- 1Department of Head and Neck Oncology, Cancer Center and State Key Laboratory of Biotherapy, West China Hospital, Sichuan University, Chengdu, China
- 2Department of Radiation Oncology, Cancer Center and State Key Laboratory of Biotherapy, West China Hospital, Sichuan University, Chengdu, China
- 3Cancer Prevention and Treatment Institute of Chengdu, Department of Oncology, Chengdu Fifth People’s Hospital (The Second Clinical Medical College, Affiliated Fifth People’s Hospital of Chengdu University of Traditional Chinese Medicine), Chengdu, China
- 4School of Clinical Medicine, Chengdu University of Traditional Chinese Medicine, Chengdu, China
Background: Tumor-infiltrating lymphocytes (TILs) have demonstrated potential as prognostic biomarkers across various cancer types. However, their prognostic implications in non-small cell lung cancer (NSCLC) remain ambiguous.
Methods: An exhaustive electronic search was executed across the Pubmed, EMBASE, Web of Science, and Cochrane Library databases to locate relevant studies published up until December 19, 2023. Studies were eligible if they assessed the association between TILs and overall survival (OS) and disease-free survival (DFS) in NSCLC patients. The OS and DFS were subsequently extracted for analysis. The prognostic significance of TILs was evaluated by calculating the Pooled Hazard Ratios (HRs) and their corresponding 95% Confidence Intervals (CIs).
Results: The meta-analysis incorporated 60 studies, which collectively included 15829 NSCLC patients. The collective analysis indicated that NSCLC patients exhibiting TILs infiltration demonstrated a significantly improved OS(HR: 0.67; 95%CI: 0.55-0.81). Subgroup analyses, based on TIL subtypes (CD8+, CD3+ and CD4+), consistently revealed a favorable prognostic impact on OS. However, it was observed that FOXP3+ was correlated with a poor OS (HR: 1.35; 95% CI: 0.87-2.11).
Conclusion: This comprehensive systematic review and meta-analysis substantiate the prognostic significance of TILs in patients diagnosed with NSCLC. Notably, elevated TILs infiltration correlates with a favorable prognosis, particularly among CD8+, CD3+ and CD4+ subtypes.
Systematic review registration: https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42023468089 PROSPERO, identifier CRD42023468089.
1 Introduction
Lung cancer stands as the most prevalent malignant neoplasm on a global scale (1), with non-small cell lung cancer (NSCLC) making up about 85% of all cases (2). The use of low-dose spiral CT has been instrumental in the early detection of lung cancer, leading to improved outcomes for patients detected at an early stage (3–5). However, some patients still face challenges such as tumor recurrence, metastasis, and mortality (6), which may be related to the characteristics of the solid pulmonary nodules, including ground-glass opacity lesions that are associated with a more favorable prognosis in early-stage NSCLC (7).The significance of tumor-infiltrating immune cells in the treatment and prognosis of NSCLC is progressively gaining recognition (8, 9). Nevertheless, the correlation between phenotypes of TILs and the local prognosis of NSCLC remains inconclusive (8). Consequently, there is a pressing need for a novel and accurate method to predict the immune biological markers of prognosis in NSCLC.
Tumor-infiltrating lymphocytes (TILs) are a heterogeneous group of cells that include T cells, B cells, natural killer (NK) cells, and dendritic cells (10), each with distinct roles in the tumor microenvironment. The presence and phenotype of TILs have been correlated with patient outcomes, suggesting that they could serve as potential biomarkers for prognosis (9, 11–15). Researches have shown that the role of tumor-infiltrating immune cells (TILs) in NSCLC is increasingly recognized for its impact on treatment and prognosis (8, 9). In the context of NSCLC, the density and composition of TILs, including CD3+, CD4+, CD8+, and FOXP3+ lymphocytes, have been studied for their association with patient survival and response to therapy. High levels of CD8+ TILs, for example, have been associated with better survival rates, while FOXP3+ regulatory T cells (Tregs) may have a suppressive effect on immune responses (16, 17).
Besides, it is clear that TILs are not only important in NSCLC but also in other cancer types such as breast cancer (18) and melanoma (19). For instance, in breast cancer, the presence of TILs within tumors has been shown to indicate an immunogenic character, with certain subtypes like triple-negative breast cancer (TNBC) and HER2-positive breast cancer demonstrating a higher number of TILs compared to hormone receptor-positive (HR+) subtypes (18). This suggests that TILs could be a common predictive marker across different cancer types.
The interaction between TILs and the tumor microenvironment is complex and can be influenced by various factors, including the tumor’s ability to evade immune detection and the presence of immunosuppressive cells (20). Understanding these dynamics is crucial for developing effective immunotherapies.
While the correlation between TIL phenotypes and local prognosis in NSCLC remains an area of active research, the body of evidence supporting the prognostic significance of TILs is growing. There is a clear need for further studies to refine the understanding of TIL subtypes and their roles in NSCLC, which will be vital for identifying novel and accurate immune biomarkers for prognostic evaluation and guiding treatment strategies in NSCLC and potentially other cancer types.
We conducted a literature search for articles on the prognostic significance of TILs, CD3+, CD4+, CD8+, and FOXP3+ lymphocytes in NSCLC. Our objective is to furnish precise biomarkers for prognostic evaluation and treatment of NSCLC.
2 Materials and methods
The systematic review and meta-analysis conformed to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines, as elucidated in Supplementary Material 1.
2.1 Registration
The research protocol was registered with PROSPERO and assigned the registration number CRD42023468089.
2.2 Literature source
All published literature published prior to December 19, 2023 were searched from databases including PubMed, EMBASE, Web of Science, and the Cochrane Library. The search terms applied in PubMed included “Lung Cancer”, “Pulmonary Neoplasms”, “Cancer of the Lung”, “Pulmonary Cancer”, “Tumor Infiltrating Lymphocyte”, “Tumor-Infiltrating Lymphocyte”, “Tumor Derived Activated Cells”. The detailed strategies for comprehensive searches are presented in Supplementary Material 2.
2.3 Study selection
As inclusion criteria, studies had to report the standard prognostic endpoints of OS and/or disease-free survival (DFS). The target population was all patients with NSCLC who had been tested for levels of TILs or other subtypes including CD3+, CD4+, CD8+, and FOXP3+ lymphocytes. Researches were mandated to be published as original articles and provide ample data necessary for the computation of the hazard ratios (HRs) and 95% CI. To uphold a satisfactory statistical rigor, studies with sample sizes below 30 were excluded. Furthermore, the following reports were excluded: (1) duplicates; (2) case reports; (3) letters, comments, or editorials; (4) reviews and/or meta-analyses; (5) guidelines, notes, or reports; (6) experimental or animal studies; (7) meeting report; (8) clinical trial registration; (9) patients without NSCLC; and (10) survival outcomes such as OS were not reported. The publication year was not restricted.
2.4 Data extraction
The following data were extracted from each study: first author’s name, year of publication, country, study design, type of publication, enrollment period, total patient count, patient age, tumor stage, histologic subtype, phenotype of TILs, geographical location, definition of enriched TILs, outcomes observed, intervention measure, and duration of follow-up.
2.5 Study quality
The evaluation of literature quality was executed utilizing the Newcastle-Ottawa Quality Assessment Scale (NOS). Two authors, QY and SL, scored each study based on the selection of study population (0 - 4 stars), comparability of study groups (0 - 2 stars), determination of outcomes for cohort studies, and exposure for case-control studies (0 - 3 stars). The total NOS scores ranged from 0 to 9 stars, with 0 indicating the lowest quality and 9 signifying the highest. Within this scale, 0 to 3 stars are indicative of low quality, 4 to 6 stars represent medium quality, and 7 to 9 stars denote high quality. Any discrepancies in the evaluation was addressed through discussion, and if necessary, a senior author (V.S.) was consulted.
2.6 Statistical analysis
Statistical analyses were conducted using STATA version 18.0 (MP-Parallel Edition). Given the observed heterogeneity in effect sizes across studies, only the random effects model was utilized for the analysis. The corresponding HR and 95% CI were subsequently summarized. Heterogeneity was assessed employing both the Cochrane Q test and the I² statistic, wherein P < 0.1 or I² > 50% signified noteworthy heterogeneity across the studies. Subgroup analyses and meta-regression was employed to explore the fundamental determinants for the observed heterogeneity. Covariates in this analysis included study quality, tumor location, and population region. Additionally, subgroup analyses were executed utilizing the aforementioned covariates. The Egger test was employed to assess publication bias, with a significance level set at P<0.1.Funnel plot was used to show the associations between HR and standard error for individual studies to assess publication bias. The trim and fill method was proposed to address any detected publication bias.
