- 1Cancer Center, The First Hospital of Jilin University, Changchun, Jilin, China
- 2Department of Pathology, The First Hospital of Jilin University, Changchun, Jilin, China
Lung enteric-type adenocarcinoma (ETAC) is a rare subtype of non-small cell lung cancer (NSCLC), comprising approximately 0.6% of all primary lung adenocarcinomas. It is characterized by a tendency for early metastasis and a prognosis comparable to that of common lung adenocarcinoma. This case report described a patient with lung-ETAC who developed gastric metastasis. The patient underwent treatment with chemotherapy and a PD-1 inhibitor, resulting in disease remission with a progression-free survival (PFS) of 8 months. The follow-up time was 13 months. This case report was aimed to enhance understanding of the biological behavior of this rare tumor and provide insights into potential future treatment strategies.
1 Introduction
Lung enteric-type adenocarcinoma (ETAC) is a rare and distinct subtype of non-small cell lung cancer (NSCLC), accounting for approximately 0.6% of all primary lung adenocarcinomas. It was firstly described by Tsao and Fraser in 1991 (1). It originates in the lungs but exhibits intestinal differentiation in its pathology. In 2015, this subtype was officially recognized in the World Health Organization (WHO) classification of lung tumors (2). The 2021 WHO classification redefined this entity as lung-ETAC. Histological diagnostic criteria require more than 50% enteric morphology (3). Due to its histological and immunohistochemical similarities to metastatic colorectal cancer (mCRC) (4), differential diagnosis often necessitates endoscopy, computed tomography (CT), and especially 18F-labeled fluoro-2-deoxyglucose positron emission tomography (18F-FDG PET-CT) to rule out primary gastrointestinal tumors (5, 6) It is an aggressive tumor with a propensity for early metastasis, often spreading to bones, liver, and lymph nodes (7), and occasionally affecting the skin, scalp, brain, pancreas, and soleus muscle (8–10). However, gastric metastasis has not been previously reported. This report described a case of lung-ETAC with gastric metastasis.
2 Case report
A 63-year-old female was admitted to the hospital in May 2021 for physical examination, which revealed a nodule in the left lower lobe of the lung. Chest CT demonstrated that the nodule was located in the lower lobe of the left lung, measuring 2.6x3.8cm (Figure 1A). Bone scan and brain CT results were normal. On May 28, 2021, the patient underwent a left-sided thoracoscopic lower lobe resection plus lymph node dissection (Figure 1B). Postoperative pathology confirmed invasive adenocarcinoma, enteric type. Immunohistochemistry (IHC) results were as follows: CKpan (+), CK7 (partially +), CK20 (+), CDX-2 (+), Ki-67 (+80%), TTF-1 (-), NapsinA (-), SATB2 (+), Villin (+), CK5/6 (-) (Figures 2B-E). The diagnosis was lung-ETAC (T2aN1M0 IIb). The patient received four courses of adjuvant chemotherapy with albumin paclitaxel and carboplatin, followed by long-term follow-up. In July 2023, the patient presented with symptoms of choking sensation on food and chest pain, and disease progression was noted. The disease-free survival (DFS) was 26 months. Chest CT revealed high-density shadows in the left lung bronchus. (Figure 1C). PET-CT confirmed these findings and identified bone and gastric sinus metastasis. Fiberoptic bronchoscopy and biopsy were performed, and the pathology of the left lung bronchus confirmed adenocarcinoma, enteric type, with PD-L1 expression at 20%. IHC results were: CDX-2 (+),SATB2 (+), CK20 (+), TTF-1 (-) (Figures 2G-J). Gastroscopy on August 28, 2023, revealed an erosion in the gastric antrum and the lesser curvature of the stomach. Pathological examination of the gastric sinuses showed poorly differentiated carcinoma. IHC results were: CK (+), CK7 (scattered +), CK20 (+), CEA (+), villin (weakly +), P53 (-), Ki-67 (+ about 20%), Syn (-), CD56 (-), CD45 (-), E-Cad (+), Her-2 (-), TTF-1 (-), Napsin-A (-) (Figures 2L-O). Genetic testing of the left lung bronchus identified an NRAS gene exon 3 mutation. Histopathological comparison by an experienced specialist revealed that the gastric and bronchial pathologies contained moderately and poorly differentiated adenocarcinomatous components similar to the postoperative lung pathology, confirming gastric metastasis. The patient was ultimately diagnosed with left lung-ETAC (cT4N3M1, stage IV) with lung, bone, and gastric sinus metastasis. The patient received six cycles of sintilimab (anti-PD1, 200mg) - albumin paclitaxe (260mg/m2), - and cisplatin (75mg/m2), the patient got a partial response after two cycles (Figure 1D), then with two courses of sintilimab (anti-PD1) immunotherapy maintenance. In March 2024, the therapeutic assessment suggested disease progression (Figure 1E) and PFS was 8 months. The patient refused further treatment. Finally, The patient received the best supportive care. At this writing, the patient remains alive but in generally poor condition. The follow-up time was 13 months.
