
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Immunol. , 24 October 2024
Sec. Cancer Immunity and Immunotherapy
Volume 15 - 2024 | https://doi.org/10.3389/fimmu.2024.1467429
This article is part of the Research Topic Community Series in Immunotherapy and Small Molecule Inhibitors as Combinational Cancer Therapeutics: Volume II View all 12 articles
Background and aims: Compared with tyrosine kinase inhibitor (TKI) monotherapy, TKI combined with PD1 can improve the therapeutic effect of liver cancer and has been widely used in clinical practice. However, there is a lack of effective biomarkers to identify patients who would benefit more from this combination therapy. Therefore, this study aimed to evaluate whether baseline lymphocyte counts can identify patients with liver cancer who would benefit from targeted immune combination therapy.
Methods: Data from patients with hepatocellular carcinoma (HCC) who received TKIs or TKIs in combination with PD1 between June 2018 and June 2020 were retrospectively collected. The patients were divided into high and low groups based on the median absolute count of peripheral lymphocytes before systemic therapy and differences in overall survival (OS) and progression-free survival (PFS) between TKI and TKI+PD1 were compared between the two groups.
Results: In total, 72 patients were included in this study, with a median follow-up of 1.5 years. Both PFS and OS in the TKI+PD1 group showed a good prognostic trend (p = 0.058 and p = 0.077, respectively). Subgroup analyses based on peripheral blood lymphocyte counts showed that the combination regimen had a significant PFS and OS advantage only in patients with high peripheral blood lymphocyte counts (p = 0.036 and p = 0.031, respectively), but not in patients with low absolute peripheral blood lymphocyte counts (p = 0.819 and p = 0.913, respectively).
Conclusions: Peripheral blood lymphocyte count is a simple and effective biomarker that can be used to identify patients with liver cancer who will benefit more from TKI+PD-1 combination therapy.
In recent years, new therapies such as targeted therapy with sorafenib/lenvatinib, and immunotherapy with PD-1/PD-L1 inhibitors have become the treatment of choice for liver cancer (1–3). Tumor vascular abnormalities lead to hypoxia and acidosis in the tumor microenvironment, which causes immunosuppression through a variety of mechanisms, and anti-angiogenic can normalize the blood vessels around the tumor and improve the microenvironment, thereby promoting the effect of immunotherapy (4, 5). Compared with the lower response rate of monotherapy, combination immunotherapy based on TKIs has shown promising efficacy in advanced liver cancer (6–9). For example, the Keynote524 studies showed that the objective response rate to lenvatinib in combination with PD1 reached 46% (8). However, many patients do not benefit from the combination regimen, which causes adverse reactions, such as hepatitis/pneumonia caused by immunotherapy, seriously reducing the patients’ quality of life of patients and affecting subsequent antitumor therapy (1). Phase III clinical trial results showed that in the lenvatinib plus pembrolizumab group, 71 (18%) of 395 patients discontinued any study treatment because of treatment-related adverse events versus 42 (11%) of 395 patients in the lenvatinib plus placebo group. The treatment-related grade 3–4 adverse events were also higher in the combination group than alone lenvatinib (243 [62%] vs 224 [57%]) (7).
Considering the toxicity and increased treatment costs of the combination regimen, identifying which patients are more suitable for the two-drug combination is a clinically meaningful direction; however, current research in this area is very limited. Lymphopenia is associated with poor prognosis in multiple cancer types and can be used to predict the efficacy of tumor immunotherapy (10–12). Therefore, in this study, we aimed to investigate whether baseline lymphocyte counts could predict the probability of benefits from targeted immune combination therapy in patients with liver cancer.
This retrospective study was conducted at Nanfang Hospital, Southern Medical University, and was approved by the Ethics Committee of Nanfang Hospital, Southern Medical University. We retrospectively analyzed patients with continuous liver cancer who received TKIs alone or TKIs in combination with PD1 at our hospital between June 2018 and June 2020. We included patients who met the following criteria: 1) age > 18 years; 2) diagnosis of liver cancer by clinical or pathological examination; 3) adequate recording of baseline blood routine tests; 4) PS score 0–2 points; and 5) the first systemic therapy was targeted therapy with lenvatinib or sorafenib, alone or in combination with PD-1 antibody. Patients were excluded if they had 1) history of organ transplantation, 2) immunodeficiency diseases, 3) incomplete medical data or loss to follow-up, 4) prior treatment with other systems, and 5) were administered immune checkpoints for second-and multiline therapy.
