- Department of General Surgery, Hepatobiliary Surgery, Shanghai Institute of Digestive Surgery, Ruijin Hospital, Shanghai Jiaotong University School of Medicine, Shanghai, China
Background: Hepatic arterial infusion chemotherapy (HAIC) with the FOLFOX regimen has demonstrated efficacy in patients with unresectable hepatocellular carcinoma (HCC). The combined targeted and immunotherapy has emerged as a first-line treatment for liver cancer. In this study, we investigated the clinical efficacy and safety of FOLFOX-HAIC in combination with targeted immunotherapy in patients with untreated, unresectable HCC.
Materials and methods: Data were collected from patients with initially unresectable HCC treated at Ruijin Hospital, affiliated with Shanghai Jiao Tong University School of Medicine, from June 2022 to June 2023. Tumor response and survival outcomes were assessed following the FOLFOX-HAIC combined with targeted immunotherapy, The safety was also evaluated through the incidence of related adverse events.
Results: A total of 51 eligible patients were recruited. The objective response rate (ORR) based on mRECIST and RECIST 1.1 criteria were 60.8% and 45.1%, respectively. The surgical conversion rate was 25.5%. The median progression-free survival (PFS) was 15.2 months. The 1-year overall survival rate was 88.2%. Adverse events were observed in 98% patients, with 23.5% experiencing grade 3 or 4 adverse events.
Conclusion: The FOLFOX-HAIC combined with targeted immunotherapy regimen is effective in patients with unresectable HCC, demonstrated by a high surgical conversion rate and manageable adverse effects. This regimen represents a potential novel first-line treatment option for HCC.
Introduction
Liver cancer is among the most malignant gastrointestinal tumors, including hepatocellular carcinoma (HCC), cholangiocarcinoma, and mixed-cell carcinoma, with HCC being the fourth leading cause of cancer-related deaths worldwide. The etiology of liver cancer is diverse, with common causes including chronic viral hepatitis (such as hepatitis B and C virus infections), alcohol-related liver disease, and non-alcoholic fatty liver disease (1–3). Many patients are diagnosed at an advanced or late stage of unresectable liver cancer, often resulting in a poor prognosis. To convert unresectable liver cancer to resectable liver cancer, it is crucial to identify effective treatment methods to improve the survival time of patients with advanced liver cancer.
Hepatic arterial infusion chemotherapy (HAIC) is recommended in Asia as an alternative option for patients with unresectable HCC (4–6). The Japanese Society of Hepatology’s HCC practice guidelines have recognized HAIC as an effective treatment for locally advanced HCC. HAIC is a liver-directed therapy that delivers high concentrations of chemotherapeutic agents directly to liver tumors via the hepatic artery, achieving a strong tumor response while minimizing excessive exposure of normal liver parenchyma to chemotherapy drugs. This approach effectively reduces tumor burden with relatively low systemic toxicity (7, 8). The combination of targeted drugs and immune checkpoint inhibitors is a first-line treatment strategy for advanced liver cancer. Targeted drugs inhibit the growth, division, and spread of tumor cells by binding to specific molecular targets associated with the tumor (9). Immunotherapy drugs enhance the recognition and elimination of tumor cells by targeting immune cells (10). Compared to single-agent therapies, combination treatments have shown significant efficacy, markedly improving patient survival. For instance, the median overall survival (mOS) with the combination of atezolizumab and bevacizumab can reach 19.2 months, the median progression-free survival (mPFS) was 6.9 months, and the objective response rate (ORR) was 30% (11).
Combining the advantages of HAIC and targeted immunotherapy, the integration of these therapies as a systemic treatment approach is still under exploration. This retrospective study examines the efficacy and safety of HAIC combined with targeted immunotherapy drugs in the treatment of advanced unresectable liver cancer.
Materials and methods
Patient information
Clinical data were collected from patients with initially unresectable HCC treated at Ruijin Hospital, affiliated with Shanghai Jiao Tong University School of Medicine, from June 2022 to June 2023. The inclusion criteria were: (1) Diagnosis of primary hepatocellular carcinoma based on pathology or imaging; (2) Tumors occupying more than 50% of the liver volume or associated with significant liver cirrhosis, multiple bilobar lesions, or invasion of major vascular structures, making it impossible to ensure negative surgical margins or zero residual disease, thus assessed as initially unresectable; (3) At least one measurable target lesion based on the Response Evaluation Criteria in Solid Tumors (RECIST) and the modified RECIST (mRECIST); (4) Completion of at least two cycles of hepatic arterial infusion chemotherapy combined with targeted immunotherapy; (5) No prior anti-HCC treatment; (6) Child-Pugh class A/B liver function and Eastern Cooperative Oncology Group (ECOG) performance status (PS) score of 0-2; (7) No significant organ dysfunction in kidneys, heart, or brain. The exclusion criteria were: (1) Incomplete clinical data; (2) Previous HCC treatments; (3) Decompensated liver function or ECOG PS score of 3-4; (4) Severe dysfunction of the heart, kidneys, or other organs, or concurrent other malignancies; (5) Intolerance or allergy to the medications; (6) Patients with extrahepatic metastases; (7)Barcelona Clinic Liver Cancer (BCLC) stage D.
Research methods
Treatment protocol
Within one week before combined treatment, patients underwent enhanced CT or MRI scans to assess imaging characteristics. Relevant laboratory and examination information, including complete blood count, biochemistry, coagulation, and tumor immunology, were recorded. Patients lay supine. After disinfection and draping in the bilateral inguinal area, the femoral artery pulse was palpated on both sides to choose an appropriate puncture site (usually on the right side) (12, 13). Using local anesthesia, the Seldinger technique was employed to puncture the femoral artery, and after successful puncture, a 4F vascular sheath was placed. A contrast catheter was inserted through the vascular sheath, and selective catheterization of the celiac trunk or superior mesenteric artery was performed. Angiography indicates no vascular anomalies within the abdominal cavity. If ectopic vessels supplying the tumor were present, after arterial embolization, a selective catheterization to the tumor-feeding artery was conducted. A microcatheter was left in the tumor-feeding artery, the Y-valve was properly fixed, the right lower limb was secured, and the patient was returned to the ward. Postoperatively in the ward. A regimen of fluorouracil combined with oxaliplatin (FOLFOX) was continuously infused through the catheter using an infusion pump: (1)Oxaliplatin: 85 mg/m² (adjusted to 130 mg/m² if the tumor diameter was greater than 10 cm) infused over 2 hours. Typically, 130 mg/m² was used for large, well-vascularized tumors, while 85 mg/m² was used for smaller, less vascularized tumors. Dosage adjustments were necessary if a large tumor shrinks. (2)Leucovorin (Calcium Folinate): 200 mg/m² infused over 2 hours. (3)Fluorouracil: 400 mg/m² infused within 15 minutes, followed by 2400 mg/m² infused over 46 hours. The next chemotherapy cycle was repeated 3 or 4 weeks later with re-catheterization. If toxicity was intolerable, treatment may be interrupted when grade 3 or 4 adverse events occurred.
