- Department of Hematology, West China Hospital, Sichuan University, Chengdu, Sichuan, China
B-cell acute lymphoblastic leukemia (B-ALL) with the MLL-AF4 fusion gene has a poor prognosis, and the mortality rate exceeds 90%, particularly in cases of extramedullary relapse (EMR). Herein, we present a case of a 46-year-old male patient who developed relapsed B-ALL with MLL-AF4. The patient initially achieved a complete remission (CR) after induction therapy and underwent haploidentical hematopoietic stem cell transplantation. Five months post-transplantation, he developed enlarged lymph nodes and subcutaneous masses. A lymph node biopsy confirmed EMR, without leukemia in the bone marrow or peripheral blood. The patient received the VCA regimen (venetoclax, chidamide, and azacitidine) and was regularly monitored through blood counts, marrow cell morphology analysis, flow cytometry, and computed tomography or positron emission tomography-computed tomography imaging. After the first VCA course, the patient achieved a second CR with only transient myelosuppression. Following two VCA courses, he received chimeric antigen receptor T-cell therapy, which led to complete metabolic remission and improved prognosis. This case underscores the potential of the VCA regimen as a bridging therapy for EMR in B-ALL with MLL-AF4, although further studies are warranted.
1 Introduction
In adult patients with B-cell acute lymphoblastic leukemia (B-ALL), induction therapy can typically exceed a complete remission (CR) rate of 90%. However, the long-term survival is only 30–60% among those treated with a standard chemotherapy with or without allogeneic hematopoietic cell transplantation (allo-HCT). Moreover, relapse is commonly reported in 25–30% of patients who have undergone allo-HCT (1–4) plausibly due to unfavorable cytogenetic changes such as MLL rearrangements (MLL-r) with t(4;11)(q21;q23) that results in the formation of the MLL-AF4 gene (also known as KMT2A-AFF1), which is associated with poor prognosis.
Research has revealed B-cell lymphoma-2 (Bcl-2) overexpression, histone deacetylation, and extensive promoter hypermethylation in cells among patients with MLL-r. We hypothesize that combining therapeutic agents targeting these three major pathogenic mechanisms will control B-ALL with MLL-r, especially in relapsed and refractory (R/R) patients. This report presents a case of B-ALL with MLL-r who developed extramedullary relapse (EMR) after undergoing allo-HCT, and was treated with the VCA regimen—a combination of venetoclax (a Bcl-2 inhibitor), chidamide (a selective histone deacetylase inhibitor), and azacitidine (a DNA demethylating agent)—which resulted in a CR.
2 Case description
A 46-year-old male patient presented with multiple enlarged lymph nodes throughout his body in June 2023. According to results of blood tests, bone marrow (BM) aspiration, and other relevant examinations, the patient was diagnosed with acute pre-B-cell lymphoblastic leukemia (Pro-B-ALL) with MLL-AF4. Chromosome karyotyping analysis revealed 45,XY,t(4;11) (q21;q23),add(5)(p15.3),der(5)t(5;12)(p15.3;q13),12 (5)/46,XY (11) (classified as the poor prognosis group as per NCCN 2021 guidelines) (5). The patient received an induction with one course of VDLP (vindesine, daunorubicin, L-asparaginase, and prednisone). Flow cytometry examinations following induction treatment revealed morphologic CR with 2.15×10−2 minimal residual disease (MRD). Since August 2023, the patient received one course of blinatumomab (35 µg ×9) as a consolidation therapy. Reexamination of the BM following consolidation therapy revealed CR with negative MRD. In September 2023, the patient with the help of his sibling sister as the donor underwent haploidentical hematopoietic stem cell transplantation with myeloablative conditioning and PTCy-based graft-versus-host disease (GvHD) prophylaxis. The engraftment was successful, and there was no apparent GvHD. The patient remained in CR with negative MRD thereafter. Considering the poor prognosis of B-ALL patients with MLL-r, the patient received blinatumomab as maintenance therapy from 3 months post-transplantation as an infusion from 9 to 18 µg/day for 7 days.