All patients provided written informed consent before undergoing systemic therapy. Sorafenib orally 400 mg 1/day. Lenvatinib 8 mg orally or at 12 mg 1/day. PD-1 inhibitor alone, camrelizumab (200 mg), toripalimab (240 mg), sintilimab(200 mg) or pembrolizumab(200 mg)once every 3 weeks as an intravenous infusion or nivolumab (3 mg/kg every 2 weeks). The reduction or discontinuation of treatment was determined by the clinician, depending on the disease status and adverse effects.
Patient baseline characteristics, such as age, sex, ECOG Score and AFP, were obtained from their electronic medical records. Hematological parameters for all patients were concentrated in the 1 week before the first systemic therapy. Evaluation of patient efficacy was based on imaging information using the mRESIST criterion, and patient survival information was collected via telephonic follow-up. OS was defined as the time from the first administration of systemic therapy to the patient’s death or loss to follow-up. PFS was defined as the time from the first administration of systemic therapy to tumor progression.
Categorical or continuous variables were compared between groups using the chi-square test or t-test. Kaplan–Meier analysis was used for OS and PFS, and the log-rank test was used for comparisons between groups. P < 0.05 was considered statistically significant. All statistical analyses were performed using the SPSS software.
In total, 72 patients were included in this study; their baseline characteristics are shown in Table 1. Most patients had hepatitis B virus (HBV) infection, BCLC stage C, Eastern Cooperative Oncology Group (ECOG) performance status of 0–1, and Child–Pugh class A. A total of 36 (50.0%) patients received concomitant local therapy, including TACE/HAIC/radiotherapy/radiofrequency ablation. Among the 72 patients, 29 received targeted immune combination therapy as first-line treatment, whereas 43 received targeted therapy alone. A higher proportion of patients in the TKI+PD-1 group received lenvatinib than those in the TKI group (37.9% vs. 14.0%, p = 0.019).