Targeted immunotherapy
After a single HAIC chemotherapy session, targeted drugs and immunotherapeutic agents were selected based on the patients’ preferences and their financial situations. Common targeted and immune combinations include: (1) Atezolizumab + Bevacizumab; (2) Sintilimab + Bevacizumab biosimilar; (3) Lenvatinib or Donafenib combined with Tislelizumab or Pembrolizumab or camrelizumab. Before or after the first HAIC session, patients were intravenously administered anti-PD-1 antibodies every 3 weeks (200-mg tislelizumab, 200-mg camrelizumab, or 200-mg pembrolizumab). For anti-angiogenesis treatment, the patients were administered 8-mg lenvatinib orally once daily, 200-mg Donafenib orally twice daily. Patients in the atezolizumab + bevacizumab group were intravenously administered atezolizumab (1200 mg) plus bevacizumab (15 mg/kg) or sintilimab (200 mg) plus bevacizumab biosimilar (IBI305) every 3 weeks. Generally, each treatment cycle lasted 3 weeks and was repeated. If severe adverse reactions occur during the combined medication period, dose reductions and appropriate extensions of the treatment cycle were considered.
Evaluation of treatment effectiveness
After two HAIC sessions, enhanced CT or MRI re-examinations were conducted, and relevant indicators such as complete blood count, biochemistry, and tumor markers were collected. Tumor efficacy was assessed according to the RECIST 1.1 and the mRECIST criteria. The assessments were as follows:Complete Response(CR); Partial Response (PR); Stable Disease (SD); Progressive Disease (PD); The Overall Response Rate (ORR) was calculated as the sum of PR and CR. The Disease Control Rate (DCR) includes PR, CR, and SD. Additionally, the surgical conversion rate and Progression-Free Survival (PFS) were evaluated.
Statistical analysis
Relevant data were analyzed using the statistical software SPSS 26.0. Measurement data following a normal distribution were expressed as mean ± standard deviation; data not following a normal distribution were expressed as median and interquartile range. Survival analysis was performed using Kaplan-Meier curves. The Cox proportional hazards regression model was used for univariate and multivariate analyses of risk factors affecting Progression-Free Survival (PFS). All statistical methods calculated the 95% confidence interval (95% CI), with a p-value < 0.05 defined as statistically significant.
Results
Clinical characteristics of patients
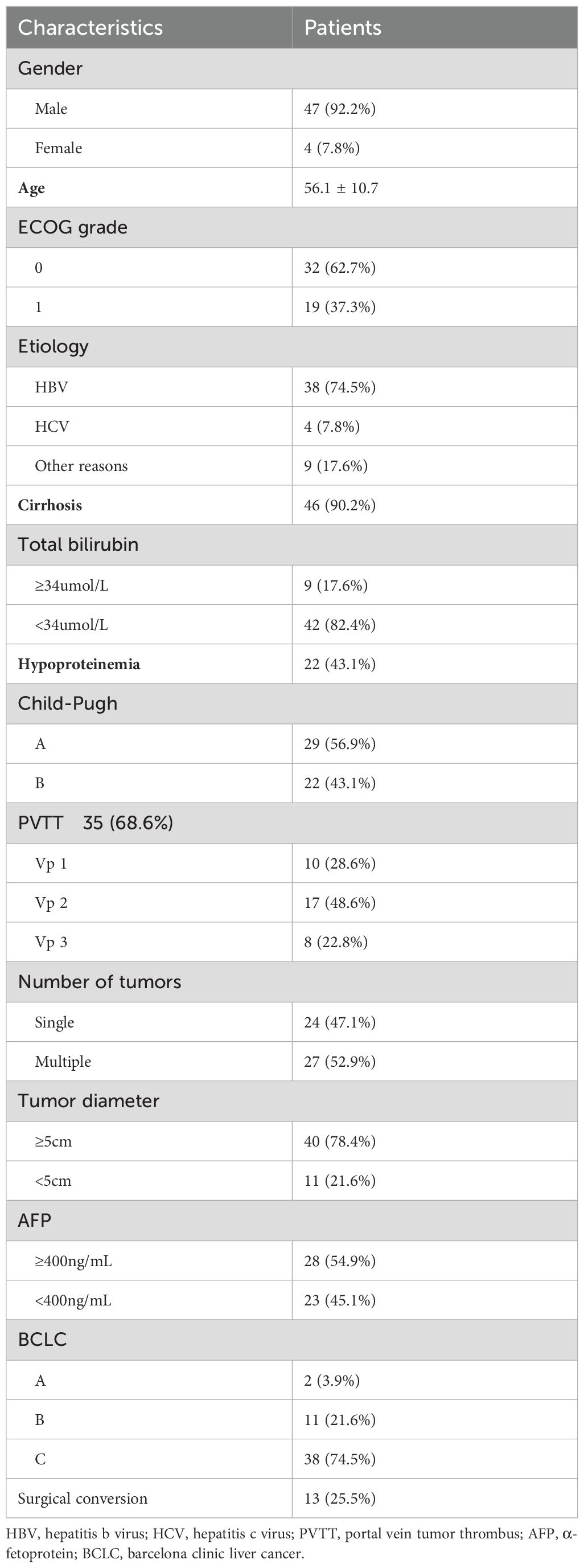
In this study, a total of 51 patients with initially unresectable liver cancer who met the criteria were included. The included patients were mostly middle-aged and elderly, predominantly male (92.2%). The mean age of the enrolled patients was 56.1 ± 10.7 years. Most patients had chronic hepatitis B virus infection (74.5%), with 46 patients (90.2%) having a background of liver cirrhosis. Upon evaluation, 40 patients (78.4%) had a maximum tumor diameter of ≥7 cm (the 7 cm threshold was used for grouping based on previous study (7) and subsequent statistical analyses, and Univariate and multivariate analyses revealed that hepatocellular carcinoma smaller than 7 cm may have a better prognosis after treatment). Additionally, 35 patients (68.6%) had portal vein tumor thrombus, and 27 patients (52.9%) had multiple tumors. All patients were in Child-Pugh grade A or B. Overall, the tumor stages were advanced with a high burden. Among them, 49 patients (96.1%) were in BCLC stages B and C. 13 patients underwent Hepatectomy for HCC after combined treatment (Table 1). A total of 151 HAIC treatments were administered, combined with molecular targeted and immunotherapy. The choice of targeted therapy and immunotherapy drugs depended on the patients’ preferences and their financial situations. Eight patients opted for lenvatinib combined with camrelizumab; two patients chose lenvatinib combined with pembrolizumab; three patients selected lenvatinib combined with tislelizumab; three patients chose donafenib combined with sintilimab; three opted for donafenib combined with pembrolizumab; seventeen patients selected atezolizumab combined with bevacizumab, and fifteen patients chose sintilimab combined with a bevacizumab biosimilar. All drugs were administered according to their standard dosages. The median interval between HAIC therapy was 26 days. The median interval between targeted immunotherapy was 23 days.
Tumor response and patient survival
According to RECIST v1.1 evaluation, the numbers of CR, PR, SD, and PD cases were 2 (3.9%), 21 (41.2%), 22 (43.1%), and 6 (11.8%), respectively. The ORR was 45.1% and the DCR was 88.2%. And according to mRECIST evaluation, the numbers of CR, PR, SD, and PD cases were 4 (7.8%), 27 (52.9%), 14 (27%), and 6 (11.8%), respectively. The ORR was 60.8% and the DCR was 88.2%. After combined treatment, 13 patients underwent liver cancer resection, with a surgical conversion rate of 25.5% (Table 2). The change in the intrahepatic target lesion size of the patients is shown in Figure 1.
