- 1Department of Urology, People’s Hospital of Deyang City, Chengdu University of Traditional Chinese Medicine, Deyang, China
- 2Department of Anesthesia & Operating room, Sichuan Provincial People’s Hospital, School of Medicine, University of Electronic Science and Technology of China, Chengdu, China
Objective: The high hemoglobin, albumin, lymphocyte, and platelet (HALP) score has been reported to be a good prognostic indicator for several malignancies. However, more evidence is needed before it can be introduced into clinical practice. Here, we systematically evaluated the predictive value of HALP for survival outcomes in patients with solid tumors.
Methods: This study was performed according to Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) and Assessing the Methodological Quality of Systematic Reviews (AMSTAR) Guidelines. In March 2024, an electronic literature search was performed for articles regarding the prognostic role of HALP in solid tumors. Data from studies with reported risk ratios (HRs) and 95% confidence intervals (CIs) were pooled in a meta-analysis. Study bias was assessed using the QUIPS tool.
Results: Of the 729 articles reviewed, 45 cohorts including data from 17,049 patients with cancer were included in the pooled analysis. The pooled results demonstrated that elevated HALP score was significantly associated with favorable overall survival (HR = 0.60, 95% CI 0.54-0.67, p < 0.01), cancer-specific survival (HR = 0.53, 95% CI 0.44- 0.64, p < 0.01), progression-free survival (HR = 0.62, 95% CI 0.54-0.72, p < 0.01), recurrence-free survival (HR = 0.48, 95% CI 0.30-0.77, p < 0.01), and disease-free survival (HR = 0.72, 95% CI 0.57-0.82, p < 0.01). Subgroup analyses based on various confounding factors further revealed the consistent prognostic impact of HALP on overall survival in patients with solid tumors.
Conclusions: Our findings suggest that high HALP is associated with better survival outcomes in patients. The HALP score is a potential prognostic biomarker in solid tumors, but it needs to be further studied whether it can improve the established prognostic model.
Introduction
Cancer is a major public health problem worldwide, placing a heavy burden on human health. According to data from the International Agency for Research on Cancer (IARC) in 2020, an estimated 19.3 million new cancer cases and nearly 10 million cancer deaths occurred worldwide (1). Despite significant advances in current cancer treatment, such as the use of immune checkpoint inhibitors and oncogene-targeted drugs, overall cancer-related mortality remains high (2). In addition, cancer treatment varies greatly among individuals, making the prognosis of different individuals significantly different (3). Therefore, there is a need for a reliable biomarker to predict survival in patients with cancer so that therapeutic strategies can be tailored to improve outcomes (4).
Tumor progression and metastasis are not only dependent on the type of tumor cells, but also inflammatory response and nutritional status play important roles in these processes (5, 6). Substantial evidence suggests that parameters reflecting nutritional and inflammatory status, including albumin and hemoglobin levels and lymphocyte and platelet counts, are critical for cancer survival (7–10). The downside of these metrics, however, is that each captures only one aspect of inflammation or nutrition (11). Further studies discovered that a combination of these parameters, including platelet-to-lymphocyte ratio (PLR), neutrophil-to-lymphocyte ratio (NLR), and prognostic nutrition index (PNI), could accurately predict patient outcome more than any single index (12–14). In addition to these well-known markers, a novel inflammatory index combining hemoglobin, albumin, lymphocyte, and platelet (HALP) has been shown to be strongly associated with the prognosis of several malignancies (15–18).
Although a series of studies have attempted to explore the use of HALP as a prognostic marker in human cancer, the results of these findings have been inconsistent (15, 17, 19–22). The advantage of meta-analyses is that they allow pooled effect sizes to be derived from the results of previous studies and thus allow for more robust conclusions to be drawn using data from a large number of patients (23). The purpose of this study was to investigate whether HALP could be a new prognostic indicator for solid tumors using meta-analysis.
Materials and methods
This meta-analysis was performed following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2020 guideline (24) and A MeaSurement Tool to Assess systematic Reviews 2 (AMSTAR 2) guideline (25). This study was also registered with PROSPERO (CRD42022334548).
Search strategy
An electronic literature search was conducted on PubMed, Ovid-Embase, Web of Science, and Cochrane Library in March 2024 for articles regarding the prognostic role of HALP in solid tumors. We used the following search terms: “hemoglobin, albumin, lymphocyte, and platelet”, “HALP”, “neoplasm”, “neoplasia”, “cancer”, “tumor”, “carcinoma” and “malignancy”. We also manually searched the literature reference list to further investigate potentially relevant studies. Discrepancies were addressed through discussion or ultimately by third-party adjudication.
Selection criteria
The criteria for inclusion of studies were as follows: (1) prospective or retrospective clinical studies; (2) studies investigating the association of pretreatment HALP with prognosis in any histologically confirmed solid tumor; (3) patients were adults 18 years of age or older; (4)cut-off values for pre-treatment HALP have been determined and divided into high and low groups; and (5) sufficient data were obtained to assess the hazard ratio (HR) and corresponding 95% confidence interval (CI) between pretreatment HALP and survival outcomes including overall survival (OS), cancer-specific survival (CSS), progression-free survival (PFS), recurrence-free survival (RFS), and/or disease-free survival (DFS). Exclusion criteria were studies categorized as reviews, conference abstracts, letters, and expert opinions. Additionally, unpublished studies, duplicate published studies, studies with insufficient survival data, and studies focusing only on hematological malignancies were excluded.
Data extraction
Two authors separately collected the following variables from the included studies: first author’s name, year of publication, country, ethnicity, study type, tumor type, tumor stage, treatment strategy, sample size, age of subjects, HALP cut-off value, analysis of survival, survival outcomes (HRs with corresponding 95% CIs for OS, CSS, PFS, RFS, and DFS), and follow-up period. Data were extracted from a multivariate analysis when survival data from a study were analyzed in two ways (univariate and multivariate analyses). Moreover, if relevant data for the article were missing, the corresponding author was contacted. If no response was received or data were not available, the article was excluded.
Methodological quality
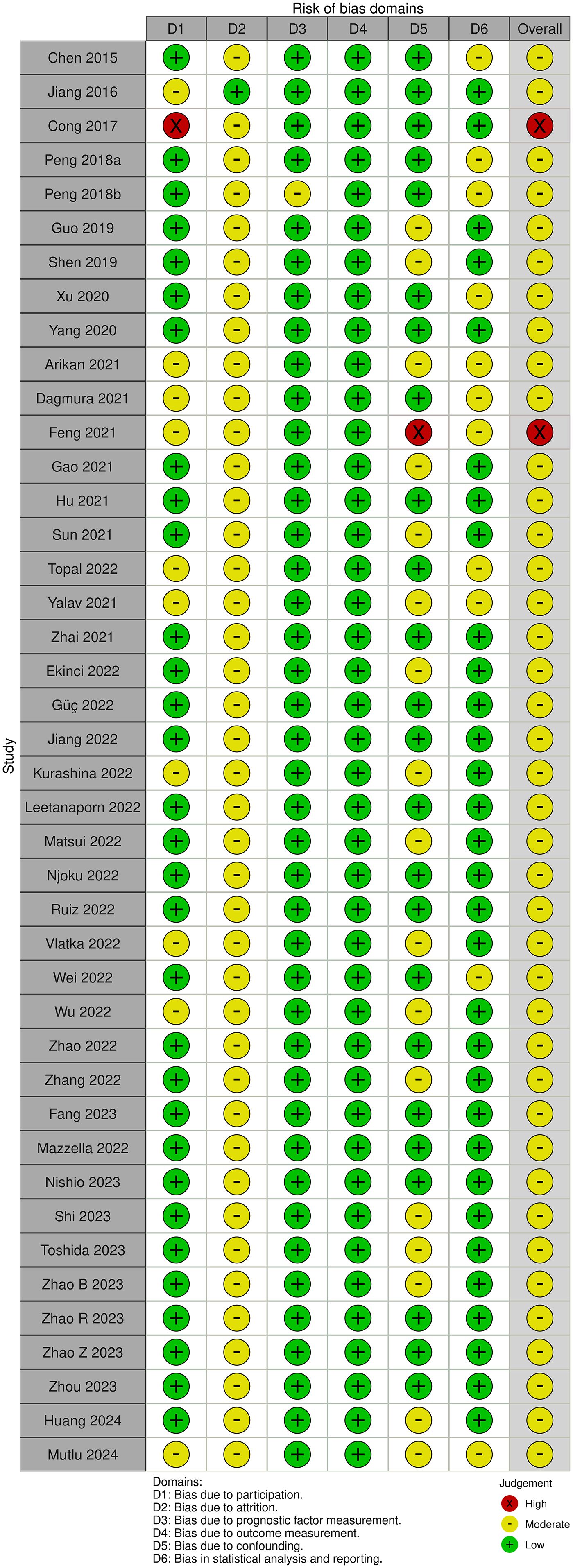
Risk of bias assessment for included studies using the Quality In Prognosis Studies (QUIPS) tool (26). This tool covers six main domains, including study population, study attrition, prognostic factor measurement, outcome measurement, study confounding, and statistical analysis and reporting. Each study was rated as high, moderate, or low risk of bias based on the description in the original study. Two reviewers independently conducted the quality assessment and all disagreements were resolved through discussion or adjudicated by a third party.
