- Medical Oncology, Institut de Cancérologie Strasbourg Europe (ICANS), Strasbourg, France
Introduction: Immune checkpoint inhibitors (ICIs) have revolutionized cancer therapy by enhancing the antitumor immune response. This case describes an 80-year-old male with synchronous multiple primary malignancies (MPMs), including lung metastatic hepatocellular carcinoma (HCC), and non-small cell lung carcinoma (NSCLC), and brain metastatic urothelial carcinoma, who was treated with dual ICI therapy.
Case presentation: The patient, with a history of diabetes, hypertension, dyslipidaemia, well-differentiated neuroendocrine duodenal tumors and micronodular exogenous cirrhosis (Child-Pugh class A), presented with a non-invasive bladder carcinoma (pT1N0M0) resected endoscopically in December 2022. Incidentally discovered hepatic and pulmonary tumors were confirmed as primary HCC and squamous cell carcinoma of the lung (cT1bN0M0, PD-L1 expression 100%), respectively. Due to the rapid progression of pulmonary metastases secondary to HCC, dual ICI therapy (durvalumab and tremelimumab) was initiated, resulting in a partial response (>30%) according to RECISTv1.1 criteria in pulmonary and hepatic lesions. After one year of ICI therapy, cerebellar syndrome due to secondary brain lesions emerged, which was confirmed as urothelial metastases. Surgical resection of the symptomatic cerebral metastases was completed with cerebral radiotherapy, and ICIs were continued. The patient is still receiving dual ICIs.
Discussion: This case highlights the crucial role of ICIs in treating MPMs. The patient’s favourable response suggests the importance of PD-L1 expression as a predictive biomarker.
Conclusion: This rare case showed dual ICI therapy efficacy across multiple malignancies. Effective multidisciplinary collaboration and biomarker evaluation are crucial for managing such complex cases.
Introduction
Multiple primary malignancies (MPM) are defined as the occurrence of two or more cancers in the same individual. The incidence of MPM ranges between 2-17%. MPM represent a significant clinical challenge because of their complex diagnostic and therapeutic implications. These malignancies can be categorized as synchronous, diagnosed simultaneously or within six months, or metachronous, diagnosed at intervals greater than six months. Risk factors for MPM include genetic predispositions, environmental exposures, previous cancer treatments, and lifestyle factors such as smoking and alcohol consumption. The increasing incidence of MPM can also be attributed to improved diagnostics and more prolonged survival of cancer patients, underscoring the need for vigilant long-term surveillance and comprehensive management strategies (1).
Over the past decade, immune checkpoint inhibitors (ICI) have revolutionized therapeutic strategies for many solid tumors by enhancing the antitumor immune response (2). These Food and Drug Administration and European Medicines Agency approved monoclonal antibodies target co-inhibitory molecules, thereby blocking the negative regulatory pathways of the immune system. ICI, widely employed in the treatment of various cancers, offer promising therapeutic options for MPM.
In the therapeutic management of metastatic hepatocellular carcinoma (HCC), ICI have demonstrated efficacy in combination with anti-angiogenic agents. The IMBrave150 trial revealed improved overall survival (OS) with the combination of atezolizumab (ICI anti-PDL1) and bevacizumab (antibody against VEGF) versus sorafenib (multi-kinase inhibitor) (HR 0.66; 95% CI 0.52–0.85; p <0.001) (3). The HIMALAYA trial reported that the combination of durvalumab (ICI anti-PDL1) and tremelimumab (ICI anti-CTLA4) also improved OS, with a median of 16.6 months, compared with 13.8 months for sorafenib in the first-line setting (4).
In the treatment of metastatic non-small cell lung carcinoma (NSCLC), immunotherapy alone or in combination with chemotherapy has shown significant efficacy. Compared with chemotherapy alone, combinations of chemotherapies, such as pembrolizumab (ICI anti-PD1) with platinum-based chemotherapy, improved survival outcomes (5, 6). In patients with a PD-L1 rate greater than 50%, pembrolizumab monotherapy has proven effective, as shown in the Keynote-042 study (7). In addition, the CheckMate-227 trial demonstrated superior OS with nivolumab (ICI anti-PD1) plus ipilimumab (ICI anti-CTLA4) compared with chemotherapy in the first-line treatment of metastatic NSCLC patients with PD-L1 expression above 1% (HR = 0.76; 95% CI 0.65–0.90) (8). The MYSTIC trial evaluated the combination of durvalumab (anti-PD-L1) and tremelimumab (anti-CTLA4) in metastatic NSCLC. The trial demonstrated a significant improvement in progression-free survival (PFS) with the combination therapy compared to chemotherapy, particularly in patients with high PD-L1 expression. However, the OS benefit was not consistently demonstrated in the initial analysis, with a HR for OS of 0.85 (95% CI 0.61–1.17) in the durvalumab plus tremelimumab arm versus chemotherapy (9).
