analysis (Figure 1). Among these studies, 7 were conducted in China, while 1 was conducted across multiple countries. A total of 5 adjunctive treatment regimens were included: TACE (transcatheter arterial chemoembolization), HAIC, RT, atezolizumab + bevacizumab, and sintilimab. In total, 1,909 subjects were enrolled in these studies (Figure 2). The basic characteristics of the included studies are presented in Supplementary Table 3.
All studies achieved complete outcome reporting with random allocation. They were all open-label studies. Except for the study by Qin et al., which had some risk in terms of randomization, the other 7 studies exhibited a low risk of bias across the 5 assessed domains (Supplementary Figure 1).
All included studies provided analyzable data on DFS. Compared to surgery alone, the postoperative adjuvant treatment regimens included demonstrated a significant benefit in DFS. In indirect comparisons, RT showed a superior effect in delaying recurrence compared to TACE (HR: 1.74; 95% CI: 1.09, 2.8) and atezolizumab combined with bevacizumab (HR: 0.56; 95% CI: 0.33, 0.92). Meanwhile, no significant statistical differences were observed between TACE, HAIC, atezolizumab combined with bevacizumab, and sintilimab (Figure 3). The Gelman-Rubin plot and kernel density estimation indicate a satisfactory convergence of the MCMC chains and demonstrate the robustness of the model (Supplementary Figure 2).
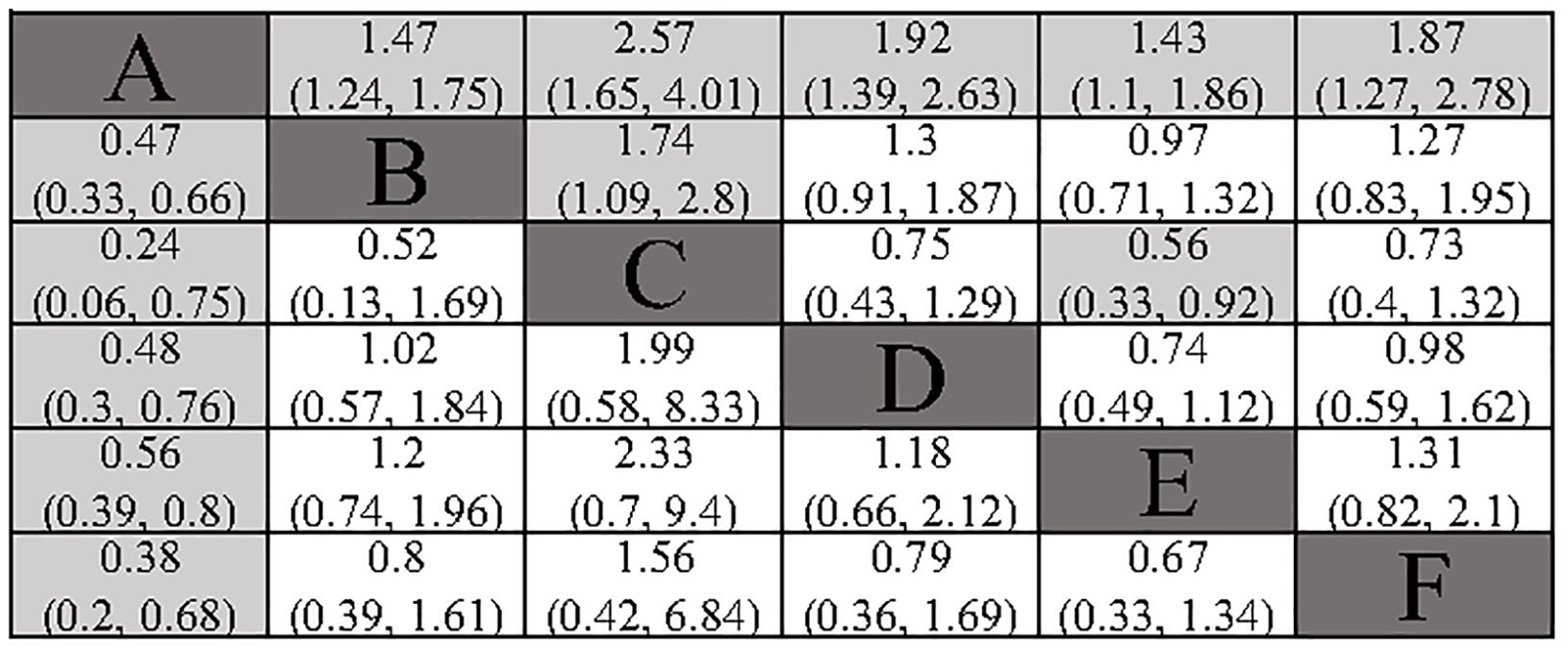
Figure 3. Summary of Network Meta-Analysis. pooled risk ratio (95% confidence interval) for DFS (upper triangle); pooled risk ratio (95% confidence interval) for 1-DFS (lower triangle). A, Radical surgery alone; B, Radical surgery + adjuvant TACE; C, Radical surgery + adjuvant RT; D, Radical surgery + adjuvant HAIC; E, Radical surgery + adjuvant atezolizumab plus bevacizumab; F, Radical surgery + adjuvant sintilimab.
All included studies provided analysable data on 1-DFS. All the adjuvant treatment regimens assessed demonstrated a significant benefit in terms of 1-DFS. In the indirect comparison, none of the included adjuvant treatment regimens showed a significant statistical difference (Figure 3). The Gelman-Rubin plot and kernel density estimation illustrate that the convergence of the MCMC chains is satisfactory and demonstrate the robustness of the model (Supplementary Figure 3).