Statistical analyses
We used software R 3.6.3 and Stata 14.0 for statistical analysis. A pooled HR with 95% CI was utilized to assess the association between pre-treatment HALP and survival outcomes. Heterogeneity between studies was estimated using Cochran’s Q test and Higgin’s I2 test, and I2 > 50% or p < 0.10 demonstrated significant heterogeneity. A random effects model was employed for the combined analysis in this meta-analysis. Moreover, any potential publication bias was evaluated by Begg’s test. We performed subgroup analyses to investigate potential sources of heterogeneity. Meta-regression analysis was conducted to assess the effect of the HALP cutoff value on the HR for OS. Subsequently, sensitivity analyses were also conducted to assess the robustness and reliability of the pooled results. Two-sided p < 0.05 was considered statistically significant.
Results
Study characteristics
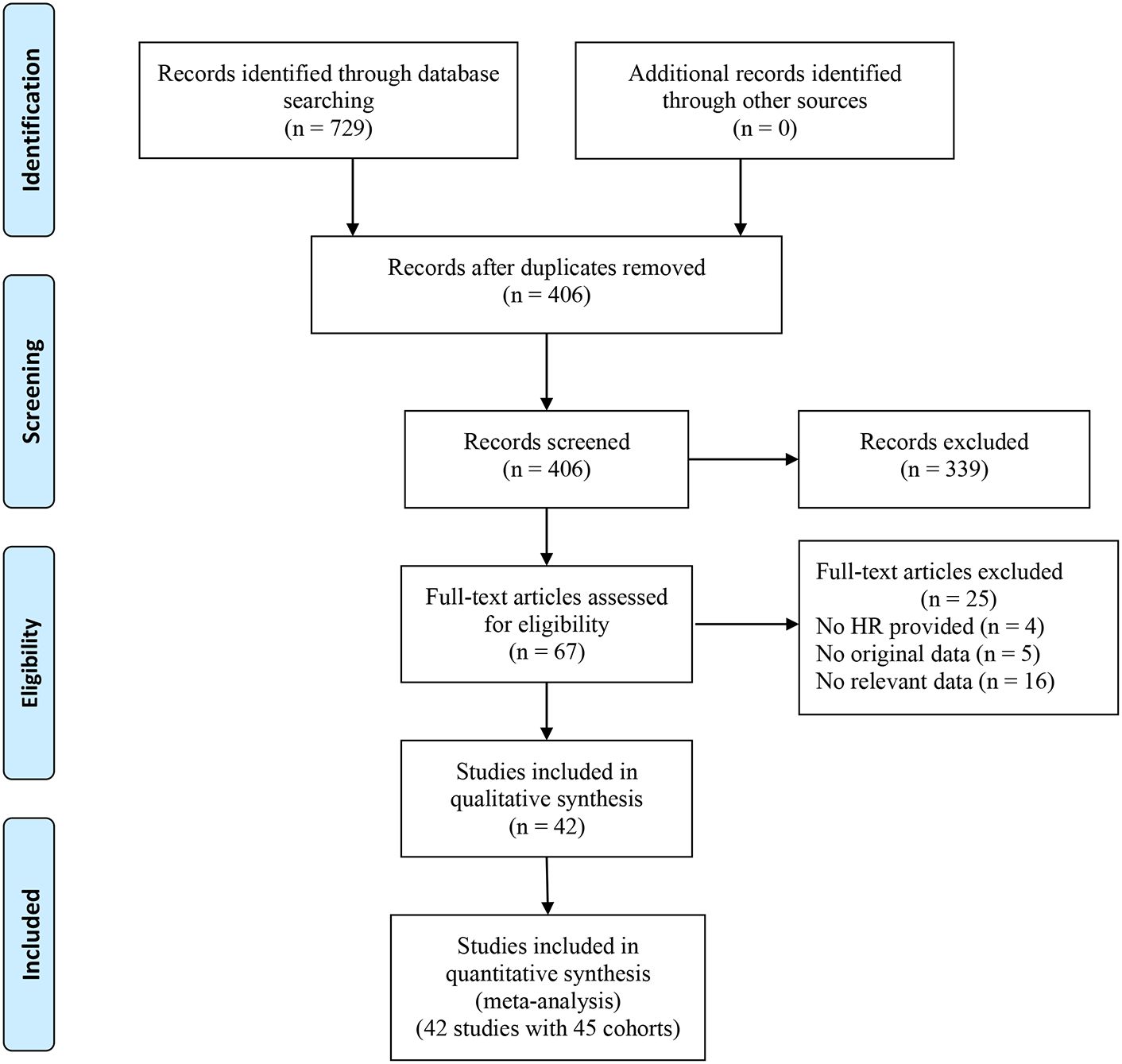
The search initially identified 729 articles, leaving 406 articles after eliminating duplicate publications. By reading the titles and abstracts, 339 articles that did not fit the main idea were excluded. The full text of 67 studies was then reviewed, and 25 studies (including 4 studies that did not provide the HR with corresponding 95% CI data, 5 studies with missing survival outcome data, and 16 studies involving patients with non-solid tumors) were excluded. Finally, 42 studies containing 17,049 patients were included in this meta-analysis (11, 15–22, 27–59). The flowchart of the study screening process is presented in Figure 1.
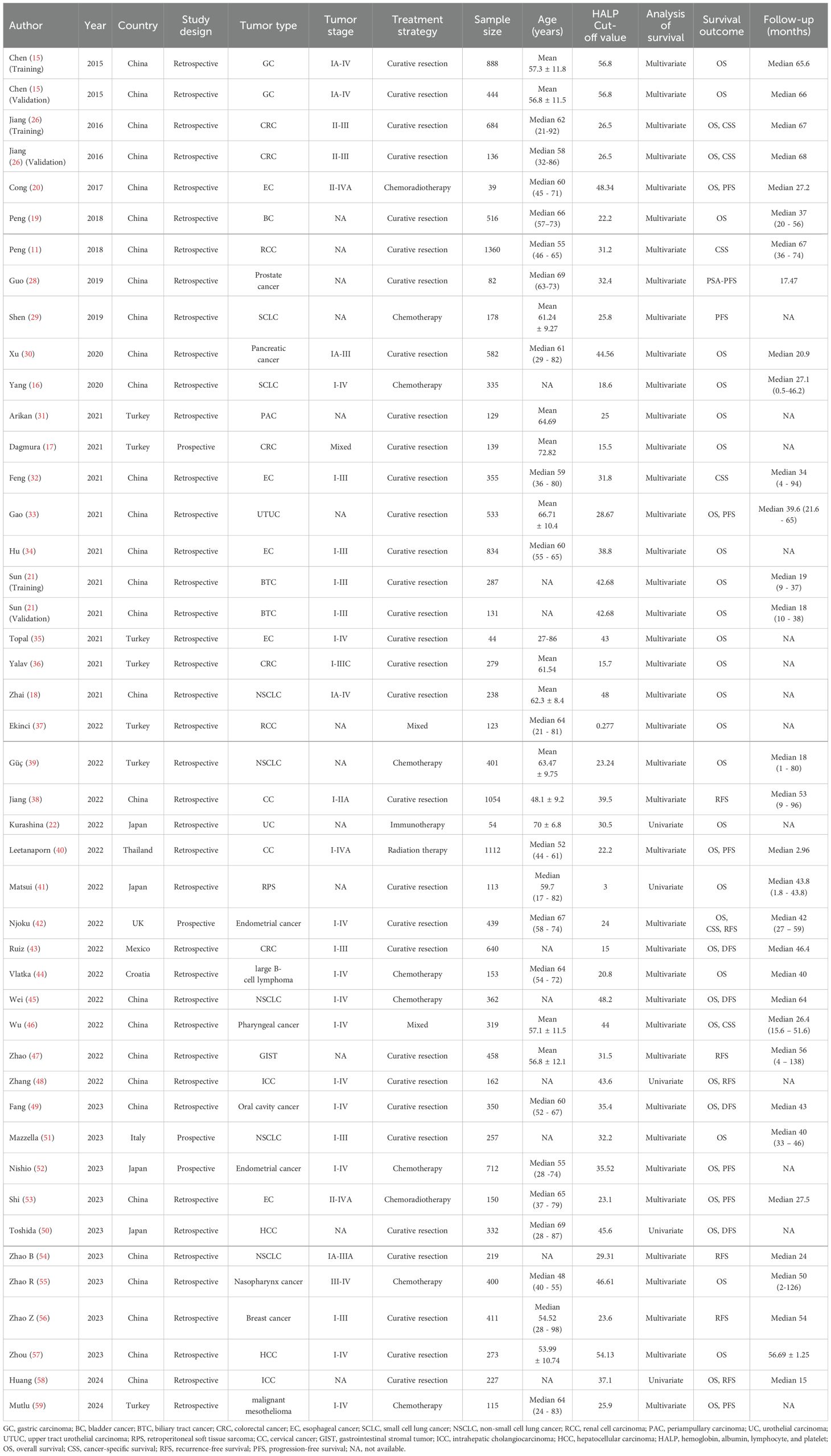
Of these 42 studies, three studies had two cohorts (training and validation cohorts) (15, 21, 27), resulting in a total of 45 cohorts included in this meta-analysis. The 29 cohorts were from China (11, 15, 16, 18–21, 27–30, 32–34, 38, 45–49, 53–58), seven from Turkey (17, 31, 35–37, 39, 59), four from Japan (22, 41, 50, 52), three from European and American countries (42, 43, 51), and one study from Thailand (40). In the included cohorts, the most common tumor type was hepatobiliary and pancreatic cancer (n = 8) (21, 30, 31, 48, 50, 57, 58), followed by gastrointestinal cancer (n = 7) (15, 17, 27, 36, 