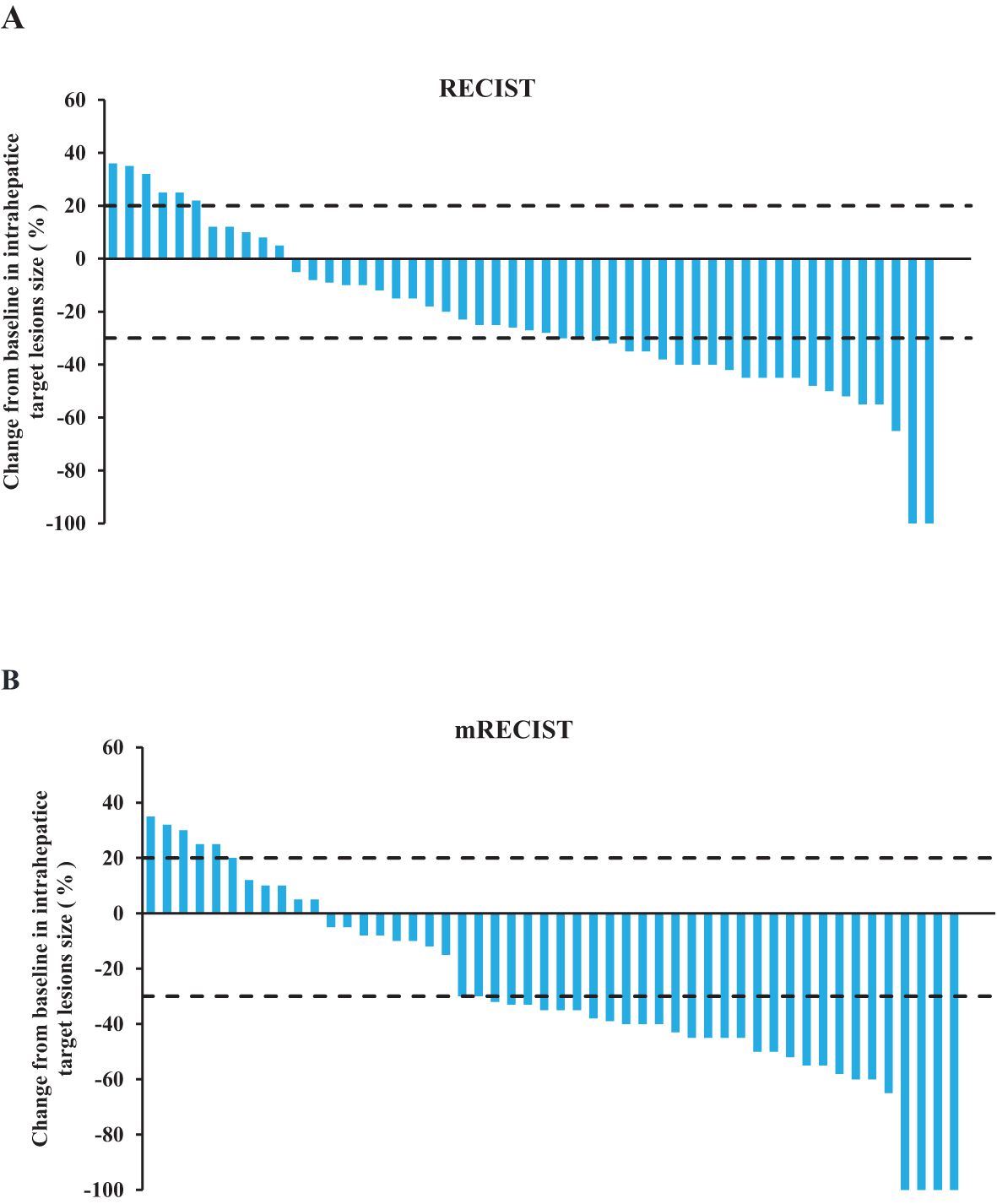
Figure 1. Tumor treatment response. (A) The best percentage change of the target lesion relative to the baseline according to RECIST v1.1. (B) The best percentage change of the target lesion relative to the baseline according to mRECIST.
Typical cases of pathological remission achieved after combination therapy
A patient with multiple intrahepatic tumors(multiple lesions in the S5/S7/S8 segments of the liver), the largest being 13.9 cm in diameter (Figure 2A), and associated major vascular cancer thrombus, had a Child-Pugh B liver function and was at BCLC stage C for the tumors. After undergoing two treatments of FOLFOX-HAIC (Figure 2B) combined with sintilimab and a biosimilar of bevacizumab, a follow-up evaluation showed significant reduction in the intrahepatic tumors. After the first treatment, the tumor was evaluated to have shrunk to 13.2cm, and after the second combination therapy, it was reduced to 10.7cm (Figure 2C), and partial remission was achieved according to mRICIST assessment. The combined treatment’s side effects were tolerable. Subsequently, a complete surgical resection was performed, and postoperative pathology revealed no residual cancer tissue, indicating a complete pathological response to the treatment (Figure 2D).
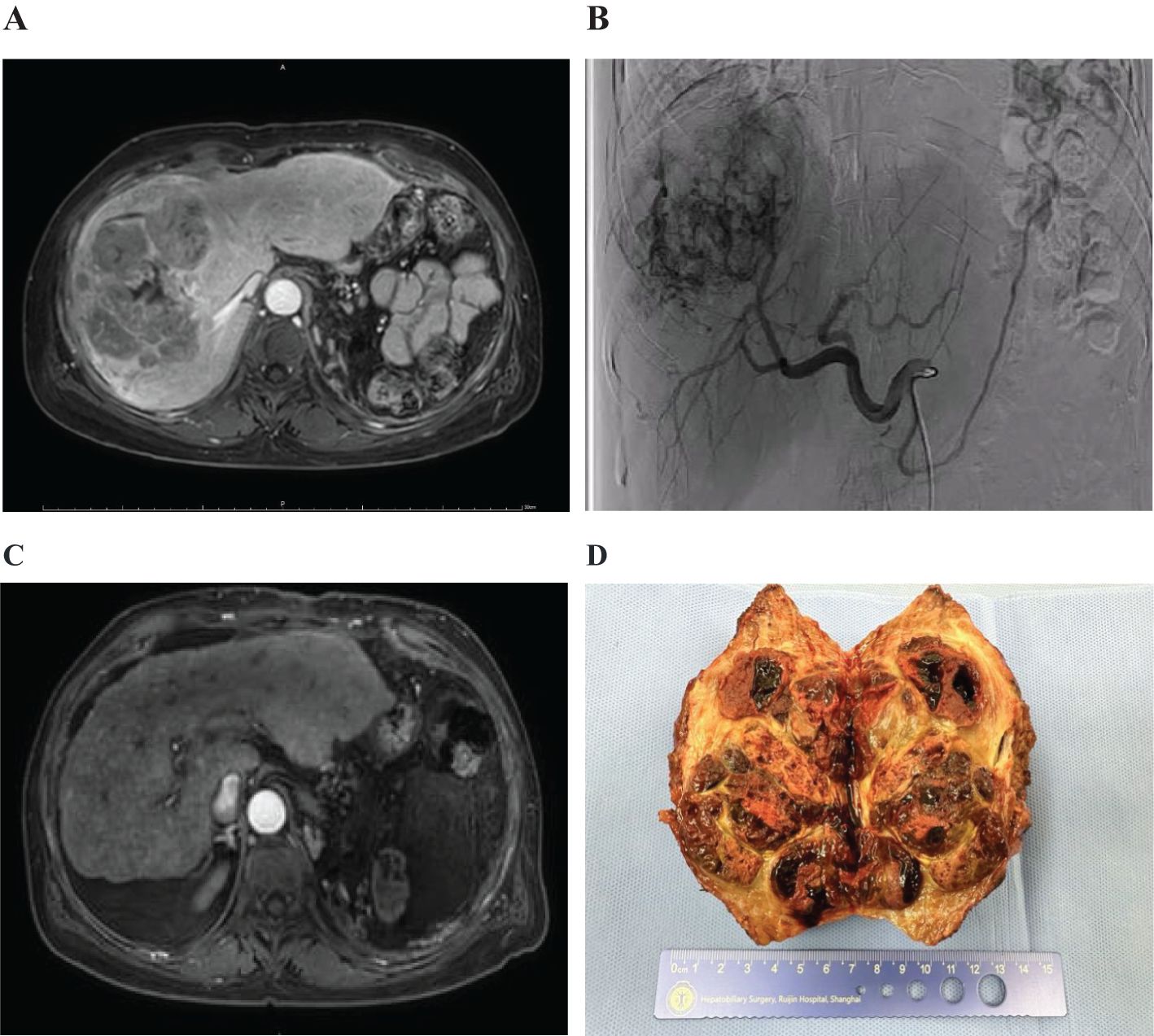
Figure 2. Imaging and pathological results of patients with hepatocellular carcinoma. (A) Multiple intrahepatic tumors were present. (B) Digital subtraction angiography (DSA) showed the tumors. (C) The lesions significantly reduced in size after treatment. (D) Postoperative gross pathological specimens were obtained.
