- 1College of Traditional Chinese Medicine, Shandong University of Traditional Chinese Medicine, Jinan, Shandong, China
- 2The First Clinical Medical College, Shandong University of Traditional Chinese Medicine, Jinan, Shandong, China
- 3School of Nursing, Shandong University of Traditional Chinese Medicine, Jinan, Shandong, China
- 4Shandong Co-Innovation Center of Classic Traditional Chinese Medicine (TCM) Formula, Shandong University of Traditional Chinese Medicine, Jinan, Shandong, China
Immunotherapy has brought hope to many breast cancer patients, but not all patients benefit from it. Quercetin (Qu), a natural product found in various sources, has anti-inflammatory and anti-tumor properties. We conducted a review of the pharmacological research of Qu in regulating anti-tumor immunity in vivo and in vitro. Qu can directly regulate the local tumor microenvironment (TME) by enhancing the activity of immune cells which includes promoting the infiltration of T cells and natural killer (NK) cells, inhibiting the recruitment of myeloid-derived suppressor cells and tumor-associated macrophages. Additionally, Qu inhibits anaerobic glycolysis in tumor cells, thereby reducing the production and transport of lactic acid. It also suppresses tumor angiogenesis by targeting the vascular endothelial growth factor (VEGF) pathway and the vitamin D pathway. Furthermore, Qu can enhance the efficacy of immunotherapy for breast cancer by modulating the systemic microenvironment. This includes inhibiting obesity-related chronic inflammation to decrease the production of inflammatory factors, regulating the composition of intestinal microbiota, and intervening in the metabolism of intestinal flora. At the same time, we also address challenges in the clinical application of Qu, such as low absorption rates and unknown effective doses. In conclusion, we highlight Qu as a natural immunomodulator that enhances immune cell activity and has the potential to be developed as an adjunct for breast cancer.
1 Background
In 2020, breast cancer has surpassed lung cancer to become the most common malignant tumor in the world, and it ranks the fifth in the total number of cancer deaths (1).With the continuous development of treatment for breast cancer, especially the emergence of immunotherapy, the survival time of patients has been greatly prolonged. However, not all patients can benefit from immunotherapy (2), one of the main reasons is the immunosuppressive tumor microenvironments (TME).
There are many studies have shown that natural products have a regulatory effect on the tumor immune microenvironment (3), which can well improve the efficiency of immunotherapy. Quercetin (Qu) is a flavonoid from a wide range of sources, mainly derived from vegetables, coffee, tea and other plants, which has the effect of anti-tumor, anti-inflammatory, antioxidant (4). In addition to treating metabolic related diseases, Qu can directly act on breast cancer cells to promote the apoptosis by regulating the oxidative stress, ferroptosis, fat metabolism, aromatase promoter activation and miRNA (5–9). The study for Qu directly acting on tumor cells has been fully summarized and reviewed in previous reviews (10, 11) (Table 1). However, in the era of immunotherapy, it is still lacking to summarize the mechanism of Qu to treat breast cancer from the perspective of regulating the immune microenvironment. In order to improve the efficiency of immunotherapy for breast cancer and promote the clinical application of Qu as immunotherapy adjuvant, it is necessary to comprehensively summarize the mechanism of Qu regulating the immune microenvironment of breast cancer. Therefore, this paper reviews the mechanism of Qu regulating immune microenvironment. The articles we reviewed were characterized by clear dosing intervals, reasonable control group design, and accurate index observation, excluding unverified dummy studies and other studies that did not meet the criteria. In addition, we also discussed the future research directions and the challenges in the clinical application of Qu as an adjuvant for breast cancer immunotherapy.
2 Qu regulates the local TME
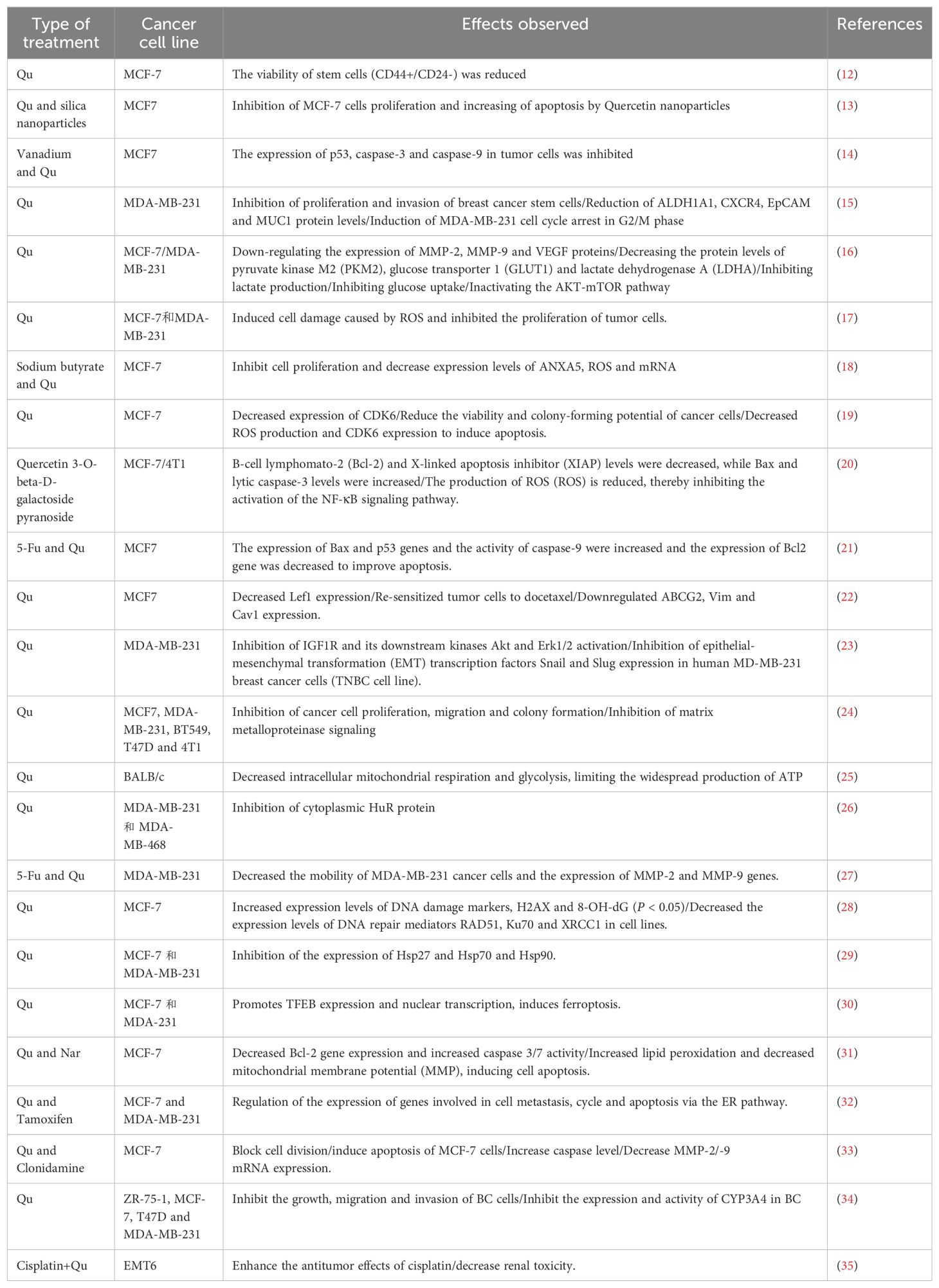
Due to the unique biological characteristics of tumor cells, the TME has the characteristics of acid-stage, hypoxia, ROS accumulation and so on, which ultimately leads to the immunosuppressive microenvironment. As a natural regulator, Qu can directly enhance the activity of effector T cells and NK cells (Table 2). Additionally, Qu inhibits the production of lactic acid by tumor cells through anaerobic glycolysis and reduces the expression of lactate transporters on the surface of tumor cells, thereby decreasing lactic acid accumulation in the TME. Qu also inhibits tumor angiogenesis not only via the VEGF pathway but also by downregulating vitamin D receptor expression and alleviating hypoxia within the TME. Finally, Qu can inhibit the accumulation of ROS, thereby mitigating their suppressive effects on anti-tumor immune cell activity (Figure 1).
2.1 Qu regulates the acidic TME
2.1.1 Lactic acid production and immunosuppression
Lactic acid acts as the end product of glycolysis in tumor cells for energy production and promotes angiogenesis and immune evasion (44). Lactic acid produced by glycolytic activity is associated with poor prognosis with triple-negative breast cancer (TNBC) patients (45). Inhibiting the glycolytic process of tumor cells and reducing the content of lactic acid in TME can inhibit the activity of Myeloid-derived suppressor cells (MDSCs), promote the killing function of effector T cells (45) and enhance the anti-tumor immunity.
2.1.2 Qu regulates the production and transport of lactic acid
Many studies have demonstrated that Qu can inhibit the glycolytic process in breast cancer cells. Compared with the control group, 30μM Qu significantly inhibited glucose uptake and lactate production in MCF-1/MDA-MB-231 cells, and decreased the levels of glycolytic-associated pyruvate kinase M2 (PKM2), glucose transporter 1 (GLUT1), and lactate dehydrogenase A (LDHA). Qu can inhibit tumor growth and metastasis by inhibiting the expression of VEGF and PKM2 in vivo (16). In addition to inhibiting glycolysis, Qu also suppresses lactate transport in breast cancer cells. Monocarboxylate transporter (MCT) is associated with extracellular acidification and is highly expressed in MDA-MB-468 and Hs578T cells, but hardly expressed in MCF-7/AZ cells. It was observed that Qu only inhibited glucose consumption and lactic acid production in MDA-MB-468 and Hs578T cells, but had no effect on the metabolic behavior of MCF-7/AZ cells. This suggests that Qu inhibited lactic acid transport in breast cancer cells, and its effect was related to MCT1 (46).
2.2 Qu regulates angiogenesis
2.2.1 Angiogenesis and immunosuppression
In TME, due to the over-expression of angiogenic factors in tumors, the tumor-associated vasculature is over-branched and disorganized, resulting in the reduction of oxygen content of TME and the promotion of immunosuppressive cell infiltration (47). Tumor-related vascular hyperplasia can further hinder the infiltration of effector T cells and promote T cell apoptosis (48).
2.2.2 Qu suppresses tumor angiogenesis
Qu can directly inhibit tumor angiogenesis by acting on VEGF. MCF-7 cells were injected into the breast fat of female BALB/c nude mice and treated with Qu (34 mg/kg) for 21 days. The results showed that Qu decreased serum VEGF expression (P < 0.01) and microvascular density (P < 0.05). Qu treatment significantly inhibited tumor calcineurin activity (inhibition rate: 62.73%) and expression of nuclear factor of activated T cells (NFAT), a pathway critical in human breast cancer angiogenesis (49). Treatment of tamoxifen-resistant MCF-7 cells with six different flavonoids showed that treatment with 30μM Qu for 2 weeks was able to stably inhibit angiogenesis in breast cancer by inhibiting the expression of hypoxia-inducing factor-1α (HIF-1α) and activating protein-1 (AP-1), which were key transcription factors for VEGF gene transcription (50).