43). Notably, only 4 cohorts were prospectively designed (17, 42, 51, 52), the rest were retrospective (11, 15, 16, 18–22, 27–41, 43–50, 53–59). Of the included cohorts, 31 cohorts underwent curative resection (11, 15, 17–19, 21, 27, 28, 30–36, 38, 41–43, 45, 47–51, 54, 56–58), 9 cohorts received adjuvant therapy (e.g., chemotherapy, radiotherapy, chemoradiotherapy, and immunotherapy) (16, 20, 22, 29, 39, 40, 53, 55, 59), and 2 cohorts received mixed treatment (37, 46). The number of patients included in the individual cohorts ranged from 39 to 1360. The cut-off value of HALP ranged from 0.277 to 56.8. thirty-seven cohorts reported associations between HALP and OS (15–22, 27, 30, 31, 33–37, 39–43, 45, 46, 48–53, 55, 57, 58), 6 cohorts investigated associations between HALP and CSS (11, 27, 32, 42, 46), 8 cohorts examined associations between HALP and PFS (20, 28, 29, 33, 40, 52, 53, 59), 7 cohorts investigated associations between HALP and RFS (38, 42, 47, 48), and 4 cohorts reported associations between HALP and DFS (43, 45, 49, 50, 54, 56, 58). The basic characteristics of the enrolled cohorts are shown in Table 1.
Quality of the studies
The study quality of each study was assessed using the QUIPS tool. QUIPS domains most commonly evaluated as low risk of bias were the prognostic factor measurement and outcome measurement, while the QUIPS domain most commonly evaluated as a moderate risk of bias was attrition. The majority of studies were judged to be moderate risk of bias and 2 studies were judged to be high risk, as illustrated in Figure 2.
Association of HALP with survival outcomes
Overall survival
Thirty-four studies comprising 37 cohorts investigated the association of HALP with OS in patients with cancer (15–22, 27, 30, 31, 33–37, 39–43, 45, 46, 48–53, 55, 57, 58). The results demonstrated that OS was significantly longer in patients with increased pretreatment HALP (HR = 0.60, 95% CI 0.54-0.67, p < 0.01), with significant heterogeneity among studies (I2 = 77%, p < 0.01) (Figure 3).
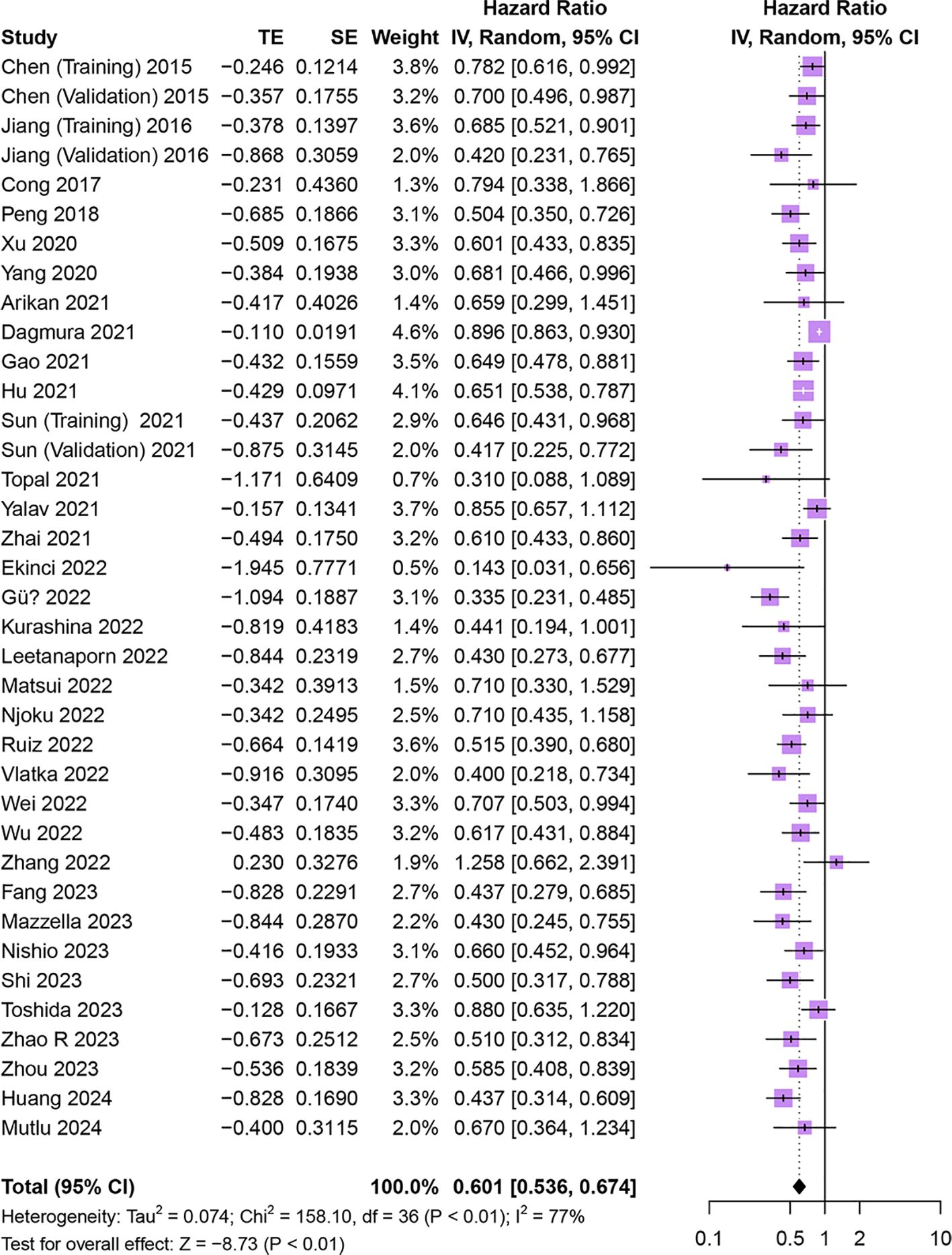
Figure 3. Forest plot showing hazard ratio for overall survival for HALP greater than or less than the cutoff value. HALP, hemoglobin, albumin, lymphocyte and platelet.