The median follow-up period was 1.5 years. By the end of follow-up time, 46 of the 72 patients had a death outcome and 64 had disease progression. Compared to the TKI treatment group, the PFS and OS of the TKI+PD1 group showed a better prognostic trend. The median PFS (mPFS) was longer in the TKI+PD1 group (3.5 months, 95%CI 1.5–5.5) than in the TKI group (2.7 months, 95%CI 2.2–3.2) [p = 0.058; Figure 1A]. The median OS was 10.2 months, (95%CI 5.7–14.6) in the TKI group and 19.9 months, (95%CI 7.3–32.5) in the TKI+PD1 group [p = 0.077; Figure 1B].
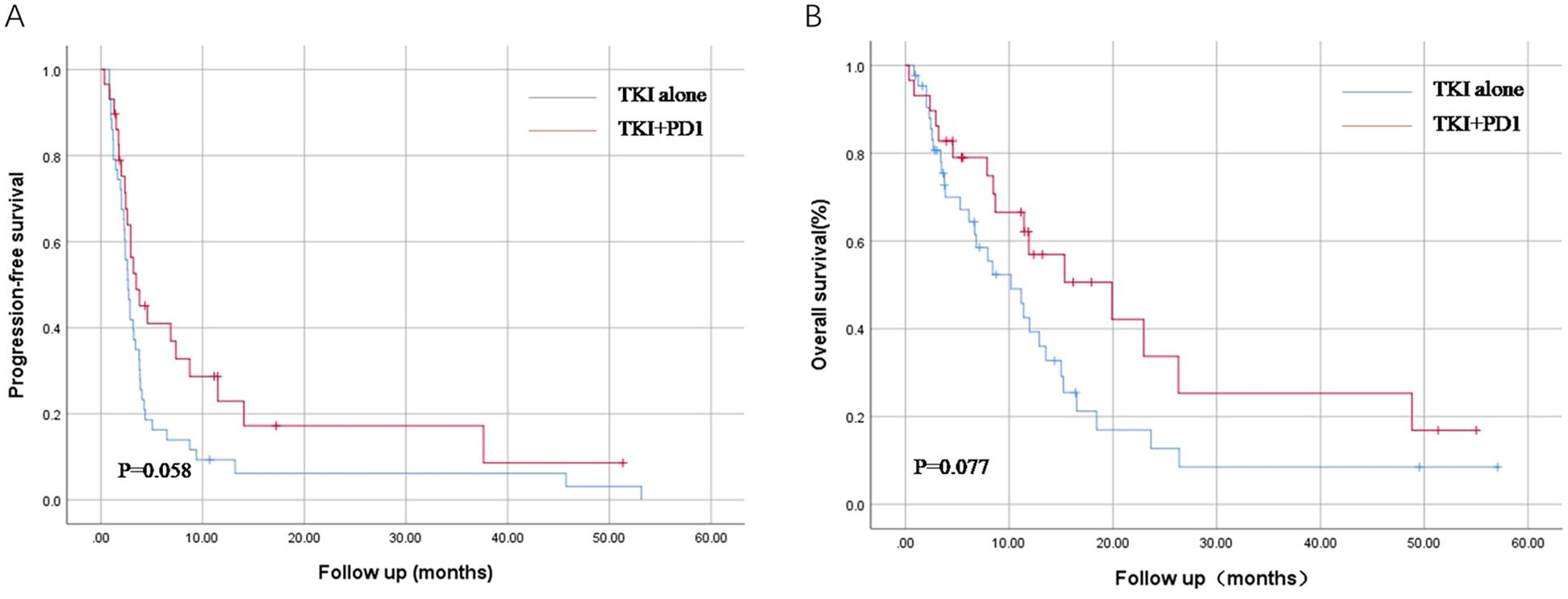
Figure 1. Kaplan–Meier curves for progression-free survival (A) and overall survival (B) in TKI alone and TKI+PD-1 group. TKIs, tyrosine Kinase Inhibitors. All statistical tests were two-sided.
Stratified analysis was performed based on the absolute peripheral blood lymphocyte count before systemic therapy. Patients were divided into high- and low-L groups based on the median absolute count of peripheral blood lymphocytes. In the high-L group, patients in the TKI+PD1 group showed longer mPFS compared with those who received TKIs [3.5 months, (95%CI 0.1–14.1) versus 2.9 months (95%CI 1.6–4.2), p = 0.036], and mOS [22.9 months, (95%CI 1.4–44.5) versus 7.9 months, (95%CI 0.1–16.0) p = 0.031; Figures 2A, B].
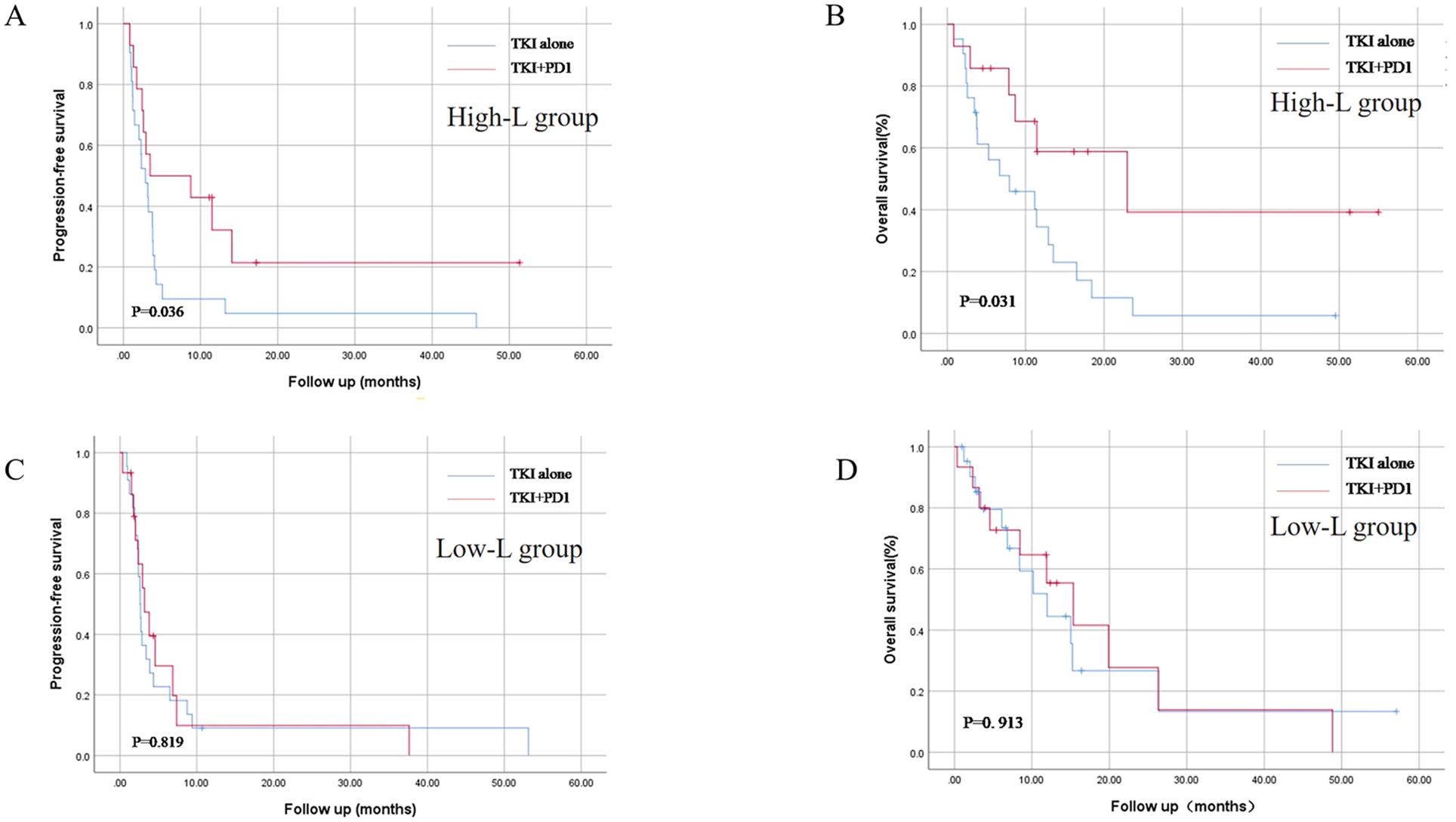
Figure 2. Kaplan–Meier curves for progression-free survival (A, C) and overall survival (B, D) in TKI alone and TKI+PD-1 group after stratification by peripheral blood lymphocyte count. TKIs, tyrosine Kinase Inhibitors. All statistical tests were two-sided.
No significant difference was found in mPFS [2.6 months, (95%CI 2.3–3.0) versus 3.2 months (95%CI 1.7–4.7), p = 0.819], and mOS [11.9 months, (95%CI 5.9–18.1) versus 15.3 months (95%CI 7.6–23.1), p = 0.913; Figures 2C, D] between TKI and TKI+PD1 use in the low-L group.