Significant advancements have also been made in the treatment of metastatic bladder cancer with the introduction of ICIs. The phase III Keynote-045 trial demonstrated the efficacy of pembrolizumab monotherapy as a second-line treatment (10). Avelumab (ICI anti-PDL1) showed a survival benefit as maintenance therapy following platinum-based chemotherapy (11). The EV-302 study, which evaluated pembrolizumab in combination with enfortumab-vedotin (antibody-drug conjugate against Nectin 4) versus chemotherapy, reported a doubling of OS in patients receiving metastatic first-line treatment (12).
In this report, we describe a patient with multiple synchronous malignancies, including metastatic HCC, NSCLC, and urothelial carcinoma, whose treatment with an ICI doublet regimen proved effective.
Case presentation
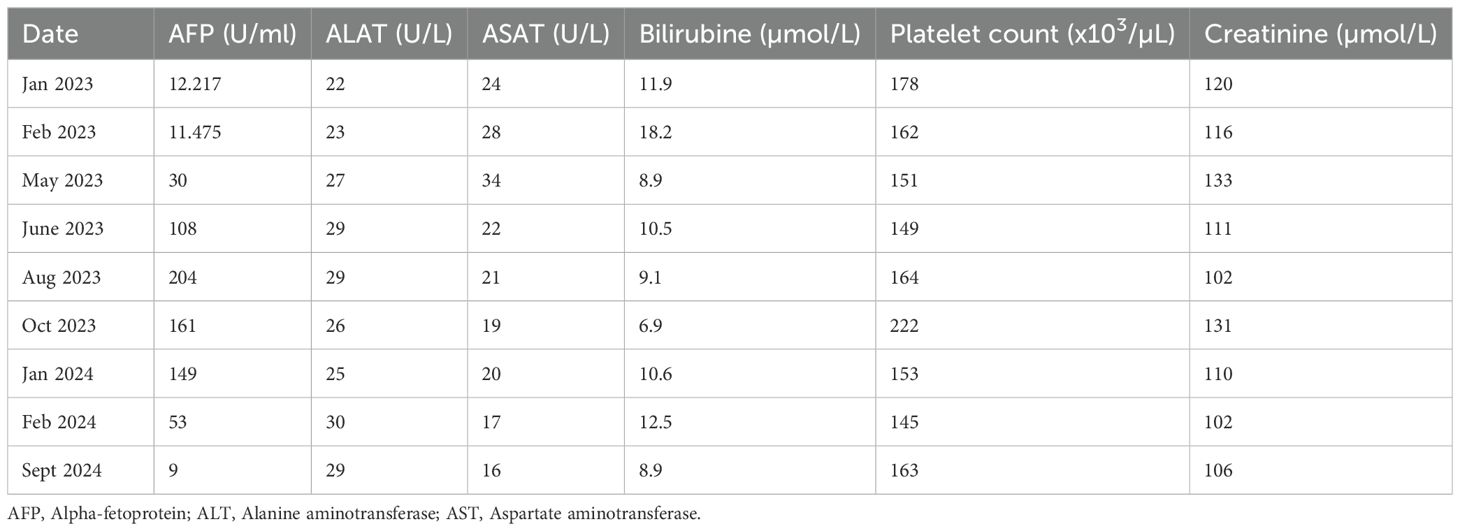
An 80-year-old male with a medical history of diabetes, hypertension, dyslipidaemia, multiple neuroendocrine duodenal tumors, and micronodular exogenous cirrhosis (classified as Child-Pugh class A) was diagnosed in December 2022 with non-invasive bladder carcinoma, classified as pT1N0M0. The patient did not receive further treatment for the bladder carcinoma. During an abdominal ultrasound performed for cirrhosis monitoring, a bilocular hepatic tumor with an encapsulated appearance was discovered in segment VI of the right liver, along with an endoportal tumor thrombosis. Subsequent 18F-FDG PET-Scan on January 2023 confirmed hypermetabolic tissue lesions suggestive of primary HCC and a suspicious spiculated pseudo-nodular lesion in the right lung. Alpha-fetoprotein (AFP) levels were elevated to 12.217 U/ml, surpassing the normal range (<10 U/ml) (Table 1). Following hepatic radioembolization in February 2023, a liver MRI revealed a mixed response with a partial response in the right liver, indicating a tumor response in the endovenous system and progression of the disease due to regional invasion of the biliary ducts (Figure 1).