In addition to the VEGF pathways, Qu may also inhibit tumor angiogenesis through other mechanisms. Overexpression of the VDR can inhibit tumor angiogenesis (51). In vivo experiments using a mouse model of Ehrlich ascites cancer with Qu (50 mg/kg) intraperitoneal administration for 15 days showed significant inhibition of peritoneal neovascularization compared to the control group. Further in vitro experiments using human breast cancer cell lines (MDA-MB-231 and BT-474) demonstrated that Qu activates VDR to inhibit angiogenesis (52). Prostaglandin E2 (PGE2) binds to its receptor EP2 to directly promote tumor angiogenesis by enhancing endothelial cell survival and motility (53), while Qu significantly inhibits PGE2 production in breast cancer cells. In addition, Qu inhibited COX-2 promoter activation of MDA-MB-231 and MCF-231 breast cancer cell lines in a dose-dependent manner at 0-300μM and 0-200μM, and inhibited COX-2-mediated angiogenesis (54).
2.3 Qu regulates reactive oxygen species
Since cancer cells are under oxidative stress for a long time, they produce a large amount of ROS. ROS is a key driver of tumorigenesis, progression, metastasis and drug resistance. However, as the research progressed, the complex and diverse roles of ROS in TME have been gradually discovered (55).
2.3.1 The dual role of ROS in anti-tumor immunity
In anti-tumor immunity, ROS is a double-edged sword. ROS can activate T cells and NK cells to kill cancer cells, and activated T cells and NK cells can increase the production of ROS, further promoting the recruitment and activation of both (56). On the other hand, elevated ROS can not only promote the infiltration and activation of immunosuppressor cells and inhibit the body’s anti-tumor immunity, including MDSCs, TAMs, and regulatory T cells (Tregs), but also inhibits T cell response by inhibiting the formation of TCR and MHC antigen complexes (57). Excessive ROS in T cells can reduce the levels of TCRζ chain and CD16ζ chain, block the activation of NF-kB, and lead to insufficient production of IFN-γ, TNF-α and IL-2 (58), thus inhibiting the activity of T cells. This contradictory effect of ROS may be related to the levels and different cell locations of ROS in TME. Continuous oxidative stress allows cancer cells to survive in high levels of ROS while maintaining cell viability, but can severely impair the activity of anti-tumor immune cells (59). The use of ROS as a therapeutic target for anti-tumor immunity largely depends on ROS levels and the tolerance of different cells to ROS. Targeting ROS in TME can be used as a therapeutic strategy to improve effector T cell function and enhance anti-tumor immunity (60).
2.3.2 Qu regulates ROS production
Although there have been some drugs in clinical experiments confirmed that the antioxidant treatment could increase the effect of breast cancer immunotherapy (61). However, the selected drugs are still few and have some side effects.
Qu exhibits antioxidant properties, which can directly enhance the accumulation of ROS in tumor cells and induce their apoptosis (10). Treating MCF-7 cell lines with 25 μmol/mL Qu or Qu solid lipid nanoparticles (QU-SLN) for 48 hours, QU-SLNs significantly increased the level of ROS in MCF-7 cells and decreased the activity of antioxidant enzymes. Qu also increased the apoptosis and necrosis, but QT-SLNs showed more significant effects (62). It was found that after being treated with Qu and its water-soluble metabolites Qu 3’ -sulfate (Q3’s) and Qu 3-glucuronide (Q3G) at a dose of 25 µM for 48 hours, MCF-7 cell survival rates were 53.0% (P < 0.01), 55.6% (P < 0.01), and 65.1% (P < 0.05), significantly lower than the untreated group (100%). The cell survival rate decreased with the increase of drug concentration, and the inhibitory effect was dose-dependent, accompanied by the accumulation of ROS. The cytotoxic effects of Qu and Q3’S were similar to 5-fluorouracil at 100 µM (63).
Qu can directly reduce the ROS content in the TME (5) and alleviate the immunosuppression caused by excessive ROS levels. Compared with the ROS scavenger N-acetyl cysteine (NAC), Qu 3-o-β-D-galactoside pyranoside decreased ROS production in 4T1 cells with increasing concentration, functioning similarly to the NAC group. Both Qu 3-O-β-D-galactoside pyranoside and NAC treatment groups showed decreased IκBα phosphorylation and p65 expression levels. Qu 3-o-β-D-galactoside pyranoside inhibited the activation of the NF-κB signaling pathway by reducing intracellular ROS production (20), alleviating the immunosuppressive microenvironment. Sequential treatment of MDA-MB 231 cells, MDA-MB 468 cells, MCF-7 cells, and A549 cells with vitamin C and Qu reduced endogenous ROS production in a dose-dependent manner (P=0.027) and decreased ROS accumulation in the TME. The effective concentration of Qu ranges from 155.1 to 232.9μM (64).
3 Qu regulates the activity of immune cells
3.1 Qu regulates T cell activity
Qu can regulate the recruitment and functional activation of effector T cells. Qu increased CXCL1-2 expression in MDA-MB-231 and MCF-7 cells in a dose-dependent manner (P < 0.05 or P < 0.01) (20μM-80μM) (65). PD-L1 in TME can inhibit the function of T cells, and Qu activates T cells by inhibiting the binding of PD-L1/PD-1. The anti-PD-L1 antibody (200ng, Santa) was used as a control, while BSA (0.5μg/ml) served as a negative control. Qu (5μM) can inhibit PD-1/PD-L1 binding in HEK293 cells, and the inhibition rate reaching as high as 90%. In MDA-MB-231 and PBMCs xenograft mouse models, 60 mg/kg of Qu significantly inhibits the proliferation of tumor cells compared with the control group (0 mg/kg of Qu) and increases the protein levels of CD8, GZMB, and IFN-γ in tumor tissues (66). Gamma delta T cells (γδT cells) are a type of innate immune cell that plays an immunomodulatory role in the immune response to many infections and immune diseases (67). Qu concentration at concentrations of 2.5μM to 5μM can significantly promote the proliferation of γδ T cells in vitro. The killing ability of γδ T cells against breast cancer (MCF-10A > MCF-10AT > MCF-7 > MDA-MB-231) was increased after treatment with 5μM of Qu. When the ratio of γδ T cells to target cells (E/T) was 10:1, the killing effect was most pronounced, and the mechanism of Qu action was related to the JAK-STAT1 signaling pathway (38).
3.2 Qu regulates NK cell activity
In addition to effector T cells, Qu can influence the activity of NK cells. In a BALB/c mouse model constructed using 4T1 cells, the diet was supplemented with 1%, 2.5%, and 5% Qu, respectively, along with 50 mg/kg cyclophosphamide. The results showed that Qu combined with cyclophosphamide increased the activity of T cells and NK cells to a greater extent, and reduced the activity of Treg cells in the breast cancer microenvironment. Notably, 2.5% Qu had the most significant effect (68). This finding partially confirms the potential of Qu in regulating the chemotactic recruitment of immune cells. K562 and SNU1 cells were treated with 50 mM Qu for 30 hours, SNU-C4 cells with 20 mM Qu for 24 hours, and then the tumor cells were co-cultured with NK cells. The experimental results demonstrated that compared with tumor cells without Qu treatment, Qu increased the susceptibility of tumor cells to the toxic effects of NK cells. The cytotoxicity was reversed by the NKG2D receptor neutralizing antibody. Qu can effectively induce NKG2D ligands on the surface of tumor cells, thereby enhancing the tumor-killing effect of NK cells (69).
4 Qu regulates obesity-related chronic inflammation
Obesity is a complex chronic inflammatory disease that affects more than one third of the world’s population (70). Adipose tissue can induce chronic inflammation and negatively regulate the body’s immune surveillance function (71). Although drugs targeting obesity-related chronic inflammation have been widely used in the adjuvant treatment of obesity-related cancers, such as anti-inflammatory steroids and non-steroidal drugs, they often bring significant adverse effects in the course of long-term use, and sometimes have life-threatening consequences (72). Qu has strong anti-inflammatory activity and its side effects are relatively manageable, which has the potential to be developed as an adjuvant for immunotherapy against obesity-related cancers.
4.1 Obesity-related chronic inflammation inhibits anti-tumor immunity
In the UK, obesity elevates the risk of breast cancer among the postmenopausal population and results in a diminished response rate to anti-tumor therapies in breast cancer patients, ultimately contributing to a poorer prognosis (73). Additionally, obesity-related chronic inflammation and metabolic syndrome have been identified as independent prognostic factors that influence treatment response rates and the risk of mortality from breast cancer (74). Obesity-related chronic inflammation is systemic, but can reach the local TME through the circulatory system to exert immunosuppressive effects and suppress the effects of breast cancer immunotherapy (75), by inducing T cell exhaustion and immunosuppressive cell recruitment. In addition, chronic inflammation is frequently linked to tumor cachexia, which significantly impacts patients’ quality of life (76).
Obesity-related chronic inflammation can promote the infiltration of TAMs and CAFs and inhibit anti-tumor immune response. The crownlike structure (CLS), which is widely present in adipose tissue, is a structure in which macrophages clear dead adipose cells, but causes macrophages to be exposed to high levels of saturated fatty acids (77). Saturated fatty acids can activate TLR4 on the surface of macrophages, induce NF-κB signaling, and inhibit anti-tumor immunity (78). Obesity also increases the activity of NLRP3 in adipose tissue (79), which promotes the infiltration of MDSCs and TAMs, forms an immunosuppressive microenvironment (80). ASCs, as the main component of adipose tissue, can differentiate into cancer-associated fibroblasts (CAFs) (81, 82). In breast cancer, CAFs are able to suppress anti-tumor immunity through their metabolism and secretion of cytokines (83).
Obesity can lead to a decrease in both the number and diversity of T cells, as well as promote T cell exhaustion (84). In obese breast cancer patients, leptin in breast adipose tissue inhibits CD8+ T cell effector function by activating STAT3-fatty acid oxidation (FAO) and inhibiting glycolysis (85). Obesity also increases the concentration of CXCL1, promotes CXCR2 mediated Fas ligand (FasL) activation, and recruitment of MDSCs, leading to apoptosis of CD8+ T cells and decreased immunotherapy efficiency (86).