Moreover, we also used the lower limit of the normal value of lymphocytes to distinguish between people with high and low lymphocytes, and we found the same phenomenon (Additional File 1: Supplementary Figure S1).
To date, there are no effective biomarkers to screen patients with cancer to identify those who are more suitable for TKI+PD1 rather than single-agent TKI use. In this study, we assessed whether peripheral blood lymphocyte count could be used as a prognostic marker for the combination regimen and found that patients with low lymphocyte counts did not receive additional benefit from the combination regimen compared with single-agent targeting. Thus, our results suggest that peripheral blood lymphocyte count can be used as a biomarker to identify patients with liver cancer who will benefit from TKI+PD-1 combination therapy.
The treatment of advanced liver cancer remains challenging, with molecularly targeted therapies such as sorafenib and lenvatinib having low response rates. Consequently, combination immunotherapy such as PD-1/PD-L1 monoclonal antibodies has become a trend in the treatment of liver cancer (1). Target-immune therapy has a higher objective response rate than single-agent targeting (6–9). Similarly, our data showed that targeted combined immunization can prolong overall patient survival, which supports the advantages of combination therapy. However, a significant proportion of patients do not benefit from additional combination therapy, and the overall toxicity of combination regimens is high. These issues have prompted clinicians to make granular treatment decisions.
Our study found that TKI+PD1did not improve the prognosis of patients with peripheral blood lymphocytopenia. Compared with the difficulty and heterogeneity of tissue biopsy, peripheral blood lymphocytes are a simple clinical test index, based only on a simple routine blood test. This can help roughly determine which patients do not require targeted immunotherapy drugs, especially considering the toxicity and cost of combination therapy.