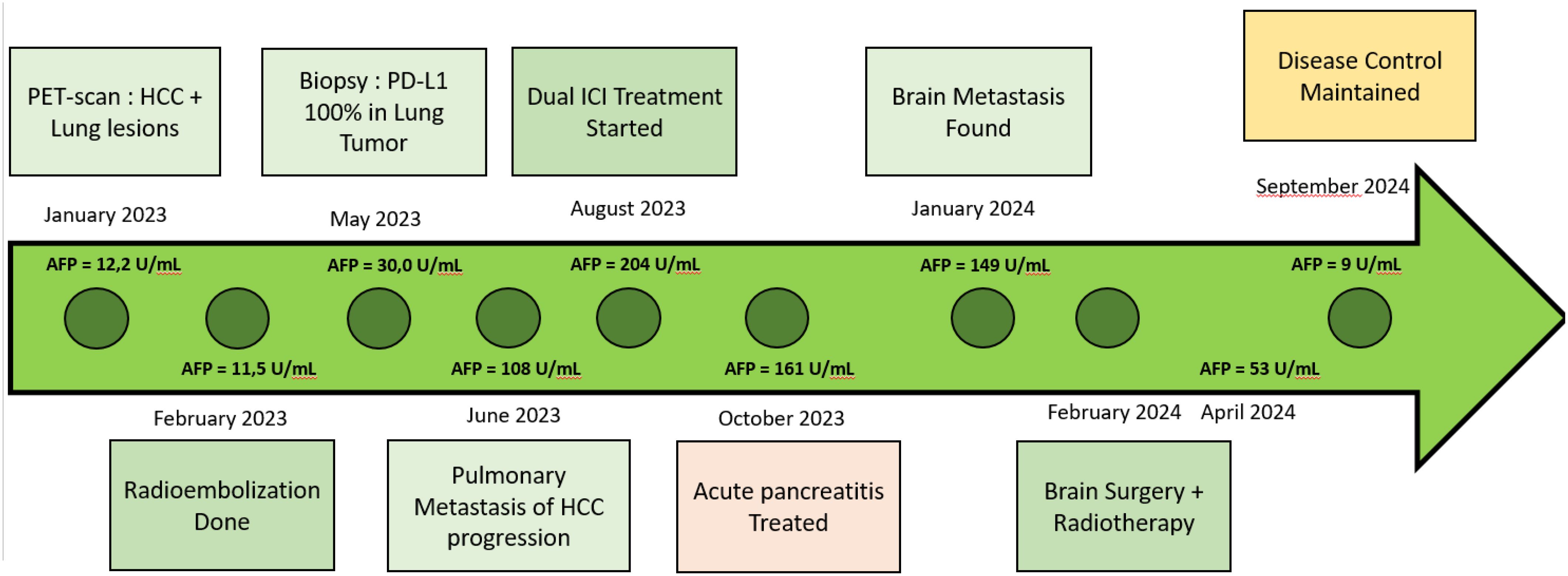
Figure 1. Timeline AFP, Alpha-fetoprotein; PDL1, programmed death ligand 1; HCC, Hepacellular carcinoma.
In May 2023, a CT-guided biopsy of the suspicious pulmonary lesion identified a squamous cell lung carcinoma in the right lower lobe, classified as cT1bN0M0 (Figure 2A), with a 100% PD-L1 expression level. The multidisciplinary team meeting recommended stereotactic radiotherapy. A thoracic CT scan in June 2023, performed prior to radiotherapy, revealed the appearance of pulmonary nodules. A lung biopsy confirmed pulmonary metastatic involvement of the HCC. The patient did not undergo stereotactic radiotherapy for his NSCLC after confirmation of metastasis. Owing to the rapid progression of pulmonary tumors secondary to hepatic carcinoma, first-line treatment with dual ICI, consisting of durvalumab and tremelimumab, was started in August 2023 (Figure 1). This treatment led to a partial pulmonary (Figure 2B), hepatic response (Figure 3), and decreased AFP level to 9 U/ml (Table 1).
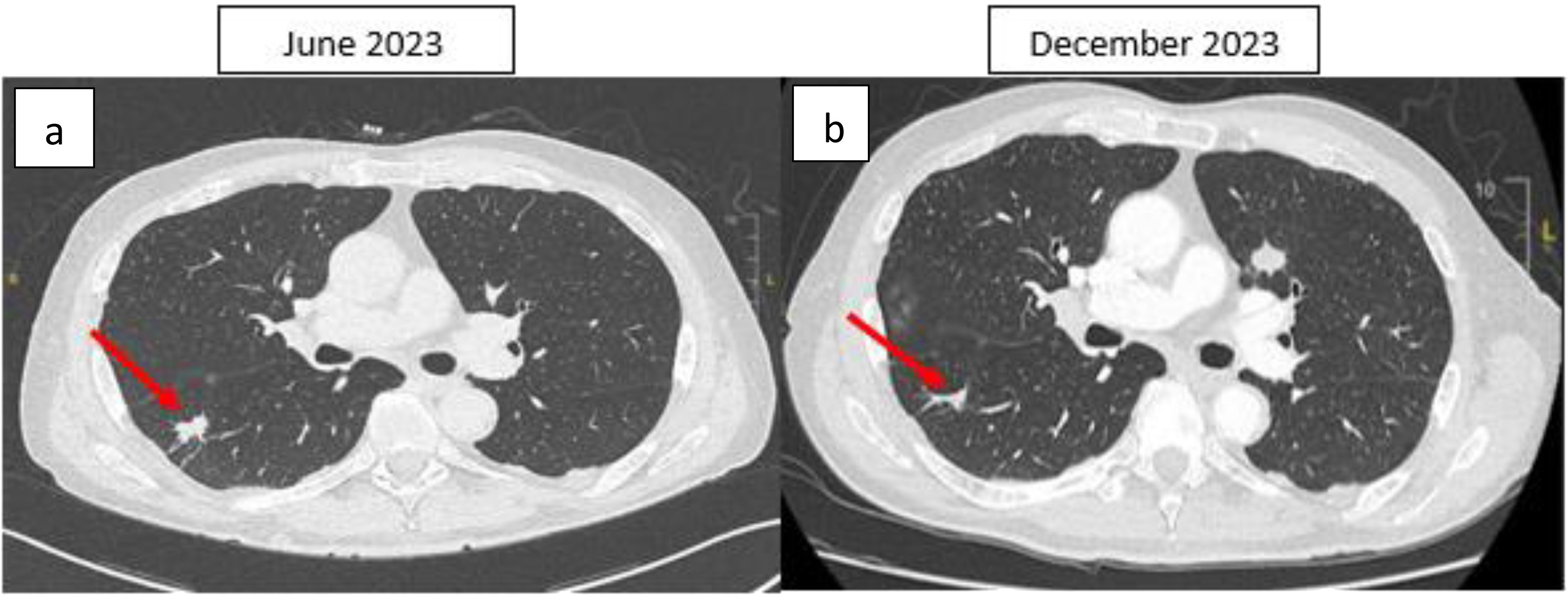
Figure 2. Thoracic CT scan, axial section: (A) Spiculated nodule in the apical segment of the right lower lobe initially measuring 20 × 11 mm, with some spicules reaching the fissural and parietal pleura. (B) After four months of ICI therapy, the nodule was reduced to 15 × 3 mm. The right lung lesion demonstrates a reduction in size between the June 2023 and December 2023 CT scans. However, a growing nodule is observed in the left lung on the December 2023 scan. This may be explained