In February 2024, 5 months post-transplantation, the patient complained of multiple enlarged lymph nodes and subcutaneous masses. A prompt biopsy of the left inguinal lymph node revealed consistent medium sized lymphocytes with a high nuclear−cytoplasmic ratio and a morphology resembling that of lymphoblasts. Immunohistochemical analysis revealed their characteristics as CD20−PAX-5+ CD19+ CD22−CD3−CD10−TdT−CD34−CD43+CD117−MPO−, Ki-67 (MIB-1) (+, 80–90%), and EBER1/2-ISH negative. Gene rearrangement analysis by polymerase chain reaction showed the presence of IgH gene clonal expansion in the target fragment range. Flow cytometry analysis of the same sample confirmed that 61.4% of nucleated cells were lymphocytes. Among them, B-cell population was approximately 61.8%, of which CD45dim+CD19dim+CD20−CD22−CD5−CD10−CD23−FMC7−CD38+Bcl-2 (+, in small amounts) TDT−HLA-DR+CD34−CD117−MPO−CD14−CD13−CD33−CD64−CD123− and mlgK (−), mlgλ (−) confirmed EMR. However, there was no evidence of leukemia in the BM or peripheral blood sample. BM cells showed complete donor chimerism. Contrast-enhanced computed tomography (CT) of the neck, chest, and abdomen (Figures 1A1, B1, C1, D1) revealed the presence of enlarged lymph nodes in bilateral submandibular spaces, adjacent to the carotid sheath and within the posterior triangle of the neck, supraclavicular regions, and axilla. The largest node was in the left inguinal fossa and measured 4.1×4.0 cm. Soft tissues were observed, adjacent to the T4 vertebra on the left side, T7-8 vertebra on the right side, and beneath the sternum. In addition, soft tissue nodules were detected in local areas of the anterior superior abdominal wall and the chest wall below the right axilla, the largest nodule being approximately 1.7×1.1 cm. Moreover, patchy nodules with ground−glass opacities and diffused distribution were scattered in both lungs, the lower lobes of which showed subpleural consolidation in the posterior basal segment (Figure 1E1).
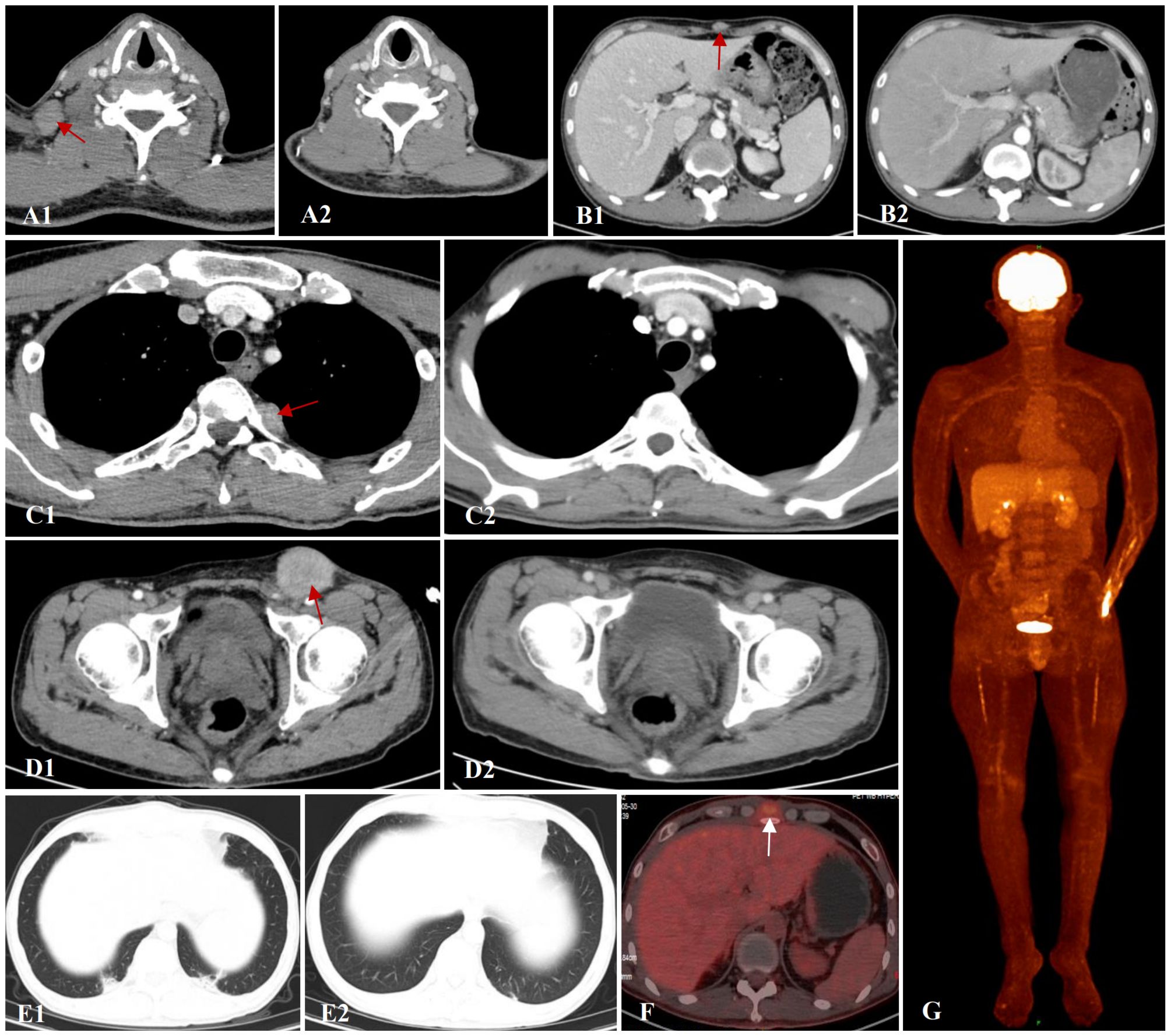
Figure 1. Contrast-enhanced CT of the neck, chest and abdomen. (A1, A2) Axillary lymph nodes before and after VCA treatment. (B1, B2) Soft tissue mass around the subxiphoid before and after VCA treatment. (C1, C2) Paravertebral soft tissue image before and after VCA treatment. (D1, D2) Soft tissue mass in the inguinal region before and after VCA treatment. (E1, E2) Pulmonary infection before and after antifungal therapy. (F, G) PET-CT revealed active 18F-FDG metabolism in the superficial fascia and xiphoid process of the right chest wall after VCA treatment.