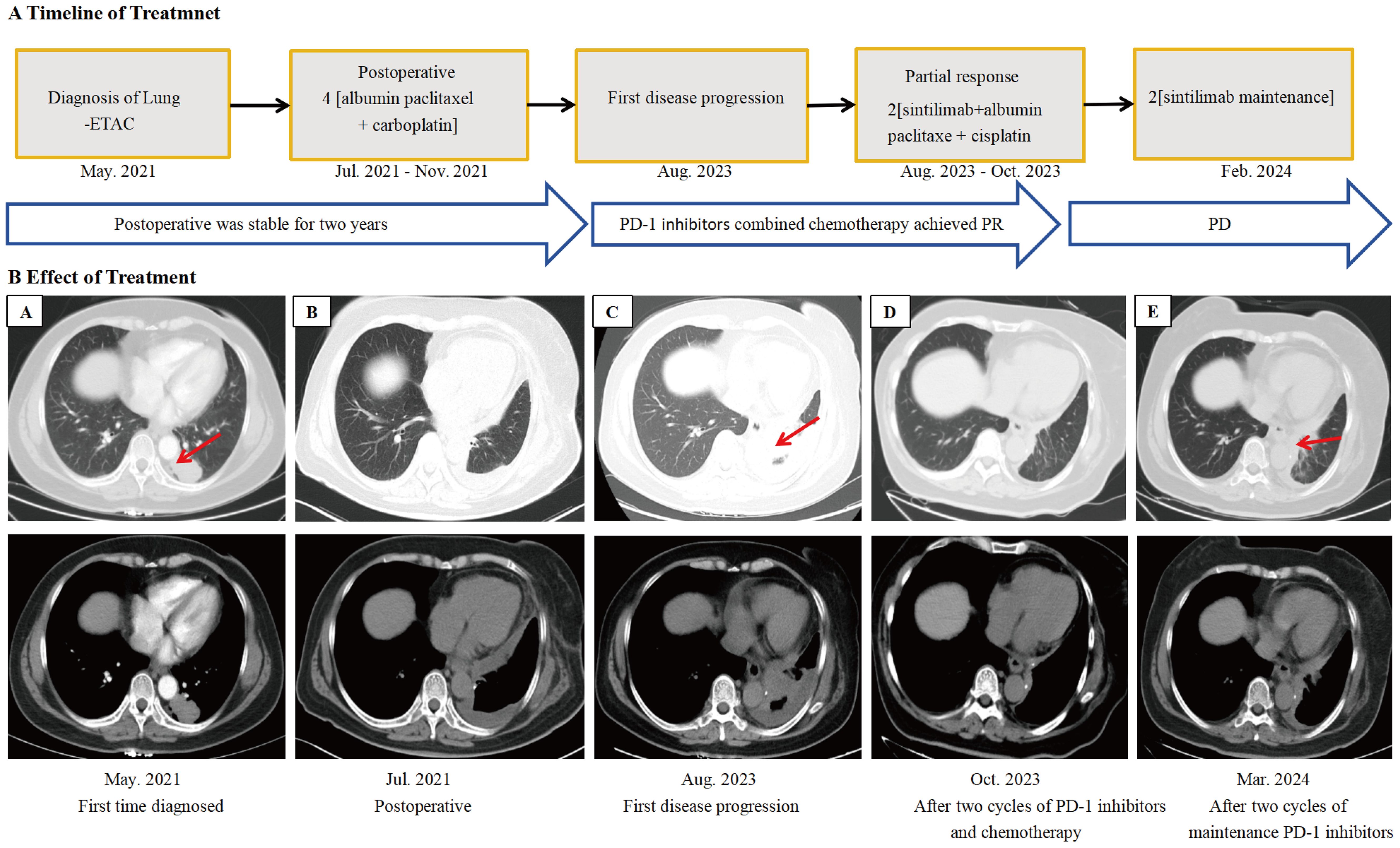
Figure 1. Imaging changes during treatment. CT images demonstrating diagnosis, treatment responses, and progression. (A) Lung-ETAC was first diagnosed (May 2021), enhanced CT scan showed a 2.6x3.8cm pulmonary nodule of the left lung. (B) Postoperative (Jul 2021). (C) Progression after postoperative, chest CT scan showed high-density shadows of bronchia in the left lung and larger than before (Aug 2023). (D) Partial remission was achieved after two courses of PD-1 inhibitors in combination with chemotherapy (Oct 2023). (E) Progression after two courses of maintenance immunotherapy (Mar 2024). PR, partial response; PD, disease progression.