All included studies reported overall survival (OS) data. Compared to surgery alone, adjuvant TACE (HR: 0.63; 95% CI: 0.50, 0.81) and adjuvant RT (HR: 0.45; 95% CI: 0.28, 0.74) demonstrated a significant OS benefit. Although adjuvant HAIC (HR: 0.57; 95% CI: 0.31, 1.04) and adjuvant sintilimab (HR: 0.51; 95% CI: 0.25, 1.01) did not reach statistical significance, they still showed a favorable trend. In the indirect comparison, RT, HAIC, and sintilimab demonstrated a superior OS benefit compared to atezolizumab plus bevacizumab (Figure 4). However, it is important to note that in the studies involving HAIC, atezolizumab plus bevacizumab, and sintilimab, the different study cohorts did not reach the OS endpoint, thereby reducing the credibility of these results. The Gelman-Rubin plot and kernel density estimation illustrate that the convergence of the MCMC chains is satisfactory and demonstrate the robustness of the model (Supplementary Figure 4).
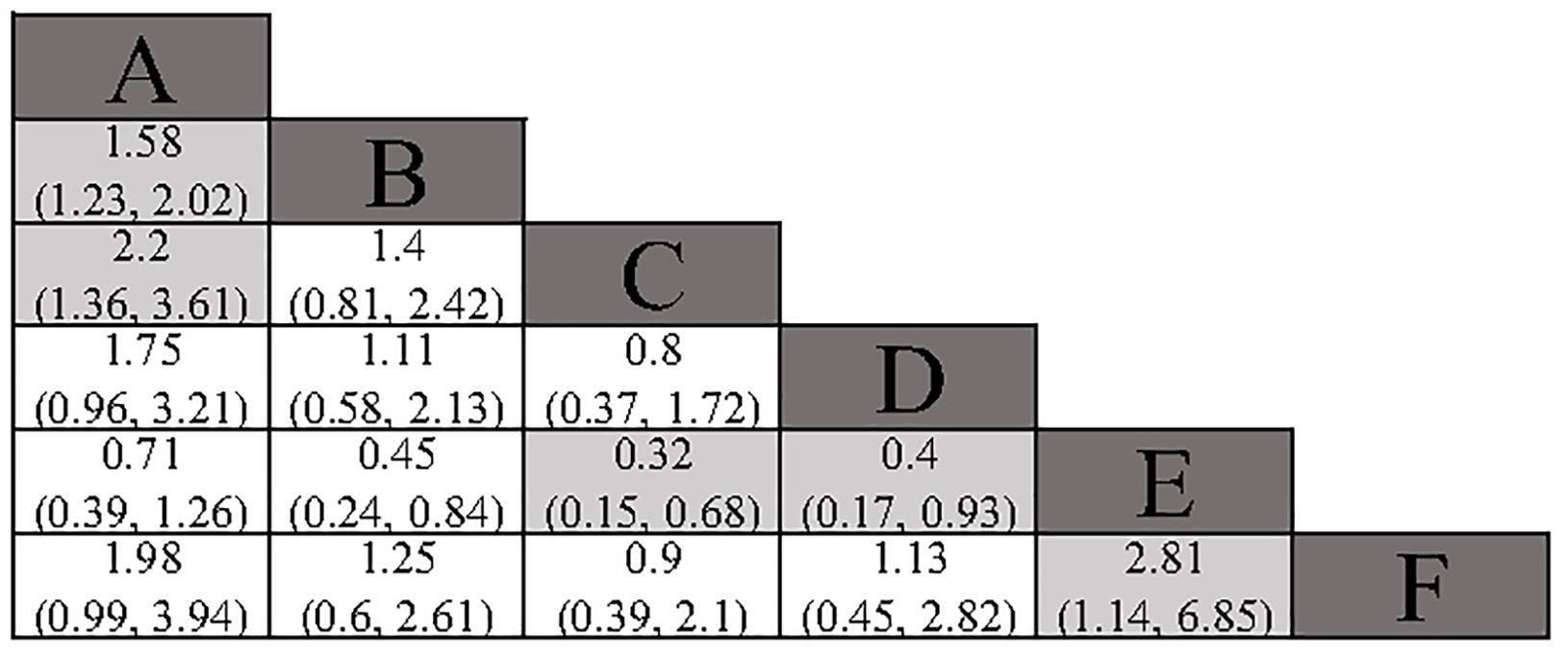
Figure 4. Summary of Network Meta-Analysis. pooled risk ratio (95% confidence interval) for OS. A, Radical surgery alone; B, Radical surgery + adjuvant TACE; C, Radical surgery + adjuvant RT; D, Radical surgery + adjuvant HAIC; E, Radical surgery + adjuvant atezolizumab plus bevacizumab; F, Radical surgery + adjuvant sintilimab.
The assessment of AEs is descriptive in nature. In the studies involving TACE, RT, and HAIC, most of the complications related to adjuvant therapy were classified as grade 1-2, indicating that the patients were generally tolerable. None of the six studies reported AEs that would change the treatment outcomes. In the IMbrave050 trial, 41% of patients in the postoperative group receiving a combination of atezolizumab and bevacizumab experienced grade 3 or 4 AEs, compared to an incidence of just 13% in the postoperative active monitoring group. Notably, there was a significant increase in the rates of hypertension, proteinuria, and decreased platelet count among those in the combination therapy group. In patients receiving postoperative treatment with atezolizumab in combination with bevacizumab, 63% experienced immune-mediated AEs of any grade, with grade 3 or 4 AEs reaching 10%. The most common events among these were hepatitis and hypothyroidism. It is noteworthy that the combination of atezolizumab and bevacizumab may increase the incidence of bleeding events. Furthermore, any grade of AEs leading to the discontinuation of atezolizumab and bevacizumab occurred in 9% of patients. In the study by Wang et al., 63.9% of patients in the sintilimab group experienced treatment-related AEs of any grade, with 12.4% experiencing grade 3 or 4 treatment-related AEs. Similar to the IMbrave050 trial, 8.1% of patients discontinued treatment due to AEs, with elevated ALT levels being a major contributing factor. Table 1 summarizes the incidence of select ≥3 AEs.
Figure 5 shows the Bayesian ranking of the different outcomes for the different treatment options. Supplementary Table S4 summarises the ranking results. The Bayesian ranking results were consistent with the results of the HR analyses. Furthermore, we conducted a comprehensive assessment of the included treatment regimens based on the SUCRA values, as presented in Table 2.