by the fact that the June 2023 scan was performed prior to the initiation of immunotherapy (which began in August 2023). As a result, the left lung nodule (HCC metastases) could have continued to progress during the interval between the scans, before the therapeutic effects of immunotherapy were fully realized. This temporal difference in treatment response may account for the observed discrepancies in lesion size. The red arrow indicates the lung tumor.
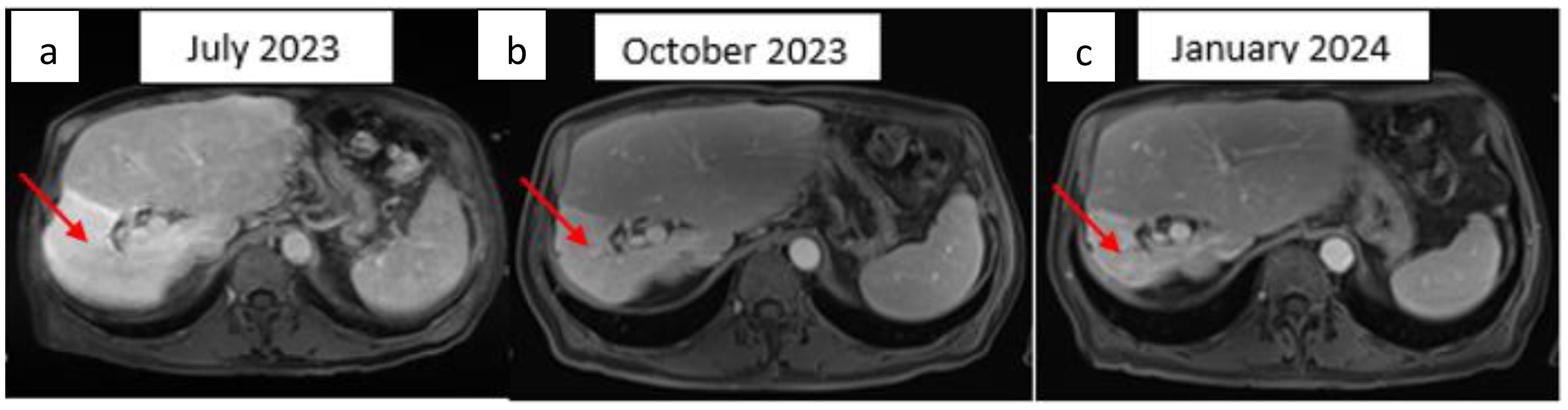
Figure 3. Hepatic T1 MRI image after gadolinium injection for three min. Axial image: (A) Bilocular hepatic tumor with an encapsulated appearance in segment VI of the right liver, initially measuring 69 × 68 × 81 mm. (B) Partial response with a 25% reduction after three months of ICI therapy. (C) Partial response with 50% reduction mm after six months of ICI therapy. The red arrow indicates the hepatic tumor.
After the patient had received four cycles of ICI with durvalumab and tremelimumab, he developed CTCAE grade I acute pancreatitis. This complication was detected based on lipase levels, which were significantly higher than the normal range. Upon diagnosis, the patient was promptly treated with corticosteroids at a dose of 1 mg/kg/day, which led to a marked improvement in his clinical condition. The patient had a complete recovery from the pancreatitis, with normalization of lipase levels within two weeks of initiating steroid therapy. Regarding immune-related adverse events (irAEs), the patient did not experience any other immune-related toxicities.