4.2 Qu treats obesity-related chronic inflammation
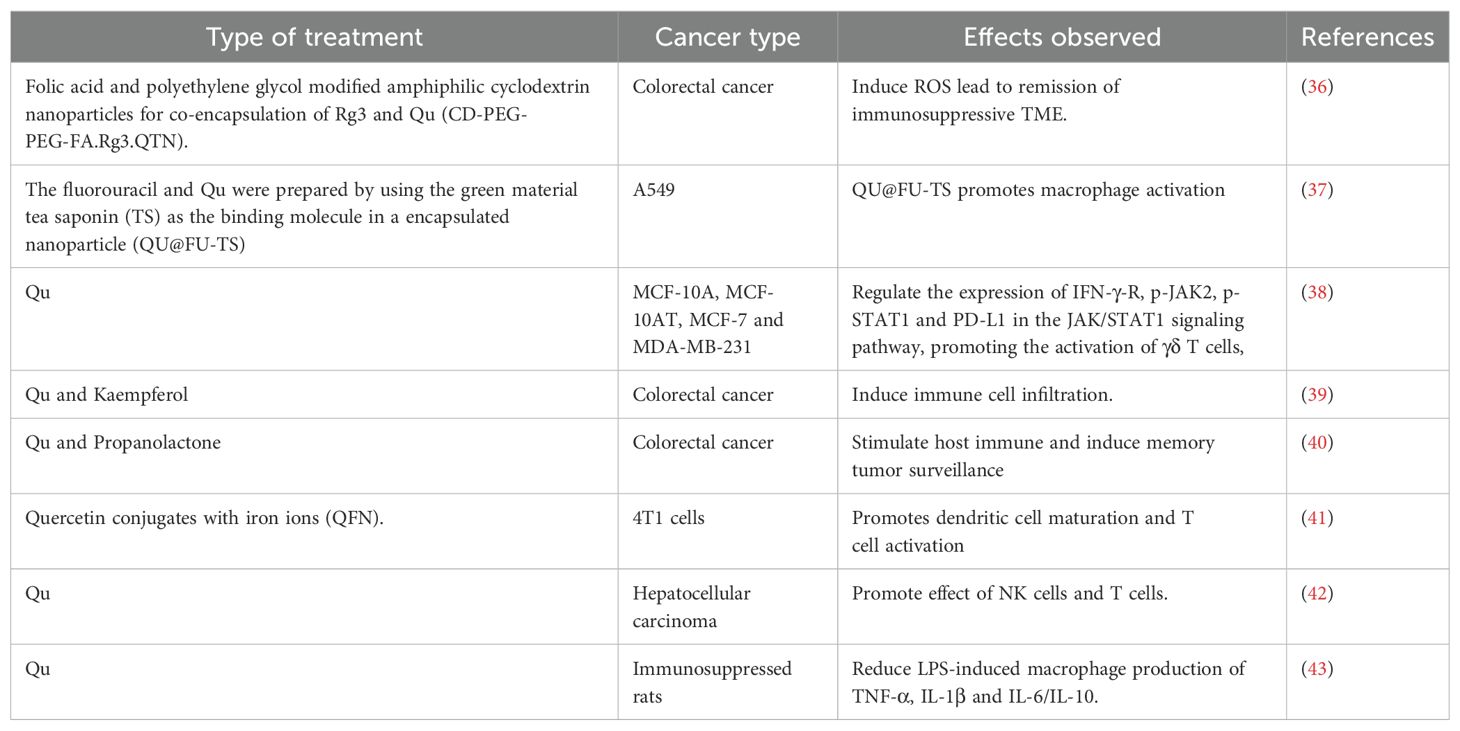
Qu improves the prognosis of breast cancer patients by inhibiting obesity-related chronic inflammation, alleviating obesity-related metabolic syndrome, and increasing treatment sensitivity (Figure 2). Treatment of adipose-derived macrophages with Qu (0, 3, 10, or 30 mM) for 5 hours inhibited the expression of inflammatory genes, such as tumor necrosis factor (TNF)-α, interleukin (IL)-6, IL-8, and IL-1β compared with untreated macrophages (87). Qu (10 mg/kg) can reverse the obesity caused by a high-calorie diet in vivo. Compared with the high-calorie diet groups, phentermine groups, and orlistat groups, Qu can significantly inhibit the expression of pro-inflammatory genes such as IL-6, IL-1β, IL-18, and TNF-α, thereby alleviating the inflammatory state caused by obesity (88). When fed 50 mg/kg, 100 mg/kg, and 200 mg/kg respectively, Qu inhibited serum IL-6 production in a dose-dependent manner compared with high-fat diet (HFD) alone, but there was no difference in the reduction of serum TNF-α between groups. Compared with the use of metformin, Qu (300 mg/kg), can assist metformin in reducing the levels of serum IL-6 and TNF-α, relieving the inflammatory response of obesity, and has good safety, with no obvious organ toxicity (89).
Obesity-related chronic inflammation can lead to the infiltration of inhibitory immune cells, promote T cell exhaustion, and diminish the effectiveness of breast cancer immunotherapy. Qu can directly influence the metabolism of adipose tissue and inhibit the development of obesity-related chronic inflammation by reducing the production of inflammatory factors and the recruitment of TAMs. This has positive implications for the clinical application of immunotherapy. Dietary Qu (17 mg/kg) for 9 weeks induced white fat remodeling and decreased IL-6 and adiponectin levels in adipose tissue in obese mice (90). Qu inhibits the infiltration of macrophages in adipose tissue to relieve chronic inflammation. After 12 weeks of supplementation with 0.1% Qu (Purity ≥98%) on the basis of HFD, Qu significantly reduced the infiltration of macrophages in adipose tissue compared with HFD alone. Qu increased the number of M2-type macrophages while decreasing the levels of pro-inflammatory cytokines TNF-α, IL-6, and MCP-1. Qu activates AMPKα1/SIRT1 signaling to inhibit the polarization and inflammation of bone marrow derived macrophages in vitro (91). In addition to feeding with a Western diet (high fat, high cholesterol, and high sucrose), 0.05% Qu was supplemented for 18 weeks. The results showed that Qu significantly inhibited the increase of serum TNF-α, leptin, and decreased the protein expression of NF-κB p65 in western diet mice. Immunohistochemistry showed that Qu inhibited Western diet-induced macrophage accumulation in adipose tissue (92).
In summary, the metabolism of adipose tissue frequently induces chronic inflammation, characterized by the accumulation of inflammatory factors and populations of white blood cells, which often inhibits the anti-tumor immune response. Tumor cells themselves also produce various chemokines to attract neutrophils, macrophages, myeloid-derived suppressor cells, and other immune cells. These cells, in turn, secrete a range of cytokines and cytotoxic mediators. Therefore, in cancer patients, regardless of obesity, chronic inflammation is prevalent and often adversely affects the response to tumor immunotherapy. Given the widespread nature of chronic inflammation in cancer patients, utilizing Qu as a routine clinical intervention to suppress chronic inflammation presents a promising treatment strategy. This approach may enhance the immunotherapy response rate and improve patient prognosis.
5 Qu regulates gut microbiota
The interaction between gut microbiota and the immune system can significantly affect the anti-tumor immune response. Therefore, the immuno-oncology-microbiome axis hypothesis was gradually formed to summarize the mechanism of gut microbiota in tumor pathogenesis and anti-tumor immunity (93). Qu can affect the anti-tumor immunity of the body by affecting the gut microbiota.
5.1 Gut microbiota regulate anti-tumor immunity
Many studies have demonstrated that the composition of intestinal flora significantly influences the effectiveness of breast cancer immunotherapy. Certain specific types of flora can even serve as biomarkers to predict the efficacy of this treatment. Qu has the ability to modulate the composition of intestinal flora, enhance its abundance, and increase the overall microbial diversity, thereby improving the effectiveness of immunotherapy. Additionally, the metabolites produced by these flora can impact the efficiency of immunotherapy by reshaping the immune microenvironment associated with breast cancer. Qu can also regulate the metabolism of intestinal flora, leading to an increased accumulation of beneficial metabolites, which in turn affects the efficacy of immunotherapy. Metabolites produced by gut microbiota get to local microenvironment of breast cancer through the circulatory system, and regulate key signaling pathways in cancer cells and various immune cells affecting the efficiency of anti-tumor immunotherapy (94). Butyrate, a metabolite of gut microbiota, improves the efficacy of anti-PD-1 immunotherapy by regulating TCR signaling in cytotoxic T cells (95). Intestinal B. pseudolongum enhances anti-tumor immunotherapy response by producing the inosine (96). Immunotherapy leads to a decrease in intestinal barrier function, and local inosine in the intestine is more likely to participate in the systemic circulation. Short chain fatty acids (SCFA) produced by Escherichia coli have cytotoxic effects on breast cancer cells and directly inhibit breast cancer cell proliferation (97). Gut microbiota and its metabolites can not only promote the immune response, but also inhibit the immune response of the body and promote the progression of tumors. In a C3-1-TAg mouse model of breast cancer, it has been observed that liver infection with Helicobacter hepaticus, a gut-resident bacterium, can induce breast cancer progression by promoting neutrophil recruitment and infiltration in TME, shaping an immunosuppressive microenvironment (98).
In addition to metabolites, extracellular polysaccharides or surface proteins in the structure of bacteria themselves can act as pathogen-associated molecular patterns (PAMPs), directly stimulating intestinal immune cells and inducing innate or adaptive immune responses to regulate anti-breast cancer immune responses. Exopolysaccharides produced by Lactobacillus delbrueckii subsp. bulgaricus OLL1073R-1 (EPS-R1) induce CCR6+CD8+T cells in mice and humans. In mice, ingestion of EPS-R1 enhanced the anti-tumor effects of anti-CTLA-4 or anti-PD-1 monoclonal antibodies against tumors expressing CCL20 (99). Immunostimulating exopolypolysaccharide (EPS) produced by Lactobacillus delbrueckii ssp. bulgaricus OLL1073R-1 enhanced activity of natural killer (NK) cell in mouse spleen cells and induced production of IFN-γ (100).