As the patient had relapsed early (within 6 months post-transplantation) from allo-SCT and blinatumomab maintenance, classical therapies such as chemotherapy or donor lymphocyte infusion may not be effective enough for remission reinduction. We recommended this patient to receive anti-CD19 chimeric antigen receptor T-cell (CAR-T) as salvage therapy. However, after his peripheral blood mononuclear cells were harvested, he experienced progressive enlargement of aforementioned lymph nodes and soft tissue masses, which necessitated an optimal bridging therapy.
On March 8, 2024, the patient received VCA regimen (venetoclax 200 mg daily on days 1-14, chidamide 30 mg twice weekly for 2 weeks, and azacitidine 100 mg daily on days 1-7) along with an aggressive antifungal treatment for pulmonary infection. Measures were taken to optimize his performance status. During the period of myelosuppression associated with VCA therapy, he suffered from grade II leukopenia and grade IV thrombocytopenia, for which platelet transfusion was performed (Figure 2A). The enlarged lymph nodes and soft tissue masses disappeared in CT scans following the first VCA course, indicative of a CR (Figures 1A2, B2, C2, D2). The pulmonary infection had resolved (Figure 1E2). On April 22, 2024, he received a second course. The period of BM suppression lasted for approximately 10 days, without any transfusion (Figure 2B). A positron emission tomography-computed tomography (PET-CT) performed on May 30, 2024, identified a soft tissue mass in the subcutaneous tissue and the tip of the sternum that exhibited increased 18F-FDG uptake (Figures 1F, G). Therefore, he underwent autologous CD19 CAR-T cell therapy with classic fludarabine/cyclophosphamide conditioning. A week later, the nodules on the right chest wall and the tip of the sternum completely disappeared. A subsequent PET-CT scan performed 10 weeks post-CD19 CAR-T cell therapy indicated complete metabolic remission (CMR). The patient remained in CR at the last follow-up. The disease timeline and treatment course of this patient is shown in Figure 3.