After six months of ICI therapy, the patient developed cerebellar syndrome, and further imaging revealed secondary brain lesions: a 17 mm lesion in the right temporal lobe and a 49 mm lesion in the left cerebellum (Figure 4). Following surgical resection of the cerebellar tumor, histological analysis revealed carcinoma proliferation with GATA3 positivity, consistent with the patient’s primary urothelial carcinoma. Immunostaining revealed a combined positive score (CPS) of 40. Post-surgery, the patient received cerebral radiotherapy to the operative bed in the left cerebellum and the right temporal lesion under stereotactic conditions. It was decided to continue with ICI, which have permitted disease control for 12 months. The patient is still receiving ICI therapy (Figure 1).
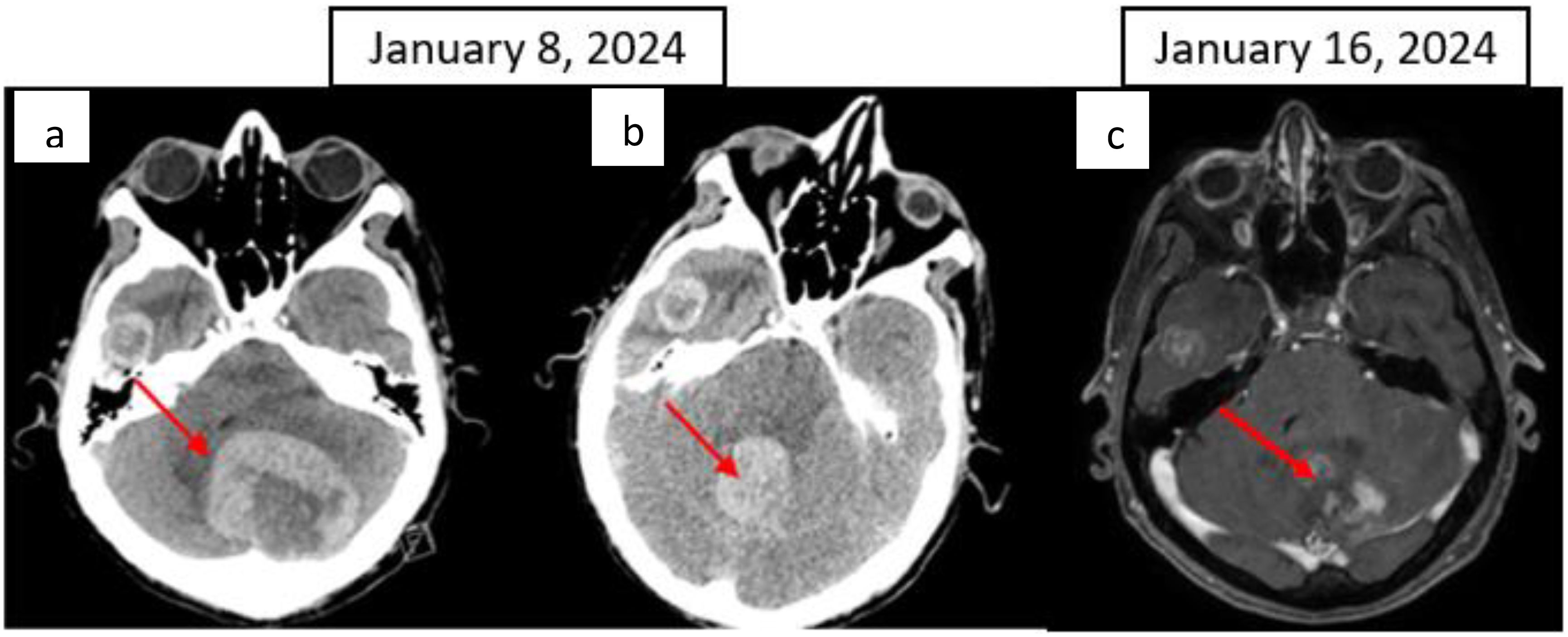
Figure 4. CT scan: (A) Left cerebellar metastasis measuring 55 × 51 mm; (B) right temporal metastasis with a necrotic centre measuring 24 × 18 mm; (C) Brain T1 MRI image after gadolinium injection, axial image. The red arrow indicates the cerebellar metastasis.
Discussion
This rare case reported an extended response to dual ICI therapy in a patient with synchronous pulmonary metastatic hepatocarcinoma, squamous lung cancer and brain metastatic urothelial carcinoma. Focal treatment for brain progression allowed the continuation of ICI therapy.