5.2 Qu regulates intestinal flora to promote anti-tumor immunity
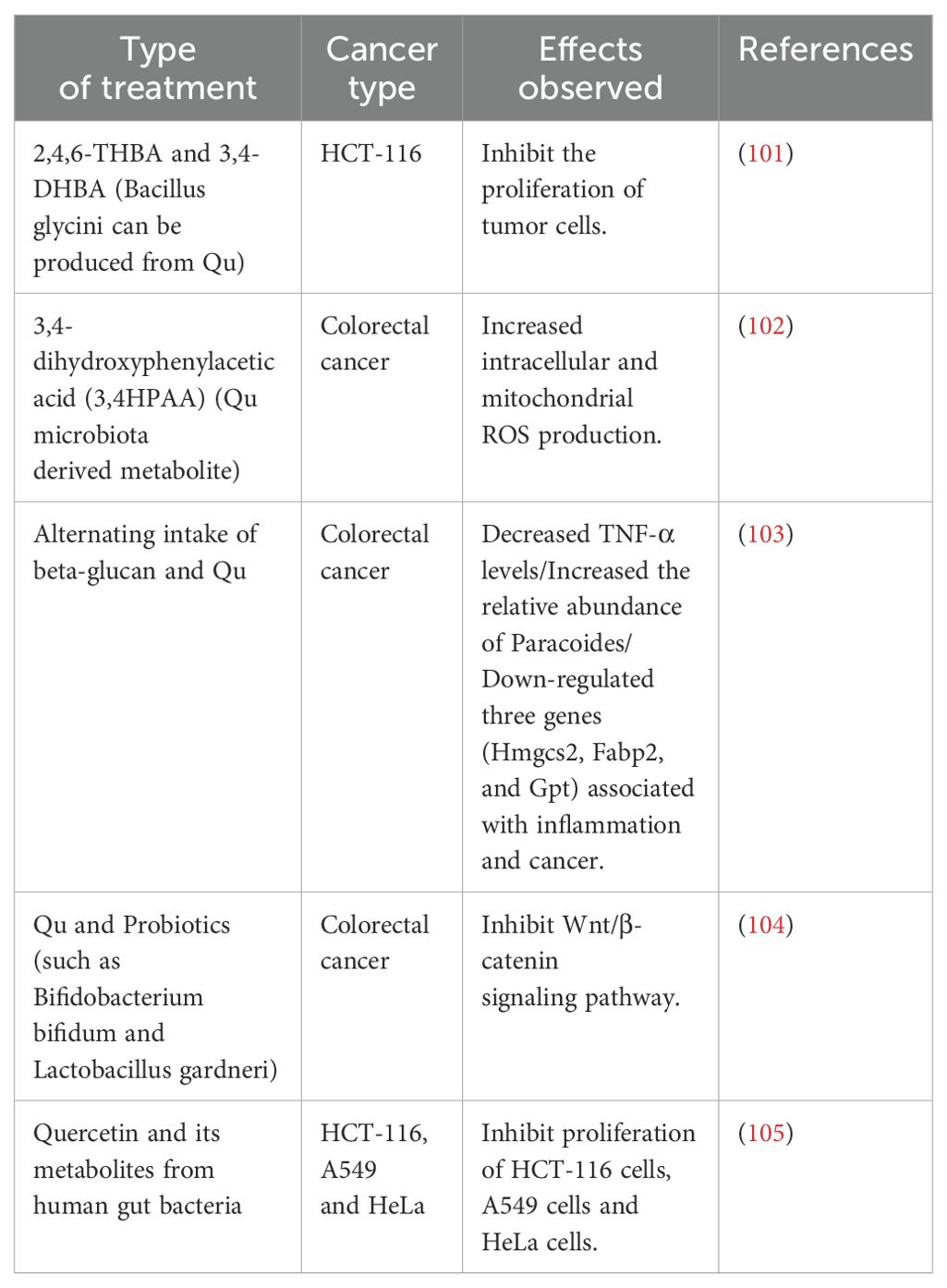
Qu can not only directly inhibit the proliferation of tumor cells by regulating intestinal flora (Table 3) but also improve the efficiency of anti-tumor immunotherapy. Qu can regulate the proportion of intestinal flora and increase the number of beneficial flora (Figure 3). In the TNBC mouse model, the addition of 2.5% Qu to the diet significantly induced Akkermansia enrichment and increased the activation of effector T cells and NK cells compared with mice without Qu supplementation (68). In a mouse model of hepatocellular carcinoma, the combination of Qu and immune checkpoint inhibitors increased the abundance of Firmicutes, Actinobacteria, and Vermilata microbiota, as well as Dubosiella and Acementia at the genus level, and increased the expression of CD8a, CD4, CD11b, IL-10, and IFN-γ in the TME compared with Qu or immune checkpoint inhibitors alone (106). In a mouse model of colitis, additional supplementation with Qu (30 mg/kg) increased the numbers of Bacteroides, Bifidobacterium, Lactobacillus, and Clostridium, and significantly decreased the numbers of Clostridium and Enterococcus (P < 0.05) (107). In the model of using antibiotics to disrupt the balance of intestinal flora, the experimental group added 0.2% Qu based on AIN-93G feeding, and the results showed that Qu increased the level of intestinal beneficial bacteria species. These included Faecalibaculum rodentium (103.13%), Enterorhabdus caecimuris (4.13%), Eggerthella lenta (4%), Roseburia hominis (1.33%), and Enterorhabdus mucosicola (1.79%). These bacteria can produce butyrate and reduce serum lipopolysaccharide and TNF-α levels (108). The mice fed with 0.05% Qu based on HFD lasted for 6 weeks. Compared with the mice in the HFD group, Qu significantly reduced liver fat and blood glucose levels. In feces, the relative abundance of Akkermansia was significantly increased (109).
Qu can directly regulate the metabolites of intestinal flora to exert anti-inflammatory effects, thereby shaping an effective anti-tumor immune microenvironment (Figure 3). Through the antibiotic induced intestinal flora dysregulation mouse model, the experimental group was fed AIN-93G diet containing 0.2% Qu for 10 days. Through the analysis of mouse feces and serum metabolites, it was clear that isorhamnetin, a Qu methylated metabolite, was the main active component in serum. Compared with antibiotic intervention alone, Qu treatment was able to increase the diversity of intestinal flora (still lower than that of the group without antibiotics) and butyrate levels (110). Compared with HFD-fed mice, mice fed with 1% Qu for 16 weeks had lower body weight and total plasma cholesterol, reduced the ratio of Firmicutes, Bacteroidetes in the stool of HFD-fed mice and increased the production of short-chain fatty acids (SCFA) (111). Studies have shown that SCFA can improve the efficiency of breast cancer immunotherapy (112).
Based on the regulatory effects of Qu on intestinal flora, clinical treatment strategies can be influenced by the following three aspects. First, the sensitivity of intestinal flora richness can predict the efficacy of immunotherapy. A higher richness of intestinal flora often correlates with a better response to immunotherapy. Therefore, in the clinical application of immunotherapy, the bacterial flora of patients can be sequenced in advance to assess their microbial composition. Based on the results of microbiota sequencing, Qu can be administered beforehand to enhance the abundance of intestinal flora, thereby improving the response rate to immunotherapy. Second, the expression of beneficial bacteria often enhances the response to immunotherapy, including species such as Ackermannia and Ruminococcus.etc. Qu has been shown to improve the expression of these beneficial bacteria, thereby increasing the efficacy of immunotherapy. In addition to utilizing natural products, the incorporation of probiotics can also enhance the recruitment of beneficial bacteria in the gut. Third, some metabolites produced by intestinal flora can boost the effectiveness of immunotherapy. Qu can promote bacterial metabolism, making its application advantageous for increasing the accumulation of bacterial metabolites, which is beneficial for the clinical application of immunotherapy. Therefore, given Qu’s impact on intestinal flora, it has the potential to be developed as an adjuvant for tumor immunotherapy.
6 Comparison of the immunomodulatory effects of Qu and other natural products
Numerous in vivo and in vitro experimental studies have demonstrated that natural products possess a positive regulatory effect on the immune system and can enhance tumor control. Due to their diverse sources and relatively low toxicity, natural products and their derivatives have become essential in the design and development of anti-tumor drugs (113).
Similar to the role of Qu, the immunomodulatory effects of various natural products and their derivatives extend beyond direct cytotoxic effects, they also play a crucial role in reshaping the immune microenvironment and inhibiting chronic inflammation. These natural products can stimulate immune cells, enhancing their activity, including CD8+ T cells, NKs, DCs, or inhibit the recruitment of immunosuppressive cells within the TME, such as MDSCs, Tregs, and TAMs, thereby blocking immunosuppression in the TME. Furthermore, in addition to their direct actions on the TME, the inhibition of chronic inflammation represents a significant mechanism through which natural products regulate anti-tumor immunity, including the suppression of signaling pathways such as NF-κB and JAK-STAT. Resveratrol is classified as a stilbene. It can inhibit the interaction between PD-1 and PD-L1, thereby enhancing the activity of cytotoxic T lymphocytes and improving the response rate to immunotherapy (114). Vanillic acid, an aromatic phenolic compound, induces macrophages to polarize into the M1 phenotype by activating the IL-6R/Janus kinase (JAK) signaling pathway, which enhances anti-tumor immunity (115). Ursolic acid, a triterpenoid compound, reduces the recruitment of MDSCs and Tregs in tumor tissue, thereby reshaping the immunosuppressive microenvironment associated with breast cancer (116). Fucose gum, a sulfated polysaccharide, enhances both adaptive and innate immune responses by boosting the activity of T cells, macrophages, DC, and NK cells (117). Anthraquinone (AQ) mitigates oxidative stress and mitochondrial damage in MCF-7 cells and inhibits the proliferation of MCF-7 breast cancer cells in a concentration-dependent manner (118).
Many natural products possess the ability to regulate anti-tumor immunity. However, the varying mechanisms of action among these products, along with the unclear effective dosages and low bioavailability in vivo, complicate the execution of clinical trials involving natural active compounds. Consequently, the efficacy of different natural products in regulating anti-tumor immunity has not been thoroughly investigated. Nevertheless, combining various natural products appears to enhance the effects of individual compounds. Specifically, the combination of curcumin, resveratrol, and Qu (RCQ) has been shown to reshape anti-tumor immunity in TNBC by modulating macrophage polarization, increasing the recruitment of effector T cells, and shifting the immune balance toward an immune-activated state (119).
7 Adjuvant application of Qu in tumor therapy
Qu has certain cytotoxic effects and demonstrates a synergistic effect when combined with chemotherapy in clinical practice. The concurrent use of Qu and docetaxel significantly inhibited cell growth and induced apoptosis in the MDA-MB-231 cell (120). The combination of docetaxel (7nM) and Qu (95μM) produced the most substantial synergistic effect, with a value of 34,268,250. In estrogen receptor-positive breast cancer, low concentrations of Qu (1-20μM) significantly inhibited tamoxifen-induced proliferation of MCF-7 cells, while higher concentrations of Qu (≥50μM) synergistically enhanced tamoxifen-induced apoptosis (32). Qu has also been shown to improve the efficacy of the gemcitabine and doxorubicin combination by downregulating the expression of HIF-1α and increasing the expression level of the apoptosis regulator p53 (121). In breast cancer cells, the combination of Qu and doxorubicin further enhanced the antitumor effect of doxorubicin (122). Qu inhibits YAP expression and its translocation to the nucleus, leading to the restoration of the Hippo pathway, which inhibits cancer progression and increases cisplatin sensitivity, thereby facilitating effective synergistic chemotherapy applications (123).
Qu and its derivatives can effectively target the regulation of the TME and support the advancement of immunotherapy. By modulating the expression of p-JAK2, p-STAT1 and PD-L1 in the JAK/STAT1 signaling pathway, Qu enhances the regulation of γδ T cells and improves the cytotoxic activity of immune cells against breast cancer (124). Qu and iron ions (QFN) were combined to create a multifunctional nano-photosensitizer. Qu released by QFN can decrease the expression of PD-L1 in tumor cells by inhibiting the phosphorylation of JAK2 and STAT3, thereby enhancing anti-tumor immunity (41). The combination of folic acid (FA) and polyethylene glycol (PEG) modified amphiphilic cyclodextrin nanoparticles (NPs) encapsulated ginsenosides and Qu into a nano-preparation (CD-PEG-FA.Rg3.QTN) along with anti-PD-L1 antibodies, significantly improves the efficacy of immunotherapy for colorectal cancer and prolongs the survival rate of animal models (36).