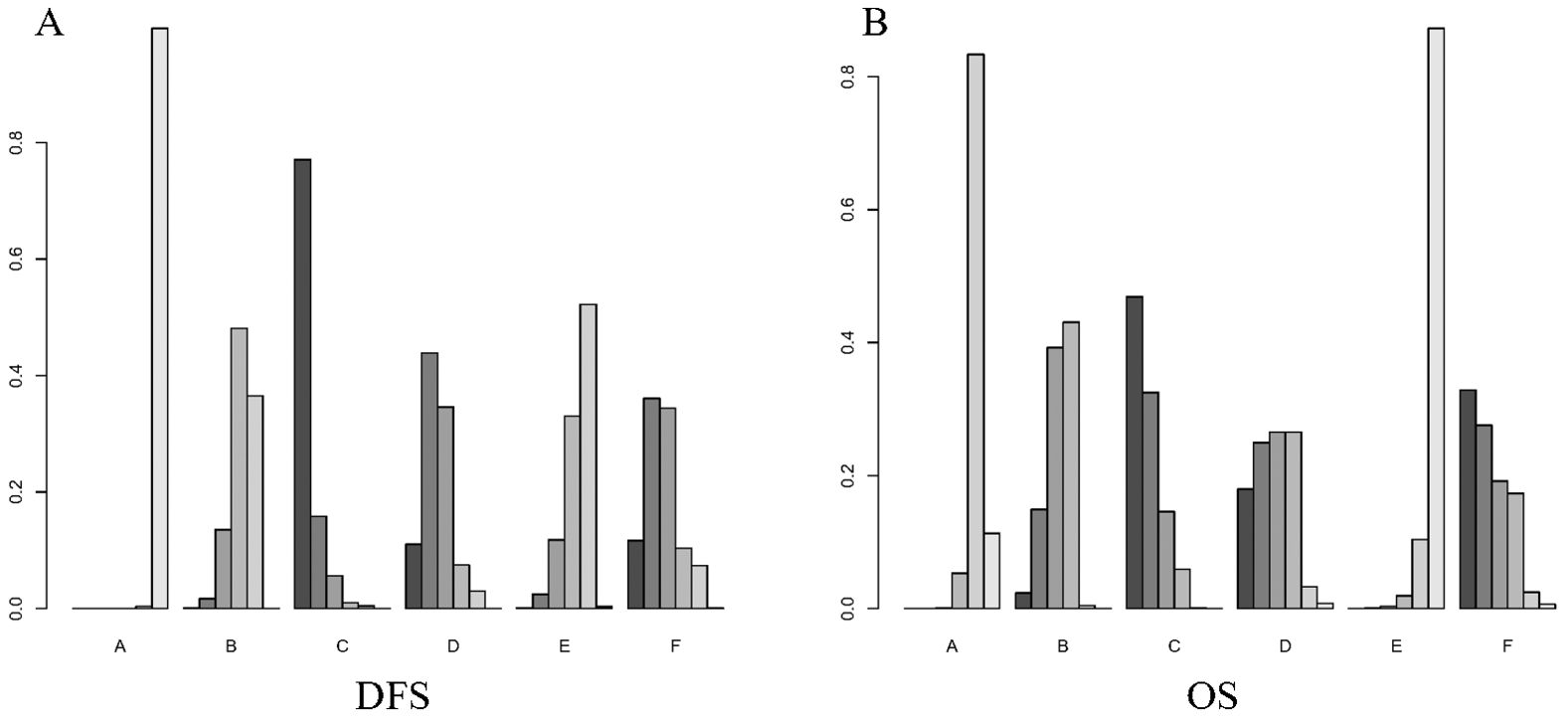
Figure 5. Bayesian ranking plot of treatment. (A) DFS; (B) OS. A, Radical surgery alone; B, Radical surgery + adjuvant TACE; C, Radical surgery + adjuvant RT; D, Radical surgery + adjuvant HAIC; E, Radical surgery + adjuvant atezolizumab plus bevacizumab; F, Radical surgery + adjuvant sintilimab.
We performed a pairwise meta-analysis of the outcomes from different treatment regimens. The results indicated that there was no significant heterogeneity in the combined DFS and OS (I² = 0%) (Figures 6, 7).
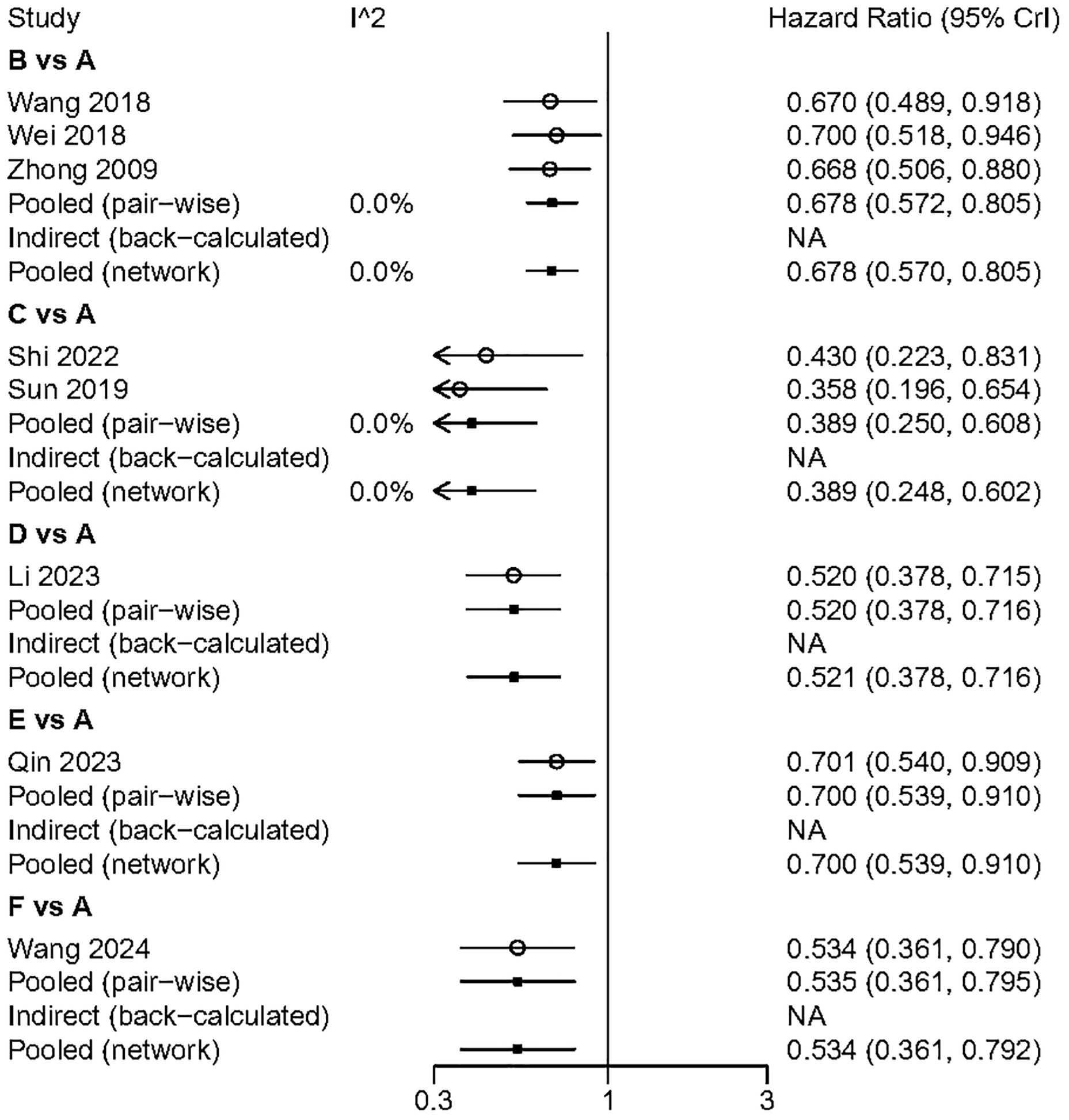