Managing MPM poses a significant clinical challenge because of their diverse tumor characteristics and treatment responses. There are currently no standard guidelines for the therapeutic management of MPM. These rare occurrences underscore the complexity in designing effective therapeutic strategies that address each cancer type without compromising patient outcomes (1). Our patient’s case involving synchronous hepatocellular carcinoma, non-small cell lung cancer, and urothelial carcinoma highlights the intricate nature of MPM and the necessity for tailored treatment approaches (13). ICI could revolutionize MPM treatment by harnessing the immune system against tumors (14). As reported in this case, treatment with durvalumab and tremelimumab demonstrated extended efficacy across multiple cancers, emphasizing the evolving role of ICI in managing complex oncological scenarios (15).
The management of MPM in an elderly patient, particularly one with significant comorbidities such as diabetes, hypertension requires a careful balance between efficacy and safety. In this case, the patient’s relatively well-preserved liver function (Child-Pugh A) and good overall performance status were key factors that allowed the administration of dual ICI, despite his advanced age. While ICI, such as durvalumab and tremelimumab, have demonstrated substantial clinical efficacy in several cancers, their use in elderly patients, particularly those with liver cirrhosis and other comorbidities, remains complex (16, 17). The absence of significant immune-related toxicities other than mild pancreatitis, which was successfully managed, underscores the feasibility of ICI therapy in this population. This favorable outcome encourages further exploration of dual ICI therapy in elderly MPM patients, as it may offer prolonged disease control, even in complex cases (18). However, it also highlights the need for close monitoring of irAEs, especially given the increased vulnerability of older adults to treatment-related toxicities (19). The patient’s favorable tolerance and response to dual therapy suggest that age and comorbidities should not be definitive contraindications to aggressive immunotherapy in MPMs. Instead, these factors should be carefully evaluated when making individualized treatment decisions (20).
While single-agent immunotherapy has proven effective in certain cancers, its use is often limited by factors such as suboptimal response rates and the development of resistance, particularly in tumors with lower mutational burden or complex immune escape mechanisms (21). For instance, monotherapy with PD-1/PD-L1 inhibitors is associated with variable responses in cancers like NSCLC and HCC (22). In contrast, combination immunotherapy has emerged as a promising strategy to overcome these limitations by targeting multiple immune checkpoints, thereby enhancing the durable immune response (23). The use of dual ICI regimens, such as durvalumab and tremelimumab, has shown improved efficacy in treating complex cases, as evidenced by our patient with MPM (24). However, while combination therapies offer increased treatment efficacy, they also come with an elevated risk of irAEs. Thus, careful patient selection and monitoring are crucial to maximize the benefits of combination immunotherapy while minimizing potential toxicities (25).
Predictive biomarkers such as PD-L1 expression and tumor mutational burden (TMB) are crucial in guiding ICI therapy decisions and predicting treatment responses across different treatment responses cancer types (26). In our study, high PD-L1 expression in lung cancer cells correlated with favourable responses to durvalumab and tremelimumab, highlighting PD-L1 as a potential biomarker for ICI efficacy prediction. Similar findings in NSCLC studies support the notion of enhanced ICI response in tumors with elevated PD-L1 expression (27, 28). However, while PD-L1 expression has shown predictive value in some cancers, its role as a reliable biomarker for combination immunotherapies, especially in HCC, remains less clear, and the evidence for its utility in guiding dual ICI treatment is still evolving. For MPM, the immune environment, with markers of response to immunotherapy, such as the CPS, has a greater impact than the tumor histological type on the introduction of 1st-line immunotherapy. Despite an initial diagnosis of non-invasive bladder cancer, our patient developed brain metastases, illustrating the complexities of managing metastatic patterns and the varying responses of metastatic sites to ICI therapy (29). Reports in the literature indicate mixed outcomes in controlling intracranial disease with ICIs, indicating both potential and limitations in treating brain metastases (30). Recent studies suggest that although ICIs can be effective in controlling extracerebral disease, the response to brain metastases may be less predictable, necessitating the combination of focal therapies such as radiotherapy to manage intracranial progression while continuing ICI treatment for systemic disease (31). Brain focal therapies remain a complementary therapeutic option to continue ICIs, especially in cases of extracerebral disease control with exclusive brain progression (32). The evolution from non-invasive bladder cancer to muscle-invasive evolution with exclusive brain metastases underscores the unpredictable nature of cancer progression and the need for close monitoring and adaptive treatment strategies tailored to the individual patient’s disease trajectory.
Conclusion
Integrating ICI into MPM management represents a transformative approach in oncology, emphasizing personalized treatment strategies and the pivotal role of immunological biomarkers in optimizing therapeutic outcomes. Further research is warranted to elucidate the underlying mechanisms governing differential ICI responses across diverse cancer types and metastatic sites, paving the way for more effective and tailored therapies for patients with complex malignancies.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
Ethical approval was not required for the study involving humans in accordance with the local legislation and institutional requirements. Written informed consent to participate in this study was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and the institutional requirements. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
NG: Writing – original draft. HB: Writing – original draft. PC: Writing – review & editing. LS: Writing – review & editing. FM-B: Writing – review & editing.