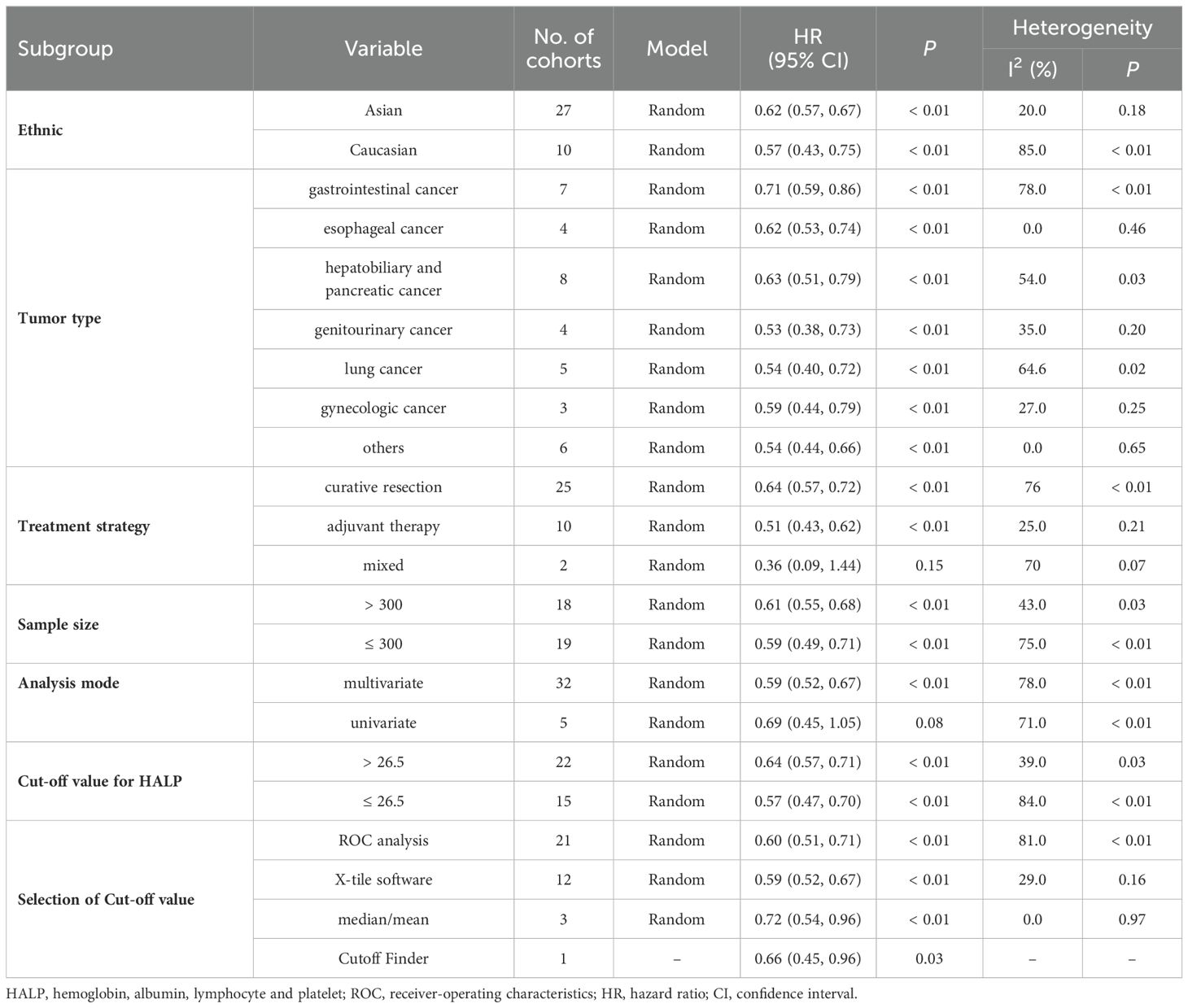
Given the significant heterogeneity between studies, we performed subgroup analyses of OS based on study ethnicity, tumor type, treatment strategy, sample size, study design, analysis mode, cut-off value, and cut-off selection method (Table 2). High pre-treatment HALP was found to be consistently associated with better OS regardless of ethnicity, tumor type, treatment strategy, sample size, cut-off value, or cut-off selection method (all p < 0.01). On subgroup analysis stratified by analysis mode, the multivariate analysis subgroup was significantly associated with longer OS (p < 0.01), while the univariate analysis subgroup was not associated with OS (p = 0.08). Furthermore, Meta-regression analysis revealed no significant association between the HALP cutoff value and the HR for OS (p = 0.401, Supplementary Data Sheet 1).
Cancer-specific survival
Five studies comprising 6 cohorts explored the association of HALP with CSS in patients with cancer (11, 27, 32, 42, 46). The results indicated that higher pretreatment HALP was associated with longer CSS in patients (HR = 0.53, 95% CI 0.44 - 0.64, p < 0.01), and there was low heterogeneity among studies (I2 = 24%, p = 0.25) (Figure 4).
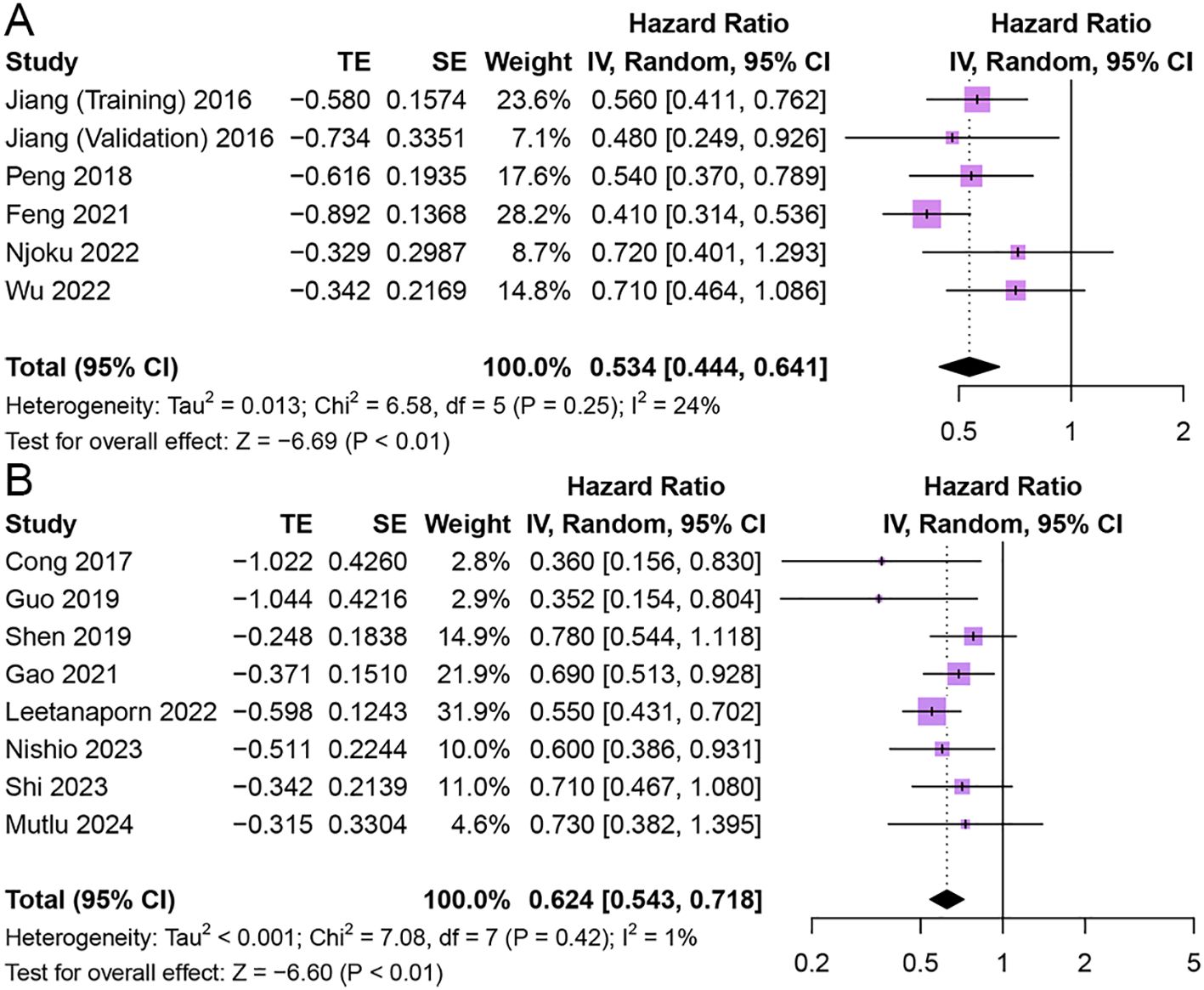
Figure 4. Forest plot showing hazard ratio for cancer-specific survival (A) and progression-free survival (B) for HALP greater than or less than the cutoff value. HALP, hemoglobin, albumin, lymphocyte and platelet.
Progression-free survival
Eight studies reported the relationship between HALP and PFS in patients with cancer (20, 28, 29, 33, 40, 52, 53, 59). The results showed that patients with elevated pretreatment HALP had better PFS (HR = 0.62, 95% CI 0.54 - 0.72, p < 0.01), with low heterogeneity between studies (I2 = 1%, p = 0.42) (Figure 4).