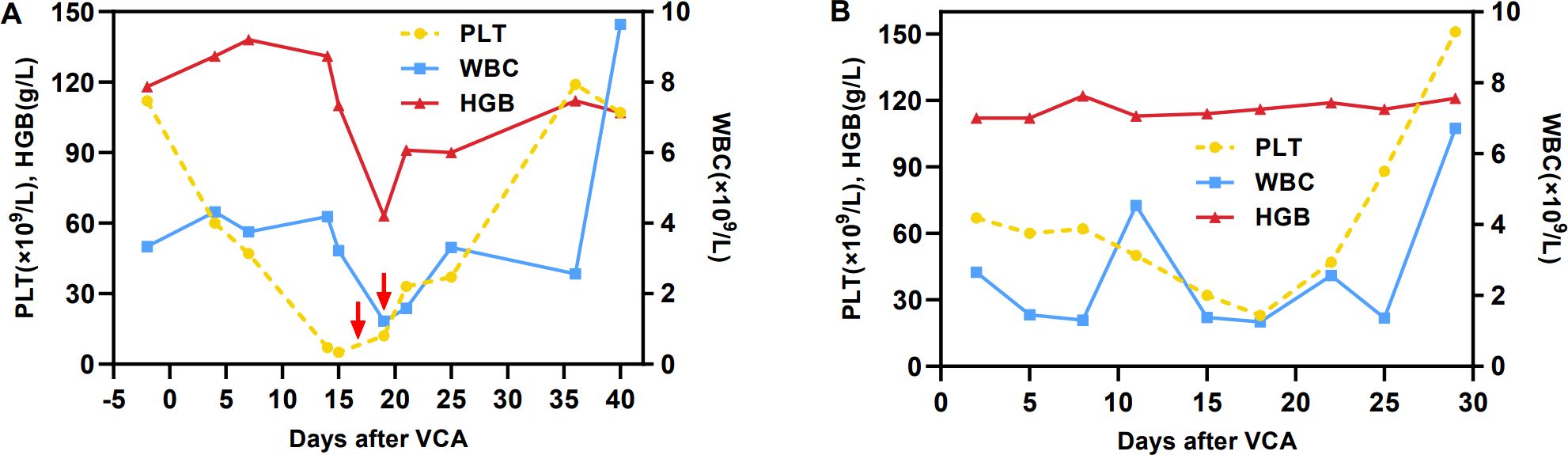
Figure 2. Hemoglobin, white blood cell and platelet counts. (A) First course, (B) second course. The red arrow indicates the infusion of one therapeutic dose of platelets.
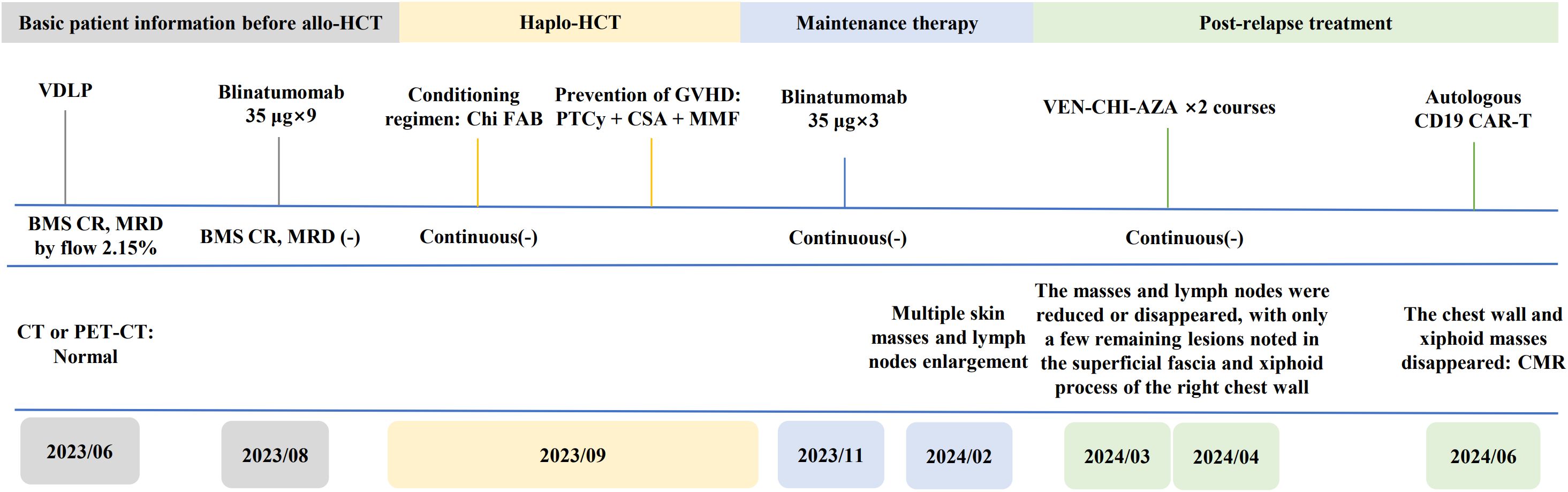
Figure 3. Timeline of the patient’s treatment and bone marrow, CT or PET-CT assessment. BMS, bone marrow smear.