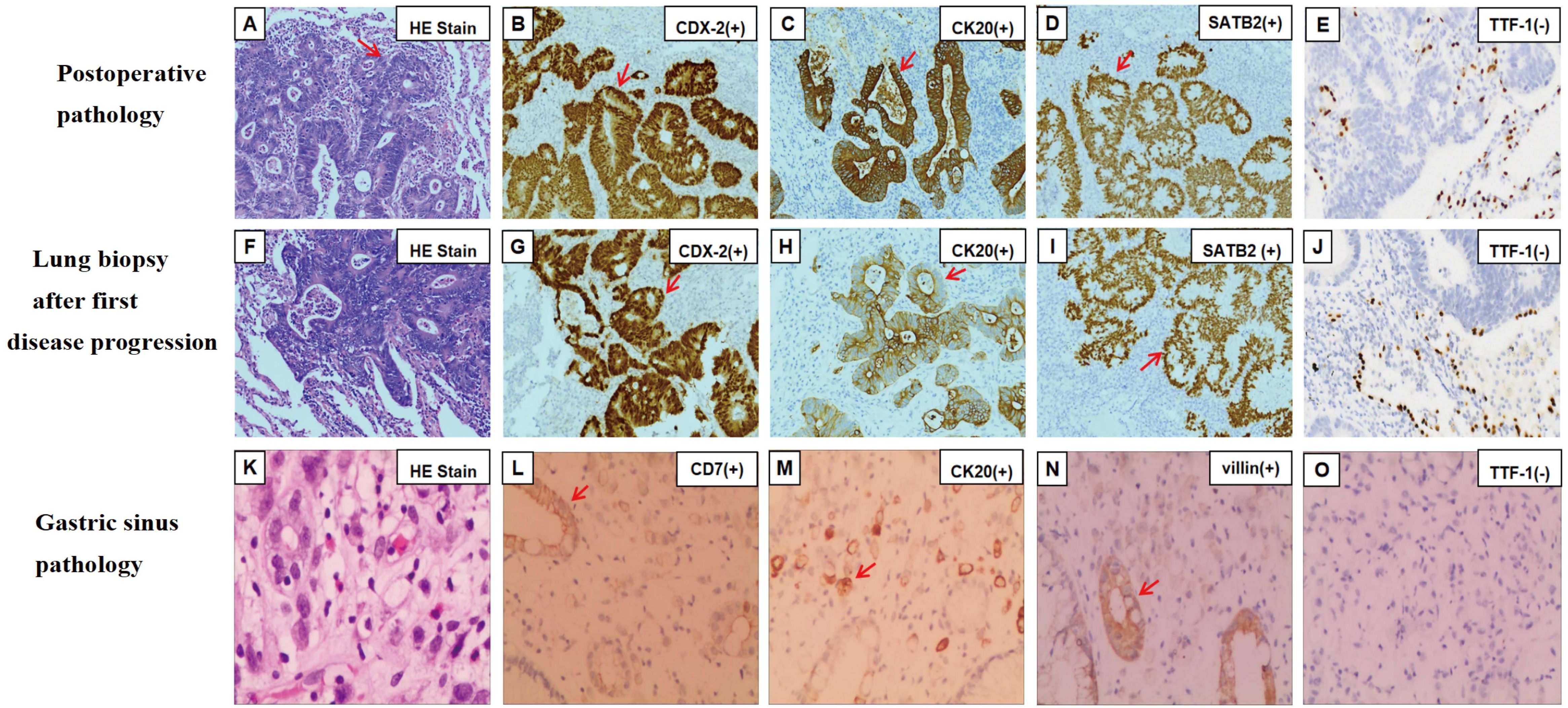
Figure 2. Hematoxylin and eosin staining and immunohistochemical findings of samples at different disease courses. (A) Postoperative pathology of left lung on hematoxylin and eosin staining. (B-E) Immunohistochemical staining was positive for CDX-2 (B), CK20 (C), and SATB2 (D). (F) Hematoxylin and eosin staining of lung biopsy after first disease progression. (G-J) Immunohistochemical staining was positive for CDX-2 (G), CK20 (H), and SATB2 (I). (K) Gastric sinus on hematoxylin and eosin staining. (L-O) Immunohistochemical staining was positive for CK7 (L), CK20 (M), villin (N). Magnification×200 (A-O). The red arrow points out positively stained cells. TTF-1, thyroid transcription factor-1; CK, cytokeratin; CDX-2, caudal-type homeobox transcription factor 2; SATB2, Special AT-Rich Sequence-Binding Protein 2.
3 Discussion
Our report described a case of primary lung-ETAC with gastric sinus metastasis, accompanied by an NRAS gene exon 3 mutation. After relapse, the patient underwent treatment with a combination of PD-1 inhibitors and chemotherapy. Six courses of this combination were administered, followed by two cycles of maintenance immunotherapy. Partial remission was achieved after two courses of PD-1 inhibitors in combination with chemotherapy, but progression was observed after two cycles of immunotherapy maintenance, as indicated by imaging assessments. The PFS was 8 months.
Among the rare subtypes of NSCLC, including enteric, fetal, and colloid types, the enteric type represented approximately 60% of these cases (11). The etiology of lung-ETAC remains unclear, with hypotheses suggesting common cancer stem cells in the mucosa of the lower respiratory and gastrointestinal tracts and the activation of the Wnt/β-catenin signaling pathway (12, 13). There was also an association found between lung-ETAC and smoking (14). Studies have shown that lung-ETAC is more prevalent in males, typically found in the right lung, and is rare in the left lung (15). In our case, the patient had no history of smoking, and the primary lesion was located in the left lung.