Recurrence-free survival
Seven studies reported the relationship between HALP and RFS in patients with cancer (43, 45, 49, 50, 54, 56, 58). The results revealed that patients with elevated pretreatment HALP had favorable RFS in patients with solid tumors (HR = 0.48, 95% CI 0.30 - 0.77, p < 0.01), with significant heterogeneity among studies (I2 = 82%, p < 0.01) (Figure 5).
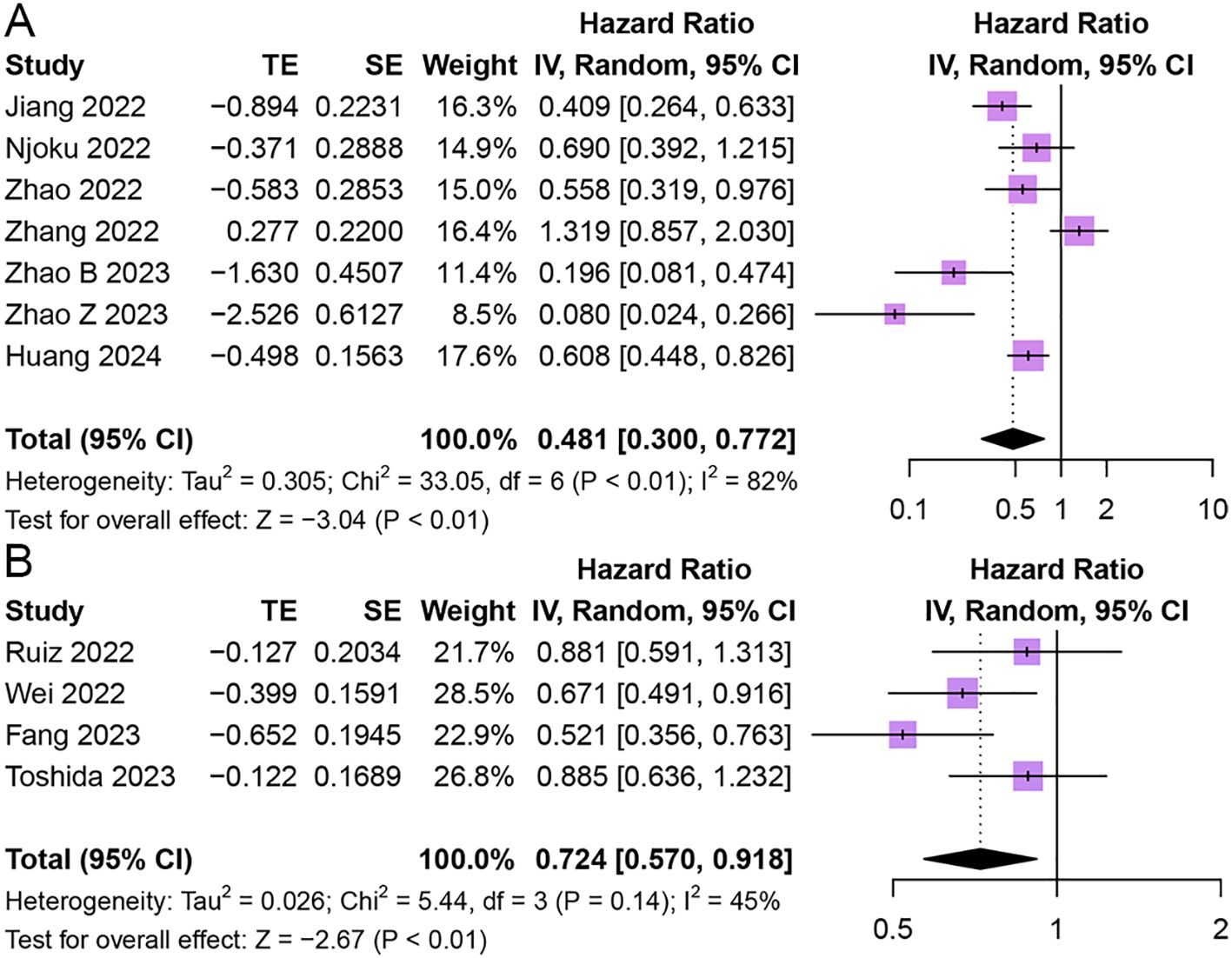
Figure 5. Forest plot showing hazard ratio for recurrence-free survival (A) and disease-free survival (B) for HALP greater than or less than the cutoff value. HALP, hemoglobin, albumin, lymphocyte and platelet.
Disease-free survival
Four studies reported the relationship between HALP and DFS in patients with cancer (43, 45, 49, 50). The results demonstrated that patients with elevated pretreatment HALP had better DFS (HR = 0.72, 95% CI 0.57 - 0.92, p < 0.01), with lower significant heterogeneity among studies (I2 = 45%, p = 0.14) (Figure 5).
Sensitivity analysis
We performed sensitivity analyses to assess the reliability of pooled HRs for OS (Supplementary Data Sheet 1). The exclusion of individual studies had no significant effect on the combined HR, confirming that the results of this meta-analysis are relatively robust and reliable.
Publication bias
The Begg’s test demonstrated that the results were not statistically significant (OS: p = 0.824), but the Begg’s funnel plots showed asymmetry between the left and right sides, which increases the likelihood of potential publication bias (Supplementary Data Sheet 1).
Discussion
To date, cancer remains the leading cause of death and a significant barrier to increasing life expectancy in all countries of the world (60). Due to the higher cost of cancer management, the establishment of reliable prognostic biomarkers is essential for predicting therapeutic outcomes and determining the patients most likely to benefit from treatment. HALP is a new score based on a combination of inflammatory and nutritional deficiency concepts that was first discovered in 2015 to predict the prognosis of patients with gastric cancer (15). Over the past few years, HALP has been successively used to evaluate survival outcomes in various malignancies. Although a recent systematic review has revealed that low pre-treatment HALP predicts a worse overall prognosis for cancer patients (61), however, there is great heterogeneity in studies investigating HALP in terms of cancer type, outcome, HALP threshold, and population of interest. Here, we conducted an updated meta-analysis based on the available literature to investigate the prognostic impact of HALP. In addition, subgroup analyses were performed to explore the influence of factors such as ethnicity, tumor type, and treatment strategy on the study results.
Evidence from the inclusion of 45 cohorts suggested that an elevated HALP was associated with better OS, CSS, PFS, and DFS in patients with solid tumors. When stratified by ethnicity, disease type, treatment strategy, sample size, and study design higher HALP was consistently an independent factor for favorable OS. Of interest, the included studies reported different HALP cut-off values for different disease types and used different methods to select HALP cut-off values. However, we observed that the prognostic impact of HALP on OS was retained across subgroups. Moreover, in subgroup analyses stratified by analysis mode, HALP scores in the multivariate analysis subgroup were independently associated with OS (Table 2). Although no significant difference in OS was observed in the univariate subgroup, it is unlikely to affect the interpretation of our results given the small number of studies included in the analysis. Notably, in this meta-analysis, we included a substantial number of retrospective studies. The subgroup analysis based on study design showed no significant difference between the data from retrospective studies and the overall results (Table 2). To some extent, this indicates that data from retrospective studies are consistent with those from other types of studies and did not introduce noticeable bias into the final comprehensive conclusion.
Furthermore, due to the heterogeneity of the studies themselves, we were unable to comprehensively assess the relationship between HALP and age or gender. As age increases, the prognosis of elderly cancer patients is generally worse. However, we observed that almost all studies accounted for patient age when performing multivariate regression or constructing nomograms. Therefore, age does not appear to influence the HALP score. Further research is needed to study HALP scores in healthy populations to accurately evaluate the correlation between HALP and age. Additionally, some studies have reported differences in baseline HALP scores between males and females, but after adjusting for gender, the HALP score remained significant (11, 15, 19, 34). Thus, based on current results, gender does not significantly affect the utility of HALP as a biomarker. In general, a more refined search method and more stringent inclusion criteria were used than in the previous systematic review (61), which dramatically improved the quality and credibility of the study.
The mechanism of the association between high HALP and favorable outcomes in cancer patients remains unclear. One potential mechanism for the prognostic impact of HALP could be the association of high HALP with inflammation and nutrition. Anemia is a well-documented cancer-related phenomenon. In chronic anemia, CD3 T lymphocytes and macrophages release pro-inflammatory cytokines such as IL-6 (62). IL-6 mediates the release of hepcidin from the liver, which inhibits iron absorption and iron release to prevent cancer cells from utilizing iron, thereby reducing erythropoiesis (63). Previous studies also have demonstrated that low hemoglobin levels were associated with adverse clinical outcomes in cancer patients, including impaired quality of life and reduced survival (64, 65). Serum albumin is a reliable indicator for assessing nutritional status and visceral protein function. Studies have reported that in the later stages of the disease, malnutrition and inflammation inhibit albumin synthesis, resulting in lower serum albumin concentrations (66). The reason for this may be due to the production of cytokines, such as IL-6, which regulate albumin production by hepatocytes (67). Furthermore, tumor necrosis factor may increase microvascular permeability, thereby increasing the passage of albumin through capillaries (68, 69). Therefore, mild or no hypoalbuminemia in the early stages of cancer, but a significant decrease in albumin levels as the disease progresses could be a good indicator of cancer prognosis (7).