3 Discussion
To our knowledge, this is the first case of R/R B-ALL with MLL-r being treated with the combination of a BCL-2 inhibitor and epigenetic modulators, leading to CR. In this patient who presented with multiple extramedullary lesions, the VCA regimen significantly reduced the tumor burden and served as a successful bridge to CD19 CAR-T cell therapy.
The prognosis of patients with MLL-AF4–positive ALL is in general poor, with a 3-year overall survival of 24% following conventional chemotherapy. Although allo-HCT serves as a curative opportunity with a survival rate exceeding 60%, the 5-year overall survival among those who relapse post-transplantation is only 8% (6, 7). Further, the prognosis is poorer among patients with EMR where the mortality is 96.6% (28/29) (7). Recent breakthroughs in immunotherapies such as anti-CD19 CAR-T therapy have demonstrated promising outcomes with a response rate of 85–100% in B-ALL patients with MLL-AF4 (8, 9). Studies have also underscored the significant activity of CAR-T therapy in EMD (10). However, this patient was ineligible for an immediate CAR-T therapy owing to poor performance status, severe pulmonary infections, rapid disease progression, which demanded 2-3 weeks for CAR-T cell preparation. A lower tumor burden at the time of CAR-T infusion is known to ameliorate long-term survival and reduce the risk of severe cytokine release syndrome and neurotoxicity (11). Therefore, a bridging therapy prior to CAR-T therapy was warranted to curb disease progression and lower tumor burden.
Common bridging therapies such as low-intensity chemotherapy and targeted agents such as inotuzumab ozogamicin (INO) and blinatumomab (12) were not suitable for this patient. Conventional chemotherapy is associated with a 64% failure rate in inducing remission among high-risk relapsed ALL patients, particularly those with KMT2A rearrangements or hypodiploidy (13). In EMD, the response rate with INO is 55–71% primarily in those with high CD22 expression (14, 15). The patient in our case, however, showed no CD22 expression. Although he was CD19 positive, the efficacy of CD3/CD19-directed bispecific T-cell engagers such as blinatumomab in EMD was poor. Numerous reports have indicated the persistent EMR among B-ALL patients treated with blinatumomab, plausibly owing to inadequate tissue penetration and T-cell recruitment to nonhematopoietic tissues (16–18). Therefore, we used the combination therapy exercising potential pharmacological synergy—VEN, CHI, and AZA—a targeted and immunomodulatory regimen with reduced toxicity.
The VEN, CHI, and AZA combination exerts synergistic antileukemic effects. Evidence suggests the high expression of Bcl-2 and MCL-1 in MLL-AF4-expressing cells (19, 20). These cells also exhibit extensive promoter hypermethylation, which leads to silencing of tumor suppressor genes and the consequent development of hematologic malignancies (21–23). Therefore, the Bcl-2 inhibitor VEN and the demethylating agent AZA exert therapeutic effects in patients with MLL-AF4 fusion gene AZA enhances the antileukemic efficacy of VEN by lowering MCL-1, which reduces the leukemic resistance to VEN (24). Moreover, VEN and AZA synergistically eliminate leukemia stem cells by reducing amino acid uptake and disrupting the tricarboxylic acid cycle via electron transport chain complex II suppression (25–27). Histone deacetylases (HDACs) participate in the generation and functioning of MLL gene rearrangement (28, 29). Inhibitors of HDACs such as CHI activate endogenous MLL and consequently eliminate active products of MLL-AF4, thereby inhibiting the proliferation and viability of t(4;11) cells (30). CHI also enhances the antileukemic activity of VEN through MCL-1 and Bcl-xL downregulation and BIM upregulation (31). The combination of VEN and demethylating agents exerts significant efficacy, is well tolerated in AML and R/R B-ALL patients, and has been incorporated into clinical guidelines for elderly and/or unfit patients with AML (32, 33). Wang B.R. et al. reported promising results with this combination among three R/R AML patients (34). To our knowledge, there are no reports describing its use for B-ALL treatment.
Following patient’s consent, we initiated the VEN, CHI, and AZA combination for this relapsed patient post-haplo-HCT based on aforementioned research findings. Surprisingly, he achieved CR immediately following the first course. After two courses, the patient received CAR-T therapy and achieved CMR. Noteworthy, this is a chemotherapy-free regimen with mild and manageable adverse effects, primarily transient pancytopenia. It is particularly suitable for those with poor performance status and uncontrolled infectious complications.