3 Results
3.1 Study characteristics
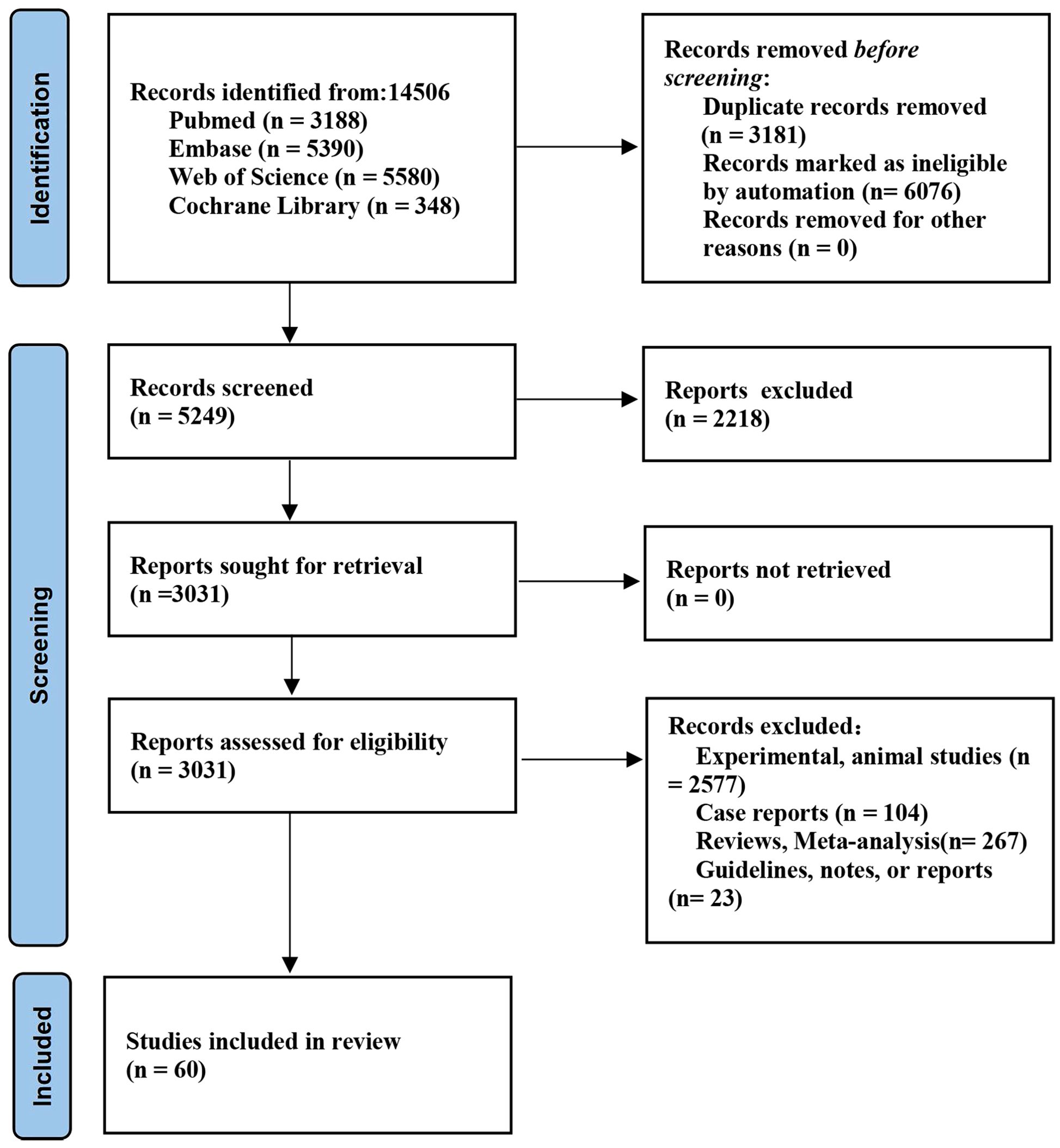
Initially, a total of 14,506 papers were identified from databases. Finally, 60 studies (11–13, 21–77) (15829 patients with NSCLC) were eligible (Figure 1). The characteristics of the incorporated studies are delineated in Supplementary Table S1. The sample size was between 30 and 1546 patients. There were 55 full-text publications and 5 abstracts. The studies were published from 2003 to 2023. 26 studies were conducted in Asia, 20 in Europe, and 8 in North America. 14 studies had moderate quality and 46 high quality (Supplementary Table S1).
3.2 Patient characteristics
The age of patients ranged from 18 to 96 years. Among the patients, 7040 were diagnosed with adenocarcinoma, 5576 with squamous cell carcinoma, and 3213 presented with an unclear pathological type. Furthermore, there were 9390 patients who were diagnosed at stage I-II, 3751 at stage III-IV, and 2688 whose stage was indeterminate.
3.2.1 CD8+ TILs
3.2.1.1 OS
28 publications involving 58 cohorts reported the prognostic significance of CD8+ TIL (11–13, 21–45). As illustrated in Figure 2, a positive correlation was observed between a heightened density of CD8+ TILs and enhanced OS in individuals diagnosed with NSCLC (HR=0.86, 95%CI:0.79-0.94, P<0.05, Figure 2A). The observed heterogeneity in this relationship was statistically significant (I2 = 81.7%, P<0.001). Subgroup analysis indicated a significant correlation between the presence of CD8+TILs in the TC location and improved OS(HR=0.78, 95%CI:0.69-0.88, P<0.001, Figure 2B). In contrast, CD8+TILs in the TS location did not exhibit a significant association with OS(HR=0.95, 95%CI:0.81-1.10, P=0.46, Figure 2B). Moreover, subgroup analysis demonstrated a positive correlation between CD8+TILs and enhanced OS in the European population(HR=0.83, 95%CI: 0.74-0.95, P=0.004, Figure 2C). However, in the Asian (HR=0.82, 95%CI: 0.66-1.02, P=0.07, Figure 2C) and American populations(HR=0.97, 95%CI: 0.89-1.07, P=0.57, Figure 2C), CD8+TILs did not yield a significant association with OS. No heterogeneity sources were identified through regression and sensitivity analyses. A notable publication bias was identified within the investigated studies (P=0.011). An iterative method was employed to estimate for the number of missing studies, which was found to be 7 (Supplementary Figure S1). Consequently, data from seven virtual studies were incorporated into the meta-analysis. The results did not demonstrate any reversal, thereby confirming the robustness of the pooled outcomes.
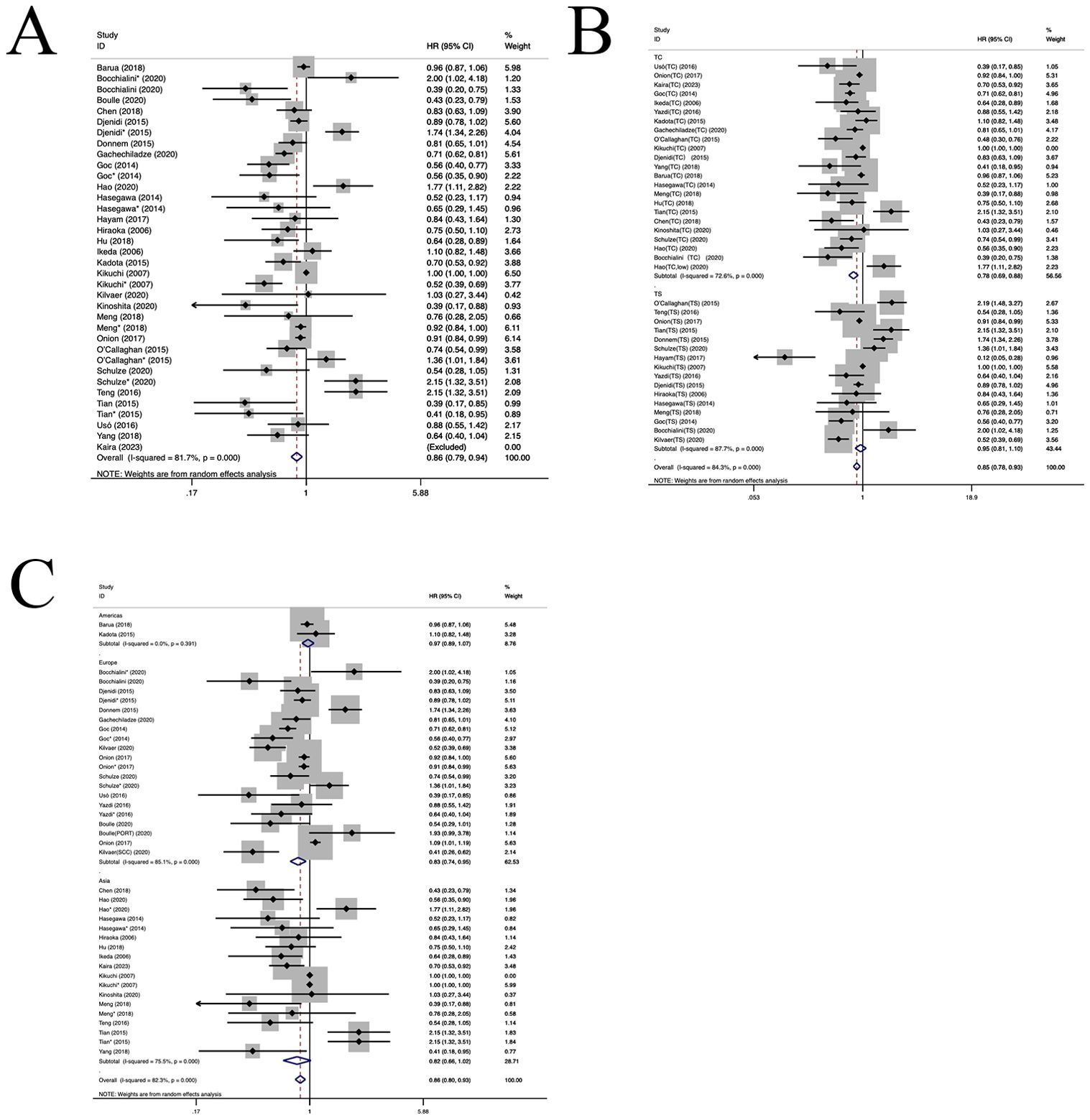
Figure 2. Forest plots of the subgroup analysis of CD8+ TILs on OS in patients with NSCLC. TILs (tumour-infiltrating lymphocytes), OS (overall survival), HRs (hazard ratios), 95% CIs (95% confidence intervals), TC (tumour compartment), TS (tumour stroma), SCC(squamous cell carcinoma), PORT(First had tumour surgical resection followed by treatment with chemotherapy and radiotherapy).* stands for TS. (A) Forest plots of the prognostic value of CD8+TILs on OS in patients with NSCLC; (B) Forest plots of the prognostic value of different locations of CD8+TILs; (C) Forest plots of the prognostic value of different populations of CD8+TILs.
3.2.1.2 DFS
Six studies (21, 22, 25, 26, 41, 46) have estimated the prognostic value of CD8+ TILs. As shown in Figure 3, a higher density of CD8+ TILs was correlated with an improved DFS in patients with NSCLC (HR=0.92, 95%CI: 0.50-1.69, P>0.05, Figure 3A), with significant heterogeneity (I2 = 89.3%, P<0.001). Subgroup analyses revealed no notable difference in DFS between the CD8+TILs in TC location(HR=0.75, 95%CI: 0.41-1.39, P=0.36, Figure 3B) and the TS location(HR=1.30, 95%CI: 0.54-3.15, P=0.56, Figure 3B). Furthermore, in the Asian population, CD8+TILs were associated with better OS(HR=0.40, 95%CI: 0.24-0.65, P<0.001, Figure 3C). However, in the European population (HR=1.27, 95%CI: 0.67-2.41, P=0.46, Figure 3C), CD8+TILs did not yield a significant association with DFS. Both regression and sensitivity analyses failed to pinpoint any sources of heterogeneity. The examined studies did not exhibit any significant evidence of publication bias (P=0.556),as well as shown in the funnel plot (Supplementary Figure S2).