Abundant evidence indicates that the inflammatory microenvironment is an important component of carcinogenesis. As the basic components of the systemic inflammatory response, platelets and lymphocytes are involved in the continuous inflammation of the tumor microenvironment (70, 71). Platelets have been reported to promote tumor growth and angiogenesis by secreting a mixture of major proangiogenic cytokines in the microcirculation of potentially prothrombotic tumors (72, 73). In addition, platelets also enhance tumor metastasis by covering circulating tumor cells to protect tumor cells from physical factors such as shear stress and host immune responses (72, 74). On the other hand, the importance of lymphocytes has been highlighted in earlier studies. It is an important component of anti-tumor immunity and can inhibit tumor proliferation and migration through cytotoxicity (70). These findings suggest that serum hemoglobin, albumin, and lymphocytes can be considered favorable factors for tumor prognosis, while platelets may be an unfavorable factor.
Over the past decade, energy and resources have been invested in developing biomarkers to help personalize treatment plans for cancer patients. The HALP score combines malnutrition factors (hemoglobin and albumin) with inflammatory response factors (lymphocyte and platelet counts). It may help identify more patients with a poor prognosis than a single index because abnormalities in any single indicator do not truly reflect the patient’s condition. In addition, HALP has even been shown to have the potential to distinguishing between benign and malignant processes (75). Therefore, we reasoned that HALP could serve as a more practical and comprehensive prognostic marker for human cancers, including gastrointestinal, lung, genitourinary tract, gynecological, among others.
Strengths and limitations
The strength of this study is that it followed international guidelines and a rigorous systematic search and bias assessment protocol were developed in advance. Additionally, this study is the up-to-date systematic review and meta-analysis on this topic and represents the available evidence. Nevertheless, some limitations should be acknowledged. First, this study analyzed aggregated data rather than individual patient data. Second, the majority of the included studies are retrospective, which increases the risk of bias. Future research should prioritize prospective study designs, especially randomized controlled trials, to confirm our conclusions with a higher level of evidence. Third, although stable results were shown in subgroup analyses stratified by treatment strategy, there was a greater heterogeneity in the treatment strategies of patients with different tumors, which could have some potential impact on the study results. Fourth, lymphocyte and platelet counts are non-specific parameters and may be affected by factors such as infection and inflammation (13). Despite most of the included studies have tried to control for these factors, the confounding effects of concurrent inflammatory conditions cannot be completely excluded. Finally, cutoff values for HALP were measured in different ways, and although we did not find a difference between the method of measurement and OS in our subgroup analysis, it is important to establish the optimal HALP cutoff value.
Conclusions
This study found that an elevated HALP was correlated with better survival in patients with solid tumors, and HALP could be used as a cost-effective prognostic biomarker. The prognostic model based on HALP deserves further investigation.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Author contributions
JL: Data curation, Methodology, Resources, Software, Writing – original draft. JZ: Data curation, Formal analysis, Methodology, Software, Writing – original draft. PW: Formal analysis, Methodology, Validation, Writing – review & editing. DL: Conceptualization, Formal analysis, Investigation, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2024.1483855/full#supplementary-material
References
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2021) 71:209–49. doi: 10.3322/caac.21660
2. Jung JH, Hwang J, Kim JH, Sim DY, Im E, Park JE, et al. Phyotochemical candidates repurposing for cancer therapy and their molecular mechanisms. Semin Cancer Biol. (2021) 68:164–74. doi: 10.1016/j.semcancer.2019.12.009
3. Jackson SE, Chester JD. Personalised cancer medicine. Int J Cancer. (2015) 137:262–6. doi: 10.1002/ijc.v137.2
4. Ekser B, Veroux M. New prognostic indicators in surgery. Int J Surg. (2019) 68:176–7. doi: 10.1016/j.ijsu.2019.07.015
5. Colotta F, Allavena P, Sica A, Garlanda C, Mantovani A. Cancer-related inflammation, the seventh hallmark of cancer: links to genetic instability. Carcinogenesis. (2009) 30:1073–81. doi: 10.1093/carcin/bgp127
6. Wiseman MJ. Nutrition and cancer: prevention and survival. Br J Nutr. (2019) 122:481–7. doi: 10.1017/S0007114518002222
7. Gupta D, Lis CG. Pretreatment serum albumin as a predictor of cancer survival: a systematic review of the epidemiological literature. Nutr J. (2010) 9:69. doi: 10.1186/1475-2891-9-69
8. Littlewood TJ. The impact of hemoglobin levels on treatment outcomes in patients with cancer. Semin Oncol. (2001) 28:49–53. doi: 10.1016/S0093-7754(01)90213-1
9. Wang S, Sun J, Chen K, Ma P, Lei Q, Xing S, et al. Perspectives of tumor-infiltrating lymphocyte treatment in solid tumors. BMC Med. (2021) 19:140. doi: 10.1186/s12916-021-02006-4
10. Haemmerle M, Stone RL, Menter DG, Afshar-Kharghan V, Sood AK. The platelet lifeline to cancer: challenges and opportunities. Cancer Cell. (2018) 33:965–83. doi: 10.1016/j.ccell.2018.03.002
11. Peng D, Zhang CJ, Tang Q, Zhang L, Yang KW, Yu XT, et al. Prognostic significance of the combination of preoperative hemoglobin and albumin levels and lymphocyte and platelet counts (HALP) in patients with renal cell carcinoma after nephrectomy. BMC Urol. (2018) 18:20. doi: 10.1186/s12894-018-0333-8
12. Li B, Zhou P, Liu Y, Wei H, Yang X, Chen T, et al. Platelet-to-lymphocyte ratio in advanced Cancer: Review and meta-analysis. Clin Chim Acta. (2018) 483:48–56. doi: 10.1016/j.cca.2018.04.023
13. Templeton AJ, McNamara MG, Šeruga B, Vera-Badillo FE, Aneja P, Ocaña A, et al. Prognostic role of neutrophil-to-lymphocyte ratio in solid tumors: a systematic review and meta-analysis. J Natl Cancer Inst. (2014) 106:dju124. doi: 10.1093/jnci/dju124
14. Yan L, Nakamura T, Casadei-Gardini A, Bruixola G, Huang YL, Hu ZD, et al. Long-term and short-term prognostic value of the prognostic nutritional index in cancer: a narrative review. Ann Transl Med. (2021) 9:1630. doi: 10.21037/atm-21-4528
15. Chen XL, Xue L, Wang W, Chen HN, Zhang WH, Liu K, et al. Prognostic significance of the combination of preoperative hemoglobin, albumin, lymphocyte and platelet in patients with gastric carcinoma: a retrospective cohort study. Oncotarget. (2015) 6:41370–82. doi: 10.18632/oncotarget.v6i38
16. Yang N, Han X, Yu J, Shu W, Qiu F, Han J, et al. Hemoglobin, albumin, lymphocyte, and platelet score and neutrophil-to-lymphocyte ratio are novel significant prognostic factors for patients with small-cell lung cancer undergoing chemotherapy. J Cancer Res Ther. (2020) 16:1134–9. doi: 10.4103/jcrt.JCRT_1066_19
17. Dagmura H, Daldal E, Okan I. The efficacy of hemoglobin, albumin, lymphocytes, and platelets as a prognostic marker for survival in octogenarians and nonagenarians undergoing colorectal cancer surgery. Cancer Biother Radiopharm. (2021) 37:955–62. doi: 10.1089/cbr.2020.4725