Funding
The authors declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted without commercial or financial relationships that could be construed as potential conflicts of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Vogt A, Schmid S, Heinimann K, Frick H, Herrmann C, Cerny T, et al. Multiple primary tumours: challenges and approaches, a review. ESMO Open. (2017) 2:e000172. doi: 10.1136/esmoopen-2017-000172
3. Cheng AL, Qin S, Ikeda M, Galle PR, Ducreux M, Kim TY, et al. Updated efficacy and safety data from IMbrave150: Atezolizumab plus bevacizumab vs. sorafenib for unresectable hepatocellular carcinoma. J Hepatol. (2022) 76:862−73. doi: 10.1016/j.jhep.2021.11.030
4. Abou-Alfa GK, Lau G, Kudo M, Chan SL, Kelley RK, Furuse J, et al. Tremelimumab plus durvalumab in unresectable hepatocellular carcinoma. NEJM Evid. (2022) 1:EVIDoa2100070. doi: 10.1056/EVIDoa2100070
5. Gandhi L, Rodríguez-Abreu D, Gadgeel S, Esteban E, Felip E, Angelis FD, et al. Pembrolizumab plus chemotherapy in metastatic non–small-cell lung cancer. N Engl J Med. (2018) 378:2078−92. doi: 10.1056/NEJMoa1801005
6. Paz-Ares L, Luft A, Vicente D, Tafreshi A, Gümüş M, Mazières J, et al. Pembrolizumab plus chemotherapy for squamous non-small-cell lung cancer. N Engl J Med. (2018) 379:2040−51. doi: 10.1056/NEJMoa1810865
7. Mok TSK, Wu YL, Kudaba I, Kowalski DM, Cho BC, Turna HZ, et al. Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or metastatic non-small-cell lung cancer (KEYNOTE-042): a randomised, open-label, controlled, phase 3 trial. Lancet Lond Engl. (2019) 393:1819−30. doi: 10.1016/S0140-6736(18)32409-7
8. Hellmann MD, Paz-Ares L, Bernabe Caro R, Zurawski B, Kim S-W, Carcereny Costa E, et al. Nivolumab plus ipilimumab in advanced non–small-cell lung cancer. N Engl J Med. (2019) 381:2020−31. doi: 10.1056/NEJMoa1910231
9. Rizvi NA, Cho BC, Reinmuth N, Lee KH, Luft A, Ahn MJ, et al. MYSTIC Investigators. Durvalumab with or wthout tremelimumab vs standard chemotherapy in first-line treatment of metastatic non-small cell lung cancer: the MYSTIC phase 3 randomized clinical trial. JAMA Oncol. (2020) 6:661–74. doi: 10.1001/jamaoncol.2020.0237
10. Bellmunt J, de Wit R, Vaughn DJ, Fradet Y, Lee JL, Fong L, et al. Pembrolizumab as second-line therapy for advanced urothelial carcinoma. N Engl J Med. (2017) 376:1015−26. doi: 10.1056/NEJMoa1613683
11. Powles T, Park SH, Voog E, Caserta C, Valderrama BP, Gurney H, et al. Avelumab maintenance therapy for advanced or metastatic urothelial carcinoma. N Engl J Med. (2020) 383:1218−30. doi: 10.1056/NEJMoa2002788
12. Powles T, Valderrama BP, Gupta S, Bedke J, Kikuchi E, Hoffman-Censits J, et al. Enfortumab vedotin and pembrolizumab in untreated advanced urothelial cancer. N Engl J Med. (2024) 390:875−88. doi: 10.1056/NEJMoa2312117
13. Chitwood H, Carey T. Managing the patient with multiple primary tumors. J Adv Pract Oncol. (2023) 14:218−21. doi: 10.6004/jadpro.2023.14.3.7
14. Zhang B, He L, Zhou C, Cheng X, Li Q, Tang Y, et al. A pancancer analysis of the clinical and genomic characteristics of multiple primary cancers. Sci Rep. (2024) 14:2367. doi: 10.1038/s41598-024-52659-3
15. Wan Y, Wang Z, Yang N, Liu F. Treatment of multiple primary Malignancies with PD-1 inhibitor camrelizumab: A case report and brief literature review. Front Oncol. (2022) 12:911961. doi: 10.3389/fonc.2022.911961
16. Rojas-Rivera J, Patel MR, Recondo G, Srivastava S, Burger HU, Pietanza MC. Safety and efficacy of immune checkpoint inhibitors in elderly patients with cancer: A systematic review. Cancer. (2020) 126:482–91. doi: 10.1002/cncr.32674
17. Piersma S, van Vliet T, Timmers L, Smorenburg CH, Groenendijk FH, Boers JE. The role of immune checkpoint inhibitors in elderly patients with advanced cancer. J Geriatr Oncol. (2019) 10:1036–43. doi: 10.1016/j.jgo.2019.05.010
18. Hodi FS, Antonia SJ, Lopez-Martin JA, Postow MA, Taylor MH, Paz-Ares L. Durvalumab plus tremelimumab versus chemotherapy in advanced cancer: A systematic review and meta-analysis. J Clin Oncol. (2023) 41:1227–35. doi: 10.1200/JCO.22.02279