The median follow-up period was 16.6 months. According to the mRECIST criteria, the OS at 6, 12, 18 months were 100%, 88.2%, and 76.4%, respectively. The median OS (mOS) was not reached. The median follow-up interval was 3.6 months (Figure 3A). The PFS at 6 and 12 months were 88.2% and 60.9%, respectively, with a median PFS (mPFS) of 15.2 months (Figure 3B). After 18 months of follow-up, a total of 12 patients died, with a survival rate of 76.4%. In the subgroup analysis of the surgery and non-surgery groups, it was found that the mOS of the non-surgery group was 17.2 months (95% CI,14.4 to 20.0), and the mPFS was 12.7 months (95% CI,10.0 to 15.4). The mOS and mPFS of the surgical group were not achieved. Two subgroup analyses revealed that there was a statistically significant difference in OS (HR,0.12, P=0.012) and PFS (HR,0.20, P=0.003) between the surgery and non-surgery groups (Figures 3C, D).
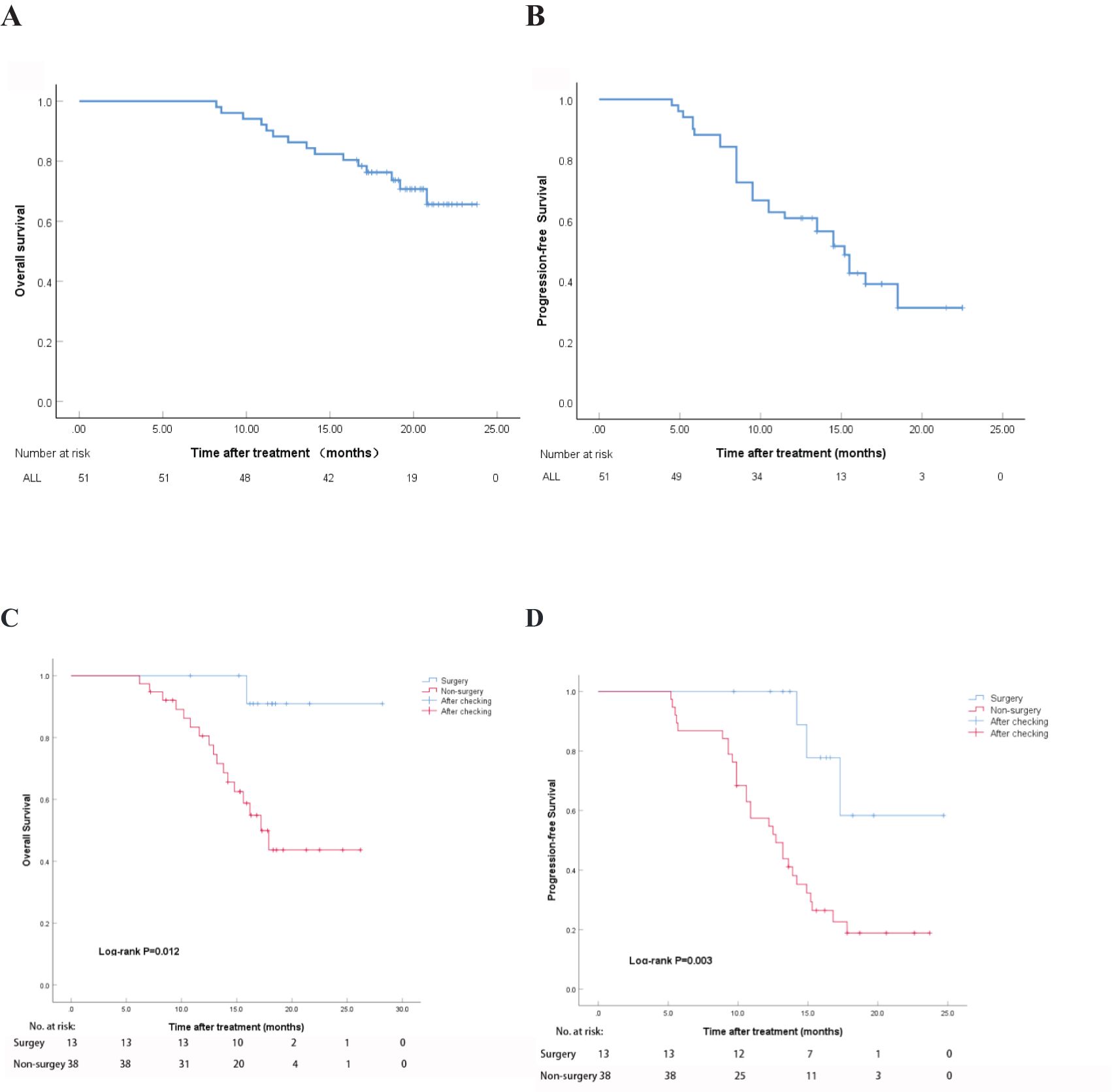
Figure 3. Overall survival time and progression-free survival period after treatment. (A) OS using mRECIST; (B) PFS using mRECIST; (C) OS in the surgery and non-surgery groups; (D) PFS in the surgery and non-surgery groups.