Figure 6. The pairwise meta-analysis of DFS. A. Radical surgery alone; B, Radical surgery + adjuvant TACE; C, Radical surgery + adjuvant RT; D, Radical surgery + adjuvant HAIC; E, Radical surgery + adjuvant atezolizumab plus bevacizumab; F, Radical surgery + adjuvant sintilimab.
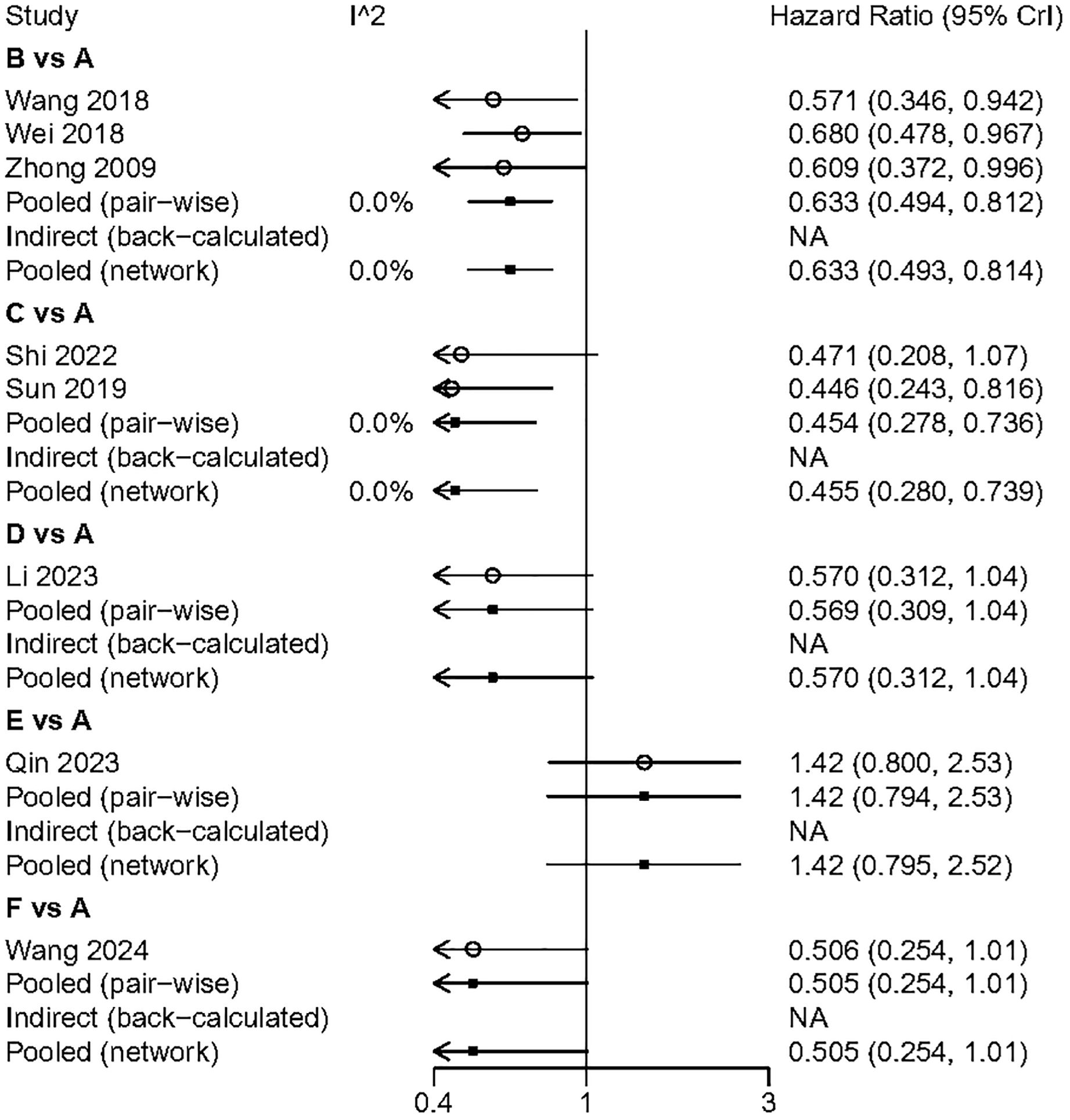
Figure 7. The pairwise meta-analysis of OS. A, Radical surgery alone; B, Radical surgery + adjuvant TACE; C, Radical surgery + adjuvant RT; D, Radical surgery + adjuvant HAIC; E, Radical surgery + adjuvant atezolizumab plus bevacizumab; F, Radical surgery + adjuvant sintilimab.