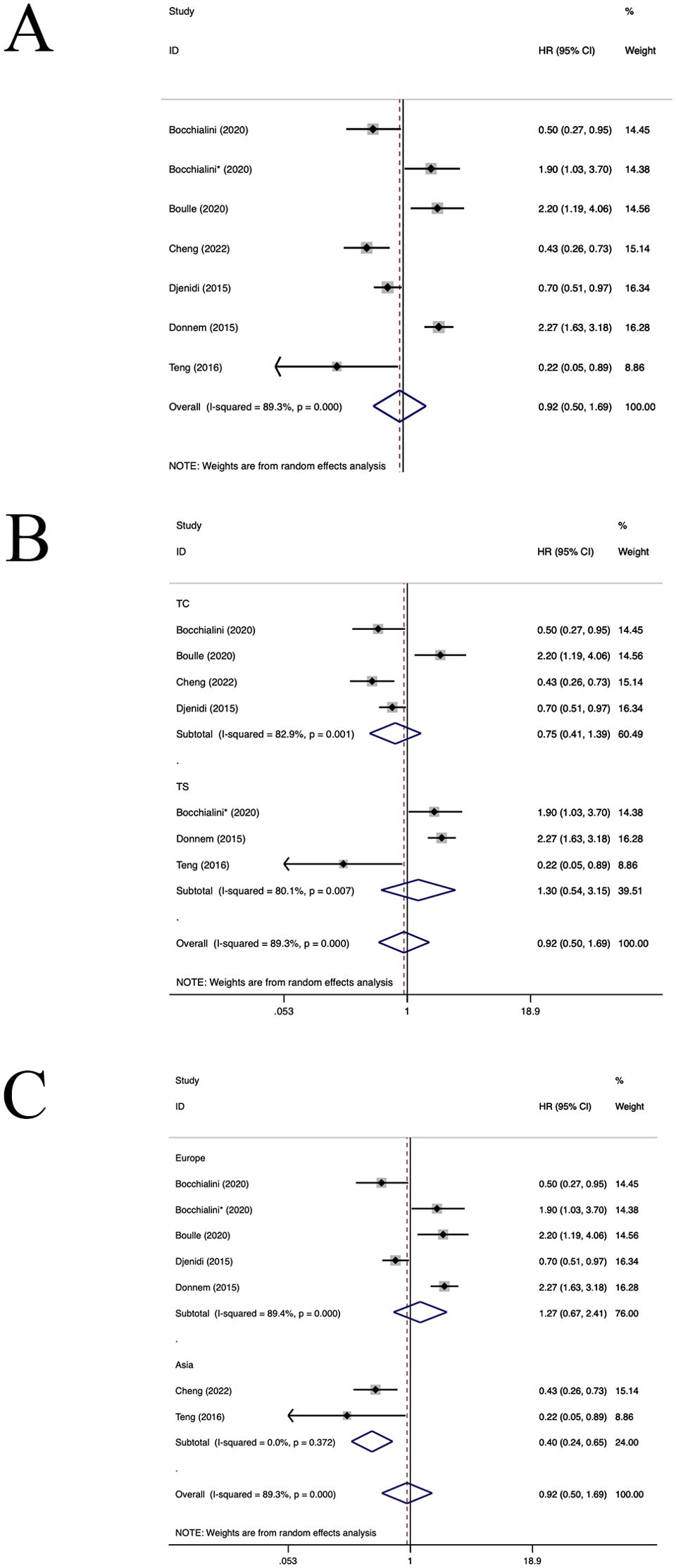
Figure 3. Forest plots of the subgroup analysis of CD8+TILs on DFS in patients with NSCLC. TILs (tumour-infiltrating lymphocytes), DFS (disease-free survival), HRs (hazard ratios), 95% CIs (95% confidence intervals), TC (tumour compartment), TS (tumour stroma).* stands for TS. (A) Forest plots of the prognostic value of CD8+TILs on DFS in patients with NSCLC; (B) Forest plots of the prognostic value of different locations of CD8+TILs; (C) Forest plots of the prognostic value of different populations of CD8+TILs.
3.2.2 FOXP3+ TILs
Twelve studies (23, 29, 30, 34–37, 40, 41, 43, 47, 48) have estimated the prognostic value of FOXP3+TILs. The aggregated findings illustrated a positive correlation between an elevated density of FOXP3+TILs and shortened OS in patients diagnosed with NSCLC (HR=1.35, 95%CI: 0.87-2.11, P>0.05, Figure 4A), with significant aheterogeneity (I2 = 88.7%, P<0.001). Subgroup analyses revealed no notable difference in OS between FOXP3+TILs in TC location (HR=1.40, 95%CI: 0.95-2.06, P=0.09, Figure 4B) and the TS location (HR=1.34, 95%CI: 0.43-4.16, P=0.61, Figure 4B). In the American and European populations, FOXP3+TILs were associated with poorer OS (American population: HR=1.46, 95%CI: 1.11-1.91, P=0.006, European population: HR=2.20, 95%CI: 1.11-4.38, P=0.02, Figure 4C). However, in the Asian and Australian populations (HR=1.27, 95%CI: 0.67-2.41, P=0.46, Figure 3C), FOXP3+TILs did not yield a significant association with OS(Asian population: HR=1.31, 95%CI: 0.75-2.30, P=0.34; Australia population: HR=0.95, 95%CI: 0.06-15.81, P=0.97, Figure 4C). Regression and sensitivity analyses did not pinpoint any heterogeneity sources. Furthermore, our analysis failed to uncover any significant signs of publication bias across the included studies(P=0.619), which is also reflected in the funnel plot (Supplementary Figure S3).
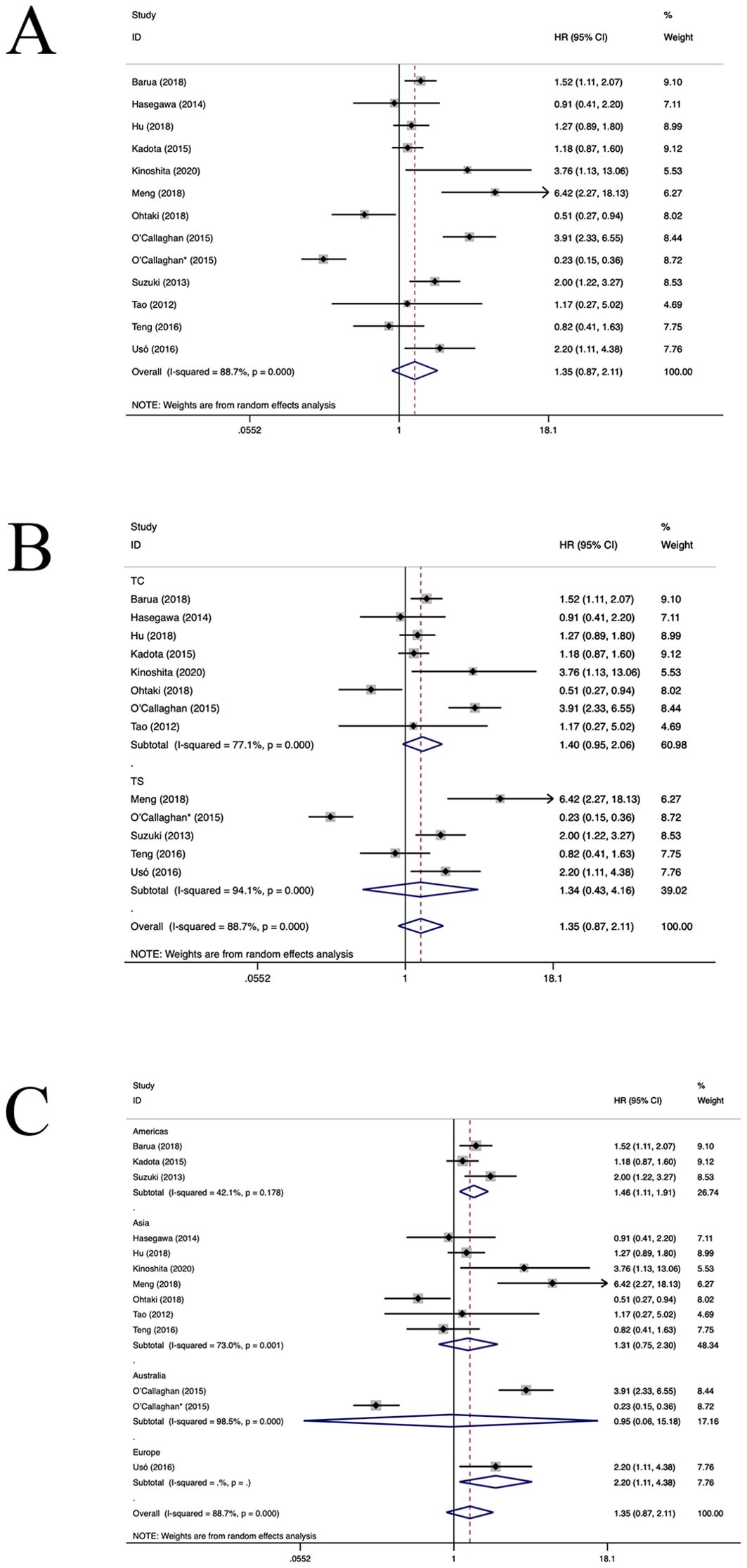
Figure 4. Forest plots of the subgroup analysis of FOXP3+TILs on OS in patients with NSCLC. FOXP3+ (forkhead box P3+),TILs (tumour-infiltrating lymphocytes), OS (overall survival), HRs (hazard ratios), 95% CIs (95% confidence intervals), TC (tumour compartment), TS (tumour stroma). * stands for TS. (A) Forest plots of the prognostic value of FOXP3+TILs on OS in patients with NSCLC; (B) Forest plots of the prognostic value of different locations of FOXP3+ TILs; (C) Forest plots of the prognostic value of different populations of FOXP3+TILs.
3.2.3 CD3+ TILs
Eleven studies assessed the prognostic significance of CD3+ TILs (26, 27, 35, 37, 42, 49–54). As depicted in Figure 5, the pooled analysis revealed that an elevated density of CD3+TILs correlated positively with improved OS for patients diagnosed with NSCLC (HR=0.84, 95%CI: 0.69-1.01, P>0.05, Figure 5A), with significant heterogeneity (I2 = x 78.8%, P<0.001). Upon further subgroup analysis, it was observed no notable difference in OS between CD3+TILs in TC location(HR=0.83, 95%CI: 0.64-1.08, P=0.16, Figure 5B) and the TS location (HR=1.00, 95%CI: 0.72-1.39, P=0.99, Figure 5B). In the European population, CD3+TILs were associated with better OS(HR=0.84, 95%CI: 0.72-0.98, P=0.02, Figure 5C). While in the Australian population(HR=1.55, 95%CI:1.04-2.32, P=0.03, Figure 5C), CD3+TILs were associated with poorer OS. Moreover, CD3+TILs did not yield a significant association with OS in the American (HR=0.69, 95%CI: 0.24-2.02, P=0.16, Figure 5C) and the Asian populations (HR=1.26, 95%CI: 0.33-4.82, P=0.73, Figure 5C). Both regression and sensitivity analyses failed to pinpoint any potential sources of heterogeneity. Moreover, our analysis did not reveal substantial indications of publication bias within the selected studies (P=0.397), which is also reflected in the funnel plot (Supplementary Figure S4).