18. Zhai B, Chen J, Wu J, Yang L, Guo X, Shao J, et al. Predictive value of the hemoglobin, albumin, lymphocyte, and platelet (HALP) score and lymphocyte-to-monocyte ratio (LMR) in patients with non-small cell lung cancer after radical lung cancer surgery. Ann Transl Med. (2021) 9:976. doi: 10.21037/atm-21-2120
19. Peng D, Zhang CJ, Gong YQ, Hao H, Guan B, Li XS, et al. Prognostic significance of HALP (hemoglobin, albumin, lymphocyte and platelet) in patients with bladder cancer after radical cystectomy. Sci Rep. (2018) 8:794. doi: 10.1038/s41598-018-19146-y
20. Cong L, Hu L. The value of the combination of hemoglobin, albumin, lymphocyte and platelet in predicting platinum-based chemoradiotherapy response in male patients with esophageal squamous cell carcinoma. Int Immunopharmacol. (2017) 46:75–9. doi: 10.1016/j.intimp.2017.02.027
21. Sun L, Guan A, Jin Y, Liu M, Xiao Y, Xu H, et al. Comparison of prognostic value of red cell-related parameters of biliary tract cancer after surgical resection and integration of a prognostic nomogram: A retrospective study. Adv Ther. (2021) 38:1227–44. doi: 10.1007/s12325-020-01595-5
22. Kurashina R, Ando K, Inoue M, Izumi K, Maruyama R, Mitani K, et al. Platelet-to-lymphocyte ratio predicts the efficacy of pembrolizumab in patients with urothelial carcinoma. Anticancer Res. (2022) 42:1131–6. doi: 10.21873/anticanres.15576
23. Takenaka Y, Oya R, Takemoto N, Inohara H. Predictive impact of sarcopenia in solid cancers treated with immune checkpoint inhibitors: a meta-analysis. J Cachexia Sarcopenia Muscle. (2021) 12:1122–35. doi: 10.1002/jcsm.12755
24. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Int J Surg. (2021) 88:105906. doi: 10.1016/j.ijsu.2021.105906
25. Shea BJ, Reeves BC, Wells G, Thuku M, Hamel C, Moran , et al. AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. Bmj. (2017) 358:j4008. doi: 10.1136/bmj.j4008
26. Hayden JA, van der Windt DA, Cartwright JL, Côté P, Bombardier C. Assessing bias in studies of prognostic factors. Ann Intern Med. (2013) 158:280–6. doi: 10.7326/0003-4819-158-4-201302190-00009
27. Jiang H, Li H, Li A, Tang E, Xu D, Chen Y, et al. Preoperative combined hemoglobin, albumin, lymphocyte and platelet levels predict survival in patients with locally advanced colorectal cancer. Oncotarget. (2016) 7:72076–83. doi: 10.18632/oncotarget.12271
28. Guo Y, Shi D, Zhang J, Mao S, Wang L, Zhang W, et al. The hemoglobin, albumin, lymphocyte, and platelet (HALP) score is a novel significant prognostic factor for patients with metastatic prostate cancer undergoing cytoreductive radical prostatectomy. J Cancer. (2019) 10:81–91. doi: 10.7150/jca.27210
29. Shen XB, Zhang YX, Wang W, Pan YY. The hemoglobin, albumin, lymphocyte, and platelet (HALP) score in patients with small cell lung cancer before first-line treatment with etoposide and progression-free survival. Med Sci Monit. (2019) 25:5630–9. doi: 10.12659/MSM.917968
30. Xu SS, Li S, Xu HX, Li H, Wu CT, Wang WQ, et al. Haemoglobin, albumin, lymphocyte and platelet predicts postoperative survival in pancreatic cancer. World J Gastroenterol. (2020) 26:828–38. doi: 10.3748/wjg.v26.i8.828
31. Arikan TB, Sozuer E, Topal U, Lale A, Yilmaz AZ. The value and prognostic significance of preoperative hemoglobin and albumin levels, and the lymphocyte and platelet count (halp) scores in predicting pancreatic fistula in patients undergoing pancreaticoduodenectomy due to periampullary region tumors. Acta Med Mediterr. (2021) 37:1341–6. doi: 10.19193/0393-6384_2021_2_206
32. Feng JF, Wang L, Yang X. The preoperative hemoglobin, albumin, lymphocyte and platelet (HALP) score is a useful predictor in patients with resectable esophageal squamous cell carcinoma. Bosn J Basic Med Sci. (2021) 21:773–81. doi: 10.17305/bjbms.2021.5666
33. Gao X, Lin B, Lin Q, Ye T, Zhou T, Hu M, et al. A HALP score-based prediction model for survival of patients with the upper tract urothelial carcinoma undergoing radical nephroureterectomy. Bosn J Basic Med Sci. (2022) 22:280–90. doi: 10.17305/bjbms.2021.6543
34. Hu SJ, Zhao XK, Song X, Lei LL, Han WL, Xu RH, et al. Preoperative maximal voluntary ventilation, hemoglobin, albumin, lymphocytes and platelets predict postoperative survival in esophageal squamous cell carcinoma. World J Gastroenterol. (2021) 27:321–35. doi: 10.3748/wjg.v27.i4.321
35. Topal U, Dal F, Sozuer E, Akyuz M, Talih T, İslam D, et al. Combination of preoperative haemoglobin and albumin levels and lymphocyte and platelet counts (HALP) in patients with oesophageal cancer. J Evol Med Dental Sci. (2021) 10:173–8. doi: 10.14260/jemds/2021/38
36. Yalav O, Topal U, Unal AG, Eray IC. Prognostic significance of preoperative hemoglobin and albumin levels and lymphocyte and platelet counts (HALP) in patients undergoing curative resection for colorectal cancer. Ann Ital Chir. (2021) 92:283–92.
37. Ekinci F, Balcik OY, Oktay E, Erdogan AP. HALP score as a new prognostic index in metastatic renal cell cancer. J Coll Phys Surg Pak. (2022) 32:313–8.
38. Jiang P, Kong W, Gong C, Chen Y, Li F, Xu L. Predicting the recurrence of operable cervical cancer patients based on hemoglobin, albumin, lymphocyte, and platelet (HALP) score and classical clinicopathological parameters. J Inflammation Res. (2022) 15:5265–81. doi: 10.2147/JIR.S383742
39. Güç ZG, Alacacıoğlu A, Kalender ME, Oflazoğlu U, Ünal S, Yıildız Y, et al. HALP score and GNRI: Simple and easily accessible indexes for predicting prognosis in advanced stage NSCLC patients. The İzmir oncology group (IZOG) study. Front Nutr. (2022) 9:905292. doi: 10.3389/fnut.2022.905292
40. Leetanaporn K, Hanprasertpong J. Predictive value of the hemoglobin-albumin-lymphocyte-platelet (HALP) index on the oncological outcomes of locally advanced cervical cancer patients. Cancer Manag Res. (2022) 14:1961–72. doi: 10.2147/CMAR.S365612
41. Matsui Y, Matsuda A, Maejima A, Shinoda Y, Nakamura E, Komiyama M, et al. The clinical significance of perioperative inflammatory index as a prognostic factor for patients with retroperitoneal soft tissue sarcoma. Int J Clin Oncol. (2022) 27:1093–100. doi: 10.1007/s10147-022-02150-8
42. Njoku K, Barr CE, Ramchander NC, Crosbie EJ. Impact of pre-treatment prognostic nutritional index and the haemoglobin, albumin, lymphocyte and platelet (HALP) score on endometrial cancer survival: A prospective database analysis. PloS One. (2022) 17:e0272232. doi: 10.1371/journal.pone.0272232
43. Calderillo Ruiz G, Lopez Basave H, Vazquez Renteria RS, Castillo Morales A, Guijosa A, Castillo Morales C, et al. The prognostic significance of HALP index for colon cancer patients in a Hispanic-based population. J Oncol. (2022) 2022:4324635. doi: 10.1155/2022/4324635
44. Vlatka P, Marko L, Stefan M, Dorian L. The hemoglobin, albumin, lymphocyte, and platelet (HALP) score is a novel prognostic factor for patients with diffuse large B-cell lymphoma. J Cancer Res Ther. (2022) 18:725–32. doi: 10.4103/jcrt.jcrt_174_21
45. Wei S, Shao J, Wang J, Wang G. The preoperative hemoglobin, albumin, lymphocyte, and platelet score is a prognostic factor for non-small cell lung cancer patients undergoing adjuvant chemotherapy: a retrospective study. Ann Transl Med. (2022) 10:457. doi: 10.21037/atm-22-1097
46. Wu CY, Lin YH, Lo WC, Cheng PC, Hsu WL, Chen YC, et al. Nutritional status at diagnosis is prognostic for pharyngeal cancer patients: a retrospective study. Eur Arch Otorhinolaryngol. (2022) 279:3671–8. doi: 10.1007/s00405-021-07222-5