Subgroup analyses of DFS results were conducted based on the age of the study cohorts, tumor differentiation, and tumor quantity (Figure 8). The results showed that both adjuvant HAIC and sintilimab provided significant DFS benefits in these patients.
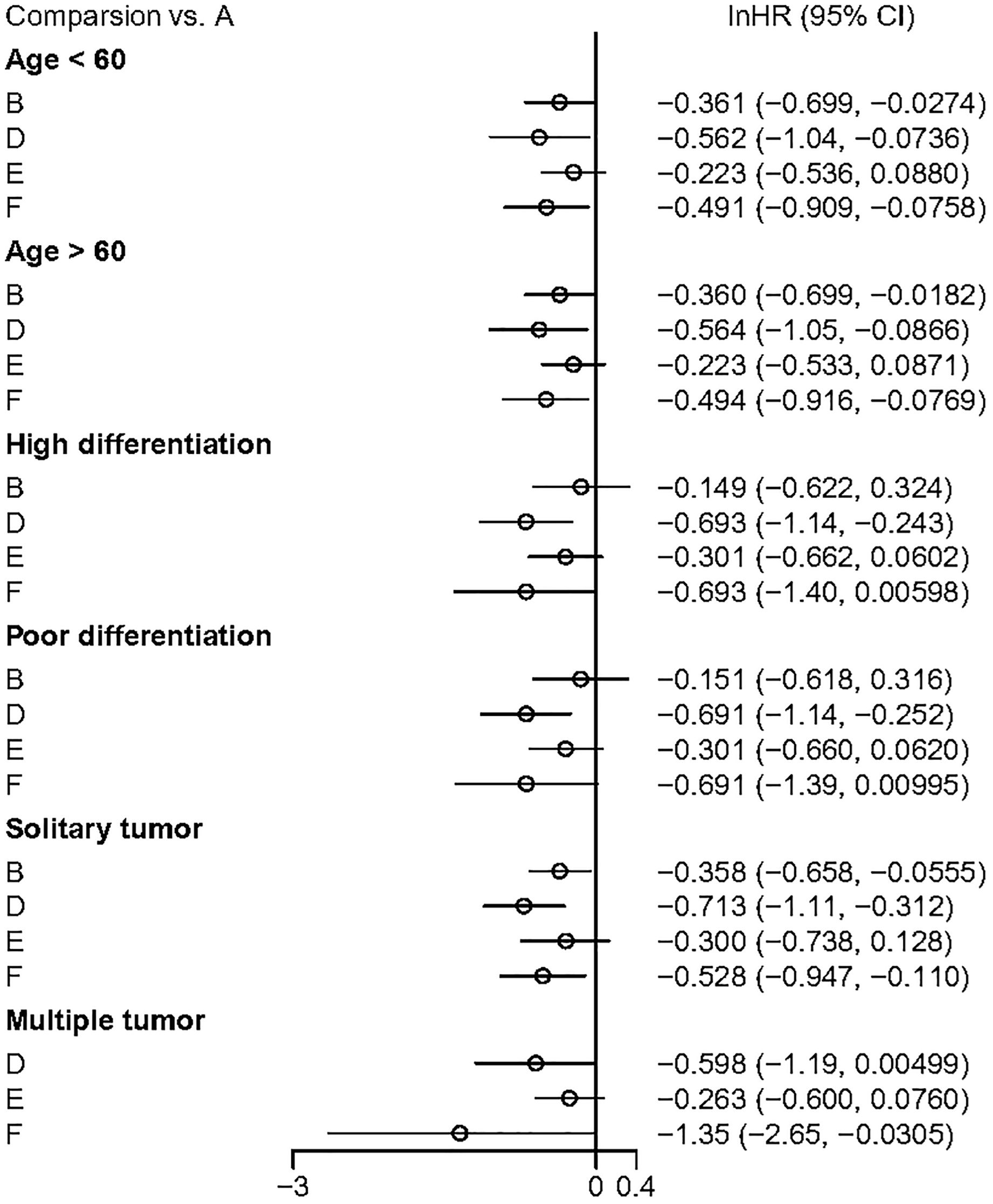
Figure 8. The outcome of subguoup analyses. A, Radical surgery alone; B, Radical surgery + adjuvant TACE; C, Radical surgery + adjuvant RT; D, Radical surgery + adjuvant HAIC; E, Radical surgery + adjuvant atezolizumab plus bevacizumab; F, Radical surgery + adjuvant sintilimab.
Network meta-regression analysis of disease-free survival (DFS) results revealed that variables such as study duration, sample size, patient age, gender, HBV infection, multiple tumours, and vascular invasion were not significantly associated with the combined DFS outcomes (Table 3).
In recent years, there has been rapid advancement in the treatment of hepatocellular carcinoma HCC. Currently, localized regional therapies and combination treatment strategies based on these local therapies are important options for patients. Endovascular therapies such as TACE and HAIC are widely used and supported by high-quality randomized controlled trials RCTs (17, 18). Combination therapies based on TACE are being extensively explored in various clinical scenarios (19). HAIC is another endovascular therapy that exerts tumor control by continuously infusing chemotherapy drugs directly into the tumor lesion. In clinical practice, HAIC is often employed to treat HCC patients with a high tumor burden and those in more advanced stages of the disease. Based on the FOLFOX chemotherapy regimen, HAIC has demonstrated significant clinical efficacy in certain patient subgroups, including those with large tumor burdens, advanced HCC, and portal vein invasion (18, 20, 21). The application of immune checkpoint inhibitors has ushered in a new era of immunotherapy for HCC (22–24). Although some guidelines recommend the combination of atezolizumab and bevacizumab as the preferred treatment for advanced HCC (25), the relatively high incidence of adverse reactions associated with this regimen remains a cause for concern (26). So far, radical tumour resection remains the primary curative treatment for patients with early-stage HCC. However, the high postoperative recurrence rate continues to be a significant factor affecting patient prognosis, particularly among those with high-risk recurrence factors. Such patients often exhibit more aggressive tumour phenotypes and biological behaviour (27, 28). Therefore, it is crucial to implement necessary measures to reduce the recurrence rate (20).