Histological features of lung-ETAC include well or moderately-differentiated gland formation and/or papillary structures. Tumor cells are usually tall-columnar with slightly darker-stained nuclei and nuclear palisading (16) (Figures 2A, F, K), similar to CRC. Previous studies have confirmed that CK20, CDX-2, SATB2, villin, and HNF4α are common biomarkers of digestive tract epithelium, and TTF-1 and NapsinA are biomarkers of the alveolar epithelium (17, 18). The diagnosis of lung-ETAC is often supported by IHC. Lung-ETAC expresses at least one enteric biomarker, such as CDX-2, CK20, villin, MUC2, HNF4α, and SATB2, while lung cellular markers like TTF-1 and NapsinA are generally absent. CK7 is usually expressed in contrast to lung adenocarcinoma (19). This was consistent with our case, where IHC analysis of the lung tissue revealed positive results for CDX-2, CK20, and villin, a partial positive result for CK7, and negative staining for TTF-1 and NapsinA. No gastrointestinal lesions were detected at the initial diagnosis, only lung lesions. KRAS, NRAS, and EGFR are common oncogenes in lung-ETAC. It is reported that the incidence of KRAS and NRAS mutations was about 31% (15, 20–22). KRAS is a genetic profile of invasive mucinous adenocarcinoma of the lung (23). In this case, the NRAS mutation was predominantly identified, the most common gene mutations in lung cancer are all negative, including EGFR, KRAS, BRAF, ALK, ROS1, and so on. Within rare oncogenes of NSCLC, NRAS mutations occur in only 0.9%, with a high frequency of codon 61 mutations (24). Mutations in KRAS/NRAS (RAS) are important biomarkers in mCRC and occur in approximately 60% of mCRCs (25). Mutant RAS proteins exert oncogenic effects through signaling pathways, with tumor cells carrying mutant RAS exhibiting a more aggressive phenotype. RAS-RAF-MEK-ERK and RAS-PI3K-AKT-mTORC are the basic signaling pathways of RAS proteins (26). The mutational status of RAS correlates with a worse prognosis. NRAS also promotes the colonization of the lungs by various tumor types in mouse models by regulating interleukin-8-related chemokine expression (27). Direct targeting of RAS proteins was previously considered impossible due to the lack of binding pockets on the protein surface; however, the approval of Lumakras (sotorasib, AMG510) for KRAS mutations has changed this perception (26). Currently, there are no targeted agents for NRAS mutations, so we opted for a combination of chemotherapy and immunotherapy based on positive PD-L1 expression.
Common extrapulmonary metastatic sites for lung cancer include lymph nodes, the brain, liver, adrenal glands, and bones. Gastric metastasis from lung cancer has been relatively rare, with incidence rates ranging from 0.19% to 5.1% (28). However, the autopsy detection rate is notably high (29, 30). The most frequently affected gastric sites are the fundus and cardia (29). It is hypothesized that gastric metastasis may be related to the stomach’s rich vascular system, which facilitates hematogenous dissemination (31). In this case, the diagnosis of lung ETAC with gastric metastasis was established following PET-CT and the integration of pathological features from both the lung and stomach. To date, gastric metastasis from lung-ETAC has not been reported. The NRAS mutation was predominantly identified in our report. Compared to lung cancer, NRAS mutations are more common in CRC, the percentage approximately 60% of mCRC, and are associated with strong aggressiveness (25, 26). It has been reported in the literature that KRAS mutations were associated with CRC metastasis (32), NRAS promotes the colonization of the lungs by various tumor types in mouse models (27). Whereas whether NRAS mutations mediate gastric metastasis needs to be further confirmed. Due to the specificity of the pathologic type, its morphological and molecular level alterations preserved features of CRC. So we speculate that this rare subtype may have a propensity to metastasize to the gastrointestinal tract.