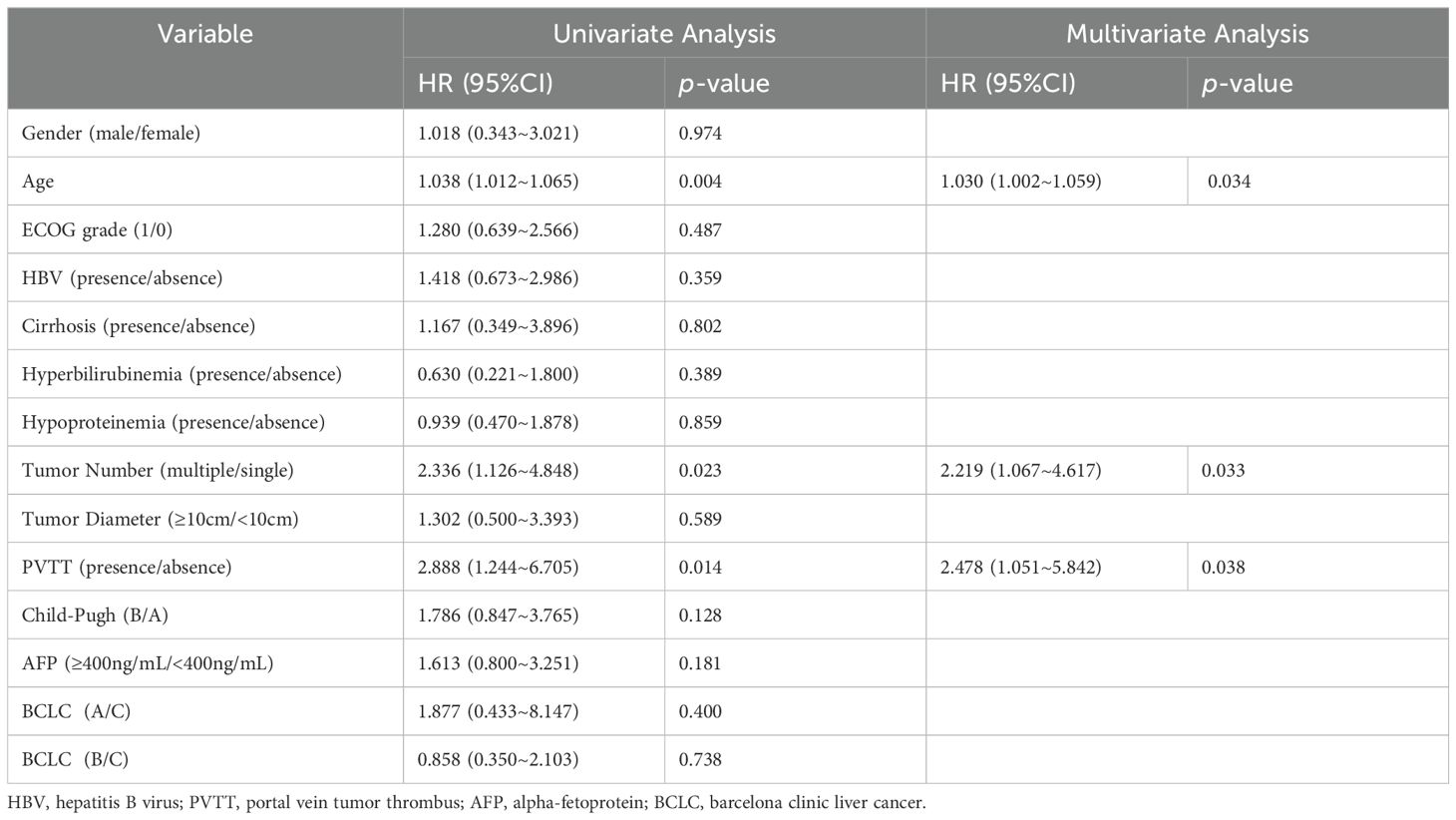
Through univariate and multivariate COX analyses of PFS, it was found that age, portal vein tumor thrombus (PVTT), and tumor number were risk factors analyzed for their impact on prognosis. Specifically, it indicated that the older patients were more likely to experience disease progression compared to the younger ones. Patients with multiple tumor lesions were more likely to experience disease progression in contrast to the patients with a single tumor. Additionally, in the subgroup analysis based on PVTT, patients with PVTT had a potentially poor prognosis, who was more likely to experience disease recurrence (Table 3).
Adverse events
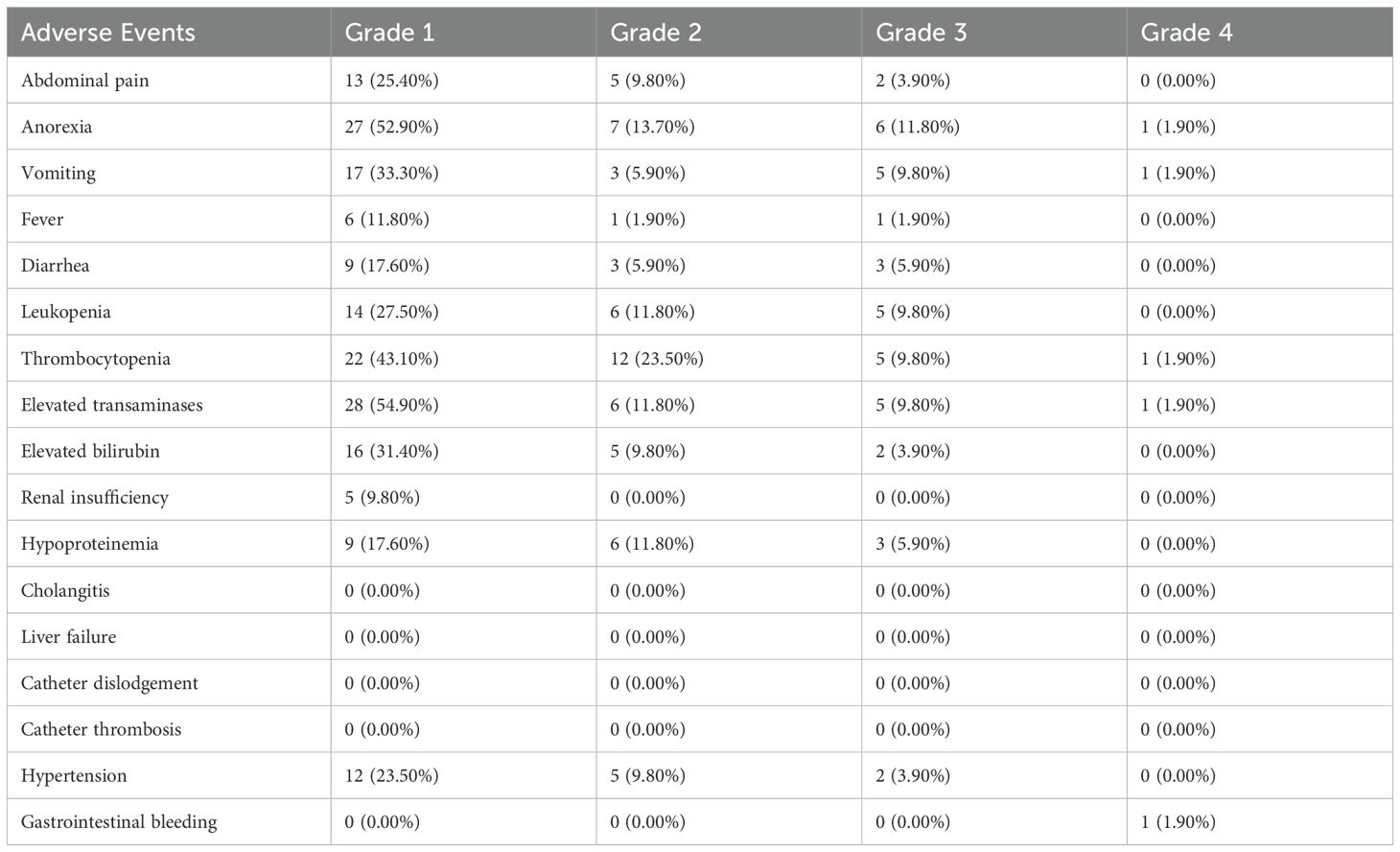
In this treatment study, adverse events related to a total of 151 HAIC treatments in 51 patients were statistically analyzed. All patients experienced adverse reactions of varying degrees (Table 4). The most common adverse reactions were elevated transaminases, thrombocytopenia, decreased albumin, abdominal pain, nausea, and vomiting. However, most patients experienced adverse reactions of grade 1-2, with no grade 5 adverse events observed. One patient (1.9%) experienced a grade 4 adverse reaction post-treatment, characterized by severe thrombocytopenia (41 x 10^9/L) accompanied by upper gastrointestinal bleeding symptoms, which improved after hemostasis via gastroscopy and platelet transfusion therapy. Twelve patients (23.5%) experienced severe post-chemotherapy reactions, including nausea, abdominal pain, vomiting, and difficulty eating, along with abnormal test indicators during repeated treatments. Chemotherapy drug perfusion was paused, and the patients were hospitalized for supportive symptomatic treatment with fluid supplementation, after which the symptoms improved, and subsequent drug perfusion chemotherapy was continued. The remaining 39 patients (74.5%) experienced grade 1-2 adverse reactions, mainly mild abdominal pain, vomiting, and elevated transaminases, which occurred during arterial infusion chemotherapy and improved with antispasmodic, analgesic, antiemetic, and liver-protective treatments. No catheter-related complications or treatment-related deaths occurred. The most common adverse reactions in patients who opted for FOLFOX-HAIC combined with lenvatinib and camrelizumab were vomiting, leukopenia, lypertension and Elevated transaminases. The most common adverse reactions in patients who selected FOLFOX-HAIC combined with atezolizumab combined with bevacizumab were anorexia, hypertension, elevated transaminases and elevated bilirubin. The most common adverse reactions in patients who chose FOLFOX-HAIC combined with sintilimab and a bevacizumab biosimilar were abdominal pain, anorexia, elevated transaminases and elevated bilirubin. Common adverse reactions in other patients include abdominal pain, anorexia, vomiting, elevated granulocytes, elevated transaminases and elevated bilirubin.