8 Discussion
Qu, as a natural product, is widely present in many fruits and vegetables. However, there are several pressing issues that need to be addressed to enhance its potential use as a drug in clinical practice.
The low oral bioavailability of Qu hinders its ability to exert effective biological activity in vivo. Many researchers have made significant progress in enhancing the bioavailability of Qu by coupling it with nanoparticles (125). Compared with free Qu treatment, AuNPs-Qu-5 treatment reduced HUVECs cell viability in a dose-dependent manner, inhibiting in vitro angiogenesis by suppressing the formation of capillary tubes in human umbilical vein endothelial cells (HUVECs) at 50μM. Gold nanoparticle-conjugated Qu (AuNPs‐Qu‐5) downregulated VEGFR-2 protein expression in HUVECs. In a DMBA-induced breast cancer rat model treated with free Qu and AuNPs‐Qu‐5, AuNPs‐Qu‐5 significantly suppressed tumor growth and prolonged the survival time of tumor-bearing rats compared with free Qu-treated rats (126). After the engineering modification of Qu, its function of promoting T cell activation becomes more significant. The Qu polyethylene glycol co-nano module (P-Qu-MTX-Fe) can effectively inhibit the recurrence of primary and metastatic breast tumors by activating anti-tumor immune responses mediated by CD8+T cells (127). When encapsulated in phospholipid complexes, Qu can be delivered directly into cells, significantly enhancing its bioavailability (128, 129). In addition to the structural modifications of Qu, extensive in vivo studies on Qu’s secondary metabolites and the development of their applications represent effective strategies to enhance Qu’s utilization rate. Many researchers have modified the structure of Qu by incorporating nanomaterials to enhance its absorption rate and biological activity (130), leading to some success in modulating anti-tumor immunity. Future research could focus on developing novel drug combinations that support anti-tumor immunity by combining immune checkpoint blockade (ICB) with Qu.
Due to the numerous targets of Qu immunomodulatory action, its low oral bioavailability, and the uncertainties surrounding effective dosages and potential toxic side effects, it is difficult to carry out clinical trials to investigate its anti-tumor effects poses significant challenges. Consequently, there is currently no clinical evidence to support the use of Qu for the prevention or treatment of cancer in humans. However, as a naturally occurring dietary compound, extensive animal studies have confirmed its efficacy and safety, indicating its potential for development as an adjuvant in tumor immunotherapy. Qu can be used as a dietary supplement to assist in the immunotherapy of tumors, but the optimal and safe dose for the tumor patient population remains to be studied (131). In vivo experiments in mice have shown that high-purity Qu, as an additive in the range of 0.05%-0.5% added to HFD, can have a better anti-inflammatory effect, with no observed toxicity. However, most of these studies focused on obese mice or colitis mouse models. Tumor mice often experience an increased metabolic load due to tumor metabolism and metastasis, so the safety of Qu in tumor patients needs further study. Building on the research results of Qu in metabolic diseases, inflammatory diseases, and intestinal flora metabolism, future research should conduct extensive in vivo experiments and large-scale clinical trials to thoroughly understand its adjuvant effect on breast cancer immunotherapy and evaluate its safety. In addition, by employing current multi-omics research methods and leveraging artificial intelligence, we can comprehensively integrate and analyze the research data related to Qu. This analysis will focus on its targets, effective dosages, and potential adverse reactions, thereby establishing a solid foundation for subsequent rigorous clinical trials. Ultimately, this will facilitate the clinical application of Qu.
In summary, this paper reviews numerous in vivo and in vitro studies to elucidate the mechanisms by which Qu modifies the immune microenvironment in breast cancer by modulating both local and systemic factors. The findings indicate that Qu, as a natural immunomodulator, has the potential to be developed as an adjuvant for breast immunotherapy. However, there remain several challenges that researchers and clinicians must address in order to expedite the development and application of Qu as an immunotherapy adjuvant.
Author contributions
LF: Visualization, Writing – original draft. DG: Writing – review & editing. TW: Writing – review & editing. HZ: Supervision, Writing – review & editing. YZ: Funding acquisition, Writing – review & editing. SW: Supervision, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by the National Natural Science Foundation of China (No.82374178), the Natural Science Foundation of Shandong Province (NO.ZR2022MH180).
Acknowledgments
Thanks to the editors and reviewers for their hard work and important comments. Thanks to BioRender.com for the drawing material.
Conflict of interest
The authors declare the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2021) 71:209–49. doi: 10.3322/caac.21660
2. Baxevanis CN, Fortis SP, Perez SA. The balance between breast cancer and the immune system: challenges for prognosis and clinical benefit from immunotherapies. Semin Cancer Biol. (2021) 72:76–89. doi: 10.1016/j.semcancer.2019.12.018
3. Zhang Y, Lou Y, Wang J, Yu C, Shen W. Research status and molecular mechanism of the traditional chinese medicine and antitumor therapy combined strategy based on tumor microenvironment. Front Immunol. (2020) 11:609705. doi: 10.3389/fimmu.2020.609705
4. Hosseini A, Razavi BM, Banach M, Hosseinzadeh H. Quercetin and metabolic syndrome: A review. Phytother Res. (2021) 35:5352–64. doi: 10.1002/ptr.7144
5. Biswas P, Dey D, Biswas PK, Rahaman TI, Saha S, Parvez A, et al. A comprehensive analysis and anti-cancer activities of quercetin in ros-mediated cancer and cancer stem cells. Int J Mol Sci. (2022) 23:11746. doi: 10.3390/ijms231911746
6. Gonzalez-Bosch C, Zunszain PA, Mann GE. Control of redox homeostasis by short-chain fatty acids: implications for the prevention and treatment of breast cancer. Pathogens. (2023) 12:486. doi: 10.3390/pathogens12030486
7. Khan SI, Zhao J, Khan IA, Walker LA, Dasmahapatra AK. Potential utility of natural products as regulators of breast cancer-associated aromatase promoters. Reprod Biol Endocrinol. (2011) 9:91. doi: 10.1186/1477-7827-9-91
8. Ahmed F, Ijaz B, Ahmad Z, Farooq N, Sarwar MB, Husnain T. Modification of Mirna Expression through Plant Extracts and Compounds against Breast Cancer: Mechanism and Translational Significance. Phytomedicine. (2020) 68:153168. doi: 10.1016/j.phymed.2020.153168
9. Zhao X, Wang X, Pang Y. Phytochemicals targeting ferroptosis: therapeutic opportunities and prospects for treating breast cancer. Pharm (Basel). (2022) 15:1360. doi: 10.3390/ph15111360
10. Wang H, Dong Z, Liu J, Zhu Z, Najafi M. Mechanisms of cancer-killing by quercetin; a review on cell death mechanisms. Anticancer Agents Med Chem. (2023) 23:999–1012. doi: 10.2174/1871520623666230120094158
11. Ezzati M, Yousefi B, Velaei K, Safa A. A review on anti-cancer properties of quercetin in breast cancer. Life Sci. (2020) 248:117463. doi: 10.1016/j.lfs.2020.117463
12. Li X, Zhou N, Wang J, Liu Z, Wang X, Zhang Q, et al. Quercetin suppresses breast cancer stem cells (Cd44(+)/cd24(-)) by inhibiting the pi3k/akt/mtor-signaling pathway. Life Sci. (2018) 196:56–62. doi: 10.1016/j.lfs.2018.01.014
13. Aghapour F, Moghadamnia AA, Nicolini A, Kani SNM, Barari L, Morakabati P, et al. Quercetin conjugated with silica nanoparticles inhibits tumor growth in mcf-7 breast cancer cell lines. Biochem Biophys Res Commun. (2018) 500:860–5. doi: 10.1016/j.bbrc.2018.04.174
14. Roy S, Banerjee S, Chakraborty T. Vanadium quercetin complex attenuates mammary cancer by regulating the P53, akt/mtor pathway and downregulates cellular proliferation correlated with increased apoptotic events. Biometals. (2018) 31:647–71. doi: 10.1007/s10534-018-0117-3
15. Wang R, Yang L, Li S, Ye D, Yang L, Liu Q, et al. Quercetin inhibits breast cancer stem cells via downregulation of aldehyde dehydrogenase 1a1 (Aldh1a1), chemokine receptor type 4 (Cxcr4), mucin 1 (Muc1), and epithelial cell adhesion molecule (Epcam). Med Sci Monit. (2018) 24:412–20. doi: 10.12659/msm.908022
16. Jia L, Huang S, Yin X, Zan Y, Guo Y, Han L. Quercetin suppresses the mobility of breast cancer by suppressing glycolysis through akt-mtor pathway mediated autophagy induction. Life Sci. (2018) 208:123–30. doi: 10.1016/j.lfs.2018.07.027
17. Chekuri S, Vyshnava SS, Somisetti SL, Cheniya SBK, Gandu C, Anupalli RR. Isolation and anticancer activity of quercetin from acalypha indica L. Against breast cancer cell lines mcf-7 and mda-mb-231. 3 Biotech. (2023) 13:289. doi: 10.1007/s13205-023-03705-w
18. Betts Z, Deveci Ozkan A, Yuksel B, Alimudin J, Aydin D, Aksoy O, et al. Investigation of the combined cytotoxicity induced by sodium butyrate and a flavonoid quercetin treatment on mcf-7 breast cancer cells. J Toxicol Environ Health A. (2023) 86:833–45. doi: 10.1080/15287394.2023.2254807