The prognosis of lung-ETAC is not well understood due to its low incidence, and it is closely related to its clinical stage. Gu et al. reported that survival times for stage III or IV patients ranged from 0 to 9 months, whereas stage I or II patients had survival times ranging from 1 to 27 months. Fassi et al. found that the median overall survival (mOS) was 56.0 months for early-stage patients and 14.0 months for those with advanced or metastatic disease, with a median DFS (mDFS) of 24 months. This was consistent with our case. Wang et al. reported that PFS for 15 patients with stage III-IV ranged from 2.0 to 17 months, with a mean PFS of 6.5 months and a median PFS (mPFS) of 6.0 months (15, 33, 34). Several factors have been identified as potentially influencing prognosis, including smoking history, Eastern Cooperative Oncology Group (ECOG) performance status, tumor size ≤ 5 cm, lymph node metastasis, and expressions of biomarkers such as CK20, CDX-2, and NapsinA (15, 34–37). Chemotherapy remains the predominant treatment approach and can achieve complete remission or stability in some cases (38–40). Lung-ETAC appears to benefit from immunotherapy as well. Chen et al. were the first to identify that the nonsynonymous tumor mutational burden (TMB) of lung-ETAC was significantly higher compared to typical pulmonary adenocarcinomas (20). Jurmeister et al. first reported positive membrane PD-L1 staining in lung-ETAC tumor cells (41). Manglaviti et al. found the efficacy of immune-checkpoint inhibitors (ICIs) remains uncertain in uncommon histologies (35). Xie et al. and Liu et al. observed the PD-L1 expression in 17.6% (3/17) and 30% (3/10) of patients, respectively (22, 42). Despite these observations, the efficacy of immunotherapy is still debated. Gastrointestinal tumor regimens such as FOLFOX have also been used due to the pathology’s specificity (15). Targeted therapies have been promising with one case of EGFR L858R + A871G mutations treated with gefitinib achieving 5 years of PFS, and another case with EGFR E19del and T790M mutations treated with osimertinib showing 3 months of PFS (43, 44). We compared similar studies, and the treatment regimens are summarized in Supplementary Table 1 (48–56). Most of the studies were treated with conventional chemotherapy with a PFS of 3-5 months. In our case, PD-L1 expression is 20%, which is moderate. In NSCLC, gastric cancer, and gastroesophageal junction tumors, PD-L1 expression might be useful as a predictive marker of response to anti-PD1 therapy (45). So the patient was treated with a combination of PD-1 inhibitor and chemotherapy after gastric metastasis, resulting in a PFS of 8 months. This confirms that PD-1 inhibitors in combination with chemotherapy are significantly better than chemotherapy. This was consistent with findings from the KEYNOTE-189, IMpower150, and ORIENT-11 studies, which showed that immunotherapy combined with chemotherapy significantly improved PFS in previously untreated patients with metastatic non-squamous NSCLC, with PFS rates of 9, 6.3, and 11.4 months, respectively (46, 47). We reviewed the literature and were unable to conclude that PD-1 inhibitors prevented gastric metastasis.
Currently, data on the treatment and prognosis of lung-ETAC have been limited, with most information derived from retrospective studies. This case highlighted the need for enhanced pathological and molecular diagnostics and calls for more comprehensive studies to better understand the clinical benefits and prognosis of lung-ETAC.
In conclusion, this study reported the first case of lung-ETAC with gastric metastasis, demonstrating the effectiveness of chemotherapy in combination with immunotherapy. However, the mechanisms of gastric metastasis need further clarification.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by Ethics Committee of the First Hospital of Jilin University. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
XL: Writing – original draft. KM: Writing – review & editing. XM: Data curation, Writing – review & editing. XZ: Investigation, Writing – review & editing. MF: Writing – review & editing, Project administration. YX: Supervision, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
The authors acknowledge the valuable contributions of specialists from the Surgery, Internal Medicine, Pathology, and Imaging Departments.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2024.1486214/full#supplementary-material
References
1. Tsao MS, Fraser RS. Primary pulmonary adenocarcinoma with enteric differentiation. Cancer. (1991) 68:1754–7. doi: 10.1002/1097-0142(19911015)68:8<1754:aid-cncr2820680818>3.0.co;2-e
2. Travis WD, Brambilla E, Nicholson AG, Yatabe Y, Austin JHM, Beasley MB, et al. The 2015 world health organization classification of lung tumors: impact of genetic, clinical and radiologic advances since the 2004 classification. J Thorac Oncol. (2015) 10:1243–60. doi: 10.1097/jto.0000000000000630
3. Nicholson AG, Tsao MS, Beasley MB, Borczuk AC, Brambilla E, Cooper WA, et al. The 2021 WHO classification of lung tumors: impact of advances since 2015. J Thorac Oncol. (2022) 17:362–87. doi: 10.1016/j.jtho.2021.11.003
4. Jurmeister P, Schöler A, Arnold A, Klauschen F, Lenze D, Hummel M, et al. DNA methylation profiling reliably distinguishes pulmonary enteric adenocarcinoma from metastatic colorectal cancer. Mod Pathol. (2019) 32:855–65. doi: 10.1038/s41379-019-0207-y
5. Hu Y, Wu D, Tian C, Wei Q, Bian Y. Diagnosis of multiple primary intestinal-type adenocarcinoma in the lung by 18F-FDG PET/CT. Clin Nucl Med. (2018) 43:693–4. doi: 10.1097/rlu.0000000000002171
6. Su H, Yang L, Shen G, Tian R. Bilateral pulmonary enteric adenocarcinoma on FDG PET/CT. Clin Nucl Med. (2023) 48:e436–7. doi: 10.1097/rlu.0000000000004749
7. Li H, Cao W. Pulmonary enteric adenocarcinoma: a literature review. J Thorac Dis. (2020) 12:3217–26. doi: 10.21037/jtd-19-4171
8. Todisco A, Internò V, Stucci LS, Ostuni C, Lovero D, D'Oronzo S, et al. Cutaneous metastasis as a primary presentation of a pulmonary enteric adenocarcinoma. Int J Biol Markers. (2019) 34:421–6. doi: 10.1177/1724600819877190
9. Sun WW, Xu ZH, Wang CF, Wu F, Cao JM, Cui PJ, et al. Pulmonary enteric adenocarcinoma with pancreatic metastasis: A case report. Oncol Lett. (2017) 13:4651–6. doi: 10.3892/ol.2017.6060
10. Suzuki T, Furusawa H, Watanabe Y, Sakashita H, Fujii S, Tasaka Y, et al. A case of pulmonary enteric adenocarcinoma with soleus muscle metastasis. Gan To Kagaku Ryoho. (2019) 46:267–70.