Discussion
Hepatocellular carcinoma is one of the most common malignant tumors. Liver resection surgery is considered the preferred treatment method for curatively treating liver cancer, offering patients a chance for long-term survival. However, approximately 80% of HCC patients are ineligible for curative surgery at initial diagnosis due to tumor burden, tumor stage, or limited liver function reserve. The challenge of downstaging tumors to render initially unresectable HCC patients eligible for surgery and extending their survival has garnered increasing attention from researchers worldwide (14). Different treatment combinations, including local treatments such as interventional therapy and ablation, as well as systemic treatments combining targeted therapy and immunotherapy, are gaining prominence in conversion therapy aimed at tumor downstaging.
HAIC is based on the pathophysiological characteristics of most malignant liver tumors. The blood supply to hepatocellular carcinoma primarily comes from the hepatic artery, while the normal liver parenchyma is mainly supplied by the portal vein. HAIC achieves a high tumor response by delivering high concentrations of chemotherapeutic drugs directly to liver tumors through the hepatic artery, thus avoiding excessive exposure of the normal liver parenchyma to the drugs. Catheter arterial infusion chemotherapy involves continuous infusion of chemotherapeutic drugs into the hepatic artery via percutaneous puncture, increasing drug concentration, reducing systemic toxicity, and enhancing tumor uptake (15). The FOLFOX regimen was proposed by Chinese researchers, who found that this regimen had an ORR and DCR of 40.8% and 77.6%, respectively. The 6-month and 12-month survival rates were 71.4% and 55.1%. Additionally, in a clinical trial involving 262 liver cancer patients, it was discovered that compared to treatment with the single-target drug sorafenib, the FOLFOX regimen resulted in a higher OS (Overall Survival) (13.9 months vs. 8.2 months) (7, 8, 16, 17).
Currently, for patients with advanced unresectable liver cancer, systemic therapy with targeted drugs combined with immunotherapy is prioritized due to its proven efficacy (18). It is recommended to use either targeted therapy alone or in combination with Programmed Death Receptor-1 (PD-1/PD-L1) immune checkpoint inhibitors as the first-line treatment or alternative therapy for advanced HCC (19–23). In the IMbrave150 study, the combination of atezolizumab and bevacizumab showed superior OS and PFS compared to the single-agent sorafenib, providing better tumor efficacy and longer survival for patients. The ORR (Overall Response Rate) for the T+A group reached 30%, indicating the advantage of combining targeted therapy with immunotherapy over single-agent targeted therapy. The median OS in the global population was 19.2 months, and the combination achieved a 6% complete response rate. The ORIENT-32 study (24) results showed that, compared to the sorafenib group, the combination of sintilimab and a bevacizumab biosimilar reduced the risk of death by 43% and the risk of progression by 43%. The ORR in the combination therapy group was also significantly higher than in the sorafenib group. According to RECIST 1.1 criteria, the ORRs were 20.3% and 4.1% for the two groups, respectively; according to mRECIST criteria, the ORRs were 23.4% and 7.1%. The KeyNote 524 study (11) revealed that the combination of lenvatinib and pembrolizumab had an ORR of 41% and a DCR (Disease Control Rate) of 86%, demonstrating reliable anti-tumor activity. The BGB-A317-211 study found that the combination of tislelizumab and lenvatinib had an ORR of 41.9% and a DCR of 85.5%. Additionally, the SHR 310 study (25) discovered that the combination of camrelizumab and apatinib (C+R) significantly prolonged PFS and OS and improved the ORR.
The triple therapy regimen combining HAIC (Hepatic Arterial Infusion Chemotherapy) with targeted therapy and immunotherapy has also shown good safety and excellent efficacy (26, 27). Existing research results indicate that the HAIC + lenvatinib + PD-1 triple therapy group achieved longer PFS (11.1 months vs. 5.1 months, P<0.001) and overall survival (not reached vs. 11 months, P<0.001) compared to the standard single targeted drug lenvatinib group. It also showed a higher DCR (90.1% vs. 72.1%, P=0.005) and objective response rate [RECIST 1.1 criteria: 59.2% vs. 9.3%, P<0.001; new criteria for evaluating solid tumors (mRECIST criteria): 67.6% vs. 16.3%, P<0.001]. Additionally, 14.1% of patients in the triple therapy group achieved CR according to the mRECIST criteria (28).
In this study, we retrospectively included 51 patients with advanced unresectable liver cancer. After receiving combined FOLFOX-HAIC targeted and immune therapy, the median mPFS was 15.2 months, and mOS had not been reached. According to the mRECIST criteria, the ORR was 60.8%, and the DCR was 88.2%. Thirteen patients (25.5%) successfully obtained the opportunity for surgery after combined therapy, among which 4 patients achieved CR. A previous retrospective study (29) of HAIC combined with targeted immune therapy indicated that among 25 patients, 15 (60.0%) achieved conversion to surgical standards; 1 person refused surgery, and the remaining 14 underwent surgical resection. Among these, 7 patients (28.0%) achieved pathological CR after resection, with a recurrence-free survival of 13.17 months. In our study, the surgical conversion rate did not reach the expected results mentioned above. This may be due to a significant portion of the enrolled patients being classified as BCLC stage C, with high tumor burden and most having PVTT (portal vein tumor thrombosis). According to Zhang et al., (30) the combination of HAIC targeted immunotherapy demonstrated a significant ORR of 54.1%, DCR of 94.6%, a Surgical conversion rate of 29.6%. Common adverse effects include pain, fatigue and abnormal liver function. These results were similar to the results in this study. Lin (31) reported that when HAIC was combined with targeted immunotherapy, it achieved an ORR of 60.4%, along with a DCR of 100% based on mRECIST criteria. The median PFS (mPFS) was 13.9 months. Adverse events of different degrees were observed in all patients after receiving the combination therapy. Abdominal pain, nausea, vomiting, increased levels of transaminases, anorexia, fatigue, and diarrhea mainly occurred in the treatment. All patients recovered within a few days. Zhang (27) reported that the combination of HAIC, camrelizumab, and apatinib demonstrated an ORR of 77.1%and a DCR of 97.1% in treating advanced HCC patients. The median PFS (mPFS) was recorded at 10.38 months which was shorter than the mPFS in this study. This year’s global multicenter clinical study EMERALD-1 (32) revealed the effectiveness and safety of TACE combined with targeted immunotherapy. We compared this study with the EMERALD-1 study and found that according to the up-to-7 criteria, patients in this study had higher tumor burden levels, with more patients exceeding the up-to-7 criteria (90.2% vs. 52.5%, p<0.001), while the objective response rate and disease control rate in this study were higher (ORR: 60.8% vs. 43.6%, p=0.024; DCR: 88.2% vs. 65.8%, p<0.001).