This network meta-analysis compared the effects of endovascular therapy, radiotherapy, and immunotherapy in patients with HCC who underwent radical surgery and presented with high-risk recurrence factors. The main findings are summarised as follows: in patients with HCC exhibiting high-risk recurrence factors, adjuvant endovascular therapy, radiotherapy, and immunotherapy all demonstrated significant efficacy in controlling recurrences. In indirect comparisons, adjuvant radiotherapy showed superior recurrence control compared to TACE and the combination of atezolizumab and bevacizumab; however, no statistically significant differences were observed between adjuvant radiotherapy and adjuvant HAIC or sintilimab. In direct comparisons regarding OS, postoperative TACE and radiotherapy exhibited improved OS outcomes compared to surgery alone. In indirect comparisons of OS, postoperative radiotherapy, HAIC, and sintilimab provided superior OS benefits compared to the combination of atezolizumab and bevacizumab. Moreover, sintilimab and the combination of atezolizumab and bevacizumab were associated with a tendency towards a higher incidence of serious AEs.
This study conducted a comprehensive analysis of the effectiveness and safety of five adjuvant therapeutic approaches by investigating three outcome indicators: DFS, OS, and AEs. Prior to initiating the research, we established an extensive search strategy, ensuring that the data incorporated were thorough and derived from high-quality randomised controlled trials, which further enhanced the credibility of the study. Additionally, we performed subgroup analyses and meta-regression analyses to provide further recommendations for clinical decision-making for this patient population.
However, this study does have limitations. Firstly, the potential variations in study protocols, patient populations, and interventions among the included studies make it challenging to draw definitive conclusions. For instance, the inclusion of different adjuvant therapies and patient cohorts with varying baseline characteristics may introduce confounding factors that cannot be fully addressed, even though network meta-regression analyses may have partially mitigated this bias. Secondly, among the eight studies included, seven were conducted in China, while only one was a multinational study. This raises concerns about the generalizability of the results to populations outside of China, as regional differences in the etiology, genetics, and healthcare practices related to hepatocellular carcinoma. Thirdly, although all studies reported OS, the studies conducted by Li et al. (11), Qin et al. (15), and Wang et al. (14) did not reach the OS endpoint. This weakens the overall conclusions regarding the survival benefits of these therapies and reduces the credibility of the results, especially concerning the indirect comparisons in the network meta-analysis. Therefore, the combined outcomes may be subject to considerable bias, and the analysis results should be interpreted with caution.
There remains ongoing debate regarding adjuvant therapy for HCC. Guidelines from Western countries and some East Asian nations do not recommend or explicitly endorse adjuvant treatment for patients with high-risk recurrence factors (29–32). Postoperative active surveillance can assist clinicians in identifying early tumor recurrences, which may be treated through subsequent radical resection. However, portal hypertension, insufficient functional reserve of the remaining liver, and technical difficulties may render repeated resections challenging and risky (33). Therefore, discussing suitable and effective adjuvant therapy options is of paramount importance.
In China, adjuvant TACE is recommended for patients to reduce recurrence rates (2). This study supports the utility of radiotherapy, HAIC, and immunotherapy in adjuvant treatment, contributing to updates in the guidelines. The findings demonstrate that external beam radiotherapy shows significant potential for the management of HCC patients with high-risk recurrence factors. With the advancement of new technologies such as three-dimensional conformal radiotherapy, intensity-modulated radiation therapy, and stereotactic body radiation therapy, it is now possible to enhance the radiation dose delivered to target areas while better sparing adjacent healthy liver tissue. This significantly facilitates the clinical application of radiotherapy techniques (9, 34). Furthermore, the results of this study demonstrate that adjuvant immunotherapy is not inferior to local regional therapies in terms of efficacy, thereby promoting the application of immunotherapy in a broader range of clinical scenarios. However, the higher incidence of adverse reactions associated with immune checkpoint inhibitors and their safety profile remain important concerns that warrant attention.
At the same time, the results of this study highlight several issues that need to be addressed in the future. Firstly, current research provides very limited data on long-term outcomes. While DFS is important, a more comprehensive exploration of overall survival over longer follow-up periods would be more beneficial. Secondly, certain adjuvant therapies, particularly the combination of atezolizumab and bevacizumab, exhibit a high incidence of adverse reactions, raising concerns regarding the safety of these treatments. The significantly increased rates of hypertension, proteinuria, and thrombocytopenia, as well as immune-mediated adverse effects such as hepatitis and hypothyroidism in these patient populations, underscore the need for a more careful risk-benefit analysis in clinical practice. Thirdly, future trials should aim to include direct head-to-head comparisons of the most promising treatments identified in this study whenever possible, to reduce the uncertainty associated with indirect comparisons. Finally, for certain therapies lacking reliable Phase III clinical trial data, further research should be conducted to reinforce the conclusions drawn from this study.