19. Yousuf M, Khan P, Shamsi A, Shahbaaz M, Hasan GM, Haque QMR, et al. Inhibiting cdk6 activity by quercetin is an attractive strategy for cancer therapy. ACS Omega. (2020) 5:27480–91. doi: 10.1021/acsomega.0c03975
20. Qiu J, Zhang T, Zhu X, Yang C, Wang Y, Zhou N, et al. Hyperoside induces breast cancer cells apoptosis via ros-mediated nf-kappab signaling pathway. Int J Mol Sci. (2019) 21:131. doi: 10.3390/ijms21010131
21. Mawalizadeh F, Mohammadzadeh G, Khedri A, Rashidi M. Quercetin potentiates the chemosensitivity of mcf-7 breast cancer cells to 5-fluorouracil. Mol Biol Rep. (2021) 48:7733–42. doi: 10.1007/s11033-021-06782-3
22. Prieto-Vila M, Shimomura I, Kogure A, Usuba W, Takahashi RU, Ochiya T, et al. Quercetin inhibits lef1 and resensitizes docetaxel-resistant breast cancer cells. Molecules. (2020) 25:2576. doi: 10.3390/molecules25112576
23. Chen WJ, Tsai JH, Hsu LS, Lin CL, Hong HM, Pan MH. Quercetin blocks the aggressive phenotype of triple-negative breast cancer by inhibiting igf1/igf1r-mediated emt program. J Food Drug Anal. (2021) 29:98–112. doi: 10.38212/2224-6614.3090
24. Hosseini SS, Ebrahimi SO, Haji Ghasem Kashani M, Reiisi S. Study of quercetin and fisetin synergistic effect on breast cancer and potentially involved signaling pathways. Cell Biol Int. (2023) 47:98–109. doi: 10.1002/cbin.11942
25. Ruidas B, Sur TK, Das Mukhopadhyay C, Sinha K, Som Chaudhury S, Sharma P, et al. Quercetin: A silent retarder of fatty acid oxidation in breast cancer metastasis through steering of mitochondrial cpt1. Breast Cancer. (2022) 29:748–60. doi: 10.1007/s12282-022-01356-y
26. Umar SM, Patra S, Kashyap A, Dev JRA, Kumar L, Prasad CP. Quercetin impairs hur-driven progression and migration of triple negative breast cancer (Tnbc) cells. Nutr Cancer. (2022) 74:1497–510. doi: 10.1080/01635581.2021.1952628
27. Roshanazadeh M, Babaahmadi Rezaei H, Rashidi M. Quercetin synergistically potentiates the anti-metastatic effect of 5-fluorouracil on the mda-mb-231 breast cancer cell line. Iran J Basic Med Sci. (2021) 24:928–34. doi: 10.22038/ijbms.2021.56559.12629
28. Karimian A, Majidinia M, Moliani A, Alemi F, Asemi Z, Yousefi B, et al. The modulatory effects of two bioflavonoids, quercetin and thymoquinone on the expression levels of DNA damage and repair genes in human breast, lung and prostate cancer cell lines. Pathol Res Pract. (2022) 240:154143. doi: 10.1016/j.prp.2022.154143
29. Kiyga E, Sengelen A, Adiguzel Z, Onay Ucar E. Investigation of the role of quercetin as a heat shock protein inhibitor on apoptosis in human breast cancer cells. Mol Biol Rep. (2020) 47:4957–67. doi: 10.1007/s11033-020-05641-x
30. An S, Hu M. Quercetin promotes tfeb nuclear translocation and activates lysosomal degradation of ferritin to induce ferroptosis in breast cancer cells. Comput Intell Neurosci. (2022) 2022:5299218. doi: 10.1155/2022/5299218
31. Rhman MA, Devnarain N, Khan R, Owira PMO. Synergism potentiates oxidative antiproliferative effects of naringenin and quercetin in mcf-7 breast cancer cells. Nutrients. (2022) 14:3437. doi: 10.3390/nu14163437
32. Xu Z, Zhao D, Zheng X, Huang B, Xia X, Pan X. Quercetin exerts bidirectional regulation effects on the efficacy of tamoxifen in estrogen receptor-positive breast cancer therapy: an in vitro study. Environ Toxicol. (2020) 35:1179–93. doi: 10.1002/tox.22983
33. Ozkan E, Bakar-Ates F. Potentiation of the effect of lonidamine by quercetin in mcf-7 human breast cancer cells through downregulation of mmp-2/9 mrna expression. Acad Bras Cienc. (2020) 92:e20200548. doi: 10.1590/0001-3765202020200548
34. Tang H, Kuang Y, Wu W, Peng B, Fu Q. Quercetin inhibits the metabolism of arachidonic acid by inhibiting the activity of cyp3a4, thereby inhibiting the progression of breast cancer. Mol Med. (2023) 29:127. doi: 10.1186/s10020-023-00720-8
35. Liu H, Lee JI, Ahn TG. Effect of quercetin on the anti-tumor activity of cisplatin in emt6 breast tumor-bearing mice. Obstet Gynecol Sci. (2019) 62:242–8. doi: 10.5468/ogs.2019.62.4.242
36. Sun D, Zou Y, Song L, Han S, Yang H, Chu D, et al. A cyclodextrin-based nanoformulation achieves co-delivery of ginsenoside rg3 and quercetin for chemo-immunotherapy in colorectal cancer. Acta Pharm Sin B. (2022) 12:378–93. doi: 10.1016/j.apsb.2021.06.005
37. Lin Z, Liu Y, Gong X, Nie F, Xu J, Guo Y. Construction of quercetin-fucoidan nanoparticles and their application in cancer chemo-immunotherapy treatment. Int J Biol Macromol. (2024) 256:128057. doi: 10.1016/j.ijbiomac.2023.128057
38. Qiu D, Yan X, Xiao X, Zhang G, Wang Y, Cao J, et al. To explore immune synergistic function of quercetin in inhibiting breast cancer cells. Cancer Cell Int. (2021) 21:632. doi: 10.1186/s12935-021-02345-5
39. Gu C, Tang L, Hao Y, Dong S, Shen J, Xie F, et al. Network pharmacology and bioinformatics were used to construct a prognostic model and immunoassay of core target genes in the combination of quercetin and kaempferol in the treatment of colorectal cancer. J Cancer. (2023) 14:1956–80. doi: 10.7150/jca.85517
40. Zhang J, Shen L, Li X, Song W, Liu Y, Huang L. Nanoformulated codelivery of quercetin and alantolactone promotes an antitumor response through synergistic immunogenic cell death for microsatellite-stable colorectal cancer. ACS Nano. (2019) 13:12511–24. doi: 10.1021/acsnano.9b02875
41. Li L, Zhang M, Liu T, Li J, Sun S, Chen J, et al. Quercetin-ferrum nanoparticles enhance photothermal therapy by modulating the tumor immunosuppressive microenvironment. Acta Biomater. (2022) 154:454–66. doi: 10.1016/j.actbio.2022.10.008
42. Mo Z, Cao Z, Yu L, Wang Y, Li P, Lin Y, et al. An integrative analysis reveals the potential mechanism between herbal medicine yinchen and immunoregulation in hepatocellular carcinoma. BioMed Res Int. (2020) 2020:8886914. doi: 10.1155/2020/8886914
43. Ezzat MI, Hassan M, Abdelhalim MA, El-Desoky AM, Mohamed SO, Ezzat SM. Immunomodulatory effect of noni fruit and its isolates: insights into cell-mediated immune response and inhibition of lps-induced thp-1 macrophage inflammation. Food Funct. (2021) 12:3170–9. doi: 10.1039/d0fo03402a
44. Choi SY, Collins CC, Gout PW, Wang Y. Cancer-generated lactic acid: A regulatory, immunosuppressive metabolite? J Pathol. (2013) 230:350–5. doi: 10.1002/path.4218
45. Li W, Tanikawa T, Kryczek I, Xia H, Li G, Wu K, et al. Aerobic glycolysis controls myeloid-derived suppressor cells and tumor immunity via a specific cebpb isoform in triple-negative breast cancer. Cell Metab. (2018) 28:87–103 e6. doi: 10.1016/j.cmet.2018.04.022
46. Morais-Santos F, Miranda-Goncalves V, Pinheiro S, Vieira AF, Paredes J, Schmitt FC, et al. Differential sensitivities to lactate transport inhibitors of breast cancer cell lines. Endocr Relat Cancer. (2014) 21:27–38. doi: 10.1530/ERC-13-0132
47. Huang Y, Kim BYS, Chan CK, Hahn SM, Weissman IL, Jiang W. Improving immune-vascular crosstalk for cancer immunotherapy. Nat Rev Immunol. (2018) 18:195–203. doi: 10.1038/nri.2017.145
48. Rahma OE, Hodi FS. The intersection between tumor angiogenesis and immune suppression. Clin Cancer Res. (2019) 25:5449–57. doi: 10.1158/1078-0432.CCR-18-1543
49. Zhao X, Wang Q, Yang S, Chen C, Li X, Liu J, et al. Quercetin inhibits angiogenesis by targeting calcineurin in the xenograft model of human breast cancer. Eur J Pharmacol. (2016) 781:60–8. doi: 10.1016/j.ejphar.2016.03.063
50. Soo Jin O, Ok K, Jong Suk L, Jung-Ae K, Mi Ra K, Hong Seok C, et al. Inhibition of angiogenesis by quercetin in tamoxifen-resistant breast cancer cells. Food Chem Toxicol. (2010) 48:3227–34. doi: 10.1016/j.fct.2010.08.028
51. Chung I, Han G, Seshadri M, Gillard BM, Yu WD, Foster BA, et al. Role of vitamin D receptor in the antiproliferative effects of calcitriol in tumor-derived endothelial cells and tumor angiogenesis in vivo. Cancer Res. (2009) 69:967–75. doi: 10.1158/0008-5472.CAN-08-2307
52. Sannappa Gowda NG, Shiragannavar VD, Puttahanumantharayappa LD, Shivakumar AT, Dallavalasa S, Basavaraju CG, et al. Quercetin activates vitamin D receptor and ameliorates breast cancer induced hepatic inflammation and fibrosis. Front Nutr. (2023) 10:1158633. doi: 10.3389/fnut.2023.1158633
53. Kamiyama M, Pozzi A, Yang L, DeBusk LM, Breyer RM, Lin PC. Ep2, a receptor for pge2, regulates tumor angiogenesis through direct effects on endothelial cell motility and survival. Oncogene. (2006) 25:7019–28. doi: 10.1038/sj.onc.1209694
54. Xiao X, Shi D, Liu L, Wang J, Xie X, Kang T, et al. Quercetin suppresses cyclooxygenase-2 expression and angiogenesis through inactivation of P300 signaling. PloS One. (2011) 6:e22934. doi: 10.1371/journal.pone.0022934
55. Marchi S, Guilbaud E, Tait SWG, Yamazaki T, Galluzzi L. Mitochondrial control of inflammation. Nat Rev Immunol. (2023) 23:159–73. doi: 10.1038/s41577-022-00760-x
56. Morris G, Gevezova M, Sarafian V, Maes M. Redox regulation of the immune response. Cell Mol Immunol. (2022) 19:1079–101. doi: 10.1038/s41423-022-00902-0
57. Cheung EC, Vousden KH. The role of ros in tumour development and progression. Nat Rev Cancer. (2022) 22:280–97. doi: 10.1038/s41568-021-00435-0