Adverse reactions caused by chemotherapeutic drugs in HAIC are relatively common, but most are mild (grades 1-2). Studies (33, 34) have found that adverse reactions to HAIC alone occur at a rate of 88.1%, with leukopenia (9%) being the most common. In this study, the total number of adverse reactions after HAIC combined with targeted immunotherapy was 46 (90.2%). Among grade 1-2 adverse reactions, thrombocytopenia and elevated transaminases were the most common, with anorexia and vomiting being the predominant reactions after chemotherapy. These patients showed improvement after conservative treatment with liver protection, antiemetics, and gastric protection, and tolerated subsequent treatments. Among grade 3 adverse reactions, anorexia, leukopenia, thrombocytopenia, and elevated transaminases were more common, and these patients were also able to maintain subsequent treatment after receiving appropriate interventions.
This study has several limitations. First, it is a retrospective, single-center study; hence, it is inevitably subject to unknown selection biases, which reduce the generalizability of the findings. Second, the small sample size and the lack of a control group diminish the reliability of the evidence and increase the comparative error. Therefore, further research with a control group in a larger population is needed. Finally, the relatively short follow-up period may reduce the quality of the observed effectiveness and affect the estimates of ORR, PFS and OS.
Conclusion
The FOLFOX-HAIC combined with targeted immunotherapy regimen is effective in patients with unresectable HCC, demonstrated by a high surgical conversion rate and manageable adverse effects. This regimen represents a potential novel first-line treatment option for HCC.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Ethics Committee of Ruijin Hospital Affiliated to Shanghai Jiao Tong University School of Medicine. The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants’ legal guardians/next of kin because the analysis used anonymous clinical data that were obtained after each patient agreed to treatment by written consent.
Author contributions
YL: Writing – original draft, Writing – review & editing. YY: Writing – original draft, Writing – review & editing. DM: Writing – review & editing. JW: Writing – review & editing. FH: Writing – review & editing. XC: Writing – review & editing. YC: Writing – original draft, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. Foundation: Beijing Medical Award Foundation (No. YXJL-2020-0191-0024).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2024.1471017/full#supplementary-material
References
1. Singal AG, Kanwal F, Llovet JM. Global trends in hepatocellular carcinoma epidemiology: implications for screening, prevention and therapy. Nat Rev Clin Oncol. (2023) 20:864–84. doi: 10.1038/s41571-023-00825-3
2. Huang DQ, El-Serag HB, Loomba R. Global epidemiology of NAFLD-related HCC: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol. (2021) 18:223–38. doi: 10.1038/s41575-020-00381-6
3. Rumgay H, Arnold M, Ferlay J, Lesi O, Cabasag CJ, Vignat J, et al. Global burden of primary liver cancer in 2020 and predictions to 2040. J Hepatol. (2022) 77:1598–606. doi: 10.1016/j.jhep.2022.08.021
4. Kudo M, Matsui O, Izumi N, Iijima H, Kadoya M, Imai Y, et al. JSH consensus-based clinical practice guidelines for the management of hepatocellular carcinoma: 2014 update by the liver cancer study group of Japan. Liver Cancer. (2014) 3:458–68. doi: 10.1159/000343875
5. Chen LT, Martinelli E, Cheng AL, Pentheroudakis G, Qin S, Bhattacharyya GS, et al. Pan-Asian adapted ESMO Clinical Practice Guidelines for the management of patients with intermediate and advanced/relapsed hepatocellular carcinoma: a TOS-ESMO initiative endorsed by CSCO, ISMPO, JSMO, KSMO, MOS and SSO. Ann Oncol. (2020) 31:334–51. doi: 10.1016/j.annonc.2019.12.001
6. 2022 KLCA-NCC Korea practice guidelines for the management of hepatocellular carcinoma. Korean J Radiol. (2022) 23:1126–240. doi: 10.3348/kjr.2022.0822
7. Li QJ, He MK, Chen HW, Fang WQ, Zhou YM, Xu L, et al. Hepatic arterial infusion of oxaliplatin, fluorouracil, and leucovorin versus transarterial chemoembolization for large hepatocellular carcinoma: A randomized phase III trial. J Clin Oncol. (2022) 40:150–60. doi: 10.1200/JCO.21.00608
8. Hamaoka M, Kobayashi T, Kuroda S, Iwako H, Okimoto S, Kimura T, et al. Hepatectomy after down-staging of hepatocellular carcinoma with portal vein tumor thrombus using chemoradiotherapy: A retrospective cohort study. Int J Surg. (2017) 44:223–8. doi: 10.1016/j.ijsu.2017.06.082
9. Michaud HA, Eliaou JF, Lafont V, Bonnefoy N, Gros L. Tumor antigen-targeting monoclonal antibody-based immunotherapy: Orchestrating combined strategies for the development of long-term antitumor immunity. Oncoimmunology. (2014) 3:e955684. doi: 10.4161/21624011.2014.955684
10. Pennock GK, Chow LQ. The evolving role of immune checkpoint inhibitors in cancer treatment. Oncologist. (2015) 20:812–22. doi: 10.1634/theoncologist.2014-0422
11. Finn RS, Qin S, Ikeda M, Galle PR, Ducreux M, Kim TY, et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med. (2020) 382:1894–905. doi: 10.1056/NEJMoa1915745
12. Song DS, Song MJ, Bae SH, Chung WJ, Jang JY, Kim YS, et al. A comparative study between sorafenib and hepatic arterial infusion chemotherapy for advanced hepatocellular carcinoma with portal vein tumor thrombosis. J Gastroenterol. (2015) 50:445–54. doi: 10.1007/s00535-014-0978-3
13. Lau WY, Lai EC. Salvage surgery following downstaging of unresectable hepatocellular carcinoma–a strategy to increase resectability. Ann Surg Oncol. (2007) 14:3301–9. doi: 10.1245/s10434-007-9549-7
14. Deng M, Cai H, He B, Guan R, Lee C, Guo R. Hepatic arterial infusion chemotherapy versus transarterial chemoembolization, potential conversion therapies for single huge hepatocellular carcinoma: a retrospective comparison study. Int J Surg. (2023) 109:3303–11. doi: 10.1097/JS9.0000000000000654
15. Lewandowski RJ, Geschwind JF, Liapi E, Salem R. Transcatheter intraarterial therapies: rationale and overview. Radiology. (2011) 259:641–57. doi: 10.1148/radiol.11081489