Nonetheless, the mechanism of peripheral blood lymphopenia in targeted immune combination therapy remains unclear. It is speculated that the mechanism may be related to the key role of lymphocytes in tumor immunity. A low peripheral blood lymphocyte count suggests a preexisting immunosuppressive state, resulting in an inadequate tumor immune response (12–14).
In contrast, patients with advanced liver cancer in the context of hepatitis B often have cirrhosis, which contributes to the development of hypersplenism, which often manifests as a decrease in the number of peripheral blood cells, including peripheral blood lymphocytes (15). The results of our association analysis showed that patients with low peripheral blood lymphocytes are often accompanied by a decrease in platelets and leukocytes. Some studies have suggested that the cellular immune function of patients with hypersplenism is severely impaired (15, 16), which may be a possible reason why peripheral lymphocytes can predict target-immune combination therapy; further experiments are needed to verify this.
Our study had the following limitations. First, this was a single-center retrospective study and the small sample size limited further subgroup analyses. Moreover, patient data was mainly based on electronic medical records and telephone follow-up, and patients who were lost to follow-up may experience a certain degree of bias. Second, the study included a combination of systemic regimens, including lenvatinib and sorafenib, which may differ in prognostic outcomes depending on the choice of the drug. In addition, some patients received concomitant local therapies. These local treatments may have a certain impact on the interpretation of the results. Based on your suggestions, we further analyzed the situation of local treatments received during the same period in different groups and found that there was no statistical difference between the subgroups in whether or not local treatment was received, which weakened the impact of this factor to a certain extent (Additional File 2: Supplementary Table S1).
In conclusion, our study revealed that peripheral blood lymphocyte count is an objective and simple indicator to identify which patients with advanced HCC should receive TKI+PD1 as a first-line systemic therapy rather than TKI alone. Properly designed prospective studies are needed to further explore these interesting findings.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
This study was approved by the Ethics Committees of Nanfang Hospital, Southern Medical University. The requirement for individual informed consent was waived by the committees.
QZ: Data curation, Formal analysis, Investigation, Methodology, Writing – original draft. LW: Data curation, Writing – review & editing. HF: Validation, Writing – original draft. YZ: Funding acquisition, Supervision, Writing – review & editing. QX: Funding acquisition, Supervision, Writing – review & editing, Conceptualization.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was funded by the National Natural Science Foundation of China (82102890), and Science and Technology Program of Guangzhou (202201010949), and Natural Science Foundation of Guangdong Province (2023A1515030044).
The authors acknowledge the assistance of their colleagues at the Nanfang Hospital, Southern Medical University.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2024.1467429/full#supplementary-material
HCC, Hepatocellular carcinoma; TKI, tyrosine kinase inhibitor; KM, Kaplan–Meier; OS, overall survival; DFS, disease free survival; CI, confidence interval; HBV, hepatitis B virus.