In this systematic review and network meta-analysis, we evaluated the efficacy and safety of various adjuvant treatment regimens for HCC patients with high-risk recurrence factors. The results indicate that adjuvant immunotherapy provides a comparable effect in delaying tumor recurrence when compared to local regional therapies; however, its safety profile remains a significant concern, necessitating the establishment of stringent usage criteria before clinical application. Adjuvant radiotherapy demonstrated superior efficacy in delaying tumor recurrence compared to adjuvant TACE, but further support from Phase III clinical trial evidence is required. Additionally, the long-term effects of HAIC and immunotherapy in this patient population warrant further investigation.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
JHL: Conceptualization, Data curation, Formal analysis, Methodology, Writing – original draft. YL: Investigation, Supervision, Writing – review & editing. YQ: Data curation, Formal analysis, Visualization, Writing – original draft. CQ: Resources, Writing – review & editing. JRL: Conceptualization, Investigation, Supervision, Writing – review & editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2024.1487353/full#supplementary-material
HAIC, hepatic arterial infusion chemotherapy; HCC, hepatocellular carcinoma; HRs, hazard ratios; MCMC, Markov Chain Monte Carlo; ORs, odds ratios; OS, overall survival; TACE, transarterial chemoembolization; RT, radiotherapy.
1. Rumgay H, Arnold M, Ferlay J, Lesi O, Cabasag CJ, Vignat J, et al. Global burden of primary liver cancer in 2020 and predictions to 2040. J Hepatol. (2022) 77:1598–606. doi: 10.1016/j.jhep.2022.08.021
2. Zhou J, Sun H, Wang Z, Cong W, Zeng M, Zhou W, et al. Guidelines for the diagnosis and treatment of primary liver cancer (2022 edition). Liver Cancer. (2023) 12:405–44. doi: 10.1159/000530495
3. Nevola R, Ruocco R, Criscuolo L, Villani A, Alfano M, Beccia D, et al. Predictors of early and late hepatocellular carcinoma recurrence. World J Gastroenterol. (2023) 29:1243–60. doi: 10.3748/wjg.v29.i8.1243
4. Xu XF, Xing H, Han J, Li ZL, Lau WY, Zhou YH, et al. Risk factors, patterns, and outcomes of late recurrence after liver resection for hepatocellular carcinoma: A multicenter study from China. JAMA Surg. (2019) 154:209–17. doi: 10.1001/jamasurg.2018.4334
5. Shen J, Wen J, Li C, Wen T, Yan L, Li B, et al. The prognostic value of microvascular invasion in early-intermediate stage hepatocelluar carcinoma: a propensity score matching analysis. BMC Cancer. (2018) 18:278. doi: 10.1186/s12885-018-4196-x
6. Zhong C, Guo RP, Li JQ, Shi M, Wei W, Chen MS, et al. A randomized controlled trial of hepatectomy with adjuvant transcatheter arterial chemoembolization versus hepatectomy alone for Stage III A hepatocellular carcinoma. J Cancer Res Clin Oncol. (2009) 135:1437–45. doi: 10.1007/s00432-009-0588-2
7. Wang Z, Ren Z, Chen Y, Hu J, Yang G, Yu L, et al. Adjuvant transarterial chemoembolization for HBV-related hepatocellular carcinoma after resection: A randomized controlled study. Clin Cancer Res. (2018) 24:2074–81. doi: 10.1158/1078-0432.CCR-17-2899
8. Wei W, Jian PE, Li SH, Guo ZX, Zhang YF, Ling YH, et al. Adjuvant transcatheter arterial chemoembolization after curative resection for hepatocellular carcinoma patients with solitary tumor and microvascular invasion: a randomized clinical trial of efficacy and safety. Cancer Commun (Lond). (2018) 38:61. doi: 10.1186/s40880-018-0331-y
9. Sun J, Yang L, Shi J, Liu C, Zhang X, Chai Z, et al. Postoperative adjuvant IMRT for patients with HCC and portal vein tumor thrombus: An open-label randomized controlled trial. Radiother Oncol. (2019) 140:20–5. doi: 10.1016/j.radonc.2019.05.006
10. Shi C, Li Y, Geng L, Shen W, Sui C, Dai B, et al. Adjuvant stereotactic body radiotherapy after marginal resection for hepatocellular carcinoma with microvascular invasion: A randomised controlled trial. Eur J Cancer. (2022) 166:176–84. doi: 10.1016/j.ejca.2022.02.012
11. Li SH, Mei J, Cheng Y, Li Q, Wang QX, Fang CK, et al. Postoperative adjuvant hepatic arterial infusion chemotherapy with FOLFOX in hepatocellular carcinoma with microvascular invasion: A multicenter, phase III, randomized study. J Clin Oncol. (2023) 41:1898–908. doi: 10.1200/JCO.22.01142
12. Li L, Wu PS, Liang XM, Chen K, Zhang GL, Su QB, et al. Adjuvant immune checkpoint inhibitors associated with higher recurrence-free survival in postoperative hepatocellular carcinoma (PREVENT): a prospective, multicentric cohort study. J Gastroenterol. (2023) 58:1043–54. doi: 10.1007/s00535-023-02018-2
13. Chen W, Hu S, Liu Z, Sun Y, Wu J, Shen S, et al. Adjuvant anti-PD-1 antibody for hepatocellular carcinoma with high recurrence risks after hepatectomy. Hepatol Int. (2023) 17:406–16. doi: 10.1007/s12072-022-10478-6
14. Wang K, Xiang YJ, Yu HM, Cheng YQ, Liu ZH, Qin YY, et al. Adjuvant sintilimab in resected high-risk hepatocellular carcinoma: a randomized, controlled, phase 2 trial. Nat Med. (2024) 30:708–15. doi: 10.1038/s41591-023-02786-7
15. Qin S, Chen M, Cheng AL, Kaseb AO, Kudo M, Lee HC, et al. Atezolizumab plus bevacizumab versus active surveillance in patients with resected or ablated high-risk hepatocellular carcinoma (IMbrave050): a randomised, open-label, multicentre, phase 3 trial. Lancet. (2023) 402:1835–47. doi: 10.1016/S0140-6736(23)01796-8