16. Lyu N, Lin Y, Kong Y, Zhang Z, Liu L, Zheng L, et al. FOXAI: a phase II trial evaluating the efficacy and safety of hepatic arterial infusion of oxaliplatin plus fluorouracil/leucovorin for advanced hepatocellular carcinoma. Gut. (2018) 67:395–6. doi: 10.1136/gutjnl-2017-314138
17. Lyu N, Kong Y, Mu L, Lin Y, Li J, Liu Y, et al. Hepatic arterial infusion of oxaliplatin plus fluorouracil/leucovorin vs. sorafenib for advanced hepatocellular carcinoma. J Hepatol. (2018) 69:60–9. doi: 10.1016/j.jhep.2018.02.008
18. Rimassa L, Finn RS, Sangro B. Combination immunotherapy for hepatocellular carcinoma. J Hepatol. (2023) 79:506–15. doi: 10.1016/j.jhep.2023.03.003
19. Cheng AL, Kang YK, Chen Z, Tsao CJ, Qin S, Kim JS, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. (2009) 10:25–34. doi: 10.1016/S1470-2045(08)70285-7
20. Qin S, Li Q, Gu S, Chen X, Lin L, Wang Z, et al. Apatinib as second-line or later therapy in patients with advanced hepatocellular carcinoma (AHELP): a multicentre, double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Gastroenterol Hepatol. (2021) 6:559–68. doi: 10.1016/S2468-1253(21)00109-6
21. Qin S, Bi F, Gu S, Bai Y, Chen Z, Wang Z, et al. Donafenib versus sorafenib in first-line treatment of unresectable or metastatic hepatocellular carcinoma: A randomized, open-label, parallel-controlled phase II-III trial. J Clin Oncol. (2021) 39:3002–11. doi: 10.1200/JCO.21.00163
22. Abou-Alfa GK, Meyer T, Cheng AL, El-Khoueiry AB, Rimassa L, Ryoo BY, et al. Cabozantinib in patients with advanced and progressing hepatocellular carcinoma. N Engl J Med. (2018) 379:54–63. doi: 10.1056/NEJMoa1717002
23. Psilopatis I, Damaskos C, Garmpi A, Sarantis P, Koustas E, Antoniou EA, et al. FDA-approved monoclonal antibodies for unresectable hepatocellular carcinoma: what do we know so far? Int J Mol Sci. (2023) 24. doi: 10.3390/ijms24032685
24. Ren Z, Xu J, Bai Y, Xu A, Cang S, Du C, et al. Sintilimab plus a bevacizumab biosimilar (IBI305) versus sorafenib in unresectable hepatocellular carcinoma (ORIENT-32): a randomised, open-label, phase 2-3 study. Lancet Oncol. (2021) 22:977–90. doi: 10.1016/S1470-2045(21)00252-7
25. Qin S, Chan SL, Gu S, Bai Y, Ren Z, Lin X, et al. Camrelizumab plus rivoceranib versus sorafenib as first-line therapy for unresectable hepatocellular carcinoma (CARES-310): a randomised, open-label, international phase 3 study. Lancet. (2023) 402:1133–46. doi: 10.1016/S0140-6736(23)00961-3
26. Mei J, Tang YH, Wei W, Shi M, Zheng L, Li SH, et al. Hepatic arterial infusion chemotherapy combined with PD-1 inhibitors plus lenvatinib versus PD-1 inhibitors plus lenvatinib for advanced hepatocellular carcinoma. Front Oncol. (2021) 11:618206. doi: 10.3389/fonc.2021.618206
27. Zhang TQ, Geng ZJ, Zuo MX, Li JB, Huang JH, Huang ZL, et al. Camrelizumab (a PD-1 inhibitor) plus apatinib (an VEGFR-2 inhibitor) and hepatic artery infusion chemotherapy for hepatocellular carcinoma in Barcelona Clinic Liver Cancer stage C (TRIPLET): a phase II study. Signal Transduct Target Ther. (2023) 8:413. doi: 10.1038/s41392-023-01663-6
28. He MK, Liang RB, Zhao Y, Xu YJ, Chen HW, Zhou YM, et al. Lenvatinib, toripalimab, plus hepatic arterial infusion chemotherapy versus lenvatinib alone for advanced hepatocellular carcinoma. Ther Adv Med Oncol. (2021) 13:17588359211002720. doi: 10.1177/17588359211002720
29. Zhang J, Zhang X, Mu H, Yu G, Xing W, Wang L, et al. Surgical conversion for initially unresectable locally advanced hepatocellular carcinoma using a triple combination of angiogenesis inhibitors, anti-PD-1 antibodies, and hepatic arterial infusion chemotherapy: A retrospective study. Front Oncol. (2021) 11:729764. doi: 10.3389/fonc.2021.729764
30. Zhang W, Zhang K, Liu C, Gao W, Si T, Zou Q, et al. Hepatic arterial infusion chemotherapy combined with anti-PD-1/PD-L1 immunotherapy and molecularly targeted agents for advanced hepatocellular carcinoma: a real world study. Front Immunol. (2023) 14:1127349. doi: 10.3389/fimmu.2023.1127349
31. Lin ZP, Hu XL, Chen D, Huang DB, Zou XG, Zhong H, et al. Efficacy and safety of targeted therapy plus immunotherapy combined with hepatic artery infusion chemotherapy (FOLFOX) for unresectable hepatocarcinoma. World J Gastroenterol. (2024) 30:2321–31. doi: 10.3748/wjg.v30.i17.2321
32. Lencioni R, Kudo M, Erinjeri J, Qin S, Ren Z, Chan S, et al. EMERALD-1: A phase 3, randomized, placebo-controlled study of transarterial chemoembolization combined with durvalumab with or without bevacizumab in participants with unresectable hepatocellular carcinoma eligible for embolization. J Clin Oncol. (2024) 42:LBA432–2. doi: 10.1200/JCO.2024.42.3_suppl.LBA432
33. Li S, Lyu N, Han X, Li J, Lai J, He M, et al. Hepatic artery infusion chemotherapy using fluorouracil, leucovorin, and oxaliplatin versus transarterial chemoembolization as initial treatment for locally advanced hepatocellular carcinoma: A propensity score-matching analysis. J Vasc Interv Radiol. (2021) 32:1267–76.e1. doi: 10.1016/j.jvir.2021.06.008
34. Pan Y, Wang R, Hu D, Xie W, Fu Y, Hou J, et al. Comparative safety and efficacy of molecular-targeted drugs, immune checkpoint inhibitors, hepatic arterial infusion chemotherapy and their combinations in advanced hepatocellular carcinoma: findings from advances in landmark trials. Front Biosci (Landmark Ed). (2021) 26:873–81. doi: 10.52586/4994
Keywords: hepatocellular carcinoma, targeted therapy, immunotherapy, combined therapy, conversion therapy, FOLFOX-HAIC, unresectable advanced cancer
Citation: Lu Y-C, Yang Y-C, Ma D, Wang J-q, Hao F-J, Chen X-x and Chen Y-j (2024) FOLFOX-HAIC combined with targeted immunotherapy for initially unresectable hepatocellular carcinoma: a real-world study. Front. Immunol. 15:1471017. doi: 10.3389/fimmu.2024.1471017
Received: 26 July 2024; Accepted: 11 November 2024;
Published: 26 November 2024.
Edited by:
Tian Yang, First Affiliated Hospital of Jilin University, ChinaReviewed by:
Shuanggang Chen, Sun Yat-sen University Cancer Center (SYSUCC), ChinaSagnik Giri, University of Arizona, United States
Copyright © 2024 Lu, Yang, Ma, Wang, Hao, Chen and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yong-jun Chen, c3dha2NoZW55akBob3RtYWlsLmNvbQ==
†These authors have contributed equally to this work and share first authorship
 Yan-Cen Lu
Yan-Cen Lu Yu-Chen Yang†
Yu-Chen Yang† Jun-qing Wang
Jun-qing Wang Feng-Jie Hao
Feng-Jie Hao Xu-xiao Chen
Xu-xiao Chen