11. Mogavero A, Bironzo P, Righi L, Merlini A, Benso F, Novello S, et al. Deciphering lung adenocarcinoma heterogeneity: an overview of pathological and clinical features of rare subtypes. Life (Basel). (2023) 13:1291. doi: 10.3390/life13061291
12. Satoh Y, Hoshi R, Tsuzuku M, Ishikawa Y, Inamura K, Horai T. Cytology of pulmonary adenocarcinomas showing enteric differentiation. Acta Cytol. (2006) 50:250–6. doi: 10.1159/000325950
13. Kishikawa S, Hayashi T, Takamochi K, Ura A, Sasahara N, Saito T, et al. Frequent nuclear β-catenin expression in pulmonary enteric-type adenocarcinoma according to the current World Health Organization criteria. Virchows Arch. (2023) 483:699–703. doi: 10.1007/s00428-023-03657-9
14. Matsushima J, Yazawa T, Suzuki M, Takahashi Y, Ota S, Nakajima T, et al. Clinicopathological, immunohistochemical, and mutational analyses of pulmonary enteric adenocarcinoma: usefulness of SATB2 and β-catenin immunostaining for differentiation from metastatic colorectal carcinoma. Hum Pathol. (2017) 64:179–85. doi: 10.1016/j.humpath.2017.04.006
15. Fassi E, Mandruzzato M, Zamparini M, Bianchi S, Petrelli F, Baggi A, et al. Clinical presentation and outcome of patients with enteric-type adenocarcinoma of the lung: A pooled analysis of published cases. Lung Cancer. (2023) 179:107176. doi: 10.1016/j.lungcan.2023.107176
16. Inamura K, Satoh Y, Okumura S, Nakagawa K, Tsuchiya E, Fukayama M, et al. Pulmonary adenocarcinomas with enteric differentiation: histologic and immunohistochemical characteristics compared with metastatic colorectal cancers and usual pulmonary adenocarcinomas. Am J Surg Pathol. (2005) 29:660–5. doi: 10.1097/01.pas.0000160438.00652.8b
17. Lugli A, Tzankov A, Zlobec I, Terracciano LM. Differential diagnostic and functional role of the multi-marker phenotype CDX2/CK20/CK7 in colorectal cancer stratified by mismatch repair status. Mod Pathol. (2008) 21:1403–12. doi: 10.1038/modpathol.2008.117
18. Kitamura H, Yazawa T, Sato H, Okudela K, Shimoyamada H. Small cell lung cancer: significance of RB alterations and TTF-1 expression in its carcinogenesis, phenotype, and biology. Endocr Pathol. (2009) 20:101–7. doi: 10.1007/s12022-009-9072-4
19. Gong J, Fan Y, Lu H. Pulmonary enteric adenocarcinoma. Transl Oncol. (2021) 14:101123. doi: 10.1016/j.tranon.2021.101123
20. Chen M, Liu P, Yan F, Xu S, Jiang Q, Pan J, et al. Distinctive features of immunostaining and mutational load in primary pulmonary enteric adenocarcinoma: implications for differential diagnosis and immunotherapy. J Transl Med. (2018) 16:81. doi: 10.1186/s12967-018-1449-z
21. Zuo Y, Zhong J, Bai H, Xu B, Wang Z, Li W, et al. Genomic and epigenomic profiles distinguish pulmonary enteric adenocarcinoma from lung metastatic colorectal cancer. EBioMedicine. (2022) 82:104165. doi: 10.1016/j.ebiom.2022.104165
22. Liu Y, Lu T, Yuan M, Chen R, Lu J, Wang H, et al. Genomic and transcriptomic insights into the precision treatment of pulmonary enteric adenocarcinoma. Lung Cancer. (2023) 179:107169. doi: 10.1016/j.lungcan.2023.03.005
23. Guo M, Tomoshige K, Meister M, Muley T, Fukazawa T, Tsuchiya T, et al. Gene signature driving invasive mucinous adenocarcinoma of the lung. EMBO Mol Med. (2017) 9:462–81. doi: 10.15252/emmm.201606711
24. Harada G, Yang SR, Cocco E, Drilon A. Rare molecular subtypes of lung cancer. Nat Rev Clin Oncol. (2023) 20:229–49. doi: 10.1038/s41571-023-00733-6
25. Loree JM, Wang Y, Syed MA, Sorokin AV, Coker O, Xiu J. Clinical and functional characterization of atypical KRAS/NRAS mutations in metastatic colorectal cancer. Clin Cancer Res. (2021) 27:4587–98. doi: 10.1158/1078-0432.Ccr-21-0180
26. Chen K, Zhang Y, Qian L, Wang P. Emerging strategies to target RAS signaling in human cancer therapy. J Hematol Oncol. (2021) 14:116. doi: 10.1186/s13045-021-01127-w
27. Giannou AD, Marazioti A, Kanellakis NI, Giopanou I, Lilis I, Zazara DE, et al. NRAS destines tumor cells to the lungs. EMBO Mol Med. (2017) 9:672–86. doi: 10.15252/emmm.201606978
28. Wang Y, Yan C, Zhang C, Yu E, Wang K, Liu X, et al. The disappearance of gastric metastasis and liver metastasis in non-small cell lung adenocarcinoma is due to osimertinib. J Cancer Res Clin Oncol. (2023) 149:16069–73. doi: 10.1007/s00432-023-05386-7
29. Burbige EJ, Radigan JJ, Belber JP. Metastatic lung carcinoma involving the gastrointestinal tract. Am J Gastroenterol. (1980) 74:504–6.