In conclusion, the successful outcomes in this case serve as a preliminary indication of applicability of the VCA regimen in controlling rapid disease progression in B-ALL patients with MLL-AF4 who experience EMR post allo-HCT, thereby providing an opportunity for subsequent treatment. However, conclusions drawn from a single case report are limited. Therefore, further clinical studies are needed to confirm the potential of the VCA regimen in B-ALL treatment.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Biomedical Research Ethics Committee, West China Hospital of Sichuan University. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
XJ: Data curation, Validation, Writing – original draft. ZL: Supervision, Validation, Writing – review & editing. YW: Supervision, Validation, Writing – review & editing. JJ: Conceptualization, Methodology, Supervision, Validation, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
We thank all reviewers who participated in the review and MJEditor (www.mjeditor.com) for its linguistic assistance during the preparation of this manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Cho H, Kim Y, Yoon JH, Lee J, Lee GD, Son J, et al. Non-inferior long-term outcomes of adults with Philadelphia chromosome-like acute lymphoblastic leukemia. Bone Marrow Transplant. (2021) 56:1953–63. doi: 10.1038/s41409-021-01253-6
2. Yoon JH, Kim HS, Min GJ, Park SS, Park S, Lee SE, et al. Cytogenetic and molecular characteristics and outcomes of adult patients with early T-cell precursor acute lymphoblastic leukemia. Eur J Haematol. (2023) 110:137–48. doi: 10.1111/ejh.13883
3. Eom KS, Shin SH, Yoon JH, Yahng SA, Lee SE, Cho BS, et al. Comparable long-term outcomes after reduced-intensity conditioning versus myeloablative conditioning allogeneic stem cell transplantation for adult high-risk acute lymphoblastic leukemia in complete remission. Am J Hematol. (2013) 88:634–41. doi: 10.1002/ajh.23465
4. Cho BS, Lee S, Kim YJ, Chung NG, Eom KS, Kim HJ, et al. Reduced-intensity conditioning allogeneic stem cell transplantation is a potential therapeutic approach for adults with high-risk acute lymphoblastic leukemia in remission: results of a prospective phase 2 study. Leukemia. (2009) 23:1763–70. doi: 10.1038/leu.2009.102
5. Brown PA, Shah B, Advani A, Aoun P, Boyer MW, Burke PW, et al. Acute lymphoblastic leukemia, version 2.2021, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. (2021) 19:1079–109. doi: 10.6004/jnccn.2021.0042
6. Spyridonidis A, Labopin M, Schmid C, Volin L, Yakoub-Agha I, Stadler M, et al. Outcomes and prognostic factors of adults with acute lymphoblastic leukemia who relapse after allogeneic hematopoietic cell transplantation. An analysis on behalf of the Acute Leukemia Working Party of EBMT. Leukemia. (2012) 26:1211–7. doi: 10.1038/leu.2011.351
7. Marks DI, Moorman AV, Chilton L, Paietta E, Enshaie A, DeWald G, et al. The clinical characteristics, therapy and outcome of 85 adults with acute lymphoblastic leukemia and t(4;11)(q21;q23)/MLL-AFF1 prospectively treated in the UKALLXII/ECOG2993 trial. Haematologica. (2013) 98:945–52. doi: 10.3324/haematol.2012.081877
8. Gardner R, Wu D, Cherian S, Fang M, Hanafi LA, Finney O, et al. Acquisition of a CD19-negative myeloid phenotype allows immune escape of MLL-rearranged B-ALL from CD19 CAR-T-cell therapy. Blood. (2016) 127:2406–10. doi: 10.1182/blood-2015-08-665547
9. Davila ML, Riviere I, Wang X, Bartido S, Park J, Curran K, et al. Efficacy and toxicity management of 19-28z CAR T cell therapy in B cell acute lymphoblastic leukemia. Sci Transl Med. (2014) 6:224ra25. doi: 10.1126/scitranslmed.3008226