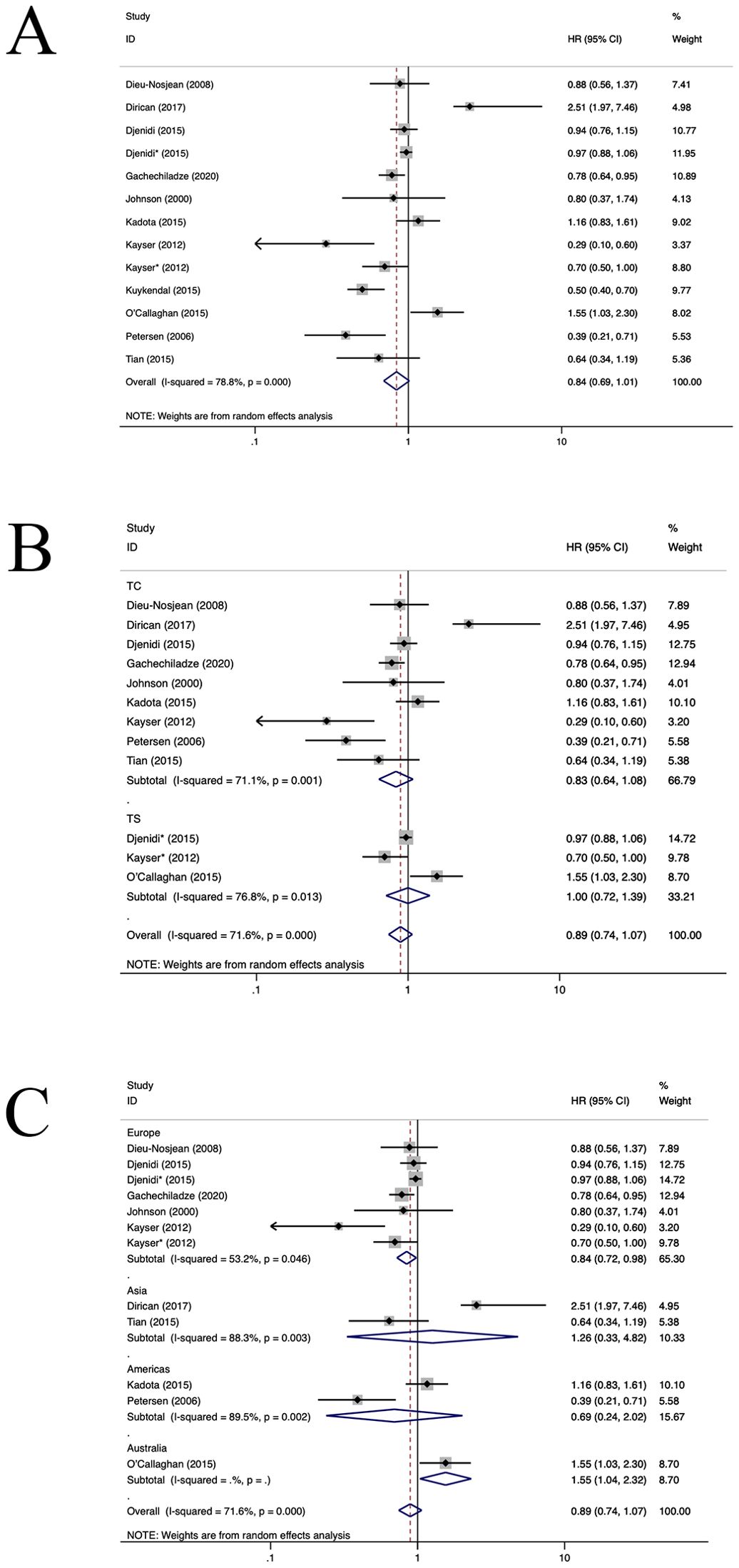
Figure 5. Forest plots of the subgroup analysis of CD3+TILs on OS in patients with NSCLC. TILs (tumour-infiltrating lymphocytes), OS (overall survival), HRs (hazard ratios), 95% CIs (95% confidence intervals), TC (tumour compartment), TS (tumour stroma). * stands for TS. (A) Forest plots of the prognostic value of CD3+TILs on OS in patients with NSCLC; (B) Forest plots of the prognostic value of different locations of CD3+TILs; (C) Forest plots of the prognostic value of different populations of CD3+TILs.
3.2.4 TILs
Ten selected articles evaluated the prognostic significance of TILs (11, 27, 55–62) (Figure 6). The findings demonstrated that an elevated density of TILs correlated with improved OS for patients with NSCLC (HR=0.67, 95%CI: 0.55-0.81, P<0.05, Figure 6A). Notably, there was statistically significant heterogeneity observed in the data (I2 = 60.0%, P<0.001). In the European population, TILs+ were correlated with an improved OS (HR=0.66, 95%CI: 0.54-0.81, P<0.001, Figure 6B). Both regression and sensitivity analyses failed to pinpoint the source of this observed heterogeneity. Furthermore, no significant publication bias was evident among the included studies (P=0.284), which is also reflected in the funnel plot (Supplementary Figure S5).
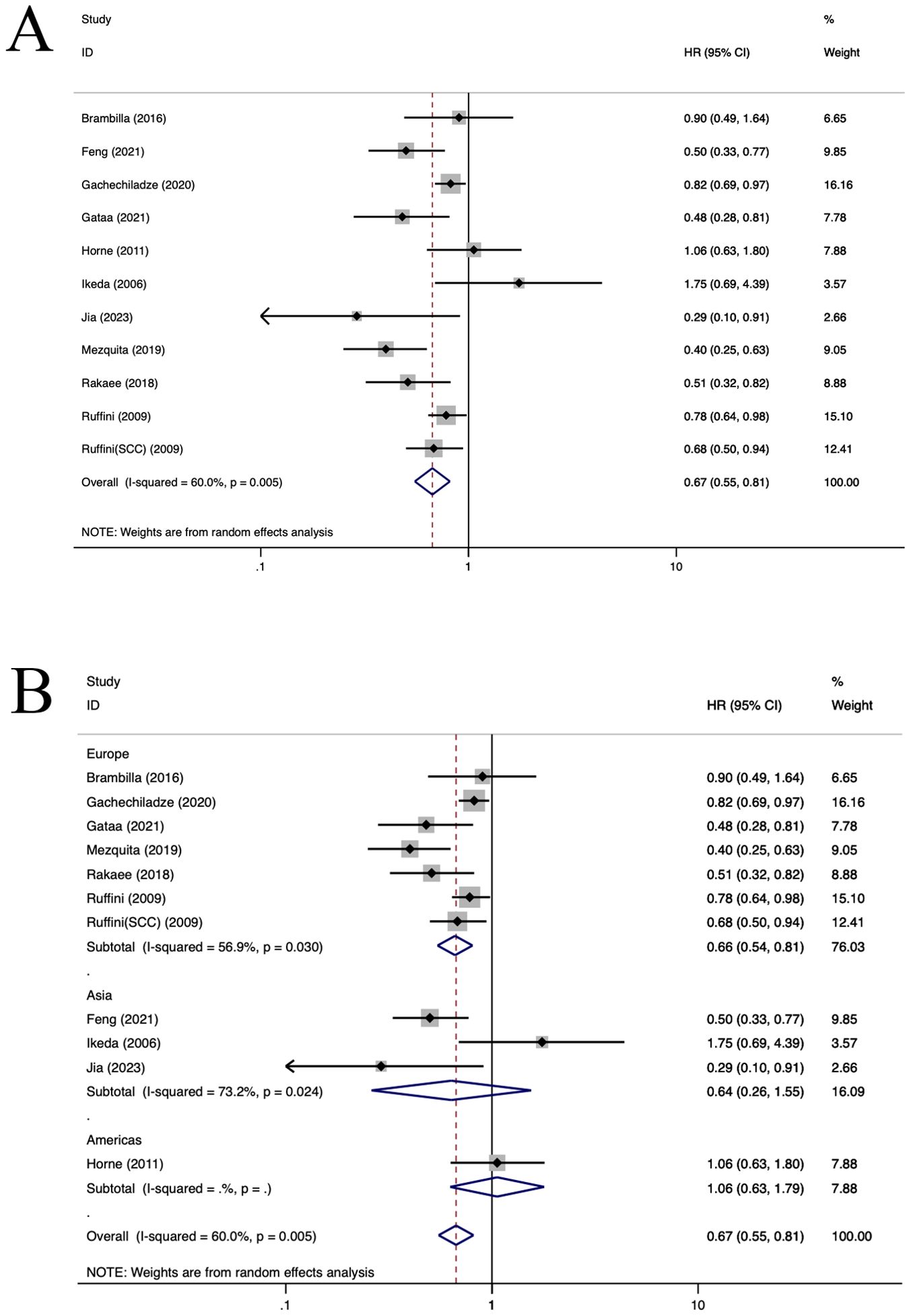
Figure 6. Forest plots of the subgroup analysis of TILs on OS in patients with NSCLC. TILs (tumour-infiltrating lymphocytes), OS (overall survival), HRs (hazard ratios), 95% CIs (95% confidence intervals), SCC(squamous cell carcinoma), TC (tumour compartment), TS (tumour stroma). (A) Forest plots of the prognostic value of TILs on OS in patients with NSCLC; (B) Forest plots of the prognostic value of different populations of TILs.
3.2.5 CD4+ TILs
Ten studies (12, 23, 29, 31, 35, 36, 40, 47, 59, 63) assessed the prognostic significance of CD4+ TILs. A positive relationship was observed between a heightened density of CD4+ TILs and enhanced OS in patients with NSCLC (HR=0.90, 95%CI: 0.77-1.05, P>0.05, Figure 7A), with significant heterogeneity (I2 = 39.2%, P=0.065). Subgroup analysis indicated that there was no notable difference in OS between CD4+TILs in TC location (HR=0.82, 95%CI: 0.64-1.04, P=0.10, Figure 7B) and the TS location(HR=0.98, 95%CI: 0.78-1.23, P=0.86, Figure 7B). In Asian, the population of CD4+TILs exhibited a longer OS (HR=0.80, 95%CI: 0.66-0.96, P=0.01, Figure 7C). No heterogeneity sources were identified through regression and sensitivity analyses. No statistically significant publication bias was identified within the study populations (P=0.181), which is also reflected in the funnel plot (Supplementary Figure S6).