47. Zhao Z, Yin XN, Wang J, Chen X, Cai ZL, Zhang B, et al. Prognostic significance of hemoglobin, albumin, lymphocyte, platelet in gastrointestinal stromal tumors: A propensity matched retrospective cohort study. World J Gastroenterol. (2022) 28:3476–87. doi: 10.3748/wjg.v28.i27.3476
48. Zhang D, Zeng H, Pan Y, Zhao Y, Wang X, Chen J, et al. Liver tumor markers, HALP score, and NLR: simple, cost-effective, easily accessible indexes for predicting prognosis in ICC patients after surgery. J Pers Med. (2022) 12:2041. doi: 10.3390/jpm12122041
49. Fang KH, Lai CH, Hsu CM, Liao CT, Kang CJ, Lee YC, et al. Prognostic impact of the hemoglobin-albumin-lymphocyte-platelet score in patients with oral cavity cancer undergoing surgery. Head Neck. (2023) 45:1558–71. doi: 10.1002/hed.27372
50. Toshida K, Itoh S, Kayashima H, Nagao Y, Yoshiya S, Tomino T, et al. The hemoglobin, albumin, lymphocyte, and platelet score is a prognostic factor for Child-Pugh A patients undergoing curative hepatic resection for single and small hepatocellular carcinoma. Hepatol Res. (2023) 53:522–30. doi: 10.1111/hepr.13885
51. Mazzella A, Maiolino E, Maisonneuve P, Loi M, Alifano M. Systemic inflammation and lung cancer: is it a real paradigm? Prognostic value of inflammatory indexes in patients with resected non-small-cell lung cancer. Cancers (Basel). (2023) 15:1854. doi: 10.3390/cancers15061854
52. Nishio S, Murotani K, Yamagami W, Suzuki S, Nakai H, Kato K, et al. Pretreatment systemic inflammatory markers predict survival in endometrial cancer: A Japanese Gynecologic Oncology Group 2043 exploratory data analysis. Gynecol Oncol. (2024) 181:46–53. doi: 10.1016/j.ygyno.2023.12.007
53. Shi Y, Shen G, Zeng Y, Ju M, Chen X, He C, et al. Predictive values of the hemoglobin, albumin, lymphocyte and platelet score (HALP) and the modified -Gustave Roussy immune score for esophageal squamous cell carcinoma patients undergoing concurrent chemoradiotherapy. Int Immunopharmacol. (2023) 123:110773. doi: 10.1016/j.intimp.2023.110773
54. Zhao B, Guo H, Wu W, Duan G. Hemoglobin, albumin, lymphocyte and platelet (HALP) score can predict the prognosis of patients with non-small cell lung cancer (NSCLC). Asian J Surg. (2023) 46:4891–2. doi: 10.1016/j.asjsur.2023.05.152
55. Zhao R, Liang Z, Chen K, Zhu X. Nomogram based on hemoglobin, albumin, lymphocyte and platelet score to predict overall survival in patients with T3-4N0-1 nasopharyngeal carcinoma. J Inflammation Res. (2023) 16:1995–2006. doi: 10.2147/JIR.S411194
56. Zhao Z, Xu L. Prognostic significance of HALP score and combination of peripheral blood multiple indicators in patients with early breast cancer. Front Oncol. (2023) 13:1253895. doi: 10.3389/fonc.2023.1253895
57. Zhou J, Yang D. Prognostic significance of hemoglobin, albumin, lymphocyte and platelet (HALP) score in hepatocellular carcinoma. J Hepatocell Carcinoma. (2023) 10:821–31. doi: 10.2147/JHC.S411521
58. Huang G, Zhang H, Yang Z, Li Q, Yuan H, Chen P, et al. Predictive value of HTS grade in patients with intrahepatic cholangiocarcinoma undergoing radical resection: a multicenter study from China. World J Surg Oncol. (2024) 22:17. doi: 10.1186/s12957-023-03281-6
59. Mutlu E, Inanc M. Prognostic significance of inflammation scores in Malignant mesothelioma. Eur Rev Med Pharmacol Sci. (2024) 28:2340–50. doi: 10.26355/eurrev_202403_35741
60. Bray F, Laversanne M, Weiderpass E, Soerjomataram I. The ever-increasing importance of cancer as a leading cause of premature death worldwide. Cancer. (2021) 127:3029–30. doi: 10.1002/cncr.v127.16
61. Xu H, Zheng X, Ai J, Yang L. Hemoglobin, albumin, lymphocyte, and platelet (HALP) score and cancer prognosis: A systematic review and meta-analysis of 13,110 patients. Int Immunopharmacol. (2023) 114:109496. doi: 10.1016/j.intimp.2022.109496
62. McCranor BJ, Kim MJ, Cruz NM, Xue QL, Berger AE, Walston JD, et al. Interleukin-6 directly impairs the erythroid development of human TF-1 erythroleukemic cells. Blood Cells Mol Dis. (2014) 52:126–33. doi: 10.1016/j.bcmd.2013.09.004
63. Ferrucci L, Semba RD, Guralnik JM, Ershler WB, Bandinelli S, Patel KV, et al. Proinflammatory state, hepcidin, and anemia in older persons. Blood. (2010) 115:3810–6. doi: 10.1182/blood-2009-02-201087
64. Cella D, Kallich J, McDermott A, Xu X. The longitudinal relationship of hemoglobin, fatigue and quality of life in anemic cancer patients: results from five randomized clinical trials. Ann Oncol. (2004) 15:979–86. doi: 10.1093/annonc/mdh235
65. Knight K, Wade S, Balducci L. Prevalence and outcomes of anemia in cancer: a systematic review of the literature. Am J Med. (2004) 116 Suppl 7A:11s–26s. doi: 10.1016/j.amjmed.2003.12.008
66. Yeun JY, Kaysen GA. Factors influencing serum albumin in dialysis patients. Am J Kidney Dis. (1998) 32:S118–125. doi: 10.1016/S0272-6386(98)70174-X
67. Pfensig C, Dominik A, Borufka L, Hinz M, Stange J, Eggert M, et al. A new application for albumin dialysis in extracorporeal organ support: characterization of a putative interaction between human albumin and proinflammatory cytokines IL-6 and TNFα. Artif Organs. (2016) 40:397–402. doi: 10.1111/aor.2016.40.issue-4
68. McMillan DC, Watson WS, O’Gorman P, Preston T, Scott HR, McArdle CS, et al. Albumin concentrations are primarily determined by the body cell mass and the systemic inflammatory response in cancer patients with weight loss. Nutr Cancer. (2001) 39:210–3. doi: 10.1207/S15327914nc392_8
69. McCarthy ET, Sharma R, Sharma M, Li JZ, Ge XL, Dileepan KN, et al. TNF-alpha increases albumin permeability of isolated rat glomeruli through the generation of superoxide. J Am Soc Nephrol. (1998) 9:433–8. doi: 10.1681/ASN.V93433
70. Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. (2008) 454:436–44. doi: 10.1038/nature07205
71. Greten FR, Grivennikov SI. Inflammation and cancer: triggers, mechanisms, and consequences. Immunity. (2019) 51:27–41. doi: 10.1016/j.immuni.2019.06.025
72. Buergy D, Wenz F, Groden C, Brockmann MA. Tumor-platelet interaction in solid tumors. Int J Cancer. (2012) 130:2747–60. doi: 10.1002/ijc.v130.12
73. Wojtukiewicz MZ, Sierko E, Hempel D, Tucker SC, Honn KV. Platelets and cancer angiogenesis nexus. Cancer Metastasis Rev. (2017) 36:249–62. doi: 10.1007/s10555-017-9673-1
74. Schlesinger M. Role of platelets and platelet receptors in cancer metastasis. J Hematol Oncol. (2018) 11:125. doi: 10.1186/s13045-018-0669-2
75. Bendari A, Bendari A, Zhong X, Geetha SD, Al-Refai R, Sham S, et al. Hemoglobin-albumin-lymphocyte-platelet (HALP) score as a predictive value of incidental prostate cancer for patients going for transurethral resection of the prostate (TURP): A single-center study. Cureus. (2024) 16:e57736. doi: 10.7759/cureus.57736
Keywords: solid tumors, HALP, biological marker, prognosis, survival
Citation: Li J, Zheng J, Wang P and Lv D (2024) Prognostic significance of hemoglobin, albumin, lymphocyte and platelet score in solid tumors: a pooled study. Front. Immunol. 15:1483855. doi: 10.3389/fimmu.2024.1483855
Received: 20 August 2024; Accepted: 15 November 2024;
Published: 18 December 2024.
Edited by:
Pengpeng Zhang, Nanjing Medical University, ChinaReviewed by:
Adolfo Martinez, General Hospital of Mexico, MexicoJianping Xiong, Peking University Third Hospital, China
Bicheng Ye, Yangzhou Polytechnic Institute, China
Copyright © 2024 Li, Zheng, Wang and Lv. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jinze Li, RHJfbGlqaW56ZUAxNjMuY29t; Dong Lv, TFY4MDA5MTlAMTYzLmNvbQ==
†These authors have contributed equally to this work
 Jinze Li
Jinze Li Jing Zheng2†
Jing Zheng2† Puze Wang
Puze Wang