30. Antler AS, Ough Y, Pitchumoni CS, Davidian M, Thelmo W. Gastrointestinal metastases from Malignant tumors of the lung. Cancer. (1982) 49:170–2. doi: 10.1002/1097-0142(19820101)49:1<170::aid-cncr2820490134>3.0.co;2-a
31. Maeda J, Miyake M, Tokita K, Iwahashi N, Nakano T, Tamura S, et al. Small cell lung cancer with extensive cutaneous and gastric metastases. Intern Med. (1992) 31:1325–8. doi: 10.2169/internalmedicine.31.1325
32. Huang D, Sun W, Zhou Y, Li P, Chen F, Chen H. Mutations of key driver genes in colorectal cancer progression and metastasis. Cancer Metastasis Rev. (2018) 37:173–87. doi: 10.1007/s10555-017-9726-5
33. Gu L, Wang XZ, Wen W, Lin J, Chen XF, Lai GX, et al. Clinical analysis of 23 patients pathologically diagnosed with primary and secondary pulmonary enteric adenocarcinoma. Chin Med J (Engl). (2019) 132:1368–9. doi: 10.1097/cm9.0000000000000266
34. Wang Q, Zhang L, Li H, Liu L, Sun X, Liu H. Clinical features and prognosis of pulmonary enteric adenocarcinoma: A retrospective study in China and the SEER database. Front Oncol. (2023) 13:1099117. doi: 10.3389/fonc.2023.1099117
35. Manglaviti S, Brambilla M, Signorelli D, Ferrara R, Lo Russo G, Proto C, et al. Immune-checkpoint inhibitors in advanced non-small cell lung cancer with uncommon histology. Clin Lung Cancer. (2022) 23:e17–28. doi: 10.1016/j.cllc.2021.06.013
36. Lee JG, Kim S, Shim HS. Napsin A is an independent prognostic factor in surgically resected adenocarcinoma of the lung. Lung Cancer. (2012) 77:156–61. doi: 10.1016/j.lungcan.2012.02.013
37. Feng C, Feng M, Gao Y, Zhao X, Peng C, Yang X, et al. Clinicopathologic significance of intestinal-type molecules' Expression and different EGFR gene status in pulmonary adenocarcinoma. Appl Immunohistochem Mol Morphol. (2019) 27:364–72. doi: 10.1097/pai.0000000000000632
38. Xu X, Chen D, Wu X, Wang Q. A pulmonary enteric adenocarcinoma patient harboring a rare EGFR exon 19 P753S mutation: Case report and review. Front Oncol. (2022) 12:988625. doi: 10.3389/fonc.2022.988625
39. Qureshi A, Furrukh M. Enteric adenocarcinoma lung: a rare presentation in an Omani woman. BMJ Case Rep. (2013) 2013:bcr2012007667. doi: 10.1136/bcr-2012-007667
40. Li HC, Schmidt L, Greenson JK, Chang AC, Myers JL. Primary pulmonary adenocarcinoma with intestinal differentiation mimicking metastatic colorectal carcinoma: case report and review of literature. Am J Clin Pathol. (2009) 131:129–33. doi: 10.1309/ajcpb04xwictferl
41. Jurmeister P, Vollbrecht C, Behnke A, Frost N, Arnold A, Treue D, et al. Next generation sequencing of lung adenocarcinoma subtypes with intestinal differentiation reveals distinct molecular signatures associated with histomorphology and therapeutic options. Lung Cancer. (2019) 138:43–51. doi: 10.1016/j.lungcan.2019.10.005
42. Xie M, Chen D, Li Y, Liu X, Kuang D, Li X. Genetic mutation profiles and immune microenvironment analysis of pulmonary enteric adenocarcinoma. Diagn Pathol. (2022) 17:30. doi: 10.1186/s13000-022-01206-7
43. Cui Y, Liang J, Kang X, Liu M, Zhang Q, Zhang H. Gefitinib, an effective treatment option for patients with pulmonary enteric adenocarcinoma harboring compound EGFR L858R and A871G mutation. Invest New Drugs. (2023) 41:787–90. doi: 10.1007/s10637-023-01401-3
44. Yang M, Yu P, He Z, Deng J. Case report: Target and immunotherapy of a lung adenocarcinoma with enteric differentiation, EGFR mutation, and high microsatellite instability. Front Immunol. (2023) 14:1266304. doi: 10.3389/fimmu.2023.1266304
45. Ganesh K, Stadler ZK, Cercek A, Mendelsohn RB, Shia J, Segal NH. Immunotherapy in colorectal cancer: rationale, challenges and potential. Nat Rev Gastroenterol Hepatol. (2019) 16:361–75. doi: 10.1038/s41575-019-0126-x