1. Llovet JM, Kelley RK, Villanueva A, Singal AG, Pikarsky E, Roayaie S, et al. Hepatocellular carcinoma. Nat Rev Dis Primers. (2021) 7(1):6. doi: 10.1038/s41572-020-00240-3
2. Zhu AX, Finn RS, Edeline J, Cattan S, Ogasawara S, Palmer D, et al. Pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib (KEYNOTE-224): a non-randomised, open-label phase 2 trial. Lancet Oncol. (2018) 19(7):940–52. doi: 10.1016/s1470-2045(18)30351-6
3. El-Khoueiry AB, Sangro B, Yau T, Crocenzi TS, Kudo M, Hsu C, et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet. (2017) 389(10088):2492–502. doi: 10.1016/s0140-6736(17)31046-2
4. Fukumura D, Kloepper J, Amoozgar Z, Duda DG, Jain RK. Enhancing cancer immunotherapy using antiangiogenics: opportunities and challenges. Nat Rev Clin Oncol. (2018) 15(5):325–40. doi: 10.1038/nrclinonc.2018.29
5. Mattei F, Kato Y, Tabata K, Kimura T, Yachie-Kinoshita A, Ozawa Y, et al. Lenvatinib plus anti-PD-1 antibody combination treatment activates CD8+ T cells through reduction of tumor-associated macrophage and activation of the interferon pathway. PloS One. (2019) 14(2):e0212513. doi: 10.1371/journal.pone.0212513
6. Finn RS, Qin S, Ikeda M, Galle PR, Ducreux M, Kim T-Y, et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. New Engl J Med. (2020) 382(20):1894–905. doi: 10.1056/NEJMoa1915745
7. Llovet JM, Kudo M, Merle P, Meyer T, Qin S, Ikeda M, et al. Lenvatinib plus pembrolizumab versus lenvatinib plus placebo for advanced hepatocellular carcinoma (LEAP-002): a randomised, double-blind, phase 3 trial. Lancet Oncol. (2023) 24(12):1399–410. doi: 10.1016/s1470-2045(23)00469-2
8. Finn RS, Ikeda M, Zhu AX, Sung MW, Baron AD, Kudo M, et al. Phase Ib study of lenvatinib plus pembrolizumab in patients with unresectable hepatocellular carcinoma. J Clin Oncol. (2020) 38:2960–70. doi: 10.1200/JCO.20.00808
9. Ren Z, Xu J, Bai Y, Xu A, Cang S, Du C, et al. Sintilimab plus a bevacizumab biosimilar (IBI305) versus sorafenib in unresectable hepatocellular carcinoma (ORIENT-32): a randomised, open-label, phase 2–3 study. Lancet Oncol. (2021) 22(7):977–90. doi: 10.1016/s1470-2045(21)00252-7
10. Chen X, Liu C, Zhang A, Wu W, Liu L, Lan Y, et al. Low absolute neutrophil count during induction therapy is an adverse prognostic factor in childhood acute lymphoblastic leukaemia. Ann Hematol. (2021) 100(9):2269–77. doi: 10.1007/s00277-021-04412-3
11. Ho WJ, Yarchoan M, Hopkins A, Mehra R, Grossman S, Kang H. Association between pretreatment lymphocyte count and response to PD1 inhibitors in head and neck squamous cell carcinomas. J ImmunoTherapy Cancer. (2018) 6(1):84. doi: 10.1186/s40425-018-0395-x
12. Ray-Coquard I, Cropet C, Van Glabbeke M, Sebban C, Le Cesne A, Judson I, et al. Lymphopenia as a prognostic factor for overall survival in advanced carcinomas, sarcomas, and lymphomas. Cancer Res. (2009) 69(13):5383–91. doi: 10.1158/0008-5472.CAN-08-3845
13. Maleki Vareki S, Garrigós C, Duran I. Biomarkers of response to PD-1/PD-L1 inhibition. Crit Rev Oncology/Hematology. (2017) 116:116–24. doi: 10.1016/j.critrevonc.2017.06.001
14. Voong KR, Feliciano J, Becker D, Levy B. Beyond PD-L1 testing-emerging biomarkers for immunotherapy in non-small cell lung cancer. Ann Trans Med. (2017) 5(18):376–76. doi: 10.21037/atm.2017.06.48
15. Yoshida H, Shimizu T, Yoshioka M, Matsushita A, Kawano Y, Ueda J, et al. The role of the spleen in portal hypertension. J Nippon Med Sch. (2023) 90(1):20–5. doi: 10.1272/jnms.JNMS.2023_90-104
Keywords: hepatocellular carcinoma, TKI, PD1, peripheral blood lymphocyte count, combination therapy
Citation: Zhao Q, Wang L, Fu H, Zhang Y and Xie Q (2024) Effect of peripheral blood lymphocyte count on the efficacy of immunotherapy combined with TKI in the treatment of advanced liver cancer. Front. Immunol. 15:1467429. doi: 10.3389/fimmu.2024.1467429
Received: 19 July 2024; Accepted: 20 September 2024;
Published: 24 October 2024.
Edited by:
Subhash Kumar Tripathi, Seattle Children’s Research Institute, United StatesReviewed by:
Arpit Mishra, Benaroya Research Institute, United StatesCopyright © 2024 Zhao, Wang, Fu, Zhang and Xie. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yuqin Zhang, NzkxMzY5NjMxQHFxLmNvbQ==; Qiankun Xie, eGllcWswNDA2QDEyNi5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.