19. Finkelmeier F, Neureiter D, Meyer C, Gonschorek A, Schlabe S, Lauer U. Immunotherapy in the elderly: Special considerations for checkpoint inhibitors. Cancers (Basel). (2020) 12:2101. doi: 10.3390/cancers12072101
20. Mehra R, Esposito A, Diaz-Beveridge R, Weber JS, Garon EB, Spira AI. Considerations in treatment of elderly patients with advanced Malignancies: The role of immunotherapy. ” Oncol Treat. (2021) 43:318–25. doi: 10.1002/ot.2562
21. Jenkins RW, Barbie DA, Flaherty KT. Mechanisms of resistance to immune checkpoint inhibitors. British J Cancer. (2018) 118:9–16. doi: 10.1038/bjc.2017.434
22. Yau T, Park JW, Finn RS, Cheng AL, Mathurin P, Edeline J, et al. Nivolumab versus sorafenib in advanced hepatocellular carcinoma (CheckMate 459): A randomised, multicentre, open-label, phase 3 trial. Lancet Oncol. (2020) 23:77–90. doi: 10.1016/S1470-2045(21)00604-5
23. Motzer RJ, Tannir NM, McDermott DF, Arén Frontera O, Melichar B, Choueiri TK, et al. CheckMate 214 Investigators. Nivolumab plus Ipilimumab versus Sunitinib in Advanced Renal-Cell Carcinoma. N Engl J Med. (2018) 378:1277–19. doi: 10.1056/NEJMoa1712126
24. Arru C, De Miglio MR, Cossu A, Muroni MR, Carru C, Zinellu A, et al. Durvalumab plus tremelimumab in solid tumors: a systematic review. Adv Therapy. (2021) 38:3674–93. doi: 10.1007/s12325-021-01796-6
25. Schneider BJ, Naidoo J, Santomasso BD, Lacchetti C, Adkins S, Anadkat , et al. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: ASCO guideline update. J Clinical Oncol: Official J Am Soc Clinical Oncol. (2016) 39:4073–126. doi: 10.1200/JCO.21.01440
26. Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. (2012) 366:2443−54. doi: 10.1056/NEJMoa1200690
27. El-Khoueiry AB, Sangro B, Yau T, Crocenzi TS, Kudo M, Hsu C, et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet Lond Engl. (2017) 389:2492−502. doi: 10.1016/S0140-6736(17)31046-2
28. Garon EB, Rizvi NA, Hui R, Leighl N, Balmanoukian AS, Eder JP, et al. Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med. (2015) 372:2018−28. doi: 10.1056/NEJMoa1501824
29. Tawbi HA, Forsyth PA, Algazi A, Hamid O, Hodi FS, Moschos SJ, et al. Combined nivolumab and ipilimumab in melanoma metastatic to the brain. N Engl J Med. (2018) 379:722−30. doi: 10.1056/NEJMoa1805453
30. Goldberg SB, Gettinger SN, Mahajan A, Chiang AC, Herbst RS, Sznol M, et al. Pembrolizumab for patients with melanoma or non-small-cell lung cancer and untreated brain metastases: early analysis of a non-randomised, open-label, phase 2 trial. Lancet Oncol. (2016) 17:976−83. doi: 10.1016/S1470-2045(16)30053-5
31. Tawbi HA, Forsyth PA, Hodi FS, Algazi AP, Hamid O, Lao , et al. Long-term outcomes of patients with active melanoma brain metastases treated with combination nivolumab plus ipilimumab (CheckMate 204): final results of an open-label, multicentre, phase 2 study. The Lancet Oncol. (2021) 22:1692–704. doi: 10.1016/S1470-2045(21)00545-3
Keywords: multiple primary malignancies, lung cancer, urothelial carcinoma, hepatocellular carcinoma, immune checkpoint inhibitor
Citation: Ghammem N, Bischoff H, Chiappa P, Somme L and Moinard-Butot F (2024) Case report: Long-term response to a combination of immune checkpoint inhibitors as a single treatment for multiple synchronous cancers: a case study. Front. Immunol. 15:1487227. doi: 10.3389/fimmu.2024.1487227
Received: 27 August 2024; Accepted: 29 November 2024;
Published: 16 December 2024.
Edited by:
Paulo Rodrigues-Santos, University of Coimbra, PortugalReviewed by:
Shuang Dong, Huazhong University of Science and Technology, ChinaFernando Mendes, Polytechnical Institute of Coimbra, Portugal
Youngnam N. Jin, Wuhan University, China
Copyright © 2024 Ghammem, Bischoff, Chiappa, Somme and Moinard-Butot. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Fabien Moinard-Butot, Zi5tb2luYXJkLWJ1dG90QGljYW5zLmV1
†These authors have contributed equally to this work and shared first authorship
 Nour Ghammem
Nour Ghammem Herve Bischoff†
Herve Bischoff† Laura Somme
Laura Somme Fabien Moinard-Butot
Fabien Moinard-Butot