16. Wang H, Zhao Q, Zhang Y, Wei J, Wang B, Zheng Z, et al. Efficacy and safety of systemic treatments for patients with recurrent/metastatic head and neck squamous cell carcinoma: A systematic review and network meta-analysis. Pharmacol Res. (2021) 173:105866. doi: 10.1016/j.phrs.2021.105866
17. Peng Z, Fan W, Zhu B, Wang G, Sun J, Xiao C, et al. Lenvatinib combined with transarterial chemoembolization as first-line treatment for advanced hepatocellular carcinoma: A phase III, randomized clinical trial (LAUNCH). J Clin Oncol. (2023) 41:117. doi: 10.1200/JCO.22.00392
18. Lyu N, Wang X, Li J-B, Lai J-F, Chen Q-F, Li S-L, et al. Arterial chemotherapy of oxaliplatin plus fluorouracil versus sorafenib in advanced hepatocellular carcinoma: A biomolecular exploratory, randomized, phase III trial (FOHAIC-1). J Clin Oncol. (2022) 40:468–80. doi: 10.1200/JCO.21.01963
19. Zhong BY, Jin ZC, Chen JJ, Zhu HD, Zhu XL. Role of transarterial chemoembolization in the treatment of hepatocellular carcinoma. J Clin Transl Hepatol. (2023) 11:480–9. doi: 10.14218/JCTH.2022.00293
20. Li QJ, He MK, Chen HW, Fang WQ, Zhou YM, Xu L, et al. Hepatic arterial infusion of oxaliplatin, fluorouracil, and leucovorin versus transarterial chemoembolization for large hepatocellular carcinoma: A randomized phase III trial. J Clin Oncol. (2022) 40:150–60. doi: 10.1200/JCO.21.00608
21. He M, Li Q, Zou R, Shen J, Fang W, Tan G, et al. Sorafenib plus hepatic arterial infusion of oxaliplatin, fluorouracil, and leucovorin vs sorafenib alone for hepatocellular carcinoma with portal vein invasion: A randomized clinical trial. JAMA Oncol. (2019) 5:953–60. doi: 10.1001/jamaoncol.2019.0250
22. Qin S, Chan LS, Gu S, Bai Y, Ren Z, Lin X, et al. LBA35 Camrelizumab (C) plus rivoceranib (R) vs. sorafenib (S) as first-line therapy for unresectable hepatocellular carcinoma (uHCC): A randomized, phase III trial. Ann Oncol. (2022) 33:S1401–2. doi: 10.1016/j.annonc.2022.08.032
23. Galle PR, Finn RS, Qin S, Ikeda M, Zhu AX, Kim TY, et al. Patient-reported outcomes with atezolizumab plus bevacizumab versus sorafenib in patients with unresectable hepatocellular carcinoma (IMbrave150): an open-label, randomised, phase 3 trial. Lancet Oncol. (2021) 22:991–1001. doi: 10.1016/S1470-2045(21)00151-0
24. Ren Z, Xu J, Bai Y, Xu A, Cang S, Du C, et al. Sintilimab plus a bevacizumab biosimilar (IBI305) versus sorafenib in unresectable hepatocellular carcinoma (ORIENT-32): a randomised, open-label, phase 2-3 study. Lancet Oncol. (2021) 22:977–90. doi: 10.1016/S1470-2045(21)00252-7
25. Singal AG, Llovet JM, Yarchoan M, Mehta N, Heimbach JK, Dawson LA, et al. AASLD Practice Guidance on prevention, diagnosis, and treatment of hepatocellular carcinoma. Hepatology. (2023) 78. doi: 10.1097/HEP.0000000000000466
26. Cappuyns S, Corbett V, Yarchoan M, Finn RS, Llovet JM. Critical appraisal of guideline recommendations on systemic therapies for advanced hepatocellular carcinoma: A review. JAMA Oncol. (2024) 10:395–404. doi: 10.1001/jamaoncol.2023.2677
27. Erstad DJ, Tanabe KK. Prognostic and therapeutic implications of microvascular invasion in hepatocellular carcinoma. Ann Surg Oncol. (2019) 26:1474–93. doi: 10.1245/s10434-019-07227-9
28. Li J, Su X, Xu X, Zhao C, Liu A, Yang L, et al. Preoperative prediction and risk assessment of microvascular invasion in hepatocellular carcinoma. Crit Rev Oncol Hematol. (2023) 190:104107. doi: 10.1016/j.critrevonc.2023.104107
29. Vogel A, Cervantes A, Chau I, Daniele B, Llovet JM, Meyer T, et al. Hepatocellular carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. (2018) 29:iv238–55. doi: 10.1093/annonc/mdy308
30. Benson AB, D'Angelica MI, Abbott DE, Anaya DA, Anders R, Are C, et al. Hepatobiliary cancers, version 2.2021, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. (2021) 19:541–65. doi: 10.6004/jnccn.2021.0022
31. Korean Liver Cancer Association (KLCA) and National Cancer Center (NCC) Korea. 2022 KLCA-NCC Korea practice guidelines for the management of hepatocellular carcinoma. Clin Mol Hepatol. (2022) 28:583–705. doi: 10.3350/cmh.2022.0294
32. Kudo M, Kawamura Y, Hasegawa K, Tateishi R, Kariyama K, Shiina S, et al. Management of hepatocellular carcinoma in Japan: JSH consensus statements and recommendations 2021 update. Liver Cancer. (2021) 10:181–223. doi: 10.1159/000514174
33. Criss CR, Makary MS. Salvage locoregional therapies for recurrent hepatocellular carcinoma. World J Gastroenterol. (2023) 29:413–24. doi: 10.3748/wjg.v29.i3.413
Keywords: hepatocellular carcinoma, TACE, HAIC, adjuvant therapy, immunotherapy, network meta-analysis
Citation: Li J, Liu Y, Qiu Y, Qu C and Li J (2024) Comparison of adjuvant treatment regimens for high-risk hepatocellular carcinoma: a Bayesian network meta analysis and systematic review. Front. Immunol. 15:1487353. doi: 10.3389/fimmu.2024.1487353
Received: 28 August 2024; Accepted: 17 October 2024;
Published: 11 November 2024.
Edited by:
Xiaojie Xu, Beijing Institute of Technology, ChinaReviewed by:
Bin-Yan Zhong, The First Affiliated Hospital of Soochow University, ChinaCopyright © 2024 Li, Liu, Qiu, Qu and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jiarui Li, bGpyQGpsdS5lZHUuY24=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.