10. Aldoss I, Khaled SK, Wang X, Palmer J, Wang Y, Wagner JR, et al. Favorable activity and safety profile of memory-enriched CD19-targeted chimeric antigen receptor T-cell therapy in adults with high-risk relapsed/refractory ALL. Clin Cancer Res. (2023) 29:742–53. doi: 10.1158/1078-0432.Ccr-22-2038
11. Park JH, Rivière I, Gonen M, Wang X, Sénéchal B, Curran KJ, et al. Long-term follow-up of CD19 CAR therapy in acute lymphoblastic leukemia. N Engl J Med. (2018) 378:449–59. doi: 10.1056/NEJMoa1709919
12. Perica K, Flynn J, Curran KJ, Rivere I, Wang X, Senechal B, et al. Impact of bridging chemotherapy on clinical outcome of CD19 CAR T therapy in adult acute lymphoblastic leukemia. Leukemia. (2021) 35:3268–71. doi: 10.1038/s41375-021-01196-3
13. Eckert C, Parker C, Moorman AV, Irving JA, Kirschner-Schwabe R, Groeneveld-Krentz S, et al. Risk factors and outcomes in children with high-risk B-cell precursor and T-cell relapsed acute lymphoblastic leukaemia: combined analysis of ALLR3 and ALL-REZ BFM 2002 clinical trials. Eur J Cancer. (2021) 151:175–89. doi: 10.1016/j.ejca.2021.03.034
14. DeAngelo DJ, Advani AS, Marks DI, Stelljes M, Liedtke M, Stock W, et al. Inotuzumab ozogamicin for relapsed/refractory acute lymphoblastic leukemia: outcomes by disease burden. Blood Cancer J. (2020) 10:81. doi: 10.1038/s41408-020-00345-8
15. Kayser S, Sartor C, Luskin MR, Webster J, Giglio F, Panitz N, et al. Outcome of relapsed or refractory acute B-lymphoblastic leukemia patients and BCR-ABL-positive blast cell crisis of B-lymphoid lineage with extramedullary disease receiving inotuzumab ozogamicin. Haematologica. (2022) 107:2064–71. doi: 10.3324/haematol.2021.280433
16. Topp MS, Kufer P, Gökbuget N, Goebeler M, Klinger M, Neumann S, et al. Targeted therapy with the T-cell-engaging antibody blinatumomab of chemotherapy-refractory minimal residual disease in B-lineage acute lymphoblastic leukemia patients results in high response rate and prolonged leukemia-free survival. J Clin Oncol. (2011) 29:2493–8. doi: 10.1200/jco.2010.32.7270
17. Daghri S, Bendari M, Belmoufid N, Yahyaoui A, Ahnach M. An unusual presentation of extramedullary relapse following blinatumomab in Philadelphia chromosome-positive acute lymphoblastic leukemia. Cureus. (2022) 14:e23262. doi: 10.7759/cureus.23262
18. Demosthenous C, Lalayanni C, Iskas M, Douka V, Pastelli N, Anagnostopoulos A. Extramedullary relapse and discordant CD19 expression between bone marrow and extramedullary sites in relapsed acute lymphoblastic leukemia after blinatumomab treatment. Curr Probl Cancer. (2019) 43:222–7. doi: 10.1016/j.currproblcancer.2018.04.006
19. Benito JM, Godfrey L, Kojima K, Hogdal L, Wunderlich M, Geng H, et al. MLL-rearranged acute lymphoblastic leukemias activate BCL-2 through H3K79 methylation and are sensitive to the BCL-2-specific antagonist ABT-199. Cell Rep. (2015) 13:2715–27. doi: 10.1016/j.celrep.2015.12.003
20. Godfrey L, Kerry J, Thorne R, Repapi E, Davies JO, Tapia M, et al. MLL-AF4 binds directly to a BCL-2 specific enhancer and modulates H3K27 acetylation. Exp Hematol. (2017) 47:64–75. doi: 10.1016/j.exphem.2016.11.003
21. Stumpel DJ, Schneider P, van Roon EH, Boer JM, de Lorenzo P, Valsecchi MG, et al. Specific promoter methylation identifies different subgroups of MLL-rearranged infant acute lymphoblastic leukemia, influences clinical outcome, and provides therapeutic options. Blood. (2009) 114:5490–8. doi: 10.1182/blood-2009-06-227660
22. Stumpel DJ, Schneider P, van Roon EH, Pieters R, Stam RW. Absence of global hypomethylation in promoter hypermethylated Mixed Lineage Leukaemia-rearranged infant acute lymphoblastic leukaemia. Eur J Cancer. (2013) 49:175–84. doi: 10.1016/j.ejca.2012.07.013