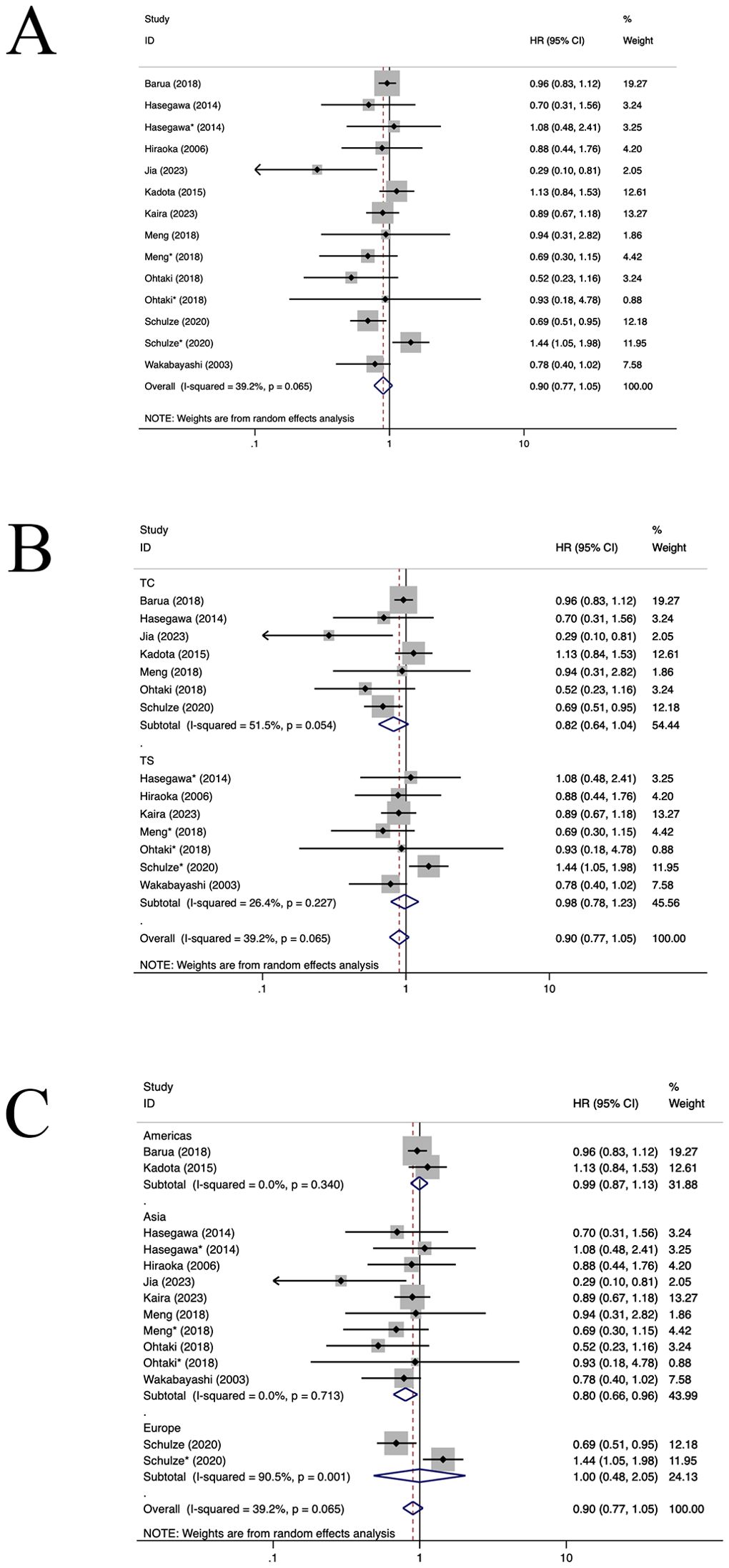
Figure 7. Forest plots of the subgroup analysis of CD4+TILs on OS in patients with NSCLC. TILs (tumour-infiltrating lymphocytes), OS (overall survival), HRs (hazard ratios), 95% CIs (95% confidence intervals), TC (tumour compartment), TS (tumour stroma). * stands for TS. (A) Forest plots of the prognostic value of CD4+TILs on OS in patients with NSCLC; (B) Forest plots of the prognostic value of different locations of CD4+TILs; (C) Forest plots of the prognostic value of different populations of CD4+TILs.
4 Discussion
Research has shown that immune cell infiltration, including TILs, plays a significant role in cancer prognosis, with some studies suggesting a link between TILs and patient survival rates in breast cancer (18), melanoma (19) and NSCLC. However, the correlation between TIL subtypes and survival remains a topic of debate (8, 23). Our study, which incorporated a comprehensive analysis of 60 articles and 15,829 NSCLC patients, utilized a random effects model for meta-analysis. Data extracted from 48 articles (11–13, 21–64, 76) were used to evaluate the prognostic role of TILs in NSCLC. Our research indicates that TILs, particularly CD8+, CD3+ and CD4+ TILs, are associated with improved prognosis in NSCLC, supporting the notion that these immune cells contribute to anti-tumor immunity.
Building upon previous meta-analyses that were limited by smaller sample sizes and a lack of analysis on the impact of different regional populations, our study expanded the sample size and conducted a thorough analysis of regional population characteristics and sources of heterogeneity. This approach allowed for a more robust and comprehensive understanding of the relationship between TILs and NSCLC prognosis. Our research demonstrated that both the TILs infiltration location and the region of population were linked with improved prognosis in NSCLC. However, the manifestation of this association varies in intensity across different subtypes of TILs. There is compelling evidence suggesting that TILs are indicative of the adaptive anti-tumor immune response, typically associated with a favorable prognosis (29). This observation aligns with our study’s findings. The subgroup analysis suggested that the spatial distribution of TILs might offer a plausible explanation for the observed heterogeneity. Additionally, we discovered that the prognosis was relatively better among individuals from the European and Asian populations, potentially attributable to racial heterogeneity across different populations. Further investigation is warranted to elucidate this phenomenon.
TIL subtypes predominantly encompass CD3+ TILs, CD4+ TILs, CD8+ TILs, and FOXP3+ TILs. The prognostic significance of CD3+ TILs in NSCLC remains debatable. Some investigations have identified an imbalance in the expression of NKG2D and NKG2A receptors on CD3+ lymphocytes. This imbalance may correlate with poor prognosis, heightened malignancy, and immune evasion in advanced cancer stages, and is implicated in the progression of lung cancer (78). However, existing evidence suggests that the infiltration of augmented CD3+ T cells independently predicts clinical benefit in patients with NSCLC (42, 64). Our study corroborated a superior prognosis associated with CD3+ TILs in NSCLC, despite no statistically significant variance. Consequently, further research is warranted to clarify the function of CD3+ TILs within the immune microenvironment of NSCLC.
Throughout the course of tumor progression, cytotoxic T lymphocytes (CTLs) may exhibit functional impairment or exhaustion, attributable to immune-related tolerance and immunosuppression within the tumor microenvironment. This can lead to adaptive immune resistance (79). The primary anti-tumor immune cells in this environment are CD4+ and CD8+ T cells (80). Prior studies have elucidated that antigen-specific CD4+ T cells contribute to anti-tumor immunity by aiding CD8+T cells (81). We have collated pertinent studies to assess the prognostic significance of CD4+TILs and CD8+TILs in NSCLC. Our findings indicate that CD4+TILs correlate with a favorable prognosis in NSCLC patients with no statistical significance, whereas CD8+TILs are associated with enhanced OS(with statistically significant), aligning with prior research outcomes (82). However, some evidence suggests that tumor-infiltrating CD4+ and CD8+ T cells do not correlate with OS or DFS. In contrast, high stromal infiltration of CD4+ T cells has been associated with improved OS in patients with NSCLC (83, 84). Contradictory conclusions from multiple studies may be attributed to the hierarchical levels of stromal-infiltrating immune cells (85). Further research is required to clarify the prognostic significance of stromal-infiltrating immune cells at different concentrations in NSCLC.
FOXP3, a significant regulatory factor for regulatory T cells, is frequently found to be aberrantly expressed in lung cancer cells (86). Recent studies have increasingly underscored the oncogenic role of FOXP3 in lung cancer. These investigations propose that FOXP3 functions as a co-activator to facilitate the Wnt-β-catenin signaling pathway and induce epithelial-mesenchymal transition, thereby promoting tumor growth and metastasis in NSCLC (16, 17). Additionally, FOXP3 may enhance the invasiveness and migratory capabilities of NSCLC cells by modulating the vascular endothelial growth factor (VEGF), EMT, and Notch1/Hes1 pathways (87). Our research also revealed an association between FOXP3 expression in NSCLC and poor prognosis. This could potentially be attributed to the potential of FOXP3 regulatory T cells to obstruct effective anti-tumor immune responses (88). Grell et al. documented a correlation associating FOXP3 positivity with reduced PFS and OS (89). However, another study suggested that tumor FOXP3 expression held prognostic value and improved survival outcomes in NSCLC (48, 82). The conflicting findings from these studies underscore the necessity for further comprehensive research into the role of FOXP3 in NSCLC.
While our meta-analysis is not the inaugural study to explore the relationship between TILs and NSCLC prognosis, it may yield divergent conclusions from prior research. Our inclusion of 60 articles and 15829 patients substantially expands upon sample sizes in previous studies. Notably, neither subgroup regression analysis nor sensitivity analysis were conducted in earlier investigations. Our findings underscore the prognostic significance of TILs in NSCLC, with a more pronounced effect observed among the European and Asian populations. Specifically, CD4+TILs in the Asian population, CD3+TILs in the European population, CD8+TILs in the Asian and European populations emerged as favorable prognostic biomarkers for NSCLC. Moreover, CD8+TILs demonstrated a marked survival advantage specifically when they infiltrated the tumor. This was evidenced by a survival benefit in the European population, as well as a significant enhancement in DFS for NSCLC in the Asian population. In contrast, the prognosis of FOXP3+TILs was not significantly improved in NSCLC across all ethnicities, and even worse in the American and European populations. These findings suggest that distinct TIL subtypes exert varied prognostic roles in NSCLC patients, rendering them viable biological markers for treatment strategies, prognostic evaluations, and recurrence or metastasis monitoring.