58. Malmberg KJ, Arulampalam V, Ichihara F, Petersson M, Seki K, Andersson T, et al. Inhibition of activated/memory (Cd45ro(+)) T cells by oxidative stress associated with block of nf-kappab activation. J Immunol. (2001) 167:2595–601. doi: 10.4049/jimmunol.167.5.2595
59. Cubillos-Ruiz JR, Bettigole SE, Glimcher LH. Tumorigenic and immunosuppressive effects of endoplasmic reticulum stress in cancer. Cell. (2017) 168:692–706. doi: 10.1016/j.cell.2016.12.004
60. Kuo CL, Chou HY, Chiu YC, Cheng AN, Fan CC, Chang YN, et al. Mitochondrial oxidative stress by lon-pycr1 maintains an immunosuppressive tumor microenvironment that promotes cancer progression and metastasis. Cancer Lett. (2020) 474:138–50. doi: 10.1016/j.canlet.2020.01.019
61. Aboelella NS, Brandle C, Kim T, Ding ZC, Zhou G. Oxidative stress in the tumor microenvironment and its relevance to cancer immunotherapy. Cancers (Basel). (2021) 13:986. doi: 10.3390/cancers13050986
62. Niazvand F, Orazizadeh M, Khorsandi L, Abbaspour M, Mansouri E, Khodadadi A. Effects of quercetin-loaded nanoparticles on mcf-7 human breast cancer cells. Medicina (Kaunas). (2019) 55:114. doi: 10.3390/medicina55040114
63. Wu Q, Needs PW, Lu Y, Kroon PA, Ren D, Yang X. Different antitumor effects of quercetin, quercetin-3’-sulfate and quercetin-3-glucuronide in human breast cancer mcf-7 cells. Food Funct. (2018) 9:1736–46. doi: 10.1039/c7fo01964e
64. Mostafavi-Pour Z, Ramezani F, Keshavarzi F, Samadi N. The role of quercetin and vitamin C in nrf2-dependent oxidative stress production in breast cancer cells. Oncol Lett. (2017) 13:1965–73. doi: 10.3892/ol.2017.5619
65. Wang F, Yuan C, Wu HZ, Liu B, Yang YF. Bioinformatics, molecular docking and experiments in vitro analyze the prognostic value of cxc chemokines in breast cancer. Front Oncol. (2021) 11:665080. doi: 10.3389/fonc.2021.665080
66. Jing L, Lin J, Yang Y, Tao L, Li Y, Liu Z, et al. Quercetin inhibiting the pd-1/pd-L1 interaction for immune-enhancing cancer chemopreventive agent. Phytother Res. (2021) 35:6441–51. doi: 10.1002/ptr.7297
67. Lee HW, Chung YS, Kim TJ. Heterogeneity of human gammadelta T cells and their role in cancer immunity. Immune Netw. (2020) 20:e5. doi: 10.4110/in.2020.20.e5
68. Manni A, Sun YW, Schell TD, Lutsiv T, Thompson H, Chen KM, et al. Complementarity between microbiome and immunity may account for the potentiating effect of quercetin on the antitumor action of cyclophosphamide in a triple-negative breast cancer model. Pharm (Basel). (2023) 16:1422. doi: 10.3390/ph16101422
69. Bae JH, Kim JY, Kim MJ, Chang SH, Park YS, Son CH, et al. Quercetin enhances susceptibility to nk cell-mediated lysis of tumor cells through induction of nkg2d ligands and suppression of hsp70. J Immunother. (2010) 33:391–401. doi: 10.1097/CJI.0b013e3181d32f22
71. Picon-Ruiz M, Morata-Tarifa C, Valle-Goffin JJ, Friedman ER, Slingerland JM. Obesity and adverse breast cancer risk and outcome: mechanistic insights and strategies for intervention. CA Cancer J Clin. (2017) 67:378–97. doi: 10.3322/caac.21405
72. Papavasileiou G, Tsilingiris D, Spyrou N, Vallianou NG, Karampela I, Magkos F, et al. Obesity and main urologic cancers: current systematic evidence, novel biological mechanisms, perspectives and challenges. Semin Cancer Biol. (2023) 91:70–98. doi: 10.1016/j.semcancer.2023.03.002
73. Campbell NJ, Barton C, Cutress RI, Copson ER. Impact of obesity, lifestyle factors and health interventions on breast cancer survivors. Proc Nutr Soc. (2023) 82:47–57. doi: 10.1017/S0029665122002816
74. Chlebowski RT, Aragaki AK, Pan K, Simon MS, Neuhouser ML, Haque R, et al. Breast cancer incidence and mortality by metabolic syndrome and obesity: the women’s health initiative. Cancer. (2024) 130:3147–56. doi: 10.1002/cncr.35318
75. Iyengar NM, Gucalp A, Dannenberg AJ, Hudis CA. Obesity and cancer mechanisms: tumor microenvironment and inflammation. J Clin Oncol. (2016) 34:4270–6. doi: 10.1200/JCO.2016.67.4283
76. Fang L, Liu K, Liu C, Wang X, Ma W, Xu W, et al. Tumor accomplice: T cell exhaustion induced by chronic inflammation. Front Immunol. (2022) 13:979116. doi: 10.3389/fimmu.2022.979116
77. Shapiro H, Pecht T, Shaco-Levy R, Harman-Boehm I, Kirshtein B, Kuperman Y, et al. Adipose tissue foam cells are present in human obesity. J Clin Endocrinol Metab. (2013) 98:1173–81. doi: 10.1210/jc.2012-2745
78. Shi H, Kokoeva MV, Inouye K, Tzameli I, Yin H, Flier JS. Tlr4 links innate immunity and fatty acid-induced insulin resistance. J Clin Invest. (2006) 116:3015–25. doi: 10.1172/JCI28898
79. Vandanmagsar B, Youm YH, Ravussin A, Galgani JE, Stadler K, Mynatt RL, et al. The nlrp3 inflammasome instigates obesity-induced inflammation and insulin resistance. Nat Med. (2011) 17:179–88. doi: 10.1038/nm.2279
80. Guo B, Fu S, Zhang J, Liu B, Li Z. Targeting inflammasome/il-1 pathways for cancer immunotherapy. Sci Rep. (2016) 6:36107. doi: 10.1038/srep36107
81. Okumura T, Ohuchida K, Kibe S, Iwamoto C, Ando Y, Takesue S, et al. Adipose tissue-derived stromal cells are sources of cancer-associated fibroblasts and enhance tumor progression by dense collagen matrix. Int J Cancer. (2019) 144:1401–13. doi: 10.1002/ijc.31775
82. Kidd S, Spaeth E, Watson K, Burks J, Lu H, Klopp A, et al. Origins of the tumor microenvironment: quantitative assessment of adipose-derived and bone marrow-derived stroma. PloS One. (2012) 7:e30563. doi: 10.1371/journal.pone.0030563
83. Gao D, Fang L, Liu C, Yang M, Yu X, Wang L, et al. Microenvironmental regulation in tumor progression: interactions between cancer-associated fibroblasts and immune cells. BioMed Pharmacother. (2023) 167:115622. doi: 10.1016/j.biopha.2023.115622
84. Harris BHL, Macaulay VM, Harris DA, Klenerman P, Karpe F, Lord SR, et al. Obesity: A perfect storm for carcinogenesis. Cancer Metastasis Rev. (2022) 41:491–515. doi: 10.1007/s10555-022-10046-2
85. Zhang C, Yue C, Herrmann A, Song J, Egelston C, Wang T, et al. Stat3 activation-induced fatty acid oxidation in cd8(+) T effector cells is critical for obesity-promoted breast tumor growth. Cell Metab. (2020) 31:148–61 e5. doi: 10.1016/j.cmet.2019.10.013
86. Gibson JT, Orlandella RM, Turbitt WJ, Behring M, Manne U, Sorge RE, et al. Obesity-associated myeloid-derived suppressor cells promote apoptosis of tumor-infiltrating cd8 T cells and immunotherapy resistance in breast cancer. Front Immunol. (2020) 11:590794. doi: 10.3389/fimmu.2020.590794
87. Overman A, Chuang CC, McIntosh M. Quercetin attenuates inflammation in human macrophages and adipocytes exposed to macrophage-conditioned media. Int J Obes (Lond). (2011) 35:1165–72. doi: 10.1038/ijo.2010.272
88. Barrios-Nolasco A, Dominguez-Lopez A, Miliar-Garcia A, Cornejo-Garrido J, Jaramillo-Flores ME. Anti-inflammatory effect of ethanolic extract from tabebuia rosea (Bertol.) dc., quercetin, and anti-obesity drugs in adipose tissue in wistar rats with diet-induced obesity. Molecules. (2023) 28:3801. doi: 10.3390/molecules28093801
89. Zhao J, Sun Y, Yuan C, Li T, Liang Y, Zou H, et al. Quercetin ameliorates hepatic fat accumulation in high-fat diet-induced obese mice via ppars. Food Funct. (2023) 14:1674–84. doi: 10.1039/d2fo03013f
90. Forney LA, Lenard NR, Stewart LK, Henagan TM. Dietary quercetin attenuates adipose tissue expansion and inflammation and alters adipocyte morphology in a tissue-specific manner. Int J Mol Sci. (2018) 19:363–74. doi: 10.3390/ijms19030895
91. Dong J, Zhang X, Zhang L, Bian HX, Xu N, Bao B, et al. Quercetin reduces obesity-associated atm infiltration and inflammation in mice: A mechanism including ampkalpha1/sirt1. J Lipid Res. (2014) 55:363–74. doi: 10.1194/jlr.M038786
92. Kobori M, Takahashi Y, Sakurai M, Akimoto Y, Tsushida T, Oike H, et al. Quercetin suppresses immune cell accumulation and improves mitochondrial gene expression in adipose tissue of diet-induced obese mice. Mol Nutr Food Res. (2016) 60:300–12. doi: 10.1002/mnfr.201500595
93. Sepich-Poore GD, Zitvogel L, Straussman R, Hasty J, Wargo JA, Knight R. The microbiome and human cancer. Science. (2021) 371:eabc4552. doi: 10.1126/science.abc4552
94. Yang Q, Wang B, Zheng Q, Li H, Meng X, Zhou F, et al. A review of gut microbiota-derived metabolites in tumor progression and cancer therapy. Adv Sci (Weinh). (2023) 10:e2207366. doi: 10.1002/advs.202207366
95. Zhu X, Li K, Liu G, Wu R, Zhang Y, Wang S, et al. Microbial metabolite butyrate promotes anti-pd-1 antitumor efficacy by modulating T cell receptor signaling of cytotoxic cd8 T cell. Gut Microbes. (2023) 15:2249143. doi: 10.1080/19490976.2023.2249143
96. Mager LF, Burkhard R, Pett N, Cooke NCA, Brown K, Ramay H, et al. Microbiome-derived inosine modulates response to checkpoint inhibitor immunotherapy. Science. (2020) 369:1481–9. doi: 10.1126/science.abc3421
97. Nakkarach A, Foo HL, Song AA, Mutalib NEA, Nitisinprasert S, Withayagiat U. Anti-cancer and anti-inflammatory effects elicited by short chain fatty acids produced by escherichia coli isolated from healthy human gut microbiota. Microb Cell Fact. (2021) 20:36. doi: 10.1186/s12934-020-01477-z