46. Yang Y, Wang Z, Fang J, Yu Q, Han B, Cang S, et al. Efficacy and Safety of Sintilimab Plus Pemetrexed and Platinum as First-Line Treatment for Locally Advanced or Metastatic Nonsquamous NSCLC: a Randomized, Double-Blind, Phase 3 Study (Oncology pRogram by InnovENT anti-PD-1-11). J Thorac Oncol. (2020) 15:1636–46. doi: 10.1016/j.jtho.2020.07.014
47. Reck M, Remon J, Hellmann MD. First-line immunotherapy for non-small-cell lung cancer. J Clin Oncol. (2022) 40:586–97. doi: 10.1200/jco.21.01497
48. Metro G, Valtorta E, Siggillino A, Lauricella C, Cenci M, Ludovini V, et al. Enteric-type adenocarcinoma of the lung harbouring a novel KRAS Q22K mutation with concomitant KRAS polysomy: a case report. Ecancermedicalscience. (2015) 9:559. doi: 10.3332/ecancer.2015.559
49. Garajová I, Funel N, Fiorentino M, Agostini V, Ferracin M, Negrini M, et al. MicroRNA profiling of primary pulmonary enteric adenocarcinoma in members from the same family reveals some similarities to pancreatic adenocarcinoma-a step towards personalized therapy. Clin Epigenet. (2015) 7:129. doi: 10.1186/s13148-015-0162-5
50. Lin LI, Xu CW, Zhang BO, Liu RR, Ge FJ, Zhao CH, et al. Clinicopathological observation of primary lung enteric adenocarcinoma and its response to chemotherapy: A case report and review of the literature. Exp Ther Med. (2016) 11:201–7. doi: 10.3892/etm.2015.2864
51. Lin L, Zhuang W, Wang W, Xu C, Chen R, Guan Y, et al. Genetic mutations in lung enteric adenocarcinoma identified using next-generation sequencing. Int J Clin Exp Pathol. (2017) 10:9583–90.
52. Prakobkit R, Churk-Nam Auyeung W, Xu L, Berry GJ. Pulmonary adenocarcinoma with enteric differentiation presenting with bronchorrhea. J Thorac Oncol. (2017) 12:e120–3. doi: 10.1016/j.jtho.2017.04.005
53. Tu LF, Sheng LY, Zhou JY, Wang XF, Wang YH, Shen Q, et al. Diagnosis and treatment of primary pulmonary enteric adenocarcinoma: Report of Six cases. World J Clin cases. (2021) 9:9236–43. doi: 10.12998/wjcc.v9.i30.9236
54. Teranishi S, Sugimoto C, Nagayama H, Segawa W, Miyasaka A, Hiro S, et al. Combination of pembrolizumab with platinum-containing chemotherapy for pulmonary enteric adenocarcinoma. Cancer Diagn Progn. (2022) 2:253–7. doi: 10.21873/cdp.10102
55. Hu CH, Shi S, Dong W, Xiao L, Zang H, Wu F. Hyperprogressive disease after immunotherapy: A case report of pulmonary enteric adenocarcinoma. Front Oncol. (2022) 12:799549. doi: 10.3389/fonc.2022.799549
Keywords: lung enteric-type adenocarcinoma, gastric metastasis, NRAS gene exon 3 mutation, chemotherapy and immunotherapy, non-small cell lung cancer
Citation: Li X, Ma K, Ma X, Zhao X, Fan M and Xu Y (2024) Lung enteric-type adenocarcinoma with gastric metastasis: a rare case report and literature review. Front. Immunol. 15:1486214. doi: 10.3389/fimmu.2024.1486214
Received: 25 August 2024; Accepted: 07 October 2024;
Published: 23 October 2024.
Edited by:
Mohd Farhan, Georgetown University Medical Center, United StatesReviewed by:
Mingming Zhao, University of Mississippi, United StatesSanthosh Shanthi Bhupathi, West Virginia University, United States
Suravi Majumder, University of Texas Health Science Center at Houston, United States
Payal Mitra, George Washington University, United States
Copyright © 2024 Li, Ma, Ma, Zhao, Fan and Xu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yinghui Xu, eHV5aW5naHVpQGpsdS5lZHUuY24=.
†These authors have contributed equally to this work
 Xiaoning Li
Xiaoning Li Kewei Ma
Kewei Ma Xiaobo Ma2
Xiaobo Ma2 Yinghui Xu
Yinghui Xu