Despite the comprehensiveness of this study, certain limitations must be acknowledged. Primarily, significant heterogeneity was observed in most of the pooled results, with no substantial differences identified through subgroup analysis or meta-regression. This heterogeneity could potentially be attributed to individual patient variations within the clinical studies, including the varying quality levels of these studies, and the differing assessment criteria for TIL density. Additionally, selective reporting of positive data in some studies could compromise the authenticity of the findings. Addressing these limitations in future research will be crucial for refining our understanding of the complex interplay between TILs and NSCLC prognosis.
Conclusions
Our research indicates that TILs, particularly CD8+, CD3+ and CD4+TILs, are associated with improved prognosis in NSCLC, supporting the notion that these immune cells contribute to anti-tumor immunity. The conflicting conclusions from various studies highlight the need for further research to elucidate the prognostic significance of TIL subtypes in NSCLC and their potential as biological markers for treatment strategies and prognostic evaluations.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Author contributions
QY: Writing – original draft, Writing – review & editing. SL: Writing – original draft, Writing – review & editing. LH: Writing – review & editing, Resources, Supervision. NC: Writing – review & editing, Project administration, Supervision.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. The study was supported by the Technological Innovation Research and Development Projects of Chengdu Science and Technology Bureau (2022-YF05-01677-SN), the Sichuan Medical Research Youth Innovation Project of Sichuan Medical Association (Q21102), and the Research Project of Chengdu Health Commission (2023108).
Acknowledgments
We would like to thank the researchers and study participants for their contributions.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2024.1476365/full#supplementary-material
Abbreviations
TILs, Tumor-infiltrating lymphocytes; NSCLC, non-small cell lung cancer; OS, overall survival; DFS, disease-free survival; CIs, Confidence Intervals; HRs, Hazard Ratios.
References
1. Siegel RL, Miller KD, Wagle NS, Jemal A. Cancer statistic. CA Cancer J Clin. (2023) 73:17–48. doi: 10.3322/caac.21763
2. Li Q, Wang R, Yang Z, Li W, Yang J, Wang Z, et al. Molecular profiling of human non-small cell lung cancer by single-cell RNA-seq. Genome Med. (2022) 14:87. doi: 10.1186/s13073-022-01089-9
3. Sugarbaker DJ, Strauss GM. Extent of surgery and survival in early lung carcinoma: implications for overdiagnosis in stage IA nonsmall cell lung carcinoma. Cancer. (2000) 89:2432–7. doi: 10.1002/1097-0142(20001201)89:11+<2432::aid-cncr17>3.3.co;2-1
4. Chen L, Fang W. A review on comparison of lobectomy and segmentectomy in the treatment of early stage non-small cell lung cancer. Zhongguo Fei Ai Za Zhi. (2019) 22:526–31. doi: 10.3779/j.issn.1009-3419.2019.08.08
5. Edelman MJ, Schuetz J. Follow-up of local (stage I and stage II) non-small-cell lung cancer after surgical resection. Curr Treat Options Oncol. (2002) 3:67–73. doi: 10.1007/s11864-002-0043-y
6. Shao W, Wang D, He J. The role of gene expression profiling in early-stage non-small cell lung cancer. J Thorac Dis. (2010) 2:89–99.
7. Rao S, Ye L, Cui X, Sun Q, Cao R, Xiao S, et al. Progress in survival prognosis of segmentectomy for early-stage non-small cell lung cancer. Zhongguo Fei Ai Za Zhi. (2020) 23:830–6. doi: 10.3779/j.issn.1009-3419.2020.102.21
8. Federico L, McGrail DJ, Bentebibel SE, Haymaker C, Ravelli A, Forget MA, et al. Distinct tumor-infiltrating lymphocyte landscapes are associated with clinical outcomes in localized non-small-cell lung cancer. Ann Oncol. (2022) 33:42–56. doi: 10.1016/j.annonc.2021.09.021
9. Gueguen P, Metoikidou C, Dupic T, Lawand M, Goudot C, Baulande S, et al. Contribution of resident and circulating precursors to tumor-infiltrating CD8(+) T cell populations in lung cancer. Sci Immunol. (2021) 6(55):eabd5778. doi: 10.1126/sciimmunol.abd5778
10. Paijens ST, Vledder A, de Bruyn M, Nijman HW. Tumor-infiltrating lymphocytes in the immunotherapy era. Cell Mol Immunol. (2021) 18:842–59. doi: 10.1038/s41423-020-00565-9
11. Ikeda S, Funakoshi N, Inagaki M, Shibata T. Clinicopathologic roles of tumor-infiltrating lymphocytes and CD8-positive lymphocytes in lung cancer imprint smears in squamous cell carcinoma and adenocarcinoma. Acta Cytol. (2006) 50:423–9. doi: 10.1159/000325986
12. Hiraoka K, Miyamoto M, Cho Y, Suzuoki M, Oshikiri T, Nakakubo Y, et al. Concurrent infiltration by CD8+ T cells and CD4+ T cells is a favourable prognostic factor in non-small-cell lung carcinoma. Br J Cancer. (2006) 94:275–80. doi: 10.1038/sj.bjc.6602934
13. Goc J, Germain C, Vo-Bourgais TK, Lupo A, Klein C, Knockaert S, et al. Dendritic cells in tumor-associated tertiary lymphoid structures signal a Th1 cytotoxic immune contexture and license the positive prognostic value of infiltrating CD8+ T cells. Cancer Res. (2014) 74:705–15. doi: 10.1158/0008-5472.Can-13-1342
14. Corredor G, Wang X, Zhou Y, Lu C, Fu P, Syrigos K, et al. Spatial architecture and arrangement of tumor-infiltrating lymphocytes for predicting likelihood of recurrence in early-stage non-small cell lung cancer. Clin Cancer Res. (2019) 25:1526–34. doi: 10.1158/1078-0432.Ccr-18-2013
15. Lopez de Rodas M, Nagineni V, Ravi A, Datar IJ, Mino-Kenudson M, Corredor G, et al. Role of tumor infiltrating lymphocytes and spatial immune heterogeneity in sensitivity to PD-1 axis blockers in non-small cell lung cancer. J Immunother Cancer. (2022) 10(6):e004440. doi: 10.1136/jitc-2021-004440
16. Yang S, Liu Y, Li MY, Ng CSH, Yang SL, Wang S, et al. FOXP3 promotes tumor growth and metastasis by activating Wnt/β-catenin signaling pathway and EMT in non-small cell lung cancer. Mol Cancer. (2017) 16:124. doi: 10.1186/s12943-017-0700-1
17. Zhu J, Li Z, Chen J, Li W, Wang H, Jiang T, et al. A comprehensive bioinformatics analysis of FOXP3 in nonsmall cell lung cancer. Med (Baltimore). (2022) 101:e32102. doi: 10.1097/md.0000000000032102
18. Ye F, Dewanjee S, Li YH, Jha NK, Chen ZS, Kumar A, et al. Advancements in clinical aspects of targeted therapy and immunotherapy in breast cancer. Mol Cancer. (2023) 22:105. doi: 10.1186/s12943-023-01805-y
19. Zhao JL, Li DD, Xie SZ, Deng XP, Wen XZ, Li JJ, et al. Nomogram for predicting prognosis of patients with metastatic melanoma after immunotherapy: A Chinese population-based analysis. Front Immunol. (2023) 13:1083840.eCollection 2022. doi: 10.3389/fimmu.2022.1083840
20. Xie JD, Liu MQ, Deng XP, Tang YH, Zheng SQ, Ou XQ, et al. Gut microbiota reshapes cancer immunotherapy efficacy: Mechanisms and therapeutic strategies. Imeta. (2024) 3:e156. doi: 10.1002/imt2.156
21. Bocchialini G, Lagrasta C, Madeddu D, Mazzaschi G, Marturano D, Sogni F, et al. Spatial architecture of tumour-infiltrating lymphocytes as a prognostic parameter in resected non-small-cell lung cancer. Eur J Cardiothorac Surg. (2020) 58:619–28. doi: 10.1093/ejcts/ezaa098
22. Boulle G, Velut Y, Mansuet-Lupo A, Gibault L, Blons H, Fournel L, et al. Chemoradiotherapy efficacy is predicted by intra-tumour CD8+/FOXP3+ double positive T cell density in locally advanced N2 non-small-cell lung carcinoma. Eur J Cancer. (2020) 135:221–9. doi: 10.1016/j.ejca.2020.04.040
23. Barua S, Fang P, Sharma A, Fujimoto J, Wistuba I, Rao AUK, et al. Spatial interaction of tumor cells and regulatory T cells correlates with survival in non-small cell lung cancer. Lung Cancer. (2018) 117:73–9. doi: 10.1016/j.lungcan.2018.01.022
24. Chen J, He Q, Liu J, Xiao Y, Xiao C, Chen K, et al. CD8+ tumor-infiltrating lymphocytes as a novel prognostic biomarker in lung sarcomatoid carcinoma, a rare subtype of lung cancer. Cancer Manag Res. (2018) 10:3505–11. doi: 10.2147/cmar.S169074
25. Donnem T, Hald SM, Paulsen EE, Richardsen E, Al-Saad S, Kilvaer TK, et al. Stromal CD8+ T-cell density—A promising supplement to TNM staging in non-small cell lung cancer. Clin Cancer Res. (2015) 21:2635–43. doi: 10.1158/1078-0432.Ccr-14-1905
26. Djenidi F, Adam J, Goubar A, Durgeau A, Meurice G, de Montpréville V, et al. CD8+CD103+ tumor-infiltrating lymphocytes are tumor-specific tissue-resident memory T cells and a prognostic factor for survival in lung cancer patients. J Immunol. (2015) 194:3475–86. doi: 10.4049/jimmunol.1402711
27. Gachechiladze M, Škarda J, Skanderová D, Überall I, Kolek V, Smičkova P, et al. Prognostic value of tumor-infiltrating lymphocytes (TILs) and their association with PD-L1 expression and DNA repair protein RAD51 in patients with resected non-small cell lung carcinoma. Lung Cancer. (2020) 147:30–8. doi: 10.1016/j.lungcan.2020.06.025
28. Hao J, Wang H, Song L, Li S, Che N, Zhang S, et al. Infiltration of CD8(+) FOXP3(+) T cells, CD8(+) T cells, and FOXP3(+) T cells in non-small cell lung cancer microenvironment. Int J Clin Exp Pathol. (2020) 13:880–8.