98. Lakritz JR, Poutahidis T, Mirabal S, Varian BJ, Levkovich T, Ibrahim YM, et al. Gut bacteria require neutrophils to promote mammary tumorigenesis. Oncotarget. (2015) 6:9387–96. doi: 10.18632/oncotarget.3328
99. Kawanabe-Matsuda H, Takeda K, Nakamura M, Makino S, Karasaki T, Kakimi K, et al. Dietary lactobacillus-derived exopolysaccharide enhances immune-checkpoint blockade therapy. Cancer Discovery. (2022) 12:1336–55. doi: 10.1158/2159-8290.CD-21-0929
100. Makino S, Sato A, Goto A, Nakamura M, Ogawa M, Chiba Y, et al. Enhanced natural killer cell activation by exopolysaccharides derived from yogurt fermented with lactobacillus delbrueckii ssp. Bulgaricus oll1073r-1. J Dairy Sci. (2016) 99:915–23. doi: 10.3168/jds.2015-10376
101. Sankaranarayanan R, Sekhon PK, Ambat A, Nelson J, Jose D, Bhat GJ, et al. Screening of human gut bacterial culture collection identifies species that biotransform quercetin into metabolites with anticancer properties. Int J Mol Sci. (2021) 22:7045. doi: 10.3390/ijms22137045
102. Catalan M, Ferreira J, Carrasco-Pozo C. The microbiota-derived metabolite of quercetin, 3,4-dihydroxyphenylacetic acid prevents Malignant transformation and mitochondrial dysfunction induced by hemin in colon cancer and normal colon epithelia cell lines. Molecules. (2020) 25:4138. doi: 10.3390/molecules25184138
103. Qi J, Yu J, Li Y, Luo J, Zhang C, Ou S, et al. Alternating consumption of beta-glucan and quercetin reduces mortality in mice with colorectal cancer. Food Sci Nutr. (2019) 7:3273–85. doi: 10.1002/fsn3.1187
104. Benito I, Encio IJ, Milagro FI, Alfaro M, Martinez-Penuela A, Barajas M, et al. Microencapsulated bifidobacterium bifidum and lactobacillus gasseri in combination with quercetin inhibit colorectal cancer development in apc(Min/+) mice. Int J Mol Sci. (2021) 22:4906. doi: 10.3390/ijms22094906
105. Zhang Z, Peng X, Zhang N, Liu L, Wang Y, Ou S. Cytotoxicity comparison of quercetin and its metabolites from in vitro fermentation of several gut bacteria. Food Funct. (2014) 5:2152–6. doi: 10.1039/c4fo00418c
106. Wu R, Xiong J, Zhou T, Zhang Z, Huang Z, Tian S, et al. Quercetin/anti-pd-1 antibody combination therapy regulates the gut microbiota, impacts macrophage immunity and reshapes the hepatocellular carcinoma tumor microenvironment. Front Biosci (Landmark Ed). (2023) 28:327. doi: 10.31083/j.fbl2812327
107. Lin R, Piao M, Song Y. Dietary quercetin increases colonic microbial diversity and attenuates colitis severity in citrobacter rodentium-infected mice. Front Microbiol. (2019) 10:1092. doi: 10.3389/fmicb.2019.01092
108. Mi W, Hu Z, Xu L, Bian X, Lian W, Yin S, et al. Quercetin positively affects gene expression profiles and metabolic pathway of antibiotic-treated mouse gut microbiota. Front Microbiol. (2022) 13:983358. doi: 10.3389/fmicb.2022.983358
109. Tan Y, Tam CC, Rolston M, Alves P, Chen L, Meng S, et al. Quercetin ameliorates insulin resistance and restores gut microbiome in mice on high-fat diets. Antioxid (Basel). (2021) 10:1251. doi: 10.3390/antiox10081251
110. Shi T, Bian X, Yao Z, Wang Y, Gao W, Guo C. Quercetin improves gut dysbiosis in antibiotic-treated mice. Food Funct. (2020) 11:8003–13. doi: 10.1039/d0fo01439g
111. Pei Y, Otieno D, Gu I, Lee SO, Parks JS, Schimmel K, et al. Effect of quercetin on nonshivering thermogenesis of brown adipose tissue in high-fat diet-induced obese mice. J Nutr Biochem. (2021) 88:108532. doi: 10.1016/j.jnutbio.2020.108532
112. He Y, Fu L, Li Y, Wang W, Gong M, Zhang J, et al. Gut microbial metabolites facilitate anticancer therapy efficacy by modulating cytotoxic cd8(+) T cell immunity. Cell Metab. (2021) 33:988–1000 e7. doi: 10.1016/j.cmet.2021.03.002
113. Ma R, Kwok HF. New opportunities and challenges of venom-based and bacteria-derived molecules for anticancer targeted therapy. Semin Cancer Biol. (2020) 80:356–69. doi: 10.1016/j.semcancer.2020.08.010
114. Verdura S, Cuyàs E, Cortada E, Brunet J, Lopez-Bonet E, Martin-Castillo B, et al. Resveratrol targets pd-L1 glycosylation and dimerization to enhance antitumor T-cell immunity. Aging (Albany NY). (2020) 12:8–34. doi: 10.18632/aging.102646
115. Zhu M, Tang X, Zhu Z, Gong Z, Tang W, Hu Y, et al. Sting activation in macrophages by vanillic acid exhibits antineoplastic potential. Biochem Pharmacol. (2023) 213:115618. doi: 10.1016/j.bcp.2023.115618
116. Zhang N, Liu S, Shi S, Chen Y, Xu F, Wei X, et al. Solubilization and delivery of ursolic-acid for modulating tumor microenvironment and regulatory T cell activities in cancer immunotherapy. J Control Release. (2020) 320:168–78. doi: 10.1016/j.jconrel.2020.01.015
117. Li Y, McGowan E, Chen S, Santos J, Yin H, Lin Y. Immunopotentiating activity of fucoidans and relevance to cancer immunotherapy. Mar Drugs. (2023) 21:128. doi: 10.3390/md21020128
118. Jayaprakash B, Suresh AR, Thiruvengadam R, Alharbi NS, Kadaikunnan S, Sankaran S, et al. Evaluation of oyster mushroom (Pleurotus ostreatus)-derived anthraquinone on the induction of apoptosis and suppression of mmp-2 and mmp-9 expression in breast cancer cells. Int J Med Sci. (2024) 21:1016–26. doi: 10.7150/ijms.93334
119. Li C, Xu Y, Zhang J, Zhang Y, He W, Ju J, et al. The effect of resveratrol, curcumin and quercetin combination on immuno-suppression of tumor microenvironment for breast tumor-bearing mice. Sci Rep. (2023) 13:13278. doi: 10.1038/s41598-023-39279-z
120. Safi A, Heidarian E, Ahmadi R. Quercetin synergistically enhances the anticancer efficacy of docetaxel through induction of apoptosis and modulation of pi3k/akt, mapk/erk, and jak/stat3 signaling pathways in mda-mb-231 breast cancer cell line. Int J Mol Cell Med. (2021) 10:11–22. doi: 10.22088/ijmcm.Bums.10.1.11
121. Hassan S, Peluso J, Chalhoub S, Idoux Gillet Y, Benkirane-Jessel N, Rochel N, et al. Quercetin potentializes the respective cytotoxic activity of gemcitabine or doxorubicin on 3d culture of aspc-1 or hepg2 cells, through the inhibition of hif-1α and mdr1. PLoS One. (2020) 15:e0240676. doi: 10.1371/journal.pone.0240676
122. Staedler D, Idrizi E, Kenzaoui BH, Juillerat-Jeanneret L. Drug combinations with quercetin: doxorubicin plus quercetin in human breast cancer cells. Cancer Chemother Pharmacol. (2011) 68:1161–72. doi: 10.1007/s00280-011-1596-x
123. Li T, Li Y. Quercetin acts as a novel anti-cancer drug to suppress cancer aggressiveness and cisplatin-resistance in nasopharyngeal carcinoma (Npc) through regulating the yes-associated protein/hippo signaling pathway. Immunobiology. (2023) 228(2):152324. doi: 10.1016/j.imbio.2022.152324
124. Picano E, Ostajic M, Varga A, Sicari R, Djordjevic-Dikic A, Nedeljkovic I, et al. Combined low dose dipyridamole-dobutamine stress echocardiography to identify myocardial viability. J Am Coll Cardiol. (1996) 27:1422–8. doi: 10.1016/0735-1097(95)00621-4
125. Ansari MA, Thiruvengadam M, Farooqui Z, Rajakumar G, Sajid Jamal QM, Alzohairy MA, et al. Nanotechnology, in silico and endocrine-based strategy for delivering paclitaxel and mirna: prospects for the therapeutic management of breast cancer. Semin Cancer Biol. (2020) 69:109–28. doi: 10.1016/j.semcancer.2019.12.022
126. Balakrishnan S, Bhat FA, Raja Singh P, Mukherjee S, Elumalai P, Das S, et al. Gold nanoparticle-conjugated quercetin inhibits epithelial-mesenchymal transition, angiogenesis and invasiveness via egfr/vegfr-2-mediated pathway in breast cancer. Cell Prolif. (2016) 49:678–97. doi: 10.1111/cpr.12296
127. Guan J, Tan X, Jiao J, Lai S, Zhang H, Kan Q, et al. Iron ion-coordinated carrier-free supramolecular co-nanoassemblies of dual DNA topoisomerase-targeting inhibitors for tumor suppression. Acta Biomater. (2022) 144:121–31. doi: 10.1016/j.actbio.2022.03.027
128. Patel G, Thakur NS, Kushwah V, Patil MD, Nile SH, Jain S, et al. Liposomal delivery of mycophenolic acid with quercetin for improved breast cancer therapy in sd rats. Front Bioeng Biotechnol. (2020) 8:631. doi: 10.3389/fbioe.2020.00631
129. Patel G, Thakur NS, Kushwah V, Patil MD, Nile SH, Jain S, et al. Mycophenolate co-administration with quercetin via lipid-polymer hybrid nanoparticles for enhanced breast cancer management. Nanomedicine. (2019) 24:102147. doi: 10.1016/j.nano.2019.102147
130. Zang X, Cheng M, Zhang X, Chen X. Quercetin nanoformulations: A promising strategy for tumor therapy. Food Funct. (2021) 12:6664–81. doi: 10.1039/d1fo00851j
Keywords: Quercetin, breast cancer, immunotherapy, natural immunomodulator, tumor immune microenvironment
Citation: Fang L, Gao D, Wang T, Zhao H, Zhang Y and Wang S (2024) From nature to clinic: Quercetin’s role in breast cancer immunomodulation. Front. Immunol. 15:1483459. doi: 10.3389/fimmu.2024.1483459
Received: 20 August 2024; Accepted: 19 November 2024;
Published: 06 December 2024.
Edited by:
Neha Garg, Banaras Hindu University, IndiaReviewed by:
Muthu Thiruvengadam, Konkuk University, Republic of KoreaGopal Patel, Sagar Institute of Science and Technology (SISTec), India
Copyright © 2024 Fang, Gao, Wang, Zhao, Zhang and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shijun Wang, cGF0aG9sb2d5QDE2My5jb20=
†These authors have contributed equally to this work and share first authorship
 Liguang Fang
Liguang Fang Dandan Gao2†
Dandan Gao2† Yanan Zhang
Yanan Zhang