23. Schafer E, Irizarry R, Negi S, McIntyre E, Small D, Figueroa ME, et al. Promoter hypermethylation in MLL-r infant acute lymphoblastic leukemia: biology and therapeutic targeting. Blood. (2010) 115:4798–809. doi: 10.1182/blood-2009-09-243634
24. Jin S, Cojocari D, Purkal JJ, Popovic R, Talaty NN, Xiao Y, et al. 5-azacitidine induces NOXA to prime AML cells for venetoclax-mediated apoptosis. Clin Cancer Res. (2020) 26:3371–83. doi: 10.1158/1078-0432.Ccr-19-1900
25. Jones CL, Stevens BM, D’Alessandro A, Reisz JA, Culp-Hill R, Nemkov T, et al. Inhibition of amino acid metabolism selectively targets human leukemia stem cells. Cancer Cell. (2018) 34:724–740.e4. doi: 10.1016/j.ccell.2018.10.005
26. Cheung LC, Aya-Bonilla C, Cruickshank MN, Chiu SK, Kuek V, Anderson D, et al. Preclinical efficacy of azacitidine and venetoclax for infant KMT2A-rearranged acute lymphoblastic leukemia reveals a new therapeutic strategy. Leukemia. (2023) 37:61–71. doi: 10.1038/s41375-022-01746-3
27. Pollyea DA, Stevens BM, Jones CL, Winters A, Pei S, Minhajuddin M, et al. Venetoclax with azacitidine disrupts energy metabolism and targets leukemia stem cells in patients with acute myeloid leukemia. Nat Med. (2018) 24:1859–66. doi: 10.1038/s41591-018-0233-1
28. Xia ZB, Anderson M, Diaz MO, Zeleznik-Le NJ. MLL repression domain interacts with histone deacetylases, the polycomb group proteins HPC2 and BMI-1, and the corepressor C-terminal-binding protein. Proc Natl Acad Sci U.S.A. (2003) 100:8342–7. doi: 10.1073/pnas.1436338100
29. Khobta A, Carlo-Stella C, Capranico G. Specific histone patterns and acetylase/deacetylase activity at the breakpoint-cluster region of the human MLL gene. Cancer Res. (2004) 64:2656–62. doi: 10.1158/0008-5472.can-03-1126
30. Ahmad K, Katryniok C, Scholz B, Merkens J, Löscher D, Marschalek R, et al. Inhibition of class I HDACs abrogates the dominant effect of MLL-AF4 by activation of wild-type MLL. Oncogenesis. (2014) 3:e127. doi: 10.1038/oncsis.2014.39
31. Chen K, Yang Q, Zha J, Deng M, Zhou Y, Fu G, et al. Preclinical evaluation of a regimen combining chidamide and ABT-199 in acute myeloid leukemia. Cell Death Dis. (2020) 11:778. doi: 10.1038/s41419-020-02972-2
32. DiNardo CD, Pratz K, Pullarkat V, Jonas BA, Arellano M, Becker PS, et al. Venetoclax combined with decitabine or azacitidine in treatment-naive, elderly patients with acute myeloid leukemia. Blood. (2019) 133:7–17. doi: 10.1182/blood-2018-08-868752
33. Othman TA, Azenkot T, Moskoff BN, Tenold ME, Jonas BA. Venetoclax-based combinations for the treatment of newly diagnosed acute myeloid leukemia. Future Oncol. (2021) 17:2989–3005. doi: 10.2217/fon-2021-0262
Keywords: relapsed/refractory, MLL-AF4, ALL, leukemia, Bcl-2 inhibitor (venetoclax), histone deacetylase inhibitor (chidamide), demethylation (azacitidine), targeted therapy
Citation: Jin X, Liu Z, Wu Y and Ji J (2025) Venetoclax in combination with chidamide and azacitidine for the treatment of relapsed/refractory B-cell acute lymphoblastic leukemia with the MLL-AF4 gene: a case report and literature review. Front. Immunol. 15:1475974. doi: 10.3389/fimmu.2024.1475974
Received: 04 August 2024; Accepted: 16 December 2024;
Published: 14 January 2025.
Edited by:
Athanasios Tragiannidis, Aristotle University of Thessaloniki, GreeceReviewed by:
Francesco Angotzi, University Hospital of Padua, ItalyJoanna Zawitkowska, Medical University of Lublin, Poland
Copyright © 2025 Jin, Liu, Wu and Ji. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jie Ji, amllamlAc2N1LmVkdS5jbg==
 Xuelian Jin
Xuelian Jin Zhigang Liu
Zhigang Liu Yu Wu
Yu Wu Jie Ji
Jie Ji