29. Hasegawa T, Suzuki H, Yamaura T, Muto S, Okabe N, Osugi J, et al. Prognostic value of peripheral and local forkhead box P3(+) regulatory T cells in patients with non-small-cell lung cancer. Mol Clin Oncol. (2014) 2:685–94. doi: 10.3892/mco.2014.299
30. Hu Z, Gu X, Zhong R, Zhong H. Tumor-infiltrating CD45RO(+) memory cells correlate with favorable prognosis in patients with lung adenocarcinoma. J Thorac Dis. (2018) 10:2089–99. doi: 10.21037/jtd.2018.03.148
31. Kaira K, Yamaguchi O, Kawasaki T, Hashimoto K, Miura Y, Shiono A, et al. Prognostic significance of tumor infiltrating lymphocytes on first-line pembrolizumab efficacy in advanced non-small cell lung cancer. Discovery Oncol. (2023) 14:6. doi: 10.1007/s12672-023-00615-4
32. Kilvaer TK, Paulsen EE, Andersen S, Rakaee M, Bremnes RM, Busund LR, et al. Digitally quantified CD8+ cells: the best candidate marker for an immune cell score in non-small cell lung cancer? Carcinogenesis. (2020) 41:1671–81. doi: 10.1093/carcin/bgaa105
33. Kikuchi E, Yamazaki K, Torigoe T, Cho Y, Miyamoto M, Oizumi S, et al. HLA class I antigen expression is associated with a favorable prognosis in early stage non-small cell lung cancer. Cancer Sci. (2007) 98:1424–30. doi: 10.1111/j.1349-7006.2007.00558.x
34. Kinoshita F, Takada K, Yamada Y, Oku Y, Kosai K, Ono Y, et al. Combined evaluation of tumor-infiltrating CD8 + and FOXP3 + Lymphocytes provides accurate prognosis in stage IA lung adenocarcinoma. Ann Surg Oncol. (2020) 27:2102–9. doi: 10.1245/s10434-019-08029-9
35. Kadota K, Nitadori JI, Ujiie H, Buitrago DH, Woo KM, Sima CS, et al. Prognostic impact of immune microenvironment in lung squamous cell carcinoma: tumor-infiltrating CD10+ Neutrophil/CD20+ Lymphocyte ratio as an independent prognostic factor. J Thorac Oncol. (2015) 10:1301–10. doi: 10.1097/jto.0000000000000617
36. Meng X, Gao Y, Yang L, Jing H, Teng F, Huang Z, et al. Immune microenvironment differences between squamous and non-squamous non-small-cell lung cancer and their influence on the prognosis. Clin Lung Cancer. (2018) 20:48–58. doi: 10.1016/j.cllc.2018.09.012
37. O’Callaghan DS, Rexhepaj E, Gately K, Coate L, Delaney D, O’Donnell DM, et al. Tumour islet FOXP3+ T-cell infiltration predicts poor outcome in nonsmall cell lung cancer. Eur Respir J. (2015) 46:1762–72. doi: 10.1183/13993003.00176-2014
38. Onion D, Isherwood M, Shridhar N, Xenophontos M, Craze ML, Day LJ, et al. Multicomponent analysis of the tumour microenvironment reveals low CD8 T cell number, low stromal caveolin-1 and high tenascin-C and their combination as significant prognostic markers in non-small cell lung cancer. Oncotarget. (2017) 9:1760–71. doi: 10.18632/oncotarget.18880
39. Hayam RE, Abdelrahman AE, Abdelgawad M, Balata S, Shabrawy ME. Prognostic significance of programmed cell death ligand 1 (PD-L1), CD8+ Tumor-infiltrating lymphocytes and p53 in non-small cell lung cancer: an immunohistochemical study. Turk Patoloji Derg. (2017) 1:211–22. doi: 10.5146/tjpath.2017.01398
40. Schulze AB, Evers G, Görlich D, Mohr M, Marra A, Hillejan L, et al. Tumor infiltrating T cells influence prognosis in stage I-III non-small cell lung cancer. J Thorac Dis. (2020) 12:1824–42. doi: 10.21037/jtd-19-3414a
41. Teng F, Meng X, Wang X, Yuan J, Liu S, Mu D, et al. Expressions of CD8+TILs, PD-L1 and FOXP3+TILs in stage I NSCLC guiding adjuvant chemotherapy decisions. Oncotarget. (2016) 7:64318–29. doi: 10.18632/oncotarget.11793
42. Tian C, Lu S, Fan Q, Zhang W, Jiao S, Zhao X, et al. Prognostic significance of tumor-infiltrating CD8+ or CD3+ T lymphocytes and interleukin-2 expression in radically resected non-small cell lung cancer. Chin Med J (Engl). (2015) 128:105–10. doi: 10.4103/0366-6999.147828
43. Usó M, Jantus-Lewintre E, Bremnes RM, Calabuig S, Blasco A, Pastor E, et al. Analysis of the immune microenvironment in resected non-small cell lung cancer: the prognostic value of different T lymphocyte markers. Oncotarget. (2016) 7:52849–61. doi: 10.18632/oncotarget.10811
44. Yang H, Shi J, Lin D, Li X, Zhao C, Wang Q, et al. Prognostic value of PD-L1 expression in combination with CD8(+) TILs density in patients with surgically resected non-small cell lung cancer. Cancer Med. (2018) 7:32–45. doi: 10.1002/cam4.1243
45. Yazdi M, van Riet S, van SChadewijk A, Fiocco M, van Hall T, Taube C, et al. The positive prognostic effect of stromal CD8+ tumor-infiltrating T cells is restrained by the expression of HLA-E in non-small cell lung carcinoma. Oncotarget. (2016) 7:3477–88. doi: 10.18632/oncotarget.6506
46. Cheng X, Wang L, Zhang Z. Prognostic significance of PD-L1 expression and CD8(+) TILs density for disease-free survival in surgically resected lung squamous cell carcinoma: a retrospective study. J Thorac Dis. (2022) 14:2224–34. doi: 10.21037/jtd-22-630
47. Ohtaki Y, Kaira K, Atsumi J, Nagashima T, Kawashima O, Ibe T, et al. Prognostic significance of PD-L1 expression and tumor infiltrating lymphocytes in large cell neuroendocrine carcinoma of lung. Am J Transl Res. (2018) 10:3243–53.
48. Tao H, Mimura Y, Aoe K, Kobayashi S, Yamamoto H, Matsuda E, et al. Prognostic potential` of FOXP3 expression `in non-small cell lung cancer cells combined with tumor-infiltrating regulatory T cells. Lung Cancer. (2012) 75:95–101. doi: 10.1016/j.lungcan.2011.06.002
49. Dieu-Nosjean MC, Antoine M, Danel C, Heudes D, Wislez M, Poulot V, et al. Long-term survival for patients with non-small-cell lung cancer with intratumoral lymphoid structures. J Clin Oncol. (2008) 26:4410–7. doi: 10.1200/jco.2007.15.0284
50. Dirican N, Karakaya YA, Gunes S, Daloglu FT, Dirican A. Association of intra-tumoral tumour-infiltrating lymphocytes and neutrophil-to-lymphocyte ratio is an independent prognostic factor in non-small cell lung cancer. Clin Respir J. (2017) 11:789–96. doi: 10.1111/crj.12417
51. Johnson SK, Kerr KM, Chapman AD, Kennedy MM, King G, Cockburn JS, et al. Immune cell infiltrates and prognosis in primary carcinoma of the lung. Lung Cancer. (2000) 27:27–35. doi: 10.1016/s0169-5002(99)00095-1
52. Kayser G, Schulte-Uentrop L, Sienel W, Werner M, Fisch P, Passlick B, et al. Stromal CD4/CD25 positive T-cells are a strong and independent prognostic factor in non-small cell lung cancer patients, especially with adenocarcinomas. Lung Cancer. (2012) 76:445–51. doi: 10.1016/j.lungcan.2012.01.004
53. Kuykendal AT, Khalil F, Haura E, Antonia SJ, Schabath M, Gabrilovich D, et al. Distribution of immune markers and their association with overall survival and time to progression in non-small-cell lung cancer (NSCLC). J Thorac Oncol. (2015) 10:S268–S9.
54. Petersen RP, Campa MJ, Sperlazza J, Conlon D, Joshi MB, Harpole DH Jr., et al. Tumor infiltrating FOXP3+ regulatory T-cells are associated with recurrence in pathologic stage I NSCLC patients. Cancer. (2006) 107:2866–72. doi: 10.1002/cncr.22282
55. Brambilla E, Le Teuff G, Marguet S, Lantuejoul S, Dunant A, Graziano S, et al. Prognostic effect of tumor lymphocytic infiltration in resectable non-small-cell lung cancer. J Clin Oncol. (2016) 34:1223–30. doi: 10.1200/jco.2015.63.0970
56. Feng W, Li Y, Shen L, Zhang Q, Cai XW, Zhu ZF, et al. Clinical impact of the tumor immune microenvironment in completely resected stage IIIA(N2) non-small cell lung cancer based on an immunoscore approach. Ther Adv Med Oncol. (2021) 13:1758835920984975. doi: 10.1177/1758835920984975
57. Gataa I, Mezquita L, Rossoni C, Auclin E, Kossai M, Aboubakar F, et al. Tumour-infiltrating lymphocyte density is associated with favourable outcome in patients with advanced non-small cell lung cancer treated with immunotherapy. Eur J Cancer. (2021) 145:221–9. doi: 10.1016/j.ejca.2020.10.017
58. Horne ZD, Jack R, Gray ZT, Siegfried JM, Wilson DO, Yousem SA, et al. Increased levels of tumor-infiltrating lymphocytes are associated with improved recurrence-free survival in stage 1A non-small-cell lung cancer. J Surg Res. (2011) 171:1–5. doi: 10.1016/j.jss.2011.03.068
59. Jia W, Guo H, Wang M, Li J, Yu J, Zhu H, et al. High post-chemotherapy TIL and increased CD4+TIL are independent prognostic factors of surgically resected NSCLC following neoadjuvant chemotherapy. MedComm. (2023) . 4:e213. doi: 10.1002/mco2.213
60. Mezquita L, Preeshagul I, Auclin E, Saravia D, Hendriks L, Rizvi H, et al. Predicting immunotherapy outcomes under therapy in patients with advanced NSCLC using dNLR and its early dynamics. Eur J Cancer. (2019) 151:211–20. doi: 10.1016/j.ejca.2021.03.011
61. Rakaee M, Kilvaer TK, Dalen SM, Richardsen E, Paulsen EE, Hald SM, et al. Evaluation of tumor-infiltrating lymphocytes using routine H&E slides predicts patient survival in resected non-small cell lung cancer. Hum Pathol. (2018) 79:188–98. doi: 10.1016/j.humpath.2018.05.017
62. Ruffini E, Asioli S, Filosso PL, Lyberis P, Bruna MC, Macrì L, et al. Clinical significance of tumor-infiltrating lymphocytes in lung neoplasms. Ann Thorac Surg. (2009) 87:365–71. doi: 10.1016/j.athoracsur.2008.10.067
63. Wakabayashi O, Yamazaki K, Oizumi S, Hommura F, Kinoshita I, Ogura S, et al. CD4+ T cells in cancer stroma, not CD8+ T cells in cancer cell nests, are associated with favorable prognosis in human non-small cell lung cancers. Cancer Sci. (2003) 94:1003–9. doi: 10.1111/j.1349-7006.2003.tb01392.x
64. Kim H, Kwon HJ, Han YB, Park SY, Kim ES, Kim SH, et al. Increased CD3+ T cells with a low FOXP3+/CD8+ T cell ratio can predict anti-PD-1 therapeutic response in non-small cell lung cancer patients. Mod Pathol. (2019) 32:367–75. doi: 10.1038/s41379-018-0142-3
65. Al-Shibli KI, Donnem T, Al-Saad S, Persson M, Bremnes RM, Busund LT. Prognostic effect of epithelial and stromal lymphocyte infiltration in non-small cell lung cancer. Clin Cancer Res. (2008) 14(16):5220–7. doi: 10.1158/1078-0432.Ccr-08-0133
66. Kilic A, Landreneau RJ, Luketich JD, Pennathur A, Schuchert MJ. Density of tumor-infiltrating lymphocytes correlates with disease recurrence and survival in patients with large non-small-cell lung cancer tumors. J Surg Res. (2011) 167:207–10. doi: 10.1016/j.jss.2009.08.029
67. Koh J, Kim S, Kim MY, Go H, Jeon YK, Chung DH. Prognostic implications of intratumoral CD103+ tumor-infiltrating lymphocytes in pulmonary squamous cell carcinoma. Oncotarget. (2017) 8:13762–9. doi: 10.18632/oncotarget.14632
68. Kose F, Canbolat T, Fındıkcıoglu A, Sedef AM, Ozdemir Y, Besen A, et al. Prognostic and predictive role of FOXP3 positive tumor infiltrating lymphocytes (TILs) in curatively resected non small cell lung cancer other than stage IA. J Oncological Sci. (2017) 3(3):102–6. doi: 10.1016/j.jons.2017.07.002
69. Lee HE, Luo L, Kroneman T, Passow MR, Del Rosario KM, Christensen MR, et al. Increased plasma cells and decreased B-cells in tumor infiltrating lymphocytes are associated with worse survival in lung adenocarcinomas. J Clin Cell Immunol. (2020) 11(1):584.
70. Mlika M, Saidi A, Mejri N, Abdennadher M, Haddouchi C, Labidi S, et al. Prognostic impact of tumor-infiltrating lymphocytes in non-small cell lung carcinomas. Asian Cardiovasc Thorac Ann. (2022) 30:177–84. doi: 10.1177/02184923211042129
71. Richardet E, Hernández P, Paradelo M, Acosta L, Molina M, Riso A, et al. EP1.03-23 update of the analysis of the status of lymphocyte infiltration in patients with NSCLC. J Thorac Oncol. (2019) 14:S962. doi: 10.1016/j.jtho.2019.08.2104
72. Shimizu K, Nakata M, Hirami Y, Yukawa T, Maeda A, Tanemoto K. Tumor-infiltrating FOXP3+ regulatory T cells are correlated with cyclooxygenase-2 expression and are associated with recurrence in resected non-small cell lung cancer. J Thorac Oncol. (2010) 5:585–90. doi: 10.1097/JTO.0b013e3181d60fd7
73. Shirasawa M, Yoshida T, Imabayashi T, Okuma K, Matsumoto Y, Masuda K, et al. Baseline PD-L1 expression and tumour-infiltrated lymphocyte status predict the efficacy of durvalumab consolidation therapy after chemoradiotherapy in unresectable locally advanced patients with non-small-cell lung cancer. Eur J Cancer. (2022) 162:1–10. doi: 10.1016/j.ejca.2021.11.013
74. Soo RA, Asuncion BR, Loh M, Fazreen Z, Sim B, Nga ME, et al. CT Combined immunophenotyping of tumor-infiltrating lymphocytes as a prognostic factor in resected patients with non-small cell lung cancer. J Clin Oncol. (2014) 32:11125–5. doi: 10.1200/jco.2014.32.15_suppl.11125
75. Souza P, Parra ER, Atanazio MJ, da Silva OB, Noleto GS, Ab’Saber AM, et al. Different morphology, stage and treatment affect immune cell infiltration and long-term outcome in patients with non-small-cell lung carcinoma. Histopathology. (2012) 61:587–96. doi: 10.1111/j.1365-2559.2012.04318.x
76. Suzuki K, Kadota K, Sima CS, Nitadori J, Rusch VW, Travis WD, et al. Clinical impact of immune microenvironment in stage I lung adenocarcinoma: tumor interleukin-12 receptor β2 (IL-12Rβ2), IL-7R, and stromal FOXP3/CD3 ratio are independent predictors of recurrence. J Clin Oncol. (2013) 31:490–8. doi: 10.1200/jco.2012.45.2052
77. Tan Q, Li H, Yu M, Tang X, Tan J, Zhang S, et al. CD45RO+Memory T lymphocytes as A candidate marker for non-small cell lung cancer. Zhongguo Fei Ai Za Zhi. (2021) 24:254–64. doi: 10.3779/j.issn.1009-3419.2021.103.05
78. Yu DP, Han Y, Zhao QY, Liu ZD. CD3+ CD4+ and CD3+ CD8+ lymphocyte subgroups and their surface receptors NKG2D and NKG2A in patients with non-small cell lung cancer. Asian Pac J Cancer Prev. (2014) 15:2685–8. doi: 10.7314/apjcp.2014.15.6.2685
79. Farhood B, Najafi M, Mortezaee K. CD8(+) cytotoxic T lymphocytes in cancer immunotherapy: A review. J Cell Physiol. (2019) 234:8509–21. doi: 10.1002/jcp.27782
80. Wu Z, Zhang C, Najafi M. Targeting of the tumor immune microenvironment by metformin. J Cell Commun Signal. (2022) 16:333–48. doi: 10.1007/s12079-021-00648-w
81. Brightman SE, Becker A, Thota RR, Naradikian MS, Chihab L, Zavala KS, et al. Neoantigen-specific stem cell memory-like CD4(+) T cells mediate CD8(+) T cell-dependent immunotherapy of MHC class II-negative solid tumors. Nat Immunol. (2023) 24:1345–57. doi: 10.1038/s41590-023-01543-9
82. Eberst G, Vernerey D, Laheurte C, Meurisse A, Kaulek V, Cuche L, et al. Prognostic value of CD4+ T lymphopenia in non-small cell lung Cancer. BMC Cancer. (2022) 22:529. doi: 10.1186/s12885-022-09628-8
83. Jackute J, Zemaitis M, Pranys D, Sitkauskiene B, Miliauskas S, Bajoriunas V, et al. The prognostic influence of tumor infiltrating FOXP3(+)CD4(+), CD4(+) and CD8(+) T cells in resected non-small cell lung cancer. J Inflammation (Lond). (2015) 12:63. doi: 10.1186/s12950-015-0108-x
84. Wei K, Wu J, Tang H. Research progress of immunotherapy in EGFR mutated non-small cell lung cancer. J Chongqing Med University. (2023) 48(11):1282–9. doi: 10.13406/j.cnki.cyxb.003365
85. Niemeijer AN, Sahba S, Smit EF, Lissenberg-Witte BI, de Langen AJ, Thunnissen E. Association of tumour and stroma PD-1, PD-L1, CD3, CD4 and CD8 expression with DCB and OS to nivolumab treatment in NSCLC patients pre-treated with chemotherapy. Br J Cancer. (2020) 123:392–402. doi: 10.1038/s41416-020-0888-5
86. Peng J, Yang S, Ng CSH, Chen GG. The role of FOXP3 in non-small cell lung cancer and its therapeutic potentials. Pharmacol Ther. (2023) 241:108333. doi: 10.1016/j.pharmthera.2022.108333
87. Li C, Wang H, Fang H, He C, Pei Y, Gai X. FOXP3 facilitates the invasion and metastasis of non-small cell lung cancer cells through regulating VEGF, EMT and the Notch1/Hes1 pathway. Exp Ther Med. (2021) 22:958. doi: 10.3892/etm.2021.10390
88. Li L, Chao QG, Ping LZ, Xue C, Xia ZY, Qian D, et al. The prevalence of FOXP3+ regulatory T-cells in peripheral blood of patients with NSCLC. Cancer Biother Radiopharm. (2009) 24:357–67. doi: 10.1089/cbr.2008.0612
89. Grell P, Borilova S, Fabian P, Selingerova I, Novak D, Muller P, et al. FOXP3 expression in tumor-infiltrating lymphocytes as potential predictor of response to immune checkpoint inhibitors in patients with advanced melanoma and non-small cell lung cancer. Cancers (Basel). (2023) 15(6):1901. doi: 10.3390/cancers15061901
Keywords: tumor-infiltrating lymphocytes, non-small cell lung cancer, prognostic implication, systematic review, meta-analysis
Citation: Yan Q, Li S, He L and Chen N (2024) Prognostic implications of tumor-infiltrating lymphocytes in non-small cell lung cancer: a systematic review and meta-analysis. Front. Immunol. 15:1476365. doi: 10.3389/fimmu.2024.1476365
Received: 05 August 2024; Accepted: 02 September 2024;
Published: 20 September 2024.
Edited by:
Xudong Zhu, University of Kentucky, United StatesReviewed by:
Yuehua Li, University of South China, ChinaPeng Liu, Sun Yat-sen University, China
Tao Zhang, The First Affiliated Hospital of Chongqing Medical University, China
Copyright © 2024 Yan, Li, He and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lang He, aGVsYW5nNzI5QDE2My5jb20=; Nianyong Chen, bl95Y2hlbkBob3RtYWlsLmNvbQ==
†These authors have contributed equally to this work
 Qin Yan
Qin Yan Shuai Li
Shuai Li Lang He
Lang He Nianyong Chen
Nianyong Chen